Abstract
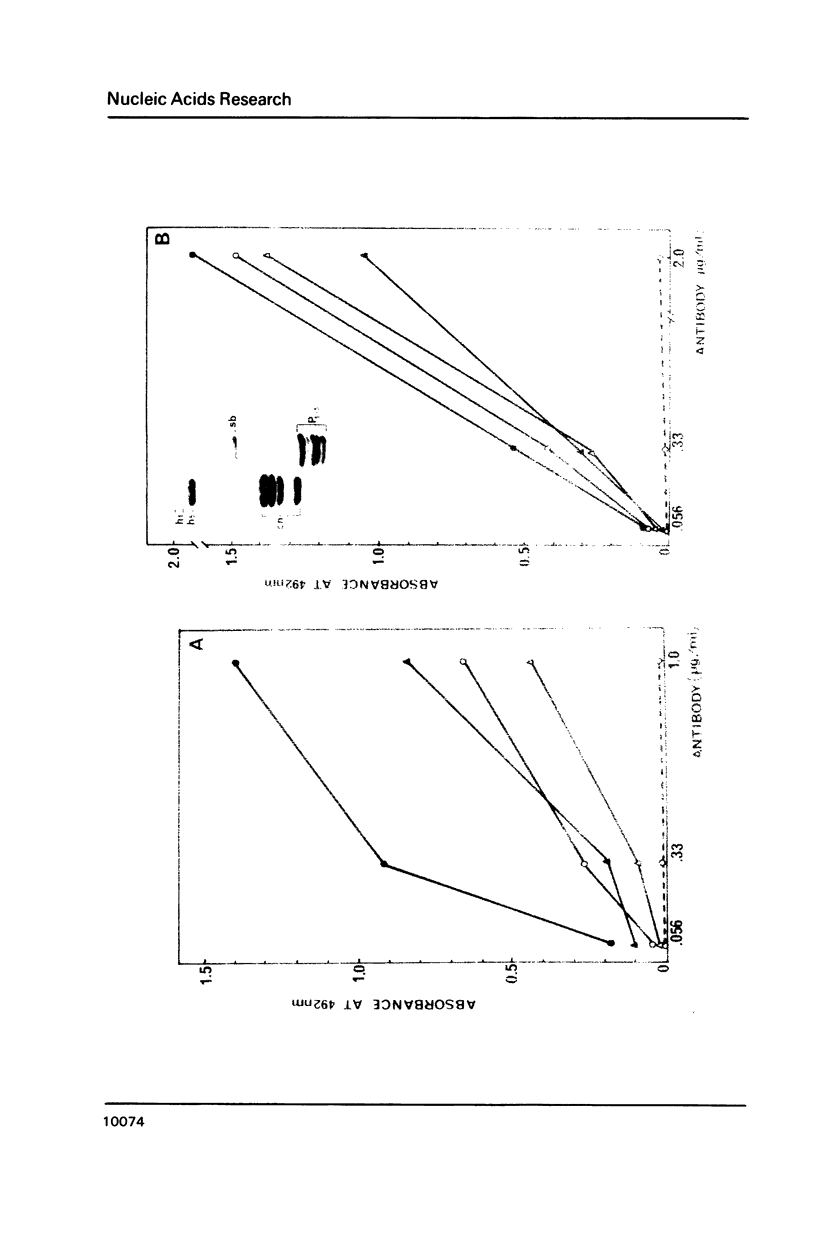
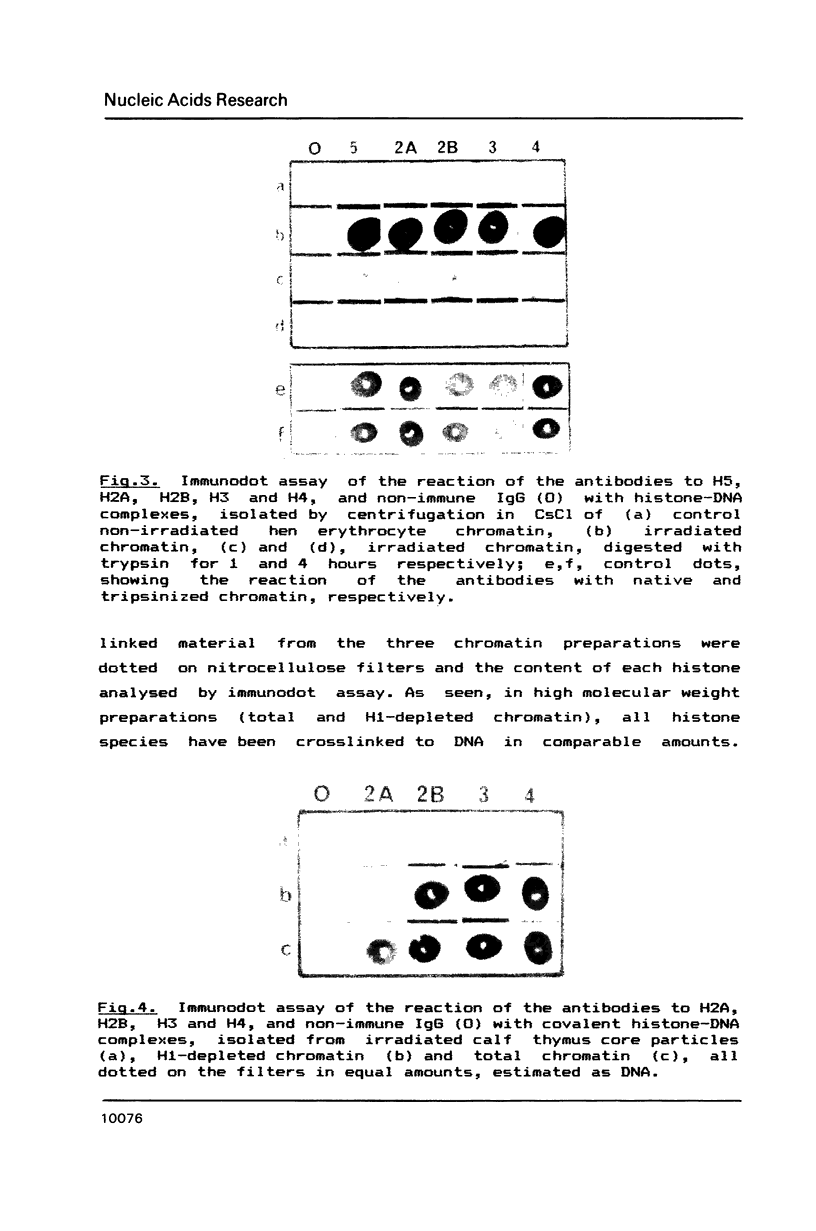
UV laser irradiation has been used to covalently crosslink histones to DNA in nuclei, chromatin and core particles and the presence of the different histone species in the covalently linked material was detected immunochemically. When nuclei were irradiated and then trypsinized to cleave the N- and C- terminal histone tails, no histones have been found covalently linked to DNA. This finding shows that UV laser-induced crosslinking of histones to DNA is accomplished via the non-structured domains only. This unexpected way of crosslinking operated in chromatin, H1-depleted chromatin and core particles, i.e. independently of the chromatin structure. The efficiency of crosslinking, however, showed such a dependence: whilst the yield of crosslinks was similar in total and H1-depleted chromatin, in core particles the efficiency was 3-4 times lower for H2A, H2B and H4 and 10-12 times lower for H3. The decreased crosslinking efficiency, especially dramatic in the case of H3, is attributed to a reduced number of binding sites, and, respectively, is considered as a direct evidence for interaction of nonstructured tails of core histones with linker DNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Angelov D., Stefanovsky VYu, Dimitrov S. I., Russanova V. R., Keskinova E., Pashev I. G. Protein-DNA crosslinking in reconstituted nucleohistone, nuclei and whole cells by picosecond UV laser irradiation. Nucleic Acids Res. 1988 May 25;16(10):4525–4538. doi: 10.1093/nar/16.10.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausio J., van Holde K. E. Histone hyperacetylation: its effects on nucleosome conformation and stability. Biochemistry. 1986 Mar 25;25(6):1421–1428. doi: 10.1021/bi00354a035. [DOI] [PubMed] [Google Scholar]
- Bavykin S. G., Usachenko S. I., Lishanskaya A. I., Shick V. V., Belyavsky A. V., Undritsov I. M., Strokov A. A., Zalenskaya I. A., Mirzabekov A. D. Primary organization of nucleosomal core particles is invariable in repressed and active nuclei from animal, plant and yeast cells. Nucleic Acids Res. 1985 May 24;13(10):3439–3459. doi: 10.1093/nar/13.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode J., Gómez-Lira M. M., Schröter H. Nucleosomal particles open as the histone core becomes hyperacetylated. Eur J Biochem. 1983 Feb 15;130(3):437–445. doi: 10.1111/j.1432-1033.1983.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Moss T., Bradbury E. M. High-resolution proton-magnetic-resonance studies of chromatin core particles. Eur J Biochem. 1978 Sep 1;89(2):475–482. doi: 10.1111/j.1432-1033.1978.tb12551.x. [DOI] [PubMed] [Google Scholar]
- Dimitrov S. I., Pashev I. G., Markov G. G. Studies on the thermal denaturation of histone-H1-depleted chromatin. Eur J Biochem. 1981 Apr;115(3):545–550. doi: 10.1111/j.1432-1033.1981.tb06237.x. [DOI] [PubMed] [Google Scholar]
- Ebralidse K. K., Grachev S. A., Mirzabekov A. D. A highly basic histone H4 domain bound to the sharply bent region of nucleosomal DNA. Nature. 1988 Jan 28;331(6154):365–367. doi: 10.1038/331365a0. [DOI] [PubMed] [Google Scholar]
- Imai B. S., Yau P., Baldwin J. P., Ibel K., May R. P., Bradbury E. M. Hyperacetylation of core histones does not cause unfolding of nucleosomes. Neutron scatter data accords with disc shape of the nucleosome. J Biol Chem. 1986 Jul 5;261(19):8784–8792. [PubMed] [Google Scholar]
- Levina E. S., Bavykin S. G., Shick V. V., Mirzabekov A. D. The method of crosslinking histones to DNA partly depurinated at neutral pH. Anal Biochem. 1981 Jan 1;110(1):93–101. doi: 10.1016/0003-2697(81)90117-2. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Dimitrov S. I., Petrov P. T. Salt-induced conformational transitions in chromatin. A flow linear dichroism study. Eur J Biochem. 1983 Jul 1;133(3):491–497. doi: 10.1111/j.1432-1033.1983.tb07491.x. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Dimitrov S. I., Tsaneva I. R., Pashev I. G. The role of histone H1 and non-structured domains of core histones in maintaining the orientation of nucleosomes within the chromatin fiber. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1021–1027. doi: 10.1016/0006-291x(84)91193-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Rau D. C., Charney E., Felsenfeld G. Orientation of the nucleosome within the higher order structure of chromatin. Cell. 1980 Nov;22(1 Pt 1):87–96. doi: 10.1016/0092-8674(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Nucleosome-nucleosome interaction in chromatin. FEBS Lett. 1979 Mar 1;99(1):123–128. doi: 10.1016/0014-5793(79)80263-x. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Russanova V. R., Dimitrov S. I., Makarov V. L., Pashev I. G. Accessibility of the globular domain of histones H1 and H5 to antibodies upon folding of chromatin. Eur J Biochem. 1987 Sep 1;167(2):321–326. doi: 10.1111/j.1432-1033.1987.tb13339.x. [DOI] [PubMed] [Google Scholar]
- Walker I. O. Differential dissociation of histone tails from core chromatin. Biochemistry. 1984 Nov 6;23(23):5622–5628. doi: 10.1021/bi00318a037. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Stein A. Folding of DNA by histones which lack their NH2-terminal regions. J Biol Chem. 1978 Jun 10;253(11):3857–3861. [PubMed] [Google Scholar]
- Zalenskaya I. A., Pospelov V. A., Zalensky A. O., Vorob'ev V. I. Nucleosomal structure of sea urchin and starfish sperm chromatin. Histone H2B is possibly involved in determining the length of linker DNA. Nucleic Acids Res. 1981 Feb 11;9(3):473–487. doi: 10.1093/nar/9.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayetz V. W., Bavykin S. G., Karpov V. L., Mirzabekov A. D. Stability of the primary organization of nucleosome core particles upon some conformational transitions. Nucleic Acids Res. 1981 Mar 11;9(5):1053–1068. doi: 10.1093/nar/9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]