Abstract
Craniofacial sutures are bone growth fronts that respond and adapt to biomechanical environments. Little is known of the role sutures play in regulating the skull biomechanical environment during patency and fusion conditions, especially how delayed or premature suture fusion will impact skull biomechanics. Tgf-β3 has been shown to prevent or delay suture fusion over the short term in rat skulls, yet the long-term patency or its consequences in treated sutures is not known. It was therefore hypothesized that Tgf-β3 had a long-term impact to prevent suture fusion and thus alter the skull biomechanics. In this study, collagen gels containing 3 ng Tgf-β3 were surgically placed superficial to the posterior interfrontal suture and deep to the periosteum in postnatal day 9 (P9) rats. At P9, P24, and P70, biting forces and strains over left parietal bone, posterior interfrontal suture, and sagittal suture were measured with masticatory muscles bilaterally stimulated, after which the rats were sacrificed and suture patency analyzed histologically. Results demonstrated that Tgf-β3 treated sutures showed less fusion over time than control groups, and strain patterns in the skulls of the Tgf-β3 treated group were different from that of the control group. While bite force increased with age, no alterations in bite force were attributable to Tgf-β3 treatment. These findings suggest that the continued presence of patent sutures can affect strain patterns, perhaps when higher bite forces are present as in adult animals.
Keywords: Cranial sutures, cranial bone, suture fusion, Tgf-β3, biomechanics
INTRODUCTION
Cranial sutures are the major sites of calvarial bone expansion (Baer, 1954; Opperman, 2000). The form of the cranial vault is modified under the influence of masticatory hypofunction or hyperfunction (Ulgen et al., 1997; Katsaros et al., 2002; Wright, 2005) and growth of the underlying brain (Kreiborg, 2000). On the other hand, in cases such as premature obliteration of sutures (craniosynostosis), the normal pattern of bone growth is altered (Jane and Persing, 1986; Burrows et al., 1995), and often the expansion of cranial bones is limited (Singhal et al., 1997; Mooney et al., 1999).
Although cranial sutures form in the absence of muscle activity (Hirabayashi et al., 1989), a number of studies support Moss’ hypothesis that the fine details of suture morphology are secondary responses to extrinsic forces, and incurred strain modes (Moss, 1957; Herring and Mucci, 1991; Anton et al., 1992; Rafferty and Herring, 1999; Byron et al., 2004; Markey et al., 2006; Markey and Marshall, 2007; Byron, 2009). In patent sutures, suture complexity and tissue structure changes over time and with a change in masticatory forces (Massler and Schour, 1951; Gross, 1961; Milch, 1966; Kokich, 1976; Miroue and Rosenberg, 1975; Saito et al., 2002; Mao, 2003; Byron et al., 2004; Wang et al., 2006b; Byron, 2009; Smith et al., 2010). The change in suture morphology before suture fusion (such as bony bridging, interdigitation, and beveling) is thus of particular interest within the context of the biomechanical environment. Masticatory muscle resection and surgical isolation and mechanical immobilization of portions of sutures result in the loss of sutural complexity (Moss, 1954; Koskinen, 1977; Foley and Kokichi, 1980), implying an association between suture patency and craniofacial biomechanics. The effects of mastication on growth of the calvaria have been studied in a variety of mammals (Behrents et al., 1978; Jaslow, 1990; Mao et al., 2003a,b; Rafferty et al., 2003). Average strain values in patent sutures are an order of magnitude higher than in adjacent regions of cortical bone (Behrents et al., 1978; Jaslow, 1990; Rafferty et al., 2003; Lieberman et al., 2004; Wang et al., 2008). Previous research has shown that posterior interfrontal sutures (IFS) and sagittal sutures (SGS) have individual strain patterns that change with age and as they fuse (Shibazaki et al., 2007). Strain patterns are related to suture fusion (Sun et al., 2004), however the question of how suture morphology and fusion affect the global biomechanics of the craniofacial skeleton has not been systematically studied.
Suture fusion is closely associated with changes in gene expression. Mutations in genes for fibroblast growth factor receptors (FGFRs), MSX2, and TWIST have all been associated with premature suture fusion (craniosynostosis) (Jabs, 2002). Mutations in transforming growth factors beta (TGF-βs) have not been associated with premature suture fusion. However, recent findings show that mutations in TGF-β receptor type II (TβR-II) are associated with craniosynostosis (Loeys et al., 2005). In vitro data have shown that Tgf-β2 and Tgf-β3 are potent regulators of suture patency (Opperman et al., 1999, 2000). Tgf-β2 added to fetal rat calvariae in culture induced the normally patent coronal sutures to fuse, while Tgf-β3 rescued these sutures from fusion. Furthermore, IFS obliteration was delayed in the short term by Tgf-β3 in vivo (Opperman et al., 2002; Chong et al., 2003). It is possible that Tgf-β3 prevented the loss of the suture mesenchyme as described in cases of craniosynostosis by Holmes et al. (2009).
While these data show that changes in strain patterns and growth factor expression are related to suture patency, it is important to determine if a change in the status of suture patency (i.e. the process of fusion and suture obliteration) alter the cranial biomechanical environment. The hypothesis to be tested is that Tgf-β3 effects long-term suture patency, and that preventing suture fusion will alter strain patterns in craniofacial sutures. To test this hypothesis, a rat cranial suture model was used, with three experimental aims. The first aim was to delineate the long-term effects of Tgf-β3 on degree of IFS bony bridging and suture morphology. The second aim was to determine whether Tgf-β3 treatment of the IFS had any effect on bite force, and the final aim was to compare the overall strain patterns across patent sagittal sutures (SGS) and fusing or non-fusing IFS and the adjacent parietal bone (PB).
MATERIAL AND METHODS
Animals
Pregnant Sprague-Dawley rats were purchased (Harlan, Indianapolis, IN, USA) and housed in the animal facility at the Baylor College of Dentistry. Of the pups born to these rats, 180 nine-day old (P9) male and female pups were used in this study (Table 1). The protocol for surgery and post-surgical observation and care followed the guidelines dictated by the Baylor College of Dentistry IACUC, and has been described previously (Shibazaki et al., 2007). All animals for this study were housed in the Animal Research Facility at Baylor College of Dentistry.
Table 1.
Numbers of animals used in experiments
| CON (n=45) | SHAM (n=45) | GEL ONLY (n=45) | β3 (n=45) | Sum | |||||
|---|---|---|---|---|---|---|---|---|---|
| Histo | Bite force & Strain | Histo | Bite force & Strain | Histo | Bite force & Strain | Histo | Bite force & Strain | ||
| Day 9 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 60 |
| Day 24 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 60 |
| Day 70 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 60 |
|
| |||||||||
| Total | 15 | 30 | 15 | 30 | 15 | 30 | 15 | 30 | 180 |
Surgery
The first author conducted all surgeries, using methods reported previously (Opperman et al., 2002). Nine days after birth (P9), rat pups were anesthetized using 3.25% Isofluorane (Butler, Dallas, TX, USA) and 900 cc/min O2 inhalant anesthetic delivered by a Foregger F500 anesthesia system via a nose cone. Animals were randomly assigned to four groups (n=45 per group). Group 1 served as non-surgical control (CON). Group 2 was used as a sham surgical control (Sham). In this group, a coronal incision through the scalp was made from ear to ear, the skin was reflected to expose the coronal and IFS, and the periosteum was loosened above the IFS by use of a periosteal elevator. No further manipulation was done prior to closing the incision. Group 3 had bovine collagen type I gel soaked with sterile phosphate-buffered saline (PBS) placed on the exposed IFS beneath the periosteum (Gel only). Group 4 received collagen gels adsorbed with 3 ng Tgf-β3 (β3). The two edges of the scalp were approximated and closed with a 6-0 silk suture (DG, New York, NY), with care taken not to damage or move the gel.
At P9, P24, and P70 (0, 15 or 61 days after surgery), rats were anesthetized by intramuscular injection (1 ml/kg body weight) of a solution containing 13 mg Rompun and 87 mg Ketamine in each ml of cocktail prior to strain and bite force measurement. After measurements were taken at each time point, the rats (n=60 per time point) were sacrificed by Halothane (Butler, Dallas, TX, USA) overdose, and tissues prepared for histology.
Histology
Sixty rats not used for bite-force or strain measurements (n=5 per time point per group) were used for histology. They were decapitated and the scalps removed. The heads were postfixed overnight in 4% paraformaldehyde and decalcified using 0.5M EDTA for two weeks (P9, P24) or for one week with microwave (P70). Different decalcification methods were required because of the much thicker P70 bones. Following decalcification, the heads were cut in half along the coronal suture, separating each into two cranial regions, one containing frontal bones with intervening fusing or non-fusing IFS and the other containing PB with intervening patent SGS. Samples were processed for paraffin embedding and sectioning at 6 μm and three sections from midway through each tissue were stained with hematoxylin and eosin (H&E). Sections were used for qualitative examination of suture morphology, and for scoring the amount of bony bridging within the sutures.
Scoring of bony bridging within the IFS
To examine the patency of each suture, 3 sections from each rat IFS and SGS (n=360 sections total) were stained with H&E, and examined using polarized light microscopy. The SGS was examined to determine if the Tgf-β3 placed over the IFS had any effect on patency of the SGS. In the cross-sections, the vertical height of the parietal and frontal bones was measured, along with the vertical height of the fused region of the suture and the vertical height of the unfused portion of the suture (Fig. 1A). These measurements were used to calculate a percentage of bony bridging in the suture. It is worth noting that this scoring method was for assessing the degree of sutural fusion along the direction of bone thickness to the effect of Tgf-β3 or lack of it, not for assessing a correlation between suture morphology (such as interdigitation, beveling, and fusion) and biomechanics; the latter needs a three dimensional image database (Markey and Marshall, 2007). A two-sample t test (for the two tailed hypotheses) was used to test the differences between the Tgf-β3 group and the rest of the groups in degrees of bony bridging and suture fusion. The significance level was set at α = 0.05.
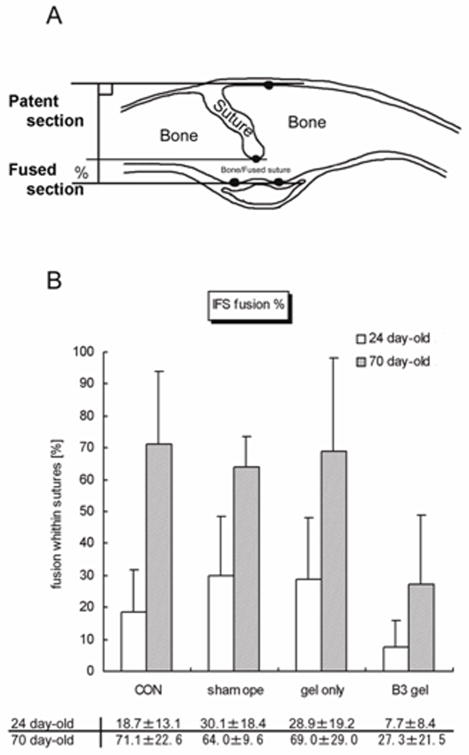
FIG. 1.
A, Diagram showing a coronal section through the calvaria with a partially endocranially fused interfrontal suture (IFS) between left and right frontal bones. The thickness of the bones was measured, and the distance between top and bottom horizontal line gives the total width of the suture. Sutures begin fusing from the endocranial surface, so the distance between the middle and bottom horizontal lines gives the width of the fused region of the suture. This scoring method assessed the degree of sutural fusion across the thickness of the bones to test the effect of Tgf-β3 or lack of it. This measurement was not meant to correlate suture morphology (such as interdigitation, beveling, and fusion) and biomechanics, which requires a three dimensional image database. B, Graph showing the percent fusion of sutures in each group.
Masticatory muscle stimulation and bite-force measurement
On the remaining 120 rats (4 groups with n=30 animals per group, and n=10 for each time point), muscle stimulation was achieved via unipolar needle electrodes (Platinum Subdermal Electrode Type E2, Grass Instrument Co., MA, USA) inserted through the skin bilaterally into each masseter muscle. Under anesthesia, a super-maximal train of pulses (120 Hz, 40 V, single train) was delivered to the muscles by a SIU5 Stimulus Isolation Unit/Solid-State Square Pulse Stimulator (Grass Instrument Co., MA, USA).
The bite force transducer consisted of two differential strain aluminum beams, 4.0 mm in thickness, mounted with four strain gages in a full bridge configuration (Shibazaki et al., 2007). After calibrating the bite force transducer, all bite force measurements were recorded by incisor biting. The strain was calibrated to force (gf) with a conversion chart made by measuring weights. The masseter muscle stimulation was coordinated with both bite force measurements and strain measurements.
IFS/SGS/PB strain measurement
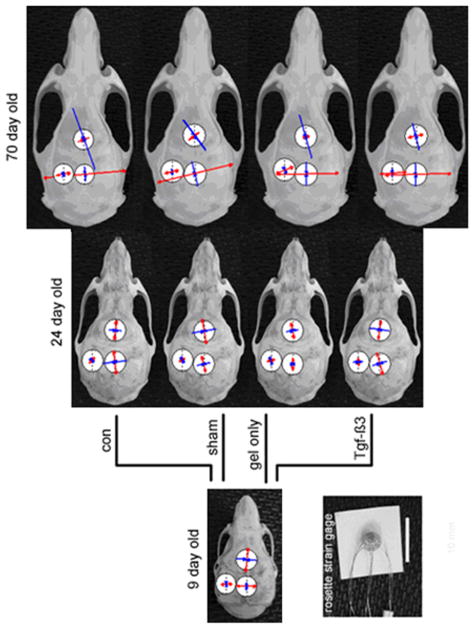
The same rats used for bite-force measurement were used for strain measurement. One strain gage was placed for each rat. After anesthesia, the skin and periosteum were incised over sites chosen for strain gage placement across the IFS or SGS, or on the PB. The exposed bony surfaces were smoothed, degreased and dried as before (Shibazaki et al., 2007). A stacked delta rosette gage (Model UFRA-1–11, Tokyo Sokki Kenkyujyo Co., Ltd., Tokyo, Japan) was bonded across the posterior part of the IFS, the anterior part of the SGS or on the PB next to the anterior part of the SGS (Fig. 2). In UFRA models, the backing plate is thinnest and more flexible than in other existing models, which is important for use on curved surfaces such as those on bone. During masseter muscle stimulation, strain signals were recorded, digitized, sampled and stored to a sensor interface (PCD-300A, Kyowa Electronic Instruments Co., Ltd., Tokyo, Japan). Values from each element of the rosette gage were analyzed, and principal maximum (tensile) and minimum (compressive) strain magnitudes and orientations were calculated using application software (DAS-100A, Kyowa Electronic Instruments Co., Ltd., Tokyo, Japan). Tensile strains were expressed as positive values and compressive strains as negative values. Peak strains were selected based on the 3rd stimulation record of the 10th biting cycle of each animal. Orientation of maximum strain (θ max) was presented using Oriana 2.0 software (Kovach Computing Services, Wales, UK).
FIG. 2.
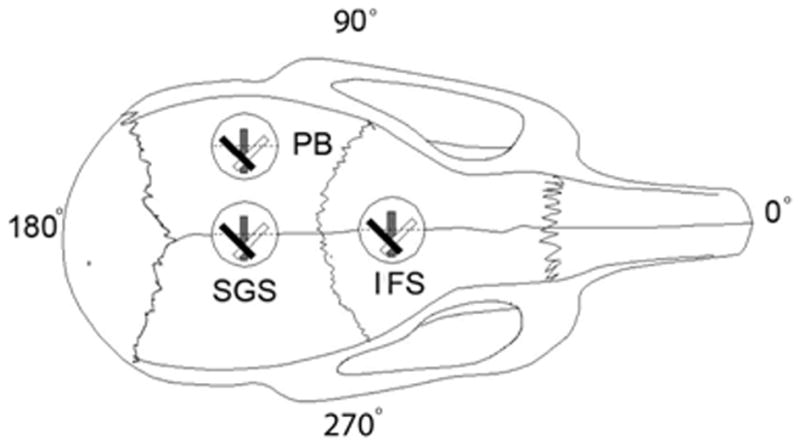
Diagram of a rat skull showing placement of stacked delta rosette gages at two sutural sites and on one bone surface. IFS, Interfrontal suture; SGS, sagittal suture; PB, parietal bone.
RESULTS
Suture morphology and degree of fusion within the IFS
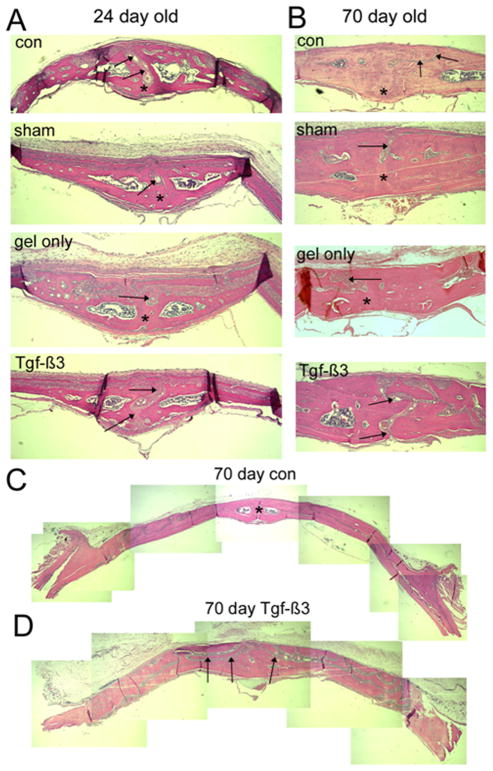
At P9, sutures from all groups remained patent (data not shown). At P24, the suture morphology of the Sham, Gel only and β3 groups were similar to that of CON sutures (Fig. 3). The IFS showed bony bridging from the dural side in all groups where fusion was noted. In the β3 group at P70, the IFS of two thirds of the animals (3 of 5) remained completely patent, and the structure of the patent sutures was highly complex, closely resembling the structure of the patent SGS at P70.
FIG. 3.
Photomicrographs of histological sections through the posterior IFS of 24 and 70 day old animals. The ectocranial (periosteal) surface is superior and endocranial (dural) surface is inferior in all micrographs. Frontal bones are to the left and right of the suture (arrows). A, IFS of 24-day animals, showing sutures (arrows) in the control (con), sham operated (sham), and Gel only groups with bony bridging on their dural aspect (asterisks) by 24 days. In all cases the sutures fused from the dura side. Sutures in the Tgf-β3 treated group remained open (arrows). B, IFS of 70-day animals, showing gradual thickening of the bones compared to the 24-day animals. Note the progressive bony bridging (asterisks) across the 70-day control, sham and gel only sutures (arrows), with the persistent patency of the Tgf-β3 treated IFS (arrows). C, Low power composite images of sections through calvariae of control and Tgf-β3 treated animals. Note the thicker bones in the Tgf-β3 treated calvaria, with the highly complex IFS (arrows) winding through the bone.
Within IFS, the amount of bony bridging at P24 and the percent fusion at P70 in the β3 group were significantly lower than in the other three groups as hypothesized (P < 0.05 at P24, P < 0.001 at P70). The percentage of bony bridging or fusion within IFS was comparable in three other groups at both ages examined.
Changes in bite force with age
The changes in bite forces were similar to those reported previously (Shibazaki et al., 2007). Bite force increased with age, and a significant increase in bite force was found between P9 and P24 in all groups (Fig. 4; Table 2). While bite force in P24 and P70 rats with Tgf-β3-treated IFS were lower than control and sham-operated groups, these differences were not significant.
FIG. 4.
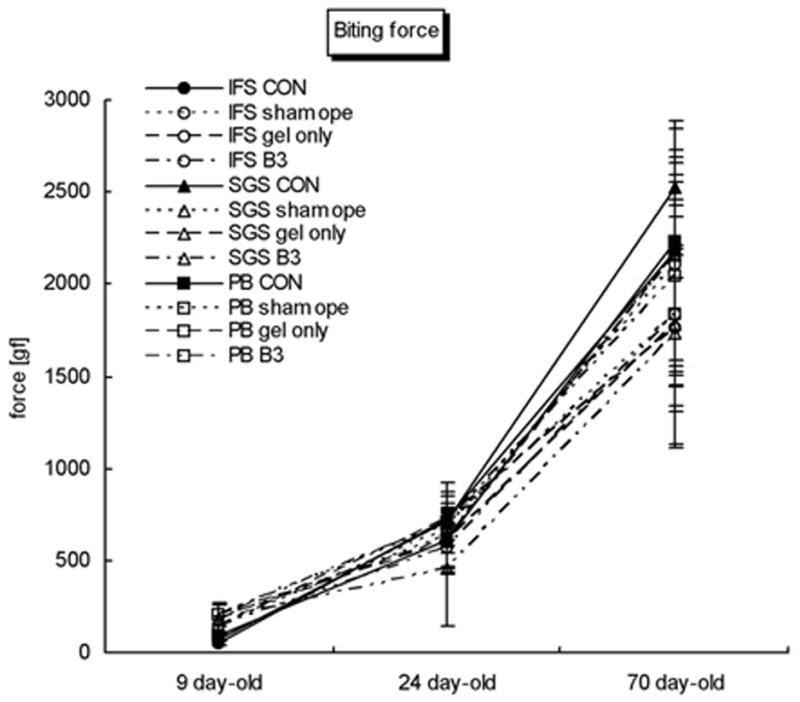
Graph showing changes in bite forces with age in all treatment groups. Change in bite force with age was significant in all groups. No significant differences in bite force were noted between groups at any age.
Table 2.
Biting force (mean ±SD)
| IFS measurement group | SGS measurement group | PB measurement group | Average | |
|---|---|---|---|---|
| Day 9 | ||||
| Con | 55±22 | 79±15 | 84±13 | 73±20 |
|
| ||||
| Day 24 | ||||
| Con | 803±118 | 690±167 | 630±141 | 707±150 |
| Sham | 677±134 | 630±163 | 695±80 | 667±124 |
| Gel only | 727±144 | 604±151 | 610±169 | 647±155 |
| β3 | 566±139 | 458±311 | 710±57 | 578±213 |
|
| ||||
| Day 70 | ||||
| Con | 2451±308 | 2585±229 | 2241±105 | 2426±255 |
| Sham | 2108±550 | 2159±574 | 1833±381 | 2033±494 |
| Gel only | 1766±426 | 1783±676 | 2160±721 | 1903±606 |
| β3 | 1840±709 | 1732±429 | 2049±549 | 1874±548 |
P<0.05 among treatments. Unit: gf (1gf = 9.80665 millinewtons)
Suture/Bone strains during incisor biting
The magnitude and orientation of maximum strain in the IFS were not significantly different between P9 and P24, were tensile in nature and were parallel to the IFS (Fig. 5A). However, a compressive force occurred perpendicular to the IFS at P70, and a significantly large compressive force was found parallel to the IFS at the same age (Fig. 6). Those changes were found in the CON, Sham, and Gel only groups. In contrast, tensile strain perpendicular to the IFS and less compressive strain parallel to the IFS were found in the β3 group at P70.
FIG. 5.
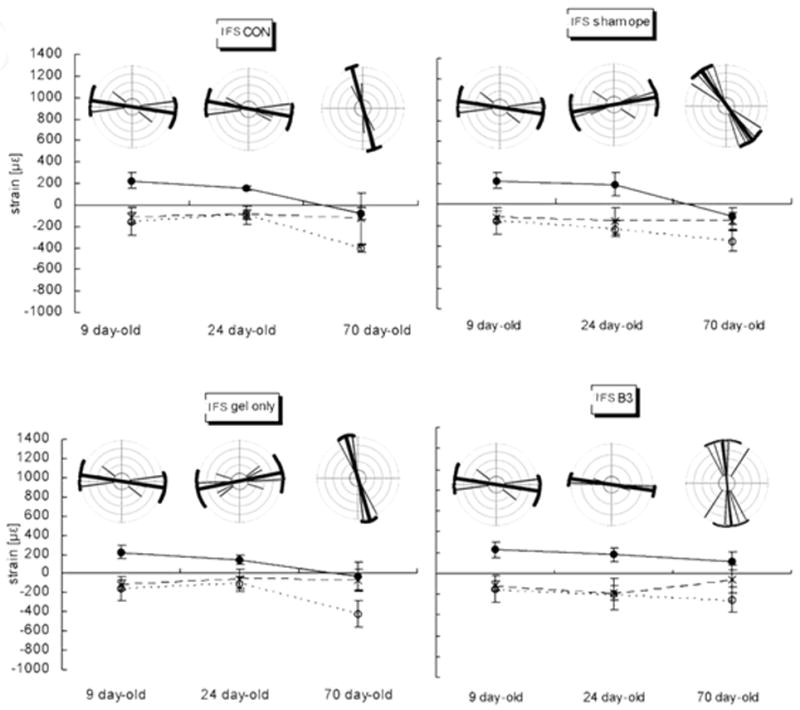
A, IFS. B, SGS. C, PB. Graphs showing magnitudes and orientations of strain at three ages in four animal groups. Mean of three strain values, the maximum strain (tension), minimum strain (compression), and the strain channel (Channel 2; Ch2) or central element of rosette gage are plotted in age groups. The orientation of maximum strain was illustrated by rose distribution generated using the Oriana program for angular statistics. The central bold line in each circle represents the mean orientation, the brackets and two thin long lines linking their ends represent the circular standard deviations. Other thin lines represent individual orientations and sample size. On the graphs below the circles, means ± standard errors of the mean are given. At all ages and sites and in all animal groups, the change in strain patterns is remarkable between age 24 days and 70 days. The changes in orientation of the maximum stiffness are also significant.
FIG. 6.
Summary of strain patterns at the three gage sites, for the three ages and four experimental groups. Red lines with open arrows – maximum or tensile strain; blue lines with closed arrows – minimum or compressive strains. The lengths of the arrows are scaled to the mean magnitude, and their orientations are the mean orientations.
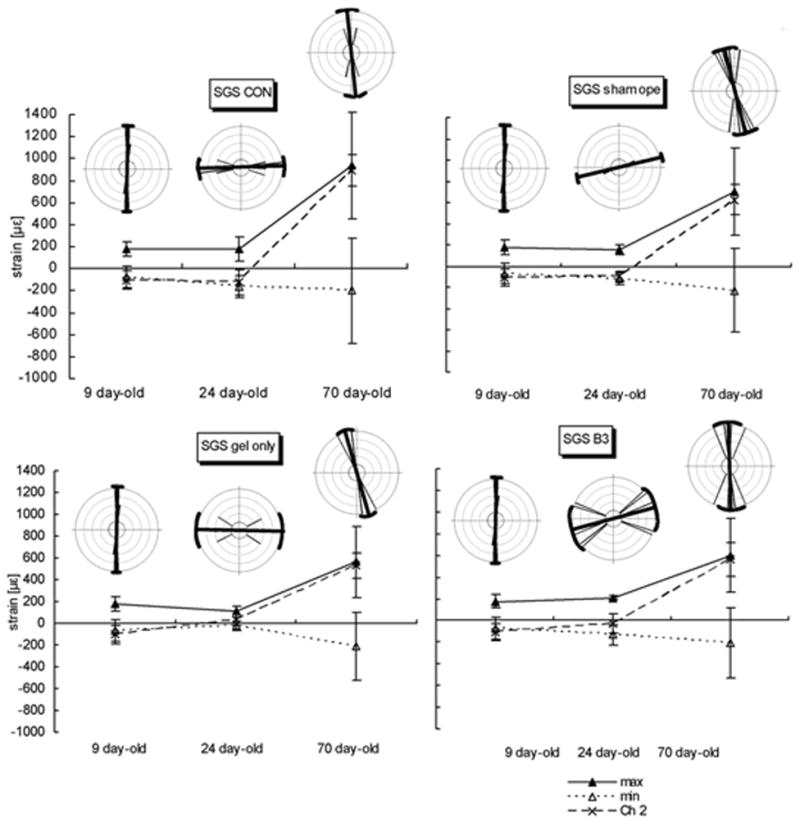
The orientation of maximum strain was not significantly different between P9 and P70 in the SGS, but the magnitude of strain at P70 was significantly higher than at P9 (Figs. 5B, 6; Table 3). Although the magnitude of tensile strain was not significantly different between P9 and P24, the orientation of strain at P24 was nearly perpendicular to that at P9. This change in orientation of strain was present in all groups.
Table 3.
Peak strain and orientation (mean±SD)
| Rat age, group | Interfrontal suture (IFS)
|
Sagittal suture (SGS)
|
Parietal bone (PB)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch 2 (με) | max (με) | min (με) | α(°) | Ch 2 (με) | max (με) | min (με) | α(°) | Ch 2 (με) | max (με) | min (με) | α(°) | |
| Day 9 | ||||||||||||
| Con | −118±74 | 224±128 | −156±50 | 173±12 | −105±64 | 177±99 | −70±80 | 89±4 | 91±34 | 101±51 | −28±66 | 94±11 |
|
| ||||||||||||
| Day 24 | ||||||||||||
| Con | −90±23 | 154±80 | −96±43 | 171±14 | −124±110 | 175±98 | −165±114 | 7±5 | 17±24 | 58±10 | −18±30 | 138±7 |
| Sham | −159±111 | 192±74 | −236±126 | 10±20 | −102±45 | 152±58 | −117±45 | 14±4 | 40±20 | 59±22 | −30±15 | 119±6 |
| Gel only | −57±50 | 141±74 | −110±102 | 11±21 | 31±53 | 107±45 | −26±48 | 179±19 | 38±66 | 71±26 | −44±44 | 108±29 |
| β3 | −190±67 | 179±154 | −257±131 | 172±6 | −29±16 | 210±97 | −132±92 | 15±30 | −46±66 | 65±63 | −85±18 | 3±34 |
|
| ||||||||||||
| Day 70 | ||||||||||||
| Con | −128±55 | −78±33 | −408±237 | 106±7 | 892±485 | 937±477 | −202±145 | 96±11 | 125±39 | 139±38 | −15±32 | 101±16 |
| Sham | −159±79 | −114±94 | −349±78 | 126±13 | 628±415 | 701±395 | −229±139 | 104±13 | 103±36 | 122±36 | 1±34 | 102±29 |
| Gel only | −66±153 | −31±135 | −419±110 | 106±9 | 528±328 | 561±311 | −211±114 | 91±14 | 131±28 | 166±10 | −33±31 | 113±11 |
| β3 | −57±92 | 118±122 | −257±131 | 95±20 | 559±339 | 602±326 | −205±156 | 92±14 | 234±100 | 239±98 | −81±75 | 93±6 |
Ch 2: the rosette gage element perpendicular to sutures, max; maximum strain, min; minimum strain, α; orientation of maximum strain . P<0.05 among treatments.
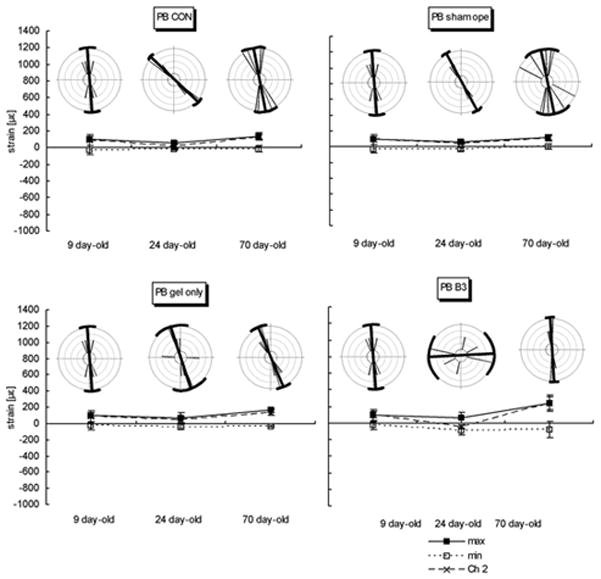
Compared to both SGS and IFS, strains in the PB were low in all groups. The orientation of maximum strain at P9 and P70 were similar to those seen in the SGS (Fig.5C). The same strain patterns in the PB at P9, P24 and P70 were found in all groups (Fig. 6).
DISCUSSION
In the rat, all of the cranial sutures remain patent throughout life except for the posterior part of the IFS (Moss, 1954). This region of the suture undergoes osseous obliteration early, and the IFS fuses by P21 (Moss, 1957). Roth et al. showed that IFS from P12 – P20 were found to be actively fusing, and by P30, this suture was completely fused, with an occasional remnant of suture connective tissue visible at the periosteal (ectocranial) surface (Roth et al., 1997). All other cranial sutures remained patent. Previous research showed that Tgf-β3 delayed IFS closure in rats, at least until P24 (Opperman et al., 2002), and that Tgf-β3-treatment of a rabbit model of delayed onset suture fusion, allowed continued bone growth up to 84 days (Chong et al., 2003). This study showed a complex but patent IFS morphology in Tgf-β3 treated rat IFS by P70, indicating that Tgf-β3 treatment not only delays, but also inhibits normal IFS fusion for extended periods of time, possibly by preventing the loss of the suture mesenchyme as described by Holmes et al. (2009).
As was previously shown, the increases in bite force between P9 and P24 likely reflect changes from a soft to hard diet during weaning (Shibazaki et al., 2007). Bite force increased dramatically between P24 and P70 in all groups (Table 2), and no differences in bite force were noted between animals treated with Tgf-β3 and control animals. The latter finding suggests that suture patency may be unrelated to bite force. However, the use of super-maximal pulses may have masked subtle changes in bite force related to changes in suture patency.
Strains parallel to the IFS were tensile at P9 and P24, and changed from tensile to compressive between P24 and P70. At P24 the Tgf-β3 IFS showed less bony bridging than controls, and the value and orientation of strain across the IFS were similar to the control groups. Since bite forces were similar between groups; this implies that strain levels were unchanged by delaying or inhibiting suture fusion. However, by P70 the Tgf-β3 treated group continued to show less bony bridging within sutures than other groups, but now the tensile forces were perpendicular to the IFS. These latter findings are similar to those of Sun et al. (2004) who found that strain patterns in the posterior IFS that remained patent changed in strain pattern from pure compression to both compression and tension with increasing age, in contrast to the unchanged strain pattern in sagittal sutures (interparietal sutures) as they fused with age (Sun et al., 2004). These findings suggest that the continued presence of patent sutures can affect strain patterns when higher bite forces are present as in adult animals.
The SGS is situated between the parietal bones, and applying bite force resulted in a tensile strain perpendicular to the SGS. However, this angle changed from positive to negative at P24, and a tensile force now occurred parallel to the SGS. These changes occurred in all groups including P70, regardless of Tgf-β3 treatment and changes in IFS suture patency. These data suggest that IFS patency does not affect SGS function, such that the IFS and SGS may work separately, and their biomechanics do not interfere or interact with each other.
Correlations between suture fusion and the global mechanics of the calvaria have been little studied, except in in silico simulations (Wang et al., 2010). In results presented here, P70 Tgf-β3 treated IFS were patent or had less bony bridging than control sutures, and the biting forces and suture/bone strain were the same as in control rats. It appears then that artificial inhibition of IFS fusion does not have an effect on masticatory function. The suture morphology of the unfused P70 IFS was highly complex, winding through the frontal bone, similar to that seen in the SGS. Understanding whether the biomechanical effects of masticatory forces affect the morphology and patency of IFS and SGS needs more longitudinal studies. In the cranium, the dura mater has been believed to regulate significant suture growth events such as fusion or maintenance of patency (Opperman et al., 1993, 1995, 1998), yet it is unclear what regulates facial sutures. In rabbits, three facial sutures, the pre-maxilla-maxillary suture, the frontonasal suture, and the zygomaticotemporal suture and their immediately adjacent sutural mineralization fronts have markedly different suture complexity, and display different elastic properties and different capacities for mechanical deformation (Mao, 2002,2003; Mao et al., 2003a,b; Radhakrishnan and Mao, 2004). The question of whether facial sutures are more directed by extrinsic mechanical forces than calvarial sutures awaits further study (Wang et al., 2012).
It is intriguing that in many reptile and mammalian species the same sutures remain patent or fuse, regardless of craniofacial shape or dimensions (Krogman, 1930; Chopra, 1957; Herring, 1993; Hershkovitz et al, 1997; Rubidge and Sidor, 2001; Sidor, 2001; Wang et al., 2006b). It seems reasonable then to pose the question as to which comes first – fusion of the IFS, or a change of muscle mechanics with age? With this IFS delayed/inhibited suture fusion model, it is suggested that suture fusion affects the biomechanics of the craniofacial skeleton rather than vice versa. Thus, additional work is necessary to understand whether biomechanics of the craniofacial skeleton can have reciprocal effects on suture morphology and fusion.
The studies of suture biology and biomechanics call for more careful longitudinal experimental investigation. It is worth to noting that the diversity of sutural morphologies (in both surface and cross-sectional views) poses a challenge to the analysis of sutural biology and biomechanics (Herring, 1993; Herring and Mucci, 1991; Rafferty and Herring, 1999; Byron et al., 2004; Markey et al., 2006; Markey and Marshall, 2007; Byron, 2009; Wang et al., 2012). In patent sutures, suture complexity (ectocranial sinuosity) and tissue structure changes over time and with a change in masticatory forces (Massler and Schour, 1951; Gross, 1961; Milch, 1966; Kokich, 1976; Miroue and Rosenberg, 1975; Saito et al., 2002; Mao, 2003; Byron et al., 2004; Wang et al., 2006b; Byron, 2009; Smith et al., 2010). Other studies demonstrated that the suture interdigitation pattern is complex and diversified, and often disconnected from the ectocranial sinuosity pattern (Markey and Marshall, 2007). These authors demonstrate well that studies of suture morphologies and their interaction with their biomechanical environments call for the use of advanced imaging techniques, such as micro-CT or high resolution radiographs to accurately reconstruct the suture morphologies in a three dimensional environment.
Multiple biologic factors may play a role in the development, morphology, and biomechanical characteristics of sutures. It is interesting that Tgf-β3-induced IFS suture patency produces suture complexity similar to the SGS suture. These findings indicate the complex interplay of growth factors and other molecular factors, biomechanical stimuli and suture patency in the craniofacial complex. This complexity calls for more coherent studies including various related fields such as fractal analysis (Yu et al., 2003), advanced imaging analysis and histological study (Markey et al., 2006; Markey and Marshall, 2007; Smith et al., 2010), in vivo and in vitro experiments (Herring and Mucci, 1991; Rafferty et al., 2003; Lieberman et al., 2004; Sun et al., 2004; Markey et al., 2006; Wang et al., 2008), investigation of the maturation of the craniofaciodental complex (Wang et al., 2006b, 2007; Wang, 2012), phylogenetic studies (Rubidge and Sidor, 2001; Sidor, 2001; Wang et al., 2006a), and computer aided simulations such as Finite Element Analysis (Kupczik et al., 2007, 2009; Farke et al., 2008; Moazen et al., 2009; Jasinoski et al., 2010a,b; Wang et al., 2010, 2012).
Acknowledgments
The authors wish to thank Ms. Jo Taylor for advice concerning the technical aspects of histological preparation of tissues. Bovine collagen type I gel (a generous gift of Amr Moursi and NeuColl, Inc., Campbell, CA). This work was supported by NIH DE15401, and by intramural grants from Showa University School of Dentistry and Baylor College of Dentistry, and partially by NSF BCS 0725141 and 0725183.
References
- Anton SC, Jaslow CR, Swartz SM. Sutural complexity in artificially deformed human (Homo sapiens) crania. J Morphol. 1992;214:321–332. doi: 10.1002/jmor.1052140307. [DOI] [PubMed] [Google Scholar]
- Baer MJ. Patterns of growth of the skull as revealed by vital staining. Human Biol. 1954;26:80–126. [PubMed] [Google Scholar]
- Behrents RG, Carlson DS, Abdelnour T. In vivo analysis of bone strain about the sagittal suture in macaca mulatto during masticatory movements. J Dent Res. 1978;57:904–908. doi: 10.1177/00220345780570091401. [DOI] [PubMed] [Google Scholar]
- Burrows AM, Mooney MP, Smith TD, Losken HW, Siegel MI. Growth of the cranial vault in rabbits with congenital coronal suture stenosis. Cleft Palate-Craniofacial Journal. 1995;32:235–246. doi: 10.1597/1545-1569_1995_032_0235_gotcvi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Byron CD. Cranial Suture Morphology and its Relationship to Diet in Cebus. J Hum Evol. 2009;57:649–655. doi: 10.1016/j.jhevol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Byron CD, Borke J, Yu J, Pashley D, Wingard CJ, Hamrick M. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec. 2004;279A:676–684. doi: 10.1002/ar.a.20055. [DOI] [PubMed] [Google Scholar]
- Chong SL, Mitchell R, Moursi AM, Winnard P, Losken HW, Bradley J, Ozerdem O, Azari K, Acarturk O, Opperman LA, Siegel MI, Mooney MP. Rescue of coronal suture fusion using transforming growth factor-beta 3 (Tgf-β3) in rabbits with delayed-onset craniosynostosis. Anat Rec. 2003;274:962–971. doi: 10.1002/ar.a.10113. [DOI] [PubMed] [Google Scholar]
- Chopra SRK. The cranial suture closure in monkeys. Proc Zool Soc Lond. 1957;128:67–112. [Google Scholar]
- Farke AA. Frontal sinuses and head-butting in goats: a finite element analysis. J Exp Biol. 2008;211:3085–3094. doi: 10.1242/jeb.019042. [DOI] [PubMed] [Google Scholar]
- Foley WJ, Kokich VG. The effects of mechanical immobilization on sutural development in the growing rabbit. J Neurosurg. 1980;53:794–801. doi: 10.3171/jns.1980.53.6.0794. [DOI] [PubMed] [Google Scholar]
- Gross J. Aging of connective tissue, the extracellular components. In: Bourne GH, editor. Structural aspects of aging. New York: Hafner; 1961. pp. 179–192. [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. The Skull. 1993;1:153–206. [Google Scholar]
- Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. Journal of Morphology. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz I, Latimer B, Dutour O, Jellema LM, Wish-Baratz S, Rothschild C, Rothschild BM. Why do we fail in aging the skull from the sagittal suture? Am J Phys Anthropol. 1997;103:393–399. doi: 10.1002/(SICI)1096-8644(199707)103:3<393::AID-AJPA8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hirabayashi S, Harii K, Sakurai A, Takaki EK, Fukuda O. An experimental study of craniofacial growth in a heterotopic rat head transplant. J Plast Reconstr Surg. 1989;5:145–150. doi: 10.1097/00006534-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Holmes G, Rothschild G, Roy UB, Deng CX, Mansukhani A, Basilico C. Early onset of craniosynostosis in an Apert mouse model reveals critical features of this pathology. Dev Biol. 2009;328:273–284. doi: 10.1016/j.ydbio.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs EW. Genetic etiologies of craniosynostosis. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Etiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 125–146. [Google Scholar]
- Jane JA, Persing JA. Neurosurgical treatment of craniosynostosis. In: Cohen MM Jr, editor. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Raven Press; 1986. pp. 249–320. [Google Scholar]
- Jasinoski SC, Rayfield EJ, Chinsamy A. Functional implications of dicynodont cranial suture morphology. J Morph. 2010a;271:705–728. doi: 10.1002/jmor.10828. [DOI] [PubMed] [Google Scholar]
- Jasinoski SC, Reddy BD, Louw KK, Chinsamy A. Mechanics of cranial sutures using the finite element method. J Biomech. 2010b;43:3104–3111. doi: 10.1016/j.jbiomech.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Jaslow CR. Mechanical properties of cranial sutures. J Biomech. 1990;23:313–21. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- Katsaros C, Berg R, Kiliaridis S. Influence of masticatory muscle function on transverse skull dimensions in the growing rat. J Orofac Orthop. 2002;63:5–13. doi: 10.1007/s00056-002-9903-0. [DOI] [PubMed] [Google Scholar]
- Kokich VG. Age changes in the human frontozygomatic suture from 20 to 95 years. Am J Orthod. 1976;69:411–430. doi: 10.1016/0002-9416(76)90209-8. [DOI] [PubMed] [Google Scholar]
- Koskinen L. Adaptive sutures. Changes after unilateral masticatory muscle resection in rats. A microscopic study. Proc Finnish Dent Soc. 1977;73:3–80. [PubMed] [Google Scholar]
- Kreiborg S. Postnatal growth and development of the craniofacial complex in premature craniosynostosis. In: Cohen MM Jr, editor. Craniosynostosis, Diagnosis, Evaluation and Management. New York: Oxford University Press; 2000. pp. 81–103. [Google Scholar]
- Krogman WM. Studies in growth changes in the skull and face of anthropoids: Ectocranial and endocranial suture closure in anthropoids and old world apes. Am J Phys Anthropol. 1930;46:315–353. [Google Scholar]
- Kupczik K, Dobson CA, Crompton RH, Phillips R, Oxnard CE, Fagan MJ, O’Higgins P. Masticatory loading and bone adaptation in the supraorbital torus of developing macaques. Am J Phys Anthropol. 2009;139:193–203. doi: 10.1002/ajpa.20972. [DOI] [PubMed] [Google Scholar]
- Kupczik K, Dobson CA, Fagan MJ, Crompton RH, Oxnard CE, O’Higgins P. Assessing mechanical function of the zygomatic region in macaques: Validation and sensitivity testing of finite element models. J Anat. 2007;210:41–53. doi: 10.1111/j.1469-7580.2006.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz GE, Yates FW, Devlin M, St Claire M. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. J Hum Evol. 2004;46:655–677. doi: 10.1016/j.jhevol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nature Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Markey MJ, Main RM, Marshall CR. In vivo cranial suture function and suture morphology in the extant fish Polypterus: Implications for inferring skull function in living and fossil fish. J Exp Biol. 2006;209:2085–2102. doi: 10.1242/jeb.02266. [DOI] [PubMed] [Google Scholar]
- Markey MJ, Marshall CR. Linking form and function of the fibrous joints in the skull: a new quantification scheme for cranial sutures using the extant fish Polypterus endlicherii. J Morph. 2007;268:89–102. doi: 10.1002/jmor.10504. [DOI] [PubMed] [Google Scholar]
- Massler M, Schour I. The growth pattern of the cranial vault in the albino rat as measured by vital staining with alizarine red S. Anat Rec. 1951;110:83–101. doi: 10.1002/ar.1091100109. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;81:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003;18:521–528. doi: 10.1359/jbmr.2003.18.3.521. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Wang X, Kopher RA. Biomechanics of craniofacial sutures: orthopedic implications. Angle Orthod. 2003a;732:128–135. doi: 10.1043/0003-3219(2003)73<128:BOCSOI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Wang X, Mooney MP, Kopher RA, Nudera JA. Strain induced osteogenesis of the craniofacial suture upon controlled delivery of low-frequency cyclic forces. Frontiers Biosci. 2003b;8:10–17. doi: 10.2741/917. [DOI] [PubMed] [Google Scholar]
- Milch RA. Aging of connective tissues. In: Schock NW, editor. Perspectives in experimental gerontology. Springfield: Charles C Thomas; 1966. pp. 109–124. [Google Scholar]
- Miroue M, Rosenberg L. MSD thesis. University of Washington; 1975. The human facial sutures: a morphological and histological study of age changes from 20 to 95 years. [Google Scholar]
- Moazen M, Curtis N, O’Higgins P, Jones MEH, Evans SE, Fagan MJ. Assessment of the role of sutures in a lizard skull: a computer modelling study. Proceedings of the Royal Society B-Biological Sciences. 2009;276:39–46. doi: 10.1098/rspb.2008.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney MP, Fellows-Mayle W, Losken HW, Dechant J, Burrows AM, Smith TD, Cooper GM, Pollack I, Siegel MI. Increased intracranial pressure after coronal suturectomy in craniosynostotic rabbits. J Craniofac Surg. 1999;10:104–110. doi: 10.1097/00001665-199903000-00003. [DOI] [PubMed] [Google Scholar]
- Moss ML. Growth of the calvaria in the rat. The determination of osseous morphology. Am J Anat. 1954;94:333–362. doi: 10.1002/aja.1000940302. [DOI] [PubMed] [Google Scholar]
- Moss ML. Experimental alteration of sutural area morphology. Anat Rec. 1957;127:569–589. doi: 10.1002/ar.1091270307. [DOI] [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Adab K, Gakunga PT. TGF-β2 and TGF-β3 regulate fetal rat cranial suture morphogenesis by regulating levels of cell proliferation and apoptosis. Dev Dynam. 2000;219:237–247. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1044>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Chhabra A, Cho RW, Ogle RC. Cranial suture obliteration is induced by removal of transforming growth factor (TGF)-β3 activity and prevented by removal of TGF-β2 activity from fetal rat calvaria in vitro. J Craniofac Genet Dev Biol. 1999;19:164–173. [PubMed] [Google Scholar]
- Opperman LA, Chhabra A, Nolen AA, Bao Y, Ogle RC. Dura mater maintains rat cranial sutures in vitro by regulating suture cell proliferation and collagen production. J Craniofac Genet Dev Biol. 1998;18:150–158. [PubMed] [Google Scholar]
- Opperman LA, Moursi AM, Sayne JR, Wintergrest AM. Transforming growth factor-beta 3 (Tgf-β3) in a collagen gel delays fusion of the rat posterior interfrontal suture in vivo. Anat Rec. 2002;267:120–130. doi: 10.1002/ar.10094. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Miner Res. 1995;10:1978–1987. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Sweeney TM, Redmon J, Persing JA, Ogle RC. Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn. 1993;198:312–322. doi: 10.1002/aja.1001980408. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, Mao JJ. Nanomechanical properties of facial sutures and sutural mineralization front. J Dent Res. 2004;83:470–475. doi: 10.1177/154405910408300607. [DOI] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW. Craniofacial sutures: Morphology, growth, and in vivo masticatory strains. J Morph. 1999;242:167–197. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW, Marshall CD. Biomechanics of the rostrum and the role of facial sutures. J Morphol. 2003;257:33–44. doi: 10.1002/jmor.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DA, Longaker MT, McCarthy JG, Rosen DM, McMullen HF, Levine JP, Sung J, Gold LI. Studies in cranial suture biology: Part I. Increased immunoreactivity for TGF-b isoforms (b1, b2, and b3) during rat cranial suture fusion. J Bone Miner Res. 1997;12:311–321. doi: 10.1359/jbmr.1997.12.3.311. [DOI] [PubMed] [Google Scholar]
- Rubidge BS, Sidor CA. Evolutionary patterns among Permo-Triassic Therapsids. Annu Rev Ecol Syst. 2001;32:449–480. [Google Scholar]
- Saito K, Shimizu Y, Ooya K. Age-related morphological changes in squamous and parietomastoid sutures of human cranium. Cells Tissues Organs. 2002;170:266–273. doi: 10.1159/000047931. [DOI] [PubMed] [Google Scholar]
- Shibazaki R, Dechow PC, Maki K, Opperman LA. Biomechanical strain and morphological changes with age in rat calvarial bone and sutures. Plastic Reconstr Surg. 2007;119:2167–2178. doi: 10.1097/01.prs.0000260705.70329.38. [DOI] [PubMed] [Google Scholar]
- Sidor CA. Simplication as a trend in synapsid cranial evolution. Evolution. 2001;58:1419–1442. doi: 10.1111/j.0014-3820.2001.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Singhal VK, Mooney MP, Burrows AM, Wigginton W, Losken HW, Smith TD, Towbin R, Siegel MI. Age related changes in intracranial volume in craniosynostotic rabbits using 3-D CT scans. Plast Reconstr Surg. 1997;100:1121–1128. doi: 10.1097/00006534-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Smith TD, Burrows AM, Dumont ER. Microanatomical assessment of nasomaxillary suture patency. Anat Rec. 2010;293:651–657. doi: 10.1002/ar.21125. [DOI] [PubMed] [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: Growth and fusion in relation to masticatory strain. Anat Rec. 2004;276A:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgen M, Baran S, Kaya H, Karadede I. The influence of masticatory hypofunction on craniofacial growth and development in rats. Am J Orthod Dentofac Orthop. 1997;111:189–198. doi: 10.1016/s0889-5406(97)70215-4. [DOI] [PubMed] [Google Scholar]
- Wang Q. Dental maturity and the ontogeny of sex-based differences in the dentofacial complex of rhesus macaques from Cayo Santiago. In: Wang Q, editor. Bones, Genetics, and Behavior of Rhesus Macaques: Macaca mulatta of Cayo Santiago and Beyond. New York: Springer; 2012. pp. 177–194. [Google Scholar]
- Wang Q, Dechow PC, Hens SM. Ontogeny and diachronic changes in sexual dimorphism in the craniofacial skeleton of Rhesus macaques from Cayo Santiago, Puerto Rico. J Hum Evol. 2007;53:350–361. doi: 10.1016/j.jhevol.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Dechow PC, Wright BW, Ross CF, Strait DS, Richmond BG, Spencer MA, Byron CD. Surface strain on bone and sutures in a monkey facial skeleton: an in vitro method and its relevance to Finite Element Analysis. In: Vinyard CJ, Ravosa MJ, Wall CE, editors. Primate Craniofacial Function and Biology. New York: Springer; 2008. pp. 149–172. [Google Scholar]
- Wang Q, Opperman LA, Havill LM, Carlson DS, Dechow PC. Inheritance of sutural pattern at the pterion in Rhesus monkey skulls. Anat Rec. 2006a;288A:1042–1049. doi: 10.1002/ar.a.20373. [DOI] [PubMed] [Google Scholar]
- Wang Q, Smith AL, Strait DS, Wright BW, Richmond BG, Grosse IR, Byron CD, Zapata U. The global impact of sutures assessed in a finite element model of a macaque cranium. Anat Rec. 2010;293:1477–1491. doi: 10.1002/ar.21203. [DOI] [PubMed] [Google Scholar]
- Wang Q, Strait DS, Dechow PC. Fusion patterns of craniofacial sutures in rhesus monkey skulls of known age and sex from Cayo Santiago. Am J Phys Anthropol. 2006b;131:469–485. doi: 10.1002/ajpa.20481. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wood S, Grosse I, Ross C, Zapata U, Byron C, Wright B, Strait D. The role of the sutures in biomechanical dynamic simulation of a macaque cranial finite element model: Implications for the evolution of craniofacial form. Anat Rec. 2012;295:278–288. doi: 10.1002/ar.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BW. Craniodental biomechanics and dietary toughness in the genus Cebus. J Hum Evol. 2005;48:473–492. doi: 10.1016/j.jhevol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Yu JC, Wright RL, Williamson MA, Braselton JP, Abell ML. A Fractal Analysis of Human Cranial Sutures. Cleft Palate Craniofac J. 2003;40:409–415. doi: 10.1597/1545-1569_2003_040_0409_afaohc_2.0.co_2. [DOI] [PubMed] [Google Scholar]