Abstract
Sterile insect release (SIR) is used to suppress insect pest populations in agro-ecosystems, but its success hinges on the performance of the released insects and prevailing environmental conditions. For example, low temperatures dramatically reduce SIR efficacy in cooler conditions. Here, we report on the costs and benefits of thermal acclimation for laboratory and field responses of codling moth, Cydia pomonella. Using a component of field fitness, we demonstrate that low temperature acclimated laboratory-reared moths are recaptured significantly more (∼2–4×) under cooler conditions in the wild relative to warm-acclimated or control moths. However, improvements in low temperature performance in cold-acclimated moths came at a cost to performance under warmer conditions. At high ambient temperatures, warm-acclimation improved field performance relative to control or cold-acclimated moths. Laboratory assessments of thermal activity and their limits matched the field results, indicating that these laboratory assays may be transferable to field performance. This study demonstrates clear costs and benefits of thermal acclimation on laboratory and field performance and the potential utility of thermal pretreatments for offsetting negative efficacy in SIR programmes under adverse thermal conditions. Consequently, the present work shows that evolutionary principles of phenotypic plasticity can be used to improve field performance and thus possibly enhance pest control programmes seeking increased efficacy.
Keywords: competitiveness, field fitness, performance, release-recapture, sterile insect release
Introduction
Sterile insect release (SIR) has been used to suppress populations of agricultural insect pests and vectors of human and animal disease with varying levels of success. The SIR method for insect control typically involves sterilization (mainly through gamma radiation) of mass-reared insects which are released into the wild for mating with their wild counterparts. Thus, the pest population produces nonviable offspring leading to population declines (Vreysen and Robinson 2010). Apart from financial, social and political issues, field performance of laboratory-reared insects probably remains one of the greatest challenges to SIR success (Enserink 2007; Terblanche and Chown 2007; Simmons et al. 2010). Codling moth (CM), Cydia pomonella (Lepidoptera: Tortricidae), is a major pest of global pome fruit production with huge economic losses suffered in cases where integrated control strategies are not implemented (Simmons et al. 2010; Vreysen et al. 2010). For CM, in particular, the lack of competitiveness of laboratory moths when compared to their wild counterparts in springtime has been a major setback (Thistlewood and Judd 2003; Judd et al. 2004, 2006a,b). Emphasis on laboratory-reared moth maintenance and quality has therefore increased in an effort to improve the efficacy of SIR programmes (Calkins and Parker 2005).
Quality of a laboratory population incorporates aspects of biological, physiological and behavioural factors. This is critical for SIR success as released moths must respond to biotic cues under field conditions and behave accordingly if a beneficial interaction (i.e. mating) is to be achieved. Research focusing on improvement of mass-reared CM quality has ranged from the inclusion of diapause in rearing (Bloem et al. 1997; Judd et al. 2006a), lower dosage of gamma radiation (Bloem et al. 1999a,b, 2001; Judd and Gardiner 2006) and the combination of both (Bloem et al. 2004). Typically, CM rearing and maintenance in SIR programmes use only constant, optimal temperatures, probably to maximize rearing productivity (Bloem et al. 2004) regardless of the environmental conditions moths will be released into. This factor in particular may negatively impact the competitiveness of CM for SIR in the field. By contrast, a less well-established method for improving CM for SIR is thermal preconditioning. This idea probably originates from Fay and Meats (1987), who suggested thermal treatment prior to release may be a potential solution to poor low temperature performance in the Queensland fruit fly Bactrocera tryoni. However, to our knowledge, no studies have demonstrated the field efficacy of such an approach in any Lepidopteran or agricultural pests.
Physiologists have long hypothesized that acclimation to a particular environment enhances performance in that environment (Prosser 1986; Hochachka and Somero 2002; Angilletta 2009). However, acclimation responses are controversial for a number of reasons, of which two are probably of primary importance to the present study. First, the beneficial acclimation hypothesis (BAH), which posits that individuals acclimated to a particular environment perform better than those which have not been given the opportunity to acclimate, has been increasingly questioned and its importance debated (e.g. Leroi et al. 1994; Huey et al. 1999; Wilson and Franklin 2002; Chown and Terblanche 2007; Angilletta 2009). Second, the link between a particular acclimation response and evolutionary fitness in the wild is not well understood (reviewed in Chown and Terblanche 2007; Ghalambor et al. 2007; Angilletta 2009). Instead, it may be costly to acclimate in certain environments (Gilchrist and Huey 2001; Loeschcke and Hoffmann 2002) which could lead to tradeoffs in thermal performance under different thermal conditions (Angilletta et al. 2002; Kristensen et al. 2008). Recent demonstrations of the costs and benefits of thermal acclimation on field performance in insects suggest that such a method may have practical benefits to the SIR programmes for CM and other insect pests. Using release-recapture in Drosophila, for example, it has recently been shown that field performance is traded-off when flies are acclimated to varying thermal regimes (Kristensen et al. 2008). Specifically, cold-acclimated flies were recaptured more than control (nonacclimated) flies suggesting strong benefits for acclimation in the field. In addition, under warmer environmental conditions, warm-acclimated flies were recaptured in higher numbers than control or cold-acclimated flies (and see Loeschcke and Hoffmann 2007; Kristensen et al. 2007). This clearly indicates an important role for phenotypic plasticity in altering behaviour and field performance (Kristensen et al. 2008). Moreover, these results imply that if similar responses were widespread among other insect taxa it could have practical value in manipulating field performance with potential improved efficacy in an SIR programme.
Here, we report laboratory and field experiments investigating the thermal physiology of performance and activity of CM. Specifically, we test the hypothesis that C. pomonella have plastic thermal physiology which leads to tradeoffs in performance depending on their immediate thermal environment. Furthermore, we assess whether there are costs and benefits of manipulating thermal environments during CM rearing for field activity in a pest control programme. First, we test if upper and lower critical thermal limits (CTmax and CTmin, respectively) for activity are altered in response to adult or developmental rearing temperature. Second, we test if short-term (within-generation) changes in rearing temperatures (4–5°C above or below growth optima) during larval development significantly improves field performance of adult CM, scored as recapture rates at pheromone traps, under a range of ambient environmental temperatures (Ta). The implications of these results are discussed in the context of C. pomonella pest control programmes and plastic thermal responses.
Material and methods
Moth rearing conditions
The CM culture used for our experiments was first established in 2004. Eggs hatched and developed (from larvae to adult) on a diet described by (Guennelon et al. 1981) in black perspex boxes in the laboratory under 12:12 (L:D) photoperiod in air-conditioned, insulated rooms at 20 ± 1, 25 ± 1, or 30 ± 1°C. On emergence, adult moths were given access to 50% sugar/water solution from eclosion until they were used in thermal tolerance assays as 24–48 h old adults. Gender and irradiation were not taken into consideration during main experiments as preliminary assays comparing critical thermal limits between males and females, and between irradiated or nonirradiated moths showed no significant differences (P > 0.05 in all cases). Owing to logistic limitations – specifically, the transportation and time required to move the moths between the laboratory where the acclimations took place, the cobalt radiator where sterilization occurs and the field site where release work was undertaken – we did not include irradiation treatments because we were initially concerned that any induced acclimation effects may have worn off rather quickly in the adults and so our trials focused mainly on the release-recapture effects of temperature in the orchards rather than the impacts of irradiation. In pilot trials undertaken in the laboratory, we found that developmental thermal acclimation effects are similar for critical thermal limits when compared between irradiated and nonirradiated adult CM (acclimation × radiation effects: F2,114 = 0.866, P = 0.42). However, it is clear that some aspects of locomotor performance of irradiated moths are generally poorer than nonirradiated moths (e.g. Judd and Gardiner 2006), and the implications of irradiation for the field performance therefore require further validation.
Thermal acclimation effects on critical thermal limits
We used a degree-day model for CM (Pitcairn et al. 1992; Howell and Neven 2000) to predict the impact of the three treatments on expected time to eclosion. Thus, starting each treatment at a different time-point (coolest treatment first, warmest treatment last), we were able to synchronize the emergence times of the acclimation groups, although all acclimation groups treated the same age 5th instar larvae at initiation. Three groups of C. pomonella (n = 2000 per group) larvae were exposed to three different ambient temperatures 20 ± 1, 25 ± 1 and 30 ± 1°C for 6 days (developmental acclimation) before eclosion, upon eclosion transferred to 25°C, and all three groups had their critical thermal limits assayed thereafter. One group of adults that developed at 25 ± 1°C was partitioned into cages at 20 ± 1, 25 ± 1 and 30 ± 1°C for 6 days (adult acclimation) before their critical thermal limits were measured. All the cages in adult acclimation provided moths with access to 50% sugar: water solution.
For the determination of CTmin and CTmax, a programmable water bath was used for regulation of water/glycol solution (1:1) flowing through an insulated, double-jacketed series of chambers (‘organ pipes’) following previously established methods (e.g. Terblanche et al. 2008). Prior to CTmin and CTmax determination, moths were chilled at 5°C for 5 min to restrict movement and allow individual placement into the chambers. Moths located individually into the chambers were given 10 min to equilibrate at 25°C and then subjected to either controlled heating or cooling at a constant rate of 0.25°C/min to determine the upper or lower critical thermal limits to activity (CTmax and CTmin, respectively). This standard protocol was followed for all critical thermal limit experiments as they are known to be affected by start temperature and rate of temperature change (Terblanche et al. 2007; Chown et al. 2009). A copper-constantan (Type T, 36 SWG) thermocouple attached to a digital thermometer (accuracy 0.01°C, Fluke 54II; Fluke UK Ltd., Norwich, UK) was inserted into the control chamber to measure and verify chamber temperatures. During ramping protocols, body temperatures of 40–50 mg flies are in equilibrium with chamber temperatures (Terblanche et al. 2007), and we therefore assumed that thermal inertia effects are limited in these similar-sized insects. The CTmin and CTmax were defined as the temperatures at which the moths lost coordinated muscle function. Each individual moth was treated as a replicate, and critical thermal limit experiments were repeated until at least twenty individuals were used per acclimation or experimental treatment group for all CTmin and CTmax experiments.
Developmental thermal acclimation effects on activity of CM
Using moths developmentally acclimated as in the critical thermal limits experiments above, we assessed the effects of these acclimations on temperature-dependent activity as an attempt to link field and laboratory estimates of performance. A programmable waterbath was used to maintain constant test temperatures of 20, 25 and 30°C on a custom built thermal arena. Ten insects from a specific acclimation group were placed on marked spots on the thermal arena and given 1 h before activity was scored as total number of individuals that moved from their start location as a percentage of the total number of moths placed on the arena per trial. Five replications of 10 individuals per acclimation group were done for each test temperature (total n = 450). Surface temperature was verified using an infrared thermometer (accuracy 0.01°C, Fluke 63; Fluke UK Ltd., Norwich, UK) before and after each replicate of these experiments.
Thermal acclimation effects on field release-recapture rates
Developmental acclimation effects on performance of adult SIR moths was investigated using field release-recapture trials broadly following the methods outlined in (Kristensen et al. 2008) but with one distinct difference. Specifically, we used a sex pheromone trapping system compared to their food bait trapping method. Developmental acclimation in these trials differed from the earlier laboratory trials on critical thermal limits and activity, mainly in that moths were acclimated for their entire egg-larval duration owing to logistic constraints on climate chambers. Laboratory-reared CM eggs were held until the 5th instar as developmental acclimation at 20 ± 1, 25 ± 1 and 30 ± 1°C and then all groups were transferred to 25°C until adult eclosion. A staggered experimental protocol was used for developmental acclimation based on the day-degree model, as before (Pitcairn et al. 1992; Howell and Neven 2000) to ensure synchronization of eclosion of moths from different acclimation temperature regimes. Adult moths from all the acclimation groups were chilled at 5°C for 5 min before being marked by different fluorescent micronized dust (Day GLO Colour Corporation, Cleveland, OH, USA). Releases of the different acclimation groups were done simultaneously within 24–48 h of eclosion on a total of 11 occasions. Treatment colours (undertaken using the fluorescent powder) were randomized between acclimation groups on each experimental day to eliminate any influence of dye colours on recapture rates.
All the field release-recapture trials were carried out in a single Rosemarie cultivar apple orchard at Stellenbosch University Experimental Farm (33°56′ S, 18°52′ E). The orchard was planted in 1998 with a 4.5 × 1.25 m (inter-row x in-row) plant spacing. A total of eight yellow delta traps baited with a pheromone lure, CM1X Biolure® with E8-E10 dodecadienol (5.25 g/kg) (Chempac, Paarl, South Africa) as the active ingredient were used. The traps were hung in a rectangular pattern around a single central release point with three traps (15 m apart) in each external row and two traps in the middle row (30 m apart) with the single release point in the middle. All traps were hung at ∼1.8 m (Thwaite and Madsen 1983) oriented along the rows and secured to reduce wind-related movement. This ensured that moths were forced to cross rows, likely by flight, to reach traps.
On each field release day, adult moths from the three different developmental thermal acclimation regimes were taken simultaneously to the field in three large (10 L) containers stored within an insulated box. All moths were released at ground-level from the central release point simultaneously. All field releases took place within 0.5 h of transportation from the laboratory to the field at 12:00 h on any given release. Each release therefore involved three groups of moths (n > 700–1000 for each thermal acclimation group). Releases were replicated at least five times until at least three successful release-recapture trials had taken place at low, intermediate and high temperatures (thus, the total number of released moths over the entire study was n ≥ 30 180). Weather forecasts (South Africa National Weather Service, http://www.weathersa.co.za) were used to ensure that moths were not released on days with extremely hot (>38°C predicted daily maximum) or rainy (>5 mm predicted) weather conditions which are known to influence trap catches (Pitcairn et al. 1990). Environmental microclimate temperatures were recorded at 30-min intervals using three Thermochron iButtons (0.5°C accuracy) (Model DS1920; Dallas Semiconductors, Maxim Integrated Products, Inc., Sunnyvale, CA, USA) located on the ground, mid tree height and upper canopy of the tree during the course of each release-recapture experiment. The traps were then monitored once every day for 3 days. On each day, Sticky Pads (Chempac) were replaced and returned to the laboratory. Scoring recapture numbers was done on the retrieved Sticky Pads with daily CM catches using a UV light in a dark room. We waited several days (typically 4–5× longer than time to starve or desiccate to death in CM) at the end of a release trial before undertaking another trial to ensure that moths caught in different releases were temporally independent.
Statistical analyses
The effects of either developmental or adult acclimation on upper or lower critical thermal limits were compared using separate One-Way ANOVAs in STATISTICA 9 (Statsoft, Tulsa, OK, USA). The dependent variable was either CTmin or CTmax, while the categorical effect of acclimation temperature (either adult or developmental) was the independent variable in these analyses. Key assumptions of ANOVA were checked and were met for homogeneity of variance and normality of data distributions. Tukey's HSD post hoc tests were used to identify statistically homogenous groups. Locomotor activity determined in adult CM in the laboratory was compared between different acclimation groups using a generalized linear model (GLZ), assuming a Poisson distribution and an identity link function, in SAS 9.1 (SAS Institute Inc., Cary, NC, USA) and correcting for overdispersion. Here, proportion of active moths relative to total moths tested was the dependent variable, while acclimation temperature and test temperature were included in the model as categorical effects.
Field data for laboratory-reared moth recapture rates were regarded as count data and analysed using GLZ which is less sensitive to homogeneity of variance and normality assumptions, as in e.g., ANOVA. Here, using a GLZ and assuming a Poisson distribution and an identity link function in SAS statistical software (Proc Genmod), with corrections for overdispersion, we investigated the effect of acclimation temperature (as a categorical variable) on recapture ratios (dependent variable) at varying average field temperatures (as a continuous variable). The Wald χ2 statistic was used to test for significant differences between acclimation groups. These GLZ analyses were run using absolute moth abundance (summed across all traps on a given day) per acclimation group relative to the total moths released per acclimation group and separately also repeated for proportion of moths recaptured in field trials relative to the intermediate (25°C) acclimation group. Thus, in the latter analysis, the intermediate (25°C) group acted as a control for variation in weather or cohort effects among release days and allowed for more standardized comparisons of temperature effects among release days. The rationale for including an intermediate group as a control is discussed in detail in previous studies (e.g. Huey et al. 1999; Sinclair and Chown 2005; Terblanche and Chown 2006; Kristensen et al. 2008) and specifically accounts for factors such as ageing, handling stress, or cohort effects between trials, and is also commonly employed in other areas of biological statistics (see Quinn and Keough 2002).
Results
Laboratory assays of critical thermal limits and temperature dependence of activity
Both upper and lower critical thermal limits (CTmax and CTmin) to activity were affected by thermal acclimation occurring either in developing larvae or adult moths (P < 0.001 in all cases, Table 1, Fig. 1). Low temperature acclimation (20°C) reduced CTmin for both developmental and adult acclimation in CM. High temperature (30°C) acclimation increased CTmin and CTmax in adult CM. For CTmin in either developmental or adult acclimation experiments, all three acclimation groups were statistically heterogeneous, with the rank order 20 < 25 < 30°C (Fig. 1C,D). By contrast, effects of developmental acclimation on CTmax differed when compared with adult acclimation effects and adults appeared less responsive to thermal acclimation for this trait of high temperature tolerance. In the developmental acclimation experiment, 20°C acclimation resulted in lower CTmax compared with 25 and 30°C acclimation (Fig. 1A). In the adult acclimation experiment, however, CTmax for 20 and 25°C acclimation groups were statistically homogeneous with the 30°C-acclimated moths having a significantly higher CTmax (Fig. 1B). Most significantly, the developmental acclimation experiments showed that physiological changes in response to the thermal rearing environment carried over to the adult stage and persisted in the age-group of moths which would typically be used for release in SIR programmes.
Table 1.
Results of One-Way ANOVAs showing the effects of developmental and adult acclimation temperature (20, 25, 30°C) on upper and lower critical thermal limits of adult codling moth. Each ANOVA was run separately and assumptions of homogeneity of variance and data normality were checked and met in all cases. Tukey's HSD post hoc test was used to separate heterogeneous groups
| Effect | SS | d.f. | F | P |
|---|---|---|---|---|
| CTmax | ||||
| Developmental acclimation | 9.9 | 2, 57 | 19.2 | <0.001 |
| Adult acclimation | 30.52 | 2, 57 | 21.78 | <0.001 |
| CTmin | ||||
| Developmental acclimation | 39.6 | 2, 57 | 66.6 | <0.001 |
| Adult acclimation | 105.6 | 2, 57 | 140.1 | <0.001 |
Figure 1.
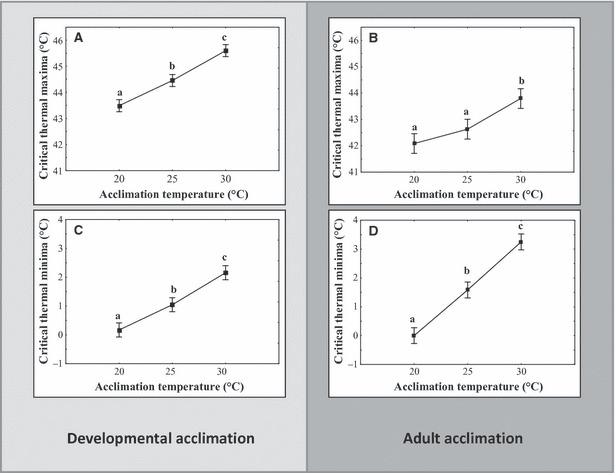
Effects of 6 days of developmental (A, C) or adult (B, D) acclimation at 20, 25 and 30°C on critical thermal limits of Cydia pomonella. Similar letters on each panel indicate statistically homogenous groups as determined by Tukeys’ HSD post hoc test (n = 20 in each group) (See Table 1 for statistics).
Laboratory assays of adult CM temperature-dependent activity showed a significant interaction between developmental acclimation and test temperature (GLZ Wald χ2 = 138.54, d.f. = 4, P < 0.0001; Fig. 2). However, the significant effect of acclimation (χ2 = 16.89, d.f. = 2, P = 0.0002) and non-significant effect test temperature (χ2 = 7.28, d.f. = 2, P = 0.262) were not significant when pooled across groups. Thus, a higher proportion of cold-acclimated (20°C) moths were active at 20°C test temperature than those acclimated at 25°C (intermediate) or 30°C (Fig. 2). However, at a 30°C test temperature, a higher proportion of 30°C-acclimated moths were active and thus, showed locomotor performance, than cold-acclimated moths.
Figure 2.
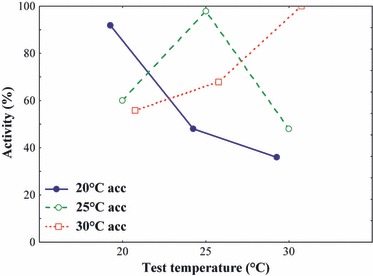
The proportion of active individuals in 1- to 2-day-old adult codling moths exposed to variation in developmental acclimation temperature (20, 25 and 30°C) at three test temperatures (20, 25 and 30°C) (n = 50 per acclimation and test temperature). Note that data are offset for clarity.
Field performance trials
The majority of moths that were recaptured were trapped within the first 24 h after release (>95%). On the second day, a maximum of eight moths were recaptured in one event with a median recapture of one moth across all trials and treatment groups. In consequence, no significant effects were detected between acclimation treatments in either the 2nd or 3rd day or both days pooled (P > 0.23 in all cases). By the third day after release, no moths were ever recaptured. Therefore, we only focused on data from the first day's recaptures for further analyses of temperature effects on moth field performance. Field release trials showed a significant interaction effect between acclimation temperature and field temperature (Table 2, Fig. 3A). Cold-acclimated moths were recaptured significantly more under cold conditions at sex pheromone traps, but not under warm conditions, and that warm-acclimated moths were recaptured in higher numbers under warm, but not cooler, conditions (Fig. 3A). Field release trials also showed significant effects of acclimation temperature on absolute recapture rates at different ambient temperatures in C. pomonella (Fig. 3A, Table 2). There is considerable variation in recapture rates among releases undertaken on different days. Hence, we also examined the possibility that this is a consequence of other varying abiotic factors, such as wind. Using a type I model least-squares regression, we examined the residual variance in the recapture rates using a range of other climate variables, most significantly, maximum and minimum wind speed, average wind speed, wind direction, and maximum and minimum daily temperature (note: all wind data were taken from a nearby orchard weather station). However, after mean temperature during the release has been accounted for, no other climate variables we examined were significant explanatory variables in our release-recapture data (P > 0.25 in all cases). It is therefore unclear what determines the variation in recapture rates among trial days, but may be related to cohort effects.
Table 2.
Results of generalized linear model (GLZ) analyses investigating the effects of developmental acclimation temperature (20, 25, 30°C; ‘Acclimation’) on the absolute numbers of recaptured moths and the proportion of recaptured moths relative to the intermediate control group (25°C acclimation temperature). Data for laboratory-reared moth recapture rates were regarded as count data and analysed using GLZ assuming a Poisson distribution and an identity link function in SAS statistical software (Proc Genmod), with corrections for overdispersion. In all cases investigated, the effect of acclimation temperature was the categorical variable and recapture ratios or absolute number captured as the dependent variable at varying average field temperatures as a continuous variable. The Wald χ2 was used to test for significant differences between acclimation groups. Significant effects are highlighted in bold font
| Effect | d.f. | Wald χ2 | P |
|---|---|---|---|
| Absolute moth recapture | |||
| Acclimation | 3 | 26.68 | <0.0001 |
| Field temperature | 1 | 23.04 | <0.0001 |
| Acclimation × field temperature | 2 | 15.13 | <0.0005 |
| Proportion moth recapture relative to control numbers | |||
| Acclimation | 3 | 63.23 | <0.0001 |
| Field temperature | 1 | 0.03 | 0.8663 |
| Acclimation × field temperature | 2 | 53.13 | <0.0001 |
Figure 3.
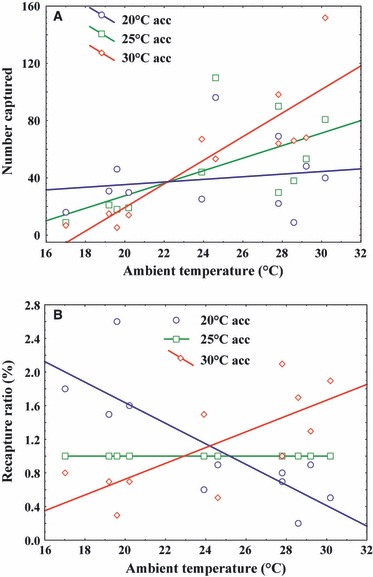
(A) Absolute and (B) relative capture success of laboratory-reared Cydia pomonella moths developmentally acclimated (at 20, 25 and 30°C) across a range of 24 h average field microclimate temperatures. (A) Acclimation temperature effects are shown for the absolute number of moths captured per acclimation group (20, 25 and 30°C) and for (B) proportion recapture success as a ratio of moths caught in the intermediate (control) group (25°C) to standardize effects across release days. Regression equations for lines of fit in plot of (A) are: 20°C: y = 1.749 ± 0.971x−25.679 ± 22.306 (R2 = 0.094, P = 0.083); 25°C: y = 3.505 ± 1.096x−62.950 ± 25.177 (R2 = 0.248, P = 0.003); 30°C: y = 5.423 ± 1.256x−103.616 ± 28.037 (R2 = 0.376, P = 0.0002) and those in plot of (B) are: 20°C y = −1.154 ± 0.717x−0.734 ± 0.226 (R2 = 0.539, P = 0.010); 30°C: y = 4.083 ± 0.704x−0.82 ± 0.191 (R2 = 0.673, P = 0.0002). All results presented are for moths captured in the first day after release.
However, to account for differences among releases, we repeated analyses adjusting for control 25°C moth recapture in each release trial. Here, using the proportion recaptured moths relative to intermediate (25°C) temperature group numbers, the effects of acclimation temperature were still prominent (Table 2, Fig. 3B). As expected, using these control-adjusted results, there are no significant effects of field average temperature as this has been standardized across releases (χ2 = 0.03, d.f. = 1, P = 0.8663). Regardless, the interaction between field average temperature and acclimation temperature is still highly significant for moth recapture rates (Table 2, Fig. 3B). As in the uncorrected results of moth recapture, this shows that warm-acclimated moths performed relatively well, and were recaptured in higher numbers, under warm conditions but poorly under cooler conditions. It also showed the opposite: cold-acclimated moths performed well in the field under cooler conditions but poorly under warmer conditions. Standardized for intermediate control group numbers, this translates to roughly a twofold difference between acclimated and non-acclimated moths in either warm or cold environmental conditions (Fig. 3B).
Discussion
These results show that rearing protocols can have dramatic effects on field performance under variable ambient temperatures in C. pomonella and that inducible plastic physiological responses are of direct relevance to CM control programmes. Indeed, poor recapture rates of mass-reared CM under low Ta have been attributed to rearing under constant optimal growth temperature (Thistlewood and Judd 2003; Judd et al. 2004, 2006b). Our results demonstrate that plastic physiological responses gained during development may improve the performance of adult CM in the field such that thermal acclimation can be undertaken during development and preconditioning need not be performed only in adults. This may prove useful for rearing protocols of insects like CM which, because ageing and starvation may compromise the fitness and thermal tolerance of insects, could result in reduced reproductive success (Emlen and Oring 1977; Bowler and Terblanche 2008). Moreover, from our laboratory assays, the magnitude of plastic responses induced during developmental acclimation appeared similar to adult acclimation effects (Fig. 1A vs B and C vs D), although adult CTmax is generally lower in older moths. In our field assays, warm- or cold-acclimated CM were recaptured almost twice as much in Ta similar to their thermal history. This may be critical in reducing SIR programme costs as fewer moths will be required to achieve the current efficacy levels. Simple calculations suggest a roughly twofold reduction in costs per hectare, or approximately double the efficacy of releasing thermally preconditioned CM. Alternatively, the same numbers could be released, possibly with increased efficacy because of increased successful mating events. Hence, SIR programmes could incorporate rearing of CM at both increased and reduced temperatures, or perhaps increased variability of temperature (e.g. Jallow and Judd 2007), to improve moth performance during releases undertaken on days with adverse thermal conditions. Further work is required, however, to strengthen these results for SIR, because, for example, we did not include irradiation treatments owing to logistic constraints. Nevertheless, it might be argued that even the inclusion of an irradiation treatment on thermally-acclimated CM does not convincingly prove the method's efficacy. For example, assessing trap recapture rates alone does not demonstrate improved SIR efficacy (even if undertaken on irradiated moths) and, instead, future work will need to demonstrate improved mating success with wild females, a reduction in wild moth populations, and decreased fruit damage. Regardless, the present results are an important demonstration of the feasibility of such an approach. Overall, it appears that thermal acclimation probably gives mass-reared CM a significant performance advantage to cope better under adverse field temperatures when released in temperatures similar to their thermal history, which will in turn allow the laboratory-reared CM to compete better with wild individuals immediately after release.
The present work can also be interpreted as providing support for the beneficial acclimation hypothesis (BAH, reviewed in Angilletta 2009; Chown and Terblanche 2007). Formally, the BAH has been defined as ‘…acclimation to a particular environment gives an organism a performance advantage in that environment…’ (Leroi et al. 1994). In light of this definition, the results of both the laboratory trials for critical thermal limits and temperature-dependent activity assays can be argued to provide support for the BAH because in all cases CM performed best in environments they were acclimated to previously, and worse in environments not previously experienced (and see Kristensen et al. 2008). For the field recapture rates, a similar conclusion can be reached from the distinct increases in recapture rates when animals were released in temperatures they had been exposed to during development, probably indicating increased flight or dispersal performance. However, this interpretation presumes that increased recapture rates is an indicator of improved performance, and vice versa, and that this may be a reasonable proxy for field fitness (and see Loeschcke and Hoffmann 2007 for discussion). This is probably a reasonable assumption given the other studies which have used similar methods and made similar assumptions for food bait traps (e.g. Kristensen et al. 2008) although verification of this assumption would be valuable. One potential weakness of this as a test of the BAH is that developmentally acclimated moths were tested as adults, as these two forms of plasticity may be argued to be fundamentally different (Wilson and Franklin 2002; Terblanche and Chown 2006; Kristensen et al. 2008; discussed in Chown and Terblanche 2007), especially if behavioural thermoregulation differs among stages and impacts acclimation responses (Marais and Chown 2008).
Unlike Kristensen et al. (2008), we assessed the effects of acclimation on CM thermal tolerance in the laboratory using a dynamic protocol (ramping temperatures as opposed to constant temperatures) and found that these results were in close agreement with the field release-recapture results. Specifically, cold-acclimated CM in our study had generally lower critical thermal limits, and vice versa for warm-acclimated moths. This suggests that dynamic protocols may be more ecologically relevant for assessing field thermal tolerance, especially if starting conditions and thermal ramping rates (heating or cooling) can be modified to match the thermal environments experienced by the insect (Terblanche et al. 2007; Chown et al. 2009; Mitchell and Hoffmann 2010; Nyamukondiwa and Terblanche 2010). Although our heating and cooling rates were not identical to those experienced in our field sites (approximately 3 times faster than natural rates) to maximize throughput of acclimation groups in our laboratory assays, the dynamic thermal tolerance protocols we used may still be more relevant to field performance than static (acute) thermal tolerance survival assays. The incongruence in results between Kristensen et al.'s (2008) laboratory assays of acclimation responses and our results for these experiments may therefore be attributed to their scoring of survival, rather than critical thermal limits, as a measure of thermal tolerance. This suggests that critical thermal limits may describe more closely the locomotor ability of the organisms to cope with diurnal changes in temperature and functional performance, and perhaps that CTL's are probably a better method for linking laboratory acclimation results with field performance (and see discussion in Chidawanyika and Terblanche in press).
The 5 min chilling at 5°C of moths for handling and sorting purposes, in conjunction with temperature variation during transportation to the field, had no effect on the benefits of acclimation, suggesting the key physiological changes acquired during development persist into the field. This may prove critical for SIR programmes which rely on transportation of moths at low temperatures (Bloem et al. 2006) to the release sites because acclimatory benefits gained take considerably longer to be lost. CM in the field may survive as adults for 2–4 weeks depending on the season (Thistlewood and Judd 2003; Tyson et al. 2008) and have re-mating capacity (Knight 2007). Therefore, longer duration of survival in adult moths probably has a direct impact on population dynamics through reproductive output, and increased survival and performance of laboratory-reared moths released into the wild can also have additional benefits to pest control programmes. Under field conditions, such ability to retain acclimatory benefits even in this laboratory-strain of CM, may help in improving CM competitiveness and longevity in the field after release, but will likely depend on the duration and intensity of novel temperatures encountered after thermal pretreatment (see discussions in Fay and Meats 1987; Jallow and Judd 2007; Nyamukondiwa and Terblanche 2010) and the impact of irradiation on performance decay over time (e.g. Judd and Gardiner 2006; and see discussions in Vreysen and Robinson 2010).
Codling moths were attracted using CM1X Biolure® with E8–E10 dodecadienol (5.25 g/kg) (Chempac) sex pheromone located within yellow delta traps (Chempac) containing Sticky Pads (Chempac) and thus probably only recaptured male moths. Despite the marked difference in lure and capture method (i.e. food vs sex pheromone) between our study and Kristensen et al.'s (2008) study, the overall results for costs and benefits of thermal acclimation on field performance remained similar. This demonstrates that an important component of SIR success, namely mating attraction, is retained under variable thermal conditions (Castrovillo and Carde 1979). In addition, time taken to locate calling females is probably an important aspect of competition between wild and mass-reared male CM (Thornhill and Alcock 1983; Andersson and Iwassa 1996; Judd et al. 2006a) and thus probably also represents an important aspect of field fitness. The results of the influence of thermal history on CM locomotor activity assays confirmed that Ta influences mobility in the laboratory. Indeed, Bloem et al. (2006) showed higher activity in moths at 25 and 20°C than 15°C in diapaused and nondiapaused CM which were reared in constant temperatures but kept under different durations of cold storage. Nevertheless, irrespective of specific differences in acclimation protocols and traits examined, our results clearly show that CM activity can be enhanced in both laboratory and field trials (Figs 2 and 3) if moths experience Ta identical to their thermal history relative to CM which have not had the opportunity to acclimate.
One important issue this work raises is the general lack of information on behavioural thermoregulation, and the use of microsites to optimize body temperatures (Tb), in adult CM in the wild. It is clear that under controlled laboratory conditions larvae and adults of CM have the ability to behaviourally thermoregulate and thereby maintain Tb within a fairly narrow range of preferred temperature (Kührt et al. 2006a,b). Moreover, many insect species can sense and respond to small increments in temperature (Chown and Terblanche 2007). CM also prefer to oviposit within a relatively narrow range of temperatures (Kührt et al. 2006b) and experience a wide range of thermal conditions in orchard microsites (Kührt et al. 2006c). However, little work to date has been undertaken for adults in the wild and it is generally poorly understood if such regulation allow moths to avoid temperature stress under natural conditions (Kührt et al. 2006b; and see discussions in Chidawanyika and Terblanche in press). Field observations showed that moths in our study clearly dispersed rapidly from the release point, using both short flights and walking, and that traps were located well within the expected daily dispersal distance of laboratory-reared moths. After 24 h, it was extremely difficult to observe released moths in the orchard, although traps captured moths relatively effectively, making the difficulties of microsite and thermoregulation work considerable. Nevertheless, this is an important area for future research as behavioural thermoregulation may partially or fully offset any potential benefits gained through laboratory acclimation. However, it will need to borne in mind that any adjustments in Tb largely dependent on microsite opportunities and operative environmental temperatures and that behavioural thermoregulation may impact on the form of acclimation responses (Stevenson 1985; Marais and Chown 2008).
In conclusion, this novel study shows plastic physiological responses of CM in response to thermal acclimation which clearly transfers to field performance. In addition, our results provide an important first demonstration that thermal acclimation during development could be a potential way to manipulate and enhance field performance of C. pomonella for pest control programmes, although further work is necessary to validate such an approach. Moreover, this study demonstrates clear costs and benefits of thermal performance depending on thermal conditions previously experienced. Finally, this study has shown the potential value and practical feasibility of thermal pretreatments for offsetting negative efficacy in the SIR programme for C. pomonella control under cooler, springtime conditions, though also under potentially adverse warmer conditions typical of some growing regions in peak summer. These results are of direct importance to CM and other pest control programmes and the evolution of thermal performance in terrestrial ectotherms.
Acknowledgments
We are grateful to Matthew Addison and Des Conlong for support and several valuable discussions and to staff at the Stellenbosch DFPT Sterile Insect Rearing Facility for providing C. pomonella. This research was supported financially by DFPT and a National Research Foundation THRIP award to Dr Pia Addison. Water baths and iButtons were purchased with funding support from Stellenbosch University's Sub-Committee B to J.S.T. We are especially grateful for constructive comments from Brent Sinclair, Jesper Sørensen, Gary Judd and several anonymous reviewers that helped improve the manuscript greatly.
Literature cited
- Andersson M, Iwassa Y. Sexual selection. Trends in Ecology and Evolution. 1996;11:53–58. doi: 10.1016/0169-5347(96)81042-1. [DOI] [PubMed] [Google Scholar]
- Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. New York: Oxford University Press; 2009. [Google Scholar]
- Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. Journal of Thermal Biology. 2002;27:249–268. [Google Scholar]
- Bloem S, Bloem KA, Fielding LS. Mass rearing and storing codling moth larvae in diapause: a novel approach to increase production for sterile insect release. Journal of the Entomological Society of British Columbia. 1997;94:75–81. [Google Scholar]
- Bloem S, Bloem KA, Carpenter JE, Calkins CO. Inherited sterility in codling moth (Lepidoptera: Tortricidae): effect of substerilising doses of radiation on insect fecundity, fertility, and control. Annals of the Entomological Society of America. 1999a;92:222–229. [Google Scholar]
- Bloem S, Bloem KA, Carpenter JE, Calkins CO. Inherited sterility in codling moth (Lepidoptera: Tortricidae): effect of substerilising doses of radiation on field competitiveness. Environmental Entomology. 1999b;28:669–674. [Google Scholar]
- Bloem S, Bloem KA, Carpenter JE, Calkins CO. Season-long releases of partially sterile males for control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), in Washington apples. Environmental Entomology. 2001;30:763–769. [Google Scholar]
- Bloem S, Carpenter JE, Bloem KA, Tomlin L, Taggart S. Effect of rearing strategy and gamma radiation on field competitiveness of mass-reared codling moths (Lepidoptera: Tortricidae) Journal of Economic Entomology. 2004;97:1891–1989. doi: 10.1603/0022-0493-97.6.1891. [DOI] [PubMed] [Google Scholar]
- Bloem S, Carpenter JE, Dorn S. Mobility of mass-reared diapaused and non diapaused Cydia pomonella (Lepidoptera: Tortricidae): effect of different constant temperatures and lengths of cold storage. Journal of Economic Entomology. 2006;99:707–713. doi: 10.1603/0022-0493-99.3.707. [DOI] [PubMed] [Google Scholar]
- Bowler K, Terblanche JS. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biological Reviews. 2008;83:339–355. doi: 10.1111/j.1469-185x.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- Calkins CO, Parker AG. Sterile insect quality. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht: Springer; 2005. pp. 269–296. [Google Scholar]
- Castrovillo PJ, Carde RT. Environmental regulation of female calling and male response periodicities in the codling moth (Laspeyresia pomonella) Journal of Insect Physiology. 1979;25:659–667. [Google Scholar]
- Chidawanyika F, Terblanche JS. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae) Journal of Insect Physiology. doi: 10.1016/j.jinsphys.2010.09.013. doi: 10.1016/j.jinsphys.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Chown SL, Terblanche JS. Physiological diversity in insects: ecological and evolutionary contexts. Advances in Insect Physiology. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown SL, Jumbam KR, Sørensen JG, Terblanche JS. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depends on methodological context. Functional Ecology. 2009;23:133–140. [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Enserink M. Welcome to Ethiopia's fly factory. Science. 2007;317:310–313. doi: 10.1126/science.317.5836.310. [DOI] [PubMed] [Google Scholar]
- Fay HAC, Meats A. The sterile insect release method and the importance of thermal conditioning before release: field-cage experiments with Dacus tryoni in spring weather. Australian Journal of Zoology. 1987;35:197–204. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gilchrist GW, Huey RB. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution. 2001;55:209–214. doi: 10.1111/j.0014-3820.2001.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Guennelon G, Audemard H, Fremond JC, Idrissi Ammari MA. Progrès réalisés dans l’élevage permanent du carpocapse (Laspeyresia pomonella L.) sur milieu artificiel. Agronomie. 1981;1:59–64. [Google Scholar]
- Hochachka PW, Somero GN. Biochemical Adaptation: Mechanisms and Process in Physiological Evolution. New York: Oxford University Press; 2002. [Google Scholar]
- Howell JF, Neven LG. Physiological development time and zero development temperature of codling moth (Lepidoptera: Tortricidae) Environmental Entomology. 2000;29:766–772. [Google Scholar]
- Huey RB, Berrigan D, Gilchrist GW, Herron JC. Testing the adaptive significance of acclimation: a strong inference approach. American Zoologist. 1999;39:323–336. [Google Scholar]
- Jallow MFA, Judd GJR. Effect of rearing strategy and handling on the quality of mass-reared codling moths Cydia pomonella (Lepidoptera: Tortricidae) Global IOBC Bulletin. 2007;3:73–76. [Google Scholar]
- Judd GRJ, Gardiner MGT. Temperature, irradiation and delivery as factors affecting spring-time flight activity and recapture of mass-reared male codling moths released by the Okanagan–Kootenay sterile insect programme. Journal of the Entomological Society of British Columbia. 2006;103:19–32. [Google Scholar]
- Judd GJR, Gardiner MGT, Thistlewood HMA. Seasonal variation in recapture of mass-reared sterile codling moth Cydia pomonella (Lepidoptera: Tortricidae): implications for control by sterile insect technique in British Columbia. Journal of the Entomological Society of British Columbia. 2004;101:29–43. [Google Scholar]
- Judd GJR, Thistlewood HMA, Gardiner MGT, Lannard BL. Is lack of mating competitiveness in spring linked to mating asynchrony between wild and mass-reared codling moths from an operational insect programme? Entomologia Experimentalis et Applicata. 2006a;120:113–124. [Google Scholar]
- Judd GJR, Cockburn S, Eby C, Gardiner MGT, Wood S. Diapause improves mating competitiveness of male codling moth mass-reared for a sterile insect programme. Entomologia Experimentalis et Applicata. 2006b;120:161–166. [Google Scholar]
- Knight AL. Multiple mating of male and female codling moth (Lepidoptera: Tortricidae) in apple orchards treated with sex pheromone. Environmental Entomology. 2007;36:157–164. doi: 10.1603/0046-225x(2007)36[157:mmomaf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kristensen TN, Loeschcke V, Hoffmann AA. Can artificially selected phenotypes influence a component of field fitness? Thermal selection and fly performance under thermal extremes. Proceedings of the Royal Society of London B. 2007;274:771–778. doi: 10.1098/rspb.2006.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. Costs and benefits of cold acclimation in field-released Drosophila. Proceedings of the National Academy of Science USA. 2008;105:216–221. doi: 10.1073/pnas.0708074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kührt U, Samietz J, Dorn S. Thermoregulation behaviour in codling moth larvae. Physiological Entomology. 2006a;30:54–61. [Google Scholar]
- Kührt U, Samietz J, Dorn S. Thermal response in adult codling moth. Physiological Entomology. 2006b;31:80–88. [Google Scholar]
- Kührt U, Samietz J, Dorn S. Effect of plant architecture and hail nets on temperature of codling moth habitats in apple orchards. Entomologia Experimentalis et Applicata. 2006c;118:245–259. [Google Scholar]
- Leroi AM, Bennett AF, Lenski RE. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proceedings of the National Academy of Science USA. 1994;105:216–221. doi: 10.1073/pnas.91.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke V, Hoffmann AA. The detrimental acclimation hypothesis. Trends in Ecology and Evolution. 2002;17:407–408. [Google Scholar]
- Loeschcke V, Hoffmann AA. Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. The American Naturalist. 2007;169:175–183. doi: 10.1086/510632. [DOI] [PubMed] [Google Scholar]
- Marais E, Chown SL. Beneficial acclimation and the Bogert effect. Ecology Letters. 2008;11:1027–1036. doi: 10.1111/j.1461-0248.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Hoffmann AA. An ecologically relevant measure of knockdown resistance with low evolvability and upper thermal limits in Drosophila. Functional Ecology. 2010;24:694–700. [Google Scholar]
- Nyamukondiwa C, Terblanche JS. Within-generation variation of critical thermal limits in adult Mediterranean and Natal fruit flies Ceratitis capitata and Ceratitis rosa: thermal history affects short term response to temperature. Physiological Entomology. 2010;35:255–264. [Google Scholar]
- Pitcairn MJ, Zalom FG, Bentley WJ. Weather factors influencing capture of Cydia pomonella (Lepidoptera: Tortricidae) in pheromone traps during overwintering flight in California. Environmental Entomology. 1990;19:1253–1258. [Google Scholar]
- Pitcairn MJ, Zalom FG, Rice R. Degree-day forecasting of generation time of Cydia pomonella (Lepidoptera: Tortricidae) populations in California. Environmental Entomology. 1992;21:441–446. [Google Scholar]
- Prosser CL. Adaptational Biology: Molecules to Organisms. New York: Wiley; 1986. [Google Scholar]
- Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Simmons GS, Suckling DM, Carpenter JE, Addison MF, Dyck VA, Vreysen MJB. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. Journal of Applied Entomology. 2010;134:261–273. [Google Scholar]
- Sinclair BJ, Chown SL. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. Journal of Experimental Biology. 2005;208:869–879. doi: 10.1242/jeb.01455. [DOI] [PubMed] [Google Scholar]
- Stevenson RD. The relative importance of behavioural and physiological adjustments controlling body temperatures in terrestrial ectotherms. The American Naturalist. 1985;126:362–386. [Google Scholar]
- Terblanche JS, Chown SL. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae) Journal of Experimental Biology. 2006;209:1064–1073. doi: 10.1242/jeb.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Chown SL. Factory flies are not equal to wild flies. Science. 2007;317:1678. doi: 10.1126/science.317.5845.1678b. [DOI] [PubMed] [Google Scholar]
- Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. Critical thermal limits depend on methodological context. Proceedings of the Royal Society of London B. 2007;274:2935–2942. doi: 10.1098/rspb.2007.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Clusella-Trullas S, Deere JA, Chown SL. Thermal tolerance in a south-east African population of the tsetse fly Glossina pallidipes (Diptera, Glossinidae): implications for forecasting climate-change impacts. Journal of Insect Physiology. 2008;54:114–127. doi: 10.1016/j.jinsphys.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Thistlewood HMA, Judd GJR. Area-wide management of codling moth, Cydia pomonella at very low densities. IOBC WPRS Bulletin. 2003;26:103–109. [Google Scholar]
- Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard University Press; 1983. [Google Scholar]
- Thwaite WG, Madsen HF. The influence of trap density, trap height, outside traps and trap design on Cydia pomonella (L.) captures with sex pheromone traps in New South Wales apple orchards. Australian Journal of Entomology. 1983;22:97–99. [Google Scholar]
- Tyson R, Newton KD, Thistlewood H, Judd G. Mating rates between sterile and wild codling moths (Cydia pomonella) in springtime: a simulation study. Journal of Theoretical Biology. 2008;254:319–330. doi: 10.1016/j.jtbi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Vreysen MJB, Robinson AS. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. Agronomy for Sustainable Development. 2010 doi: 10.1051/agro/2010009. [Google Scholar]
- Vreysen MJB, Carpenter JE, Marec F. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaues) (Lepidoptera Tortricidae) to facilitate expansion of field application. Journal of Applied Entomology. 2010;134:165–181. [Google Scholar]
- Wilson RS, Franklin CE. Testing the beneficial acclimation hypothesis. Trends in Ecology and Evolution. 2002;17:66–70. [Google Scholar]


