Summary
Schistosomiasis is a parasitic disease of significant medical and veterinary importance in many regions of the world. Recent shifts in global health policy have led towards the implementation of mass chemotherapeutic control programmes at the national scale in previously ‘neglected’ countries such as those within sub-Saharan Africa. Evolutionary theory has an important role to play in the design, application and interpretation of such programmes. Whilst celebrating the rapid success achieved to date by such programmes, in terms of reduced infection prevalence, intensity and associated human morbidity, evolutionary change in response to drug selection pressure may be predicted under certain circumstances, particularly in terms of the development of potential drug resistance, evolutionary changes in parasite virulence, transmission and host use, and/or competitive interactions with co-infecting pathogens. Theoretical and empirical data gained to date serve to highlight the importance of careful monitoring and evaluation of parasites and their hosts whenever and wherever chemotherapy is applied and where parasite transmission remains.
Keywords: control, disease, monitoring, schistosomiasis
Introduction
Schistosomiasis is a chronic and debilitating disease which affects millions of people, particularly the rural poor in the developing world. Of some 600 million people at risk, an estimated 200 million are infected, more than half of which are symptomatic and at least 20 million exhibit severe disease manifestations (King et al. 2005). Schistosomes, the causative agents, are parasitic bloodflukes (Phylum: Platyhelminth; Class: Trematoda) with indirectly transmitted life-cycles involving obligatory alternation of generations between sexual reproduction in a mammalian host and asexual reproduction within a molluscan (freshwater snail) host (Fig. 1). The free-living molluscan intermediate host-infecting larval stages, miracidia, arise from eggs passed in faeces or urine of the mammalian definitive host which hatch in freshwater. Following asexual replication in the intermediate host, cercariae are released which are infective to the mammalian host, when the hosts come into contact with infected water. Cercariae actively invade skin, transform to schistosomula and then migrate through the blood vasculature to the final maturation site, the hepatic portal and mesenteric venous systems for Schistosoma mansoni (prevalent in sub-Saharan Africa and South America) and Schistosoma japonicum (prevalent in South-East Asia), and the blood vessels of the urogenital system for Schistosoma haematobium (prevalent in sub-Saharan Africa). Of the three main schistosome species of public health importance, infection with S. japonicum and S. mansoni is associated with chronic hepatic and intestinal fibrosis, whilst S. haematobium infections can lead to ureteric and bladder fibrosis, calcification of the urinary tract and bladder cancer due to the retention of schistosome eggs within tissues and inflammatory response to them (Vennervald et al. 2000).
Figure 1.
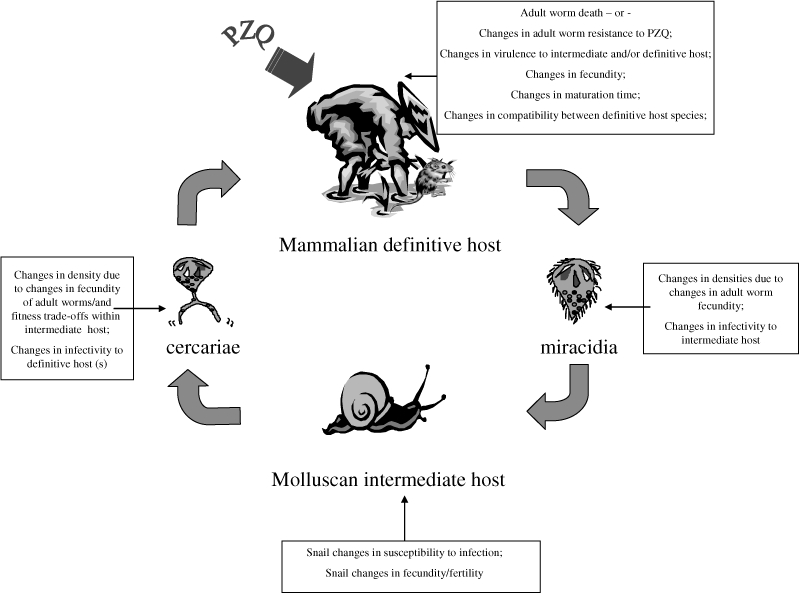
The schistosome life-cycle incorporating some of the potential effects of mass chemotherapy schistosomiasis control programmes on the different parasite life stages.
Current schistosome control programmes
There is currently no effective vaccine against human schistosomiasis and interruption of the transmission cycle through mollusciciding, biological control of the intermediate snail hosts and health education have generally proved insufficient control methods on their own (Fenwick and Webster 2006). In areas where schistosomiasis is highly endemic therefore, the present goal is to mitigate the burden of the disease by controlling morbidity (Fenwick and Webster 2006). Chemotherapy with praziquantel (PZQ) is the mainstay for schistosomiasis morbidity control, and will remain the drug of choice for the foreseeable future; the 54th World Health Assembly recently set a target of treating at least 75% of school-age children in areas with high schistosomiasis burdens by 2010 (Colley et al. 2001). Linked to this objective, the Schistosomiasis Control Initiative (SCI), established in 2002 with funding from the Bill & Melinda Gates Foundation, has assisted six sub-Saharan African countries (Burkina Faso, Mali, Niger, Tanzania including Zanzibar, Uganda and Zambia) to develop and implement sustainable schistosomiasis morbidity control programmes. The strategy included the donation of PZQ for mass treatment for schistosomiasis and co-administering, where appropriate, albendazole for soil-transmitted helminthiasis (STH, caused by the nematodes Ascaris, Trichuris and hookworm), combined with the provision of health education. Over the 5 years of the SCI program to date, more than 43 million treatments have been delivered in the six countries. Furthermore, the most recent 1.5 million were treated within an integrated program covering an additional set of neglected tropical diseases (NTDs) which include lymphatic filariasis, onchocerciasis and the bacterial disease trachoma. In all countries the impact of annual treatments on the prevalence and intensity of schistosome and STH infection has been impressive (Koukounari et al. 2007; Zhang et al. 2007). Likewise, a range of morbidity indicators, such as anaemia, liver, spleen and bladder pathologies, have all also been significantly reduced as a response to treatment (Koukounari et al. 2006a,b, 2007; Webster et al. 2007a).
The role of evolution in schistosomiasis control
In both the design and evaluation of the success of such programmes it is very important to consider the parasites and hosts as active and dynamic forces subject to evolutionary pressures. Evolutionary theory must therefore play a role both in the monitoring and evaluation, and in predicting the longer-term impact, of these and future programmes. The recent shift towards mass antischistosome chemotherapy may be expected to lead to intensive and prolonged new selection pressures on the parasites in particular, leading to a range of genotypic and phenotypic changes over space and time. The history of the use of drugs to treat infection shows clearly that in the vast majority of cases, if the selective pressure exerted by the drug is sufficiently strong at the population level, resistant strains of the infectious agent evolve and prosper. The emergence of resistance is almost always (particularly where resistance is a recessive trait) sigmoid in form over time, where little evidence for resistance is observed for many years before exponential growth in frequency towards a plateau. The magnitude of the plateau depends on many factors including the intensity of selection and the relative fitness of wild type and resistant organisms. The time scale of emergence and gene frequency change depend on the intensity of the selective pressure and the generation time of the pathogen, being a few days for many viruses to many months to years for complex multi-cellular organisms such as helminths.
Previous studies have shown that, in the laboratory at least, a range of different specific selective pressures imposed on schistosomes can produce rapid changes in parasite infectivity, fecundity, virulence (here defined as harm to the host), drug resistance and population genetic structure within only a few generations (Fallon and Doenhoff 1994; Davies and Webster 2001; Davies et al. 2001; Webster 2001; Webster and Davies 2001; Webster et al. 2001, 2003, 2004, 2007b; Gower and Webster 2004, 2005). Such selection pressures may also be predicted to influence the dynamics of inter-specific parasite interactions such as those between different species of schistosomes and between schistosomes and other co-infecting parasites, some of which are also being targeted in these control programmes (Ernould et al. 1999; Fleming et al. 2006). Parasite evolution can be a key obstacle in the development of any effective disease control programmes. Whilst drug resistance is the most obvious example, the possibility that pathogen life history characteristics, in particular virulence, but also parasite infectivity, diversity, population subdivision and transmission strategies, could also evolve under field conditions is beginning to attract the attention of evolutionary biologists (Ewald 1983; Woolhouse et al. 2002). Knowledge on how the phenotype and genotype of parasite populations may change in response to such selective pressures may therefore have important implications for the long-term success of control programmes.
Impact of mass chemotherapy on schistosome parasites
Evolution of drug resistance
Anthelmintic resistance is defined as a heritable change in a population of worms that enables their survival after chemotherapeutic treatment regimes that have previously proved to be effective against the same species and stage(s) of infection. Resistance emerges and establishes as the selective advantage of the drug resistant phenotype exceeds that of the wild type at the population level, which includes within the host and within the host population. Both populations are of relevance as a strongly resistant phenotype will only prosper if it retains an ability to transmit between hosts. Suboptimal drug concentrations, to those defined as optimal in clinical trials, can pertain as a consequence of the pharmacokinetics and pharmacodynamics of the drug mediated by host genetic background, adherence to recommended drug regimens and other factors such immune-incompetence and nutritional state of the host. Natural selection is the driving force in all biological systems and with population level application of drug treatments the risk of parasites evolving various ways to combat the impact of the drug on survival and reproduction will always exist.
The reliance of human helminth control programmes on a small number of drugs and the limited investment in antiparasitic discovery by pharmaceutical companies makes the control of these diseases highly vulnerable to the emergence and spread of drug resistance (Fenwick and Webster 2006). In parasites of veterinary importance, reports of resistance have been made in every livestock host and to every anthelmintic class. In some locations multi-drug resistance has spread to such an extent that it has jeopardized the small ruminant industry (Prichard et al. 2007). The public health community must learn from current problems in the field of veterinary medicine and seek to protect the effectiveness of existing safe and efficacious human antihelminthic drugs (and the investment incurred to develop them), particularly as there are generally few or no alternatives to be expected in the near future. This may be particularly pertinent for antischistosome control, since there is currently only PZQ available (Fenwick and Webster 2006). Furthermore, there is ample evidence of the ability of the schistosome parasites to evolve drug resistant mechanisms under field conditions, as has already become apparent in the case of oxamaniquine, which was in wide use prior to the development and use of PZQ (Katz et al. 1991; Cioli et al. 1993; Fallon and Doenhoff 1994; Fallon et al. 1995; Bonesso-Sabadini and De Souza Dias 2002).
Fortunately, to date, there is no evidence to suggest that schistosomes have already developed and/or established resistance to PZQ under field conditions, even within China despite its widespread use for over 20 years (Doenhoff et al. 2002; Doenhoff and Pica-Mattoccia 2006). However, clinical investigations carried out, primarily in Egypt and Senegal on S. mansoni (Ismail et al. 1999; Gryseels et al. 2001), the occasional reports of individual failures of PZQ in treatment of travellers with schistosomiasis (Alonso et al. 2006), and the results from a range of laboratory studies and successful artificial-selection of resistance lines, continue to raise concerns about the existence or development of potential PZQ resistance in the future (Doenhoff et al. 2002). A further cause for potential concern is the fact that the mode of action of PZQ, and the mechanisms and/or genetics of resistance in laboratory strains, still remains to be elucidated. Recent work has, however, indicated an important role for voltage-gated calcium channels, in particular the variant β subunit (Greenberg 2007) to PZQ sensitivity, although other laboratory studies (Valle et al. 2003) suggest that multiple mechanisms of resistance may be possible. This is an important consideration in predicting and monitoring the impact of mass control on the evolution of drug resistance, as such a phenotype could be predicted to emerge in different populations through varying mutations which may have different competitive advantages.
The likelihood and rate of drug resistance emerging is dependent on the underlying genetics of resistance, the frequency of resistance genes in the population, the generation time of the parasite and the strength of selection pressure imposed. One determinant of the strength of selection for drug resistance is the proportion of the population in refugia at the time of treatment. Refugia provide a pool of susceptible genes that can dilute the resistant genes in the population, and are now considered critical in the management of drug resistance in the small ruminant industry (Van Wyk 2001). For example, high levels of multi-drug resistance to albendazole, tetramisole and ivermectin were detected in the nematode parasites (predominantly Haemonchus contortus and Trichostronglyus spp.) of a goat flock in Eastern Ethiopia but not in a separately managed sheep flock in the same area, that had high levels of refugia due to communal grazing of the sheep with other animals (cattle, goats, camels and horses). Moreover it was demonstrated that drug resistance in the goat flock could be reversed by introducing communal grazing with the susceptible sheep population (Sissay et al. 2006). For human mass chemotherapy treatment of schistosomes such refugia are naturally present, and could presumably, hypothetically at least, be used in a similar way if PZQ-resistance was to emerge in human populations. The sizes of refugia are unknown but are likely to be high under current treatment schedules. PZQ is short-acting and generally given only annually (in contrast to the, up to, bimonthly frequency commonly used in animal husbandry) and a significant proportion of parasites will be in untreated individuals [such as adults or preschool children (Odogwu et al. 2006)], alternative reservoir host species (see below), the intermediate snail hosts or as free-living stages. Moreover, immature schistosomes are not susceptible to PZQ action (Gönnert and Andrews 1977), and thus may also act as refugia. Size of refugia might, however, be expected to differ between schistosome species since S. japonicum, for example, is thought to have multiple domestic and wildlife reservoir hosts, in contrast to, for example, S. haematobium, which is essentially a human parasite. Such high levels of refugia for schistosomes suggests that the likelihood of drug resistance and rate of spread will be low, contrasting markedly with for example the filarial nematodes, Onchocerca volvolus (the causative agent of river blindness) and Wuchereria bancrofti and Brugia malayi (causative agents of lymphatic filariasis), where there is some concern of reduced efficacy of Ivermectin (IVM) in humans in some areas (Osei-Atweneboana et al. 2007). These parasites are thought to have low levels of refugia as they have no free-living stages or reservoir hosts in their life-cycle, and only a small proportion of the population at any one time being present in their insect vector.
Another critical component of the strength of selection for the development of drug resistance will be the fitness of drug resistant individuals compared to susceptible individuals in the absence of drug pressure. Studies in Egypt have shown no increase of drug failure despite 10 years of therapeutic pressure in villages where there had previously been treatment failures due to the presence of worms with decreased sensitivity to PZQ (Ismail et al. 1999; Botros et al. 2005). One possible explanation as to why and how these resistant worms have not become a more significant portion of the schistosome population is an inherent ‘cost of resistance’, whereby resistant genotypes are less fit than their susceptible counterparts in the absence of drug pressure, thereby limiting their establishment and spread. In all other pathogens that have been studied intensively under drug pressure, reduced fitness of the resistant strains in the absence of drug pressure has been recorded in the early stages of the emergence of resistance. Subsequently, however, especially in bacteria, compensatory mutations or genetic changes occur to increase the fitness of the resistant variants relative to wildtype (Levin et al. 2000; Orr 2006). Drug resistant parasites may incur costs such as a lower reproduction rate, especially in the schistosome system where parasites may be required to co-adapt successfully to both their intermediate and definitive hosts. Indeed, resistant strains have been shown in the laboratory to exhibit reduced cercarial output and/or increased virulence to their intermediate host compared to susceptible strains, with some reverting to susceptibility with repeated lifecycle passage under no treatment (Lamberton 2005). Mathematical models of selection and evolution have recently been applied to the issue of the development of anthelmintic resistance. Such models have identified, for instance, those parameters which will be most important in determining the impact of mass chemotherapy, and therefore those which should be most closely monitored when evaluating this impact. The change in frequency of resistant strains has been predicted to depend on the interplay between their relative fitness, the degree of selection pressure exerted by the drug treatments and even the life-cycle stages on which density dependence operates within the host-parasite system to regulate parasite abundance (Feng et al. 2001; Churcher et al. 2006). One such model has predicted that if parasite reproduction and virulence are linked to resistance, then drug treatment may lead to the establishment of multiple parasite strains with many different levels of resistance, though resistance levels are expected to remain relatively low when resistance has a cost to the parasites (Feng et al. 2001). Other models have however suggested that, even with inherent costs of resistance, higher chemotherapeutic treatment rates, as are now being dispensed within sub-Saharan Africa, might allow both parasites with lower drug resistance to successfully invade and co-existence between susceptible and resistant parasite strains (Xua et al. 2005). The study of models that meld population genetics and transmission dynamics is in its infancy. Much biological complexity is poorly understood (such as whether the prime resistance gene will be dominant or recessive) and as such the predictive capability of these models is limited at present. To improve their predictive power, more needs to be understood about the mechanisms of resistance and relative fitness properties throughout the complex life cycles of schistosomes. Our current ignorance therefore demands that careful monitoring for the potential evolution of such resistance is part of all mass chemotherapy based control programmes.
Our ability to detect the emergence of anthelmintic resistance and to monitor its spread has indeed been declared as a priority (Prichard et al. 2007). Monitoring should include in vitro and in vivo drug efficacy assays, pharmacokinetic analyses and ideally the monitoring of molecular markers associated with resistance to PZQ (Southgate et al. 2005). A variety of in vitro and in vivo assays are available, although whether they are always good indicators of the susceptibility-resistance status of parasites has been questioned (Southgate et al. 2005). An alternative promising method of investigating the possibility of drug resistance in helminth infections of humans is based on the notion that if rare resistance-conferring alleles were already present in untreated parasite populations, their frequency would increase with chemotherapeutic selection pressure. To test this notion it is necessary to: (i) identify polymorphic genes that are undergoing treatment-induced selection; (ii) demonstrate that the favoured alleles may confer a fitness advantage to the parasites carrying them in the presence of the drug; and (iii) link observed (normal and suboptimal) patterns in response to treatment with parasite phenotype and genotype before and after repeated treatment. As drug resistance is a genetic trait, molecular (DNA) markers perhaps hold the greatest promise for identifying the development of drug resistance to anthelminthics, and hence enable further monitoring and subsequent management of any spread of such resistance. The early stages of an effect of treatment would be manifested as a change in frequency and/or diversity of alleles linked to resistance. For example, one recent study reported changes in gene diversity, heterozygosity and in the number of markers not in Hardy–Weinberg equilibrium in 28 genetic markers spanning the P-gylcoprotein locus – which is thought to be linked to ivermectin (IVM) sensitivity – of four O. volvulus populations from the Volta Region of Ghana. The authors thereby concluded that the use of IVM for onchocerciasis control has imposed strong selection on O. volvulus populations (Ardelli et al. 2006). This conclusion should not surprise us – it is to be expected in all mass treatment programmes for all pathogens. However, for many systems, such as schistosomes and PZQ, molecular markers linked to potential resistance genes are currently not available. An international consortium has recently been formed to develop anthelmintic resistance single nucleotide polymorphism (SNP) markers for detecting anthelmintic resistance (Prichard et al. 2007). Such markers would ideally be mechanistically associated with the drug resistance phenotype (informative), but could simply be strongly associated without a mechanistic link (noninformative). Development of such markers will however be a lengthy progress, particularly given the lack of mechanistic knowledge concerning PZQ action or resistance. Furthermore the association study design used to identify informative SNPs requires the existence of two populations, one that exhibits a particular phenotype and one that does not, and hence makes such studies difficult in schistosome populations where drug resistance is not well characterized. This leaves noninformative markers as an option to detect the emergence of resistance, although the large amount of genetic diversity present in schistosome populations indicates that a very large number of noninformative SNPs would be necessary in order to find any significant associations with resistance phenotypes (Rollinson et al. 1997).
In the absence of such currently available molecular markers linked to resistance/susceptibility traits, population genetic studies with neutral markers such as microsatellites can provide valuable information about population genetic changes linked to the emergence of drug resistance. These include whether infections of previously treated people represent treatment failures (and therefore putatively ‘resistant parasites’) or new infections, enabling identification of sources of re-infection, as well as providing important understanding of how microevolutionary forces, such as migration, gene flow and genetic drift, might affect the evolution of drug resistance. Studying movement in parasites and hosts is important for understanding the evolution of drug resistance. For example, the widespread occurrence of drug-resistant Plasmodium falciparum was shown to result from the recent spread of a few selected alleles, rather than from the independent evolution of new resistance alleles in multiple locations (Nair et al. 2003; Roper et al. 2003). Indeed one recent study has reported that gene diversity in the P-glycoprotein locus in O. volvolus was reduced even in worms sampled from people in neighbouring untreated regions, and suggested that movements of IVM-treated worms by the fly vector could be responsible (Ardelli et al. 2006). Such investigations have recently begun in the schistosome system following the development of microsatellite markers for the three major species of human schistosomes: S. mansoni (Durand et al. 2000; Blair et al. 2001), S. japonicum (Shrivastava et al. 2003) and S. haematobium (Golan et al. 2007). For example, in an initial study, adult worms from seven populations of S. mansoni from Kenya were examined using five microsatellite markers with a high level of genetic diversity detected suggesting that the strong genetic structure observed was a result of limited gene flow and large population sizes (Agola et al. 2006). An interesting hypothesis to emerge from this study was that limited gene flow between populations might hinder the development of drug resistance. Large scale population genetic studies as part of the monitoring and evaluation of mass human chemotherapy programmes should investigate a range of factors. These include the genetic diversity, presence and nature of population structure and gene flow in human helminth populations over space and time, estimates of effective population sizes from allele frequency data and any changes mediated by mass treatment, in order to determine the impact of treatment on the parasite population as a whole. A key problem militating against studies of schistosomes has, however, been that adult worms cannot be directly sampled from living hosts, because of their location deep within the blood vessels surrounding the intestine and bladder. Traditional indirect sampling involving passage through laboratory animals in order to obtain parasite material for analysis presents significant ethical and practical drawbacks, and may result in sampling biases such as bottlenecking processes and/or host-induced selection pressures. Recent new methodologies involving advances in room temperature DNA storage and mutliplexing are allowing direct genotyping of individual field collected larvae from infected people (Shrivastava et al. 2005; Gower et al. 2007). These new methodologies, which have obvious ethical and biological advantages, represent an enormous reduction in the logistical effort required in assaying schistosome populations. For the first time there is now the opportunity to collect samples from a large number of infected human individuals at multiple locations.
Evolution of parasite life histories
The evolution of drug resistance is only one dimension of potential parasite adaptation to human intervention through mass chemotherapy. Parasite life-history traits, including a parasite’s survivorship and fecundity within a host, will evolve in response to selection and their evolution will be constrained by trade-offs between traits (Paterson and Barber 2007). Large scale use of chemotherapeutic agents against human helminth disease is likely to result in traits other than those associated with anthelminthic resistance being under potentially strong selection – the ultimate prerequisite being heritable variation on which selection can act. It has been suggested, for example, that when anthelmintics reduce adult life expectancy, natural selection will favour parasites that mature earlier (Medley 1994; Poulin 1998) allowing parasites to produce eggs before the host is given anthelminthics but which would result in worms which were smaller and less fecund. Conversely, Skorping and Read (1998) argued that parasitic nematodes with larvae in host tissues (poorly susceptible to treatments) and adults in the gastrointestinal tract (highly susceptible to treatment) should postpone maturity, become larger and more fecund. These hypotheses were investigated in Teladorsagia cicumcinta worms infecting sheep, where resistance to one group of antheminthics, the benzimidazoles, is controlled by a point mutation on the β-tubulin gene, allowing easy genotyping of worms into resistant or susceptible categories within individual isolates over a series of drug treatments (Leignel and Cabaret 2001). The authors reported that adult worms became larger when subjected to massive chemotherapy, in agreement with Skorping and Read’s (1998) hypothesis. Differences in schistosome susceptibility to PZQ has also been documented and one hypothesis has been a delayed maturation rate in Senegal (Fallon et al. 1997) although it is not known if this could be due to previous PZQ exposure.
Another key component of parasite fitness which has attracted attention in theoretical studies is the evolution of parasite-induced harm to the host, or virulence (Bull 1994). Virulence can in some circumstances represents a cost to parasites, through reducing the duration of infection and thus transmissibility, and is thus a key source of selection via its impact on reproductive success. The virulence of parasites is of importance in schistosomiasis control, as the aim of mass chemotherapy control programs is the reduction of parasite-induced host morbidity. Drug treatment with PZQ, in the absence of drug resistance, will directly reduce parasite infection intensity (i.e. the numbers of parasites present) but evolutionary changes in parasite virulence characteristics might affect, either positively or negatively, the impact of such effects on human morbidity.
A considerable body of theory has been developed to explain how nonzero levels of virulence can evolve and be maintained as a result of parasite adaptive evolution where, for example, increased virulence is concomitant with increased parasite reproduction despite a reduced duration of infection. Current theory predicts that though virulence is costly to the parasite, it may be maintained or promoted where the fitness costs of being virulent are offset by benefits such as increased transmission, dominance in intra-host competitive interactions, or the ability to withstand host defences (May and Anderson 1983; Frank 1996). Such models most often assume that virulence is a direct side-effect of increased parasite reproduction, but that such increased reproduction also increases the probability of transmission to new hosts, or parasite competitive ability (Bull 1994), resulting in a trade-off between traits and an ‘optimum’ virulence level that maximizes parasite fitness. This has led to ideas of virulence management (Dieckmann et al. 2002) and specific predictions about, for instance, public health strategies which could prompt the evolution of benign parasites (Ewald 1994) or create the conditions which would favour more virulent pathogens (Gandon et al. 2001). For example, Porco and colleagues extended the adaptive trade-off model to consider the effect of chemotherapeutic treatment on such trade-offs (Porco et al. 2005). They considered that treatment exerts selective pressure by reducing the duration of an infection, and hence opportunities for continued reproduction, thereby reducing the value of duration-increasing strategies to the pathogen and favouring pathogen strategies that maximize the rate of transmission. Thus when virulence is subject to such a trade-off, treatment can lead to an increase in optimal virulence. In other scenarios, however, such as if there is a trade-off between virulence and host activity levels, which may determine opportunities for contacts with other susceptible hosts, they concluded that treatment decreases the optimal virulence. Theoretical studies based on parasite adaptive evolution have also proposed multiple or mixed genotype infections as a further mechanism to promote the maintenance and evolution of virulence in parasite populations, as intra-host competition can favour high virulence parasite strains of lower potential fitness than less virulent strains wherever they have a local growth or transmission advantage (Anita et al. 1994; May and Nowak 1994). This is because competition between strains may select for genotypes that exploit host resources sooner, regardless of whether they would produce fewer new infections in the single genotype situation (Bremermann and Pickering 1983), and may be directly observed as an increase in the virulence of mixed infections, or changes in optimal virulence levels over evolutionary time. Some authors have proposed that treatment may reduce the incidence of multiple infections, and thus select for lower virulence [e.g. (Galvani et al. 2003)]. However, an important point to consider is that observed virulence will be a product of both parasite and host factors (Taylor et al. 1998). Host defence against genetically heterogeneous infections may require more host resources or such defence may be less effective (Morand and Harvey 2000; Moret and Schimd-Hempel 2000). A rise in virulence not necessarily based on parasite adaptation, or conversely, parasite diversity itself may be an important component of the development of adaptive immunity and hence protection from severe disease.
A feature of schistosome epidemiology is that infection intensity and disease severity vary considerably between geographic areas and between individuals within the same region whether due to age and duration of exposure, host or parasite genetic background and/or host behaviour. Laboratory studies, albeit currently limited to S. mansoni, have demonstrated that such variation can be attributed in some instances to repeatable differences in parasite strain (Nelson and Saoud 1968; Kassim et al. 1979; Chunge et al. 1995; Thiongo et al. 1997) and there is preliminary evidence (from RAPD data) of an association of specific parasite genotypes with more intense pathology in S. haematobium (Brouwer et al. 2003). In the laboratory, schistosome traits including infectivity, rates of replication, transmission and virulence in both the definitive and intermediate hosts have been demonstrated not only to have a genetic basis, but to be sometimes genetically correlated with rapid responses to artificial selection clearly documented (Davies et al. 2001; Gower and Webster 2004; Webster et al. 2004, 2007b). Different, and sometimes opposing, selective pressures acting on parasites within the intermediate and definitive hosts have been identified. These have been postulated to be crucial to the evolution of pathogens with multi-host lifecycles (Gandon 2004) and might be expected to promote polymorphisms in traits via fluctuating selection, whereby different genotypes, or selection for different alleles, will be favoured in the different obligatory host stages. Likewise, infection of hosts by multiple strains of parasite genotypes has been shown to be associated with exacerbated host morbidity as a result of intra-host parasite competition (Davies et al. 2002). However, intra-host competition has also been documented to favour strains of varying virulence phenotype in this system (Gower and Webster 2005). Thus evolutionary alterations in schistosome life history characteristics in response to selection imposed by mass chemotherapy programs may well be likely, although the direction and strength of selection in endemic field situations is unknown. For example, if treatment were to favour schistosome evolutionary strategies that maximize transmission, an increase in egg production and concomitantly an increase in the morbidity of residual or untreated infections might be predicted. Conversely, treatment may reduce the incidence of multiple infections, and hence select for reduced parasite virulence.
In the field, and in the absence of genetic markers for parasite traits such as virulence and fecundity, the impact of mass chemotherapy on general parasite life-history traits could potentially be monitored through the integration of clinical measures and prevalence data with population genetic analyses using microsatellite markers. Such data may be valuable in monitoring relationships between prevalence, intensity and morbidity measures and associations of particular genotypes or degrees of genetic diversity in clinical phenotypes (such as population subdivision between areas or individuals with differing morbidity or infection intensity, which would suggest that parasite strain variability may be a potential contributing factor) and the effect of chemotherapeutic treatment on such associations. However, detecting alterations of parasite reproductive strategies is particularly difficult in schistosomes, as adult worm burdens cannot be assessed in living hosts, although population genetic studies may give some indications of changes having occurred. For example, a reduction in the genetic diversity of infections of similar intensity might be evidence of decreases in per capita egg production.
All organisms, including parasites, have limited resources that can be allocated to various functions such as growth, survival and reproduction. Maximizing fitness (lifetime reproductive success) may involve trade-offs between such traits. Strategies of resource allocation that maximize fitness can differ between benign and stressful environments, including that of stresses imposed by chemotherapy (Buckling et al. 1997). Consequently natural selection often favours phenotypic alteration of reproductive effort in response to stress. For example, increased reproductive effort, termed fecundity compensation, should follow cues associated with a decreased probability of future survival or reproduction – this has been observed in the enhanced egg production occurring in infected crickets (Adamo 1999), infected Daphnia (Chadwick and Little 2005) and snails that have been exposed to castrating parasites (Minchella and LoVerde 1981; Blair and Webster 2006). Secondly, if conditions change such that one life history stage becomes relatively more vulnerable than another, increased investment into the least vulnerable stage is predicted [the ‘safe harbour’ hypothesis (Shine 1978)] Similar parasite life history counter-adaptations may be associated with the stresses imposed by human intervention strategies such as chemotherapy. For example, a subcurative dose of the antimalarial chloroquine has been demonstrated to cause malaria parasites to divert resources from within host asexual replication to the production of transmission stages (gametocytes) in a Plasmodium chabaudi laboratory model (Buckling et al. 1997). These alterations lead to correlated changes in infectivity to mosquitoes, with the consequence that chloroquine treatment had no effect on the proportion of mosquitoes infected. Whilst such phenotypic plasticity may be less common in more complex multicellular organisms such as the helminth parasites, recent experimental studies of the fish trematode, Coitocaecum parvum, have suggested that they too can use information about transmission opportunities to trigger life history alterations. In this example accelerated development and precocious maturity of larvae in intermediate hosts was observed in the absence of either chemical cues emanating from the fish definitive host or the presence of high intermediate host mortality (Poulin 2003). Recent laboratory studies of S. mansoni have also shown that when intermediate hosts are stressed due, for example, to starvation, the resulting reduced parasite success in that intermediate host stage appears to be subsequently traded-off with increased parasite investment and subsequent success within the definitive host stage (C. M. Gower, L. Blair and J. P. Webster, unpublished data). Such studies further indicate that there may be phenotypic plasticity in this system that may aid maintenance of transmission in response to stress. If such mechanisms do exist, it might be predicted that human treatment would promote an increase in the reproductive importance of the snail component of the life history, and therefore, that the force of transmission may not be reduced as much as expected by reference to reductions in the intensity and prevalence of human infections. This further underlines the importance of morbidity reduction as the major goal of current schistosomiasis control programmes.
Impact of mass chemotherapy on interactions between different species of schistosome and between schistosomes and other parasites
In the developing world co-infections and polyparasitism within humans appear to be the rule rather than the exception. For example, co-infections between S. mansoni and S. haematobium have been reported in an increasing number of foci in sub-Saharan Africa (Cunin et al. 2000, 2003a; Garba et al. 2004) and also with species of STHs (Brooker et al. 2000; Tcheum Tchuente et al. 2003; Fleming et al. 2006). Inter-specific interactions between parasites are expected to occur when there are co-infections. One of the main characteristics of these interactions relates to whether they result in a positive or negative association between the abundances of different parasite species within the host. Facilitation refers to positive interactions, where infection of a host by one parasite species enables infection of that host by a second parasite species which would otherwise not be able to infect. Negative interactions occur due to inter-specific competition: the competition between two species of parasites co-infecting a host for limited niches and/or resources. Both types of interaction may be mediated by the host immune system through either suppression of the immune response allowing facilitation or stimulation of a immune response in the case of competition (Schad 1996). Such synergistic or antagonistic interactions of co-infections may clinically affect individuals and co-endemicity may affect the epidemiology of the parasites (Keusch and Migasena 1982). Polyparasitism may also be likely to have a large impact on both the parasites and the host involved as it may affect parasite growth, maturation, reproductive success and survival along with the susceptibility, morbidity and immune response of the host (Keusch and Migasena 1982).
The effects of interactions between S. haematobium and S. mansoni on parasite dynamics were originally investigated in the field, where studies in Cameroon and Senegal revealed that in areas of overlap S. mansoni eggs were being excreted in urine samples of humans as opposed to the usual faecal route (Ratard et al. 1991). This indicated that male S. haematobium worms and female S. mansoni worms (which cannot successfully hybridize) were pairing heterospecifically and migrating to the urinary oviposition site, resulting in S. mansoni-shaped eggs in the urine (through parthenogenesis). Laboratory studies revealed that S. haematobium males compete with, and are more successful than, S. mansoni males in pairing with S. mansoni females (Webster et al. 1999). When such competition exists in a system there is the risk of exclusion, either partially or totally, of one species by another and such mating competition has been implicated in limiting the distribution of another less common schistosome species, S. intercalatum, due to competitive interactions with S. mansoni (Tchuem Tchuente et al. 1995). However, the outcome of such competition is difficult to predict as it relies on several factors relating to the individual parasite-life histories. These include the relative infectivity of the parasites, length of life-cycle (shorter for S. mansoni), sex ratio (more female biased for S. mansoni) and male competitive pairing ability (greater for S. haematobium). Indeed dominance in mating competition may in some cases result in a reduction in fecundity for the dominant species due to the fact that hybridization does not occur (Webster et al. 1999). In areas of overlap between S. mansoni and S. haematobium in Egypt, for example, S. mansoni has been observed to be replacing S. haematobium and at least in this focus S. mansoni appears to be competitively dominant (Adel-Wahab et al. 1993). However, interactions between schistosomes and co-infecting STHs have not been so extensively studied. In many reports of the levels of co-occurrence for these two groups of parasites in the same individuals it appears that the parasites may be positively associated with each other and therefore undergoing positive inter-specific interactions such as facilitation of infection of one species by the other or synergistic effects on the host immune response once co-infected (Tcheum Tchuente et al. 2003; Fleming et al. 2006). Competitive interactions have, however, been identified despite this, through for example the observation of reduced infection intensities for Ascaris lumbricoides and S. mansoni in co-infections in comparison to individual infections (Fleming et al. 2006). In all such work based on field observations, care must be taken, nevertheless, in the interpretations of simple correlations between the abundances of different parasite species within hosts. Many factors other than competition or facilitation could influence such patterns, including the nutritional state or immunocompetence of the host, host behaviour and the environment of the host population.
Mass chemotherapy may be predicted to impact on such complex dynamic interspecific interactions, especially if the selection pressure differs between the parasite species. For example effects will be observed if PZQ efficacy is different for S. mansoni and S. haematobium or if the efficacy of PZQ on schistosome infections is different to that of albendazole for STHs. Indeed, as regards the former, whilst PZQ works on all species of schistosome infecting humans,, there is some evidence of differential susceptibility, where potential tolerance (or even resistance) to S. mansoni has been detected but not for S. haematobium (Southgate et al. 2005). Moreover, as S. mansoni and S. haematobium differ in the generation times/length of time to maturation, and as juveniles schistosomes are not susceptible to PZQ, this too could potentially lead to differences between these two species. Such differences could affect the order of establishment of the parasites within their human hosts [a factor known to be very important in determining the outcome of inter-specific competition (Cosgrove and Southgate 2002)]. Also, if for example, PZQ resistance was to develop in one schistosome species (say S. mansoni) it could not be passed between the species (due to their inability to hybridize). One could hypothesize that in conditions of high drug imposed selection this could lead to S. mansoni having increased competitive dominance over S. haematobium in areas of overlap. However in conditions of reduced drug imposed selection (possibly due to the identification of such drug resistance in the S. mansoni species), S. haematobium could be competitively dominant due to inherent costs in S. mansoni’s drug resistance. A comparable scenario could also be imagined with relation to drug resistance emerging for either schistosomes or STHs. Such theories, although somewhat speculative at this stage, highlight the importance of monitoring and evaluating the impact of mass chemotherapy control programmes on such inter-specific interactions. Limited evidence is just emerging of possible competitive interactions. For example, one study found an increase of intestinal schistosomiasis after PZQ treatment in a S. haematobium and S. mansoni mixed focus in Senegal (Ernould et al. 1999). In one village, where the two parasites were present before treatment, the disappearance of the urinary schistosomiasis after treatment was concomitant with a dramatic increase of intestinal schistosomiasis: S. mansoni egg excretion was seven times higher than before treatment. The direct cause of this change is unknown. However possible reasons include lack of activity of PZQ against S. mansoni in this focus, a reduction in mating competition, within which S. haematobium is dominant (Webster et al. 1999), and/or the fact that it was a developing (epidemic) focus.
Inter-specific interactions may also be predicted to directly impact morbidity, as well as affecting the prevalence and intensity of human infections. For example, positive associations between mixed infections of different species of schistosomes and of schistosomes and STHs, and increases in parasite infection intensity have been observed (Brooker et al. 2000; Tcheum Tchuente et al. 2003; Fleming et al. 2006). However, associated increases in morbidity have not yet been determined. One study on interactions between S. mansoni and S. haematobium has identified a potential effect of inter-specific interactions on host morbidity. This study did not support the speculative evolutionary theory which predicts that more diverse infections are more costly to the host (Anita et al. 1994; Bonhoeffer and Nowak 1994; Nowak and May 1994; van Baalen and Sabelis 1995; May and Nowak 1995; Frank 1996). Instead it reported a lowering of S. mansoni induced morbidity (hepatomegaly and splenomagaly morbidity) in mixed infections with S. haematobium relative to that observed for S. mansoni single infections (Cunin et al. 2003b). The authors suggest that this lowering effect on liver morbidity could be due to S. haematobium males mating with S. mansoni females with the eggs from such couplings passing to the urinary tract (the S. mansoni females will lay parthenogenic eggs shaped like normal S. mansoni eggs and the S. haematobium male will carry the female to the normal S. haematobium egg-laying site, i.e. the urinary tract, resulting in S. mansoni-shaped eggs in urine), thereby reducing the amount of classical S. mansoni induced morbidity. The consequence of typical S. haematobium-associated bladder pathology was not, however, investigated in this preliminary study on mixed species infections, and further work to evaluate this is underway (Gouvras et al. in press). The effects of chemotherapeutic treatment on such inter-specific interactions between parasites generally may well be predicted to have implications for the morbidity induced by such mixed infections on human hosts, due to a general lowering effect of treatment on infection prevalences of each, or one or other, of the separate parasites species.
Co-evolutionary interactions between schistosomes and their molluscan intermediate host
Mass chemotherapy programs might also be likely to affect co-evolutionary interactions between helminths and their hosts, particularly in those with part of the lifecycle in short-lived intermediate hosts, whose generation times are roughly similar to those of their infecting parasites, such as schistosomes and their freshwater snail hosts. Snail-trematode compatibility is a highly specific relationship, often at the population or strain levels for both participants (Lo and Lee 1995; Webster and Woolhouse 1998; Webster 2001; Webster and Davies 2001). This specificity has the important practical effect of limiting medically important trematodes such as schistosomes to geographic areas occupied by compatible snails (van der Knapp and Loker 1990). Studies have demonstrated both that snail resistance to schistosome infection is a heritable genetic trait (Lo and Lee 1995; Webster and Woolhouse 1998; Webster 2001; Webster and Davies 2001) and that schistosome infection presents a significant fitness cost to host snails in terms of increased mortality and reduced fertility (Woolhouse 1989; Webster and Woolhouse 1999). However, natural host populations often exhibit considerable genetic variability in resistance to parasitism. Co-evolutionary theory suggests that one potential mechanism for maintaining such diversity is a trade-off between fitness costs associated with resistance and fitness costs associated with parasitism (May and Anderson 1983; Fritz and Simms 1992; Frank 1994). Examples of such as a cost of resistance in snails to their schistosomes, in terms of reduced egg fertility but not necessarily fecundity, have been demonstrated (Webster and Woolhouse 1999). Selection for susceptible snails, mediated by the high costs of resistance, also has wider implications for schistosomiasis control measures. Most importantly, if a reduction in net transmission rates due to mass chemotherapy favours an increase in the susceptibility of the snail population, then this may increase the likelihood of the re-emergence of the disease should control measures be relaxed. This could serve to reinforce any increased susceptibility of the human population due to loss of, or a reduction in, acquired immunity.
Impact of mass chemotherapy on human hosts
The potential impacts of mass chemotherapy programmes on parasites and intermediate hosts are also important in relation to their overall effect on infection intensity and virulence of schistosomes within their human host. Mass chemotherapy control programmes may, however, also be predicted to impact on human hosts directly. It is now widely accepted that humans exhibit an acquired immunity to schistosome infections which develops slowly as individuals’ age and accumulate exposure experiences. In schistosome endemic areas, human infection levels typically increase during childhood, peak during adolescence and decline during adulthood (Gryseels et al. 1995; Mutapi et al. 2006), a pattern which is in part attributed to cumulative exposure to infection and the development of gradually acquired resistance to schistosomes, and in part due to behavioural changes with age that are related to exposure to infection. Some parasite antigens that are thought to play some part in stimulating such protective acquired immunity are located beneath the worm tegument of adult schistosomes and are therefore only available to the immune systems when the tegument is damaged or worms die (Mutapi 2001). Adult schistosome worms are known to live for, on average, between 3 and 7 years. If these antigens beneath the worm tegument are the key stimulants of acquired immunity, this observation on life expectancy may be linked with the fact that it takes several years for infected children to ‘acquire’ resistance (Mutapi 2001). The potential effects of mass chemotherapy on host acquired resistance have been extensively studied. Mass chemotherapy control programmes may be predicted to prevent the development of naturally acquired resistance to infection, and may therefore have detrimental effects, at least in terms of the proportion of humans infected, if control ceases and individuals continued to be highly infected at older ages without having developed any acquired immunity during childhood. Similar concerns apply for the control of many parasitic infections such as the malarial parasites. However, studies to date indicate that the exposure of the host immune system to parasite antigens through the damage and subsequent killing of adult schistosome worms by the action of PZQ actually stimulates a form of acquired immunity itself. This ‘chemotherapy-induced’ acquired immunity has been shown to be different to naturally acquired immunity (Correa-Oliveira et al. 2000; van den Biggelaar et al. 2002), possibly due to differences between the large amounts of antigens from adult worms released over a small time period directly into the host bloodstream due to PZQ action. In contrast, in the absence of treatment smaller amounts of various antigens are released over a long period of time through the skin, lungs, liver and the blood from adult and juvenile worms (Mutapi 2001). There is ongoing debate as to the effectiveness of such treatment-acquired-immunity in comparison to naturally acquired immunity in relation to the amount and duration of protection that it provides (van den Biggelaar et al. 2002). Nevertheless, it may well be of value to monitor the effectiveness of such treatment-acquired immunity in relation to mass chemotherapy control programmes over space and time. One way to do this may be to measure reinfection rates by age group, discounting for the herd impact of mass treatment, to see if this drug induced immunity reduces establishment of the adult worms.
Mass chemotherapy schistosomiasis control programmes do not only involve the administration of chemotherapy, but also the provision of health education. Health education may be predicted to have a large effect on humans if it achieves its aim of changing human water-contact behaviour. Two facets of human water-contact behaviour are important; human defecation and urination into water containing schistosome intermediate host snails and human contact with water containing infective schistosome cercariae. Changes in these two facets of water contact are likely to have different relative effects in terms of human exposure to schistosomes, as well as broader aspects of the transmission dynamics of schistosome populations. A complete cessation in contact with water containing cercariae would prevent human schistosome infection. However, considering the current infrastructure in communities with endemic schistosomiasis this is not a likely outcome of such control programmes. Instead some degree of reduction in both of these types of water contact is possible and they would be expected to reduce prevalence and infection intensity for human hosts in general, and thereby also potentially impact the selective pressures imposed upon parasite virulence and/or other life history traits.
Impact of mass chemotherapy relating to nonhuman definitive hosts
Although mass chemotherapy programmes are specifically directed at reducing human morbidity, they may have implications for other definitive hosts of schistosomes. For example, mass chemotherapy may be important both in terms of their effects on these hosts (which may potentially be important livestock) but also for their role previously discussed as refugia and also as reservoirs for re-infection of human hosts. The number of nonhuman definitive hosts vary greatly between the schistosome species with S. haematobium being almost exclusively a human parasite, S. mansoni infecting humans, rodents and primates (Morand et al. 1996; Muller-Graf et al. 1997) and S. japonicum infecting humans and a very wide range of nonhuman hosts (Wang et al. 2006). The importance of nonhuman hosts as both sources of reinfection, and conversely as refugia, will depend on whether the parasite population is panmitic or whether parasite strains are present which are circulating independently within hosts of different types. For example, cross-infections have been demonstrated to be rare between Ascaris nematodes of humans and pigs in sympatric areas (Anderson and Jaenike 1997). Thus, although infections of humans are unlikely to occur from pig-derived parasite populations, these populations won’t act as natural refugia either unless at some point they come into contact with human–derived worms. Wang and colleagues also used microsatellite markers to examine S. japonicum across a range of host species in China and reported that parasites were circulating between humans and all the domestic animals species studied, although with some strain structure present and greatest potential transfer between humans and bovines, at least within the marshland regions (Wang et al. 2006).
Mass chemotherapy treatment may be predicted to impact on the infection of nonhuman definitive hosts as any ‘human adapted strains’ would be killed via PZQ treatment in humans giving any ‘nonhuman host’ adapted strains a distinct competitive advantage. It is likely therefore, that there will be strong selective pressure for further adaptation of schistosomes to their nonhuman hosts which may have detrimental effects for these hosts. Conversely for human hosts different scenarios may be predicted relating to the extent to which schistosomes adapt to transmission in these nonhuman hosts. If PZQ treatment in human hosts imposes a very strong selection pressure on parasites to adapt to their nonhuman hosts, then schistosomes may adapt to solely infect these nonhuman hosts. Indeed there appears to be clear evidence of this already happening in response to differing environmental selective pressures being imposed on schistosomes in Guadeloupe, with the recent adaptation of S. mansoni (back) to its rodent host (Theron 1984; Theron et al. 1992) and an associated potential decrease in risk of further human infection. With intermediate levels of selection pressure imposed by PZQ treatment it may conversely be predicted that schistosome infections within nonhuman hosts may act as reservoirs for human re-infection. Indeed there are data to suggest this may already be occurring within both S. mansoni (Muller-Graf et al. 1997) and S. japonicum (D. Lu, J. Rudge, T. Wang and J. P.Webster, unpublished data).
Conclusions and recommendations
Recently implemented mass-chemotherapeutic control programmes have been shown to have a rapid and dramatic impact on reducing schistosome prevalence, intensity and subsequent human host morbidity. However, as control efforts gather pace across sub-Saharan Africa the wider availability of PZQ may exacerbate the risk of the emergence of drug-resistant parasites. This provides a strong incentive to extend monitoring and surveillance and to increase investigation of the population biology and genetics of potentially resistant parasites and the fine detail of resistance mechanisms. Unfortunately to date there are very limited empirical data available relating to any natural genetic diversity in schistosome, or indeed any helminth, populations or how any of the variations observed in the few published studies influence the biology of the parasite and its clinical impact on humans or animals. This situation for helminths is in marked contrast to that pertaining for most other important pathogens of humans – especially the viruses and bacteria – where large international databases exist recording sequence variation in isolates from different clinical and epidemiological settings. Given the growth in interest in the community based control of helminth infections by chemotherapy, much greater attention needs to be directed towards recording genetic diversity in a systematic manner in databases that also reflect epidemiological and treatment experience. Records of sequence variation need to take account of the fact that over the last 40 years schistosome research has been over-dependent on laboratory-reared parasites repeatedly passaged through a variety of mouse strains, which we know may not represent the field population (Shrivastava et al. 2005; Gower et al. 2007).
Evolutionary theory has a role in the design, application and interpretation of such programmes. It is perhaps most important here in terms of identifying where change may occur in the host parasite system as a result of PZQ treatment and therefore allowing us to design effective monitoring processes to identify if and in which direction these changes actually occur (Table 1). Whilst there may well be currently limited evidence (and/or studies performed to look for it) that evolutionary changes in natural schistosome or host populations have occurred as a result of current PZQ control efforts, it is axiomatic that such changes are expected under the intensity of selection created by community based control interventions. However, their relative importance to the success of control programmes will be determined by the speed of parasite adaptive evolution, and the genetic variation present upon which selection may act, which may itself be reduced by mass chemotherapy treatment. In foci where local eradication is possible, schistosomes may not evolve sufficiently rapidly to undergo substantial life history adaptation before elimination. However, it must be considered, that the major focus of current schistosomiasis control programs is morbidity, rather than transmission control, and thus evolutionary considerations are greater in this context than perhaps in control programs attempting parasite eradication. The imperative to control these debilitating infections using the best tool available (i.e. chemotherapy) is obvious. However, we do suggest that consideration of control measures from an evolutionary standpoint is of importance given the history of the impact of wide scale chemotherapeutics on the evolution of resistance across a very wide range of organisms. Continued monitoring and evaluation of parasites and their hosts whenever and wherever transmission remains must become an essential arm of intervention. For schistosomes, concerns over the evolution and rapid spread of resistance are somewhat less than for most pathogens for reasons explained in this review but especially the large predicted refugia and fitness costs involved. Nevertheless, it must not be forgotten that schistosomes have already evolved resistance to oxamniquine (Katz et al. 1991; Cioli et al. 1993; Fallon and Doenhoff 1994; Fallon et al. 1995; Bonesso-Sabadini and De Souza Dias 2002). Schistosome control programmes such as that of the SCI in sub-Saharan Africa have, wherever logistically and financially feasible, already integrated such detailed evolutionary/resistance monitoring and evaluation of host and parasite factors. In addition to the parasite prevalence, intensity and subsequent parasite-induced morbidity levels closely monitored in each population, SCI monitors the genotype and phenotype of the parasite at baseline, prior to the new chemotherapeutic intervention, and then, both in the population overall, but specifically in identifiable age-structure longitudinal cohorts of children over space, time and treatment history, to observe the effect on epidemiological patterns and parasite genetic diversity.
Table 1.
Specific example predictions for the potential effect of mass chemotherapy schistosomiasis control programmes on parasites and their hosts and the ways in which each of these may be monitored.
| Prediction | |
|---|---|
| Drug resistance may develop | Monitoring of drug efficacy and population genetic surveys. Development of molecular markers |
| Treatment may alter optimal parasite virulence; treatment may reduce incidence of multiple infections | Patterns of relationship of intensity, genetic diversity and morbidity before and after treatment |
| Treatment may alter competitive balance or interactions with other infections | Alterations in epidemiological patterns pre- and post-treatment in terms of intensity and prevalence of coinfections |
| Treatment may promote investment in other stages of the schistosome lifecycle | Monitoring of snail infection rates and prevalences; might predict no difference in force of infection even with decreasing human infection |
It is unethical to withhold treatment for individuals in order to obtain ‘control’ data of parasites over space and time within such mass control programmes. However, by examining the parasites, either repeatedly within countries prior to mass drug implementation, and the cercariae released from the sympatric snail intermediate host populations, and in conjunction with the miracidia released from the new 6–7-year-old child recruits at each site and over each year, it is possible to elucidate the potential impact on the parasite populations. Much still remains to be achieved, and indeed the requirement of novel molecular markers for PZQ-resistance are central to an improved understanding. We recommend that where at all possible all future large scale human and indeed animal helminthiasis-control programmes initiate detailed monitoring and evaluation of host and parasite genetic diversity factors from their onset. In making this recommendation we fully recognize the cost implications but the trend in sequencing costs at least give optimism that this type of monitoring can be without detriment to the resources directed at morbidity control.
Acknowledgments
We are very grateful to Professors Roy Anderson and Alan Fenwick, Drs Michelle Tseng, Poppy Lamberton and Tom Churcher, together with three anonymous referees for valuable comments on the text. The authors were funded by grants from the Royal Society, the Wellcome Trust (grant no. 063774), the Bill and Melinda Gates Foundation and the European Union (contact no. 032203).
Literature cited
- Adamo SA. Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Animal Behaviour. 1999;57:117–124. doi: 10.1006/anbe.1998.0999. [DOI] [PubMed] [Google Scholar]
- Adel-Wahab FM, Yosery A, Narooz S, Esmat G, El Hak E, Nasif S, Stickland GT. Is Schistosoma mansoni replacing Schistosoma haematobium in Fayoum? American journal of tropical medicine and hygiene. 1993;49:697–700. doi: 10.4269/ajtmh.1993.49.697. [DOI] [PubMed] [Google Scholar]
- Agola LE, Mburu DN, DeJong RJ, Mungai BN, Muluvi GM, Njagi EN, Loker ES, Mkoji GM. Microsatellite typing reveals strong genetic structure of Schistosoma mansoni from localities in Kenya. Infection, Genetics and Evolution. 2006;6:484–490. doi: 10.1016/j.meegid.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Alonso D, Munoz J, Gascon J, Valls ME, Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. American Journal of Tropical Medicine and Hygiene. 2006;74:342–344. [PubMed] [Google Scholar]
- Anderson TJ, Jaenike J. Host specificity, evolutionary relationships and macrogeographic differentiation among Ascaris populations from humans and pigs. Parasitology. 1997;115:325–342. doi: 10.1017/s0031182097001339. [DOI] [PubMed] [Google Scholar]
- Anita R, Levin BR, May RM. Within host population dynamics and the evolution and maintenance of microparasite virulence. American Naturalist. 1994;144:457–472. [Google Scholar]
- Ardelli BF, Guerriero SB, Prichard RK. Ivermectin imposes selection pressure on P-glycoprotein from Onchocerca volvulus: linkage disequilibrium and genotype diversity. Parasitology. 2006;132:375–386. doi: 10.1017/S0031182005008991. [DOI] [PubMed] [Google Scholar]
- Van Baalen M, Sabelis MW. The dynamics of multiple infection and the evolution of virulence. American Naturalist. 1995;146:881–910. [Google Scholar]
- Van Den Biggelaar AHJ, Borrmann S, Kremsner P, Yazdanbakhsh M. Immune responses induced by repeated treatment do not result in protective immunity to Schistosoma haematobium: Interleukin (IL)-5 and IL-10 responses. Journal of Infectious Diseases. 2002;186:1474–1482. doi: 10.1086/344352. [DOI] [PubMed] [Google Scholar]
- Blair L, Webster JP. Dose-dependent schistosome-induced mortality and morbidity risk elevates host reproductive effort. Journal of Evolutionary Biology. 2006;20:54–64. doi: 10.1111/j.1420-9101.2006.01230.x. [DOI] [PubMed] [Google Scholar]
- Blair L, Webster JP, Barker GC. Isolation and characterization of polymorphic microsatellite markers in Schistosoma mansoni from Africa. Molecular Ecology Notes. 2001;1:93–95. [Google Scholar]
- Bonesso-Sabadini PI, De Souza Dias LC. Altered response of strain of Schistosoma mansoni to oxamniquine and praziquantel. Memorias de Instituto Oswaldo Cruz. 2002;97:381–385. doi: 10.1590/s0074-02762002000300019. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer S, Nowak MA. Mutation and the evolution of virulence. Proceedings of the Royal Society of London Series B. 1994;258:133–140. [Google Scholar]
- Botros S, Sayed H, Amer N, El-Ghannam M, Bennett JL, Day TA. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. International Journal for Parasitology. 2005;35:787–791. doi: 10.1016/j.ijpara.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Bremermann HJ, Pickering J. A game-theoretical model of parasite virulence. Journal of Theoretical Biology. 1983;100:411–426. doi: 10.1016/0022-5193(83)90438-1. [DOI] [PubMed] [Google Scholar]
- Brooker S, Miguel EA, Moulin S, Luoba AI, Bundy DAP, Kremer M. Epidemiology of single and multiple species of helminth infections among school children in Busai District, Kenya. East African Medical Journal. 2000;77:157–161. doi: 10.4314/eamj.v77i3.46613. [DOI] [PubMed] [Google Scholar]
- Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ. Urinary tract pathology attributed to Schistosoma haematobium: does parasite genetics play a role? American Journal of Tropical Medicine and Hygiene. 2003;68:456–462. [PubMed] [Google Scholar]
- Buckling AGJ, Taylore LH, Carloton JMR, Read AF. Adaptive changes in Plasmodium transmission strategies following chloroquine chemotherapy. Proceedings of the Royal Society of London Series B. 1997;264:553–559. doi: 10.1098/rspb.1997.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ. Perspective : virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Chadwick W, Little TJ. A parasite-mediated life-history shift in Daphnia magna. Proceedings of the Royal Society (London) Series B. 2005;272:505–509. doi: 10.1098/rspb.2004.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunge RN, Karumba N, Ouma JH, Thiongo FW, Sturrock RF, Butterworth AE. Polyparasitism in two rural communities with endemic Schistosoma mansoni infection in Machakos district, Kenya. Journal of Tropical Medicine and Hygiene. 1995;98:440–444. [PubMed] [Google Scholar]
- Churcher TS, Filipe JAN, Basanez M-G. Density dependence and the control of helminth parasites. Journal of Animal Ecology. 2006;75:1313–1320. doi: 10.1111/j.1365-2656.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- Cioli D, Pica-Mattoccia L, Archer S. Drug resistance in schistosomes. Parasitology Today. 1993;9:162–166. doi: 10.1016/0169-4758(93)90138-6. [DOI] [PubMed] [Google Scholar]
- Colley DG, LoVerde PT, Savioli L. Infectious disease. medical helminthology in the 21st century. Science. 2001;293:1437–1438. doi: 10.1126/science.1060733. [DOI] [PubMed] [Google Scholar]
- Correa-Oliveira R, Caldas IR, Martins-Filho OA, Queiroz CC, Lambertucci JR, Cunha-Melo JR, Silveira AS, et al. Analysis of the effects of treatment of human Schistosoma mansoni infection on the immune response of patients in endemic areas. Acta Tropica. 2000;77:141–146. doi: 10.1016/s0001-706x(00)00127-3. [DOI] [PubMed] [Google Scholar]
- Cosgrove CL, Southgate VR. Mating interactions between Schistosoma mansoni and S. margrebowiei. Parasitology. 2002;125:233–243. doi: 10.1017/s0031182002002111. [DOI] [PubMed] [Google Scholar]
- Cunin P, Griffet A, Poste B, Djibrilla K, Martin PM. Epidemic Schistosoma mansoni in a known Schistosoma haematobium area. Transactions of the Royal Society for Tropical Medicine and Hygiene. 2000;94:657–660. doi: 10.1016/s0035-9203(00)90221-9. [DOI] [PubMed] [Google Scholar]
- Cunin P, Tcheum Tchuente LA, Poste B, Djibrilla K, Martin PMV. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Tropical Medicine and International Health. 2003a;8:1110–1117. doi: 10.1046/j.1360-2276.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- Cunin P, Tcheum Tchuente LA, Poste B, Djibrilla K, Martin PMV. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Tropical Medicine and International Health. 2003b;8:1110–1117. doi: 10.1046/j.1360-2276.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- Davies CM, Webster JP. A genetic trade-off of virulence and transmission in a snail-schistosome system. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95:248. [Google Scholar]
- Davies CM, Webster JP, Woolhouse MEJ. Trade-offs in the evolution of virulence of schistosomes – macroparasites with an indirect life-cycle. Proceedings of the Royal Society (London), Series B. 2001;268:251–257. doi: 10.1098/rspb.2000.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CM, Fairbrother E, Webster JP. Mixed strain schistosome infections of snails and the evolution of virulence. Parasitology. 2002;124:31–38. doi: 10.1017/s0031182001008873. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Metz JAJ, Sabelis MW, Sigmund K. Adaptive Dynamics of Infectious Diseases: In Pursuit of Virulence Management. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Reviews in Anti-Infection Therapies. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- Durand P, Sire C, Theron A. Isolation of microsatellite markers in the digenetic trematode Schistosoma mansoni from Guadeloupe island. Molecular Ecology. 2000;9:997–998. doi: 10.1046/j.1365-294x.2000.00939-4.x. [DOI] [PubMed] [Google Scholar]
- Ernould JC, Ba K, Sellin B. Increase of intestinal schistosomiasis after praziquantel treatment in a Schistosoma haematobium and Schistosoma mansoni mixed focus. Acta Tropica. 1999;73:143–152. doi: 10.1016/s0001-706x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- Ewald PW. Host-parasite relations, vectors, and the evolution of disease severity. Annual Review of Ecology and Systematics. 1983;14:465–485. [Google Scholar]
- Ewald PW. The Evolution of Infectious Diseases. Oxford: Oxford University Press; 1994. [Google Scholar]
- Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. American Journal of Tropical Medicine and Hygiene. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Hamilton JV, Doenhoff MJ. Efficacy of treatment of murine Schistosoma mansoni infections with praziquantel and oxamniquine correlates with infection intensity: role of host antibody. Parasitology. 1995;111:59–66. doi: 10.1017/s003118200006460x. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Mubarak JS, Fookes RE, Niang M, Butterworth AE, Sturrock RF, Doenhoff MJ. Schistosoma mansoni: maturation rate and drug susceptibility of different geographic isolates. Experimental Parasitology. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- Feng Z, Curtis J, Minchella DJ. The influence of drug treatment on the maintenance of schistosome genetic diversity. Mathematical Biology. 2001;43:52–68. doi: 10.1007/s002850100092. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Webster JP. Schistosomiasis – challenges for control, treatment and drug resistance. Current Opinion in Infectious Disease. 2006;19:577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- Fleming F, Brooker S, Geiger SM, Caldas IR, Correa-Oliveira R, Hotez PJ, Bethony JM. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Tropical Medicine and International Health. 2006;11:56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Frank SA. Coevolutionary genetics of hosts and parasites with quantative inheritance. Evolutionary Ecology. 1994;8:74–94. [Google Scholar]
- Frank SA. Models of parasite virulence. Quarterly Review of Biology. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Fritz RS, Simms EL, editors. Plant Resistance to Herbivores and Pathogens. University of Chicago Press; 1992. [Google Scholar]
- Galvani AP, Coleman RM, Ferguson NM. The maintenance of sex in parasites. Proceedings of the Royal Society (London) Series B. 2003;270:19–28. doi: 10.1098/rspb.2002.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S. Evolution of multi-host pathogens. Evolution. 2004;58:455–469. [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Garba A, Labbo R, Tohon Z, SIdiki A, Djibrilla A. Emergence of Schistosoma mansoni in the Niger river valley, Niger. Transactions of the Royal Society for Tropical Medicine and Hygiene. 2004;98:296–298. doi: 10.1016/S0035-9203(03)00070-1. [DOI] [PubMed] [Google Scholar]
- Golan R, Gower CM, Emory AM, Rollinson D, Webster JP. Isolation and characterization of the first polymorphic microsatellite markers for Schistosoma haematobium and their application in multiplex reactions of larval stages. Molecular Ecology Notes. 2007 doi: 10.1111/j.1471-8286.2007.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönnert R, Andrews P. Praziquantel, a new board-spectrum antischistosomal agent. Zeitschrift fur Parasitenkunde. 1977;52:129–150. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- Gouvras A, Norton AJ, Garba A, Lamine MA, Fenwick A, Webster JP. The impact of single and mixed Schistosoma mansoni and Schistsosoma haematobium infections on parasite population structure and human host morbidity. Transactions of the Royal Society for Tropical Medicine and Hygiene [Google Scholar]
- Gower CM, Webster JP. Fitness of indirectly-transmitted pathogens: restraint and constraint. Evolution. 2004;58:1178–1184. doi: 10.1111/j.0014-3820.2004.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Gower CM, Webster JP. Intra-specific competition and the evolution of virulence in a parasitic trematode. Evolution. 2005;59:544–553. [PubMed] [Google Scholar]
- Gower CM, Shrivastava J, Lamberton PHL, Rollinson D, Emory A, Webster BL, Kabatereine NB, Webster JP. Development and application of an ethical and epidemiologically appropriate assay for the multi-locus microsatellite analysis of Schistosoma mansoni. Parasitology. 2007;134:523–536. doi: 10.1017/S0031182006001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RM. Molecular target of the antischistosomal drug praziquantel. Future Microbiology. 2007;2:265–268. [Google Scholar]
- Gryseels B, Stelma F, Talla I. Immuno-epidemiology of Schistosoma mansoni infections in a recently exposed community in Senegal. Memorias do Insitituto Oswaldo Cruz. 1995;90:271–276. doi: 10.1590/s0074-02761995000200025. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guisse F, Van Lieshout L, Faye D, et al. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Tropical Medicine and International Health. 2001;6:864–873. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. American Journal of Tropical Medicine and Hygiene. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Kassim OO, Cheever AW, Richards CS. Schistosoma mansoni: mice infected with different worm strains. Experimental Parasitology. 1979;48:220–224. doi: 10.1016/0014-4894(79)90102-4. [DOI] [PubMed] [Google Scholar]
- Katz N, Rocha RS, De Souza CP, Coura Filho P, Bruce JI, Coles GC, Kinoti GK. Efficacy of alternating therapy with oxamniquine and praziquantel to treat Schistosoma mansoni in children following failure of first treatment. American Journal of Tropical Medicine and Hygiene. 1991;44:509–512. doi: 10.4269/ajtmh.1991.44.509. [DOI] [PubMed] [Google Scholar]
- Keusch GT, Migasena P. Biological implications of polyparasitism. Reviews of Infectious Diseases. 1982;4:880–882. doi: 10.1093/4.4.880. [DOI] [PubMed] [Google Scholar]
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Van Der Knapp WPW, Loker ES. Immune mechanisms in trematode-snail interactions. Parasitology Today. 1990;6:175–182. doi: 10.1016/0169-4758(90)90349-9. [DOI] [PubMed] [Google Scholar]
- Koukounari A, Fenwick A, Whawell S, Kabatereine N, Kazibwe F, Tukahebwa E, Stothard R, et al. Morbidity indicators of Schistosoma mansoni: relationship between infection and anaemia in Ugandan schoolchildren before and after praziquantel and albendazole chemotherapy. American Journal of Tropical Medicine and Hygiene. 2006a;75:278–286. [PubMed] [Google Scholar]
- Koukounari A, Sacko M, Gabrielli A, Landouré A, Dembelé R, Clements A, Whawell S, et al. Assessment of ultrasound morbidity indicators for schistosomiasis in the context of large-scale programmes illustrated with experiences from Malian children. American Journal of Tropical Medicine and Hygiene. 2006b;75:1042–1052. [PubMed] [Google Scholar]
- Koukounari A, Gabrielli AF, Touré S, Bosque-Oliva E, Zhang Y, Donnelly CA, Fenwick A, Webster JP. Urinary schistosomiasis infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. Journal of Infectious Diseases. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- Lamberton PHL. Adaptation and evolution of Schistosoma spp. in response to chemotherapeutic pressure. Transactions of the Royal Society for Tropical Medicine and Hygiene. 2005;99:948. [Google Scholar]
- Leignel V, Cabaret J. Massive use of chemotherapy influences life traits of parasitic nematodes in domestic ruminants. Functional Ecology. 2001;15:569–574. [Google Scholar]
- Levin BR, Perrot V, Walker N. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics. 2000;154:985–997. doi: 10.1093/genetics/154.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CT, Lee KM. Schistosoma japonicum, zoophilic strain in Oncomelania hupensis chiui and O.h. formosana : miracidal penetration and comparative histology. Journal of Parasitology. 1995;81:708–713. [PubMed] [Google Scholar]
- May RM, Anderson RM. Epidemiology and genetics in the coevolution of parasites and hosts. Proceedings of the Royal Society of London Series B. 1983;219:281–313. doi: 10.1098/rspb.1983.0075. [DOI] [PubMed] [Google Scholar]
- May RM, Nowak MA. Superinfection, metapopulation and the evolution of diversity. Journal of Theoretical Biology. 1994;170:95–114. doi: 10.1006/jtbi.1994.1171. [DOI] [PubMed] [Google Scholar]
- May RM, Nowak MA. Coinfection and the evolution of virulence. Proceedings of the Royal Society of London Series B. 1995;261:209–215. doi: 10.1098/rspb.1995.0138. [DOI] [PubMed] [Google Scholar]
- Medley GF. Chemotherapy. In: Scott ME, Smith G, editors. Parasitic and Infectious Diseases: Epidemiology and Ecology. San Diego, CA: Academic Press; 1994. pp. 141–157. [Google Scholar]
- Minchella DJ, LoVerde PT. A cost of increased early reproductive effect in the snail Biomphalaria glabrata. American Naturalist. 1981;118:876–881. [Google Scholar]
- Morand S, Harvey P. Mammalian metabolism, longevity and parasite species richness. Proceedings of the Royal Society (London) Series B. 2000;267:1993–2003. doi: 10.1098/rspb.2000.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S, Manning SD, Woolhouse MEJ. Parasite-host coevolution and geographic patterns of parasite infectivity and host susceptibility. Proceedings of the Royal Society (London) Series B. 1996;263:119–128. doi: 10.1098/rspb.1996.0019. [DOI] [PubMed] [Google Scholar]
- Moret Y, Schimd-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Muller-Graf CDM, Collins DA, Packer C, Woolhouse MEJ. Schistosoma mansoni infection in a natural population of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology. 1997;115:621–627. doi: 10.1017/s0031182097001698. [DOI] [PubMed] [Google Scholar]
- Mutapi F. Heterogeneities in anti-schistosome humoral responses following chemotherapy. Trends in Parasitology. 2001;17:518–524. doi: 10.1016/s1471-4922(01)02118-3. [DOI] [PubMed] [Google Scholar]
- Mutapi F, Mduluza T, Gomez-Escobar N, Gregory WF, Fernandez JC, Midizi N, Maizels RM. Immuno-epidemiology of human Schistosoma haematobium infection: preferential IgG3 antibody responsiveness to a recombinant antigen dependent on age and parasite burden. BMC Infectious Diseases. 2006;6 doi: 10.1186/1471-2334-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Willaims JT, Brockman A, Paiphum L, Mayxay M, Newton PN, Guthmann J-P, et al. A selective sweep driven by pyrimethamine treatment in southeast asian malaria parasites. Molecular Biology and Evolution. 2003;20:1526–1536. doi: 10.1093/molbev/msg162. [DOI] [PubMed] [Google Scholar]
- Nelson GS, Saoud MFA. A comparison of the pathogenecity of two geographical strains of Schistosoma mansoni in rhesus monkeys. Journal of Helminthology. 1968;17:339–362. doi: 10.1017/s0022149x00017946. [DOI] [PubMed] [Google Scholar]
- Nowak MA, May RM. Superinfection and the evolution of parasite virulence. Proceedings of the Royal Society of London Series B. 1994;255:81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- Odogwu SE, Ramamurthy NK, Kabaterenine NB, Kazibwe F, Tukahebwa E, Webster JP, Fenwick A, Stothard JR. Schistosoma mansoni in infants (aged <3 years) along the Ugandan shoreline of Lake Victoria. Annals of Tropical Medicine and Parasitology. 2006;100:315–326. doi: 10.1179/136485906X105552. [DOI] [PubMed] [Google Scholar]
- Orr HA. The distribution of fitness effects among beneficial mutations in Fisher’s geometric model of adaptation. Journal of Theoretical Biology. 2006;238:279–285. doi: 10.1016/j.jtbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- Paterson S, Barber R. Experimental evolution of parasite life-history traits in Strongyloides ratti (Nematoda) Proceedings of the Royal Society (London) Series B. 2007;274:1467–1474. doi: 10.1098/rspb.2006.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porco TC, Lloyd-Smith JO, Gross KL, Galvani AP. The effect of treatment on pathogen virulence. Journal of Theoretical Biology. 2005;233:91–102. doi: 10.1016/j.jtbi.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. Evoluiotionary Ecology of Parasites: From Individuals to Communities. London: Chapman & Hall; 1998. [Google Scholar]
- Poulin R. Information about transmission opportunities triggers a life-history switch in a parasite. Evolution. 2003;57:2899–2903. doi: 10.1111/j.0014-3820.2003.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Prichard RK, Samson-Himmelstjerna V, Blackhall WJ. Foreword: towards markers for anthelmintic resistance in helminths of importance in animal and human health. Parasitology. 2007;134:1073–1076. doi: 10.1017/S0031182007000078. [DOI] [PubMed] [Google Scholar]
- Ratard RC, Ndamkou CN, Kouemeni LE, Ekani Bessala MM. Schistosoma mansoni eggs in urine. Journal of Tropical Medicine and Hygiene. 1991;94:348–351. [PubMed] [Google Scholar]
- Rollinson D, Kaukas A, Johnston DA, Simpson AJ, Tanaka M. Some molecular insights into schistosome evolution. International Journal for Parasitology. 1997;27:11–28. doi: 10.1016/s0020-7519(96)00169-5. [DOI] [PubMed] [Google Scholar]
- Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- Schad GA. Immunity, competition and natural regulation of helminth populations. American Naturalist. 1996;100:359–364. [Google Scholar]
- Shine R. Propagule size and parental care: the ‘safe harbour’ hypothesis. Journal of Theoretical Biology. 1978;75:417–424. doi: 10.1016/0022-5193(78)90353-3. [DOI] [PubMed] [Google Scholar]
- Shrivastava J, Barker G, Johansen MV, Xiaonong Z, Aligui GD, McGarvey ST, Webster JP. Isolation and characterisation of polymorphic DNA microsatellite markers from Schistosoma japonicum. Molecular Ecology Notes. 2003;3:406–408. [Google Scholar]
- Shrivastava J, Gower CM, Balolong EJ, Wang TP, Qian BZ, Webster JP. Population genetics of multi-host parasites - the case for molecular epidemiological studies of Schistosoma japonicum using naturally sampled larval stages. Parasitology. 2005;131:617–626. doi: 10.1017/S0031182005008413. [DOI] [PubMed] [Google Scholar]
- Sissay MM, Asefa A, Uggla A, Waller PJ. Anthelmintic resistance of nematode parasites of small ruminants in eastern Ethiopia: exploitation of refugia to restore anthelmintic efficacy. Veterinary Parasitology. 2006;135:337–346. doi: 10.1016/j.vetpar.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Skorping A, Read AF. Drugs and parasites: global experiments in life history evolution. Ecology Letters. 1998;1:10–12. [Google Scholar]
- Southgate VR, Rollinson D, Tchuem Tchuenté LA, Hagan P. Towards control of schistosomiasis in sub-Saharan Africa. Journal of Helminthology. 2005;79:181–185. doi: 10.1079/joh2005307. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Mackinnon MJ, Read AF. Virulence of mixed-clone and single-clone infections of the rodent malaria Plasmodium chabaudi. Evolution. 1998;52:583–591. doi: 10.1111/j.1558-5646.1998.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Tcheum Tchuente LA, Behnke JM, Gilbert FS, Southgate VR, Vercruysee J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Tropical Medicine and International Health. 2003;8:975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Southgate VR, Imbert-Establet D, Jourdane J. Change of mate and mating competition between males of Schistosoma intercalatum and S. mansoni. Parasitology. 1995;110(Pt 1):45–52. doi: 10.1017/s0031182000081038. [DOI] [PubMed] [Google Scholar]
- Theron A. Early and late emergence patterns of Schistosoma mansoni cercariae : Ecological significance in transmission to human and murine hosts. Journal of Parasitology. 1984;70:652–655. [PubMed] [Google Scholar]
- Theron A, Pointier JP, Morand S, Imbert-EStablet D, Borel G. Long-term dynamics of natural populations of Schistosoma mansoni among Rattus rattus in patchy environment. Parasitology. 1992;104:291–298. doi: 10.1017/s0031182000061734. [DOI] [PubMed] [Google Scholar]
- Thiongo FW, Madsen H, Ouma JH, Andreassen J, Christensen NO. Host-parasite relationships in infections with two Kenyan isolates of Schistosoma mansoni in NMRI mice. Journal of Parasitology. 1997;83:330–332. [PubMed] [Google Scholar]
- Valle C, Troiani AR, Festucci A, Pica-Mattoccia L, Liberti P, Wolstenholme A, Francklow K, et al. Sequence and level of endogenous expression of calcium channel beta subunits in Schistosoma mansoni displaying different susceptibilities to praziquantel. Molecular and Biochemical Parasitology. 2003;130:111–115. doi: 10.1016/s0166-6851(03)00171-3. [DOI] [PubMed] [Google Scholar]
- Van Wyk JA. Regugia – overlooked as perhaps the most potent factor concerning the development of antheminthic resistance. The Onderstepoort Journal of Veterinary Research. 2001;68:55–67. [PubMed] [Google Scholar]
- Vennervald BJ, Kahama AI, Reimert CM. Assessment of morbidity in Schistosoma haematobium infection: current methods and future tools. Acta Tropica. 2000;77:81–89. doi: 10.1016/s0001-706x(00)00116-9. [DOI] [PubMed] [Google Scholar]
- Wang T-P, Shrivastava J, Johansen MV, Zhang ZK, Webster JP. Does multiple hosts mean multiple parasites?: population genetic structure of Schistosoma japonicum between definitive host species. International Journal for Parasitology. 2006;36:1317–1325. doi: 10.1016/j.ijpara.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Webster JP. Compatibility and sex in a snail-schistosome system. Parasitology. 2001;122:423–432. doi: 10.1017/s0031182001007442. [DOI] [PubMed] [Google Scholar]
- Webster JP, Davies CM. Coevolution and compatibility in the Snail-Schistosome system. Parasitology. 2001;123:S41–S56. doi: 10.1017/s0031182001008071. [DOI] [PubMed] [Google Scholar]
- Webster JP, Woolhouse MEJ. Selection and strain specificity of compatibility between snail intermediate hosts and their parasitic schistosomes. Evolution. 1998;52:1627–1643. doi: 10.1111/j.1558-5646.1998.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Webster JP, Woolhouse MEJ. Cost of Resistance: relationship between reduced fertility and increased resistance in a Schistosoma host-parasite system. Proceedings of the Royal Society, (London), Series B. 1999;266:391–396. [Google Scholar]
- Webster BL, Southgate VR, Tchuem Tchuente LA. Mating interactions between Schistosoma haematobium and S. mansoni. Journal of Helminthology. 1999;73:351–356. [PubMed] [Google Scholar]
- Webster JP, Davies CM, Hoffman JI, Ndamba J, Noble LR, Woolhouse MEJ. Population genetics of Biomphalaria pfeifferi in the Zimbabwean highveld: implications for co-evolutionary theory. Annals of Tropical Medicine and Parasitology. 2001;95:203–214. doi: 10.1080/00034980120041062. [DOI] [PubMed] [Google Scholar]
- Webster JP, Hoffman J, Berdoy MEL. Parasite resistance and mate choice – battle of the genders in a simultaneous hermaphrodite. Proceedings of the Royal Society (London) Series B. 2003;270:1481–1485. doi: 10.1098/rspb.2003.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Gower CM, Blair L. Do hosts and parasites coevolve?: empirical support from the Schistosoma system. American Naturalist. 2004;164:S33–S51. doi: 10.1086/424607. [DOI] [PubMed] [Google Scholar]
- Webster JP, Koukounari A, Lamberton PHL, Stothard JR, Fenwick A. The identification, application and evaluation of potential schistosome-associated morbidity markers within large-scale mass chemotherapy programmes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007a in press. [Google Scholar]
- Webster JP, Shrivastava J, Johnson P, Blair L. Is host-schistosome coevolution going anywhere? BMC Evolutionary Biology. 2007b;7 doi: 10.1186/1471-2148-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse MEJ. The effect of schistosome infection on the mortality rates of Bulinus globulus and Biomphalaria pfeifferi. Annals of Tropical Medicine and Parasitology. 1989;83:137–141. doi: 10.1080/00034983.1989.11812321. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the coevolution of pathogens and their hosts. Nature Genetics. 2002;32:569–577. doi: 10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- Xua D, Curtis J, Feng Z, Minchella DJ. On the role of schistosome mating structure in the maintenance of drug resistant strains. Bulletin of Mathematical Biology. 2005;67:1207–1226. doi: 10.1016/j.bulm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Koukounari A, Kabatereine N, Fleming F, Kazibwe F, Tukahebwa E, Webster JP, Fenwick A. Parasitological impact of two-year chemotherapy on schistosomiasis and soil-transmitted helminthiasis in Uganda. BMC Medicine. 2007;5:27. doi: 10.1186/1741-7015-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


