Abstract
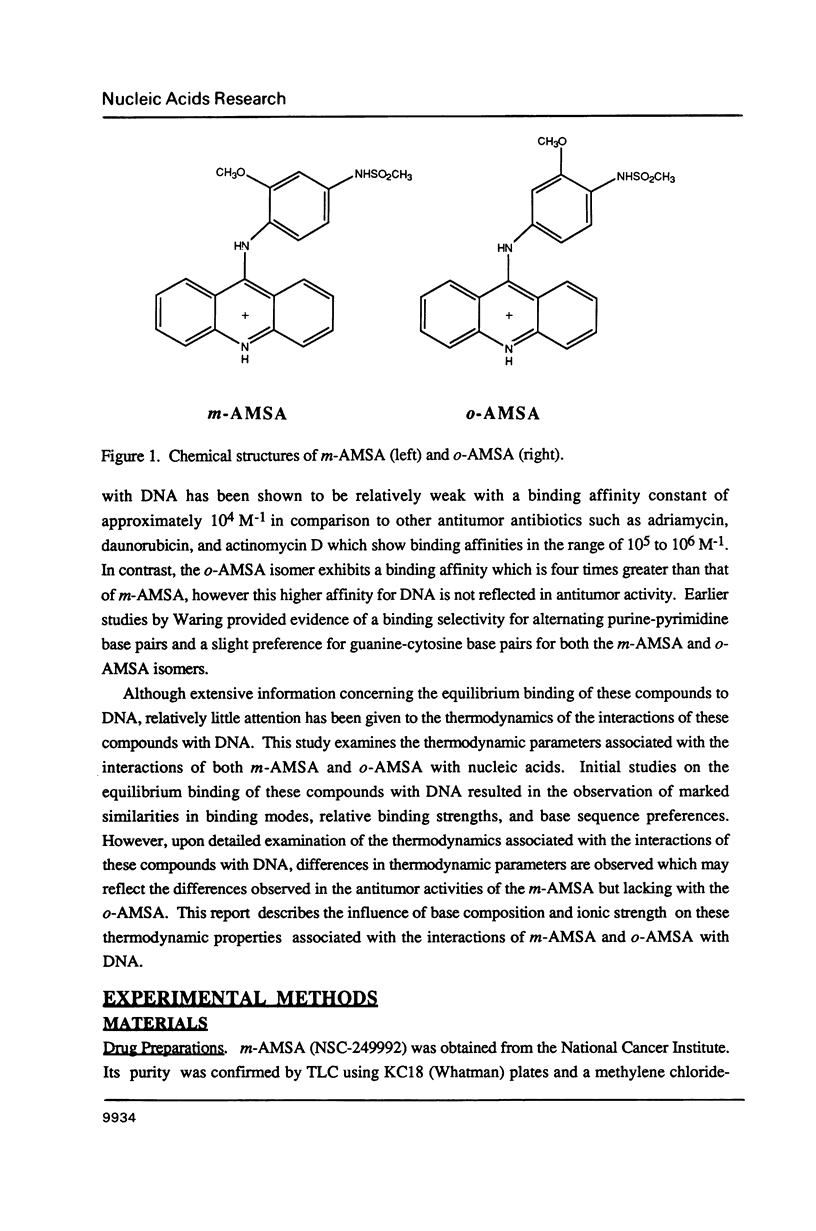
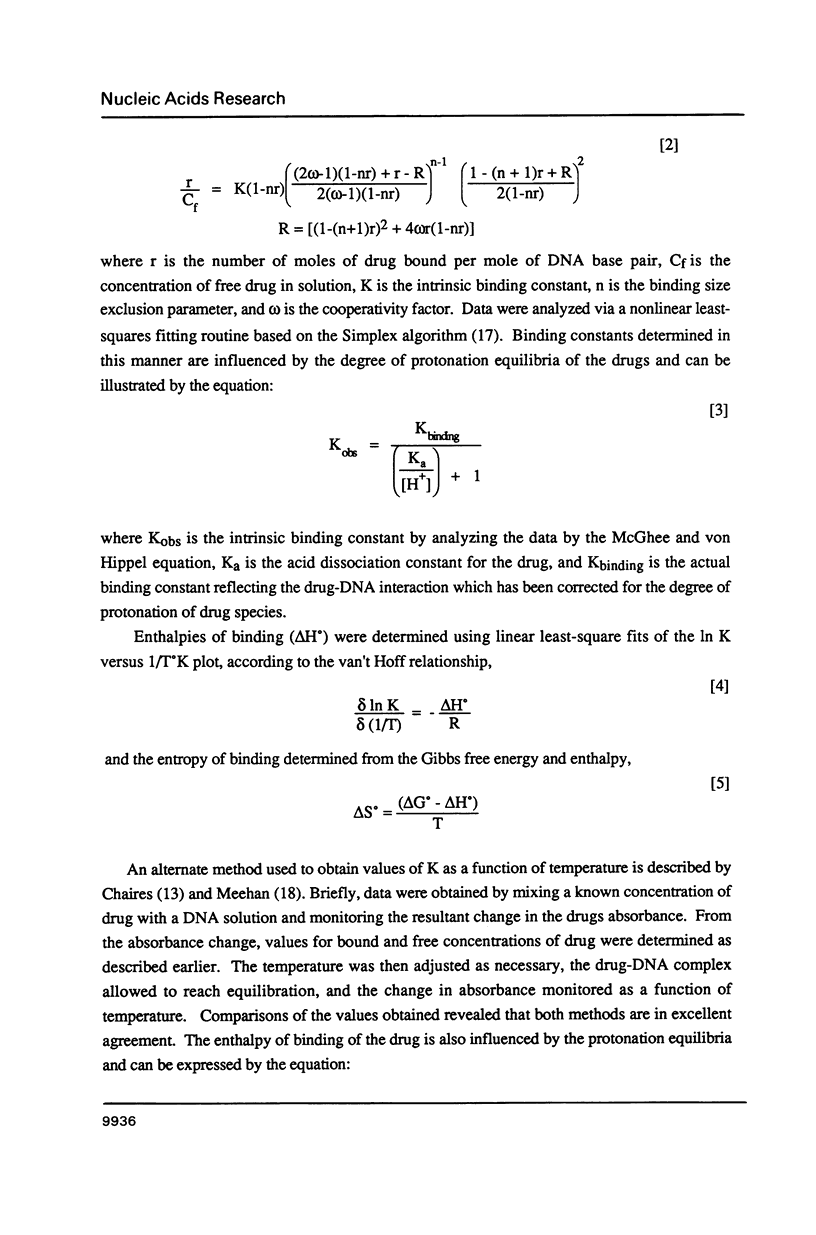
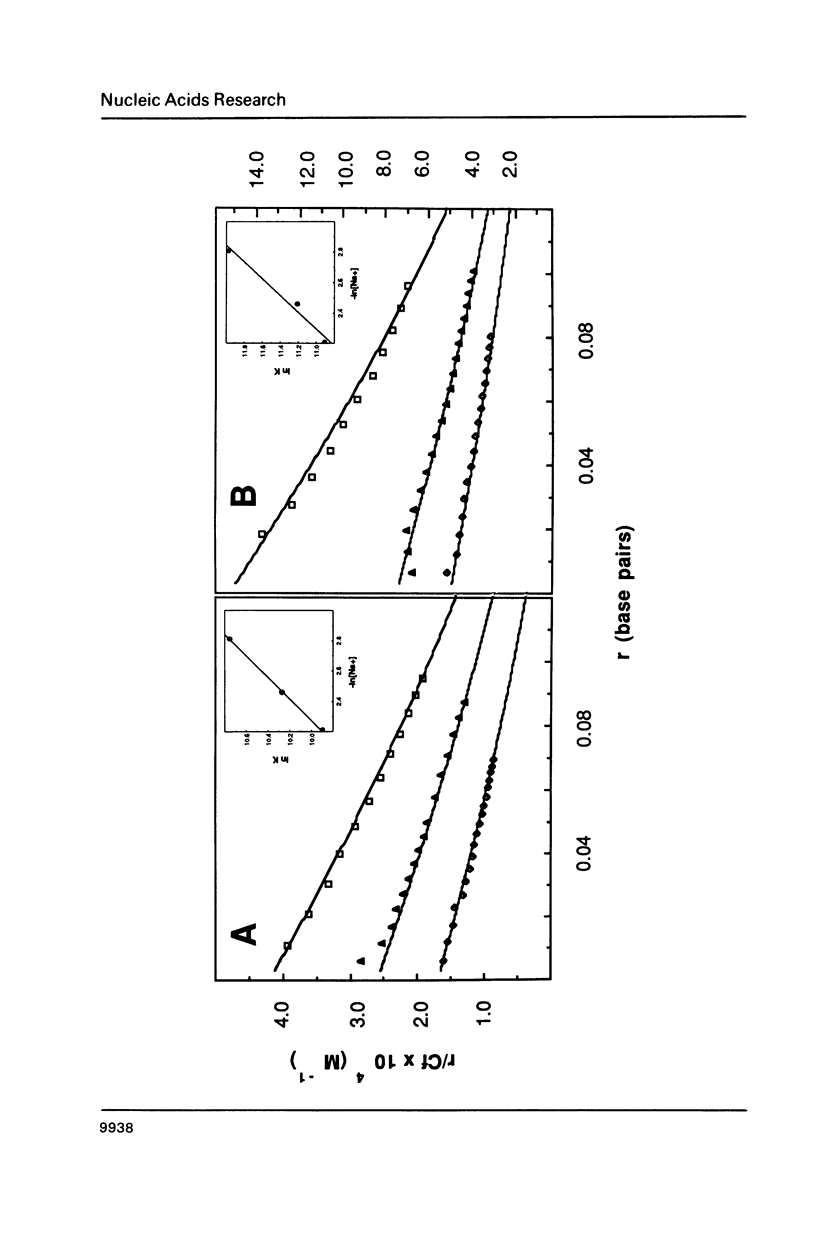
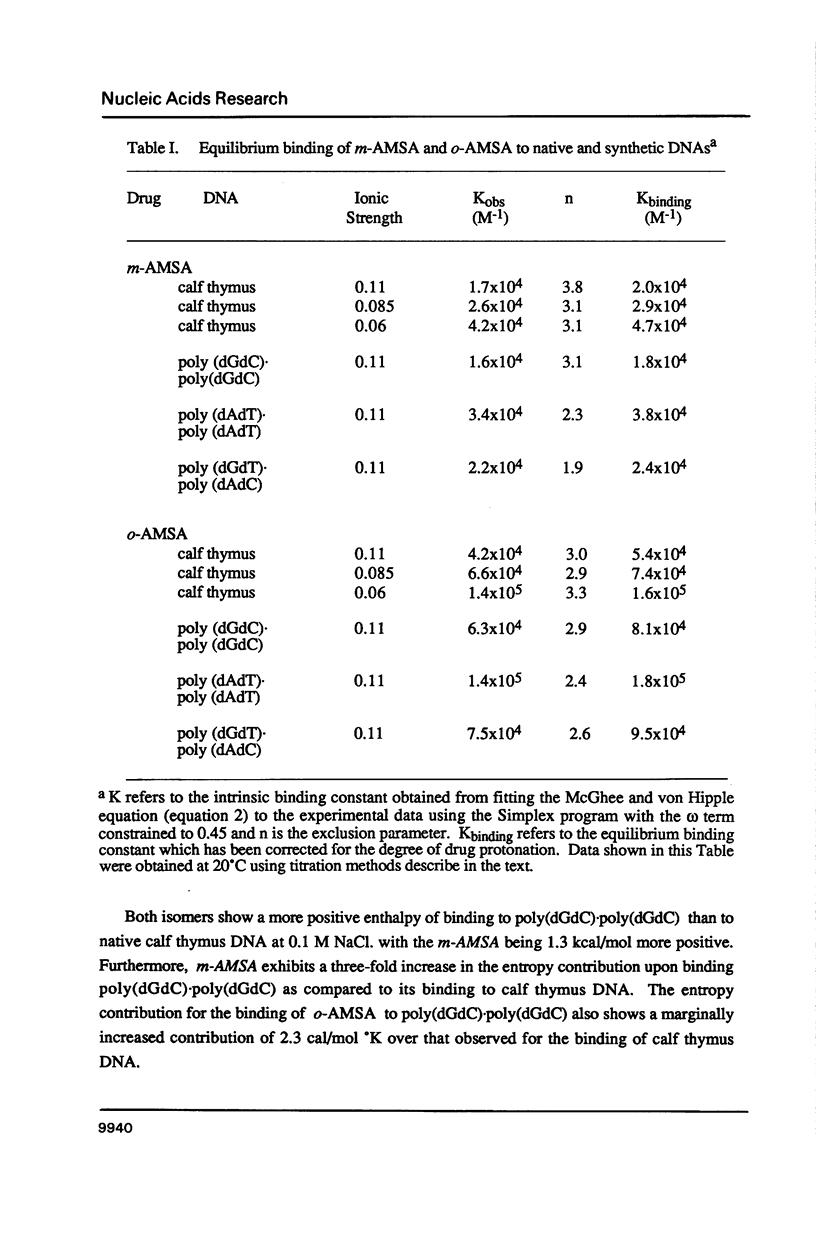
The equilibrium binding of the antitumor agent m-AMSA and its biologically inactive analog o-AMSA to native and synthetic DNAs are compared over a wide range of ionic strengths and temperatures. Although o-AMSA binds DNA with a higher affinity than m-AMSA it is not effective as an antitumor agent. Both m-AMSA and o-AMSA bind DNA in an intercalative manner. Indepth investigations into the thermodynamic parameters of these interactions reveal the interaction of m-AMSA with DNA to be an enthalpy driven process. In contrast, the structurally similar but biologically inactive o-AMSA binds DNA through an entropy driven process. The differences in thermodynamic mechanisms of binding between the two isomers reveal that the electronic and/or steric factors resulting from the position of the methoxy substituent group on the anilino ring directs the DNA binding properties of these compounds and ultimately the biological effectiveness as an antitumor agent.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain B. F., Atwell G. J., Denny W. A. Potential antitumor agents. 16.4'-(Acridin-9-ylamino)methanesulfonanilides. J Med Chem. 1975 Nov;18(11):1110–1117. doi: 10.1021/jm00245a013. [DOI] [PubMed] [Google Scholar]
- Chaires J. B. Thermodynamics of the daunomycin-DNA interaction: ionic strength dependence of the enthalpy and entropy. Biopolymers. 1985 Feb;24(2):403–419. doi: 10.1002/bip.360240208. [DOI] [PubMed] [Google Scholar]
- Hopkins H. P., Jr, Wilson W. D. Enthalpy and entropy changes for the intercalation of small molecules to DNA. II. Ethidium and propidium fluoride. Biopolymers. 1987 Aug;26(8):1347–1355. doi: 10.1002/bip.360260810. [DOI] [PubMed] [Google Scholar]
- Legha S. S., Gutterman J. U., Hall S. W., Benjamin R. S., Burgess M. A., Valdivieso M., Bodey G. P. Phase 1 clinical investigation of 4'-(9-acridinylamino)methanesulfon-m-anisidide (NSC 249992), a new acridine derivative. Cancer Res. 1978 Nov;38(11 Pt 1):3712–3716. [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calorimetric determination of base-stacking enthalpies in double-helical DNA molecules. Biopolymers. 1982 Nov;21(11):2185–2194. doi: 10.1002/bip.360211107. [DOI] [PubMed] [Google Scholar]
- Marsoni S., Wittes R. Clinical development of anticancer agents--a National Cancer Institute perspective. Cancer Treat Rep. 1984 Jan;68(1):77–85. [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Covey J. M., Kerrigan D., Markovits J., Pham R. DNA unwinding and inhibition of mouse leukemia L1210 DNA topoisomerase I by intercalators. Nucleic Acids Res. 1987 Aug 25;15(16):6713–6731. doi: 10.1093/nar/15.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Covey J., Kerrigan D., Mattes W., Markovits J., Kohn K. W. Role of DNA intercalation in the inhibition of purified mouse leukemia (L1210) DNA topoisomerase II by 9-aminoacridines. Biochem Pharmacol. 1987 Oct 15;36(20):3477–3486. doi: 10.1016/0006-2952(87)90329-7. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Mattern M. R., Schwartz R. E., Zwelling L. A., Kohn K. W. Changes in deoxyribonucleic acid linking number due to treatment of mammalian cells with the intercalating agent 4'-(9-acridinylamino)methanesulfon-m-anisidide. Biochemistry. 1984 Jun 19;23(13):2927–2932. doi: 10.1021/bi00308a012. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Rewcastle G. W., Denny W. A., Wilson W. R., Baguley B. C. In vitro and in vivo assessment of activity of new anilino-substituted analogues of amsacrine against Lewis lung carcinoma. Anticancer Drug Des. 1986 Nov;1(3):215–222. [PubMed] [Google Scholar]
- Schneider E., Lawson P. A., Ralph R. K. Inhibition of protein synthesis reduces the cytotoxicity of 4'-(9-acridinylamino)methanesulfon-m-anisidide without affecting DNA breakage and DNA topoisomerase II in a murine mastocytoma cell line. Biochem Pharmacol. 1989 Jan 15;38(2):263–269. doi: 10.1016/0006-2952(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Shimer G. H., Jr, Wolfe A. R., Meehan T. Equilibrium binding of benzo[a]pyrene tetrol to synthetic polynucleotides: sequence selectivity, thermodynamic properties, and ionic strength dependence. Biochemistry. 1988 Oct 4;27(20):7960–7966. doi: 10.1021/bi00420a055. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA-binding characteristics of acridinylmethanesulphonanilide drugs: comparison with antitumour properties. Eur J Cancer. 1976 Dec;12(12):995–1001. doi: 10.1016/0014-2964(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Lopp I. G. Analysis of cooperativity and ion effects in the interaction of quinacrine with DNA. Biopolymers. 1979 Dec;18(12):3025–3041. doi: 10.1002/bip.1979.360181210. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Baguley B. C., Wakelin L. P., Waring M. J. Interaction of the antitumor drug 4'-(9-acridinylamino)methanesulfon-m-anisidide and related acridines with nucleic acids. Mol Pharmacol. 1981 Sep;20(2):404–414. [PubMed] [Google Scholar]
- Winton E. F., Hearn E. B., Vogler W. R., Johnson L., Logan T., Raney M. Amsacrine in refractory adult acute leukemia: a pilot study of the Southeastern Cancer Study Group. Cancer Treat Rep. 1983 Nov;67(11):977–980. [PubMed] [Google Scholar]



