Abstract
Angiogenesis has not been extensively studied in Parkinson’s disease (PD) despite being associated with other neurodegenerative disorders. Post-mortem human brain tissues were obtained from subjects with pathologically confirmed Parkinson’s disease (PD) and progressive supranuclear palsy (PSP), a rapidly progressing Parkinsonian-like disorder. Tissues were also obtained from subjects with incidental Lewy body disease (iLBD) who had Lewy bodies in the substantia nigra pars compacta (SNpc) but had not been diagnosed with PD and age-matched controls without Lewy body pathology. The SNpc, putamen, locus ceruleus (LC) and midfrontal cortex were examined for integrin αvβ3, a marker for angiogenesis, along with vessel number and activated microglia. All parkinsonian syndromes had greater αvβ3 in the LC and the SNpc, while only PD and PSP subjects had elevated αvβ3 in the putamen compared to controls. PD and PSP subjects also had increases in microglia number and activation in the SNpc suggesting a link between inflammation and clinical disease. Microglia activation in iLBD subjects was limited to the LC, an area involved at an early stage of PD. Likewise, iLBD subjects did not differ from controls in αvβ3 staining in the putamen, a late area of involvement in PD. The presence of αvβ3 reactive vessels in PD and its syndromes is indicative of newly created vessels that have not likely developed the restrictive properties of the blood brain barrier. Such angiogenic vessels could contribute to neuroinflammation by failing to protect the parenchyma from peripheral immune cells and inflammatory or toxic factors in the peripheral circulation.
Keywords: angiogenesis, Parkinson’s disease, incidental Lewy body disease, progressive supranuclear palsy, integrin αvβ3, microglia
Introduction
Parkinson’s disease (PD) is a progressive neuroinflammatory disease characterized by slowness of movement, rigidity, postural instability, and resting tremor (Fahn and Przedborski, 2000). These clinical manifestations of disease result from a marked loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc) and reduced projections to the caudate and putamen (striatum) as well as neurodegenerative changes in other brain regions including the locus ceruleus (LC). Degenerating neurons contain Lewy Bodies, a histological hallmark of PD composed of cytoplasmic inclusions containing α-synuclein and other aggregated proteins. PD patients also have increased numbers of activated microglia in disease-afflicted areas indicating neuroinflammation (Croisier et al., 2005; McGeer and McGeer, 2008; McGeer et al., 1988; Whitton, 2007; Zhang et al., 2005). Upon activation, microglia release pro-inflammatory cytokines including tumor necrosis factor-alpha (TNFα), interleukin (IL)-1β and transforming growth factor-β (Whitton, 2007) that are thought to participate in DA neuron death.
Many of the cytokines released by activated microglia in PD are not only pro-inflammatory, but are also pro-angiogenic (Naldini and Carraro, 2005; Pogue and Lukiw, 2004). Likewise the pro-angiogenic molecule, Vascular Endothelial Growth Factor (VEGF) is elevated in the SNpc of PD patients (Wada et al., 2006; Yasuda et al., 2007). It is therefore not surprising that Faucheux and colleagues showed an increase in the number of stained nuclei of endothelial cells in the SN of PD patients consistent with an increase in vessel density, but the increase in endothelial cell nuclei was not observed in the ventral tegmental area, an area not affected in PD (Faucheux et al., 1999). In addition, Barcia and colleagues observed increased numbers of blood vessels in close proximity to degenerating DA neurons in the SN of non-human primates, which correlated with increased VEGF expression (Barcia et al., 2005). Taken together, these data suggest that angiogenic changes may accompany the pathophysiological processes underlying PD.
Angiogenesis may also be associated with blood brain barrier (BBB) dysfunction (Barcia et al., 2004). In the periphery, newly created angiogenic vessels are leaky due to their numerous fenestrea, widened inter-endothelial junctions, abnormal endothelial cell shape, and discontinuous or absent basement membrane (Baluk et al., 2004). In the brain, immature vessels likely lack the full characteristics of the BBB, including the development of tight junctions, recruitment of pericytes, and the formation of a glial limitans. Thus, angiogenesis may compromise the BBB, which could contribute to ongoing neuroinflammation by allowing peripheral molecules and immune cells access to brain parenchyma. Indeed, we and others have shown that the BBB is dysfunctional in a variety of animal models of PD resulting in punctate leakage of FITC-labeled albumin (FITC-LA) and other tracers (Carvey et al., 2005; Carvey et al., 2009; Chen et al., 2008; Westin et al., 2006). Our group as well as others also demonstrated increased entry of drugs (Carta et al., 2006; Carvey et al., 2005; Westin et al., 2006) as well as peripheral immune cells (Benner et al., 2008; Brochard et al., 2009; Reynolds et al., 2010) in animal models of PD suggested alterations in barrier integrity. Interestingly, the punctate areas of FITC-LA leakage present in the SN and striatum of a toxin-induced animal model of PD co-localized with the angiogenic marker (β3 integrin) indicating an association between angiogenesis and BBB dysfunction (Carvey et al., 2005). Therefore, expression of integrins could be used to assess angiogenesis and possible BBB integrity in patients.
To assess angiogenic vessels in PD autopsy material we used integrin αvβ3 as an angiogenic marker. αvβ3 is not expressed on patent vessels, but is dramatically increased on angiogenic vessels (Brooks et al., 1994a; Brooks et al., 1994b; Brooks, 1996; Folkman, 2004; Friedlander et al., 1995). Indeed, antibodies to either the β3 subunit or to the αvβ3 heterodimer have been used to measure angiogenesis in a variety of conditions including abdominal aneurysm, ovarian cancer, retinopathy, myocardial infarction, and cortical stroke (Kalinowski et al., 2008; Lahdenranta et al., 2007; Paik et al., 2004; Wei et al., 2001; Willmann et al., 2008). In this study we used an antibody to the αvβ3 heterodimer that we have previously used to identify angiogenic vessels in postmortem tissues from human subjects with Alzheimer’s disease (AD) (Desai et al., 2009). In an effort to determine if angiogenesis was present early in the disease process, we also assessed angiogenesis in tissues from subjects with incidental Lewy Body disease (iLBD). Although there remains some controversy concerning the staging of PD by Lewy Body pathology (Kalaitzakis et al., 2008), iLBD is thought to represent pre-clinical PD, as these subjects have Lewy Bodies upon autopsy but lack clinical symptoms of PD (DelleDonne et al., 2008; Dickson et al., 2008). We also examined autopsy tissue from subjects with Progressive Supranuclear Palsy (PSP), a more rapidly progressing parkinsonian disorder with atypical clinical features including vertical gaze and pseudobulbar palsy (Steele et al., 1964). PSP is marked by significant neuronal degeneration, protein aggregates in affected brain regions, and neuroinflammation (Ishizawa and Dickson, 2001). By examining a range of Parkinsonian disorders, we demonstrate that angiogenesis is not only present in PD patients, but also in iLBD and PSP suggesting that angiogenic changes are an element of the neurodegenerative process.
Materials and Methods
Human Subjects
Deceased and autopsied subjects were from the Rush Alzheimer’s disease center including the Religious Orders Study, the Clinical Cores (Bennett et al., 2006b). The Religious Orders Study and Clinical Cores are longitudinal clinical-pathological studies of aging and AD (Bennett et al., 2006a). Participants of the Religious Orders Study are older Catholic nuns, priests, or brothers from the Chicago area and about 40 additional sites throughout the country who enroll without known dementia and agree to brain donation at the time of death. Participants of the Religious Orders Study and Clinical Core have annual clinical evaluations, which include medical history, neurological examination, neuropsychological performance testing and diagnostic classification. All Religious Orders Study subjects sign an Anatomical Gift Act donating his/her brain to Rush investigators at the time of death. The next of kin for the clinic patients and clinical core participants sign autopsy consents at the time of death. The Institutional Review Board of Rush University Medical Center approved all the procurement procedures for tissues used in this study.
Group Criteria
Nine PD cases, 6 iLBD, 4 PSP and 10 control cases were evaluated (Table I). Prior to our receiving the tissues, all cases were evaluated for the presence of Lewy bodies in 6-μm sections from the SNpc using α-synuclein immunohistochemistry (Schneider et al., 2007; Schneider et al., 2009; Schneider et al., 2009; Wilson et al., 2010). PD subjects had moderate to severe neuronal loss in the SNpc, Lewy Bodies in the SNpc and were clinically diagnosed with PD. iLBD cases were identified as having Lewy Bodies in the SNpc upon autopsy and were not clinically diagnosed with PD. None of the iLBD cases had neocortical Lewy Bodies. PSP subjects fulfilled standard criteria for pathologic diagnosis including degeneration in multiple brain regions with neurofibrillary tangles and neuropil threads in the basal ganglia and brain stem (Hauw et al., 1994). One PSP subject also had Lewy bodies in the substantia nigra. Any drug regimen taken by the subjects was noted during annual examination. Age-matched control cases were selected based on lack of neurofibrillary tangles and Lewy Bodies in the substantia nigra. A board-certified neuropathologist blindly reviewed all sections for diagnosis and provided sections for this study in a blinded fashion.
Table 1.
Demographics of Subjects
| Control | iLBD | PD | PSP | |
|---|---|---|---|---|
| Sex | 6F/4M | 3F/3M | 1F/8M | 2F/2M |
| Age at death | 81.9 ± 2.9 | 84.0 ± 3.4 | 81.1 ± 2.2 | 87.6 ± 4.9 |
| Post mortem Interval (hours) | 11.4 ± 3.5 | 5.1 ± 1 | 7.3 ± 1.8 | 5.3 ± 0.5 |
| PD clinical diagnosis (years) | NA | NA | 5 ± 1* | 4.5 ± 2** |
| Lewy Bodies | − | + | + | NA |
Data expressed as Mean ±SEM
Duration of disease information available on 8/9 subjects
2 PSP patients had clinical diagnosis of PD but upon autopsy were found to have pathology characteristic of PSP
Post-mortem Brain Tissue Processing
Brains of deceased subjects were removed in a standard fashion, weighed, cut into 1 cm-thick coronal slabs, digitally photographed, fixed, and stored (Bennett et al., 2006a). The location, age, and volume of all macroscopic infarctions were recorded. Tissues were fixed for at least 72 hour in 4% paraformaldehyde and stored in cryoprotectant. The following regions were dissected into 0.5-cm-thick blocks and embedded in paraffin, cut at 40μm, and used for integrin αvβ3 and microglia immunohistochemistry: one hemisection of substantia nigra at the level caudal to the exit of the 3rd nerve (region of the decussation of the superior cerebellar peduncles), pars compacta (area 9),.one hemisection of the mid to upper midbrain including locus ceruleus, one section of putamen immediately posterior to the anterior commissure, and for the mid frontal cortex, Brodman area 9/46, middle frontal gyrus
Immunohistochemistry
In general, seven to eight sections for each brain region for each subject were available. Alternating sections were used for the microglia studies and for the αvβ3 studies. Sections underwent antigen unmasking and were incubated with primary antibody overnight. An antibody that recognizes an epitope present when the αv and β3 integrin subunits are associated (Clone BV3; 1:100; Abcam; Cambridge, MA) was used to label endothelial cells undergoing angiogenesis. This antibody is specific for the αvβ3 integrin heterodimer and does not recognize other β3 or αv heterodimers. We have previously used this antibody to verify that angiogenesis was associated with Alzheimer’s disease and found that staining was limited to vessels in the brain (Desai et al., 2009).
Activated microglia were identified using an antibody against MHC Class II molecule located on the cell surface (HLA-DR, -DQ, -DP, Clone Cr3/43, 1:100, Dako, Glostrup, Denmark). Immunostaining was performed using the avidin-biotin-peroxidase method (ABC Elite; Vector, Burlingame, CA ) with 3,3 diaminobenzidine (DAB) as the chromagen for integrin αvβ3, and activated microglia.
Assessment of αvβ3
Integrin αvβ3 immunoreactivity was visualized using a Leitz Fluovert FU microscope (Leitz; Wetzlar, Germany) in the SNpc, putamen, locus ceruleus and midfrontal cortex. Within each region, 100% of the delineated tissue section was scanned for vessels in a 4 × 5 mm counting frame at 25x magnification. Previous studies indicated that vessels had a range of staining (Desai et al., 2009). Rather than rely on an arbitrary decision as to what constituted an angiogenic vessel, or institute a semi-quantitative scale for rating positive vessels, an optical density protocol was used (Desai et al., 2009). Pictures of the areas within the section were taken using brightfield microscopy and the images were opened in NIH image J (Image J 1.36b; National Institutes of Health, Bethesda, MD). All longitudinal blood vessels were identified. Vessels perpendicular to the section (cross-sectional vessels) were often stained, but were not counted to avoid any false positives due to edge effects around potential holes in the tissue. All longitudinal vessels were traced using the free-hand tracing tools available in NIH Image J and the integrated optical density was determined for the area constrained by the vessel tracing.. The tracing of the vessel was moved to an area of the section free of vessels and pigmented cells, and the optical density of that area was taken as a background. The background density was subtracted from the vessel density. The vessel densities were averaged for all the vessels within a section and then across the 3–4 sections for each brain region for a given subject.
Vessels were counted in the same images used above for the αvβ3 optical density measurements. As with optical density determinations, only longitudinal vessels, regardless of length, were counted. Each ramification was counted as an individual vessel. Vessels were counted in the entire tissue section on a 2D plane of the saved jpeg image. Since vessels were not counted within the thickness of the tissue, a volume measurement could not be assessed. However, this method reduces the possibility of counting cross-sectional, tortuous or non-continuous vessels multiple times through the thickness of the section. In order to determine the vascular number, the number of vessels was summed across three or four sections for each patient in each brain region. The total number of vessels was divided by the total area evaluated for vessels to yield a measure of vessel number/mm2.
Microglia Immunohistochemistry and Assessment
Microglia cell counts were assessed in the same brain regions used for integrin αvβ3 immunohistochemistry. An Olympus BX60 microscope with a computer-controlled motorized stage, high sensitivity HV-C20 CCD video camera (Hitachi, Japan) and StereoInvestigator software version 5.1 (MicroBrightField, Colchester, VT) was used to estimate microglia cell counts and volume of the sampling region in equidistant serial sections. Activated microglia were determined by MHC Class II immunoreactivity and by the more compact phagocytic morphology characteristic of activated microglia, stage C & D as identified in Kanaan et al. (Kanaan et al., 2008). The number of activated microglia were counted within the thickness of the tissue section under an Olympus 100x objective using the unbiased three-dimensional counting adapted from Gunderson (Williams and Rakic, 1988). The areas of interest were delineated at low power magnification prior to random sampling.. Approximately 10–20% of the region was quantified using a 200μm × 200μm counting frame. Due to the limited number of human post-mortem brain tissue sections, between three to four sections were available for each subject. Therefore, the total number of activated microglia and the volume of tissue evaluated was divided to obtain a density measurement. Density of activated microglia was expressed as total number/mm3.
Statistics
Before preceding with ANOVA to analyze the αvβ3, vessel count, microglia, age at death and PMI data, a Bartlett statistic and a Kolmogorov and Smirnov test were used to determine if the data had equal standard deviations (SD) and a Gaussian distribution required by ANOVA. Demographics were compared using ANOVA. PMI required the use of a non-parametric Kruskal Wallis test. In the case of the αvβ3 integrated densities and vessel numbers, a log transformation was applied and the Bartlett test confirmed equal SD and the Kolmogorov and Smirnov test confirmed a Gaussian distribution. One-Way ANOVA was used to determine within group and between group variations for each brain area. If the one-way ANOVA was significant, a Dunnett Multiple Comparison Test was used post hoc to determine if PD, iLBD, and PSP differed from control.
Results
Demographics
The mean age at death was 81.9± 2.9 years for controls. PD cases were of a similar age as controls while iLBD, and PSP cases were slightly older (Table 1) albeit not significantly different from control subjects (F(3,25) =0.62, p=0.61; Table 1). The average PMI for the patient groups was 6.2 hours and for controls, 11.4 hours. PMI was higher in controls, most likely to due to two patients with PMIs greater than 20 hours. However, there were no significant differences in PMI across the groups (H=2.85, p=0.42). None of the Control or iLBD subjects had a clinical diagnosis of PD (Table 1). For PD subjects, the average duration of the diagnosis was 5 ± 1 years with a range of 1 to 10 years before death (Table 1). Note that two of the PSP patients had a clinical diagnosis of PD but were included in the PSP group as they had pathology characteristic of PSP upon autopsy.
Angiogenesis
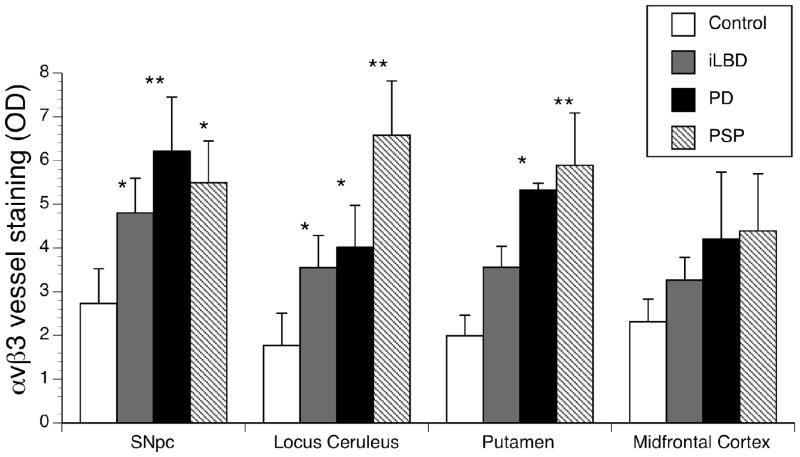
In order to determine whether vessels were actively undergoing angiogenesis, we assessed integrin αvβ3 immunoreactivity, an adhesion molecule present on endothelial cells of angiogenic vessels (Brooks, 1996) (Fig. 1). Significant changes in αvβ3 immunoreactivity were present in the SNpc (F(3,19) =5.67, p<0.01), putamen (F(3,16) =6.07, p<0.01), and locus ceruleus (F(3,19) =5.73, p<0.01), but not midfrontal cortex (F(3,17) =0.82, p=0.49) (Fig 2). All significant differences were then subjected to post hoc analyses using Dunnett’s multiple comparison tests to determine which groups differed from control. In the SNpc, PD (p<0.01), iLBD (p<0.05) and PSP (p<0.05) exhibited increased αvβ3 immunoreactivity relative to age-matched controls (Fig 2). In the putamen both PD (p<0.05) and PSP (p<0.01) exhibited significantly greater αvβ3 immunoreactivity than controls. iLBD subjects showed an intermediate level of enhanced αvβ3 reactivity in the putamen, but it did not reach statistical significance (fig 2). In the LC, PD (p<0.05), iLBD (p<0.05) and PSP (p<0.01) had significantly more αvβ3 immunoreactivity than controls (fig 2). Although αvβ3 immunoreactivity was not significantly altered in the midfrontal cortex, it is interesting to note that PSP patients exhibited a marked increase in immunoreactivity. Taken together, these results indicate that angiogenesis as measured by αvβ3 reactivity is ongoing in all three Parkinsonian disorders, but there are differences in the localization of αvβ3 reactivity.
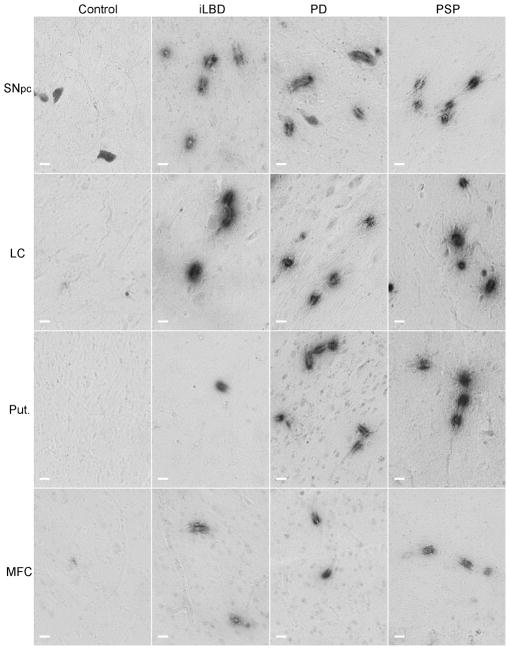
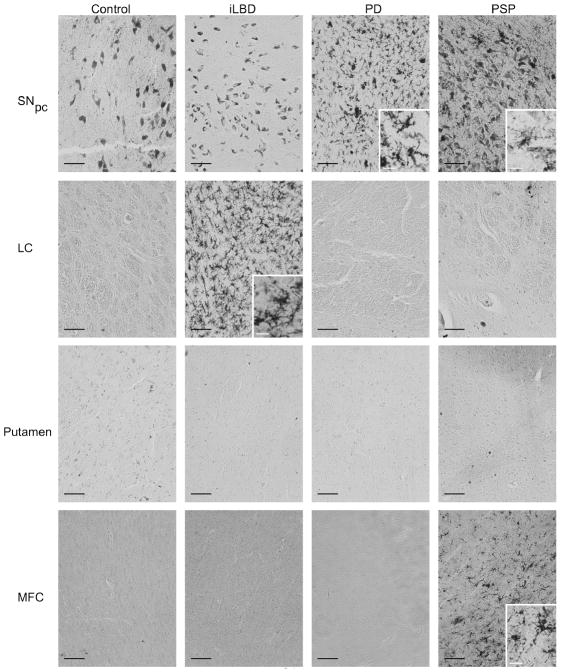
Fig 1. Integrin αvβ3 staining in post-mortem human brain tissue.
Endothelial cells of human post-mortem brain tissue were labeled with mouse anti-human integrin αvβ3 antibody and visualized with the chromagen DAB. Integrin αvβ3 reactive vessels are shown in post-mortem tissues from non-pathological controls, incidental Lewy Body Disease (iLBD), Parkinson’s disease (PD) and progressive supranuclear palsy (PSP) subjects. Note the distinct pattern of staining along vessels. In most cases only a small portion of the longitudinal vessel is stained. In other cases the vessels are perpendicular resulting in a ring of staining. The grey cells seen in the SNpc are neuromelanin-containing cells that are evident in unstained tissues and could be distinguished from the αvβ3 staining by stain color (in the original color images), and by morphology. Black scale bars = 100 μm.
Fig 2. Intensity of αvβ3 staining in post-mortem human brain tissue samples.
Integrin αvβ3 immunoreactivity was quantified as optical density (OD) of the vessels (see Methods). Integrin αvβ3 immunoreactivity was significantly greater in all Parkinsonian conditions compared with controls in the SNpc, and locus ceruleus. In the putamen, only PD and PSP were different than control. In the Midfrontal Cortex, there was variability and none of the differences reached statistical significance. Data is expressed as Mean ± SEM. Statistical significance was determined for each area using ANOVA with Dunnett test for mean comparison to control with * (p<0.05) and ** (p<0.01).
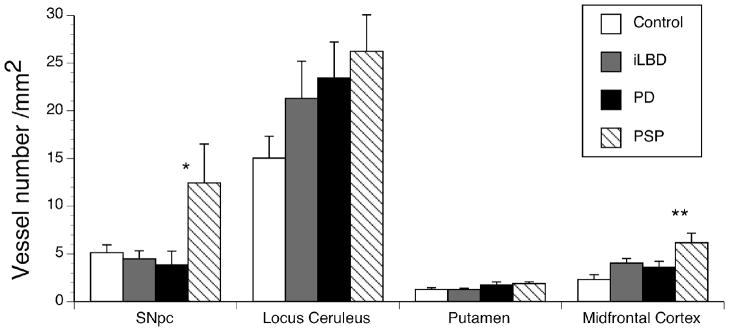
Although angiogenesis is a dynamic process and vessels may develop or regress, the numbers of vessels in each brain region was counted to determine if prior angiogenic activity resulted in an increased number of vessels. Although vessel number appeared to increase in all brain areas (Fig. 3), only changes in the SNpc (F (3,19)=3.22, p<0.05) and MFC (F (3,17) =5.66, p< 0.01) were statistically significant. Dunnett’s multiple comparison tests indicated that only the PSP subjects had a greater number of vessels than controls in both the SNpc (p<0.05) and in the MFC (p<0.01). Although there was evidence of increased vessel number in the LC in all three Parkinsonian conditions (Fig. 3), the overall data did not reach significance (F (3,19) = 2.72, p=0.07).
Fig 3. Vessel number in post-mortem human tissue samples.
Vessel numbers were counted under bright field microscopy as described in the Methods. PSP subjects had increased vessel number/mm2 in the SNpc and MFC. iLBD subjects had a small but significant increase in vessel number/mm2 in the MFC. Vessel number/mm2 appeared increased in the LC for all Parkinsonian disorders but did not reach statistical significance. Data was expressed as Mean ± SEM. Statistical significance was determined using ANOVA with Dunnett test for mean comparison to control with * (p<0.05) and ** (p<0.01).
Activated microglia
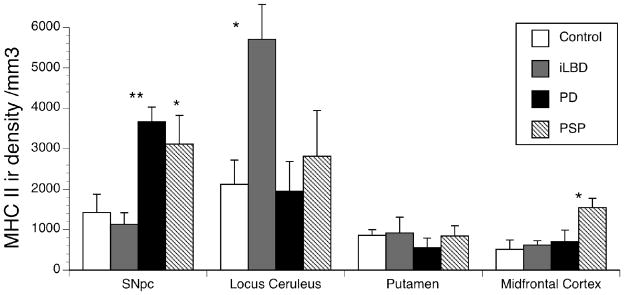
To assess the degree of neuroinflammation in each group, the number of activated microglia cells, identified by MHC Class II immunoreactivity, was determined in each brain region using stereology. Activated microglia were identified using a scale established previously (Kanaan et al., 2008). The number of activated microglia was significantly different in the SNpc (F(3,17) =6.99, p<0.01), LC (F(3,16) =4.21, p<0.05), MFC (F(3,18) =3.56, p<0.05), but not in the putamen (F(3,16) =0.46, p=0.71) (fig 5). These changes were disease specific as PD (p<0.01) and PSP (p<0.05) had more activated microglia in the SNpc while iLBD did not differ from control (fig 5). In the LC, only the preclinical iLBD cases had more activated microglia than controls (p<0.05) (fig 5). In the MFC, only the PSP subjects had a significant increase in microglia (p<0.05) (fig 5).
Fig. 5. Stereological assessment of Microglial density.
Stereology was used to determine the number of activated microglia in a given region. Density was obtained by dividing total number of activated microglia by volume of interest (refer to Methods). The number of activated microglia was significant for PD in the SNpc. PSP subjects showed an increase in both the SNpc and the MFC. iLBD subjects had an increase in the LC. Data is expressed as Mean ± SEM. Statistical significance was determined using ANOVA with Dunnett test for mean comparison to control with * (p<0.05) and ** (p<0.01).
Discussion
Angiogenesis has been observed in a variety of neurodegenerative diseases and their animal models including Alzheimer’s disease (AD) (Desai et al., 2009; Pogue and Lukiw, 2004; Schultheiss et al., 2006; Thirumangalakudi et al., 2006; Vagnucci and Li, 2003) and multiple sclerosis (MS) (Holley et al., 2010; Kirk et al., 2004; Roscoe et al., 2009). Angiogenesis has also been observed in animal models of PD (Barcia et al., 2005) and both increases in endothelial cell counts (Faucheux et al., 1999) and the presence of angiogenic factors (Wada et al., 2006; Yasuda et al., 2007) are consistent with its presence in human PD. The data presented here indicates that angiogenesis, as measured by αvβ3 reactivity, is present in human PD. Integrin αvβ3 reactivity was present (Fig 2) in the disease-affected areas (Braak et al., 2004) but was sparse in the MFC, an area not significantly involved in PD. Likewise, αvβ3 positive vessels were present in iLBD suggesting that it may appear in the preclinical period of PD. Unlike PD and PSP subjects, iLBD subjects had an intermediate level of angiogenesis that was not statistically different from controls in the putamen, an area affected in the later stages of PD. In addition, angiogenesis does not appear dependent on microglia activation, as angiogenesis was present in the SNpc of iLBD without microglia activation. In PD, angiogenesis may not have progressed to new vessel formation, as increases in vessel number/mm2 were not observed but the methods used may not have been sensitive enough to detect all such microvascular changes. The more rapidly progressing PSP subjects also had wide spread angiogenesis and areas of higher vessel number2 were detected. Changes in angiogenesis and microglia and vessel number/mm2 varied with the disorder and brain region and are discussed here within the context of the Braak staging of PD progression (Braak et al., 2004). Although there were some findings that were surprising, much of the data is consistent with the idea that angiogenesis is an event that accompanies the neurodegenerative changes associated with Parkinsonian disorders.
αvβ3 reactivity was used as a marker for angiogenesis as it is absent on patent vessels and expressed on angiogenic endothelial cells undergoing cell division and migration (Brooks et al., 1994a; Brooks et al., 1994b; Brooks, 1996; Folkman, 2004; Friedlander et al., 1995). The interpretation of angiogenesis also rests on the specificity of αvβ3 reactivity for angiogenic vessels and not spurious epitopes present on other cells in the brain. Although integrins mediate cell attachment to the matrix, and a variety of integrin receptors are present in the brain, the β3 integrin does not appear to be expressed in the brain (Pinkstaff et al., 1999). Further, a β3 monoclonal antibody does not react with autopsy brain tissue (Akiyama et al., 1991). Despite these findings, integrin αvβ3 expression was reported on cultured oligodendrocyte precursor cells, but it was absent on the day of isolation and only present after two to ten days in culture (Milner et al., 1997). Because αvβ3 is commonly upregulated in cultured cells (Horton, 1990), its expression was likely a response to tissue culture. The absence of β3 in brain tissue rules out the expression of the αvβ3 heterodimer. However, this does not mean that other related molecules are not present. For example, the αv subunit is expressed in the brain in conjunction with β5 and has been localized to activated microglia that express αvβ5 but not αvβ3 (Milner and Campbell, 2003). Because of this, the use antibodies to αv or polyclonal antibodies to the vitronectin receptor(s) are not appropriate measures for angiogenesis. Several researchers have used a β3 integrin antibody to identify angiogenesis in animal models (Baluk and McDonald, 2008; Carvey et al., 2005; Schultheiss et al., 2006). More specific antibodies that recognize an epitope present only when the αv and β3 integrin subunits are associated identify angiogenesis in human tissues (Baluk and McDonald, 2008). In this study, and another (Desai et al., 2009), we have found that an antibody that recognizes the αvβ3 shared epitope results in staining that is limited to vessels in brain autopsy tissue.
Subjects with PD had greater reactivity to the αvβ3 antibody in areas associated with PD pathology including the LC, SNpc, and putamen when compared with control subjects (fig 2). In addition to increases in angiogenesis, PD subjects also had greater numbers of activated microglia compared with controls in the SNpc (fig 4&5) confirming the findings of multiple investigators (Croisier et al., 2005; McGeer and McGeer, 2008; McGeer et al., 1988; Whitton, 2007; Zhang et al., 2005).
Fig 4. Activated microglia in post-mortem human tissue samples.
Activated microglia were labeled using an antibody to MHC class II antigen and visualized with the chromagen DAB. In the SNpc, there were neuromelanin-containing cells, as evident in the control condition. Microglia could be distinguished form neuromelanin containing cells by size and morphology. The black scale bar = 100 μm. Insets in the panels were taken at higher magnification and show microglia morphology typical of the microglia in that condition. White scale bars in the insets are 20 μm.
To determine if angiogenesis was present early in the disease process, we examined tissues from subjects with iLBD. These subjects had Lewy bodies, a histo-pathological hallmark of PD, but did not have clinical symptoms (DelleDonne et al., 2008; Dickson et al., 2008). The distribution of Lewy Bodies in iLBD mimics that of PD in the lower brainstem and anterior olfactory nucleus and this distribution reflects the staging system of PD progression proposed by Braak (Braak and Del Tredici, 2009; Braak and Del Tredici, 2010; Braak et al., 2003; Braak et al., 2004). Although there is some controversy concerning the Braak staging in PD (Kalaitzakis et al., 2008), the similarities between Lewy Body expression in PD and iLBD and the evidence that there is intermediate DA neuron loss in the SN of iLBD patients has led to the suggestion that iLBD may represent preclinical PD (DelleDonne et al., 2008; Dickson et al., 2008). The αvβ3 and microglia data in the present study is consistent with the idea that iLBD represents a preclinical PD progression. Thus, iLBD subjects had significant increases in αvβ3 in the LC and SNpc compared to controls, but unlike PD subjects they did not differ from control subjects in the putamen. These findings are consistent with Braak staging of PD as the LC is involved in stage 2, while the SNpc involvement begins later in stage 3 and then progresses until there is nearly complete dopamine loss in the putamen producing motor symptoms in stage 4 (Braak et al., 2004). Unlike PD subjects, iLBD subjects did not have significant increases in activated microglia in the SNpc suggesting less neuroinflammation. Thus, compared to PD patients the iLBD subjects had fewer activated microglia in the SNpc and less αvβ3 staining in the putamen, suggesting less dysfunction in the areas involved later in the disease process. Interestingly, PD patients did not exhibit increased microglia in the LC whereas iLBD subjects did (fig 4&5). While we did not observe increased microglial staining in the LC of PD subjects, others have observed a weak microglial proliferation in the LC of PD subjects (Bertrand et al., 1997). It is possible that the lack of immunostaining for microglia in the LC of PD patients (fig 4) is because the microglia lost MHC Class II immunoreactivity, underwent apoptosis (Streit, 2006) or migrated to the SNpc as part of disease progression. However, we cannot rule out the possibility that iLBD has a distinct pathology from PD.
To determine if angiogenesis was a feature of a rapidly progressing Parkinsonian disorder, angiogenesis was also evaluated in autopsy materials from subjects with PSP. PSP subjects have motor deficits similar to PD subjects, and are often initially diagnosed with PD (Josephs and Dickson, 2003; Rajput et al., 1991). Indeed, two of the PSP subjects in this study were initially diagnosed with PD but were found to have PSP upon autopsy. PSP is a distinct disorder with differing pathology and more rapid progression (Armstrong et al., 2007). Like PD, there was an increase in both αvβ3 and microglia reactivity in the SNpc (fig 4&5). Thus, both PD and PSP had activated microglia in the SNpc, but the preclinical subjects with Lewy bodies had αvβ3 without activated microglia in this area. In PSP patents there were also changes in angiogenesis and microglia in the MFC that were not observed in PD patents. Although αvβ3 was elevated in the MFC but not significantly different from controls, PSP subjects had an increase in vessel number/mm2 consistent with past angiogenesis in the MFC as well as an increase in activated microglia in an area involved in PSP, but not PD pathology. The finding of activated microglia in the SNpc and MFC is consistent with observations of microglia activation in a previous PET imaging study of PSP subjects (Gerhard et al., 2006). It is not known if the widespread angiogenic activity observed in the PSP subjects would contribute to the multi-region degeneration and atrophy seen in PSP (Armstrong et al., 2007).
No firm conclusions can be drawn about the relationship between angiogenesis and microglia activation. We had expected that angiogenesis would result from the inflammatory agents released by activated microglia. However, our results indicate that angiogenesis is more wide spread than activated microglia making it unlikely that angiogenesis is dependent on microglia activation. In an animal model of PD, treatment with L-DOPA can cause angiogenesis {Lindgren et al., 2009, Neuropsychopharmacology, 34, 2477-88}, independent of inflammatory response. Thus, some of the angiogenesis found in the PD patients may be related to pharmacotherapy. However, iLBD subjects were not diagnosed with PD nor treated with L-DOPA. Thus it may be that angiogenesis precedes microglia activation, as the preclinical iLBD subjects were positive for angiogenesis in the SNpc without a significant increase in activated microglia. Likewise, the appearance of activated microglia occurred in areas either positive for αvβ3 or areas that had increased vessel number indicative of past angiogenesis. While these results suggest that angiogenesis would precede microglia activation, any conclusions regarding the relationship between angiogenesis and microglia activation are based on the assumption that both αvβ3 and activated microglia are static markers and what is present at autopsy is indicative of what occurred over a lifetime. Integrin αvβ3 is a marker for ongoing angiogenesis should be lost as a vessel matures, as αvβ3 is not found on patent vessels (Brooks et al., 1994a; Brooks et al., 1994b; Brooks, 1996; Folkman, 2004; Friedlander et al., 1995). Even vessel number may be misleading as angiogenesis is a dynamic process with vessels being formed and pruned depending on the relative concentration of angiogenic and antiangiogenic factors (Baluk et al., 2004; Hanahan and Folkman, 1996). As a result, the evidence present at autopsy is a snapshot and may be insufficient to determine whether angiogenesis precedes microglia activation or vice-versa.
It is interesting that PSP subjects were the only group to show an increase in vessel number in the SNpc. This may be a matter of timing since PSP is a rapidly progressing disease and angiogenesis may have occurred earlier and progressed further to allow for the creation of new vessels. However, if it is a matter of timing, then vessels should be evident in the LC owing to its early involvement in disease progression. There is some support for this as all of the Parkinsonian disorders exhibited increased vessel number/mm2 relative to controls in the LC, but the increases in vessel number /mm2 were not statistically significant (fig 3). It is possible that the high vessel number/mm2 present in the aged controls may preclude significant increases via a ceiling effect. Another issue is that we may not have had the sensitivity to detect subtle changes in vessel number. A limitation of this study is that we did not have additional tissues dedicated to counting vessel density. Rather, vessels were identified in the course of assessing the brightfield αvβ3 stained images. A vessel specific stain was not used because it would interfere with the assessment of optical density of the αvβ3 stained vessels. Thus, vessel density and in particular, the number of small vessels may be under-estimated as we may have missed vessels that would have been visible if stained. Barcia et al. examined stained vessels and found an increase in vessel staining in the SNpc of MPTP treated monkeys that was attributed to microangiogenesis (Barcia et al., 2005). Another study examined endothelial nuclei and found an increase in the SNpc of PD compared with controls (Faucheux et al., 1999), a result consistent with increased vessel number, but this result would also be consistent with endothelial cell division occurring during angiogenesis.
Irrespective of whether our assay was sensitive enough to assess an increase in vessel density, the expression of αvβ3 in post mortem tissue suggests an ongoing process of angiogenesis. Angiogenesis may be a positive response to injury, restoring circulation and providing necessary oxygenation and nutrients from the blood. Normally astrocytes respond to a variety of stimuli including hypoxia by releasing VEGF (Kenneth and Rocha, 2008; Schmid-Brunclik et al., 2008) a potent angiogenic factor. However, the newly created angiogenic vessels must undergo a period of maturation orchestrated by a sequence of pro-angiogenic factors (Jain, 2003). Further, the creation of the BBB requires an increase in tight junctions between endothelial cells, the deposition of matrix proteins, recruitment of pericytes, and the presence of astroglial end feet. Thus, the newly created vessels are likely to lack the highly differentiated structure of the BBB. As such, these vessels may not efficiently restrict immune cells entry to the brain parenchyma. Experiments using mouse chimeras indicate that bone marrow cells migrate into the SNpc and adopt the phenotype of activated microglia in MPTP treated mice (Kokovay and Cunningham, 2005; Rodriguez et al., 2007). Further, the infiltration of CD4 and CD8 T cells is enhanced by MPTP treatment (Brochard et al., 2009; Kurkowska-Jastrzebska et al., 1999) and MPTP-induced DA loss is reduced in mice lacking CD 4 cells (Brochard et al., 2009). In humans, LFA–1 positive leukocytes were detected in the SN of PD subjects to a greater extent than controls (Miklossy et al., 2006) and elevated numbers of CD4 and CD8 T cells were seen in PD subjects (Brochard et al., 2009). All of the above results are consistent with the enhanced entry of peripheral cells into parenchyma in PD and its animal models.
The release of VEGF by astrocytes may have multiple effects on the brain. While nanomolar concentrations of VEGF are neuroprotective (Jin et al., 2002; Yasuhara et al., 2004; Yasuhara et al., 2005), the acute administration of VEGF in concentrations above 100 ng/day has deleterious effects (Croll et al., 2004; Yasuhara et al., 2005); including an immediate increase in vessel permeability followed by a breakdown of the BBB as evidenced by decreased astroglial endfeet, deposition of plasma proteins, the extravasation of leukocytes (Croll et al., 2004), and degeneration of DA neurons adjacent to the injection site (Rite et al., 2007). Elevations of VEGF have been found in the SNpc and in the striatium of PD subjects (Wada et al., 2006; Yasuda et al., 2007). In addition, Barcia et al. found that MPTP treatment increased VEGF in the SNpc of monkeys and that this was associated with angiogenesis (Barcia et al., 2005). These results led Barcia to speculate that these newly created vessels would facilitate the entry of peripheral toxins and pro-inflammatory cytokines.
The idea that the BBB may be dysfunctional in PD remains controversial. A recent MRI study using a gadolinium tracer failed to find a breach in the BBB of MPTP-treated macaques (Astradsson et al., 2009). In addition, the BBB of PD subjects is assumed to be intact as the widely used AAAD inhibitors, carbidopa and benserazide, do not appear to compromise levodopa therapy. In fact, carbidopa was developed based on its inability to cross the BBB while blocking peripheral decarboxylase (Celesia and Wanamaker, 1976). However, there is evidence that benserazide can enter the brain and affect levodopa metabolism (Jonkers et al., 2001; Shen et al., 2003) and that the enhanced entry of levodopa into the striatium is associated with dyskinesias (Carta et al., 2006; Westin et al., 2006). In addition, the BBB is dysfunctional in a variety of animal models of PD resulting in punctate leakage of FITC-labeled albumin and other tracers (Carvey et al., 2005; Carvey et al., 2009; Chen et al., 2008; Chung et al., 2010; Westin et al., 2006). Using a β3 antibody, we determined that angiogenesis colocalized with these areas of punctate FITC-albumin leakage indicating an association between angiogenesis and a localized BBB dysfunction (Carvey et al., 2005).
In tumor biology, the continued exposure to angiogenic factors such as VEGF can lead to “pathological” angiogenesis (Jain, 2003; Nagy et al., 2008). Such pathological vessels may lack pericytes, are continually leaky and can actually raise interstitial pressure, which then impedes the delivery of oxygen and nutrients (Jain, 2005). If VEGF expression is causing pathological angiogenesis and restricting oxygen delivery, it may be perpetuating its own release, as the expression of VEGF by astrocytes is at least in part driven by hypoxia (Kenneth and Rocha, 2008; Schmid-Brunclik et al., 2008). Such a feed forward mechanism could drive chronic angiogenesis, and result in exposure to peripheral toxins, cytokines and recruitment of immune cells. Such a mechanism may underlie the association of angiogenesis with various neurodegenerative diseases. In such a scenario, angiogenesis is a response to the precipitating lesion and should have a specific localization. In line with this supposition, we have determined that the expression of αvβ3 is highest in the MFC in AD subjects (Desai et al., 2009) while this area did not differ from control in iLBD, PD or PSP subjects (fig 2).
Given the observational nature of autopsy data we cannot determine from our data if angiogenesis perpetuates the inflammatory response or if it is secondary to these events. Future studies in animal models are needed to determine if manipulating angiogenesis can affect BBB dysfunction, the entry of peripheral toxins or immune cells and ultimately DA neuron loss. If angiogenesis plays such a role in neuroinflammation then interfering with angiogenesis may provide a new avenue for addressing neuroinflammation in PD and a variety of progressive neurological disorders.
Acknowledgments
This work was supported by RO1AI51619 (BH), K08AG00084 (JAS), P30AG10161 (JAS), R01AG15819 (JAS), NS052414 (PMC) and the Kenneth Douglas Foundation (PMC). We thank Dr. Sue Leurgans for her statistical expertise. We thank the nuns, priests, and brothers from the following groups participating in the Religious Orders Study: Archdiocesan priests of Chicago, Dubuque, and Milwaukee; Benedictine Monks, Lisle, IL, and Collegeville, MN; Benedictine Sisters of Erie, Erie, PA; Benedictine Sisters of the Sacred Heart, Lisle, IL; Capuchins, Appleton, WI; Christian Brothers, Chicago, IL, and Memphis, TN; Diocesan priests of Gary, IN; Dominicans, River Forest, IL; Felician Sisters, Chicago, IL; Franciscan Handmaids of Mary, New York, NY; Franciscans, Chicago, IL; Holy Spirit Missionary Sisters, Techny, IL; Maryknolls, Los Altos, CA and Maryknoll, NY; Norbertines, DePere, WI; Oblate Sisters of Providence, Baltimore, MD; Passionists, Chicago, IL; Presentation Sisters, Dubuque, BVM., IA; Servites, Chicago, IL; Sinsinawa Dominican Sisters, Chicago, IL, and Sinsinawa, WI; Sisters of Charity, B.V.M., Chicago, IL, and Dubuque, IA; Sisters of the Holy Family, New Orleans, LA; Sisters of the Holy Family of Nazareth, Des Plaines, IL; Sisters of Mercy of the Americas, Chicago and Aurora, IL, Erie, PA; Sisters of St. Benedict, St. Cloud and St. Joseph, MN; Sisters of St. Casimir, Chicago, IL; Sisters of St. Francis of Mary Immaculate, Joliet, IL; Sisters of St. Joseph of LaGrange, LaGrange Park, IL; Society of Divine Word, Techny, IL; Trappists, Gethsemani, KY, and Peosta, IA; Wheaton Franciscan Sisters, Wheaton, IL. We thank T. Colvin and J. Bach, Religious Orders Study Coordinators; data and analytic programmers, Karen Skish, and Yu Li for technical assistance and the Rush Alzheimer’s Disease Center laboratory staff.
Footnotes
Disclosure Statement: There are no actual or potential conflicts of interest.
Literature Cited
- Akiyama H, Kawamata T, Dedhar S, McGeer PL. Immunohistochemical localization of vitronectin, its receptor and beta-3 integrin in Alzheimer brain tissue. J Neuroimmunol. 1991;32:19–28. doi: 10.1016/0165-5728(91)90067-h. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. Progressive supranuclear palsy (PSP): a quantitative study of the pathological changes in cortical and subcortical regions of eight cases. J Neural Transm. 2007;114:1569–1577. doi: 10.1007/s00702-007-0796-3. [DOI] [PubMed] [Google Scholar]
- Astradsson A, Jenkins BG, Choi JK, Hallett PJ, Levesque MA, McDowell JS, Brownell AL, Spealman RD, Isacson O. The blood-brain barrier is intact after levodopa-induced dyskinesias in parkinsonian primates--evidence from in vivo neuroimaging studies. Neurobiol Dis. 2009;35:348–351. doi: 10.1016/j.nbd.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, McDonald DM. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bautista V, Sanchez-Bahillo A, Fernandez-Villalba E, Faucheux B, Poza y Poza M, Fernandez Barreiro A, Hirsch EC, Herrero MT. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J Neural Transm. 2005;112:1237–1248. doi: 10.1007/s00702-004-0256-2. [DOI] [PubMed] [Google Scholar]
- Barcia C, Emborg ME, Hirsch EC, Herrero MT. Blood vessels and parkinsonism. Front Biosci. 2004;9:277–282. doi: 10.2741/1145. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006a;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006b;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Dymecki J. Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson’s disease. Folia Neuropathol. 1997;35:80–86. [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Pathophysiology of sporadic Parkinson’s disease. Fortschr Neurol Psychiatr. 2010;78(Suppl 1):S2–4. doi: 10.1055/s-0029-1245179. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC. Role of integrins in angiogenesis. Eur J Cancer. 1996;32A:2423–2429. doi: 10.1016/s0959-8049(96)00381-4. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochem. 2006;96:1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111:291–314. doi: 10.1111/j.1471-4159.2009.06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia GG, Wanamaker WM. L-dopa-carbidopa: combined therapy for the treatment of Parkinson’s disease. Dis Nerv Syst. 1976;37:123–125. [PubMed] [Google Scholar]
- Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem. 2008;107:1147–1157. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH, Jin BK. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010;43:225–232. doi: 10.5483/bmbrep.2010.43.4.225. [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Ransohoff RM, Cai N, Zhang Q, Martin FJ, Wei T, Kasselman LJ, Kintner J, Murphy AJ, Yancopoulos GD, Wiegand SJ. VEGF-mediated inflammation precedes angiogenesis in adult brain. Exp Neurol. 2004;187:388–402. doi: 10.1016/j.expneurol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm. 2009;116:587–597. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- Fahn S, Przedborski S. Parkinsonism. 2000:679–693. [Google Scholar]
- Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet. 1999;353:981–982. doi: 10.1016/S0140-6736(99)00641-8. [DOI] [PubMed] [Google Scholar]
- Folkman J. Endogenous angiogenesis inhibitors. Apmis. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Trender-Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord. 2006;21:89–93. doi: 10.1002/mds.20668. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Holley JE, Newcombe J, Whatmore JL, Gutowski NJ. Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Neurosci Lett. 2010;470:65–70. doi: 10.1016/j.neulet.2009.12.059. [DOI] [PubMed] [Google Scholar]
- Horton M. Vitronectin receptor: tissue specific expression or adaptation to culture? Int J Exp Pathol. 1990;71:741–759. [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–657. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers N, Sarre S, Ebinger G, Michotte Y. Benserazide decreases central AADC activity, extracellular dopamine levels and levodopa decarboxylation in striatum of the rat. J Neural Transm. 2001;108:559–570. doi: 10.1007/s007020170056. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. Controversies over the staging of alpha-synuclein pathology in Parkinson’s disease. Acta Neuropathol. 2008;116:125–8. doi: 10.1007/s00401-008-0381-3. author reply 129–31. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobrucki LW, Meoli DF, Dione DP, Sadeghi MM, Madri JA, Sinusas AJ. Targeted imaging of hypoxia-induced integrin activation in myocardium early after infarction. J Appl Physiol. 2008;104:1504–1512. doi: 10.1152/japplphysiol.00861.2007. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Age and region-specific responses of microglia, but not astrocytes, suggest a role in selective vulnerability of dopamine neurons after 1-methyl–4-phenyl-1,2,3,6-tetrahydropyridine exposure in monkeys. Glia. 2008;56:1199–1214. doi: 10.1002/glia.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- Kirk S, Frank JA, Karlik S. Angiogenesis in multiple sclerosis: is it good, bad or an epiphenomenon? J Neurol Sci. 2004;217:125–130. doi: 10.1016/j.jns.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl–4-phenyl–1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Sidman RL, Pasqualini R, Arap W. Treatment of hypoxia-induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. Faseb J. 2007;21:3272–3278. doi: 10.1096/fj.07-8273com. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM–1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Milner R, I, Campbell L. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- Milner R, Frost E, Nishimura S, Delcommenne M, Streuli C, Pytela R, Ffrench-Constant C. Expression of alpha vbeta3 and alpha vbeta8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia. 1997;21:350–360. [PubMed] [Google Scholar]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med. 2004;36:524–533. doi: 10.1038/emm.2004.67. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue AI, Lukiw WJ. Angiogenic signaling in Alzheimer’s disease. Neuroreport. 2004;15:1507–1510. doi: 10.1097/01.wnr.0000130539.39937.1d. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism--a prospective study. Can J Neurol Sci. 1991;18:275–278. doi: 10.1017/s0317167100031814. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T Cells Attenuate Th17 Cell-Mediated Nigrostriatal Dopaminergic Neurodegeneration in a Model of Parkinson’s Disease. J Immunol. 2010 doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rite I, Machado A, Cano J, Venero JL. Blood-brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem. 2007;101:1567–1582. doi: 10.1111/j.1471-4159.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- Roscoe WA, Welsh ME, Carter DE, Karlik SJ. VEGF and angiogenesis in acute and chronic MOG((35–55)) peptide induced EAE. J Neuroimmunol. 2009;209:6–15. doi: 10.1016/j.jneuroim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Schmid-Brunclik N, Burgi-Taboada C, Antoniou X, Gassmann M, Ogunshola OO. Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R864–73. doi: 10.1152/ajpregu.00536.2007. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss C, Blechert B, Gaertner FC, Drecoll E, Mueller J, Weber GF, Drzezga A, Essler M. In vivo characterization of endothelial cell activation in a transgenic mouse model of Alzheimer’s disease. Angiogenesis. 2006 doi: 10.1007/s10456-006-9030-4. [DOI] [PubMed] [Google Scholar]
- Shen H, Kannari K, Yamato H, Arai A, Matsunaga M. Effects of benserazide on L-DOPA-derived extracellular dopamine levels and aromatic L-amino acid decarboxylase activity in the striatum of 6-hydroxydopamine-lesioned rats. Tohoku J Exp Med. 2003;199:149–159. doi: 10.1620/tjem.199.149. [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving The Brain Stem, Basal Ganglia And Cerebellum With Vertical Gaze And Pseudobulbar Palsy, Nuchal Dystonia And Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimers Dis. 2006;10:111–118. doi: 10.3233/jad-2006-10114. [DOI] [PubMed] [Google Scholar]
- Vagnucci AHJ, Li WW. Alzheimer’s disease and angiogenesis. Lancet. 2003;361:605–608. doi: 10.1016/S0140-6736(03)12521-4. [DOI] [PubMed] [Google Scholar]
- Wada K, Arai H, Takanashi M, Fukae J, Oizumi H, Yasuda T, Mizuno Y, Mochizuki H. Expression levels of vascular endothelial growth factor and its receptors in Parkinson’s disease. Neuroreport. 2006;17:705–709. doi: 10.1097/01.wnr.0000215769.71657.65. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Westin JE, Lindgren HS, Gardi J, Nyengaard JR, Brundin P, Mohapel P, Cenci MA. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J Neurosci. 2006;26:9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, Rosenberg J, Gambhir SS. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248:936–944. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Fukuda-Tani M, Nihira T, Wada K, Hattori N, Mizuno Y, Mochizuki H. Correlation between levels of pigment epithelium-derived factor and vascular endothelial growth factor in the striatum of patients with Parkinson’s disease. Exp Neurol. 2007;206:308–317. doi: 10.1016/j.expneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19:1494–1504. doi: 10.1111/j.1460-9568.2004.03254.x. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Shingo T, Muraoka K, wen Ji Y, Kameda M, Takeuchi A, Yano A, Nishio S, Matsui T, Miyoshi Y, Hamada H, Date I. The differences between high and low-dose administration of VEGF to dopaminergic neurons of in vitro and in vivo Parkinson’s disease model. Brain Res. 2005;1038:1–10. doi: 10.1016/j.brainres.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]