Abstract
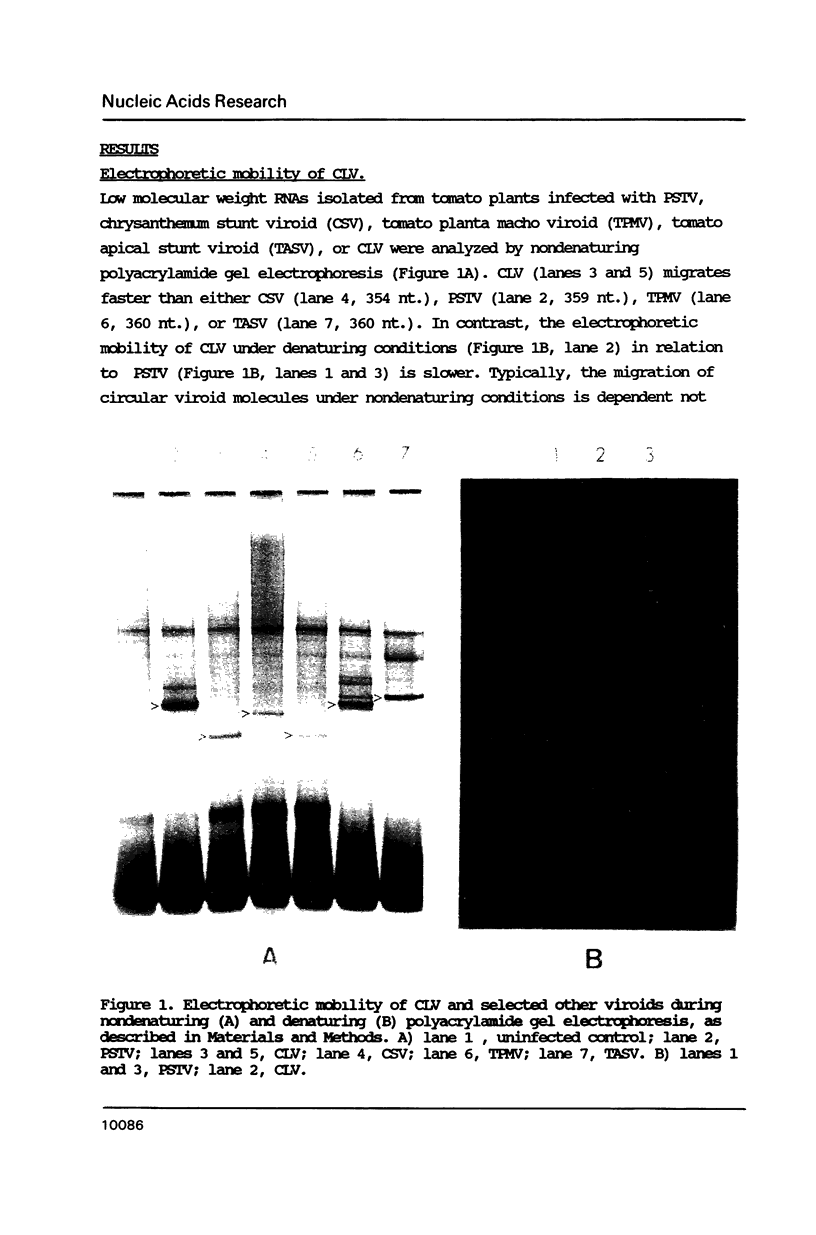
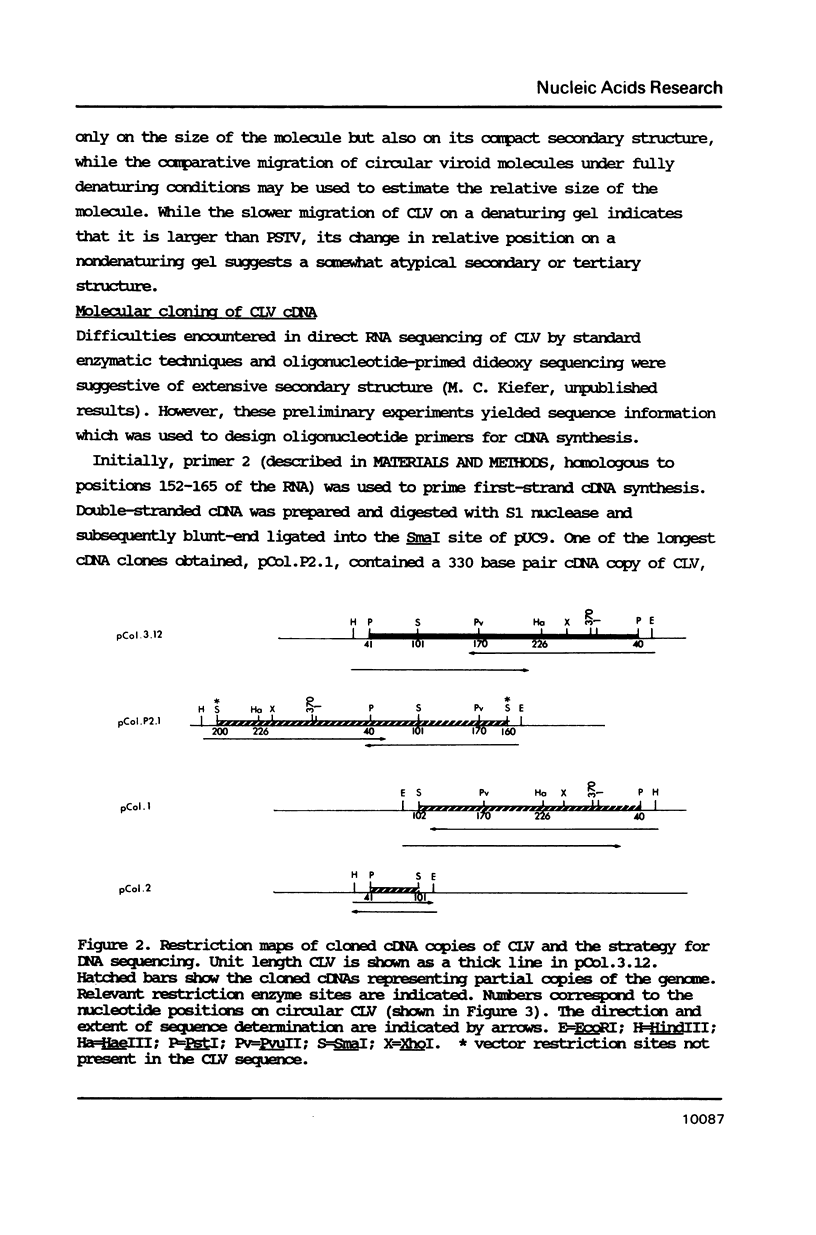
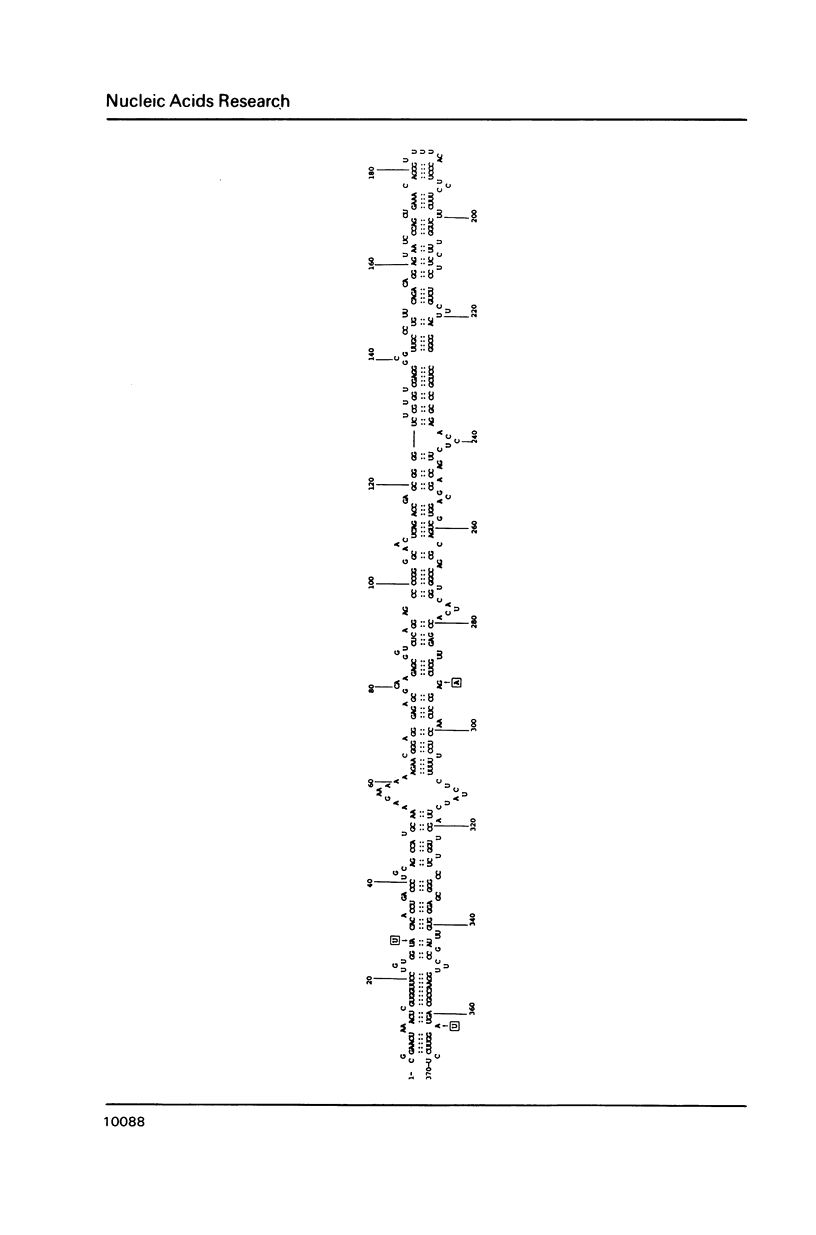
The Columnea latent viroid (CLV) occurs latently in certain Columnea erythrophae plants grown commercially. In potato and tomato, CLV causes potato spindle tuber viroid (PSTV)-like symptoms. Its nucleotide sequence and proposed secondary structure reveal that CLV consists of a single-stranded circular RNA of 370 nucleotides which can assume a rod-like structure with extensive base-pairing characteristic of all known viroids. The electrophoretic mobility of circular CLV under nondenaturing conditions suggests a potential tertiary structure. CLV contains extensive sequence homologies to the PSTV group of viroids but contains a central conserved region identical to that of hop stunt viroid (HSV). CLV also shares some biological properties with each of the two types of viroids. Most probably, CLV is the result of intracellular RNA recombination between an HSV-type and one or more PSTV-type viroids replicating in the same plant.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Parker M. W. The presence of a copper/zinc superoxide dismutase in the bacterium Photobacterium leiognathi: a likely case of gene transfer from eukaryotes to prokaryotes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):149–152. doi: 10.1073/pnas.82.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroid processing: a model involving the central conserved region and hairpin I. Proc Natl Acad Sci U S A. 1986 Jan;83(1):58–62. doi: 10.1073/pnas.83.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Riesner D. Viroids: a class of subviral pathogens. Angew Chem Int Ed Engl. 1980;19(4):231–243. doi: 10.1002/anie.198002313. [DOI] [PubMed] [Google Scholar]
- Hammond R. W., Diener T. O., Owens R. A. Infectivity of chimeric viroid transcripts reveals the presence of alternative processing sites in potato spindle tuber viroid. Virology. 1989 Jun;170(2):486–495. doi: 10.1016/0042-6822(89)90440-6. [DOI] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. Fish to bacterium gene transfer. Science. 1985 Mar 1;227(4690):1020–1020. doi: 10.1126/science.227.4690.1020. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic Acid hybridization. Science. 1981 Aug 7;213(4508):670–672. doi: 10.1126/science.213.4508.670. [DOI] [PubMed] [Google Scholar]
- Puchta H., Ramm K., Sänger H. L. Molecular and biological properties of a cloned and infectious new sequence variant of cucumber pale fruit viroid (CPFV). Nucleic Acids Res. 1988 Aug 25;16(16):8171–8171. doi: 10.1093/nar/16.16.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H., Ramm K., Sänger H. L. Nucleotide sequence of a hop stunt viroid isolate from the German grapevine cultivar 'Riesling'. Nucleic Acids Res. 1988 Mar 25;16(6):2730–2730. doi: 10.1093/nar/16.6.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H., Ramm K., Sänger H. L. The molecular structure of hop latent viroid (HLV), a new viroid occurring worldwide in hops. Nucleic Acids Res. 1988 May 25;16(10):4197–4216. doi: 10.1093/nar/16.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaian M. A., Koltunow A. M., Krake L. R. Isolation of three viroids and a circular RNA from grapevines. J Gen Virol. 1988 Feb;69(Pt 2):413–422. doi: 10.1099/0022-1317-69-2-413. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., Hataya T., Shikata E. Complete nucleotide sequence of a viroid isolated from Etrog citron, a new member of hop stunt viroid group. Nucleic Acids Res. 1988 Jan 11;16(1):347–347. doi: 10.1093/nar/16.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., Uyeda I., Shikata E., Ohno T., Okada Y. Nucleotide sequence of cucumber pale fruit viroid: homology to hop stunt viroid. Nucleic Acids Res. 1984 Apr 25;12(8):3427–3434. doi: 10.1093/nar/12.8.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Mühlbach H. P., Sänger H. L. Linear oligomeric potato spindle tuber viroid (PSTV) RNAs are accurately processed in vitro to the monomeric circular viroid proper when incubated with a nuclear extract from healthy potato cells. EMBO J. 1987 Aug;6(8):2173–2183. doi: 10.1002/j.1460-2075.1987.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]