Abstract
Understanding the evolutionary processes that have shaped existing patterns of genetic diversity of reef-building corals over broad scales is required to inform long-term conservation planning. Genetic structure and diversity of the mass-spawning hard coral, Acropora tenuis, were assessed with seven DNA microsatellite loci from a series of isolated and discontinuous coastal and offshore reef systems in northwest Australia. Significant subdivision was detected among all sites (FST = 0.062, RST = 0.090), with the majority of this variation due to genetic differentiation among reef systems. In addition, genetic divergence was detected between the coastal and offshore zones that cannot be adequately explained by geographic distance, indicating that transport of larvae between these zones via large-scale oceanic currents is rare even over time frames that account for connectivity over multiple generations. Significant differences in the amount of genetic diversity at each system were also detected, with higher diversity observed on the lower latitude reefs. The implications are that these reef systems of northwest Australia are not only demographically independent, but that they will also have to rely on their own genetic diversity to adapt to environmental change over the next few decades to centuries.
Keywords: Acropora tenuis, gene flow, genetic connectivity, Leeuwin Current, management unit, mass-spawning corals, Northwest Australia, reef-building corals
Introduction
Coral reefs around the world are being degraded by an increasing range of disturbances which operate over various spatial and temporal scales (Nyström et al. 2000; Pandolfi et al. 2003). The ability of coral reefs to not only recover from, but also to adapt to, altered disturbance regimes is highly dependent upon the pattern and strength of connections among populations via dispersal of larvae – the mechanisms and consequences of which also vary in space and time. Contemporary dispersal of large numbers of larvae is important for the demographic recovery and persistence of populations and often operates over local scales (Gaines et al. 2007). Conversely, because geographic isolation among populations will eventually lead to unique genetic lineages, weaker historical connections are important for evolutionary processes affecting species distributions, genetic diversification, and adaptation (Fraser and Bernatchez 2001; Moritz 2002; Bowen and Roman 2005). Therefore, to mitigate the impacts of different disturbances on coral systems, an understanding of the multiple temporal and spatial scales of connectivity is required to inform appropriate management strategies (Kinlan et al. 2005; Cowen et al. 2007).
In the far northwest of Australia (NWA), recent genetic work on corals has focused on elucidating patterns of local-scale dispersal that primarily influence short-term persistence of populations (e.g. Whitaker 2004; Underwood et al. 2007, 2009), but consideration also needs to be given to how corals will respond to climate change and other anthropogenic impacts over the next few decades to centuries. This longer-term conservation planning requires an investigation of the evolutionary processes that have shaped the distribution of genetic diversity of coral reefs over broad scales (van Oppen and Gates 2006; Rocha et al. 2007).
Within the region of NWA, corals form discontinuous reef systems that are either coastal reefs adjacent to the mainland, or are isolated offshore reefs located along the margin of the continental shelf in the Timor Sea (Fig. 1). The continental shelf is more than 100 km wide north and east of Ningaloo Reef, and presumably small changes in sea-level would have altered the distribution of coral reefs that fringed this section of the mainland. For example, at low sea levels during the last glacial period (from about 110–18 000 years ago), most of the continental shelf was exposed, and any fringing coral reefs would have been much closer to the offshore reefs. However, there is currently no evidence pertaining to the existence of such fringing reefs, but offshore reefs probably existed during the late Tertiary–Quaternary (Collins 2002). In addition to sea level variation, changes in oceanic circulation and temperature undoubtedly influenced the distribution of coral reef species (Wyrwoll et al. in press). Therefore, while large changes in geographic position of fringing reefs must have occurred in recent geological history, it is not obvious whether the offshore reefs may also have come and gone, or whether genetic connections between the offshore and coastal zones were maintained during these changes.
Figure 1.
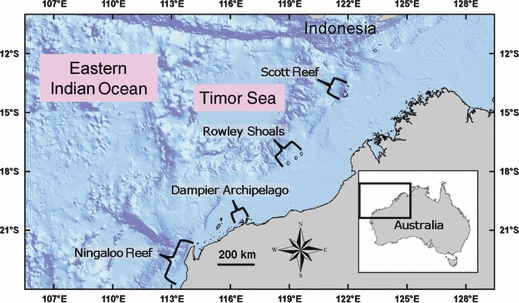
Map of northern Western Australia, showing the bathymetry of the continental shelf and the coastal and offshore coral reefs where genetic samples of A. tenuis were collected.
Until recently, it has been assumed that the offshore systems of NWA and the coastal systems further south and west are strongly connected by a poleward flowing current which originates in the Indonesian Throughflow and moves through the Timor Sea along the continental shelf margin (e.g. Veron 1995; Nof et al. 2002). This flow is strongest in autumn (April/May) (Holloway and Nye 1985; Holloway 1995), during the time of the major mass-spawning of corals and was thought to create unidirectional gene flow between the regionally separated coral reef systems (Simpson 1991). However, in the Timor Sea, this south westerly flow is broad and weak relative to the Leeuwin Current further south (Holloway 1995), and seasonal south-west winds induce a reversal of the current to the north-east in Spring (October/November) (Cresswell et al. 1993). Furthermore, recent oceanographic studies have not been able to identify a direct connection between these water masses and the Leeuwin Current which begins in earnest near the Ningaloo Reef coast at 22°S (Domingues et al. 2007; D'Adamo et al. in press).
Biogeographic evidence also indicates that connectivity between the coastal and offshore zones may be limited. Qualitative differences in species composition between these zones have been observed not only for scleractinian corals, but also for many marine floral and faunal species (B. Wilson, personal communication). Additionally, there is also a quantitative relationship of latitude with the distribution of scleractinian species, whereby species richness declines in systems that are further south (Veron 1995); this may be due to a lack of connectivity or to changes in habitat suitability for non-generalist species.
Patterns of genetic diversity within a species that occurs throughout this region would provide insights into the degree of historical connections between coastal and offshore reefs, systems and zones of NWA; large differences in genetic structure and diversity in abundant populations (i.e. large effective population sizes) from the offshore and coastal systems or zones would suggest that the long-term isolation has been an important influence on biodiversity in this region. Underwood et al. (2009) explored connectivity in the mass-spawning coral Acropora tenuis and the brooding coral Seriatopora hystrix within and between the two offshore systems of Scott Reef and Rowley Shoals, and showed that ecologically relevant gene flow is restricted between systems, between reefs within each system, and even within some reefs. However, while differences between the two systems were significant, the level of this differentiation for the mass-spawner was only moderate (FST = 0.034), suggesting that they are connected by rare dispersal events. Further research is required to assess whether the coastal systems in NWA are differentiated by similar levels.
In this study, I addressed the hypothesis that populations of a reef-building coral species with planktonic dispersal are panmictic in the NWA region. To this end, the distribution of genetic variation of microsatellite DNA markers in the mass-spawning coral Acropora tenuis (Dana) was measured to infer patterns of connectivity among reef systems in the offshore (Scott Reef and Rowley Shoals) and coastal (Dampier Archipelago and Ningaloo Reef) zones of NWA. The Dampier Archipelago is separated from the Rowley Shoals by a similar distance (∼400 km) as Rowley Shoals is from Scott Reef (Fig. 1). Ningaloo Reef is the other major coastal system of coral reefs in NWA, and the northern tip of this system is located about 300 km south-west of the Dampier Archipelago (Fig. 1). Thus, these two coastal systems provide an excellent opportunity to test whether genetic differences between the offshore and coastal zones are greater than differences within the two zones, unconfounded by the extent of geographic separation. Additionally, to gain further insight into degree of isolation, effective population size and the importance of asexual versus sexual reproduction, I test whether genetic and genotypic (clonal) diversities vary between the high-latitude, offshore reefs and the low latitude, coastal reefs.
Materials and methods
Study species
Acropora tenuis is a scleractinian coral with branching growth form that is abundant throughout the Indo-Pacific. As a reef-building coral, it helps form the three-dimensional structure of these ecosystems. Species of the genus Acropora dominate Indo-Pacific reefs, and sexual reproduction by broadcast spawning in most acroporids provides the opportunity for widespread dispersal. Buoyant eggs and sperm are released into the water column during synchronized mass-spawning events, and the entire larval development phase of days to weeks, is spent in the plankton (Wallace 1999). The maximum time larvae can survive in the water column (and still be competent to settle and metamorphose) affects the capacity for long-distance dispersal, and broadcast spawners have a wide range of maximum competence periods. For example, in one laboratory experiment, Acropora pulchra larvae could not successfully settle after 14 days, while Acropora valida were still competent after 110 days (Baird 2004). Maximum competencies of A. tenuis larvae are in the mid-range for mass-spawned larvae, with larvae surviving and settling up to 69 days in the laboratory (Nishikawa et al. 2003). Consequently, broad-scale patterns of connectivity of A. tenuis among the offshore and coastal reefs of NWA should be generally applicable to many mass-spawning corals.
Sampling, genotyping and analysis of clonal structure
Samples from 1156 colonies of Acropora tenuis were collected from six or seven sites at each of the Scott Reef, Rowley Shoals, Ningaloo Reef and the Dampier Archipelago (Fig. 1). More than 40 colonies were sampled from most sites (Table 1). Collections at each site were made within a 300 m by 50 m belt, and the location of each colony was recorded. The size of each colony was also recorded as the longest lineal dimension. Some differences existed between physical characteristics of the sites on the offshore and coastal reefs. On the offshore reefs of Scott Reef and Rowley Shoals, sample sites occurred on steep reef slopes. At the Dampier Archipelago, sample sites were located on reef slopes with much shallower gradients. At Ningaloo Reef, sites were either located on the reef crest or in lagoon channels, both of which are relatively flat. However, strong hydrodynamic connections to the outside of the reef are maintained through wind- and wave-generated currents (residence times within the lagoon are on the order of hours; Hearn and Parker 1988). These differences in habitat are unlikely to have influenced the genetic structure and diversity measured in this study because there is no significant local barrier to larval transfer between the areas of reef where the sites were located and the waters beyond (such as might occur in an enclosed lagoon). Furthermore, microsatellite markers are generally considered neutral (Estoup and Angers 1998), and therefore, habitat based selection is unlikely to have influenced genetic structure across seven loci.
Table 1.
Numbers of samples (N), unique multilocus genotypes (Ng), genotypic richness (Ng:N), and mean (mean LLD) and standard deviation (SD LLD) of the longest lineal dimension in centimetres of colonies of Acropora tenuis from sites in northwest Australia and the Great Barrier Reef
| System | Site | N | Ng | Ng:N | Mean LLD | SD LLD |
|---|---|---|---|---|---|---|
| Scott Reef | SL1 | 49 | 48 | 0.98 | 27.1 | 12.2 |
| SL2 | 32 | 32 | 1.00 | 33.5 | 11.2 | |
| SL4 | 50 | 49 | 0.98 | 28.7 | 13.9 | |
| SL5 | 49 | 49 | 1.00 | 37.1 | 15.2 | |
| SS1 | 48 | 46 | 0.96 | 16.1 | 8.1 | |
| SS2 | 50 | 49 | 0.98 | 22.9 | 33.4 | |
| SS3 | 23 | 22 | 0.96 | 23.8 | 11.0 | |
| Total | 301 | 295 | ||||
| Average | 0.98 | 27.0 | 7.0 | |||
| Rowley Shoals | RS1_1 | 50 | 50 | 1.00 | 25.7 | 11.6 |
| RS1_S | 49 | 49 | 1.00 | 31.3 | 17.2 | |
| RS2_2 | 50 | 50 | 1.00 | 34.1 | 15.2 | |
| RS2_S | 50 | 50 | 1.00 | 32.5 | 17.7 | |
| RS3_3 | 49 | 48 | 0.98 | 34.6 | 12.1 | |
| RS3_S | 27 | 27 | 1.00 | 26.6 | 10.6 | |
| Total | 275 | 274 | ||||
| Average | 1.00 | 30.8 | 3.8 | |||
| Dampier Archipelago | DCZ1 | 50 | 47 | 0.94 | 156.4 | 142.7 |
| DEN1 | 50 | 43 | 0.86 | 84.1 | 70.1 | |
| DEN2 | 25 | 12 | 0.48 | 130.4 | 139.1 | |
| DEN3 | 50 | 31 | 0.62 | 37.4 | 20.8 | |
| DWL1 | 22 | 21 | 0.95 | 38.7 | 19.7 | |
| DWL2 | 50 | 40 | 0.80 | 88.4 | 57.2 | |
| Total | 247 | 194 | ||||
| Average | 0.78 | 89.2 | 47.9 | |||
| Ningaloo Reef | NIN 1 | 50 | 29 | 0.58 | 61.9 | 46.4 |
| NIN 3 | 50 | 46 | 0.92 | 67.8 | 53.9 | |
| NIN 4 | 50 | 49 | 0.98 | 57.5 | 45.0 | |
| NIN 5 | 50 | 44 | 0.88 | 58.4 | 38.9 | |
| NIN 6 | 49 | 49 | 1.00 | 48.0 | 22.8 | |
| NIN 7 | 49 | 47 | 0.96 | 29.0 | 16.7 | |
| NIN 8 | 35 | 34 | 0.97 | 49.8 | 27.2 | |
| Total | 333 | 298 | ||||
| Average | 0.90 | 53.2 | 12.6 |
Care was taken to collect from a colony that was physically distinct and more than 1.5 m from other sampled colonies. This sampling regime, which was applied consistently at all sites, minimized multiple collections of the same genet that may have been produced through asexual fragmentation or propagation (ramets). Therefore, this provided an underestimate (but an unbiased underestimate) of the real contribution of asexual reproduction. Clonality was measured by calculating the proportion of unique multilocus genotypes as (Ng:N) at each site. The probability that two individual samples from the same site shared a multilocus genotype by chance, PID (calculated in GenAlEx v6), was very low, ranging from 3.3 × 10−6 to 2.7 × 10−4, with an average of 7.5 × 10−5. Further, the vast majority of samples that shared the same genotype were collected within 10 m of each other, providing solid evidence that each sample that shared the same diploid genotype were clone mates (ramets) belonging to the same genet. Therefore, apart from the analysis of clonal structure, each unique multilocus genotype was included only once in the subsequent analysis to ensure assessments of genetic structure and diversity were based on sexually produced colonies. Of the 1156 samples that were collected, 1061 had unique multilocus genotypes (final sample sizes at each site are given in Table 1).
The genotyping procedure was as follows. Genomic DNA was extracted with a DNeasy extraction kit for animal tissue (Qiagen, Melbourne, Australia) or by a standard salting out protocol (Digital Appendix S(‘0-9’+)). Data were collected from seven microsatellite loci developed from a genomic DNA library from Acropora millepora (van Oppen et al. 2007). Five of these loci were optimized for A. millepora and described by van Oppen et al. (2007), and two loci were optimized specifically for A. tenuis. Two multiplex polymerase chain reactions (PCRs) were performed per individual using fluorescently labelled primers (details of multiplex PCR, loci characteristics and GenBank accession numbers are given in Underwood et al. 2009). PCR products were analysed on a MegaBACE 1000 capillary sequencer (Amersham Biosciences, Sydney, Australia), and the resulting electropherograms were scored using the program MegaBACE GENETIC PROFILER 2.2 (Amersham Biosciences). To minimize genotyping errors, all automated scorings of alleles were checked manually, and uncertainties were cleared by re-amplification and comparison. Alleles were scored as the size of the PCR product in base pairs. A genotyping error rate of 2.68% was estimated per reaction at each loci according to Bonin et al. (2004), as the ratio between the observed number of allelic differences and the total number of allelic comparisons when 24 genotypes were repeated. This error rate is unlikely to effect significantly the results presented below because of the large sample sizes, and more importantly, because the analyses were applied at the level of population structure, but not at individual identity (Bonin et al. 2004). Tests for Hardy–Weinberg and linkage disequilibrium were conducted using FSTAT v2.9.3 (Goudet 1995), and significance levels were adjusted with sequential Bonferroni correction for multiple tests when P < 0.05. The Hardy–Weinberg test was based on 1000 permutations of alleles among individuals within sites and overall all sites using the inbreeding coefficient FIS.
Genetic structure analysis
To explore the historical genetic connections among sites and systems, an analysis of molecular variance (AMOVA) was used to measure the proportion of genetic variation that is geographically structured (Excoffier et al. 1992). This analysis partitioned the amount of genetic variation within and among sites with respect to different alleles (FST), and on the sum of squared size differences of the alleles, assuming a stepwise model of mutation (RST). Because the relative accuracy of FST and RST will vary according to the levels of population divergence and my data cover a range of levels, I present both statistics as recommended by Balloux and Lugon-Moulin (2002). Analysis was performed with GenAlEx v6 (Peakall and Smouse 2006) in three stages. First, the proportion of variation among sites within systems (FSR and RSR) was calculated with data from each system only. Second, the proportion of variation within systems (FSR and RSR), among systems (FRTA and RRTA), between the coastal and the offshore zone (FRTB and RRTB), and among all sites (FST and RST), was also calculated relative to the total variance of all the WA sites. Lastly FST and RST were calculated from the complete data set. Tests for statistical significance for all estimates were based on 1000 random permutations.
To quantify further the relationships among sites, I calculated three genetic distance measures between pairs of sites because they are calculated with independent methods: DLR, which compares the likelihoods of complete multilocus genotypes in two populations (Paetkau et al. 1995); DS, Nei's standard genetic distance (Nei 1972); and pairwise FST. DLR and DS are complimentary and independent estimates of genetic distance and performed well in studies that evaluated the effectiveness of different genetic distances (Takezaki and Nei 1996; Paetkau et al. 1997). DLR and DS were calculated with the online calculator found at (http://www2.biology.ualberta.ca/jbrzusto/Doh.php). Because differences in genetic diversity can influence the accuracy of DLR and DS (Paetkau et al. 1997), but FST is calculated relative to the observed variance in allelic frequencies and is therefore less affected by genetic diversity, pairwise FST estimates were also calculated as an additional and independent measure of genetic distance (in GenAlEx v6). To illustrate these genetic relationships among sites, I used a multidimensional approach; Principal Coordinates Analysis plots were constructed using GenAlEx v6 from the three pairwise genetic distances (matrices are given in Digital Appendix S(‘0-9’+)). Although it is common to use cluster analysis to present data from genetic distance matrices in the form of bifurcating trees, these trees often oversimplify the relationships between study areas (Paetkau et al. 1999) and have the potential to force patterns that do not exist (Zink and Barrowclough 2008). In contrast, Principal Coordinates Analysis has no a priori assumptions about structure.
Genetic diversity analysis
Genetic diversity measures were calculated with FSTAT v2.9.3 (Goudet 1995) as an unbiased estimate of gene diversity ‘HSK; described by Nei (1987)’ and allelic richness (RS) per locus and site. These measures adjust for unequal sample sizes. Average gene diversity and allelic richness at each northwest Australian system were calculated across all sites within each system. Significant differences in both measures of diversity among the WA systems (comparison among groups of samples) were tested by 1000 permutations of a randomized data set. While it is possible that, because markers were screened for polymorphism in samples collected from Scott Reef and Rowley Shoals, there may be some ascertainment bias towards higher levels of genetic diversity in these offshore reefs, this is unlikely to affect significantly my results for two reasons. First, in the development stage, I selected markers that were polymorphic (i.e. more than two alleles), but did not preferentially select highly polymorphic markers. Second, gene diversity and expected heterozygosity were high at a site on the Great Barrier Reef compared to the NWA sites (J.N. Underwood, unpublished data), providing an independent confirmation with samples that were not used in the initial marker development that this bias was not strong.
Results
Microsatellite marker characteristics
All seven loci were polymorphic at all sites except one (Amil2_010 at site Den2), and loci had an average of between 3 and 11 alleles per site and average expected heterozygosity of 0.5 across all sites and loci. Large and significant deficits of heterozygotes were detected in many populations and at most loci, but the coastal sites of Ningaloo Reef and the Dampier Archipelago exhibited markedly fewer departures (less than a third) from Hardy-Weinberg equilibrium compared with the offshore sites at the Scott Reef and Rowley Shoals systems (Digital Appendix S(‘0-9’+)). Further, no loci showed heterozygote deficits at all sites, providing evidence that null alleles were either not widespread, or varied in frequency. The common occurrence of heterozygote deficits in corals (Ayre and Dufty 1994; Ayre and Hughes 2000; Gilmour 2002; Mackenzie et al. 2004; Whitaker 2004; Nishikawa and Sakai 2005; Underwood et al. 2007), and marine species in general (Tracey et al. 1975; Johnson and Black 1982, 1984; Andrade and Solferini 2007) suggests that biological factors associated with spatial or temporal admixture and nonrandom mating within sites are the main determinant of these patterns. In particular, significant differentiation among size classes of A. tenuis was detected at many sites (J.N. Underwood, unpublished data), suggesting that Wahlund effects from mixing genetically distinct cohorts in the one sample are probably a major cause of the observed heterozygote deficits (e.g. Johnson and Black 1984). Therefore, because methods that estimate null alleles (e.g. MICRO-CHECKER; van Oosterhout et al. 2004) assume that heterozygote deficits are caused by null alleles only (van Oosterhout et al. 2006), but the evidence points to predominantly biological causes, I have not used these methods here. The heterozygote deficits were not accompanied by linkage disequilibrium among loci; of the 567 tests between loci at each site, only one remained significant after Bonferroni correction.
Clonal structure
Differences in clonal structure were detected between coastal and offshore sites. In contrast to the offshore reefs of Rowley Shoals and the Scott Reef systems, where the proportion of unique multilocus genotypes (Ng:N) was equal to or greater than 0.96 at all sites, Ng:N was less than 0.90 at 6 of the 13 coastal sites at the Dampier Archipelago and Ningaloo Reef (Table 1). Sites DEN2 and DEN3 from Dampier and NIN1 from Ningaloo exhibited particularly low Ng:N ratios of 48%, 62% and 58% respectively. Because the sampling regime was similar at all sites, these results show that asexual fragmentation has made a substantial contribution to recruitment in A. tenuis communities at these sites, even though sampled individuals were separated by more than 1.5 m. Size of colonies that were sampled also differed among sites; Dampier had on average larger colonies than Ningaloo, while colonies at Rowley Shoals and Scott Reef were smaller than those at Ningaloo (Table 1).
Genetic structure
Replicate (clonal) genotypes were excluded from the AMOVA analysis, which detected significant subdivision at all levels (Table 2), providing evidence of strong geographic structure of A. tenuis populations in NWA. Overall FST and RST values were 0.062 and 0.090. A large amount of this genetic variation was attributed to differentiation among systems (FRTA = 0.046, RRTA = 0.072), and most of this was due to differences between the coastal and offshore zones (FRTB = 0.041 and RRTB= 0.070). The Principal Coordinates analyses of the pairwise distances of DLR (Fig. 2A), DS (Fig. 2B) and FST (Fig. 2C) highlighted the differences between the coastal sites and offshore zones, with the coastal and offshore sites separating into two distinct clusters. These plots also showed that the genetic distances between the Ningaloo sites and the offshore sites were generally smaller compared with distances between the Dampier and offshore sites.
Table 2.
Hierarchical AMOVA calculated in GenAlEx with respect to different alleles (FST) and the sum of squared size differences of the alleles (RST)
| Scott | Rowleys | Dampier | Ningaloo | All | |
|---|---|---|---|---|---|
| FSR: within systems | 0.020*** | 0.025*** | 0.007* | 0.016*** | 0.017*** |
| FRTA: among systems | 0.046*** | ||||
| FRTB: among zones | 0.041*** | ||||
| FST: among all sites | 0.062*** | ||||
| RSR: within systems | 0.021*** | 0.031*** | 0.019** | 0.011** | 0.020*** |
| RRTA: among systems | 0.072*** | ||||
| RRTB: among zones | 0.070*** | ||||
| RST : among all sites | 0.090*** |
The proportion of variance was estimated in two steps: first, among sites within systems (FSR and RSR) calculated with data from each system only and with data from all northwest Australian sites; and second, among systems (FRTA and RRTA), between coastal (Dampier and Ningaloo) and offshore (Scott and Rowleys) zones (FRTB and RRTB) and among all sites (FST and RST) relative to the total variance of all the northwest Australian sites.
Tests for statistical significance were based on 1000 random permutations. Levels of statistical significance for the F- and R- values are indicated by *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
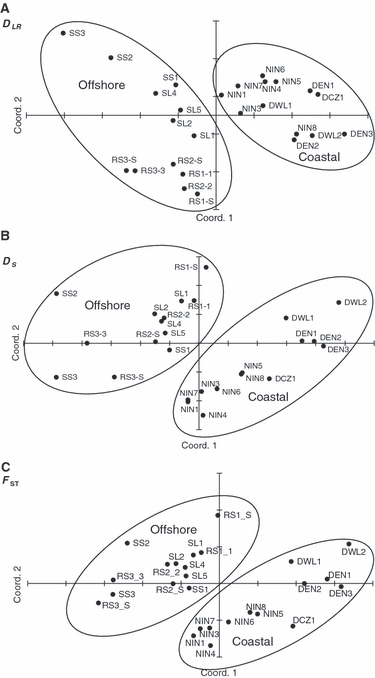
Plots of Principal Coordinate Analysis calculated in GenAlEx v6 from standardised distance matrix derived from pairwise DLR (A), DS (B) and FST (C) estimates between Acropora tenuis sites in northwest Australia. Site names begin with the first letter of each system. The first two axes explain 71% of the variation for DLR, 82% for DS and 89% for FST.
Genetic diversity
Gene diversity (HSK) and allelic richness (RS) varied significantly among the four coral reef systems (P = 0.001), and both measures showed congruent patterns. Specifically, Scott Reef had the highest genetic diversities, the Rowley Shoals and Ningaloo systems exhibited intermediate levels, and the Dampier sites had lowest diversities of all systems (Fig. 3).
Figure 3.
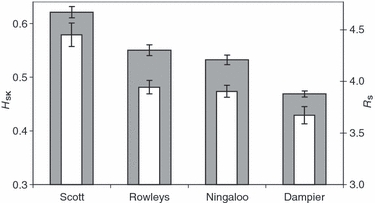
Average gene diversity (HSK; dark bars) and allelic richness (RS; light bars) of Acropora tenuis calculated across sites at each reef system in northwest Australia. Estimates account for unequal sample sizes and error bars are ±standard errors.
Discussion
Geographic discontinuity and genetic structure
Pronounced genetic differences in the mass-spawning coral Acropora tenuis were detected between the offshore and coastal reefs of NWA. While significant genetic subdivision was detected at all levels of analysis, most of the variation is due to differences between the coastal and offshore zones of NWA rather than to differences between systems within zones, or sites within systems (Table 2). Further, all three measures of genetic distance showed a congruent and clear divergence between the offshore and coastal sites (Fig. 2). Therefore, because geographic distances between zones are comparable to distances between systems within zones, these results show that the genetic connections between the offshore and coastal reefs via oceanic currents are much weaker than that expected if they were simply part of a discontinuous set of reefs.
Recent oceanographic studies have measured and modelled patterns of currents in the region of NWA and support the inference from the genetic data that connections are limited between the offshore and coastal reefs. Of seven satellite-tracked drifters (‘Davis’ type drifters that float in near the sea surface and are used to ascertain movement of the water column) released from the offshore reefs of NWA during the time of the mass-spawning in autumn and spring, most either drifted between the offshore systems or were moved off in a northwesterly direction, and none drifted within 150 km of the mainland coast (Gilmour et al. in press). Furthermore, a simulated particle model suggested that the Leeuwin Current draws much of its water from sources to the north and west of Ningaloo, and could not identify a continuous poleward current originating from the Indonesian Throughflow that flows along continental shelf of NWA (Domingues et al. 2007). Domingues et al. (2007) also indicated that particles in this region of the NWA are probably subject to intense vertical mixing from internal waves which is likely to compromise further the ability of long-distance transport of larvae via this route. Finally, average current speeds in this region are relatively slow, typically 0.2 ms−1 (Holloway 1995), which may mean that larvae produced at the Rowley Shoals would take a minimum of 40 days to get to the Dampier Archipelago, or more than 65 days to get to Ningaloo Reef. Even if larvae were to travel between the systems in a straight line, periods of 1–2 months are beyond the optimal competency periods of A. tenuis (Nishikawa et al. 2003), and most coral larvae (Wilson and Harrison 1998; Miller and Mundy 2003; Nozawa and Harrison 2005). Thus, the genetic data presented here, combined with the oceanographic evidence, indicate that dispersal of coral larvae between the offshore zone of Scott Reef and Rowley Shoals, and the coastal zone of the Dampier Archipelago and Ningaloo Reef, is absent over ecological time and rare over time frames that account for connectivity over many generations.
Evidence from other marine organisms suggests the potential for stronger connectivities over broad scales in NWA than those found here for A. tenuis. For example, levels of subdivision among populations of four species of fish, six bivalves, three gastropods, and one urchin, in this region were all less than in A. tenuis (Johnson et al. 1993). Although the geographic scales of these studies were similar or even greater than the present study, no systems were sampled that were as far offshore as Scott Reef and Rowley Shoals (i.e. on the edge of the continental shelf). Therefore, given that the majority of the geographic subdivision among A. tenuis populations was accounted for by differences between these offshore systems and the coastal systems, the results of Johnson et al. (1993) are not directly comparable to those presented here. However, the expectation is that coral reef species, such as fish that have planktivorous larvae with longer pelagic larval durations and a greater swimming ability than coral larvae, will have more extensive genetic connections among the coastal and offshore systems compared with those presented here for A. tenuis.
Geographic discontinuity and clonal structure
In addition to sexual reproduction that involves external fertilization of gametes, asexual reproduction in acroporid corals occurs through vegetative fragmentation via physical disturbance (Wallace 1999). High levels of clonality have been detected in several broadcast spawning corals (e.g. Resing and Ayre 1985; Ayre and Willis 1988; Ayre and Hughes 2000), and this is likely to be an important means of local proliferation in some systems (Wallace 1985). The findings of Baums et al. (2006) in their rigorous investigation of clonality in Acropora palmata in the western Atlantic are of particular relevance to this study. These researchers not only detected differences in clonality between genetically distinct provinces, but also found more clonality on inshore reefs that had a larger shelf area compared with offshore reefs. In the present study, more than 98% of A. tenuis colonies sampled from the offshore systems of Rowley Shoals and Scott Reef were produced sexually. In contrast, even though colonies were also sampled at distances of greater than 1.5 m on the coastal reefs, there clonal structuring was evident at all of the Dampier sites and at half of the Ningaloo sites (Table 1). Because offshore sites were located on steep reef slopes, while the coastal sites occurred on reefs with much shallower gradients, it is probable that coral fragments produced by storm or wave action are washed off the offshore reefs into deep water. Conversely, fragments are more likely to be retained at the coastal sites with flatter topographies, providing the opportunity for re-attachment during calmer conditions. Also congruent with Baums et al. (2006), colonies were larger at the coastal sites that exhibited clonal structure compared with the offshore sites (Table 1); this may provide a source of fragments if exposed to physical disturbances that are not strong enough to completely break up the large colonies.
Although a comprehensive investigation of the mechanisms that influence clonality in A. tenuis is beyond the scope of this study, the data presented demonstrates that asexual reproduction makes an important contribution to recruitment on some coastal reefs of NWA. This result may have important ramifications for the population dynamics of these reefs. For example, work on other partially clonal organisms suggests that populations with low clonal diversity recover slower after extreme climatic events (Reusch et al. 2005) and are more vulnerable to disease (Schmid 1994; Zhu et al. 2000).
Geographic discontinuity and genetic diversity
Significant differences in genetic diversity of A. tenuis were detected among the northwest Australian systems; Scott Reef has the highest gene diversity and expected heterozygosity, while Rowley Shoals and Ningaloo Reef have intermediate levels, and the Dampier Archipelago has the lowest (Fig. 3). Thus genetic diversity declined with increasing latitude, reflecting the decline in species diversity of scleractinian corals in NWA (Veron 1995). These results not only support the conclusion that connectivity is limited among reef systems, but also shed light on the degree of isolation, effective population sizes and histories of disturbance of these systems (see below).
The attenuation of genetic diversity with latitude observed here is not mirrored in tropical eastern Australia, where no differences in genetic diversity were detected along 1200 km of interconnected reefs of the Great Barrier Reef (Ayre and Hughes 2000). Both regions are dominated by poleward flowing warm-water currents, and sample areas in the two studies span almost identical degrees of latitude. However, reduced genetic diversity was observed in isolated, subtropical coral reefs south of the Great Barrier Reef (Ayre and Hughes 2004; Miller and Ayre 2008). Therefore, it seems that differences in genetic diversity detected in the present study are also influenced by the physical isolation of the NWA reef systems. In particular, because the continental shelf is relatively wide off the Dampier Archipelago (Fig. 1), exposure of these reefs to oceanic currents is likely to be limited or absent. This may explain why the Dampier sites exhibit the lowest genetic diversity (Fig. 3), and largest genetic distances from offshore sites (Fig. 2). In addition, because waters where A. tenuis occurs in the Dampier Archipelago are highly turbid compared with the other systems, effective population sizes of are likely to be smaller due to limitations on their depth distribution; this would also contribute to the low diversities and increased differentiation observed here.
The patterns of genetic diversity detected here also show that the catastrophic bleaching at Scott Reef in 1998, which killed more than 90% of corals surveyed (Smith et al. 2008), did not have a major effect on genetic diversity of A. tenuis. Given that reef patches at Scott Reef are genetically differentiated (Underwood et al. 2009), and that the vast majority of sampled colonies recruited after the bleaching (based on colony sizes), recovery must have been facilitated locally by corals that survived the bleaching on reef patches within the system. Therefore, a major bottleneck was avoided, and genetic diversity retained within the Scott Reef system. This conclusion is also supported by genetic data from the brooding coral Seriatopora hystrix, which also showed that the majority of postbleaching recruits came from the local area (Underwood et al. 2007), and that gene diversity was significantly higher at Scott Reef than at Rowley Shoals for this species (HSK at Scott Reef = 0.40, HSK at Rowley Shoals = 0.32; J.N. Underwood, unpublished data). Interestingly, the relative high level of genetic diversity at Scott Reef was equivalent to that observed from a reef in the central region of the Great Barrier Reef (J.N. Underwood, unpublished data), and is possibly a result of ecologically rare but evolutionarily important gene flow from the highly diverse reefs of Indonesia. Sampling from Indonesia is required to test this hypothesis.
Management implications
Placing these results in a management context, the genetic structure and diversity of the reef-building coral Acropora tenuis in NWA demonstrate that these reefs are a unique set of demographically independent systems that rely primarily on their own metapopulation dynamics for their population maintenance and for the generation of their genetic diversity. Therefore, recovery of coral populations in these systems will not be facilitated by input of exogenous recruits. In particular, the Dampier Archipelago seems to be the most isolated and genetically depauperate of the NWA systems, and is probably highly vulnerable to disturbances. Furthermore, the historical isolation of the coastal and offshore zones, in combination with the unique environments of each system (for example; the turbid, low energy water of the Dampier Archipelago, the colder, high energy waters of Ningaloo, or the clear, warm water of the offshore reefs), implies that each zone harbours a unique component of the genetic diversity of this coral reef species. Therefore, these reefs require preservation not only on ecological, but also on evolutionary, grounds. Effective conservation of these reefs will require the implementation of strategies that increase the resilience of coral communities within each system to cope with potential large-scale disturbances associated with climate change in the short term by reducing localized anthropogenic pressures such as elevated sediment loads, overfishing or habitat fragmentation. If such strategies are successful at each system, the unique genetic building blocks of this species will be preserved, which will provide the greatest opportunity for adaptation and survival in a rapidly changing environment over the next few decades to centuries.
Acknowledgments
Many thanks to Mike Johnson, Luke Smith, Madeleine van Oppen and James Gilmour for their supervision and support throughout this study. Thanks also to Ric and Kylie Gleadell, Jess Soma and Nat Rosser, who provided enthusiastic and enjoyable assistance in the field. The Department of Environment and Conservation (WA) and the Northwest Research Association provided field equipment and accommodation. The School of Animal Biology population genetics crew at the University of Western Australia, Fred Allendorf and two anonymous reviewers provided constructive comments on this manuscript. David Ayre and Michael Hellberg also gave valuable feedback on this work as part of the examination of my PhD thesis. The authors acknowledge the financial support of Woodside Energy Limited as operator of the Browse LNG Development Limited in the conduct of this research. The University of Western Australia and the Australian Coral Reef Society provided additional funding for this project.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Digital Appendix S1. Protocol for extracting DNA from coral fragments.
Digital Appendix S2. Details of the seven Acropora tenuis microsatellite markers from offshore reefs in NWA (a) and coastal reefs in NWA (b). Given are the number of alleles (A), the proportion of expected (HE) heterozygotes per locus and site, and the FIS calculated for each locus and each site (FIS All loci); numbers in bold indicate significant deviations from Hardy–Weinberg Equilibrium because of heterozygote deficits (P < 0.05) after sequential Bonferroni correction. Also given are average number of alleles per locus (A All loci), average expected heterozygosity for all loci at each site (HE All loci) and the number of private alleles (PVA) at each site.
Digital Appendix S3. Genetic distances of DLR, DS and FST between pairs of sites of the mass-spawning coral Acropora tenuis from Scott Reef, Rowley Shoals, Dampier Archipelago and Ningaloo Reef in northwest Australia.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature cited
- Andrade SC, Solferini VN. Fine-scale genetic structure overrides macro-scale structure in a marine snail: nonrandom recruitment, demographic events or selection? Biological Journal of the Linnean Society. 2007;91:23–36. [Google Scholar]
- Ayre DJ, Dufty S. Evidence for restricted gene flow in the viviparous coral Seriatopora hystrix on Australia's Great Barrier Reef. Evolution. 1994;48:1183–1201. doi: 10.1111/j.1558-5646.1994.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Ayre DJ, Hughes TP. Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution. 2000;54:1590–1605. doi: 10.1111/j.0014-3820.2000.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Ayre DJ, Hughes TP. Climate change, genotypic diversity and gene flow in reef-building corals. Ecology Letters. 2004;7:273–278. [Google Scholar]
- Ayre DJ, Willis BL. Population structure in the coral Pavona cactus: clonal genotypes show little phenotypic plasticity. Marine Biology. 1988;99:495–505. [Google Scholar]
- Baird AH. The ecology of coral larvae: settlement patterns, habitat selection and the length of the larval phase. Journal & Proceedings of the Royal Society of New South Wales. 2004;137:43. [Google Scholar]
- Balloux F, Lugon-Moulin N. The estimation of population differentiation with microsatellite markers. Molecular Ecology. 2002;11:155–165. doi: 10.1046/j.0962-1083.2001.01436.x. [DOI] [PubMed] [Google Scholar]
- Baums IB, Miller MW, Hellberg ME. Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecological Monographs. 2006;76:503–519. [Google Scholar]
- Bonin A, Bellemain E, Bronken Eidesen P, Pompanon F, Brochmann C, Taberlet P. How to track and assess genotyping errors in population genetics studies. Molecular Ecology. 2004;13:3261–3273. doi: 10.1111/j.1365-294X.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- Bowen BW, Roman J. Gaia's Handmaidens: the Orlog Model for conservation biology. Conservation Biology. 2005;19:1037–1043. [Google Scholar]
- Collins LB. Tertiary foundations and quaternary evolution of the coral reef systems of Australias North West Shelf. In: Keep M, Moss SJ, editors. The Sedimentary Basins of Western Australia 3: Proceedings of the Petroleum Exploration Society of Australia. Perth, Australia: Petroleum Exploration Society of Australia; 2002. pp. 129–152. [Google Scholar]
- Cowen RK, Gawarkiewicz G, Pineda J, Thorrold SR, Werner FE. Population connectivity in marine systems an overview. Oceanography. 2007;20:3–21. [Google Scholar]
- Cresswell G, Frische A, Peterson J, Quadfasel D. Circulation in the Timor Sea. Journal of Geophysical Research. 1993;98:14379–14389. [Google Scholar]
- D'Adamo N, Fandry C, Buchan SJ, Domingues C. Northern sources of the Leeuwin Current, and associated circulation on the North West Shelf. Journal of the Royal Society of Western Australia. in press. [Google Scholar]
- Domingues CM, Maltrud ME, Wijffels SE, Church JA, Tomszak M. Simulated Lagrangian pathways between the Leeuwin Current System and the upper-ocean circulation of the southeast Indin Ocean. Deep-Sea Research Part II Topical Studies in Oceanography. 2007;54:797–817. [Google Scholar]
- Estoup A, Angers B. Microsatellites and minisatellites for molecular ecology: theoretical and empirical considerations. In: Carvalho G, editor. Advances in Molecular Ecology. Amsterdam: IOS Press; 1998. pp. 55–86. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction sites. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DJ, Bernatchez L. Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Molecular Ecology. 2001;10:2741–2752. [PubMed] [Google Scholar]
- Gaines SD, Gaylord B, Gerber LRG, Hastings A, Kinlan BP. Connecting places. The ecological consequences of dispersal in the sea. Oceanography. 2007;20:90–99. [Google Scholar]
- Gilmour J. Substantial asexual recruitment of mushroom corals contributes little to population genetics of adults in conditions of chronic sedimentation. Marine Ecology Progress Series. 2002;235:81–91. [Google Scholar]
- Gilmour JP, Smith LD, Brinkman RM. Biannual spawing, rapid larval development and evidence of self-seeding for corals on an isolated system of reefs. Marine Biology. in press. [Google Scholar]
- Goudet J. Fstat (version 1.2): a computer program to calculate F-statistics. Journal of Heredity. 1995;86:485–486. [Google Scholar]
- Hearn CJ, Parker IN. Hydrodynamic processes on the Ningaloo coral reef, Western Australia. Proceedings of the 6th International Coral Reef Symposium. 1988;2:497–502. [Google Scholar]
- Holloway PE. Leeuwin current observations on the Australian North West Shelf, May–June 1993. Deep-Sea Research Part I Oceanographic Research Papers. 1995;42:285–305. [Google Scholar]
- Holloway PE, Nye HC. Leeuwin Current and wind distributions on the southern part of the Australian North West Shelf between January 1982 and July 1983. Australian Journal of Marine and Freshwater Research. 1985;36:123–137. [Google Scholar]
- Johnson MS, Black R. Chaotic genetic patchiness in an intertidal limpet, Siphonaria sp. Marine Biology. 1982;70:157–164. [Google Scholar]
- Johnson MS, Black R. The Wahlund effect and the geographical scale of variation in the intertidal limpet Siphonaria sp. Marine Biology. 1984;79:295–302. [Google Scholar]
- Johnson MS, Hebbert DR, Moran MJ. Genetic analysis of populations of northwestern Australian fish species. Marine and Freshwater Research. 1993;44:673–685. [Google Scholar]
- Kinlan BP, Gaines SD, Lester SE. Propagule dispersal and the scales of marine community process. Diversity and Distributions. 2005;11:139–148. [Google Scholar]
- Mackenzie JB, Munday PL, Willis BL, Miller DJ, Van Oppen MJH. Unexpected patterns of genetic structuring among locations but not colour morphs in Acropora nasuta (Cnidaria; Scleractinia) Molecular Ecology. 2004;13:9–20. doi: 10.1046/j.1365-294x.2003.02019.x. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Ayre DJ. Protection of genetic diversity and maintenance of connectivity among reef corals within marine proteced areas. Conservation Biology. 2008;22:1245–1254. doi: 10.1111/j.1523-1739.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- Miller K, Mundy C. Rapid settlement in broadcast spawning corals: implications for larval dispersal. Coral Reefs. 2003;22:99–106. [Google Scholar]
- Moritz C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Systematic Biology. 2002;51:238–254. doi: 10.1080/10635150252899752. [DOI] [PubMed] [Google Scholar]
- Nei M. Genetic distance between populations. American Naturalist. 1972;106:283–292. [Google Scholar]
- Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press; 1987. [Google Scholar]
- Nishikawa A, Sakai K. Genetic connectivity of the scleractinian coral Goniastrea aspera around the Okinawa Islands. Coral Reefs. 2005;24:318–323. [Google Scholar]
- Nishikawa A, Masaya K, Kazuhiko S. Larval settlement rates and gene flow of broadcast-spawning (Acropora tenuis) and planula-brooding (Stylophora pistillata) corals. Marine Ecology Progress Series. 2003;256:87–97. [Google Scholar]
- Nof D, Pichevin T, Sprintall J. “Teddies” and the origin of the Leeuwin Current. Journal of Physical Oceanography. 2002;32:2571–2588. [Google Scholar]
- Nozawa Y, Harrison PL. Temporal settlement patterns of larvae of the broadcast spawning reef coral Favites chinensis and the broadcast spawning and brooding reef coral Goniastrea aspera from Okinawa, Japan. Coral Reefs. 2005;24:274–282. [Google Scholar]
- Nyström M, Folke C, Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends in Ecology & Evolution. 2000;15:413–417. doi: 10.1016/s0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout C, Hutchinson W, Wills D, Shipley P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes. 2004;4:535–538. [Google Scholar]
- Van Oosterhout C, Weetman D, Hutchinson W. Estimation and adjustment of microsatellite null alleles in nonequilibrium populations. Molecular Ecology Notes. 2006;6:255–256. [Google Scholar]
- Van Oppen MJH, Gates RD. Conservation genetics and the resilience of reef-building corals. Molecular Ecology. 2006;15:3863–3883. doi: 10.1111/j.1365-294X.2006.03026.x. [DOI] [PubMed] [Google Scholar]
- Van Oppen MJH, Underwood JN, Muirhead AN, Peplow L. Ten microsatellite loci for the reef-building coral Acropora millepora (Cnidaria, Scleractinia) from the Great Barrier Reef, Australia. Molecular Ecology Notes. 2007;7:436–438. [Google Scholar]
- Paetkau D, Calvert W, Sterling I, Strobeck C. Microsatellite analysis of population structure in Canadian polar bears. Molecular Ecology. 1995;4:347–354. doi: 10.1111/j.1365-294x.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Waits LP, Clarkson PL, Craighead L, Strobeck C. An empirical evaluation of genetic distance statistics using microsatellite data from bear (Ursidae) populations. Genetics. 1997;147:1943–1957. doi: 10.1093/genetics/147.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau D, Amstruo SC, Born EW, Calvert W, Derocher AE, Garner GW, Messier F, et al. Genetic structure of the world's polar bear populations. Molecular Ecology. 1999;8:1571–1584. doi: 10.1046/j.1365-294x.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resing JM, Ayre DJ. The usefulness of tissue grafting bioassays as an indicator of coral identity in Scerlactinian corals. Proceeding of the 5th International Coral Reef Symposium. 1985;6:75. [Google Scholar]
- Reusch BH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha LA, Craig MT, Bowen BW. Phylogeography and the conservation of coral reef fishes. Coral Reefs. 2007;26:501–512. [Google Scholar]
- Schmid B. Effects of genetic diversity in experimental stands of Solidago altissima: evidence for the potential role of pathogens as selective agents in plant-populations. Journal of Ecology. 1994;82:165–175. [Google Scholar]
- Simpson CJ. Mass spawning of corals on Western Australian reefs and comparisons with the Great Barrier Reef. Journal of the Royal Society of Western Australia. 1991;74:85–91. [Google Scholar]
- Smith LS, Gilmour JP, Heyward AJ. Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs. 2008;27:197–205. [Google Scholar]
- Takezaki N, Nei M. Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics. 1996;144:389–399. doi: 10.1093/genetics/144.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey ML, Bellet NF, Gravem CD. Excess allozyme homozygosity and breeding population structure in the mussel Mytilus californianus. Marine Biology. 1975;32:303–311. [Google Scholar]
- Underwood JN, Smith LD, Van Oppen MJH, Gilmour JP. Multiple scales of genetic connectivity in a brooding coral on isolated reefs following catastrophic bleaching. Molecular Ecology. 2007;16:771–784. doi: 10.1111/j.1365-294X.2006.03187.x. [DOI] [PubMed] [Google Scholar]
- Underwood JN, Smith LD, Van Oppen MJH, Gilmour JP. Ecologically relevant dispersal of a brooding and a broadcast spawning coral at isolated reefs: implications for managing community resilience. Ecological Applications. 2009;19:18–29. doi: 10.1890/07-1461.1. [DOI] [PubMed] [Google Scholar]
- Veron JEN. Corals in Space and Time. Sydney: University of New South Wales; 1995. [Google Scholar]
- Wallace C. Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Marine Biology. 1985;88:217–233. [Google Scholar]
- Wallace C. Staghorn Corals of the World: A Revision of the Coral Genus Acropora. Collingwood, Vic: CSIRO Publishing; 1999. [Google Scholar]
- Whitaker K. Non-random mating and population genetic subdivision of two broadcasting corals at Ningaloo Reef, Western Australia. Marine Biology. 2004;144:593–603. [Google Scholar]
- Wilson JR, Harrison PL. Settlement-competency periods of larvae of three species of scleractinian corals. Marine Biology. 1998;131:339–345. [Google Scholar]
- Wyrwoll K-H, Greenstein B, Kendrick G, Chen G-S. The paleoceanography of the Leeuwin Current. Journal of the Royal Society of Western Australia. in press. [Google Scholar]
- Zhu YY, Chen HR, Fan JH, Wang YY, Li Y, Chen JB, Fan JX, et al. Genetic diversity and disease control in rice. Nature. 2000;406:718–722. doi: 10.1038/35021046. [DOI] [PubMed] [Google Scholar]
- Zink RM, Barrowclough GF. Mitochondrial DNA under siege in avian phylogeography. Molecular Ecology. 2008;17:2107–2121. doi: 10.1111/j.1365-294X.2008.03737.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


