Abstract
Gillnet fisheries are strongly size-selective and seem to produce changes in size at maturity for exploited fishes. After Word War II, large-scale gillnet fisheries targeted Pacific salmon (Oncorhynchus spp.) in the high seas area of the North Pacific and the Bering Sea, but these fisheries were closed in 1993. To assess the effects of this high seas gillnet fishery (and its closing) on size at maturity, we examined long-term trends in size at 50% probability of maturing (L50) for chum salmon (O. keta) from three populations in Hokkaido, Japan. The L50 trends were statistically different among rivers, but showed similar temporal patterns with decreases in the 1970s and early 1980s and increases after the 1985 brood year. While fishery-induced evolution seemed largely responsible for this temporal change in L50 during the fishing period, natural selection and phenotypic plasticity induced by environmental changes could contribute to the increases in L50 after the relaxation of fishing pressure.
Keywords: fishery-induced evolution, gillnet selectivity, high seas fishery, maturation threshold, ocean growth, phenotypic plasticity
Introduction
Fisheries can cause evolutionary changes toward smaller body sizes and younger ages at maturity (reviewed by Law 2000). This can occur through at least two different mechanisms. One mechanism is an increase in total mortality as a result of adding fishing mortality to natural mortality. This increased total mortality can select for earlier maturity, and therefore a smaller size at maturity (Reznick and Ghalambor 2005). A second mechanism is size-selective mortality that occurs because various fishing methods can be biased toward the capture of certain phenotypes (Stokes et al. 1993). The prevalence of fishery-induced evolutionary change, as a result of some combination of these mechanisms, has been revealed through decreases in fish size or age at maturity during periods vulnerable to fisheries (e.g., Ricker 1981, 1995; Rijnsdorp 1993; Trippel 1995; Olsen et al. 2004).
If a decrease in size or age at maturity is the result of fishery-induced evolution, an increase size or age at maturity would be expected owing to natural selection after the relaxation of fishing pressure, such as would result from fisheries closures or moratoria. For example, recent studies of pike (Esox lucius L.) show that natural selection and fishery selection often act in opposite directions, which causes somatic growth to increase after the relaxation of fishing pressure (Carlson et al. 2007; Edeline et al. 2007). However, increases or recoveries of maturation size after fishery closures or moratoria have rarely been reported (cf., Olsen et al. 2004). Thus, we examined whether the maturation size of chum salmon [Oncorhynchus keta (Walbaum)] increased after the closure of a high seas fishery.
Japanese high seas fisheries targeting Pacific salmon (Oncorhynchus spp.) were conducted in the central North Pacific and the Bering Sea. Chum salmon caught in the Bering Sea fishery area appeared to originate from Russia and Japan (Ishida et al. 1989). These high seas salmon fisheries developed in the 1950–1970s, decreased in the 1980s, and were closed in 1993 because of enforcement of the Convention for the Conservation of Anadromous Stocks in the North Pacific. This act prohibits salmon harvesting in international waters of the North Pacific and adjacent seas, with the exception of research activities. High seas salmon fishermen used gill nets with ≥121-mm mesh sizes (Harris 1989). Gillnet fisheries are size-selective and can provide a strong selective force for the evolution of life history traits (Handford et al. 1977; Ricker 1981).
Over the last few decades, the size at maturity of chum salmon has decreased in many populations (reviewed by Bigler et al. 1996). These trends are often interpreted as plastic responses of somatic growth rates resulting from environmental changes or density dependence (Ishida et al. 1993; Helle and Hoffman 1998; Kaeriyama 1998). For example, ocean somatic growth of chum salmon decreased in the 1970s and 1980s, when chum salmon abundance in the North Pacific Ocean increased (Fukuwaka et al. 2007). Under such variable conditions, organisms might be selected for particular plastic responses to specific environmental conditions, i.e., different ‘reaction norms’ (Stearns 1992; Chapter 6). Of most interest, here is the probability of maturity at a given size and age, the so-called probabilistic maturation reaction norm (PMRN). Recent work has shown how these PMRNs can be used to deduce evolutionary change while controlling for plastic variation in growth rates (Heino et al. 2002; Dieckmann and Heino 2007). We applied this method to recent changes in maturation size in Japanese chum salmon, as the change might be the result of adaptive evolution (Morita et al. 2005). Note that size at maturity (average body size of mature individuals) and PMRN (usually the reaction norm midpoint at the 50% maturation probability) are different measures and could show opposite trends (Morita and Fukuwaka 2007).
Thus, the objectives of this study were to describe temporal changes in maturation sizes for chum salmon before and after the closure of the high seas gillnet fishery. We specifically use the PMRN method to compare these changes to temporal changes in fishery-induced selection and natural selection. However, temporal changes in the PMRN will not necessarily provide a reliable indicator of genetic change, because PMRNs can change plastically with somatic growth (Morita and Fukuwaka 2006, 2007; Law 2007). One way to lessen this problem might be to compare observed changes to those expected based on natural selection (Swain et al. 2007). To this end, we compared the observed PMRNs to estimates of optimal maturation thresholds, with and without fishing mortality.
Materials and methods
Hatchery chum salmon populations
We examined chum salmon populations in the Chitose River (a tributary of the Ishikari River), the Nishibetsu River, and the Tokachi River, all in Hokkaido, Japan. The Chitose River is located in central Hokkaido and discharges into the Sea of Japan, near where a salmon hatchery was built in 1888. The Nishibetsu River is located in eastern Hokkaido and discharges into the Nemuro Strait between the Sea of Okhotsk and the Pacific Ocean, near a salmon hatchery built in 1890. The Tokachi River is located in eastern Hokkaido and discharges into the Pacific Ocean, near a salmon hatchery built in 1899. Chum salmon populations in these three rivers/hatcheries are genetically isolated from each other (Okazaki 1982). Little information is available on natural spawning populations in these three rivers, although a small number of carcasses (<100−1), potentially from naturally spawning adults, have been observed in the upper reaches of the Chitose River (Ito et al. 2005). Regardless, we consider our study populations to be of hatchery origin, as is the case for nearly all Japanese chum salmon (Hiroi 1998).
After release from hatcheries to rivers, Japanese chum salmon migrate widely in the North Pacific and adjacent waters (Fig. 1). The oceanic migration of chum salmon is a feeding migration, with individuals feeding on zooplankton such as jellyfish, ctenophores, and crustaceans (Quinn 2005). Japanese chum salmon are distributed in the Sea of Okhotsk during their first summer of ocean life, but move to the Bering Sea for their second and subsequent summers (Urawa et al. 2001). Somatic growth rates are highest during the summer (Ishida et al. 1998), and annual natural mortality coefficients (i.e., the instantaneous mortality rate caused by sources other than fisheries) range from 0.156 to 0.316 year−1 during offshore stages (Parker 1962; Ricker 1964, 1976). In the Bering Sea, immature and maturing chum salmon were vulnerable to the high seas fishery, which was size-selective (more details are provided below). In chum salmon, onset of the maturation process should occur at 3–5 years of age at or before the spring preceding the final year of ocean life (T. A. Onuma, H. Katsumata, K. Makino, M. Fukuwaka, P. Swanson and A. Urano, unpublished manuscript). During spawning migrations toward natal rivers, maturing chum salmon were vulnerable to coastal fisheries that primarily used trap nets. We assume that the coastal trap nets were not size-selective because the mesh sizes (≤105 mm in Hokkaido) were too small for maturing chum salmon to escape.
Figure 1.
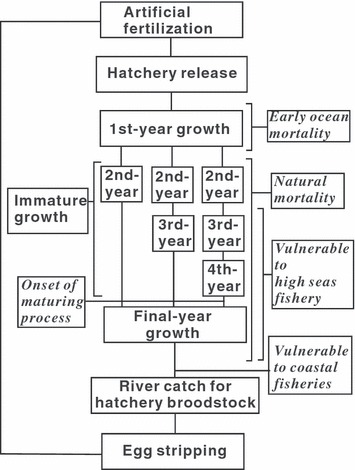
Schematic diagram of the life history of hatchery-reared chum salmon.
After entering their natal river, most fish were collected in a fish weir set to capture hatchery broodstock. The numbers of adult chum salmon caught for hatchery broodstock in the Chitose and Nishibetsu Rivers increased from the 1970s to the 1990s, showing a similar temporal trend to that for the total catch of Asian chum salmon. Catches in the Tokachi River were relatives stable over this period [Fig. 2; Hokkaido Salmon Hatchery (HSH) (1972–1997a); National Salmon Resources Center (NSRC) 1998–2004a]. Because they are semelparous, adult chum salmon were killed before egg or sperm stripping. Stripped eggs were artificially fertilized with sperm and incubated in a salmon hatchery. Juveniles were reared with dry pellet feed and released from the hatchery to the river in the spring of the subsequent year at ca. 5 cm fork length. The number of juveniles released into the Chitose River was stable from the late 1970s to the 1990s, and releases into the Nishibetsu and Tokachi Rivers were stable from the 1970s to the 1990s (Fig. 2). This stability was largely because of fixed hatchery carrying capacities, determined by the size and number of fish at release and the amount of available water (Websters 2001). Adult salmon caught in rivers exceeding hatchery capacity were not used for breeding. Although artificial selection or domestication selection is certainly possible in hatcheries (McLean et al. 2005; reviewed by Waples 1991, 1999; Reisenbichler and Rubin 1999), such information is not available for our study populations.
Figure 2.
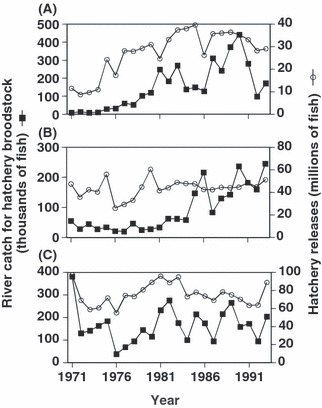
Numbers of hatchery broodstock caught in rivers and releases of chum salmon in the Chitose River (A), Nishibetsu River (B), and Tokachi River (C), 1971–1993.
High seas salmon fisheries in the North Pacific included two main types. The mothership fishery involved approximately 40 catcher boats that delivered salmon to a factory ship (i.e., the mothership), which then produced canned, salted, and frozen salmon. In contrast, land-based fisheries involved individual fishing boats that delivered salmon to a base port for processing. In the Bering Sea and central North Pacific, the mothership fishery operated from 1952 to 1989 and then changed to a land-based fishery from 1990 to 1993. In the western North Pacific, a land-based fishery predominated from 1952 to 1993. The best fishing season for chum salmon in the mothership fishery was in July (Peterson 1974). During the summer, Japanese populations accounted for a high proportion of chum salmon in the Bering Sea (Ishida et al. 1989; Seeb et al. 2004). Chum salmon catches from the high seas fisheries in the central North Pacific and the Bering Sea accounted for 0.5–33% of total Asian chum salmon catches, including the high seas fishery in the western North Pacific and the coastal and riverine catches, during 1969–1991 (Fig. 3; Eggers et al. 2003). In the 1960s and 1970s, chum salmon catches in the central North Pacific and the Bering Sea were larger than before. While catches in the central North Pacific and the Bering Sea decreased after 1979, catches in other areas, mainly coastal fisheries, increased rapidly in the 1980s.
Figure 3.
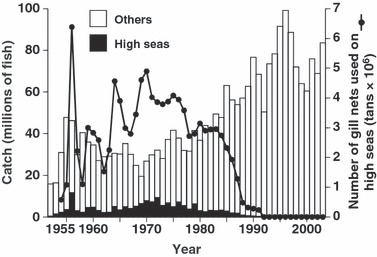
Chum salmon catches in Asia (bars) and number of gill nets used in the high seas area (line) from 1952 to 2003. The solid bar indicates the catch in the high seas area east of 170°E. The open bar indicates catches along the Asian coasts, in rivers, and in offshore areas west of 170°E. The number of gill nets used during 1952 and 1953 was not available. One tan (panel) of gill net is ca. 50 m in length and ca. 7 m in height.
Estimation of threshold size for maturation
To estimate mean threshold size for maturation of chum salmon, we estimated fork length at 50% maturity, L50, from adult chum salmon returning to the three rivers. We examined only females because fecundity selection acted on female optimal maturation threshold. Returning females were caught in rivers from 1976 to 1997, mainly using fish weirs. In total, 6911 females were sampled; each individual fork length (cm) was measured, and a scale was collected from the region between the dorsal and anal fins and near the lateral line. Fish age was determined by counting the number of annuli on a scale.
We estimated L50 using back-calculated fork lengths at immature and maturing ages because the timing of the onset of maturity is nearly coincident with the timing of annulus formation (winter to spring) (Fukuwaka 1998; Campbell et al. 2003; T. A. Onuma, H. Katsumata, K. Makino, M. Fukuwaka, P. Swanson and A. Urano, unpublished manuscript). Scale radii, from the center of focus to every annulus and to the edge of the scale, were measured to the nearest micrometer along the longest axis of the scale using a video micrometer system. The biological intercept method was used to estimate fork length at each ocean age (Campana 1990). In the biological intercept back-calculation, we used a 4-cm fork length and a 114 μm scale radius as the base points (Fukuwaka and Kaeriyama 1994).
To estimate L50, we used the PMRN method with a generalized linear model (GLM) with maturity as a binary dependent variable (i.e., mature or immature), back-calculated fork length and age as continuous independent variables, and brood year as a categorical independent variable (Heino et al. 2002). In this estimation, we assumed a quasibinomial distribution of error, allowing a larger variance than the nominal variance of a binomial distribution because of the overdispersion of maturity variables (McCullagh and Nelder 1989; R Development Core Team 2007). We used the statistical package R version 2.5.1 (The R Foundation for Statistical Computing) for the estimations (R Development Core Team 2007). The model equation was
| 1 |
where αb was the intercept for brood year b, and β1 and β2 were slopes (Heino et al. 2002). Age-specific L50 was calculated as
| 2 |
Confidence limits for L50 were estimated using the delta method from the covariance matrix of parameters.
When immature fork lengths are back-calculated from scales of mature fish, the estimated L50 can be biased by mortality suffered during immature ages (Morita et al. 2005). Thus, to correct the bias because of natural and fishing mortality, the maturity variable was weighted by estimating the number of fish alive at a specific age per number of samples, assuming that the exploitation rate of coastal fisheries was constant and uniform:
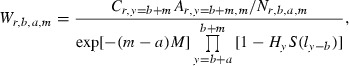 |
3 |
where
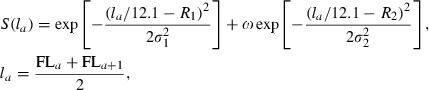 |
Wr,b,a,m was weighted for fish age at maturity m for brood year b at river r and back-calculated age a. Cr,y was the number of fish caught in river r in year y; Ar,y,m was the proportion of fish of age at maturity m caught in year y and river r; Nr,b,a,m was the number of samples of age at maturity m for brood year b at river r and back-calculated age a; M was the natural mortality coefficient for ocean life; Hy was the encounter rate of fish to gill nets; q was the catchability coefficient; Ey was fishing effort in tans (or panels) of gill nets for year y; S(l) was a binormal selection function of 121-mm mesh gillnets for fork length l; R1, R2, σ1, σ2, ω were parameters in the binormal gillnet selection function (Millar and Fryer 1999); and FLa was the back-calculated fork length for age a.
To calculate the weight at maturity variable, river catches, and age compositions for female chum salmon during returning years were obtained from HSH (1972–1997a, 1996–1997b) and NSRC (1998–2004a, 1998–2004b). The number of tans of gill nets used east of 170°E by the high seas fishery was obtained from the International North Pacific Fisheries Commission (INPFC 1958–1996). For gill net selection parameters, we used R1 = 5.36, R2 = 32.2, σ1 = 0.496, σ2 = 4.88, and ω = 28700 (M. Fukuwaka, T. Azumaya, N. Davis and T. Nagasawa, unpublished manuscript).
To confirm that estimated temporal changes of L50 were not artifacts because of estimation biases with assumed mortalities, we examined some cases with realistic ranges of natural mortality and fishing mortality. We used three values of M (i.e., 0.1, 0.2, and 0.3) and brood year-specific M values calculated from the river catch-at-age data. The abundance of chum salmon was largely affected by the coastal environment during early ocean life (Fukuwaka and Suzuki 2002; Mueter et al. 2002). However, natural ocean mortality could not be rejected as a determinant of chum salmon abundance. Thus, in addition to three constant values of M, we calculated brood year-specific M values, assuming that freshwater and early coastal survival was uniform and the exploitation rates of the coastal fisheries were the ratio of the coastal catch to the sum of the coastal and river catches in each region of Hokkaido.
For cases of different fishing mortalities, we used three values of q: 5.0 × 10−8, 7.5 × 10−8, and 1.0 × 10−7. The mean estimate of q for the period from 1954 to 1991 was 4.71 × 10−8 (range 1.81 × 10−8 to 1.35 × 10−7), assuming that all catches in the high seas fishery were reported, no non-catch losses occurred, fishing gear selected only maturing fish, and all maturing fish were caught along the Asian coast and in rivers. However, the values may be underestimates because violations of the high seas fishery regulation were observed (Fredin et al. 1977) and estimates of non-catch fishing mortality by gill nets were less than 50% for immature fish and approximately 25% for maturing fish (Ricker 1976).
Tests for differences among rivers and temporal trends
To test for differences among rivers or temporal trends in L50 values, we used GLM and ANOVA. To test for differences among river populations, we used the significance of the interaction terms with rivers included in the model:
| 4 |
To test for temporal trends in L50 before and after the closure of the high seas fishery, we assessed significant differences in the slopes for brood years as continuous variables between the period before the 1985 brood year and the period since the 1985 brood year (i.e., the significance of the interaction term brood × period) in the model:
| 5 |
where subscript p indicates either the period before the 1985 brood year or since the 1985 brood year. In these tests, we used a quasibinomial GLM (McCullagh and Nelder 1989; R Development Core Team 2007). The significances of the main effects and interaction effects were tested using F-statistics because the likelihood ratio test could not be used in the quasibinomial GLM. The rate of L50 change in haldanes was estimated in standard deviations per generation using coefficients in the equation (5) (Hendry and Kinnison 1999):
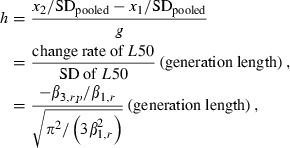 |
where x2 and x1 were the mean trait values at two different times, SDpooled was the pooled standard deviation, and g was the number of generation (years divided by generation length). Generation length was the average age at maturity for parents by river and by period, which was calculated from the age composition and the number of broodstocks used in the hatcheries.
Direction of selection
To examine the effect of fishery-induced selection on observed L50, we compared the direction of selection and response to selection. The direction of selection on a brood year-specific L50 was the sign of the difference between the brood year-specific optimal maturation threshold and the observed L50:
where Lm was the optimal maturation threshold. The response to selection was the sign of the difference between the observed L50 and the L50 4 years later:
because most female chum salmon mature when 4 years old (ocean age 3 corresponded to 4 years old at maturity). We used the Ives–Gibbons correlation coefficient for testing between these dichotomous variables, which ranges from −1 to 1 (Zar 1999). The significance of the coefficient was tested using a binomial test with a null hypothesis H0: P = 0.5. When the direction corresponds to the response perfectly, the correlation coefficient is 1, and the test rejects the null hypothesis. When the direction corresponds to the response in half of the cases, the correlation coefficient is 0, and the test does not reject the null hypothesis. However, when the direction conflicts with the response perfectly, the correlation coefficient is −1, and the test rejects the null hypothesis.
To predict optimal maturation thresholds, we used the integral projection model for the life history of hatchery-reared chum salmon (Appendix A). We estimated the optimal brood year-specific intercept for the maturation function αb with fixed values of β1 and β2 by maximizing the net reproductive rate, R0, because density dependence acted at hatchery capacity. To examine the effect of fishing selection on L50, we estimated optimal maturation thresholds under two alternative assumptions: presence and absence of fishing mortality. While temporal changes in optimal maturation threshold with fishing mortality reflected offshore mortality, including natural and fishing mortalities, values of optimal maturation threshold without fishing reflected the effect of somatic growth because R0 was determined by mortality and fecundity.
Results
Estimated L50 increased after the 1985 brood year, while it had decreased before the 1985 brood year (Fig. 4). In the late 1980s, the fishing effort by the high seas fishery decreased sharply (Fig. 3). Although we only used mature fish caught in rivers that had experienced size-selective fishing mortality on the high seas to estimate L50, the temporal correspondence was not an artifact of the L50 estimation because our calculations were weighted using fishing effort and similar temporal trends were observed for L50 values with different values of q. In examining cases with different values of q or M, L50 did differ among cases but changed similarly through time. The maturity function and estimated L50 values also differed among river populations (P < 0.001 for interaction terms of αbr and β1,r, but P = 0.101 for the interaction term of β2,r in GLM equation (4) in the case of q = 7.5 × 10−8 and M = 0.2). However, a similar temporal trend in L50, that is, decreasing before the 1985 brood year and increasing after the 1985 brood year, was observed (P < 0.001 for the interaction term of β3,rp in GLM equation (5), the same sign of β3,rp among river populations in both periods, plus before the 1985 brood year and minus after the 1985 brood year). The rate of phenotypic change in L50 estimated from GLM coefficients was −0.214 haldanes (generation length = 3.57 years) for the Chitose River, −0.170 haldanes (generation length = 3.80 years) for the Nishibetsu River, and −0.089 haldanes (generation length = 4.07 years) for the Tokachi River before the 1985 brood year (12 years), and 0.782 haldanes (generation length = 3.90 years) for the Chitose River, 0.527 haldanes (generation length = 3.93 years) for the Nishibetsu River, and 0.746 haldanes (generation length = 4.36 years) for the Tokachi River after the 1985 brood year (8 years) when q = 7.5 × 10−8 and M = 0.2. Size at maturity (i.e., average fork length of mature individuals) for 4-year-old fish decreased throughout the study period that differed from the temporal pattern of L50.
Figure 4.
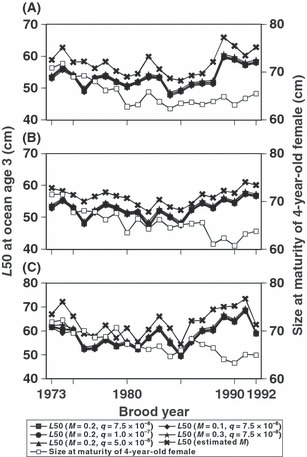
Estimated L50 for female chum salmon at ocean age 3 (i.e., 4 years old) under assumptions of M and q values (see ‘Materials and methods’) and size at maturity (i.e., average fork length of mature individuals) for 4 year-olds from the 1973 to 1992 cohorts from the Chitose River (A), Nishibetsu River (B), and Tokachi River (C) populations. Not all results from possible combinations of different values of M and q are shown because estimates of other combinations were similar to values presented in this figure.
The optimal maturation threshold under the assumption of the presence of fishing mortality increased until the mid 1980s, reflecting decreased high seas fishing effort (Fig. 5). Estimated L50 values when q = 7.5 × 10−8 and M = 0.2 were larger than the optimal maturation threshold until the mid 1980s. After 1985, estimated L50 values were often smaller than the optimal maturation threshold. The optimal maturation threshold under the assumption of the absence of fishing mortality decreased until the 1989 brood year but increased after the 1989 brood year, reflecting temporal changes in immature growth. The temporal changes in estimated L50 at ocean age 3 did not correspond to either the change in optimal maturation threshold without fishing mortality (r = −0.180 to −0.005, p = 0.448 to 0.982, n = 20) or to the change in immature growth at ocean age 2 (r = −0.326 to −0.125, p = 0.161 to 0.599, n = 20). These indicate that observed L50 was not only affected by optimal phenotypic plasticity induced by changes in somatic growth but also by fishing mortality.
Figure 5.
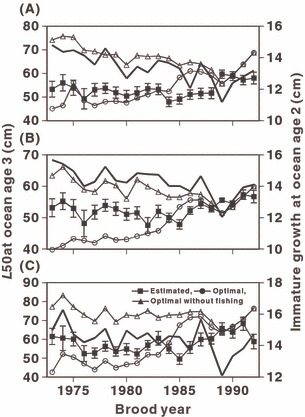
Estimated L50, optimal L50, optimal L50 without high seas fishing at ocean age 3 (thin lines), and weighted average of immature growth at ocean age 2 (thick line) under the assumption of M = 0.2 and q = 7.5 × 10−8 for 1973–1992 cohorts from the Chitose River (A), Nishibetsu River (B), and Tokachi River (C) populations of female chum salmon. Vertical bar indicates 95% confidence interval of estimated L50.
In two cases, q = 7.5 × 10−8 or q = 1.0 × 10−7 and M = 0.2, the estimated direction of selection corresponded to the observed temporal changes in L50 (Table 1). In all other cases, the estimated direction of selection did not correspond to the observed response of L50.
Table 1.
Ives–Gibbons correlation coefficients (rn) and significance levels (P) between the direction of selection and the sign of the response of L50 estimated under assumptions of M and q values (see ‘Materials and methods’).
| Chitose River | Nishibetsu River | Tokachi River | |||||
|---|---|---|---|---|---|---|---|
| M | q | rn | P | rn | P | rn | P |
| 0.2 | 5.0 × 10−8 | 0.176 | 0.166 | 0.778 | <0.001 | 0.000 | 0.407 |
| 0.2 | 7.5 × 10−8 | 0.647 | <0.01 | 0.556 | <0.01 | 0.444 | <0.05 |
| 0.2 | 1.0 × 10−7 | 0.765 | <0.001 | 0.444 | <0.05 | 0.333 | <0.05 |
| 0.1 | 7.5 × 10−8 | −0.059 | 0.500 | 0.444 | <0.05 | 0.000 | 0.407 |
| 0.3 | 7.5 × 10−8 | 0.176 | 0.166 | 0.111 | 0.240 | 0.222 | 0.119 |
| Estimated | 7.5 × 10−8 | 0.125 | 0.227 | 0.000 | 0.402 | 0.000 | 0.402 |
The predicted optimal maturation threshold was sensitive to assumed natural and fishing mortalities, whereas changes in the estimated L50 were smaller because of different values of assumed mortalities (Fig. 4). This indicates that the interpretation of temporal changes in L50 for chum salmon is dependent on the assumptions of natural or fishing mortalities.
Discussion
Here, we showed an increase in the maturation size threshold for chum salmon after the closure of high seas gillnet fishery. That is, chum salmon tended to mature at larger ages and larger sizes. Observed rates of phenotypic change during the recovery (0.527–0.782 haldanes over 8 years, i.e., 1.83–2.05 generations) were similar to previously reported rates of contemporary phenotypic change (Hendry and Kinnison 1999) but were lower than the change in similarly-estimated maturation thresholds for Atlantic cod (Gadus morhua L.) during intense fishing (Olsen et al. 2004). A size-structured consumer-resource model that considered reduced food competition and size-selectivity caused by harvesting, predicted multiple alternative ecological and evolutionary stable states (ESSs) for size at maturity (de Roos et al. 2006). In such a case, protracted and high fishing pressure (i.e., 40 years, ca. 25 generations, and a 60% exploitation rate) could cause a shift to a new ESS, which might then prolong the reduced size at maturity, even during a moratorium. The exploitation rate of the high seas salmon fishery on Asian chum salmon was 33% at its maximum, assuming the fishery caught only maturing fish. Although we did not assess alternative ESSs for chum salmon maturation thresholds, the actual duration of this high-intensity fishing does not seem to have been sufficient to have prevented recovery of the maturation threshold after fishing ceased. However, despite the recovery in maturation thresholds, the observed average size at maturity has either remained stable or has decreased (Fig. 4). Such contrasts between phenotypic and genetic responses to environmental change appear common (Merilä et al. 2001; Gienapp et al. 2008), and probably reflect opposing influences of natural selection and the plastic influence of environmental conditions. For chum salmon, it seems that selection favors maturity at larger sizes but ocean conditions are still leading to slower growth, thus resulting in no increase in observed mean body size.
Although our results are consistent with an interpretation of evolutionary responses to the cessation of a size-selective high seas fishery, other explanations must be considered. The coastal trap net fishery is one such possibility, particularly because it had a very high exploitation rate throughout the study period. We suggest that this fishery was not the cause of changes in maturation size thresholds, primarily because the mesh size of the traps (≤105 mm in Hokkaido) is too small for mature chum salmon to escape. We further suggest that the overall increases in size-independent mortality owing to this fishery are not driving the observed trends. Harvesting both immature and mature individuals can reduce size and age at maturity, but harvesting only mature individuals can increase size and age at maturity in iteroparous organisms (Law and Grey 1989; Heino 1998; Ernande et al. 2004). Pacific salmon, however, are semelparous and, in this case, there is no theoretical reason to suspect that increasing size-independent mortality for adults alone (Fig. 3) would lead to selection for larger size at maturity. We confirmed this expectation by finding that changes in coastal exploitation rates do not affect optimal maturation threshold values (result not shown).
Another possible explanation for changes in maturation thresholds is hatchery effects. Here, we considered that a stable hatchery capacity leads to fecundity selection as a countering force to fishery-induced selection on maturation thresholds (Ratner and Lande 2001). In a growing population, selection favors younger age at maturity because individuals maturing at younger ages can have a larger contribution to the future population than individuals maturing at older ages (Stearns 1992). If somatic growth rate does not change, selection toward a younger age at maturity leads to a decrease in the maturation threshold. It is therefore worth considering whether production changes in the hatcheries might have shifted from a period of population growth, thus favoring younger age at maturity, to a stable population at carrying capacity, thus favoring older age at maturity. Until 1985, hatchery releases increased and the maturation threshold decreased in the Chitose River. However, maturation thresholds in the Nishibetsu and Tokachi Rivers also decreased during the same period, whereas hatchery releases there showed no temporal trends. Thus, the decrease in maturation threshold until the mid 1980s may not have been induced by temporal changes in hatchery releases. Although other aspects of domestication or artificial selection on size at maturity may occur in these hatchery populations, any such effects are unknown.
Finally, changes in maturation thresholds might be the result of phenotypic plasticity induced by environmental change. Indeed, the maturation threshold (L50) for chum salmon changes plastically with somatic growth rate, a reaction norm that may be adaptive (Morita et al. 2005; Morita and Fukuwaka 2006). In general, historical trends in PMRNs will never be able to fully exclude the possibility of correlated, but unmeasured, environmental drivers (Law 2007; Morita and Fukuwaka 2007). Additional support for evolutionary responses might therefore be gained by showing that observed changes in maturation thresholds are well-predicted by observed selection (Swain et al. 2007) or by optimality models (present study). Here, we found that temporal changes in L50 mostly corresponded to predictions with respect to changes in fishing but not to predictions with respect to changes in immature growth or optimal maturation thresholds without fishing. This correspondence between changes in fishing and changes in both predicted and observed maturation thresholds supports our conclusion that the observed trends are at least partly driven by genetic adaptations to changes in fishing pressure. Swain et al. (2007) reached a similar conclusion based on their analysis of selection on, and phenotypic change in, size at age in Gulf of St Lawrence cod stocks.
Why does the cessation of fishing lead to the recovery of life history parameters in some stocks, such as Japanese chum salmon (this study), but not in others, such as some cod stocks (Olsen et al. 2004)? We suggest that the difference may be related to trophic levels. For Atlantic cod off southern Labrador and eastern Newfoundland, which prey on fish or macroinvertebrates, the maturation threshold has begun to recover after fishing ceased but population sizes remain very low (Olsen et al. 2004, 2005; Frank et al. 2005). Maladaptation of life history traits induced by fisheries was cited as one of the causes for this prolonged depression in abundance (Olsen et al. 2004, 2005; Walsh et al. 2006). A contrasting situation is found in plankton-feeding chum salmon, which are showing recovery in maturation thresholds and have a very high stock abundance (Eggers et al. 2003; Fukuwaka et al. 2007; Morita and Fukuwaka 2007). Similarly, planktivorous Norwegian spring-spawning herring (Clupea harengus L.) have shown full recovery of maturation thresholds and stock condition following the relaxation of fishing (Engelhard and Heino 2004). We suggest that these trophic level differences lead to different genetic and plastic bases for maturation thresholds and therefore different evolutionary responses following changes in fishing pressure. For example, lower trophic levels may be more strongly affected by temporal and spatial fluctuations in primary and secondary productivity (Beamish and Bouillon 1993; Robinson and Ware 1999; Hutchings 2000; Hutchings and Baum 2005). Under these conditions, genetic variation in maturation thresholds might be maintained, thereby allowing more rapid responses to changes in selection.
In summary, fishery-induced evolution seemed largely responsible for the temporal change in the maturation threshold for Japanese chum salmon during the fishing period, whereas natural selection and phenotypic plasticity likely contributed to changes that occurred after the relaxation of fishing. Ocean growth and size at maturity of chum salmon decreased with increased abundance in the 1970s to mid 1990s, which seemed to result from density dependence (Ishida et al. 1993; Bigler et al. 1996). More recently, the sizes of adults caught in rivers and ocean growth have increased significantly, and the chum salmon stock level has remained at a historic high (Helle and Hoffman 1998; Eggers et al. 2003; Fukuwaka et al. 2007; Morita and Fukuwaka 2007). Helle and Hoffman (1998) suggested that the change in association between abundance and growth was caused by a change in ocean carrying capacity for salmon. Changing somatic growth can induce plastic changes in size and age at maturity (Morita et al. 2005; Morita and Fukuwaka 2007). Because of partial genetic control of size at maturity in Pacific salmon (Smoker et al. 1994; Gall and Neira 2004), environmentally induced changes in somatic growth can also induce evolutionary changes in size at maturity through natural selection.
Acknowledgments
We thank A. Hendry for invaluable comments and suggestions to earlier versions of the manuscript and for the invitation to contribute to the Special Issue. We also thank R. Waples for the invitation, and the staff of the National Salmon Resources Center, Fisheries Research Agency, for their careful collection of data and samples.
Appendix A. Estimation of optimal threshold size at maturation for female chum salmon
To predict the optimal threshold size at maturation by brood year, we used the integral projection model modified for the life history of hatchery reared chum salmon (Fig. 1) (Ellner and Rees 2006). The number of chum salmon in a fork length class xj at time t + 1 was
where w was the width of the fork length class, m was the number of fork length classes covering the range of possible fork lengths, Pb was the survival and growth rate from a class xi to a class xj for brood year b, and Fb was the production of female juveniles in a class xj by a female in a class xi for brood year b. The optimal maturation function was computed by numerical maximization of the net reproductive rate, R0, because hatchery capacities limited juvenile releases in early life. R0 equals the average per capita life time juvenile production of a cohort of newborns.
Survival and growth rate was
where sb(xi) was the annual survival rate at fork length xi, m(xi, a) was the maturation rate at fork length xi and age a, and gb(xj, xi, a) was the transition rate due to immature growth from fork length class xi to xj at age a. The survival rate function includes natural mortality and fishing mortality as in equation (3):
The production of female juveniles from a female in fork length class xi was
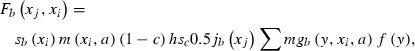 |
where c was the exploitation rate of coastal fisheries, h was the hatchery survival from the egg stripping to the release, sc was the freshwater and coastal survival of released juveniles, jb(xj) was the fork length distribution of ocean age 1 fish, mgb(y, xi, a) was the transition rate from fork length class xi to y at age a due to final year growth, and f(y) was the fecundity function at final fork length y. Functions and parameters used in the model are shown in Table A1. We estimated growth parameters by brood year from back-calculated fork lengths weighted by the estimated number of fish alive at a specific age per number of samples shown in the ‘Materials and methods’.
Literature cited
Ellner, S. P., and M. Rees. 2006. Integral projection models for species with complex demography. American Naturalist 167:410–428.
Table A1.
| Demographic process | Function or parameters | Calculation method |
| Ocean natural mortality | M = 0.1, 0.2, and 0.3 | |
| Catchability coefficient | q = 0 (without fishing mortality), 5.0 × 10−8, 7.5 × 10−8, and 1.0 × 10−7 (with fishing mortality) | |
| Maturation rate | logit(m) = αb + β1x + β2a | GLM with weights using different values of M and q |
| Immature growth | xj − xi = αb + β1xi + β2a + e | ANOVA of back-calculated growth with weights using different values of M and q |
| Exploitation rate of coastal fisheries | c = 0.809 (CH), 0.948 (NI), and 0.931 (TO) | 1 − Geometric mean [upstream migration rate (UMR), 1976–1997]. UMR = river catch/(river catch + coastal catch) in the region was obtained from HSH (1977–1997a) and NSRC (1998a) |
| Hatchery survival | h = 0.9 | |
| Freshwater and coastal survival | Number of ocean age 1 fish/number of released juveniles | VPA using different values of M and q |
| Fork length distribution of ocean age 1 fish | Mean xj + e | Average of back-calculated fork length with weights using different values of M and q |
| Final year growth | y − xi = αb + β1xi + β2a + e | ANOVA of back-calculated growth with weights using different values of M and q |
| Fecundity | log10f = −2.81 + 3.47 log10y (CH), log10f = −1.61 + 2.76 log10y (NI), and log10f = −3.07 + 3.55 log10y (TO) | Geometric linear regression using the authors’ unpublished data |
Literature cited
- Beamish RJ, Bouillon DR. Pacific salmon production trends in relation to climate. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:1002–1016. [Google Scholar]
- Bigler BS, Welch DW, Helle JH. A review of size trends among North Pacific salmon (Oncorhynchus spp.) Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:455–465. [Google Scholar]
- Campana SW. How reliable are growth back-calculations based on otoliths? Canadian Journal of Fisheries and Aquatic Sciences. 1990;47:2219–2227. [Google Scholar]
- Campbell B, Dickey JT, Swanson P. Endocrine changes during onset of puberty in male spring chinook salmon, Oncorhynchus tshawytscha. Biology of Reproduction. 2003;69:2109–2117. doi: 10.1095/biolreprod.103.020560. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, Fletcher JM, James JB, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamics tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the USA. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers DM, Irvine JR, Fukuwaka M, Karpenko VI. Catch trends and status of North Pacific salmon (NPAFC Doc. No. 723, Rev. 3) Vancouver: North Pacific Anadromous Fish Commission; 2003. http://www.npafc.org. Accessed 1 April 2007. [Google Scholar]
- Engelhard GH, Heino M. Maturity changes in Norwegian spring-spawning herring Clupea harengus: compensatory or evolutionary responses? Marine Ecology Progress Series. 2004;272:245–256. [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London Series B. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- Fredin RA, Major RL, Bakkala RG, Tanonaka GK. Pacific Salmon and the High Seas Salmon Fisheries of Japan (Processed Report) Seattle: US Department of Commerce, National Oceanic and Atmospheric Administration, Northwest and Alaska Fisheries Center; 1977. [Google Scholar]
- Fukuwaka M. Scale and otolith patterns prove growth history of Pacific salmon. North Pacific Anadromous Fish Commission Bulletin. 1998;1:190–198. [Google Scholar]
- Fukuwaka M, Kaeriyama M. A back-calculation method for estimating individual growth of juvenile chum salmon by scale analysis. Scientific Reports of the Hokkaido Salmon Hatchery. 1994;48:1–9. [Google Scholar]
- Fukuwaka M, Suzuki T. Early sea mortality of mark-recaptured juvenile chum salmon in open coastal waters. Journal of Fish Biology. 2002;60:3–12. [Google Scholar]
- Fukuwaka M, Azumaya T, Nagasawa T, Starovoytov AN, Helle JH, Saito T, Hasegawa E. Trends in abundance and biological characteristics of chum salmon. North Pacific Anadromous Fish Commission Bulletin. 2007;4:35–43. [Google Scholar]
- Gall GAE, Neira R. Genetic analysis of female reproduction traits of farmed coho salmon (Oncorhynchus kisutch. Aquaculture. 2004;234:143–154. [Google Scholar]
- Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. Climate change and evolution: disentangling environmental and genetic responses. Molecular Ecology. 2008;17:167–178. doi: 10.1111/j.1365-294X.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- Handford P, Bell G, Reimchen T. A gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Harris CK. Seattle: University of Washington; 1989. The Effect of International Treaty Changes on Japan’s High Seas Salmon Fisheries, with Emphasis on their Catches of North American Sockeye Salmon, 1972–1984. PhD Dissertation. [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1971–1982. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Helle JH, Hoffman MS. Changes in size and age at maturity of two North American stocks of chum salmon (Oncorhynchus keta) before and after a major regime shift in the North Pacific Ocean. North Pacific Anadromous Fish Commission Bulletin. 1998;1:81–89. [Google Scholar]
- Hendry AP, Kinnison MT. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hiroi O. Historical trends of salmon fisheries and stock conditions in Japan. North Pacific Anadromous Fish Commission Bulletin. 1998;1:23–27. [Google Scholar]
- Hokkaido Salmon Hatchery (HSH) Jigyo seiseki-sho. Sapporo: Hokkaido Salmon Hatchery, Fisheries Agency of Japan; 1972-1997a. [Google Scholar]
- Hokkaido Salmon Hatchery (HSH) Database on Biological Assessment of Chum Salmon Populations in Japan 1994, 1995. Sapporo: Hokkaido Salmon Hatchery, Fisheries Agency of Japan; 1996-1997b. [Google Scholar]
- Hutchings JA. Collapse and recovery of marine fishes. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Baum JK. Measuring marine fish biodiversity: temporal changes in abundance, life history and demography. Philosophical Transactions of the Royal Society of London Series B. 2005;260:315–338. doi: 10.1098/rstb.2004.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International North Pacific Fisheries Commission (INPFC) Statistical Yearbook 1952–1992. Vancouver: International North Pacific Fisheries Commission; 1958-1996. [Google Scholar]
- Ishida Y, Ito S, Takagi K. Stock identification of chum salmon Oncorhynchus keta from their maturity and scale characters. Nippon Suisan Gakkaishi. 1989;55:654–656. [Google Scholar]
- Ishida Y, Ito S, Kaeriyama M, McKinnell S, Nagasawa K. Recent changes in age and size of chum salmon (Oncorhynchus keta) in the North Pacific Ocean and possible causes. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:290–295. [Google Scholar]
- Ishida Y, Ito S, Ueno Y, Sakai J. Seasonal growth patterns of Pacific salmon (Oncorhynchus spp.) in offshore waters of the North Pacific Ocean. North Pacific Anadromous Fish Commission Bulletin. 1998;1:66–80. [Google Scholar]
- Ito T, Nakajima M, Shimoda K. Abundance of salmon carcasses at the upper reach of a fish trap. Ecological Research. 2005;20:87–93. [Google Scholar]
- Kaeriyama M. Dynamics of chum salmon, Oncorhynchus keta, populations released from Hokkaido, Japan. North Pacific Anadromous Fish Commission Bulletin. 1998;1:90–102. [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:249–251. [Google Scholar]
- Law R, Grey DB. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology. 1989;3:343–359. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd edn. London: Chapman & Hall; 1989. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Nonrandom, size- and timing-biased breeding in a hatchery population of steelhead trout. Conservation Biology. 2005;19:446–454. [Google Scholar]
- Merilä J, Sheldon BC, Kruuk LEB. Explaining stasis: microevolutionary studies in natural populations. Genetica. 2001;112–113:199–222. [PubMed] [Google Scholar]
- Millar RB, Fryer RJ. Estimating the size-selection curves of towed gears, traps, nets and hooks. Reviews in Fish Biology and Fisheries. 1999;9:89–116. [Google Scholar]
- Morita K, Fukuwaka M. Does size matter most? The effect of growth history on probabilistic reaction norm for salmon maturation. Evolution. 2006;60:1516–1521. [PubMed] [Google Scholar]
- Morita K, Fukuwaka M. Why age and size at maturity have changed in Pacific salmon. Marine Ecology Progress Series. 2007;335:289–294. [Google Scholar]
- Morita K, Morita SH, Fukuwaka M, Matsuda H. Rule of age and size at maturity of chum salmon (Oncorhynchus keta): implications of recent trends among Oncorhynchus spp. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:2752–2759. [Google Scholar]
- Mueter FJ, Peterman RM, Pyper BJ. Opposite effects of ocean temperature on survival rates of 120 stocks of Pacific salmon (Oncorhynchus spp.) in northern and southern areas. Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:456–463. [Google Scholar]
- National Salmon Resources Center (NSRC) Gyomu houkoku-sho. Sapporo: National Salmon Resources Center; 1998-2004a. [Google Scholar]
- National Salmon Resources Center (NSRC) Database on Biological Assessment of Chum Salmon Populations in Japan, 1996–2003. Sapporo: National Salmon Resources Center; 1998-2004b. [Google Scholar]
- Okazaki T. Genetic study on population structure in chum salmon (Oncorhynchus keta. Bulletin of the Far Seas Fisheries Research Laboratory. 1982;19:25–116. [Google Scholar]
- Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Lilly GR, Heino M, Morgan MJ, Brattey J, Dieckmann U. Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:811–823. [Google Scholar]
- Parker RR. Estimations of ocean mortality rates for Pacific salmon (Oncorhynchus. Journal of the Fisheries Research Board of Canada. 1962;19:561–589. [Google Scholar]
- Peterson AE. Atlas of catch and fishing effort, Japanese mothership salmon fishery, 1956–1970. International North Pacific Fisheries Commission Bulletin. 1974;30:81–107. [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle: University of Washington Press; 2005. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. http://www.R-project.org. Accessed 28 August 2007. [Google Scholar]
- Ratner S, Lande R. Demographic and evolutionary responses to selective harvesting in populations with discrete generations. Ecology. 2001;82:3093–3104. [Google Scholar]
- Reisenbichler RR, Rubin SP. Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES Journal of Marine Science. 1999;56:459–466. [Google Scholar]
- Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:791–801. [Google Scholar]
- Ricker WE. Ocean growth and mortality of pink and chum salmon. Journal of the Fisheries Research Board of Canada. 1964;21:905–931. [Google Scholar]
- Ricker WE. Review of the rate of growth and mortality of Pacific salmon in salt water, and noncatch mortality caused by fishing. Journal of the Fisheries Research Board of Canada. 1976;33:1483–1524. [Google Scholar]
- Ricker WE. Changes in the average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Ricker WE. Trends in the average size of Pacific salmon in Canadian catches. Canadian Special Publication of Fisheries and Aquatic Sciences. 1995;121:593–602. [Google Scholar]
- Rijnsdorp AD. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. [DOI] [PubMed] [Google Scholar]
- Robinson CLK, Ware DM. Simulated and observed response of the southwest Vancouver Island pelagic ecosystem to oceanic conditions in the 1990s. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:2433–2443. [Google Scholar]
- De Roos AM, Boukal DS, Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proceedings of the Royal Society of London Series B. 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeb LW, Crane PA, Kondzela CM, Wilmot RL, Urawa S, Varnavskaya NV, Seeb JE. Migration of Pacific rim chum salmon on the high seas: insight from genetic data. Environmental Biology of Fishes. 2004;69:21–36. [Google Scholar]
- Smoker WW, Gharrette AJ, Stekoll MS, Joyce JE. Genetic analysis of size in an anadromous population of pink salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl. 1):9–15. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society of London Series B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippel EA. Age at maturity as a stress indicator in fisheries. Bioscience. 1995;45:759–771. [Google Scholar]
- Urawa S, Ueno Y, Ishida Y, Seeb LW, Crane PA, Abe S, Davis ND. A migration model of Japanese chum salmon during early ocean life. North Pacific Anadromous Fish Commission Technical Report. 2001;2:1–2. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Waples RS. Genetic interactions between hatchery and wild salmonids: lessons from the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48(Suppl. 1):124–133. [Google Scholar]
- Waples RS. Dispelling some myths about hatcheries. Fisheries. 1999;24:12–21. [Google Scholar]
- Websters H. Production. In: Wedemeyer GA, editor. Fish Hatchery Management. 2nd edn. Bethesda: American Fisheries Society; 2001. pp. 31–89. [Google Scholar]
- Zar JH. Biostatistical Analysis. 4th edn. Upper Saddle River: Prentice Hall; 1999. [Google Scholar]


