Abstract
Negatively stained influenza virions sometimes show irregular morphology and are often referred to as pleomorphic. However, this irregular morphology has not been visualized when ultrathin-section transmission and scanning electron microscopies are used. This study focused on the effects of ultracentrifugation on influenza A virion morphology, as negative staining often involves ultracentrifugation to concentrate or purify virions. The morphologies of unfixed, glutaraldehyde-fixed and osmium tetroxide-fixed virions were quantitatively compared before and after ultracentrifugation, and it was found that, without chemical fixation, approximately 30 % of virions were altered from oval to irregular shapes following ultracentrifugation. By contrast, most glutaraldehyde-fixed virions remained uniformly elliptical, even after ultracentrifugation. When a virus with an 11 aa deletion at the C terminus of its M2 cytoplasmic tail was ultracentrifuged, its morphology was appreciably deformed compared with that of the wild-type virus. These results demonstrate that the native morphology of influenza A virions is regular but is disrupted by ultracentrifugation, and that the cytoplasmic tail of M2 is important for virion integrity.
Introduction
Influenza A virus is an enveloped virus with a segmented, negative-sense ssRNA genome that encodes at least 11 proteins (Palese & Shaw, 2007). Its virions are generally spherical or elliptical and about 100 nm in diameter; occasionally, they are filamentous reaching >20 µm in length, and sometimes they are irregular in shape. They possess many membrane-spanning glycoproteins, haemagglutinin (HA) and neuraminidase (NA), and small amounts of an ion channel protein (M2) on their surface. The membrane protein (M1), which binds to the lipid envelope, is thought to maintain virion structure (Palese & Shaw, 2007). Previous studies have shown that single amino acid substitutions in M1 alter the virion morphology from filamentous to spherical, and vice versa (Bourmakina & García-Sastre, 2003; Elleman & Barclay, 2004; Roberts et al., 1998). Deletion of the cytoplasmic tail of M2, HA or NA also alters virion morphology from spherical/elliptical to irregular (Iwatsuki-Horimoto et al., 2006; Jin et al., 1997). Thus, the interactions among M1, M2, HA and NA are important for the formation and preservation of the characteristic virion shapes.
The virion structures of influenza A viruses have been studied extensively using various electron microscopies. When observed by ultrathin-section transmission electron microscopy (TEM) or scanning electron microscopy (SEM), influenza virions appear spherical or elliptical (Bächi et al., 1969; Compans & Dimmock, 1969; Morgan et al., 1956, 1961; Nayak et al., 2009; Noda et al., 2006). In contrast, negatively stained influenza virions often show varied morphologies from virion to virion, including irregularly shaped virions (Almeida & Waterson, 1967; Almeida et al., 1967; Ruigrok et al., 1986; Stevenson & Biddle, 1966; Wrigley, 1979). This discrepancy could be due to artefacts caused during sample preparation for negative staining, as artefacts can be introduced into fragile biological samples on air drying (Nermut & Frank, 1971; Ruigrok et al., 1992). However, we cannot exclude other possibilities. In this study, the effects of ultracentrifugation on influenza virion morphology were evaluated as a potential cause of changes in virion morphology, as sample preparation for negative staining often involves ultracentrifugation of virions.
Results
Budding virions observed by ultrathin-section TEM
When influenza A virions budding from infected cells are observed by ultrathin-section TEM, they are uniformly elliptical or spherical (Bächi et al., 1969; Boulan & Sabatini, 1978; Compans & Dimmock, 1969; Nayak et al., 2004, 2009). To confirm whether ultrathin-sectioned virions of A/Puerto Rico/8/34 (PR8) strain are also uniformly elliptical, virion budding from allantoic membrane cells was subjected to ultrathin-section TEM. As observed in Fig. 1(a), the virions budding from the apical plasma membranes were elliptical and almost uniform in shape, although they had slightly different lengths.
Fig. 1.
Budding PR8 virions observed by ultrathin-section TEM. (a) A representative electron micrograph of PR8 virions budding from the cells of allantoic membranes. Uniformly oval virions can be seen budding from the apical plasma membrane. (b) Representative electron micrographs of ultrathin-sectioned virions. The respective shape complexity values are given below the photos. (c) Proportion of shape complexity values of ultrathin-sectioned virions. In total, 216 virions were assessed. Bars, 500 nm (a); 100 nm (b).
Next, to quantify the shapes of the virions visualized by ultrathin-section TEM, we randomly picked longitudinally sectioned virions containing rod-like ribonucleoprotein (RNP) complexes (Fig. 1b), whose long axes were thought to be entirely present within the ultrathin sections (50 nm thick), probably representing the size and shape of the whole virion. Virions that did not contain any RNPs were excluded, because they were probably sectioned at the edge and would therefore not have intact shapes. For descriptive purposes, the shape complexity values, which numerically classify the pleomorphicity of virions, were calculated as described in Methods and divided into three groups: 1.0–1.399, 1.4–1.8 and >1.8. In general, the 1.0–1.399 group contained spherical or elliptical particles, the 1.4–1.8 group had elongated or irregular-shaped virions and the >1.8 group had extremely irregular virions (Fig. 1). Of the ultrathin-sectioned PR8 virions, 94.9 % were spherical or elliptical and fell into the 1.0–1.399 group, whereas 5.1 % were elongated and classified in the 1.4–1.8 group (Fig. 1b, c). No virion was irregular in shape. Our observations indicated that most virions observed by ultrathin-section TEM were uniform in shape.
Released virions observed by SEM
Next, we examined virions that were budding and released from the cells of allantoic membranes using SEM. The virions that budded from these cells were homogeneous in shape (Fig. 2a), similar to those observed by ultrathin-section TEM (Fig. 1a). Released virions bound to chicken erythrocytes were also observed by SEM (Fig. 2b), and 197 virions, selected at random, were subjected to morphological measurements. As in the case of ultrathin-section TEM, most of the virions were round or oval, and their complexity values fell into the 1.0–1.399 group (Fig. 2c, d). About 7 % of the virions had an elongated shape and were categorized in the 1.4–1.8 group, and no virion was irregular in shape (Fig. 2c, d). These results suggested that most virions observed by SEM were round or elliptical in shape and were morphologically homogeneous.
Fig. 2.
PR8 virions observed by SEM. (a) A representative electron micrograph of PR8 virions budding from the cells of allantoic membranes. (b) A representative electron micrograph of virions adsorbed onto a chicken erythrocyte, which was found with allantoic membrane cells under an SEM field. Elliptical and spherical virions can be seen. (c) A higher magnification of the representative electron micrographs of released virions on chicken erythrocytes. Respective shape complexity values are given below the photos. (d) Proportion of shape complexity values of virions visualized by SEM. In total, 197 virions were examined. Bars, 500 nm (a, b); 100 nm (c).
Negatively stained virions
In previous reports, negatively stained virions were often heterogeneous and sometimes showed irregular shapes (Almeida & Waterson, 1967; Almeida et al., 1967; Ruigrok et al., 1986; Stevenson & Biddle, 1966; Wrigley, 1979), which were generally thought to be artefacts introduced during the air-drying step of negative staining (Nermut & Frank, 1971; Ruigrok et al., 1992). To identify steps other than air drying that may cause changes in virion morphology, we focused on ultracentrifugation, as this step is often performed to purify or concentrate virions before negative staining. To evaluate the effect of ultracentrifugation on virion shape, we prepared virions that were unfixed or fixed with glutaraldehyde (GLA) or osmium tetroxide (OsO4). Subsets of specimens were observed directly by negative staining with 2 % phosphotungstic acid solution, and the rest were subjected to ultracentrifugation at 90 000 g for 1.5 h at 4 °C. In all samples, with or without ultracentrifugation, spike proteins composed of HA and NA were clearly visible on the virion surfaces (Fig. 3a–f). Most of the virions without ultracentrifugation had an oval shape, but a small number of morphologically irregular virions were also observed (Figs 3a–c and 4a–c). Without ultracentrifugation, ~90 % of the virions were classified in the 1.0–1.399 group, whilst 6–10 % were elongated and classified in the 1.4–1.8 group (Fig. 3a–c and g–i). Some deformed virions that were classified in the >1.8 group were found in the unfixed sample without ultracentrifugation (Figs 3a, g and 4a–c); similar virions were not observed in the GLA- or OsO4-fixed samples (Fig. 3b, c, h, i).
Fig. 3.
Negatively stained PR8 virions. Representative electron micrographs of unfixed virions (a), OsO4-fixed virions (b), GLA-fixed virions (c), unfixed and ultracentrifuged (UC) virions (d), OsO4-fixed and UC virions (e) and GLA-fixed and UC virions (f). The respective shape complexity values are given below the photos. The proportion of shape complexity values for samples (a)–(f) is shown in (g)–(l), respectively. The total number of analysed virions is shown beneath the graphs. Bars, 100 nm.
Fig. 4.
Examples of irregular-shaped PR8 virions with complexity values >1.8. (a–c) Unfixed and non-ultracentrifuged virions; (d–g) unfixed and ultracentrifuged virions. Bars, 100 nm.
Following ultracentrifugation, unfixed virions showed various irregular amoeba-like shapes (Fig. 3d). Overall, 21.3 % fell into the 1.4–1.8 group and 9.1 % were in the >1.8 group (Figs 3j and 4d–g). However, virions fixed with GLA prior to ultracentrifugation did not show significant changes in morphology and most had an elongated shape (Fig. 3f, l). Although OsO4-fixed virions were slightly altered into a round shape after ultracentrifugation, most were homogeneous, and morphologically irregular virions were not observed (Fig. 3e, k). These results indicated that ultracentrifugation affects virion morphology and that the proportion of morphologically irregular virions increases after ultracentrifugation unless virions are first chemically fixed.
Statistical analysis of the morphological changes caused by ultracentrifugation
To examine the morphological changes caused by ultracentrifugation quantitatively, the mean complexity values of each specimen were calculated using the perimeters and areas of each virion, as described in Methods. There were no significant differences in the mean complexity values among unfixed, GLA-fixed and OsO4-fixed virions without ultracentrifugation (P>0.01; Table 1), suggesting that negative staining itself does not cause significant morphological changes. Following ultracentrifugation, the mean complexity value of unfixed virions increased significantly compared with that of unfixed virions without ultracentrifugation (P<0.01; Table 1), indicating that the morphology of unfixed virions was significantly affected by ultracentrifugation. The mean complexity value of the OsO4-fixed virions decreased significantly after ultracentrifugation, indicating that these virions were more spherical compared with those without ultracentrifugation (P<0.01; Fig. 3b, e and Table 1). However, the mean complexity value of ultracentrifuged GLA-fixed virions was not statistically different from that of GLA-fixed virions without ultracentrifugation (P>0.01; Table 1), suggesting that GLA fixation preserves virion morphology, whether ultracentrifugation is carried out or not.
Table 1. Quantitative comparison of PR8 viral morphologies.
| Sample | Mean complexity value/P-value |
| Mean complexity values for:* | |
| Unfixed | 1.233±0.162 (n = 218) |
| OsO4 | 1.248±0.099 (n = 209) |
| GLA | 1.229±0.112 (n = 215) |
| Unfixed+UC | 1.380±0.305 (n = 197) |
| OsO4+UC | 1.196±0.104 (n = 205) |
| GLA+UC | 1.251±0.067 (n = 200) |
| Ultrathin-section TEM | 1.268±0.071 (n = 216) |
| SEM | 1.262±0.086 (n = 197) |
| Comparison of mean complexity values† | |
| Unfixed vs OsO4 | P = 0.266 |
| Unfixed vs GLA | P = 0.739 |
| OsO4 vs GLA | P = 0.062 |
| Unfixed vs unfixed+UC | P = 4.779E−9 |
| OsO4 vs OsO4+UC | P = 2.233E−8 |
| GLA vs GLA+UC | P = 2.277E−2 |
Mean complexity values (±sd) and the total number of PR8 virions analysed for each sample are shown.
Comparison of the mean complexity values between the samples indicated. Statistical analyses were carried out using a non-parametric t-test. Statistically significant results (P<0.01) are shown in bold.
Morphology of an M2 tail deletion mutant as observed by TEM
Previously, we examined the role of the M2 cytoplasmic tail in viral morphology, by demonstrating that an 11 aa deletion from the C terminus of the M2 cytoplasmic tail does not affect virus growth, packaging efficiency of nucleoprotein (NP), or virion structure (Iwatsuki-Horimoto et al., 2006). Here, to determine whether the M2 cytoplasmic tail is important for maintaining virion integrity, wild-type influenza A/WSN/33 (WSN) and a mutant virus that lacked the C-terminal 11 aa of M2 (M2Δ11) were generated using reverse genetics (Iwatsuki-Horimoto et al., 2006; Neumann et al., 1999). The viruses were propagated once in Madin–Darby canine kidney (MDCK) cells and purified by ultracentrifugation. Western blotting showed that the ratios of the viral structural proteins HA, NP, M1 and M2 were comparable between the wild-type and M2Δ11 viruses (Fig. 5a, b). Ultrathin-section TEM also demonstrated that there was no morphological difference between the two viruses, and rod-like RNPs were observed in longitudinally sectioned virions, as reported previously (Fig. 5c, d, left panels); the eight RNP segments were arranged in a specific pattern in cross-sectioned virions (Fig. 5c, d, right panels) (Noda et al., 2006).
Fig. 5.
Comparison of WSN wild-type and M2Δ11 virions. (a, b) Western blot analysis of the structural proteins of the two viruses. (a) Viral proteins HA, NP and M1 were separated by SDS-PAGE under non-reducing conditions and detected with an anti-influenza virus rabbit polyclonal antibody (R309). (b) Under reducing conditions, monomeric M2 proteins were detected with an anti-M2 mAb (14C2). (c, d) WSN wild-type and M2Δ11 virions observed by ultrathin-section TEM. Rod-like RNPs can be seen in all of the virions. Bars, 100 nm.
Comparison of morphological changes in wild-type WSN and M2Δ11 virions following ultracentrifugation
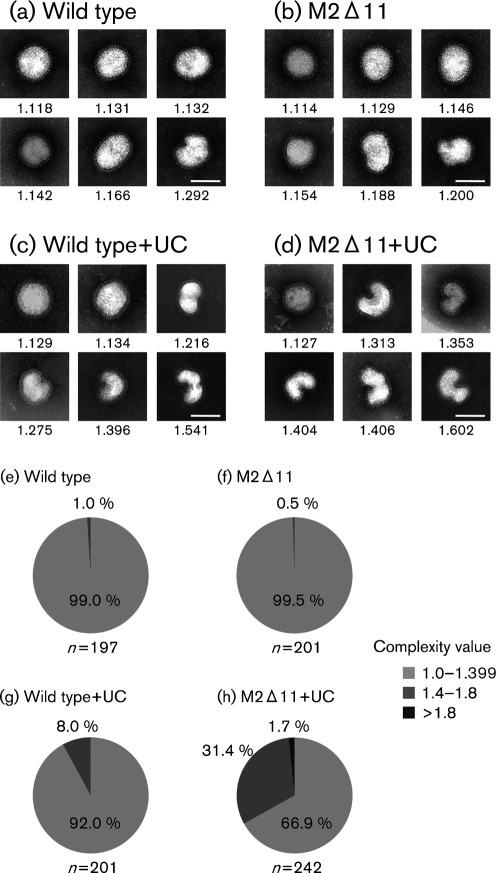
To test the effect of the 11 aa deletion from the C terminus of the M2 cytoplasmic tail on virion integrity, wild-type and M2Δ11 virions were examined by negative staining. The WSN virions were rounder than the PR8 virions, as reported previously (Nayak et al., 2009). Without ultracentrifugation, both wild-type and M2Δ11 virions were uniformly spherical or elliptical, although a few irregular-shaped virions were observed (Fig. 6a, b). The proportion of shape complexity values was similar between wild-type and M2Δ11 viruses without ultracentrifugation, and 1.0 or 0.5 % of the wild-type and M2Δ11 virions, respectively, fell into the 1.4–1.8 group (Fig. 6e, f). The mean complexity values of the wild-type and M2Δ11 virions were similar, and there was no significant difference between the two viruses (P>0.01; Table 2). As was the case with PR8 virions, both the unfixed WSN wild-type and M2Δ11 virions changed their morphology after ultracentrifugation (Fig. 6c, d). Importantly, the structures of the M2Δ11 virions were substantially deformed after ultracentrifugation and most virions had irregular shapes (Fig. 6d). A total of 31.4 % fell into the 1.4–1.8 group and 1.7 % were in the >1.8 group, whereas 8.0 % of the wild-type virions were in 1.4–1.8 group (Fig. 6g and h). Following ultracentrifugation, the mean complexity values of both the wild-type and M2Δ11 virions were significantly increased (Table 2). Moreover, the mean complexity value of the ultracentrifuged M2Δ11 virions was significantly greater than that of the wild-type virions (Table 2), indicating that the morphological changes in the M2Δ11 virions as a result of ultracentrifugation were much greater than those in the wild-type virions.
Fig. 6.
Negatively stained WSN virions. Representative electron micrographs of WSN wild-type virions (a), M2Δ11 virions (b), ultracentrifuged (UC) wild-type virions (c) and UC M2Δ11 virions. The proportion of shape complexity values for samples (a)–(d) is shown in (e)–(h), respectively. Respective shape complexity values are given below the panels. The total number of analysed virions is shown beneath the graphs. Bars, 100 nm.
Table 2. Quantitative comparison of the morphology of WSN virus.
| Sample | Mean complexity value/P-value |
| Mean complexity values for:* | |
| Wild type | 1.167±0.076 (n = 197) |
| M2Δ11 | 1.159±0.060 (n = 201) |
| Wild type+UC | 1.217±0.106 (n = 201) |
| M2Δ11+UC | 1.353±0.165 (n = 242) |
| Comparison of mean complexity values† | |
| Wild type vs M2Δ11 | P = 0.199 |
| Wild type vs wild type+UC | P = 8.200E−8 |
| M2Δ11 vs M2Δ11+UC | P = 2.770E−46 |
| Wild type+UC vs M2Δ11+UC | P = 8.887E−23 |
Mean complexity values (±sd) and the total number of analysed WSN wild-type and M2Δ11 virions are shown.
Comparison of the mean complexity values between the samples indicated. Statistical analyses were carried out as described in Table 1. Statistically significant results (P<0.01) are shown in bold.
Discussion
It is widely accepted that typical influenza A virions are spherical, elliptical or filamentous, but that sometimes irregular-shaped virions are observed following negative staining. Such morphological features reflect the pleomorphism of influenza virions (Le Ru et al., 2010; Stevenson & Biddle, 1966). Here, we found that virion morphology is significantly affected by ultracentrifugation, which is often involved in the negative-staining process, resulting in the generation of morphologically irregular virions. However, the morphology of virions fixed with GLA was relatively conserved, even after ultracentrifugation.
When virions fixed with GLA, a reagent that cross-links adjacent proteins (Bozzola & Russell, 1998), were ultracentrifuged, they maintained their uniformly spherical or oval shapes. In these virions, membrane-bound M1 molecules, which form a layer underneath the lipid envelope (Burleigh et al., 2005; Harris et al., 2001), were tightly cross-linked to each other by GLA, presumably ensuring that the virion shape was conserved (Fig. 3f, l). In contrast, when virions fixed with OsO4 were ultracentrifuged, the virions tended to change to round rather than irregular structures (Fig. 3e, k). This morphological change probably reflects the fact that OsO4 fixes mainly lipid membranes rather than proteins (Bozzola & Russell, 1998), which is insufficient to maintain the protein–protein interactions of the transmembrane proteins and M1 when faced with the physical impact of ultracentrifugation, although the lipid fixation protects the viral envelope from complete deformation into an irregular shape. For ultrathin-section TEM and SEM of budding virions, sample processing begins with GLA and OsO4 chemical fixation, and does not involve ultracentrifugation. Therefore, the virion structures are relatively conserved during sample processing, and irregular virions were not found on ultrathin-section TEM and SEM analyses (Figs 1 and 2). Taken together, we suggest that ultracentrifugation is a major cause of morphological artefacts with unfixed influenza virions and that the native structure of influenza virions is uniformly spherical, elliptical or filamentous.
A recent study showed that the M2 cytoplasmic tail interacts directly with M1 through aa 71–76 in M2 and plays an essential role in viral assembly (Chen et al., 2008). Because the mutant virus tested in this study still possessed the ability to bind to M1, the deletion did not affect virion morphology or the incorporation of M1 or other viral proteins into virions (Fig. 5). Nevertheless, the integrity of the mutant virions following ultracentrifugation was considerably decreased (Fig. 6 and Table 2), suggesting that the C-terminal 11 aa of M2 are involved in interactions with viral components (e.g. M1). The fragility of the M2 tail mutant may explain its suboptimal growth compared with that of wild-type virus (Iwatsuki-Horimoto et al., 2006).
Concentration and purification of specimens by ultracentrifugation facilitate structural analyses when using cryoelectron microscopy or negative-staining electron microscopy. However, their artefactual effects, especially on fragile samples such as enveloped virions, must be considered. Although cryoelectron microscopy, which is used to observe native structures of unfixed, unstained and frozen hydrated specimens, has been used to document morphologically irregular influenza virions in purified samples (Booy et al., 1985; Fujiyoshi et al., 1994; Harris et al., 2006; Nayak et al., 2009), such virions are unlikely to reflect the native structure of those virions due to sample processing involving ultracentrifugation. The artefactual effects caused by ultracentrifugation should be considered fully when undertaking detailed morphological analyses of enveloped viruses, including influenza viruses.
Methods
Viruses.
A/Puerto Rico/8/34 (H1N1; PR8) strain was inoculated into 10-day-old chicken embryos at 37 °C and the allantoic fluid was collected 2 days after inoculation. 293T cells were propagated in Dulbecco’s modified Eagle’s medium containing 10 % FBS. MDCK cells were cultured in minimum essential medium containing 5 % newborn calf serum. All cell cultures were supplemented with penicillin/streptomycin solution (100×; Wako Pure Chemical Industries) and maintained at 37 °C in an atmosphere of 5 % CO2. A/WSN/33 (H1N1; WSN) wild-type virus and its mutant virus, which had an 11 aa deletion from the C terminus of its M2 cytoplasmic tail (M2Δ11), were generated in 293T cells by reverse genetics, as described previously (Iwatsuki-Horimoto et al., 2006; Neumann et al., 1999).
Fixation and ultracentrifugation.
Allantoic fluid or culture supernatant of infected MDCK cells was centrifuged at 780 g for 5 min to remove debris. Aliquots of PR8 virions were fixed with GLA or OsO4 at a final concentration of 2.5 or 0.5 %, respectively, for 1 h at 4 °C. All WSN virions were unfixed. The fixed or unfixed samples were ultracentrifuged through a 20 % (w/w) sucrose cushion at 90 000 g for 1.5 h at 4 °C. The pelleted virions were then suspended in PBS.
Negative staining.
Virions were adsorbed to Formvar-coated copper mesh grids, negatively stained with 2 % phosphotungstic acid solution and air dried. Digital images of virions were taken with a Tecnai F20 electron microscope (FEI) at 80 or 200 kV.
Ultrathin-section TEM.
At 24 h after inoculation with PR8 virus, the chorioallantoic membranes of embryonated eggs were harvested and washed with PBS. MDCK cells were inoculated with WSN wild-type or M2Δ11 virus and incubated at 37 °C for 24 h. These samples were pre-fixed with 2.5 % GLA in 0.1 M cacodylate buffer (pH 7.4) for 1 h at 4 °C. They were then washed with the same buffer and post-fixed with 2 % OsO4 in the same buffer for 1 h at 4 °C. After dehydration through a series of ethanol gradients followed by propylene oxide, the samples were embedded in Epon 812 resin mixture (TAAB Laboratories) and polymerized at 70 °C for 2 days. Ultrathin sections (50 nm) were stained with 2 % uranyl acetate in 70 % ethanol and Reynold’s lead solution, and examined with a Tecnai F20 electron microscope, as above.
SEM.
Allantoic membranes were fixed as described for ultrathin-section TEM. The fixed membranes were dehydrated with a series of ethanol gradients, followed by t-butanol, and dried in an ES-2030 freeze dryer (Hitachi High-Technologies). The specimens were then coated with OsO4 using an HPC-1S osmium coater (Vacuum Device) and examined with an S-4200 microscope (Hitachi High-Technologies).
Western blotting.
Virions, purified by ultracentrifugation through a 20 % (w/w) sucrose cushion, were suspended in 2× Tris/glycine SDS sample buffer (Invitrogen) with or without DTT at a final concentration of 100 mM and incubated for 5 min at 95 °C. The samples were subjected to SDS-PAGE on Tris/glycine gels (Any kD Mini-PROTEAN TGX gel; Bio-Rad Laboratories) and then transferred to Immobilon-P transfer membranes (Millipore). The membranes were treated with a commercial blocking buffer (Blocking One; Nacalai Tesque) and incubated with primary antibodies, an anti-influenza virus rabbit polyclonal antibody (R309; prepared in house) or an anti-M2 (14C2) mAb (a gift from Dr Robert A. Lamb, Northwestern University, IL, USA) at 4 °C overnight. Antibody–antigen complexes were detected using an immunodetection kit (ABC kit; Vector Laboratories) and Immunostain HRP-1000 (Konica Minolta Medical and Graphic).
Quantification of virion shapes.
Approximately 200 virions with a length of <300 nm were randomly picked for quantification. Their perimeters were traced and their boundary lengths and areas measured using ImageJ software (http://rsbweb.nih.gov/ij/). The values for shape complexity, which are defined as the ratio of (perimeter)2/4π (area), were calculated as described elsewhere (Satoh et al., 1985). The complexity value theoretically is 1.0 for round virions and becomes larger for elongated or distorted virions.
Acknowledgements
We thank Dr Susan Watson for editing the manuscript and Eiryo Kawakami and Ryo Takano for discussion. We also thank Dr Robert A. Lamb for providing the anti-M2 (14C2) mAb. This work was supported by grants-in-aid from the Japan Society for the Promotion of Science, by grants-in-aid for Specially Promoted Research and for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, by ERATO (Japan Science and Technology Agency) and by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases.
References
- Almeida J. D., Waterson A. P. (1967). Some observations on the envelope of an influenza virus. J Gen Microbiol 46, 107–110 [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Waterson A. P., Drewe J. A. (1967). A morphological comparison of Bittner and influenza viruses. J Hyg (Lond) 65, 467–474 10.1017/S0022172400046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. (1969). Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol 4, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy F. P., Ruigrok R. W., van Bruggen E. F. (1985). Electron microscopy of influenza virus. A comparison of negatively stained and ice-embedded particles. J Mol Biol 184, 667–676 10.1016/0022-2836(85)90312-2 [DOI] [PubMed] [Google Scholar]
- Boulan E. R., Sabatini D. D. (1978). Asymmetric budding of viruses in epithelial monolayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A 75, 5071–5075 10.1073/pnas.75.10.5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourmakina S. V., García-Sastre A. (2003). Reverse genetics studies on the filamentous morphology of influenza A virus. J Gen Virol 84, 517–527 10.1099/vir.0.18803-0 [DOI] [PubMed] [Google Scholar]
- Bozzola J. J., Russell L. D. (1998). The mechanism of chemical fixation. In Electron Microscopy, 2nd edn, pp. 20–22 Edited by McKean B. L. Massachusetts: Jones and Bartlett Publishers [Google Scholar]
- Burleigh L. M., Calder L. J., Skehel J. J., Steinhauer D. A. (2005). Influenza A viruses with mutations in the M1 helix six domain display a wide variety of morphological phenotypes. J Virol 79, 1262–1270 10.1128/JVI.79.2.1262-1270.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. J., Leser G. P., Jackson D., Lamb R. A. (2008). The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding. J Virol 82, 10059–10070 10.1128/JVI.01184-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. (1969). An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology 39, 499–515 10.1016/0042-6822(69)90098-1 [DOI] [PubMed] [Google Scholar]
- Elleman C. J., Barclay W. S. (2004). The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321, 144–153 10.1016/j.virol.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Fujiyoshi Y., Kume N. P., Sakata K., Sato S. B. (1994). Fine structure of influenza A virus observed by electron cryo-microscopy. EMBO J 13, 318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A., Forouhar F., Qiu S., Sha B., Luo M. (2001). The crystal structure of the influenza matrix protein M1 at neutral pH: M1–M1 protein interfaces can rotate in the oligomeric structures of M1. Virology 289, 34–44 10.1006/viro.2001.1119 [DOI] [PubMed] [Google Scholar]
- Harris A., Cardone G., Winkler D. C., Heymann J. B., Brecher M., White J. M., Steven A. C. (2006). Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci U S A 103, 19123–19127 10.1073/pnas.0607614103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki-Horimoto K., Horimoto T., Noda T., Kiso M., Maeda J., Watanabe S., Muramoto Y., Fujii K., Kawaoka Y. (2006). The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J Virol 80, 5233–5240 10.1128/JVI.00049-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Leser G. P., Zhang J., Lamb R. A. (1997). Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J 16, 1236–1247 10.1093/emboj/16.6.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ru A., Jacob D., Transfiguracion J., Ansorge S., Henry O., Kamen A. A. (2010). Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine 28, 3661–3671 10.1016/j.vaccine.2010.03.029 [DOI] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Moore D. H. (1956). Structure and development of viruses observed in the electron microscope. III. Influenza virus. J Exp Med 104, 171–182 10.1084/jem.104.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Hsu K. C., Rifkind R. A., Knox A. W., Rose H. M. (1961). The application of ferritin-conjugated antibody to electron microscopic studies of influenza virus in infected cells. I. The cellular surface. J Exp Med 114, 825–832 10.1084/jem.114.5.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Hui E. K., Barman S. (2004). Assembly and budding of influenza virus. Virus Res 106, 147–165 10.1016/j.virusres.2004.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Balogun R. A., Yamada H., Zhou Z. H., Barman S. (2009). Influenza virus morphogenesis and budding. Virus Res 143, 147–161 10.1016/j.virusres.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Frank H. (1971). Fine structure of influenza A2 (Singapore) as revealed by negative staining, freeze-drying and freeze-etching. J Gen Virol 10, 37–51 10.1099/0022-1317-10-1-37 [DOI] [PubMed] [Google Scholar]
- Neumann G., Watanabe T., Ito H., Watanabe S., Goto H., Gao P., Hughes M., Perez D. R., Donis R., et al. (1999). Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96, 9345–9350 10.1073/pnas.96.16.9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Sagara H., Yen A., Takada A., Kida H., Cheng R. H., Kawaoka Y. (2006). Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439, 490–492 10.1038/nature04378 [DOI] [PubMed] [Google Scholar]
- Palese P., Shaw M. L. (2007). Orthomyxoviridae: the viruses and their replication. In Fields Virology, 5th edn, pp. 1647–1689 Edited by Knipe D. M., Howley P. M. Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- Roberts P. C., Lamb R. A., Compans R. W. (1998). The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology 240, 127–137 10.1006/viro.1997.8916 [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Wrigley N. G., Calder L. J., Cusack S., Wharton S. A., Brown E. B., Skehel J. J. (1986). Electron microscopy of the low pH structure of influenza virus haemagglutinin. EMBO J 5, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R. W., Hewat E. A., Wade R. H. (1992). Low pH deforms the influenza virus envelope. J Gen Virol 73, 995–998 10.1099/0022-1317-73-4-995 [DOI] [PubMed] [Google Scholar]
- Satoh H., Ueda T., Kobatake Y. (1985). Oscillations in cell shape and size during locomotion and in contractile activities of Physarum polycephalum, Dictyostelium discoideum, Amoeba proteus and macrophages. Exp Cell Res 156, 79–90 10.1016/0014-4827(85)90263-0 [DOI] [PubMed] [Google Scholar]
- Stevenson J. P., Biddle F. (1966). Pleomorphism of influenza virus particles under the electron microscope. Nature 212, 619–621 10.1038/212619a0 [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. (1979). Electron microscopy of influenza virus. Br Med Bull 35, 35–38 [DOI] [PubMed] [Google Scholar]