Abstract
We review the results of a series of experiments involving Aedes aegypti and its microsporidian parasite Vavraia culicis to illustrate how intra-specific competition and parasitism shape life history traits. More specifically these experiments showed that some major components of virulence are host condition-dependent in this system, while others are not. We also briefly discuss the ways through which V. culicis modifies the physiological functioning of its host. We discuss the implications of these results for studies of host – parasite interactions in general and propose ways through which our studies could contribute to vector control and management programs.
Keywords: Aedes, mosquitoes, microsporidia, resistance, Vavraia, virulence
Introduction
Host–parasite interactions have attracted increasing attention during the past 30 years for several reasons: they require study because of their relevance to public health and economic growth; they constitute intriguing ecological and evolutionary systems worthy of study for their own sake, and they are involved in the evolution of other traits, such as, reproductive mode.
Laboratory systems, although in no way sufficient, have proved extremely helpful in deepening our understanding of ecological and evolutionary processes, through the careful examination of the effects of specific factors that they allow us to manipulate and investigate.
In this paper, we review the results of several experiments we performed on a model host–parasite system and propose an integrated discussion of their implications, in particular for interpreting and designing experiments for field systems. We also discuss how our results may contribute towards designing vector control programs.
Study system
We used the yellow-fever mosquito Aedes aegypti as host and the microsporidium Vavraia culicis as parasite. The mosquito A. aegypti is the major vector of dengue and yellow fever. It is a subtropical mosquito, whose larvae grow in natural or artificial containers (Southwood et al. 1972). It is a very well-studied organism: its ecology is known in detail (Christophers 1960), it has been a model organism in insect physiology (Clements 1992), and its full genome has been recently published (Nene et al. 2007). It can be easily raised in the laboratory, and its eggs can be stored on filter paper for at least a few months and can be hatched synchronously within 1 h. The combination of these characteristics makes it a convenient species to work with when interested in ecological or evolutionary experiments at the organism level.
In all the studies mentioned in this paper, we used a population provided by Dr. A.B. Falloux (Pasteur Institute, Paris) and originally sampled in Tingua, Brazil and provided by Ricardo Lourenço de Oliveira of the Instituto Oswaldo Cruz (Rio de Janeiro, Brazil). We maintain it in the laboratory using 3000 reproductive adults. The choice of this population was guided by the fact that it was the only population available to use which had recently been isolated from the field (two generations previously), that had been initiated by a large number of founders, and did not contain sex ratio distorters. Indeed, several populations of A. aegypti harbour sex-ratio distorters (Wood and Newton 1991) and their interactions with mosquito life-history traits have been poorly investigated. Another factor known to interact with life history traits is insecticide resistance (Lenormand et al. 1999) and we verified that our population is susceptible to organophosphate insecticides (V. Corbel LIN, IRD Montpellier, pers. comm.). Unless otherwise mentioned, in all of our experiments, mosquitoes were grown in individual vials to avoid confounding density effects (Agnew et al. 2002). The life history traits we typically measured were preadult survival, developmental time (time to pupation/emergence), adult body size, measured through either wing length or dry weight at death, and adult longevity until death by starvation. The latter measure can be seen as an indication of the amount of resources an individual acquired as a larva, because in our experiments adults did not receive any food and pupae do not feed.
Like all microsporidia, V. culicis is an obligate endocellular parasite. None of the documented forms of invertebrate immune responses is able to suppress or clear infections by these intracellular parasites. Vavraia culicis naturally infects several mosquito species (Weiser 1980; Becnel et al. 2005; Andreadis 2007). It has a direct life cycle: mosquito larvae get infected by ingesting spores while feeding. The spores first infect the mosquito gut epithelial cells, and the infection spreads to other gut and fat body cells. Infection may cause physical damage when spore-laden cells eventually rupture. This microsporidium is transmitted only horizontally and its spores do not resist desiccation (Kelly et al. 1981). It is thus important for the within-site transmission of this parasite that infected hosts remain in the water and die as either larvae or pupae. Because of this, mosquito developmental time and preadult survival are important traits determining the outcome of infection for host and parasite in this system.
The microsporidia we used were first isolated in Florida from A. albopictus larvae (Fukuda et al. 1997) and provided to us by Dr. J. J. Becnel (United States Department of Agriculture, Gainesville, FL, USA). This is the only isolate of V. culicis currently available. Vavra and Becnel (2007) provided information on the origin and ultrastructure of this isolate and its relationship to other isolates of V. culicis. The microsporidia used in our studies were propagated once in larvae of the lepidopteran Spodoptera littoralis. In all of the experiments discussed here, newly hatched larvae were exposed to parasites during their first 24 h only, so as to synchronize infections.
To summarize, this system offers many advantages. The host has very well-known genetics, physiology and ecology which potentially allow detailed mechanistic studies to be performed; it has a relatively short generation time, and it is possible to synchronize generations and store eggs for at least several months. Moreover, it is an important human disease vector, which potentially adds applied relevance to the studies undertaken. The parasite has a direct life cycle, is relatively easy to manipulate and can be stored at 4°C for years.
Virulence
We investigated how infection by V. culicis affected several life history traits of A. aegypti. In a first study (Bedhomme et al. 2004), we looked at how the parasite’s virulence was influenced by host condition, which was manipulated through the amount of food provided to host larvae. By incorporating the effects of the environment on the outcome of this host–parasite interaction, this study provides a fuller description of what could be called the direct cost of infection. In a second study, we looked at how parasitism and intraspecific competition interact in shaping host life history traits (Bedhomme et al. 2005). This latter study revealed that parasitized hosts are weaker competitors, thus unveiling indirect costs of infection through the modification of competitive ability.
Direct costs of infection
The aims of this experiment, fully reported in Bedhomme et al. (2004), were twofold: (i) to investigate how host condition affected the expression of virulence and (ii) to see whether host condition affects the within host growth of the parasite.
We manipulated host condition through the amount of food provided to host larvae. The food gradient we used ranged from amounts low enough that virtually no larva made it to the adult stage, to levels where almost all the larvae became adults.
This study showed that the expression of virulence did not depend on host-condition for adult traits. We found significant effects of infection: infected individuals took a longer time to become adults, were smaller and lighter adults, and lived for a shorter time as adults than uninfected individuals. We also found intuitive effects of food on these life history traits: individuals developed faster, were larger and lived longer when they had received greater amounts of food. However, we did not find any significant interactions between these two factors, suggesting that the cost of infection on these traits is independent of host condition.
The only trait on which virulence depended on host condition was the probability of emergence. As Fig. 1 shows, the probability of emergence increased from almost 0 to almost 100% across the food gradient we used. However, infected and uninfected individuals reacted differently to increased food: indeed, while at (relatively) low or (relatively) very high food levels larvae managed to develop into pupae equally badly or equally well, at intermediate food levels infected larvae had a significantly lower probability to reach the adult stage than uninfected larvae. The decrease in probability of pupation due to infection can be as large as 30%.
Figure 1.
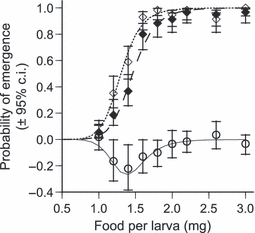
Probability of emergence of control and infected hosts along a gradient of larval food availability. Open diamonds represent uninfected larvae and filled diamonds represent larvae infected with Vavraia culicis. The dotted line is the predicted curve for uninfected treatments and the dashed line is for infected treatments. Circles represent the difference between infected and uninfected treatments. Error bars in each case are 95% confidence intervals (re-drawn from Bedhomme et al. 2004).
To address the second issue of whether the parasite’s within-host growth was affected by host condition, we looked at how a measure of spore production depended on the amount of food each individual had received as a larva. Our measure of spore production took into account the effect of the food treatment on host longevity, and thus time available for spore production. The analysis was separated into two, according to the amount of food provided to host larvae, because of the effect of this factor on host development. Indeed, as previously discussed, under low food conditions most hosts die as larvae, while under relatively high food conditions almost all of them become adults. The first part of the analysis thus indicates how host food condition affects parasite growth in larvae, and the second part in adults. Our analysis revealed a positive relationship between spore production and host condition in both cases (Fig. 2). The reproductive output of the parasite is thus increased as host condition improves, indicating that within host growth of V. culicis is resource dependent.
Figure 2.
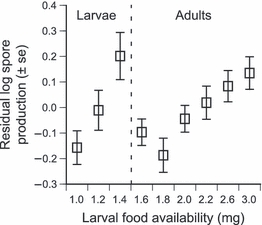
Spore production in individuals dying as larvae (food treatments 1.0–1.4 mg) and as adults (food treatments 1.6–3.0 mg) as a function of the amount of food available to host larvae. The figure reports the residuals of the regression of the number of spores produced by individual at each food treatment on the age of the individual when it died (re-drawn from Bedhomme et al. 2004).
Indirect costs
We then looked at how parasitism and intraspecific competition among hosts could interact in shaping host life history traits (Bedhomme et al. 2005). To this end we slightly modified our basic design, by growing two individuals in each vial. These two individuals could be both infected, both uninfected, or one infected and one uninfected. Small amounts of food were added on a daily basis to help ensure that both individuals would complete their development and emerge as adults; this also enabled us to identify the gender of both competitors.
To uncover the interactions of parasitism and competition, we looked at how developmental time of a focal individual depended on its own infection status and on the infection status of its competitor (Fig. 3). We thus found that infected individuals competing with another infected individual (the +/+ case of Fig. 3) developed faster than infected individuals competing with a healthy individual (the +/− case), indicating that infected individuals are weaker competitors. The corollary was true as well: healthy individuals developed faster when they were in competition with an infected individual (the −/+ case) than when they were in competition with another healthy individual [the −/− case]. Infection thus induces an indirect cost, through the modification of the competitive ability of the hosts [Fig. 3, arrows (i) and (iii)]. Note, these indirect costs are greater than those observed when comparing the developmental time of infected and uninfected larvae when reared with a competitor of the same infection status [Fig 3. arrow (ii)]. This result has several important consequences, which we discuss later.
Figure 3.
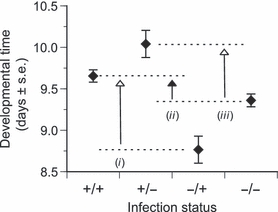
Developmental time (age at pupation) of individuals as a function of their own status (first symbol) and of the infection status of their competitor (second symbol). For example, +/− refers to the case where we are interested in the developmental time of infected individuals when in competition with healthy uninfected individuals. The closed arrow (ii) indicates what is commonly measured as the direct cost of infection. The two open arrows, (i) and (iii), indicate the indirect costs of competition (re-drawn from Bedhomme et al. 2005).
How virulence arises
After having investigated the phenotypic effects of infection on host life history traits, we tried to study the mechanistic basis of virulence. To this end, we followed two approaches. The first consisted in quantifying the consequences of infection in terms of resource depletion (Rivero et al. 2007). We thus compared the amount of lipids, proteins, sugars and glycogen in 5-day-old infected and uninfected larvae. The second approach consisted in characterizing the effects of infection on the proteome of A. aegypti (Biron et al. 2005).
Host resource depletion by the parasite
One very likely cause of virulence is host resource depletion by the parasite. This is especially likely for microsporidia, which are obligate parasites lacking mitochondria and thus heavily rely on the host cell to obtain the energy necessary for their growth (Agnew et al. 2003). We thus undertook a study aiming at quantifying the effects of infection of A. aegypti larvae by V. culicis in terms of various types of resources, namely proteins, lipids, sugars and glycogen (Rivero et al. 2007).
As in previous experiments, infection led to a smaller host body size. After controlling for this effect, we found that infected larvae had significantly less lipids, glycogen and sugars. Because it is possible to transform the quantities of all these types of resources in energy units, we were able to estimate that under our experimental conditions infection resulted in a 20% decrease of total energy.
We then asked for the cause of this decrease in resources. A first possibility was that infected larvae feed less. We measured feeding rates and found no difference between infected and healthy larvae. A second possibility was that infected larvae use resources either to repair the damage caused by the infection or to fight infection. To assess whether this occurred we compared the metabolic rate of infected and healthy larvae. We again found no difference, indicating that if such processes are elicited by infection they do not lead to the energetic losses we documented. We thus conclude that the energy deficit of infected individuals is most likely diverted by the parasite for its own growth.
Effect of infection on host proteome
To characterize the effects of infection on the A. aegypti larval proteome, we also used another treatment, hypoxia; this allowed us to distinguish between general stress responses and more specific reactions to infection (Biron et al. 2005). By analysing the reaction of young and older larvae, corresponding to early and late stages of infection, we were further able to analyse the temporal pattern of proteome modification. We limited our analysis to presence/absence effects because we lacked objective criteria for linking quantitative differences in protein spot size to functional differences. We are thus aware that our analysis is quite crude and that we likely miss many more subtle effects.
We found that stresses such as hypoxia, infection and their combination, caused a general decrease in the number of spots, which most probably indicates that stressed individuals shut down functions. Older larvae reacted most strongly to infection, in terms of the percentage of spots whose expression was affected, while younger larvae reacted most to the combined stresses and to hypoxia. Relatively few spots were specifically induced by infection (approx. 6% out of a total of about 900 spots). Identification of the protein spots specifically induced or suppressed by infection revealed that many of the identified proteins with known functions are directly or indirectly involved in defense against microorganisms. Further, the comparison of specifically affected, i.e. induced or suppressed, proteins in younger and older larvae revealed an interesting pattern: several of the proteins differentially expressed in younger larvae are expressed at the peritrophic matrix, i.e. at the very locus where infection is initiated, and others are involved in antimicrobial defense, while most of the proteins differentially expressed in older larvae are involved in maintaining physiological or metabolic functions. Thus, the temporal pattern of differential protein expression is compatible with the advance of infection: in younger larvae, proteins exhibiting differential expression are involved in antibacterial defense and expressed at the locus of initiation of the infection, the gut; in older larvae, when the infection is well advanced and host tissues are experiencing physical damage proteins involved in maintenance are differentially expressed.
Finally, our analysis revealed that the parasite is potentially manipulating the host’s immune system, through the modification of the nitric oxide synthase (NOS) cascade. Nitric oxide is a molecule involved in many functions, including defense against parasites (Rivero 2006). The NOS cascade leads either to the production of nitric oxide, which is toxic to parasites (and can be toxic to the host as well at higher concentrations), or to the production of polyamines. Vincendeau et al. (2003) proposed that several vertebrate parasites could actively manipulate this metabolic pathway diverting it towards the synthesis of polyamines and away from the synthesis of nitric oxide. They would thus benefit doubly, both from the nonproduction of the toxic molecule and from the production of the polyamines which they can use directly for their own metabolism. Our results indicate the specific suppression of enzymes active in the cascade towards nitric oxide production and the specific induction of enzymes involved in the cascade leading to polyamine production. It is worth noting that while microsporidia are able to produce such polyamines themselves, it has been shown that they preferentially use the polyamines produced by the host (Bacchi et al. 2003).
Discussion/perspectives
The integration of the findings of all the above described studies leads towards a coherent understanding of this host–parasite system and will hopefully stimulate analogous studies in field systems.
There is an increasing realization that the outcome of host–parasite interactions is influenced by the environment (Agnew and Koella 1999; Brown et al. 2000; Elliot et al. 2002; Dybdahl and Krist 2004; Krist et al. 2004; Jokela et al. 2005; Mitchell et al. 2005; Tseng 2006). The first experiment reviewed here showed that one component of parasite virulence is host condition dependent: preadult survival. This is a key trait in this system, as it is obviously strongly related to host fitness (only those hosts who become adults can reproduce) and to parasite transmission, and hence parasite fitness. This dependence of virulence on the environment renders the cost of parasitism, and hence the strength of selection on any trait potentially affected by parasitism variable in space. How important this is calls for an empirical evaluation in field systems. Theoretical investigations have already shown that spatial variability of the strength of selection in host–parasite interactions may significantly affect, for example, local adaptation (Nuismer 2006).
Our results indicate that under relatively good environmental conditions, the mosquito will come out with relatively little harm from this microsporidian infection, while the microsporidium will see its transmission largely compromised because almost all of the hosts leave the aquatic environment. On the contrary, in relatively intermediate quality environments the mosquito will suffer greatly from the reduction of its preadult survival, while precisely through the same trait, the microsporidium will enjoy high transmission. Thus, under any given environmental condition the reproductive capacity and population size of one of the antagonists will be severely limited, thus constraining its capacity to adapt. If these considerations can be extrapolated to field conditions at all, in this or another system, they indicate that within a given site we cannot have co-evolution but only adaptive evolution of the antagonist that comes out relatively unharmed. Whether co-evolution may occur in such systems is therefore a matter of how fine grained the environment is with respect to environmental quality. This will not only depend on the spatial distribution of environmental quality but also on the relative spatial scales of reproduction of the antagonist species, i.e. whether mating occurs at the scale of a single site or a group of more or less distant sites, and the scale of selection (Boots and Sasaki 1999, 2000; Boots and Mealor 2007). We do not know of any system where the outcome of infection depends on environmental conditions, and where information on how fine-grained the environment is, is available.
Bize et al. (2008) have recently pointed out that parasite fitness may depend on host quality in a nonmonotonic way. Poor condition hosts may be less able to defend themselves, but may offer only a small gain to the parasite because, e.g. they have very limited resources to offer. Good condition hosts may represent a larger ‘reward’ but may be much riskier to conquer. In our mosquito–microsporidium system, due to the effects of host condition on parasite within host growth and on parasite transmission, intermediate conditions for host larval growth apparently maximize parasite fitness.
Measures of costs of parasitism typically involve the comparison of traits of infected populations to that of uninfected populations, equivalent to the comparison between the +/+ and the −/− cases in Fig. 3 [arrow (ii)], or all the comparisons in the Direct costs of infection section. Our analysis of the experiment on the interaction between parasitism and competition shows that not only indirect costs exist, through the modification of host competitive ability, but also that these costs may even be larger than the direct costs [Fig. 3, arrows (i) and (iii)]. Because these costs are typically overlooked, it is highly likely that our understanding of at least the ecological functioning of host–parasite interactions is extremely partial.
Moreover, these indirect costs are prevalence dependent, as they only arise when infected individuals compete with uninfected individuals. Because this will occur with high probability when the prevalence of infection is low, such costs may help the evolution of resistance mechanisms with constitutive costs. Indeed, the evolution of such mechanisms is problematic when disease prevalence is low, as because of low prevalence their benefits are seldom experienced while their costs are always expressed. The existence of indirect costs of infection, such as the ones we describe, increases the potential benefits of resistance to infection at low prevalence. This may facilitate the evolution of such resistance mechanisms, and thus indirect costs may also impact upon the evolutionary dynamics of host–parasite interactions.
The resource accumulation experiment highlighted at least one of the ways through which the parasite achieves its virulence: host resource depletion. Microsporidia rely exclusively on their hosts to acquire the energy necessary for their reproduction, and even on the host cell machinery as they lack mitochondria. The decrease in host resources caused by infection is thus not surprising. What is perhaps more interesting is that this resource depletion affects the phenotypic expression of host traits directly relevant to parasite transmission, such as, preadult survival and developmental time.
The proteome study yielded several very interesting results, such as, a temporal pattern of protein expression compatible with the advance of infection within individual hosts. It also opens lines of research through the identification of proteins whose expression pattern is specifically affected by infection, and whose genetic variability can thus be analysed in field samples and related to the prevalence of disease. But perhaps the most interesting and unexpected results were those pertaining to the manipulation and activation of components of the host’s immune system by the parasite.
As already mentioned above, the possibility that parasites may manipulate the NOS system of their hosts and benefit doubly by this manipulation has already been raised (Vincendeau et al. 2003). The possibility that such a manipulation exists in this system, as suggested by the proteome expression evidence, can be explicitly tested. Indeed, it is possible to up-regulate or down-regulate the NOS cascade and observe the direct effects of such a manipulation on the course and development of the infection (Rivero 2006).
The finding that several mosquito immune system components are specifically induced by the microsporidian infection was unexpected, because none of the known insect immune system components are able to clear such infections. We can offer two nonmutually exclusive explanations. The first is that mosquitoes feel they are sick and try to fight infection with what they have. The second is that they are fighting against opportunistic infections which may arise in their gut following the damaging effects of microsporidian entry into epithelial cells. Whatever the cause, these results indicate that despite their inefficiency against them, microsporidia elicit an immune response from mosquitoes. The ‘opportunistic infections’ hypothesis raises some potentially very intriguing interactions between unrelated microorganisms affecting host traits in nonintuitive and as yet unaccounted for ways. For all that we know the phenotypic effects we observe following microsporidian infection could be due to some, potentially large or small, extent to the concomitant development of opportunistic microbial infections. Such interactions would introduce variability in the outcome of infection.
In addition to the research perspectives outlined above, we believe this experimental system offers the opportunity to deepen our understanding, through carefully designed experiments, of topics such as the consequences of polyparasitism, the synchronous infection of a given host individual by several parasite species (Hotez et al. 2006; Utzinger and de Savigny 2006), on host and parasite evolution and ecology, or the effects of other environmental gradients, such as, temperature. This system also offers itself to experimental evolution and selection experiments on host–parasite issues.
Microsporidia, in general (Sweeney and Becnel 1991; Becnel and Johnson 2000; Andreadis 2007), and V. culicis more specifically (Reynolds 1970, 1972; Kelly et al. 1981), have been considered as potential mosquito control agents for a long time. While the use of some microsporidia is considered promising [e.g. Edhazardia aedis to control A. aegypti (Becnel and Johnson 2000)], V. culicis is not regarded in general as a promising vector control agent. This appreciation is largely based on the fact that most studies on the effects of this microsporidium on mosquito hosts failed to reveal significant effects on the probability of emergence (Reynolds 1970; Agnew et al. 1999). This, per se, is a strange result for a parasite which, given its life cycle, must make at least most of its transmission by keeping its host into the water, and the best way to do this is by imposing preadult mortality. This discrepancy between intuition and experimental results was one of the reasons that motivated the studies reviewed in section Virulence. In particular, our study on the virulence reaction norm with host condition (Bedhomme et al. 2004) revealed significant larval mortality effects when the environment was relatively harsh for the host, but not when the environment was relatively benign. The study on the indirect effects of infection through the modification of the competitive ability also revealed conceptually more subtle, but potentially significant costs of the infection which were previously unnoticed, and which occur under relatively harsh environmental conditions of intraspecific competition. We thus feel that it is important that laboratory evaluations of vector control agents include several environments, and in particular environments relevant to field conditions. We realize that it may be difficult to identify and reproduce in the laboratory relevant field conditions. One potential approach would be to use laboratory conditions that yield values of key life history traits for uninfected mosquitoes similar to those observed in the field. Our feeling is that the environments used in laboratories are often very benign. For example, the few field estimates available on the average time to adulthood in A. aegypti are of the order of 20 days [18 days for a population in Thailand (Southwood et al. 1972); 24 days for a population in Florida (Wijeyaratne et al. 1974)], which is still much longer than what we get in what we view as harsh conditions [<10 days; see Fig. 2 of Bedhomme et al. (2004) and Fig. 3 of this paper]. A corollary of this, is that human interventions which make the environment harsher for target organisms will not only directly affect their population sizes, but may also generate conditions in which their parasites thrive.
Acknowledgments
Our research was funded by CNRS and IRD. YM acknowledges funding from grant ANR-06-MIME-033-01.
Literature Cited
- Agnew P, Koella JC. Life history interactions with environmental conditions in a host–parasite relationship and the parasite’s mode of transmission. Evolutionary Ecology. 1999;13:67–89. [Google Scholar]
- Agnew P, Bedhomme S, Haussy C, Michalakis Y. Age and size at maturity of the mosquito Culex pipiens infected by the microsporidian parasite Vavraia culicis. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:947–952. [Google Scholar]
- Agnew P, Hide M, Sidobre C, Michalakis Y. A minimalist approach to investigating the effects of density-dependence on selected life-history traits of the mosquito Aedes aegypti. Ecological Entomology. 2002;27:396–402. [Google Scholar]
- Agnew P, Becnel JJ, Ebert D, Michalakis Y. Symbiosis of Microsporidia and insects. In: Bourtzis K, editor. Insect Symbiosis. Boca Raton, FL: CRC Press LLC; 2003. pp. 145–163. [Google Scholar]
- Andreadis TG. Microsporidian parasites of mosquitoes. Journal of the American Mosquito Control Association. 2007;23:3–29. doi: 10.2987/8756-971X(2007)23[3:MPOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bacchi CJ, Yarlett N, Weiss LM. Polyamine metabolism in the Microsporidia. Biochemical Society Transactions. 2003;31:420–423. doi: 10.1042/bst0310420. [DOI] [PubMed] [Google Scholar]
- Becnel JJ, Johnson MA. Impact of Edhazardia aedis (Microsporidia:Culicosporidae) on a seminatural population of Aedes aegypti (Diptera:Culicidae) Biological Control. 2000;18:39–48. [Google Scholar]
- Becnel JJ, White SE, Shapiro AM. Review of microsporidia-mosquito relationships: from the simple to the complex. Folia Parasitologica. 2005;52:41–50. [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Virulence reaction norms across a food gradient. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:739–744. doi: 10.1098/rspb.2003.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Vital Y, Sidobre C, Michalakis Y. Prevalence-dependent costs of parasite virulence. PLoS Biol. 2005;3 doi: 10.1371/journal.pbio.0030262. e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron DG, Agnew P, Marche L, Renault L, Sidobre C, Michalakis Y. Proteome of Aedes aegypti larvae in response to infection by the intracellular parasite Vavraia culicis. International Journal for Parasitology. 2005;35:1385–1397. doi: 10.1016/j.ijpara.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Bize P, Jeanneret C, Klopfenstein A, Roulin A. What makes a host profitable? Parasites balance host nutritive resources against immunity. American Naturalist. 2008;171:107–118. doi: 10.1086/523943. [DOI] [PubMed] [Google Scholar]
- Boots M, Mealor M. Local interactions select for lower pathogen infectivity. Science. 2007;315:1284–1286. doi: 10.1126/science.1137126. [DOI] [PubMed] [Google Scholar]
- Boots M, Sasaki A. ‘Small worlds’ and the evolution of virulence: infection occurs locally and at a distance. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:1933–1938. doi: 10.1098/rspb.1999.0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots M, Sasaki A. The evolutionary dynamics of local infection and global reproduction in host–parasite interactions. Ecology Letters. 2000;3:181–185. [Google Scholar]
- Brown MJF, Loosli R, Schmid-Hempel P. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos. 2000;91:421–427. [Google Scholar]
- Christophers SR. Aedes aegypti (L.). The Yellow Fever Mosquito. Its Life History, Bionomics and Structure. Cambridge: Cambridge University Press; 1960. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Volume 1: Development, Nutrition and Reproduction. London: Chapman & Hall; 1992. [Google Scholar]
- Dybdahl MF, Krist AC. Genotypic vs. condition effects on parasite-driven rare advantage. Journal of Evolutionary Biology. 2004;17:967–973. doi: 10.1111/j.1420-9101.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- Elliot SL, Blanford S, Thomas MB. Host–pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proceedings. Biological Sciences. 2002;269:1599–1607. doi: 10.1098/rspb.2002.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Willis OR, Barnard DR. Parasites of the Asian tiger mosquito and other container-inhabiting mosquitoes (Diptera:Culicidae) in northcentral Florida. Journal of Medical Entomology. 1997;34:226–233. doi: 10.1093/jmedent/34.2.226. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Medicine. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J, Taskinen J, Mutikainen P, Kopp K. Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. Oikos. 2005;108:156–164. [Google Scholar]
- Kelly JF, Anthony DW, Dillard CR. A laboratory evaluation of the microsporidian Vavraia culicis as an agent for mosquito control. Journal of Invertebrate Pathology. 1981;37:117–122. [Google Scholar]
- Krist AC, Jokela J, Wiehn J, Lively CM. Effects of host condition on susceptibility to infection, parasite developmental rate, and parasite transmission in a snail-trematode interaction. Journal of Evolutionary Biology. 2004;17:33–40. doi: 10.1046/j.1420-9101.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- Lenormand T, Bourguet D, Guillemaud T, Raymond M. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature. 1999;400:861–864. doi: 10.1038/23685. [DOI] [PubMed] [Google Scholar]
- Mitchell SE, Rogers ES, Little TJ, Read AF. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution; International Journal of Organic Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuismer SL. Parasite local adaptation in a geographic mosaic. Evolution. 2006;60:24–30. [PubMed] [Google Scholar]
- Reynolds DG. Laboratory studies of the microsporidian Plistophora culicis (Weiser) infecting Culex pipiens fatigans Wied. Bulletin of Entomological Research. 1970;60:339–349. doi: 10.1017/S0007485300040852. [DOI] [PubMed] [Google Scholar]
- Reynolds DG. Experimental introduction of a microsporidian into a wild population of Culex pipiens fatigans Wied. Bulletin of the World Health Organization. 1972;46:807–812. [PMC free article] [PubMed] [Google Scholar]
- Rivero A. Nitric oxide: an antiparasitic molecule of invertebrates. Trends In Parasitology. 2006;22:219–225. doi: 10.1016/j.pt.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Rivero A, Agnew P, Bedhomme S, Sidobre C, Michalakis Y. Resource depletion in Aedes aegypti mosquitoes infected by the microsporidia Vavraia culicis. Parasitology. 2007;134:1355–1362. doi: 10.1017/S0031182007002703. [DOI] [PubMed] [Google Scholar]
- Southwood TR, Murdie G, Yasuno M, Tonn RJ, Reader PM. Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bulletin of the World Health Organization. 1972;46:211–226. [PMC free article] [PubMed] [Google Scholar]
- Sweeney AW, Becnel JJ. Potential of microsporidia for the biological-control of mosquitoes. Parasitology Today. 1991;7:217–220. doi: 10.1016/0169-4758(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Tseng M. Interactions between the parasite’s previous and current environment mediate the outcome of parasite infection. American Naturalist. 2006;168:565–571. doi: 10.1086/507997. [DOI] [PubMed] [Google Scholar]
- Utzinger J, De Savigny D. Control of neglected tropical diseases: integrated chemotherapy and beyond. PLoS Medicine. 2006;3:e112. doi: 10.1371/journal.pmed.0030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavra J, Becnel JJ. Vavraia culicis Weiser, 1977 revisited: the Florida reference isolate. Folia Parasitologica. 2007;54:259–271. [PubMed] [Google Scholar]
- Vincendeau P, Gobert AP, Daulouede S, Moynet D, Mossalayi MD. Arginases in parasitic diseases. Trends in Parasitology. 2003;19:9–12. doi: 10.1016/s1471-4922(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Weiser J. Geneva: World Health Organisation; 1980. Data Sheet on the Biological Control Agent VavraiaPleistophoraculicis (Weiser 1946) pp. 1–5. [Google Scholar]
- Wijeyaratne PM, Seawright JA, Weidhaas DE. Development and survival of a natural population of Aedes aegypti. Mosquito News. 1974;34:36–42. [Google Scholar]
- Wood RJ, Newton ME. Sex-ratio distortion caused by meiotic drive in mosquitoes. American Naturalist. 1991;137:379–391. [Google Scholar]


