Abstract
The study of fisheries-induced evolution is a research field which is becoming recognized both as an important and interesting problem in applied evolution, as well as a practical management problem in fisheries. Much of the research in fisheries-induced evolution has focussed on quantifying and proving that an evolutionary response has taken place, but less effort has been invested on the actual processes and traits underlying capture of a fish by a fishing gear. This knowledge is not only needed to understand possible phenotypic selection associated to fishing but also to help to device sustainable fisheries and management strategies. Here, we draw attention to the existing knowledge about selectivity of fishing gears and outline the ways in which this information could be utilized in the context of fisheries-induced evolution. To these ends, we will introduce a mathematical framework commonly applied to quantify fishing gear selectivity, illustrate the link between gear selectivity and the change in the distribution of phenotypes induced by fishing, review what is known about selectivity of commonly used fishing gears, and discuss how this knowledge could be applied to improve attempts to predict evolutionary impacts of fishing.
Keywords: evolution, fisheries, Gadus morhua, gear selectivity, management, retention probability, selection, selectivity curve
Introduction
Fisheries-induced evolution has recently emerged as a new and rapidly developing subfield within evolutionary biology (e.g. Law 2000; Heino and Godø 2002; Dieckmann and Heino 2007; Kuparinen and Merilä 2007; Law 2007). Interest in the evolutionary effects of fishing stem from the observation that age and size at maturation have been decreasing in several heavily exploited fish stocks – an expected adaptive response to increased pre-adult and adult mortality (e.g. Heino and Godø 2002; Law 2000). The primary focus in studies of fisheries-induced evolution has been placed on disentangling growth related changes in maturation (considered to reflect variation of environmental origin) from shifts of the maturation reaction norms (considered to reflect evolutionary responses to fishing) (Heino et al. 2002; Olsen et al. 2004; Engelhart and Heino 2004; Dieckmann and Heino 2007). In contrast, little effort has been invested in this context in quantifying the fundamental components of any evolutionary response: heritability (h2) and intensity of selection (S). The main reason for this appears to be that under the prevailing harvesting rates the intensity of directional selection is anticipated to be substantial, and that heritability estimates for relevant life-history traits in fish have been assumed to be at least moderate (e.g. Law 2000, 2007). Hence, evolutionary responses are expected to be seen although the h2 and S have rarely been quantified (e.g. Law 2000, 2007; Heino and Godø 2002). This is understandable because quantifying heritability in natural fish populations can be challenging (e.g. Kuparinen and Merilä 2007; Heino et al. 2008), though successful examples do exist (Smoker et al. 1994; Hard et al. 1999; DiBattista et al. in press), and the same difficulties has also been considered to apply for quantifying selection (Heino and Godø 2002; Haugen and Vøllestad 2001). More recently, debate on the plausibility and interpretability of reaction norm analyses has increased the interest towards alternative and/or complementary approaches to investigate evolutionary changes in fish life-histories (Law 2007; Marshall and McAdam 2007; Morita and Fukuwaka 2007). In particular, as put by Law (2007)‘the quantitative analyses of whether rates of change are consistent with likely heritabilities and selection differentials caused by fishing, allowing for change in the environment, is a critical issue needing more research’.
Any analyses comparing changes in fish life-histories with selectivity regimes induced by fisheries are heavily constrained by the lack of estimates of fisheries-induced selection for fitness related traits, such as growth rate, body size or timing and size at maturation (Law 2007; Fenberg and Roy 2008). In fact, only a handful of studies have provided selection differential estimates (reviewed in Hard et al. 2008), with the most detailed ones being estimated from length-at-age trajectories back-calculated from otoliths or opercular bones (Sinclair et al. 2002; Carlson et al. 2007; Edeline et al. 2007; Swain et al. 2007). As such detailed long-term phenotypic data are rarely available, indirect methods for estimating how fishing alters the distribution of phenotypes in the target population would be welcome for understanding potential selection regimes associated to fishing at least as a first step ‘as shown by Darimont et al. (2009)’. Even if fairly rough, such estimates could provide insights into the magnitude and interannual variation in selection pressures and whether those weaken during the course of exploitation as expected if phenotypic variability is being lost or a new optimum is being approached (Haugen and Vøllestad 2001), and for comparing changes in the distribution of phenotypes induced by alternative fishing methods and strategies.
In general, selection associated to fishing is generated by the selectivity and intensiveness of fishing (e.g. Heino et al. 2002; Law 2007; Hard et al. 2008). Selection towards early maturation can arise directly due to increased fishing mortality, but fishing is in most cases (virtually always) at least to some extent also size-selective by targeting larger individuals due to minimum landing sizes and gear regulations. This will alter size distribution in a population, and the opportunity for selection for body size (and for correlated traits) then depends on the overall harvest rates, i.e. the proportion of population removed by fishing. Evolutionary change in growth due to size-selective mortality was empirically demonstrated in Atlantic silverside (Menidia menidia) by keeping overall harvest rates and population density constant but varying selectivity (Conover and Munch 2002). The experimental set-up was rather extreme since the size distribution was truncated so that the remaining fish were the largest or smallest 10% of the population. Nevertheless, the results still highlight the importance of not only focussing on the overall harvest rates, but also on the size selectivity of fishing as it was shown to induce evolutionary shifts in the size distribution of the study population within just four generations (Conover and Munch 2002). Size-selectivity of fishing gear is an extensively studied area in fisheries sciences due to the simple fact that most modern fisheries aim at improving gear-selectivity to minimize by-catch, catches of undersized fish and to maximize catch per unit effort (CPUE) for target species.
Here, our aim is to draw attention to the existing knowledge of selectivity of fishing gears and how that information could be utilized to assess how fishing alters phenotypic composition of the target population. To these ends, we will introduce a mathematical framework commonly applied to quantify fishing gear selectivity, illustrate the link between gear selectivity and changes in the distribution of phenotypes due to fishing, and review how much is known about selectivity of typical fishing gears. We then discuss how knowledge of fishing gear selectivity could be utilized in the context of fisheries-induced evolution.
From fishing gear selectivity to changes in the distribution of phenotypes
In principle, fisheries-induced changes in the distribution of phenotypes in the targeted population could simply be measured by comparing the mean trait value of catch with that in the nonfished (nonselected) population, but data needed for this are difficult to obtain. A way to approach the problem would be to utilize knowledge of how the applied fishing gear selects individuals, to indirectly assess how fished population might differ from the unfished one.
Selectivity of fishing is traditionally described in terms of body length. Clearly, this is not necessarily the trait determining whether a fish is captured or not, but because of being most easily measurable and strongly correlated with the other traits affecting capture probability (such as girth or mouth size), it has become established as the primary phenotypic measure in quantification of selectivity. Selectivity of fishing is generally partitioned into three length specific probabilities: (i) availability of fish for fisheries, (ii) contact of fish with the fishing gear, and (iii) fish retention by the gear (Millar and Fryer 1999). The overall fish length distribution in a catch is determined jointly by these three probabilities, but most of practical research has focussed on quantifying only fish retention, so that in typical terminology this probability as a function of length is identified as a ‘selectivity curve’. There are two common shapes for this curve: logistic and dome-shaped. The logistic selectivity curve can be formulated as (Millar and Fryer 1999):
| (1) |
where r(l) is the retention probability of a fish of length l, a and b are shape parameters, so that 50% retention probability is reached at length −a/b, and δ is a parameter inducing asymmetry to the curve about −a/b. In general, the retention probability increases with fish body length, so that this shape is best suited for towed fishing gears, such as trawls and seines. For gillnets, which capture fish by wedging or entangling, a dome-shaped selectivity curve is considered the most appropriate. A typical functional form for a selection curve of this type is a Gaussian curve (Millar and Fryer 1999):
| (2) |
where r(l) and l are as above, μ is the length at which the curve peaks and σ is standard deviation describing the width of the curve about its peak. Other choices for curve formulation would be e.g. bi-normal or gamma functions. For a dome-shaped curve, the retention probability is considered relative. This means that rather than giving the exact retention probability, the curve gives the relative retention probability compared to the length class fully selected by fisheries, i.e. compared to length μ, so that r(μ) is scaled to 1.
The selectivity curves (eqns 1 and 2) can be estimated by comparing size distributions in catches using different mesh or hook sizes, by assuming that retention probability only depends on the relative difference between fish size and mesh/hook size (i.e. the principle of geometric similarity) and, in case of eqn 2, that gears with different mesh/hook sizes are equally efficient in catching fish of the modal length. The selectivity framework described above, and a widely used statistical method for estimation of r(l) were formulated by Millar and Fryer (1999). In comparison to direct retention probability estimates obtained by using underwater cameras, this indirect estimation method has turned out accurate (Grant et al. 2004).
Commercially important fish stocks are being regularly monitored by survey studies in which abundance, age and length structure of the stock, weight–length relationship, as well as age and length specific maturity ogives (i.e. proportion of mature individuals) are being estimated (e.g. Jennings et al. 2001; Evans and Grainger 2002). Bridged together with the selectivity curve of the applied gear and catch size, this survey-based knowledge of fish stock demography provides a basis for estimating the changes fishing might have induced to the distribution of phenotypes in the harvested population. By assuming that fish of different sizes contacted the fishing gear in the same proportions that they were abundant in the stock (i.e. each fish has the same probability of getting into a contact with the gear), then for a catch of weight c taken by one gear it holds
| (3.1) |
so that
| (3.2) |
where w(li) is weight of fish in the length class li, n(li) is the number of fish in the length class li, and i is the length class index. For a dome-shaped selectivity curve, F is the fishing mortality for the length class best selected by the fishing gear, i.e. the length class at which the selectivity curve peaks. Fishing mortality in each length class is then r(li)F, so it is reduced in proportion to the relative retention success of the fishing gear in the considered length class (Williams and Shertzer 2004). For a logistic selectivity curve the length specific mortality is calculated similarly, but the interpretation of F is slightly different: it is an asymptote for mortality experienced by the largest length classes. Once length class specific fishing mortality rates are known, the demographic structure of the catch can be calculated directly in terms of all demographic variables known for each length class. Specifically, based on maturity ogives, the characteristics of reproducing individuals removed by fisheries can be estimated, and provide a proxy of how those phenotypes that remain to reproduce differ from the captured ones. Provided that relative probabilities of fish phenotypes being available to fisheries and coming into contact with the gear are known, then the assumption of fish coming into contact with the gear randomly can be relaxed and information about the availability and contact can be plugged into the retention probability r(l) (Millar and Fryer 1999).
The equations above and a case-study example in Box 1 sketch the way in which the well-established framework for quantifying fishing gear selectivity can be utilized to estimate what kind of changes in the distribution of phenotypes in the targeted population might be associated to fishing. Undoubtedly, these calculations should be viewed as approximations, as in reality several other factors and processes (e.g. schooling behaviour, activity and boldness of a fish) affect capture, but in the absence of detailed information about these it can be hard to incorporate them accurately to the gear selectivity curve. Before entering into a discussion about these complexities, potential ways to deal with them, and applicability of the knowledge of fishing gear selectivity in the context of fisheries-induced evolution, we first provide a brief review of the studies on fishing gear selectivity to identify major components and mechanisms underlying overall patterns of selectivity induced by fishing gears.
Box 1 Gillnet and trawl selection in the Baltic cod – an example.
Baltic cod (Gadus morhua) stock is a representative example of a fish stock that has declined to seriously low numbers due to intensive fishing (ICES 2007). In the Baltic cod fishery, individuals are mainly caught by gillnets and trawls, but little is known about selective pressures these gears might induce to the cod population. To investigate this, we estimated what kind of changes fishing with a 105 mm diamond mesh gillnet and a 140 mm cod end trawl would induce in the length at maturity in Baltic eastern cod stock (subdivision 25–32). The length structure of this population in its unfished state is shown in Fig. 1, and the selectivity curves for the considered gillnet and trawl are given in Fig. 2 (both derived from Kuikka et al. 1999; see this publication for more details). In general, the gillnet most efficiently selects individuals about 45–65 cm in length, and the selection has a sharp peak in about 50 cm length class, whereas the trawl best captures large individuals, thus yielding a selectivity curve increasing as a function of length (Fig. 2). Changes in the mean length at maturity induced by the gears were estimated for catch sizes 50 000 t, 100 000 t and 150 000 t, which cover the typical catches by the two gears during 1994–1998 (ICES 2007, Kuikka et al. 1999). In addition to estimating changes induced by individual gears, we also estimated how mean length at maturity would change if catch quota was evenly allocated to both of the gears. The calculations were done using eqn 3.1 and 3.2, and the weight–length relationship w(l) = 0.01l3 (Kuikka et al. 1999).
Figure 1.
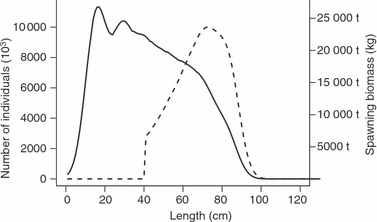
Structure of the unfished Baltic cod stock (eastern subdivision) as described in Kuikka et al. (1999). Length specific distribution of individuals is shown by the solid line and the distribution of spawning biomass in the population by dashed line.
As expected, for all the gear scenarios the magnitude of change in the length at maturity increased with increasing fishing effort (Table 2). For the gillnet, shift was towards larger size at maturation (Table 2), as the gillnet was only able to capture smaller individuals in the spawning stock, thus leaving larger length classes fairly unexploited (Figs 1 and 2). In contrast, the trawl was particularly efficient in capturing large individuals and thus decreased mean length at maturity, but at the same time it captured a wider range of lengths (Fig. 2), so that the change was not as large as in the case of the gillnet (Table 2). The combination of gillnet and trawl slightly increased length at maturity, but the magnitude of this shift was much lower than those for the gillnet alone (Table 2).
Table 2.
Change in mean length at maturity of Baltic cod induced by using only gillnet, only trawl or a combination of these gears
| Change in mean length (cm)* | ||||
|---|---|---|---|---|
| Catch (kg) | Harvest rate (%) | Gillnet | Trawl | Gillnet and trawl† |
| 50 000 t | 5.6 | 0.9 | −0.3 | 0.3 |
| 100 000 t | 11.1 | 2.0 | −0.6 | 0.6 |
| 150 000 t | 16.7 | 3.5 | −0.9 | 1.1 |
Difference in the mean length of mature individuals before and after fishing.
Both gears caught half of the total catch.
The estimated changes in the length at maturity suggest that fisheries shift a phenotypic mean value to different directions and at different relative magnitudes depending on the selectivity curve(s) of the fishing gear(s). If being introduced to an unfished cod population, trawling would shift distribution of phenotypes towards smaller length at maturity, whereas large individuals would become disproportionally abundant in the spawning stock in the presence of gillnet fishing. It should be noted however, that the latter result would only apply for a short while, until high mortality at intermediate sizes would start to reduce the number of individuals entering into the greater length classes. To investigate such interactions, and possible evolutionary trends induced by fishing, the selectivity patterns arising from fishing should be estimated for a sequence of years and the dynamics of the population should be simulated (see the section ‘Fishing gear selectivity in an evolutionary context’).
General shapes of selectivity curves typically arise from the capture mechanisms (Millar and Fryer 1999), so that the pattern of selectivity associated with an individual gear may not be easy to change. However, as illustrated by the combination of gillnets and trawls in cod fishery (Table 2), overall change in the distribution of phenotypes in the targeted population can be modified by allocating fishing effort between different gears. Designing a combination of gears so that unwanted changes would not arise might therefore provide a way to minimize evolutionary risks associated with fishing.
Flesh around the bones of the fishing gear selectivity framework
As already noted above, most of the field studies looking at gear selectivity have focussed on estimating gear and species-specific retention probability curves to describe how effectively fish are being captured if coming into contact with the gear. The most well studied gears are trawls, longlines and gillnets of which trawls and longlines are generally characterized by logistically shaped selectivity curves (e.g. Huse et al. 1999; Zuur et al. 2001) whereas dome-shaped selectivity curves apply to gillnets (e.g. Huse et al. 1999; Madsen et al. 1999; Stergiou and Erzini 2002). Comparison of the ranges of fish lengths each of these gears select has revealed that trawls generally capture the widest range of lengths (Fabi et al. 2002; Stergiou et al. 2002). For instance, in the case of Greenland halibut, immature fish are most abundant in trawl catches (Nedreaas et al. 1993). These features associated with trawling are unfortunate, as minimum landing sizes are aimed to correspond with length at first reproduction and narrow selectivity would best minimize the possibility of catching undersized fish that are to be discarded (e.g. MacLennan 1995; Fabi et al. 2002). Notably, trawling may induce substantial mortality also among the fish small enough to escape through the mesh due to injuries and stress: e.g. in Baltic herring this so-called postcapture mortality is expected to even exceed 70% in some length classes (Suuronen et al. 1996).
As suggested by varying shapes of the estimated retention probability curves, mechanisms and processes underlying capture – and also fish getting into contact with the gear – are not always the same, leading to differences in traits under selection by different gears. For example, comparative studies have demonstrated that fast-growing individuals in younger age classes and slow growing individuals in older classes are overrepresented in trawl catches (Huse et al. 1999). While the former one undoubtedly arises from slow growing individuals in younger age classes being able to escape through meshes, the latter one is a result of the largest individuals being able to avoid an approaching trawl due to their better swimming ability (Huse et al. 2000), which is also reflected in larger trawls (i.e. faster moving ones) being better at catching large fish than small trawls (Bethke et al. 1999). Accordingly, trawl selectivity may not remain constant over a year, but it can vary due to seasonal changes in swimming ability arising, for instance, from changes in water temperature (Özbilgin et al. 2005, 2006). Another aspect typically related to most gear operating with nets is that despite retention probabilities being expressed as a function of body length, the actual trait affecting fish being wedged or captured by the mesh is body girth. Therefore, the probability of becoming captured can be influenced by gonad development or body condition if these traits affect girth (Huse et al. 1999, 2000; Jørgensen et al. 2006; Santos et al. 2006). This in turn can lead to higher exploitation rates for mature than immature individuals of the same length, particularly among younger fish (Huse et al. 2000).
A fundamental difference in bait and net-based fishing practices is that fish feeding behaviour becomes one of the central determinants of the capture process in bait fishing (Stoner 2004). This is reflected in observations that longlines catch cod of lower condition than gillnets, probably due to increased feeding motivation of individuals in poor condition (Huse et al. 2000). At the gear level, size-selectivity of longline is regulated by hook size, so that longline retains a wide range of fish sizes above a certain threshold size (Huse et al. 2000; Stergiou et al. 2002). Large individuals are particularly vulnerable to longline fisheries, as they come disproportionally often into contact with the gear by winning the competition over bait due to their enhanced swimming ability (Huse et al. 1999, 2000; Woll et al. 2001; Stergiou et al. 2002). In recreational angling fisheries bait attack rates have been found to be associated with metabolic rate, aggression and parental care (Cooke and Cowx 2006; Cooke et al. 2007), so that selection against these traits might be also expected for baited fishing gears. Removal of dominant individuals can also negatively affect juvenile fitness, if the latter learn from dominant adults vital behavioural traits related to e.g. migration and predator avoidance (Shumway 1999). Moreover, as active, aggressive individuals typically grow faster, selection on behavioural traits may induce selection towards lower growth rates (Biro and Post 2008; Uusi-Heikkilä et al. 2008). This does not apply only for baited gear, as fast growth is associated with increased movement activity and boldness, leading to fast growing individuals coming more frequently into contact with e.g. gillnets than slow growing ones (Biro and Post 2008).
Within the fishing gear selectivity framework, markedly less attention has been focussed on the processes affecting fish availability to fisheries, than has been focussed on retention probabilities curves and on traits related to the probability of fish getting into contact with a gear. Fish are known to migrate ontogenically to deep water, so features of the gear and depth at which it is applied have been shown to affect size distribution in both longline (Ward 2008) and trawl (Jacobson et al. 2001) catches. This can even result in the overall selectivity of trawling being dome-shaped (Jacobson et al. 2001) despite the logistic shape of the retention probability curve, which describes selectivity among fish entering the gear. In contrast to what is known about the vertical distributions, any attempt to estimate spatial (i.e. horizontal) availability as a function of phenotypes is challenging as it can be affected by several factors of which little is known (Erzini et al. 2003). However, the spatial availability component is of similar importance to the overall fishing selectivity as are the contact, retention and vertical availability components. For example, fisheries targeting anadromous fish at their spawning migration can induce substantial selection on age at maturation if early and late maturing components of the spawning stock migrate at different times and are unequally exploited (e.g. Consuegra et al. 2005; Cooke and Cowx 2006; Hard et al. 2008). Similar patterns of selection may also arise if fishing is concentrated at spawning grounds (e.g. Kuparinen and Merilä 2007). A particularly intriguing question related to fish availability to fisheries is the potential role of behavioural traits: bolder individuals may be more abundant on areas better accessible for fisheries (Uusi-Heikkilä et al. 2008), and schooling tendency of an individual can determine availability to fishing vessels targeting schools (Parrish 1999). Selection on such traits can strongly affect not only population viability, but also future fisheries because if frequently encountered phenotypes are removed, phenotypes exhibiting e.g., increased hiding tendency would become more abundant. As a consequence, catching fish is expected to get increasingly difficult (Uusi-Heikkilä et al. 2008).
Fishing gear selectivity in an evolutionary context
Knowledge of the selectivity of the applied fishing gear together with target stock demography provide means to approximate how fishing might shift the distribution of phenotypes in a stock over one fishing season (Box 1). However, to assess possible evolutionary responses to fishing, impacts of such fisheries-induced changes in the distribution of phenotypes and their demographic consequences should be known over several generations. Evolutionary dynamics of harvested fish stocks have frequently been investigated using simulation models incorporating complicated ecological features, such as density-dependent growth or body size dependent fecundity (e.g. Heino 1998; Ratner and Lande 2001; Ernande et al. 2004;de Roos et al. 2006; Andersen et al. 2007). However, in these approaches fisheries-induced mortality rates have typically been assumed to be simply constants at lengths exceeding some threshold (e.g. Heino 1998; Ratner and Lande 2001; Ernande et al. 2004; de Roos et al. 2006). Viewed in the light of how much is known about the selectivity of common fishing gears, obvious synergy benefit would arise if information about actual selectivity curves would be incorporated to simulation approaches designed to predict evolutionary responses to fishing. In particular, such simulations could be applied to quantitatively investigate what kind of patterns of selection might arise from different harvesting rates and selectivity curves of the fishing gears. The theoretical simulation study by Williams and Shertzer (2004) provides a pioneering example of this kind: the authors utilized a logistic fishing selectivity curve (eqn 1, Fig. 2) to predict associated selection differentials on growth traits, by calculating fisheries-induced mortality rates for length classes in a similar way as formulated above (eqn 3.1 and 3.2, and Box 1). More recently, similar approach was also taken by Hilborn and Minte-Vera (2008).
Figure 2.
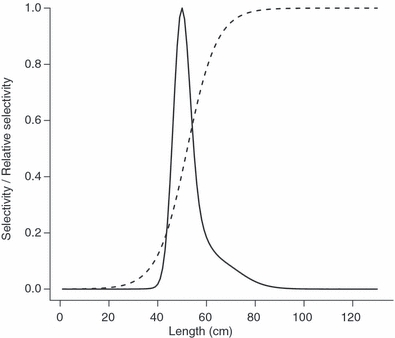
Selectivity curves for 105 mm diamond mesh gillnet (solid line) and 140 mm cod end trawl (dashed line) for the Baltic cod fishery.
One remarkable feature in studies predicting evolutionary responses to fishing is that large individuals are commonly assumed to experience higher fishing mortality than smaller ones (e.g. Andersen et al. 2007). However, selectivity curves induced by e.g. gillnets are clearly dome-shaped (e.g. Madsen et al. 1999), and also the trawl avoidance and vertical distribution of large fish suggests lower fishing mortality in largest size classes (e.g. Huse et al. 2000; Jacobson et al. 2001). In addition, dome-shaped mortality patterns and selection differentials have also been empirically estimated e.g. for Atlantic cod (Sinclair et al. 2002; Swain et al. 2007). In the presence of such disruptive selection, the direction to which phenotypes are expected to evolve is not at all obvious (Rueffler et al. 2006), but depends on the interplay of population demography and fishing intensity (Gårdmark and Dieckmann 2006). To understand the range of ways in which fishing might affect the targeted population, it would therefore be of particular interest to assess what kind of population level consequences might be induced by fishing gears with dome-shaped selectivity curves.
Typically, fisheries aim at minimizing the catch of undersized fish, due to which gears with narrow selectivity ranges are generally considered preferable as they allow focusing fishing effort sharply on legal length classes (e.g. MacLennan 1995; Fabi et al. 2002; Stergiou et al. 2002). However, adjusting fisheries to maximize CPUE in the short term is likely to be in conflict with long-term ecological and evolutionary management goals. Fishing with narrow selectivity ranges targets a few cohorts heavily, leading to variability in yield due to annual variability in recruitment. If stabilized annual yields are strived for, this would be better achieved by fishing with wide selectivity ranges so that more year classes are available to fisheries (MacLennan 1995). This would also prevent fisheries from truncating weak year classes even more. In the presence of a logistic gear selectivity curve, steepness of the curve has been shown to be associated with large selection differentials on growth rates (Williams and Shertzer 2004), suggesting that narrow selectivity range is not at all optimal from an evolutionary perspective either (Kuparinen and Merilä 2007). Therefore, attention should be focussed on assessing how gear selectivity curves – traditionally evaluated only in terms of the amount of undersized fish caught (e.g. Madsen et al. 1999; Harley et al. 2000; Huse et al. 2000; Fabi et al. 2002) – will alter distribution of phenotypes in the targeted population (e.g. as illustrated in eqn 3.1 and 3.2, and Box 1), and what kind of selectivity regimes these might induce to fitness related traits ‘e.g. using the models by Williams and Shertzer (2004) and Hilborn and Minte-Vera (2008)’. Incorporating these perspectives to existing modelling approaches to predict long-term demographic consequences of alterations in fishing effort and gear selectivity (e.g. Kvamme and Kuldbrandsen Føysa 2004) might provide a platform for a balanced comparison of ecological, evolutionary and economical impacts of alternative fisheries management strategies.
So far, we have only discussed effects of fishing on the target population. However, when investigating possible evolutionary shifts in exploited fish stocks, the role of natural selection cannot be overlooked. Recent findings in a pike population followed over a long time period suggest that natural and fisheries-induced selection can act in opposing directions (Carlson et al. 2007), so that the direction to which phenotypes eventually shift depends on the relative strengths of these two sources of selection (Edeline et al. 2007). Particularly sexual selection may act against fisheries-induced selection, if individuals targeted by fisheries are still overwhelmingly favoured in mating, leading to realized selection being much weaker that that induced by fishing in the first place (Hutchings and Rowe 2008). These findings stress the fact that natural selection should be incorporated into the analyses and predictions of fisheries-induced evolution or, at the very least, uncertainty arising from omitting it should be acknowledged.
Conclusions
We have drawn attention to existing knowledge about fishing gear selectivity (summary in Table 1), how it could be utilized in quantifying how fishing shapes the distribution of phenotypes in the targeted population (e.g. Box 1), and further incorporated to approaches to assess evolutionary impacts of fishing (e.g. Ratner and Lande 2001; Williams and Shertzer 2004). This synergy should provide researchers with the means to better understand and predict the potential evolutionary consequences of fishing and different harvesting strategies. The approaches discussed here are by no means competing with the currently used methods to investigate phenotypic shifts in exploited populations, such as the probabilistic reaction norms (Heino et al. 2002; Barot et al. 2004). Rather, we suggest a more rigorous investigation of changes in phenotype distributions and selective regimes associated with different fishing methods and strategies as a complementary approach which can provide information needed for predicting evolutionary responses to fishing and for understanding underlying causes of long-term phenotypic trends seen in exploited populations. Likewise, such an approach is needed for the development of evolutionary sustainable fisheries management (Heino and Godø 2002). In this respect the role of gear selectivity is substantial as it is the component in fishing that can be relatively easily regulated.
Table 1.
A summary of traits selected by three typical fishing gears and general shapes of the gear selectivity curves
| Traits selected by the gear | |||
|---|---|---|---|
| Gear | Selectivity curve* | Contact | Retention |
| Gillnet | Dome-shaped | Fast growth, boldness | Girth |
| Trawl | Logistic shape | Low swimming speed, slow escapement reaction | Girth |
| Longline | Logistic shape | High swimming speed, increased feeding motivation | Mouth size |
Typically characterized with a curve giving retention probability as a function of fish body length.
Merging theory and empirical knowledge of traditional fisheries selectivity research into the fields of fisheries-induced evolution should be beneficial for many reasons. First, the framework for quantifying fisheries selectivity provides selectivity curves for numerous fishing gears and species that can be readily applied to estimate and/or predict immediate changes in phenotype distributions induced by different gears, gear combinations and harvest rates (Box 1). Information about gear selectivity can also be incorporated to simulation approaches to predict selection differentials arising from fishing (Williams and Shertzer 2004; Hilborn and Minte-Vera 2008) and possible evolutionary changes in the targeted population (e.g. Ratner and Lande 2001, de Roos et al. 2006). Due to general flexibility of the gear selectivity framework, also additional information about e.g., behavioural traits playing a role in gear selection (Biro and Post 2008; Uusi-Heikkilä et al. 2008) can be easily incorporated. Secondarily, relying partly on the same tools and theory is likely to bring traditional fisheries management and evolutionary biologists closer to each other and generate interdisciplinary discussion. These kind of interactions are urgently needed as so far those responsible for practical fisheries management have not been too convinced about fisheries-induced evolution being a great concern (Kuparinen and Merilä 2007), which is clearly manifested in the fact that fisheries management still rely on minimum size regulations as a primary tool despite its potentially detrimental selective effects (Fenberg and Roy 2008).
Acknowledgments
We would like to thank Hannu Lehtonen for comments on the earlier version of the manuscript, and John Loehr for linguistic corrections. Our study was funded by the University of Helsinki and Academy of Finland.
Literature cited
- Andersen KH, Farnsworth KD, Thygesen UH, Beyer JE. The evolutionary pressure from fishing on size at maturation of Baltic cod. Ecological Modelling. 2007;204:246–252. [Google Scholar]
- Barot S, Heino M, O'Brien L, Dieckmann U. Estimating reaction norms for age and size at maturation when age at first reproduction is unknown. Evolutionary Ecology Research. 2004;6:659–678. [Google Scholar]
- Bethke B, Arrhenius F, Cardinale M, Håkansson N. Comparison of the selectivity of three pelagic sampling trawls in a hydroacoustic survey. Fisheries Research. 1999;44:15–23. [Google Scholar]
- Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2919–2922. doi: 10.1073/pnas.0708159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, Fletcher JM, James JB, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yield over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Consuegra S, Garcia de Leaniz C, Serdio A, Verspoor E. Selective exploitation of early running fish may induce genetic and phenotypic changes in Atlantic salmon. Journal of Fish Biology. 2005;67:129–145. [Google Scholar]
- Cooke SJ, Cowx IG. Contrasting recreational and commercial fishing: searching for common issues to promote unified conservation of fisheries resources and aquatic environments. Biological Conservation. 2006;28:93–108. [Google Scholar]
- Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. Physiological and behavioral consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiological and Biochemical Zoology. 2007;80:480–490. doi: 10.1086/520618. [DOI] [PubMed] [Google Scholar]
- Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace other agents of trait change in wild. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:952–954. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBattista JD, Feldheim KA, Garant D, Gruber SH, Hendry AP. Evolutionary potential of a large marine vertebrate: quantitative genetic parameters in a wild population. Evolution. doi: 10.1111/j.1558-5646.2008.00605.x. In press, in press; doi: 10.1111/j.1558-5646.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population a driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart G, Heino M. Maturity changes in Norwegian spring-spawning herring Clupea harengus: compensatory or evolutionary responses? Marine Ecology Progress Series. 2004;272:245–256. [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptation changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London, Series B. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzini K, Concalves JMS, Bentes L, Lino PG, Ribeiro J, Stergiou KI. Quantifying the roles of competing static gears: comparative selectivity of longlines and monofilament gill nets in a multi-species fishery of the Algarve (southern Portugal) Scientia Marina. 2003;67:341–352. [Google Scholar]
- Evans D, Grainger R. Gathering data for resource monitoring and fisheries management. In: Hart PJB, Reynolds JD, editors. Handbook of Fish Biology and Fisheries. Oxford: Blackwell Science; 2002. pp. 84–102. [Google Scholar]
- Fabi G, Sbrana M, Biagi F, Grati F, Leonori I, Sartot P. Trammel net and gillnet selectivity for Lithognathus mormyrus (L., 1758), Diplodus annularis (L. 1758) and Mullus barbatus (L., 1758) in the Adriatic and Ligurian seas. Fisheries Research. 2002;54:375–388. [Google Scholar]
- Fenberg PB, Roy K. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Molecular Ecology. 2008;17:209–220. doi: 10.1111/j.1365-294X.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- Gårdmark A, Dieckmann U. Disparate maturation adaptations to size-dependent mortality. Proceedings of the Royal Society B. 2006;273:2185–2192. doi: 10.1098/rspb.2006.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GC, Radomski P, Anderson CS. Using underwater video to directly estimate gear selectivity: the retention probability for walleye (Sander vitreus) in gill nets. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:168–174. [Google Scholar]
- Hard JJ, Winans GA, Richardson JC. Phenotypic and genetic architecture of juvenile morphometry in Chinook salmon. Journal of Heredity. 1999;90:597–606. [Google Scholar]
- Hard JJ, Gross MR, Heino M, Hilborn R, Kope RG, Law R, Reynolds JD. Evolutionary consequences of fishing and their implications for salmon. Evolutionary Applications. 2008;2:388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley SJ, Millar RB, McArdle BH. Examining the effects of changes in the minimum legal sizes used in the Hauraki Gulf snapper (Pagrus auratus) fishery in New Zealand. Fisheries Research. 2000;45:179–187. [Google Scholar]
- Haugen TO, Vøllestad LA. A century of life-history evolution in grayling. Genetica. 2001;112–113:475–491. [PubMed] [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1971–1982. [Google Scholar]
- Heino M, Godø OR. Fisheries-induced selection pressures in the context of sustainable fisheries. Bulletin of Marine Science. 2002;70:639–656. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Heino M, Baulier L, Boukal DS, Dunlop ES, Eliassen S, Enberg K, Jørgenssen C, et al. Evolution of growth in Gulf of St. Lawrence cod? Proceedings of the Royal Society B. 2008;275:1111–1112. doi: 10.1098/rspb.2007.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn R, Minte-Vera CV. Fisheries-induced changes in growth rates in marine fisheries: Are they significant? Bulletin of Marine Science. 2008;83:95–105. [Google Scholar]
- Huse I, Gundersen AC, Nedreaas KH. Relative selectivity of Greenland halibut (Reinhardtius hippoglossoides, Walbaum) by trawls, longlines and gillnets. Fisheries Research. 1999;44:75–93. [Google Scholar]
- Huse I, Løkkeborg S, Soldal AV. Relative selectivity in trawl, longline and gillnet fisheries for cod and haddock. ICES Journal of Marine Science. 2000;57:1271–1282. [Google Scholar]
- Hutchings JA, Rowe S. Consequences of sexual selection for fisheries-induced evolution: an exploratory analyses. Evolutionary Applications. 2008;1:129–136. doi: 10.1111/j.1752-4571.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICES. 2007. Report of the ICES Advisory Committee on Fishery Management, Advisory Committee on the Marine Environment and Advisory Committee on Ecosystems, 2007. ICES Advice. Books 8, 147pp.
- Jacobson LD, Brodziak J, Rogers J. Depths distributions and time-varying bottom trawl selectivities for Dover sole (Microstomus pacificus), sablefish (Anoplopoma fimbria), and thornyheads (Sebastolobus alascanus and S. altivelis) in a commercial fishery. Fishery Bulletin. 2001;99:309–327. [Google Scholar]
- Jennings S, Kaiser MJ, Reynolds JD. Marine Fisheries Ecology. Oxford: Blackwell Science; 2001. [Google Scholar]
- Jørgensen T, Ingólfsson ÓA, Graham N, Isaksen B. Size-selection of cod by rigid grids–Is anything gained compared to diamond mesh codends only? Fisheries Research. 2006;79:337–348. [Google Scholar]
- Kuikka S, Hildén M, Gislason H, Hansson S, Sparholt H, Varis O. Modeling environmentally driven uncertainties in Baltic cod (Gadus morhua) management by Bayesian influence diagrams. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:629–641. [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology and Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kvamme C, Kuldbrandsen Føysa K. Assessing the effects on stocks of selectivity changes in a fishery. Fisheries Research. 2004;69:283–292. [Google Scholar]
- Law R. Fishing, selection and phenotypic evolution. ICES Journal of Marine Sciences. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- MacLennan DN. Gear selectivity and the variation of yield. ICES Journal of Marine Science. 1995;52:827–836. [Google Scholar]
- Madsen N, Holst R, Wileman D, Moth-Poulsen D. Size selectivity of sole gill nets fished in the North Sea. Fisheries Research. 1999;44:59–73. [Google Scholar]
- Marshall CT, McAdam BJ. Integrative perspectives on genetic and environmental effects on maturation can reduce potential error of inference. Marine Ecology Progress Series. 2007;335:301–310. [Google Scholar]
- Millar RB, Fryer RJ. Estimating the size-selection curves of towed gears, traps, nets and hooks. Reviews in the Fish Biology and Fisheries. 1999;9:89–116. [Google Scholar]
- Morita K, Fukuwaka M. Why age and size at maturity have changed in Pacific salmon. Marine Ecology Progress Series. 2007;335:289–294. [Google Scholar]
- Nedreaas KH, Soldal AV, Bjordal Å. Performance and biological implications of a multi-gear fishery for Greenland halibut (Reinhardtius hippoglossoides. Journal of Northwest Atlantic Fishery Science. 1993;19:59–72. [Google Scholar]
- Olsen ME, Heino M, Lilly GR, Morgam MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Özbilgin H, Tosunoglu Z, Tokac A, Metin G. Seasonal variation in trawl codend selectivity for annular sea bream (Diplodus annularis L., 1758) Turkish Journal of Veterinary & Animal Sciences. 2005;29:959–965. [Google Scholar]
- Özbilgin H, Ferro RST, Robertson JHB, Holtrop G, Kynoch RJ. Seasonal variation in trawl codend selection of northern North Sea haddock. ICES Journal of Marine Science. 2006;63:737–748. [Google Scholar]
- Parrish JK. Using behavior and ecology to exploit schooling fishes. Environmental Biology of Fishes. 1999;55:157–181. [Google Scholar]
- Ratner S, Lande R. Demographic and evolutionary responses to selective harvesting in populations with discrete generations. Ecology. 2001;82:3093–3104. [Google Scholar]
- De Roos AM, Boukal DS, Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proceeding of the Royal Society of London, Series B. 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffler C, Van Dooren TJM, Leimar O, Abrams PA. Disruptive selection and then what? Trends in Ecology and Evolution. 2006;21:238–245. doi: 10.1016/j.tree.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Santos MN, Canas A, Lino PG, Monteiro CC. Length-girth relationships for 30 marine fish species. Fisheries Research. 2006;78:368–373. [Google Scholar]
- Shumway CA. A neglected science: applying behavior to aquatic conservation. Environmental Biology of Fishes. 1999;55:183–201. [Google Scholar]
- Sinclair AF, Swain DP, Hanson JM. Measuring changes in the direction and magnitude of size-selective mortality in commercial fish population. Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:361–371. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS, Joyce JE. Genetic analyses of size in an anadromous population of pink salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:9–15. [Google Scholar]
- Stergiou KI, Erzini K. Comparative fixed gear studies in the Cyclades (Aegean Sea): size selectivity of small-hook longlines and monofilament gill nets. Fisheries Research. 2002;58:25–40. [Google Scholar]
- Stergiou KI, Moutopoulos DK, Erzini K. Gill net and longlines fisheries in Cyclades waters (Aegean Sea): species composition and gear competition. Fisheries Research. 2002;57:25–37. [Google Scholar]
- Stoner AW. Effects of environmental variables of fish feeding ecology: implications for the performance of baited fishing gear and stock assessment. Journal of Fish Biology. 2004;65:1445–1471. [Google Scholar]
- Suuronen P, Erickson DL, Orrensalo A. Mortality of herring escaping from pelagic trawl codends. Fisheries Research. 1996;25:305–321. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Heikkilä S, Wolter C, Klefoth T, Arlinghaus R. A behavioral perspective on fisheries-induced evolution. Trends in Ecology and Evolution. 2008;23:419–421. doi: 10.1016/j.tree.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Ward P. Empirical estimates of historical variations in the catchability and fishing power of pelagic longline fishing gear. Reviews in Fish Biology and Fisheries. 2008;18:409–426. [Google Scholar]
- Williams EH, Shertzer KW. Effects of fishing on growth traits: a simulation analyses. Fisheries Bulletin. 2004;103:392–403. [Google Scholar]
- Woll AK, Boje J, Holst R, Gundersen AC. Catch rates and hook and bait selectivity in longline fishery Greenland halibut (Reinhardtius hippoglossoides, Walbaum) at East Greenland. Fisheries Research. 2001;51:237–246. [Google Scholar]
- Zuur G, Fryer RJ, Ferro RST, Tokai T. Modeling the size selectivities of a trawl codend and an associated square mesh panel. ICES Journal of Marine Science. 2001;58:657–671. [Google Scholar]


