Abstract
Identification of genes regulated by the ferric uptake regulator (Fur) protein has provided insights into the diverse mechanisms of adaptation to iron limitation. In the soil bacterium Bacillus subtilis, Fur senses iron sufficiency and represses genes that enable iron uptake, including biosynthetic and transport genes for the siderophore bacillibactin and uptake systems for siderophores produced by other organisms. We here demonstrate that Fur regulates hmoA (formerly yetG), which encodes a haem monooxygenase. HmoA is the first characterized member of a divergent group of putative monooxygenases that cluster separately from the well-characterized IsdG family. B. subtilis also encodes an IsdG family protein designated HmoB (formerly YhgC). Unlike hmoA, hmoB is constitutively expressed and not under Fur control. HmoA and HmoB both bind haemin in vitro with approximately 1 : 1 stoichiometry and degrade haemin in the presence of an electron donor. Mutational and spectroscopic analyses indicate that HmoA and HmoB have distinct active site architectures and interact differently with haem. We further show that B. subtilis can use haem as an iron source, but that this ability is independent of HmoA and HmoB.
Introduction
Almost all bacteria require iron for growth. In most aerobic environments, however, iron availability is limited by the formation of highly insoluble hydroxides. To overcome this scarcity, bacteria have developed several mechanisms for iron acquisition (Andrews et al., 2003). Many bacteria secrete low-molecular-mass iron chelators called siderophores that have a high affinity for ferric iron. Iron–siderophore complexes are transported through high-affinity uptake systems to the cytoplasm, where iron is removed from the complex either by reduction or by cleavage of the ferri–siderophore complex using an esterase.
The ability of bacteria to use haem as an iron source has been best studied in human pathogens (Skaar & Schneewind, 2004). These organisms face a significant challenge, since almost all iron in human tissues is tightly sequestered by haemoproteins and by high-affinity iron binding proteins such as transferrin and lactoferrin. Indeed, the withholding of iron from invading organisms is an important component of the innate immune system (Cornelissen, 2003). Bacterial pathogens have developed elaborate systems to acquire iron from the host, including expression of high-affinity siderophores, transferrin receptors, and proteins implicated in haem and haemoprotein utilization. Although best documented for pathogens, several free-living Rhizobium species can also acquire iron from haem (Noya et al., 1997). In Bradyrhizobium japonicum, haem uptake genes have been characterized (Nienaber et al., 2001) and two haem-degrading monoxygenases have been identified (Puri & O’Brian, 2006).
The soil bacterium Bacillus subtilis serves as a model organism for the investigation of iron homeostasis (Baichoo et al., 2002; Moore & Helmann, 2005). B. subtilis has several routes to acquire iron including synthesis and import of the high-affinity siderophore bacillibactin (BB) (May et al., 2001), although laboratory strains (B. subtilis 168 and its derivatives) fail to synthesize the intact siderophore due to a mutation in the gene encoding the phosphopantetheinyltransferase Sfp. Upon binding iron, the Fe–BB complex binds with high affinity to the substrate binding protein FeuA (Miethke et al., 2006), and is transported through the ATP binding cassette (ABC) transport system FeuBC in conjunction with the YusV ATPase (Ollinger et al., 2006). In addition to its own siderophore, B. subtilis pirates other siderophores through expression of specific uptake systems, efficiently utilizes ferric citrate, and has an oxidase-dependent elemental iron transport (OfeT-type) system (Grosse et al., 2006; Ollinger et al., 2006). It has been shown that haemin can rescue the growth of a B. subtilis hemA mutant, which is unable to synthesize haem (Miczák, 1977). Moreover, exogenously added haemin can restore cytochrome c function to ccdA mutant cells (Schiött et al., 1997). To our knowledge, however, it has never been shown that B. subtilis can use haem as an iron source.
Here we demonstrate that B. subtilis can utilize haem as an iron source and that its genome encodes two haem monooxygenase homologues (HmoA and HmoB). HmoB belongs to the well-characterized IsdG family and is not regulated by iron, while the Fur-regulated HmoA protein represents a previously uncharacterized subgroup of monooxygenases that is also found in several pathogenic bacteria. Although both HmoA and HmoB are haem binding proteins, they are not required for iron utilization from added haem and their in vivo role remains to be resolved.
Methods
Strain construction and growth conditions.
All B. subtilis strains used in this study are derivatives of CU1065 (trpC2 attSPβ sfp0) and are listed in Table 1. For selection, antibiotics were added at the following concentrations: erythromycin (1 µg ml−1) and lincomycin (25 µg ml−1) [for selecting for macrolide–lincosamide–streptogramin B (MLS) resistance], spectinomycin (100 µg ml−1), chloramphenicol (10 µg ml−1), kanamycin (15 µg ml−1), tetracycline (5 µg ml−1) and neomycin (10 µg ml−1). Growth curves were determined using a Bioscreen C MBR system with OD600 measurements every 10 min for 24 h.
Table 1. Strains used in the study.
| Strain | Genotype | Source or reference |
| CU1065 | trpC2 attSPβ sfp0 | Laboratory stock |
| HB2501 | CU1065 fur : : kan | Fuangthong & Helmann (2003) |
| HB8402 | CU1065 hmoA : : spc | This study |
| HB8404 | CU1065 SPβc2Δ2 : : Tn917 : : ϕ (hmoA-cat-lacZ) | This study |
| HB8405 | HB2501 SPβc2Δ2 : : Tn917 : : ϕ (hmoA-cat-lacZ) | This study |
| HB8412 | CU1065 hmoB : : tet | This study |
| HB8413 | CU1065 hmoA : : spc hmoB : : tet | This study |
| HB8419 | CU1065 SPβc2Δ2 : : Tn917 : : ϕ (hmoB-cat-lacZ) | This study |
| HB8420 | HB2501 SPβc2Δ2 : : Tn917 : : ϕ (hmoB-cat-lacZ) | This study |
| HB11078 | CU1065 ywbLMN : : kan | This study |
| HB11080 | CU1065 hmoA : : spc ywbLMN : : kan | This study |
| HB11081 | CU1065 hmoB : : tet ywbLMN : : kan | This study |
| HB11082 | CU1065 hmoA : : spc hmoB : : tet ywbLMN : : kan | This study |
| HE8253 | E. coli BL21(DE3) pLysS with hmoA in pET16b | This study |
| HE8255 | E. coli BL21(DE3) pLysS with hmoB in pET16b | This study |
| HE8261 | E. coli BL21(DE3) pLysS with hmoA R6A in pET16b | This study |
| HE8262 | E. coli BL21(DE3) pLysS with hmoA R6N in pET16b | This study |
| HE8263 | E. coli BL21(DE3) pLysS with hmoB N70A in pET16b | This study |
| HE8264 | E. coli BL21(DE3) pLysS with hmoB N70R in pET16b | This study |
Routine molecular biology procedures were carried out using Escherichia coli DH5α for DNA cloning as described by Sambrook & Russell (2001). Isolation of B. subtilis chromosomal DNA, transformation and specialized SPβ transduction were performed as described by Cutting & VanderHorn (1990). Restriction enzymes, DNA ligase and DNA polymerases were used according to the manufacturer’s instructions (New England Biolabs). Primers used are listed in Supplementary Table S1.
B. subtilis was grown in Luria–Bertani (LB) medium or in a MOPS-based iron starvation minimal medium (Chen et al., 1993). Metals were added from filter-sterilized stocks before inoculation. Haemin was dissolved in 0.1 M NaOH and neutralized by adding an equal volume of 1.0 M Tris buffer (pH 7.5) (Tullius et al., 2011). Haemin solutions were treated overnight with 5 % (w/v) Chelex-100 to remove contaminating iron and filter-sterilized.
Mutants were constructed using long-flanking-homology PCR (LFH-PCR) as described previously (Butcher & Helmann, 2006). To construct cat–lacZ transcriptional fusions, regulatory regions were amplified from genomic DNA by PCR and cloned as HindIII–BamHI fragments into pJPM122 (Slack et al., 1993). The resulting constructs were linearized with ScaI and transformed into ZB307A (Zuber & Losick, 1987) selecting for neomycin resistance. A SPβ-transducing lysate was prepared by heat induction and used to transduce different strains as indicated (Slack et al., 1993). β-Galactosidase activity was assayed using a modification of the procedure of Miller (1972), as described by Bsat et al. (1998).
DNA binding assays.
Fur was purified as described previously (Bsat & Helmann, 1999). The hmoA promoter region was amplified from B. subtilis chromosomal DNA by PCR using a [γ-32P]ATP-labelled primer, and electrophoretic mobility shift assays (EMSAs) were performed using DNA (<100 pM) essentially as described previously (Baichoo & Helmann, 2002).
Expression and purification of HmoA and HmoB.
The hmoA and hmoB coding sequences were amplified from the B. subtilis 168 genome using PCR, cloned into pET16B (Novagen) and used to transform E. coli BL21(DE3) pLysS (Novagen). For overexpression, cells were grown in LB with appropriate antibiotics at 37 °C to OD600 0.4. After addition of 1 mM IPTG, cells were further incubated for 2 h and harvested by centrifugation. Purification of His-tagged proteins was done using PrepEase His-tagged protein purification resin (USB) according to the manufacturer’s instructions. Purified proteins were dialysed against 50 mM Tris/HCl (pH 8), 150 mM NaCl, 5 % (v/v) glycerol and stored at −20 °C.
Haemin binding to HmoA and HmoB.
The binding of haemin to HmoA and HmoB was monitored spectrophotometrically in 50 mM Tris/HCl (pH 8) and 150 mM NaCl, as described by Skaar et al. (2006). Haemin was added to wild-type and mutant proteins in a 4 : 1 molar ratio. The resulting proteins were repurified using PrepEase His-tagged protein purification resin and dialysed against 50 mM Tris/HCl (pH 8), 150 mM NaCl, 5 % (v/v) glycerol. Some precipitate was observed after dialysis, which was removed by centrifugation.
Haemin degradation assay.
Haemin degradation was assayed using ascorbate as reductant in 50 mM Tris/HCl (pH 8) and 150 mM NaCl buffer. Ascorbic acid-dependent degradation of haem bound to HmoA or HmoB was monitored in the presence and absence of catalase, as described by Skaar et al. (2006). Site-directed mutagenesis of hmoA and hmoB was done using PCR and overlap extension (Ho et al., 1989). Different concentrations were used for each protein, since some precipitation occurred during dialysis of the haemin-bound proteins and the resulting concentrations were remeasured after removing the precipitates.
Results and Discussion
Haem serves as an iron source for B. subtilis
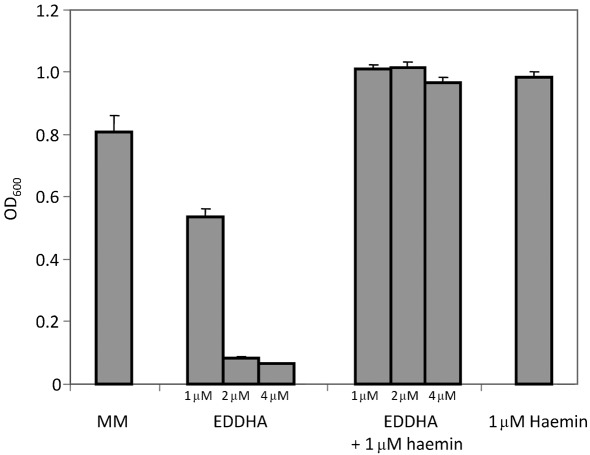
To test whether B. subtilis can use haemin as an iron source, the wild-type strain CU1065 (which is sfp0 and cannot produce bacillibactin) was grown in iron-limiting minimal medium containing the strong iron chelator ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) (EDDHA) (Fig. 1). Whereas 1 µM EDDHA caused an almost 50 % reduction in cell density, 2 and 4 µM EDDHA completely abolished growth. As shown previously (Ollinger et al., 2006), growth inhibition by EDDHA can be overcome by addition of siderophores with a higher affinity for iron than EDDHA (e.g. bacillibactin; Ollinger et al., 2006). The addition of 1 µM haemin restored growth to normal levels even in the presence of 4 µM EDDHA (Fig. 1). Thus, haemin provides a form of iron that can be utilized by B. subtilis in the presence of the strong Fe(III) chelator EDDHA, which binds ferric iron with an affinity similar to many hydroxamate siderophores (Ollinger et al., 2006; Wong et al., 1983).
Fig. 1.
B. subtilis uses haemin as an iron source. Cells were grown in minimal medium supplemented with 1, 2 or 4 µM EDDHA in the absence or presence of 1 µM haemin overnight at 37 °C. The results are the mean and sd of at least three independent replicates.
At least two distinct pathways for the utilization of haem-bound iron have been described (Anzaldi & Skaar, 2010; Létoffé et al., 2009). In many organisms, haem is transported into the cell, where cleavage of the porphyrin ring by haem monooxygenases facilitates iron release. An alternative pathway in E. coli has been described in which iron is removed from surface-bound haemin and imported by the EfeUOB system (Létoffé et al., 2009). The E. coli EfeUOB system was originally described as an acid-induced, low-pH Fe(II) uptake pathway (Cao et al., 2007; Grosse et al., 2006) homologous to the B. subtilis YwbLMN elemental iron uptake system (Ollinger et al., 2006). These elemental iron uptake systems share similarities with the Saccharomyces cerevisiase Ftr1p/Fet3p copper oxidase-dependent iron uptake pathway. We hypothesized that the YwbLMN (EfeUOB-type) pathway might be responsible for haem utilization in B. subtilis. However, a ywbL : : kan mutant grew as well as the wild-type strain using haemin as an iron source (in the presence of EDDHA), suggesting that B. subtilis may instead (or additionally) use a haem monooxygenase-dependent pathway.
Identification of two candidate haem monooxygenases in B. subtilis
The B. subtilis genome encodes two candidate haem monooxygenases (YetG and YhgC) with similarity to Staphylococcus aureus IsdG (Skaar et al., 2004) (see Supplementary Fig. S1). Based on the results presented herein, we rename YetG as HmoA and YhgC as HmoB. Neither the hmoA nor the hmoB gene had been previously linked to iron homeostasis. HmoA and HmoB are annotated in the B. subtilis genome as a putative monooxygenase and conserved hypothetical protein, respectively.
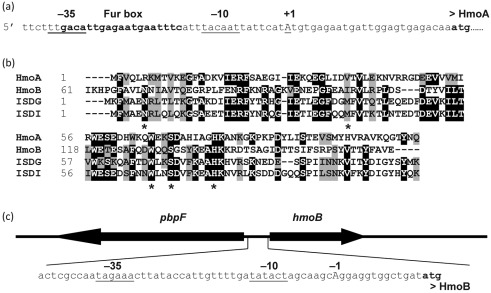
Comparison of HmoA with IsdG and other homologous proteins suggests that HmoA starts with a methionine corresponding to position 18 within the originally annotated protein sequence. This reassignment reveals a candidate σA-type promoter and overlapping Fur box (Fig. 2a). Analysis of transcripts from this region using 5′-random amplification of cDNA ends (RACE) confirms that this promoter drives expression of hmoA: the dominant cDNA product detected corresponded to initiation at an adenine residue 26 nt upstream of the reassigned ATG start codon. HmoA is 33 % identical (61 % similar) to S. aureus IsdG (Supplementary Fig. S1).
Fig. 2.
HmoA and HmoB regulatory region of the haem monooxygenases. (a) Operator region of the hmoA gene with −35 and −10 promoter elements, and the experimentally determined +1 start site indicated by underlined upper-case type. The Fur box is indicated by bold type. (b) Amino acid sequence alignment of HmoA and HmoB from B. subtilis with IsdG and IsdI from S. aureus. Residues suggested to bind haem are indicated by asterisks. (c) Organization of the pbpF–hmoB intergenic region, showing the putative divergent promoter for hmoB.
Unlike hmoA, the hmoB gene is not associated with an apparent Fur box. Sequence analyses and tiling array transcriptome datasets (Rasmussen et al., 2009) suggest that hmoB is constitutively expressed as a monocistronic transcript initiated from a σA-type promoter element (Fig. 2c). HmoB is 25 % identical (42 % similar) to HmoA and 33 % identical (61 % similar) to S. aureus IsdG (Supplementary Fig. S1).
Expression of HmoA is regulated by Fur and iron
Genes involved in iron uptake are frequently regulated by the ferric uptake repressor Fur (Bsat et al., 1998; Lee & Helmann, 2007). Under iron sufficiency, Fur binds to inverted repeat sequences in the operator regions of iron-regulated genes (Bsat et al., 1998; Bsat & Helmann, 1999). The Fur regulon has been extensively characterized using transcriptome analysis (Baichoo et al., 2002), and the Fur binding site defined by mutational and DNA binding studies (Baichoo & Helmann, 2002).
The presence of a candidate Fur box upstream of the reannotated hmoA (yetG) coding sequence (Fig. 2a) suggests that HmoA is a member of the Fur regulon. Indeed, analysis of previous microarray data showed that hmoA is derepressed sixfold in a fur mutant and induced 8.5-fold under conditions of iron deficiency in the presence of dipyridyl (Baichoo et al., 2002). However, in this previous study the hmoA gene was not assigned as a direct target of Fur regulation due, in retrospect, to the misannotation of the gene and the resulting lack of an associated upstream Fur box.
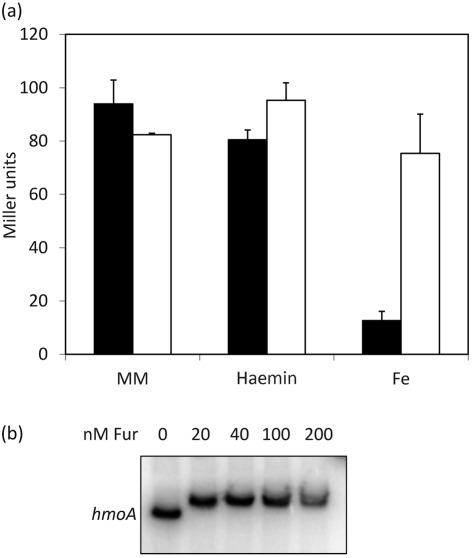
To further investigate the regulation of the hmoA gene, we cloned the putative promoter and regulatory region to generate an ectopically integrated reporter fusion (carried within the SPβ prophage). This hmoA–cat–lacZ transcriptional fusion was highly expressed in iron starvation minimal medium and was fully derepressed in a fur null mutant (Fig. 3a). While hmoA expression was repressed by addition of 100 µM iron, it was not affected by addition of 1 µM haemin (Fig. 3a). In an EMSA, purified Fur bound with high affinity [dissociation constant (Kd) <20 nM] to the hmoA regulatory region (Fig. 3b). Together with the previous transcriptome data (Baichoo et al., 2002), these results demonstrate that hmoA is a member of the Fur regulon. Unlike hmoA, hmoB was constitutively expressed and not regulated by Fur or iron. Addition of haem did not affect expression of either hmoA or hmoB (data not shown).
Fig. 3.
hmoA is under Fur regulation. (a) Regulation of hmoA as determined using a hmoA–cat–lacZ fusion. Cells of the wild-type strain (black bars) or a fur mutant (white bars) were grown in minimal medium (MM) or MM containing either 100 µM Fe or 1 µM haemin. The results are the mean and sd of at least three independent replicates. (b) EMSA showing binding of Fur to the hmoA promoter region.
HmoA and HmoB proteins bind haem
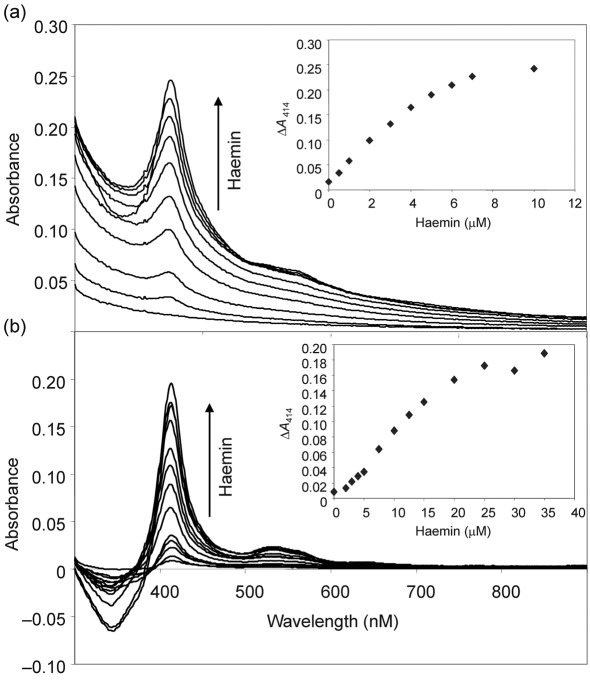
Haem monooxygenases bind haemin in vitro and, in the presence of a suitable reductant, haem is oxidatively degraded (Frankenberg-Dinkel, 2004; Skaar et al., 2004; Wilks & Schmitt, 1998). HmoA and HmoB were overexpressed and purified from E. coli as His-tagged fusion proteins. Both proteins as purified showed very low absorbance in the range characteristic of haem, indicating that they are largely free of contaminating haem (Fig. 4). Addition of increasing concentrations of haemin to HmoA or HmoB at pH 8.0 increased the difference absorption spectrum (relative to haemin added to buffer) with a maximum at 414 nm, and a broad peak at either 560 nm (HmoA) or 543 nm (HmoB) (Fig. 4a, b). These titration studies suggest that both proteins bind haemin with approximately 1 : 1 stoichiometry (Fig. 4a, b, insets). These results are consistent with what was found for the haem monooxygenases IsdG from Bacillus anthracis and S. aureus (Skaar et al., 2004, 2006). To ensure that the haem binding was not affected by the His-tag used for purification, the tag (2.5 kDa) was cleaved from the protein sequence using factor Xa followed by dialysis using 6–8 kDa dialysis tubes. Binding of haem to the resulting native HmoA and HmoB proteins was indistinguishable from binding to His-tagged proteins (data not shown).
Fig. 4.
HmoA and HmoB bind and degrade haem. Difference absorption spectroscopy of haemin binding to 8 µM HmoA (a) and to 20 µM HmoB (b). Haemin was added to both the sample and reference cuvettes and the differences in absorbance were recorded. Differential absorption showed that both proteins bind haemin with approximately 1 : 1 stoichiometry (insets).
HmoA and HmoB behave as haem monooxygenases
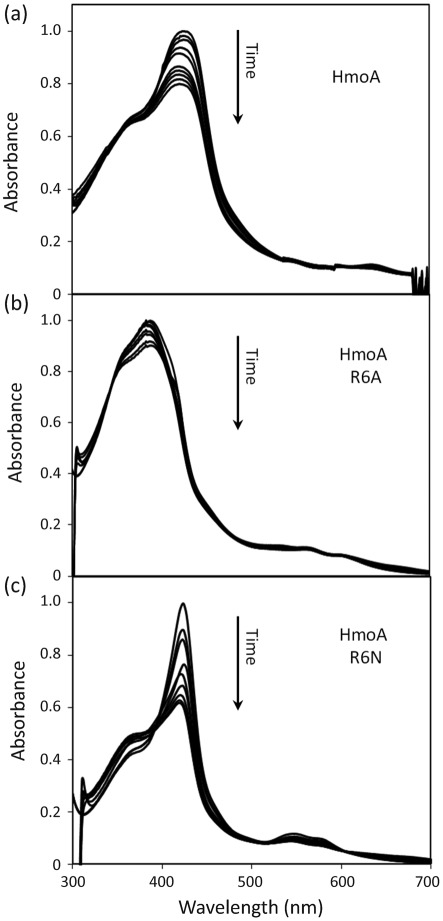
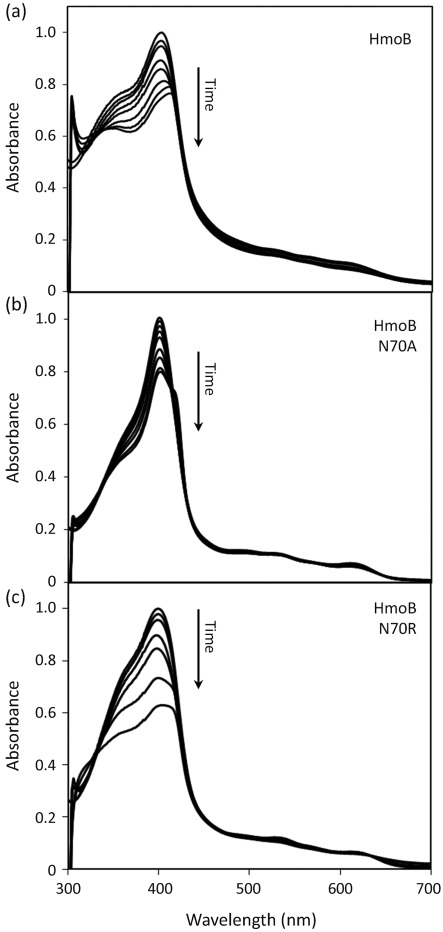
Degradation of haem in the presence of an electron donor can be monitored by the decrease in the characteristic haem absorption peak (Wilks, 2002). Using absorption spectroscopy in the presence of ascorbate, both the haemin–HmoA (Fig. 5a) and haemin–HmoB (Fig. 6a) complexes displayed a time-dependent loss of haem absorbance. Their overall rate of degradation was similar to that reported for IsdG from B. anthracis and S. aureus (Skaar et al., 2004, 2006), but with HmoA and HmoB the reaction did not result in a complete loss of haemin absorption. This may mean that the reaction did not go to completion or that the product retains absorption in this region. To ensure that this reaction was not the result of non-enzymic degradation of haem (which can occur through a coupled oxidation reaction triggered by hydrogen peroxide; Sigman et al., 2001), the same reaction was carried out in the presence of catalase and similar results were obtained (in the presence of catalase in a 0.5 : 1 ratio with HmoA–haemin or HmoB–haemin). These results indicate that both HmoA and HmoB bind haemin in an approximately 1 : 1 stoichiometry, and that in the presence of reductant, the haemin is chemically altered, as reflected by the decrease of absorbance.
Fig. 5.
Absorption spectra of haemin complexes of 9 µM HmoA wild-type (a), 13 µM HmoA R6A (b) and 13 µM HmoA R6N (c), after addition of ascorbic acid. Spectra were taken immediately before addition of ascorbic acid and then every 10 min. All maximum absorption values (0.56, 0.93 and 0.804, respectively) were normalized to 1.0 for clearer comparison.
Fig. 6.
Absorption spectra of haemin complexes of 6 µM HmoB wild-type (a), 15 µM HmoB N70A (b) and 6 µM HmoB N70R (c), after addition of ascorbic acid. Spectra were taken immediately before addition of ascorbic acid and then every 10 min. All maximum absorption values (0.494, 1.0 and 0.548, respectively) were normalized to 1.0 for comparison.
HmoA is a founding member of an uncharacterized subfamily of Pfam03992 proteins
S. aureus possesses two paralogous haem monooxygenases, IsdG and IsdI, which have been characterized both functionally (Skaar et al., 2004) and structurally (Lee et al., 2008). Significant sequence similarity is apparent between HmoA, HmoB and the IsdG and IsdI enzymes (Fig. 2b). These proteins all belong to the antibiotic biosynthesis monooxygenase or ABM domain family (pfam03992), and the true enzymic activity has only been established for a few selected examples.
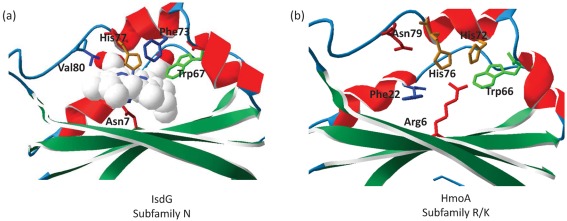
The IsdG and IsdI proteins are dimeric enzymes (Lee et al., 2008). The haem binding site in IsdG was identified on the basis of mutational analysis as involving residues N7, W67 and H77 (Wu et al., 2005). This critical NWH triad is conserved in HmoB but, surprisingly, only partially conserved in HmoA: the residue corresponding to asparagine 7 (N7) is substituted with arginine (Fig. 2b). HmoB differs from other homologous proteins in that it contains an additional 60 aa N-terminal domain with no similarity to known motifs.
To further investigate the possible implications of the N to R substitution within the putative active site, we modelled the HmoA sequence on the IsdG crystal structure (Fig. 7). The overall structure of the haem binding pocket, as visualized in IsdG, is conserved in HmoA. Not surprisingly, the HmoA H76 (equivalent to IsdG H77) is also predicted to be spatially conserved, consistent with its reported role in the binding of the ferric ion of the haem group. However, the essential IsdG N7 residue (Wu et al., 2005), is positionally substituted with R6, as predicted from the sequence alignment (Fig. 2b). Since N7 is predicted to interact directly with the haem iron in the active site and is essential for enzymic function (Wu et al., 2005), this substitution is surprising. The replacement of N7(IsdG) with R6(HmoA) suggests that the details of substrate binding or catalysis may differ between the IsdG/HmoB type enzymes and HmoA.
Fig. 7.
(a) Structure of S. aureus haem-bound IsdG (Lee et al., 2008), showing residues involved in haem binding and enzymic activity. (b) Structure of HmoA modelled on IsdG using swiss-model (Arnold et al. 2006), showing conservation of the haem binding pocket except for IsdG N7. The sequence identity between the two proteins was 57.55 %, with a QMEAN6 score of 0.66 and a QMEAN Z score of −1.34.
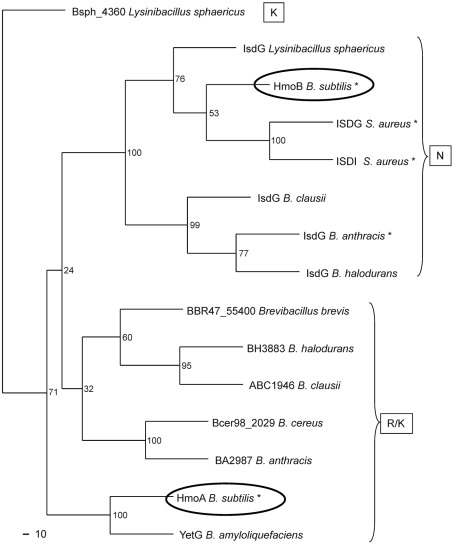
We conducted a multiple sequence alignment and phylogenetic analyses to determine whether this asparagine residue is present in any other pfam03992 proteins (Fig. 8). All known IsdG-like haem monooxygenases (including HmoB) form one cluster, whereas HmoA and related proteins form a distinct cluster. Interestingly, there is a perfect correspondence between these two protein families (as defined by alignments based on the full-length protein sequences) and the presence of either a conserved asparagine (IsdG/HmoB subfamily) or an arginine or lysine residue (HmoA subfamily) (Fig. 8). This suggests that a substitution of this active site residue occurred early during the divergence of these two protein groups. To our knowledge, HmoA is the first member of this second group to be characterized. Thus, the results presented here lead to the hypothesis that other members of this HmoA subfamily (including, for example, B. anthracis BA2987) may also function as haem monooxygenases, albeit with possible differences in the mechanisms of haem recognition and degradation relative to the better-studied IsdG family proteins.
Fig. 8.
Phylogenetic analysis of haem monooxygenases using clustal w and phylip programs at the PhylOgenetic WEb Repeater (POWER) server using standard options (Lin et al., 2005). Asterisks indicate proteins with demonstrated activity. Letters within boxes identify subgroups based on amino acid identity at the first position of the catalytic triad (see text).
Mutational analysis of the variable Asn residue of the conserved NWH catalytic triad
The roles of the residues corresponding to IsdG N7 in HmoA (R6) and HmoB (N70) were tested by using site-directed mutagenesis either to truncate the corresponding side chain (by mutation to A) or to substitute with the amino acid found in the other subfamily. The resulting HmoA R6A and R6N and HmoB N70A and N70R mutant proteins were purified and tested for haem binding as described above for wild-type proteins. All four mutant proteins bound haem with approximately 1 : 1 stoichiometry, as noted for wild-type HmoA and HmoB. Before, an N7A mutant of S. aureus IsdG has been shown to still bind haem but to have lost all capacity for haem degradation (Wu et al., 2005). In contrast, both the HmoA (R6A) and HmoB (N70A) mutants retained the ability to degrade haem (Figs 5b and 6b), albeit at a reduced rate relative to the wild-type. Moreover, the HmoA R6N and HmoB N70R mutants retained activity which was, in each case, at least as great as that for the corresponding wild-type proteins. In addition, there was a change in the spectrum of the degradation reaction that was more pronounced for HmoA mutants (Fig. 5) than for HmoB mutants (Fig. 6). This might reflect a change in the nature of the haem–protein interaction or in the resulting degradation products. We conclude that either residue (R or N) at this position can support near wild-type rates of haem degradation (Figs 5c and 6c).
Consistent with earlier studies of haem monooxygenases (Kunkle & Schmitt, 2007; Puri & O’Brian, 2006; Skaar et al., 2004, 2006; Skaar & Schneewind, 2004), we observed apparent degradation of the bound haem substrate as monitored spectroscopically, but we have been unable to demonstrate turnover (catalytic activity). Moreover, and despite several attempts, we have been unable to recover sufficient quantities of the degradation product for chemical analysis, apparently due to very tight binding to the protein. Similar results have been reported for other haem monooxygenases (Puri & O’Brian, 2006; Skaar et al., 2004, 2006). This suggests that an additional factor may be needed in vivo to dissociate the degradation product from the protein. Recently, sufficient amounts of product were recovered from IsdG- and IsdI-dependent haemin degradation for structural characterization. The resulting products, designated staphylobilins, were identified as 5-oxo-δ-bilirubin and 15-oxo-β-bilirubin (Reniere et al., 2010).
Growth on haem is not dependent on HmoA and HmoB
To determine whether hmoA and hmoB play a role in haem utilization by B. subtilis, single and double mutants were constructed by replacing the relevant coding sequences with an antibiotic gene cassette (Table 1). Since the phenotypes of haem utilization mutants are often very subtle (typically, quite small differences in growth rate and/or colony size have been reported; Reniere & Skaar, 2008), we tested the mutants in both liquid and solid media supplemented with EDDHA (to induce iron stress) and/or with haemin. In these assays, neither the hmoA and hmoB single mutants nor the hmoA hmoB double mutant displayed robust and reproducible phenotypes relative to the wild-type. A number of possible explanations could account for these negative results. It is frequently observed that iron uptake pathways are redundant, in which case a lack of HmoA and HmoB may not preclude haem utilization. For example, it is possible that haem is not internalized under these growth conditions (the iron may be removed outside the cells and then transported) (Létoffé et al., 2009). However, even a triple mutant lacking hmoA, hmoB and the ywbLMN operon (homologous to the efeUOB operon implicated in uptake of haem-associated iron in E. coli; Létoffé et al., 2009) was still able to grow using haem as an iron source. We also considered the possibility that hmoA and hmoB might be involved in haem detoxification, but we did not observe any differences in sensitivity to high levels of haem. To date, haem detoxification systems for intracellular haem seem to be closely correlated with a lifestyle that involves exposure to or growth within haem-rich environments such as mammalian blood (Skaar, 2010). It is also possible that HmoA and HmoB function primarily as haem chaperones or delivery proteins, and that their abilities to apparently degrade haem in vitro are not related to their in vivo function. Finally, we considered the possibility that these proteins might be involved in utilization of other types of metalloporphyrins, although no evidence to support this idea has emerged. Thus, the physiological roles of HmoA and HmoB remain unclear, although the direct regulation of HmoA by Fur certainly supports the notion that utilization of haem as an iron source is one function.
Concluding remarks
Bacterial haem monooxygenases degrade haem and thereby facilitate iron acquisition from this ubiquitous cofactor. The product of haem degradation has, in most cases, not been well defined but is generally presumed to be biliverdin. Haem monooxygenases fall into two major families. The first is characterized by a GXXXG motif, a conserved histidine residue required for activity (Wilks, 2002) and a HemeO superfamily motif. Members of this family have been characterized in several bacteria, including Neisseria spp. (Zhu et al., 2000), Corynebacterium diphtheriae (Wilks & Schmitt, 1998) and Pseudomonas aeruginosa (Ratliff et al., 2001). The second family of bacterial haem monooxygenases are part of the large and diverse family of Pfam03992 proteins that share an ABM motif (Puri & O’Brian, 2006; Skaar et al., 2004, 2006; Wu et al., 2005). As shown here, HmoA and HmoB also contain ABM motifs (Marchler-Bauer et al., 2007), but define two distinct subfamilies. HmoA and other members of this subfamily (Fig. 1b) contain a basic amino acid (R or K) in place of the N7 in IsdG (Wu et al., 2005). Importantly, members of the HmoA subfamily are found in the human pathogens B. anthracis and Bacillus cereus, where they may play a role in iron acquisition during infection. The overall sequence conservation with B. anthracis and S. aureus IsdG (Skaar et al., 2006), and the retention of most of the known active site residues, suggests a mode of activity similar to IsdG (Reniere et al., 2010). Indeed, both HmoA and HmoB bind haemin with approximately 1 : 1 stoichiometry, and the characteristic haemin absorbance decreases upon addition of reductant. In addition, transcription of hmoA is regulated by Fur, consistent with a role in iron acquisition. Together, these results support a model in which HmoA, and perhaps also HmoB, functions to oxidatively release iron from haem. Ultimately, further biochemical and physiological studies will be required to define the nature of the haem degradation product(s) and the role of HmoA and HmoB in cell physiology.
Supplementary Material
Acknowledgements
We would like to thank Dr Eric Skaar for his advice and for sharing unpublished information. This work was supported by a grant from the National Institutes of Health (GM059323).
Abbreviations:
- EDDHA
ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid)
- EMSA
electrophoretic mobility shift assay
Footnotes
A supplementary figure, showing percentage similarities and identities among putative haem monooxygenases, and a supplementary table, listing oligonucleotides used in this study, are available with the online version of this paper.
References
- Andrews S. C., Robinson A. K., Rodríguez-Quiñones F. (2003). Bacterial iron homeostasis. FEMS Microbiol Rev 27, 215–237. 10.1016/S0168-6445(03)00055-X [DOI] [PubMed] [Google Scholar]
- Anzaldi L. L., Skaar E. P. (2010). Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect Immun 78, 4977–4989. 10.1128/IAI.00613-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J., Schwede T. (2006). The swiss-model workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201. 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- Baichoo N., Helmann J. D. (2002). Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J Bacteriol 184, 5826–5832. 10.1128/JB.184.21.5826-5832.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichoo N., Wang T., Ye R., Helmann J. D. (2002). Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol Microbiol 45, 1613–1629. 10.1046/j.1365-2958.2002.03113.x [DOI] [PubMed] [Google Scholar]
- Bsat N., Helmann J. D. (1999). Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo. J Bacteriol 181, 4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsat N., Herbig A., Casillas-Martinez L., Setlow P., Helmann J. D. (1998). Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol 29, 189–198. 10.1046/j.1365-2958.1998.00921.x [DOI] [PubMed] [Google Scholar]
- Butcher B. G., Helmann J. D. (2006). Identification of Bacillus subtilis σw-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol 60, 765–782. 10.1111/j.1365-2958.2006.05131.x [DOI] [PubMed] [Google Scholar]
- Cao J., Woodhall M. R., Alvarez J., Cartron M. L., Andrews S. C. (2007). EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157 : H7. Mol Microbiol 65, 857–875. 10.1111/j.1365-2958.2007.05802.x [DOI] [PubMed] [Google Scholar]
- Chen L., James L. P., Helmann J. D. (1993). Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol 175, 5428–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen C. N. (2003). Transferrin-iron uptake by Gram-negative bacteria. Front Biosci 8, d836–d847. 10.2741/1076 [DOI] [PubMed] [Google Scholar]
- Cutting S. M., VanderHorn P. B. (1990). Genetic analysis. In Molecular Biological Methods for Bacillus, pp. 27–74. Chichester: Wiley. [Google Scholar]
- Frankenberg-Dinkel N. (2004). Bacterial heme oxygenases. Antioxid Redox Signal 6, 825–834. [DOI] [PubMed] [Google Scholar]
- Fuangthong M., Helmann J. D. (2003). Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis. J Bacteriol 185, 6348–6357. 10.1128/JB.185.21.6348-6357.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse C., Scherer J., Koch D., Otto M., Taudte N., Grass G. (2006). A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol Microbiol 62, 120–131. 10.1111/j.1365-2958.2006.05326.x [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Kunkle C. A., Schmitt M. P. (2007). Comparative analysis of hmuO function and expression in Corynebacterium species. J Bacteriol 189, 3650–3654. 10.1128/JB.00056-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Helmann J. D. (2007). Functional specialization within the Fur family of metalloregulators. Biometals 20, 485–499. 10.1007/s10534-006-9070-7 [DOI] [PubMed] [Google Scholar]
- Lee W. C., Reniere M. L., Skaar E. P., Murphy M. E. (2008). Ruffling of metalloporphyrins bound to IsdG and IsdI, two heme-degrading enzymes in Staphylococcus aureus. J Biol Chem 283, 30957–30963. 10.1074/jbc.M709486200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Heuck G., Delepelaire P., Lange N., Wandersman C. (2009). Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc Natl Acad Sci U S A 106, 11719–11724. 10.1073/pnas.0903842106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Lin F. K., Lin C. H., Lai L. W., Hsu H. J., Chen S. H., Hsiung C. A. (2005). POWER: PhylOgenetic WEb Repeater – an integrated and user-optimized framework for biomolecular phylogenetic analysis. Nucleic Acids Res 33 (Web Server), W553–W556. 10.1093/nar/gki494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Anderson J. B., Derbyshire M. K., DeWeese-Scott C., Gonzales N. R., Gwadz M., Hao L., He S., Hurwitz D. I., et al. (2007). CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res 35 (Database issue), D237–D240. 10.1093/nar/gkl951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. J., Wendrich T. M., Marahiel M. A. (2001). The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J Biol Chem 276, 7209–7217. 10.1074/jbc.M009140200 [DOI] [PubMed] [Google Scholar]
- Miczák A. (1977). Porphyrin and corrinoid mutants of Bacillus subtilis. J Bacteriol 131, 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke M., Klotz O., Linne U., May J. J., Beckering C. L., Marahiel M. A. (2006). Ferri-bacillibactin uptake and hydrolysis in Bacillus subtilis. Mol Microbiol 61, 1413–1427. 10.1111/j.1365-2958.2006.05321.x [DOI] [PubMed] [Google Scholar]
- Miller J. H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Moore C. M., Helmann J. D. (2005). Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol 8, 188–195. 10.1016/j.mib.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Nienaber A., Hennecke H., Fischer H. M. (2001). Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol Microbiol 41, 787–800. 10.1046/j.1365-2958.2001.02555.x [DOI] [PubMed] [Google Scholar]
- Noya F., Arias A., Fabiano E. (1997). Heme compounds as iron sources for nonpathogenic Rhizobium bacteria. J Bacteriol 179, 3076–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger J., Song K. B., Antelmann H., Hecker M., Helmann J. D. (2006). Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol 188, 3664–3673. 10.1128/JB.188.10.3664-3673.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S., O’Brian M. R. (2006). The hmuQ and hmuD genes from Bradyrhizobium japonicum encode heme-degrading enzymes. J Bacteriol 188, 6476–6482. 10.1128/JB.00737-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S., Nielsen H. B., Jarmer H. (2009). The transcriptionally active regions in the genome of Bacillus subtilis. Mol Microbiol 73, 1043–1057. 10.1111/j.1365-2958.2009.06830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M., Zhu W., Deshmukh R., Wilks A., Stojiljkovic I. (2001). Homologues of neisserial heme oxygenase in Gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J Bacteriol 183, 6394–6403. 10.1128/JB.183.21.6394-6403.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere M. L., Skaar E. P. (2008). Staphylococcus aureus haem oxygenases are differentially regulated by iron and haem. Mol Microbiol 69, 1304–1315. 10.1111/j.1365-2958.2008.06363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere M. L., Ukpabi G. N., Harry S. R., Stec D. F., Krull R., Wright D. W., Bachmann B. O., Murphy M. E., Skaar E. P. (2010). The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol Microbiol 75, 1529–1538. 10.1111/j.1365-2958.2010.07076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. (2001). Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Schiött T., Throne-Holst M., Hederstedt L. (1997). Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J Bacteriol 179, 4523–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman J. A., Wang X., Lu Y. (2001). Coupled oxidation of heme by myoglobin is mediated by exogenous peroxide. J Am Chem Soc 123, 6945–6946. 10.1021/ja015776u [DOI] [PubMed] [Google Scholar]
- Skaar E. P. (2010). The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6, e1000949. 10.1371/journal.ppat.1000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar E. P., Schneewind O. (2004). Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect 6, 390–397. 10.1016/j.micinf.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Skaar E. P., Gaspar A. H., Schneewind O. (2004). IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J Biol Chem 279, 436–443. 10.1074/jbc.M307952200 [DOI] [PubMed] [Google Scholar]
- Skaar E. P., Gaspar A. H., Schneewind O. (2006). Bacillus anthracis IsdG, a heme-degrading monooxygenase. J Bacteriol 188, 1071–1080. 10.1128/JB.188.3.1071-1080.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Sonenshein A. L. (1993). Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol 175, 4605–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius M. V., Harmston C. A., Owens C. P., Chim N., Morse R. P., McMath L. M., Iniguez A., Kimmey J. M., Sawaya M. R., et al. (2011). Discovery and characterization of a unique mycobacterial heme acquisition system. Proc Natl Acad Sci U S A 108, 5051–5056. 10.1073/pnas.1009516108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. (2002). Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal 4, 603–614. 10.1089/15230860260220102 [DOI] [PubMed] [Google Scholar]
- Wilks A., Schmitt M. P. (1998). Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J Biol Chem 273, 837–841. 10.1074/jbc.273.2.837 [DOI] [PubMed] [Google Scholar]
- Wong G. B., Kappel M. J., Raymond K. N., Matzanke B., Winkelmann G. (1983). Coordination chemistry of microbial iron transport compounds. 24. Characterization of coprogen and ferricrocin, two ferric hydroxamate siderophores. J Am Chem Soc 105, 810–815. 10.1021/ja00342a027 [DOI] [Google Scholar]
- Wu R., Skaar E. P., Zhang R., Joachimiak G., Gornicki P., Schneewind O., Joachimiak A. (2005). Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J Biol Chem 280, 2840–2846. 10.1074/jbc.M409526200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Wilks A., Stojiljkovic I. (2000). Degradation of heme in Gram-negative bacteria: the product of the hemO gene of neisseriae is a heme oxygenase. J Bacteriol 182, 6783–6790. 10.1128/JB.182.23.6783-6790.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. (1987). Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol 169, 2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.