Abstract
Large portions of anadromous salmonid habitat in the western United States has been lost because of dams and other blockages. This loss has the potential to affect salmonid evolution through natural selection if the loss is biased, affecting certain types of habitat differentially, and if phenotypic traits correlated with those habitat types are heritable. Habitat loss can also affect salmonid evolution indirectly, by reducing genetic variation and changing its distribution within and among populations. In this paper, we compare the characteristics of lost habitats with currently accessible habitats and review the heritability of traits which show correlations with habitat/environmental gradients. We find that although there is some regional variation, inaccessible habitats tend to be higher in elevation, wetter and both warmer in the summer and colder in the winter than habitats currently available to anadromous salmonids. We present several case studies that demonstrate either a change in phenotypic or life history expression or an apparent reduction in genetic variation associated with habitat blockages. These results suggest that loss of habitat will alter evolutionary trajectories in salmonid populations and Evolutionarily Significant Units. Changes in both selective regime and standing genetic diversity might affect the ability of these taxa to respond to subsequent environmental perturbations. Both natural and anthropogenic and should be considered seriously in developing management and conservation strategies.
Keywords: dams, differential habitat loss, evolutionary trajectory, genetic variation, Oncorhynchus
Introduction
Loss of habitat and its attendant consequences have been implicated as the largest threat to endangered species in the United States (Wilcove et al. 1998), and the loss of habitat is seen as the major cause of extinctions (Ehrlich and Ehrlich 1981; Wilson et al. 2003). World-wide habitat loss is enormous; up to one-half of the earth’s land surface has been transformed by human activity (Vitousek et al. 1997) and more than two-thirds of some ecosystems (Mediterranean and temperate forests and woodlands) have been converted to human uses (Millennium Ecosystem Assessment (MA) (2005).
Studies investigating the impact of lost habitat have usually focused on demographic or ecological effects including extinction risk (Fahrig 2001; Seabloom et al. 2002), species richness (Goodsell and Connell 2002; Helm et al. 2006), population trends (Browne and Hecnar 2007; Rannap et al. 2007) or range restriction (Benson and Chamberlain 2007). However, the loss of habitat can potentially affect the evolutionary trajectories of affected species in significant ways. First, a biased loss of particular habitat types might substantially alter the selective regime a species experiences. If a species shows local adaptation to particularly habitats, habitat loss will reduce the variety of traits displayed by a species; if the loss of primary habitat displaces a species into less favorable or previously unutilized habitats with novel selective regimes, survival will require new phenotypes to emerge and spread (Miller and Sadro 2003). When there is considerable phenotypic plasticity underlying trait expression, individual response patterns (‘norms of reaction’) themselves can adaptively evolve as the benefit of responses for some habitats are lost (Scheiner 1993, 2002, and references therein).
Secondly, even when habitat loss is not biased, the reduction in habitat area and changes in its distribution in space can affect the potential for future evolution by altering the level and distribution of genetic variation. A large reduction in carrying capacity will reduce the effective population size, which both enhances the effects of random genetic drift and limits the potential for adaptation to new conditions. The impact of lower population size will be particularly severe if migration among populations is also reduced as a result of blockages. This combination of habitat loss and fragmentation can reshape the dynamic balance between gene flow, genetic drift and natural selection.
Anadromous salmonids, which travel between freshwater and marine habitats, offer a prime example of how habitat loss can alter evolutionary trajectories. Pacific salmon and steelhead (Oncorhynchus spp.) have been excluded from large portions of historically accessible habitats in the western United States, either by passage barriers or by large-scale changes in habitat quality. In fact, nearly 45% of the area historically available to these fishes in the contiguous United States is now inaccessible (Fig. 1). This loss of habitat is clearly reflected in their current status – over half of the Pacific anadromous salmonid Evolutionarily Significant Units (ESUs) in the contiguous United States are listed as endangered or threatened under the Endangered Species Act (NMFS 1992). ESUs in this context are defined by two criteria: (i) they must be substantially reproductively isolated from other conspecific units, and (ii) they must represent an important component of the evolutionary legacy of the species. ESUs can be listed as ‘distinct population segments’ under the Endangered Species Act (Waples 1995). In addition, habitat loss might affect evolutionary trajectories because a number of salmonid life history traits, including spawn timing and run timing vary adaptively with environmental parameters such as elevation, temperature and hydrology (Quinn 2005; Beechie et al. 2006; Achord et al. 2007).
Figure 1.
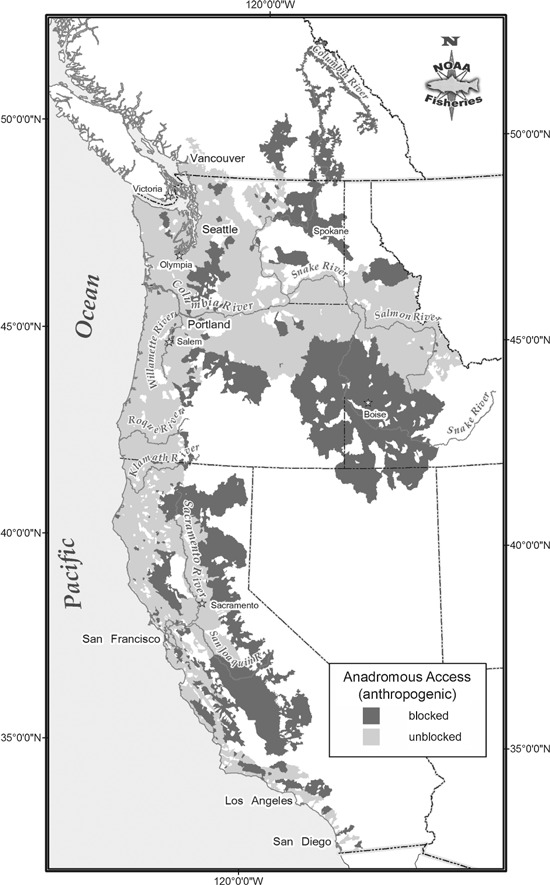
Area in the western United States (and portions of British Columbia, Canada in the Columbia River drainage) that were historically accessible to anadromous fishes. Area that is currently blocked by anthropogenic barriers is marked in dark gray.
The associations of salmonid life histories with environmental characteristics can result from either genetically-based adaptive evolution or individual phenotypic plasticity. In phenotypically plastic traits, environment-trait associations reflect immediate environmental responses rather than local genetic adaptation, and selective change might be buffered in complex ways (Sultan 2007). Accordingly, in this paper we focus on heritable traits likely to be strongly influenced by the altered selection pressures that result from biased patterns of habitat loss. Adaptive evolution, which tailors organisms to their local environment, is likely a strong driver of habitat-specific fitness-related traits (e.g., life history traits) and is the net effect of selection and inheritance. The so-called breeder’s equation: R = h2 × S, is a heuristic model for this process. The quantity S, is the selection differential or the change in the mean phenotypic value within a generation, which can also be expressed as the slope of a regression of relative fitness on the values of the character (Brodie et al. 1995). The term h2, called the narrow-sense heritability, is the proportion of the phenotypic variance in a trait that is due to the additive effect of genes (e.g., Roff 1997). The quantity R, the response to selection, is the net change in the mean phenotypic value across generations (Falconer and Mackay 1996). The breeder’s equation is useful for focusing attention on the two components of selective change – the selective regime and the level of genetic variation.
To date, studies have examined the demographic consequences of habitat losses to salmonids (e.g., loss of capacity), but the possible evolutionary consequences of these habitat losses have not been considered. In this paper, we first examine the potential for selective change of salmonids as a result of habitat loss. Specifically, we (i) quantify the loss of habitat in the western United States, comparing the environmental characteristics of accessible habitats and now inaccessible habitats to reveal changes in selective regime or selection differential; (ii) summarize what is known about the heritability of ecologically important traits and their associations with habitat characteristics; and (iii) present case studies that show how particular habitat losses can affect the response or evolution of salmonid populations through altered selective regimes. Finally, we discuss nonselective change that might be caused in salmonid populations by rendering large portions of habitat inaccessible and discuss implications for conservation biology in general.
Habitat loss and selective change for salmonids
Biased loss of habitats – a change in selective regime
Historically, anadromous salmonids utilized freshwater habitats in the western United States (excluding Alaska) from the coast inland to Montana and Nevada totaling nearly 633 000 km2. Large-scale blockages in this region have left only about 56% of that area (∼355 000 km2) accessible (Fig. 1). Dams constructed for irrigation and hydroelectric power generation are one of the largest culprits in blocking access for these fishes, but culverts and river engineering have also reduced the amount of habitat that anadromous fishes can use (Furniss et al. 1991; NRC 1996). Modification to currently accessible rivers and their surrounding landscapes has also changed environmental conditions in rivers, sometimes rendering them uninhabitable. For example, in the Grande Ronde basin, in the Snake River drainage, low-lying wide flood-plain habitats have been channelized and are currently much warmer than historically, precluding occupancy during many months of the year (McIntosh et al. 1994, 2000).
A qualitative perusal suggests that neither the loss of habitats nor the change in habitats appears to have been uniformly distributed across habitat types. Rather, lowlands have been drained, and estuaries channelized and diked. In addition, the upper reaches of larger rivers have tended to be blocked, potentially resulting in loss of access to particular kinds of habitat. For example, access to nearly 50% of the habitat previously occupied by Chinook salmon (Oncorhynchus tshawytscha) and steelhead (Oncorhynchus mykiss) in the Snake River drainage is now blocked (NRC 1996); these blocked areas in the upper reaches appear to be drier than other regions of the Snake River basin. In contrast, inaccessible regions in the Puget Sound, in the Willamette Valley, and other locations throughout Oregon, Washington, California and Idaho contain unique habitats, many of these in the lower portions of river basins. From 1870 to 1970, for example, an estimated 77% of the 10 500 ha of tidal swamps and 63% of the 6500 ha of tidal marshes around the mouth of the Columbia River were diked or filled (Sherwood et al. 1990), obliterating overwintering area for coho (Tschaplinski and Hartman 1983) and rearing habitat for chum, Chinook salmon, and sea-run cutthroat (Healey 1991; Salo 1991; Simenstad et al. 1992). Complete habitat characterizations are not available across the western USA, but environmental data allow us to compare and contrast general characteristics of accessible and inaccessible habitats.
Methods
We used a geographic information system (GIS) based analysis to determine characteristics of subwatersheds within river systems of the western United States (Washington, Idaho, Oregon, California, and Nevada) and contiguous portions of British Columbia, Canada that were historically accessible to anadromous salmonids. To define the overall geographic area and its sub-units, we merged existing GIS watershed features from three separate datasets (The California Interagency Watershed Map, California Interagency Watershed Mapping Committee and Department of Water Resources 2004; British Columbia Ministry of Environment 2005; National Marine Fisheries Service, NOAA Fisheries 2006) into a seamless layer with shared attributes.
We defined historically accessible areas by identifying areas with documented past presence of anadromous salmonids, current resident forms of anadromous salmonids, or predicted historical occupation estimated using landscape characteristics. These data and analyses have been developed by NOAA Fisheries Technical Recovery Teams (Interior Columbia Basin, Puget Sound, Lower Columbia Willamette, Oregon Coast, Southern Oregon and Northern California Coast, Central Valley, Central and Southern California Coast) and local agencies and recently compiled in a single database (NWFSC 2007). In California and portions of southern Oregon, we incorporated species-specific intrinsic potential analyses to identify previously occupied subwatersheds (Lindley et al. 2006b; Williams et al. 2006). Current distributions of resident species were used to represent historical conditions only in portions of British Columbia where no assessments of previous anadromy were available, and where native rainbow trout and kokanee (freshwater resident O. nerka) populations were clearly identified (BCME 2006). To compensate for anthropogenic habitat loss within these data, we compared current distributions to GIS features representing dams and reservoirs. If an anthropogenic feature was found at the limit of current distribution, we considered upstream watersheds as historically accessible. Historical accessibility was truncated at natural barriers, as compiled by federal, state, and local agency sources (ODFW 2004; CALFISH 2006; Northwest Fisheries Science Center 2007) and reaches at (200 m) with a stream gradient ≥20% (ICTRT 2007). In cases where GIS data had not been developed or was not provided, we geo-referenced hardcopy maps found within report documents.
Within the historically accessible areas, we defined areas that are anthropogenically blocked and currently inaccessible using blockage data compiled by technical recovery teams (Lindley et al. 2006b; Williams et al. 2006; NWFSC 2007). Because this project encompasses a large area and data availability was limited, this analysis focused on large blockages such as dams rather than smaller-scale blockages such as culverts. While dams do cause a number of other changes to the environment that can result in selection, we restrict this discussion to selection as a result of habitat losses.
Large watersheds in the USA have been divided hierarchically into subwatersheds based on hydrologic characteristics (USEPA/USGS 2005). For this analysis, we used subwatersheds delineated by 6th-field Hydrologic Unit Code (HUC-6) in the United States (CWMP/CDWR 2004; NOAA Fisheries 2006) and approximate equivalents for Canadian territory (Environment Canada 1994). We recorded habitat and climate characteristics for each historically accessible subwatershed, focusing on attributes associated with stream temperature, morphology and flow, as salmonid phenotypic traits are correlated with both these factors (see below). These attributes included mean stream elevation, mean January minimum air temperature, and mean July maximum air temperature, mean stream gradient and mean annual precipitation. In addition, we quantified level IV ecoregions (USEPA 2007) occurred within the boundaries of each subwatershed; we used these ecoregions as proxies for overall habitat characteristics as they identify areas of similar climate and landscape characteristics (Omernik 1987; USEPA 2007).
We also examined potential anthropogenic stresses to streams to identify habitats likely affected by changes in temperature, flow regime or other characteristics important for salmonid phenotypic trait expression (Gregory and Bisson 1997; McIntosh et al. 2000; Allen 2004). We used the USEPA Environmental Monitoring and Assessment Program monitoring data (USEPA 2005) as a gridded vector point layer with 3 km spacing and assigned each feature to its underlying sub-basin. Specific impacts included: the percent of stream length located adjacent to human-impacted land cover types (Anderson et al. 1976); nitrogen and phosphorous loads; road density; and, the percentage of land cover types within each hydrological unit that were developed for agricultural or urban uses.
We compared the characteristics of inaccessible and accessible habitats at two scales: (i) across the entire contiguous western United States; and (ii) within major geographic regions that each support separate ESUs: Puget Sound, Olympic Peninsula/Washington Coast, Lower Columbia/Willamette, Mid-Columbia, Upper Columbia, Snake River, Oregon Coast, Southern Oregon and Northern California Coast, Central Valley, Central and Southern California Coast. We used simple two-sample comparison tests of the subwatersheds within each area. We normalized some data sets by applying square root (elevation, gradient) and natural logarithm (precipitation, percent of human-impacted land cover types) transformations prior to applying two-sided t-tests. When the compared samples’ variances were unequal (Levene’s test) we applied Welch’s approximate t-test (Zar 1999). For those data sets for which no suitable normalizing transformation was found, we used the distribution-independent Wilcoxon (Mann–Whitney) rank sum test (Zar 1999). We applied the false discovery rate correction to the P-values to account for multiple comparisons (Benjamini and Hochberg 1995) and the global significance level, α, was set to 0.05.
Results
Across the western United States, we found significant differences between accessible and inaccessible areas in every metric (Table 1). Because these data were non-normally distributed, we plotted medians and 95% confidence intervals. As such the figures do not visually emphasize the statistical differences in the data. At this gross scale, areas that are currently inaccessible are significantly higher in elevation, are colder in the winter, warmer in the summer, and drier than those that continue to be accessible to anadromous salmonids. They also have somewhat lower gradient (Table 1, Fig. 2). This might be due in part to the exclusion of extremely high gradient (and thus unusable) habitat from the analysis. In addition, a number of EPA ecoregions present in inaccessible areas were not represented at all in available portions of large basins (Table 2). Finally, for every measure of human impact – nitrogen and phosphorus loading, road density, and % of anthropogenically altered land – blocked areas had a significantly lower value than areas that are currently accessible (Table 1, Fig. 2), meaning that previously used, but now inaccessible habitats are less affected by human activities and associated changes in flow, temperature and other characteristics.
Table 1.
Habitat characteristics for accessible and inaccessible areas in the western contiguous United States. The number of subwatersheds considered for each characteristic is denoted ‘n’.
| Accessible | Inaccessible | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Median | Min. | Max. | n | Median | Min. | Max. | n | Test | Test statistic | P-value |
| Elevation [Mean elevation (m) of reach segments]* | 425 | 0 | 2682 | 6541 | 994 | 0 | 3150 | 2060 | t | 40.09 | <0.001 |
| Gradient [Mean gradient or slope of reach segments]†,‡ | 0.06 | 0 | 0.55 | 4805 | 0.06 | 0 | 0.503 | 3878 | t | 3.22 | 0.001 |
| Precipitation [Mean annual precipitation (cm)]§,¶ | 106 | 17 | 489 | 8151 | 64 | 17 | 383 | 602 | t | 27.42 | <0.001 |
| Jan. min. temp. [Mean minimum January air temperature (°C)]§,¶ | −0.57 | −16.73 | 9.04 | 5247 | −4.98 | −16.75 | 7.23 | 3509 | w | 6966863 | <0.001 |
| July max. temp. [Mean maximum July air temperature (°C)]§,¶ | 26.69 | 13.85 | 37 | 5999 | 29.36 | 12.86 | 37.43 | 2754 | t | 25.30 | <0.001 |
| Percent human-impacted (Percent of land cover classified as human-developed)* | 0.02 | 0 | 0.99 | 2286 | 0.01 | 0 | 0.96 | 1150 | w | 7466189 | <0.001 |
| Nitrogen loading [Nitrogen export coefficient (kg/ha/year)]*,** | 2.14 | 0 | 6.43 | 4731 | 1.74 | 0 | 7.16 | 3745 | w | 11136858 | <0.001 |
| Phosphorus loading [Phosphorus export coefficient (kg/ha/year)]*,** | 0.22 | 0 | 1.62 | 4731 | 0.19 | 0 | 1.72 | 3745 | w | 11533153 | <0.001 |
| Road density (km × km−2)* | 1.05 | 0 | 13.24 | 4132 | 0.91 | 0 | 9.43 | 22 | w | 9561634 | <0.001 |
| Percent stream length (Percent of stream length adjacent to human land use)†† | 0.01 | 0 | 0.99 | 2033 | 0.005 | 0 | 0.95 | 1120 | w | 8066700 | <0.001 |
‡USGS (2005).
§NRC (2001).
¶SCAS (2003).
Figure 2.
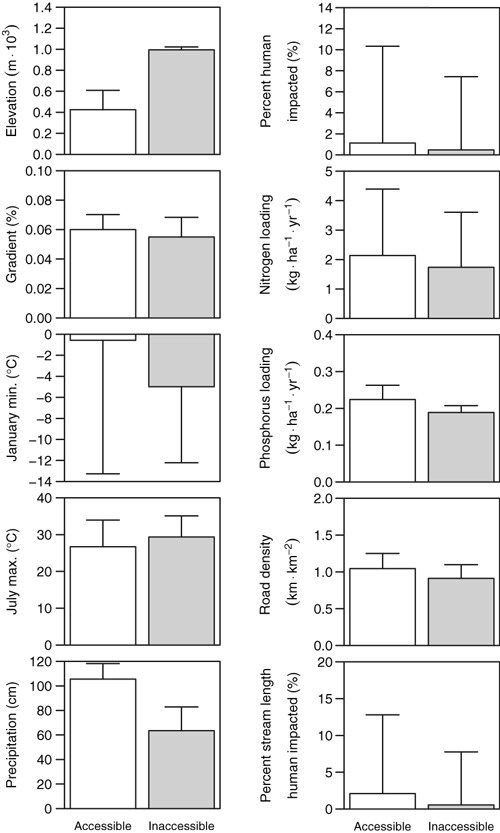
Physical characteristics and measures of anthropogenic impacts compared between basins of the West Coast (see Table 3) that are accessible (white rectangles) to anadromous salmonids with those that are no longer accessible (gray rectangles). All values shown are medians with error bars depicting 95% confidence intervals. Statistical tests were conducted for some characteristics on transformed variables and were significant for each of the comparisons shown (P < 0.05).
Table 2.
USEPA Level IV Ecoregions (Omernik 1987) accessible and inaccessible areas of three steelhead ESUs in the Pacific Northwest, and km2 of inaccessible habitat.
| Level III ecoregions | Accessible | Inaccessible | Km2 inaccessible |
|---|---|---|---|
| Middle Columbia Steelhead ESU | |||
| Cascade Crest Montane Forest | × | × | 320 |
| Cascade Subalpine/Alpine | × | ||
| Chiwaukum Hills and lowlands | × | ||
| Cold Basins | × | × | 75 |
| Continental Zone Highlands | × | ||
| Deep Loess Foothills | × | ||
| Deschutes River Valley | × | × | 2497 |
| Deschutes/John Day Canyons | × | ||
| Grand Fir Mixed Forest | × | × | 571 |
| High Lava Plains | × | 460 | |
| John Day/Clarno Highlands | × | × | 1958 |
| John Day/Clarno Uplands | × | × | 4776 |
| Loess Islands | × | ||
| Maritime-Influenced Zone | × | × | 256 |
| Melange | × | ||
| Mesic Forest Zone | × | ||
| North Cascades Highland Forests | × | × | 438 |
| North Cascades Subalpine/Alpine | × | × | 123 |
| Oak/Conifer Foothills | × | × | 350 |
| Pleistocene Lake Basins | × | × | 107 |
| Pluvial Lake Basins | × | 1058 | |
| Ponderosa Pine/Bitterbrush Woodland | × | × | 1276 |
| Pumice Plateau Forest | × | 216 | |
| Subalpine-Alpine Zone | × | ||
| Umatilla Dissected Uplands | × | × | 657 |
| Umatilla Plateau | × | × | 659 |
| Western Cascades Montane Highlands | × | ||
| Yakima Folds | × | ||
| Yakima Plateau and Slopes | × | ||
| Total (n) | 26 | 17 | 15 797 |
| Puget Sound Steelhead ESU* | |||
| Cascade Subalpine/Alpine | × | ||
| Central Puget Lowland | × | ||
| Eastern Puget Riverine Lowlands | × | ||
| Eastern Puget Uplands | × | ||
| Fraser Lowland | × | ||
| High Olympics | × | × | 382 |
| Low Olympics | × | × | 381 |
| North Cascades Highland Forests | × | × | 241 |
| North Cascades Lowland Forests | × | × | 935 |
| North Cascades Subalpine/Alpine | × | ||
| Olympic Rainshadow | × | ||
| Southern Puget Praries | × | ||
| Volcanics | × | × | 234 |
| Western Cascades Lowlands and Valleys | × | × | 1306 |
| Western Cascades Montane Highlands | × | 1446 | |
| Total (n) | 14 | 7 | 4925 |
| Upper Columbia Steelhead ESU* | |||
| Channeled Scablands | × | × | 2416 |
| Chelan Tephra Hills | × | ||
| Chiwaukum Hills and Lowlands | × | ||
| Granitic Selkirk Mountains | × | 165 | |
| Inland Maritime Foothills and Valleys | × | 59 | |
| Loess Islands | × | 3121 | |
| North Cascades Highland Forests | × | ||
| North Cascades Subalpine/Alpine | × | ||
| Northern Idaho Hills and Low Relief Mtns | × | 67 | |
| Okanogan-Colville Xeric Valleys and Foothills | × | 4725 | |
| Okanogan Drift Hills | × | × | 430 |
| Okanogan Highland Dry Forest | × | 2771 | |
| Okanogan Pine/Fir Hills | × | × | 684 |
| Okanogan Valley | × | × | 787 |
| Palouse Hills | × | 1333 | |
| Pasayten/Sawtooth Highlands | × | ||
| Pleistocene Lake Basins | × | × | 1185 |
| Spokane Valley Outwash Plains | × | 1155 | |
| Wenatchee/Chelan Highlands | × | ||
| Western Okanogan Semiarid Foothills | × | × | 693 |
| Western Selkirk Maritime Forest | × | 103 | |
| Yakima Folds | × | ||
| Total (n) | 13 | 15 | 19 694 |
| Grand total | 53 | 39 | 40 416 |
Excluding portions in Canada.
Interestingly, however, the results for the entire USA West coast are not uniform across all geographic regions (Fig. 3). (Complete details of these analyses for both the entire area and geographic regions are available online: see Supplementary material) The inaccessible areas of geographic regions that included large river systems (Sacramento, Skagit, Klamath and Columbia Rivers), were significantly different from accessible areas. Inaccessible areas in nearly all of these regions were all higher in elevation and cooler than accessible areas, although the inaccessible areas in the Snake River were warmer and not significantly different in elevation than currently accessible areas in the Snake. In addition, the accessible areas of more southerly and interior recovery domains (Snake River, Southern Oregon and Northern California Coast, Central Valley and Central and Southern California Coast) receive significantly more precipitation than the inaccessible regions, whereas the accessible regions of more northerly and coastal recovery domains tended to be significantly drier than the currently blocked areas (Fig. 3). These results suggest that the changes in selective regime and potential evolutionary impacts on affected salmonid populations might be region or ESU-specific.
Figure 3.
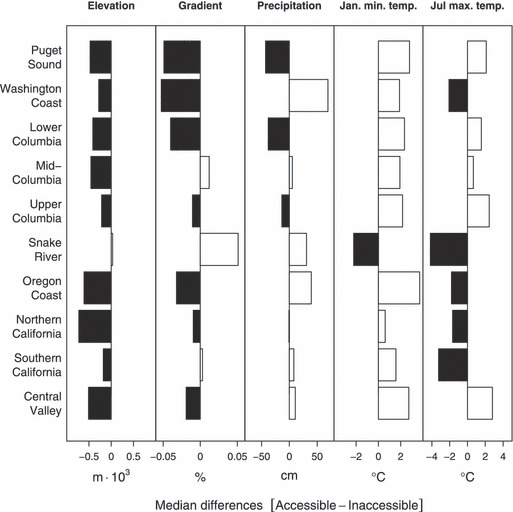
Median differences between basins that are accessible and inaccessible to anadromous salmonids for selected physical characteristics within 10 West Coast geographic regions. Black boxes indicate where inaccessible areas were greater and white boxes indicate areas where accessible areas were greater for a given characteristic.
Biological relevance
Many studies correlate environmental gradients with variation in salmonid life history and morphological traits, suggesting that diversity in these traits reflects adaptive evolutionary responses to local selective pressures (Brannon et al. 1981; Taylor 1990; Quinn and Buck 2001; Quinn 2005). With respect to our questions about potential evolutionary consequences for salmonids, many studies identify correlations between fitness-related traits and either stream temperatures (Brannon 1987; Beacham and Murray 1989; Unwin et al. 2000; Hodgson and Quinn 2002) or stream flows (Smith 1969; Beacham and Murray 1989; Quinn et al. 2001). For instance, median spawning date across populations of Chinook salmon from California to Alaska is positively related to average incubation temperatures (e.g., fig. 8-1, Quinn 2005). In fact, a difference of 2°C can be associated with differences in spawning dates of 2 weeks to over a month (Myers et al. 1998). Moreover, within systems, small changes in spring temperatures drive interannual variation in the timing of smolt migration (e.g., fig. 12-4, Quinn 2005). On average, accessible and inaccessible areas of geographic regions that support salmonid ESUs differed by 2°C in air temperature (Fig. 3 and see Appendix S1). Obviously, differences in air temperature do not translate directly to differences in water temperature, but they are tightly correlated, suggesting that this magnitude of difference is ecologically (and thus selectively) relevant for these fishes.
Stream flows also affect fitness components. For instance, peak flows are negatively and exponentially correlated with egg-to-fry survival in the Cedar River, WA, USA (e.g., fig. 8-6, Quinn 2005), meaning that small changes in flow are associated with very large reductions in egg-to-fry survival, especially at the lower range of natural flow rates. Direct measures of flow across the entire western USA were not available, but measures of precipitation and gradient – both of which are correlated with flow – differ significantly between accessible and inaccessible areas of geographic regions. The differences in average precipitation between accessible and inaccessible areas ranged from −42 cm (inaccessible > accessible) in Puget Sound to 31 cm in the Snake River (accessible areas > inaccessible areas). Such differences are large enough that the flow conditions experienced in the accessible and inaccessible regions are likely to be different. Another important difference is that in areas of low precipitation, many stream reaches are dry in summer and salmon adapt to these conditions through life history strategies that avoid late summer dry periods (e.g., out-migration in spring as age-0 smolts or movement to other reaches during summer). Together, these differences in temperature and flow-related parameters are substantial enough that it is reasonable to posit a non-negligible selection differential.
Heritability of ecologically important traits
A selection differential, such as is likely (above), however, is only one of two things required for an evolutionary response to be elicited. The affected traits must also be heritable for adaptive evolution to occur. In fact, many salmonid phenotypic traits display significant narrow-sense heritabilities although their levels of heritability are quite varied [see recent reviews by Carlson and Seamons (2008) and Garcia de Leaniz et al. (2007)]. These values lead us to expect that they can respond to selection but the net the rate of response of different traits will vary widely. It is important to recognize that traits with low narrow-sense heritabilities can still be underlain by a substantial amount of additive genetic variance; heritability is a proportion and if there is substantial phenotypic plasticity in a triat or only weak canalization, then the denominator will be such that the proportion will appear small. We take the demonstration of significant heritability to indicate a realistic potential for future evolutionary change. Hereafter, we focus on a subset of heritable physiological and life-history traits that have clear connections to the environmental parameters we have examined above.
Selection on salmon body size is related to variation in stream size and flow. Larger fish can better maintain position in larger streams, and also are favored by the positive relationship between female body size and fecundity (reviewed in Quinn 2005). In addition, deeper scour of bed material in large rivers (Montgomery et al. 1999; R. S. Waples, G. R. Pess and T. J. Beechie, unpublished manuscripts) should select for larger and older fish so that nests can be excavated to a depth that will minimize scour-induced egg mortality (Steen and Quinn 1999). Indeed, larger, older fish tend to be found in larger streams with stronger flow (e.g., Quinn et al. 2001). Both body size and age-at-maturity, which affects size-at-age, have positive heritabilities (Carlson and Seamons 2008; Table 3) and so should respond to these selective pressures.
Table 3.
Narrow-sense heritability estimates for life history and morphological traits with connections to environmental parameters in the Pacific salmon. We present median heritability for studies providing separate estimates for sexes, strains/lines, cohorts, or populations. Sample sizes for each study are presented in parentheses. ‘Broodstock’ and ‘treatment’ were defined following Carlson and Seamons (2008), where broodstock represents the recent history of the population and treatment represents the setting where the heritabilities were estimated. See original studies for individual estimates and details on significance and estimation methods.
| Trait | Study | Species | Broodstock | Treatment | h2 (n) |
|---|---|---|---|---|---|
| Hatching time | Sato (1980) | O. kisutch | Unknown | Hatchery | 0.261 (1) |
| McIntyre and Blanc (1973) | O. mykiss | Unknown | Hatchery | 0.115 (2) | |
| Kinnison et al. (1998) | O. tshawytscha | Wild | Hatchery | 0.140 (2) | |
| Run timing | Dickerson et al. (2005) | O. gorbuscha | Wild | Wild | 0.029 (8) |
| Smoker et al. (1994) | O. gorbuscha | Wild | Sea-ranched | 0.225 (6) | |
| Smoker et al. (1998) | O. gorbuscha | Wild | Sea-ranched | 0.285 (2) | |
| Quinn et al. (2000) | O. tshawytscha | Wild | Sea-ranched | 1.260 (1) | |
| Maturation timing | Quinn et al. (2000) | O. tshawytscha | Wild | Sea-ranched | 1.070 (4) |
| Spawn timing | Gall and Neira (2004) | O. kisutch | Farmed | Farmed | 0.240 (1) |
| Neira et al. (2006a) | O. kisutch | Farmed | Farmed | 1.110 (4) | |
| Siitonen and Gall (1989) | O. mykiss* | Hatchery | Hatchery | 0.540 (2) | |
| Su et al. (1997) | O. mykiss* | Hatchery | Hatchery | 0.842 (3) | |
| Su et al. (1999) | O. mykiss* | Hatchery | Hatchery | 0.739 (6) | |
| Wilson et al. (2003) | O. mykiss* | Farmed | Unknown | 0.062 (6) | |
| Age-at-maturity | Iwamoto et al. (1984)† | O. kisutch | Sea-ranched | Hatchery | 0.133 (6) |
| Silverstein and Hershberger (1992)† | O. kisutch | Sea-ranched | Hatchery | 0.050 (1) | |
| Gjerde and Gjedrem (1984) | O. mykiss | Farmed | Farmed | 0.160 (3) | |
| McKay et al.(1986) | O. mykiss* | Hatchery | Hatchery | 0.210 (1) | |
| Sylvén and Elvingson (1992) | O. mykiss | Unknown | Farmed/Hatchery | 0.020 (2) | |
| Hankin et al. (1993) | O. tshawytscha | Sea-ranched | Sea-ranched | 0.490 (9) | |
| Heath et al. (1994)† | O. tshawytscha | Farmed | Farmed | 0.750 (8) | |
| Heath et al. (2002)† | O. tshawytscha | Farmed | Farmed | 0.650 (2) | |
| Gjerde (1984) | S. salar | Unknown | Farmed | 0.570 (2) | |
| Gjerde et al. (1994) | S. salar | Farmed | Farmed | 0.120 (2) | |
| Gjerde and Gjedrem (1984) | S. salar | Farmed/Wild | Farmed | 0.140 (3) | |
| Standal and Gjerde (1987) | S. salar | Hatchery | Farmed | 0.125 (2) | |
| Wild et al. (1994)† | S. salar | Farmed | Farmed | 0.145 (8) | |
| Length-at-maturity | Dickerson et al. (2005) | O. gorbuscha | Wild | Wild | 0.060 (8) |
| Funk et al. (2005) | O. gorbuscha | Wild | Sea-Ranched | 0.395 (2) | |
| Smoker et al. (1994) | O. gorbuscha | Wild | Sea-ranched | 0.250 (18) | |
| Gall and Neira (2004) | O. kisutch | Farmed | Farmed | 0.330 (1) | |
| Silverstein and Hershberger (1995) | O. kisutch | Hatchery | Hatchery | 0.260 (1) | |
| Gjerde and Gjedrem (1984) | O. mykiss | Farmed | Farmed | 0.160 (1) | |
| Gjerde and Gjedrem (1984) | S. salar | Farmed/Wild | Farmed | 0.350 (1) | |
| Mass-at-maturity | Smoker et al. (1994) | O. gorbuscha | Wild | Sea-Ranched | 0.000 (18) |
| Gall and Neira (2004) | O. kisutch | Farmed | Farmed | 0.290 (3) | |
| Neira et al. (2006a) | O. kisutch | Farmed | Farmed | 0.135 (2) | |
| Neira et al. (2006b) | O. kisutch | Farmed | Farmed | 0.395 (2) | |
| Silverstein and Hershberger (1995) | O. kisutch | Hatchery | Hatchery | 0.190 (1) | |
| Crandall and Gall (1993a) | O. mykiss* | Hatchery | Hatchery | 0.230 (14) | |
| Crandall and Gall (1993b) | O. mykiss* | Hatchery | Hatchery | 0.580 (2) | |
| Gall and Huang (1988) | O. mykiss* | Hatchery | Hatchery | 0.200 (1) | |
| Su et al. (1997) | O. mykiss* | Hatchery | Hatchery | 0.135 (3) | |
| Jónasson and Gjedrem (1997) | S. salar | Sea-ranched | Hatchery/sea-ranched | 0.250 (4) | |
| Jónasson et al. (1997) | S. salar | Sea-ranched | Sea-ranched | 0.290 (5) | |
| Anadromy | Thrower et al. (2004) (smoltification) | O. mykiss | Wild | Wild | 0.726 (3) |
| Theriault et al. (2007) | S. fontinalis | Wild | Wild | 0.560 (89) |
Estimates generated for resident (nonanadromous) O. mykiss.
Focused on early maturity in males.
Temperature regime strongly affects development rate and so, not surprisingly, the timing of egg hatching and the timing of fry emergence from the gravel are temperature dependent. Adults are expected to breed at a time of year that, given the long-term average local temperature regime, will lead to fry emerging during a period that maximizes their growth and survival (Brannon 1987; Webb and McLay 1996; Quinn 2005). Previous research has demonstrated that populations breeding in colder areas (higher latitude, higher altitude) typically arrive on the breeding grounds and breed earlier in the year than populations breeding in relatively warmer/milder areas (Quinn 2005). Many traits affect the timing of fry emergence including the timing of arrival to the breeding grounds (‘run timing’), timing of maturation, and the timing of spawning. Because all of these traits have positive narrow-sense heritabilities (Table 3), they are expected to respond to this temperature-induced selection.
Temperature regime also appears to affect spawn timing both by stabilizing selection acting on the time of fry emergence (discussed above) and by selection to avoid high stream temperatures during the spawning period (Beer and Anderson 2001). Populations occupying high elevation streams experience colder incubation temperatures and longer incubation times, so earlier spawning favors earlier fry emergence in spring that coincides with favorable environmental conditions (Beacham and Murray 1987; Brannon 1987). In contrast, fish spawning in low elevation reaches can face high summer stream temperatures and be forced to spawn later when stream temperatures are lower (Beer and Anderson 2001). The observation that salmon spawn across a wide range of temperatures and the fact that narrow-sense heritabilities for spawn timing are sometimes extremely large (Table 3), together suggest that spawn timing might evolve quickly in response to changing local conditions.
Selective responses to loss of habitat: case studies
Clearly, salmonid habitat has not been lost at random, but rather has been rendered inaccessible in a biased fashion. Moreover, the factors that differ between accessible and inaccessible habitats are associated with heritable variation in morphology and life history. Unfortunately, no study has looked explicitly at the selection imposed by differential habitat loss. However, there are a number of situations in which selection because of habitat loss appears to have occurred.
Change in run and spawn-timing – Lemhi River Chinook salmon
The Lemhi River sits in a high elevation glacial valley in central Idaho. Surrounded by steep peaks, its wide, flat valley historically hosted wide, braided channels (Konrad 2006). This Chinook salmon population is thought to have included fish that returned to the Columbia Basin in both the late spring (spring-run) and summer (summer-run) (Bjornn 1978). While it is not entirely certain that summer-run fish occupied the Lemhi basin, nearby rivers with similar attributes do contain both life history types (ICTRT 2003). Both spring and summer run fish in this ESU rear in fresh water for approximately 1 year before migrating to the ocean (Folmar and Dickhoff 1980; Myers et al. 1998; Gustafson et al. 2007), but spring-run fish return to fresh water and spawn earlier in the year than do summer-run fish (Bjornn 1978; Groot and Margolis 1991, Quinn 2005). In addition, spring-run fish tend to spawn earlier in smaller, higher elevation tributary habitats, while summer-run fish tend to spawn in larger streams and rivers (Feist et al. 2003; Interior Columbia Technical Recovery Team 2003). Exact spatial and temporal boundaries between the two types, however, have not been documented (Interior Columbia Technical Recovery Team 2003). In 1907, a hydroelectric dam constructed at the lower end of the Lemhi River diverted all its flow to a powerhouse on the Salmon River and prevented passage during the summer-run (Bjornn 1978; SBT/NPT/IDFG 2004). This barrier was removed in the 1950s (Furness 1989; B. Smith, USDA Forest Service, pers. comm.), but ongoing water withdrawals increase the river temperature and de-water stretches of the lower river, often rendering it uninhabitable for salmonids (NPCC 2004).
Although current conservation efforts in the area are working to address these and other issues, there are no summer-run fish currently in this population as a direct result of initial habitat blockage and subsequent habitat modifications. The truncation of run and spawn timing in this population in response to this inadvertent selection depletes both the phenotypic and genetic (as these traits can be highly heritable) diversity in this population, and might affect its resilience to environmental fluctuations (Hilborn et al. 2003).
Selection for a resident life-history – Central Valley steelhead
The Sacramento and San Joaquin river systems drain much of the Sierra mountain range and the expansive Central Valley of California, joining in the San Francisco Bay Delta and entering the Pacific Ocean under the Golden Gate Bridge. The two systems historically supported spatially extensive populations of steelhead, but recent estimates suggest that approximately 80% of the natural spawning and rearing habitat is now inaccessible because of impassable dams (McEwan 2001; Lindley et al. 2006a). In particular, the higher gradient, upstream reaches steelhead prefer for spawning are virtually absent now in Central Valley rivers (Fig. 1), although some high gradient habitat is still accessible in small tributary creeks of the northern Sacramento River. All steelhead currently present in the Central Valley system are considered to be winter run, or ocean maturing. McEwan (2001) provides some evidence that summer run (stream maturing) steelhead were present prior to dam construction but have since been extirpated because of the loss of access to holding habitats in upper reaches. Most steelhead populations remaining in the system now spawn and rear in low gradient mainstem habitats that differ markedly in substrate composition, current velocity, temperature, and volume compared with historical spawning habitats.
There is extensive evidence that formerly anadromous populations of O. mykiss have residualized and become established as residents above passage barriers in California rivers (Gall and Bentley 1990). Although otolith microchemistry studies have demonstrated that anadromous progeny can be derived naturally from resident parents and vice versa (Zimmerman and Reeves 2000; Ruzycki et al. 2003), there does appear to be a maternal effect, with anadromous mothers more often giving rise to anadromous offspring than resident mothers (Ruzycki et al. 2003; Thrower et al. 2004). In addition, in a study that compared survival of all possible resident by anadromous crosses, the return survival of resident-origin progeny that migrated was markedly reduced compared with that of crosses in which one or both parents were anadromous fish (Thrower et al. 2004). Heritability of smolting in this species (Thrower et al. 2004) and of anadromy in brook trout (Theriault et al. 2007) have been shown to be quite high (Table 3) in comparison with other life history traits (Carlson and Seamons 2008). As anadromous offspring from individuals above a barrier cannot contribute to subsequent generations, we would expect selection to eliminate the anadromous life history above barriers.
There is, in fact, general consensus that an anadromous to resident switch is far more likely than the reverse, again suggesting eventual loss of the anadromous life history without continual interbreeding of the two types (Waples et al. 2001).
In addition to the forced residualization of O. mykiss populations in tributaries above barriers, some Central Valley populations below barriers also appear to have a higher than expected proportion of residents. Tailwater sections below dams often develop deep pools with moderated temperatures and unnaturally high levels of food availability, conditions conducive to very rapid growth for juvenile salmonids. Steelhead are predicted to follow the general life history model developed by Thorpe et al. (1998). Under this model, emigration is a life history option selected when environmental conditions no longer meet the energetic needs of the individual. To compete effectively and be successful in mating, migration to the ocean with its superior growth opportunity is presumably necessary when freshwater food supplies are relatively low. However, fish that can grow rapidly in fresh water avoid the risks associated with migration, and in such a situation, anadromy would be expected to be selected against. As an example, a 35 km reach of the Upper Sacramento River below Keswick Dam has developed a renowned recreational fishery for resident rainbow trout. According to Dean (2005), this population dramatically increased following dam construction and subsequent temperature controls that maintain water temperatures at around 13°C. Analysis of adult scales confirmed that the large fish in this system are residents, with only one fish out of 101 showing evidence of a marine growth period (Dean 2005). Prior to dam construction, this reach likely had low flows and high temperatures during summer, presumably favoring an anadromous life history, but current, high productivity conditions likely select against anadromy and for residency.
To summarize, impassable barriers have impacted Central Valley steelhead populations in at least two major ways. First, formerly anadromous populations upstream of barriers have necessarily adopted a resident life history or have been extirpated (summer run). And, second, habitats below barriers have changed to become more conducive to the resident life history even though ocean migration is possible. The loss and change of habitat because of blockages in the Central Valley might thus have substantial evolutionary consequences as a result of an altered selection regime. In particular, the selective pressure against an anadromous life history reduces the variability of life history expression in the population(s). Likewise, the segregation of upstream and downstream habitats likely inhibits natural fluctuations in life history expression associated with juveniles rearing across a spectrum of environmental conditions. Any buffering against the impact of environmental perturbations, that is provided by this diversity of life histories will have been reduced especially in the freshwater environments. As emphasized by McEwan (2001), the interdependence of the different life histories has presumably contributed to the success and persistence of populations throughout a highly variable range of habitats and environmental conditions in California. Steelhead are plastic, so alternatively, the changes exerted by a biased loss of habitats might alter the norms of reaction for life history plasticity. This, in turn, could also affect the populations’ ability to respond to future environmental perturbations or changes. In addition, selection against anadromy might reduce gene flow between geographic regions, as steelhead migrate between populations. This could also strongly affect the genetic variation within these populations.
Reduction in rearing time – Puget Sound coho salmon
Coho salmon (Oncorhynchus kisutch) in Puget Sound migrate downstream to overwinter in lower mainstem floodplain and delta habitats. Historically, this typically occurred during the high flow season after rearing in upper areas for a year (Quinn 2005). Like Central Valley steelhead, this ESU is showing a reduction in a previously common life history strategy (age-1 out-migration) with a concomitant increase in a previously rare phenotype (age-0 out-migration) in response to loss of habitat.
Historic reconstruction of riverine landscapes in the North Puget Sound (Beechie et al. 2001; Pess et al. 2002) have quantified the loss of floodplain and delta off-channel habitats in Puget Sound since the turn of the 20th century. Over 50% of the floodplain habitats and over 70% of the estuarine environment have been either filled or disconnected from the Skagit and Stillaguamish River systems since the turn of the 20th century (collective basin area 10 040 km2) (Beechie et al. 2001; Pess et al. 2002). In addition, one of the main geomorphic drivers that create side channel and slough habitats, channel migration of the main river across the floodplain, has been significantly reduced due to physical constraints by diking and levees along the main stem (Pess et al. 2002). This change in habitat has dramatically reduced the carrying capacity of these systems for overwintering coho. This, in turn, might lead to changes in the proportion of juvenile coho that overwinter for up to 1 year versus those that leave as age-0 smolts. In fact, recent work by Bennett (2006) has shown that up to 50% of a coho population can outmigrate as age-0 smolts in watersheds where off-channel habitats are minimal.
Age-at-smoltification in salmonids appears to have a positive narrow-sense heritability (Table 3), although data are somewhat limited. A heritability of approximately 0.5 combined with the loss of habitats conducive to a more typical, overwintering life history strategy suggest that biased habitat loss is exerting a selective influence on the population and that the increase in age-0 smolts could be a response to that selection. Such a change could have an effect on overall population viability, since age-0 coho smolts are typically smaller than age-1 smolts. If such differences in size and related survival translate into a reduction in fitness of the individuals, the productivity and viability of the population might be compromised. Additional work clarifying fitness consequences of such life history and morphological changes is clearly needed.
Habitat loss and nonselective change for salmonids
Passage barriers have two effects that can create important evolutionary consequences even in the absence of any direct changes in selective regime. First, large reductions in habitat area can reduce system capacity. The immediate evolutionary concern would be whether this loss of capacity creates a reduction in the effective population size (Ne) sufficient to affect the level of genetic variation in local populations or the ESU as a whole (Wayne et al. 1991; Alter et al. 2007; Willi et al. 2007). Reduced levels of genetic variation can accelerate the emergence of inbreeding effects and limit a population’s ability to adapt to novel conditions in the future (Futuyma 2005; Willi et al. 2006). If local populations are completely closed to migration and gene flow from other populations, this concern could be justified. However, with any appreciable dispersal among populations, the actual level of genetic variation that will be maintained in a local population will approach that in the ESU as a whole (Slatkin 1987; Strobeck 1987; Lande 1992) and the reduction in capacity would have to be catastrophic over the entire ESU to create a genetic crisis.
In this light, the second potential effect of passage barriers, the disruption of connections among local populations, is probably much more important. For one, if barriers preclude some populations from exchanging migrants with others, then the system as a whole will be broken into isolated subsystems, each of which will sustain lower overall levels of genetic variation than would have been sustained in the system as a whole, even for the same total numbers of adult fish (Whitlock and Barton 1997). In this case, the genetic consequences of a substantial reduction in capacity in one or more of the subsystems will not be mitigated by the re-introduction of variation through migration and the prospects for genetic concerns will increase substantially. The problem can be exacerbated if there are local population extinctions and passage barriers restrict the number of populations from which recolonizing individuals are drawn (Slatkin 1987; Whitlock and Barton 1997; Waples 2002).
These impacts of habitat loss have not been well-studied, but may be widespread because there are many situations in which much of the historical habitat capacity has been removed. For example, the Snake River fall Chinook salmon ESU, which once spawned from the confluence of the Snake with the Columbia River upstream 990 km to Shoshone Falls is now confined to the area below the Hells Canyon Dam complex at river km 397, downstream of areas in which nearly all historical spawning activity was reported (Evermann 1896; CBIC 1957; Haas 1965; Fulton 1970; Van Hyning 1973; Lavier 1976). Historical abundance estimates for this ESU do not exist, but they likely numbered in the hundreds of thousands, given catch estimates of 3–9 million pounds (1.4–4.1 million kg) annually (Waples et al. 1991; Williams et al. 2008). Over the last 20 years the number of wild spawners has ranged from 78 to about 5000 (ICTRT, unpublished data, derived from Fish Passage Center data); a recent study by Williams et al. (2008) estimated the Ne/generation for this population as approximately 1000 for the 1960s–1990s. Clearly, there has been a decrease in effective population size for this ESU of several orders of magnitude. With a decrease of this magnitude, rare and not-so-rare alleles have almost certainly been lost, and homozygosity within the population is almost certainly greater than it was historically. How much of the final effect is attributable to numerical changes and how much to disruptions of the historical population structure cannot be determined but the net effect has been dramatic. While this example might be an extreme one, it is not unique, and the genetic effects of habitat loss and passage barriers deserve greater attention.
Discussion
Evolutionary consequences of anthropogenic impacts on salmonids are only now beginning to be examined in the conservation literature. Those evolutionary effects that have been considered to date tend to be the result of direct intentional or unintentional selection (e.g. harvest and hydropower effects Ricker 1981; Quinn and Adams 1996; Achord et al. 2007). However, more ‘passive’ selection exerted by differential loss of habitat types and nonselective change because of large reductions in carrying capacity can also exert profound evolutionary effects that are relevant for conservation.
We evaluated loss of habitats due to large-scale anthropogenic barriers and showed that this loss is not random. Rather, habitats with unique characteristics have been differentially blocked and rendered inaccessible to anadromous fishes. Specifically, in the contiguous western United States, blocked areas are higher in elevation, more extreme in temperature, and receive more precipitation than areas that are currently accessible. In addition, the magnitude of human impacts varied between inaccessible and accessible areas; blocked areas were typically less impacted than currently accessible areas. The agricultural and urban land uses tracked in this study are also commonly associated with changes in stream flow patterns and temperature. At a more regional level, there were some differences between geographic areas in these patterns, suggesting that the direction of selection can vary from region to region.
Both the magnitude of habitat loss and the differential loss of specific types of habitats have evolutionary implications. The biased loss of habitat types can result in a dramatically altered selective regime for affected populations. In the case of genetically based trait variation, such biased habitat loss in turn can select against particular phenotypes and, in extreme cases, can result in local or regional extirpation of particular phenotypes. The loss of primary habitat area could also result in colonization of novel environments or increased reliance on marginal habitat either of which might exert new selective pressures resulting in phenotypic change. Such altered selective regimes can also lead to changes in individual norms of reaction (plasticity patterns), including possible loss of adaptive plasticity for response to inaccessible habitats. These selective reductions of genetic diversity can potentially affect the long-term ability of populations to respond adaptively to either natural or anthropogenic environmental change.
In addition, habitat blockage can have evolutionary effects apart from these selective changes. In particular, a substantive reduction in capacity associated with anthropogenic barriers can reduce the effective population size and consequently genetic variation within the population. Blockages and extirpations can compound this effect by fragmenting populations and thus severely disrupting natural patterns of gene flow. Together, these effects can all result in less diversity at a variety of levels.
Less genetic and phenotypic diversity at the population, ESU and species level could compromise the ability of these groups to weather large-scale environmental fluctuations in the future. Loss of particular genotypes and phenotypes via directional selection in altered habitat regimes is likely to be of particular concern in a world with changing climates, where the potential for response to novel environments is crucial for survival. Indeed, areas that are no longer inhabited by anadromous salmonids tend, in general, to have warmer summers than those that are currently accessible. Phenotypes and genotypes (and potentially norms of reaction) selected for in these warmer areas have been lost, potentially hampering the ability of these species to adapt to a change in climates.
We have used salmonids to illustrate the range of potential effects of habitat loss on the evolutionary trajectories of populations and species. However, these effects are not confined to salmonids, fishes or freshwater habitats. Virtually all taxa are losing habitat as human uses of natural landscapes expand (Vitousek et al. 1997; Kerr and Deguise 2004). Human uses do not tend to be distributed randomly across habitat types, suggesting that the biased loss of habitats we found for salmonids also applies to other species. In addition, the ‘passive’ effects of reduced effective population size and altered population structure and reduced genetic diversity are also likely to be replicated in other species. In fact, a recent study has shown that highways and other anthropogenic constructs blocking migration of bighorn sheep have resulted in a rapid and dramatic decline in genetic diversity in their populations (Epps et al. z2005). Similarly, butterfly populations in Finland subject to loss of habitat have shown a decrease in effective population size and an increase in extinction risk (Saccheri et al. 1998). Together, these observations suggest that the effects of loss of habitat are likely to be more diverse and potentially of longer-term impact than are commonly accounted for. Conservation strategies that address habitat loss should consider the evolutionary impacts of that loss as well as the demographic effects, and in particular seek to maintain or restore natural patterns of phenotypic and genotypic variability.
Acknowledgments
We would like to acknowledge participants of the ‘Evolutionary Changes and Salmon’ workshop in Seattle, Washington in December 2006 for insightful discussion. Jon Moore, Jon Honea, Robin Waples and two anonymous reviewers provided extremely helpful reviews of earlier drafts of this paper.
Supporting Information
Literature cited
- Achord S, Zabel RW, Sandford BP. Migration timing, growth, and estimated parr-to-smolt survival rates of wild Snake River spring–summer Chinook salmon from the Salmon River Basin, Idaho, to the Lower Snake River. Transactions of the American Fisheries Society. 2007;136:142–154. [Google Scholar]
- Allen JD. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology, Evolution and Systematics. 2004;35:257–284. [Google Scholar]
- Alter SE, Rynes E, Palumbi SR. DNA evidence for historic population size and past ecosystem impacts of gray whales. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15162–15167. doi: 10.1073/pnas.0706056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, Hardy EE, Roach JT, Witmer RE. Geological Survey Professional Paper 964. Washington D.C.: United States Government Printing office; 1976. A land use and land cover classification for use with remote sensor data; p. 28. http://landcover.usgs.gov/pdf/anderson.pdf [accessed 20 March 2008] [Google Scholar]
- Beacham TD, Murray CB. Adaptive variation in body size, morphology, egg size, and developmental biology of chum salmon (Oncorhynchus keta) in British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1987;44:244–261. [Google Scholar]
- Beacham TD, Murray CB. Variation in developmental biology of sockeye salmon (Oncorhynchus nerka) and Chinook salmon (O. tshawytscha) in British Columbia. Canadian Journal of Zoology. 1989;67:2081–2089. [Google Scholar]
- Beechie TJ, Collins BD, Pess GR. Holocene and recent geomorphic processes, land use and salmonid habitat in two north Puget Sound river basins. In: Dorava JB, Montgomery DR, Fitzpatrick F, Palcsak B, editors. Geomorphic Processes and Riverine Habitat, Water Science and Application. Washington, DC: American Geophysical Union; 2001. pp. 37–54. [Google Scholar]
- Beechie TJ, Ruckelshaus MH, Buhle E, Fullerton A, Holsinger L. Hydrologic regime and the conservation of salmon life history diversity. Biological Conservation. 2006;130:560–572. [Google Scholar]
- Beer WN, Anderson JJ. Effect of spawning day and temperature on salmon emergence: interpretations of a growth model for Methow River Chinook. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:943–949. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Bennett TR. Movement, Growth, and Survival of Juvenile Coho and Trout in the East Twin River. Seattle, WA: Washington University of Washington; 2006. [Google Scholar]
- Benson JF, Chamberlain MJ. Space use, survival, movements, and reproduction of reintroduced Louisiana black bears. Journal of Wildlife Management. 2007;71:2393–2403. [Google Scholar]
- Bjornn TC. Survival, Production, and Yield of Trout and Chinook Salmon in the Lemhi River, Idaho. Idaho, Moscow: Idaho Department of Fish and Game Bulletin 27, Idaho Cooperative Fishery Research Unit, University of Idaho; 1978. p. 57. [Google Scholar]
- Brannon EL. Mechanisms stabilizing salmonid fry emergence timing. Canadian Special Publication of Fisheries and Aquatic Sciences. 1987;96:120–124. [Google Scholar]
- Brannon EL, Quinn TP, Lucchetti GL, Ross BD. Compass orientation of sockeye salmon fry from a complex river system. Canadian Journal of Zoology. 1981;59:1548–1553. [Google Scholar]
- British Columbia Ministry of Environment. British Columbia Watershed Atlas. Victoria, BC: British Columbia Ministry of Environment; 2005. [ESRI shapefile], 1:50,000, Available at: http://www.env.gov.bc.ca/fish/watershed_atlas_maps/index.html [accessed 20 March 2008] [Google Scholar]
- British Columbia Ministry of Environment. British Columbia Historical Fish Distribution [ESRI shapefile] 2006. Victoria, BC. Available at: http://www.env.gov.bc.ca/fish/fiss/maps/fissfish2ftp.html [accessed 20 March 2008]
- Brodie ED, Moore AJ, Stern FJ. Visualizing and quantifying natural selection. Trends in Ecology and Evolution. 1995;10:313–318. doi: 10.1016/s0169-5347(00)89117-x. [DOI] [PubMed] [Google Scholar]
- Browne CL, Hecnar SJ. Species loss and shifting population structure of freshwater turtles despite habitat protection. Biological Conservation. 2007;138:421–429. [Google Scholar]
- CALFISH. A California Cooperative Anadromous Fish and Habitat Data Program. California Fish Passage Assessment Database Project [ESRI shapefile] 2006. Sacramento, CA. URL: http://www.calfish.org/FishDataandMaps/DataDownloads/tabid/90/Default.aspx (accessed on January 2008)
- California Interagency Watershed Mapping Committee and Department of Water Resources. 2004. [E00 file]. California Interagency Watershed Map of 1999 (updated 2004). Sacramento, CA. Available at: http://www.env.gov.bc.ca/fish/watershed_atlas_maps/index.html [accessed 20 March 2008]
- California Watershed Mapping Committee and Department of Water Resources (CWMP/CDWR) 2004. The California Interagency Watershed Map of 1999 (updated 2004) [computer file]. Sacramento, CA. Available WEB: http://cain.nbii.gov/calwater (accessed on 6 December, 2006)
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbia Basin Interagency Committee. 1957. Inventory of streams and proposed improvements for development of the fishery resources of the Columbia River Basin – Part II. Columbia River Basin Fishery Program, 100p. Available Environmental and Technical Services Division, NMFS, 911 N.E. 11th Ave., Room 620, Portland, OR 97232.
- Crandell PA, Gall GAE. The effect of sex on heritability estimates of body weight determined from data on individually tagged rainbow trout (Oncorhynchus mykiss. Aquaculture. 1993a;113:47–55. [Google Scholar]
- Crandell PA, Gall GAE. The genetics of age and weight at sexual maturity based on individually tagged rainbow trout (Oncorhynchus mykiss. Aquaculture. 1993b;117:95–105. [Google Scholar]
- Dean M. Collection and Analysis of Scales From Rainbow Trout of the Sacramento River near Redding, CA. Sacramento, CA: California Department of Fish and Game; 2005. Unpublished report. [Google Scholar]
- Dickerson BR, Willson MF, Bentzen P, Quinn TP. Heritability of life history and morphological traits in a wild pink salmon population assessed by DNA parentage analysis. Transactions of the American Fisheries Society. 2005;134:1323–1328. [Google Scholar]
- Ehrlich PR, Ehrlich AH. Extinction: The Causes and Consequences of the Disappearance of Species. New York, NY: Random House; 1981. [Google Scholar]
- Environment Canada. Canadian Monthly Climate Data and 1961-1990 Normals on CD-ROM, Version 3.0E. Downsview, ON: Environment Canada, Atmospheric Environment Service; 1994. [Google Scholar]
- Epps CW, Palsboll PJ, Wehausen JD, Roderick GK, Ramey RR, McCullough DR. Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecology Letters. 2005;8:1029–1038. [Google Scholar]
- Evermann BW. A preliminary report upon salmon investigations in Idaho in1894. U.S. Fisheries Commission, Bulletin. 1896;15:253–284. [Google Scholar]
- Fahrig L. How much habitat is enough? Biological Conservation. 2001;100:65–74. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Essex, UK: Prentice Hall; 1996. [Google Scholar]
- Feist BE, Steel EA, Pess GR, Bilby RE. The influence of scale on salmon habitat restoration priorities. Animal Conservation. 2003;6:271–282. [Google Scholar]
- Folmar LC, Dickhoff WW. The parr–smolt transformation (smoltification) and seawater adaptation in salmonids: a review of selected literature. Aquaculture. 1980;21:1–37. [Google Scholar]
- Fulton LA. Spawning area and abundance of steelhead trout and coho, sockeye, and chum salmon in the Columbia River basin – past and present. 1970. Special Scientific Report – Fisheries No. 618. U.S..DOC, NOAA, NMFS. pp. 37. [Google Scholar]
- Funk WC, Tyburczy JA, Knudsen KL, Lindner KR, Allendorf FW. Genetic basis of variation in morphological and life-history traits of a wild population of pink salmon. Journal of Heredity. 2005;96:24–31. doi: 10.1093/jhered/esi009. [DOI] [PubMed] [Google Scholar]
- Furness M. Electric power in Salmon. In: Magoon T, Crosby M, editors. Patchwork: Pieces of Local History. Salmon ID: Salmon High School; 1989. pp. 55–63. [Google Scholar]
- Furniss MJ, Roelofs TD, Yee CS. Road construction and maintenance. American Fisheries Society Special Publication. 1991;19:297–323. [Google Scholar]
- Futuyma DJ. Evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Gall GAE, Bentley B. Genetic isolation of steelhead rainbow trout in Kaiser and Redwood Creeks, California. California Fish and Game. 1990;76:216–223. [Google Scholar]
- Gall GAE, Huang N. Heritability and selection schemes for rainbow trout: body weight. Aquaculture. 1988;73:43–56. [Google Scholar]
- Gall GAE, Neira R. Genetic analysis of female reproduction traits of farmed coho salmon (Oncorhyncus kisutch. Aquaculture. 2004;234:143–154. [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Response to individual selection for age at sexual maturity in Atlantic salmon. Aquaculture. 1984;38:229–240. [Google Scholar]
- Gjerde B, Gjedrem T. Estimates of phenotypic and genetic parameters for carcass traits in Atlantic salmon and rainbow trout. Aquaculture. 1984;36:97–110. [Google Scholar]
- Gjerde B, Simianer H, Refstie T. Estimates of genetic and phenotypic parameters for body-weight, growth-rate and sexual maturity in Atlantic salmon. Livestock Production Science. 1994;38:133–143. [Google Scholar]
- Goodsell PJ, Connell SD. Can habitat loss be treated independently of habitat configuration? Implications for rare and common taxa in fragmented landscapes. Marine Ecology-Progress Series. 2002;239:37–44. [Google Scholar]
- Gregory SV, Bisson PA. Degradation and loss of anadromous fish habitat in the Pacific Northwest. In: Stouder DJ, Bisson PA, Naiman RJ, editors. Pacific Salmon and Their Ecosystems: Status and Future Options. New York, NY: Chapman and Hall; 1997. pp. 277–314. [Google Scholar]
- Groot C, Margolis L. Pacific Salmon Life Histories. Vancouver, BC: University of British Columbia; 1991. [Google Scholar]
- Gustafson RG, Waples RS, Myers JM, Weitkamp LA, Bryant GJ, Johnson OW, Hard JJ. Pacific salmon extinctions: quantifying lost and remaining diversity. Conservation Biology. 2007;21:1009–1020. doi: 10.1111/j.1523-1739.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- Haas JB. Fishery problems associated with Brownlee, Oxbow, and Hells Canyon Dams on the middle Snake River. Portland, OR: Oregon Department of Fish and Wildlife; 1965. p. 95. Fish Commission Oregon, Investigational Rep. No. 4. [Google Scholar]
- Hankin DG, Nicholas JW, Downey TW. Evidence for inheritance of age at maturity in Chinook salmon, Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:347–358. [Google Scholar]
- Healey MC. The life history of Chinook salmon (Oncorhynchus tshawytscha. In: Margolis CGroot,andL., editor. Life History of Pacific Salmon. Vancouver, BC: University of British Columbia Press; 1991. pp. 311–393. [Google Scholar]
- Heath DD, Devlin RH, Heath JW, Iwama GK. Genetic, environmental and interaction effects on the incidence of jacking in Oncorhynchus tshawytscha (chinook salmon) Heredity. 1994;72:146–154. [Google Scholar]
- Heath DD, Rankin L, Bryden CA, Heath JW, Shrimpton JM. Heritability and Y-chromosome influence in the jack male life history of chinook salmon (Oncorhynchus tshawytscha. Heredity. 2002;89:311–317. doi: 10.1038/sj.hdy.6800141. [DOI] [PubMed] [Google Scholar]
- Helm A, Hanski I, Partel M. Slow response of plant species richness to habitat loss and fragmentation. Ecology Letters. 2006;9:72–77. doi: 10.1111/j.1461-0248.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- Hilborn R, Quinn TP, Schindler DE, Rogers DE. Biocomplexity and fisheries sustainability. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6564–6568. doi: 10.1073/pnas.1037274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S, Quinn TP. The timing of adult sockeye salmon migration into freshwater: adaptations by populations to prevailing thermal regimes. Canadian Journal of Zoology. 2002;80:542–555. [Google Scholar]
- Interior Columbia Technical Recovery Team. 2003. Independent Populations of Chinook, Steelhead, and Sockeye for Listed Evolutionarily Significant Units Within the Interior Columbia River Domain. Working draft report available at: http://www.nwfsc.noaa.gov/trt/col_docs/independentpopchinsteelsock.pdf.
- Interior Columbia Technical Recovery Team. 2007. Viability Criteria for Application to Interior Columbia Basin Salmonid ESUs. Review draft available at: http://www.nwfsc.noaa.gov/trt/trt_documents/ictrt_viability_criteria_reviewdraft_2007_complete.pdf.
- Iwamoto RN, Alexander BA, Hershberger WK. Genotypic and environmental-effects on the incidence of sexual precocity in coho salmon (Oncorhynchus kisutch. Aquaculture. 1984;43:105–121. [Google Scholar]
- Jónasson J, Gjedrem T. Genetic correlation for body weight of Atlantic salmon grilse between fish in sea ranching and land-based farming. Aquaculture. 1997;157:205–214. [Google Scholar]
- Jónasson J, Gjerde B, Gjedrem T. Genetic parameters for return rate and body weight of sea-ranched Atlantic salmon. Aquaculture. 1997;154:219–231. [Google Scholar]
- Kerr JT, Deguise I. Habitat loss and the limits to endangered species recovery. Ecology Letters. 2004;7:1163–1169. [Google Scholar]
- Kinnison MT, Unwin MJ, Hershberger WK, Quinn TP. Egg size, fecundity, and development rate of two introduced New Zealand chinook salmon (Oncorhynchus tshawytscha) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1946–1953. [Google Scholar]
- Konrad C. Location and timing of river-aquifer exchanges in six tributaries to the Columbia River in the Pacific Northwest of the United States. Journal of Hydrology. 2006;328:444–470. [Google Scholar]
- Lande R. Neutral theory of quantitative genetic variance in an island model with local extinction and recolonization. Evolution. 1992;46:381–389. doi: 10.1111/j.1558-5646.1992.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Lavier DC. Production of Wild Fish-Contribution to Escapement. Portland, OR: Environmental and Technical Services Division, NMFS; 1976. p. 25. Investigative Reports of Columbia River Fisheries Project. Prepared for Pacific Northwest Regional Commission. [Google Scholar]
- Lindley ST, Schick R, Agrawal A, Goslin M, Pearson T, Mora E, Anderson JF, et al. Historical population structure of Central Valley steelhead and its alteration by dams. San Francisco Estuary and Watershed Science. 2006a;4:1–19. [Google Scholar]
- Lindley ST, Schick R, Agrawal A, Goslin M, Pearson TE, Mora E, Anderson JJ, et al. Historical population structure of Central Valley steelhead and its alteration by dams. San Francisco Estuary and Watershed Science. 2006b;4:21. [Google Scholar]
- McEwan D. Central Valley Steelhead. In: Brown RL, editor. Contributions to the Biology of Central Valley Salmonids. San Diego CA: Scripps Institutions of Oceanography Library; 2001. pp. 1–45. Fish Bulletin 179. Available at: http://repositories.cdlib.org/sio/lib/fb/179 [accessed 20 March 2008] [Google Scholar]
- McIntosh BA, Sedell JR, Smith JE, Wissmar RC, Clarke SE, Reeves GH, Brown LA. Management history of eastside ecosystems: changes in fish habitat over 50 years, 1935 to 1992. Portland, OR: Department of Agriculture, Forest Service, Pacific Northwest Research Station; 1994. p. 55. Gen. Tech. Rep. PNW-GTR-321. U.S. [Google Scholar]
- McIntosh BA, Sedell JR, Thurow RF, Clarke SE, Chandler GL. Historical changes in pool habitats in the Columbia River Basin. Ecological Applications. 2000;10:1478–1496. [Google Scholar]
- McIntyre JD, Blanc JM. A genetic analysis of hatching time in steelhead trout (Salmo gairdneri. Journal of the Fisheries Research Board of Canada. 1973;30:137–139. [Google Scholar]
- McKay LR, Ihssen PE, Friars GW. Genetic parameters of growth in rainbow trout, Salmo gairdneri, as a function of age and maturity. Aquaculture. 1986;58:241–254. [Google Scholar]
- Millennium Ecosystem Assessment (MA) Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press; 2005. [Google Scholar]
- Miller BA, Sadro S. Residence time and seasonal movements of Juvenile Coho Salmon in the Ecotone and Lower Estuary of Winchester Creek, South Slough, Oregon. Transactions of the American Fisheries Society. 2003;132:546–559. [Google Scholar]
- Montgomery DR, Beamer EM, Pess GR, Quinn TP. Channel type and salmonid spawning distribution and abundance. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:377–387. [Google Scholar]
- Myers JM, Kope R, Bryant G, Teel D, Lierheimer L, Wainright T, Grant W, et al. 1998. p. 443. Status review of chinook salmon from Washington, Idaho, Oregon, and California. U.S. Dept. Commer., NOAA Tech. Memo. NMFS-NWFSC-35. Available at: http://www.nwfsc.noaa.gov/publications/techmemos/tm35/index.htm.
- National Marine Fisheries Service, Northwest Regional Office (NOAA Fisheries) 2006. Northwest Regional HUC 6 Watershed Boundaries [ESRI shapefile]. Portland, OR.
- Natural Resources Canada (NRC) Sault Ste. Marie, Ontario: Canadian Forest Service; 2001. Monthly mean minimum and maximum temperature and precipitation averages (from Mckenney et al. 2001) [computer file] [Google Scholar]
- Neira R, Díaz NF, Gall GAE, Gallardo JA, Lhorente JP, Alert A. Genetic improvement in coho salmon (Oncorhynchus kisutch). II: Selection response for early spawning date. Aquaculture. 2006a;257:1–8. [Google Scholar]
- Neira R, Díaz NF, Gall GAE, Gallardo JA, Lhorente JP, Manterola R. Genetic improvement in coho salmon (Oncorhynchus kisutch). I: Selection response and inbreeding depression on harvest weight. Aquaculture. 2006b;257:9–17. [Google Scholar]
- NMFS. Endangered and threatened species; threatened status for Snake River spring/summer Chinook salmon, threatened status for Snake River fall Chinook salmon. Federal Register. 1992;57:14653–14662. 78 (22 April 1992) [Google Scholar]
- NOAA Fisheries. Northwest Region HUC 6 Watershed Boundaries [computer file] Portland, OR: NOAA Fisheries Regional Office; 2006. [Google Scholar]
- Northwest Fisheries Science Center. Northwest Salmonid ESA Populations [SDE geodatabase] Seattle, WA: National Oceanic and Atmospheric Administration, Northwest Fisheries Science Center; 2007. [Google Scholar]
- Northwest Power Planning Council. 2004. Salmon River Subbasin Plan. Columbia River Basin Fish and Wildlife Program. Portland, OR. [Available at: http://www.nwcouncil.org/fw/subbasinplanning/salmon/plan/]. [accessed 20 October 2007]
- NRC. Upstream: Salmon and Society in the Pacific Northwest. Washington, DC. pp. 452: National Academy Press; 1996. [Google Scholar]
- Omernik JM. Ecoregions of the conterminous United States. Map (scale 1:7,500,000) Annals of the Association of American Geographers. 1987;77:118–125. [Google Scholar]
- Oregon Department of Fish and Wildlife. 2004. Oregon Barriers [ESRI shapefile]. Salem, OR. URL: http://nrimp.dfw.state.or.us/nrimp/default.aspx?pn=fishbarrierdata (accessed on January 2008)
- Pess GR, Montgomery DR, Beechie TJ, Holsinger L. Anthropogenic alterations to the biogeography of salmon in Puget Sound. In: Montgomery DR, Booth DB, editors. Restoration of Puget Sound Rivers. Seattle, WA: University of Washington Press; 2002. pp. 129–154. [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle, WA: University of Washington Press; 2005. [Google Scholar]
- Quinn TP, Adams DJ. Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology. 1996;77:1151–1162. [Google Scholar]
- Quinn TP, Buck GB. Size- and sex-selective mortality of adult sockeye salmon: bears, gulls, and fish out of water. Transactions of the American Fisheries Society. 2001;130:995–1005. [Google Scholar]
- Quinn TP, Unwin MJ, Kinnison MT. Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced Chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Wetzel L, Bishop S, Overberg K, Rogers DE. Influence of breeding habitat on bear predation and age at maturity and sexual dimorphism of sockeye salmon populations. Canadian Journal of Zoology. 2001;79:1782–1793. [Google Scholar]
- Rannap R, Lohmus A, Jakobson K. Consequences of coastal meadow degradation: the case of the natterjack toad (Bufo calamita) in Estonia. Wetlands. 2007;27:390–398. [Google Scholar]
- Reckhow KH, Beaulac MN, Simpson JT. Modeling Phosphorus Loading and Lake Response Under Uncertainty: A Manual and Compilation of Export Coefficients. Washington, DC: Office of Water Regulations and Standards, U.S. Environmental Protection Agency; 1980. USEPA 440/5-80-011. [Google Scholar]
- Ricker WE. Changes in the average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Roff DA. Evolutionary Quantitative Genetics. New York, NY: Chapman and Hall; 1997. [Google Scholar]
- Ruzycki JR, Flesher MW, Carmichael RW, Eddy DL. Lower Snake River Compensation Plan: Oregon Evaluation Studies-Steelhead Life History, Genetics, and Kelt Reconditioning. La Grande, OR: Oregon Department of Fish and Wildlife; 2003. p. 57. [Google Scholar]
- Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I. Inbreeding and extinction in a butterfly metapopulation. Nature. 1998;392:491–494. [Google Scholar]
- Salo EO. Life history of chum salmon (Oncorhynchus keta. In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. Vancouver, BC: University of British Columbia Press; 1991. pp. 231–310. [Google Scholar]
- Sato R. Variations in hatchability and hatching time, and estimation of the heritability for hatching time among individuals within the strain of coho salmon (Oncorhynchus kisutch. Bulletin of the National Research Institute of Aquaculture. 1980;1:21–28. [Google Scholar]
- Spatial Climate Analysis Service (SCAS) Corvallis, OR: Oregon State University; 2003. United States average monthly or annual maximum temperature and mean precipitation, 1971–2000 [computer file] Available WEB: http://www.ocs.orst.edu/prism/products [accessed 6 December 2006] [Google Scholar]
- Spatial Climate Analysis Service (SCAS) United States average monthly or annual maximum temperature and mean precipitation, 1971-2000[computer file] Corvallis, OR: Oregon State University; 2003. Available WEB: http://www.ocs.orst.edu/prism/products [accessed on December 6, 2006] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics. 1993;24:35–68. [Google Scholar]
- Scheiner SM. Selection experiments and the study of phenotypic plasticity. Journal of Evolutionary Biology. 2002;15:889–898. [Google Scholar]
- Seabloom EW, Dobson AP, Stoms DM. Extinction rates under nonrandom patterns of habitat loss. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11229–11234. doi: 10.1073/pnas.162064899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CR, Jay DA, Harvey RB, Simenstad CA. Historical changes in the Columbia River estuary. Progress in Oceanography. 1990;25:299–352. [Google Scholar]
- Siitonen L, Gall GAE. Response to selection for early spawn date in rainbow trout, Salmo gairdneri. Aquaculture. 1989;78:153–161. [Google Scholar]
- Silverstein JT, Hershberger WK. Precocious maturation in coho salmon (Oncorhynchus kisutch): estimation of the additive genetic variance. Journal of Heredity. 1992;83:282–286. [Google Scholar]
- Silverstein JT, Hershberger WK. Genetics of size and growth-rate through sexual maturity in fresh-water-reared coho salmon (Oncorhynchus kisutch. Theoretical and Applied Genetics. 1995;90:733–739. doi: 10.1007/BF00222141. [DOI] [PubMed] [Google Scholar]
- Simenstad CA, Day DA, Sherwood CR. Impacts of watershed management on land-margin ecosystems: the Columbia River Estuary. In: Naiman RJ, editor. Watershed Management: Balancing sustainability and environmental change. New York, NY: Springer-Verlag; 1992. pp. 266–306. [Google Scholar]
- Slatkin M. The average number of sites separating DNA sequences drawn from a subdivided population. Theoretical Population Biology. 1987;32:42–44. doi: 10.1016/0040-5809(87)90038-4. [DOI] [PubMed] [Google Scholar]
- Smith SB. Reproductive isolation in summer and winter races of steelhead trout. In: Northcote TG, editor. Symposium on Salmon and Trout in Streams. Vancouver, BC: University of British Columbia; 1969. pp. 21–38. MacMillan Lectures in Fisheries. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS, Joyce JE. Genetic analysis of size in an anadromous population of pink salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:9–15. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS. Genetic variation of return date in a population of pink salmon: a consequence of fluctuating environment and dispersive selection? Alaska Fishery Research Bulletin. 1998;5:46–54. [Google Scholar]
- Standal M, Gjerde B. Genetic variation in survival of Atlantic salmon during the sea-rearing period. Aquaculture. 1987;66:197–207. [Google Scholar]
- Steen RP, Quinn TP. Egg burial depth by sockeye salmon (Oncorhynchus nerka): implications for survival of embryos and natural selection on female body size. Canadian Journal of Zoology. 1999;77:836–841. [Google Scholar]
- Strobeck C. Average number of nucleotide differences in a sample from a single subpopulation: a test for population subdivision. Genetics. 1987;117:149–153. doi: 10.1093/genetics/117.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su GS, Liljedahl LE, Gall GAE. Genetic and environmental variation of female reproductive traits in rainbow trout (Oncorhynchus mykiss. Aquaculture. 1997;154:115–124. [Google Scholar]
- Su GS, Liljedahl LE, Gall GAE. Estimates of phenotypic and genetic parameters for within-season date and age at spawning of female rainbow trout. Aquaculture. 1999;171:209–220. [Google Scholar]
- Sultan S. Development in context: the timely emergence of eco-devo. Trends in Ecology and Evolution. 2007;22:575–582. doi: 10.1016/j.tree.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Sylvén S, Elvingson P. Comparison of rainbow trout (Oncorhynchus mykiss) strains for body weight, length and age at maturity in different Swedish production systems. Aquaculture. 1992;104:37–50. [Google Scholar]
- Taylor EB. Environmental correlates of life-history variation in juvenile Chinook salmon, Oncorhynchus tshawytscha (Walbaum) Journal of Fish Biology. 1990;37:1–17. [Google Scholar]
- Theriault V, Garant D, Bernatchez L, Dodson JJ. Heritability of life-history tactics and genetic correlation with body size in a natural population of brook charr (Salvelinus fontinalis. Journal of Evolutionary Biology. 2007;20:2266–2277. doi: 10.1111/j.1420-9101.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- Thorpe JE, Mangel M, Metcalfe NB, Huntingford FA. Modeling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evolutionary Ecology. 1998;12:581–599. [Google Scholar]
- Thrower FP, Hard JJ, Joyce JE. Genetic architecture of growth and early life-history transitions in anadromous and derived freshwater populations of steelhead. Journal of Fish Biology. 2004;65:286–307. [Google Scholar]
- Tschaplinski PJ, Hartman GF. Winter distribution of juvenile coho salmon (Oncorhynchus kisutch) before and after logging in Carnation Creek, British Columbia, and some implications for overwinter survival. Canadian Journal of Fisheries and Aquatic Sciences. 1983;40:452–461. [Google Scholar]
- United States Environmental Protection Agency (USEPA) Level IV Ecoregions. 2007. Available at: http://www.epa.gov/wed/pages/ecoregions/level_iv.htm (accessed on 2 October 2007)
- Unwin MJ, Quinn TP, Kinnison MT, Boustead NC. Divergence in juvenile growth and life history in two recently colonized and partially isolated Chinook salmon populations. Journal of Fish Biology. 2000;57:943–960. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) 2005. Environmental Monitoring and Assessment Program [computer file]. EPA/ORD/NER/ESD Landscape Ecology Branch, Las Vegas, NV. ( http://www.epa.gov/esd/land-sci/emap_west_browser/pages/wemap_download.htm [accessed on 6 December, 2006]) [PubMed]
- U.S. Environmental Protection Agency, and U.S. Geological Survey (USEPA/USGS) 2005. National Hydrography Dataset (NHDPlus) [computer file]. Available at: http://www.horizon-systems.com/nhdplus/ [accessed 20 March 2008]
- United States Geological Survey (USGS) Digital Elevation Models (DEMs) 2005. Available at: http://edc.usgs.gov/products/elevation/dem.html [accessed on December 6, 2006] [Google Scholar]
- United States Geological Survey (USGS) Digital Elevation Models (DEMs) 2005. Available at: http://edc.usgs.gov/products/elevation/dem.html [accessed 6 December 2006] [Google Scholar]
- Van Hyning JM. Factors affecting the abundance of fall chinook salmon in the Columbia River. Fish Commission of Oregon Research Report. 1973;4:1–87. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human Domination of Earth’s Ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Waples RS. Evolutionary significant units and the conservation of biological diversity under the Endangered Species Act. In: Nielsen JL, editor. Evolution and the Aquatic Ecosystem: Defining Unique Units in Population Conservation. Bethesda, Maryland: American Fisheries Society; 1995. pp. 8–27. [Google Scholar]
- Waples RS. Definition and estimation of effective population size in the conservation of endangered species. In: Beissinger SR, McCullogh DR, editors. Population Viability Analysis. Chicago, IL: University of Chicago Press; 2002. pp. 147–168. [Google Scholar]
- Waples RS, Jones RP, Jr, Beckman BR, Swan GA. Status Review for Snake River Fall Chinook Salmon. 1991. U.S. Dep. Commer., NOAA Tech. Memo. NMFS F/NWC-201. 73p. [Google Scholar]
- Waples RS, Gustafson RG, Weitkamp LA, Myers JM, Johnson OW, Busby PJ, Hard JJ, et al. Characterizing diversity in salmon from the Pacific Northwest. Journal of Fish Biology. 2001;59:1–41. [Google Scholar]
- Wayne RK, Lehman N, Girman D, Gogan PJP, Gilbert DA, Hansen K, Peterson RO, et al. Conservation Genetics of the Endangered Isle-Royale Gray Wolf. Conservation Biology. 1991;5:41–51. [Google Scholar]
- Webb JH, McLay HA. Variation in the time of spawning of Atlantic salmon (Salmo salar) and its relationship to temperature in the Aberdeenshire Dee, Scotland. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:2739–2744. [Google Scholar]
- Whitlock MC, Barton NH. The effective size of a subdivided population. Genetics. 1997;146:427–441. doi: 10.1093/genetics/146.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States. BioScience. 1998;48:607–615. [Google Scholar]
- Wild V, Simianer H, Gjoen HM, Gjerde B. Genetic parameters and genotype X environment interaction for early sexual maturity in Atlantic salmon (Salmo salar. Aquaculture. 1994;128:51–65. [Google Scholar]
- Willi Y, Van Buskirk J, Hoffman AA. Limits to the adaptive potential of small populations. Annual Review of Ecology, Evolution and Systematics. 2006;37:433–458. [Google Scholar]
- Willi Y, Van Buskirk J, Schmid B, Fischer M. Genetic isolation of fragmented populations is exacerbated by drift and selection. Journal of Evolutionary Biology. 2007;20:534–542. doi: 10.1111/j.1420-9101.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- Williams TH, Bjorkstedt EP, Duffy W, Hillemeier D, Kautsky G, Lisle T, McCain M, et al. 2006. Historical population structure of coho salmon in the Southern Oregon / Northern California Coasts Evolutionarily Significant Unit. NOAA Technical Memo. NMFS-SWFSC-390.
- Williams JG, Zabel RW, Waples RS, Hutchings JA, Connor WP. Potential for anthropogenic disturbances to influence evolutionary change in the life history of a threatened salmonid. Evolutionary Applications. 2008;1:271–285. doi: 10.1111/j.1752-4571.2008.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, McDonald G, Moghadam HK, Herbinger CM, Ferguson MM. Marker-assisted estimation of quantitative genetic parameters in rainbow trout, Oncorhynchus mykiss. Genetical Research. 2003;81:145–156. doi: 10.1017/s0016672302006055. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 4th edn. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Zimmerman CE, Reeves GH. Population structure of sympatric anadromous and nonanadromous Oncorhynchus mykiss: evidence from spawning surveys and otolith microchemistry. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:2152–2162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


