Abstract
Captive breeding programs are increasingly being initiated to prevent the imminent extinction of endangered species and/or populations. But how well can they conserve genetic diversity and fitness, or re-establish self-sustaining populations in the wild? A review of these complex questions and related issues in salmonid fishes reveals several insights and uncertainties. Most programs can maintain genetic diversity within populations over several generations, but available research suggests the loss of fitness in captivity can be rapid, its magnitude probably increasing with the duration in captivity. Over the long-term, there is likely tremendous variation between (i) programs in their capacity to maintain genetic diversity and fitness, and (ii) species or even intraspecific life-history types in both the severity and manner of fitness-costs accrued. Encouragingly, many new theoretical and methodological approaches now exist for current and future programs to potentially reduce these effects. Nevertheless, an unavoidable trade-off exists between conserving genetic diversity and fitness in certain instances, such as when captive-bred individuals are temporarily released into the wild. Owing to several confounding factors, there is also currently little evidence that captive-bred lines of salmonids can or cannot be reintroduced as self-sustaining populations. Most notably, the root causes of salmonid declines have not been mitigated where captive breeding programs exist. Little research has also addressed under what conditions an increase in population abundance due to captive-rearing might offset fitness reductions induced in captivity. Finally, more empirical investigation is needed to evaluate the genetic/fitness benefits and risks associated with (i) maintaining captive broodstocks as either single or multiple populations within one or more facilities, (ii) utilizing cryopreservation or surrogate broodstock technologies, and (iii) adopting other alternatives to captive-rearing such as translocations to new habitats. Management recommendations surrounding these issues are proposed, with the aim of facilitating meta-analyses and more general principles or guidelines for captive-breeding. These include the need for the following: (i) captive monitoring to involve, a priori, greater application of hypothesis testing through the use of well-designed experiments and (ii) improved documentation of procedures adopted by specific programs for reducing the loss of genetic diversity and fitness.
Keywords: captive breeding, genetic diversity, domestication selection, reintroduction, conservation, salmon
Introduction
Because of increasing environmental impacts from human activities, a growing number of captive breeding programs are being initiated to salvage endangered species and/or populations from extinction (IUCN 1998, 2006; Seddon et al. 2007; Frankham 2008). Historically, many of these programs have been met with considerable difficulty (Philippart 1995; Snyder et al. 1996; Wolf et al. 1996; Frankham 2008). Yet, despite the extensive resources and labor that captive breeding programs require, few studies have thoroughly investigated the following: (i) how well current captive breeding procedures might recover endangered populations, (ii) to what extent particular genetic factors might hinder or help the success of captive breeding, and (iii) alternative solutions to captive breeding for endangered species and/or population recovery.
Here, I critically investigate these issues as they pertain to how well captive breeding programs involving fish hatcheries can conserve salmonid diversity, a group of well-studied and socio-economically important fish species native to the northern hemisphere. In a time when the remarkable diversity within salmonid species has been recognized legally for its import to species’ persistence and adaptability (Waples 1995; Irvine et al. 2005), salmonid populations in many regions of their native ranges are experiencing unprecedented population declines and/or low levels of natural recruitment. Human activities implicated in salmonid declines include overexploitation, habitat loss from logging, agriculture, damming and urbanization, environmental change related to climate warming, stocking of hatchery fish and negative interactions with their wild counterparts, and the introduction of non-native or invasive species (Lassuy 1995; NRC 1996; Parrish et al. 1998; Myers et al. 2004). Population declines and habitat fragmentation are often so severe that natural recolonization of habitats via dispersal (‘straying’) is difficult (O'Reilly and Doyle 2007). Consequently, captive breeding programs involving hatcheries have become widely-used tools in an attempt to prevent population extinctions or reintroduce extirpated populations (Berejikian et al. 2004; Flagg et al. 2004a,b; Pollard and Flagg 2004; O'Reilly and Doyle 2007).
The general uses and goals of hatcheries in salmonids are varied (Waples et al. 2007; Naish et al. 2008). For instance, ‘hatchery augmentation programs’ are a century-old management tool and aim to increase the abundance of populations solely for fishery opportunities (Naish et al. 2008). For the purposes of this review, however, and to avoid confusion, I categorize two other ‘types’ of hatchery programs below that either (i) aim to restore extirpated or endangered populations, or (ii) rehabilitate declining or threatened populations. Indeed, it is these conservation-oriented programs that are most relevant to consider in the context of the capacity of hatcheries to conserve biodiversity, particular in the context of (i) which is the focus of the review.
‘Captive breeding programs’, broadly speaking, serve to use hatcheries to maintain populations that are unable to survive in the wild for at least a portion of their lifecycle (Utter and Epifanio 2002). The proximate goal of these programs is to prevent imminent extinction of declining species or populations. Their ultimate goal is to maintain the genetic diversity and fitness within populations until the threats to them are removed and they can be reintroduced as self-sustaining populations (Utter and Epifanio 2002; Pollard and Flagg 2004). These programs have been recently advanced and the most extensively applied in Europe and North America.
‘Supplementation programs’, on the other hand, involve the intentional demographic integration of hatchery and natural production, with the goal of improving the status of an existing natural population (Waples et al. 2007). Such programs have been used in many regions but most extensively in Western North America (Naish et al. 2008). Here they are used to mitigate losses in declining or threatened populations from human activities and/or environmental changes.
In reality, the definitions, uses, and goals of these programs represent a continuum along which the status of populations may range anywhere from being threatened to extirpated (and ultimately, rendering the species extinct) (Fig. 1). In some cases then, it may be hard to distinguish the exact moment when a supplementation program has become a captive breeding program, or vice-versa (Fig. 1). In addition, programs within these categories may vary considerably between hatchery facilities, in terms of (i) the procedures that they adopt to improve the chances that the program will achieve its goals and (ii) the duration or life-history stage of hatchery-rearing (Table 1; see Appendix 1 for literature search details). For instance, only recently have a number of procedures been feasible or recognized for mitigating a myriad of genetic risks in the hatchery that might affect the success of captive breeding programs (Table 2). Live-gene banking programs of Atlantic salmon in Norway and eastern North America (see Box 1; Table 1) are good examples of ‘current’ captive breeding programs that accommodate many of these new procedures to protect populations that are at the extreme of the continuum outlined in Fig. 1; that is, populations that are extirpated or facing imminent extinction.
Figure 1.
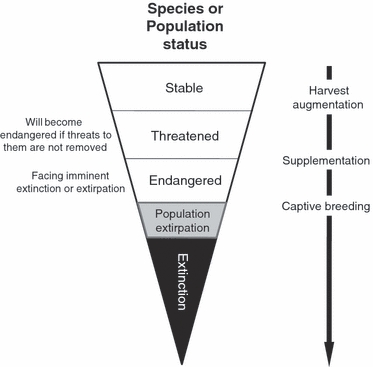
The continuum of different types of hatchery programs (‘harvest supplementation’, ‘supplementation’, and ‘captive-breeding’) in relation to the status of a species or population. The designation of different programs to specific points along the continuum is not intended to be prescriptive.
Table 1.
Commonalities and differences between and within categories of hatchery programs, depending on the salmonid species and/or particular geographic location. X=majority or all
| Characteristic of the program | Traditional hatchery augmentation | Supplementation (e.g., Chinook salmon) | Supplementation (e.g., steelhead, western USA, Canada) | Captive-breeding (winter-run Chinook salmon, California) | Captive-breeding (Pacific salmon, western USA, Canada) | Live gene banking (Atlantic salmon, Norway) | Live gene banking (Atlantic salmon, Canada) |
|---|---|---|---|---|---|---|---|
| Use of local populations for generating broodstocks | Some | Some | X | X | X | X | |
| Hatchery release as eyed-embryos | Some | Some | |||||
| Hatchery release as unfed fry in the wild | Some | Some | X | ||||
| Hatchery release as several week-old fry in the wild | Some | Some | X | ||||
| Hatchery release as parr or presmolts in the wild | Some | Some | X | ||||
| Hatchery release as smolts in the wild | X | X | X | X | Most | Some | |
| Free mate choice – release of adults (*captured as wild juveniles) | Some | Some* | |||||
| Adult broodstock always retained in captivity | X | Some | |||||
| Release at optimal dates and sizes | Some | Some | Some | X | Some | X | X |
| Pedigree information used to prevent kinship matings | Some | Some | X | Some | X | X | |
| Relatedness estimates of founders to prevent kinship matings | Some | Some | Some | X | X | ||
| Fish grown at ‘natural’ growth trajectories before release | X | ||||||
| Equalization of family sizes in captivity and at release | X | Some | X | X | |||
| Balanced sex ratios when breeding | Some | Some | X | Some | X | X | |
| Recovery of offspring from each spawned adult at each spawning | X | ||||||
| Sperm cryopreservation | ? | Some | X | X |
Undoubtedly, individual programs within each category differ in the particular procedures adopted and in the proportion of the broodstock to which each procedure is applied. Detailed comparisons of harvest augmentation and captive-breeding programs in Pacific salmon can be found in Flagg et al. (2004b). See also O'Reilly and Doyle (2007) for a description of live-gene banking in Atlantic salmon.
Table 2.
Examples of means for reducing genetic and other risks associated with captive breeding programs.
| Means for reducing genetic or other risks associated with captive breeding programs | Outcome/benefit | References |
|---|---|---|
| Minimize generations in captivity | Reduces domestication selection to captivity Reduces the potential loss of genetic diversity in captivity | Frankham et al. (2002); Frankham (2008) |
| Minimize intentional selection in captivity (e.g., large adult size, early spawning adults) | Reduces domestication selection to captivity Early release of offspring; use broodstock with wild exposure or from the wild | Miller and Kapuscinski (2003); Frankham et al. (2002); O'Reilly and Doyle (2007) |
| Use local populations for captive breeding and/or supplementation | Reduces the loss of adaptation to local environments Prevents outbreeding depression | Brannon et al. (2004) |
| Restrict captive-rearing to life-history stages where natural mortality in the wild is not as severe | May reduce domestication selection in captivity | P. O'Reilly, DFO Halifax, Canada, personal communication |
| Maintain Ne as high as possible | Reduces the loss of genetic diversity in captivity | Ryman and Stahl (1981); Tave (1984); Allendorf and Ryman (1987); Withler (1988); Eknath and Doyle (1990); Allendorf (1993); Doyle et al. (2001); Frankham et al. (2002); Campton (2004); Rodriguez-Ramilo et al. (2006); O'Reilly and Doyle (2007); Wedekind et al. (2007) |
| Start the initial captive broodstock with as many genetically-diverse founders as possible | ||
| Equalize founder representation in the initial captive broodstock | ||
| Equalize family sizes in captivity and at time of release | ||
| Equalize sex ratios at spawning | ||
| Recover offspring from each spawning adult at each sampling event before release at in each spawning year | ||
| Equalize captive population sizes across generations | ||
| Maximize captive generation length | ||
| Do not carry out mixed-sperm fertilizations | ||
| Allow free mate choice rather than conduct random matings | May improve offspring quality and retention of fitness | Fleming (1994); Wedekind 2002; Berejikian et al. (2004); Pitcher and Neff (2007) |
| Minimize family variance in the captive component relative to the wild component of the population | Potentially increases Ne of the whole population | Ryman and Laikre (1991); Hedrick et al. (2000a,b); Wang and Ryman (2001) |
| Apply sperm cryopreservation techniques, or surrogate broodstock technologies | Maximizes generation length, thereby potentially reducing the loss of genetic diversity and loss of fitness (from domestication selection or a relaxation of natural selection) in captivity | Okutsu et al. (2007); O'Reilly and Doyle (2007) |
| Greater naturalization of the captive environment (for physiological, morphological and behavioural conditioning) | Improves survival chances upon exposure to the wild | Maynard et al. (1996, 2004); Braithwaite and Salvanes (2005); Salvanes and Braithwaite (2005) |
| Estimate relatedness among founders and use this information, as well as employ pedigree analyses, to minimize/avoid kin matings | Reduces inbreeding and retains genetic diversity | Fernandez and Caballero (2001); Fernandez et al. (2003); Hansen and Jensen (2005); Herbinger et al. (2006); O'Reilly and Doyle 2007; Kozfkay et al. (2008) |
| Optimal releases of captive-reared individuals into the wild (e.g. at proper times, body sizes, water temperatures) | Improves survival chances upon exposure to the wild | Miller and Kapuscinski (2003); Brannon et al. (2004); O'Reilly and Doyle (2007) |
| Grow captive-reared individuals at ‘natural’ rates of growth | Decreases sex ratio skews brought on my early male maturation | Larsen et al. (2004) |
| Monitor success of released captive-bred individuals | Feedback for improvement of captive-breeding programs | Flagg et al. (2004a); O'Reilly and Doyle (2007) |
| Delay maturation of individuals in captivity | Extend generation length in captivity | Frankham et al. (2002) |
Discussions of many of these with specific respect to salmonid fishes can also be found in Miller and Kapuscinski (2003), Reisenbichler et al. (2003), Flagg et al. (2004b) and O'Reilly and Doyle (2007).
Box 1. Glossary of terms used throughout the review.
Allelic richness – A measure of genetic diversity, usually expressed as the mean number of alleles found at multiple gene loci; otherwise known as allelic diversity.
Effective population size - The size of a stable, randomly mating population that would have the same rate of gene loss or increase in inbreeding as the real population (size N). All finite populations are inbred to some degree and generally do not choose mates at random, so Ne is typically 1/10 N or less (Frankham 1995). Frankham (1995) reviewed the factors that reduce Ne relative to N and found that fluctuating population sizes, variance in family sizes and unequal sex ratios are the most important factors driving Ne/N downwards.
Domestication selection – For the purposes of this review, this term is defined broadly following Currens and Busack (1995) and Waples (1999). Domestication selection firstly relates to genetic changes in a captive population resulting directly or indirectly from either intentional or nonintentional selection within the captive breeding environment. It also relates to temporary relaxation of selection in the captive environment which might not lead to genetic change in the captive environment but which would otherwise occur in the wild (Waples 1999). In other words, domestication selection can be any change in the selection regime of a cultured population relative to that experienced by the natural population (Waples 1999). Also known as, broadly speaking, genetic adaptation (reviewed in Frankham 2008).
Genetic drift - Stochastic fluctuations in allele frequencies or loss of rare alleles due to the random sampling of gametes at each generation.
Heterozygosity – A measure of genetic diversity. Having different alleles at one or more corresponding gene loci.
Inbreeding - A regime of reproduction that implicates the union of related gametes (gametes sharing a common ancestor).
Inbreeding depression- A reduction in the fitness of offspring from the mating of related individuals.
Live-gene banking program – A form of captive breeding program that (i) involves multiple generations of captive breeding to protect populations that are at immediate risk from extinction and (ii) implements a number of procedures from Table 2 to minimize genetic and fitness-related risks associated with captive breeding or rearing (O'Reilly and Doyle 2007). Typically, and by necessity, most if not all of the population is housed under captive conditions for at least a part of the species’ lifecycle.
Local source population – The creation of a captive-bred population from a particular wild population that is then reintroduced into the same environment (e.g., river) occupied by that wild population, for the purposes of re-establishing or supplementing the wild population.
Neutral genetic markers- DNA technologies targeting and amplifying genomic regions (gene loci) that are not subject to natural selection (i.e., that are selectively neutral). Genetic differentiation within or between populations can be evaluated using neutral genetic markers, to evaluate the relative roles of genetic drift, gene flow and/or mutation in population differentiation, or to identify family relationships (kinship) between individuals within populations. Genetic differentiation at neutral genetic markers is common in salmonids, including at small geographic scales (e.g., within large river systems, between geographically proximate lakes) (Taylor 1991; Garcia de Leaniz et al. 2007). Genetic differentiation at neutral genetic markers is also sometimes positively correlated with phenotypic or life-history trait differentiation in salmonids, suggesting that selection has played a role in driving the differentiation at these traits (e.g., Fraser and Bernatchez 2005). However, in general, it would appear that differentiation at neutral genetic markers is often a poor proxy for adaptive genetic differentiation between and/or within populations (e.g., Reed and Frankham 2001).
Nonlocal source population – The creation of a captive-bred population from a particular wild population that is then reintroduced into a different environment (e.g., river) than that of the wild population from which it was derived, for the purposes of re-establishing or supplementing the wild population; similar to the use of the term ‘out-of-basin hatchery stock’ in the primary literature (Brannon et al. 2004; Araki et al. 2007b).
Outbreeding depression – A reduction of fitness in the offspring (hybrids) of crosses between divergent populations. Outbreeding depression can occur either through the disruption of intrinsic interactions between genes or disruption of extrinsic interactions between genes and the environment (reviewed by Edmands 2007). Outbreeding depression in hatchery–wild hybrids through the disruption of extrinsic interactions between genes and the environment would be expected primarily if differential selective pressures drive population differentiation. Conversely, outbreeding depression in hatchery–wild hybrids through the disruption of intrinsic interactions between genes would be expected if the ancestral wild population of the hatchery strain and the other wild populations were historically isolated. In reality, both mechanisms might act simultaneously, especially if the hatchery fish originate from a nonlocal source population.
Owing to these considerations, I pay careful attention throughout the review to distinguish how differences between supplementation and captive breeding programs may affect interpretations of the capacity of the latter, the predominant focus of the review, to conserve biodiversity. Similarly, wherever possible, the review is careful to discuss how conclusions drawn from previous captive breeding programs may change in the context of ‘current’ captive breeding programs such as live-gene banking that adopt procedures to minimize genetic risks. Additionally, unless otherwise stated, the term ‘wild’ refers to fish born in the wild, regardless of the origin of their parents. ‘Hatchery’ or ‘hatchery-reared’ refers to fish born and raised in the hatchery during some portion of their lifecycle, regardless of the origin of their parents, but where details of the hatchery-rearing process were unknown. Conversely, ‘captive’, ‘captive-bred’ or ‘captive-reared’ refers to fish born and raised in the hatchery (or in ‘captivity’) during some portion of their lifecycle, regardless of the origin of their parents, but where some information was available to describe how genetic risks of hatchery-rearing were mitigated.
Bearing these considerations in mind, I take stock of the capacity of captive breeding programs involving hatcheries to conserve salmonid biodiversity by addressing the following questions and related issues. First, I very briefly consider why genetic diversity between and within salmonid populations is important to conserve. Second, I review and weigh the evidence that salmonid captive breeding programs are capable of maintaining both genetic diversity and fitness within populations. Third, I summarize available information that captive-reared lines of salmonids can be successfully reintroduced into the wild as self-sustaining populations if and when the threats imposed on them are removed. Fourth, because some degree of wild fitness may be unavoidably lost in captivity, I explore theoretical grounds for whether a demographic boost from increased population abundance can offset such fitness reductions. Fifth, I evaluate whether single or multiple facilities are required to more effectively carry out captive breeding programs involving hatcheries (from a genetic and fitness perspective). Finally, I consider whether technical alternatives to captive breeding programs might be used to conserve salmonid biodiversity. Importantly, while the review focuses on salmonid fishes, these same questions are directly relevant to the assessments of captive breeding programs for many other threatened/endangered species.
Genetic diversity among and within populations: important to conserve?
The conservation of genetic diversity within species is a hallmark of contemporary conservation biology (reviewed in: Soulé 1987;Ryder 1986; Crandall et al. 2000; Fraser and Bernatchez 2001; Frankham et al. 2002; Moritz 2002; Frankham 2005). This is true of salmonid biodiversity conservation as well (references in Table 3), and the costs of not conserving genetic diversity are also embodied within the precautionary approach to salmonid fisheries management (Dodson et al. 1998; Garcia de Leaniz et al. 2007). The motivation behind conserving genetic diversity stems from a number of important functions that genetic diversity serves, or is believed to serve, to biodiversity maintenance both among and within populations (Table 3). These functions include (i) maximizing the potential for species/populations to evolve to cope with environmental change, (ii) providing the raw material that natural selection acts upon to generate diversification, and (iii) influencing both ecosystem recovery following disturbances and community species richness (Table 3). Indeed, the consequences of reduced genetic diversity are strongly purported to reinforce demographic/environmental processes and together drive species extinctions (Lande 1995; Spielman et al. 2004).
Table 3.
Evidence that genetic diversity between and within populations is important to conserve, as well as functions of genetic diversity within and between salmonid populations and their biological and/or human benefits.
| Evidence that genetic diversity is important to biodiversity maintenance | Species | References |
|---|---|---|
| Reduced genetic diversity and associated inbreeding within populations are associated with an increased risk of extinction | Butterflies | Saccheri et al. (1998) |
| Elevated extinction risk in populations with higher rates of inbreeding (lower genetic diversity) than in populations with lower rates of inbreeding (higher genetic diversity) | Plants, fruitflies | Newman and Pilson (1997); Bijlsma et al. (2000) |
| Molecular (e.g., allelic) variation has significant effects on population growth rate | Butterflies | Hanski and Saccheri (2006) |
| Quantitative (e.g., body size) variation has significant effects on population growth rate | Sheep | Pelletier et al. (2007) |
| Higher genetic diversity within species enhances ecosystem recovery following disturbances | Sea grass | Reusch et al. (2005) |
| Higher genetic diversity within species increases community species richness | Plants | Booth and Grime (2003) |
| Function of salmonid genetic diversity | Salmonid references |
|---|---|
| Maximizes the potential for species to respond to environmental change Protects the progenitors of future biodiversity (e.g., new species) Reduces the likelihood of extinction | Utter (1981); Waples (1991a, 1995); Ryman et al. (1995),Bernatchez (1995); Taylor (1999); see also Bowen (1999),Waples (1995); Dodson et al. (1998) |
| Direct/indirect benefits of conserving salmonid genetic diversity | |
| Long-term species persistence | Utter (1981); Waples (1991a); Ryman et al. (1995); Taylor (1999) |
| Short-term population viability | Dodson et al. (1998) |
| Maintenance of natural evolutionary processes | Waples (1991a, 1995); Dodson et al. (1998) |
| Protection of different habitats, and potentially ecosystem functioning | Waples (1991a, 1995); Allendorf et al. (1997) |
| Maintenance of local adaptations | Waples (1991a, 1995); Dodson et al. (1998) |
| Maintenance of ecosystem stability | Riddell (1993) |
| Permits humans to understand how salmonid biodiversity arises | Taylor (1999) |
| Development of proper restoration guidelines if some natural systems are conserved | Riddell (1993); Fraser and Bernatchez (2008) |
| Potential future resources for humans | Waples (1991a); Fraser et al. (2006) |
| Potential future resources for aquaculture programs | O'Reilly and Doyle (2007) |
Salmonids are well-studied in terms of the degree to which genetic diversity is partitioned between and within populations. To date, however, the vast majority of these studies have been based on neutral genetic markers (Box 1). The scale and the extent to which genetic diversity in salmonids is adaptive remain poorly understood. Nevertheless, conservation of salmonid genetic diversity is strongly advocated because several indications suggest that adaptive divergence via natural selection may be important in salmonid diversification, and that it can vary with habitat heterogeneity and/or environmental stability (Taylor 1991; Garcia de Leaniz et al. 2007). On the other hand, how best to prioritize intraspecific diversity, both in salmonids and in general, is still a matter of considerable debate (Allendorf et al. 1997; Currens et al. 1998; Wainwright and Waples 1998;Fraser and Bernatchez 2001; Moritz 2002; Wood and Gross 2008).
Can captive breeding programs involving hatcheries conserve genetic diversity within populations?
Given it is commonly accepted that genetic diversity both within and between populations is important to conserve, it is relevant to consider whether or not captive breeding programs can maintain genetic diversity. For the most concerning situations involving the extirpation or near-extirpation of populations in the wild, captive broodstocks may be unavoidably small owing to a lack of space for housing fish or a limited number of remaining wild founders to initiate captive lines. Captive broodstocks will therefore have a low effective population size (Ne) (Box 1). Smaller Ne populations, in the absence of gene flow, lose genetic diversity at a much higher rate through genetic drift (Box 1) than large Ne populations (reviewed in Frankham et al. 2002; Keller and Waller 2002; but see Willi et al. 2006). Relative to larger Ne populations, smaller Ne populations are also more susceptible to inbreeding and its associated effects (inbreeding depression) (Box 1), if they have not been small over long histories to have effectively purged deleterious, recessive alleles (Leberg and Firmin 2008). This is a common situation for many captive-bred species that have often experienced rapid declines related directly or indirectly to human activities. In theory, a well-managed captive breeding program implementing a number of procedures (e.g., Table 2; see below) can generate a ratio of Ne to census size (N) of a population that exceeds one (Frankham et al. 2002). Usually though, Ne will be less than N owing to three variables: unequal sex ratios, variation in family sizes and, particularly, fluctuating population sizes, that drive down the ratio of Ne/N (Frankham 1995), including in salmonids (Waples 2002a; Ardren and Kapuscinski 2003; Araki et al. 2007a). There is consequently a consensus that the more these effects are reduced in a captive breeding program (see Table 2), and the larger the Ne, the more successful that captive breeding program will be at maintaining genetic diversity (Frankham et al. 2002; Koljonen et al. 2002; McLean et al. 2004, 2007; O'Reilly and Doyle 2007; Frankham 2008).
One relevant question to ask is, how many generations can Ne of typical salmonid captive breeding programs maintain genetic diversity? Frankham et al. (2002) have argued that the retention of 90% of genetic diversity (e.g. allelic richness, heterozygosity; Box 1) over a 100-year period in captivity should be a targeted conservation goal. This time period would equate to 25–33 generations for most captive-reared salmonids, and stems from the timeframe when human population growth is expected to decline and increases in wild habitat may become available (Soulé et al. 1986). In an analogous situation, Franklin (1980) and Frankel and Soulé (1981) also argued that a decrease in mean heterozygosity of 1% per generation (i.e., an inbreeding rate of 1%) due to low Ne was an acceptable rate of loss of diversity in livestock breeding programs. However, there is currently no empirically or theoretically justifiable answer to the question ‘how much genetic diversity is enough to conserve a species or population?’ Additionally, a rate of loss of heterozygosity of 1% per generation might be acceptable in benign agricultural environments but has not been tested on captive-reared salmonids or other fishes that will be released into the wild (Naish et al. 2008). In reality, the goal of any captive breeding program should be perhaps to conserve as much genetic diversity as possible. Relationships between genetic diversity and population viability are also complex and likely vary between species and populations within species (Tallmon et al. 2004). Therefore, conservation hope should not be abandoned if a population has lost, say, 20% or more of its genetic diversity over 100 years of captive-rearing; it might, of course, still be reintroduced successfully into the wild. Indeed, there is at least one report of a successful introduction of salmonid populations into previously unoccupied habitat despite limited genetic diversity and low Ne (Koskinen et al. 2002). Consequently, the present review applies the aforementioned yardsticks cautiously and with these points in mind when interpreting results from empirical or theoretical studies on salmonids.
Ne in the context of salmonid biology
Theoretical works initially developed to characterize the rate of loss of genetic diversity expected over time in populations of finite size referred to Ne per generation and were based on species with discrete generations in which there was 100% turnover each generation (Waples 1990, 2002a; Waples and Teel 1990). In reality and, like many other organisms, salmonids have overlapping generations in which individuals from several different-year classes might contribute to a population's gene pool annually. However, relative to species from which historical modeling of overlapping generations was derived (Felsenstein 1971; Hill 1972), many salmonids also differed because they die after breeding. This is the case for semelparous Pacific salmon (Oncorhynchus spp.) and for populations of other salmonids with a low degree of iteroparity (e.g., Atlantic salmon, Salmo salar). Waples (1990) discussed how such a characteristic causes a complete turnover in the breeding population each year rather than the gradual transition of most overlapping generation models. In other words, short-term genetic change in many salmonids is more a function of the effective number of breeders per year, Nb, and not generational Ne (Waples 1990). If Nb remains stable across years, then Ne per generation is equivalent to Nb multiplied by the average age of breeders, or generation length g, and Ne = gNb accurately predicts the rate to which genetic diversity may be lost through genetic drift in an isolated, captive-reared population.
Waples (1990) simulated the loss of heterozygosity and allelic diversity that might be accrued over 100 years in isolated salmonid populations with Nb of 24, 50, and 100, under a Wright-Fisher model with random mating and separate sexes. For a salmonid with a 4-year generation time, these Nb values would translate into generational Ne values of roughly 100, 200, and 400. Relative to discrete population models, only slight deficiencies in heterozygosity occurred in the early years of simulations when Nb was small, by the extent (g + 1)/(8Nb) (details in Waples 1990). Thus, according to this model (and similar to discrete generation theoretical models: Hartl and Clark 1989), even populations of Nb = 24 would be capable of retaining ≍88% of initial heterozygosity over a 100-year period (Waples 1990); in other words, only ≍0.5% of heterozygosity would be lost per generation in captivity in a salmonid with a 4-year generation time.
For reasons that are unclear, in scenarios involving small versus large Nb (Nb = 24 vs. 50 or 100), small Nb populations also lost rarer alleles more readily than that predicted by the discrete generation model in the early generations, but this effect disappeared after about 10 generations (details in Waples 1990). Unless Nb was small (Nb = 24), few alleles of frequency >10% would be lost even over a 100-year period, but rarer alleles (with frequencies of 2% or 5%) would be lost at a much higher rate. For instance, an isolated population of Nb = 24 could expect to lose ≍47% of alleles with frequencies ≤5% in 100 years, whereas a population of Nb = 50 or 100 would only lose ≍20% or ≍3% of such alleles, respectively, over the same time period (Waples 1990). Such greater losses of allelic diversity relative to heterozygosity are consistent with a wide body of theory (e.g., Allendorf 1986; Leberg 1992; Luikart et al. 1998). Waples (1990) simulations also assumed an age structure of 50% age 4 breeders, and 25% age 3 and age 5 breeders, but changing the age structure had little effect on the outcomes.
Based on these results, Waples (1990) recommended that maintaining Nb = 100 in salmonid hatchery broodstocks per year would be sufficient to preserve most alleles for tens of generations. Put another way however, the model suggested that for a salmonid with a 4-year generation time, 90% of the initial rarer allelic diversity (frequencies≤5%) could still be retained after about 8, 17, and >25 captive generations for populations of Nb = 24, 50 and 100, respectively (see Waples 1990). Thus, the model also suggested that a smaller Nb (24–50) might be reasonably tolerated in captive breeding programs for shorter time periods than 100 years (e.g., 30–60 years).
Reducing the rate of loss of genetic diversity in captivity predicted from theoretical expectations
Encouragingly, there are means by which to reduce the rate of loss of genetic diversity based on the theoretical considerations outlined above (e.g., a random mating, idealized population), even if the captive broodstock census size is low. Ideally though, it is better to start with as large a founder captive population as possible (Allendorf and Ryman 1987;Frankham et al. 2002).
One simple and widely recognized approach is to ensure that each individual contributes exactly the same number of progeny to the next generation. Assuming that, for example, an individual of one sex is bred with a single individual of the opposite sex, equalized family sizes from these matings yield a rate of of inbreeding and genetic drift that is roughly half those generated from random contributions of parents in an idealized population (Wright 1938; Wang 1997). In other words, equalization of family sizes effectively doubles Ne relative to a randomly mated population. The few experimental tests carried out on this topic with fruitflies have supported theoretical predictions (Borlase et al. 1993; Rodriguez-Ramilo et al. 2006). For instance, Rodriguez-Ramilo et al. (2006) compared the genetic diversity of captive-lines with equalized versus random contributions after 38 generations at constant size (N = 20 or 100) and environmental conditions. After 38 generations, they found that ‘equalized’ lines retained 23–36% more allelic diversity (at four microsatellite loci) than ‘randomized’ lines. With respect to salmonids, more recently instated captive breeding programs, such as live-gene banking programs of Atlantic salmon, attempt to balance sex ratios and equalize family sizes not only within captivity but also at the time of release into the wild (O'Reilly and Doyle 2007; P. O'Reilly, Department of Fisheries and Oceans, Halifax, personal communication; see also Hedrick et al. 2000a,b; Moyer et al. 2007). Live-gene banking programs also attempt to recover at least one offspring per spawned adult repeatedly at each spawning, in each spawning year, and at each sampling event to maximize the retention of genetic diversity of individuals (P. O'Reilly, Department of Fisheries and Oceans, Halifax, personal communication).
Another more sophisticated and recommended approach is to use pedigree or molecular genetic marker data to minimize mean inbreeding or kinship (coancestry) coefficients between parents before generating every new captive generation (Ballou and Lacy 1995; Caballero and Toro 2000, 2002; Fernandez et al. 2003, 2004; Wang 2004). Salmonid spawnings based on minimizing mean kinship are now being carried out in a number of captive breeding programs (e.g., Flagg et al. 2004a; Hansen and Jensen 2005; O'Reilly and Doyle 2007; Kozfkay et al. 2008). Currently, however, little empirical research in salmonids or other taxa has been conducted to assess to what degree genetic diversity can be more effectively retained with these additional measures relative to theoretical expectations, in terms of their long-term effectiveness. For instance, over four generations and constant population size, Montgomery et al. (1997) compared the amount of genetic diversity retained in replicate populations of fruitflies where either kinship was minimized or randomized between breeders (based on six microsatellite loci and seven allozyme loci). The authors found that minimum kinship replicates retained significantly greater levels of allelic richness and heterozygosity than randomized replicates, although diversity in randomized replicates was still 94–95% that of minimized kinship replicates. On the other hand, minimizing kinship in captive populations of endangered species/populations might yield greater benefits than this experimental work would suggest because female fruitflies in this study were restricted to a single mating. Namely, the reuse of under-represented individuals in successive generations would allow them to make greater genetic contributions to successive captive generations (Montgomery et al. 1997). Finally, it is worth noting that some measures for minimizing kinship require detailed pedigree information (e.g., Toro et al. 1999; Wang 2001a; but see Wang 2004) which may not be available in some situations.
One potential caveat of strategies that minimize kinship is that they often assume captive broodstock founders are unrelated and not inbred (Rudnick and Lacy 2008), although with DNA techniques, it is now possible to at least estimate founder relationships (Gautschi et al. 2003; Russello and Amato 2004). If founders are related or inbred, maximizing Ne by equaling the genetic contributions of captive breeders will only exacerbate the effects caused by a nonrepresentative sampling of the ancestral gene pool within the captive broodstock (Ebanhard 1995; Doyle et al. 2001). This is important to consider in many salmonids for two reasons. First, related family members within populations may not be distributed randomly at various stages of the life cycle (Hansen et al. 1997; Fraser et al. 2005). Second, sampling collections for captive broodstock purposes may be restricted in time and spatial coverage (Herbinger et al. 2006).
Recent modeling suggests that while the potential benefits from knowing founder relationships probably vary on a case-by-case basis, minimizing kinship within a captive broodstook under traditional founder assumptions could still generate near optimal results (Rudnick and Lacy 2008). Yet, Doyle et al. (2001) illustrated an empirical example in which a greater level of genetic diversity was recovered (and thus retained) within a small captive population generated from related founders, characteristics likely of many captive breeding programs (Utter 1998; Hedrick et al. 2000a,b,c; O'Reilly and Doyle 2007). Notably, using relatedness estimates based on DNA markers and minimum kinship analyses, Doyle et al. (2001) carried out compensatory matings in a captive population of sea bream (Pagurus major), wherein subsets of founders from under-represented lineages were preferentially mated to increase their contribution. Relative to random subsets of breeders of equal size, preferentially-mated subsets of breeders had a lower mean coancestry and they generated an offspring gene pool with greater heterozygosity and allelic diversity (Doyle et al. 2001). While genetic diversity of the random subsets was still 96% of preferentially-mated subsets, the results suggested a means by which to also reduce the rate of inbreeding and genetic drift predicted from theoretical considerations of Ne, by accounting for the genetic nature of founders.
Empirical Nb and Ne in captive salmonid populations
Table 4 provides available estimates of Nb and Ne and levels of genetic diversity mainly at highly polymorphic, nuclear DNA loci (microsatellite loci) in a number of salmonid hatchery/captive breeding programs (see Appendix 1 for literature search details). Because of their high polymorphism, microsatellite loci currently represent the most widely used DNA technologies to detect whether losses of genetic diversity have occurred within captive breeding programs.
Table 4.
Estimated effective number of breeders (Nb), effective population sizes (Ne), and genetic diversity within broodstocks of captive breeding programs of salmonids, from studies involving mainly polymorphic nuclear DNA markers (microsatellite DNA loci) (95% confidence intervals for Nb/Ne are in parentheses, where estimated).
| Species | Population | Program | L | Broodstock Nb | Broodstock Ne | He | A | Ga | Comments or caveats | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Salmonids | ||||||||||
| Atlantic salmon | Teno | S | 9 | 8b | 32 (17–55) | 0.759 | 10.3 | 1 | 1.7% and 8.2% loss of He and A per generation, respectively | Koljonen et al. (2002) |
| Tornionjoki | S | 9 | 60b | 238 (89-∞) | 0.702 | 10.1 | 2 | 1.0% and 1.6% loss of He and A per generation, respectively | Koljonen et al. (2002) | |
| Iijoki | C | 9 | NA | NA | 0.679 | 8.0 | 5 | Founded from 500 individuals | Koljonen et al. (2002) | |
| Oulojoki | C | 9 | NA | NA | 0.682 | 6.6c | 10 | Koljonen et al. (2002) | ||
| Teno | S | 7 | 11b | 44 (27–228) | 0.750 | 10.4 | 1 | 2.5% and 4.8% loss of He and A per generation, respectively | Saisa et al. (2003) | |
| Iijoki | C | 7 | 20b | 79 (48–149) | 0.680 | 9.1 | 5 | 0.1% and 0.9% loss of He and A per generation, respectively | Saisa et al. (2003) | |
| Oulojoki | C | 7 | NA | NA | 0.660c,d | 7.1c | 10 | 0.7% and 1.5% loss of He and A per generation, respectively | Saisa et al. (2003) | |
| Big Salmon ‘A’ | C | 9 | NA | NA | 0.822 | 12.1 | 1 | Number of founders was chiefly 24 adults | Herbinger et al. (2006) | |
| Big Salmon ‘B’ | C | 9 | 73 | 270e | 0.852 | 17.4 | 0 | Genetic diversity of founder population for live-gene banking | Herbinger et al. (2006) | |
| Stewiacke | C | 9 | 69 | 255e | 0.846 | 19.1 | 0 | Genetic diversity of founder population for live-gene banking | Herbinger et al. (2006) | |
| Philip | F | 11 | 21–42b | 102–207f | 0.610 | 6.8 | 6f | 0.6% and 5.4% loss of He and A per generation, respectively | Innes and Elliott (2006) | |
| Sellas | S | 6 | 16 | 64g | 0.797 | 12.2 | 0 | Machado-Schiaffino et al. (2007) | ||
| Cares | S | 6 | 58 | 232g | 0.783 | 10.3 | 0 | Machado-Schiaffino et al. (2007) | ||
| Connecticut | S/C | 9 | 49b | 194 (159–232) | 0.741 | NA | 5h | Only observed heterozygosity presented Only 1 generation between temporal samples for estimating Ne from the method of Wang (2001a) | Spidle et al. (2004) | |
| Chinook salmon | North Fork | S | 14 | >333i | >1000 | 0.837 | 13.5 | 4 | 0.0% and 0.2% loss of He and A per generation, respectively Supplementation program for an already abundant wild population | Eldridge and Killebrew (2007) |
| Sacremento | S/H | 21j | 133(68–355) | 399k | NA | NA | ? | Linkage disequilibrium data | Bartley et al. (1992) | |
| Sacremento-winter-run | S/C | 20j | 89(45–266) | 267k | NA | NA | 0 | Linkage disequilibrium data | Bartley et al. (1992) | |
| Sacramento-winter-run | S/C | 5–18 | 15–54l | Ne based on demographic data | Hedrick et al. (1995) | |||||
| Sacramento-winter-run | S/C | 7 | 18–32 | 54–95m | NA | NA | 0 | Genetic diversity of founder population for captive breeding | Hedrick et al. (2000b) | |
| Cedar Creek | S/H | 11 | 33k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Cole (spring) | S/H | 1151 | 3453k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Cole (fall) | S/H | 35 | 105k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Elk | S/H | 279 | 837k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Fall Creek | S/H | 55 | 165k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Rock Creek | S/H | 96 | 288k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Salmon Creek | S/H | 80 | 240k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Trask (Spring) | S/H | 139 | 417k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Trask (Fall) | S/H | 172 | 516k | ? | Maximum Nb based on demographic data (1979–1984) | Waples and Teel (1990) | ||||
| Rainbow trout | Shasta | S/H | 7j | 36(13–113) | 108n | NA | NA | 8 | Linkage disequilibrium data | Bartley et al. (1992) |
| Lake trout | Lewis Lake | S/C | 9 | NA | NA | 0.448 | 3.1 | ? | Unequal sex ratios; mixed milt fertilizations | Page et al. (2005) |
| Isle Royale | S/C | 9 | 105 (40–307) | 630o | 0.410 | 3.3 | 0 | Genetic diversity of founder population for captive breeding | Page et al. (2005) | |
| Apostle Islands | S/C | 9 | 115 (45–322) | 690o | 0.411 | 3.2 | 0 | Genetic diversity of founder population for captive breeding | Page et al. (2005) | |
| Green Lake | S/H | 3 | 20 (8–41) | 120o | 0.538 | 3.7 | ? | Unequal sex ratios; mixed milt fertilizations | Page et al. (2005) | |
| Seneca | S/H | 3 | 48(18–124) | 288o | 0.629 | 5.0 | ? | Unequal sex ratios; mixed milt fertilizations | Page et al. (2005) | |
| Other species | ||||||||||
| Bream Pagrus major | Japan | S | 4 | NA | 63.7 | 0.867 | 29.8 | 0 | Genetic diversity of founder population for stock enhancement | Perez-Enriquez et al. (1999) |
| Iberian wolf (Canus lupus signatus) | Iberian Peninsula | C | 13 | NA | NA | 0.460 | 4.6 | 2 | Founded from 15 individuals 0.0% and 3.0% loss of He and A per generation, respectively | Ramirez et al. (2006) |
| Mallorcan midwife toad (Alytes muletensis) | Europe | C | 10 | NA | NA | 0.500c | 3.0c | 8 | 2.3% and 6.4% loss of He and A per generation, respectively Founded from 14 individuals | Kraaijeveld-Smit et al. (2006) |
Where available, the rate of loss of genetic diversity per generation is noted. L = number of microsatellite DNA loci screened. Program type: S, supplementation; C, captive-breeding; H, harvest augmentation. F, farmed/aquaculture. He, expected heterozygosity; A, mean numbers of alleles per locus (uncorrected across studies for sample size); G, number of generations in captivity; NA, not available.
Generations refer to the number of generations that had taken place in the hatchery at the time the study was conducted. Note, however, that programs within the same category (e.g., ‘supplementation’ or ‘hatchery augmentation’) may differ.
Estimates based on values of Ne, from the relationship Ne = 4Nb (Waples 1990), assuming a 4-year generation time for Atlantic salmon.
Significant reduction in He or A in the captive broodstock relative to the wild population or initial founding captive generation.
Statistically significant decreases in heterozygosity were detected at two of seven loci.eEstimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), based on a 3.7 year generation time for inner Bay of Fundy Atlantic salmon (COSEWIC 2006b)
Based on a 4-year generation time in Atlantic salmon, and allelic frequency variance between an ancestral wild population and its farmed broodstock counterpart over a 24-year period.
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 4-year generation time for Atlantic salmon.
Based on a 4-year generation time in Atlantic salmon.
Estimates based on values of Ne, from the relationship Ne = 4Nb (Waples 1990), assuming a 3-year generation time for Chinook salmon.
Allozyme loci.
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 3-year generation time for Chinook salmon.
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 3-year generation time for Chinook salmon. The range provided is based on Nb from three different years (1991–1993; Hedrick et al. 1995).
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 3-year generation time for Chinook salmon. The range provided is based on Nb from two different years (1994–1995; Hedrick et al. 1995).
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 3-year generation time for rainbow trout.
Estimates based on values of Nb, from the relationship Ne = 4Nb (Waples 1990), assuming a 6- year generation time for lake trout (Page et al. 2005).
Several points and caveats of the studies in Table 4 are worth noting that might make generalities difficult with respect to what constitute typical Nb and Ne values in salmonid captive broodstocks. First, Nb and Ne estimates were derived from systems involving supplementation and/or captive breeding. In the case of supplementation programs, information was often lacking on whether broodstocks were being supplemented each generation with wild-caught individuals, or whether they were being entirely regenerated from previous generations of the captive broodstock (isolated from the wild). Second, it is uncertain in some cases whether a loss of genetic diversity might be attributable to low captive Ne or whether it was related to captive founder effects, because levels of genetic diversity in the captive broodstock were compared to those of the wild population and not to the initial founding captive broodstock. These differences may affect interpretations of the rate at which genetic diversity is lost over time in captive broodstocks. Third, details were lacking in many programs to assess what types of procedures were employed to minimize reductions in Ne in the hatchery, so the results may not always be directly applicable to current captive breeding programs (Table 1) adopting procedures in Table 2. Fourth, Nb and Ne were estimated from different methods, and in particular cases, some of the underlying assumptions of these methods were violated (Table 4). Similarly, Nb and Ne point estimates in some cases had fairly wide confidence intervals, and many had no confidence intervals at all (Table 4). Fraser et al. (2007a) have recently found that many commonly used methods for estimating Ne do not always generate correlated Ne estimates in salmonids. Finally, conversions of Nb estimates to a generational Ne estimate assumed that each year's breeding population contributed equally to the next generation regardless of the number of breeders (Waples 1990, 2002a). However, Waples (2002b) showed that variability in Nb can substantially reduce generational Ne, especially within semelparous salmonids, and this reduction in Ne is in addition to reductions in the ratio of Nb/N in individual years.
Keeping these caveats in mind, it may not be overly surprising that point estimates of Nb and Ne for salmonid broodstocks vary considerably (Nb: 5–133; Ne = 15–690, with the exception of two populations having estimates much larger than these ranges but where it is evident that the supplementation deviated from realistic Nb/Ne for captive-breeding programs) (Table 4). Assuming that these estimates reflect true values, only crude generalities can be made regarding the capacity of salmonid captive breeding programs to conserve genetic diversity. For instance, across all populations of different species (n = 26), only eight broodstocks (31%) would fulfill Waples (1990) recommendation of Nb = 100 (Table 4). Additionally, of the 26 broodstocks, about 15 (58%) and 21 (81%) would also fulfill a minimum Nb = 50 and 24, respectively (Table 4). These results suggest that a considerable number of salmonid captive breeding programs might still lose a ‘fair’ proportion of their allelic diversity within 30–60 years of existence (≍8–15 generations; see above), perhaps unless several of the procedures in Table 2 are instated which might subdue these losses. Interestingly, in only one captive population (Oulujoki) was a statistically significant reduction in heterozygosity and allelic richness detected (Table 4). However, this population had existed for 10 generations in captivity, and declines in genetic diversity over time that were not statistically significant could very well reflect (i) the fewer number of generations accumulated in these captive breeding programs, and/or (ii) a limitation in statistical power to detect significant changes in genetic diversity owing to insufficient sample sizes and/or the modest numbers of loci employed (see Luikart et al. 1998). For example, in two of six captive populations where data on the rate of loss of genetic diversity per generation existed, a loss of 4.8–8.2% of allelic diversity per generation was estimated (Teno, Philip; Table 4).
Other considerations: captive-reared and wild population components
The above discussion has dealt with cases where populations have become extirpated or nearly extirpated from the wild. In such cases most, if not all, remaining population members are involved in captive breeding. These are relevant cases to consider in the context of the capacity of captive breeding programs to conserve genetic diversity. Nevertheless, during the process towards successful reintroduction into the wild, at some point there will be both wild and captive-reared components to the population. Likewise, when a wild population is experiencing drastic declines and a decision is made to prevent its extinction by supplementing the wild population with captive-reared individuals, the population will comprise these same two components.
Ryman and Laikre (1991) modeled the potential increase or reduction in Ne (even if N of the population is increased), and thus the potential for the rate of loss of genetic diversity to be diminished or magnified, that might occur when captive-reared individuals are released into a wild population over a single generation. In particular, Ryman and Laikre (1991) focused on how captive releases might lead to a reduction in Ne. In such a situation, the reproductive rate of the captive-component of the population is favored so the variance in family size increases in the population, thereby decreasing the ratio of Ne/N. Among other things, the model assumed discrete generations, that captive-reared and wild fish had equal probabilities of breeding in the wild, and that the number of offspring produced by either wild or captive breeder components was distributed binomially. For salmonids, such assumptions are likely violated in many cases (Waples 1990; Wang and Ryman 2001; see below). Nevertheless, the model made an important conclusion. For situations where the wild population was small, and thus, most likely to go extinct, supplementation with captive-reared fish could especially lead to a serious reduction of genetic diversity of the overall population through a reduction of Ne (Ryman and Laikre 1991). This concern was also most prominent when only a few captive-reared individuals were used in attempts to recover populations (Ryman and Laikre 1991).
On a positive note, however, in perhaps the only detailed salmonid captive breeding program to effectively apply the Ryman and Laikre method, supplementation does not appear to have reduced genetic diversity in a small, wild population, and it perhaps increased Ne (Hedrick et al. 1995, 2000a,b). For instance, Hedrick et al. (2000b) found that supplementation of endangered, winter-run Chinook salmon led to apparent increases in the lower and upper bound of Nb of 16–81% and 2–11%, respectively, for two different run years. Estimation of Nb in this study made several assumptions; most notably that survival and return of released (captive-reared) individuals were random. Nevertheless, using genotypic pedigree information to examine the representation of different captive-reared families in returning breeders, Hedrick et al. (2000a) were able to show that the numbers of returning individuals were within 93.6% and 78.2% of expected values. The results implied that if Nb of the wild population had not been increased with a captive-rearing component, it had at least not been greatly reduced (Hedrick et al. 2000a,b). Importantly, this program attempted to equalize the contributions of captive breeders by breeding each male and female as evenly as possible and by releasing the captive offspring generated from different families as evenly as possible (Hedrick and Hedgecock 1994; Hedrick et al. 1995, 2000a,b).
More recent models and simulations have evaluated under what conditions supplementing a wild population over multiple generations could be either beneficial or detrimental, in terms of increasing or reducing Ne, and related effects such as the rate of inbreeding and genetic drift (Waples and Do 1994; Wang and Ryman 2001; Duchesne and Bernatchez 2002). Both Wang and Ryman (2001) and Duchesne and Bernatchez (2002) found that supplementation did not result in either substantial reductions in Ne or increases in inbreeding under certain conditions. For instance, in species where the variance in family size in the wild component was much larger than binomial variance (as may be common in salmonids, e.g., Hedrick and Hedgecock 1994), supplementation could be favorable for increasing Ne, at least in the first generation (Wang and Ryman 2001). In some circumstances, family size variance in the captive component might even be manipulated (i.e., reduced) to offset high family size variance in the wild (Wang and Ryman 2001). In addition, Duchesne and Bernatchez (2002) found that scenarios, where the rate of inbreeding with supplementation either remained stable or declined (relative to a control of no supplementation), were generally those involving a larger than smaller captive N. In fact, captive N was more important in determining the effect of supplementation on inbreeding than (i) the degree to which the captive component (or population) was ‘refreshed’ with breeders from the wild component, or (ii) the generational duration of supplementation (Duchesne and Bernatchez 2002).
Nevertheless, the outcomes generated by these models often changed considerably depending on the demographic scenario employed or the underlying assumptions. Wang and Ryman (2001) found that supplementation could only increase N and Ne if the increase in N was substantial and continuous, in which case, elevated rates of inbreeding and genetic drift could ensue. The boost in Ne over multiple generations was in part due to the increase in N which compensated for the effects of the enlarged variance in the genetic contributions between individuals in the whole population that arose from initial supplementation (Wang and Ryman 2001). For the early stages of many captive breeding programs, however, such continual census size increase scenarios may be too optimistic (Waples and Do 1994; Duchesne and Bernatchez 2002). Similarly, this model did not explore how declining populations could affect genetic diversity outcomes (Wang and Ryman 2001), which is another realistic situation in which decisions to initiate captive breeding programs are based. Additionally, and particularly for smaller populations, initial supplementation in the first couple of generations could be detrimental to wild Ne, given the negative demographic effect of sampling the wild population to generate a captive broodstock. Just to potentially overcome such an initial setback, captive rearing and successful supplementation (i.e., an increase in N) would have to be carried out over several more generations (Wang and Ryman 2001; Duchesne and Bernatchez 2002; see also Waples and Do 1994). Finally, neither Wang and Ryman (2001) nor Duchesne and Bernatchez (2002) examined how reduced reproductive success in captive-reared individuals (see below) could affect genetic diversity outcomes in supplemented populations.
Collectively, few generalizations can be currently made with respect to scenarios wherein both captive and wild components are involved in the (i) supplementation of a severely declining population or (ii) reintroduction of an extirpated one. The outcomes of supplementation are difficult to predict based on current modeling, empirical tests of the predictions of these models are very limited, and outcomes may be specific to particular captive breeding programs (Waples 1999; Duchesne and Bernatchez 2002; Naish et al. 2008).
Summary
While empirical and theoretical studies both suggest that most salmonid captive breeding programs can maintain genetic diversity over several captive generations, considerable uncertainty remains with respect to the capability of many programs to maintain genetic diversity over the longer-term. In part, this is because many of the procedures for maintaining captive Ne (Table 2) have only been implemented recently in most salmonid captive breeding programs, often after considerable time had passed since the programs were initiated. Thus, the apparent low Ne in some captive broodstocks might easily be avoided today through the use of such procedures. On the other hand, despite the plethora of procedures available to reduce the loss of genetic diversity in captivity through the maintenance of Ne (Table 2), few have been systematically evaluated for long-term effectiveness. In any event, the varying Nb and Ne estimates of different broodstocks in Table 4 suggest that the capacity of captive breeding programs to maintain genetic diversity in endangered salmonids will likely be case-specific.
Although it is clearly important to maintain genetic diversity within captive-bred/reared populations, a main caveat of Table 4 studies is that they are all based on neutral genetic diversity. Standing levels of neutral genetic diversity may not be a good correlate of quantitative genetic diversity (Reed and Frankham 2001), and the level of either can depend on many factors other than population size (Willi et al. 2006). Recent studies suggest that, on average, quantitative genetic variation may not be lost within small populations as rapidly as neutral genetic diversity, but that levels of quantitative genetic variation can be highly variable among small populations (Willi et al. 2006). Similarly, putatively neutral microsatellite loci are located in parts of the genome that are not subject to natural selection. As a result, allelic characteristics at these loci they may have little or no relationship to survival and fitness, and they tell us nothing about genetic changes at quantitative traits that might be occurring in the captive environment (Reed and Frankham 2001; McKay and Latta 2002). Consequently, even if levels of neutral genetic diversity can be sufficiently maintained in captivity, caution must be exercised in interpreting such data for risk assessment and the ability of captive breeding programs to maintain fitness, a subject treated in detail in the next section.
Can captive breeding programs involving hatcheries conserve fitness within populations?
A lengthy, two-sided debate surrounds the use of harvest augmentation, supplementation and captive breeding programs to either increase salmonid harvest levels, give a demographic boost to declining, at-risk populations, or to recover endangered salmonid populations, respectively. The debate is especially contentious with respect to whether or not hatchery- or captive-rearing, in general, can maintain attributes other than genetic diversity, namely fitness.
A first predominant perspective argues that hatchery- or captive-rearing has negative impacts on the long-term persistence and fitness of wild salmonids. Under this view, hatchery- or captive-rearing leads to unavoidable genetic changes within hatchery-raised salmonids, chiefly through domestication selection (Box 1). Domestication selection results in a fitness reduction when hatchery- or captive-reared fish are then introduced into the wild and breed with wild fish. Such domestication selection can be reduced (Table 2), but it cannot be eliminated entirely (Hindar et al. 1991; Waples 1991b, 1999; Fleming and Gross 1993; Campton 1995; Currens and Busack 1995; Snyder et al. 1996; Reisenbichler and Rubin 1999; Fleming and Petersson 2001; Frankham 2008). Theoretical work also suggests that domestication selection in the hatchery could have significant fitness consequences for a wild population in the case of supplementation programs, even if local, wild-born fish are used to generate hatchery fish each generation (Lynch and O'Hely 2001; Ford 2002; Reisenbichler et al. 2003; Theodorou and Couvet 2004; Goodman 2005). A corollary to this perspective is that hatchery programs, particularly hatchery augmentation and supplementation programs which have been the main focus of the debate, generally fail in their objective of maintaining fitness and of contributing to the natural productivity of wild salmonid populations (Reisenbichler and Rubin 1999; Fleming and Petersson 2001; Reisenbichler et al. 2003).
A second and alternative perspective argues that hatchery- or captive-rearing of salmonids can maintain fitness within populations and play an important role in the supplementation of declining or recovery of endangered salmonid populations (Brannon et al. 2004). A corollary to this perspective is that the genetic risks associated with hatchery- or captive-rearing have been overstated. First, proponents of this view argue that, aside from theoretical studies on these genetic risks, the purported long-term effects of hatchery- or captive-rearing have little or no empirical basis (Incerpi 1996; Rensel 1997; Brannon et al. 2004). Second, in many cases, apparent effects on wild populations have not been differentiated from the effect of management decisions involving the misuse of the hatchery fish (Campton 1995; Rensel 1997; Brannon et al. 2004). Most notably, in many instances, hatchery fish from nonlocal rather than local source populations (Box 1) were stocked into large geographic regions without consideration that they may not have been adapted to those areas (Brannon et al. 2004).
To objectively evaluate the comparative strength of these divergent perspectives in the context of salmonid captive breeding programs, the evidence for each one must firstly be carefully sifted and presented (see Appendix 1 for details of the literature search). Particularly relevant are hatchery- or captive-rearing programs where (i) wild-born broodstock (parents of hatchery fish) are collected from a local river each generation, large numbers of their offspring are raised under captive conditions for a period of time, then released into the same local river, and where (ii) the lifetime fitness performance of the returning hatchery-born adults (or their wild-born offspring) versus wild adults can be directly evaluated in the wild. Under these conditions, one can most legitimately address the likelihood that current captive breeding procedures involving hatcheries will conserve fitness within populations.
Laboratory studies
Table 5 summarizes 30 laboratory studies that evaluated whether hatchery-rearing resulted in genetic changes in hatchery relative to wild salmonids. This list of studies by no means should be viewed as exhaustive as undoubtedly, some other studies have been inadvertently overlooked. The studies in Table 5 were not carried out in the wild, so they only address the potential for genetic changes incurred from captive breeding to have negative impacts on the persistence and adaptability of wild salmonids. Additionally, many of these studies have been based on traditional supplementation practices (see Table 1; footnotes of Table 5) and not necessarily on current captive breeding program procedures.
Table 5.
Laboratory studies that have provided evidence for genetic changes or that found no evidence of genetic changes in phenotypic traits between hatchery-reared and wild populations of salmonid fishes.
| H origin | Artificial selection? | Program | Hatchery gens.a | Species | Trait | Change | Other comments and/or caveats | Reference |
|---|---|---|---|---|---|---|---|---|
| Local | No | S | 1 | Chinook salmon | Predator avoidance | H<W | 2.2% reduction in survival | Fritts et al. (2007) |
| Localb | No | S/H | 4–7 | Rainbow trout | Predator avoidance | H<W | 16.3–28.9% reduction in survival | Berejikian (1995) |
| Local | No | S/H | 5 | Brown trout | Antipredator response | H<W | Ferno and Jarvi (1998) | |
| Local | No | S/H | >5c | Brown trout | Antipredator response | H<W | Johnsson et al. (1996) | |
| Local | No | S | 1 | Chinook salmon | Aggression | 0 | Large number of comparisons (n = 97 to 276, depending on the type of competition) | Pearsons et al. (2007) |
| Local | No | S/H | >5c | Brown trout | Aggression | 0 | Large number of comparisons (n = 287) | Johnsson et al. (1996) |
| Localb | No | S/H | 4–7 | Rainbow trout | Aggression | H> <Wd | Berejikian et al. (1996) | |
| Local | No | S/H | >5c | Brown trout | Juvenile growth rate | H>W | Johnsson et al. (1996) | |
| Local | No | S | 0 | Rainbow trout | Length/weight | H>W | Not reared under common conditions | Kostow (2004) |
| Variance in length/weight | H<W | Not reared under common conditions | ||||||
| Variance in age | H<W | Not reared under common conditions | ||||||
| Local | No | S | 4–6e | Chinook salmon | Female egg size | H<W | Egg size reduction in two populations with considerable supplementation (28–43%); no change in egg size in two other populations with low supplementation (4–16%) | Heath et al. (2003) |
| Genetic effects were not disentangled from environmental effects on egg size | (see Beacham 2003; Fleming et al. 2003) | |||||||
| Local | No | S | 1 | Brown trout | Juvenile growth rate | H>W | Differences were small | Dahl et al. (2006) |
| Local | No | S/H | <1f | Brown trout | Antipredator response | H<W | Not reared under common conditions | Dellefors and Johnsson (1995) |
| Local | No | S/H | >25g | Coho salmon | Aggression | H>W | Not reared under common conditions | Rhodes and Quinn (1998) |
| Local | Yes | H | >10h | Brook trout | Juvenile growth rate | H>W | Hatchery fish were selected for growth | Vincent (1960) |
| Local | Yes | H | >10h | Brook trout | Wariness | H<W | Hatchery fish were selected for growth | Vincent (1960) |
| Locali | Yes | F | 5–7 | Atlantic salmon | Antipredator response | H<W | Hatchery fish were farmed | Johnsson et al. (2001) |
| Local | Yes | F | 1 | Atlantic salmon | Body morphology | H≠W | Hatchery fish were farmed | Fleming et al. (1994) |
| Nonlocal | No | S | 4–5j | Coho salmon | Body morphology | H≠W | Hatchery fish: smaller heads, more streamlined bodies Different ancestral origin of hatchery fish | Swain et al. (1991) |
| Nonlocal | No | S | 2–3k | Rainbow trout | Adult body size | H>W | Inadvertent selection of larger body size | McLean et al. (2005) |
| Nonlocal | No | S | 2–3k | Rainbow trout | Adult run-timing | H≠W | Inadvertent selection of earlier-run timing females for fulfilling hatchery recruitment requirements | McLean et al. (2005) |
| Nonlocal | No | S | ? | Atlantic salmon | Juvenile growth rate | H>W | Kallio-Nyberg and Koljonen (1997) | |
| Nonlocal | No | S | 4–5j | Coho salmon | Aggression | H>W | Swain and Riddell (1990) | |
| Nonlocal | No | H | ? | Atlantic salmon | Aggression | H<W | Norman (1987) | |
| Nonlocal | No | H | ? | Brook trout | Aggression | H>W | Moyle (1969) | |
| Nonlocal | No | S/H | 1–2 | Brown trout | Antipredator response | H<W | Not reared under common conditions | Alvarez and Nicieza (2003) |
| Nonlocal | No | S/H | 1 | Coho salmon | Male spawning performance | H<W | Not reared under common conditions | Fleming and Gross (1993) |
| Nonlocal | No | S | ? | Chinook salmon | Male spawning performance | H<W | Not reared under common conditions | Chebanov and Riddell (1998) |
| Nonlocal | No | S | ? | Chinook salmon | Female spawning performance | H<W | Not reared under common conditions | Chebanov and Riddell (1998) |
| Nonlocal | Yes | F | 5–7 | Atlantic salmon | Male spawning performance | H<W | Not reared under common conditions | Fleming et al. (2000) |
| Nonlocal | Yes | S/H | >5 | Rainbow trout | Antipredator response | H<W | Hatchery fish were selected for growth | Johnsson and Abrahams (1991) |
| Nonlocal | Yes | S | >15l | Masu salmon | Antipredator response | H<W | Chemically simulated predator attack | Yamamoto and Reinhardt (2003) |
| Nonlocal | Yes | F | 5–7 | Atlantic salmon | Antipredator response | H<W | Hatchery fish were farmed | Einum and Fleming (1997) |
| Nonlocal | Yes | F | 5–7 | Atlantic salmon | Antipredator response | H<W | Hatchery fish were farmed | Fleming and Einum (1997) |
| Nonlocal | Yes | F | 4–7 | Atlantic salmon | Juvenile growth rate | H>W | Hatchery fish were farmed | McGinnity et al. (2003) |
| Nonlocal | Yes | S | >15l | Masu salmon | Juvenile growth rate | H>W | Hatchery fish were selected for growth | Yamamoto and Reinhardt (2003) |
| Nonlocal | Yes | S | >15l | Masu salmon | Aggression | 0 | Hatchery fish were selected for growth | Yamamoto and Reinhardt (2003) |
Hatchery and wild populations were compared under common environmental conditions, unless otherwise noted. Statistical significance was based on P < 0.05, unless otherwise noted.
Program type: S, supplementation; C, captive-breeding; H, harvest augmentation; F, farmed/aquaculture. H<W, hatchery population showed reduced aggression/antipredator response/predator avoidance/growth rate/egg size etc. relative to the wild population; H>W, hatchery population showed greater aggression/antipredator response/predator avoidance/growth rate/egg size etc. relative to the wild population; H≠W, hatchery population shifted in other traits from the wild population (details of the main shifts provided); 0, no change observed; ?, not presented or with insufficient detail.
Hatchery generations refer to the number of generations in which the hatchery program involving a local or nonlocal hatchery population had taken place at the time the study was conducted. Note, however, that the hatchery programs themselves might differ. In some cases, naturally-spawning adults are collected from the wild; their offspring are raised in hatcheries for a period of time and then released back into the wild. In other cases, particularly traditional hatchery programs, hatchery fish are regenerated from captive broodstock that were maintained solely in hatcheries. Particularly for older hatchery programs (i.e., more generations), details regarding whether the latter was involved were not always clear.
Hatchery population originated from a wild population that was very geographically close to the wild population used in comparisons. The authors acknowledge that the existence of genetically distinct subpopulations within the same drainage system might have been a confounding effect for their comparison of hatchery and wild steelhead (Berejikian 1995; Berejikian et al. 1996)
Based on a generation time of 3.8 years for anadromous brown trout.
Direction of change depended on age. Newly emergent fry: H<W; 105 days postemergence: H>W.
Based on a generation time of 3.0 years for Chinook salmon.
Not stated in the article how long hatchery fish have been generated for this river. However, in this study, the important aspect was the comparison between some fish born and reared in the hatchery and those that had wild exposure for the first 1+ year of life (see Dellefors and Johnsson 1995).
Based on 100 years of hatchery propagation on the same wild population.
Based on a generation time of 3.0 years for brook trout.
Authors acknowledge that the farmed strain was derived principally from the same wild population where comparisons were being made.
Hatchery populations had been established for four to five generations for five of six hatchery populations, and two generations in one case.
Based on a generation time of 3.0 years for anadromous steelhead.
Based on a 2-year generation time for this masu salmon population (Yamamoto and Reinhardt 2003).
Of the 30 studies comparing hatchery and wild fish in Table 5, only five compared hatchery fish derived from the same local population as the wild fish, and without confounding environmental and genetic differences or some degree of intentional artificial selection in the hatchery, which is not a typical element of captive breeding programs (see Table 5 footnotes). Of these five studies, three compared traits in hatchery and wild salmonids after one generation of captive breeding (Dahl et al. 2006; Fritts et al. 2007; Pearsons et al. 2007). Despite ample statistical power, only small, albeit significant, genetic differences were detected in two of three studies. Most significantly was a 2.2% reduction in survival of first-generation hatchery Chinook salmon relative to wild fish when exposed to natural predators (Fritts et al. 2007). In another study, trait differences that had been detected under hatchery conditions were not found when comparing hatchery and wild fish in the wild (Dahl et al. 2006). The other two studies compared traits in hatchery and wild salmonids after four to six generations of captive rearing (Johnsson et al. 1996; Ferno and Jarvi 1998). Genetic differences were detected in three of four trait comparisons for juvenile growth rate and antipredator response. Finally, as expected, clear genetic differences between hatchery and wild fish were also detected when hatchery fish were nonlocal or had experienced intentional selection (Table 5).
Field studies
Table 6 summarizes 20 studies that have directly evaluated the fitness performance of hatchery and wild salmonid fishes in the wild, with one additional study comparing fitness between fish with different degrees of captive-rearing (Carofinno et al. 2008), and another study comparing the fitness between wild fish of local and nonlocal origin (McGinnity et al. 2004). Again, this list of studies by no means should be viewed as exhaustive as undoubtedly, some other studies have been inadvertently overlooked. Likewise, many of these studies have been based on common supplementation practices rather than current captive breeding procedures (see Table 1; footnotes of Table 6).
Table 6.
Field studies within natural environments that have evaluated the fitness performance of hatchery and wild salmonid fishes
| H origin | Program | G | Species (Reference) | Performance comparison | Major Result | Other comments or potential caveats |
|---|---|---|---|---|---|---|
| Local | S | 2a | Rainbow trout (Reisenbichler and McIntyre 1977) | Egg to smolt survival in four different study streams, for hatchery, wild and F1 hatchery-wild crosses | Wild crosses had significantly greater survival than hatchery fish in 2 of 4 streams (up to double the survival rate)b | Some downstream migrating fish escaped in high water conditions |
| Hatchery-wild hybrids equaled or exceeded the survival and growth performance of wild fish in 2 of 4 streams | ||||||
| Local | S/H | <1 | Chinook salmon (Unwin 1997) | Early life history to adult survival in hatchery fish released at 8–12 months of age, vs. wild fish | Lower survival rates in hatchery fish relative to their initial size advantage upon release | Based on hatchery rearing environment; not necessarily indicative of genetic change Hatchery fish were larger than wild fish when released into the wild |
| Local | S/H | <1 | Coho salmon (Rhodes and Quinn 1999) | Early life history to smolt survival in two streams between a test group of fish exposed to 3 months of hatchery rearing postemergence before release into streams and a test group of the same genetic source of fish released at emergence | No difference in survival between the hatchery and wild test groups | Hatchery fish were 10% larger than wild fish when released into the wild |
| Local | S | <1 | Brown trout (Bohlin et al. 2002) | Age 1 to 2 survival in a stream between a test group of fish hatchery reared for one year before release and a test group of the same genetic source of fish released at emergence | No difference in survival between the hatchery and wild test groups | Hatchery fish were 20–40% larger than wild fish when released into the wild |
| Local | S | 7 | Brown trout (Dannewitz et al. 2003) | Egg to smolt survival in an experimental stream | No differences in survival, growth, condition and smolt age between hatchery and wild fish, despite genetic differences in life-history traits and behaviour under laboratory conditions | Each generation, hatchery fish were reared until 2 years of age and then released; only returning adult hatchery fish were used to produce the next generation Wild population was mainly composed of hatchery predecessors |
| Local | S | 20 | Coho salmon (Ford et al. 2006) | Offspring produced by hatchery and wild fish | No differences mean offspring numbers produced by hatchery or wild fish | Wild population was mainly composed of hatchery predecessors |
| Local | S | 1 | Brown trout (Dahl et al. 2006) | Age 1–2 survival and growth in a stream between hatchery fish, wild fish hand their F1 hybrids after hatchery rearing for one year under common conditions | No differences in survival and growth and body morphology between hatchery and wild fish, or their hybrids | Wild population composed of a large degree of hatchery ancestry (Palm et al. 2003) |
| Local | S | 1 | Rainbow trout (Araki et al. 2007b) | Lifetime performance between supplemental hatchery and wild fish and their F1 hybrids | No significant differences in reproductive success between supplemental hatchery fish and wild fish | Supplemental hatchery fish had wild-born parents, and were released into the wild as juveniles (1 year-old smolts) |
| No significant differences in reproductive success between supplemental hatchery/wild hybrids and wild fish | ||||||
| Local | S | 2 | Rainbow trout (Araki et al. 2007c) | Lifetime performance between supplemental hatchery-wild crosses and wild crosses with different histories of captive-rearing in the previous generation | Reduced reproductive success in hatchery-wild fish relative to wild fish (60%) | All crosses had wild-born parents (but grandparents of the hatchery-wild fish had also experienced captive-rearing), and were released into the wild as juveniles (1 year-old smolts) |
| Local | S | <1c | Rainbow trout (Carofinno et al. 2008) | Fry to smolt survival of fish raised in the hatchery to the fry stage from (i) parents raised in the hatchery to the fry stage (Ha); (ii) parents raised in the hatchery to age 1 (Hb) | Reduced survival in Hb relative to Ha fish (64–75%) | Potential for maternal effects to affect the performance of Ha versus Hb fish |
| Nonlocal | S/H | 4–6d | Coho salmon (Nickelson et al. 1986) | Comparison of run-timing and reproductive success between hatchery and wild salmon in 15 streams | Hatchery fish produced fewer juveniles than wild fish in 15 streams Earlier return of adults to rivers in stocked streams | Nonlocal hatchery fish stocked at larger sizes than wild fish; stocking densities were higher than natural densities; hatchery population origin had earlier run-timing |
| Nonlocal | S/H | 8.6 | Rainbow trout (Chilcote et al. 1986; Leider et al. 1990) | Reproductive success between hatchery and wild adults | Poorer reproductive success in hatchery relative to wild fish | Hatchery fish were intentionally selected for earlier run-timing and larger size |
| Nonlocal | S/H | ? | Rainbow trout (Chilcote 2003) | Relationship between wild population productivity and the proportion of hatchery fish in each of 12 mixed populations | Negative relationship between population productivity (recruits per spawner) and the proportion of the population comprised of hatchery fish (12 hatchery/wild populations over 15 years) | Nonlocal hatchery fish predominantly, based on conventional stocking Not a causal relationship – many hatchery programs were in areas where freshwater habitat was severely degradated |
| Nonlocal | S | >10e | Rainbow trout (Kostow et al. 2003). | Lifetime performance between a nonlocal hatchery strain and wild fish | Reduced survival in hatchery fish relative to wild fish (33%) | Not raised in common environments; hatchery fish raised to smolt stage in the hatchery |
| Nonlocal | S | 10f | Rainbow trout (Miller et al. 2004) | Early life history to age 1+ survival between hatchery fish, wild fishe, and their F1 hybrids in four geographic proximate streams | Reduced survival in hatchery fish relative to wild fish (21%) in three of four streams over two sampling years | |
| Nonlocal | S | >10e | Rainbow trout (Araki et al. 2007b) | Lifetime performance between two traditional, nonlocal hatchery strains and wild fish | Reduced reproductive success in both traditional hatchery strains relative to wild fish (6–11% and 31–45%, respectively, in different years | Traditional hatchery fish had hatchery-born parents, and were released into the wild as young juveniles or smolts |
| Nonlocal | S | 0 | Atlantic salmon (McGinnity et al. 2004) | Lifetime performance between nonlocal and local wild fish in the latter's home environment | Reduced reproductive (lifetime) success in the nonlocal relative to local wild fish (35%) | Nonlocal/local wild fish raised under common environmental conditions from the eyed-embryo stage; potential for environmental maternal effects from different populations to affect offspring performance |
| Nonlocal | F | 5–7 | Atlantic salmon (Fleming et al. 2000) | Lifetime performance between farmed and wild fish, and their F1 hybrids | Reduced survival in farmed fish (16% relative to wild fish) | Hatchery fish were farmed under intentional artificial selection |
| Nonlocal | F | 4–7 | Atlantic salmon (McGinnity et al. 2003) | Lifetime performance between farmed and wild fish, and their F1 and F2 generation hybrids | Reduced survival in farmed fish (2% relative to wild fish); hybrids had lower survival relative to wild fish (27–89%) | Hatchery fish were farmed under intentional artificial selection |
| Nonlocal | S | 5g | Rainbow trout (McLean et al. 2004) | Lifetime reproductive success between hatchery and wild females | Reduced reproductive success in hatchery females relative to wild females in two different years (4.4–7.0%) | |
| Nonlocal | H | 12.5 | Brown trout (Hansen 2002) | Lifetime reproductive success between hatchery and wild fish | Reduced reproductive success of hatchery fish relative to wild fish (9.4%) | Reproductive success inferred from assignment of wild-caught individuals to wild and hatchery components of the populations, and levels of hatchery stocking |
G, number of hatchery generations as in Table 5. Program type: S, supplementation; C, captive-breeding; H, harvest augmentation; F, farmed/aquaculture.
Most hatchery fish used were no more than two generations, but there were no details in the study as to how many exceeded this.
Consistent survival differences were only found in two of four streams over multiple sampling periods.
Based on a generalized hatchery strain initiated in the late 1960s and a generation time of 3 years in rainbow trout (Miller et al. 2004).
Based on a 3.0-year generation time in coho salmon.
Details not provided in the paper, but was assumed based on the use of a generalized hatchery strain that was initiated in the 1950s (Araki et al. 2007b).
Wild fish were naturalized trout that had been originally introduced from the Pacific Northwest into the Laurentian Great Lakes (Miller et al. 2004).
Details not provided in the paper, but was assumed based on the use of a generalized hatchery strain that was initiated at the earliest in the 1970s (McLean et al. 2004).
Of these 20 studies comparing hatchery and wild fish in Table 6, nine compared hatchery fish derived from the same local population as the wild fish. Of these nine studies, three detected survival differences between hatchery and wild fish (Reisenbichler and McIntyre 1977; Unwin 1997; Araki et al. 2007c). However, the lifetime performance of second generation hatchery and wild fish in Reisenbichler and McIntyre (1977) differed in only two of four stream comparisons (where hatchery fish survival was lower), and the Unwin (1997) study was confounded by rearing hatchery fish for 8–12 months in captivity before release into the wild (from Brannon et al. 2004; Table 6). In addition, all studies finding no survival differences must be considered with caution because (i) hatchery fish were larger than wild fish when released into the wild (Rhodes and Quinn 1999; Bohlin et al. 2002), (ii) hatchery fish comprised much of the ‘wild’ population for many generations before studies were undertaken (Dannewitz et al. 2003; Ford et al. 2006), (iii) hatchery-wild performance comparisons were not carried out over the entire life cycle (Dahl et al. 2006), or (iv) hatchery fish had temporarily different rearing environments than wild fish (Araki et al. 2007b; discussed in detail below) (Table 5). On the other hand, unanimously, hatchery fish had inferior fitness when they were nonlocal or had been under intentional selection (Table 6).
To date, Araki et al. (2007b,c) are the only studies that have evaluated whether a supplementation program with some analogous features to many current captive breeding programs can provide a boost to the size of a wild population without fitness costs over one or two generations. Based on steelhead trout (Oncorhynchus mykiss), the program used wild-born broodstock (parents of hatchery fish) that were collected each generation and from which more numbers of offspring were raised in a hatchery for a period of time before being released into the same local river as 1-year old, juvenile smolts. The program also used DNA pedigree information to avoid kinship matings of hatchery-reared fish, and hatchery-reared individuals were released into the wild at ‘normal’ body sizes and dates conducive to survival, features that should reduce the genetic risks posed to wild populations from captive-rearing (Table 2).
For a single generation, Araki et al. (2007b) compared the reproductive success of returning, wild adults to that of local hatchery adults and of adults from a nonlocal (‘traditional’) hatchery strain raised under hatchery conditions for several generations. Consistent with what would be expected if captive breeding programs use local broodstock and minimize the time that individuals are kept in captivity, the authors found (i) no differences in reproductive success between local hatchery fish and wild fish, (ii) no differences in reproductive success between local hatchery-wild crosses and wild-wild crosses, but (iii) lower reproductive success in nonlocal hatchery fish relative to wild fish. The results were therefore encouraging because they suggested that short-term captive-rearing programs of one generation might be capable of generating fish with quasi-equal fitness to that of wild fish. Still, Araki et al. (2007b) acknowledged that despite having reasonable statistical power (80%), they might have failed in some cases to detect up to 10–15% lower reproductive success in local hatchery relative to wild fish. The study also could not rule out the possibility that initial differences in rearing environments between the local hatchery and wild fish affected the former's fitness performance in the wild (Araki et al. 2007c).
Araki et al. (2007c) avoided this problem by comparing the reproductive success of captive-reared individuals with different histories of captive breeding in the previous generation. Specifically, they compared captive-reared wild × wild crosses with captive-reared hatchery × wild crosses (again, from the same population; egg-to-juvenile stage captive rearing). Each type of crosses shared the same generation in captivity under a common rearing environment, but the hatchery × wild crosses had half their genome from a captive-bred parent that had also experienced a generation of captive rearing. The two chief results of the study were as follows. First, the captive-reared hatchery × wild fish had only 55–60% of the wild fitness (reproductive success) of the captive-reared wild × wild fish (Araki et al. 2007c). Second, relative to pure wild fish with no history of captive-rearing, and born and returning from sea in the same years (a replication of Araki et al. 2007b), captive-reared wild × wild fish and hatchery × wild fish had only 60% and 31% of the fitness of pure wild fish (Araki et al. 2007c).
The results of Araki et al. (2007c) suggested that a considerable degree of fitness may be lost within captive-bred populations after one or two generations of captive rearing. However, confidence intervals around point estimates of reproductive success were large in both Araki et al. (2007b,c). This might account for the conflicting conclusions regarding whether one generation of captive-rearing leads to or does not lead to a loss of fitness in the wild. In addition, there are nuances of the study's species/supplementation program that might have affected fitness estimates, or that make the study's results difficult to apply to other salmonids or other captive breeding programs (see below). First, steelhead trout often exhibit alternative reproductive ecotypes in the form of anadromous and nonanadromous (‘resident’) fish within the same river system (including the Araki study system). Araki et al. (2007a,b) could only account for the fitness of anadromous individuals, but it was apparent that nonanadromous males were the fathers of many anadromous offspring. If anadromous, hatchery-reared fish generate a greater proportion of nonanadromous offspring than anadromous wild fish, or vice-versa, then the relative fitness of hatchery-reared anadromous fish relative to wild fish in these studies would have been underestimated or overestimated, respectively. Second, steelhead are often raised in hatcheries for a whole year to achieve a body size conducive to smoltifying which will increase survival chances in the wild (Araki et al. 2008). This period of time in the hatchery is greater than in other salmonids (e.g., Chinook, chum, and pink salmon), where smoltifying can occur either just after emergence or several months in freshwater, and so there may be more time for fitness effects to arise (Araki et al. 2008). Third, in many cases, hatchery- or captive-rearing programs for steelhead trout, and Chinook and coho salmon, accelerate growth rates and smoltification to achieve larger yearling smolts (Mahnken et al. 1982; Dickhoff et al. 1995; ODFW and USFWS 1996; Kostow 2004), but this may not apply to other species. Fourth, results from supplementation or captive breeding programs that raise juveniles to the smolt stage in salmonids (e.g., steelhead) might not be applicable to programs that involve adult or very early life-history stage releases, such as recently initiated Canadian live-gene banking programs for Atlantic salmon (see below).
A final informative study is that of Carofinno et al. (2008), also on steelhead and based on fish derived from the same population and raised in the hatchery to the fry stage. The authors compared early life-history (fry stage)-to-smolt survival in the wild of fish derived from parents that had been raised in the hatchery to the fry stage (Ha) versus fish derived from parents raised in the hatchery to age 1(Hb). Fish with Hb parents had a 25–36% lower survival rate than fish with Ha parents (Carofinno et al. 2008). These results were consistent with the hypothesis that the duration of time in the hatchery environment may increase the opportunities for domestication selection and hence reduce the fitness of fish released into the wild. However, it was unclear to what degree maternal effects might have affected the survival of fry from the two groups. Namely, the study assumed that the extra year of hatchery-rearing in mothers of Hb fish had a negligible effect on their own offspring's survival relative to mothers of Ha fish (Carofinno et al. 2008).
Alternative mechanisms to domestication selection
More recent simulations have shown that the severe loss of fitness in captive-reared steelhead trout (Araki et al. 2007c; Carofinno et al. 2008) could, in fact, be explained by domestication selection alone, although these simulations inevitably made a number of assumptions (discussed in Araki et al. 2008). Other mechanisms associated with the captive-breeding process (some already alluded to) might also contribute to fitness declines, but these await empirical testing or exploration in salmonids or other taxa.
First, manipulations during captive-rearing or breeding could elicit unusually high chromosomal abnormalities or epigenetic changes in salmonids, and thereby affect offspring fitness, (O'Reilly and Doyle 2007; Araki et al. 2008). Epigenetic changes such as alterations to DNA or mutations that affect gene regulation have been recently shown to have considerable effects in mammals (Guerrero-Bosagna et al. 2005; Jirtle and Skinner 2007; Reik 2007).
Second, deleterious mutations might accumulate in captive breeding programs that rear juvenile life-history stages because survival from egg to smolt stages in salmonids is typically 85–95% in hatcheries but only 1–5% in the wild (Reisenbichler et al. 2004). Araki et al. (2008) have argued that mutation accumulation is an unlikely explanation, at least in the first few generations of captive breeding. For instance, typical rates of mutation, including in salmonids, are too low to generate large fitness effects over such short time-periods. Still, even though a procedure such as equalizing family sizes has genetic and fitness benefits (i.e., it halves the rate of inbreeding, genetic drift, and domestication selection), it does not prevent within-family selection (Rodriguez-Ramilo et al. 2006). The procedure still has the potential to increase the likelihood that new mutations arising during the captive-breeding program will become fixed from domestication selection, in this case, because of a relaxation of natural selection in the captive environment (Bryant and Reed 1999; Rodriguez-Ramilo et al. 2006). Nevertheless, the only empirical treatment of this topic involving fruitflies suggests that this may not be a great concern, even for large captive populations and long periods of captivity (Rodriguez-Ramilo et al. 2006).
Third, maternal effects are common in early life history traits of salmonids (Einum and Fleming 1999; Heath et al. 1999; Perry et al. 2005). These effects might influence the fitness of captive-reared fish if their mothers had experienced a period of time in the hatchery, as environmental variation in the captive environment (relative to the natural environment) may elicit plastic changes in reproductive investment. For instance, female salmonids raised in hatcheries tend to exhibit smaller egg sizes than wild females that are not necessarily genetically based (Jonsson et al. 1996; Fleming et al. 2003), and depending on environmental conditions, smaller salmonid offspring generated from smaller eggs may have reduced fitness (Einum and Fleming 1999, 2000).
Finally, prevention of free mate choice for adults during captive-breeding might reduce the fitness of captive-reared offspring (Berejikian et al. 2004). This may specifically inhibit sexual selection and the benefits gained from mating with differentiated partners in genes associated with improved immune responses [e.g. major histocompatibility (MHC) genes]. Indeed, in several salmonids, it appears that males and females seek out partners with maximal or at least intermediate MHC dissimilarity (Landry et al. 2001; Foresberg et al. 2007).
Other qualifications with applying current knowledge to ‘current’ captive breeding programs
Although currently lacking critical empirical assessment in any salmonid (or to my knowledge any other organism besides fruitflies), captive breeding programs adopting many recent procedures (see details in Table 2) might reduce the severity of domestication selection or captive generations in a number of ways that could mitigate fitness reductions in captivity. These procedures may be especially invaluable to programs dealing with the last remaining wild founders from a population that has become extirpated from the wild, given that some domestication selection in capacity is likely unavoidable in such cases.
For instance, Atlantic salmon live-gene banking programs recently initiated in eastern Canada have individuals raised mainly or solely in the wild up to the end of juvenile stages, with the captive phase being the marine (subadult-adult) stage of the lifecycle because salmon are unable to survive in the wild at this stage for currently unknown reasons (O'Reilly and Doyle 2007). In salmonids, wild exposure at the juvenile stages may be especially effective at reducing domestication selection, because this is a stage when mortality in the wild is especially high (Waples 1999; Quinn 2005).
These same programs also equalize family sizes in captivity and at the time of release into the wild (O'Reilly and Doyle 2007). Theoretical and empirical studies (King 1965; Allendorf 1986, 1993; Frankham et al. 2000; Allendorf and Luikart 2007) support that this procedure alone should halve domestication selection. However, the only empirical study conducted to date (on fruitflies) did not find that the procedure minimized the loss of fitness upon the return of populations into the wild (Frankham et al. 2000). Additionally, an inherent trade-off exists in subsequently equalizing family size following a period of exposure to the wild environment. While this may increase levels of neutral genetic diversity in the successive captive broodstock, it may negate the fitness benefits accrued to the population from having natural selection disproportionately favour some families more than others during the period of wild exposure (Box 2). Such a trade-off is perhaps one of the most perplexing issues facing captive breeding programs that attempt to conserve both genetic diversity and fitness, given that conserving each has its merits (Box 2).
Box 2. Trade-offs between conserving genetic diversity and fitness: equalize family sizes following wild exposure?
Owing to its potential advantages for reducing domestication selection in captivity, there is growing interest in having captive-bred individuals exposed to the wild for at least some portion of the lifecycle (e.g., Hebdon et al. 2004; O'Reilly and Doyle 2007). However, following a period of wild exposure, an unavoidable trade-off exists between retaining genetic diversity and fitness when generating the new captive broodstock. Casual arguments for conserving genetic diversity versus fitness might proceed as follows, and striking a balance between them may very well depend on the specific case:
‘Genetic diversity’: equalization of family sizes following wild exposure is essential to maximize the retention of genetic diversity when generating the new captive broodstock.
‘Fitness’: but equalizing family sizes following wild exposure would reduce (in theory, halve) the fitness benefits accrued in the wild if some family genotypes are disproportionately favored over others by natural selection. It is individuals from these better-surviving families that should be used disproportionately to generate the new captive broodstock.
‘Genetic diversity’: but this assumes that the families with higher survival at the life-history stage exposed to the wild (e.g., juvenile) would also have higher survival at other stages (e.g., adult). One cannot rule out that inter-family survival varies at different life history stages. Additionally, even with equalizing family sizes after wild exposure, the benefits of exposing genotypes within families to natural selection would still be gained. Furthermore, the disproportionate use of individuals from better-surviving families for generating the new broodstock would result in an irreversible loss of genetic diversity. Some families would be under-represented and others potentially not represented at all. Such diversity may be important for the population to respond to future environmental change.
‘Fitness’: perhaps, but there is uncertainty in what the future environmental conditions might be for the reintroduced captive population. Disproportionately using individuals from families with a greater fitness performance is most in line with what existing conditions in the wild can support. This practice should improve the likelihood that the reintroduced population will become self-sustaining.
‘Genetic diversity’: perhaps, but there may be temporal variability in selective pressures within the wild environment. Captive-bred families favored by natural selection in the wild this year or the next might not be those favored several years or a decade down the road.
Cryopreserved sperm obtained from males in the founder or early generations of captivity could also be used to fertilize female eggs in subsequent generations (Sonesson et al. 2002; discussed in detail below). This practice could mitigate the loss of fitness in captivity due to domestication selection or the relaxation of natural selection in captivity, by minimizing captive generations before reintroduction in the wild. The technique has been initiated in recently commenced live-gene banking programs of Atlantic salmon in Norway and Canada (O'Reilly and Doyle 2007), but like any tool, it has disadvantages that merit consideration as well (discussed below).
Allowing captive-reared adults, or adults that have had some degree of captive-rearing, to also breed in the wild and thus have free mate choice, may generate offspring that have benefitted from sexual selection and whose parents have had exposure to natural breeding conditions and breeding grounds (Berejikian et al. 2004; O'Reilly and Doyle 2007). One potential constraint of the procedure is that it requires the capture of some offspring from the wild to produce the next captive generation, and this may be resource/labour intensive. The procedure is currently being attempted as part of some Pacific salmon captive breeding programs (Berejikian et al. 2004) and Atlantic salmon live-gene banking programs in eastern Canada (P. O'Reilly, DFO, Halifax, Canada, personal communication).
Increasingly, hatchery-rearing procedures or environments are also being modified to more closely resemble the natural environment. Modifications include reduced juvenile densities, overhead or submerged cover, naturally coloured substrate, antipredator behavior conditioning, subsurface rather than overhead feeding, and even net-pen rearing in natural environments (Maynard et al. 1996, 2004; Hebdon et al. 2004; Reisenbichler 2004). Reisenbichler (2004) pointed out that the effects of seminatural environments on potentially reducing domestication selection have not been empirically tested in salmonids, and he discussed two potential approaches for assessing this.
Summary
Considerable uncertainty remains with respect to the short- and long-term fitness effects of captive breeding in salmonids, despite the numerous laboratory and field studies conducted to date on the performance of hatchery-reared and wild salmonids. Most of these studies are not relevant to the question of whether captive breeding programs adopting current procedures (Table 2) can recover endangered populations and conserve fitness: they either used nonlocal hatchery strains in comparisons with wild fish or hatchery strains that had undergone artificial selection, their experimental design could have affected the performance of hatchery fish, and/or they did not truly examine the outcomes of current captive breeding procedures.
The most relevant studies to date also appear to have had limited statistical power to make general conclusions regarding whether or not one generation of captive-rearing can reduce fitness in the wild (Araki et al. 2007b,c). Caveats aside, the studies of Araki et al. (2007c) and Carofinno et al. (2008) do raise concerns that captive breeding has at least the potential to substantially reduce fitness within wild populations after only 1 year (i.e., within a single generation) to up to two generations of captive-rearing, at one or more life-history stages. Furthermore, as discussed by Hard (1995) and Waples (1999), the power of even the most ambitious monitoring programs to statistically detect a captive-breeding effect on phenotypic and life history traits is likely very low because natural variability in the same traits is very high. This means that the effects of captive-breeding might only be detected long after considerable harm to wild fish has occurred (Waples 1999). On the other hand, for several reasons, the rate to which fitness was lost in Araki et al. (2007c) (10–40%, generation one; another 40%, generation two) might not be a general phenomenon in other salmonid populations or species, or in captive-breeding programs such as live-gene banking (see below). As a result, clearer resolution of the magnitude of potential fitness effects of captive-breeding/rearing awaits further study.
Interestingly, fitness reductions in hatchery-reared salmonids detected in laboratory studies were not as strong as the Araki study (2.2–29%, over one to four generations of hatchery-rearing: Fritts et al. 2007; Berejikian 1995; see Table 5 for caveats). This provides a cautionary note that laboratory studies, especially those not considering correlational selection between traits by evaluating only one or a few traits separately, likely underestimate the degree to which fitness is reduced in the wild from the captive-breeding/rearing process (e.g., Hard 1995, 2004; Knudsen et al. 2006).
Studies involving nonlocal hatchery fish also suggest that fitness reductions will become elevated with increasing generations of manipulation or rearing in the captive environment (see also Araki et al. 2008; Carofinno et al. 2008). Indeed, many of the poorest performances of hatchery fish relative to wild fish involved nonlocal hatchery strains that had been in captivity for greater than five generations or that had undergone intentional artificial selection (e.g., McGinnity et al. 2003; McLean et al. 2004; Miller et al. 2004; Araki et al. 2007b; Table 6).
Finally, a major issue meriting further debate and study pertains to the trade-offs between maintaining genetic diversity and fitness of captive broodstocks (Box 2; see also the section below on whether to use single versus multiple facilities to conserve genetic diversity and fitness). For instance, there are clear fitness benefits to exposing individuals to existing conditions in the wild for some period of their lifecycle. There are also clear benefits to equalizing family sizes after a period of wild exposure to maintain neutral genetic diversity. Yet, this may also reduce the fitness benefits that were accrued during the period of wild exposure.
Can captive-reared lines be reintroduced successfully as self-sustaining populations if/when the threats are removed?
Reintroduction attempts of a variety of captive-reared endangered species or populations into the wild have historically had mixed success (Griffith et al. 1989; Wolf et al. 1996, 1998; Fischer and Lindenmayer 2000; Frankham 2008). Wolf et al. (1996) found that 53% of avian and mammalian reintroductions were successful in leading to apparently self-sustaining populations (Box 3), whereas another global review of 145 reintroduction programs of captive-bred animals, mainly vertebrates, found only 16 cases (11%) of successfully established wild populations (Beck et al. 1994). However, owing to the earlier dates in which a considerable portion of the studies within these reviews were conducted, many of these reintroduction attempts might have failed because the reintroduction programs did not account for all the prerequisites for success identified in later documentation, such as mitigating the factors originally leading to extirpation, behavioural deficiencies of the released animals, or improper release dates (e.g., IUCN 1998; acknowledged in Beck et al. 1994; Snyder et al. 1996; Wolf et al. 1996). Additionally, it has only been widely recognized more recently that domestication selection may affect reintroduction success (Frankham et al. 2002; Frankham 2008). Thus, many historical captive breeding programs probably did not adopt procedures to reduce domestication selection or the loss of genetic diversity in captivity (see Table 2).
Box 3. When is a reintroduction ‘successful’?
Seddon (1999) summarized a variety of definitions that have been considered regarding what constitutes a successful reintroduction. The definitions put forth have included (i) breeding by the first-wild born generation, (ii) a breeding population with recruitment exceeding adult death rates for 3 years, (iii) an unsupported wild population of a minimum of 500 individuals, (iv) establishment of a self-sustaining population (Griffith et al. 1989; Beck et al. 1994; Sarrazin and Barbault 1996). Evidently, the applicability of any one criterion might be limited depending on the life history characteristics of the species targeted for reintroduction (Seddon 1999).
For the purposes of this review, I consider a salmonid reintroduction to be successful if it leads to the establishment of a self-sustaining population in the native species’ range. I define a self-sustaining population as a population that persists for multiple generations in the absence of any human intervention, such as supplementation, artificial habitat enhancement or any degree of captive breeding or genetic modification. In many ways, this definition is most in line with one of the ultimate goals of captive-breeding programs; that is, to re-establish a species in an area which was once part of its historical range (IUCN 1998). The definition is also formulated with the hope that self sustainability will represent the long-term persistence of the reintroduced species, but does not assume that self sustainability is equated with long-term persistence. For instance, a salmonid population could be reintroduced as a self-sustaining population for several generations, but then a new threat might render it no longer viable (e.g., climate change, introduced pathogens).
Bearing these caveats in mind, I reviewed cases where reintroductions of salmonids have been attempted and whether these were successful in generating self-sustaining populations if/when the threats imposed on them were removed (Box 3; see also Appendix 1). I also considered this issue from four additional contexts. First, was there any evidence that hatchery-reared fish in supplementation programs provided net long-term benefits to wild salmonid populations? These programs differ somewhat from reintroducing captive-reared salmonids into formerly occupied habitats, but they provide another context for assessing the potential for captive-reared lines to translate into self-sustaining populations. Second, do general patterns of successful/unsuccessful transplants of salmonids within and outside of their native ranges shed light on why reintroductions of endangered salmonids within their species’ ranges might succeed or fail? Third, how can one improve the chances of successful reintroduction if the wild environment has changed by the time the captive population can be reintroduced? Fourth, was there any indication that particular salmonid species or life-history types may be more difficult to reintroduce successfully?
Summary of salmonid reintroductions in native ranges using hatchery- or captive-reared fish
Table 7 summarizes cases in which hatchery-or captive-reared salmonids have been used to reintroduce extirpated or ‘near-extirpated’ populations into previously occupied habitats, the vast majority of which were anadromous or with other complex life-histories (e.g., lake migratory). This list of studies by no means should be viewed as exhaustive as undoubtedly, some other systems have been inadvertently overlooked. There is a species bias, with Atlantic and Chinook salmon representing 18 of 31 of the ‘population systems’. In 16 of 31 population systems, captive-breeding programs are too recent to assess whether they will ultimately be successful or not in translating into self-sustaining populations. In six of the remaining 15 systems, reintroductions have been unsuccessful at generating self-sustaining populations. Reintroduction failures have occurred even after 30 years of reintroduction attempts in some cases (Table 7). Reintroduction failure over this timeframe might not be too surprising given that many historical programs probably did not adopt procedures that are implemented in current captive breeding programs (Table 2). However, the list of reintroduction failures also includes two captive breeding programs that incorporate many of these procedures (e.g., Atlantic salmon in Maine; winter-run Chinook salmon, California). Importantly, not all of the obvious factors that were likely contributing to reintroduction failure had been removed in any of these six systems, regardless of whether current captive breeding procedures had or had not been adopted. While these factors were often multifaceted, it is noteworthy that environmental changes to habitat were implicated in all six systems with unsuccessful reintroductions (Table 7).
Table 7.
Characteristics of salmonid reintroductions involving hatchery- or captive-reared fish.
| Species and population/region | Life-history type | Population status | Self-sustaining population? | Years of restoration/ reintroduction efforts | Factors involved or reputed to being involved in reintroduction successes or failures | References |
|---|---|---|---|---|---|---|
| Atlantic salmon – Lake Ontario (Canada/USA) | Lake migratory | Extirpated | No | 30–50 | Environmental changes in juvenile and adult habitats | McKenna and Johnson (2005); Scott et al. (2005a,b); COSEWIC 2006a |
| Competition with nonindigenous salmonids | ||||||
| Inadequate knowledge of a proper source population for reintroduction | ||||||
| Atlantic salmon – Inner Bay of Fundy (Nova Scotia, New Brunswick, Canada) | Anadromous | Near-extirpated | ?b | 6–10a | Environmental changes in juvenile and adult habitats | COSEWIC 2006b; Claytor et al. (2006); O'Reilly and Doyle (2007) P. O'Reilly, personal communication; Fraser et al. (2007b) |
| Interactions with farmed salmon | ||||||
| Loss of immigration for persistence from larger surrounding populations | ||||||
| Anadromous | Near-extirpated | ? | 9–10a | Reintroduction attempts started in 2001- | ||
| Stewiacke | Anadromous | Near-extirpated | ? | 9–10a | Reintroduction attempts started in 2001- | |
| Big Salmon | Anadromous | Extirpated | ? | 7–9a | Reintroduction attempts started in 2005- | |
| Great Village | Anadromous | Extirpated | ? | 8a | Reintroduction attempts started in 2005- | |
| Economy | Anadromous | Near-extirpated | ? | 8a | Reintroduction attempts started in 2002- | |
| Gaspereau | Anadromous | Near-extirpated | ? | 6–7 | Live gene banking started in 2002- | |
| Upper Salmon Pointe Wolfe | Anadromous | Extirpated | Yes | 1 | Reintroduction in 1984 following dam removal | Fraser et al. (2007b) |
| Natural straying from nearby, healthy populations | ||||||
| Atlantic salmon – Outer Bay of Fundy (New Brunswick, Canada) | Anadromous | Near-extirpated | ? | 1–3 | Environmental changes in adult habitats Lack of behavioural training Interactions with farmed salmon | Carr et al. (2004) |
| Atlantic salmon – Maine (USA) (eight populations) | Anadromous | Extirpated | Noc | >20a | Environmental changes in juvenile and adult habitats | Baum (1997); U.S. Atlantic Salmon Assessment Committee (2005) |
| Interactions with farmed salmon Dams | ||||||
| Atlantic salmon – Connecticut R. (New England, USA) | Anadromous | Extirpated | Yesd | 30a,d | Hydroelectric dams | Spidle et al. (2004); Ward et al. (2008) |
| Atlantic salmon – Thames R. (UK) | Anadromous | Extirpated | Yese | 28e | Pollution, shipping locks | U.K. Environment Agency (2006a, 2006b, 2007) |
| Atlantic salmon – Rhine R. (France, Germany) | Anadromous | Extirpated | Nof | >15 | Environmental changes to juvenile habitats | Gerlier and Roche (1998) |
| Hydroelectric dams, pollution | ||||||
| Atlantic salmon – Meuse R. (Belgium, France, Netherlands) | Anadromous | Extirpated | Nog | >11 | Hydroelectric dams | Prignon et al. (1999) |
| Atlantic salmon – Norway | Anadromous | Extirpated | Yesh | 3–12 | Artificial liming of rivers to reduce acidification from acid rain | Hesthagen and Larsen (2003) |
| Atlantic salmon – (Eidfjord, Norway) | Anadromous | Near-extirpated | ? | 5–17a | Introduced pathogen, Gyrodactylus salaris | Gausen (1993); data from O'Reilly and Doyle (2007) |
| Bejarelva | Anadromous | Near-extirpated | ? | 14–17a | Reintroduction attempts started in 1998–2001 | |
| Ranaelva | Anadromous | Near-extirpated | ? | 6–16a | Reintroduction attempts started in 2005–2010 | |
| Rossaga | Anadromous | Near-extirpated | ? | 5–16a | Reintroduction attempts started in 2005–2010 | |
| Fusta | Anadromous | Near-extirpated | ? | 12–14a | Reintroduction attempts started in 2010–2015 | |
| Vefsna | Anadromous | Near-extirpated | ? | 10–14a | Reintroduction attempts started in 2010–2015 | |
| Atlantic salmon – (Haukvik, Norway) | Anadromous | Near-extirpated | ? | 4–19a | Introduced pathogen, Gyrodactylus salaris | Gausen (1993); data from O'Reilly and Doyle (2007) |
| Skibutnelva | Anadromous | Near-extirpated | ? | 4–13a | Reintroduction attempts - unknown | |
| Namsen | Anadromous | Near-extirpated | ? | 13–18a | Reintroduction attempts - unknown | |
| Figga | Anadromous | Near-extirpated | ? | 16–18a | Reintroduction attempts started in 2003–2012 | |
| Steinkjerelva | Anadromous | Near-extirpated | ? | 10–19a | Reintroduction attempts started in 2003–2008 | |
| Ogna | Anadromous | Near-extirpated | ? | 9–19a | Reintroduction attempts started in 2003–2008 | |
| Batnfjordselva | Anadromous | Near-extirpated | ? | 9–19a | Reintroduction attempts started in 1995–2001 | |
| Driva | Anadromous | Near-extirpated | ? | 16–18a | ||
| Mana | Anadromous | Near-extirpated | ? | 16–18a | Reintroduction attempts started in 1995–2004 | |
| Innfjordelva | Anadromous | Near-extirpated | ? | 7–15a | Reintroduction attempts started in 2007–2011 | |
| Rauma | Anadromous | Near-extirpated | ? | 15–19a | Reintroduction attempts started in 2007–2011 | |
| Aurelva | Anadromous | Near-extirpated | ? | 18a | Reintroduction attempts started in 1995–1998 | |
| Eidsdalselva | Anadromous | Near-extirpated | ? | 16–19a | Reintroduction attempts started in 1995–2004 | |
| Norddalselva | Anadromous | Near-extirpated | ? | 15–17a | Reintroduction attempts started in 1996–2004 | |
| Valdalselv | Anadromous | Near-extirpated | ? | 16–19a | Reintroduction attempts started in 1995–2004 | |
| Atlantic salmon – (Bjerka, Norway) | Anadromous | Near-extirpated | ? | 11–16a | Introduced pathogen, Gyrodactylus salaris | Gausen (1993); data from O'Reilly and Doyle (2007) |
| Hydroelectric dams, acidification, sea lice, interactions with farmed salmon | ||||||
| Jolstra | Anadromous | Near-extirpated | ? | 11–16a | Reintroduction attempts started in 1999- | |
| Flekke/Guddal | Anadromous | Near-extirpated | ? | 9–14a | Reintroduction attempts started in 2002- | |
| Laerdalselva | Anadromous | Near-extirpated | ? | 4–16a | Reintroduction attempts started in 2006–2010 | |
| Aroyelva | Anadromous | Near-extirpated | ? | 4–16a | Reintroduction attempts started in 1998- | |
| Ekso | Anadromous | Near-extirpated | ? | 4–14a | Reintroduction attempts started in 2000- | |
| Vosso | Anadromous | Near-extirpated | ? | 7–17a | Reintroduction attempts started in 1999- | |
| Loneelva | Anadromous | Near-extirpated | ? | 10–17a | Reintroduction attempts started in 1999–2004 | |
| Oselva | Anadromous | Near-extirpated | ? | 13–16a | Reintroduction attempts started in 2001–2002 | |
| Eidfjordvassdraget | Anadromous | Near-extirpated | ? | 4–14a | Reintroduction attempts started in 2002- | |
| Etneelva | Anadromous | Near-extirpated | ? | 12–17a | Reintroduction attempts started in 1999- | |
| Coaster brook trout, Salvelinus fontinalis, Upper Great Lakes (Canada, USA) | Lake migratory | Near-extirpated | ? | 5–10 | Habitat degradation, overexploitation, competition with nonindigenous salmonids | Volkman et al. (2004) |
| Aurora trout, Salvelinus fontinalis timagamiensis– (Ontario, Canada) | Nonanadromous | Extirpated | Yes | >30 | Reduced acidification in lakes from previously high levels of acid rain | COSEWIC (2000) |
| Lake trout, Salvelinus namaycush, Upper Great Lakes (Canada/USA) | Nonanadromous | Extirpated | No | >30 | Environmental changes to spawning and nursery habitat Invasive sea lamprey predation | Krueger and Ihssen (1995); Page et al. (2005); Bronte et al. (2007) |
| Coho salmon – Yakima R. (WA, USA) | Anadromous | Near-extirpated | Yesi | 10–20 | Habitat restoration Improved adult feeding area conditions Harvest rate reductions | Bosch et al. (2007) |
| Coho salmon – Scott Cr. (CA, USA) | Anadromous | Near-extirpated | ? | >5 | ? | Berejikian et al. (2004) |
| Chinook salmon – winter-run, Sacremento R. (CA, USA) | Anadromous | Near-Extirpated | Noj | >16a | Environmental changes to juvenile and estuarine habitats Interactions with nonnative species | Yoshiyama et al. (2000); Hedrick et al. (1995, 2000a,b); Brown and Michniuk (2007) |
| Chinook salmon – spring-run, Clearwater River (ID, USA) | Anadromous | Extirpated | Yesk | ? | Dam removal | ISRP (2003) |
| Chinook salmon – spring-run, Umatilla River (OR, USA) | Anadromous | Extirpated | ? | 20l | Environmental changes to juvenile and spawning habitats | Rowan (1997); discussed in Waples and Drake (2004) |
| Chinook salmon – Grand Ronde River, Snake River basin (OR, USA) | Anadromous | Near-extirpated | ? | >10 | Environmental changes to juvenile and spawning habitats; hydroelectric dams | Berejikian et al. (2004) |
| Chinook salmon – Salmon River, Snake River basin (ID, USA) | Anadromous | Near-extirpated | ? | >10 | Environmental changes to juvenile and spawning habitats; hydroelectric dams | Berejikian et al. (2004) |
| Chinook salmon – Tucannon River, Columbia River basin (WA, USA) | Anadromous | Near-extirpated | ? | >8 | Environmental changes to juvenile and spawning habitats; hydroelectric dams | Berejikian et al. (2004) |
| Sockeye salmon - Upper Fraser River (British Columbia, Canada) | Anadromous | Extirpated | Yes | 10–60 | Intact spawning and juvenile habitat Source populations with similar ecological and genetic characteristics as extirpated populations Dam removal Natural straying from nearby healthy populations | Withler et al. (2000) |
| Sockeye salmon – Sakinaw Lake (British Columbia, Canada) | Anadromous | Near-extirpated | ? | 5a,m | Environmental changes to juvenile and spawning habitats, incidental harvest in mixed-stock fisheries | COSEWIC (2003a, 2006c) |
| Sockeye salmon – Cultus Lake (British Columbia, Canada) | Anadromous | Near-extirpated | ? | 7a | Shifts in migration timing, incidental harvest in mixed-stock fisheries | COSEWIC (2003b) |
| Sockeye salmon – Redfish Lake, Snake River (ID, USA) | Anadromous | Near-extirpated | Yesn | 17a | Environmental changes to juvenile and spawning habitats, dams | Flagg et al. (1995, 1999, 2004a); Kozfkay et al. 2008) |
| Steelhead (rainbow trout) – East Vancouver Island (British Columbia, Canada) | Anadromous | Near-extirpated | ? | ? | ? | Berejikian et al. (2004) |
| Steelhead (rainbow trout)- Hamma Hamma River (WA, USA) | Anadromous | Near-extirpated | ? | ? | ? | Berejikian et al. (2004) |
| Chum salmon – Hood River (WA, USA) | Anadromous | Near-extirpated | Yeso | 12 | Habitat enhancement | Hood Canal Coordinating Council (2005) |
| Atlantic whitefish (Nova Scotia, Canada) | Nonanadromous/anadromous | Near-extirpated | ? | 3a | Dams, invasive species | Edge and Gilhen (2001); D.F.O. (2006) |
Note that the factors involved or reputed to being involved in reintroduction successes or failures may differ from those (or some of those) involved in the original extirpation/near-extirpation of the wild population.
?, too early to assess if reintroduction is leading to a self-sustaining population.
Recently established live gene banking program or captive breeding program (with some procedures adopted from Table 2) as part of a recovery initiative.
Live gene banking still involved (into its third generation); total wild adult abundance less than a few hundred as of 2006.
Poor adult returns in many rivers; viability of populations still dependent on supplementation up to 2005.
Adult returns of mean N ≍ 200 between 1976 and 1996 (based on smolt- and fry-stocked returns); reintroduction efforts started in 1967 but did not use a geographically proximate source population until 1976. Still dependent on supplementation: ‘not close to sustainability yet’ (Spidle et al. 2004; p. 263).
Adult returns from reintroductions of mean N ≍ 200–300 between 1980 and 2004; N = 0 (2005); N = 2 (2006). Still dependent on supplementation.
Poor adult returns; supplementation still involved.
Poor adult returns; supplementation still involved.
Re-establishment in eight of nine extirpated rivers; population persistence believed to depend on supplementation.
Supplementation/reintroduction still involved.
Supplementation/ reintroduction still involved.
Successful reintroduction of several tributaries following removal of Lewiston Dam.
Natural spawning adults for the first time in over a century but still dependent on supplementation.
Poor adult returns in first two years of rehabilitation through supplementation (N = 99, 2004; N = 27, 2005).
Adult return of 312 hatchery-derived individuals in the eighth to eleventh years of captive breeding (1999–2002). Adult returns of N = 316 (2005) and N = 367 (2006) (Kozfkay et al. 2008); supplementation still involved.
Reintroduction of two extirpated rivers with hatchery fish; supplementation ended in 2004.
Conversely, there were no obvious habitat limitations in the nine population systems, where captive-breeding has led to apparently self-sustaining populations. Yet, in one case, artificial liming of rivers was required to reduce acidification (induced by acid rain) so that Atlantic salmon populations inhabiting them could be self-sustaining (Hesthagen and Larsen 2003). In another case, successful reintroduction of sockeye salmon populations might have been driven by dispersal and gene flow from neighboring, healthy wild populations and not necessarily by captive-reared fish (Withler et al. 2000; see also Pointe Wolfe River Atlantic salmon, inner Bay of Fundy: Fraser et al. 2007b). In four other cases, reintroduced populations might be becoming self-sustaining but they are all still dependent on supplementation (Spidle et al. 2004; U.K. Environment Agency 2006b, 2007, Bosch et al. 2007; Kozfkay et al. 2008).
Consequently, there is little long-term evidence regarding whether captive-reared salmonids can or cannot be reintroduced as self-sustaining populations. This is either because (i) captive breeding programs that adopt a multitude of procedures to reduce domestication selection and the rate of loss of genetic diversity in captivity have been initiated too recently to assess the performance of captive releases in the wild, (ii) reintroduction failures were confounded by not having other threats removed that likely impeded reintroduction success, most notably, habitat loss or change, (iii) apparently successful reintroductions may have been confounded by other factors which could explain the success besides captive-breeding (e.g., natural recolonization, artificial habitat manipulations), or because (iv) reintroduction attempts involving captive-breeding programs are still undergoing supplementation, making it difficult to assess whether the reintroduced populations have truly become self-sustaining. Overall, however, and based on the duration of even more ‘modern’ programs’ (e.g., Hedrick et al. 2000a,b; Flagg et al. 2004a; O'Reilly and Doyle 2007), it would appear that a minimum of 15–20 years will be likely necessary to potentially achieve the conservation goal of establishing a self-sustaining salmonid population. This estimate is based on the realistic amount of time required to initiate a captive-breeding program, carry out reintroduction attempts, and monitor postrelease success after multiple generations.
Additional contexts: supplementation programs
Waples et al. (2007) recently conducted a meta-analysis of 22 major supplementation programs from the Pacific Northwest, specifically examining their ability to provide net long-term benefits to wild Pacific salmon populations. Most programs (17 of 22) used hatchery fish from the local wild population for supplementation, but their data had not previously been summarized and published in the primary literature. For net long-term benefits to occur, Waples et al. (2007) argued that evidence was needed showing that hatchery fish could survive and spawn in the wild, produce viable progeny, and thus contribute to the natural population. Again, this situation is somewhat different from that of reintroducing captive-reared salmonids in an attempt to generate self-sustaining populations into formerly occupied habitats – it more typifies the situation where a captive-breeding program is initiated to supplement a rapidly declining population. Also, Waples et al. (2007) did not examine what procedures (e.g., Table 2) were employed in specific supplementation programs to reduce the potential effects of hatchery-rearing. Bearing these caveats in mind, the major conclusions from the meta-analysis were as follows. First, many supplementation programs have achieved a measure of short-term success in terms of boosting overall numbers of fish, either through high survival of broodstock and/or increases in the number of returning hatchery (captive-bred) adults compared to the wild population (Waples et al. 2007; see also Sharma et al. 2006; Berejikian et al. 2008). Second, in the long-term, and in parallel to the observations and conclusions above, there is considerable uncertainty regarding the ability of supplementation programs to provide net long-term benefits to wild salmonid populations. As a result, these authors highlighted that the lack of empirical demonstration that supplementation provides net long-term benefits to wild salmonids should be a cautionary note to those considering initiating new programs or continuing existing ones (Waples et al. 2007: p. 396).
Species transplants
In light of threats such as habitat degradation that have not been removed and are likely impeding current reintroduction efforts, transplants within and outside of the species’ range of different salmonids provide another context to consider the potential for captive-reared lines to be reintroduced as self-sustaining populations. In a review of anadromous Pacific salmon transplants, all of which would have involved some form of hatchery-rearing, Withler (1982) found no undisputed example of a successful transplantation within a species’ range where there were no obvious physical barriers to natural dispersal. When natural, physical barriers were apparent and removed within species’ ranges, successful transplantations have occurred (Federenko and Shepherd 1986; Burger et al. 2000; Withler et al. 2000; Hendry 2001; Koskinen et al. 2002; Mullins et al. 2003; Thrower et al. 2004). In addition, transplants of hatchery-reared, anadromous salmonids outside of salmonid species’ ranges have been successful at times (Waugh 1980; Crawford 2001; Pascual et al. 2001; Quinn et al. 2001; Pascual and Ciancio 2007; Soto et al. 2007).
These patterns are interesting for two reasons. First, successful introduction of salmonids outside of species’ ranges in the past 30–50 years (and even 100 years) suggests that the historical failure of some reintroductions within salmonid species’ ranges over the same timeframe cannot be solely attributed to poorly developed hatchery- or captive-rearing techniques at the time. Second, where salmonids have historically been capable of dispersing naturally, they have colonized all habitats currently suitable to them. Thus, within their species’ ranges, if anadromous salmonids are not present within a system, there is likely a good reason why they are not (Quinn 2005). An emerging conclusion is that the long-term recovery of endangered salmonids within their species’ ranges is unlikely with captive breeding/rearing, unless the factors that contributed to their initial decline are addressed concurrently. Thus, given the uncertainty about whether the underlying causes of salmonid declines can be identified or remedied, an important societal question meriting debate is, when does one initiate and/or terminate captive breeding?
Wild environment changes
The wild environment of captive salmonid populations might also change dramatically by the time fish can be reintroduced. For instance, there is evidence that the Bay of Fundy, Canada, a region with a number of endangered Atlantic salmon populations, is undergoing ecosystem changes (COSEWIC 2006b). The environment of the Bay may therefore be very different than that of say 15 to 20 years before its salmon populations collapsed, and these changes could have been the major reason for the collapse in the first place (COSEWIC 2006b). Krueger et al. (1991) and Frankham (2008) have suggested that in such a circumstance, the crossing of all captive individuals and/or subpopulations prior to reintroduction would result in a reintroduced population with maximum genetic diversity. Such an approach would presumably lead to a greater likelihood of that captive population evolving the capacity to respond to environmental change. To date, however, no empirical studies (on any species) have addressed this possibility (Frankham 2008), though research on this topic has recently been initiated within live-gene banking programs for Atlantic salmon populations in eastern Canada (P. O'Reilly, DFO, Halifax, personal communication). Still, one potential risk of this approach is that it could lead to an increase in straying to nontarget areas and thereby potentially affect other native populations. For instance, interbreeding of individuals between pink salmon populations resulted in increased straying rates to surrounding populations (Bams 1976). In addition, and especially if the crosses will be carried out at a hierarchical level greater than subpopulations (e.g., at the population level), such a consideration would have to consider the geographic scale at which the crosses were being made and the potential for evolutionary and/or adaptive divergence to exist between the populations. For instance, the advantages of generating greater genetic diversity in the released individuals might be outweighed by the possible disadvantages of outbreeding depression from mixing populations (reviewed in Edmands 2007).
Species and/or life history differences in the chances of successful reintroduction?
Currently, there appears to be insufficient quantitative data on salmonid reintroductions to discern whether different species or life-history types vary in their chances of being successfully reintroduced into previously occupied habitats (Table 7). However, if the ability of a salmonid species to be introduced successfully outside of its native range reflects its ability to be reintroduced into previously occupied parts of its native range, then two points merit consideration. First, anadromous populations, followed by lake migratory populations, may on average be more difficult to reintroduce than freshwater, resident populations. For instance, reviews of salmonid introductions suggest that anadromous salmonid populations do not transplant as well as freshwater species, perhaps because of their more complex requirements in having intricate life histories across multiple environments (Withler 1982; Allendorf and Waples 1996; Utter 2000). Factors involved in freshwater salmonid declines might also be easier to rectify than those occurring across environments utilized by anadromous populations. Second, species such as rainbow trout and brown trout might be easier on average to reintroduce than species such as Atlantic salmon or several other Pacific salmon species, the former having been successfully introduced in many regions throughout the world where the latter have not (Quinn 2005; references therein; Crawford and Muir 2008).
One caveat of these predictions is that they assume the potential fitness consequences of captive-rearing are uniform across species and captive-breeding programs (or even life-history variants within species). But as previously mentioned, this is likely not the case. A sensible but untested hypothesis is that captive-breeding programs elicit the greatest reductions in fitness in species or populations with the greatest life-history and habitat differences between captive and natural conditions (Reisenbichler 2004).
Can the demographic increase to population abundance from captive breeding outweigh the loss of fitness in captivity?
Even captive breeding programs that adopt some procedures to reduce genetic changes during captive-breeding/rearing might result in substantial fitness reductions within wild populations after one or a few generations (Araki et al. 2007c). In other words, no matter how good the intentions, it would appear that as yet, humans have not generated a group of captive-bred/reared fish that on average will perform equally to wild fish once they are released into the wild. On the other hand, it appears that some supplementation programs, at least those involving juvenile releases, can achieve a measure of short-term success in terms of boosting overall numbers of fish (Waples et al. 2007; Berejikian et al. 2008). It would also seem that many salmonid populations with long histories of intense supplementation have not become extinct or severely reduced in abundance. If fitness can be reduced so much and so rapidly by domestication selection, why have not many of these populations experienced rapid declines? Thus, an unresolved enigma in evaluating the likelihood that captive breeding programs can translate into self-sustaining salmonid populations, is whether, and how, increases to population abundance (N) provided by captive-rearing could offset reduced fitness in the wild of captive-reared fish and their progeny. Interestingly, there are numerous examples of the ability of salmonids to evolve rapidly in the wild over several generations (Haugen and Vollestad 2000; Hendry et al. 2000; Quinn et al. 2001; Koskinen et al. 2002). Certainly, then, the possibility exists that a reintroduced population based on captive-reared fish could re-adapt to the wild environment under a similar timeframe.
Consider firstly a simple scenario where the original threats that led to the extirpation of a wild population have been removed and a one-time reintroduction of the captive-reared population is implemented. Owing to inevitable domestication selection in captivity, the captive-reared population has experienced a shift away from the wild optimum in quantitative trait variation related to fitness. Thus, it is now maladapted to the wild environment. Gomulkiewicz and Holt (1995) introduced a model examining conditions under which selection might prevent extinction of the captive-bred population upon reintroduction (Fig. 2). They considered whether such a population could evolve a sufficiently positive intrinsic growth rate (r) at abundance (N) below carrying capacity (K) before extinction from demographic stochasticity took place. Gomulkiewicz and Holt (1995) did not consider density-dependent effects but assumed that extinction risk was elevated below a threshold, critical population size (Nc). In the context of attempting to reintroduce populations with captive-reared fish, the major implication of the model is that an initially maladapted reintroduced population with a negative growth rate could evolve a positive growth rate without going extinct, provided that: (i) genetic diversity was sufficiently high, (ii) fish were not too maladapted initially, and (iii) initial N was large relative to Nc to allow the reintroduced population to persist long enough for evolution to occur (Fig. 2). Note that these conclusions are also consistent with those in previous sections relating to the importance of maintaining as high a Ne as possible in captivity (Frankham et al. 2002), and maximizing genetic diversity in the captive-release generation (i.e., just before reintroduction; Frankham 2008). Note also, however, that there is an inherent tension between keeping Ne (and genetic diversity) as high as possible and reducing domestication selection in captivity, a subject treated in detail in the next section.
Figure 2.
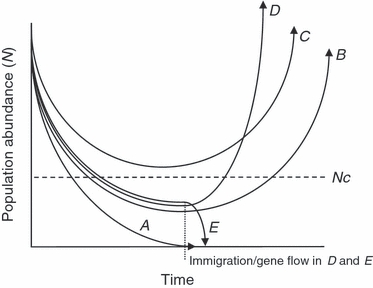
Potential relationships between reintroduced population abundance and extinction risk with or without evolution by natural selection, modified from Gomulkiewicz and Holt (1995) (see also Kinnison and Hairston 2007). Population growth is density-independent and Nc represents a threshold abundance below which extinction risk is high. Without evolution, or when evolution cannot achieve replacement in the absence of gene flow, reintroduced populations decline to extinction (A). Evolution is insufficient to prevent the reintroduced population from being at a high risk of extinction, but it allows the population to avoid extinction if the population persists (B). Evolution is sufficient to prevent the population from being at a high risk of extinction (C). Immigration and resultant gene flow allows the evolving population to avoid extinction more rapidly (D) than in its absence (B). Immigration and resultant gene flow increases the susceptibility of extinction to the evolving population (E) than in its absence. All cases assume the same reduction in wild fitness within the captive-bred population before reintroduction.
Gomulkiewicz and Holt's (1995) model thus also assumed that mechanisms exist that allow for positive population growth despite reintroduction of maladapted individuals, and similarly, that at some point following the initial drop in N from K, evolutionary contributions to population growth would not be countered by density-dependent factors (Gomulkiewicz and Holt 1995; Tufto 2001; Kinnison and Hairston 2007). Unfortunately, empirical assessments of these assumptions are currently very limited in salmonids. For instance, analogous to reintroducing maladapted, captive-bred fish to a previously occupied habitat, Kinnison and Hairston (2007) and Kinnison et al. (2008) noted how founding or postfounding contributions might influence evolution and resultant population growth in salmon during colonisation of new habitat.
While it is easy to envision that evolution within a maladapted, reintroduced population could be sufficient in and of itself to result in a self-sustaining population, in many cases this might not happen before the reintroduced population succumbs to extinction through demographic stochasticity (a delay in ‘A’ from Fig. 2). Under what conditions, then, could repeated reintroduction events increase the likelihood of successful overall reintroduction? On one hand, recurrent immigration from a maladapted, captive-reared source could demographically rescue a young, reintroduced population because the population literally never becomes extinct (Holt 1993). The infusion of genetic diversity through ‘low’, constant gene flow (perhaps even only one or two migrants per generation), particularly in the early stages of reintroduction, might also generate the novel variation required by selection to shift a population's growth from negative to positive, as well as to offset traditional problems associated with small population size (e.g., inbreeding, genetic drift) (Fig. 2D; Gomulkiewicz and Holt 1995; Tufto 2001; Tallmon et al. 2004; Kinnison and Hairston 2007). Indeed, repeated influxes of immigrants have apparently been involved in some successful introductions or species invasions (Lambrinos 2004; Roman and Darling 2007). On the other hand, immigrants would in general be maladapted to the local environment and resultant gene flow with the reintroduced population as it grows might constrain the effects of ongoing selection (Fig. 2E; Gomulkiewicz and Holt 1995; Kinnison and Hairston 2007). As a rough guide based on Gomulkiewicz and Holt (1995), the reciprocal of the time a population first reaches low densities (Nc) following the initial reintroduction could be used as the frequency of gene flow episodes required for population persistence due to regular immigration or introductions. In short, assessments of the relative degree to which these opposing effects might affect reintroduction success are sorely needed.
Can single hatchery facilities maintain genetic diversity and fitness, or are multiple facilities required?
Whether single or multiple facilities are required to maintain both genetic diversity and fitness in captive breeding programs of endangered salmonids raises some important trade-offs to be factored in for biodiversity conservation. On one hand, to avoid significant losses of genetic diversity in captivity, captive populations must be kept at sufficiently large Ne to slow the rate of loss of genetic diversity due to the genetic consequences of small Ne (Frankham et al. 2002). This suggests that the following three options could be sufficient to maintain genetic diversity: (i) a single large population, maintained at a single hatchery facility (‘Option 1’), (ii) several small populations mixed frequently at a single hatchery facility (to effectively comprise one large population of identical Ne: ‘Option 2’), or (iii) several small populations mixed frequently between multiple facilities (to effectively comprise one large population of identical Ne: ‘Option 3’).
Yet, paradoxically, larger Ne populations respond more readily to selection than smaller Ne populations, all else being equal (Robertson 1960; Weber and Diggins 1990; Allendorf and Luikart 2007). That is, a large Ne facilitates adaptation by minimizing genetic drift, whereas a small Ne increases genetic drift, which can hinder adaptation (Crow and Kimura 1970). Consequently, while a larger Ne is more advantageous than a smaller Ne in the wild (larger Ne populations will on average be more capable of responding to environmental change than smaller Ne populations), it might be disadvantageous in captivity (larger Ne populations may become more adapted than smaller Ne populations to the captive environment). Having multiple small, isolated populations, maintained at either a single hatchery facility (‘Option 4’) or at multiple hatchery facilities (‘Option 5’), could thus be a better means of reducing the loss of fitness in captivity. Nevertheless, Options 4–5 must be tempered with the fact that in small Ne populations, one gets more genetic drift, in addition to some selection. In other words, both large and small Ne captive ‘options’ represent genetic changes from the wild population state. Thus, a key issue for accommodating fitness and genetic diversity is not only the degree to which a captive population becomes adapted to the hatchery environment, but also the degree to which the selective regimes differ between the captive and wild environment. If the difference in selective regimes can be reduced considerably, at some point a large Ne captive population (‘Options 1–3’) could be the way to go, because it would retain considerably more genetic diversity while at the same time not becoming too adapted to the captive environment relative to small Ne captive populations (‘Options 4–5’).
To throw more complexity into the different options, however, some theory (Kimura and Crow 1963; Nei and Takahata 1993; see also Waples 2002b) predicts that Options 4–5 could also result in the maintenance of more overall genetic diversity and increase the overall Ne compared to Options 1–3. This would only happen if no extinctions of the small populations occurred (Kimura and Crow 1963; Nei and Takahata 1993; Lande 1995; Toro and Caballero 2005). Yet, such extinctions can arise in small captive breeding programs (e.g., Snyder et al. 1996; Toro and Caballero 2005), and indeed, all else being equal, small populations are more likely to go extinct than large ones. Thus, unless there is some means to avoid these captive population extinctions altogether, the potential genetic diversity benefits of Options 4–5 might not be realized.
Based on all of these considerations, it has been suggested that a ‘best’ overall option might be an intermediate one (e.g., a compromise between Options 2/4 or 3/5). For salmonids, this would involve the maintenance of several small populations in captivity at one or multiple hatchery facilities, with translocations occurring only every several generations (see Margan et al. 1998; Frankham 2008).
Empirical evidence
To my knowledge, no empirical studies have tested whether the potential advantages of utilizing several small, isolated captive breeding populations with periodic mixture are upheld in salmonid captive breeding programs. In fact, only one empirical study has addressed theoretical predictions relating to the general ‘single-large versus several-small’ captive population issue, using fruit flies (Drosophila spp.) as a model (Margan et al. 1998). These authors generated replicate populations and compared the genetic diversity and reproductive fitness of populations with the following N compositions: (i) 50 vs. 2 × 25, (ii) 100 vs. 2 × 50 vs. 4 × 25, and (iii), 500 vs. 2 × 250 vs. 4 × 100 + 2 × 50 vs. 8 × 25 + 6 × 50. Margan et al. (1998) maintained all of these populations separately at their indicated sizes for 50 generations (including subdivided populations). The N compositions involving population subdivision (e.g., 2 × 25, 2 × 50 etc.) were subsequently pooled and all populations were maintained an additional 8 to 10 generations prior to evaluating their fitness and genetic diversity. The authors found that the ‘several-small with periodic mixing’ captive breeding population option was more advantageous than the ‘single-large with no mixing’ option. Namely, cases involving subdivided populations that were then pooled, when compared to single large populations of equivalent total size, had lower inbreeding levels, significantly higher or similar reproductive fitness, and higher levels of genetic diversity (i.e., heterozygosity) (Margan et al. 1998).
Summary
There is only very limited empirical research to suggest that maintaining several small isolated populations with periodic mixing may be more effective at reducing losses of genetic diversity and fitness than maintaining a single large population. Periodic mixing might also reduce the risks associated with regular translocations (e.g., the introduction of infectious diseases). This raises the possibility that a compromise between either Options 2/4 or Options 3/5 (i.e., several small, isolated populations with periodic mixing, housed in either a single hatchery facility or multiple hatchery facilities, respectively) might be the best way to maintain endangered salmonid populations in captivity. Again, though, the tentative conclusion here is based on the assumption that no extinctions of the small populations occur in captivity.
Although Frankham (2008) recently acknowledged that such a fragmentation regime had considerable merit, he did not recommend its application, perhaps because of the limited research on the subject. I now consider some potential pros and cons of these options as they may pertain to salmonids. For example, relative to a mix of Options 2/4 (single facility), a mix of Options 3/5 (multiple facilities) could also act as a safeguard against catastrophes such as extreme weather, water shortages or fires (Margan et al. 1998; Frankham 2008). However, in theory, catastrophes like disease outbreaks might still be contained at the same hatchery facility with Options 2/4. In addition, relative to Options 3/5, the use of a single hatchery facility with Options 2/4 would not require translocations between facilities when periodic mixing was required. This might have advantages in reducing (i) financial costs associated with translocations, (ii) the stresses that translocations impose on animals (depending on the life-history stage of salmonid being translocated), and (iii) the potential asynchrony that might arise in breeding times and embryonic developmental times by using multiple facilities that realistically vary in their thermal regimes (i.e., from different water sources).
Assuming either ‘several-small-occasional mixing’ approach is adopted in salmonids (Options 2/4 or 3/5), substantial uncertainty remains with respect to its implementation, as only generalized recommendations have been discussed in the primary literature. A first recommendation is that the small populations should not be so small that rapid inbreeding (and loss of genetic diversity) arises.
A second recommendation, based on the results of Margan et al. (1998), is that the genetic benefits of using small isolated populations might increase with the number of small populations involved. For instance, relative to a single large population of N = 100, four replicates of N = 25 subsequently pooled together led to a ≍60% increase in fitness under simulated wild conditions and a ≍41% increase in genetic diversity (heterozygosity), whereas pooling of two replicates of N = 50 led to ≍28% and ≍17% increases. Thus, further splitting populations in captivity might accrue greater fitness/genetic diversity benefits but might also require (i) more space and resources to house endangered populations, (ii) more risk of extinction of some captive populations, and/or (iii) more frequent translocations to offset inbreeding and the loss of genetic diversity. Consequently, decisions to adopt such a strategy would have to weigh such benefits against their added financial costs, perhaps especially for (i) given the kind of space required to house adult salmonids.
Finally, it is difficult to gauge how long the small populations should be maintained before pooling them. Again, any extinction of the small populations will counteract the benefits of the ‘several-small-occasional mixing’ strategy, and if left too long, rapid inbreeding will ensue in small populations (Margan et al. 1998; Toro and Caballero 2005). Inbreeding thresholds in salmonids are poorly characterized within species (Wang et al., 2002) and likely vary among populations. Yet, available data indicate that the fitness effects of inbreeding might be considerable in salmonid populations (at a minimum of a half-sibling inbreeding coefficient) without long histories of small population size (Pante et al. 2001; Myers et al. 2001; but see Su et al. 1996). As an overall cautionary approach, Margan et al. (1998) suggested monitoring inbreeding levels each generation and using as low an inbreeding threshold as possible to avoid extinction of the individual small populations. This may be unachievable in some cases unless pedigree information is available.
Are there technical alternatives to hatchery facilities for conserving genetic diversity and fitness?
Preceding summaries of certain sections in this review have suggested that salmonid captive-breeding programs may be unsuccessful in many cases because the root or purported causes of population decline or extirpation have not been mitigated. This implies that technical alternatives to hatchery facilities for conserving genetic diversity and fitness will also be unsuccessful unless at least some of the root causes of salmonid extirpation are corrected. Nevertheless, such technical alternatives may have practical utility in particular circumstances for conserving biodiversity.
Sperm cryopreservation
O'Reilly and Doyle (2007) recently reviewed the potential for cryopreservation techniques to reduce losses of genetic diversity and fitness in long-term live-gene banking programs. Namely, cryopreserved sperm obtained from salmonid males in the founder or early generations of captivity could be used to fertilize female eggs in subsequent generations (Sonesson et al. 2002). Because it can keep the genes within sperm largely intact for long periods of time (hundreds to thousands of years; Stoss and Refstie 1983), sperm cryopreservation has several advantages for biodiversity conservation. First, it could conserve a large proportion of the genetic variation in the founder generation of live-gene banking programs (up to 50%), as alleles from founder females would be represented in the sperm of first generation males (Sonesson et al. 2002). Second, the technique could minimize inbreeding and reduce domestication selection to captivity, as half of the gametes contributing to later generations would be obtained from individuals collected originally from the wild, or that had experienced only a single generation of captive rearing (O'Reilly and Doyle 2007). Importantly, sperm cryopreservation techniques have been developed for a wide variety of endangered salmonids (e.g., Stoss and Refstie 1983; Piironen 1993; Lahnsteiner et al. 1996; Kusuda et al. 2005; see also Harvey 1993; Lahnsteiner 2000 and O'Reilly and Doyle 2007 for details of techniques).
Sperm cryopreservation is not without its disadvantages. Because of its reduced viability relative to fresh sperm, more sperm than might be available through cryopreservation storage could be required to produce ample numbers of individuals that will in turn ensure modest numbers of mature adults for a live-gene banking program (O'Reilly and Doyle 2007). Thus, cryopreserved sperm could not be depended upon to produce the last live-gene banking generation intended for release into the wild. Also, significant genetic divergence might occur between the founder and prerelease or release generations in live-gene banking programs (O'Reilly and Doyle 2007). This could lead to outbreeding depression (Box 1) in the release generations of a program if the cryopreserved sperm was not used within a few generations (O'Reilly and Doyle 2007). Similarly, the wild environment might simply change during the generations of cryopreservation such that release generations may be maladapted to the wild by the time they are released. Finally, sperm cryopreservation cannot be viewed as a true alternative to hatcheries because it is necessarily dependent on breeding and rearing facilities.
Androgenesis
Techniques to preserve female eggs or fertilized embryos have not been developed for salmonids, so Thorgaard and Cloud (1993) and O'Reilly and Doyle (2007) reviewed two methods for reconstituting original wild populations from cryopreserved sperm. Either cryopreserved sperm from an extirpated population can be used to fertilize eggs from a nearby healthy population, or embryos can be produced with all-paternal inheritance (androgenesis). The latter involves obtaining unfertilized eggs from females of a nearby extant donor population that are then irradiated to inactivate their genetic material, and then fertilizing them using cryopreserved sperm from the original native (extirpated) population (Thorgaard and Cloud 1993; O'Reilly and Doyle 2007). The resulting androgenic diploids consist of DNA solely derived from the original native population by repressing the first cleavage division (Thorgaard and Cloud 1993). Overall, these methods require considerable time and labour to reconstitute the original native gene pool, and suitable nearby extant populations may not be available to carry them out. Additionally, some introgression of genetic material from the original native population is unavoidable, and maternal genetic material (mtDNA and any sex-linked nuclear DNA: nDNA) is lost (O'Reilly and Doyle 2007). Genetic changes associated with multiple generations of captive breeding and rearing will also arise when producing the final generation of juveniles intended for release into the wild. Finally, for androgenesis, the treatment used to block cleavage greatly reduces the survival of embryos, so additional crosses would likely be necessary with this method to retain heterozygosity and wild fitness. Therefore, these methods cannot be viewed as complete alternatives to captive breeding in salmon biodiversity conservation because they still require some captive breeding/rearing to be effective.
Surrogate broodstock technologies
The most promising technical alternatives to captive breeding for conserving endangered salmonids are very recently developed surrogate broodstock technologies (reviewed in Okutsu et al. 2007). These technologies involve the transplantation of primordial germ cells or spermatogonia from a target species into a related species, wherein the related species can then produce both viable sperm and eggs of the target species (Okutsu et al. 2007). Okutsu et al. (2007) carried out such a procedure by injecting cryopreserved rainbow trout (Oncorhynchus mykiss) spermatogonia into newly developing, triploid (sterile) masu salmon embryos (Oncorhynchus masou). The authors were able to raise the injected masu salmon to maturity at which time the adults produced viable trout gametes. A total of 55% of the trout spermatogonia died under cryopreservation, and only 10% of the triploid salmon females had trout eggs that could be fertilized by triploid salmon males carrying trout sperm. Nevertheless, intriguingly, the surrogated sperm and eggs when mixed created an F1 generation of normal trout, and this generation was subsequently able to produce a normal F2 generation of trout.
For biodiversity conservation, the implication of Okutsu et al. (2007) work is that it is possible to generate individuals of an endangered or extirpated salmonid population (in the case, provided the primordial germ cell tissue was collected prior to extirpation) using a widely available surrogate species. Thus, it may be possible to maximize generation length ‘in captivity’ by (i) preserving most if not all of the genetic diversity within an endangered population initially brought into captivity for several generations and (ii) preventing substantial fitness reductions in captivity before generating that population again in the future when the threats posed to it have been removed.
I foresee five potential limitations of the technique. First, it is currently unclear how well the technique will work when adopted on different target and surrogate species of salmonids. The success rate of surrogate broodstock technologies might vary among species (or even within species), or be considerably lower when using other species. Second, as in the case of sperm cryopreservation, the wild environment might simply change during the generations of cryopreservation such that captive-release generations may be unable to track selective changes in the wild by the time they are released. Third, the maternal environment of the surrogate might affect the performance of offspring. Fourth, there is potentially a political danger that efforts to protect endangered species habitat may be diminished if it is viewed that species can be brought back at any given future date. Fifth, chemicals and treatments involved in both surrogate broodstock technologies and sperm cryopreservation might generate epigenetic changes in captive-bred individuals. Epigenetic changes, such as alterations to DNA or mutations that affect gene regulation, have been recently shown to have considerable effects in mammals (Guerrero-Bosagna et al. 2005; Jirtle and Skinner 2007; Reik 2007). These changes might not be readily apparent in the hatchery environment but could have important fitness consequences when returning hatchery-fish into the wild (P. O'Reilly, DFO Halifax, personal communication). Overall, such risks would have to be addressed if these techniques are to be considered sole alternatives to captive-breeding in endangered species restoration.
Translocations to new habitats
Other alternatives to hatcheries for conserving species such as endangered anadromous salmonids might include (A) translocation to landlocked freshwater habitat, (B) transfer to other rivers that enter the sea, or (C) some mixture of artificial or semi-natural breeding from adult releases into natural river habitat, and then exclusive rearing of juveniles in freshwater and rearing of adults in sea pens, especially for those populations where marine survival is negligible. For instance, alternative (A) has been successful in generating new populations that act as safeguards against species extinction for endangered subspecies of cutthroat trout (Oncorhynchus clarki spp.) in western North America (Young et al. 2002). Alternative (A) has also recently been adopted in a live-gene banking program of Atlantic whitefish (Coregonus huntsmani), wherein individuals have been introduced into a lake that shares many environmental features of the species’ traditional range (A. Cook, Dalhousie University, personal communication). However, though alternatives (A) and (B) do not necessarily require the extent of labour or resources as hatcheries, they may not be feasible in some cases. These alternatives also generate a host of new challenges/issues to deal with. First, alternative (A) might not be applicable to some semelparous salmonids which show less evidence that they can support freshwater landlocked populations (but see Laurentian Great Lakes chinook and pink salmon; Crawford 2001). Second, alternatives (A) or (B) also might not be justifiable if the endangered salmonid is nonnative and thus has the potential to impact native fauna, or if populations of the same species already exist there and interbreeding might occur. Third, both alternatives (A, B) would also face similar challenges to restoring the ‘original’ fitness of the endangered population. This is because the new environments, perhaps especially alternative A, might lead to potentially irreversible evolutionary change, or at least shifts in phenotypic trait distributions of populations. Finally, alternative (C) would likely still require some degree of hatchery support to assist in the artificial spawning of fish and to ensure a good representation of genetic diversity through the generations. It, therefore, cannot be viewed as a complete alternative to captive-breeding/rearing.
Conclusions
This review on the extent to which captive breeding programs can conserve salmonid biodiversity reveals numerous trends and uncertainties. It also has several implications for ongoing salmonid captive breeding programs. Many of these implications are directly relevant to the assessments of captive breeding programs in other taxa, especially for species with indeterminate growth, high fecundities, or complex migratory lifecycles (e.g., other fishes, amphibians, and insects):
Encouragingly, for most captive breeding programs, neutral (and perhaps quantitative) genetic diversity within populations can be sufficiently maintained in captivity for several generations. However, tremendous variation likely exists among programs in their capacity to retain genetic diversity over the longer-term because: (i) adopted procedures for maintaining high Nb/N or Ne/N ratios in captivity vary among programs and (ii) Nb/Ne estimates of different captive broodstocks vary widely and are sometimes small. Uncertainty over the longer-term also exists because programs adopting many procedures to reduce the loss of genetic diversity are still young, and these procedures have not been systemically evaluated for long-term effectiveness in salmonids (and very rarely in other taxa). There is, nevertheless, great scope for current and future salmonid captive breeding programs to reduce the rate of loss of genetic diversity in captivity (Table 2).
Perhaps more importantly however, is that even with proper care, the captive environment may lead to unavoidable genetic changes and/or wild fitness changes in quantitative traits. In other words, maintenance of a large Ne captive broodstock does not necessarily ensure the retention of genetic diversity pertaining to fitness. Though limited, the most relevant research suggests that quantitative genetic changes are likely manifested more rapidly than losses of overall neutral genetic diversity in captivity. Fitness losses may potentially arise even within one generation, or after one or two generations of captive-breeding/rearing. There is also some indication that the magnitude of fitness loss increases as the duration in captivity increases. Yet, tremendous variation likely exists between different programs, species and populations within species with respect to the type and magnitude of fitness-related costs that can be accrued each generation from captive-breeding/rearing. Clearer resolution of the magnitude of potential fitness effects of captive breeding/rearing and their overall risks to wild populations awaits further investigation, especially over the longer-term.
There is an unavoidable trade-off between reducing domestication selection during captive-rearing by having a period of wild exposure, and maintaining genetic diversity by equalizing family sizes of wild-exposed individuals when generating new broodstocks. What should be considered optimal in this regard merits serious discussion.
Mechanisms reducing fitness in captivity and in the offspring of captive-wild matings are likely multifaceted, affecting behavior, swimming performance, imprinting, stress responses, growth, run-timing, developmental stability, developmental time to hatch, embryo size, maternal reproductive investment, body morphology and age-at-maturity, all of which may be linked to fitness. Identification of such mechanisms in specific cases could suggest ways to improve the chances of successful reintroduction in the long term.
Owing to several confounding factors, there is currently little empirical evidence that captive-reared lines of salmonids can or cannot be reintroduced as self-sustaining populations. However, a wide body of circumstantial evidence supports that captive breeding programs alone will not be sufficient to re-establish endangered salmonids within their species’ ranges, unless the factors contributing to their initial decline are concurrently addressed (see also Frazer 1992; Meffe 1992; Flagg et al. 1995, 2004a; Snyder et al. 1996; Waples and Drake 2004; Waples et al. 2007).
Based on the duration of more ‘modern’ captive breeding programs (e.g., Hedrick et al. 2000b; Flagg et al. 2004a; O'Reilly and Doyle 2007), a minimum of 15–20 years will likely be necessary to potentially achieve the conservation goal of re-establishing a self-sustaining salmonid population in the wild, in a previously occupied habitat within the species’ native range.
Research is sorely needed on whether the demographic advantages of increasing population abundance via captive breeding can outweigh the genetic disadvantages of losing fitness in captivity.
There are biological pros and cons to maintaining captive broodstocks as either single or multiple populations within one or more hatchery facilities. This is especially the case when the objective is to retain both their genetic diversity and fitness. There is currently little empirical support for any one approach, but there are several sound reasons for favouring multiple populations and periodic mixing, housed in multiple facilities (e.g., to reduce the risk of catastrophes).
As potential technical alternatives to conserving salmonid genetic diversity, surrogate broodstock technologies may hold the most promise in the future, but as yet have not been tested in a real-world conservation situation. Thus, for practical reasons, cryopreserved sperm may be a more useful means of retaining genetic diversity. However, both surrogate and cryopreservation methods require some level of captive breeding and therefore cannot be viewed as a replacement for captive breeding. Other alternatives include translocations to new habitats, which may be available in some cases but for several biological reasons must also be considered with caution.
Management recommendations
As illustrated by a review of salmonid fishes, ongoing, in-depth research and evaluation of existing captive breeding programs is needed to facilitate proper-decision making on when, where, and how such programs might be most useful for conserving biodiversity in the future. In a parallel situation, an independent scientific panel also recently identified three key principles for the reform of traditional hatchery programs (Mobrand et al. 2005; see also Waples 1999; Waples and Drake 2004; Flagg et al. 2004b; Waples et al. 2007; Naish et al. 2008). First, the goals of each program needed to be explicitly stated. Second, the programs had to be scientifically defensible. Third, the programs had to be capable of adapting to new information as it came in (Mobrand et al. 2005). Such principles may easily apply to captive breeding programs as well.
For instance, the specific management goals of particular captive-breeding programs or their captive-rearing practices have not always been readily apparent. Following Mobrand et al. (2005), I suggest that the goals of captive breeding programs be more specific and related to ‘success’, beyond preventing the imminent extinction of the target population. This might include (i) scientific research results relating to the maintenance of genetic diversity and fitness within captive-bred populations, and re-establishment of self-sustaining populations in the wild, (ii) knowledge generated for decision-making regarding the initiation or continued-monitoring of particular programs, and (iii) endangered species/population education through public outreach.
In addition, to date, salmonid captive breeding research has not always been structured to gain reliable knowledge for maintaining genetic diversity and fitness or generating self-sustaining populations in the wild. Inadequate experimentation in captive salmonids is likely explained by three reasons. First, many procedures which might reduce the loss of genetic diversity and fitness in captivity have only been recently adopted in most programs. Second, in dealing with endangered populations, there are inherent trade-offs between preventing extinction, having replicated controlled experiments over multiple generations, and ensuring sufficient adult returns and/or families to carry out such studies effectively or simultaneously. Third, salmonids require several years to reach maturity and ample space for captive-rearing. In some cases, additional space for multiple generations of experimentation may not be feasible. Nevertheless, where feasible, there is a critical need for captive breeding manipulations and monitoring to include, a priori, greater application of hypothesis testing through the use of well-designed experiments. In this regard, analogous guidelines for carrying out effective experimentation in salmonid supplementation programs or in general reintroductions might be very useful (see Waples 1999; Reisenbichler 2004; Waples and Drake 2004; Seddon et al. 2007; Armstrong and Seddon 2008). On a positive note as well, many new procedures and theoretical models are available to tackle challenges related to conserving genetic diversity and fitness, and they await testing in terms of their long-term effectiveness (e.g., Fernandez and Caballero 2001;Wang and Ryman 2001; Duchesne and Bernatchez 2002; Fernandez et al. 2003, 2004; Vales-Alonso et al. 2003; Wang 2004; Rodriguez-Ramilo et al. 2006).
Inferences gained to date by salmonid captive breeding programs have also been largely based on a case-by-case basis. Furthermore, many publications have not included details of procedures adopted to reduce the rate of loss of genetic diversity and fitness in captivity (but see Flagg et al. 2004a; O'Reilly and Doyle 2007). Clearly, differences between captive breeding programs might demand evaluations on a case-by-case basis within the context of program goals (Waples 1999; Berejikian et al. 2004). On the other hand, the origin of more general principles and guidelines for effective salmonid captive breeding and reintroduction might only be achievable if the documentation of procedures adopted is improved. Similarly, the knowledge generated by captive-breeding programs could be made more accessible to governmental, nongovernmental and academic researchers as well as to policy-makers. Perhaps encouragement to publish timely, peer-reviewed literature would be a means of ensuring that (i) captive-breeding programs adhere to evaluating their goals, (ii) knowledge from captive-breeding programs can be integrated for meta-analyses, and (iii) captive-breeding procedures can be modified if new information suggests that this would improve the effectiveness of programs. Such points may be especially pertinent for endangered species/populations, where time is indeed of the essence.
As previously mentioned, a myriad of procedures are now available for potentially slowing the rate of loss of genetic diversity and fitness in captivity. But many of these will likely demand additional resources and labour to carry them out effectively. There is consequently an imminent need to know and prioritize which procedures might simultaneously work best towards achieving captive breeding goals while keeping cost-benefit ratios as low as possible. For example, one major trade-off exists between the potentially greater productivity accrued from the release of older and larger juveniles versus the presumed genetic and ecological benefits of egg/early life-history releases (Berejikian et al. 2004). In this regard, referral to cost-benefit analysis guidelines developed for more traditional hatchery or supplementation programs might be very useful (e.g., Waples 1999; Waples and Drake 2004; McKinlay et al. 2004; see also Naish et al. 2008).
A final comment on uncertainty
It is encouraging that salmonid captive breeding programs can clearly fulfill the proximate goal of preventing the imminent extinction of an endangered species or population (Flagg et al. 2004a; O'Reilly and Doyle 2007). Nevertheless, a central conclusion of this review for both salmonids and other taxa is that considerable uncertainty remains regarding the ability of captive breeding to realize its ultimate goals: maintaining genetic diversity and fitness over the long-term and re-establishing populations into previously occupied habitat within species’ native ranges. In a parallel situation on traditional hatchery program reform for salmonids, Waples (1999) pointed out that improved research would not by itself be sufficient because it would not resolve all uncertainties, but of equal importance, that much key information would likely not be available for many years. Waples (1999) therefore argued that it was essential to develop workable methods for dealing with uncertainty.
Three points are worth noting in this regard in the case of salmonids. First, the number of endangered salmonid populations is already substantial (e.g., Canada's Species at Risk Act; U.S. Endangered Species Act) and will most likely increase in the future from human activities. Second, although research on several species is now underway that will improve existing captive-breeding programs, such research generally takes a decade to complete (e.g., Araki et al. 2007c). Third, this review points to a minimum duration of 15–20 years for captive-breeding programs to potentially re-establish self-sustaining populations in the wild. Consequently, now might be a good time to ask similar critical questions that previous authors have (sensuWaples 1999). For instance, where should the burden of proof lie given the inevitable uncertainty? Should captive breeding programs be used persistently because they can prevent imminent extinction (thus preventing, in the short-term, irreversible losses of diversity)? Or, conversely, should they be used only very cautiously given the uncertainty in the long-term of (i) whether they can conserve genetic diversity/fitness or re-generate self-sustaining populations and (ii) whether underlying causes of salmonid declines can be remedied? In this case, allocation of resources might be placed in potentially more cost-effective long-term strategies, such as in situ preservation of other populations. In the end, the benefits and risks of initiating, continuing, or terminating a captive breeding program from a management perspective can only be weighed from (i) estimating the probabilities of different possible outcomes and (ii) careful consideration of the potential consequences of being wrong (Currens and Busack 1995; Waples 1999).
Acknowledgments
I thank J. Rice and K. Houston of the Environment and Biodiversity Branch, Department of Fisheries and Oceans (DFO), Ottawa, Canada, for the invitation and financial support to write this review. The contents of the review were improved considerably from the discussions, suggestions and comments of A. Calvert, R. Doyle, I. Fleming, M. Ford, J. Gibson, M. Hansen, J. Hutchings, D. McKinley, S. O'Neill, P. O'Reilly, F. Palstra, A. Talbot, G. Thorgaard, E. Verspoor, R. Waples, L. Weir, R. Withler, two anonymous reviewers and by round-table discussions at DFO's Biodiversity Facility Workshop in Ottawa. I am especially grateful to P. O'Reilly for insights and comments on the use of live-gene banking in Canada. I am also grateful to H. Araki, B. Berejikian, M. Ford, K. Naish, R. Reisenbichler, R. Waples and P. O'Reilly, who also kindly provided relevant articles of theirs that were either in book chapters or in press at the time of writing this review.
Appendix 1. Literature search details
Roberts et al. (2006) recently established ‘systematic review’ guidelines for review papers in conservation, ecology, and environmental management. They suggested that a comprehensive and documented search strategy be included to reduce bias in review papers and to facilitate updating in light of further advances. To address questions throughout the review relating to how well captive breeding programs conserve salmonid biodiversity, I performed a rigorous literature search for primary, peer-reviewed journal articles in Web of Science™, ICES Journal of Marine Science and Google Scholar™ search engines. After a first collection of literature was made, relevant literature cited within these articles was collected. In addition, major authors of peer-reviewed articles involving relevant key words were searched in databases to ensure that all related works were researched. Wherever necessary, major contributing authors were contacted directly for article reprints or PDFs. The following 84 search terms (in alphabetical order) or combinations thereof were used to find relevant primary literature for various review sections:
‘allelic diversity’, ‘allelic richness’, ‘androgenesis’, ‘artificial supplementation’, ‘atlantic whitefish’, ‘biodiversity’, ‘biodiversity conservation’, ‘biological diversity’, ‘Canada’, ‘captive’, ‘captive-bred’, ‘captive breeding’, ‘captive breeding program’‘captive breeding programme’, ‘captive-reared’, ‘captive-rearing’, ‘char’, ‘charr’, ‘coancestry’, ‘Coregonus’, ‘COSEWIC’, ‘cryopreservation’, ‘cryopreserved’, ‘domestication’, ‘domestication selection’, ‘embryo’, ‘effective population size’, ‘endangered’, ‘enhancement’, ‘environmental change’, ‘epigenetic’, ‘extirpated’, ‘extirpation’, ‘fitness’, ‘function’, ‘gamete’, ‘genetic’, ‘genetic adaptation’, ‘genetic diversity’, ‘genetic drift’, ‘genetic variability’, ‘genetic variation’, ‘Great Lakes’, ‘hatcheries’, ‘hatchery’, ‘heterozygosity’, ‘inbred’, ‘inbreeding’, ‘inbreeding coefficient’, ‘lake trout’, ‘lifetime performance’, ‘live gene bank’, ‘live gene banking’, ‘Oncorhynchus’, ‘population’, ‘recovery initiative’, ‘recovery initiatives’, ‘re-established’, ‘re-establish’, ‘rehabilitated’, ‘rehabilitation’, ‘reintroduced’, ‘reintroduction’, ‘restore’, ‘restoration’, ‘Sacremento River’, ‘Salmo’, ‘salmon’, ‘salmonid’, ‘Salvelinus’, ‘self-sustaining’, ‘sperm’, ‘stock’, ‘stock enhancement’, ‘supplementation’, ‘supportive breeding’, ‘supportive breeding program’, ‘supportive breeding programme’, ‘supportive rearing’, ‘temporal data’, ‘trout’, ‘USA’.
Literature cited
- Allendorf FW. Delay of adaptation to captive breeding by equalizing family size. Zoo Biology. 1986;5:181–190. [Google Scholar]
- Allendorf FW. Delay of adaptation to captive breeding by equalizing family size. Conservation Biology. 1993;7:416–419. [Google Scholar]
- Allendorf FW, Luikart G. Conservation and the Genetics of Populations. UK: Blackwell Publishing; 2007. [Google Scholar]
- Allendorf FW, Ryman N. Genetic management of hatchery stocks. In: Ryman N, Utter F, editors. Population Genetics and Fishery Management. Seattle, WA: University of Washington Press; 1987. pp. 141–159. [Google Scholar]
- Allendorf FW, Waples RS. Conservation and genetics of salmonid fishes. In: Avise JC, Hamrick JL, editors. Conservation Genetics: Case Histories From Nature. New York: Chapman and Hall; 1996. pp. 238–280. [Google Scholar]
- Allendorf FW, Bayles D, Bottom DL, Currens KP, Frissell CA, Hankin D, Lichatowich JA, et al. Prioritizing Pacific salmon stocks for conservation. Conservation Biology. 1997;11:140–152. [Google Scholar]
- Alvarez D, Nicieza AG. Predator avoidance behaviour in wild and hatchery-reared brown trout: the role of experience and domestication. Journal of Fish Biology. 2003;63:1565–1577. [Google Scholar]
- Araki H, Waples RS, Ardren WR, Cooper B, Blouin MS. Effective population size of steelhead trout: influence of variance in reproductive success, hatchery programs, and genetic compensation between life-history forms. Molecular Ecology. 2007a;16:953–966. doi: 10.1111/j.1365-294X.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- Araki H, Ardren WR, Olsen E, Cooper B, Blouin MS. Reproductive success of captive-bred steelhead trout in the wild: evaluation of three stocking programs in the Hood River. Conservation Biology. 2007b;21:181–190. doi: 10.1111/j.1523-1739.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- Araki H, Cooper B, Blouin MS. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science. 2007c;318:100–103. doi: 10.1126/science.1145621. [DOI] [PubMed] [Google Scholar]
- Araki H, Berejikian BA, Ford MJ, Blouin MS. Fitness of hatchery-reared salmonids in the wild. Evolutionary Applications. 2008;1:342–355. doi: 10.1111/j.1752-4571.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardren WR, Kapuscinski AR. Demographic and genetic estimates of effective population size (Ne) reveals genetic compensation in steelhead trout. Molecular Ecology. 2003;12:35–49. doi: 10.1046/j.1365-294x.2003.01705.x. [DOI] [PubMed] [Google Scholar]
- Armstrong DP, Seddon PJ. Directions in reintroduction biology. Trends in Ecology and Evolution. 2008;23:20–25. doi: 10.1016/j.tree.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Ballou J, Lacy RC. Identifying genetically important individuals for management of genetic diversity in pedigreed populations. In: Ballou J, Gilpin M, Foose L, editors. Population Management for Survival and Recovery: Analytical Methods and Strategies for Small Population Conservation. New York: Columbia University Press; 1995. pp. 76–111. [Google Scholar]
- Bams RA. Survival and propensity for homing as affected by presence or absence of locally adapted paternal genes in two transplanted populations of pink salmon (Oncorhynchus gorbuscha. Journal of the Fisheries Board of Canada. 1976;33:2716–2725. [Google Scholar]
- Bartley D, Bagley M, Gall G, Bartley B. Use of linkage disequilibrium data to estimate effective size of hatchery and natural fish populations. Conservation Biology. 1992;6:365–375. [Google Scholar]
- Baum E. Maine Atlantic Salmon: A National Treasure. Maine: Atlantic Salmon Unlimited, Hermon; 1997. [Google Scholar]
- Beacham TD. Comment on “Rapid evolution of egg size in captive salmon”. Science. 2003;302:59d. doi: 10.1126/science.1086625. [DOI] [PubMed] [Google Scholar]
- Beck BB, Rapaport LG, Stanley Price MR, Wilson AC. Reintroduction of captive-born animals. In: Olney PJS, Mace GM, Feistner ATC, editors. Creative Conservation: Interactive Management of Wild and Captive Animals. London: Chapman and Hall; 1994. pp. 265–286. [Google Scholar]
- Berejikian BA. The effects of hatchery and wild ancestry and experience on the relative ability of steelhead trout fry (Oncorhynchus mykiss) to avoid a benthic predator. Canadian Journal of Fisheries and Aquatic Sciences. 1995;52:2476–2482. [Google Scholar]
- Berejikian BA, Mathews SB, Quinn TP. Effects of hatchery and wild ancestry and rearing environments on the development of agonistic behavior in steelhead trout (Oncorhynchus mykiss) fry. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:2004–2014. [Google Scholar]
- Berejikian BA, Flagg T, Kline P. Release of captively reared adult anadromous salmonids for population maintenance and recovery: biological trade-offs and management considerations. American Fisheries Society Symposium. 2004;44:233–245. [Google Scholar]
- Berejikian BA, Johnson T, Endicott R, Lee J. Increases in steelhead red abundance resulting from two conservation hatchery strategies in the Hamma Hamma River, WA. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:754–764. [Google Scholar]
- Bernatchez L. A role for molecular systematics in defining evolutionary significant units. American Fisheries Society Symposium. 1995;17:114–132. [Google Scholar]
- Bijlsma R, Bundgaard J, Boerema AC. Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. Journal of Evolutionary Biology. 2000;13:502–514. [Google Scholar]
- Bohlin T, Sundstrom LF, Johnsson JI, Hojesjo I, Pettersson J. Density-dependent growth in brown trout: effects of introducing wild and hatchery fish. Journal of Animal Ecology. 2002;71:683–692. [Google Scholar]
- Booth RE, Grime JP. Effects of genetic impoverishment on plant community diversity. Journal of Ecology. 2003;91:721–730. [Google Scholar]
- Borlase SC, Loebel DA, Frankham R, Nurthen RK, Briscoe DA, Daggard GE. Modeling problems in conservation genetics using captive Drosophila populations: consequences of equalization of family sizes. Conservation Biology. 1993;7:122–131. [Google Scholar]
- Bosch WJ, Newsome TH, Dunnigan JL, Hubble JD, Neeley D. Evaluating the feasibility of reestablishing a Coho Salmon population in the Yakima River, Washington. North American Journal of Fisheries Management. 2007;27:198–214. [Google Scholar]
- Bowen BW. Preserving genes, species, or ecosystems? Healing the fractured foundations of conservation policy. Molecular Ecology. 1999;8:S5–S10. doi: 10.1046/j.1365-294x.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Braithwaite VA, Salvanes AGV. Environmental variability in the early rearing environment generates behaviourally flexible cod: implications for rehabilitating wild populations. Proceedings of the Royal Society B – Biological Sciences. 2005;272:1107–1113. doi: 10.1098/rspb.2005.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EL, Amend DF, Cronin MA, Lannon JE, LaPatra S, McNeil WJ, Noble RE, et al. The controversy about salmon hatcheries. Fisheries. 2004;29:12–30. [Google Scholar]
- Bronte CR, Holey ME, Madenjian CP, Jonas JL, Claramunt RM, McKee PC, Toneys ML, et al. Relative abundance, site fidelity, and survival of adult lake trout in Lake Michigan from 1999 to 2001: implications for future restoration strategies. North American Journal of Fisheries Management. 2007;27:137–155. [Google Scholar]
- Brown LR, Michniuk D. Littoral zone assemblages of the alien-dominated Sacremento-San Joaquin Delta, California 1980–1983 and 2001–2003. Estuaries and Coasts. 2007;30:186–200. [Google Scholar]
- Bryant EH, Reed DH. Fitness decline under relaxed selection in captive populations. Conservation Biology. 1999;7:122–131. [Google Scholar]
- Burger CV, Scribner KT, Spearman WJ, Campton DO. Genetic contribution of three introduced life history forms of sockeye salmon to colonization of Frazer Lake, Alaska. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:2096–2111. [Google Scholar]
- Caballero A, Toro MA. Interrelations between effective population size and other pedigree tools for the management of conserved populations. Genetical Research. 2000;75:331–343. doi: 10.1017/s0016672399004449. [DOI] [PubMed] [Google Scholar]
- Caballero A, Toro MA. Analysis of genetic diversity for the management of conserved subdivided populations. Conservation Genetics. 2002;3:289–299. [Google Scholar]
- Campton DE. Genetic effects of hatchery fish on wild populations of Pacific salmon and steelhead: what do we really know? American Fisheries Society Symposium. 1995;15:71–80. [Google Scholar]
- Campton DE. Sperm competition in salmon hatcheries: the need to establish genetically-benign spawning protocols. Transactions of the American Fisheries Society. 2004;133:1277–1289. [Google Scholar]
- Carofinno DC, Miller LM, Kapuscinski AR, Ostazecki JJ. Stocking success of local-origin fry and impact of hatchery ancestry: monitoring a new steelhead stocking program in a Minnesota tributary to Lake Superior. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:309–318. [Google Scholar]
- Carr JW, Whoriskey F, O'Reilly P. Efficacy of releasing captive reared broodstock into an imperilled wild Atlantic salmon population as a recovery strategy. Journal of Fish Biology. 2004;65(Suppl A):38–54. [Google Scholar]
- Chebanov NA, Riddell BE. The spawning behavior, selection of mates, and reproductive success of Chinook salmon (Oncorhynchus tshawyscha) spawners of natural and hatchery origins under conditions of joint spawning. Journal of Ichthyology. 1998;38:517–526. [Google Scholar]
- Chilcote MW. Relationship between natural productivity and the frequency of wild fish in mixed spawning populations of wild and hatchery steelhead (Oncorhynchus mykiss. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1057–1067. [Google Scholar]
- Chilcote MW, Leider SA, Loch JJ. Differential reproductive success of hatchery and wild summer-run steelhead under natural conditions. Transactions of the American Fisheries Society. 1986;115:727–735. [Google Scholar]
- Claytor R, Boudreau P, Currie T, Gibson J, Goff T, Jones R, Katopodis C, et al. Conceptual Facility to BYPASS ATLANTIC SALMON SMOLTS at the Tobique Narrows Dam. Ottawa, Canada: Department of Fisheries and Oceans; 2006. Expert opinion 2002/02. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Update Status Report on Aurora Trout (Salvelinus Fontinalis Timagamiensis) in Canada. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2000. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Update Status Report on Sockeye Salmon (Oncorhynchus Nerka) Sakinaw Population in Canada. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2003a. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Update Status Report on Sockeye Salmon (Oncorhynchus Nerka) Cultus Population in Canada. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2003b. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Update Status Report on Atlantic Salmon (Salmo Salar) of Lake Ontario. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2006a. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Update Status Report on Atlantic Salmon (Salmo Salar) Inner Bay of Fundy Populations. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2006b. [Google Scholar]
- COSEWIC. COSEWIC Emergency Assessment of the Sakinaw Lake Population of Sockeye Salmon, Oncorhynchus nerka. Ottawa: Committee of the Status of Endangered Wildlife in Canada; 2006c. [Google Scholar]
- Crandall KA, Bininda-Emonds ORP, Mace GM, Wayne RK. Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution. 2000;15:290–295. doi: 10.1016/s0169-5347(00)01876-0. [DOI] [PubMed] [Google Scholar]
- Crawford SS. Salmonine Introductions to the Laurentian Great Lakes: An Historical Review and Evaluation of Ecological Effects. Ottawa, 205pp: National Research Council Canada Monograph Series, NRC Research Press; 2001. Canadian Special Publications in Fisheries and Aquatic Sciences 132. [Google Scholar]
- Crawford SS, Muir AM. Reviews in Fish Biology and Fisheries. 2008. Global Introductions of Salmon and Trout of the Genus Oncorhynchus: 1870–2007. In press. [Google Scholar]
- Crow JF, Kimura M. An Introduction to Population Genetics Theory. New York, NY. 591 pp: Harper and Row; 1970. [Google Scholar]
- Currens KP, Busack CA. A framework for assessing genetic vulnerability. Fisheries. 1995;20:24–31. [Google Scholar]
- Currens KP, Allendorf FW, Bayles D, Bottom DL, Frissell CA, Hankin D, Lichatowich JA, et al. Conservation of Pacific salmon: response to Wainright and Waples. Conservation Biology. 1998;12:1148–1149. [Google Scholar]
- Dahl J, Pettersson E, Dannewitz J, Jarvi T, Lof AC. No difference in survival, growth and morphology between offspring of wild-born, hatchery and hybrid brown trout (Salmo trutta. Ecology of Freshwater Fish. 2006;15:388–397. [Google Scholar]
- Dannewitz J, Petersson E, Prestegaard T, Jarvi T. Effects of sea-ranching and family background on fitness traits in brown trout Salmo trutta reared under near-natural conditions. Journal of Applied Ecology. 2003;40:241–250. [Google Scholar]
- Dellefors C, Johnsson JI. Foraging under risk of predation in wild and hatchery-reared juvenile sea trout (Salmo trutta L.) Nordic Journal of Freshwater Research. 1995;70:31–37. [Google Scholar]
- D.F.O. Recovery Strategy for the Atlantic Whitefish (Coregonus Huntsmani) in Canada. Ottawa, Canada: Department of Fisheries and Oceans; 2006. Species At Risk Act Recovery strategy series. [Google Scholar]
- Dickhoff WW, Beckman BR, Larsen DA, Mahnken CVW, Schreck CB, Sharp C, Zaugg WS. Quality assessment of hatchery-reared spring chinook smolts in the Columbia River basin. American Fisheries Society Symposium. 1995;15:292–302. [Google Scholar]
- Dodson JJ, Gibson RJ, Cunjak RA, Friedland KD, Garcia de Leaniz C, Gross MR, Newbury R, et al. Elements in the development of conservation plans for Atlantic salmon (Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55(Suppl 1):312–323. [Google Scholar]
- Doyle RW, Perez-Enriquez R, Takagi M, Taniguchi M. Selective recovery of founder diversity in aquaculture broodstocks and captive, endangered fish populations. Genetica. 2001;111:291–304. doi: 10.1023/a:1013772205330. [DOI] [PubMed] [Google Scholar]
- Duchesne P, Bernatchez L. An analytical investigation of the dynamics of inbreeding in multi-generation supportive breeding. Conservation Genetics. 2002;3:47–60. [Google Scholar]
- Ebanhard T. Conservation breeding as a tool for saving animal species from extinction. Trends in Ecology and Evolution. 1995;11:438–443. doi: 10.1016/s0169-5347(00)89176-4. [DOI] [PubMed] [Google Scholar]
- Edge TA, Gilhen J. Updated status report on the endangered Atlantic Whitefish, Coregonus huntsmani. Canadian Field Naturalist. 2001;115:635–652. [Google Scholar]
- Edmands S. Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Molecular Ecology. 2007;16:463–475. doi: 10.1111/j.1365-294X.2006.03148.x. [DOI] [PubMed] [Google Scholar]
- Einum S, Fleming IA. Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. Journal of Fish Biology. 1997;50:634–651. [Google Scholar]
- Einum S, Fleming IA. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proceedings of the Royal Society of London Biological Sciences. 1999;266:2095–2100. [Google Scholar]
- Einum S, Fleming IA. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar. Evolution. 2000;54:628–639. doi: 10.1111/j.0014-3820.2000.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Eknath AE, Doyle RW. Effective population size and the rate of inbreeding in aquaculture of Indian major carps. Aquaculture. 1990;85:293–305. [Google Scholar]
- Eldridge WH, Killebrew K. Genetic diversity over multiple generations of supplementation: an example from Chinook salmon using microsatellite and demographic data. Conservation Genetics. 2007;9:13–28. [Google Scholar]
- Federenko AY, Shepherd BG. Review of Salmon Transplant Procedures and Suggested Transplant Guidelines. 1986. Canadian Technical Reports for Fisheries and Aquatic Sciences 1479, Vancouver, BC, 144pp. [Google Scholar]
- Felsenstein J. Inbreeding and variance effective numbers in populations with overlapping generations. Genetics. 1971;68:581–597. doi: 10.1093/genetics/68.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Caballero A. A comparison of management strategies for conservation with regard to population fitness. Conservation Genetics. 2001;2:121–131. [Google Scholar]
- Fernandez J, Toro MA, Caballero A. Fixed contribution designs vs. minimization of global coanscestry to control inbreeding in small populations. Genetics. 2003;165:885–894. doi: 10.1093/genetics/165.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Toro MA, Caballero A. Managing individuals’ contributions to maximize the allelic diversity maintained in small, conserved populations. Conservation Biology. 2004;18:358–367. [Google Scholar]
- Ferno A, Jarvi T. Domestication genetically alters the anitpredator behaviour of anadromous brown trout (Salmo trutta) – dummy predator experiment. Nordic Journal of Freshwater Research. 1998;74:95–100. [Google Scholar]
- Fischer J, Lindenmayer DB. An assessment of the published results of 22 animal relocations. Biological Conservation. 2000;96:1–11. [Google Scholar]
- Flagg TA, Mahnken CVW, Johnson KA. Captive broodstocks for recovery of Snake River sockeye salmon. In: Schramm HL, Piper RG, editors. Uses and Effects of Cultured Fishes in Aquatic Ecosystems. Bethesda, MD: American Fisheries Society Symposium; 1995. pp. 81–90. [Google Scholar]
- Flagg TA, McAuley MC, Fairgrieve WT. Status of Redfish Lake Sockeye Salmon Broodstocks at NMFS January Through December 1999. Seattle: Resource enhancement and utilization technologies division, National Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Association; 1999. [Google Scholar]
- Flagg TA, McAuley MC, Kline PA, Powell MS, Taki D, Gislason JC. Application of captive broodstocks to preservation of ESA-listed stocks of Pacific salmon: Redfish Lake Sockeye Salmon Case example. American Fisheries Society Symposium. 2004a;44:387–400. [Google Scholar]
- Flagg TA, Mahnken CVW, Iwamoto RN. Conservation hatchery protocols for Pacific salmon. American Fisheries Society Symposium. 2004b;44:603–619. [Google Scholar]
- Fleming IA. Captive breeding and the conservation of wild salmon populations. Conservation Biology. 1994;8:886–888. [Google Scholar]
- Fleming IA, Einum S. Experimental tests of genetic divergence of farmed from wild Atlantic salmon due to domestication. ICES Journal of Marine Science. 1997;54:1051–1063. [Google Scholar]
- Fleming IA, Gross MR. Breeding success of hatchery and wild coho salmon (Oncorhynchus kisutch) in competition. Ecological Applications. 1993;3:230–245. doi: 10.2307/1941826. [DOI] [PubMed] [Google Scholar]
- Fleming IA, Petersson E. The ability of released, hatchery salmonids to breed and contribute to the natural productivity of wild populations. Nordic Journal of Freshwater Research. 2001;75:71–98. [Google Scholar]
- Fleming IA, Jonsson B, Gross MR. Phenotypic divergence of sea-ranched, farmed and wild salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:2808–2824. [Google Scholar]
- Fleming IA, Hindar K, Mjolnerod IB, Jonsson B, Balstad T, Lamberg A. Lifetime success and interactions of farm salmon invading a native population. Proceedings of the Royal Society of London, Series B – Biological Sciences. 2000;267:1517–1524. doi: 10.1098/rspb.2000.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming IA, Einum S, Jonsson B, Jonsson N. Comment on “Rapid evolution of egg size in captive salmon”. Science. 2003;302:59d. doi: 10.1126/science.1084695. [DOI] [PubMed] [Google Scholar]
- Ford MJ. Selection in captivity during supportive breeding may reduce fitness in the wild. Conservation Biology. 2002;16:815–825. [Google Scholar]
- Ford MJ, Fuss H, Boelts B, LaHood E, Hard J, Miller JB. Changes in run timing and natural smolt production in a naturally spawning coho salmon (Oncorhynchus kisutch) population after 60 years of intensive hatchery supplementation. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2343–2355. [Google Scholar]
- Foresberg LA, Dannewitz J, Petersson E, Grahm M. Influence of genetic similarity in the reproductive success and mate choice of brown trout – females fishing for optimal MHC dissimilarity. Journal of Evolutionary Biology. 2007;20:1859–1869. doi: 10.1111/j.1420-9101.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- Frankel OH, Soulé ME. Conservation and Evolution. Cambridge, England. 327 pp: Cambridge University Press; 1981. [Google Scholar]
- Frankham R. Effective population size – adult population sizes in wildlife: a review. Genetical Research. 1995;66:95–107. doi: 10.1017/S0016672308009695. [DOI] [PubMed] [Google Scholar]
- Frankham R. Genetics and extinction. Biological Conservation. 2005;126:131–140. [Google Scholar]
- Frankham R. Genetic adaptation to captivity. Molecular Ecology. 2008;17:325–333. doi: 10.1111/j.1365-294X.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- Frankham R, Manning H, Margan SA, Briscoe DA. Does equalization of family size reduce genetic adaptation to captivity? Animal Conservation. 2000;3:357–363. [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. UK: Cambridge University Press; 2002. [Google Scholar]
- Franklin IA. Evolutionary changes in small populations. In: Soule ME, Wilcox BA, editors. Conservation Biology, and Evolutionary-Ecological Perspective. Sunderland, MA: Sinauer Associates; 1980. pp. 135–150. [Google Scholar]
- Fraser DJ, Bernatchez L. Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Molecular Ecology. 2001;10:2741–2752. [PubMed] [Google Scholar]
- Fraser DJ, Bernatchez L. Adaptive migratory divergence among sympatric brook charr populations. Evolution. 2005;59:611–624. doi: 10.1554/04-346. [DOI] [PubMed] [Google Scholar]
- Fraser DJ, Bernatchez L. Ecology, evolution and the conservation of lake-migratory brook trout: a perspective from pristine populations. Transactions of the American Fisheries Society. 2008 in press. [Google Scholar]
- Fraser DJ, Duchesne P, Bernatchez L. Migratory charr schools exhibit population and kin associations beyond juvenile stages. Molecular Ecology. 2005;14:3133–3146. doi: 10.1111/j.1365-294X.2005.02657.x. [DOI] [PubMed] [Google Scholar]
- Fraser DJ, Coon T, Dion R, Prince MR, Bernatchez L. Integrating traditional and evolutionary knowledge in biodiversity conservation: a population level case study. Ecology and Society. 2006;11:4. [Google Scholar]
- Fraser DJ, Hansen MM, Tessier N, Ostergaard S, Legault M, Bernatchez L. Comparative estimation of effective population sizes and temporal gene flow in two contrasting population systems. Molecular Ecology. 2007a;16:3866–3889. doi: 10.1111/j.1365-294X.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- Fraser DJ, Jones MW, McParland TL, Hutchings JA. Loss of historical immigration and the unsuccessful rehabilitation of extirpated salmon populations. Conservation Genetics. 2007b;8:527–546. [Google Scholar]
- Frazer N. Sea turtle conservation and halfway technology. Conservation Biology. 1992;6:179–184. [Google Scholar]
- Fritts AL, Scott JL, Pearsons TN. The effects of domestication on the relative vulnerability of hatchery and wild spring chinook salmon (Oncorhynchus tshawytscha) to predation. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:813–818. [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Gausen D. The Norwegian Gene Bank Programme for Atlantic salmon (Salmo salar. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York: Plenum Press; 1993. pp. 181–187. [Google Scholar]
- Gautschi B, Jabob G, Negro JJ. Analysis of relatedness and determination of the source of founders in the captive bearded vulture, Gypaetus barbatus, population. Conservation Genetics. 2003;4:479–490. [Google Scholar]
- Gerlier M, Roche P. A radiotelemetry study of the migration of Atlantic salmon (Salmo salar L.) and sea trout (Salmo trutta L.) in the upper Rhine. Hydrobiologia. 1998;371/372:283–293. [Google Scholar]
- Gomulkiewicz R, Holt RD. When does evolution by natural selection prevent extinction? Evolution. 1995;49:201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Goodman D. Selection equilibrium for hatchery and wild spawning fitness in integrated breeding programs. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:374–389. [Google Scholar]
- Griffith B, Scott JM, Carpenter JW, Reed C. Translocations as a species conservation tool: status and strategy. Science. 1989;245:477–480. doi: 10.1126/science.245.4917.477. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Sabat P, Valladares L. Environmental signaling and evolutionary change: can exposure of pregnant mammals to environmental estrogens lead to epigentically-induced changes in embryos? Evolution and Development. 2005;7:341–350. doi: 10.1111/j.1525-142X.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- Hansen MM. Estimating the long-term effects of stocking domesticated trout into wild brown trout (Salmo trutta) populations: an approach using microsatellite DNA analysis of historical and contemporary samples. Molecular Ecology. 2002;11:1003–1015. doi: 10.1046/j.1365-294x.2002.01495.x. [DOI] [PubMed] [Google Scholar]
- Hansen MM, Jensen LF. Sibship within samples of brown trout (Salmo trutta) and implications for supportive breeding. Conservation Genetics. 2005;6:297–305. [Google Scholar]
- Hansen MM, Nielsen EE, Mensberg K-LD. The problem of sampling families rather than populations: relatedness among individuals in samples of juvenile brown trout (Salmo trutta, L.) Molecular Ecology. 1997;6:469–474. [Google Scholar]
- Hanski I, Saccheri I. Molecular-level variation affects population growth in a butterfly metapopulation. PLOS Biology. 2006;4:719–726. doi: 10.1371/journal.pbio.0040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard JJ. Genetic monitoring of life-history characters in salmon supplementation: problems and opportunities. American Fisheries Society Symposium. 1995;15:212–225. [Google Scholar]
- Hard JJ. Evolution of Chinook salmon life history under size-selective harvest. In: Hendry AP, Stearns SC, editors. Evolution Illuminated: Salmon and Their Relatives. New York: University Press; 2004. pp. 316–337. [Google Scholar]
- Hartl DL, Clark AG. Principles of Population Genetics. Sunderland, MA: Sinauer Associates Inc; 1989. [Google Scholar]
- Harvey B. Cryopreservation of fish spermatozoa. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York: Plenum Press; 1993. pp. 175–178. [Google Scholar]
- Haugen TO, Vollestad LA. Population differences in early-life history traits in grayling. Journal of Evolutionary Biology. 2000;13:897–905. [Google Scholar]
- Heath DD, Fox CW, Heath JW. Maternal effects of offspring size: variation through early development of chinook salmon. Evolution. 1999;53:1605–1611. doi: 10.1111/j.1558-5646.1999.tb05424.x. [DOI] [PubMed] [Google Scholar]
- Heath DD, Heath JW, Bryden CA, Johnson RM, Fox CW. Rapid evolution of egg size in captive salmon. Science. 2003;299:1738–1740. doi: 10.1126/science.1079707. [DOI] [PubMed] [Google Scholar]
- Hebdon JL, Kline P, Taki D, Flagg TA. Evaluating reintroduction strategies for Redfish Lake sockeye salmon captive broodstock progeny. American Fisheries Society Symposium. 2004;44:401–413. [Google Scholar]
- Hedrick PW, Hedgecock D. Effective population size in winter-run chinook salmon. Conservation Biology. 1994;8:890–902. [Google Scholar]
- Hedrick PW, Hedgecock D, Hamelberg S. Effective population size in winter-run chinook salmon. Conservation Biology. 1995;9:615–624. doi: 10.1093/jhered/91.2.112. [DOI] [PubMed] [Google Scholar]
- Hedrick PW, Hedgecock D, Hamelberg S, Croci SJ. The impact of supplementation in winter-run Chinook salmon on effective population size. Journal of Heredity. 2000a;91:112–116. doi: 10.1093/jhered/91.2.112. [DOI] [PubMed] [Google Scholar]
- Hedrick PW, Rashbrook VK, Hedgecock D. Effective population size in winter-run chinook salmon based on microsatellite analysis of returning spawners. Canadian Journal of Fisheries and Aquatic Sciences. 2000b;57:2368–2373. [Google Scholar]
- Hedrick PW, Dowling TE, Minckley WL, Tibbets CA, Demarais BD, Marsh PC. Establishing a captive broodstock for the endangered Bonytail chub (Gila elegans. Journal of Heredity. 2000c;91:35–39. doi: 10.1093/jhered/91.1.35. [DOI] [PubMed] [Google Scholar]
- Hendry AP. Adaptive divergence and the evolution of reproductive isolation in the wild: an empirical demonstration using introduced sockeye salmon. Genetica. 2001;112:515–534. [PubMed] [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Herbinger CM, O'Reilly PT, Verspoor E. Unravelling first-generation pedigrees in wild endangered salmon populations using molecular genetic markers. Molecular Ecology. 2006;15:2261–2275. doi: 10.1111/j.1365-294X.2006.02923.x. [DOI] [PubMed] [Google Scholar]
- Hesthagen T, Larsen BM. Recovery and establishment of Atlantic salmon, Salmo salar, in limed Norwegian rivers. Fisheries Management and Ecology. 2003;10:87–95. [Google Scholar]
- Hill WG. Effective size of populations with overlapping generations. Theoretical Population Biology. 1972;3:278–289. doi: 10.1016/0040-5809(72)90004-4. [DOI] [PubMed] [Google Scholar]
- Hindar K, Ryman N, Utter F. Genetic effects of cultured fish on natural fish populations. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:945–957. [Google Scholar]
- Holt RD. Ecology at the mesoscale: the influence of regional processes on communities. In: Ricklefs R, Schluter D, editors. Species Diversity in Ecological Communities: Historical and Biogeographical Perspectives. Chicago, Illinois: University of Chicago Press; 1993. pp. 77–88. [Google Scholar]
- Hood Canal Coordinating Council. Hood Canal and Eastern Strait of Juan de Fuca Summer Chum Salmon Recovery Plan. Belfair, Washington, 15 pp: Hood Canal Coordinating Council; 2005. Executive summary. [Google Scholar]
- Incerpi A. Hatchery-bashing: a useless pastime. Fisheries. 1996;21:28. [Google Scholar]
- Independent Scientific Review Panel. Review of Idaho Supplementation Studies. Portland, Oregon: Independent Scientific Review Panel; 2003. Document no. 2003-8. [Google Scholar]
- Innes BH, Elliott NG. Genetic diversity in a Tasmanian hatchery population of Atlantic salmon (Salmo salar L.) compared with its Canadian progenitor population. Aquaculture Research. 2006;37:563–569. [Google Scholar]
- Irvine JR, Gross MR, Wood CC, Holtby LB, Schubert ND, Amiro PG. Canada's Species at Risk Act: An opportunity to protect “endangered” salmon. Fisheries. 2005;30:11–19. [Google Scholar]
- IUCN. Guidelines for reintroductions. 1998. http://www.iucn.org/themes/ssc/publications/policy/reinte/ (accessed 15 May 2008) [Google Scholar]
- IUCN. IUCN red list of threatened species. 2006. http://www.iucnredlist.org/ (accessed 15 May 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson JI, Abrahams MV. Interbreeding with domestic strain increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss) – an experimental study. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:243–247. [Google Scholar]
- Johnsson JI, Petersson E, Jönsson E, Björnsson BT, Järvi T. Domestication and growth hormone alter antipredator behaviour and growth patterns in juvenile brown trout, Salmo trutta. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:1546–1554. [Google Scholar]
- Johnsson JI, Hojesjo J, Fleming IA. Behavioural and heart rate responses to predation risk in wild and domesticated Atlantic salmon. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:788–794. [Google Scholar]
- Jonsson B, Jonsson N, Fleming IA. Does early growth cause a phenotypically plastic response in egg production of Atlantic salmon? Functional Ecology. 1996;10:89–96. [Google Scholar]
- Kallio-Nyberg I, Koljonen ML. The genetic consequence of hatchery rearing on life-history traits of the Atlantic salmon (Salmo salar L.): a comparative analysis of sea-ranched salmon with wild and reared parents. Aquaculture. 1997;153:207–224. [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends in Ecology and Evolution. 2002;17:230–241. [Google Scholar]
- Kimura M, Crow JF. The measurement of effective population number. Evolution. 1963;17:279–288. [Google Scholar]
- King JL. The effects of litter culling-or family planning-on the rate of natural selection. Genetics. 1965;51:425–429. doi: 10.1093/genetics/51.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Kinnison MT, Unwin MJ, Quinn TP. Eco-evolutionary versus habitat contribution to invasion: experimental evaluation in the wild. Molecular Ecology. 2008;17:405–414. doi: 10.1111/j.1365-294X.2007.03495.x. [DOI] [PubMed] [Google Scholar]
- Knudsen CM, Schroder SL, Busack CA, Johnston MV, Pearsons TN, Bosch WJ, Fast DE. Comparison of life history traits between first-generation hatchery and wild Upper Yakima River Spring Chinook salmon. Transactions of the American Fisheries Society. 2006;135:1130–1144. [Google Scholar]
- Koljonen ML, Tahtinen J, Saisa M, Koskiniemi J. Maintenance of genetic diversity of Atlantic salmon (Salmo salar) by captive breeding programmes and the geographic distribution of microsatellite variation. Aquaculture. 2002;212:69–92. [Google Scholar]
- Koskinen MT, Haugen TO, Primmer CR. Contemporary fisherian life-history evolution in small salmonid populations. Nature. 2002;419:826–830. doi: 10.1038/nature01029. [DOI] [PubMed] [Google Scholar]
- Kostow KE. Differences in juvenile phenotypes and survival between hatchery stocks and a natural population provide evidence for modified selection due to captive breeding. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:577–589. [Google Scholar]
- Kostow KE, Marshall AR, Phelps SR. Naturally spawning hatchery steelhead contribute to smolt production but experience low reproductive success. Transactions of the American Fisheries Society. 2003;132:780–790. [Google Scholar]
- Kozfkay CC, Campbell MR, Heindel JA, Baker DJ, Kline P, Powell MS, Flagg TA. Conservation Genetics. 2008. A genetic evaluation of relatedness for broodstock management of captive, endangered Snake River sockeye salmon, Oncorhynchus nerka. In press. [Google Scholar]
- Kraaijeveld-Smit FJL, Griffiths RA, Moore RD, Beebee TJC. Captive breeding and the fitness of reintroduced species: a test of the responses to predators in a threatened amphibian. Journal of Applied Ecology. 2006;43:360–365. [Google Scholar]
- Krueger CC, Ihssen PE. Review of genetics of lake trout in the great lakes: History, molecular genetics, physiology, strain comparisons, and restoration management. Journal of Great Lakes Research. 1995;21(Suppl 1):348–363. [Google Scholar]
- Krueger CC, Gharrett AJ, Dehring TR, Allendorf FW. Genetic aspects of fisheries rehabilitation programs. Canadian Journal of Fisheries and Aquatic Sciences. 1991;38:1877–1881. [Google Scholar]
- Kusuda S, Koide N, Kawamula H. Cryopreservation diluents for spermatozoa of Sakhalin taimen Hucho perryi. Fisheries Science. 2005;71:293–298. [Google Scholar]
- Lahnsteiner F. Semen cryopreservation in the salmonidae and in the Northern pike. Aquaculture Research. 2000;31:245–258. [Google Scholar]
- Lahnsteiner F, Weismann T, Patzner R. Cryopreservation of semen of the grayling (Thymallus thymallus) and the Danube salmon (Hucho hucho. Aquaculture. 1996;144:265–274. [Google Scholar]
- Lambrinos JG. How interactions between ecology and evolution influence invasion dynamics. Ecology. 2004;85:2061–2070. [Google Scholar]
- Lande R. Breeding plans for small populations based on the dynamics of quantitative genetic variance. In: Ballou JD, Gilpin M, Foose TJ, editors. Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Population Conservation. New York: Columbia University Press; 1995. pp. 318–340. [Google Scholar]
- Landry C, Garant D, Duchesne P, Bernatchez L. ‘Good genes as heterozygosity’– the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar. Proceedings of the Royal Society of London Biological Sciences. 2001;268:1279–1285. doi: 10.1098/rspb.2001.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DA, Beckman BR, Cooper KA, Barrett D, Johnson M, Swanson P, Dickhoff WW. Assessments of high rates of precocial male maturation in a spring Chinook salmon supplementation hatchery programme. Transactions of the American Fisheries Society. 2004;133:98–120. [Google Scholar]
- Lassuy DR. Introduced species as a factor in extinction and endangerment of native fish species. In: Schramm HL, Piper RG, editors. Uses and Effects of Cultured Fishes in Aquatic Ecosystems. Bethesda, MD: American Fisheries Society; 1995. pp. 391–396. American Fisheries Society Symposium 15. [Google Scholar]
- Leberg PL. Effects of population bottlenecks on genetic diversity as measured my allozyme electrophoresis. Evolution. 1992;46:477–494. doi: 10.1111/j.1558-5646.1992.tb02053.x. [DOI] [PubMed] [Google Scholar]
- Leberg PL, Firmin BD. Role of inbreeding depression and purging in captive breeding and restoration programmes. Molecular Ecology. 2008;17:334–343. doi: 10.1111/j.1365-294X.2007.03433.x. [DOI] [PubMed] [Google Scholar]
- Leider SA, Hulett PL, Loch JJ, Chilcote MW. Electrophoretic comparison of the reproductive success of naturally spawning transplanted and wild steelhead trout through the returning adult stage. Aquaculture. 1990;88:239–252. [Google Scholar]
- Luikart G, Allendorf FW, Cornuet JM, Sherwin WB. Distortion of allele frequency distributions provides a test for recent population bottlenecks. Journal of Heredity. 1998;89:238–247. doi: 10.1093/jhered/89.3.238. [DOI] [PubMed] [Google Scholar]
- Lynch M, O'Hely M. Captive breeding and the genetic fitness of natural populations. Conservation Genetics. 2001;2:363–378. [Google Scholar]
- Machado-Schiaffino G, Dopico E, Garcia-Vazquez E. Genetic variation losses in Atlantic salmon stocks created for supportive breeding. Aquaculture. 2007;264:59–65. [Google Scholar]
- Mahnken C, Prentic E, Waknitz W, Sims NG, Williams J. The application of recent smoltifcation research to public hatchery releases: an assessment of size/time requirements for Columbia River hatchery coho (Oncorhynchus kisutch. Aquaculture. 1982;28:251–268. [Google Scholar]
- Margan SH, Nurthen RK, Montgomery ME, et al. Single large or several small? Population fragmentation in the captive management of endangered species. Zoo Biology. 1998;17:467–480. [Google Scholar]
- Maynard DJ, Flagg TA, Mahnken CVW, Schroder SL. Natural rearing technologies for increased postrelease survival of hatchery-reared salmon. Bulletin of National Research Institute of Aquaculture. 1996;2:71–77. [Google Scholar]
- Maynard DJ, Flagg TA, Iwamoto RN, Mahnken CVW. A review of recent studies investigating seminatural release strategies as a tool for increasing Pacific salmon postrelease survival. American Fisheries Society Symposium. 2004;44:573–584. [Google Scholar]
- McGinnity P, Prodohl P, Ferguson A, Hynes R, O'Maoileidigh N, Baker N, Cotter D, et al. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proceedings of the Royal Society of London, Series B – Biological Sciences. 2003;270:2443–2450. doi: 10.1098/rspb.2003.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnity P, Prodohl P, O'Maoileidigh N, Hynes R, Cotter S, Baker N, O'Hea B, Ferguson A. Differential lifetime success and performance of native and non-native Atlantic salmon examined under common environmental conditions. Journal of Fish Biology. 2004;65(Suppl A):175–187. [Google Scholar]
- McKay JK, Latta RG. Adaptive population divergence: markers, QTL and traits. Trends in Ecology and Evolution. 2002;17:285–291. [Google Scholar]
- McKenna JE, Johnson JH. Juvenile rainbow trout production in New York tributaries of Lake Ontario: implications for Atlantic salmon restoration. North American Journal of Fisheries Management. 2005;25:391–403. [Google Scholar]
- McKinlay DD, Lehmann S, Bateman J, Cook R. Pacific salmon hatcheries in British Columbia. American Fisheries Society Symposium. 2004;44:57–75. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Differential reproductive success of sympatric, naturally spawning hatchery and wild steelhead, Oncorhynchus mykiss. Environmental Biology of Fishes. 2004;69:359–369. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Nonrandom, size- and timing-biased breeding in a hatchery population of steelhead trout. Conservation Biology. 2005;19:446–454. [Google Scholar]
- McLean JE, Seamons TR, Dauer MB, Bentzen P, Quinn TP. Variation in reproductive success and effective number of breeders in a hatchery population of steelhead trout (Oncorhynchus mykiss): examination by microsatellite-based parentage analysis. Conservation Genetics. 2007;9:295–304. [Google Scholar]
- Meffe GK. Techno-arrogance and halfway technologies: salmon hatcheries on the Pacific coast of North America. Conservation Biology. 1992;5:320–324. [Google Scholar]
- Miller LM, Kapuscinski AR. Genetic guidelines for hatchery supplementation programmes. In: Hallerman EM, editor. Population Genetics: Principles and Applications for Fisheries Scientists. Bethesda, MD: American Fisheries Society; 2003. pp. 329–355. [Google Scholar]
- Miller LM, Close T, Kapuscinski AR. Lower fitness of hatchery and hybrid rainbow trout compared to naturalized populations in Lake Superior tributaries. Molecular Ecology. 2004;13:3379–3388. doi: 10.1111/j.1365-294X.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Mobrand LE, Barr J, Blankenship L, Campton DE, Evelyn TTP, Flagg TA, Mahnken CV, et al. Hatchery reform in Washington State: principles and emerging issues. Fisheries. 2005;30:11–22. [Google Scholar]
- Montgomery ME, Ballou JD, Nurthen RK, England PR, Briscoe DA, Frankham R. Minimizing kinship in captive breeding programs. Zoo Biology. 1997;16:377–389. [Google Scholar]
- Moritz C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Systematic Biology. 2002;51:238–254. doi: 10.1080/10635150252899752. [DOI] [PubMed] [Google Scholar]
- Moyer GR, Blouin MS, Banks MA. The influence of family-correlated survival on NbN for progeny from integrated multi- and single-generation hatchery stocks of coho salmon (Oncorhynchus kisutch. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:1258–1265. [Google Scholar]
- Moyle PB. Comparative behaviour of young brook trout of domestic and wild origin. Progressive Fish Culturist. 1969;31:51–56. [Google Scholar]
- Mullins CC, Bourgeois CE, Porter TR. Opening up new habitat: Atlantic salmon (Salmo salar L.) enhancement in Newfoundland. In: Mills D, editor. Salmon at the Edge. Oxford: Blackwell Science Ltd; 2003. pp. 200–221. [Google Scholar]
- Myers JM, Heggelund PO, Hudson G, Iwamoto RN. Genetics and broodstock management of coho salmon. Aquaculture. 2001;197:43–62. [Google Scholar]
- Myers RA, Levin SA, Lande R, James FC, Murdoch WW, Paine RT. Hatcheries and endangered salmon. Science. 2004;303:1980–1980. doi: 10.1126/science.1095410. [DOI] [PubMed] [Google Scholar]
- Naish KA, Taylor JE, Levin PS, Quinn TP, Winton JR, Huppert J, Hilborn R. An evaluation of the effects of conservation and fishery enhancement hatcheries on wild populations of salmon. Advances in Marine Biology. 2008;53:61–194. doi: 10.1016/S0065-2881(07)53002-6. [DOI] [PubMed] [Google Scholar]
- Nei M, Takahata N. Effective population size, genetic diversity, and coalescence time in subdivided populations. Journal of Molecular Evolution. 1993;37:240–244. doi: 10.1007/BF00175500. [DOI] [PubMed] [Google Scholar]
- Newman D, Pilson D. Increased probability of extinction due to decreased genetic effective population size: Experimental populations of Clarkia pulchella. Evolution. 1997;51:354–362. doi: 10.1111/j.1558-5646.1997.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Nickelson TE, Solazzi MF, Johnson SL. Use of hatchery coho salmon (Oncorhynchus kisutch) presmolts to rebuild wild populations in Oregon coastal streams. Canadian Journal of Fisheries and Aquatic Sciences. 1986;43:2443–2449. [Google Scholar]
- Norman L. Stream Aquarium Observations of Territorial Behavior in Young Salmon (Salmo Salar) of Wild and Hatchery Origin, Vol. 2. Sweden: Salmon Research Institute; 1987. p. 7. Salmon Research Institute Report. [Google Scholar]
- N.R.C. (National Research Council) Upstream: Salmon and Society in the Pacific Northwest. Washington DC: National Academy Press; 1996. [Google Scholar]
- O'Reilly P, Doyle RW. Live gene banking of endangered populations of Atlantic salmon. In: Verspoor E, Stradmeyer L, Nielsen JL, editors. The Atlantic Salmon: Genetics, Conservation and Management. The Netherlands: Springer; 2007. pp. 346–380. [Google Scholar]
- Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science. 2007;317:1517. doi: 10.1126/science.1145626. [DOI] [PubMed] [Google Scholar]
- Oregon Department of Fish and Wildlife (ODFW) and US Fish and Wildlife Service (USFWS) Integrated Hatchery Operations Team – Operation Plans for Anadromous Fish Production Facilities in the Columbia River Basin. II. Portland Oregon: Bonneville Power Association; 1996. Oregon. BPA Project No. 92-043. [Google Scholar]
- Page KS, Scribner KT, Bast D, Holey ME, Burnham-Curtis MK. Genetic evaluation of a Great Lakes lake trout hatchery program. Transactions of the American Fisheries Society. 2005;134:872–891. [Google Scholar]
- Palm S, Dannewitz J, Jarvi T, Petersson E, Prestegaard T, Ryman N. Lack of molecular genetic divergence between sea-ranched and wild sea trout (Salmo trutta. Molecular Ecology. 2003;12:2057–2071. doi: 10.1046/j.1365-294x.2003.01892.x. [DOI] [PubMed] [Google Scholar]
- Pante MJR, Gjerde B, McMillan I. Effect of inbreeding on body weight at harvest in rainbow trout, Oncorhynchus mykiss. Aquaculture. 2001;192:201–211. [Google Scholar]
- Parrish DL, Behnke RJ, Gephard SR, McCormick SD, Reeves GH. Why aren't there more Atlantic salmon (Salmo salar)? Canadian Journal of Fisheries and Aquatic Sciences. 1998;55(Suppl 1):281–287. [Google Scholar]
- Pascual MA, Ciancio JE. Introduced salmonids in Patagonia: risks, uses and a conservation paradox. In: Bert TM, editor. Ecological and Genetic Implications of Aquaculture Activities. The Netherlands: Springer; 2007. pp. 333–353. [Google Scholar]
- Pascual MA, Bentzen P, Riva Rossi C, Mackey G, Kinnison M, Walker R. First documented case of anadromy in a population of introduced rainbow trout in Patagonia, Argentina. Transactions of the American Fisheries Society. 2001;130:53–67. [Google Scholar]
- Pearsons TN, Fritts AL, Scott JL. The effects of domestication on competitive dominance of juvenile spring chinook salmon (Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:803–812. [Google Scholar]
- Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T. The evolutionary demography of ecological change: Linking trait variation and population growth. Science. 2007;315:1571–1574. doi: 10.1126/science.1139024. [DOI] [PubMed] [Google Scholar]
- Perez-Enriquez R, Takagi M, Taniguchi N. Genetic variability and pedigree tracing of a hatchery-reared stock of red sea bream (Pagrus major) used for stock enhancement, based on microsatellite DNA markers. Aquaculture. 1999;173:413–423. [Google Scholar]
- Perry GML, Audet C, Bernatchez L. Maternal genetic effects on adaptive divergence between anadromous and resident brook charr during early life-history. Journal of Evolutionary Biology. 2005;18:1348–1361. doi: 10.1111/j.1420-9101.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- Philippart JC. Is captive breeding an effective solution for the preservation of endemic species? Biological Conservation. 1995;72:281–295. [Google Scholar]
- Piironen J. Cryopreservation of sperm from brown trout (Salmo trutta m-lacustris L) and Arctic charr (Salvelinus alpinus L) Aquaculture. 1993;116:275–285. [Google Scholar]
- Pitcher TE, Neff BD. Genetic quality and offspring performance in Chinook salmon: implications for supportive breeding. Conservation Genetics. 2007;8:607–616. [Google Scholar]
- Pollard HA, Flagg TA. Guidelines for use of captive broodstocks in recovery efforts for Pacific salmon. American Fisheries Society Symposium. 2004;44:333–345. [Google Scholar]
- Prignon C, Micha JC, Rimbaud G, Phillipart JC. Rehabilitation efforts for Atlantic salmon in the Meuse River Basin area. Hydrobiologia. 1999;410:69–77. [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle WA: University of Washington Press; 2005. [Google Scholar]
- Quinn TP, Kinnison MT, Unwin MJ. Evolution of Chinook salmon (Oncorhynchus tshawytscha) population in New Zealand: patterns, rates and process. Genetica. 2001;112:493–513. [PubMed] [Google Scholar]
- Ramirez O, Altet L, Ensenat C, Vila C, Sanchez A, Ruiz A. Genetic assessment of the Iberian wolf Canis lupus signatus captive breeding program. Conservation Genetics. 2006;7:861–878. [Google Scholar]
- Reed DH, Frankham R. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution. 2001;55:1095–1103. doi: 10.1111/j.0014-3820.2001.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Reisenbichler RR. Uncertainty and research needs for supplementing wild populations of anadromous Pacific salmon. American Fisheries Society Symposium. 2004;44:263–275. [Google Scholar]
- Reisenbichler RR, McIntyre JD. Genetic differences in growth and survival of juvenile hatchery and wild steelhead trout, Salmo gairdneri. Journal of the Fisheries Research Board of Canada. 1977;34:123–128. [Google Scholar]
- Reisenbichler RR, Rubin SP. Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES Journal of Marine Science. 1999;56:459–466. [Google Scholar]
- Reisenbichler RR, Utter FM, Krueger CC. Genetic concepts and uncertainties in restoring fish populations and species. In: Wissmar RC, Bisson PA, editors. Strategies for Restoring River Ecosystems. Bethesda, MD: American Fisheries Society; 2003. pp. 149–183. [Google Scholar]
- Reisenbichler RR, Rubin S, Wetzel L, Phelps S. Natural selection after release from a hatchery stock leads to domestication in steelhead, Oncorhynchus mykiss. In: Leber M, Kitada S, Blankenship HL, Svasand T, editors. Stock Enhancement and sea Ranching. Oxford, UK: Blackwell Publishing Ltd; 2004. pp. 371–384. [Google Scholar]
- Rensel J. Comment on March 1997 North Pacific International Chapter of the American Fisheries Society plenary session: incorporating hatcheries in ecosystem management. North Pacific International Chapter AFS Newsletter. 1997;19:14–15. [Google Scholar]
- Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of United States of America. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Quinn TP. Factors affecting the outcome of territorial contests between hatchery and naturally reared coho salmon parr in the laboratory. Journal of Fish Biology. 1998;53:1220–1230. [Google Scholar]
- Rhodes JS, Quinn TP. Comparative performance of genetically similar hatchery and naturally reared juvenile coho salmon in streams. North American Journal of Fisheries Management. 1999;19:670–677. [Google Scholar]
- Riddell BE. Spatial organization of Pacific salmon: what to conserve? In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York: Plenum Press; 1993. pp. 23–37. [Google Scholar]
- Roberts PD, Stewart GB, Pullin AS. Are review articles a reliable source of evidence to support conservation and environmental management? A comparison with medicine. Biological Conservation. 2006;132:409–423. [Google Scholar]
- Robertson A. A theory of limits in artificial selection. Proceedings of the Royal Society of London, Series B – Biological Sciences. 1960;153:234–249. [Google Scholar]
- Rodriguez-Ramilo ST, Moran P, Caballero A. Relaxtion of selection with equalization of parental contributions in conservation programs: an experimental test with Drosophila melanogaster. Genetics. 2006;172:1043–1054. doi: 10.1534/genetics.105.051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, Darling JA. Paradox lost: genetic diversity and the success of species invasions. Trends in Ecology and Evolution. 2007;22:454–464. doi: 10.1016/j.tree.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Rowan GD. Umatilla Hatchery Satellite Facilities Operation and Maintenance: Annual Report 1996. Portland, OR: Bonneville Power Administration, Public Information Center. CKPS-1; 1997. [Google Scholar]
- Rudnick JA, Lacy RC. Conservation Genetics. 2008. The impact of assumptions about founder relationships on the effectiveness of captive breeding strategies. In press. [Google Scholar]
- Russello A, Amato G. Ex situ population management in the absence of pedigree information. Molecular Ecology. 2004;13:2829–2840. doi: 10.1111/j.1365-294X.2004.02266.x. [DOI] [PubMed] [Google Scholar]
- Ryder OA. Species conservation and systematics – the dilemma of subspecies. Trends in Ecology and Evolution. 1986;1:9–10. [Google Scholar]
- Ryman N, Laikre L. Effects of supportive breeding on the genetically effective population size. Conservation Biology. 1991;5:325–329. [Google Scholar]
- Ryman N, Stahl G. Genetic perspectives of the identification and conservation of Scandinavian stocks of fish. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1562–1575. [Google Scholar]
- Ryman N, Utter F, Laikre L. Protection of intraspecific biodiversity of exploited fishes. Reviews in Fish Biology and Fisheries. 1995;5:417–446. [Google Scholar]
- Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I. Inbreeding and extinction in a butterfly metapopulation. Nature. 1998;392:491–494. [Google Scholar]
- Saisa M, Koljonen ML, Tahtinen J. Genetic changes in Atlantic salmon stocks since historical times and the effective population size of a long-term captive breeding programme. Conservation Genetics. 2003;4:613–627. [Google Scholar]
- Salvanes AGV, Braithwaite VA. Exposure to variable spatial information in the early rearing environment generates asymmetries in social interactions in cod (Gadus morhua. Behavioural Ecology and Sociobiology. 2005;59:250–257. [Google Scholar]
- Sarrazin F, Barbault B. Reintroduction: challenges and lessons for basic ecology. Trends in Ecology and Evolution. 1996;11:474–478. doi: 10.1016/0169-5347(96)20092-8. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Kosick R, Clement M, Noakes DLG. Nest site selection and spawning by captive bred Atlantic salmon, Salmo salar, in a natural stream. Environmental Biology of Fishes. 2005a;74:309–321. [Google Scholar]
- Scott RJ, Judge KA, Ramster K, Noakes DLG. Interactions between naturalised exotic salmonids and reintroduced Atlantic salmon in a Lake Ontario tributary. Ecology of Freshwater Fish. 2005b;14:402–405. [Google Scholar]
- Seddon PJ. Persistence without intervention: assessing success in wildlife reintroductions. Trends in Ecology and Evolution. 1999;14:503. doi: 10.1016/s0169-5347(99)01720-6. [DOI] [PubMed] [Google Scholar]
- Seddon PJ, Armstrong DP, Maloney RF. Developing the science of reintroduction biology. Conservation Biology. 2007;21:303–312. doi: 10.1111/j.1523-1739.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Morishima G, Wang S, Talbot A, Gilbertson L. An evaluation of the Clearwater River supplementation program in western Washington. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:423–437. [Google Scholar]
- Snyder NFR, Derrickson SR, Beissinger SR, et al. Limitations of captive breeding in endangered species recovery. Conservation Biology. 1996;10:338–348. [Google Scholar]
- Sonesson AK, Goddard ME, Meuwissen TH. The use of frozen semen to minimize inbreeding in small populations. Genetical Research. 2002;80:27–30. doi: 10.1017/s0016672302005712. [DOI] [PubMed] [Google Scholar]
- Soto D, Arismendi I, Di Prinzio C, Jara F. Establishment of Chinook salmon (Oncorhynchus tshawytscha) in Pacific basins of southern South America and its potential ecosystem implications. Revista Chilena de Historia Natural. 2007;80:81–98. [Google Scholar]
- Soulé ME. Viable Populations for Conservation. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- Soulé ME, Gilpin W, Conway W, Foose T. The millennium ark: how long a voyage, how many staterooms, how many passengers? Zoo Biology. 1986;5:101–113. [Google Scholar]
- Spidle AP, King TL, Letcher BH. Comparison of genetic diversity in the recently founded Connecticut River Atlantic salmon population to that of its primary donor stock, Maine's Penobscot River. Aquaculture. 2004;236:253–265. [Google Scholar]
- Spielman D, Brook WB, Frankham R. Most species are not driven to extinction before genetic factors impact them. Proceedings of the National Academy of Sciences of United States of America. 2004;101:15261–15264. doi: 10.1073/pnas.0403809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoss J, Refstie T. Short-term storage and cryopreservation of milt from Atlantic salmon and sea trout. Aquaculture. 1983;30:229–236. [Google Scholar]
- Su GS, Liljedahl LE, Gall GAE. Effects of inbreeding on growth and reproductive traits in rainbow trout (Oncorhynchus mykiss. Aquaculture. 1996;142:139–148. [Google Scholar]
- Swain DP, Riddell BE. Variation in agonistic behavior between newly emerged juveniles from hatchery and wild populations of coho salmon, Oncorhynchus kisutch. Canadian Journal of Fisheries and Aquatic Sciences. 1990;47:566–571. [Google Scholar]
- Swain DP, Riddell BE, Murray CB. Morphological differences between hatchery and wild populations of coho salmon (Oncorhynchus kisutch)- environmental versus genetic origin. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:1783–1791. [Google Scholar]
- Tallmon DA, Luikart G, Waples RS. The alluring simplicity and complex reality of genetic rescue. Trends in Ecology and Evolution. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Tave D. Effective breeding efficiency: and index to quantify the effects that different breeding programs and sex ratios have on inbreeding and genetic drift. Progressive Fish Culturist. 1984;46:262–268. [Google Scholar]
- Taylor EB. A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture. 1991;98:185–207. [Google Scholar]
- Taylor EB. Species pairs of north temperate freshwater fishes: taxonomy, evolution, and conservation. Reviews in Fish Biology and Fisheries. 1999;9:299–324. [Google Scholar]
- Theodorou K, Couvet D. Introduction of captive breeders to the wild: harmful or beneficial? Conservation Genetics. 2004;5:1–12. [Google Scholar]
- Thorgaard GH, Cloud JG. Reconstitution of genetic strains of salmonids using biotechnical approaches. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York: Plenum Press; 1993. pp. 189–196. [Google Scholar]
- Thrower F, Guthrie C, Nielsen J, Joyce J. A comparison of genetic variation between an anadromous steelhead, Oncorhynchus mykiss, population and seven derived populations sequestered in freshwater for 70 years. Environmental Biology of Fishes. 2004;69:111–125. [Google Scholar]
- Toro MA, Caballero A. Characterization and conservation of genetic diversity in subdivided populations. Philosophical Transactions of the Royal Society of London. 2005;360:1367–1378. doi: 10.1098/rstb.2005.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro M, Silio L, Rodriganez J, Rodriguez C, Fernandez J. Optimal use of genetic markers in conservation programmes. Genetics, Selection and Evolution. 1999;31:255–261. [Google Scholar]
- Tufto J. Effects of releasing maladapted individuals: a demographic evolutionary model. American Naturalist. 2001;158:331–340. doi: 10.1086/321987. [DOI] [PubMed] [Google Scholar]
- U.K. Environment Agency. History of Salmon in the Thames. Rotherham, U.K.. 3pp: U.K. Environment Agency; 2006a. [Google Scholar]
- U.K. Environment Agency. Thames Salmon Rehabilitation Scheme: 2005 Work Activities. Rotherham, U.K.. 3pp: U.K. Environment Agency; 2006b. [Google Scholar]
- U.K. Environment Agency. Thames Salmon Rehabilitation Scheme: 2006 Work Activities. Rotherham, U.K.. 2pp: U.K. Environment Agency; 2007. [Google Scholar]
- Unwin MJ. Fry-to-adult survival of natural and hatchery-produced chinook salmon (Oncorhynchus tshawytscha) from a common origin. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1246–1254. [Google Scholar]
- U.S. Atlantic Salmon Assessment Committee. Annual Report of the U.S. Atlantic Salmon Assessment Committee, Report 17-2004 Activities. Woods Hole, MA: U.S. Atlantic Salmon Assessment Committee; 2005. [Google Scholar]
- Utter FM. Biological criteria for definition of species and distinct intraspecific populations of anadromous salmonids under the U.S. Endangered Species Act of 1973. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1626–1635. [Google Scholar]
- Utter FM. Genetic problems of hatchery-reared progeny released into the wild and how to deal with them. Bulletin of Marine Science. 1998;62:623–640. [Google Scholar]
- Utter FM. Patterns of subspecific anthropogenic introgression in two salmonid genera. Reviews in Fish Biology and Fisheries. 2000;10:265–279. [Google Scholar]
- Utter FM, Epifanio J. Marine aquaculture: genetic potentialities and pitfalls. Reviews in Fish Biology and Fisheries. 2002;10:265–279. [Google Scholar]
- Vales-Alonso J, Fernandez J, Gonzalez-Castano FJ, Caballero A. A parallel optimization approach for controlling allele diversity in conservation schemes. Mathematical Biosciences. 2003;183:161–173. doi: 10.1016/s0025-5564(03)00037-3. [DOI] [PubMed] [Google Scholar]
- Vincent RE. Some influences of domestication upon three stocks of brook trout (Salvelinus fontinalis Mitchill) Transactions of the American Fisheries Society. 1960;89:35–52. [Google Scholar]
- Volkman ET, Pangle KL, Rajchel DA, Sutton TM, Bast DR. Comparison of hatchery performance attributes among three strains of age-0 coaster brook trout. North American Journal of Aquaculture. 2004;66:278–284. [Google Scholar]
- Wainwright TC, Waples RS. Prioritizing Pacific salmon stocks for conservation: a response to Allendorf et al. Conservation Biology. 1998;12:1144–1147. [Google Scholar]
- Wang J. More efficient breeding systems for controlling inbreeding and effective size in animal populations. Heredity. 1997;79:191–199. doi: 10.1038/hdy.1997.204. [DOI] [PubMed] [Google Scholar]
- Wang J. Optimal marker-assisted selection to increase the effective size of small populations. Genetics. 2001a;154:475–479. doi: 10.1093/genetics/157.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. A pseudo-likelihood method for estimating effective population size from temporally placed samples. Genetical Research. 2001b;78:243–257. doi: 10.1017/s0016672301005286. [DOI] [PubMed] [Google Scholar]
- Wang J. Monitoring and managing genetic variation in group breeding populations without individual pedigrees. Conservation Genetics. 2004;5:813–825. [Google Scholar]
- Wang J, Ryman N. Genetic effects of multiple generations of supportive breeding. Conservation Biology. 2001;15:1619–1631. [Google Scholar]
- Wang S, Hard JJ, Utter FM. Salmon inbreeding: a review. Reviews in Fish Biology and Fisheries. 2002;11:301–319. [Google Scholar]
- Waples RS. Conservation genetics of Pacific salmon. II. Effective population size and the rate of loss of genetic variability. Journal of Heredity. 1990;81:267–276. [Google Scholar]
- Waples RS. Pacific salmon and the definition of “species” under the Endangered Species Act. Marine Fisheries Review. 1991a;53:11–22. [Google Scholar]
- Waples RS. Genetic interactions between hatchery and wild salmonids: lessons from the Pacific Northwest. Canadian Journal Fisheries and Aquatic Sciences. 1991b;48:124–133. [Google Scholar]
- Waples RS. Evolutionarily significant units and the conservation of biological diversity under the Endangered Species Act. In: Nielsen JL, editor. Evolution and the Aquatic Ecosystem: Defining Unique Units in Population Conservation. American Fisheries Society Symposium 17. Bethesda, MD: American Fisheries Society; 1995. pp. 8–27. [Google Scholar]
- Waples RS. Dispelling some myths about hatcheries. Fisheries. 1999;24:12–21. [Google Scholar]
- Waples RS. Effective size of fluctuating salmon populations. Genetics. 2002a;161:783–791. doi: 10.1093/genetics/161.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples RS. Definition and estimation of effective population size in the conservation of endangered species. In: Beissinger SR, McCullough DR, editors. Population Viability Analysis. Chicago, IL: University of Chicago Press; 2002b. pp. 147–168. [Google Scholar]
- Waples RS, Do C. Genetic risk associated with supplementation of Pacific salmonids – captive broodstock programmes. Canadian Journal Fisheries and Aquatic Sciences. 1994;51:310–329. [Google Scholar]
- Waples RS, Drake J. Risk/benefit considerations for marine stock enhancement: a Pacific salmon perspective. In: Lber KM, Kitada S, Blankenship H, Svasand T, editors. Stock Enhancement and Sea Ranching: Developments, Pitfalls and Opportunities. Oxford: Blackwell; 2004. pp. 260–306. [Google Scholar]
- Waples RS, Teel DJ. Conservation genetics of Pacific salmon. I. Temporal changes in allele frequency. Conservation Biology. 1990;4:144–156. [Google Scholar]
- Waples RS, Ford MJ, Schmitt D. Empirical results of salmon supplementation in the Northeast Pacific: A preliminary assessment. In: Bert TM, editor. Ecological and Genetic Implications of Aquaculture Activities. Dordrecht, Netherlands: Kluwer Academic Publishers; 2007. pp. 383–403. [Google Scholar]
- Ward DM, Nislow KH, Holt LK. Do native species limit survival of reintroduced Atlantic salmon to historic rearing streams? Biological Conservation. 2008;141:146–152. [Google Scholar]
- Waugh GD. Salmon in New Zealand. In: Thorpe JE, editor. Salmon Ranching. London, England: Academic Press; 1980. pp. 277–303. [Google Scholar]
- Weber KE, Diggins LT. Increased selection response in larger populations II.Selection for ethanol vapor resistance in Drosophila melanogaster, at two population sizes. Genetics. 1990;125:585–597. doi: 10.1093/genetics/125.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind C. Sexual selection and life-history decisions: implications for supportive breeding and the management of captive populations. Conservation Biology. 2002;16:1204–1211. [Google Scholar]
- Wedekind C, Rudolfsen G, Jacob A, Urbach D, Muller R. The genetic consequences of hatchery-induced sperm competition in a salmonid. Biological Conservation. 2007;137:180–188. [Google Scholar]
- Willi Y, Van Buskirk J, Hoffmann AA. Limits to the adaptive potential of small populations. Annual Review in Ecology and Systematics. 2006;37:433–458. [Google Scholar]
- Withler FC. Transplanting Pacific Salmon. 1982. Canadian Technical Reports in Fisheries and Aquatic Sciences, 1079, Nanaimo, BC, 27 pp. [Google Scholar]
- Withler RE. Genetic consequences of fertilizing Chinook salmon (Oncorhynchus tshawytshca) eggs with pooled milt. Aquaculture. 1988;68:15–25. [Google Scholar]
- Withler RE, Le KD, Nelson RJ, Miller KM, Beacham TD. Intact genetic structure and high levels of genetic diversity in bottlenecked sockeye salmon (Oncorhynchus nerka) populations of the Fraser River, British Columbia, Canada. Canadian Journal Fisheries and Aquatic Sciences. 2000;57:1985–1998. [Google Scholar]
- Wolf CM, Griffith B, Reed C, Temple SA. Avian and mammalian translocations: Update and reanalysis of 1987 survey data. Conservation Biology. 1996;10:1142–1154. [Google Scholar]
- Wolf CM, Garland T, Griffith B. Predictors of avian and mammalian translocation success: reanalysis with phylogenetically independent contrasts. Biological Conservation. 1998;86:243–255. [Google Scholar]
- Wood CC, Gross MR. Elemental conservation units: communicating extinction risk without dictating targets for protection. Conservation Biology. 2008;22:36–47. doi: 10.1111/j.1523-1739.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- Wright S. Size of population and breeding structure in relation to evolution. Science. 1938;87:430–431. [Google Scholar]
- Yamamoto T, Reinhardt UG. Dominance and predator avoidance in domesticated and wild masu salmon, Oncorhynchus masu. Fisheries Science. 2003;69:88–94. [Google Scholar]
- Yoshiyama RM, Gerstung ER, Fisher FW, Moyle PB. Chinook salmon in the California Central Valley: an assessment. Fisheries. 2000;25:6–20. [Google Scholar]
- Young MK, Harig AL, Rosenlund B, Kennedy C. Recovery History of Greenback Cutthroat Trout: Population Characteristics, Hatchery Involvement, and Bibliography. Fort Collins, CO: United States Department of Agriculture Forest Service; 2002. General Technical Report. RMRS-GTR-88WWW. [Google Scholar]


