Abstract
Data were collected at a wastewater treatment plant (WWTP) in Burlington, Vermont, USA, (serving 30,000 people) to assess the relative contribution of CSO (combined sewer overflow) bypass flows and treated wastewater effluent to the load of steroid hormones and other wastewater micropollutants (WMPs) from a WWTP to a lake. Flow-weighted composite samples were collected over a 13 month period at this WWTP from CSO bypass flows or plant influent flows (n = 28) and treated effluent discharges (n = 22). Although CSO discharges represent 10% of the total annual water discharge (CSO plus treated plant effluent discharges) from the WWTP, CSO discharges contribute 40–90% of the annual load for hormones and WMPs with high (>90%) wastewater treatment removal efficiency. By contrast, compounds with low removal efficiencies (<90%) have less than 10% of annual load contributed by CSO discharges. Concentrations of estrogens, androgens, and WMPs generally are 10 times higher in CSO discharges compared to treated wastewater discharges. Compound concentrations in samples of CSO discharges generally decrease with increasing flow because of wastewater dilution by rainfall runoff. By contrast, concentrations of hormones and many WMPs in samples from treated discharges can increase with increasing flow due to decreasing removal efficiency.
Introduction
Combined sewer overflows (CSOs) are present in the United States,1 Europe2 and other parts of the world. CSOs include combined collection system of sewage and rainfall runoff. Flow in these combined systems can exceed the capacity of WWTPs (wastewater treatment plants) during stormflows so that mixtures of untreated sewage and stormwater runoff are released to receiving waters. In the US, over 700 cities have CSOs, and they are most commonly located on the east coast, Great Lakes, and Pacific northwest.1 CSOs have been identified as important sources of polycyclic aromatic hydrocarbons, organochlorine compounds, nutrients, and chemical oxygen demand.2−5
Recent studies have identified CSOs as a potential source of wastewater micropollutants (WMPs) to receiving waters,6−13 with substantially elevated WMP concentrations occurring in urban waters following CSO discharges.9,12,14−16 For example, caffeine concentrations in urban streams in Switzerland were higher than in treated WWTP effluent, and stream concentrations were highest during stormflow conditions and lowest during baseflow conditions.17 Loads for ibuprofen have been found to be up to 100 times greater in CSO discharges than in treated effluents.18 Patterns in the enantiomer fraction of the chiral pharmaceutical propranolol in several freshwater sites and a coastal bay indicated that CSO discharges and bypass flows were the largest source of propranolol to coastal waters.9 Furthermore, CSOs constitute a more important source of bacteria than treated WWTP discharges.19
Benotti and Brownawell’s model7 of CSOs as a source of WMPs to an urban estuary indicates that CSO discharges can have concentrations 10 or more times greater than treated wastewater. This model predicts that lack of treatment is a more important factor than dilution by rainfall runoff for compounds with high removal efficiencies; thus, this model predicts that for compounds with high removal efficiencies, higher concentrations can be expected in CSO flows compared to treated WWTP flows. Conversely, for compounds that are not efficiently removed by WWTP processes, CSO discharge concentrations should be more similar to treated discharge concentrations, as dilution by runoff should be a more important factor than the lack of treatment. Although this model assumes constant removal rates by a WWTP, removal rates can be expected to decrease for caffeine, propranolol, and estrogens, as flows increase (and residence times decrease) through a WWTP because of greater water-volume inputs during storms.9,17,20 Thus, hormones and other WMPs that are efficiently removed by treatment during normal flows (resulting in low or nondetectable concentrations in treated effluent) might have substantially elevated concentrations in both CSO bypass and treated effluent flows during storms due to high flow rates.
Consistent with these expectations, concentrations of caffeine and other compounds, which were generally efficiently removed during wastewater treatment, were greater in CSO bypass flows and during stormflow in urban streams receiving CSO discharge than in treated effluents in Burlington, Vermont, USA.12 In contrast to efficiently removed compounds, the highest concentrations for galaxolide (a poorly removed compound) occurred in treated effluents, with lower concentrations in CSO discharges.12 The presence of estrogens in CSO discharges has been suggested as a cause of endocrine disruption in fish,21 but this study21 did not directly measure estrogen concentrations. Little is known about the relative contribution of CSO discharges to hormone loads in the environment, although the occurrence of peak concentrations of estrone (E1) and 17β-estradiol (E2) in an urban stream during stormflows has been attributed to CSO discharges.22 Limited attention has been paid to characterizing the importance of CSOs as a source of hormones to the aquatic environment despite increasing documentation describing the sources and effects of estrogens on biologic populations.23−25 In part, this is due to difficulties in collecting representative samples and measuring flows during these short-duration, highly variable discharges.9,10 The few studies that have included direct sampling of CSO flows have relied on data obtained from a limited number of grab samples.9,12 Quantitative assessment of loads from CSO discharges requires collection of flow data for CSO and the treated effluent and flow-weighted samples collected over the duration of rainstorms.
This study assesses the relative contribution of CSO bypass flows and treated wastewater effluents to the load of steroid hormones and other WMPs from a WWTP to a lake and relates these contributions to the removal efficiency by WWTP processes over a range of flows. Comparison of annual loads from treated wastewater with respect to removal efficiencies provides a quantitative assessment of the relation between degree of removal and the importance of CSO discharges as an environmental source of individual hormones and WMPs. The effect of higher flows on removal efficiencies in the WWTP are also assessed, along with implications for high flows as a disproportionate source of compounds from treated effluents.
Experimental Methods
Analytical Methods and Method Performance
Concentration data are based on two methods for unfiltered samples: a hormone method and an organic wastewater compound (OWC) method.
The hormone method determines 20 analytes including estrogens, androgens, and additional compounds.26 Detection limits for hormones ranged from 0.4 to 4 ng/L. Reporting levels of 100 ng/L for bisphenol A (BPA) and 2000 ng/L for 3-beta-coprostanol (COP) and cholesterol (CHO) were adjusted because of laboratory blank limitations. Twelve hormone method compounds with sufficient detection frequency (55% or greater) to allow computation of CSO bypass loads are discussed in this paper: three estrogens, E1, E2, and estriol (E3); six androgens, 11-keto testosterone (11-K), androstenedione (ADSD), cis-androsterone (CAND), dihydrotestosterone (DHT), epitesosterone (EPI), and testosterone (TES); two sterols, COP and CHO, and BPA (Table S2 in Supporting Information). Additional method details are provided in the Supporting Information and elsewhere.26
Mean method recoveries from spike reagent water for the estrogens and androgens discussed in this paper were between 90 and 115%, with relative standard deviations (RSDs) from 10 to 18% (Table S2). Mean recoveries for BPA were 126% (26% RSD). Mean recoveries for CHO and COP in these reagent-water spikes were higher (about 168%), as were RSDs (about 38%) and were the result of unusually low (≤10% for most) cholesterol-d7 isotope-dilution standard recoveries in reagent-water only matrices (including blank samples) as discussed in Foreman and others26 and the Supporting Information. Two analytes analyzed using the hormone method were detected in one or more of the 23 field blanks at concentrations above the detection limit: COP in two blanks at <2600 ng/L and CHO in four blanks at <6000 ng/L. All concentrations of COP <5000 ng/L and concentrations of CHO <12,000 ng/L were censored in field samples. The median relative percent difference in analyte concentrations was 14% for the 18 replicate samples analyzed using the hormone method.
The OWC method employs continuous liquid–liquid extraction with dichloromethane of unfiltered 1-L water samples.27 Extracts are analyzed for 69 compounds by gas chromatography with electron impact mass spectrometry operated in full scan mode. Six analytes from the OWC method had sufficient detection frequency to allow computation of loads and are discussed in this paper: benzophenone (BEP), beta-sitosterol (SIT), caffeine (CAF), galaxolide (GAL), tris(2-butoxyethyl)phosphate (TBEP), and triclosan (TCS). Reporting levels applied in this study to these compounds are 200 ng/L, except for SIT (800 ng/L). The six compounds analyzed by the OWC method along with COP, CHO, and BPA are collectively referred to as WMPs in the remainder of this paper to help distinguish them from estrogens and androgens. The six compounds analyzed using the OWC method had median spike recoveries of 80% and median RSD (relative standard deviations) of 8.5% for reagent, surface, and groundwater spikes.27 Recoveries for these spikes ranged from 62 to 105%, with RSDs between 5 and 16%. Recoveries for SIT were generally lower (median of 55%) and more variable (median RSD = 22%) than the other five compounds included in the OWC method. None of the compounds analyzed using the OWC method had a detection reported in any of the seven blank samples collected during the study. Median relative percent difference for the six replicates collected during the study was 13%. Additional details on quality assurance and method performance data for this method are available in the Supporting Information.
Sampling Network
Samples were collected from the Main Burlington WWTP in Burlington, Vermont, USA, between November 2007 and December 2008 in order to characterize the concentrations of hormones and WMPs during wet- and dry-weather conditions. This WWTP serves approximately 30,000 people and has a design capacity of 0.2 m3/s. When the capacity of this WWTP is exceeded during stormflows, untreated flows are discharged to the receiving waters; such flows are referred to as CSO bypass flows. The availability of flow data for both CSO bypass flows and treated plant effluent flow at this WWTP allowed collection of flow-weighted samples required for calculating annual loads for target analytes.
Samples collected from the Plant Effluent (PE; n = 22) represent water discharged from the plant following all physical, biological, and chemical treatment steps (Figure S1) at this WWTP. PE samples were used to calculate treated plant effluent loads as indicated below. Samples of untreated wastewater were collected from two sites — Plant Influent (PI) and Combined Sewer Effluent (CSE). Unlike PE samples, samples from PI and CSE sites receive no biological treatment and minimal physical treatment. PI samples (n = 18) were collected between the grit removal and primary settling treatment steps (Figure S1). CSE samples (n = 10) represent CSO bypass flow from the WWTP during CSO events; treatment of these flows is limited to vortex separation for large particle removal and disinfection. CSO bypass loads are based on data from all CSE samples and data from select PI samples as explained below. For the period December 1, 2007 through November 30, 2008, CSO bypass occurred on 37 days in response to daily precipitation ranging from 0.2 to 4.1 cm. Treated plant effluent flow over this period ranged from 116 L/s to 516 L/s and averaged 202 L/s. CSO bypass flows represented 10% of the total annual flow (sum of treated plant effluent plus CSO bypass flows) discharged from this plant during this period. Samples were collected during both stormflow and baseflow (nonstorm) conditions from November 2007 through December 2008 approximately every four to six weeks, depending on the occurrence of stormflows. Sample information including dates, collection method, duration of composite collection, flow rates, and analytical methods used are provided in Table S1. The location of sample sites can be found in the Supporting Information (Figure S1).
Rapid changes in flow that can occur during stormflows and diurnal variability in analyte concentrations require the collection of flow-weighted samples for all sample types.28 Most of the PE and PI samples were collected as flow-weighted composites of 24 1-L samples collected hourly over a day. PE sample collection lagged PI sample collection by the hydraulic residence time of the WWTP (4 h), thus it was possible to compute removal efficiency of the compounds by comparison of concentrations in the PE and PI samples (as detailed in the Supporting Information). CSE samples were generally flow-weighted composites of 1-L samples collected every 15–30 min over the duration of storm events; these events ranged from <1 h to nearly 6 h. Composites for all sample sites were based on flow proportional volumes taken from each individual 1-L sample. This approach is similar to sampling frequencies and approaches that have been shown to result in low (<10%) variation due to sampling for compounds such as caffeine that are commonly used and excreted.28 This approach is suitable for this study due to the widespread use/and or excretion of the hormones and other compounds included in this study.29−35 Samples were kept chilled on ice or frozen (hormones) until processing, and samples collected downstream of chemical treatment for disinfection were treated with ascorbic acid (see Analytical Methods section in the SI).
Load and Removal Rate Efficiency Estimates
Concentration data from both the PI and CSE samples were used for load calculations of CSO bypass because the PI samples were collected over a wider range of flows than the CSE samples and so are essential for representing concentrations over the entire range of CSO bypass flows. CSO bypass flow loads were calculated for all 18 compounds discussed in this paper. Treated plant effluent loads were calculated for all of these compounds except the five compounds (E2, 11-K, DHT, EPI, TES) that had detection frequencies for PE samples less than 50%.
Treatment removal efficiencies for all 18 compounds were computed by comparing concentrations in PI samples with PE samples, as detailed in the Supporting Information. A 4 h lag time was used for all storms because the actual change in hydraulic retention time was not known until the end of sampling. Use of the 4 h lag likely resulted in including a greater proportion of water for the PE samples from the end of the event. Calculations indicate this lag resulted in around 10% of the total sample volume composite shifting from the early part of the event to the latter portion for the highest treated effluent flows sampled. Thus, the removal efficiencies for stormflows may be biased somewhat high, and differences in removal efficiencies between baseflow and stormflow may be greater than indicated in these results. Load estimates are not affected by a constant lag because loads are estimated on concentration-discharge relations, which are not affected by the lag between PI and PE samples, as they reflect the concentration corresponding to the flows that occurred over the sample period.
Concentration-discharge relations were used to calculate treated plant effluent and CSO bypass loads using a tobit regression. Tobit regression was used due to the large degree of censored (nondetected) data for some of the compounds.36 Details on samples and approaches used to determine loads are available in the Supporting Information, as are the tobit regression statistical results for treated plant effluent and CSO bypass loads (Table S4A,S4B).
Results and Discussion
Comparison of Concentrations between CSE/PI and PE Samples
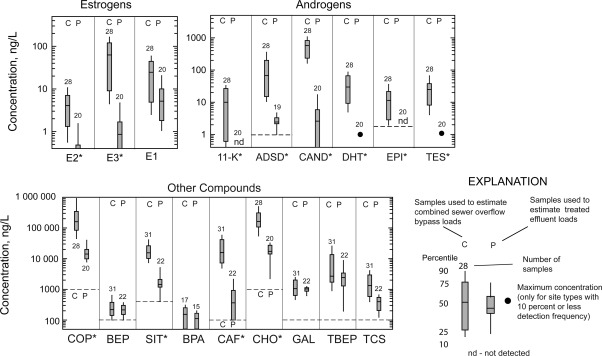
As suggested by the Benotti and Brownawell model,7 all 12 compounds (11-K, ADSD, CAND, CAF, CHO, COP, DHT, E2, E3, EPI, SIT, TES) with high removal efficiencies (>90%) had higher concentrations in CSE and PI samples compared to PE samples (Kruskal–Wallis test, p < 0.05; Figures 1, 2, S5). Concentrations in CSE and PI samples generally range from 1 to 10 ng/L for E2 and 10–100 ng/L for E3, whereas concentrations in PE samples usually are <0.5 ng/L for E2 and 2 ng/L for E3. Androgen concentrations in CSE and PI samples are generally >10 times greater than in PE samples; ADSD and CAND concentrations are highest in CSE and PI samples (generally ranging from 50 to 500 ng/L) but typically low (<5 ng/L) in PE samples. Concentrations of the hormones in these samples and estimated removal efficiencies are similar to those previously reported.17,29,37−39 The high removal efficiencies for estrogens reflect the 10–12 day solid retention time (SRT) maintained at the WWTP; comparable removal efficiencies have been reported for other activated sludge WWTPs operated under similar SRTs.40
Figure 1.
Concentrations of compounds in samples collected from Main Burlington wastewater treatment plant November 2007 to December 2008. An asterisk following the name of a compound indicates that median concentrations for the two types of samples are significantly different by the nonparametric Kruskal–Wallis test at the 0.05 level. For most analytes, the x-axis corresponds to a concentration equal to half of the reporting level; nondetected values are plotted at this level. For some analytes, a dashed line denotes half of the method reporting limit.
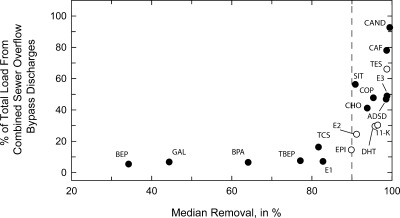
Figure 2.
Median percent removal and percent of total load from combined sewer overflow bypass flow from Main Burlington Vermont wastewater treatment plant samples, 2007–2008. An open circle denotes those compounds that had a treated plant effluent load calculated using 1/2 of the reporting level as the concentration for all samples because the compound was not detected above the method reporting level in treated plant effluent samples frequently enough to compute loads.
The remaining six compounds (BEP, BPA, E1, GAL, TBEP, and TCS) had <90% median removal efficiencies; for most of these compounds, concentrations in CSE and PI samples are not significantly different from PE samples (Kruskal-Wallace test, p < 0.05; Figures 1, 2, S5); this finding is also consistent with the Benotti and Brownawell model.7 The only exception to this generalization is for E1 and TCs; median removal efficiencies for E1 and TCS are higher (around 80%) and less variable than the removals of BEP, BPA, GAL, and TBEP (34–77% median removal; Figure 2). E1 concentrations in CSE and PI samples generally range from 5 to 20 ng/L, and those in PE samples range from 2 to 10 ng/L. E1 is a known metabolite of E2 in activated sludge systems;41 so concurrent production and degradation of E1 would result in higher E1 concentrations in PE samples relative to CSE and PI samples than for E2 or E3. Removal efficiencies for E1 during treatment reportedly are lower than for other estrogens.40,41 Concentrations in these samples and removal efficiencies for TCS and GAL are similar to those reported elsewhere.13,33,34,37,42,43
Concentration-Discharge Relation Used To Determine Loads
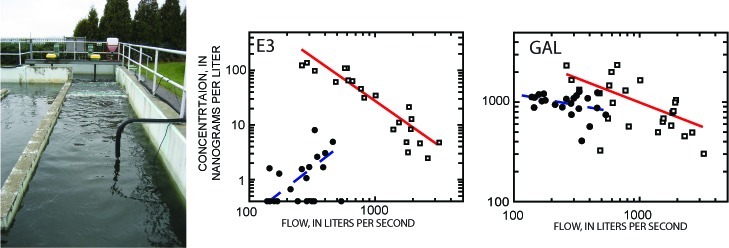
Hormone and WMP concentrations in CSE and PI samples generally decreased with increasing discharge (Figures 3, S2A, S2B), reflecting that dilution of wastewater by storm runoff is the main process controlling concentrations of these compounds in CSE and PI samples. This is similar to the pattern of decreasing total Kjeldahl nitrogen concentration with increasing flow observed in a CSO study;44 this decrease was attributed to the dilution of untreated sewage with storm runoff. A concentration-discharge relation that is controlled solely by the dilution of untreated wastewater by stormflow runoff (assuming that the compound is not present in the stormwater) will have a slope of −1. Five compounds (TES, E2, BPA, CAF, and TCS) in the current study have slopes between −0.8 and −1.2 (Tables S4A, S4B), suggesting that the major factor controlling the concentrations of these compounds in CSE and PI samples is dilution of untreated sewage by stormflow runoff.
Figure 3.
Concentration as a function of discharge for select hormones and wastewater micropollutants in Main Burlington Vermont wastewater treatment plant samples November 2007 to December 2008. These lines are tobit regression lines used to determine loads from combined sewer overflow bypass and treated plant effluent discharges. For all analytes except caffeine, nondetected values are plotted along the x-axis at a value corresponding to 1/2 of the reporting level concentration. For caffeine, nondetections are plotted at 100 ng/L (1/2 of the reporting level).
Seven compounds (TBEP, BEP, CHO, COP, GAL, CAND, and SIT), however, have concentration-discharge slopes of >−0.7 for CSE and PI samples (Tables S4A, S4B), indicating that their concentrations at higher flows are greater than predicted by stormwater dilution. This group includes all four compounds (CHO, COP, GAL, and SIT) that have high log Kow values (>6). Patterns of TSS transport in CSOs reflects a complex mixture of solids derived from wastewater, solids stored in sewage pipes, and solids derived from storm runoff.45 During stormflows, the proportion of flow from wastewater is diminished, and the proportion of flow from road runoff increases. Solids deposited in the sewer system during low flows have been found to be mobilized by high flows,2 resulting in enhanced transport of hydrophobic compounds by CSO discharges.5 The results of the current study are consistent with the hypothesis that solids stored in sewers between events are mobilized during stormflow, resulting in the observed lack of dilution of the four compounds with high log Kow values during CSO discharges.
Although the cause of the lack of dilution for the compounds in this group that have log Kow < 6 is unclear, other factors may result in the observed lack of dilution. The lack of dilution for SIT might also partially reflect contributions of this plant sterol from terrestrial plant debris washed into the storm sewer system.46 Transport associated with sediment particles is not as likely for TBEP (log Kow = 3.0), yet TBEP is used in synthetic rubber and plastics production and has been detected in snow.47 Thus, the low degree of dilution for TBEP may reflect the contribution of road surface runoff containing TBEP that occurs during stormflows. The lack of substantial dilution (regression slope of −0.34; Figure S2A, Table S4B) of CAND in CSE and PI samples with increasing discharge is not clear. One possibility is that the amount of CAND associated with suspended particles contributed from storm runoff might be greater than predicted from its octanol–water partition coefficient relative to the other hormones (Table S2). Another possibility is that CAND is being formed, for example, by oxidation of 17α- or β-isomers of 5α-androstane-3α,17-diol (not determined in this study but present in urinary excretions30) via enzymatic processes comparable to those that form E1 from E2.48
Several androgens (11-K, ADSD, EPI, DHT) and two estrogens (E1 and E3) have CSE and PI sample concentrations that are lower than predicted by dilution at higher flows (concentration-discharge slopes ←1.2; Table S4B). The reason for this is not clear, but for androgens may be because these compounds are degradation products of other androgens (especially testosterone) that might not be formed as effectively during high flow conditions as they are during low flow conditions.29,30
By contrast, concentrations of compounds in PE samples are not affected by dilution, as concentrations of many of these compounds significantly increase with flows (Figures 3, S2A, S2B). In general, the slope of the concentration-discharge curve for PE samples is more positive for compounds with high removal efficiencies (including CAF, CAND, COP, SIT, CHO) than for compounds with low removal efficiencies (BEP, GAL, BPA, TBEP). A significant increase in concentration with increasing flows occurs for many compounds for treated flows (see Table S4A) despite the decrease in concentration observed for untreated (CSE and PI) samples with increasing discharge (Table S4B). The observed lack of dilution in PE samples reflects the importance of decreased treatment efficiency (Figures S6A, S6B) that occurs for many compounds with increasing flows. This decreased treatment efficiency probably reflects less efficient biological treatment that occurs when hydraulic retention times lessen during higher flows.
Annual Loads for Treated and CSO Flows
Loads calculated using these data show that CSO bypass flow contributes a substantial proportion of total annual load for many hormones and WMPs (Figures 2, S5), particularly for those that are efficiently removed during wastewater treatment. Total annual load from this WWTP is equal to the sum of the load for the treated plant effluent and the load from the CSO flow bypass. CAF and CAND, compounds with the highest median removal efficiencies (98–99%), also had the highest proportion of total annual load from CSO bypass flow (81 and 93%, respectively). The other five compounds (SIT, CHO, COP, ADSD, and E3) having high removal efficiencies and sufficient detection frequency for treated load estimates had CSO bypass loads representing 43–61% of the total annual loads. These proportions are much higher than the proportion of total annual water discharge as CSO bypass flow (10%). The finding that most of the annual load (>80%) of caffeine from this WWTP is attributable to CSO bypass flows supports the hypothesis that CSOs can be a major source of caffeine to receiving waters.17 These results demonstrate the importance of quantifying concentrations of hormones and other compounds in bypass flows to provide a more accurate assessment of contaminant loads from CSOs relative to the contributions from WWTP discharges.
Assessment of the importance of CSO bypass loads for five compounds with high removal efficiencies (EPI, E2, 11-K, DHT, TES) was hampered by their low (<50%) detection frequency in treated samples (Figure 1; Table S2). Consequently, treated plant effluent load estimates for these compounds are based on using a concentration equal to 50% of the reporting level. This load estimate should be considered an upper bound on treated plant effluent loads and is used only to give a general comparison between CSO bypass loads and treated plant effluent loads; it is likely that the fraction of annual load discharged by CSO discharges for these five compounds is substantially greater than depicted in Figures 2 and S5. Median removals for EPI, E2, and 11-K range from 90–96%, and the estimate of total load from CSO bypass flows ranges from 17–29% (Figure 2). Median removal amounts for DHT and TES were higher (96% and 99%, respectively), as was the proportion of total annual load from CSO bypass flows (35% and 70%, respectively). Thus, even for compounds with conservative (high) treated plant effluent load estimates, CSO bypass flows appear to contribute disproportionally to the total annual load discharged from the Main Burlington WWTP to Lake Champlain.
CSO bypass flows are not as important for annual loads for compounds that are poorly removed during wastewater treatment. The remaining six compounds included in this study (BEP, BPA, E1, GAL, TBEP, and TCS) had <90% median removal efficiencies, and all but TCS (19%) had <10% contributions of total annual load from CSO bypass flows (Figure 2). These findings are in agreement with the Benotti and Brownawell7 model and observations in Germany14 that untreated discharges resulting from CSO events contribute a disproportionate load for compounds that are efficiently removed by wastewater treatment but are less important for compounds that are not efficiently removed.
CSOs have been shown to adversely affect water quality,49−51 and discharges of nutrients, pathogens, and other contaminants during CSO events have been linked to acute water-quality issues such as fish kills52 and to chronic water-quality issues in urban streams.14 For the Main Burlington WWTP, there were 37 CSO bypass flow discharges over the year studied, with discharges lasting from around 1 to 10 h. Although these flows are sporadic and of short duration, results of the current study show that concentrations of hormones and many WMPs in CSO bypass flows were up to 10 times higher than those from treated plant effluent and contribute a substantial portion of the total mass discharged to the receiving water. Thus, models of WWTP discharge that do not account for CSO discharges can severely underestimate the environmental occurrence of certain emerging contaminants.9 This is particularly true for hormones and other WMPs that are effectively removed during wastewater treatment.
The importance of hormone and WMP discharges from CSO bypass flows is dependent on the characteristics of the receiving waters. In settings such as Lake Champlain, where a CSO bypass flow discharges into a large body of water with a high degree of mixing, ecological effects might be mitigated by rapid dilution compared to other settings. Biota in urban estuaries, such as Jamaica Bay in New York,7 or other locations with receiving waters having comparatively small volume and limited mixing potential might be substantially affected by CSO discharges. In such settings, high-concentration pulses of hormones discharged to the receiving water after a CSO event might adversely affect fish reproduction, especially if they occur during key spawning or growth development periods. In addition, CSO pulses can be a major source of endocrine-disrupting compounds to sediments in receiving waters, and so may affect fish reproduction52 and enter the food chain through benthic organisms.53 Concentrations of individual estrogens in CSO bypass flows in this study commonly range from 2 to 20 ng/L (Figure 1), well within the range of concentrations (around 1 ng/L) associated with reproductive disruption in fish.24 Other sources of untreated wastewater may have similar effects as CSO bypass flow; interconnections between sewer pipes and storm drains and leaky sewer pipes also are potential sources of untreated wastewater to streams.16,54 Based on these results, assessments of sources and ecological effects of hormones and WMPS should consider CSO bypass discharges and other sources of untreated wastewater.
Importance of High Flows for Treated Plant Effluent Loads
Previous studies have shown lower removal efficiencies for hormones and other WMPs with decreased hydraulic residence time in a WWTP.9,20,38,54 Indeed, removal efficiencies significantly decreased with increasing PE flow for nine (COP, SIT, CAF, CHO, CAND, E3, GAL, TBEP, TCS) of the thirteen compounds having sufficient data to allow for this comparison (Figures S6A, S6B). Reduced hydraulic residence times during storm events have at least two possible influences on removal efficiency: decreased exposure time for degradation by the activated sludge (as appears the case for caffeine) and decreased removal of solids material (as appears the case for CHO, COP, and SIT, which can be expected to have substantial particle-phase concentrations). During high flows, the Main Burlington WWTP operates in contact stabilization mode in order to reduce the amount of solids lost from the system. This is done to maintain the 10 to 12 day SRT that the plant operates in during high flow events. Although TSS concentrations in PE samples increase with flows from around 1 to 10 mg/L, concentrations are still far less than the 100–200 mg/L concentrations associated with untreated sewage samples (Figure S3).
Although days with treated plant effluent flows greater than the WWTP’s design capacity (>325 L/s) only constitute around 11% of the annual treated plant water discharge, these high flows make a disproportionate contribution to annual treated plant loads for several compounds. The proportion of annual treated plant load represented by high flows (>325 L/s) ranged from 20 to 45% for several compounds (E3, CAND, COP, SIT, CAF, CHO, and TBEP; Figure S7). The higher proportions of treated plant effluent loads associated with high flows reflect the substantial decrease in removal efficiency with increasing flows; all these compounds have a slope >1 for the log–log relation between concentration and plant effluent flow (Table S4A, S4B). These results demonstrate that even if CSO bypass flows did not occur at this WWTP, a disproportionate amount of the annual load of some hormones and WMPs would occur at higher flows due to decreased removal efficiencies.
Acknowledgments
The staff of the Main Burlington WWTP offered open access and invaluable help in installing equipment and collecting samples throughout this study; Stephen Roy and Tim Grover in particular provided valuable assistance. Dan Edwards, Elizabeth Nystrom, John Byrnes, and Laura Medalie helped to install equipment and collect and process samples. Paul Stackelberg assisted in statistical analysis. Ed Kolodziej and Paul Bradley provided useful comments on a draft of the manuscript.
Supporting Information Available
Text that gives sampling details and additional information on results and 5 tables and 8 figures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- U.S. Environmental Protection Agency, National Pollutant Discharge Elimination System (NPDES) Combined Sewer Overflows. http://cfpub.epa.gov/npdes/home.cfm?program_id=5 (accessed March 5, 2012).
- Gasperi J.; Garnaud S.; Rocher V.; Moilleron R. Priority pollutants in wastewater and combined sewer overflow. Sci. Total Environ. 2008, 407, 263–272. [DOI] [PubMed] [Google Scholar]
- Alp E.; Melching C. S.; Zhang H.; Lanyon R. Effectiveness of combined sewer overflow treatment for dissolved oxygen improvement in the Chicago Waterways. Water Sci. Technol. 2007, 56, 215–222. [DOI] [PubMed] [Google Scholar]
- Casadio A.; Maglionico M.; Bolognesi A.; Artina S. Toxicity and pollutant impact analysis in an urban river due to combined sewer overflows loads. Water Sci. Technol. 2010, 61, 207–215. [DOI] [PubMed] [Google Scholar]
- Eganhouse R. P.; Sherblom P. M. Anthropogenic organic contaminants in the effluent of a combined sewer overflow: impact on Boston Harbor. Mar. Environ. Res. 2001, 51, 51–74. [DOI] [PubMed] [Google Scholar]
- Ellis J. B. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [DOI] [PubMed] [Google Scholar]
- Benotti M. J.; Brownawell B. J. Distributions of pharmaceuticals in an urban estuary during both dry- and wet-weather conditions. Environ. Sci. Technol. 2007, 41, 5795–5802. [DOI] [PubMed] [Google Scholar]
- Cantwell M. G.; Wilson B. A.; Zhu J.; Wallace G. T.; King J. W.; Olsen C. D.; Burgess R. M.; Smith J. P. Temporal Trends of triclosan contamination in dated sediment cores from four urbanized estuaries: Evidence of preservation and accumulation. Chemosphere 2010, 78, 347–352. [DOI] [PubMed] [Google Scholar]
- Fono L.; Sedlak D. L. Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters. Environ. Sci. Technol. 2005, 39, 9244–9252. [DOI] [PubMed] [Google Scholar]
- Bester K.; Scholes L.; Wahlberg C.; McArdell C. S. Sources and mass flows of xenobiotics in urban water cycles – an Overview on current knowledge and data gaps. Water, Air, Soil, Pollut.: Focus 2008, 8, 407–423. [Google Scholar]
- Miller T. R.; Heidler J.; Shillrud S. N.; Delaquil A.; Ritchie J. C.; Mihalic J. N.; Bopp R.; Halden R. U. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediment. Environ. Sci. Technol. 2008, 42, 4570–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.; Chalmers A. Wastewater effluent, combined sewer overflows, and other sources of organic compounds to Lake Champlain. J. Am. Water Resour. Assoc. 2009, 45, 45–57. [Google Scholar]
- Radke M.; Ulrich H.; Wurm C.; Kunkel U. Dynamics and attenuation of acidic pharmaceuticals along a river stretch. Environ. Sci. Technol. 2010, 44 (8), 2968–2974. [DOI] [PubMed] [Google Scholar]
- Weyrauch P.; Matzinger A.; Pawlowsky-Reusing E.; Plume S.; von Seggern D.; Heinzmann B.; Schroeder K.; Rouault P. Contribution of Combined sewer overflows to trace contaminant loads in urban streams. Water Res. 2010, 44, 4451–4462. [DOI] [PubMed] [Google Scholar]
- Musolff A.; Leschik S.; Reinstorf F.; Strauch G.; Schirmer M. Micropollutant loads in the urban water cycle. Environ. Sci. Technol. 2010, 44 (13), 4877–4883. [DOI] [PubMed] [Google Scholar]
- Boyd G. R.; Palmeri J. M.; S. Zhang S.; Grimm D. A. Pharmaceutical and personal care products and endocrine disrupting chemicals in stormwater canals and Bayou St. John in New Orleans, Louisiana, US. Sci. Total Environ. 2004, 333, 137–148. [DOI] [PubMed] [Google Scholar]
- Buerge I. J.; Poiger T.; Muller M. D.; Buser H. R. Combined sewer overflows to surface waters detected by the anthropogenic marker caffeine. Environ. Sci. Technol. 2006, 40 (13), 4096–4102. [DOI] [PubMed] [Google Scholar]
- Buser H.-R.; Poiger T.; Muller T. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33 (13), 4096–4102. [Google Scholar]
- Rechenburg A.; Koch C.; Classen T.; Kistmann T. C. Impact of sewage treatment plants and combined sewer overflow basins on the microbiological quality of surface water. Water Sci. Technol. 2006, 54 (3), 95–99. [DOI] [PubMed] [Google Scholar]
- Schlusener M. P.; Bester K. Behavior of steroid hormones and conjugates during wastewater treatment – A comparison of three sewage treatment plants. Clean 2008, 36 (1), 25–33. [Google Scholar]
- Johnson L. L.; Lomax D. P.; Myers M. S.; Olson O. P.; Sol S. Y.; O’Neill S. M.; West J.; Collier T. K. Xenoestrogen exposure and effects in English sole (Parophyrys vetulus) from Puget Sound, WA. Aquat. Toxic. 2008, 88, 29–38. [DOI] [PubMed] [Google Scholar]
- Pailler J.-Y.; Guignard C.; Meyer B.; Iffly J.-F.; Pfister L.; Hoffmann L.; Krein A. Behaviour and fluxes of dissolved antibiotics, analgesics and hormones during flood events in a small heterogeneous catchment in the Grand Duchy of Luxembourg. Water, Air, Soil Pollut. 2009, 203, 79–98. [Google Scholar]
- Kolodziej E. P.; Gray J. L.; Sedlak D. L. Quantification of Steroid Hormones with Pheromonal Properties in Municipal Wastewater Effluent. Environ. Toxicol. Chem. 2003, 22 (11), 2622–2629. [DOI] [PubMed] [Google Scholar]
- Vajda A. M.; Barber L. B.; Gray J. L.; Lopez E. M.; Woodling J. D.; Norris D. O. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ. Sci. Technol. 2008, 42 (9), 3407–3414. [DOI] [PubMed] [Google Scholar]
- Lange R.; Hutchinson T. H.; Croudace C. P.; Siegmund F.; Schweinfurth H.; Hampe P.; Panter G. H.; Sumpter J. P. Effects of the synthetic estrogen 17-alpha-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2011, 20, 1216–1227. [DOI] [PubMed] [Google Scholar]
- Foreman W. T.; Gray J. L.; ReVello R. C.; Lindley C. E.; Losche S. A.; Barber L. B.. Determination of steroid hormones and related compounds in filtered and unfiltered water by solid-phase extraction, derivatization, and gas chromatography with tandem mass spectrometry; USGS Tec. Meth. b. 5; ch. B6, 2012, 52 p. [Google Scholar]
- Zaugg S. D.; Smith S. G.; Schroeder M. P.. Determination of wastewater compounds in whole water by continuous liquid–liquid extraction and capillary-column gas chromatography/mass spectrometry; USGS Tec. Meth. b. 5; ch. B4, 2006, 40 p. [Google Scholar]
- Ort C.; Lawrence M. G.; Reungoat J.; Mueller J. F. Sampling for PPCPS in wastewater systems: Comparison of different sampling modes and optimization strategies. Environ. Sci. Technol. 2010, 44, 6289–6296. [DOI] [PubMed] [Google Scholar]
- Chang H; Wan Y.; Wu S.; Fan Z.; Hu J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 632–40. [DOI] [PubMed] [Google Scholar]
- Liu Z. H.; Kanjo Y.; Mizutani S. Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: A review. Sci. Total Environ. 2009, 407, 4975–4985. [DOI] [PubMed] [Google Scholar]
- Silva C. P.; Otero M.; Esteves V. Processes for the elimination of estrogenic steroid hormones from water: A review. Environ. Pollut. 2012, 165, 38–58. [DOI] [PubMed] [Google Scholar]
- Bellet V.; Hernandez-Raquet G.; Dagnino S.; Seree L.; Pardon P.; Bancon-Montiny C.; Fenet H.; Creusot N.; Ait-Aissa S.; Cavailles V.; Budzinski H.; Antignac J.-P.; Balaguer P. Occurrence of androgens in sewage treatment plants influents is associated with antagonist activities on other steroid receptors. Water Res. 2012, 46, 1912–1922. [DOI] [PubMed] [Google Scholar]
- McAvoy D. C.; Schatowitz B.; Jacob M.; Hauk A.; Eckhoff W. S. Measurement of triclosan in wastewater treatment systems. Environ. Toxicol. Chem. 2002, 21, 1323–1329. [PubMed] [Google Scholar]
- Nakada N.; Tanishima T.; Shinohara H.; Kiri K.; Takada H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [DOI] [PubMed] [Google Scholar]
- Buerge I. J.; Poiger T.; Muller M. D.; Buser H. R. Caffeine, anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [DOI] [PubMed] [Google Scholar]
- Helsel D. R.; Hirsch R. M.. Statistical Methods in Water Resources Techniques of Water Resources Investigations. USGS Tech. Water Res. Inv., Book 4, Chap. A3, 2002;522 p. http://pubs.usgs.gov/twri/twri4a3/ (accessed March 6, 2012).
- Heidler J.; Halden R. U. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42, 6324–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. C.; Aerni H.-R.; Gerritse A.; Gilbert W.; Hylland K.; Juergens M.; Nakari T.; Pickering A.; Suter J.J.-F.; Svenson A.; Wettstein F. E. Comparing steroid estrogen, and nonylphenol content across a range of European sewage plants with different treatment and management practices. Water Res. 2005, 39, 47–58. [DOI] [PubMed] [Google Scholar]
- Muller M.; Rabenoelina F.; Balaguer P.; Patureau D.; Lemenach K.; Budzinski H.; Barcelo D.; López de Alda M.; Kuster M.; Delgenès J.-P.; Hernandez-Raquet G. Chemical and biological analysis of endocrine-disrupting hormones and estrogenic activity in an advanced sewage treatment plant. Environ. Toxicol. Chem. 2008, 27, 1649–1658. [DOI] [PubMed] [Google Scholar]
- Clara M.; Kreuzinger N.; Strenn B.; Gans O.; Kroiss H. The solids retention time – a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Kanjo Y.; Mizutani S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment – physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [DOI] [PubMed] [Google Scholar]
- Miege C.; Choubert J. M.; Ribeiro L.; Eusebe M.; Coquery M. Fate of pharmaceuticals and personal care products in wastewater treatment plants – Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [DOI] [PubMed] [Google Scholar]
- Reiner J., L.; Berset J. D.; Kannan K. Mass flow of polycyclic musks in two wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2007, 52, 451–547. [DOI] [PubMed] [Google Scholar]
- Kafi M; Gasperi J; Lorgeoux C; Moilleron R; Gromaire M. C.; Chebbo G. Spatial variability of the characteristics of conveyed wet weather pollutant loads in Paris. Water Res. 2008, 42, 539–549. [DOI] [PubMed] [Google Scholar]
- Gasperi J.; Garnaud S.; Rocher V.; Moilleron R. Priority pollutants in surface waters and settleable particles within a densely urbanised area: Case study of Paris (France). Sci. Total Environ. 2009, 407, 2900–2908. [DOI] [PubMed] [Google Scholar]
- Pedersen J.; Yeager M.; Suffet I. Xenobiotic organic compounds in runoff from fields irrigated with treated wastewater. J. Agric. Food Chem. 2003, 51, 1360–1372. [DOI] [PubMed] [Google Scholar]
- Marklund A.; Andersson B.; Haglund P. Traffic as a source of organophosphorus flame retardants and plasticizers in snow. Environ. Sci. Technol. 2005, 39, 3555–3562. [DOI] [PubMed] [Google Scholar]
- Ternes T. A.; Kreckel P.; Mueller J. Behavior and occurrence of estrogens in municipal sewage treatment plants - II. Aerobic batch experiments with activated sludge. Sci. Total Environ. 1999, 225, 91–99. [DOI] [PubMed] [Google Scholar]
- Wilkison D. H.; Armstrong D. A.; Hampton S. A.. Character and trends of water quality in the Blue River Basin, Kansas City Metropolitan Area, Missouri and Kansas, 1998 through 2007; USGS 733; Sci. Inv. Rep. 2009–5169, 2009, 211 p. [Google Scholar]
- Even S.; Mouchel J.-M.; Servais P.; Flipo N.; Poulin M.; Blanc S.; Chabanel M.; Paffoni C. Modeling the impacts of combined sewer overflows on the river Seine water quality. Sci. Total Environ. 2007, 375, 140–151. [DOI] [PubMed] [Google Scholar]
- Gasperi J.; Gromaire M. C.; Kafi M.; Moilleron R.; Chebbo G. Contributions of wastewater, runoff and sewer deposit erosion to wet weather pollutant loads in combined sewer systems. Water Res. 2010, 44, 5875–5886. [DOI] [PubMed] [Google Scholar]
- Keiter S.; Rastall A.; Kosmehl T.; Wurm K.; Erdinger L.; Braunbeck T.; Hollert H. Ecotoxicological assessment of sediment, suspended matter and water samples in the upper Danube River - A pilot study in search for the causes for the decline of fish catches. Environ. Sci. Pollut. Res. Int. 2006, 13, 308–319. [DOI] [PubMed] [Google Scholar]
- Ferguson P.; Bopp F.; Chillrud S.; Aller R.; Brownawell B. Biogeochemistry of nonylphenol ethoxylates in urban estuarine sediments. Environ. Sci. Technol. 2003, 37, 3499–3506. [DOI] [PubMed] [Google Scholar]
- Kuroda K.; Murakami M.; Oguma K.; Muramatsu Y.; Takada H.; Takizawa S. Assessment of groundwater pollution in Tokyo using PPCPs as sewage markers. Environ. Sci. Technol. 2012, 46, 1455–1464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.