Abstract
Viruses are commonly investigated as vector systems for gene therapy. To be effective, virus-mediated gene-delivery systems require the presence of specific virus receptors to enter the target cell. One example is adenovirus and its primary receptor is the coxsackievirus and adenovirus receptor (CAR). Madin–Darby canine kidney (MDCK) cells have become a choice model system for studying CAR and adenovirus infection due to their ability to polarize rapidly into an epithelium with high transepithelial resistance. We show here that, whilst MDCK cells are resistant to adenovirus infection and hence appear functionally CAR-deficient, polarized MDCK cells express significant levels of CAR sequestered on the basolateral surface, where it is inaccessible for virus infection. Thus, although a cell type may be resistant to adenovirus infection, it is impossible to know whether it is due to a deficiency, as both CAR absence and inaccessibility are barriers to adenovirus-mediated gene transfer.
The coxsackievirus and adenovirus receptor (CAR) is known to have several functions. It acts as a common receptor for two unrelated types of virus (coxsackie B viruses and most adenoviruses) and also as a cell-adhesion molecule that localizes primarily to junctional adhesion complexes on the basolateral side of polarized epithelial cells. The quantity and accessibility of CAR on the cell surface are two primary determinants for the transduction efficiency of adenovirus, and pose major limitations to efficient adenovirus-mediated gene transfer in polarized cells in vitro and in vivo (Arnberg, 2009; Cohen et al., 2001b; Hutchin et al., 2000; Walters et al., 1999, 2001). Methods to overcome this limitation are essential to the clinical success of this vector and have been the source of over 500 peer-reviewed publications.
Recently, two papers by Zhong et al. (2010, 2011) described a new strategy to increase the transduction efficiency of adenovirus vectors by encapsulating the vectors in anionic liposomes via a calcium-induced phase-change method. This strategy is akin to, but more efficacious than, complexes formed with cationic lipids. The initial investigation was performed with well-designed experiments on three different cell lines: CHO-K1 cells are known to be CAR-deficient (Coyne & Bergelson, 2005; Excoffon et al., 2007) and hence are resistant to adenovirus infection, whereas A549 cells express CAR and are susceptible to adenovirus infection. Based on their experiments, Zhong et al. (2010) concluded that the adenovirus-resistant Madin–Darby canine kidney (MDCK) cell line is CAR-deficient. Although anionic liposomes increased transduction in all three cell lines, improvement was greatest in cells resistant to adenovirus infection. Here we show that, similar to A549 cells, MDCK cells express CAR. However, MDCK cells are resistant to adenovirus infection due to the formation of tight junctions, restricting CAR to the basolateral surface of these cells.
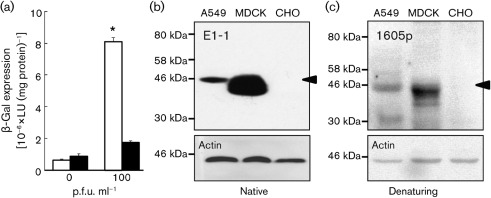
We first compared the amount of virus transduction in MDCK cells (ATCC CCL-34) with that in A549 cells (ATCC CCL-185). Cells were seeded in 24-well dishes at 2×105 cells per well and infected 48 h post-seeding with human adenovirus serotype 5 containing the β-galactosidase gene (Ad–β-Gal; University of Iowa Vector Core, Iowa City, IA, USA) at an m.o.i. of 0 or 100 for 1 h at 37 °C (n = 6 replicates, n = 3 experiments, representative results shown). Cells were lysed 24 h later and β-Gal expression (Galacto-Light Plus System; Applied Biosystems) and protein concentration (Bio-Rad Protein Assay) were determined. Consistent with the findings of Zhong et al. (2010) and others (Arnberg, 2009; Arnberg et al., 2000; Davison et al., 2001; Tamanini et al., 2006), A549 cells are highly susceptible to adenovirus infection and show a high level of β-Gal expression (Fig. 1a). In contrast, MDCK cells are highly resistant to adenovirus infection and do not show a significant increase in β-Gal activity over background, and approximately 10-fold less β-Gal expression than A549 cells, despite the high dose of adenovirus. Several possible explanations exist to account for this difference, e.g. a lack of CAR or other adenovirus co-receptors in MDCK cells, or differential rates of endocytosis or endosomal acidification between cell lines. Based on the basolateral localization of CAR observed previously (Cohen et al., 2001a, b; Walters et al., 2001), we hypothesized that MDCK cells express endogenous CAR, but it is inaccessible for apical virus infection due to sequestration on the basolateral surface.
Fig. 1.
Adenovirus infection and Western blot detection of CAR. (a) β-Gal expression in A549 (empty bars) and MDCK (filled bars) cells infected with Ad–β-Gal 2 days post-seeding. *P<0.003 for A549 compared with MDCK cells at an m.o.i. of 100. (b, c) Western blot analysis of CAR expression in lysates from A549, MDCK and CHO cells. Membranes were blotted with (b) anti-CAR E1-1 (native-gel electrophoresis) and β-actin or (c) anti-CAR 1605p (denaturing-gel electrophoresis) and β-actin. The CAR-specific band is identified by an arrowhead.
In order to determine whether MDCK cells express CAR, the primary receptor for adenovirus entry, we performed Western blotting to detect CAR in whole-cell lysates of MDCK cells, seeded at 1×106 cells per 10 cm dish and grown for 48 h, by using CAR-specific antibodies, as described previously (Excoffon et al., 2007). CHO-K1 cells (ATCC CCL-61), which are known to be CAR-deficient and resistant to virus infection, served as our negative control for Western blotting. A549 cells served as our positive control. Protein was extracted from A549, MDCK and CHO-K1 cells. The blots were subjected to two distinct CAR-specific antibodies (Fig. 1b, c). In contrast to CAR-negative CHO-K1 cells, both A549 and MDCK cells showed a CAR-specific band at approximately 46 kDa when probed with CAR antibodies directed to either the extracellular domain [E1-1 (Fig. 1b); Santa Cruz Antibodies; n = 2 experiments] or the intracellular domain [1605p (Fig. 1c) (Excoffon et al., 2005, 2007); n = 3 experiments]. Blots were stripped (25 mM glycine, 1 % SDS, pH 2) and reprobed with β-actin antibody (Applied Biosystems) to confirm equal protein loading (Fig. 1b, c, respective lower panels). Quantitative measures of CAR protein relative to actin, using ImageJ pixel quantification, demonstrate that, under these conditions, MDCK cells have approximately 20-fold more CAR than A549 cells. These data show that MDCK cells express significant protein levels of CAR and suggest that receptor deficiency is not the cause of the failure of adenovirus to infect MDCK cells.
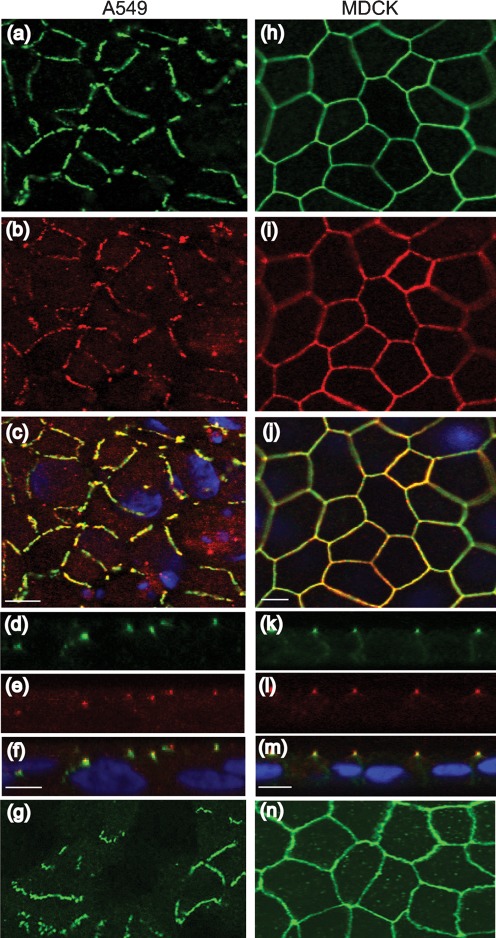
The tight junctions of polarized epithelial cells pose a formidable barrier to pathogen entry when the primary receptor for the pathogen is sequestered on the basolateral surface. In order to determine the localization of CAR, A549 and MDCK cells were fixed and subjected to immunocytochemistry as described previously (Excoffon et al., 2005) with antibodies directed against CAR (1605p or E1-1) and the tight-junction protein ZO-1 (Invitrogen) (Fig. 2). CAR-specific (green) and ZO-1-specific (red) staining was observed in both A549 and MDCK cells. However, in contrast to the smooth ‘chickenwire’ outline surrounding the MDCK cells (Fig. 2h–j, n), staining for CAR and ZO-1 in A549 cells was discontinuous (Fig. 2a–c, g). Similar localization of CAR was observed with both antibodies (1605p, Fig. 2a, h; E1-1, Fig. 2g, n). This suggests that, whilst tight junctions are well-formed in MDCK cells, tight-junction proteins are present in A549 cells, but do not appear to form contiguous sealed junctions. When viewed within the x–z plane, the apical junctions of MDCK cells were dotted with red ZO-1 staining, followed by a region of ZO-1 and CAR overlap (yellow) and green CAR staining extending along the basolateral junctions (Fig. 2k–m), suggesting a high degree of polarization. A549 cells showed some regions of overlap between ZO-1 and CAR (Fig. 2d–f, yellow), some regions with no overlap (red or green alone) and some regions where the CAR staining appeared above ZO-1, suggesting that it may be accessible on the cell surface for adenovirus infection. These data indicate that, in contrast to the polarization of MDCK cells, A549 cell polarization appears to be incomplete.
Fig. 2.
Immunocytochemistry for CAR (green) and ZO-1 (red) 2 days post-seeding. Confluent A549 cells were stained for CAR (a, d, 1605p; g, E1-1), ZO-1 (b, e) and merge (c, f, nuclei shown in blue) in the x–y (a–c, g) and x–z (d–f) planes. Confluent MDCK cells were stained for CAR (h, k, 1605p; n, E1-1), ZO-1 (i, l) and merge (j, m, nuclei shown in blue) in the x–y (h–j, n) and x–z (k–m) planes. Magnification: 60× oil immersion confocal microscopy. Bar, 10 µm.
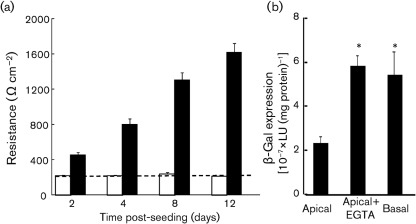
When epithelial cells polarize, significant biochemical and morphological changes culminate in the formation of a tight junction, which defines separate apical and basolateral surfaces of the cell. Transepithelial resistance (TER) measures the degree to which ions are able to cross the tight-junction barrier and correlates with the degree of polarization (Blikslager et al., 2007; Karp et al., 2002). To determine whether MDCK or A549 cells develop functional tight junctions, cells were seeded at 2.5×105 cells per well on 12 mm diameter polyester Millicell filters with a pore size of 0.4 µm (Millicell Cell Culture Inserts; Millipore). Medium on the apical surface of the cells was removed every alternate day in order to establish and maintain an air–liquid interface. TER was measured every alternate day with a chopstick ohmmeter (World Precision Instruments) (n = 6 replicates, n = 3 experiments, representative results shown). We found that there was a rapid increase in TER in MDCK cells (Fig. 3a, filled bars), suggesting the development of tight junctions, as well as definition of the apical and basolateral surfaces. This is consistent with the contiguous staining of ZO-1 and CAR around the upper part of MDCK epithelial cells (Fig. 2h–j, n) and is similar to other polarized epithelial cell types (Cohen et al., 2001b; Hutchin et al., 2000; Walters et al., 1999, 2002). Moreover, x–z sections show that CAR staining extends to the basolateral surface (Fig. 2k), demonstrating sequestration of CAR on the basolateral membrane, where it is inaccessible for apical adenovirus binding and infection. This is in contrast to A549 cells, which, although epithelial in nature, are squamous, do not have contiguous ZO-1 or CAR staining (Fig. 2a–c, g), do not attain any measure of TER above background (dotted line, Fig. 3a) and are more susceptible to adenovirus infection (Fig. 1a).
Fig. 3.
MDCK cells develop TER, which is a barrier to adenovirus infection. (a) A549 (empty bars) and MDCK (filled bars) cells were seeded on Millicell filters. Only MDCK cells attained TER above background (dotted line), indicating polarization. (b) Polarized MDCK cells were infected with Ad–β-Gal (m.o.i. of 100) from the apical surface after either mock pre-treatment (apical) or EGTA treatment (apical+EGTA), or from the basolateral surface (basal). *P<0.01 compared with apical infection.
To confirm that the basolateral nature of CAR is a barrier to adenovirus infection, MDCK cells polarized for 4 days on Millicell filters were infected for 1 h with Ad–β-Gal (m.o.i. of 100, Fig. 3b; n = 5–6 replicates, n = 6 experiments) from the apical surface with or without EGTA pre-treatment, or infected from the basolateral surface, as described previously (Excoffon et al., 2005; Walters et al., 2002). Ten minutes of 8 mM EGTA (25 mM HEPES, 150 mM NaCl) treatment prior to infection resulted in a TER drop from approximately 918±113 Ω cm−2 to <300 Ω cm−2, indicating disruption of tight-junction integrity. Significantly more adenovirus infection was observed under both the EGTA and basolateral conditions in comparison to apical treatment (P<0.01 compared with apical infection), indicating that increased access to CAR on the basolateral surface enhanced transduction. A similar increase in virus transduction was observed by Zhong et al. (2010) when confluent MDCK cells were treated with 1 mM EDTA during a 4 h adenovirus inoculation. These data are also consistent with those for primary human airway epithelial cells treated transiently with 8 mM EGTA (Walters et al., 1999, 2002).
Many studies have investigated CAR expression in MDCK cells by transient or stable transfection with CAR expression constructs, potentially leading to the conclusion that MDCK cells are CAR-deficient (Cohen et al., 2001a; Tamanini et al., 2006). However, MDCK cells do in fact express a robust amount of endogenous CAR that is sequestered from the apical surface upon confluence and polarization (Fig. 2). We have recently described an eight-exon isoform of the human CAR (CAREx8), which localizes to the apical region and surface of polarized human airway epithelia (Excoffon et al., 2010). The localization of this isoform is distinct from that of the most abundant isoform, which terminates at the end of the seventh exon (CAREx7) and is localized basolaterally in polarized epithelia. The isoform-specific transcript for CAREx7 is approximately 11 times more abundant than the CAREx8 transcript in MDCK cells and approximately nine times more abundant in A549 cells (data not shown). This is similar to the ratio in primary airway epithelia (Excoffon et al., 2010) and suggests that, although CAREx8 is a minor isoform, it could alter apical virus infection if expressed on the apical surface. A549 cells are highly susceptible to adenovirus infection and have some CAR staining above the tight-junction protein ZO-1, as well as some diffuse intracellular CAR staining, potentially representing CAREx8. In contrast, the low adenovirus susceptibility and a lack of apical immunofluorescence staining in MDCK cells suggest that CAREx8 is not present on the apical surface of MDCK cells (Figs 1, 2). Interestingly, Zhong et al. (2010) showed that adenovirus complexed with anionic lipids in the presence of EDTA resulted in significantly improved transduction of MDCK cells over EDTA alone. These data suggest that anionic lipids are able to overcome the epithelial barrier. The fact that this improvement occurs without major inflammation in vivo (Zhong et al., 2011) is an important step forward for a novel class of adenovirus formulations that may yield a significant advance for adenovirus-based, and potentially other virus-based, therapeutics for epithelial disorders.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases, award no. R15AI090625.
References
- Arnberg N. (2009). Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19, 165–178 10.1002/rmv.612 [DOI] [PubMed] [Google Scholar]
- Arnberg N., Kidd A. H., Edlund K., Olfat F., Wadell G. (2000). Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J Virol 74, 7691–7693 10.1128/JVI.74.16.7691-7693.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikslager A. T., Moeser A. J., Gookin J. L., Jones S. L., Odle J. (2007). Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87, 545–564 10.1152/physrev.00012.2006 [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Gaetz J., Ohman T., Bergelson J. M. (2001a). Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J Biol Chem 276, 25392–25398 10.1074/jbc.M009531200 [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Shieh J. T., Pickles R. J., Okegawa T., Hsieh J. T., Bergelson J. M. (2001b). The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A 98, 15191–15196 10.1073/pnas.261452898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C. B., Bergelson J. M. (2005). CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev 57, 869–882 10.1016/j.addr.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Davison E., Kirby I., Whitehouse J., Hart I., Marshall J. F., Santis G. (2001). Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by αvβ1 integrin and can be modulated by changes in β1 integrin function. J Gene Med 3, 550–559 10.1002/jgm.223 [DOI] [PubMed] [Google Scholar]
- Excoffon K. J., Traver G. L., Zabner J. (2005). The role of the extracellular domain in the biology of the coxsackievirus and adenovirus receptor. Am J Respir Cell Mol Biol 32, 498–503 10.1165/rcmb.2005-0031OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon K. J., Gansemer N., Traver G., Zabner J. (2007). Functional effects of coxsackievirus and adenovirus receptor glycosylation on homophilic adhesion and adenoviral infection. J Virol 81, 5573–5578 10.1128/JVI.02562-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon K. J., Gansemer N. D., Mobily M. E., Karp P. H., Parekh K. R., Zabner J. (2010). Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One 5, e9909 10.1371/journal.pone.0009909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin M. E., Pickles R. J., Yarbrough W. G. (2000). Efficiency of adenovirus-mediated gene transfer to oropharyngeal epithelial cells correlates with cellular differentiation and human coxsackie and adenovirus receptor expression. Hum Gene Ther 11, 2365–2375 10.1089/104303400750038471 [DOI] [PubMed] [Google Scholar]
- Karp P. H., Moninger T. O., Weber S. P., Nesselhauf T. S., Launspach J. L., Zabner J., Welsh M. J. (2002). An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188, 115–137 [DOI] [PubMed] [Google Scholar]
- Tamanini A., Nicolis E., Bonizzato A., Bezzerri V., Melotti P., Assael B. M., Cabrini G. (2006). Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol 80, 11241–11254 10.1128/JVI.00721-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W., Grunst T., Bergelson J. M., Finberg R. W., Welsh M. J., Zabner J. (1999). Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 274, 10219–10226 10.1074/jbc.274.15.10219 [DOI] [PubMed] [Google Scholar]
- Walters R. W., van’t Hof W., Yi S. M., Schroth M. K., Zabner J., Crystal R. G., Welsh M. J. (2001). Apical localization of the coxsackie–adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J Virol 75, 7703–7711 10.1128/JVI.75.16.7703-7711.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W., Freimuth P., Moninger T. O., Ganske I., Zabner J., Welsh M. J. (2002). Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110, 789–799 10.1016/S0092-8674(02)00912-1 [DOI] [PubMed] [Google Scholar]
- Zhong Z., Shi S., Han J., Zhang Z., Sun X. (2010). Anionic liposomes increase the efficiency of adenovirus-mediated gene transfer to coxsackie–adenovirus receptor deficient cells. Mol Pharm 7, 105–115 10.1021/mp900151k [DOI] [PubMed] [Google Scholar]
- Zhong Z., Han J., Wan Y., Zhang Z., Sun X. (2011). Anionic liposomes enhance and prolong adenovirus-mediated gene expression in airway epithelia in vitro and in vivo. Mol Pharm 8, 673–682 10.1021/mp100404q [DOI] [PubMed] [Google Scholar]