Abstract
Objective
This study was performed to investigate the impact of HAART versus no HAART and nucleoside free versus nucleoside containing HAART on the efficacy and safety of pegylated interferon and ribavirin therapy for the treatment of chronic HCV infection in HIV/HCV co-infected patients. In addition a control group of HCV mono-infected patients undergoing anti-HCV therapy was evaluated.
Methods
Multicenter, partially randomized, controlled clinical trial. HIV-negative and -positive patients with chronic HCV infection were treated with pegylated interferon alfa-2a and ribavirin (800 - 1200 mg/day) for 24 - 48 weeks in one of four treatment arms: HIV-negative (A), HIV-positive without HAART (B) and HIV-positive on HAART (C). Patients within arm C were randomized to receive open label either a nucleoside containing (C1) or a nucleoside free HAART (C2).
Results
168 patients were available for analysis. By intent-to-treat analysis similar sustained virological response rates (SVR, negative HCV-RNA 24 weeks after the end of therapy) were observed comparing HIV-negative and -positive patients (54% vs. 54%, p = 1.000). Among HIV-positive patients SVR rates were similar between patients off and on HAART (57% vs. 52%, p = 0.708). Higher SVR rates were observed in patients on a nucleoside free HAART compared to patients on a nucleoside containing HAART, though confounding could not be ruled out and in the intent-to-treat analysis the difference was not statistically significant (64% vs. 46%, p = 0.209).
Conclusions
Similar response rates for HCV therapy can be achieved in HIV-positive and -negative patients. Patients on nucleoside free HAART reached at least equal rates of sustained virological response compared to patients on standard HAART.
Keywords: HIV, HCV, interferon, nucleoside, HAART
Introduction
In Europe up to 33% of the HIV-positive patients are co-infected with hepatitis C virus (HCV) [1,2]. In HCV/HIV co-infected patients liver-related disease has emerged as a leading cause of morbidity and mortality [3]. The progression of chronic HCV infection to liver cirrhosis, liver failure and development of hepatocellular carcinoma is substantially accelerated in HIV/HCV co-infected compared to HCV mono-infected individuals [4]. Successful treatment of chronic hepatitis C infection with pegylated interferon and ribavirin combination therapy has been shown to stop progression of fibrosis and prevent liver related disease and death [5], but treatment of HCV in HIV coinfected individuals is complicated by additive drug toxicities of ribavirin and the nucleoside reverse transcriptase inhibitors didanosine [6,7], zidovudine [8-11] and stavudine [12]. Furthermore, competitive intracellular phosphorylation of abacavir and ribavirin has recently been hypothesized to further compromise the efficacy of HCV treatment in the coinfected host [13-15], although this is absent using weight based doses of ribavirin [14-16].
In the current study we aimed to investigate the impact of HIV-1 infection, nucleoside containing and nucleoside free HAART on the efficacy and safety of pegylated interferon and ribavirin combination therapy for the treatment of chronic HCV infection.
Methods
Study design
Fifty HIV-seronegative and 118 HIV-seropositive patients with chronic HCV infection were enrolled into this multicenter, prospective, partially randomized, controlled trial (Figure 1). HIV-positive patients with a CD4 cell-count > 300/μl, an HIV-RNA < 40.000 copies/ml and no indication for HAART received anti-HCV therapy without concomitant HAART. HIV-positive patients that either required to be started on HAART or who were already receiving HAART were randomized prior to commencing anti-HCV therapy into one of two groups: nucleoside containing HAART (C1) or nucleoside free HAART (C2). If randomization required a change in the HAART regimen or if a patient was newly commenced on HAART, a 12 week lead-in phase preceded the initiation of anti-HCV therapy to ensure stable HAART and to differentiate adverse events caused by HAART and anti-HCV therapy. Due to the risk of severe pancreatitis under concomitant ribavirin therapy, didanosine was not allowed as part of HAART. Practitioners were free to construct a nucleoside-free HAART based on the patient's genotypic resistance assay and HAART history. A boosted double protease inhibitor or non-nucleoside reverse transcriptase inhibitor plus boosted protease inhibitor were recommended as options for a nucleoside-free HAART. The study had been reviewed by the ethics committee of Bonn University and was conducted in agreement with good clinical practice and the declaration of Helsinki and its subsequent revisions.
Figure 1.
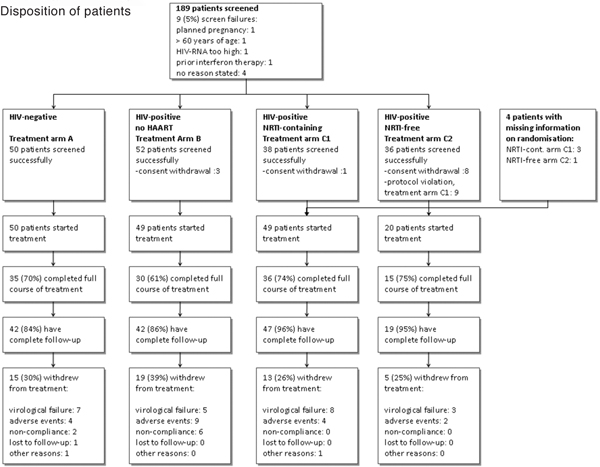
Allocation to treatment and follow-up. Data shown as numbers (%) of patients.
Definitions and major inclusion/exclusion criteria
Chronic HCV infection was defined as 2 positive HCV-RNA tests at least 6 month apart. In order to be eligible for enrollment, patients had to be naïve to interferon-based anti-HCV therapy, with positive serum HCV-RNA and elevated alanine aminotransferase (ALT) at screening, to be between 18 and 60 years of age and given written informed consent. Women or men with female partners of child-bearing age not willing to use two contraceptive measures (one of these had to be a barrier method) and pregnant or breast-feeding women were excluded from participation. Patients with advanced liver cirrhosis (CHILD-Pugh score > 6) or signs of liver decompensation were not allowed to participate in this trial. Hepatitis B virus co-infection, chronic alcohol or illicit drug abuse, hemochromatosis, other acute or chronic liver disease and contraindications for pegylated interferon/ribavirin combination therapy were further exclusion criteria.
Anti-HCV therapy
All patients were treated with pegylated interferon alfa-2a 180 μg given once weekly subcutaneously. Ribavirin was dosed 800 mg/day for genotype 2 and 3 infections and weight adapted (< 75 kg bodyweight 1000 mg/day, ≥ 75 kg 1200 mg/day) for genotype 1 and 4 infections, divided in two doses per os. HIV-positive patients on HAART received 800 mg ribavirin/day regardless of HCV-genotype. Duration of therapy was 48 weeks for genotype 1 and 4 infections and 24 weeks for genotype 2 and 3 infections. The study protocol was amended twice in order to account for recent results of APRICOT [17], ACTG A5071 [18] and the Ribavic [19] trial, leading to updated treatment recommendations for HIV/HCV co-infection [20]. Amendment I (March 2004) prolonged duration of therapy for HIV/HCV co-infected patients with genotype 2 or 3 infections to 48 weeks and amendment II (October 2004) prescribed weight-based ribavirin for HIV-infected patients with genotype 1 and 4 infections and issued a warning on the concomitant use of zidovudine and ribavirin and associated higher rates of anemia.
Virological response
Response to treatment was defined as early virological response (EVR, decay of HCV-RNA of at least 2 log10 at week 12 of treatment), end of treatment response (ETR, negative HCV-RNA at the end of treatment) and sustained virological response (SVR, negative HCV-RNA 24 weeks after the end of therapy). Levels of HCV-RNA were measured locally with approved commercial assays, i.e. Versant HCV bDNA V3.0 (Versant, Bayer Diagnostics, Tarrytown, NY, USA), COBAS Amplicor HCV Monitor V2.0 (Roche Diagnostics, Indianapolis, IN, USA), or Abbott RealTime Assay (Abbott Molecular Inc., Des Plaines, IL, USA). A common lower cut-off of 600 IU/ml HCV-RNA was set for analysis and a negative HCV-RNA was defined as any value < 600 IU/ml. HCV genotypes were determined locally with the approved and commercially available INNO-LiPA HCV II (Innogenetics, Gent, Belgium).
Statistics
The recruitment goal for the study was 200 patients in 4 study arms (50 patients per arm). Primary endpoint of the study was the difference of the rate of SVR within study arms C1 and C2 by intent-to-treat analysis. The study was powered to detect a 25% difference in the rate of SVR with a p < 0.05 and power of 80%. Analysis was performed intent-to-treat or astreated, classifying missing information as equal to failure. Non-parametric tests were used for statistical comparison, multivariate regression analysis was performed using a logistic binary regression with a conditional forward model. A two-sided p-value < 0.05 was considered statistically significant.
Results
Demographic characteristics of patients
Overall 189 patients were screened. Twenty-one subjects did not meet inclusion/exclusion criteria or withdrew consent prior start of anti-HCV therapy leaving 168 patients for final analysis (Figure 1). Despite a 1:1 randomization within treatment arm C, an uneven proportion of patients received at least one dose of study medication, i.e. 49 patients within the treatment arm C1 and 20 patients within treatment arm C2 started treatment. This was mainly due to unwillingness of patients randomized to a nucleoside free HAART to change to or start a nucleoside free HAART (withdrawal of consent, n = 8) or protocol violation and proceeding with a nucleoside containing HAART despite being randomized to a nucleoside free HAART (n = 9). Comparing HIV-negative with HIV-positive patients at baseline age, serum levels of alanine aminotransferase (ALT) and HCV-RNA and HCV genotype distribution were similar between HIV-positive and HIV-negative patients (Table 1).
Table 1.
Baseline characteristics of patients according to treatment arm
| HIV-negative | HIV-positive | |||
|---|---|---|---|---|
| Arm A | No HAART Arm B |
NRTI-containing Arm C1 |
NRTI-free Arm C2 |
|
| n = 50 | n = 49 | n = 49 | n = 20 | |
| Age [years]** | 41 (25 - 55) | 39 (24 - 47) | 42 (30 - 47) | 43 (34 - 61) |
| Male sex* [%] | 60 | 74 | 76 | 85 |
| Transmission risk [%] | ||||
| IVDU | 40 | 33 | 25 | 5 |
| Blood products | 10 | 2 | 8 | - |
| Sexual | 8 | 2 | 10 | 10 |
| other | 8 | 2 | - | - |
| unknown/missing | 34 | 61 | 57 | 85 |
| HCV-genotype [%] | ||||
| 1 | 52 | 51 | 51 | 50 |
| 2 | 6 | 8 | 4 | 5 |
| 3 | 38 | 27 | 31 | 40 |
| 4 | - | 8 | 10 | 5 |
| other | 4 | 4 | 2 | - |
| untypable | - | 2 | 2 | - |
| HCV-RNA [IU/ml, log10] | 5.7 (4.5 - 6.8) | 5.7 (4.1 - 6.7) | 5.5 (4.4 - 6.8) | 5.9 (4.3 - 7.3) |
| ≥ 500 000 IU/ml [%] | 56 | 57 | 41 | 65 |
| ALT [IU/l] | 61 (21 - 179) | 68 (17 - 212) | 71 (30 - 221) | 75 (26 - 231) |
| HAART [%] | ||||
| PI/NNRTI/3 × NUC | - | - | 57/29/12 | 95/5/0 |
| AZT | 33 | - | ||
| d4T | 18 | - | ||
| ABC | 31 | - | ||
| 3TC/FTC | 98 | - | ||
| TDF | 47 | - | ||
| HIV-RNA [copies/ml, log10]** | - | 3.9 (1.9 - 4.9) | 1.7 (1.7 - 3.5) | 1.7 (1.7 - 4.5) |
| CD4-cellcount | ||||
| [/μl]** | - | 580 (301 - 1042) | 491 (222 - 781) | 390 (152 - 846) |
| [%] | - | 27 (15 - 41) | 27 (13 - 40) | 22 (16 - 41) |
* Statistically significant difference comparing arm A with arms B and C (HIV-positive vs. HIV-negative)
** Statistically significant difference comparing arm B versus C1 versus C2
Data shown as percent of patients or median (95% range). NRTI nucleos(t)ide reverse transcriptase inhibitor, IVDU intravenous drug abuse, other double infections, e.g. genotype 1 and 2 infection; PI protease inhibitor containing HAART, NNRTI non-nucleoside reverse transcriptase inhibitor containing HAART, 3xNUC HAART based on at least 3 nucleos(t)ides, AZT zidovudine, d4T stavudine, ABC abacavir, TDF tenofovir DF
Comparing HIV-positive patients across treatment arms as treated, patients without HAART were younger than patients on HAART (39 vs. 42 vs. 43 years, p = 0.006) and had higher absolute CD4-cellcounts (580/μl vs. 491/μl vs. 390/μl, p = 0.008), in part reflecting the natural course of HIV-infection (Table 1). Level of ALT and HCV-RNA and HCV genotype distribution did not vary significantly across HIV-positive treatment groups. Patients in the nucleos(t)ide reverse transcriptase inhibitor (NRTI) free arm were mostly on double protease inhibitor HAART (n = 17), two patients received lopinavir/ritonavir monotherapy and one patient was on lopinavir/ritonavir and efavirenz combination therapy. Patients in the NRTI-containing arm received a protease inhibitor based HAART in 57% of cases; NRTIs most frequently used were lamivudine or emtricitabine (98%), followed by tenofovir (47%), zidovudine (33%) or abacavir (31%).
Treatment characteristics
Patients were recruited in Germany from 13 tertiary care centers and 14 private practices. The recruitment period lasted 5 years with the first patient starting treatment in August 2002 and the last patient in November 2007. Recruitment of patients was slow for the treatment arm C and accordingly there was a significant difference in the proportion of patients recruited within the first half of the recruitment period (2002 - 2004) compared to the second half (2005 - 2007). HIV-negative patients were all recruited within the first period, whereas 38% of HIV-positive patients were recruited within the second period (p < 0.001, Table 2). Recruitment times varied also across HIV-positive treatment arms with arm B recruiting fastest (80% of patients within first period vs. arm C1 55% vs. arm C2 30%, p < 0.001). Due to differences in recruitment times we assessed whether there was any difference in the treatment according to the protocol amendments I and II. As per protocol HIV-negative patients were treated different than HIV-positive patients and accordingly all except one HIV-negative patients with HCV genotype 1/4 infection received weight based ribavirin and all patients with HCV genotype 2/3 infections were treated for 24 weeks only. This was significantly different from HIV-positive patients (Table 2). Among HIV-positive patients, no marked differences with regard to the use of weight based ribavirin for the treatment of HCV genotype 1/4 infections or prolonged treatment of HCV genotype 2/3 infections were observed.
Table 2.
Anti-HCV Treatment characteristics of patients according to treatment arm
| HIV-negative | HIV-positive | |||
|---|---|---|---|---|
| No HAART | NRTI-containing | NRTI-free | ||
| Arm A | Arm B | Arm C1 | Arm C2 | |
| n = 50 | n = 49 | n = 49 | n = 20 | |
| Weight adapted RBV * (GT1/4 infections only) | 96 | 81 | 77 | 82 |
|
Treatment duration 48 weeks (GT 2/3 infections only) * |
0 | 35 | 35 | 67 |
| Enrollment period */** | ||||
| 2002 - 2004 | 100 | 80 | 55 | 30 |
| 2005 - 2007 | - | 20 | 43 | 70 |
* Statistically significant difference comparing arm A with arms B and C (HIV-positive vs. HIV-negative)
** Statistically significant difference comparing arm B versus C1 versus C2
Data shown as percent of patients
NRTI nucleos(t)ide reverse transcriptase inhibitor, GT HCV genotype, RBV ribavirin
Treatment modifications and adverse events
Adverse events were common among all study participants, however, only 19 patients discontinued anti-HCV therapy because of interferon or ribavirin associated adverse events (Table 3). HIV-negative patients suffered less often from moderate or severe leucopenia (WHO grade 3/4) compared to HIV-positive patients (0% vs. 9%, p = 0.034), however, they also more often received dose reductions of pegylated interferon or ribavirin (22% vs. 10%, p = 0.048 and 28% vs. 16%, trend p = 0.087). Among HIV-positive patients mild ALT elevations, anemia and leucopenia (all WHO grade 1/2) were more common among patients on HAART compared to patients without HAART (Table 3). However, dose reductions of pegylated interferon or ribavirin and premature treatment discontinuation due to adverse events were not different among HIV-positive patients.
Table 3.
Treatment modifications and selected adverse events under therapy
| HIV-negative | HIV-positive | |||
|---|---|---|---|---|
| No HAART | NRTI-containing | NRTI-free | ||
| Arm A | Arm B | Arm C1 | Arm C2 | |
| n = 50 | n = 49 | n = 49 | n = 20 | |
| Elevations serum ALT [%] | ||||
| Grade 1/2** | 64 | 61 | 78 | 90 |
| Grade 3/4 | - | 10 | 6 | 5 |
| Anemia [%] | ||||
| Grade 1/2** | 10 | 2 | 22 | 5 |
| Grade 3/4 | - | - | - | - |
| Maximum Hb loss [g/dl] | 3.1 (1.1 - 5.1) | 3.0 (0.6 - 5.3) | 2.9 (0.8 - 5.7) | 3.3 (0.8 - 4.4) |
| Leucopenia [%] | ||||
| Grade 1/2** | 40 | 25 | 69 | 55 |
| Grade 3/4* | 0 | 4 | 12 | 10 |
| Thrombocytopenia [%] | ||||
| Grade 1/2 | 44 | 35 | 51 | 55 |
| Grade 3/4 | 4 | - | 10 | 5 |
| Clinical adverse events [%] | ||||
| Grade 1/2 | 70 | 63 | 63 | 90 |
| Grade 3/4 | 12 | 18 | 8 | 5 |
| Dose reduction PegIFN [%]†* | 22 | 8 | 14 | 5 |
| % of total dose received | 84 | 87 | 81 | 88 |
| Dose reduction RBV [%]† | 28 | 8 | 25 | 15 |
| % of total dose received | 86 | 81 | 79 | 90 |
| Treatment discont. AEs [%] | 8 | 18 | 8 | 10 |
* Statistically significant difference comparing arm A with arms B and C (HIV-positive vs. HIV-negative)
** Statistically significant difference comparing arm B versus C1 versus C2
†Percent of patients who were dose reduced for pegylated interferon (PegIFN) or ribavirin (RBV) and respective percent of the cumulative dose received.
Data shown as percent of patients or median (95% range)
NRTI nucleos(t)ide reverse transcriptase inhibitor, ALT serum alanine aminotransferase, Treatment discont. AEs Treatment discontinuation due to adverse events.
Virological response
Overall an early virological response (at least a 2-log decay of HCV-RNA at week 12) was observed in 77% of patients. An end of treatment response (negative HCV-RNA at the end of treatment, ETR) was reached in 70% and was sustained in 54% of patients (SVR, sustained virological response, negative HCV-RNA 24 weeks after the end of treatment). Rates of SVR were comparable comparing HIV-negative and HIV-positive patients (54% vs. 54%, p = 1.000, Figure 2a). HIV-positive patients treatment response rates were higher in patients on NRTI-free HAART compared to those with NRTI-containing HAART, which was statistically significant with regard to SVR in the as-treated analysis (75% vs. 43%, p = 0.019, Figure 2a). Taking into account protocol violations and analyzing response rates according to intent-to-treat (analyzed as randomized, screen failures excluded, missing = failure) a 20% difference in the rate of response between nucleosidefree and nucleoside containing HAART was maintained, though the difference was no longer statistically significant (NRTI-free 62% vs. NRTI containing 44%, p = 0.209, Figure 2b).
Figure 2.
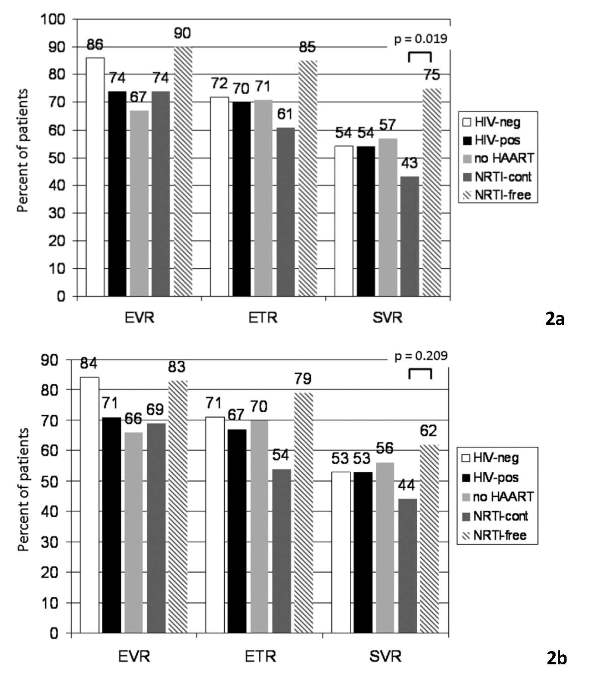
2a. Rates of virological response according to treatment group, as treated, missing = failure. EvR early virological response (at least 2 log decay of Hcv-RnA at week 12), ETR end of treatment response (negative Hcv-RnA at the end of treatment), SvR sustained virological response. 2b. Rates of virological response according to treatment group, intent-to-treat, missing = failure. EvR early virological response (at least 2 log decay of Hcv-RnA at week 12), ETR end of treatment response (negative Hcv-RnA at the end of treatment), SvR sustained virological response.
Potential confounders and post-hoc analysisof treatment Arm C1
Because treatment arms differed with regard to baseline characteristics we performed a separate analysis to investigate into the effect of potential confounders among HIV-positive patients. Patient characteristics found to be different at baseline across HIV-positive treatment arms and other known potential confounders were included in this uni- and multivariate analysis (Table 4). HCV-genotype 1 or 4, a high HCV viral load at baseline, taking an NRTI containing HAART or having to stop anti-HCV therapy prematurely due to adverse events were all associated with reduced odds of achieving SVR. Patients treated within treatment arm C1 who were on an NRTI containing regimen, had only 30% the odds of achieving SVR compared to the other patients receiving no or NRTI free HAART (95% CI 10% - 90%, p = 0.025).
Table 4.
Adjusting effects for possible confounders - SVR, HIV-positive patients
| SVR | No SVR | Uni-variate | Multi-variate | Multivariate | |
|---|---|---|---|---|---|
| n = 64 | n = 54 | p-value | p-value | Odds-ratio (95% CI) | |
| Age [years] | 40 (27 - 54) | 41 (27 - 48) | 0.566 | - | - |
| Male sex [%] | 75 | 78 | 0.662 | - | - |
| Transm. risk IVDU [%] | 20 | 30 | 0.709 | - | - |
| HCV GT 1 or 4 [%] | 50 | 76 | 0.002 | 0.001 | 0.2 (0.1 - 0.5) |
| HCVRNA ≥ 5 × 105 IU/ml[%] | 42 | 63 | 0.014 | 0.009 | 0.3 (0.1 - 0.7) |
| ALT [IU/l] | 63 (19 - 190) | 80 (25 - 250) | 0.368 | - | - |
| Weight adapted RBV [%] | 88 | 73 | 0.237 | - | - |
| Tx duration 48 wks [%] | 47 | 27 | 0.309 | - | - |
| Enrollment period [%] | |||||
| 2002 - 2004 | 56 | 67 | 0.253 | - | - |
| 2005 - 2007 | 44 | 32 | |||
| Treatment arm [%] | |||||
| B (no HAART) | 44 | 39 | |||
| C1 (NRTI-cont.) | 33 | 52 | 0.047 | 0.025 | 0.3 (0.1 - 0.9)* |
| C2 (NRTI free) | 23 | 9 | |||
| HIV-RNA [copies/ml, log10] | 1.7 (1.7 - 4.8) | 1.7 (1.7 - 4.8) | 0.372 | - | |
| CD4-cellcount | |||||
| [/μl] | 487 (236 - 1042) | 542 (222 - 831) | 0.828 | - | |
| [%] | 27 (15 - 41) | 26 (12 - 41) | 0.364 | - | |
| Anemia [%] | |||||
| Grade 1/2 | 11 | 11 | 1.000 | . | |
| Grade 3/4 | - | - | |||
| Leucopenia [%] | |||||
| Grade 1/2 | 47 | 41 | 0.578 | - | |
| Grade 3/4 | 9 | 7 | 0.753 | ||
| Thrombocytopenia [%] | |||||
| Grade 1/2 | 47 | 43 | 0.712 | - | |
| Grade 3/4 | 5 | 6 | 1.000 | ||
| Clinical adverse events [%] | |||||
| Grade 1/2 | 67 | 69 | 1.000 | - | |
| Grade 3/4 | 11 | 13 | 0.781 | ||
| Dose reduction PegIFN [%] | 11 | 9 | 1.000 | ||
| Dose reduction RBV [%] | 13 | 20 | 0.215 | - | |
| Treatm. discont. AEs [%] | 5 | 24 | 0.003 | 0.004 | 0.1 (0 - 0.5) |
Data shown as percent of patientsor median (95% range); *treatment arm C1 compared to B and C2 (reference) SVR sustained virological response, IVDU intravenous drug abuse, GT HCV genotype; ALT serum alanine aminotransferase, RBV ribavirin, Tx Treatment, wks weeks, NRTI nucleos(t)ide reverse transcriptase inhibitor, Treatment discont. AEs Treatment discontinuation due to adverse events.
In order to further understand the significantly reduced rate of SVR of patients within treatment arm C1 we analyzed this group of patients with regard to the outcome SVR (Table 5). Given the limitation of small groups, only HCV genotype 1 or 4 infection and the level of HCV-RNA were significantly associated with reaching SVR. While patients with HCV genotype 1 or 4 infection only reached an SVR in 29% of patients, an SVR was reached in 71% of patients with HCV genotype 2 or 3 infections (p = 0.007). Likewise, the level of HCV-RNA was significantly higher in patients without SVR (5.7 vs. 5.1 log, p = 0.001). However, some characteristics showed marked differences (20 or more percent difference) whilst not being statistically significant different. Among these were the use of weight adapted ribavirin in case of genotype 1 or 4 infections (100% vs. 68%), a longer duration of therapy in case of genotype 2 or 3 infection (42% vs. 20%) and the use of abacavir containing HAART (19% vs. 39%). Indeed applying post-hoc power analysis, the power to detect a significant difference with regard to the use of abacavir was only 24% and thus further studies particularly addressed to investigate into these issues are warranted.
Table 5.
Post-hoc analysis treatment arm C1 with regard to SVR
| SVR | No SVR | p-value | |
|---|---|---|---|
| n = 21 | n = 28 | ||
| Age [years] | 41 (31 - 47) | 42 (27 - 47) | 0.732 |
| Male sex [%] | 81 | 71 | 0.733 |
| Transmission risk IVDU [%] | 19 | 29 | 0.844 |
| HCV genotype 1 or 4 [%] | 43 | 79 | 0.007 |
| HCV-RNA [IU/ml, log10] | |||
| HCV-RNA ≥ 500 000 IU/ml [%] | 5.1 (4 - 6) | 5.7 (4.7 - 7.2) | 0.001 |
| 24 | 54 | 0.019 | |
| ALT [IU/l] | 72 (31 - 206) | 71 (27 - 243) | 0.949 |
| Weight adapted RBV, GT 1/4 infections only [%] | 100 | 68 | 0.141 |
| Tx duration 48 wks, GT 2/3 infections only [%] | 42 | 20 | 0.600 |
| Enrollment period 2002 - 2004 [%] | 52 | 57 | 0.771 |
| HAART [%] | |||
| 3 × NRTI | 10 | 14 | 0.688 |
| AZT | 24 | 39 | 0.359 |
| d4T | 19 | 18 | 1.000 |
| ABC | 19 | 39 | 0.210 |
| TDF | 52 | 43 | 0.572 |
| HIV-RNA > Lod [%] | 4 | 14 | 0.369 |
| CD4-cellcount [/μl] | 470 (268 - 685) | 524 (222 - 781) | 0.521 |
| Elevations serum ALT [%] | |||
| Grade 1/2 | 76 | 79 | 1.000 |
| Grade 3/4 | - | 11 | 0.250 |
| Anemia [%] | |||
| Grade 1/2 | 24 | 21 | 1.000 |
| Grade 3/4 | - | - | - |
| Leucopenia [%] | |||
| Grade 1/2 | 57 | 61 | 1.000 |
| Grade 3/4 | 14 | 11 | 1.000 |
| Thrombocytopenia [%] | |||
| Grade 1/2 | 57 | 46 | 0.567 |
| Grade 3/4 | 9 | 11 | 1.000 |
| Clinical adverse events [%] | |||
| Grade 1/2 | 67 | 61 | 0.769 |
| Grade 3/4 | 10 | 7 | 1.000 |
| Dose reduction PegIFN [%]† | 10 | 18 | 0.436 |
| % of total dose received | 87 | 81 | |
| Dose reduction RBV [%]† | 14 | 32 | 0.179 |
| % of total dose received | 91 | 78 | |
| Treatment discont. AEs [%] | - | 14 | 0.125 |
†Percent of patients who were dose reduced for pegylated interferon (PegIFN) or ribavirin (RBV) and respective percent of the cumulative dose received.
Data shown as percent of patients (95% Confidence Interval) or median (95% range). SVR sustained virological response, IVDU intravenous drug abuse, ALT serum alanine aminotransferase, RBV ribavirin, GT HCV genotype, Tx Treatment, wks weeks, NRTI nucleos(t)ide reverse transcriptase inhibitor, AZT zidovudine, d4T stavudine, ABC abacavir, TDF tenofovir DF, Lod Level of detection, Treatment discont. AEs Treatment discontinuation due to adverse events.
Discussion
In this large, prospective and partially randomized study we were able to show, for the first time in a head-to-head comparison, that anti-HCV therapy may be applied to HIV-positive patients with the same efficacy as to HIV-negative patients. The SVR rate of 54%, as observed among HIV-negative patients in our trial, is well within the range reported from the registrational trials of pegylated interferon alfa-2a and -2b, who reported SVR-rates of 54% [21] and 56% [22], when given in combination with ribavirin.
The overall rate of SVR reached among HIV-positive patients within our study is one of the highest reported in HIV-positive patients [17-19,23-28]. In part, the high response rates observed can be accounted for by the introduction of weight based ribavirin for the treatment of genotype 1 and 4 infections and prolonged treatment of genotype 2 and 3 infections for 48 weeks, which is in contrast to earlier studies and observational cohorts [17-19,23-25]. Indeed, when comparing our results with more recent trials and cohorts who have used weight-based ribavirin and longer treatment schedules [26-28] our results are within the range reported by others (54% vs. 44 - 50%).
The study was designed to examine different treatment settings of HIV infection and their impact on the treatment efficacy and safety of pegylated interferon and ribavirin therapy. Our results show that patients not receiving HAART achieved similar response rates compared to patients currently treated with HAART (57% vs. 52%) while suffering less often from anemia and leucopenia. Patients on HAART showed marked differences in rates of response. Patients on NRTI-free HAART achieved significantly more often SVR compared to patients on NRTI-containing regimens (75% vs. 43%, p = 0.019). Even though the difference in SVR is impressive caveats on this finding must be issued, particularly in the nucleoside free arm as those patients achieved even higher SVR rates than HIV-negative patients. Despite a 1:1 randomization to the HAART treatment arm, there was an uneven distribution of patients who actually started on a nucleoside free and nucleoside containing HAART. Reason for this was mainly withdrawal of consent of patients randomized to the nucleoside free treatment arm and protocol violation of patients who were treated with nucleoside containing HAART despite being randomized to NRTI free HAART. This may have introduced a bias leaving only highly motivated patients in this treatment arm. Despite taking potential confounders into account we could not adjust for adherence as we did not capture this data and therefore our findings to this regard must be considered with caution. However, even taking the nine patients with protocol violation into account, in the intent-to-treat analysis there was still an almost 20% difference in the rate of SVR between patients on NRTI containing and NRTI free HAART. In addition, it is quite suggestive that a nucleoside free HAART may be of potential benefit, as higher rates of toxicity have been reported in patients receiving didanosine, zidovudine or stavudine and SVR rates were compromised by concomitant therapy with abacavir. Recently Pineda et al. investigated into the impact of different HAART regimens on the rate of SVR in a large observational retrospective cohort. In this study an NNRTI or PI based HAART with tenofovir or stavudine plus lamivudine as nucleoside backbone showed significantly higher response rates (44% vs. 29%) compared to other nucleoside combinations [29]. These observations are in line with our study, though the group of patients on a nucleoside containing HAART was too small to detect a potential benefit of a tenofovir containing nucleoside backbone as part of HAART. In summary, our study revealed that a nucleoside-free HAART is feasible in the context of anti-HCV therapy and that it was at the least not disadvantageous for our patients. On the contrary, nucleoside-free HAART may offer great improvements of treatment response rates, which should be further studied in future treatment trials.
Conclusion
With modern pegylated interferon and ribavirin therapy similar response rates may be achieved among HIV-negative and HIV-positive patients. While pegylated interferon and ribavirin therapy without HAART led to fewer hematologic adverse events, rates of sustained virological response were similar between HIV-positive patients on HAART and off HAART. Avoiding nucleosides as part of HAART for the duration of anti-HCV therapy may offer great opportunities to further enhance treatment response rates and should be explored in more detail in future studies.
References
- Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, Chaplinskas S, Peters L, Karlsson A, Katlama C, Toro C, Kupfer B, Vogel M, Lundgren J. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–1344. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]
- Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, Kirk O, Phillips A, Ledergerber B, Lundgren J. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- Rosenthal E, Pialoux G, Bernard N, Pradier C, Rey D, Bentata M, Michelet C, Pol S, Perronne C, Cacoub P. Liver-related mortality in human-immunodeficiencyvirus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 Study) J Viral Hepat. 2007;14:183–188. doi: 10.1111/j.1365-2893.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- Martin-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, Gonzalez A, Rockstroh J, Asensi V, Miralles P, Laguno M, Moreno L, Giron JA, Vogel M, Garcia-Samaniego J, Nunez M, Romero M, Moreno S, de la Cruz JJ, Soriano V. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis. 2004;38:128–133. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellon JM, Gonzalez-Garcia J. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- Bani-Sadr F, Carrat F, Pol S, Hor R, Rosenthal E, Goujard C, Morand P, Lunel-Fabiani F, Salmon-Ceron D, Piroth L, Pialoux G, Bentata M, Cacoub P, Perronne C. Risk factors for symptomatic mitochondrial toxicity in HIV/hepatitis C virus-coinfected patients during interferon plus ribavirin-based therapy. J Acquir Immune Defic Syndr. 2005;40:47–52. doi: 10.1097/01.qai.0000174649.51084.46. [DOI] [PubMed] [Google Scholar]
- Laguno M, Milinkovic A, de Lazzari E, Murillas J, Martinez E, Blanco JL, Lonca M, Biglia A, Leon A, Garcia M, Larrousse M, Garcia F, Miro JM, Gatell JM, Mallolas J. Incidence and risk factors for mitochondrial toxicity in treated HIV/HCV-coinfected patients. Antivir Ther. 2005;10:423–429. [PubMed] [Google Scholar]
- Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat. 2006;13:683–689. doi: 10.1111/j.1365-2893.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Bani-Sadr F, Goderel I, Penalba C, Billaud E, Doll J, Welker Y, Cacoub P, Pol S, Perronne C, Carrat F. Risk factors for anaemia in human immunodeficiency virus/hepatitis C virus-coinfected patients treated with interferon plus ribavirin. J Viral Hepat. 2007;14:639–644. doi: 10.1111/j.1365-2893.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- Nunez M, Ocampo A, Aguirrebengoa K, Cervantes M, Pascual A, Echeverria S, Asensi V, Barreiro P, Garcia-Samaniego J, Soriano V. Incidence of anaemia and impact on sustained virological response in HIV/HCVcoinfected patients treated with pegylated interferon plus ribavirin. J Viral Hepat. 2008;15:363–369. doi: 10.1111/j.1365-2893.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- Mira JA, Lopez-Cortes LF, Merino D, Arizcorreta-Yarza A, Rivero A, Collado A, Rios-Villegas MJ, Gonzalez-Serrano M, Torres-Tortoso M, Macias J, Valera-Bestard B, Fernandez-Fuertes E, Giron-Gonzalez JA, Lozano F, Pineda JA. Predictors of severe haematological toxicity secondary to pegylated interferon plus ribavirin treatment in HIV-HCV-coinfected patients. Antivir Ther. 2007;12:1225–1235. [PubMed] [Google Scholar]
- Bani-Sadr F, Lapidus N, Melchior JC, Ravaux I, Bensalem M, Rosa I, Cacoub P, Pol S, Perronne C, Carrat F. Severe weight loss in HIV/HCV-coinfected patients treated with interferon plus ribavirin: incidence and risk factors. J Viral Hepat. 2008;15:255–260. doi: 10.1111/j.1365-2893.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- Bani-Sadr F, Denoeud L, Morand P, Lunel-Fabiani F, Pol S, Cacoub P, Perronne C, Carrat F. Early virologic failure in HIV-coinfected hepatitis C patients treated with the peginterferon-ribavirin combination: does abacavir play a role? J Acquir Immune Defic Syndr. 2007;45:123–125. doi: 10.1097/QAI.0b013e318040b2b6. [DOI] [PubMed] [Google Scholar]
- Mira JA, Lopez-Cortes LF, Barreiro P, Tural C, Torres-Tortosa M, de Los Santos Gil I, Martin-Rico P, Rios-Villegas MJ, Hernandez-Burruezo JJ, Merino D, Lopez-Ruz MA, Rivero A, Munoz L, Gonzalez-Serrano M, Collado A, Macias J, Viciana P, Soriano V, Pineda JA. Efficacy of pegylated interferon plus ribavirin treatment in HIV/hepatitis C virus co-infected patients receiving abacavir plus lamivudine or tenofovir plus either lamivudine or emtricitabine as nucleoside analogue backbone. J Antimicrob Chemother. 2008;62:1365–1373. doi: 10.1093/jac/dkn420. [DOI] [PubMed] [Google Scholar]
- Vispo E, Barreiro P, Pineda JA, Mira JA, Maida I, Martin-Carbonero L, Rodriguez-Novoa S, Santos I, Lopez-Cortes LF, Merino D, Rivero A, Soriano V. Low response to pegylated interferon plus ribavirin in HIV-infected patients with chronic hepatitis C treated with abacavir. Antivir Ther. 2008;13:429–437. [PubMed] [Google Scholar]
- Laufer N, Laguno M, Perez I, Cifuentes C, Murillas J, Vidal F, Bonet L, Veloso S, Gatell JM, Mallolas J. Abacavir does not influence the rate of virological response in HIV-HCV-coinfected patients treated with pegylated interferon and weight-adjusted ribavirin. Antivir Ther. 2008;13:953–957. [PMC free article] [PubMed] [Google Scholar]
- Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J, Sette H Jr, Passe S, De Pamphilis J, Duff F, Schrenk UM, Dieterich DT. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B, Colquhoun D, Nevin T, Harb G, van der Horst C. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, Morand P, Goujard C, Pialoux G, Piroth L, Salmon-Ceron D, Degott C, Cacoub P, Perronne C. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Alberti A, Clumeck N, Collins S, Gerlich W, Lundgren J, Palu G, Reiss P, Thiebaut R, Weiland O, Yazdanpanah Y, Zeuzem S. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005;42:615–624. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Berenguer J, Gonzalez-Garcia J, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, Hernando A, Sanz J, Tural C, Ortega E, Mallolas J, Santos I, Miralles P, Montes ML, Bellon JM, Esteban H. Pegylated interferon {alpha}2a plus ribavirin versus pegylated interferon {alpha}2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. J Antimicrob Chemother. 2009;63:1256–1263. doi: 10.1093/jac/dkp106. [DOI] [PubMed] [Google Scholar]
- Mira JA, Gutierrez-Valencia A, Gil Ide L, Merino D, Rivero A, Rios-Villegas MJ, Delgado M, Gonzalez-Serrano M, Collado A, Torres-Tortosa M, Omar M, Lopez-Ruz MA, Macias J, Arponen S, Pineda JA. Efficacy and safety of pegylated interferon plus ribavirin in HIV and hepatitis C virus-coinfected patients with advanced immunosuppression. Clin Infect Dis. 2009;49:e84–91. doi: 10.1086/605677. [DOI] [PubMed] [Google Scholar]
- Voigt E, Schulz C, Klausen G, Goelz J, Mauss S, Schmutz G, Jessen H, Weitner L, Mutz A, Schranz D, Rockstroh JK, Kaad Study G. Pegylated interferon alpha-2b plus ribavirin for the treatment of chronic hepatitis C in HIV-coinfected patients. J Infect. 2006;53:36–42. doi: 10.1016/j.jinf.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Laguno M, Cifuentes C, Murillas J, Veloso S, Larrousse M, Payeras A, Bonet L, Vidal F, Milinkovic A, Bassa A, Villalonga C, Perez I, Tural C, Martinez-Rebollar M, Calvo M, Blanco JL, Martinez E, Sanchez-Tapias JM, Gatell JM, Mallolas J. Randomized trial comparing pegylated interferon alpha-2b versus pegylated interferon alpha-2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology. 2009;49:22–31. doi: 10.1002/hep.22598. [DOI] [PubMed] [Google Scholar]
- Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez-Tapias JM, Bargallo X, Garcia-Criado A, de Lazzari E, Larrousse M, Leon A, Lonca M, Milinkovic A, Gatell JM, Mallolas J. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- Nunez M, Miralles C, Berdun MA, Losada E, Aguirrebengoa K, Ocampo A, Arazo P, Cervantes M, de Los Santos I, San Joaquin I, Echeverria S, Galindo MJ, Asensi V, Barreiro P, Sola J, Hernandez-Burruezo JJ, Guardiola JM, Romero M, Garcia-Samaniego J, Soriano V. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23:972–982. doi: 10.1089/aid.2007.0011. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Mira JA, Gil Ide L, Valera-Bestard B, Rivero A, Merino D, Giron-Gonzalez JA, Rios-Villegas MJ, Gonzalez-Serrano M, Collado A, Garcia-Garcia JA, Carrillo-Gomez R, Lopez-Cortes LF, Gomez-Mateos J. Influence of concomitant antiretroviral therapy on the rate of sustained virological response to pegylated interferon plus ribavirin in hepatitis C virus/HIV-coinfected patients. J Antimicrob Chemother. 2007;60:1347–1354. doi: 10.1093/jac/dkm373. [DOI] [PubMed] [Google Scholar]


