Abstract
Accumulating data indicate that hatchery fish have lower fitness in natural environments than wild fish. This fitness decline can occur very quickly, sometimes following only one or two generations of captive rearing. In this review, we summarize existing data on the fitness of hatchery fish in the wild, and we investigate the conditions under which rapid fitness declines can occur. The summary of studies to date suggests: nonlocal hatchery stocks consistently reproduce very poorly in the wild; hatchery stocks that use wild, local fish for captive propagation generally perform better than nonlocal stocks, but often worse than wild fish. However, the data above are from a limited number of studies and species, and more studies are needed before one can generalize further. We used a simple quantitative genetic model to evaluate whether domestication selection is a sufficient explanation for some observed rapid fitness declines. We show that if selection acts on a single trait, such rapid effects can be explained only when selection is very strong, both in captivity and in the wild, and when the heritability of the trait under selection is high. If selection acts on multiple traits throughout the life cycle, rapid fitness declines are plausible.
Keywords: adaptation, captive breeding, conservation genetics, selection
Introduction
Captive breeding is broadly defined as breeding and raising organisms in captive environments for at least part of their life cycle. This idea is now widely applied to the restoration and supplementation of many declining wild populations (Cuenco et al. 1993; Olney et al. 1994; Frankham et al. 2002). To date, however, little is known about the extent to which captive-reared individuals actually contribute to the restoration of wild populations. Theoretical studies suggest that captive-reared organisms might be genetically inferior to wild ones in natural environments as a consequence of domestication, which could hinder the recovery of wild populations (Lynch and O’ Hely 2001; Ford 2002; Frankham et al. 2002).
Salmonid species (Salmo and Oncorhynchus spp.) are one of the most intensively propagated species in hatchery stocking programs (Lackey et al. 2006; Williams 2006). In the following discussion we use the phrase ‘the wild’ to refer to natural stream environments. We use the term ‘wild’ to refer to fish born and reared in a natural environment (regardless of parentage), and the term ‘hatchery’ to refer to fish that were created by artificial crosses and raised in captivity through the juvenile stage before being released. Although most hatchery programs are meant to produce fish for harvest, an increasing number of hatchery programs now have the explicit mission of restoring declining wild populations (Fleming and Petersson 2001; Berejikian and Ford 2004). While there have been long and extensive discussions about whether the hatchery stocking can really contribute to conservation programs (e.g. Ryman and Utter 1987), general conclusions have not yet been reached. Some programs have increased the number of adults that spawn in the wild (Berejikian et al. in press), but increases in wild population productivity or even wild production have not been documented. Furthermore, accumulating evidence suggests that domesticated hatchery fish often exhibit differences from wild fish in predator avoidance and agonistic behavior (reviewed by Reisenbichler and Rubin 1999) and suffer low reproductive success in the wild (reviewed by Berejikian and Ford 2004), and that these changes can occur rapidly (Salmon Recovery Science Review Panel 2004; Araki et al. 2007a,b). Thus, the effects of hatchery fish on wild populations remain an open question and a topic of major concern.
In this review, we first summarize studies that have evaluated the relative fitness of hatchery and wild salmonid fish. We also use quantitative genetic theory to evaluate under what conditions domestication selection alone is a sufficient explanation for rapid fitness declines that have been observed by some studies. We conclude that selection alone can be a sufficient explanation, either when it operates on several traits throughout the life cycle or when extremely strong selection works on a single trait with very high heritability. We discuss the traits under selection that could cause the observed fitness declines. While there is a need for a comprehensive consideration of whether supplementation hatchery programs are worth operating in general (Waples and Drake 2004), in this review we focus more narrowly on just the fitness effects of hatchery rearing.
Summary of studies evaluating fitness of hatchery fish in the wild
We identified 14 completed studies that evaluated the fitness of hatchery fish relative that of wild fish spawning in the same habitat (Table 1). Eleven out of 14 are on Pacific salmonid spp. (Oncorhynchus), and three on Atlantic salmonid spp. (Salmo). Nine of them are on steelhead trout (Oncorhynchus mykiss), and six of them measured lifetime fitness (typically adult-to-adult reproductive success). Here we use the term ‘relative fitness’ to mean the fitness of hatchery fish (either lifetime or some component) relative to that of wild fish spawning in the same habitat.
Table 1.
Conditions, methodologies and estimated relative fitness (RF) in studies that compared the relative fitness hatchery and wild salmonids. Genetic effects are presumed where hatchery and natural adults were artificially spawned and the fitness of the resulting offspring was compared (assumes environmentally-mediated maternal effects of rearing from egg to smolt have no effect on offspring fitness). All paternal effects are also assumed to be genetic (assuming no grandparental maternal effects). Genetic and environmental effects are considered confounded where hatchery-born and wild-born fish are directly compared because they experienced very different juvenile environments. The duration of the hatchery fish in captivity is expressed in the number of generations in captivity (NGC), which was approximated as years of hatchery operation divided by modal age at sexual maturity. In integrated programs, where either wild fish are spawned in the hatchery or hatchery-origin fish spawn in the natural environment, the ancestry of hatchery and wild fish may differ by only a single generation, even if the duration of the hatchery program is much longer.
| I. Completed Study | Species | Life History segment | Method | Effect on RF | NGC | RF* |
|---|---|---|---|---|---|---|
| Broodstock of nonlocal origin | ||||||
| Chilcote et al. (1986) Leider et al. (1990) | Steelhead (Oncorhynchus mykiss) | Lifetime | Group genetic mark | Confounded | 6 | 0.13 |
| Fleming and Gross (1993) | Coho (O. kisutch) | Adult-to-fry | Individual behavior | Confounded | 5 | (m) 0.62 (f) 0.82 |
| McLean et al. (2003) | Steelhead | Lifetime | Mixed stock analysis | Confounded | 10+ | 0.02–0.11 |
| McLean et al. (2004) | Steelhead | Adult-to-smolt | Mixed stock analysis | Confounded | 10+ | 0.04–0.07 |
| Araki et al. (2007a) | Steelhead (winter-run) | Lifetime | Pedigree | Confounded | 10+ | (m) 0.06 (f) 0.11 |
| Araki et al. (2007a) | Steelhead (summer run) | Lifetime | Pedigree | Confounded | 10+ | (m) 0.35 (f) 0.37 |
| Scenario 2: Local origin | ||||||
| Reisenbichler and McIntyre (1977) | Steelhead | Egg-to-parr | Group genetic mark | Genetic | 2 | 0.8 |
| Reisenbichler and Rubin (1999) | Steelhead | Fry to age-1 | Group genetic mark | Genetic | 6 | 0.8 |
| Fleming et al. (1997) | Atlantic salmon (Salmo salar) | Adult to fry | Individual behavior | Environment | 1 | (m) 0.48 (f) ∼1.0 |
| Dannewitz et al. (2004) | Brown trout (S. trutta) | Egg-to-parr | Pedigree | Genetic | 7 | 1.27 |
| McGinnity et al. (2004) | Atlantic salmon | Egg-to-adult | Pedigree | Genetic | 5 | ∼1.0 |
| Dahl et al. (2006) | Brown trout | Parr to parr (1 year in stream channel) | Nose tag | Genetic | 7 | ∼1.0 |
| Ford et al. (2006) | Coho | Adult-to-smolt | Pedigree | Confounded | 25 | (m) 1.01 (f) 0.74 |
| Araki et al. (2007a,b) | Steelhead (winter-run, integrated) | Lifetime | Pedigree | Confounded | 1 | (m) 0.70 (f) 0.88 |
| 2 | (m) 0.32 (f) 0.30 | |||||
| Genetic | 1 vs 2† | (m) 0.55 (f) 0.55 | ||||
m, male, f, female, when the relative fitness (RF) was estimated separately for each sex of parent.
Hatchery fish having one wild parent and one first-generation hatchery parent (NGC-2) compared to hatchery fish having two wild parents (NGC-1).
The origin and management of the salmonid broodstocks (parents of hatchery fish) vary substantially among the studied systems and are expected to affect the relative fitness of hatchery fish. The hatchery population can be founded from either the wild population that inhabits the location of release (local hatchery stock), or from a different river than the one into which the stock is released (nonlocal hatchery stock). Hatchery populations can be perpetuated solely by spawning hatchery-origin fish (segregated broodstock), or by spawning a combination of hatchery and wild fish (integrated broodstock). In both cases hatchery and wild fish often will be spawning in the natural environment, unless hatchery fish are intentionally excluded from spawning by weirs or traps.
In Table 1 we find that the relative fitness (RF) between hatchery fish and wild fish is generally lower than one, indicating that hatchery rearing generally has negative effects on RF. In the seven studies in which RF was estimated separately for males (fathers) and females (mothers), point estimates show that RF in males was smaller than that in females in four studies, about equal in two, and larger in one. Thus, while hatchery females tend to have higher RF in the wild than hatchery males, Table 1 shows no evidence for a large sex-specific bias in RF. In addition, Araki et al. (2007b) showed that the second generation hatchery fish from hatchery-born fathers (grandfathers of wild-born descendants) had about the same reproductive success that those from hatchery-born mothers (grandmothers of wild-born descendants) had in the wild, suggesting no obvious grandmaternal/grandpaternal effects of hatchery rearing on RF.
Segregated broodstocks of nonlocal origin
The most complete information on RF of hatchery and wild salmonids comes from five studies of nonlocal, segregated hatchery steelhead populations. All of these studies indicate very low relative fitness of the hatchery fish (Lifetime RF = 0.02–0.37, Table 1). One study of coho salmon representing a similar broodstock management scenario (i.e. domesticated stock compared to a nonlocal wild population) used behavioral measures and estimated reduced relative breeding success (males 0.62; females 0.82) that was similar to the adult-to-subyearling relative fitness of steelhead (75–78%) in the one study that measured it (Leider et al. 1990). These segregated hatchery stocks have been managed as more or less ‘closed’ populations for 5–10 generations (Table 1), during which time there has been little genetic input from the wild population(s).
Hatchery and wild fish experience very different environments as juveniles. Differences in fitness or other characteristics between hatchery and wild adults can therefore be due to either genetic differences, or differences caused by rearing in different environments as juveniles, or a mixture of these two effects. However, several lines of evidence suggest that genetic effects contribute to the lowered fitness of hatchery fish. Firstly, the offspring of naturally spawning hatchery fish have been found to have lower survival to smolting and lower survival from smolting to adulthood (Chilcote et al. 1986; Leider et al. 1990; Kostow et al. 2003). One cannot rule out an environmental effect passed down through the gametes of the hatchery parents, but this seems less likely than, say an environmental effect on the mating and spawning success of the hatchery parents themselves. Secondly, Araki et al. (2007a) showed that ‘traditional’ (nonlocal, segregated) hatchery stocks exhibited substantially lower relative fitness than hatchery fish produced by wild fish (in a ‘supplementation’ program discussed below), when each was compared to the same wild population. Because both stocks experienced hatchery environments and were compared against the same wild population, the difference between them is likely to be genetic in origin. However, the two comparisons were done in different years so this conclusion remains tentative. Finally, evidence of a negative correlation between relative fitness and generations in captivity (Salmon Recovery Science Review Panel 2004; Araki et al. 2007b) again points to a genetic effect because environmental effects are not expected to accumulate over generations.
Segregated broodstocks of local-origin
An additional study of Atlantic salmon conducted as a ‘common garden’ experiment concluded that offspring of a locally derived hatchery population that had been completely segregated from the wild population for about five generations exhibited survival equal to offspring of the wild fish from egg to adult (McGinnity et al. 2004). Offspring of fish captured from an adjacent (i.e. nonlocal) population had reduced survival in both freshwater and seawater portions of the life cycle, emphasizing the importance of local adaptation in determining fitness. The apparent lack of a genetic effect of approximately five generations of hatchery propagation on egg-to-adult fitness in the locally derived hatchery population did not include an assessment of breeding performance. The locally derived hatchery population did exhibit some growth differences in the hatchery and apparent divergence in life history characteristics (e.g. age-at-smoltification and maturity).
Integrated broodstocks of local origin
The nonlocal, segregated hatchery stocks in the above studies were derived from different geographic regions than the wild populations to which they were compared. Thus, any fitness difference could be due to local adaptation or to the effects of hatchery production. The effects of hatchery propagation, per se, can be investigated by studying the fitness of hatchery fish derived from the same local population to which they are compared. We have summarized data from four such populations, two steelhead, one coho and one brown trout (Salmo trutta, Table 1).
The survival of offspring of hatchery brown trout stocked into experimental channels as embryos or as juveniles did not differ from that of wild fish from the same source population, even though the hatchery line had been in production for seven generations (Dannewitz et al. 2004; Dahl et al. 2006). Similarly, the relative fitness of a hatchery coho salmon stock did not differ significantly from that of wild coho salmon, even though the hatchery population had been in operation for approximately 25 generations (Ford et al. 2006). However, in both these populations hatchery fish had been predominating on the spawning grounds for many years, so the ‘wild’ populations in these cases probably consisted largely of hatchery fish from previous generations. All we can say here is that on average the hatchery fish in these two studies experienced the hatchery environment for one more generation than the wild fish. Or put another way, the wild fish were in the wild for at least one full generation, even if their ancestors had substantial hatchery background.
Evidence of reduced fitness in local-origin integrated hatchery populations comes from two studies on steelhead in experimental streams. Survival from egg to parr, or from fry to 1 year of age, of offspring of hatchery fish in enclosures in streams was approximately 80% that of offspring of wild fish (Reisenbichler and McIntyre 1977; Reisenbichler and Rubin 1999). The hatchery fish in these studies had been propagated artificially for 2–6 generations. Both types of fish were created via crosses in the hatchery, so the differences are probably genetic. Similarly, hatchery steelhead derived from a wild parent and a hatchery parent showed lower reproductive fitness than that of hatchery fish derived from two wild parents in each of 3 years of samples (Araki et al. 2007b). In this case overall reproductive fitness in the former hatchery fish was only 55% of the fitness in the latter hatchery fish (Table 1). This study also eliminated the confounding effects of captive rearing because all fish were spawned artificially, reared in the same environment and released on the same date. When reproductive success of these two types of hatchery fish were compared with that of wild fish (rather than with that of each other), the relative fitness of the first generation fish was 70–88% and that of the second generation fish was only around 30% (Table 1). Again, differences between wild-born and hatchery-born individuals are confounded by the different environments they experienced, but the substantial difference between first and second generation hatchery fish suggests a rapid and cumulative genetic effect of hatchery culture during the first few generations of captive rearing.
Limitations of existing data
Even with the recent accumulations of new data above, it is too early to draw a strong conclusion about whether supplementation programs in general are helping or harming the wild populations, for the following reasons: First, the precision of the point estimates of relative fitness in above studies is limited, and we typically have low power to detect a biologically significant difference in fitness between hatchery fish and wild fish (Araki and Blouin 2005; Araki et al. 2007a,b). A fitness difference of only a few percent will have strong effects on the fate of a population over the course of many generations (e.g. Crow and Kimura 1970), but even the largest studies rarely have power to detect fitness differences of less than 10–15% (Araki et al. 2007a,b). Second, data on the relative fitness of hatchery fish compared to wild fish are heavily biased towards steelhead. Most of the other species studied (brown trout; Atlantic salmon, Salmo salar; and coho salmon) share similar characteristics as steelhead; in particular, they reside in freshwater for at least their first year of life before they migrate to the ocean. This characteristic leads fishery managers to rear these species in hatcheries for at least a full year, usually until the fish change physiologically for the ocean migration (smoltification). In addition, most natural populations of steelhead smolt at age 2 or 3, which is usually not practical in a hatchery. So most steelhead hatcheries accelerate the growth of their fish and release them after 1 year. Sometimes it is necessary to advance adult spawn timing to achieve the goal of creating smolts within a year. Other species, such as some populations of Chinook salmon, migrate to sea after just a few months of rearing in freshwater, and chum salmon (O. keta) and pink salmon (O. gorbuscha) begin their migration to sea directly after emergence from the gravel. Hatchery populations of these species spend less time in freshwater and therefore might be less affected by the hatchery environment than species that spend longer in artificial environments. Many of the new relative fitness studies that we are aware of focus on Chinook salmon, on newly founded hatchery populations, or on hatcheries that incorporate wild born fish into the hatchery broodstock each generation (e.g. Chiwawa River Chinook by A. Murdoch and M. J. Ford, in prepration). Furthermore, in most of these studies, the relative proportions of cultured fish and wild fish spawning naturally, and the histories of the broodstocks, are also better known than in the studies reviewed above. Third, although estimating the relative fitness of hatchery fish is important, it is not sufficient for evaluating the effectiveness of supplementation. Long-term studies comparing the demographic performance of supplemented and unsupplemented populations are also important, and can provide a more complete understanding of the overall impacts of supplementation. For example, several studies have found widespread negative correlations between natural population productivity and intensity of hatchery production (e.g. Chilcote 2003; Nickelson 2003; Hoekstra et al. 2007). The results of these studies, which primarily focus on segregated and often nonlocal hatchery populations, are consistent with the finding that such hatchery populations can negatively impact natural populations. Similar analyses of the long-term demographic effects of supplementation should also be conducted to complement shorter-term studies of relative fitness.
Mechanisms of fitness decline
The data from our review suggest that the fitness of hatchery fish declines with increasing generations in the hatchery, although confounding factors such as locality of the broodstock and interchange between the hatchery and wild populations inhibit a firm conclusion. In studies on steelhead, however, the fitness decline has been shown to occur extremely rapidly – within the first generation or two of hatchery culture in some cases (Table 1). There are several potential mechanisms by which captive rearing could cause the fitness decline, but no studies have empirically examined the mechanism of observed declines of hatchery fish fitness in detail. Potential explanations that have been proposed for why hatchery fish are less fit than wild fish in nature are: (i) Deleterious mutation accumulation. In particular, survival from egg to smolt is usually 85–95% in hatcheries versus 1–5% in the wild (Reisenbichler et al. 2004). Thus, relaxed purifying selection during the egg-to-smolt stage is expected to result in the accumulation of new mutations that are effectively neutral in the hatchery but deleterious in the wild (Lynch and O’ Hely 2001). (ii) Inbreeding depression due to small broodstock sizes (reviewed by Wang et al. 2002). (iii) Domestication selection, in which positive selection for adaptation to the hatchery environment comes at the expense of adaptation to the natural environment (e.g. Ford 2002).
Relaxed purifying selection, coupled with the accumulation of new mutations, almost certainly contributes to the low fitness of multi-generation hatchery stocks (Lynch and O’ Hely 2001). But relaxed selection owing to a single generation of hatchery culture seems an unlikely explanation for dramatic declines in fitness unless salmon carry an extraordinary standing genetic load (e.g. Launey and Hedgecock 2001). Typical rates of mutation to deleterious mutations are around one mutation per genome per generation, and the average effect of such a mutation in the heterozygous state is around 2% (Lynch et al. 1999). Thus, it should take at least a few generations for the effects of new mutations to accumulate. There is no evidence that salmon have unusually high mutation rates (e.g. Steinberg et al. 2002). One possible explanation is that the hatchery environment somehow induces a large increase in the deleterious mutation rate, but so far no data exist to suggest this is happening.
Inbreeding depression is a reduction in fitness associated with mating between relatives, and can be caused by either an increase in homozygosity of recessive deleterious alleles or a reduction in heterosis (reviewed by Charlesworth and Charlesworth 1987). Unlike mutation accumulation, inbreeding can potentially lead to fitness declines in a few generations or even a single generation because it operates on variation that is already present in a population. Inbreeding between close relatives (inbreeding coefficient of 0.25; sib mating or equivalent) has been shown to reduce survival rates by ∼10–30% in salmonids (reviewed by Wang et al. 2002). Hatchery programs sometimes have small breeding populations and low effective population sizes (Waples and Teel 1990), so inbreeding depression can be a contributing factor to low hatchery fish fitness in some cases. However, based on the studies reviewed by Wang et al. (2002), the level of inbreeding in hatcheries would need to be unrealistically high in order to explain the fitness decline of ∼30%/generation reported by Araki et al. (2007b). Inbreeding depression also cannot explain the fitness decline in the Hood River steelhead because crosses between two hatchery-born parents were avoided in this hatchery program (Araki et al. 2007b). Therefore, although inbreeding depression can be a contributing factor to low fitness of hatchery fish, it is unlikely to be the primary factor for the fitness declines observed in some studies.
The last and most likely explanation for the rapid fitness decline is domestication selection. Domestication selection has long been known to be a strong evolutionary force intentionally changing the characteristics of captive-reared organisms, and unintentional selection is likely to occur in typical supplementation programs as well. The fact that after just one or two generations hatchery fish perform better than wild fish in hatchery environments (Reisenbichler et al. 2004) also points to positive selection, rather than to some generalized genomic deterioration (relaxation of purifying selection). Nevertheless, it is worth asking whether even strong selection could generate declines as rapid as those described in Araki et al. (2007b). Here we examine the conditions necessary for selection to generate fitness declines of >30% per generation of hatchery rearing, as suggested by the results of Araki et al. (2007b). Our goal is to explore whether selection alone is a plausible explanation for such declines in fitness.
Opportunity for selection – where in the life cycle?
In addressing this question, it is important to distinguish between selection within a generation that changes the distribution of phenotypes, and the response to selection that leads to genetic change across generations (Arnold and Wade 1984). Araki et al.’s (2007a,b) study of the Hood River steelhead illustrates the importance of this distinction. One result of this study was that naturally spawning hatchery fish that had two wild parents (C[W × W] fish) had relative fitness of 70–88% that of wild fish. The second main result was that hatchery fish with one wild parent and one first generation hatchery parent (C[C × W]) had relative fitness of ∼60% that of C[W × W] fish. In other words, the addition of one half a genome with one additional generation of exposure to the hatchery resulted in 30–40% decline in fitness.
In order to address whether selection alone can explain these fitness declines, it is useful to schematically illustrate when and where such selection could potentially occur. In Fig. 1, we can see that for the wild (W) versus C[W × W] relative fitness comparison, hatchery selection could conceptually occur anytime from the point of broodstock collection in generation 0 to the time of adult returns in generation 1 (solid line). Conceptually, it is easy to see that selection solely within generation 1 could be responsible for reduced reproductive success of the C[W × W] fish. In other words, the reduced fitness of the C[W × W] fish could be due solely to a selection-induced change in phenotypic distributions, without necessarily any genetic response to that selection. In addition, it is likely that hatchery rearing and release strategies could produce purely environmental, nonselective changes in trait distributions that could also result in lowered fitness of the C[W × W] fish. For example, hatchery fish might tend to return to spawning locations near their point of release, and if these locations happen to be in poor quality spawning habitat then the relative fitness of the hatchery fish would be reduced compared to wild fish spawning in higher quality habitat.
Figure 1.
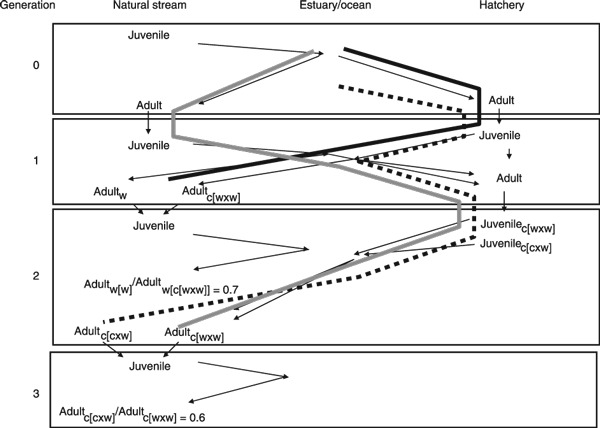
Illustration of the relative fitness comparisons made by Araki et al. (2007a,b) and the spatial and temporal opportunities for domestication selection to occur. Thin arrows indicate where a fish moves over the course of its lifecycle. The thick solid line illustrates where and when in the lifecycle selection could act to reduce the fitness of C[W × W] fish (captive progeny of two wild parents) compared to wild fish. The gray and dashed lines illustrate differences in the lifecycle (and hence opportunities for differential selection) of C[W × W] and C[C × W] fish, respectively. See text for details.
In contrast, the second major result reported by Araki et al. (2007b)– the reduction of fitness of C[C × W] compared to C[W × W] – must involve a heritable change in fitness between generations. This can be seen by comparing how the generational pathways differ between these two types of fish (gray and dashed lines in Fig. 1). Since the two types of fish experience identical selective environments in generation 2, the difference in fitness must be the result of selection (or some other effect) that occurred in generation 0 or 1 and was transferred to generation 2 (Fig. 1).
Even from the simplified view presented in Fig. 1, it is easy to see that there are many potential opportunities for selection caused by hatchery propagation to lead to changes in trait distributions of hatchery bred fish. Selection could occur on adults at the time of broodstock collection, while holding in the hatchery prior to spawning, and in the choice of fish to be spawned. Selection on eggs and juveniles could occur directly at any point prior to release. Additional selection attributed to the hatchery experience could also occur after the time of release if trait distributions at the time of release differ from what they would have been had the fish been produced in the wild (Reisenbichler et al. 2004). Conceptually, one could construct a quantitative genetic model (Lande and Arnold 1983; Arnold and Wade 1984; Falconer and Mackay 1996; Ford 2002) and try to determine whether the observed change in fitness was consistent with the model predictions. However, to realistically model even the simple situation illustrated in Fig. 1 would involve a model of selection on multiple traits at multiple life stages, which would require estimation of a large number of parameters for which we have limited or no data, such as the strength of selection on each of many traits in multiple environments, and the genetic and phenotypic covariance matrix for all of the traits.
Fortunately, we do not need to attempt to evaluate such a detailed model to assess the plausibility of selection as a mechanism for the 30% reduction in fitness/generation found by Araki et al. (2007b). Instead, we directly evaluated the plausibility of such a change in mean fitness by estimating the opportunity for selection from the observed variance in reproductive success reported by several studies. The opportunity for selection, I, is the variance in individual fitness within a generation, and is also equal to the population level change in mean fitness within a generation due to selection (Crow 1958; Arnold and Wade 1984). We first evaluate the plausibility of selection as a mechanism for the ∼30% change in mean fitness observed in first generation of the Araki et al. (2007b) study (W versus C[W × W]).
The estimated opportunity for selection on fish varied considerably among studies and between the sexes: the variance in individual fitness averaged 4.5 for males and 3.5 for females (Table 2). Note that the mean fitness in each case is scaled to 1, so it is clear that there is ample opportunity for changes in mean fitness of 30% or larger due to selection within a generation. For example, in the Chinook salmon study reported in Table 2 (Ford, unpublished data), a 30% reduction in mean fitness would result from truncation selection against the 17% of the population that had highest fitness in the wild. Although the large variance in fitness reported in these studies is not necessarily due to natural selection, this result is consistent with strong selection potentially acting on populations and suggests that there is sufficient variance in fitness among individuals within a population for selection to play a significant role in the short-term evolution of salmonids.
Table 2.
Reported opportunities for selection (variance in individual relative fitness) for salmon populations.
| I | |||
|---|---|---|---|
| Males* | Females† | Species | Reference |
| 2.7 | 1.5 | Chinook salmon | Ford (unpublished data) |
| 5.7 | 4.5 | Coho salmon | Ford et al. (2006) |
| 3.8–8.0 | 2.6–8.7 | Steelhead | Araki et al. (2007c) |
| 1.3 | 0.1 | Coho salmon | Fleming and Gross (1994) |
Mean (SD) Values
4.5 (2.4);
3.5 (3.0).
Next, we evaluate the plausibility of selection as an explanation for the inherited 30% decline in fitness per generation estimated by Araki et al. (2007b). Using the mean value of I in Table 2, heritability of relative fitness would need to be >0.07 (0.3/4.5) for males and >0.09 (0.3/3.5) for females to explain the 30% fitness decline, assuming selection attributed to the hatchery operated directly on the ‘trait’ of relative fitness in the wild. Note that the lower the heritability, however, the stronger selection would need to be to achieve the observed change in mean fitness, and the strength of selection implied by heritabilities at the low end of the range seems unrealistic. Although Fisher’s (1958) fundamental theory of natural selection predicts that the heritability of fitness itself should be zero, this prediction assumes a population at equilibrium in a stable environment and with no mutational input. The few studies that have estimated the heritability of total fitness did indeed find estimates indistinguishable from zero, but 95% confidence intervals around those estimates include the values above (Gustafsson 1986; Kruuk et al. 2000). Thus the heritabilities for fitness required for selection to generate the observed fitness declines in steelhead are plausible (see also Carlson and Seamons 2008). Because selection due to hatchery exposure would actually operate on a series of traits that are correlated to an unknown degree with relative fitness in the wild, the heritability of each trait actually under selection due to hatchery exposure would need to be larger than these minimum values. But as we demonstrate in the following section, even selection on a single trait does not require implausibly large heritabilities or selection coefficients.
Selection – single-trait model
Here we assume reduced fitness resulted from selection on a single quantitative trait. We used a quantitative genetic model (Fig. 2) to evaluate the parameter space in which selection could result in a >30% genetic fitness decline in naturally spawning offspring from first-generation hatchery-born parents. In other words, here we are asking: just how extreme would values of key parameters have to be for selection on a single trait to cause 30% declines after a single generation? Domestication selection is considered to work during captive rearing, in which a quantitative trait (e.g. growth rate) is selected differently from in the wild (and so the optimal trait value is shifted from that in the natural environment). We assume truncation selection for this step, in which only fish with trait values above a threshold are viable (Fig. 2). Such traits under selection in a hatchery are not well understood yet, although some candidate traits exist (see below). After hatchery fish are released into the wild, they are allowed to produce natural-born offspring. At this step, viability selection will work against the trait selected in captivity. To measure the strength of natural selection in this step, we used a Gaussian model of stabilizing selection, following Lande (1976) and Ford (2002). So to summarize, we begin with individuals adapted at equilibrium under stabilizing selection to a natural environment. They experience strong truncation selection in the novel (hatchery) environment, which has a different fitness optimum from the natural environment. Their offspring are then exposed to the original, natural environment, and experience strong, directional selection back towards the original optimum phenotype. We ask under what conditions this offspring generation will suffer mortality >30%.
Figure 2.
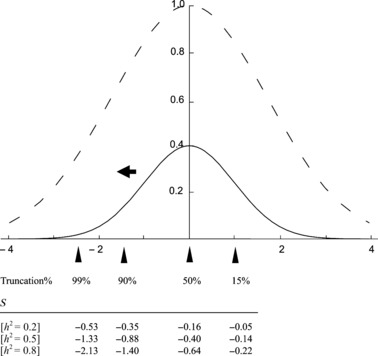
A quantitative genetic model of stabilizing selection after one generation of truncation due to domestication. The solid line represents phenotypic distribution of a quantitative trait at the equilibrium state under stabilizing selection (standard normal distribution), and the dotted line represents relative fitness of individuals with the corresponding trait values (x-axis) when ω2 = 2 (variance of the adaptive landscape: ω2 + 1 = 3. see Estes and Arnold 2007). Four different levels of truncation are considered and an arrowhead represents the truncation point (Truncation%) in each case such that all fish with trait values to the left of the arrows are viable. Selection differentials (S) after one generation of hatchery rearing and natural reproduction of hatchery fish are shown with three different levels of heritability (h2 = 0.2, 0.5, 0.8).
Before selection, we assume that the wild population is at an equilibrium state at which fitness in the wild is maximized for individuals with phenotypic value (z) equal to zero (Fig. 2). For simplicity, the standard normal phenotypic distribution, N(0, 1), is considered at this state. Therefore the mean trait value is zero and the phenotypic variance is one in the wild population. This assumption should not restrict our results because any phenotypic variables that are distributed normally can be easily standardized. In this model, we also assume that the selection function acting on this trait is also Gaussian with mean zero and variance ω2 + 1, (Ford 2002; Estes and Arnold 2007). Because ω2 determines how tightly phenotypic variation is restricted by stabilizing selection around the optimal value, it represents the strength of natural selection in the wild (smaller ω2 represents stronger stabilizing selection).
We assume that broodstock are collected at random from the wild population and that the number of broodstock is large enough to represent the phenotypic distribution in the wild. After hatchery fish are created, they are subject to truncation selection favoring the trait values adaptive to the captive environment. For comparison, we consider four different levels of truncation selection (LT = 0.15, 0.50, 0.90, and 0.99), which determine the truncation points (T) in the phenotypic distribution (Fig. 2). The selection differential (S) between the natural-born offspring from the hatchery fish and those from the natural-born fish can be obtained from the breeder’s equation (Lynch and Walsh 1998) as
| 1 |
where h2 is the realized heritability and 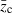 is the mean trait value (shifted from zero) after truncation selection and before reproduction.
is the mean trait value (shifted from zero) after truncation selection and before reproduction. 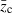 is calculated as (Lynch and Walsh 1998)
is calculated as (Lynch and Walsh 1998)
| 2 |
where p(x) is the probability density function of N(0, 1) in this case. When ω2 >> h2, we can also obtain the relative fitness (RF) of the offspring from hatchery-born fish to those from natural-born fish (from Lande 1976; Eqn (3) in Ford (2002)) as
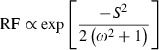 |
3 |
Thus, in the simplest model, RF is determined by only three parameters, the level of truncation in a hatchery (T), the realized heritability (h2), and strength of stabilizing selection in the wild (ω2). In Fig. 3 we show the relationship between RF and ω2 with three different levels of heritability (h2 = 0.2, 0.5, 0.8). It is intuitively obvious that RF is low when the strength of natural selection is strong, the level of truncation in a hatchery is high, and the heritability is high. However, Fig. 3 shows that all three conditions are required to explain a >30% fitness decline following a single generation of captive rearing if selection acts only on a single trait. For example, low levels of truncation (LT ≤ 0.5) cannot explain the >30% fitness decline, and neither can ω2 > 5 or h2 ≤ 0.2. Thus, the conditions necessary to explain the >30% fitness decline per generation due to selection on a single trait are fairly extreme (e.g. h2 of various traits in salmonid species are generally lower than 0.5. Carlson and Seamons 2008). On the other hand, if all these conditions are met, then Fig. 3 illustrates that the fitness decline can be very severe (even >50%) within a generation. In the following sections we discuss how realistic each condition is for hatchery fish.
Figure 3.
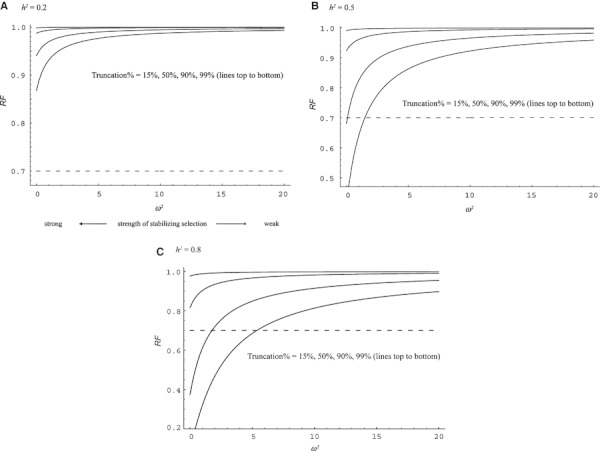
Relationship between relative fitness (RF) and strength of natural selection (ω2). Small ω2 represents strong selection in the wild. RF at different ω2 was calculated from Eqn (3) and shown with four different levels of truncation and three different levels of heritability (A–C).
Level of truncation due to domestication selection
It is commonly known that hatchery fish from wild broodstocks are difficult to rear in a hatchery and have higher mortality rates than those from traditional hatchery stocks (J. Gidley personal communication). This is probably due to larger phenotypic variation and stronger genetic maladaptation in hatchery fish from wild broodstocks than in those from traditional hatchery stocks. The latter is consistent with the model of domestication selection with displaced optimum because the selective shift of the trait values in hatcheries should be most pronounced at the first generation (when the population values are the furthest from the optimal value in captivity). Highly crowded conditions typical of hatcheries might also be a part of the reasons for the strong domestication selection (Frankham 2008). Even so, however, typical mortality rates of hatchery fish are only 5–15% during captive rearing (e.g. Reisenbichler et al. 2004). According to the above analysis, this level of mortality by itself cannot explain the >30% fitness decline even if all the mortality in a hatchery is due to domestication selection. However, as is illustrated in Fig. 1, selection could also occur at the broodstock collection and spawning phases, as well. Another possible explanation is that the domestication selection in a hatchery is correlated with natural selection on hatchery fish after the release (Reisenbichler et al. 2004). For example, mortality rate from smolt to adult is often >95% in natural environments. Thus, if some juvenile traits strongly influence survival after release, they may well be the targets of domestication selection, even though it does not create a high mortality in a hatchery. Similarly, the ability to successfully spawn could somehow be influenced by traits expressed during the hatchery phase of the life cycle. So in summary, viability selection in the hatchery itself is not sufficient to explain the observed fitness declines. On the other hand, correlated selection on traits influencing phenotypes during the high-mortality oceanic phase, or perhaps during spawning, is a plausible mechanism.
Heritability
There is a large body of data on estimates of the realized heritability on various traits in various species. Although estimated h2 values vary widely, the h2 of life history traits tend to be low compared with h2 on other traits (i.e. behavioral, physiological, and morphological traits), as expected on theoretical grounds (Mousseau and Roff 1987; Carlson and Seamons 2008). However, some fitness-related traits can have high heritabilities. For example, heritabilities of egg size ≥0.6–0.8 have been reported in salmonids (Su et al. 1997; Kinnison and Hendry 2001; Einum et al. 2004). Furthermore, the heritability of a trait depends on the environment in which it is expressed. Thus, fitness-related traits that have low heritability in the natural environment could conceivably be highly heritable in the novel environment of a hatchery. Thus, although the high heritabilities necessary for this model to work (h2 = 0.5 or 0.8) would be surprising for traits closely tied to fitness in a stable natural environment, they are not out of the question.
Strength of stabilizing selection in the wild
The strength of natural selection is in the wild has been the subject of extensive study. For natural selection on phenotypic traits, Kingsolver et al. (2001) performed a meta-analysis on selection gradients from 63 studies including 62 species in the wild. Estes and Arnold (2007) revisited their data to estimate ω2. According to these studies, the strength of stabilizing selection is generally very strong in wild populations (a modal value of ω2 = 3.21). The ω2 we assumed (Fig. 2) is well in this range, so this part of our model is very plausible.
Candidate traits under selection
We showed above that, given the high survival in hatcheries, viability selection during the hatchery phase of the life cycle is unlikely to produce the rapid fitness declines observed in some studies. Thus, viability selection during the ocean phase, or fecundity or sexual selection during the spawning phase, are also likely targets of selection leading to domestication. Reisenbichler et al. (2004) reported that ocean survival is highly correlated with body size at the smolt stage, and that strong selection acts on body size at release. In the high-food and predator-free hatchery environment, this survival difference should select for high growth rate in hatchery juveniles, perhaps via a combination of physiological and behavioral changes. But an excessively high growth rate is often maladaptive in natural environments (Arendt 1997).
Selection for high growth rate in the hatchery and associated consequences for fitness are likely most severe for steelhead, which typically spend 2 or 3 years in freshwater before migrating to sea, and for which we have the most compelling evidence of rapid fitness loss. Nearly all steelhead hatcheries, including the Hood River Hatchery (the subject of Araki et al. 2007b), rear and release smolts as yearlings. The problem is that hatcheries typically have difficulty rearing juveniles from wild broodstock (stocks that have not yet been domesticated) to a threshold smolt size (about 150 mm) in 1 year, but release all fish regardless of size, providing the opportunity for intense selection against slow growing individuals (as demonstrated in Reisenbichler et al. 2004). Traits associated with rapid growth in the hatchery, such as standard metabolic rate, might be selected against in nature (brown trout: Alvarez and Nicieza 2005). Growth rate has also been shown to correlate positively with aggression at the both individual and population level (Lahti et al. 2001), and high levels of aggression in domesticated steelhead populations are associated with risk taking behavior (Johnsson and Abrahams 1991) and reduced ability to avoid predators (Berejikian 1995). Therefore, when those hatchery fish reproduce in a natural setting, their offspring could have substantially lower fitness (Biro et al. 2004; Sundstrom et al. 2005).
How the process of broodstock collection and artificial spawning might influence subsequent spawning success is less obvious. It is known that hatchery managers sometimes inadvertently and nonrandomly select which fish to use in crosses (McLean et al. 2005). Also, broodstocks are often kept in holding pens for weeks or months before being used, and the mortality and morbidity induced has been shown to be nonrandom (Ford et al., 2008). Hatchery broodstock management has been the subject of considerable scrutiny in recent years, and guidelines have been developed to maximize effective population size, and reduce intentional and unintentional artificial selection (e.g. Campton 2005). However, artificial spawning in the hatchery almost certainly results in relaxation of mate selection (Blanchfield and Ridgway 1999; Berejikian et al. 2000; de Gaudemar et al. 2000), intra-sexual competition, and natural selection on traits such as body size, egg size, fecundity and spawn timing and location (van den Berghe and Gross 1989; Fleming and Gross 1994; Einum and Fleming 2000a,b). In addition to selection on the broodstock, viability selection on their offspring during the oceanic phase (as discussed above) could conceivably result in surviving phenotypes that are disadvantaged during reproduction to the extent that key behavioral traits under selection (e.g. aggressiveness or dominance) are affected.
Possible other mechanisms
We have shown that the conditions necessary for domestication selection alone to generate fitness declines like those observed in studies such as Araki et al. (2007b) are possible when certain conditions are met. Nevertheless, it is worth considering other explanations. We dismissed mutation accumulation as the explanation for effects appearing during the first one or two generations because typical rates of mutation to deleterious alleles and their fitness effects are too small. However, an enhanced mutation rate owing to the hatchery environment is one possibility. This could include a higher rate of chromosomal abnormalities. While there is no evidence of a high rate of chromosomal abnormality in salmonids, aneuploidy in germ cells has long been known to cause meiotic errors that can result in infertility in both plants and animals (e.g. Shi and Martin 2001; Henry et al. 2005; Hall et al. 2006). Another intriguing possibility is heritable epigenetic changes induced by the hatchery environment. Epigenetic changes, such as alternations in DNA or histone methylation, have been shown to affect an individual’s phenotypes in a heritable manner (e.g. Reik et al. 2001; Jirtle and Skinner 2007; Reik 2007). It is therefore conceivable that rearing in a hatchery environment during the early part of the salmon lifecycle could cause epigenetic changes that might eventually affect fitness of the individuals and their offspring. Note that domestication selection and more exotic mechanisms such as enhanced mutation rates or epigenetic effects are not mutually exclusive. Indeed, one can imagine such effects acting in concert to cause a plunge in fitness in the second generation of culture. Research on the traits under domestication selection, and on possible alternative mechanisms that cause fitness declines, will be an important new direction as we search for ways to improve hatchery programs.
Conclusion
A review of studies to date shows that older, nonlocal stocks generally perform worse than local stocks having experienced fewer generations in the hatchery. In some cases, even old stocks had fitness indistinguishable from that of wild fish, but in most of those situations hatchery fish had contributed high proportions of the natural breeding population for many years, making it likely that no ‘wild’ population remained. More studies are needed, particularly on salmonids other than steelhead and on hatchery programs that are still in their earliest phases. Several studies in progress on other salmonid species should soon provide additional data points. One surprising recent result is large declines in fitness of steelhead during the first two generations of hatchery culture. We showed that domestication selection is a plausible explanation for such large declines, especially if such selection operates on several traits throughout the life cycle. Other mechanisms, such as an enhanced mutation rate, relaxation selection, chromosomal abnormality, and epigenetic effects, might also contribute to the observed declines in fitness.
Our review indicates that salmonids appear to be very susceptible to fitness loss while in captivity. The degree of fitness loss appears to be mitigated to some extent by using local, wild fish for broodstock, but we found little evidence to suggest that it can be avoided altogether. The general finding of low relative fitness of hatchery fish, combined with studies that have found broad scale negative associations between the presence of hatchery fish and wild population performance (e.g. Hoekstra et al. 2007), should give fisheries managers pause as they consider whether to include hatchery production in their conservation toolbox.
Acknowledgments
We thank Steven Arnold, Hidenori Tachida, Michael Lynch, Robin Waples and Jeff Hard for useful discussions or comments on previous drafts of this manuscript. This research was funded by contracts to M.S.B. from the Bonneville Power Administration and the ODFW.
Literature cited
- Alvarez D, Nicieza AG. Is metabolic rate a reliable predictor of growth and survival of brown trout (Salmo trutta) in the wild. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:643–649. [Google Scholar]
- Araki H, Blouin MS. Unbiased estimation of relative reproductive success of different groups: evaluation and correction of bias caused by parentage assignment errors. Molecular Ecology. 2005;14:4097–4109. doi: 10.1111/j.1365-294X.2005.02689.x. [DOI] [PubMed] [Google Scholar]
- Araki H, Ardren WR, Olsen E, Cooper B, Blouin MS. Reproductive success of captive-bred steelhead trout in the wild: evaluation of three hatchery programs in the hood river. Conservation Biology. 2007a;21:181–190. doi: 10.1111/j.1523-1739.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- Araki H, Cooper B, Blouin MS. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science. 2007b;318:100–103. doi: 10.1126/science.1145621. [DOI] [PubMed] [Google Scholar]
- Araki H, Waples RS, Ardren WR, Cooper B, Blouin MS. Effective population size of steelhead trout: influence of variance in reproductive success, hatchery programs, and genetic compensation between life-history forms. Molecular Ecology. 2007c;16:953–966. doi: 10.1111/j.1365-294X.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- Arendt JD. Adaptive intrinsic growth rates: an integration across taxa. Quarterly Review of Biology. 1997;72:149–177. [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection: theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Berejikian BA. The effects of hatchery and wild ancestry and experience on the ability of steelhead trout fry (Onchorhynchus mykiss) to avoid a benthic predator. Canadian Journal of Fisheries and Aquatic Sciences. 1995;52:2476–2482. [Google Scholar]
- Berejikian BA, Ford MJ. Review of the Relative Fitness of Hatchery and Natural Salmon. Seattle, WA: Northwest Fisheries Science Center; 2004. p. 28. U.S. Dept. Commer., NOAA Tech. MemoNMFS-NWFSC-61. [Google Scholar]
- Berejikian BA, Tezak EP, LaRae AL. Female mate choice and spawning behavior of chinook salmon under experimental conditions. Journal of Fish Biology. 2000;57:647–661. [Google Scholar]
- Berejikian BA, Johnson T, Endicott R, Lee J. Hamma River, WA: Canadian Journal of Fisheries and Aquatic Sciences; Increases in Steelhead Redd Abundance Resulting from Two Conservation Hatchery Strategies in the Hamma. In press. [Google Scholar]
- Van Den Berghe EP, Gross MR. Natural selection resulting from female breeding competition in a Pacific salmon (Coho: Oncorhynchus kisutch. Evolution. 1989;43:125–140. doi: 10.1111/j.1558-5646.1989.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Biro PA, Abrahams MV, Post JR, Parkinson EA. Predators select against high growth rates and risk-taking behavior in domestic trout populations. Proceedings of the Royal Society Series B. 2004;271:2233–2237. doi: 10.1098/rspb.2004.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchfield PJ, Ridgway MS. The cost of peripheral males in a brook trout mating system. Animal Behavior. 1999;57:537–544. doi: 10.1006/anbe.1998.1014. [DOI] [PubMed] [Google Scholar]
- Campton DE. Sperm competition in salmon hatcheries: the need to institutionalize genetically benign spawning protocols. Transactions of the American Fisheries Society. 2005;134:1495–1498. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Chilcote MW. Relationship between natural productivity and the frequency of wild fish in mixed spawning populations of wild and hatchery steelhead (Oncorhynchus mykiss. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1057–1067. [Google Scholar]
- Chilcote MW, Leider SA, Loch JJ. Differential reproductive success of hatchery and wild summer-run steelhead under natural conditions. Transactions of the American Fisheries Society. 1986;115:726–735. [Google Scholar]
- Crow JF. Some possibilities for measuring selection intensities in man. Human Biology. 1958;30:1–13. [PubMed] [Google Scholar]
- Crow JF, Kimura M. An Introduction to Population Genetics Theory. New York: Harper & Row; 1970. [Google Scholar]
- Cuenco ML, Barkman TWH, Mundy PR. The use of supplementation to aid in natural stock restoration. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York: Plenum Press; 1993. pp. 269–294. [Google Scholar]
- Dahl J, Petersson E, Dannewitz J, Järvi T, Löf AC. No difference in survival, growth and morphology between offspring of wild-born, hatchery and hybrid brown trout (Salmo trutta. Ecology of Freshwater Fish. 2006;15:388–397. [Google Scholar]
- Dannewitz J, Petersson E, Dahl J, Prestegaard T, Löf AC, Järvi T. Reproductive success of hatchery-produced and wild-born brown trout in an experimental stream. Journal of Applied Ecology. 2004;41:355–364. [Google Scholar]
- Einum S, Fleming IA. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature. 2000a;405:565–567. doi: 10.1038/35014600. [DOI] [PubMed] [Google Scholar]
- Einum S, Fleming IA. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar. Evolution. 2000b;54:628–639. doi: 10.1111/j.0014-3820.2000.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Einum S, Kinnison MT, Hendry AP. Evolution of egg size and number. In: Hendry AP, Stearns SC, editors. Evolution Illuminated: Salmon and Their Relatives. Oxford/New York: Oxford University Press; 2004. pp. 127–153. [Google Scholar]
- Estes S, Arnold SJ. Resolving the paradox of stasis: models with stabilizing selection explain evolutionary divergence on all timescales. American Naturalist. 2007;169:227–244. doi: 10.1086/510633. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Essex: Pearson; 1996. [Google Scholar]
- Fisher RA. The Genetical Theory of Natural Selection. New York: Dover; 1958. [Google Scholar]
- Fleming IA, Gross MR. Breeding success of hatchery and wild coho salmon (Oncorhynchus kisutch) in competition. Ecological Applications. 1993;3:230–245. doi: 10.2307/1941826. [DOI] [PubMed] [Google Scholar]
- Fleming IA, Gross MR. Breeding competition in a Pacific salmon (Coho: Oncorhynchus kisutch): measures of natural and sexual selection. Evolution. 1994;48:637–657. doi: 10.1111/j.1558-5646.1994.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Fleming IA, Petersson E. The ability of released hatchery salmonids to breed and contribute to the natural productivity of wild populations. Nordic Journal of Freshwater Research. 2001;75:71–98. [Google Scholar]
- Fleming IA, Lamberg A, Jonsson B. Effects of early experience on the reproductive performance of Atlantic salmon. Behavioral Ecology. 1997;8:470–480. [Google Scholar]
- Ford MJ. Selection in captivity during supportive breeding may reduce fitness in the wild. Conservation Biology. 2002;16:815–825. [Google Scholar]
- Ford MJ, Fuss H, Boelts B, LaHood E, Hard JJ, et al. Changes in run timing and natural smolt production in a naturally spawning coho salmon (Oncorhynchus kisutch) stream after 60 years of intensive hatchery supplementation. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2343–2355. [Google Scholar]
- Ford MJ, Fuss H, Boelts B, LaHood E, Hard JJ, Miller J. Estimates of natural selection in a salmon population in captive and natural environments. Conservation Biology. 2008 doi: 10.1111/j.1523-1739.2008.00965.x. in press. [DOI] [PubMed] [Google Scholar]
- Frankham R. Genetic adaptation to captivity in species conservation programs. Molecular Ecology. 2008;17:325–333. doi: 10.1111/j.1365-294X.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- Frankham R, Briscoe DA, Ballou JD. Introduction to Conservation Genetics. Cambridge/New York: Cambridge University Press; 2002. [Google Scholar]
- De Gaudemar B, Bonzom JM, Beall E. Effects of courtship and relative mate size on sexual motivation in Atlantic salmon. Journal of Fish Biology. 2000;57:502–515. [Google Scholar]
- Gustafsson L. Lifetime reproductive success and heritability: empirical support for Fisher’s fundamental theorem. American Naturalist. 1986;128:761–764. [Google Scholar]
- Hall H, Hunt P, Hassold T. Meiosis and sex chromosome aneuploidy: how meiotic errors cause aneuploidy; how aneuploidy causes meiotic errors. Current Opinion in Genetics and Development. 2006;16:323–329. doi: 10.1016/j.gde.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Henry IM, Dilkes BP, Young K, Watson B, Wu H, Comai L. Aneuploidy and genetic variation in the Arabidopsis thaliana triploid response. Genetics. 2005;170:1979–1988. doi: 10.1534/genetics.104.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra JM, Bartz KK, Ruckelshaus MH, Moslemi JM, Harms TK. Quantitative threat analysis for management of an imperiled species-Chinook salmon (Oncorhynchus tshawytscha. Ecological Applications. 2007;17:2061–2073. doi: 10.1890/06-1637.1. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson JI, Abrahams MV. Interbreeding with domestic strain increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss) – an experimental study. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:243–247. [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, et al. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hendry AP. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica. 2001;112-113:145–164. [PubMed] [Google Scholar]
- Kostow KE, Marshall AR, Phelps SR. Naturally spawning hatchery steelhead contribute to smolt production but experience low reproductive success. Transactions of the American Fisheries Society. 2003;132:780–790. [Google Scholar]
- Kruuk LEB, Clutton-Brock TH, Slate J, Pemberton JM, Brotherstone S, Guinness FE. Heritability of fitness in a wild mammal population. Evolution. 2000;97:698–703. doi: 10.1073/pnas.97.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey RT, Lach D, Duncan S. Salmon 2100: The Future of Wild Pacific Salmon. Bethesda, MD: American Fisheries Society; 2006. [Google Scholar]
- Lahti K, Laurila A, Enberg K, Piironen J. Variation in aggressive behaviour and growth rate between populations and migratory forms in the brown trout, Salmo trutta. Animal Behavior. 2001;62:935–944. [Google Scholar]
- Lande R. Natural selection and random genetic drift in phenotypic evolution. Evolution. 1976;30:314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;36:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Launey S, Hedgecock D. High genetic load in the Pacific oyster Crassostrea gigas. Genetics. 2001;159:255–265. doi: 10.1093/genetics/159.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leider SA, Hulett PL, Loch JJ, Chilcote MJ. Electrophoretic comparison of the reproductive success of naturally spawning transplanted and wild steelhead trout through the returning adult stage. Aquaculture. 1990;88:239–252. [Google Scholar]
- Lynch M, O’ Hely M. Captive breeding and the genetic fitness of natural populations. Conservation Genetics. 2001;2:363–378. [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Lynch M, Blanchard J, Houle D, Kibota T, Schultz S, Vassilieva L, Willis J. Perspective: spontaneous deleterious mutation. Evolution. 1999;53:645–663. doi: 10.1111/j.1558-5646.1999.tb05361.x. [DOI] [PubMed] [Google Scholar]
- McGinnity P, Prodohl P, Maoileidigh NO, Hynes R, Cooper D, et al. Differential lifetime success and performance of native and non-native Atlantic salmon examined under communal natural conditions. Journal of Fish Biology. 2004;65(Suppl. A):173–187. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Differential reproductive success of sympatric, naturally spawning hatchery and wild steelhead trout (Oncorhynchus mykiss) through the adult stage. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:433–440. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Differential reproductive success of sympatric, naturally spawning hatchery and wild steelhead trout (Oncorhynchus mykiss. Environmental Biology of Fish. 2004;69:359–369. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Nonrandom, size- and timing-biased breeding in a hatchery population of steelhead trout. Conservation Biology. 2005;19:446–454. [Google Scholar]
- Mousseau TA, Roff DA. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Nickelson TE. The influence of hatchery coho salmon (Oncorhynchus kisutch) on the productivity of wild coho salmon populations in Oregon coastal basins. Canadian Journal of Aquatic and Fisheries Sciences. 2003;60:1050–1056. [Google Scholar]
- Olney PJS, Mace GM, Feistner A. Creative Conservation: Interactive Management of Wild and Captive Animals. London/New York: Chapman & Hall; 1994. [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Reisenbichler RR, McIntyre JD. Genetic differences in growth and survival of juvenile hatchery and wild steelhead trout, Salmo gairdneri. Journal of Fisheries Research Board of Canada. 1977;34:123–128. [Google Scholar]
- Reisenbichler RR, Rubin S. Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES Journal of Marine Science. 1999;56:459–466. [Google Scholar]
- Reisenbichler RR, Rubin S, Wetzel L, Phelps S. Natural selection after release from a hatchery leads to domestication in steelhead, Oncorhynchus mykiss. In: Leber M, Kitada S, Blankenship HL, Svåsand T, editors. Stock Enhancement and Sea Ranching. Oxford: Blackwell Publishing Ltd; 2004. pp. 371–384. [Google Scholar]
- Ryman N, Utter F. Population Genetics and Fishery Management. Seattle, WA: University of Washington Press; 1987. [Google Scholar]
- Salmon Recovery Science Review Panel. Report for Meeting Held 30 August–2 September 2004. Seattle, WA: National Marine Fisheries Service, Northwest Fisheries Science Center; 2004. http://www.nwfsc.noaa.gov/trt/rsrp_docs/rsrpreportsept30-2004b.pdf. [Google Scholar]
- Shi Q, Martin RH. Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction. 2001;121:655–666. doi: 10.1530/rep.0.1210655. [DOI] [PubMed] [Google Scholar]
- Steinberg EK, Lindner KR, Gallea J, Maxwell A, Meng and J, Allendorf FW. Rates and patterns of microsatellite mutations in pink salmon. Molecular Biology and Evolution. 2002;19:1198–1202. doi: 10.1093/oxfordjournals.molbev.a004177. [DOI] [PubMed] [Google Scholar]
- Su G-S, Liljedahl LE, Gall GAE. Genetic and environmental variation of female reproductive traits in rainbow trout (Oncorhynchus mykiss. Aquaculture. 1997;154:115–124. [Google Scholar]
- Sundstrom LF, Lohmus M, Devlin RH. Selection on increased intrinsic growth rates in coho salmon, Oncorhynchus kisutch. Evolution. 2005;59:1560–1569. [PubMed] [Google Scholar]
- Wang S, Hard JJ, Utter F. Salmonid inbreeding: a review. Reviews in Fish Biology and Fisheries. 2002;11:301–319. [Google Scholar]
- Waples RS, Drake J. Risk/benefit considerations for marine stock enhancement: A pacific salmon perspective. In: Leber KM, Kitada S, Blankenship HL, Svåsand T, editors. Stock Enhancement and Sea Ranching. Oxford: Blackwell Scientific Press; 2004. pp. 260–306. [Google Scholar]
- Waples RS, Teel DJ. Conservation genetics of pacific salmon I. Temporal changes in allele frequency. Conservation Biology. 1990;4:144–156. [Google Scholar]
- Williams RN. Return to the River: Restoring Salmon to the Columbia River. Amsterdam/Boston, MA: Elsevier Academic Press; 2006. [Google Scholar]


