Abstract
Introduced species and infectious diseases both independently pose challenges for the preservation of existing biodiversity. However, native species or disease hosts are by no means ‘unarmed’ when faced with novel environmental challenges, provided that adequate adaptive genetic variation exists to mount effective evolutionary responses. In this study, we examined the consequences of the recently introduced parasite and causative agent of whirling disease (Myxobolus cerebralis) in a wild rainbow trout (Oncorhynchus mykiss) population from Harrison Lake, Montana (USA). Consistent with the parasite’s age-specific effects, juvenile rainbow trout recruitment into Harrison Lake was substantially reduced following parasite detection in 1995. However, experimental data suggest that natural selection has rapidly reduced whirling disease susceptibility within the population over time. The rapid observed temporal change in resistance patterns argues that the standing genetic variation for parasite resistance facilitated this process. Our findings ultimately underscore the importance of preserving genetic diversity to ensure that species of economic importance or of conservation concern have maximal chances for persistence in future changing environments.
Keywords: invasive species, Myxobolus cerebralis, natural selection, parasite, resistance, salmonid, whirling disease
Introduction
Human activities have directly and indirectly facilitated the spread of both nonnative species (Mack et al. 2000) and infectious diseases (Daszak et al. 2000). The biological ramifications of this process are well established: introduced species or diseases can pose serious threats to taxa of economic importance or of conservation concern once established in new geographical regions or hosts. Historically, most research has focused on understanding the primary factors that facilitate nonnative invasions (Lee 2003) or pathogen host switches (Dobson and Foufopoulos 2001; Antia et al. 2003). In contrast, syntheses of the evolutionary responses of established species to selective pressures imposed by introduced species or emergent disease are only recently being developed (Altizer et al. 2003; Strauss et al. 2006), with few cases documenting the evolutionary responses of species to the combined challenges posed by an introduced disease.
The introduced parasite and causative agent of salmonid whirling disease (Myxobolus cerebralis Hofer, 1903) is an excellent example of a recently introduced disease in the western USA. Whirling disease originates from Europe, where it was considered to be a mild parasite of native brown trout (Salmo trutta Linnaeus, 1758) that spread to rainbow trout (Oncorhynchus mykiss Walbaum, 1792) introduced from the USA in the 1900s to supplement aquaculture industries (Hoffman 1990). Trans-Atlantic fish transport facilitated parasite introduction to the eastern USA in the 1950s, where it subsequently spread throughout the country over the next few decades (Bartholomew and Reno 2002). Following its establishment in western North America during the 1970s and 1980s, the parasite has had substantial negative impacts on wild and managed salmonid fisheries, particularly in the Intermountain West region (Nehring and Walker 1996; Vincent 1996). The parasite can infect diverse salmonid species with varying levels of severity (Hedrick et al. 1999), raising concerns over protection and management of native species and posing substantial challenges to commercial hatchery operations (House 2006).
The clinical signs of whirling disease primarily reflect the progressive degradation of fish cartilaginous tissues by parasite spores (El-Matbouli et al. 1995), which results in myriad cranial and skeletal deformities and the characteristic ‘whirling’ swimming behavior observed in heavily infected individuals (see http://whirlingdisease.montana.edu/movies/default.htm for short films documenting whirling behavior). Cartilaginous tissues are abundant in juveniles, but are reduced via ossification as fish age. The ramifications of parasite infection are therefore much more pronounced in juveniles relative to adults (Ryce et al. 2004, 2005), although adults can also be infected with minimal consequences and act as carriers for parasite spores. Combined, these factors have lead to the recognition that reductions in juvenile recruitment, coupled with positive detection of M. cerebralis, are the hallmark of whirling disease-impacted fisheries (Nehring and Walker 1996).
Whirling disease was first detected in a wild, self-sustaining rainbow trout population from Harrison Lake, Montana, USA, in 1995 (E.R. Vincent, pers. comm.). Although rainbow trout are considered to be among the most susceptible salmonid species (Hedrick et al. 1999; Vincent 2002), experimental parasite exposure studies have illustrated that the Harrison Lake population is more resistant to whirling disease than other rainbow trout strains (Vincent 2002; Wagner et al. 2006). Rainbow trout are a relatively long-lived species, with adults 10+ years of age often encountered in annual spawning traps at Harrison Lake. Consequently, the extant age-structured population at Harrison Lake contains a nearly complete record of the history of parasite exposure, and further provides unique opportunities to investigate temporal processes that have occurred following parasite establishment.
In this investigation, given that whirling disease primarily affects juvenile fish, we report changes in juvenile rainbow trout recruitment patterns in Harrison Lake following parasite establishment. Furthermore, because experimental parasite exposure studies designed to compare disease susceptibility of strains or family groups are most commonly performed using juvenile fish (Hedrick et al. 1999; Schisler et al. 2006; Wagner et al. 2006), we likewise characterized the extent of tissue damage observed in parasite-exposed progeny (n = 629) of 11 pairs of adult fish ranging from 2 years to upwards of 11 years of age to better understand the changes in resistance patterns within the population over time.
Materials and methods
Quantifying recruitment
Juvenile recruitment in the Harrison Lake population has been monitored every year since 1981 by personnel from Montana Fish, Wildlife, and Parks. Annual spawning events at Harrison Lake occur in Willow Creek (a permanent stream inlet to the lake). A ‘spawning trap’ located at the Willow Creek inlet facilitated direct enumeration of all reproductive adult individuals each year. When in operation, the spawning trap is a complete barrier to all upstream movement of spawning rainbow trout. The trap was placed into the stream prior to any rainbow trout spawning activity (late February to early March) and was operated for the entire spawning period during each year of this study. All fish attempting to enter the stream inlet were diverted by the trap into a self-loading live cage where they were individually tagged, weighed, and measured prior to being released upstream. In our investigation, we used the number of 2-year-old male fish returning to spawning grounds each year as a surrogate value quantifying recruitment success and juvenile survival from the previous generation. Females were not counted, as they generally do not reach sexual maturity or spawn until 3 years of age.
Parasite exposure experiments
Eleven male and 11 female rainbow trout adults of different ages were randomly collected from spawning traps at Harrison Lake in April 2005 and initially matched based on size to establish parental pairs of relatively similar age. Parental ages (and birth years) were later established based on tag number records or by microscopic examination of replicate scale samples (Campbell and Arthur 1953). Sperm was stripped from each male and used to fertilize eggs from its pre-assigned partner. At 60 days posthatch (600 degree-days C), fry were exposed to 1000 mature M. cerebralis triactinomyxon spores per fish using the procedure outlined in Vincent (2002) and Wagner et al. (2006). With the exception of family groups 2, 3, 4, and 6 (Table 1), two replicate exposures were performed on split progeny samples from each parental pair. Fish were sacrificed 90 days (900 degree-days C) following infection and individually prepared for histological sectioning and analysis using the MacConnell–Baldwin scale (Hedrick et al. 1999; Baldwin et al. 2000). The MacConnell–Baldwin scale is used to assign tissue damage scores ranging from 0 (no tissue damage) to 5 (severe histological damage) and is among the most widely used procedures for characterizing severity of whirling disease infections (see Baldwin et al. 2000; Hedrick et al. 1999 for detailed descriptions of scoring categories). Histological scores from the MacConnell–Baldwin scale have been independently shown to be positively correlated with M. cerebralis spore loads in rainbow trout (Schisler et al. 2006; Wagner et al. 2006). All histological analyses were performed blindly on a contractual basis at the Washington Animal Disease Diagnostic Laboratory at Washington State University. A summary of parental ages and numbers of progeny examined from each family group are provided in Table 1.
Table 1.
Birth years of male and female parents and numbers of progeny examined for whirling disease damage from each family group.
| Family number | Male birth year | Female birth year | Midparental birth year | Number of progeny examined |
|---|---|---|---|---|
| 1 | 1999 | 1995 | 1997 | 72 |
| 2 | 2003 | 2002 | 2002.5 | 17 |
| 3 | 2002 | 2002 | 2002 | 17 |
| 4 | 2001 | 2001 | 2001 | 35 |
| 5 | 2000 | 2001 | 2000.5 | 98 |
| 6 | 1996 | 1997 | 1996.5 | 25 |
| 7 | 1998 | 1998 | 1998 | 76 |
| 8 | 1997 | 1995 | 1996 | 57 |
| 9 | 2003 | 2002 | 2002.5 | 82 |
| 10 | 2002 | 2002 | 2002 | 74 |
| 11 | 1997 | 1994 | 1995.5 | 76 |
Data were analyzed via linear regression where male parent age, female parent age, and midparental ages (expressed in terms of birth year) were used as the independent variable and average histology scores from each family group were used as the response variable. Exact tests for homogeneity of histology score frequencies (using the computer program RxC; http://www.marksgeneticsoftware.net) found no significant differences between replicates from individual family groups (P > 0.05). Consequently, mean histology scores for all pooled progeny from each family group were used in regression analyses. For simplicity, we report correlation coefficients and P-values instead of regression equations in our results. In rainbow trout, infection severity is primarily a function of fish age and size at the time of infection (Wolf 1986; Markiw 1991; Ryce et al. 2005) where smaller, younger fish exhibit more severe clinical symptoms, have greater spore loads, and show more histological damage. Furthermore, female age (and size by extension) is also positively correlated with egg size and juvenile growth rates in rainbow trout (Pitman 1979). In our study, female parent ages were not significantly correlated with average progeny size at the time of exposure (r = 0.205, P = 0.545), nor was the small variation in mean pre-exposure body length of progeny from different families (range <1.4 cm) associated with average histological scores (r = 0.042, P = 0.905).
Results
Juvenile recruitment within the Harrison Lake population demonstrated a marked decline following Whiling disease establishment (Fig. 1). However, our use of parasite exposure experiments suggested that temporal changes in resistance patterns have occurred since that time (Fig. 2). In our investigation, progeny of fish from younger cohorts suffered less tissue damage (i.e. had lower histology scores) relative to progeny from older cohorts when exposed to the whirling disease parasite. Measures of progeny tissue damage showed significant negative associations with midparent birth years (r = −0.767, P <0.006), male parent birth years (r = −0.768, P < 0.006), and female parent birth years (r = −0.723, P = 0.012).
Figure 1.
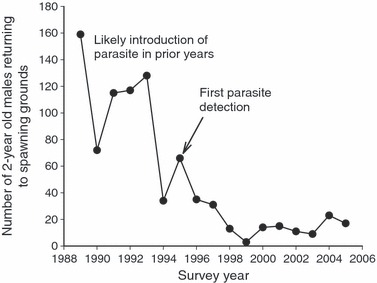
Number of 2-year-old rainbow trout males captured during annual spawning runs between 1989 and 2005.
Figure 2.
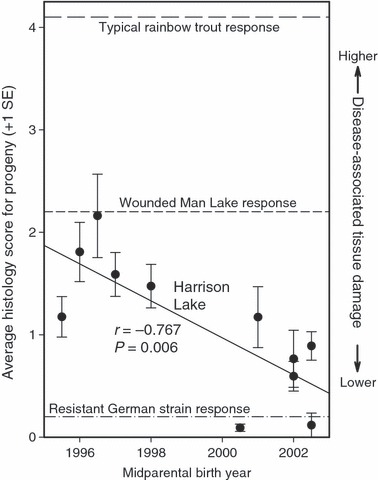
Average histology scores for Harrison Lake progeny groups as a function of midparental birth year. Histology scores of relevant comparison groups described in the Discussion (‘typical’ rainbow trout, a resistant German hatchery strain, and rainbow trout from Wounded Man Lake, Montana) are also indicated.
Discussion
Among exposure studies performed using methodologies similar to those used in this investigation (fish aged ∼600 degree-days C prior to exposure; 1000 tams/fish exposure concentrations), histology score values typically observed in rainbow trout are on the order of ∼4 on a scale from 0 to 5 (Vincent 2002; Wagner et al. 2006; DuBey et al. 2007). In this investigation, analyses revealed scores <2 in 10 of the 11 family groups analyzed (Fig. 2). These findings confirm the enhanced resistance of Harrison Lake individuals previously suggested by Vincent (2002) and Wagner et al. (2006).
Consistent with observed effects in other whirling disease-impacted rainbow trout populations (Nehring and Walker 1996; Vincent 1996), recruitment data suggest that whirling disease reduced juvenile survival in Harrison Lake (Fig. 1). Field studies conducted as early as 1996 revealed highly infected individuals within the population (Vincent 2002), and we suggest that multiple, nonexclusive processes may have influenced juvenile mortality rates following parasite establishment. For example, juvenile mortality may be directly caused by the disease, as experimental laboratory assays have shown that mortality of parasite-exposed fish can be higher than in nonexposed control individuals (Schisler et al. 2000). Furthermore, reduced swimming performance in infected individuals (Ryce et al. 2005; DuBey et al. 2007) may also increase mortality in the wild if food acquisition is impaired or if infected individuals are more likely to be preyed upon.
Our analyses indicate that Harrison Lake fish have become more resistant to whirling disease over time, as progeny of younger fish manifest less parasite-induced tissue damage relative to older fish from the population (Fig. 2). Although it has been proposed that phenomena such as parasite avoidance may minimize the effects of whirling disease in natural populations (Allendorf et al. 2001), our results instead suggest that Darwinian natural selection (i.e. differential survival and reproduction of individuals with different genetically determined phenotypes) is in the process of reducing parasite susceptibility within Harrison Lake. We suggest the following as a plausible scenario for processes that have occurred after parasite introduction. When the parasite was first introduced prior to 1995, large numbers of individuals were likely able to avoid infection, primarily due to low initial parasite levels within the population. Over time, progeny from each successive generation were exposed to increasing parasite loads, and consequently, greater juvenile mortality ensued (Fig. 1). Despite high mortality rates, some juveniles from each generation nonetheless survived to reach reproductive age and produce progeny in successive generations. Our experimental data indicate that surviving juveniles were likely selected based on their ability to resist parasites (Fig. 2). In most salmonid species, older (larger) fish tend to actively dominate annual spawns (Esteve 2005). Therefore, in the current age structured Harrison Lake population, older (more susceptible) individuals are producing more progeny than their younger (more resistant) counterparts. As the more resistant young from the population age and become dominant during annual spawning events, we predict that recruitment patterns may be restored within the population to become on par with levels observed prior to parasite establishment.
In addition to the Harrison Lake population, two additional rainbow trout populations are known to show resistance to whirling disease. The most highly resistant is a German hatchery strain that has been cultivated in the presence of the parasite for ∼100 years (Hedrick et al. 2003). Prior exposure studies of this population using experimental conditions similar to ours (but with even higher parasite doses; 2000 tams/fish) yielded typical lesion scores on the order of ∼0.2 (Schisler et al. 2006). A genetic basis for this strain’s resistance was demonstrated in exposure studies using F1 progeny from crosses with a common susceptible strain (Schisler et al. 2006). In these experiments, histology scores and spore loads of hybrid progeny were generally intermediate to comparable values obtained from progeny of intra-strain crosses. The second resistant population inhabits Wounded Man Lake, Montana (USA), which was established in 1981 from the same source (Lake DeSmet, Wyoming, USA) used to establish Harrison Lake in 1977 (Wagner et al. 2006; documentation available from E. R. Vincent). No instances of whirling disease were known in either Montana or Wyoming at the time of original population establishment (Bartholomew and Reno 2002). Although more resistant to whirling disease than most other rainbow trout populations (Wagner et al. 2006), the Wounded Man Lake population has no history of parasite exposure. Because Harrison Lake and Wounded Man Lake fish share recent common ancestry, the Wounded Man Lake population provides insights regarding resistance levels of the Harrison Lake population prior to parasite establishment. Our findings corroborate this notion, as histology scores for progeny of older Harrison Lake cohorts approximate values observed in Wounded Man Lake fish (Wagner et al. 2006; Fig. 2). Furthermore, given the moderate resistance observed in the related Wounded Man Lake population and rapid response observed within Harrison Lake, our results also indicate that standing genetic variation for resistance (as opposed to de novo mutations) facilitated this process. Our findings therefore reiterate a central tenet of conservation biology, that of preservation of both biodiversity and genetic variation within species, to ensure that both economically important taxa and wildlife species of conservation concern are adequately armed to face the challenge of changing environments.
The Harrison Lake population may represent an excellent ‘natural laboratory’ for furthering our understanding of the natural selection process, as the genealogy of the current population reflects the cumulative effect of over 13 years of parasite exposure. Future investigations including both the Harrison Lake population and the resistant German hatchery rainbow trout strain (Hedrick et al. 2003; Schisler et al. 2006) may provide excellent opportunities to elucidate the genetic basis of disease resistance in salmonids, leading to the development of new resistant hatchery strains and providing federal, state, and local agencies with new tools for fisheries management in western North America.
Acknowledgments
We thank Montana Fish, Wildlife and Parks personnel for fish trap operation and Elizabeth MacConnell for supplying triactinomyxons used in exposure experiments. Comments by R. Waples, E. Brodie Jr., M. Pfrender, and two anonymous reviewers greatly improved the content of this manuscript. Support for this project was provided by funding from the Whirling Disease Foundation and the Whirling Disease Initiative.
Literature cited
- Allendorf FW, Spruell P, Utter FM. Whirling disease and wild trout: Darwinian fisheries management. Fisheries. 2001;26:27–29. [Google Scholar]
- Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends in Ecology and Evolution. 2003;18:589–596. [Google Scholar]
- Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TJ, Vincent ER, Silflow RM, Stanek D. Myxobolus cerebralis infection in rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) exposed under natural stream conditions. Journal of Veterinary Diagnostic Investigation. 2000;12:312–321. doi: 10.1177/104063870001200403. [DOI] [PubMed] [Google Scholar]
- Bartholomew JL, Reno PW. The history and dissemination of Whirling disease. American Fisheries Society Symposium. 2002;29:3–24. [Google Scholar]
- Campbell RS, Arthur W. Impressions of fish scales in plastic. Journal of Wildlife Management. 1953;17:218–219. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBey RJ, Caldwell CA, Gould WR. Relative susceptibility and effects on performance of Rio Grande cutthroat trout and rainbow trout challenged with Myxobolous cerbralis. Transactions of the American Fisheries Society. 2007;136:1406–1414. [Google Scholar]
- El-Matbouli M, Hoffmann RW, Mandok C. Light and electron microscopic observations on the route of the triactinomyxon–sporoplasm of Myxobolus cerebralis from epidermis into the rainbow trout (Oncorhynchus mykiss) cartilage. Journal of Fish Biology. 1995;46:919–935. [Google Scholar]
- Esteve M. Observations of spawning behaviour in Salmoninae: Salmo, Oncorhynchus, and Salvelinus. Reviews in Fish Biology and Fisheries. 2005;15:1–21. [Google Scholar]
- Hedrick RP, McDowell TS, Mukkatira K, Georgiadis MP, MacConnell E. Susceptibility of selected inland salmonids to experimentally induced infections with Myxobolus cerebralis, the causative agent of whirling disease. Journal of Aquatic Animal Health. 1999;11:330–439. [Google Scholar]
- Hedrick RP, McDowell TS, Marty GD, Fosgate GT, Mukkatira K, Myklebust K, El-Matbouli M. Susceptibility of two strains of rainbow trout (one with suspected resistance to whirling disease) to Myxobolus cerebralis infection. Diseases of Aquatic Organisms. 2003;55:37–44. doi: 10.3354/dao055037. [DOI] [PubMed] [Google Scholar]
- Hoffman GL. Myxobolus cerebralis, a worldwide cause of salmonid whirling disease. Journal of Aquatic Animal Health. 1990;2:30–37. [Google Scholar]
- House D. Report confirms Utah fish sales gutted, The Salt Lake Tribune, October 18th 2006. Utah: Salt Lake City; 2006. [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends in Ecology and Evolution. 2003;17:386–391. [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. [Google Scholar]
- Markiw ME. Whirling disease: earliest susceptible age of rainbow trout to the triactinomyxid of Myxobolus cerebralis. Aquaculture. 1991;92:1–6. [Google Scholar]
- Nehring RB, Walker PG. Whirling disease in the wild: the new reality for the Intermountain West. Fisheries. 1996;21:28–30. [Google Scholar]
- Pitman RW. Effects of female age and egg size on growth and mortality in rainbow trout. Progressive Fish Culturist. 1979;41:202–204. [Google Scholar]
- Ryce EK, Zale AV, MacConnell E. Effects of fish age and parasite dose on the development of whirling disease in rainbow trout. Diseases of Aquatic Organisms. 2004;59:225–233. doi: 10.3354/dao059225. [DOI] [PubMed] [Google Scholar]
- Ryce EKN, Zale AV, MacConnell E, Nelson M. Effects of fish age versus size on the development of whirling disease in rainbow trout. Diseases of Aquatic Organisms. 2005;63:69–76. doi: 10.3354/dao063069. [DOI] [PubMed] [Google Scholar]
- Schisler GJ, Bergersen EP, Walker PG. Effects of multiple stressors on morbidity and mortality of fingerling rainbow trout infected with Myxobolus cerebralis. Transactions of the American Fisheries Society. 2000;129:859–865. [Google Scholar]
- Schisler GJ, Myklebust KA, Hedrick RP. Inheritance of Myxobolus cerebralis resistance among F1-generation crosses of whirling disease resistant and susceptible rainbow trout strains. Journal of Aquatic Animal Health. 2006;18:109–115. [Google Scholar]
- Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecology Letters. 2006;9:357–374. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Vincent ER. Whirling disease and wild trout: the Montana experience. Fisheries. 1996;21:32–33. [Google Scholar]
- Vincent ER. Relative susceptibility of various salmonids to whirling disease with emphasis on rainbow and cutthroat trout. American Fisheries Society Symposium. 2002;29:109–115. [Google Scholar]
- Wagner EJ, Wilson C, Arndt R, Goddard P, Miller M, Hodgson A, Vincent R, et al. Evaluation of disease resistance of the Fish Lake-DeSmet and Harrison Lake strains of rainbow trout exposed to Myxobolus cerebralis. Journal of Aquatic Animal Health. 2006;18:128–135. [Google Scholar]
- Wolf K. Salmonid whirling disease: status in the United States, 1985. Journal of Wildlife Diseases. 1986;22:295–299. doi: 10.7589/0090-3558-22.2.295. [DOI] [PubMed] [Google Scholar]


