Abstract
The polyomavirus JC (JCV) infects glial cells and causes progressive multifocal leukoencephalopathy (PML). We described a novel JCV-variant with a 10 bp deletion in the C terminus of the VP1 capsid protein, JCVGCN1. This mutant was associated with lytic infection of cerebellar granule cell neurons and cerebellar atrophy in an human immunodeficiency virus/PML patient. This condition, also observed independently from PML, was named JCV granule cell neuronopathy (JCV GCN). We characterized JCV mutations in cerebrospinal fluid (CSF) of four other JCV GCN patients, and reviewed the literature on 10 reported cases. The strain from one patient harboured the identical GCN1-deletion, while the other patients had novel mutations in the same area, named JCVGCN2–4, causing variable changes in VP1 structure. One patient also had wild-type JCV in the CSF. To study the mechanisms leading to JCV GCN, we compared viral replication kinetics from JCVGCN1 with the prototype JCVMad1, the PML isolate JCVHWM and the prototype JCVMad1D engineered with the GCN1-deletion. While all strains replicated at low levels in the medulloblastoma cell line DAOY from a cerebellar neuronal tumour, JCVMad1 replicated better in astroglial SVG cells than JCVMad1D or JCVGCN1 and all strains replicated at higher levels in COS-7 kidney cells, suggesting that the GCN1-deletion confers a disadvantage for viral growth in central nervous system white matter. The GCN1-deletion remained stable after 100 days in culture and VP1 protein was produced in all cell lines, indicating that JCVGCN1 is replication-competent in vitro. These data highlight an important and previously overlooked aspect of JCV-pathogenesis. Detection of GCN-type JCV strains in CSF may help clinicians diagnose JCV GCN.
Introduction
Infection with the polyomavirus JC (JCV) is widespread and 50–86 % of healthy adults are JCV seropositive (Knowles et al., 2003; Weber et al., 1997). The virus remains quiescent in immunocompetent people, but reactivates in the setting of immunosuppression. It is the aetiological agent of progressive multifocal leukoencephalopathy (PML), a demyelinating disease of the central nervous system (CNS) caused by productive and lytic infection of oligodendrocytes and astrocytes (Tan & Koralnik, 2010). PML usually occurs in patients with AIDS (Berger et al., 1987; Miller et al., 1982), haematological malignancies (Shimizu et al., 1999) and organ transplant recipients (Yogo et al., 1991), as well as individuals with autoimmune diseases treated by immunomodulatory medications (Carson et al., 2009; Clifford et al., 2010; Korman et al., 2009; Schwab et al., 2009).
In the lesions of PML, productive infection can be readily demonstrated in oligodendrocytes and astrocytes. We and others have also observed that JCV can infect neurons. We reported productive and lytic JCV infection of cerebellar granule cell neurons in a 39-year-old human immunodeficiency virus (HIV)-seropositive patient with cerebellar atrophy who also had classic lesions of PML in the white matter of both frontal lobes, but no demyelinating lesions in the cerebellum (Du Pasquier et al., 2003; Tyler, 2003). JCV infection of granule cell neurons was confirmed in another HIV-seropositive patient with cerebellar atrophy and no clinical or radiological evidence of PML. This patient had a severe cerebellar syndrome characterized by progressive alteration of coordination, gait instability and ataxia. This novel syndrome, distinct from PML, was named JCV granule cell neuronopathy (JCV GCN) (Koralnik et al., 2005). JCV GCN was subsequently reported as well by others in HIV-positive and -negative patients throughout the world, with or without PML (Table 1) (Du Pasquier et al., 2003; Bustamante et al., 2009; Granot et al., 2009; Hecht et al., 2007; Koralnik et al., 2005; Otis & Moral, 2005; Shin et al., 2008; Tan & Brew, 2009).
Table 1. Clinical characteristics of all reported JCV GCN cases.
MA, Massachusetts; CA, California; NY, New York; cART, Combined Antiretroviral Therapy; na, not applicable.
| Case no. | Geographical location | Age (years) | Gender | Underlying diagnosis | PML | JCV strains | VP1 C terminus gene PCR product size (bp)* | Clinical evolution since onset of neurological symptoms | Reference |
| 1 | Boston, MA | 39 | M | HIV+ | + | JCVGCN1, JCVHWM | 104, 114 | Died 6 months later | Du Pasquier et al. (2003) |
| 2 | New York City, NY | 43 | F | HIV+ | − | JCVGCN2 | 108 | Wheel-chair dependent, on cART, 13 years later | Koralnik et al. (2005) |
| 3 | San Francisco, CA | 15 | M | CD-40 ligand deficiency | + | JCVGCN3, JCVSF | 131, 114 | Worsening of cerebellar syndrome. PML lesions in frontal lobes after 21 months with CSF JC viral load 12×106 cps ml−1 and died after 23 months | Hecht et al. (2007) |
| 4 | Sao Paulo, Brazil | 25 | F | HIV+ | − | JCVGCN4 | 104 | Died 4 months later | |
| 5 | Sao Paulo, Brazil | 34 | F | HIV+ | − | JCVGCN1 | 104 | Lost to follow-up 18 months later | |
| 6 | Springfield, MA | 52 | F | HIV+ | + | na | na | na | Otis & Moral (2005) |
| 7 | Seoul, South Korea | 37 | F | HIV+ | − | na | na | Bed-ridden 3 years later | Shin et al. (2008) |
| 8 | Sydney, Australia | 49 | F | Sarcoidosis | + | na | na | Died | Granot et al. (2009) |
| 9 | Sydney, Australia | 42 | M | HIV+ | − | na | na | Stopped cART and died 2 years later | Tan & Brew (2009) |
| 10 | Providencia, Chile | 37 | M | HIV+ | + | na | na | Alive with severe cerebellar dysfunction 2 years later | Bustamante et al. (2009) |
Using PCR primers CJS2465 and CJR2578 described in Dang & Koralnik (2006).
Whereas the JCV-coding region is extremely conserved the hypervariable non-coding-regulatory region (RR) has been associated with neurotropism and neurovirulence. Full-length molecular characterization of the strain, named JCVGCN1, infecting granule cell neurons in our initial patient, showed a 10 bp out of frame mutation in the C terminus of the VP1 major capsid protein, leading to an alteration of the last 13 aa sequence (Dang & Koralnik, 2006). To investigate further the pathogenic mechanism leading to JCV infection of granule cell neurons, we characterized JCV VP1 sequence variation of four additional JCV GCN patients from the USA and Brazil, and studied the phenotype of JCVGCN1 in vitro. Each patient had either identical or similar mutations in the JCV VP1 C terminus, and the JCVGCN1 molecular clone was replication-competent in several cell lines in vitro, but displayed different characteristics than undeleted JCV strains.
Results
JCV strains with mutated VP1 C terminus gene (GCN-type JCV) can be found in every JCV GCN case
The clinical characteristics of the JCV GCN cases we have investigated are listed in Table 1 in chronological order of discovery. The clinical presentation of cases 1–3 has been reported in detail (Dang & Koralnik, 2006; Du Pasquier et al., 2003; Hecht et al., 2007; Koralnik et al., 2005; Tyler, 2003), but the authors of each publication were contacted to provide an update on the clinical outcome of their patients.
Case report patient #4 (Table 1)
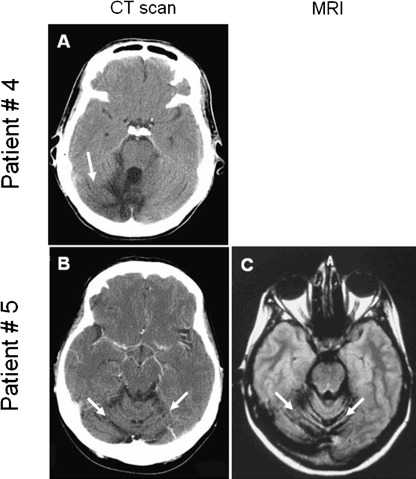
A 25-year-old HIV-infected Brazilian woman with multi-drug resistance to antiretrovirals presented with 2 months history of right-sided clumsiness and unsteady gait. Neurological examination showed a right cerebellar syndrome. Her absolute CD4+ T-cell count was 3 mm−3 and the plasma HIV viral load was 478 130 copies ml−1 (5.7 log10). A computed tomography (CT) scan of the brain demonstrated right cerebellar atrophy (Fig. 1a). Magnetic resonance imaging (MRI) confirmed that this patient exclusively had cerebellar atrophy without PML lesions in cerebellum (data not shown). Cerebrospinal fluid (CSF) analysis was normal and qualitative PCR performed in a clinical laboratory was negative for JCV DNA. Quantitative PCR (QPCR) performed at the Virology Laboratory at Instituto de Medicina Tropical (Sao Paulo, Brazil) was positive for JCV, establishing the diagnosis of JCV GCN. She was treated with antiretroviral medications and 2 months later, the neurological examination was stable, CD4+ T-cell count was up to 24 mm−3 and the HIV plasma viral load was down to 916 copies ml−1 (2.9 log10). However, she died soon thereafter from community-acquired pneumonia 4 months after the onset of neurological symptoms. A post-mortem examination was not performed.
Fig. 1.
Brain imaging studies of two HIV-seropositive patients with JCV GCN. Head CT scan of patient #4 in Table 1 (a) and head CT and MRI of patient #5 in Table 1 (b and c) showing cerebellar atrophy (arrows) and no demyelinating lesions of PML.
Case report patient #5 (Table 1)
A 34-year-old HIV-infected Brazilian woman with a history of poor compliance to antiretrovirals presented with 6 months history of dizziness, unsteady gait and right-sided clumsiness, as well as recent cough and fever. Neurological examination showed a predominantly right cerebellar syndrome. Her absolute CD4+ T-cell count was 116 cells mm−3 and HIV plasma viral load was 47 492 copies ml−1 (4.7 log10). A CT scan of the brain demonstrated isolated cerebellar atrophy (Fig. 1b) confirmed by MRI (Fig. 1c). CSF analysis was normal and qualitative PCR was negative for JCV DNA. QPCR performed at the Virology Laboratory at Instituto de Medicina Tropical (Sao Paulo, Brazil) was positive for JCV, however, consistent with the diagnosis of JCV GCN. She was restarted on cART and 9 months after her initial presentation, she had stable CT alterations, but her CD4+ T-cell count had risen to 221 mm−3 and her HIV viral load became undetectable (<50 copies ml−1). She was lost to follow up 18 months after onset of neurological symptoms.
Among 10 reported JCV GCN cases, we could access clinical samples of five cases (1, 2, 3, 4 and 5; Table 1). The JCV VP1 C terminus of each patient was PCR-amplified, cloned and sequenced as described previously (Dang & Koralnik, 2006). GCN-type JCV could be detected in every JCV GCN case available for testing and were named sequentially JCVGCN1 to JCVGCN4. Among these five cases, three (#2, 4 and 5) had no white matter lesions at presentation and therefore, no evidence of PML in addition to JCV GCN (Table 1). GCN-type JCV was the only JCV strain detected in these JCV GCN cases without PML lesions. Alignment of DNA and primary protein sequences of all GCN types, the reference strain JCVMad1 and the JCVHWM found in the hemispheric white matter of our initial patient are shown in Fig. 2.
Fig. 2.
Alignment of DNA and amino acid sequences of VP1 C terminus from JCV strains identified from different JCV GCN cases. The nucleotide and amino acid position numbers correspond to JCVMad1 prototype sequence. HWM, Hemispheric white matter; GCN1–4, granule cell neurons strains; SF, San Francisco. Dashed lines denote sequence homology. Line interruption denotes deletion. The sequence underlined and in italics in JCVGCN3 is the one that is duplicated in the 17 bp insert at nt 2502.
As reported previously, JCVGCN1 has an out of frame 10 nt mutation, resulting in an alteration of the sequence of the last 13 aa of the protein and was found in both our initial case #1 (Table 1) from Boston (Du Pasquier et al., 2003) as well as case #5 from Brazil. JCVGCN4 from Brazil has the same 10 bp deletion as JCVGCN1 starting at nt 2503 and ending at nt 2512 in the VP1 gene. In addition, JCVGCN4 has five single nucleotide mutations after the 10 bp deletion, leading to 4 aa changes compared with JCVGCN1. JCVGCN2 from New York City has a 6 bp in-frame deletion from nt 2511 to 2515, resulting in the loss of aa 348 and 349. Finally, JCVGCN3 from San Francisco has a duplication of a 17 bp sequence inserted at nt 2502, leading to a premature stop codon and a truncated VP1 protein lacking the last 9 aa. This patient also had a wild-type JCV strain in his CSF, named JCVSF.
JCV containing the GCN1 mutation are replication competent in vitro
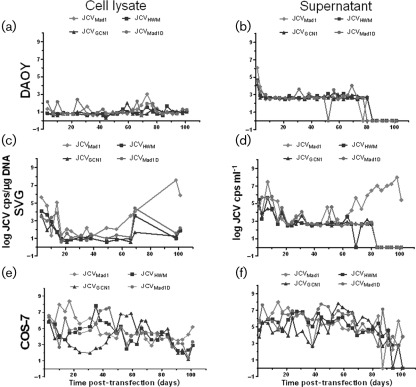
To determine whether mutation of JCV VP1 C terminus altered the kinetics of JCV replication in vitro we initially focused on the GCN1 mutation found in our index patient as well as one Brazilian case (Table 1). Prototype JCVMad1, PML isolate JCVHWM, JCVGCN1 and prototype JCVMad1D engineered with the GCN1 deletion were transfected in medulloblastoma cell line DAOY, a tumour arising from the granule cell layer of the cerebellum. In addition, these strains were also transfected in the permissive human astroglial cell line SVG and the monkey kidney cell line COS-7, which have both been transformed with the JCV-related simian virus 40 (SV40) T Ag, and therefore can sustain JCV replication. Cellular and supernatant DNA were harvested over a period of 100 days. Control experiments showed that DpnI digestion of input viral DNA resulted in complete digestion of nucleic acids, insuring that QPCR only amplified packaged viral DNA. Viral replication kinetics of the various JCV strains in different cell lines was measured by QPCR and is shown in Fig. 3. We used a regression model with pairwise contrast and Bonferroni correction for multiple comparisons to analyse differences in viral kinetics over time between the four viral strains in each cell line, and for each strain in the three different cell lines.
Fig. 3.
JCVGCN1 is replication-competent in vitro. Detection of the viral load of undeleted JCVMad1, JCVHWM, as well as JCVGCN1 and JCVMad1D with GCN1 deletion in the cell lysate and supernatant of transfected DAOY (a, b), SVG (c, d) and COS-7 (e, f) cells. DAOY, Human medulloblastoma cell line; SVG, human astroglial cell line; COS-7, monkey kidney cell line.
The DAOY cells could sustain replication of all four JCV strains at a similar low level, and allowed productive infection that could be detected in the supernatant throughout the experiment. The presence of the GCN1 mutation did not confer any significant advantage or disadvantage compared to the non-deleted strains (Fig. 3a, b). Conversely, in SVG cells however, JCVMad1 had the highest peak viral load of all four viral strains reaching 8 logs both in the cells and supernatant. JCVMad1 viral load was higher overtime in both cell lysate and supernatant compared with JCVGCN1 (P = 0.004 and P = <0.0001). Conversely, JCVMad1D engineered with the GCN1 deletion had similar viral load overtime in cell lysate and supernatant as JCVGCN1 (P = 1.0 and P = 1.0) and lower replication levels than the undeleted JCVMad1 (P = 0.09 an P = 0.003) (Fig. 3c, d).
Finally, all four JCV strains could replicate at significantly higher levels overtime in COS-7 cells compared with the other two cell lines. But the JCVGCN1 initially had the slowest replication kinetic compared with the three other strains and a lower viral load in cell lysate than JCVMad1 (P = 0.03), (Fig. 3e, f).
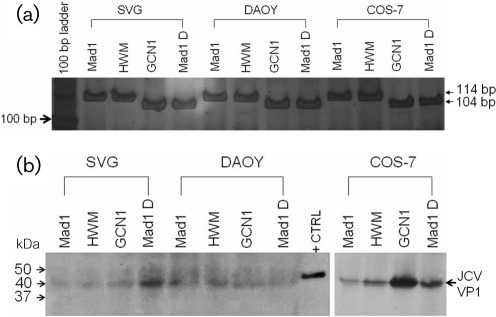
GCN1 mutation remains stable overtime in vitro
To determine whether the GCN1 mutation persisted over time in various cell lines we amplified the VP1 gene fragment encompassing the mutated area from the cellular DNA after 30 passages in vitro. Compared to the undeleted JCVMad1 and JCVHWM, the JCVGCN1 and JCVMad1D mutation remained stable after 100 days in culture (Fig. 4a) and the size of the amplified fragment was the same as the one amplified from the input virus (not shown). Furthermore, the JCV VP1 protein could be detected by Western blot from cell lysates of all three cell lines infected with either strain, indicating a complete replication cycle and the presence of mature viral particles in these cells (Fig. 4b).
Fig. 4.
The GCN1 deletion is stable and the VP1 protein is expressed in vitro. (a) PCR amplification of VP1 C terminus gene using primers CJS2465 and CJR2578. Electrophoresis was performed on 10 % TBE polyacrylamide gel followed by silver staining as described previously (Dang & Koralnik, 2006). Result shows that both undeleted JCV strains, JCVMad1 and JCVHWM (114 bp fragment) as well as JCVGCN1 and JCVMad1D harbouring the GCN1 deletion (104 bp fragment) remained stable after 30 passages in vitro in all cell lines. (b) Western blot detection of a 40 kDa VP1 protein from each JCV strain in all cell lines. DAOY, Human medulloblastoma cell line; SVG, human astroglial cell line; COS-7, monkey kidney cell line; +CTRL, positive control using SV40 virion.
Discussion
Although JCV GCN is a recently discovered CNS condition, it has now been recognized in patients from the USA (Du Pasquier et al., 2003; Hecht et al., 2007; Koralnik et al., 2005; Otis & Moral, 2005), Australia (Granot et al., 2009; Tan & Brew, 2009), Chile (Bustamante et al., 2009), South Korea (Shin et al., 2008), and in this report, Brazil. JCV GCN patients were from both genders, their age ranged from 15 to 52 years and all were immunosuppressed, either in the context of HIV infection, congenital or inflammatory conditions. Among all five JCV GCN cases reported in this study and five described by others (Bustamante et al., 2009; Granot et al., 2009; Otis & Moral, 2005; Shin et al., 2008; Tan & Brew, 2009), half of the cases had co-existent white matter lesions at the time of presentation (case #1, 3, 6, 8 and 10; Table 1). This indicates that JCV GCN can occur separately from, or in association with, PML.
Although clinically recognizable cases of JCV GCN remain rare, the prevalence of JCV infection of granule cell neurons is likely underestimated. A histopathological survey of archival cerebellar samples showed evidence of JCV infection in granule cell neurons of up to 51 % of HIV-seropositive PML patients and 3 % of HIV-seropositive individuals without PML (Wüthrich et al., 2009). This infection occurred regardless of whether classic PML lesions were also present in the nearby cerebellar white matter. It is therefore possible that many AIDS patients presenting with cerebellar atrophy and a cerebellar syndrome indeed have JCV GCN (Tagliati et al., 1998).
Interestingly, we found three novel GCN-type mutations, all occurring in the same area of the VP1 C terminus as the initial GCN1 deletion (Dang & Koralnik, 2006). JCV-coding region is highly conserved, and these changes had never been described in more than 400 full-length viral sequences deposited in GenBank. It is therefore extremely unlikely that they could have occurred by chance. Furthermore, mutations found in all but the GCN2 variant lead to the loss of a β-pleated sheet in this area as modelled by Expert Protein Analysis System (EXPASY) (data not shown). These changes may probably have important consequences on the structure of the viral capsid, which is formed by 72 pentamers of the VP1 protein, associated on their inner surface with either the VP2 or VP3 protein. Indeed, this region forms the principal interpentamer contact, since each pentamer extends five invading arms to surrounding pentamers and receives five invading arms in return (Stehle et al., 1996). Of note, this area is likely to be exposed on the exterior surface of the capsid, but in a position near the base of the canyons between pentamer knobs, which is remote from the known receptor-binding site on the surface of the pentamers (Neu et al., 2010).
Since mature viral particles were seen by electron microscopy in granule cell neurons of our initial patient (Du Pasquier et al., 2003) these changes may not prevent the formation of the capsid, but rather may influence viral tropism or post-entry events, including uncoating, transport and replication of JCV DNA, which could potentially enable productive infection in granule cell neurons.
Of note, the GCN-type mutations were not located in areas of single nucleotide mutations, leading to amino acid substitutions of the VP1 protein described in a recent study (Gorelik et al., 2011).
Regardless of the exact pathogenetic mechanism, our data suggest that the detection of GCN-type JCV will potentially help clinicians diagnose JCV GCN, using the PCR primers encompassing the area of interest. Interestingly, the patient with JCVGCN3 also had an undeleted wild-type JCV strain in his CSF. The concomitant presence of deleted and undeleted JCV strains was also present in our initial case (Dang & Koralnik, 2006; Du Pasquier et al., 2003), as well as in 25 % of CSF and 68 % of PBMC samples from PML patients, suggesting that the deletion may arise outside of the CNS, perhaps at the level of the bone marrow (Tan et al., 2009).
Studies of JCV pathogenesis in neurons are challenging since JCV only infects humans, and no suitable human neuronal cell line is available. We therefore used for the first time a cell line derived from a human medulloblastoma, a neuronal tumour arising from the cerebellar granule cell layer. The viral load of all JCV strains remained low in this cell line. However, the fact that JCV could replicate at all in cells of neuronal origin which, unlike SVG or COS-7, were not previously transformed and immortalized with the SV40 T-Ag gene, is remarkable. We had initially postulated that the isolates with GCN1 deletion would have a selective advantage in this cell line, which was not verified experimentally. These results highlight the difficulties associated with modelling neuronal infection by JCV in vitro. However, our data indicate that the GCN1 mutation does not prevent expression of the VP1 protein, and that the deletion remains stable over a long period of time in vitro, without reverting to wild-type. Furthermore, detection of the VP1 protein by Western blot in DAOY cell lysate indicates that despite a low level of viral replication, a full replicative cycle occurred in these cells with production of mature viral particles.
As expected, the prototype JCVMad1 outperformed other JCV strains in astroglial cells, whereas the introduction of the GCN1 deletion in this strain conferred the same phenotype as JCVGCN1. These results are consistent with the fact that an undeleted VP1 protein provides an advantage for replication in glial cells. It is not clear why JCVHWM isolated from a PML lesion, did not replicate significantly better than JCVGCN1 in this cell line. Both viral isolates have a similar RR with tandem repeat pattern (Dang & Koralnik, 2006), consistent with CNS-adapted strains of JCV. However, JCVHWM also has a unique in-frame deletion of 18 bp in the C terminus of the T-Ag gene, with loss of 6 aa (648–653) (Dang & Koralnik, 2006), which may dampen its replication in SVG cells. Finally, all viral strains replicated at higher levels in COS-7 cells, consistent with the fact that the kidney is a major compartment harbouring JCV and excretion in the urine is a common occurrence in healthy and immunosuppressed individuals alike (Arthur et al., 1989; Kitamura et al., 1990; Koralnik et al., 1999; Markowitz et al., 1993).
Altogether, these data underscore the genetic diversity of JCVGCN variants and shed light on an important and previously overlooked aspect of JCV pathogenesis.
Methods
This study was approved by the BIDMC institutional review board. Patients consent was obtained according to institutional IRB guidelines.
Construction of JCVGCN1.
Due to the low viral copy number, the full-length sequence of JCVGCN1 in the cerebellum sample of our index patient had been previously characterized after sequencing multiple PCR-amplified fragments covering the entire JCV genome (GenBank accession no. DQ875212) (Dang & Koralnik, 2006; Du Pasquier et al., 2003). Considering that JCVGCN1 and the JCV strain isolated from the hemispheric white matter of this patient, called JCVHWM obtained by long PCR amplification (GenBank accession no. DQ875211), shared the same backbone and differ only in the RR, the C terminus of VP1-coding region, and the C terminus of the T-coding region (Dang & Koralnik, 2006), we used JCVHWM genome to reconstruct a full-length clone of JCVGCN1. The DNA template corresponding to JCVGCN1 RR located between two NcoI sites (nt 5014–5169–1–334, 490 bp, JCVNcoI fragment) was synthesized by BlueHeron. JCVGCN1 RR target sequence was PCR amplified using primer pair: JGCNco5 (5′-GGAATTCCATGGATTCCTC-3′, GGAATT-4958–4970 of JCVHWM) and JGCNco3 (5′-CTTGTGCTTTGTTTACTT-3′, 257–240 reverse complement sequence of JCVHWM, designed by ‘Primer Express/version 2.0’; Applied Biosystems). The PCR product was cloned into TA vector (Invitrogen) and sequenced. One positive clone was selected to proceed to the next step. Plasmid containing the JCVNcoI fragment was purified from selected positive clone and was digested by NcoI (New England Biolabs) to free the JCVNcoI fragment. The JCVNcoI fragment was recovered from agarose gel (QIAquick Gel Extraction kit; Qiagen) and used to replace the corresponding sequence of JCVHWM. The JCV strain obtained after this step was a new JCV strain that had the backbone of JCVHWM and RR of JCVGCN1, this strain was called JCVGCN1-RR.
DNA template of partial JCVGCN1 genome containing the deleted VP1 gene and intact C terminus of T gene with two single restriction enzyme sites of PacI and Bsu36I (nt 2369–3056. JCVHWM sequence) was also synthesized by BlueHeron (nt 2097–3107, JCVHWM sequence, 1099 bp) and named PGVP1-T. Primer pair: JV2For (5′-AAGATATTTTGGGACACTAACAGGAGGAGA-3′, 2097–2126 of JCVHWM) and JV2Rev (5′-TTCAGGGCATGGCATAAGCAACC-3′, 3187–3165 RC sequence of JCVHWM, designed by ‘Primer Express/version 2.0’) were used to amplify partial JCVGCN1 sequence containing the C terminus of both VP1 and T gene from PGVP1-T. The PCR product was then cloned into TA vector, screened and sequenced. The plasmid containing the PGVP1-T fragment was purified and digested by PacI (2369 of JCVHWM) (Bilcock et al., 1999; Hacioglu et al., 1997) and Bsu36I (3056 of JCVHWM) (New England Biolabs). The 696 bp PGVP1-T fragment containing 10 bp VP1 deletion and intact C terminus of the T gene was recovered from agarose gel (QIAquick Gel Extraction kit; Qiagen) and then used to replace the counterpart of JCVGCN1-RR to obtain the full-length genome of JCVGCN1.
Construction of JCVMad1D.
A similar strategy was used to obtain JCVMad1D, a mutant of the JCV reference strain JCVMad1 containing the 10 bp deletion in the VP1 gene found in JCVGCN1. DNA template containing the VP1 gene of JCVMad1D with 10 bp deletion in the C terminus was synthesized by BlueHeron (nt 2336–3806 of JCVMad1 strain with 10 bp deletion between 2503 and 2512). Primer pair: Mad1D5 (5′-AACCCCTACCCAATTTCTTTCCTTC-3′, 2336–2360 of JCVMad1 strain) and Mad1D3 (5′-GCTTATGGGCATGTACTTAGACTTTCAG-3′, 3806–3779 RC sequence of JCVMad1, designed by ‘Primer Express/version 2.0’) were used to amplify the synthesized template. The PCR product was cloned into TA vector and sequenced. Target JCVMad1D DNA sequence was freed from the vector using PacI (position 2373 of JCVMad1) and PstI (position 3359 of JCVMad1) (New England Biolabs). The 977 bp target band (with 10 bp VP1 deletion) was recovered from agarose gel (QIAquick Gel Extraction kit; Qiagen) and was then used to replace the counterpart of JCVMad1 to obtain full-length genome of JCVMad1D.
Plasmids containing full-length genome of different JCV strains were used to transform Escherichia coli INV110 for large-scale preparation, leading to methylation of DpnI sites.
In vitro transfection of four JCV strains into three different cell lines.
In the in vitro transfection experiment, a total of four JCV strains; JCVMad1, JCVMad1D, JCVHWM, JCVGCN1 were used to transfect separately three different cell lines: human astroglial SVG (purchased from ATCC), simian kidney cell COS-7 (purchased from ATCC) and human medulloblastoma DAOY (a generous gift from Dr Scott L Pomeroy, Children’s Hospital, Boston, MA, USA). Plasmids containing the full-length JCV genome were digested with EcoRI (New England Biolabs), the 5 kb band was recovered from the agarose gel using QIAquick Gel Extraction kit (Qiagen). Transfection was performed with a viral per cell ratio of 105 viral genome copies per cell using Effectene transfection reagents from Qiagen in a six-well culture plate. Cells transfected with the JCV genome were then transferred from a six-well plate to P25 flask 3 days post-transfection, and to P175 flasks 6 days post-transfection. JCV-transfected cells were passaged into a new P175 flask at a ratio of 1 : 5 every 3.5 days, and DNA was extracted from one-fifth of the total cell number at each passage (QIAamp DNA Blood Mini kit; Qiagen). Supernatant was collected at each passage and stored at −20 °C. DNA was extracted from the supernatant using QIAamp DNA Blood Mini kit after cell culture was terminated. The experiment ended after 100 days and 30 passages in vitro.
QPCR detection of JC viral load in the in vitro transfection experiment.
DNA samples extracted from both JCV-transfected cells and the corresponding supernatant were quantified by SmartSpec Plus spectrophotometer (Bio-Rad). To remove any remaining contaminating input JCV DNA, samples were digested with DpnI (New England Biolabs) in a volume of 30 µl at 37 °C overnight. Since there are two DpnI sites (GAmTC, nt 841–844 and 970–973) located within the QPCR target sequence (nt 792–992), any methylated DNA sequence from input viral genome would be digested and could not be used as PCR template. After heat inactivation of DpnI at 80 °C for 20 min, 0.5 µg digested DNA was used as template in QPCR analysis. Primer pair: JCD792 (5′-TGGTGGTAGTGCTATTGCTCAGTT-3′, position 792–815 of JCVMad1 amplifying a conserved sequence in all four JCV strains, and JCD992 (5′-GACGGGCCCCAATGTCTAG-3′, reverse complement sequence of position 974–992 were used for QPCR detection. Probe JCVD824 (6FAM-AGATTTTTTGCTGACTGGGATCATAAAGTTTCAACA-TAMRA, position 824–858 of JCV Mad1 strain, all four strains have the same sequence; Applied Biosystems) was used in QPCR. QPCR was performed using standard TaqMan assay condition on ABI 7300 Real-time PCR System. JCVMad1 complete genome cloned in pUC18 vector was used as standard. The range of standard curve is from one copy to one million copies.
Statistical analysis of JC viral load in vitro.
We assessed the distribution of viral load for each of the four virus strains within the three cell lines. We calculated the mean, standard deviation, median, minimum and maximum for each of the viral load distributions. Based on the asymmetry, and non-normality of these distributions, we used the logarithm transformation for the viral load. The log-transformed viral load exhibited symmetry and near normality. Therefore, we used the log-transformed viral load data for multiple regression models. To compare the viral load among the four virus strains in each cell line, we used a regression model where the dependent variable is the log viral load, and the independent variables were the four-level indicator variable for the type of virus, and the time of sample collection. The regression model yielded the adjusted mean level of log viral load for each virus. We then used the model linear contrasts to compute the six pairwise mean differences for the four viruses. We applied Bonferroni adjustment for multiple comparisons, and reported the adjusted P-values for the pairwise comparisons. Similarly, we used the same modelling and testing technique to compare the mean log viral load of each virus strain among the three different cell lines. All of these analyses were conducted using the SAS/STAT version 9 software.
PCR amplification and detection of mutated C terminus of JCV VP1 gene.
PCR amplification of JCV VP1 C terminus was performed with primers CJS2465 and CJR2578 and amplified sequences were visualized by 10 % TBE PAGE and silver staining as described previously (Dang & Koralnik, 2006). The amplified fragments were cloned in TA vector (Invitrogen) and up to eight clones per sample were sequenced.
Western blot of JCV VP1 protein.
VP1 capsid protein was detected in the cell lysate by Western blot. Based on the viral load data from QPCR, JCV-positive cell samples with a high JCV viral load around mid-point through the experiment were selected for each viral strain for Western blot analysis. A total of three million JCV-transfected cells were resuspended in 100 µl 1× PBS, then added 50 µl Laemmli Sample Buffer from Bio-Rad, boiled for 5 min. Each boiled sample (15 µl) was loaded onto a 10-well precast SDS-gel from Bio-Rad. Cell lysate (1.5 µl) of SV40-transfected monkey fibroblast diluted to a final volume of 15 µl was used as positive control. JCV VP1 protein was detected by mouse mAb anti-SV40 VP1 protein – PAB597 (generous gift from Dr Walter Atwood), which cross-reacts with JCV VP1 protein. The presence of the protein was revealed with HRP-conjugated goat anti-mouse Ab (GAM-HRP) (Bio-Rad).
Acknowledgements
This study was supported by the NINDS (National Institute of Neurological Disorders and Strokes) R01 NS047029, R56 NS/AI 041198 and K24 NS060950 to I. J. K.; NIH U01 MH083501 to S. M. We want to thank Dr Scott L. Pomeroy from Children’s Hospital in Boston for his generous gift of the DAOY cell line and Dr Maria Cristina D. Fink from Virology Laboratory at Instituto de Medicina Tropical, Sao Paulo University, for the CSF samples from two Brazilian patients included in this study. We also want to thank Dr Walter Atwood for his generous gift of PAB597 Ab.
References
- Arthur R. R., Dagostin S., Shah K. V. (1989). Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol 27, 1174–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. R., Kaszovitz B., Post M. J., Dickinson G. (1987). Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med 107, 78–87 [DOI] [PubMed] [Google Scholar]
- Bilcock D. T., Daniels L. E., Bath A. J., Halford S. E. (1999). Reactions of type II restriction endonucleases with 8-base pair recognition sites. J Biol Chem 274, 36379–36386 10.1074/jbc.274.51.36379 [DOI] [PubMed] [Google Scholar]
- Bustamante F., Luis Cartier R., Manuel Lavados M. (2009). Atrofia cerebelosa por el virus JC en un paciente con SIDA. Rev Chin Neurro-Psiquiat 47, 222–227 [Google Scholar]
- Carson K. R., Evens A. M., Richey E. A., Habermann T. M., Focosi D., Seymour J. F., Laubach J., Bawn S. D., Gordon L. I. & other authors (2009). Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840 10.1182/blood-2008-10-186999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D. B., De Luca A., Simpson D. M., Arendt G., Giovannoni G., Nath A. (2010). Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 9, 438–446 10.1016/S1474-4422(10)70028-4 [DOI] [PubMed] [Google Scholar]
- Dang X., Koralnik I. J. (2006). A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol 87, 2533–2537 10.1099/vir.0.81945-0 [DOI] [PubMed] [Google Scholar]
- Du Pasquier R. A., Corey S., Margolin D. H., Williams K., Pfister L. A., De Girolami U., Mac Key J. J., Wüthrich C., Joseph J. T., Koralnik I. J. (2003). Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology 61, 775–782 [DOI] [PubMed] [Google Scholar]
- Gorelik L., Reid C., Testa M., Brickelmaier M., Bossolasco S., Pazzi A., Bestetti A., Carmillo P., Wilson E. & other authors (2011). Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis 204, 103–114 10.1093/infdis/jir198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot R., Lawrence R., Barnett M., Masters L., Rodriguez M., Theocharous C., Pamphlett R., Hersch M. (2009). What lies beneath the tent? JC-virus cerebellar granule cell neuronopathy complicating sarcoidosis. J Clin Neurosci 16, 1091–1092 10.1016/j.jocn.2008.07.091 [DOI] [PubMed] [Google Scholar]
- Hacioglu E., Basim H., Stall R. E. (1997). Optimized conditions of PacI and SwaI for genomic analysis of X. axonopodis pv. vesicatoria by PFGE. Biotechniques 22, 1026–1028 [DOI] [PubMed] [Google Scholar]
- Hecht J. H., Glenn O. A., Wara D. W., Wu Y. W. (2007). JC virus granule cell neuronopathy in a child with CD40 ligand deficiency. Pediatr Neurol 36, 186–189 10.1016/j.pediatrneurol.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Kitamura T., Aso Y., Kuniyoshi N., Hara K., Yogo Y. (1990). High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J Infect Dis 161, 1128–1133 10.1093/infdis/161.6.1128 [DOI] [PubMed] [Google Scholar]
- Knowles W. A., Pipkin P., Andrews N., Vyse A., Minor P., Brown D. W., Miller E. (2003). Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 71, 115–123 10.1002/jmv.10450 [DOI] [PubMed] [Google Scholar]
- Koralnik I. J., Boden D., Mai V. X., Lord C. I., Letvin N. L. (1999). JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52, 253–260 [DOI] [PubMed] [Google Scholar]
- Koralnik I. J., Wüthrich C., Dang X., Rottnek M., Gurtman A., Simpson D., Morgello S. (2005). JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 57, 576–580 10.1002/ana.20431 [DOI] [PubMed] [Google Scholar]
- Korman B. D., Tyler K. L., Korman N. J. (2009). Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol 145, 937–942 10.1001/archdermatol.2009.175 [DOI] [PubMed] [Google Scholar]
- Markowitz R. B., Thompson H. C., Mueller J. F., Cohen J. A., Dynan W. S. (1993). Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis 167, 13–20 10.1093/infdis/167.1.13 [DOI] [PubMed] [Google Scholar]
- Miller J. R., Barrett R. E., Britton C. B., Tapper M. L., Bahr G. S., Bruno P. J., Marquardt M. D., Hays A. P., McMurtry J. G., III & other authors (1982). Progressive multifocal leukoencephalopathy in a male homosexual with T-cell immune deficiency. N Engl J Med 307, 1436–1438 10.1056/NEJM198212023072307 [DOI] [PubMed] [Google Scholar]
- Neu U., Maginnis M. S., Palma A. S., Ströh L. J., Nelson C. D., Feizi T., Atwood W. J., Stehle T. (2010). Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe 8, 309–319 10.1016/j.chom.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis C. N., Moral L. A. (2005). Images in pathology: granule cell loss in AIDS-associated progressive multifocal leukoencephalopathy. Int J Surg Pathol 13, 360 10.1177/106689690501300409 [DOI] [PubMed] [Google Scholar]
- Schwab N. J. C. U., Fox R. J., Huang Y. H., Schneider-Hohendorf T., Stenner M.-P, Welch W., Kieseier B. C, Monoranu C. M., Staugaitis S. M. & other authors (2009). Fatal progressive multifocal leukoencephalopathy associated with efalizumab therapy: insights into the role of leukointegrin aLb2 in JC virus control. Multiple Sclerosis Journal 15, 271–277 [Google Scholar]
- Shimizu N., Imamura A., Daimaru O., Mihara H., Kato Y., Kato R., Oguri T., Fukada M., Yokochi T. & other authors (1999). Distribution of JC virus DNA in peripheral blood lymphocytes of hematological disease cases. Intern Med 38, 932–937 10.2169/internalmedicine.38.932 [DOI] [PubMed] [Google Scholar]
- Shin H. W., Kang S. Y., Sohn Y. H. (2008). JC viral infection-related cerebellar degeneration as the first manifestation of AIDS. Eur Neurol 59, 205–207 10.1159/000114048 [DOI] [PubMed] [Google Scholar]
- Stehle T., Gamblin S. J., Yan Y., Harrison S. C. (1996). The structure of simian virus 40 refined at 3.1 Å resolution. Structure 4, 165–182 10.1016/S0969-2126(96)00020-2 [DOI] [PubMed] [Google Scholar]
- Tagliati M., Simpson D., Morgello S., Clifford D., Schwartz R. L., Berger J. R. (1998). Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology 50, 244–251 [DOI] [PubMed] [Google Scholar]
- Tan I. L., Brew B. J. (2009). Possible JCV granular cell neuronopathy in a patient with HIV infection. Neurology 73, 1598–1599 10.1212/WNL.0b013e3181c0d6cb [DOI] [PubMed] [Google Scholar]
- Tan C. S., Koralnik I. J. (2010). Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 9, 425–437 10.1016/S1474-4422(10)70040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. S., Dezube B. J., Bhargava P., Autissier P., Wüthrich C., Miller J., Koralnik I. J. (2009). Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J Infect Dis 199, 881–888 10.1086/597117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K. L. (2003). The uninvited guest: JC virus infection of neurons in PML. Neurology 61, 734–735 [DOI] [PubMed] [Google Scholar]
- Weber T., Trebst C., Frye S., Cinque P., Vago L., Sindic C. J., Schulz-Schaeffer W. J., Kretzschmar H. A., Enzensberger W. & other authors (1997). Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis 176, 250–254 10.1086/514032 [DOI] [PubMed] [Google Scholar]
- Wüthrich C., Dang X., Westmoreland S., McKay J., Maheshwari A., Anderson M. P., Ropper A. H., Viscidi R. P., Koralnik I. J. (2009). Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol 65, 742–748 10.1002/ana.21619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Hara K., Iida T., Taguchi F., Tajima A., Kawabe K., Aso Y. (1991). Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol 65, 2422–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]