Abstract
Harvesting of marine resources raises concerns about how to identify and preserve biocomplexity, including the diversity of life histories found within and among wild populations of a species. In order to fully accomplish this, there is a need to elucidate the underlying causes of phenotypic variation, and how this variation responds to environmental changes. In general, both evolutionary (genetic) and nonevolutionary (plastic) responses may occur. Plastic responses to environmental change are expected to shift the phenotype along a reaction norm, while an evolutionary response is expected to shift the reaction norm itself. Here, we assess the maturation patterns of coastal Atlantic cod (Gadus morhua) in Skagerrak, where studies using neutral markers have revealed genetically differentiated populations of this harvested fish within tens of kilometres of coastline. Our results suggest that physiological state prior to the spawning season, as well as juvenile growth, both influence the probability of completing sexual maturation at a given age. Furthermore, our results point towards a spatial structuring of this plasticity (i.e. the maturation reaction norms) comparable with population connectivity inferred from neutral markers. We argue that such fine-scale biocomplexity calls for a Darwinian approach to fisheries management.
Keywords: Atlantic cod, biocomplexity, condition, genetic structure, maturation reaction norm, phenotypic plasticity
Introduction
Throughout the world, fish stocks are threatened by overharvesting, and there is growing concern that fishing also has negative effects on stock productivity through evolutionary processes (Conover and Munch 2002; Reznick and Ghalambor 2005). Fishing may erode genetic variation (Hutchinson et al. 2003) and select for traits associated with low or unstable productivity, such as reduced age and body size at maturation in otherwise long-lived species (Olsen et al. 2004a), and also reduced somatic growth rates (Swain et al. 2007). Biocomplexity, including intraspecific diversity in fitness-related traits such as patterns of migration, maturation and spawning, is expected to improve fisheries sustainability (Hilborn et al. 2003). For example, biocomplexity in Pacific salmon is manifested by a network of local populations, where overall stock productivity is maintained through periods of shifting environmental conditions because certain geographic- and life-history components perform well under some environmental conditions whereas other components perform well under other environmental conditions (Hilborn et al. 2003). By tailoring fishery effort to target the most productive components at any given point in time, all geographic- and life-history components are conserved, which ensures the long-term sustainability of the overall stock complex (Hilborn et al. 2003).
Biocomplexity may be defined along three axes: spatial heterogeneity, connectivity and historical contingencies (Cadenasso et al. 2006). The spatial component of biocomplexity may involve adaptations to local environmental conditions (Hilborn et al. 2003). Indeed, such spatially structured evolution depends on the interplay between heritability (history), gene flow (connectivity) and local selection regimes (heterogeneity) (Lenormand 2002; Garant et al. 2007). The marine environment should offer ample opportunities for homogenizing gene flow through passive dispersal of eggs and larvae with ocean currents and active movement of older individuals (Waples 1998). Yet, studies on neutral molecular markers have shown that marine species with highly mobile life stages can be structured into local populations on a surprisingly small scale (e.g. Jorde et al. 2007). Retention of pelagic early life stages and homing of adult individuals have been identified as important mechanisms influencing the scale of population connectivity in marine systems (Jones et al. 1999; Thorrold et al. 2001; Cowen et al. 2006). Evidence for locally adapted marine populations is emerging (Conover and Present 1990; Bricelj et al. 2005; Marcil et al. 2006), but little is known about the spatial scales of adaptive variation and how this is associated with the spatial scales of gene flow (but see, Hutchings et al. 2007).
Life-history traits are often highly plastic in response to environmental change (Alm 1959). Thus, a major challenge is to separate genetic influences from environmental influences on phenotypic variation. For this purpose, reaction norms, defined as the set of phenotypes produced by a genotype over a range of environmental conditions (Schmalhausen 1949), are helpful. A plastic response to environmental change is expected to shift the phenotype along the reaction norm, whereas an evolutionary change in life history is expected to alter the reaction norm itself (Ernande et al. 2004). Recently, the reaction norm approach has been applied to long-term survey data in order to explore whether fisheries are causing evolutionary changes in fish life histories. As predicted from theory (Stearns and Koella 1986; Ernande et al. 2004), observations from several fish stocks indicate that maturation reaction norms have shifted towards younger age and smaller size (Barot et al. 2004a; Olsen et al. 2004a) in response to size-selective harvesting.
Here, we assess the complexity of maturation patterns in Atlantic cod (Gadus morhua) from the Norwegian Skagerrak coast. Earlier work on maturation patterns in fishes focus mainly on quantifying the probability of maturing at a certain age and body length (e.g. Olsen et al. 2004a; but see, Grift et al. 2007). Age and body length together determine the growth trajectory of the fish and hence this probabilistic maturation reaction-norm approach (Heino et al. 2002; Dieckmann and Heino 2007) describes phenotypic plasticity in maturation linked to any feature of the environment that influences growth (e.g. temperature and food availability). Still, age and body length are not expected to explain all variation in maturation (Morita and Fukuwaka 2006), and a consistent trend in the environment that is not captured by variation in growth might bias maturation reaction norms estimated from field data (Law 2007). Therefore, we have added fish condition as an additional dimension in the reaction norm, realizing that animals should not start reproducing when their physiological state (condition) is below a certain critical level (McNamara and Houston 1996). We acknowledge that maturation reaction norms derived from field data will inevitably be associated with some uncertainty, as compared with common garden studies performed under standardized laboratory conditions, but we will argue that the probabilistic maturation reaction-norm approach is nevertheless valuable for studies on biocomplexity.
Skagerrak coastal cod are a good candidate for identifying biocomplexity because there is evidence for local populations on a scale of only a few tens of kilometres, revealed as genetic differentiation in presumed selectively neutral loci (Knutsen et al. 2003; Jorde et al. 2007). Using the probabilistic maturation reaction norm approach, we report evidence for spatial structuring of coastal cod life histories associated with patterns in population structure inferred from neutral markers. We argue that management strategies should aim to protect such diversity in fitness-oriented traits that are likely to influence stock productivity: that is, a Darwinian approach to fisheries science and management (Conover and Munch 2002; Jørgensen et al. 2007).
Methods
The Skagerrak coastal cod
Coastal Skagerrak cod have a relatively short life cycle, and may spawn already at an age of 2 years and a body length of about 30 cm (Olsen et al. 2004b). Traditional analyses of the proportion of mature fish at a specific age and body length (maturity ogives) have identified significant spatial variation in life histories (Olsen et al. 2004b), but it is unknown to what extent the observed phenotypic variation merely reflects plastic responses to environmental differences or genetic responses resulting from evolutionary processes. Coastal cod from eastern parts of Skagerrak have suffered a near collapse during the past 20 years, presumably due to local overfishing (Svedäng and Bardon 2003).
Sampling of coastal cod maturation data
Coastal cod were sampled for maturation data during an annual autumn survey (November–December) with trammel gillnets within five regions of the Norwegian Skagerrak coast (Table 1, Fig. 1). Each year (2001–2006), the same set of gillnets (45 mm mesh size) was set at the same well-defined sites. The nets were set in the evening and hauled during the morning, fishing for about 15 h in shallow near-shore waters (5–15 m depth). Gender and maturity stage were determined for each sampled fish via macroscopic inspections of the gonads. Total body length was measured to the nearest centimetre. Liver wet weight was measured to the nearest 0.1 g. Somatic weight was not measured. Age was determined from otoliths (Dannevig 1933). A fish was considered to be age 0 the year of hatching, age 1 the next year, and so on. Because the Skagerrak coastal cod spawn mainly in late winter, we added 1 year to the age at capture (in the autumn) to obtain age during spawning season.
Table 1.
Skagerrak coastal cod sampled for life history characteristics: sample sizes (N), mean age (range), mean total body length (range) and mean liver wet weight (range).
| Sampling site | N | Age (years) | Length (cm) | Liver weight (g) |
|---|---|---|---|---|
| Mandal | 535 | 2.3 (1–8) | 43 (17–88) | 41 (1–261) |
| Høvåg | 848 | 2.2 (1–9) | 41 (17–88) | 35 (1–280) |
| Kragerø | 552 | 2.2 (1–10) | 35 (16–78) | 21 (1–181) |
| Vasser | 302 | 2.0 (1–9) | 31 (14–92) | 13 (1–186) |
| Fredrikstad | 242 | 1.8 (1–5) | 31 (14–75) | 10 (1–103) |
Figure 1.
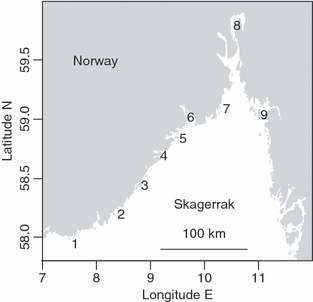
Study area. Coastal cod sampled from the Norwegian Skagerrak coast: Mandal (1), Høvåg (2), Bjelland (3), Risør (4), Kragerø (5), Grenland (6), Vasser (7), Oslo (8) and Fredrikstad (9). Site 1, 2, 5, 7 and 9 were sampled for life history traits, while site 2, 3, 4, 6, 8 and 9 were sampled for neutral markers.
Statistical modelling of cod maturation data
Condition is defined as the energy available for life-history decisions such as growth and maturation (Koops et al. 2004). Because Atlantic cod store most of their lipid energy reserves in the liver (Lambert and Dutil 1997), we therefore used liver weight as a measure of the fish’s condition.
We modelled plasticity in maturation of age 2 cod only – the youngest age at which we observed maturing fish (with the exception of three individuals that may have matured at age 1). Older fish with maturing gonads could be either recruit-spawners or repeat-spawners, and including such fish could confound analyses on the actual maturation process. Because the age distribution of the coastal cod was dominated by young fish (Table 1) these omissions do not seriously reduce the samples available for analyses. Note that, in some cases, maturation probability can be estimated even if age at first reproduction is unknown but this method is not robust when fewer than about 100 individuals are sampled from a cohort at a certain age (Barot et al. 2004b). The method was therefore not applicable to our small samples of older cod. Also, by focussing on age 2 cod, we avoid the potential confounding effect of skipped spawning, referring to the phenomenon that sexually mature fish do not necessarily reproduce every year (Rideout and Rose 2006). A mature fish that skips a spawning season might be wrongly classified as a juvenile. The probability of maturing, m, was estimated using a logistic regression model:
| 1 |
where s denotes the site, g the gender, LT the total body length and WL the liver wet weight. Sample site and gender were modelled as factors, while body length and liver weight were modelled as linear effects. Further details about using a logistic regression approach for estimating maturation reaction norms are provided by Heino et al. (2002). Model (1) was selected based on its AIC score (Burnham and Anderson 1998) when compared with a set of a priory candidate models (Table 2).
Table 2.
Comparison of logistic regression models for estimating maturation probability (m) of 2-year-old Skagerrak coastal cod, showing the explanatory variables and interaction effects (model structure), the residual deviance (dev), the residual degrees of freedom (Df) and the AIC value of each model.
| Model structure | Dev | Df | AIC |
|---|---|---|---|
| SLT + GWL + LTWL + S + G + LT + WL | 1124.3 | 1286 | 1152.3 |
| SG + SLT + SWL + GLT + GWL + LTWL + S + G + LT + WL | 1111.4 | 1277 | 1157.4 |
| SLT + GWL + S + G + LT + WL | 1137.9 | 1287 | 1163.9 |
| SLT + LTWL + S + G + LT + WL | 1127.0 | 1287 | 1153.0 |
| SLT + LTWL + S + LT + WL | 1161.4 | 1288 | 1185.4 |
| GWL + LTWL + S + G + LT + WL | 1135.8 | 1290 | 1155.8 |
| GWL + LTWL + G + LT + WL | 1277.2 | 1294 | 1289.2 |
| SLT + S + G + LT + WL | 1139.4 | 1288 | 1163.4 |
| SLT + S + G + LT | 1170.2 | 1289 | 1192.2 |
| SLT + S + LT | 1200.6 | 1290 | 1220.6 |
| GWL + S + G + LT + WL | 1161.7 | 1291 | 1179.7 |
| GWL + G + LT + WL | 1294.2 | 1295 | 1304.2 |
| LTWL + S + G + LT + WL | 1138.1 | 1291 | 1156.1 |
| LTWL + S + LT + WL | 1171.1 | 1292 | 1187.1 |
| LTWL + LT + WL | 1304.8 | 1296 | 1312.8 |
| S + G + LT + WL | 1162.5 | 1292 | 1178.5 |
| S + G + LT | 1184.7 | 1293 | 1198.7 |
| S + LT + WL | 1192.7 | 1293 | 1206.7 |
| G + LT + WL | 1296.5 | 1296 | 1304.5 |
| S + LT | 1214.0 | 1294 | 1226.0 |
| G + LT | 1304.2 | 1297 | 1310.2 |
| LT + WL | 1318.1 | 1297 | 1324.1 |
| LT | 1325.4 | 1298 | 1329.4 |
The response variable is logit(m/1 − m). Explanatory variables are site of capture (S), gender (G), total body length (LT) and liver wet weight (WL). S and G are modelled as factors while LT and WL are modelled as linear effects. The model selected for inference is shown in bold (model 1). A global model containing all two-way interaction effects is shown for reference (model 2). Models without an effect of LT on m were considered biologically unrealistic and hence not included among the candidate models.
Sampling and analyses of cod population genetic data
Earlier studies on the Skagerrak cod have provided evidence for fine-scale neutral genetic population structure; based on the genetic analysis of mature cod collected during several years (Knutsen et al. 2003; Jorde et al. 2007). Here, our aim is (i) to identify potential regions of genetic discontinuity (barriers) along this coastline and (ii) to compare these regions with the spatial pattern of adaptive structure revealed by the estimated maturation reaction norms. The genetic analyses of population connectivity along the Norwegian Skagerrak coast were based on data from Knutsen et al. (2003). Briefly, cod from six localities (Table 3) were sampled during the spawning season (January–March) in 2000 and scored for genetic variability at 10 microsatellite loci (Gmo2, Gmo3, Gmo19, Gmo34, Gmo35, Gmo36, Gmo37, Gmo132, Tch12 and Tch13). The genetic samples are thus from the same coastline from which the present life-history samples are derived (Fig. 1). Here, we reanalyse these microsatellite DNA data in order to test for potential heterogeneity in the strength of the neutral genetic structure along the coastline. First, we used the exact test in the genepop software (Raymond and Rousset 1995) to test each locality for genetic difference from the other five sample sites pooled together, one locus at a time and jointly over all 10 loci. Second, genetic differences among all pairs of samples were calculated using estimator θ of Weir and Cockerham (1984), both separately for each locus and averaged over all 10 loci (Table 4). The resulting matrices with pairwise estimates of genetic differences were applied in the barrier software (Manni et al. 2004) to detect and visualize geographic regions of genetic discontinuity, i.e. ‘barriers’ to gene flow. The relative strength of each barrier is evaluated from the rank of barriers calculated from the matrix of average θ estimates (Table 4), and we report the three strongest barriers (labelled a, b and c, in order of rank). This number of barriers was chosen because preliminary analyses indicated that only three barriers have any support in the data. The statistical support for the three barriers were evaluated by bootstrapping over loci, i.e. we used 10 single-locus matrices as input for barrier and reported the number of times each of the three barriers were included among the three strongest ones. This number represents the number of loci supporting each barrier (Jørgensen et al. 2005). Note that the maturation data and the microsatellite data were not collected from the exact same localities, but rather as independent samples throughout this coastline (Fig. 1). Accordingly, we do not aim to infer about genetic differences in maturation patterns directly from the neutral genetic population structure. Rather, our aim is to compare the spatial scales of adaptive and neutral divergence, because these processes do not necessarily occur on similar temporal and spatial scales (Conover et al. 2006).
Table 3.
Summary statistics for genetic samples, with average estimates over 10 microsatellite loci.
| Sampled locality | Sample size | Average a | Average H | Average FIS | P-value |
|---|---|---|---|---|---|
| (2) Høvåg | 100 | 12.7 | 0.687 | −0.0019 | 0.183 |
| (3) Bjelland | 92 | 13.3 | 0.692 | 0.0163 | 0.289 |
| (4) Risør | 101 | 13.1 | 0.691 | −0.0038 | 0.390 |
| (6) Grenland | 100 | 12.8 | 0.668 | 0.0085 | 0.482 |
| (8) Oslo | 109 | 13.5 | 0.679 | −0.0033 | 0.225 |
| (9) Fredrikstad | 109 | 13.1 | 0.684 | 0.0313 | 0.076 |
a, number of alleles per locus; H, gene diversity (expected heterozygosity); FIS, departure from Hardy–Weinberg genotype proportions [P-values calculated using exact probability test in genepop (Raymond and Rousset 1995) and summed over loci using Fisher’s procedure]. Data are from Knutsen et al. (2003).
Table 4.
Estimated genetic differentiation at 10 microsatellite loci (θ: Weir and Cockerham 1984) among pairs of genetic samples, and tests for allele frequency differences [exact tests for population differentiation in genepop (Raymond and Rousset 1995) summed over loci using Fisher’s procedure].
Results
Considering age 2 cod, the probability of maturing at that age depended on both body length and liver weight (Fig. 2, Table 5). The effects of body length and liver weight were not additive, as seen in the nonlinearity of the isolines of specific maturation probabilities (Fig. 2). For small-to-intermediate age 2 cod (up to about 40 cm), there was a strong and positive effect of increasing liver weight on the probability of maturing, although the exact shape of this relationship depended on geographic location and gender (Fig. 2, Table 5). For larger age 2 cod, the effect of increasing liver weight appeared less clear (Fig. 2). The probability of maturing at age 2 was shifted towards small body size and poor liver weight in northeast Skagerrak as compared with southwest Skagerrak, while cod from the two sample sites in the central region of this coastline had intermediate and similar maturation schedules (Fig. 2). Cod from the two sites in the southwest region also had quite similar maturation schedules, and different from other regions (Fig. 2). Condition of cod was quite poor in northeast Skagerrak as compared with southwest Skagerrak (Fig. 2). The probability of maturing at age 2 was shifted towards small body size and poor liver weight in males as compared with females (Fig. 2).
Figure 2.
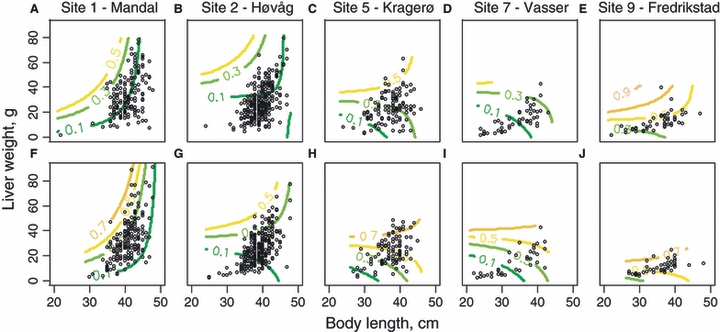
Maturation reaction norms estimated for 2-year-old female and male coastal cod, showing the predicted maturation probability (model 1, contour lines) and the observed body length and liver weight (○) for each site: (A) Mandal females, (B) Høvåg females, (C) Kragerø females, (D) Vasser females, (E) Fredrikstad females, (F) Mandal males, (G) Høvåg males, (H) Kragerø males, (I) Vasser males and (J) Fredrikstad males.
Table 5.
Parameter estimates, with SE, of the model (1) selected for inference about coastal cod maturation probability.
| Model term | Parameter estimate | SE | P-value |
|---|---|---|---|
| Intercept | −3.30 | 1.63 | 0.04 |
| SMandalLT | −0.078 | 0.079 | 0.32 |
| SLillesandLT | 0.099 | 0.076 | 0.19 |
| SKragerøLT | 0.103 | 0.065 | 0.11 |
| SVasserLT | 0.138 | 0.092 | 0.13 |
| GMalesWL | 0.018 | 0.011 | 0.11 |
| LTWL | −0.005 | 0.002 | 0.0009 |
| SMandal | 0.677 | 3.028 | 0.82 |
| SLillesand | −6.663 | 2.909 | 0.02 |
| SKragerø | −5.038 | 2.429 | 0.04 |
| SVasser | −6.708 | 3.432 | 0.05 |
| GMales | 0.395 | 0.337 | 0.24 |
| LT | 0.066 | 0.045 | 0.14 |
| WL | 0.245 | 0.065 | 0.0002 |
Explanatory variables are site of capture (S), gender (G), total body length (LT) and liver wet weight (WL). The Hvaler site and female cod were coded as zero in the model.
We also attempted to fit our maturation model (1) to the data on age 3 cod. However, this caused problems with parameter identifiability due to sparse data. As an alternative, we fitted a simper model containing only additive effects of length and liver weight (pooling males, females and sampling sites). This model indicated that there was still an overall positive effect of both length (0.052, SE = 0.021) and liver weight (0.011, SE = 0.004) on the probability of spawning (as differentiated from the probability of maturing, see Methods section).
The analyses of genetic differentiation with the barrier software indicate that the strongest genetic discontinuity separates the western localities (1 and 2) from the remaining ones, because two barriers (a and c) were located in this region (Fig. 3A) and were supported by 3 and 6 loci, respectively. The third barrier (b) appears in the eastern part of the coastline, separating the easternmost locality (9) from the others. Although this barrier has fairly low bootstrap support (3 loci), the easternmost sample is significantly different from the others, as judged by the low P-value in tests for allele frequency heterogeneity (P = 0.035, for the joint test over all 10 loci). The allele frequency tests also ‘explain’ the other barriers and identify site 2 in the southwest and site 6 in the central region as particularly divergent at microsatellite loci (Psite2 = 0.007, Psite6 = 0.014).
Figure 3.
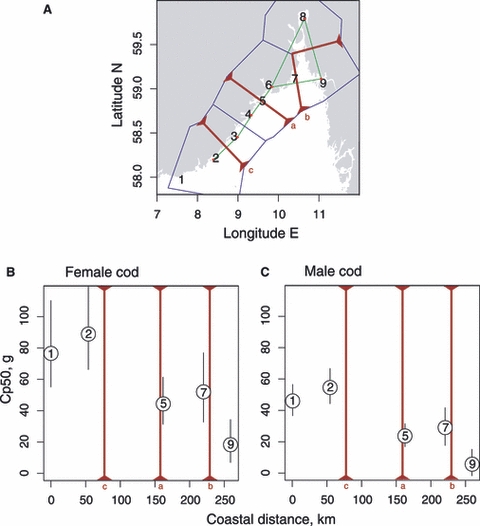
Genetic discontinuity, or ‘barriers’ to gene flow (red bars) in coastal cod, letters indicating relative strength (A) and numbers indicating sampling localities (Fig. 1). Maturation reaction norm midpoints (Cp50s), referring to the site-specific condition (liver weight) where a 50% probability of maturing is reached for 2-year-old female (B) and male (C) cod at mean body length (38 cm); ‘barriers’ to gene flow (red bars) superimposed.
There is an apparent association between spatial genetic structuring in the microsatellite data and the spatial pattern of maturation reaction norms (Fig. 3B–C). Specifically, the estimated maturation reaction norms of cod from the two westernmost localities (1 and 2) are similar to each other and differ significantly from the remaining localities (Fig. 3B–C). The two westernmost localities are also separated from all other localities to the east by two microsatellite barriers (Fig. 3B–C). Likewise, the outlying maturation reaction norm estimated for the easternmost site (9) is in agreement with the estimated barrier to gene flow separating this area from the remaining part of the coast (Fig. 3B–C).
Discussion
We have assessed biocomplexity in coastal Atlantic cod, a broadcast-spawning marine fish, and found evidence for a fine-scale spatial diversity in life histories that is associated with patterns of population connectivity based on neutral genetic markers. By estimating probabilistic maturation reaction norms (Heino et al. 2002; Dieckmann and Heino 2007) statistically controlling for environmental plasticity in growth (size at age) as well as energy reserves (liver weight 2–3 months before spawning), our finding is indicative of an underlying genetic component to the spatial complexity in maturation patterns. We discuss to what extent confounding environmental factors could still have influenced our results.
We found an association between spatial structure in life histories and population structure inferred from independent data on microsatellite DNA. Note that we did not formally correlate gene flow and adaptive divergence (Hendry and Taylor 2004). This is because of the complexity of gene flow in this system, where a component of gene flow is probably from oceanic North Sea cod and into the coastal populations (as planktonic larvae) rather than among coastal populations (Knutsen et al. 2004; Stenseth et al. 2006). We presume, however, that observed genetic differences among coastal populations imply some degree of isolation, from both the North Sea and from other coastal populations. Spawning and retention of eggs and larvae within fjord basins sheltered from coastal currents is a likely mechanism for maintaining genetic differentiation among the local populations (Knutsen et al. 2007), in combination with high site fidelity and natal homing of older cod (Espeland et al. 2007; Svedäng et al. 2007). The finding that cod from two pairs of neighbouring localities had very similar maturation reaction norms indicates that we have roughly identified the spatial scale of biocomplexity along this coastline. Our data thus suggest that life-history divergence is maintained on a scale of tens of kilometres in coastal cod, in agreement with estimates of local population ranges calculated from microsatellite DNA divergence patterns and capture–recapture data (Jorde et al. 2007). This life-history divergence is maintained despite gene flow suggested to constitute up to nearly 10% of the local age 0 populations (Stenseth et al. 2006). Earlier studies on spatial patterns of adaptive divergence in marine organisms have focussed on broader spatial patterns (Conover and Present 1990; Bricelj et al. 2005; Olsen et al. 2005; Marcil et al. 2006), although in the tropical sea anemone (Condylactis gigantean) there is evidence for adaptive genetic differentiation among phenotypes on reef habitats only 5 km apart (Stoletzki and Schierwater 2005). A recent study on Atlantic herring (Clupea harengus) reported evidence of correlated neutral genetic structure and spawning seasons and spawning location in the North Sea (Ruzzante et al. 2006). Our study differs from that by Ruzzante et al. (2006) by assessing biocomplexity on a smaller spatial scale and by statistically controlling for known environmental influences on life histories, so that the remaining pattern is indicative of a genetic component. Also, a recent common garden laboratory study on Atlantic cod from the northwest Atlantic have revealed population differences in early life-history plasticity on a broader spatial scale (Hutchings et al. 2007).
Cod from northeast Skagerrak has suffered a near collapse in abundance during the past 20 years, presumably due to overfishing (Svedäng and Bardon 2003). A displacement of the maturation reaction norms towards small body size and poor condition in northeast Skagerrak induced by high fishing pressure would fit predictions from theory (Stearns and Koella 1986; Ernande et al. 2004). Still, the relative importance of natural versus anthropogenic causes of life-history divergence in coastal cod remains largely unknown. Note also that the heritability of maturation reaction norms in Atlantic cod remains to be quantified, but there is some evidence for additive genetic variation in fish phenotypic plasticity (Haugen and Vøllestad 2000; Hutchings et al. 2007).
The shapes of the estimated reaction norms indicate that phenotypic plasticity influences the maturation probability of cod through both somatic growth history and current energetic state. Specifically, an increased liver weight is associated with a marked increase in the probability of sexual maturation of small and young cod. This finding that suggests a state-based approach is needed in order to understand how individuals optimize their life histories (McNamara and Houston 1996). An improvement in the energy available for life-history decisions (i.e. the condition) apparently speeds up the life history of the coastal cod through reproduction at a young age. For comparison, Marteinsdottir and Begg (2002) found a positive effect of condition on the probability of being mature in Atlantic cod. Recently, Grift et al. (2007) modelled the life-history transition from the juvenile to the mature state (as distinguished from the probability of being mature) in North Sea plaice (Pleuronectes platessa), and found a positive effect of condition on maturation probability. Plaice condition was estimated from data on body length and total weight sampled during the spawning season, and the apparent condition of spawners will therefore likely be elevated due to the inclusion of gonad weight in total body weight. Our study adds to these findings by estimating condition directly from liver weight – the main energy store in gadoids – measured some months ahead of spawning. We also found that the estimated maturation reaction norms of male cod were shifted towards small body size and poor liver weight, as compared with female reaction norms. Hence, male cod apparently have a (genetic) tendency to mature relatively early in life even in the face of poor environmental conditions (see also, Olsen et al. 2005). It is likely that selection pressures differ between females and males. For female cod, a large body size will offer a fitness advantage through improved fecundity and offspring quality (Trippel 1998). Also, Skjæraasen et al. (2006) found a marked positive effect of energy reserves 3–4 months before spawning on the fecundity of Norwegian coastal cod. For male cod, the fitness advantage of a large size appears less clear (Rakitin et al. 2001), although there is evidence that sperm production is positively linked to food availability and condition (Yoneda and Wright 2005).
The maturation reaction norms could be influenced by other sources of plasticity, in addition to size at age and energy reserves. In fact, the original maturation reaction norms based on body size and age were termed probabilistic, as age and size will typically not explain all variation in maturation (Heino et al. 2002; Dieckmann and Heino 2007). For instance, recent growth history, rather than size at age, could influence the maturation probability (Morita and Fukuwaka 2006). However, we consider it likely that energy reserves prior to spawning (accounted for in the present study) will capture much of the same environmental influences as recent growth history. Also, bias will only become a problem if there is a consistent trend in the environment over time or between localities that is not captured by the reaction norm. Common garden studies, where fish are raised under standardized laboratory conditions, are generally regarded as the most powerful technique for inferring about plasticity and evolution (e.g. Conover and Present 1990; Hutchings et al. 2007). For comparison, the probabilistic maturation reaction norm approach will involve added uncertainty about confounding environmental variables, but, on the other hand, this method allows for comparing life histories of long-lived species in a wide range of natural situations that are difficult or impossible to reproduce under controlled conditions. Furthermore, the depleted state of many marine fish stocks, including Atlantic cod in the North Sea region (Cook et al. 1997), have encouraged us to ask how new statistical tools can help to generate knowledge from data that are already at hand.
Our study highlights the need to manage biocomplexity within harvested marine species such as the coastal cod. As argued by Hilborn et al. (2003), Wroblewski et al. (2005) and Ruzzante et al. (2006), sustainability may be compromised by a generalized management regime not accounting for intraspecific genetic diversity. In particular, our study supports Hutchings et al. (2007), pointing out the need to manage adaptive phenotypic plasticity in life histories. Selection pressures imposed by humans might affect genetic diversity both directly through selective (or nonselective) harvesting and indirectly through environmental change. If so, what would then be the most appropriate management actions? Ferrière et al. (2004) advocates the need for evolutionary conservation biology where the evolutionary process itself is managed, because this is what will ensure the maintenance of diversity in the long run. For coastal cod, this would mean controlling harvest regimes so as to minimize maladaptive genetic effects of harvesting on the local populations, and also to protect the diversity of coastal environments where the local populations are found. There are probably several ways in which these goals could be reached, but Berkley et al. (2004) concludes that the best way to ensure old-growth age structure (i.e. selection) and complex spatial structure in populations of groundfish is through interconnected networks of marine reserves. Similarly, a modelling study by Baskett et al. (2005) suggests that marine reserves can indeed help to counter maladaptive fisheries-induced evolutionary changes in traits such as age and size at maturation. Management approaches that operate on larger spatial scales (i.e. an overall reduction of fishing pressure) might help to counter unwanted genetic changes but still involves a risk of depleting local gene pools. In addition, managers could shape the selectivity of fisheries through gear regulations (e.g. mesh size of gillnets). Wroblewski et al. (2005) argue that a combination of fisher’s ecological-knowledge, investigative science and a management system that promotes local community stewardship may help to protect the diversity of coastal cod stocks. As an example, Wroblewski et al. (2005) describe the process towards the establishment of a marine protected area encompassing a local inshore cod stock in Gilbert Bay, Canada.
In conclusion, this study presents evidence for small-scale biocomplexity in a harvested marine fish, the Atlantic cod, and advocates the need for a Darwinian perspective on fisheries management (Conover and Munch 2002) aimed at protecting such diversity.
Acknowledgments
This work was financed by the Research Council of Norway and through the EU Interreg IIIA initiative. We thank S. M. Carlson and A. Hendry for valuable comments, and K. Enersen and S. E. Enersen for skilful technical assistance during sampling and preparation of data.
Literature cited
- Alm G. Connection between maturity, size and age in fishes. Report of the Institute for Freshwater Research Drottningholm. 1959;40:5–145. [Google Scholar]
- Barot S, Heino M, O’Brien L, Dieckmann U. Long-term trend in the maturation reaction norm of two cod stocks. Ecological Applications. 2004a;14:1257–1271. [Google Scholar]
- Barot S, Heino M, O’Brien L, Dieckmann U. Estimating reaction norms for age and size at maturation when age at first reproduction is unknown. Evolutionary Ecology Research. 2004b;6:659–678. [Google Scholar]
- Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecological Applications. 2005;15:882–901. [Google Scholar]
- Berkley SA, Hixon MA, Larson RJ, Love MS. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries. 2004;29:23–32. [Google Scholar]
- Bricelj VM, Connell L, Konoki K, MacQuarrie SP, Scheuer T, Catterall WA, Trainer VL. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature. 2005;434:763–767. doi: 10.1038/nature03415. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Inference. New York: Springer; 1998. [Google Scholar]
- Cadenasso ML, Pickett STA, Grove JM. Dimensions of ecosystem complexity: heterogeneity, connectivity and history. Ecological Complexity. 2006;3:1–12. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Conover DO, Present TMC. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia. 1990;83:316–324. doi: 10.1007/BF00317554. [DOI] [PubMed] [Google Scholar]
- Conover DO, Clarke LM, Munch SB, Wagner GN. Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. Journal of Fish Biology. 2006;69(Suppl. C):21–47. [Google Scholar]
- Cook RM, Sinclair A, Stefánsson G. Potential collapse of North Sea cod stocks. Nature. 1997;385:521–522. [Google Scholar]
- Cowen RK, Paris CB, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- Dannevig A. On the age and growth of the cod (Gadus callarias L.) from the Norwegian Skagerrack coast. Report on Norwegian Fishery and Marine Investigations. 1933;4:1–145. [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London Series B. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland SH, Gundersen AF, Olsen EM, Knutsen H, Gjøsæter J, Stenseth NC. Home range and elevated egg densities within an inshore spawning ground of coastal cod. ICES Journal of Marine Science. 2007;64:920–928. [Google Scholar]
- Ferrière R, Dieckmann U, Couvet D. Evolutionary Conservation Biology. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Garant D, Forde SE, Hendry AP. The multifarious effects of dispersal and gene flow on contemporary adaptation. Functional Ecology. 2007;21:434–443. [Google Scholar]
- Grift RE, Heino M, Rijnsdorp AD, Kraak SBM, Dieckmann U. Three-dimensional maturation reaction norms for North Sea plaice. Marine Ecology Progress Series. 2007;334:213–224. [Google Scholar]
- Haugen TO, Vøllestad LA. Population differences in early life-history traits in grayling. Journal of Evolutionary Biology. 2000;13:897–905. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Taylor EB. How much of the variation in adaptive divergence can be explained by gene flow? An evaluation using lake-stream stickleback pairs. Evolution. 2004;58:2319–2331. doi: 10.1111/j.0014-3820.2004.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Hilborn R, Quinn TP, Schindler DE, Rogers DE. Biocomplexity and fisheries sustainability. Proceedings of the National Academy of Sciences of the USA. 2003;100:6564–6568. doi: 10.1073/pnas.1037274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Swain DP, Rowe S, Eddington JD, Puvanendran V, Brown JA. Genetic variation in life-history reaction norms in a marine fish. Proceedings of the Royal Society of London Series B. 2007;274:1693–1699. doi: 10.1098/rspb.2007.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson WF, Van Oosterhout C, Rogers SI, Carvalho GR. Temporal analysis of archived samples indicates marked genetic changes in declining North Sea cod (Gadus morhua. Proceedings of the Royal Society of London Series B. 2003;270:2125–2132. doi: 10.1098/rspb.2003.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GP, Milicich MJ, Emslie MJ, Lunow C. Self-recruitment in a coral reef fish population. Nature. 1999;402:802–804. [Google Scholar]
- Jorde PE, Knutsen H, Espeland SH, Stenseth NC. Spatial scale of genetic structuring in coastal cod Gadus morhua and geographic extent of local populations. Marine Ecology Progress Series. 2007;343:229–237. [Google Scholar]
- Jørgensen HBH, Hansen MM, Bekkevold D, Ruzzante DE, Loeschcke V. Marine landscapes and population genetic structure of herring (Clupea harengus L.) in the Baltic Sea. Molecular Ecology. 2005;14:3219–3234. doi: 10.1111/j.1365-294X.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Knutsen H, Jorde PE, André C, Stenseth NC. Fine-scaled geographic population structuring in a highly mobile marine species: the Atlantic cod. Molecular Ecology. 2003;12:385–394. doi: 10.1046/j.1365-294x.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- Knutsen H, André C, Jorde PE, Skogen MD, Thuróczy E, Stenseth NC. Transport of North Sea cod larvae into the Skagerrak. Proceedings of the Royal Society of London Series B. 2004;271:1337–1344. doi: 10.1098/rspb.2004.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen H, Olsen EM, Ciannelli L, Espeland SH, Knutsen JA, Simonsen JH, Skreslet S, Stenseth NC. Egg distribution, bottom topography and small scale population structure in a coastal marine system. Marine Ecology Progress Series. 2007;333:249–255. [Google Scholar]
- Koops MA, Hutchings JA, McIntyre TM. Testing hypotheses about fecundity, body size and maternal condition in fishes. Fish and Fisheries. 2004;5:120–130. [Google Scholar]
- Lambert Y, Dutil J-D. Can simple condition indices be used to monitor and quantify seasonal changes in the energy reserves of Atlantic cod (Gadus morhua)? Canadian Journal of Fisheries and Aquatic Sciences. 1997;54(Suppl 1):104–112. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends in Ecology and Evolution. 2002;17:183–189. [Google Scholar]
- Manni F, Gùerard E, Heyer E. Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by “Monmonier’s algorithm”. Human Biology. 2004;76:173–190. doi: 10.1353/hub.2004.0034. [DOI] [PubMed] [Google Scholar]
- Marcil J, Swain DP, Hutchings JA. Countergradient variation in body shape between two populations of Atlantic cod (Gadus morhua. Proceedings of the Royal Society of London Series B. 2006;273:217–223. doi: 10.1098/rspb.2005.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteinsdottir G, Begg GA. Essential relationships incorporating the influence of age, size and condition on variables required for estimation of reproductive potential in Atlantic cod Gadus morhua. Marine Ecology Progress Series. 2002;235:235–256. [Google Scholar]
- McNamara JM, Houston AI. State-dependent life histories. Nature. 1996;380:215–221. doi: 10.1038/380215a0. [DOI] [PubMed] [Google Scholar]
- Morita K, Fukuwaka M-A. Does size matter most? The effect of growth history on probabilistic reaction norm for salmon maturation. Evolution. 2006;60:1516–1521. [PubMed] [Google Scholar]
- Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004a;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Knutsen H, Gjøsæter J, Jorde PE, Knutsen JA, Stenseth NC. Life-history variation among local populations of Atlantic cod from the Norwegian Skagerrak coast. Journal of Fish Biology. 2004b;64:1725–1730. [Google Scholar]
- Olsen EM, Lilly GR, Heino M, Morgan MJ, Brattey J, Dieckmann U. Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:811–823. [Google Scholar]
- Rakitin A, Ferguson MM, Trippel EA. Male reproductive success and body size in Atlantic cod Gadus morhua L. Marine Biology. 2001;138:1077–1085. [Google Scholar]
- Raymond M, Rousset F. GENEPOP (Version-1.2) Population genetics software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:791–801. [Google Scholar]
- Rideout RM, Rose GA. Suppression of reproduction in Atlantic cod Gadus morhua. Marine Ecology Progress Series. 2006;320:267–277. [Google Scholar]
- Ruzzante DE, Mariani S, Bekkevold D, André C, Mosegaard H, Clausen LAW, Dahlgren TG, et al. Biocomplexity in a highly migratory pelagic marine fish, Atlantic herring. Proceedings of the Royal Society of London Series B. 2006;273:1459–1464. doi: 10.1098/rspb.2005.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhausen II. Factors of Evolution: the Theory of Stabilizing Selection. Philadelphia: Blakiston; 1949. [Google Scholar]
- Skjæraasen JE, Nilsen T, Kjesbu OS. Timing and determination of potential fecundity in Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:310–320. [Google Scholar]
- Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits: prediction of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Stenseth NC, Jorde PE, Chan K-S, Hansen E, Knutsen H, André C, Skogen MD, Lekve K. Ecological and genetic impact of Atlantic cod larval drift in the Skagerrak. Proceedings of the Royal Society of London Series B. 2006;273:1085–1092. doi: 10.1098/rspb.2005.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletzki N, Schierwater B. Genetic and color morph differentiation in the Caribbean sea anemone Condylactis gigantean. Marine Biology. 2005;147:747–754. [Google Scholar]
- Svedäng H, Bardon G. Spatial and temporal aspects of the decline in cod (Gadus morhua L.) abundance in the Kattegat and eastern Skagerrak. ICES Journal of Marine Science. 2003;60:32–37. [Google Scholar]
- Svedäng H, Righton D, Jonsson P. Migratory behaviour of Atlantic cod Gadus morhua: natal homing is the prime stock-separating mechanism. Marine Ecology Progress Series. 2007;345:1–12. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society of London Series B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorrold SR, Latkoczy C, Swart PK, Jones CM. Natal homing in a marine fish metapopulation. Science. 2001;291:297–299. doi: 10.1126/science.291.5502.297. [DOI] [PubMed] [Google Scholar]
- Trippel EA. Egg size and viability and seasonal offspring production of young Atlantic cod. Transactions of the American Fisheries Society. 1998;127:339–359. [Google Scholar]
- Waples RS. Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species. Journal of Heredity. 1998;89:438–450. [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Wroblewski J, Neis B, Gosse K. Inshore stocks of Atlantic cod are important for rebuilding the east coast fishery. Coastal Management. 2005;33:411–432. [Google Scholar]
- Yoneda M, Wright PJ. Effect of temperature and food availability on reproductive investment of first-time spawning male Atlantic cod, Gadus morhua. ICES Journal of Marine Science. 2005;62:1387–1393. [Google Scholar]


