Abstract
Size-selective fishing, environmental changes and reproductive strategies are expected to affect life-history traits such as the individual growth rate. The relative contribution of these factors is not clear, particularly whether size-selective fishing can have a substantial impact on the genetics and hence on the evolution of individual growth rates in wild populations. We analysed a 25-year monitoring survey of an isolated population of the Alpine whitefish Coregonus palaea. We determined the selection differentials on growth rate, the actual change of growth rate over time and indicators of reproductive strategies that may potentially change over time. The selection differential can be reliably estimated in our study population because almost all the fish are harvested within their first years of life, i.e. few fish escape fishing mortality. We found a marked decline in average adult growth rate over the 25 years and a significant selection differential for adult growth, but no evidence for any linear change in reproductive strategies over time. Assuming that the heritability of growth in this whitefish corresponds to what was found in other salmonids, about a third of the observed decline in growth rate would be linked to fishery-induced evolution. Size-selective fishing seems to affect substantially the genetics of individual growth in our study population.
Keywords: artificial selection, contemporary evolution, Coregonus, salmonid, selection differential
Introduction
Human activities have caused phenotypic changes in many ecosystems (Palumbi 2001; Smith and Bernatchez 2008). These changes can be rapid, with large modifications occurring within decades only (Thompson 1998; Hendry and Kinnison 1999; Stockwell et al. 2003; Hairston et al. 2005). In many fish populations, for instance, significant shifts in life-history traits have been described. These shifts include maturation at smaller age or size (Heino et al. 2002; Grift et al. 2003), elevated reproductive effort (Yoneda and Wright 2004) and changes in individual growth rate (Handford et al. 1977; Ricker 1981; Thomas and Eckmann 2007). Many of these phenotypic changes may be linked to fishery-induced evolution. In experiments, the systematic removal of larger fish indeed decreases the mean weight of descendants (Conover and Munch 2002) and impacts various life-history traits (Walsh et al. 2006; Hutchings and Rowe 2008). See Jorgensen et al. (2007) for a review on phenotypic traits for which evolutionary changes are likely, and Hard et al. (2008) for a discussion of evolutionary consequences of fishing on salmon.
Several conditions are mandatory for evolution to occur, and fishing on wild populations usually fulfils all these conditions. First, fishing-induced mortality can be very high and may exceed natural mortality by far more than 100% (Rijnsdorp 1993; Mertz and Myers 1998; Jackson et al. 2001). Second, fishing is typically selective with regard to size (Myers and Hoenig 1997; Fukuwaka and Morita 2008). Third, heritable variance has been found for many life-history traits in fish and can be as large as 0.5 (Theriault et al. 2007). Fishing has therefore been called a ‘large-scale experiment in life-history evolution’ (Rijnsdorp 1993; Law 2000; Stokes and Law 2000).
There is, however, much controversy regarding the relative importance of fishery-induced evolution as compared to the impact of phenotypic plasticity in response to environmental change (Hilborn 2006). It is often questioned whether significant genetic changes over conservation-relevant periods of time are frequent, as discussed in Smith and Bernatchez (2008). Phenotypic plasticity is important in fish (Thorpe 1998; Crozier et al. 2008), and many alleged adaptations could indeed be environmentally induced phenotypic responses rather than genetic changes (Gienapp et al. 2008; Hendry et al. 2008). Changes in eutrophication (Gerdeaux and Perga 2006), salinity (Ricker 1981), temperature (Thresher et al. 2007), competition (Lorenzen and Enberg 2002), or large-scale ocean regime shifts (Pearcy 1992; Thresher et al. 2007) can have an immediate impact on phenotypic traits, particularly life-history traits, without necessarily changing the genetics of a population.
The relative importance of both fishery-induced evolution and phenotypic plasticity is thus a key issue that needs to be addressed (Law 2000, 2007; Smith and Bernatchez 2008). To date, only few studies have tried to separate the effects of fishery-induced evolution and environment-induced changes on individual growth rate. A major problem in such studies is that additive genetic effects can be correlated with long-term changes in, for example, population density, water temperature, or phosphorus concentration and hence productivity of typical freshwater habitats (Hutchings and Fraser 2008). Classical statistical tools such as multiple regressions can therefore be problematic. Recently Swain et al. (2007) used back-calculated length at age 4 (from otolith measurements) to determine the difference in growth between parental and offspring generations of Atlantic cod (Gadus morhua) in the Gulf of St.-Lawrence. The authors found significant length at age differences between the generations and concluded that these differences indicate genetic change in growth, i.e. that they may reveal genetic effects of size-selective fishing. Heino et al. (2008) discuss several potential limitations to Swain et al.’s approach, especially that their approach did not account for potential changes in reproductive strategies ‘see Swain et al. (2008) for a further discussion’. Growth rate is indeed influenced by at least three different life-history traits (Heino et al. 2008): (i) growth capacity, i.e. the ability of fish to transform energy intake into body mass, (ii) the maturation schedule, and (iii) the reproductive investment, i.e. the ratio of gonad mass to somatic mass. In the case of selection against fast growers, resource reallocation from growth capacity to reproductive investment is likely (Gadgil and Bossert 1970; Heino et al. 2008), and any observed change in growth rate could therefore be linked to a change in reproductive investment or in maturation schedule rather than, or in addition to, selection against fast growers.
In this study, we applied the method of Swain et al. (2007) to estimate selection differential in a population of the Alpine whitefish Coregonus palaea, Fatio 1890 (a freshwater salmonid). We also determined potential indices of resource reallocation over an observational period of 25 years, and we used a growth metric that takes the whole lifespan of the fish into account (i.e. the whole period under fishing-induced selection). Our study population lives in a small and shallow lake. The population is isolated, i.e. no fish from other populations have been introduced into the lake during the observational period, and migration, which can also potentially affect the estimation of the selection differentials, is impossible. Fishing mortality is very high, i.e. most fish are harvested in their first years of life and old individuals are scarce (95% of the fish where caught before the age of 8 years, whereas the oldest individual in the sample was 13+). Moreover, fishing effort can be considered constant and uniform over the study period: for the duration of the study, two fishermen have been harvesting, following regulations that have not significantly changed since 1960. Fisheries data on yield since the late fifties do not show any directional trend with regard to the total whitefish yield. We can thus assume a relatively constant selection differential over several whitefish generations. This is consistent with our estimates of the selection differentials (see Results). Heritability of growth has been studied in various other salmonids and found to be significant (Theriault et al. 2007; Carlson and Seamons 2008). The specific situation of our study population therefore allows us to estimate the evolutionary consequences of fishing-induced selection on growth within the range of the existing heritability estimates.
Methods
The study population is confined to the Lake Joux, Switzerland (lat = 46.63°N, long = 6.28°E, 9.5 km2, maximum depth = 32 m). In the course of a monitoring programme that started in 1980, a total of 1654 fish were sampled from large catches (on average 75 fish caught each year, ±37 SD). Mean age of the sampled fish was 4.7 years (±1.4 SD). Fish were sampled each year except between 1997 and 2002 when fishing occurred but no monitoring was done. The catches were taken during the spawning season (November and December) at the spawning site with nylon gill nets of 40, 45 and 50 mm mesh size. The total number of eggs that were collected for supplementary breeding was recorded every year. For size measurement, males and females were pooled because no sexual size dimorphism seems to exist in this species. Total body length was measured in millimetres and scales were taken from above the lateral line between the dorsal and adipose fins for subsequent age determination and back-calculation of previous body lengths. On 719 fish, scale radius and annulus radii, i.e. the distances from the nucleus to the subsequent annuli, were measured using an ocular micrometre for length measurements. Probably due to the high altitude of Lake Joux and the marked temperature differences between summer and winter, annuli on scales are pronounced and allow for easy estimates of fish age and annuli lengths. We back-calculated the length at previous ages of each fish according to the method of Finstad (2003). This method is based on a multiple regression of fish scale including the age and length of the fish. We used a logarithm transformation of fish length and annulus length. From the resulting length-at-age back-calculations, we computed the following two-parameter logarithmic growth curve for each fish:
where Li(t) is the back-calculated length of each fish at age t, α0i the back-calculated length at age 1, and αti the logarithmic growth of each fish. Parameter αti represents the length increase per time unit on a logarithmic scale. We estimated the parameters α0i and αti for each fish from the back-calculated lengths:
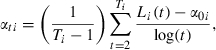 |
where Ti is the age of each fish at capture.
For each fish, we calculated the length-at-age with two different methods: with the back-calculations and with our logarithmic model. We then assessed the goodness-of-fit of our growth model with an analysis of variance of all the back-calculated lengths as dependant variable and the theoretical lengths fitted with the two-parameter logarithmic model as independent variable (ANOVA: d.f. = 3369, r 2 = 0.98, P < 0.0001). This model has several advantages: first, it has fewer parameters than other growth models such as a three-parameter von Bertalanffy model. Second, the interpretation of the two parameters is very intuitive: α0 represents the length at age 1 (i.e. can be understood as juvenile growth) and αt represents growth after age 1, i.e. approximates adult growth. Third, all the sampled fish are taken into account. With a single size-at-age measure as used in Swain et al. (2007), all the fish younger than the reference age are discarded from the analysis. This can result in a biased estimation of selection differentials that is linked to size-selective fishing, especially if growth varies among cohorts. Moreover a single size-at-age measure is more subject to environmental influence in particular years. This problem is probably less significant in our model as the parameter αt takes into account growth over several years, i.e. we expect the variance in our growth measure to be smaller than with a single size-at-age measure. Finally it has been shown for Arctic charr (Salvelinus alpinus), a freshwater salmonid, that a two-parameter log-linear growth model provides a fit that is at least as good as the von Bertalanffy growth model (Rubin and Perrin 1990).
To detect a potential change in growth parameters over time, α0 and αt were averaged for each cohort and a linear regression was calculated. Average growth parameters were the dependant variables and the birth year of the cohort the independent variable. We were interested in the relative growth difference (in %) between two generations and therefore calculated the average relative change over the generations as the observed change in both parameters divided by the average growth parameter and multiplied by the generation time. The average generation time over the generations was estimated according to Stearns (1992) and was calculated over the whole sample for simplicity, without taking the cohorts into account:
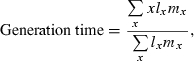 |
where x is the age class of the fish, lx the probability of survival to age x, and mx the fecundity of age class x. Fecundity was estimated as the probability (P) of being mature at age x times the mean length (L) of the fish in the age class x cubed (mx = PL3), assuming that fecundity is proportional to the length cubed of the fish (Clark and Bernard 1992).
As a potential indicator for resource reallocation from growth to reproduction, we estimated the average reproductive investment of all the females captured during the spawning season as the proportional volume of egg per female. All females were captured on spawning grounds and were therefore mature. Some females had already partially spawned, i.e. our measure of reproductive investment underestimates the total eggs production. However the magnitude of this error is not likely to change over time. We used the average volume of eggs per reproducing female of each spawning season and divided this value by the mean length cubed of the fish ‘the allometric relationship between weight and length was: weight = exp(−12.04 + 3.06*ln(length))’. We also estimated the age at maturation for each fish according to Rijnsdorp and Storbeck (1995). This method assumes that growth, i.e. the yearly size increment, is maximal and linear when the fish is immature and decreases after the fish becomes mature because some resources are invested into reproduction instead of growth. We therefore interpret the gap between large and small yearly length increases as the timing of maturation. We used linear regressions to test for linear trends over time in these two measures.
The expected response to selection (R), i.e. the change in growth rate expected if only selection by fishing is occurring under a constant environment, was estimated from the breeder's equation (Falconer and Mackay 1996):
where h2 is the heritability of growth traits and s the selection differential, i.e. the mean difference of a trait between the actual reproducers (the fish surviving to reproduction), and the whole population. Heritability estimates for growth rate in fish range approximately from 0.1 to 0.5 (Law 2000; Garcia de Leaniz et al. 2007; Swain et al. 2007; Theriault et al. 2007; Carlson and Seamons 2008). In this study, we used an intermediate heritability (h2 = 0.3) and two extreme ones (h2 = 0.1 and h2 = 0.5).
The selection differential s was determined for each age class within each cohort by comparing the reproducers, i.e. the fish caught in subsequent years and at older age, with all the fish of that particular age class. We then estimated the average selection differential in every single cohort born in year k, 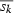 , as the mean of the selection differentials calculated for each age class. This mean took into account the number of fish in each age class and the relative contribution of each age class to reproduction, i.e. the average fecundity of the age class.
, as the mean of the selection differentials calculated for each age class. This mean took into account the number of fish in each age class and the relative contribution of each age class to reproduction, i.e. the average fecundity of the age class.
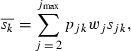 |
where pjk is the proportion of fish born in year k reproducing at age j and wj is a weighting parameter for each age class j. To account for the differential contribution of each age class within each cohort due to differences in fecundity, the weighting parameter was set as the cube of the average length of the fish within the age class. sjk is the selection differential for age class j within cohort k and is estimated as:
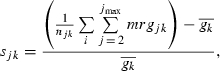 |
where njk is the number of mature fish born in year k reproducing at age j, m is the maturation status and equals 1 if j ≥ age at maturation and 0 otherwise, r is the reproductive status and equals 1 if age at maturation < j ≤ age at capture, and 0 otherwise, and 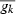 is the average growth parameter (α0 or αt) of all the fish born in year k. The size selectivity of nonfishing induced mortality was considered negligible compared to fishing selection for the estimation of selection differential.
is the average growth parameter (α0 or αt) of all the fish born in year k. The size selectivity of nonfishing induced mortality was considered negligible compared to fishing selection for the estimation of selection differential.
To disentangle the change in growth due to fishery-induced selection from the change due to environmental variation, we postulated that both factors were additive, i.e. that the observed growth change was equal to the sum of genetic (estimated by h2s) and phenotypic plasticity. To simplify, we do not take into account a potential interaction between environment and genotype. The fraction of change due to fishery-induced selection was finally calculated as h2s divided by the total observed change in growth.
All analyses were carried out on the open-access statistical software ‘r’ (R Development Core Team 2008). Population means are presented as mean ± standard deviation. All P-values are two-tailed.
Results
We did not observe any significant linear change in resource reallocation from growth to reproduction over the observational period. Neither the maturation schedule, estimated by the mean age at maturation, nor the fecundity, estimated by the proportional volume of eggs per fish, seems to change consistently over time (maturation schedule: t21 = −0.08, P = 0.94, Fig. 1A; fecundity: t16 = 1.32, P = 0.20, Fig. 1B; the years 2000 and 2001 cannot be considered outliers in Fig. 1A as tested with Cook's distances). The potential periodicity observed in fecundity (Fig. 1B) may be linked to intra-specific competition between age classes (Naceur and Büttiker 1999).
Figure 1.
Indicators of resource reallocation from growth to reproduction: (A) reproduction schedule as the mean age at maturation for each cohort and (B) reproductive investment as the mean proportion (in %) of egg volume per female each year of capture during spawning season.
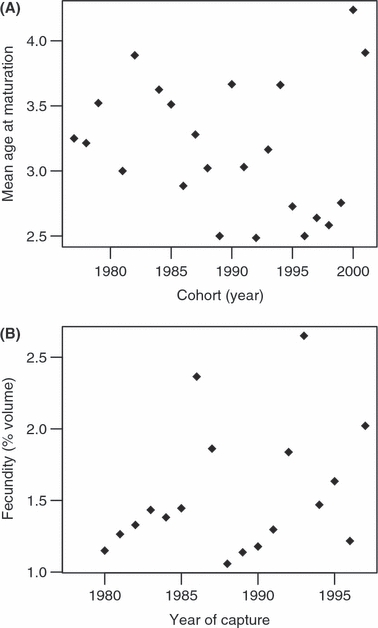
Length at age 1 (α0) did not linearly change over time (t18 = −0.34, P = 0.74, Fig. 2A). However, logarithmic growth (αt) declined by −0.94 ± 0.36% per year (t18 = −2.6, P = 0.017, Fig. 2B). The average generation time, i.e. the average age difference between parents and offspring was estimated to be 4.67 years. The relative growth change per generation is then −4.37 ± 1.66%.
Figure 2.
Growth parameters over time: (A) average length at age 1 (α0) and (B) average logarithmic growth (αt). The cohort is specified by the year of birth. The lines give the regressions.
Selection differentials on parameter α0, i.e. the difference in growth between reproducers and the whole population, did not change linearly over time (linear regression: t21 = 0.50, P = 0.62), neither did the selection differentials on parameter αt (linear regression: t21 = 1.02, P = 0.32). Moreover, no clear trend was found with these parameters. We therefore considered each sk as an independent estimation of an average selection differential s over the whole period with a precision that depends on the number of fish on which the estimation is based. As the number of observations per cohort varied, a weighted t-test, with a weighting proportional to the number of fish in each cohort, was used to test whether s was significantly different from zero.
The selection differential for length at age 1 (α0) was not significantly different from zero (t22 = −0.87, P = 0.39, Fig. 3A). However, the selection differential for the logarithmic growth (αt) was significantly negative: s = −4.93 ± 1.23% (t22 = −4.02, P < 0.001, Fig. 3B).
Figure 3.
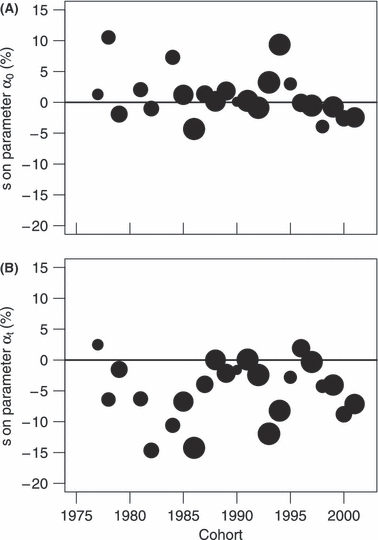
Selection differentials (s) estimated for each cohort. (A) s for length at age 1 (α0), and (B) s for logarithmic growth (αt). The width of the circle corresponds to the number of fish within each cohort.
Assuming that the heritability of growth is h2 = 0.3, with R = −4.37 ± 1.67%, and s = −4.93 ± 1.23%, the proportion of logarithmic growth decrease (αt) due to fishery-induced selection was estimated to be 33.8%. With the two extreme scenarios (i.e. heritability h2 = 0.1 and 0.5), this proportion would be 11.3% and 56.2% respectively.
Discussion
We studied a salmonid population that has been monitored for 25 years. The population is closed to migration and under a fishing pressure that can be considered constant over the observational period. The fishing pressure is strong and most fish that reach maturity seem to be eventually harvested. We therefore consider this population ideal for testing the potential effects of fishery-induced evolution of individual growth rates, a topic that has received much attention recently. We described individual growth with the two parameters α0 and αt. The first parameter α0 describes juvenile growth in the first year of life when the fish are under no direct fishing pressure, whereas the second parameter αt describes the growth trajectories at later ages and at times when selection by fishing is relevant. We found no evidence that α0 changed over the last 25 years. However αt declined significantly during this time.
Changes in individual growth rates over time can be due to fishing-induced evolution, to ecological changes (e.g. temperature, water phosphorus concentration, population density), to a change in life history such as reallocation of resources from growth to reproduction, or to any combination of these possible causes (Heino et al. 2008). Obviously, any increase in energy allocation to reproduction is expected to slow down growth (Heino and Kaitala 1999). A change in the timing of maturation or in fecundity will therefore change individual growth rates (Stearns 1992). However, we found no evidence for a change in maturation schedule or reproductive strategies in our study population. We therefore concentrate our discussion on the importance of fishing-induced evolution relative to ecological changes over time.
To study fishing-induced evolution, we need to understand the selection induced by fishing, i.e. we need good estimates of the selection differentials. Selection differentials measure the difference in a phenotypic trait between the mean of a population and the mean of the individuals selected to be parents of the next generation. Such phenotypic differences are expected to have a strong genetic component if the fish share the same environmental history. Immigrating individuals and fish escaping harvesting, both common in marine populations, could bias the estimation of selection differentials. In our study population, however, there is no migration and few fish escape eventual harvesting. This, combined with a constant fishing pressure (see Introduction), allows us to determine the selection differentials probably more accurately than analogous determinations in open marine populations. We found that the change in αt (the growth trajectories at later ages) is around 1% per year or about 4% per generation, but there was no significant change in α0 (juvenile growth in the first year of life).
Our analyses are however simplifications in several respects. First, we assumed that genetic and ecological factors have additive effects on individual growth rates and that genotype–environment interactions are negligible. Second, we did not apply nonlinear models because of lack of statistical power. Although we have data of >20 cohorts, only about 75 fish were sampled per cohort, and direct parent–offspring comparisons were not possible like in, for example, Grant and Grant (1995) who studied micro-evolutionary responses to directional selection by sampling and assigning parentage to each individual of a population. However, we believe that our model assumptions still lead to useful results in our case because the selection differential can be assumed to vary around an average that does not change over time (the fishing pressure and the reproductive strategies did not seem to change), and nonlinear responses to selection would therefore be somewhat surprising. These assumptions are supported by our data (see Fig. 2).
The evolutionary change in αt that we observed may be somewhat underestimated because slow growers are more likely to reproduce and die before being caught, i.e. natural mortality may be inversely proportional to size (Conover 2007) and slow growing fish are harvested at an older age because fishing targets fish above a certain size. If we assume that the heritability of growth rates in our study population is about the average of what has been described for salmonids so far (i.e. h2 = 0.3), we conclude that about a third of the decrease in αt is directly linked to fishing-induced genetic changes in the population. The systematic removal of larger and older fish therefore seems to significantly affect the evolution of individual growth rates in the whitefish of Lake Joux.
The fact that no growth decrease could be observed in juveniles may be surprising. Although there is no fishing pressure on small fish, juvenile and adult growth are likely to be genetically correlated (Lande and Arnold 1983; Walsh et al. 2006). Moreover, everything being equal, juveniles fish that are small may attain a lesser size than large ones and may therefore be likely to suffer less from fishing selection. A possible reason for the observed absence of a decrease might be that α0 is a single length-at-age measure and therefore more strongly influenced by environmental factors than adult growth (αt) that is an average over several years. A possible genetic decrease could therefore be masked by a plastic response to a changing environment. Temperature is known to have a significant impact on juvenile growth (Malzahn et al. 2003; Coleman and Fausch 2007; Gunther et al. 2007). Competition between juveniles and adult may also change with changing average adult size. Finally, there could also be an adaptive response linked to resources reallocation, with more energy invested for juvenile growth to increase juvenile survival and less in adult growth, the status quo in juvenile growth could be the maximal viable growth.
The marked decrease in the growth parameter αt could potentially have negative consequences for the population. There is now mounting evidence that artificial selection such as size-selective harvesting reduces the average viability in some populations (Fenberg and Roy 2008). Several specific consequences may arise from the removal of large fish, and even if these issues are controversial (Carlson et al. 2008), a precautionary approach should be taken when managing evolving fish stocks (Francis and Shotton 1997). First, large and fast-growing individuals may be of higher genetic quality than small and slow-growing individuals (Birkeland and Dayton 2005). A systematic removal of high quality adults could therefore result in an increase of the average genetic load in a population. Second, as large females usually produce larger offspring of higher viability (Trippel 1995; Walsh et al. 2006), a decrease in growth could impair the recruitment and consequently the long-term yield of the population. Third, as females in some species prefer to mate with large males (Hutchings and Rowe 2008), increased mortality of large fish could have an impact on sexual selection and therefore on mating behaviour. Fourth, nonrandom mortality could decrease the genetic diversity of the population and make it more vulnerable to environmental changes or diseases (Jones et al. 2001).
To conclude, we found that the large selection differentials imposed by size-selective fishing can significantly change the genetics of a population. Our data suggest that fishery-induced evolution can be rapid. This needs to be taken into account by population managers (Stokes and Law 2000; Ashley et al. 2003; Smith and Bernatchez 2008).
Acknowledgments
We thank the Service de la Faune et de Forêts and the Service des Eaux, Sols et Assainissement for providing the monitoring data, the Swiss National Science Foundation for funding, and D. Boukal, S. Cotton, G. Evanno, S. Guduff, A. Jacob, N. Perrin, B. von Siebenthal, T. Szekely Jr, D. Urbach, L. Bernatchez and two anonymous referees for discussion or constructive comments on the manuscript.
Literature cited
- Ashley MV, Wilson MF, Pergams ORW, O'Dowd DJ, Gende SM, Brown JS. Evolutionarily enlightened management. Biological Conservation. 2003;111:115–123. [Google Scholar]
- Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends in Ecology & Evolution. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Olsen EM, Vollestad LA. Seasonal mortality and the effect of body size: a review and an empirical test using individual data on brown trout. Functional Ecology. 2008;22:663–673. [Google Scholar]
- Clark JH, Bernard DR. Fecundity of humpback whitefish and least cisco in the Chatanika River, Alaska. Transactions of the American Fisheries Society. 1992;121:268–273. [Google Scholar]
- Coleman MA, Fausch KD. Cold summer temperature regimes cause a recruitment bottleneck in age-0 Colorado River cutthroat trout reared in laboratory streams. Transactions of the American Fisheries Society. 2007;136:639–654. [Google Scholar]
- Conover DO. Fisheries – nets versus nature. Nature. 2007;450:179–180. doi: 10.1038/450179a. [DOI] [PubMed] [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Hendry AP, Lawson PW, Quinn TP, Mantua NJ, Battin J, Shaw RG, et al. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evolutionary Applications. 2008;1:252–270. doi: 10.1111/j.1752-4571.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Essex, UK: Benjamin Cummings; 1996. [Google Scholar]
- Fenberg PB, Roy K. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Molecular Ecology. 2008;17:209–220. doi: 10.1111/j.1365-294X.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- Finstad AG. Growth backcalculations based on otoliths incorporating an age effect: adding an interaction term. Journal of Fish Biology. 2003;62:1222–1225. [Google Scholar]
- Francis RICC, Shotton R. ‘‘Risk’’ in fisheries management: a review. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1699–1715. [Google Scholar]
- Fukuwaka M-A, Morita K. Increase in maturation size after the closure of a high seas gillnet fishery on hatchery-reared chum salmon Oncorhynchus keta. Evolutionary Applications. 2008;1:376–387. doi: 10.1111/j.1752-4571.2008.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil M, Bossert WH. Life historical consequences of natural selection. The American Naturalist. 1970;104:1. [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Gerdeaux D, Perga M-E. Changes in whitefish scales delta C-13 during eutrophication and reoligotrophication of subalpine lakes. Limnology and Oceanography. 2006;51:772–780. [Google Scholar]
- Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. Climate change and evolution: disentangling environmental and genetic responses. Molecular Ecology. 2008;17:167–178. doi: 10.1111/j.1365-294X.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. Predicting microevolutionary responses to directional selection on heritable variation. Evolution. 1995;49:241–251. doi: 10.1111/j.1558-5646.1995.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Grift RE, Rijnsdorp AD, Barot S, Heino M, Dieckmann U. Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Marine Ecology Progress Series. 2003;257:247–257. [Google Scholar]
- Gunther SJ, Moccia RD, Bureau DP. Patterns of growth and nutrient deposition in lake trout (Salvelinus namaycush), brook trout (Salvelinus fontinalis) and their hybrid, F-1 splake (Salvelinus namaycush × Salvelinus fontinalis) as a function of water temperature. Aquaculture Nutrition. 2007;13:230–239. [Google Scholar]
- Hairston NGJ, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. [Google Scholar]
- Handford P, Bell G, Reimchen T. Gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Hard JJ, Gross MR, Heino M, Hilborn R, Kope RG, Law R, Reynolds JD. Evolutionary consequences of fishing and their implications for salmon. Evolutionary Applications. 2008;1:388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M, Kaitala V. Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. Journal of Evolutionary Biology. 1999;12:423–429. [Google Scholar]
- Heino M, Dieckmann U, Godo OR. Estimating reaction norms for age and size at maturation with reconstructed immature size distributions: a new technique illustrated by application to Northeast Arctic cod. ICES Journal of Marine Science. 2002;59:562–575. [Google Scholar]
- Heino M, Baulier L, Boukal DS, Dunlop ES, Eliassen S, Enberg K, Jorgensen C, et al. Evolution of growth in Gulf of St Lawrence cod? Proceedings of the Royal Society B: Biological Sciences. 2008;275:1111–1112. doi: 10.1098/rspb.2007.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. International Journal of Organic Evolution. 1999;53:1637–1654. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Rowe S. Consequences of sexual selection for fisheries-induced evolution: an exploratory analysis. Evolutionary Applications. 2008;1:129–136. doi: 10.1111/j.1752-4571.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jones MW, McParland TL, Hutchings JA, Danzmann RG. Low genetic variability in lake populations of brook trout (Salvelinus fontinalis): a consequence of exploitation? Conservation Genetics. 2001;2:245–256. [Google Scholar]
- Jorgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Ecology – managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- Lorenzen K, Enberg K. Density-dependent growth as a key mechanism in the regulation of fish populations: evidence from among-population comparisons. Proceedings of the Royal Society of London Series B: Biological Sciences. 2002;269:49–54. doi: 10.1098/rspb.2001.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malzahn AM, Clemmesen C, Rosenthal H. Temperature effects on growth and nucleic acids in laboratory-reared larval coregonid fish. Marine Ecology Progress Series. 2003;259:285–293. [Google Scholar]
- Mertz G, Myers RA. A simplified formulation for fish production. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:478–484. [Google Scholar]
- Myers RA, Hoenig JM. Direct estimates of gear selectivity from multiple tagging experiments. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1–9. [Google Scholar]
- Naceur N, Büttiker B. La palée du lac de Joux: statistiques de pêche des reproducteurs; âge, croissance et fécondité. Bulletin de la Société Vaudoise des Sciences Naturelles. 1999;86:273–296. [Google Scholar]
- Palumbi SR. Evolution – humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Pearcy WG. Ocean Ecology of North Pacific Salmonids. Seattle: Washington Sea Grant Program: Distributed by the University of Washington Press; 1992. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Development Core Team; 2008. [Google Scholar]
- Ricker WE. Changes in the average size and average age of pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Rijnsdorp A. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. [DOI] [PubMed] [Google Scholar]
- Rijnsdorp AD, Storbeck F. Determining the onset of sexual maturity from otoliths of individual female North Sea plaice, Pleuronectes platessa L. In: Secor DH, Dean JM, Campana SE, editors. Recent Developments in Fish Otolith Research. Columbia: University of South Carolina Press; 1995. pp. 271–282. [Google Scholar]
- Rubin J-F, Perrin N. How does the body-scale model affect back-calculated growth: the example of arctic charr, Salvelinus alpinus (L.), of Lake Geneva (Switzerland) Aquatic Sciences. 1990;52:287–295. [Google Scholar]
- Smith TB, Bernatchez L. Evolutionary change in human-altered environments. Molecular Ecology. 2008;17:1–8. doi: 10.1111/j.1365-294X.2007.03607.x. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Stokes K, Law R. Fishing as an evolutionary force. Marine Ecology Progress Series. 2000;208:307–309. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolution of growth in Gulf of St Lawrence cod: reply to Heino et al. Proceedings of the Royal Society B: Biological Sciences. 2008;275:1113–1115. doi: 10.1098/rspb.2007.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault V, Garant D, Bernatchez L, Dodson JJ. Heritability of life-history tactics and genetic correlation with body size in a natural population of brook charr (Salvelinus fontinalis. Journal of Evolutionary Biology. 2007;20:2266–2277. doi: 10.1111/j.1420-9101.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- Thomas G, Eckmann R. The influence of eutrophication and population biomass on common whitefish (Coregonus lavaretus) growth – the Lake Constance example revisited. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:402–410. [Google Scholar]
- Thompson JN. Rapid evolution as an ecological process. Trends in Ecology & Evolution. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- Thorpe JE. Salmonid life-history evolution as a constraint on marine stock enhancement. Bulletin of Marine Science. 1998;62:465–475. [Google Scholar]
- Thresher RE, Koslow JA, Morison AK, Smith DC. Depth-mediated reversal of the effects of climate change on long-term growth rates of exploited marine fish. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7461–7465. doi: 10.1073/pnas.0610546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippel EA. Age at maturity as a stress indicator in fisheries. BioScience. 1995;45:759–771. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Wright PJ. Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhua in the northern North Sea and Scottish west coast. Marine Ecology Progress Series. 2004;276:237–248. [Google Scholar]


