Abstract
Although evolutionary change within most species is thought to occur slowly, recent studies have identified cases where evolutionary change has apparently occurred over a few generations. Anthropogenically altered environments appear particularly open to rapid evolutionary change over comparatively short time scales. Here, we consider a Pacific salmon population that may have experienced life-history evolution, in response to habitat alteration, within a few generations. Historically, juvenile fall Chinook salmon (Oncorhynchus tshawytscha) from the Snake River migrated as subyearlings to the ocean. With changed riverine conditions that resulted from hydropower dam construction, some juveniles now migrate as yearlings, but more interestingly, the yearling migration tactic has made a large contribution to adult returns over the last decade. Optimal life-history models suggest that yearling juvenile migrants currently have a higher fitness than subyearling migrants. Although phenotypic plasticity likely accounts for some of the change in migration tactics, we suggest that evolution also plays a significant role. Evolutionary change prompted by anthropogenic alterations to the environment has general implications for the recovery of endangered species. The case study we present herein illustrates the importance of integrating evolutionary considerations into conservation planning for species at risk.
Keywords: anthropongenic disturbance, evolutionary change, life-history change, salmonid
Introduction
Human impacts to natural ecosystems profoundly affect the earth’s biota (Diamond 1989; Vitousek et al. 1997; World Commission on Dams (WCD) (2000); Foley et al. 2005), but until recently, most evaluations have focused on ecological and demographic consequences to the affected species and populations while ignoring evolutionary responses to anthropogenic effects. Yet, abundant evidence now exists that evolution can occur relatively rapidly, and within one human life time (Hendry et al. 2000; Kinnison and Hendry 2001; Quinn et al. 2001; Grant and Grant 2006). Further, rapid ecological changes associated with anthropogenic alteration of natural ecosystems can promote contemporary evolution, with unanticipated consequences. For example, bacteria affecting human health, and pests that target commercially important crops, have rapidly evolved immunity to the application of antibiotics and pesticides (Palumbi 2001). The harvest of large plants caused evolutionary change to a snow lotus plant prized for its medicinal use, leading to an increased risk of extinction (Law and Salick 2005). Fishing (Hutchings and Fraser 2008) and hunting (Coltman 2008) have also been implicated as agents of human-induced evolutionary change. Clearly, if evolutionary changes in populations occur (or might occur) in response to anthropogenic changes to their environments, conservation scientists and managers need to consider them when developing conservation strategies; otherwise, well-intentioned actions might prove ineffective or even harmful (Stockwell et al. 2003). Fortunately, scientists have begun to address the relative neglect of contemporary evolutionary processes and their potential conservation and management implications (e.g. Smith and Bernatchez 2008 and references cited therein).
In this paper, we consider how contemporary evolution associated with major human-induced ecological changes can have profound implications for the conservation and management of fall Chinook salmon (Oncorhynchyus tshawytscha) from the Snake River in the northwestern USA – a species that is listed as threatened under the US Endangered Species Act (ESA). In the last few decades, in association with major ecological changes attributable to the construction of hydroelectric dams on the Snake River (Raymond 1979; Raymond 1988), this population has experienced relatively rapid phenotypic changes in juvenile life history. We consider how the consequences of alternative future management actions might dramatically differ, depending on how much of the phenotypic change is due to evolution (as opposed to phenotypic plasticity), and whether future ecological conditions will more closely resemble the historical template or the current (anthropogenically altered) system. We begin by reviewing some background information that describes the ecosystem in which this population evolved and how human development has changed it. Next, we summarize the historical migration tactics of this population and recent evidence that they have changed. We use two models to characterize the contrasting selective regimes in the current and historical periods and their consequences for the expression of juvenile life history. Finally, we discuss the implications of our results for future conservation and management of the population.
Background information
Distribution and abundance
The Snake River (Fig. 1) is the largest tributary of the Columbia River and drains most of Idaho and parts of Washington, Oregon, Montana, Wyoming and Nevada. Historically, Snake River fall Chinook salmon spawned as far upstream as Augur Falls, an impassible barrier approximately 965 Rkm from the mouth of the Snake River (Parkhurst 1950), with adult production estimated as high as 500 000 annually (Craig and Hacker 1940; Fulton 1968; Chapman 1986). As early as the late 1800s, populations began to decline from over fishing and dam construction in the upper reaches of the river (Evermann 1896). Construction of Swan Falls Dam in 1901 limited the upstream migration to approximately Rkm 715, with the core spawning area occurring in the 40-km reach of river between the dam and Marsing, Idaho (Connor et al. 2002). By the mid-1950s, annual escapements of fall Chinook salmon to the Snake River had declined to less than 30 000 fish. With the construction of Brownlee Dam in the Hells Canyon area (1958), access to the remaining core spawning and rearing areas was cut off. Between 1961 and 1975, six more major dams were constructed – two within Hells Canyon and four in the lower Snake River. In all, dam construction inundated 62% of the remaining free-flowing lower Snake River, leaving only a 173-km stretch upstream of Lower Granite Reservoir for spawning. This remnant habitat probably had opportunistically spawning subpopulations, but large-scale historical use by a self-sustaining population has never been confirmed.
Figure 1.
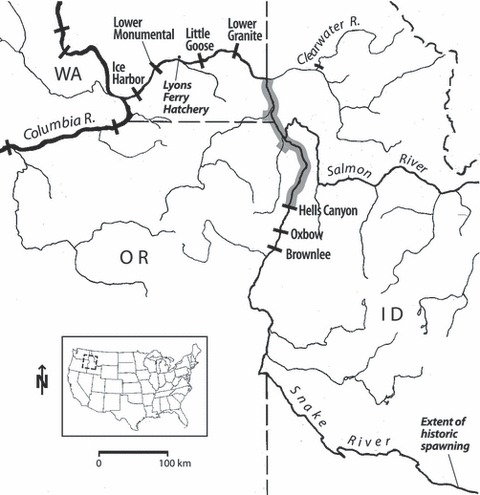
Snake River Basin showing the present spawning area of fall Chinook salmon in the main-stem Snake River and tributaries (shaded) versus the extent of the historical core area of spawning upstream of Brownlee Dam.
Abundance of Snake River fall Chinook salmon declined to less than 1000 fish per year after 1975, and they were listed as threatened under the ESA in 1992, after returns of wild fish hit a nadir of less than 100 adults. With improved ocean conditions in the late 1990s and the initiation of large-scale supplementation efforts from Lyons Ferry Hatchery (Fig. 1; see Bugert et al. 1995 for stock details), population abundance rebounded somewhat. In the mid-2000s, estimated returns of adults over Lower Granite Dam that originated from naturally spawning fall Chinook salmon ranged from approximately 3000 to 4000 fish annually.
Historical life history
In most Chinook salmon populations, juveniles begin migration to sea either shortly after emergence in the spring (subyearling migration tactic) or undergo a full year of growth in freshwater before migrating to sea as a yearling (yearling migration tactic). Generally, populations in the southern portion of the range exhibit the former tactic, and populations in the northern portion exhibit the latter tactic (Healey 1991). Brannon et al. (2004) speculated that growth opportunity primarily determines the choice of tactic – individuals need to attain a minimum size at the time of migration to survive in the marine environment, and populations achieve this minimum size in either one freshwater growing season under conditions of high growth opportunity or two freshwater growing seasons under conditions of low growth opportunity. This explanation comports with models for life-history expression in male Atlantic salmon, Salmo salar, that mature either at a small size in fresh water (as parr) or at a considerably larger size, following a seaward migration (as anadromous males). Adoption of either the nonmigratory parr tactic or the migratory anadromous male tactic appears to be conditional upon the attainment of a threshold body size, growth rate and/or physiological condition (Hutchings and Myers 1994; Thorpe et al. 1998; Aubin-Horth et al. 2006), a hypothesis that dates from the mid-1980s (Leonardsson and Lundberg 1986; Myers and Hutchings 1986; Thorpe 1986). The threshold that triggers the nonmigration/migration tactic is thought to differ genetically within and among populations (Hazel et al. 1990; Hutchings and Myers 1994; Thomkins and Hazel 2007; but see Gross 1996). Thus, within a population, temporal changes in the incidence of a specific migration tactic may be a product of phenotypic plasticity (caused by environmentally induced variation in growth rate/body size) or evolution (caused by a selection response in the value of the threshold).
The alternative life histories in Chinook salmon are further characterized by a suite of traits: yearling juveniles are more aggressive, better swimmers and respond differently to photoperiod than their subyearling counterparts (Healey 1991). These traits have a genetic basis (Taylor 1988; Taylor 1990; Clarke et al. 1992), and populations are typically dominated by one type or the other (Waples et al. 2004). In the Columbia River basin, subyearling migrants are typically associated with populations that spawn and rear in mainstem rivers and return to freshwater in the fall (hence the designation); in contrast, a genetically distinct lineage (Waples et al. 2004) of spring Chinook salmon populations from the interior Columbia River basin typically migrate as yearlings and spawn and rear in cooler tributaries at higher elevations. Multiple lines of evidence support the conclusion that historically Snake River fall Chinook salmon had a subyearling juvenile migration tactic. Researchers that studied fall Chinook salmon life history in the historical core production area (below Swan Falls Dam) only observed subyearling migrants (Bjornn 1960; Mains and Smith 1964; Krcma and Raleigh 1970). This area was relatively warm during incubation and early rearing because of geothermic inflow and a high desert climate. Consequently, growth opportunity was high relative to other Chinook salmon spawning areas and likely promoted a subyearling migration tactic (Connor et al. 2002; Connor and Burge 2003). Fish that grew fast within the mainstem river had sufficient size to migrate with the late spring/early summer high flows (Mains and Smith 1964). Fourth, mean daily temperatures during July ranged from 20°C to 23°C in the historical core production area, which would have increased predation (Vigg and Burley 1991), disrupted physiological processes (Mesa et al. 2002) and likely reduced levels of smoltification along with decreased growth (Marine and Cech 2004). Therefore, an advantage existed for fall Chinook salmon juveniles to move seaward as subyearlings. Finally, only 3% of the fall-run adults sampled for scales during the 1960–1969 Columbia River gill net fisheries, which included Snake River fall Chinook salmon, had scale patterns indicative of the yearling migration tactic (Young and Robinson 1974).
Present life history
Contemporary Snake River fall Chinook salmon exhibit migration tactics that differ from their historical counterparts, and this may have resulted from several anthropogenic disturbances. Construction and operation of Brownlee Dam changed water temperatures between Hells Canyon and where the Salmon River enters the Snake River. Water temperatures are now warmer in the fall and cooler in the spring (Ebel and Koski 1968). Because Chinook salmon spawn at declining water temperatures (Miller and Brannon 1982), the changed fall water temperatures may have delayed spawning and cooler spring temperatures reduced growth of juveniles. Further, some of the extant spawning areas in the lower part of the remnant spawning area are now cooler than the area above Brownlee Dam because inflow from high elevation tributaries cools the mainstem temperatures. As a consequence, fry in the extant spawning areas now emerge from the gravel later in the spring than their historic counterparts, juveniles grow more slowly and begin seaward movement on a later time schedule than had been observed for fish in the historical core spawning area (Krcma and Raleigh 1970; Connor et al. 2002; Connor and Burge 2003). Further, by the mid-1970s, seaward migrating fall Chinook salmon also had to pass four hydropower dams along the lower Snake River in eastern Washington to reach the Columbia River. The reservoirs created by these dams decreased water velocity and delayed seaward passage of migrants. Whereas historically peak passage of fall Chinook salmon subyearlings through the lower Snake River in eastern Washington was in June (Mains and Smith 1964), the peak passage of fall Chinook salmon through this river reach is now observed from early to mid-July (Connor et al. 2002;Smith et al. 2003). The overall change in juvenile life-history timing caused by dam construction is a factor for migration tactic selection. Young salmon must achieve high growth rates and develop physiologically in synchronization with seasonal changes in water velocity, water temperature and photoperiod to exhibit the subyearling tactic (e.g. Dickhoff et al. 1997; Beckman and Dickhoff 1998; Connor et al. 2001). If this synchronization does not occur by spring or early summer; subyearlings tend to cease active migration, delay seaward movement and exhibit the yearling tactic (Connor et al. 2002, 2005).
Larger size at the initiation of seaward movement likely provides yearling migrants with a survival advantage. Scale readings from naturally produced Snake River fall Chinook salmon sampled during their upstream migration at Lower Granite Dam from brood years 1994 through 2002 (incomplete returns for BY 2002; Connor et al. 2005 and subsequent unpublished data) indicated that an average of 54% (range 24–82%) of the total returning adult females to Lower Granite Dam had migrated to sea as yearlings. Because an estimated 30% of the return now migrates into the Clearwater River with 77% having a yearling migration tactic (no historical information on this population exists, as a dam constructed in 1927 at the mouth of the Clearwater River extirpated adult returns), we weighted the Lower Granite Dam estimate of females with a yearling migration tactic by the Clearwater River percentage to derive an estimated return to the Snake River spawning area of 44.1%.
In the following sections, we provide some analyses to estimate total numbers of yearling smolts from all sources (upstream and downstream of Lower Granite Dam).
Life-history models
We performed two modeling exercises to estimate the relative fitness of the yearling and subyearling migration tactics. The modeling exercises were based primarily on detailed demographic data for adult Snake River fall Chinook salmon that had been intercepted at the adult trap at Lower Granite Dam between 1999 and 2006, before being transported to Lyons Ferry Hatchery (Fig. 1). At the hatchery, the gender and length of each fish were determined, and scales were sampled. Based on subsequent scale reading, natural fish were distinguished from hatchery fish, total age was identified and age at ocean entry (subyearling versus yearling) was determined (see Connor et al. 2005 for methods). The combined population of wild spawners sampled at Lyons Ferry Hatchery consisted of approximately 70% spawners destined for the Snake River and 30% to the Clearwater River. Based on scale samples taken from adults on the Clearwater spawning grounds, the age-class distribution for adults that came from subyearling or yearling juvenile migrants was the same as for the combined population sampled at Lyons Ferry Hatchery; however, 76% came from yearling juveniles. We, therefore, weighted the overall adult returns by the Clearwater River proportions to estimate the expected proportion of Snake River adults that came from subyearling and yearling migrants. We estimated fecundity of females from their length and an egg–length relationship derived by Galbreath and Ridenour (1964).
The analyses are based on the Euler–Lotka equation (Lotka 1959) for individuals maturing at ages 3–6:
| 1 |
where x refers to the age at maturity, r is the fitness, lx,y the survival through age x for migration tactic type y and m the corresponding fecundity (see Stearns 1992 for examples of applications of this equation to populations). Based on age-specific survival and fecundity estimates, we solved for fitness, ry, for each migration tactic type.
The first analysis examined how relative fitness of yearling migrants to subyearling migrants varied in response to ranges in life-stage-specific survival that characterize the uncertainty in these parameters. We varied survival across two juvenile life stages and early ocean survival. In this analysis, we estimated a separate fitness for each age-at-maturity and migration tactic and determined relative fitness for fish of the same age-at-maturity.
The goal of the second analysis was to estimate the relative fitness (for all age classes combined) of individuals adopting the yearling versus subyearling migration tactic. We related relative fitness to a key, but unknown parameter – the proportion of juveniles adopting each migration tactic. After we specified this parameter, we could determine the relative survival (and consequently relative fitness) of each life-history type based on the proportion of returning adults known to have adopted each life-history type as juveniles.
Method 1
The primary purpose of this analysis was to identify the range of survival probabilities that would differentially favor individuals that adopt the yearling and subyearling smolt-migration tactics. Life tables delineate the age-specific survival probabilities and fecundity for individuals adopting either the subyearling or yearling tactic and returning to spawn after 1, 2 or 3 years at sea (Tables 1, 2). Irrespective of the tactic adopted, individuals are assumed to have the same survival probabilities from the egg stage to the time they emigrate from the Snake River (Semigrant = 0.10) and in the ocean as subadults (Ssea = 0.80 per annum). There are two key differences in the life tables. The first is the term Sriver that represents the probability that a smolt survives the period (approximately 1 year) during which it resides in freshwater. This parameter was allowed to vary between 0.2 and 0.8. The second difference in the life tables is the survival probability experienced during the migration of smolts through the Columbia River to the sea. This parameter, Smigration, ranged between 0.05 and 0.25 for subyearling smolts [based on unpublished passive integrated transponder (PIT)-tag and acoustic-tag data]. Among yearling smolts, for which a larger size may be associated with higher survival immediately prior to and/or shortly after entry to the ocean, Smigration was increased by a factor τ ranging between 1 (same survival as subyearlings) and 3 (three times the survival of subyearlings; Faulkner et al. 2007 estimated 61% for yearlings in 2006) (Table 2).
Table 1.
Life table for subyearling smolts returning after 2–4 years at sea (x = 3–5, respectively).
| Life stage | Time period (monthyear) | Stage-specific survival, Sx | Age at maturity (x) | Age-specific fecundity (mx) |
|---|---|---|---|---|
| Egg to emigration from Snake River | Nov0–Apr1 | Semigrant = 0.1 | 0 | |
| Smolt migration | May1–Oct1 | Smigration = 0.05–0.25 | 0 | |
| Subadult | Nov1–Oct2 | 0.80 | 0 | |
| Subadult | Nov2–Oct3 | 0.80 | 3 | 3868 |
| Subadult | Nov3–Oct4 | 0.80 | 4 | 5132 |
| Subadult | Nov4–Oct5 | 0.80 | 5 | 5741 |
Table 2.
Life table for yearling smolts returning after 1–3 years at sea (x = 3–5, respectively).
| Life stage | Time period (monthyear) | Stage-specific survival (Sx) | Age at maturity (x) | Age-specific fecundity (mx) |
|---|---|---|---|---|
| Egg to emigration from Snake River | Nov0–Apr1 | Semigrant = 0.1 | 0 | |
| River residence | May1–Apr2 | Sriver = 0.2–0.8 | 0 | |
| Smolt migration | May2–Oct2 | Smigration = 0.05–0.25 | 0 | |
| Subadult | Nov2–Oct3 | 0.80 | 3 | 3209 |
| Subadult | Nov3–Oct4 | 0.80 | 4 | 4671 |
| Subadult | Nov4–Oct5 | 0.80 | 5 | 5474 |
To bound the range of survival probabilities, we used estimated smolt-to-adult return ratios (SARs) developed from fall Chinook salmon tagged with PITs (Prentice et al. 1990) and released between 1995–2000 for a study to evaluate juvenile migration, survival and timing (Smith et al. 2003). The juvenile fish were automatically detected at dams during the downstream migration; likewise, automatic detection of adults occurred as they passed through detectors at Lower Granite Dam. We also used data (unpublished NMFS studies) from fish PIT-tagged in 2001 and released similarly to the earlier Smith et al. (2003) studies. We grouped juvenile detections at Lower Snake River dams into three categories: (i) fish detected between June and August, (ii) fish detected in September and October (most detection facilities at dams ceased operation by the end of October) and (iii) fish detected the following spring. We assigned adult PIT-tagged fish detected at Lower Granite Dam to their respective juvenile outmigration years, by category, then divided the totals for each outmigration year, by category, by the number of juveniles detected in the outmigration associated with each category. We then took the geometric mean of these annual PIT-tag estimates to develop relative rates of return for fish migrating as juveniles during the three different time periods.
Method 2
Based on the demographic data for returning adults, we derived the following terms: Px,y is the proportion of returning adults of life history type y (y = 0 denotes subyearling migrant and y = 1 denotes yearling migrant) returning at age x. These terms sum to 1.0 within life-history types. Py is the proportion of returning adults of migration tactic type y. 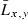 is the mean length (cm) of individuals of migration tactic type y and return age x
is the mean length (cm) of individuals of migration tactic type y and return age x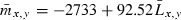 is the mean fecundity of individuals of migration tactic type y and return age x.
is the mean fecundity of individuals of migration tactic type y and return age x.
Estimating model terms for the Euler–Lotka equation required several steps. First, we developed a simple model based on the key life-history terms (Fig. 2), where P refers to the adult probabilities and p the juvenile probabilities. We assumed a common survival, SJ = 0.1, during the early juvenile period, corresponding to egg deposition to shortly after emergence. Next, we specified a juvenile to adult survival for the entire population, ST = 0.01, based roughly on PIT tag data. We note that the values of ST and SJ are not critical for the overall conclusions of the analysis. We defined the proportion of juveniles destined to adopt each life-history tactic as p0 for the proportion of individuals destined to adopt the subyearling migration tactic and p1 for the proportion of individuals destined to adopt the yearling migration tactic. We then define a juvenile to adult survival rate (Sy) for each life-history type. This period encompasses juvenile rearing, downstream migration, ocean residence and return migration.
Figure 2.
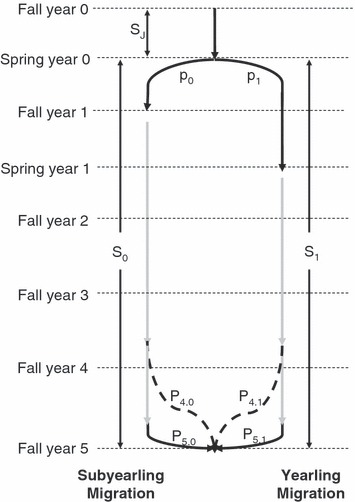
Schematic diagram of life-history stages for Snake River fall Chinook salmon, showing the differences in life-history stages between those juveniles that take a subyearling tactic versus those with a yearling tactic. P refers to adult probabilities and p refers to juvenile probabilities.
Although we estimated p0 below, this estimation required several assumptions. Thus, we developed our model such that the relative fitness of the two tactics is expressed as a function of p0. In this way, we could examine the response of relative fitness to varying values of p0. Once we specified p0, we could calculate adult survival rate (Sy) for each life-history type based on the proportion of adults that adopted each migration tactic (P0 and P1) and the overall adult survival rate for the entire population, ST, as follows.
First we note that overall adult survival is
| 2 |
Next, we express the proportion of adults returning with the subyearling migration tactic as
| 3 |
Rearranging terms in equation (3), we obtain:
| 4 |
We calculated S1 in a similar manner.
As noted above, Snake River fall Chinook salmon are semelparous and mature at several ages. Therefore, to implement the Euler–Lotka equation, we needed to estimate survival through each age-at-maturity by migration tactic, Sx,y (equivalent to lx,y in the Euler–Lotka equation), and the proportion of fish breeding by age, bx,y. To do this, we first assumed that survival of adults in the ocean (SO) was 0.8, an assumption commonly made in Chinook salmon life-cycle models (e.g. Kareiva et al. 2000; Zabel et al. 2006). We then expressed juvenile to adult survival (for individuals maturing at ages 3–5) as:
| 5 |
Note that all individuals of each tactic have a common survival through the third year, and survival in subsequent years was determined by proportioning remaining to breed and ocean survival.
Based on equation (5), Sy [calculated from equations (2) and (3)], and age at return data, we estimated S3,y and the bx,y terms. We then used these terms to determine survival through all age classes. Finally, we modified the Euler–Lotka equation to reflect that only a proportion of the adults (ages 3–6) breed at a given age:
| 6 |
We then calculated the relative fitness (of the yearling migration tactic to the subyearling migration tactic) as rREL = r1/r0. We note again that this relative fitness is a function of the (unobserved) proportion of juveniles adopting each life-history type. Therefore, we calculated rREL as a function of p0.
To simplify the presentation of the results, we calculated the ratio of the fitness associated with the yearling tactic relative to that associated with the subyearling tactic, for individuals maturing at ages 3–5 years. When this ratio exceeds 1, individuals adopting the yearling smolt-migration tactic have higher fitness than those with the subyearling tactic.
Estimation of migrant proportions
Method 2 required an estimate of the proportion of smolts in the outmigration destined to enter the ocean as subyearlings and yearlings. We based this estimate on juvenile fall Chinook salmon collected and PIT tagged in their rearing areas on the Snake River across the migration season (Connor and Burge 2003). PIT-tagged individuals were detected in juvenile fish bypass systems at three downstream sites: Lower Granite Dam (site 1), Little Goose Dam (site 2) and Lower Monumental Dam (site 3, see Fig. 3). We used these data to account for the fates of all fish.
Figure 3.
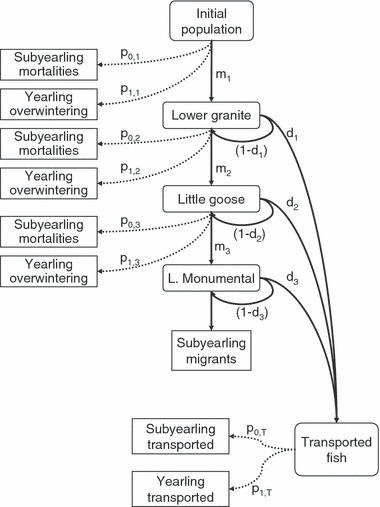
Schematic diagram of estimated probabilities of migrating and surviving detection probabilities, and proportions of fish in different migratory categories for Snake River fall Chinook salmon.
We first separated annual releases (1998–2004) into four sequential release groups per year with an equal number of fish in each group (28 release groups with between 302 and 1369 individuals per group). We then used standard methods (Cormack 1964; Jolly 1965; Seber 1965; Smith et al. 2003) to calculate the detection probability (di) at each site i and the joint probability of active migration and survival (mi) to each site for each release cohort (note that ‘loss’ of fish can occur by either mortality or by yearling migrants ceasing migration). During 1998–2004, the management strategy was to collect all fish in bypass systems and load them onto barges or trucks for transport to a release site downstream of Bonneville Dam (the last dam in the Columbia River Basin hydropower system). Therefore, di represents the proportion of individuals in the population that passed each dam and was subsequently transported. We also estimated mi for each of the releases from point of release to the tailrace of Lower Granite Dam (m1), from the tailrace of Lower Granite Dam to the tailrace of Little Goose Dam (m2) and from the tailrace of Little Goose Dam to the tailrace of Lower Monumental Dam (m3). For each dam, we calculated a mean di and mi across years (Table 3). We assumed that the members of the first cohort were actively migrating juveniles destined for a subyearling migration based on the observation that nearly all PIT-tagged fish detected early the migration season and later observed as an adult are subyearling migrants (W. Connor, unpublished data). We thus equated the mi for each year’s first cohort to survival of actively migrating subyearlings and termed this S0,i. We estimated the mean proportion of yearlings ceasing migration in each river segment i that overwintered as:
Table 3.
Data (top three rows) used to generate estimated proportions of juveniles destined for yearling and subyearling migration (bottom two rows).
| River segment number | ||||||
|---|---|---|---|---|---|---|
| To Lower Granite | Lower Granite to Little Goose | Little Goose to Lower Monumental | Migrant below Lower Monumental | Transported | Total | |
| Joint probability of migrating and surviving (mi) | 0.473 | 0.793 | 0.741 | |||
| Subyearling survival (S0,i) | 0.598 | 0.856 | 0.846 | |||
| Detection probability (di) | 0.560 | 0.629 | 0.490 | |||
| Proportion of subyearlings in each category (p0,i) | 0.318* | 0.0278* | 0.0083* | 0.0231† | 0.360‡ | 0.737 |
| Proportion of yearlings in each category (p1,i) | 0.209¶ | 0.0153¶ | 0.0076¶ | 0.0313‡ | 0.263 | |
Mortality.
Migrants below Lower Monumental Dam.
Transported below Bonneville Dam.
Overwintering.
| 7 |
We then used these probabilities to estimate the fate of all fish. For instance, for a fish to overwinter in Lower Granite reservoir, it had to migrate to Lower Granite Dam (m1), not pass via the bypass system (1 − d1), and then cease to migrate (p1,1). Thus, multiplying these terms together yields the proportion of fish overwintering in Lower Granite Pool.
We made the following assumptions :
All members of the wild subyearling population that had actively migrated and survived to the tailrace of Lower Monumental became subyearling ocean entrants. This assumption is based on observations from acoustically tagged fish (unpublished NMFS data), where nearly all fish tagged above Lower Monumental Dam as subyearlings and subsequently detected, appear to have an active downstream migration to Bonneville Dam.
Based on the reading of adult scales, approximately 0.08 of the transported subyearlings survived to over winter in freshwater or the estuary downstream of Bonneville Dam and entered saltwater the following spring as yearlings (unpublished NMFS data).
Our above analyses entailed some assumptions and thus may have some limitations. We estimated that conservatively, at most about 26% of juveniles had a yearling migration tactic. We have no direct measures for this value, but it considerably exceeds the <5% proportion of fish with a yearling migration from the total population of PIT-tagged fish observed. However, the observed fish represent only the survivors of the population with the yearling migration tactic. Based on PIT-tagged fish, the adult return rates of fish that migrate as subyearlings in the fall were similar to that for fish that migrated the following spring, suggesting that over-wintering survival was high. We recognize that most of the subyearling migrants were collected at dams on the Snake River and transported to below Bonneville Dam. However, analyses of data on fall Chinook salmon transported from the Snake River indicate that transported fish do not return at rates different from migrant fish (Williams et al. 2005). Therefore, removing fish from the river should not bias our two modeling analyses.
Results
Subyearling and yearling migrants produced adults that returned with similar age proportions (based on scales of 549 wild females Table 4) and did not differ significantly in mean age at return (two-sided t-test: t = |0.999|, P = 0.318). Subyearling migrants thus spent approximately one more year in the ocean, and yearling migrants essentially substituted 1 year of freshwater growth for 1 year of growth in the ocean. For a given age, adults from subyearling migrants were approximately 4.2-cm longer (overall mean across all ages) at return compared with yearling migrants (two-sided t-test: t = |5.82|, P < 0.001). In addition, yearling migrants returned to Lower Granite Dam approximately 1 week later than subyearling migrants (two-sided t-test: t = |3.204|; P = 0.00014).
Table 4.
Combined demographic information for Snake River subyearling and yearling migrants, BY 1994–2002.
| Subyearling migrants (N = 258 adults) | Yearling migrants (N = 291 adults) | ||||||
|---|---|---|---|---|---|---|---|
| Age at return | 3 | 4 | 5 | 3 | 4 | 5 | 6 |
| Proportion (Px,y) | 0.048 | 0.504 | 0.447 | 0.025 | 0.524 | 0.436 | 0.015 |
Mean length (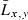 ) (cm) ) (cm) |
71.1 | 85.1 | 91.6 | 64.6 | 80.2 | 87.7 | 88.8 |
Fecundity (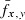 ) ) |
3868 | 5132 | 5741 | 3209 | 4671 | 5474 | 5492 |
| Overall proportion (Py)* | 0.559 | 0.441 | |||||
Adjusted for Clearwater River spawners
Method 1
PIT-tagged fish do not provide absolute estimates of SARs. Comparatively, however, the geometric mean return rates for PIT-tagged fish released between 1995 and 2001 were 0.24% for fish detected in June–August, 1.42% for fish detected in September and October and 1.65% for fish detected the following spring. The difference between these rates provided an estimated range of the maximum difference in survival for yearlings versus subyearlings over all parameters representing juvenile life history.
The life-cycle model identified survival conditions that favored the yearling smolt-migration tactic over the subyearling tactic. Results are plotted for Smigration = 0.05 as the fitness ratios were insensitive to the range of estimates considered for this parameter (0.05–0.25). The relative fitness of the yearling tactic increased with the survival advantage conferred to yearling smolts as a consequence of their larger size, i.e. τ (Fig. 4). For the range in values of τ considered here (1–3), a marginal increase in τ from unity will favor the yearling tactic at high in-river survival probabilities (Fig. 4A). At intermediate levels of in-river survival (Fig. 4B), a value of τ between 1.5 and 2 will favor the yearling tactic. The subyearling tactic is favored when the probability of in-river survival by yearling smolts is relatively low, irrespective of τ (Fig. 4C). The value of τ needed to favor the yearling tactic increased slightly as age at maturity declines, but the effect was small.
Figure 4.
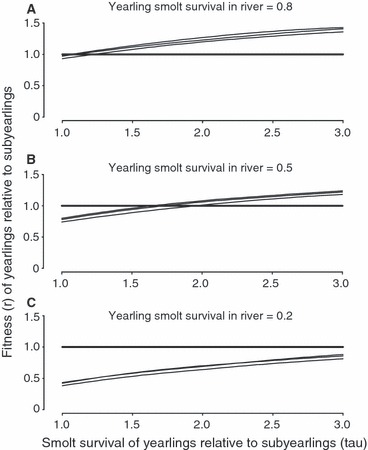
The estimated fitness of Snake River fall Chinook salmon that adopt the yearling tactic relative to those that adopt the subyearling tactic as a function of the smolt survival of yearlings relative to that of subyearlings, at three levels of yearling smolt overwinter survival probability (Sriver). Three relative-fitness functions are presented in each panel, one for individuals maturing at ages 3–5 years. In each case, the fitness function for individuals maturing at age 5 has the highest elevation whereas that for individuals maturing at age 3 has the lowest elevation.
The relative fitness of the yearling tactic increases with the probability of yearling in-river survival, Sriver (Fig. 5). When the survival of yearling smolts is three times that of subyearlings, the yearling tactic will be favored when Sriver exceeds approximately 0.3, irrespective of age at maturity (Fig. 5A). At intermediate levels of τ, Sriver needs to exceed approximately 0.5 to favor the selection of the yearling tactic (Fig. 5B). Under those circumstances when the smolt survival probability of yearlings is equal to that of subyearlings, the subyearling tactic will always be favored across the range of in-river yearling survival probabilities considered here (Fig. 5C).
Figure 5.
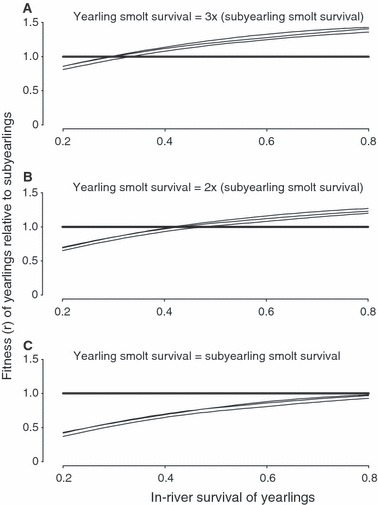
The estimated fitness of Snake River fall Chinook salmon that adopt the yearling tactic relative to those that adopt the subyearling tactic as a function of yearling smolt overwinter mortality (Sriver) for τ (ratio of yearling smolt survival relative to that of subyearlings). Three relative-fitness functions are presented in each panel, one for individuals maturing at ages 3–5 years. In each case, the fitness function for individuals maturing at age 5 has the highest elevation whereas that for individuals maturing at age 3 has the lowest elevation.
Method 2
The relationship between relative fitness (rREL) and the proportion of fish adopting a subyearling migration tactic (p0) is quite steep (Fig. 6). rREL is 1.0 for p0 slightly greater than 0.6, but rises to approximately 2.0 for p0 = 0.85. We emphasize that this is not a functional relationship and does not imply that relative fitness will vary in the future according to the proportion of fish adopting each migration tactic. Instead the relationship states that, given the known return rates of the two migration tactics, the relative fitness is a function of the (unknown) proportion of fish adopting each tactic. In other words, if a relatively large proportion of individuals adopted one tactic but a relatively small proportion of individuals returned that had adopted the tactic, then the tactic had relatively poor fitness. Thus, understanding p0 is crucial for understanding the relative fitness of the two migration tactics.
Figure 6.
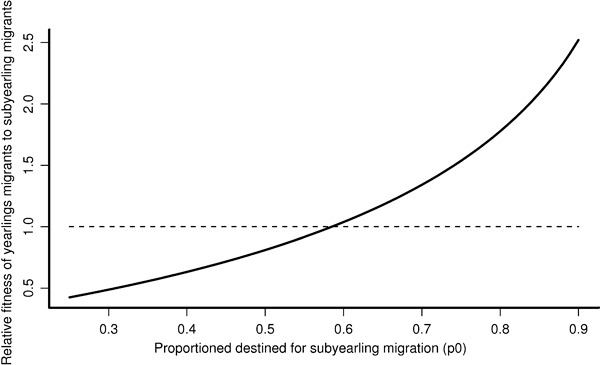
The relative fitness of the yearling smolt tactic compared with the percentage of the outmigration that has a subyearling tactic during the downstream migration.
Based on PIT-tag data and several assumptions, we estimated that 73.7% of the juvenile migrants adopted the subyearling tactic and 26.3% adopted the yearling tactic. This range in values for p0 corresponds to a relative fitness of approximately 1.5, thus indicating that yearling migrants have fitness greater than subyearling migrants.
Discussion
The migration tactics of Snake River fall Chinook salmon have changed over time. The incidence of yearling smolts was so low historically that none were observed migrating from the Snake River in the 1950s and early 1960s. Scales obtained from fish taken in the Columbia River fall Chinook salmon fishery in the 1960s indicated that fewer than 3% of returning adults had migrated to the sea as yearlings. At present, adult females (comprising approximately 44% of the adult run) originated as juveniles for which the incidence of the yearling migration tactic is approximately 23%. Although the juvenile yearling migration tactic is high compared with historical conditions, the percentage of yearling migrants in the outmigration is less than the percentage of adults that returned from fish with the yearling migration tactic. The adaptation of a yearling migration tactic represents a substantial change from historical conditions and may represent random drift, plasticity or evolutionary change, as we discuss below.
Evolution towards yearling ocean entry
Three major anthropogenic actions have changed conditions in ways that may have favored the yearling life history. First, dam construction displaced spawners into relatively cool habitat in the margins of the stock’s historical range. Consequently, fry emerge later and grow slower and become smolts later than in the historical spawning area. Second, after becoming smolts, fish now encounter low water velocities in the reservoirs in lower Snake River as a result of construction of four dams in the 1960s and 1970s. This leads to even later smolt migration timing and lower migration rates. Historically, water temperatures in the Snake River during the summer were likely too high for juvenile salmon to grow and survive, so selection against late migrants may have existed. Since the listing of Snake River fall Chinook salmon under ESA, however, cool water has been released into Lower Granite Reservoir from an upstream reservoir located in the Clearwater River drainage (1992 to present). Although the cool-water releases have not increased velocity sufficiently to allow fish to migrate downstream on their historical time schedule, they do provide thermal layers in Lower Granite Reservoir that appear to be optimal for growth and long-term survival. Connor et al. (2005) concluded that the cool-water releases enabled, or at least enhanced, the opportunity for juveniles to survive to a yearling size prior to ocean entry.
The simulations indicate that for Snake River fall Chinook salmon, the greater the in-river survival of yearling smolts, in both absolute terms and relative to that of subyearlings (assumed here to be associated with larger body size), the more likely that the yearling tactic will be associated with higher fitness than the subyearling tactic. Although one might argue that such a result is self-evident, such a conclusion overlooks the trade-offs associated with changes to life-history traits. In the present case, the increased survival associated with the yearling tactic is associated with costs to fitness reflected by smaller size at maturity and lower fecundity (Table 4).
Our simulations show that the yearling tactic is favored across a wide range of biologically reasonable values for Sriver and τ. This suggests that the higher incidence of yearling smolts in recent years results from a decreased survival cost to remaining in fresh water rather than migrating immediately to the ocean. If in-river survival probabilities for yearling smolts were historically near or below the low range of the estimates of Sriver considered here (i.e. 0.2), then our simulations indicate that the subyearling tactic is favored independently of the survival benefits conferred by a larger size at ocean entry, at least up to τ = 3 (Fig. 4C). However, as the prospects for survival in the Columbia River for yearling smolts increase, so do the fitness benefits associated with this tactic.
Three factors could, in theory, explain the recent shift toward a yearling migration tactic: (i) random drift, (ii) plasticity or (iii) evolution. Using available spawner-recruit data (unpublished, NOAA Fisheries) and the method described by Waples (2002), we estimated that the harmonic mean effective population size per generation (Ne) for Snake River fall Chinook salmon for brood years 1964–1991 was approximately 1000. In a breeding population of this size, genetic drift would be too slow to explain the observed in the relatively short time considered in this study (50 years or about 12 generations).
Shifts in smolt age suggest a possible plastic response to changing conditions for growth if individuals must achieve a specific size threshold for smolting to occur (as proposed for Atlantic salmon –Hutchings and Myers 1994; Thorpe et al. 1998). This would imply that fast growing individuals would adopt the subyearling tactic, whereas slow growing individuals would adopt the yearling tactic. If Sriver was historically low because of warm summer temperatures, we could reasonably conclude that (i) subyearlings would have a relatively small threshold smolt size because of the substantive survival benefits of entering the ocean as early as possible and (ii) most slow growers died before reaching the ocean because there was no suitable habitat to support river residence. As discussed above, current conditions promote later fry emergence and slower growth, which would cause more juveniles to fail to achieve the threshold size to smolt as subyearlings. Moreover, river conditions now appear more amenable for over-summer survival, so that a much larger fraction of the fish that remain in the river survives to migrate as yearling smolts. In combination, these two factors could increase the fraction of yearling smolts without requiring genetic change.
The third possible explanation involves evolution. Two conditions are required for adaptive evolution to occur: the trait under consideration must be heritable, and a selective differential for the trait must exist between environments or across time in the same environment. Considerable evidence exists for heritable variation in life-history traits in Pacific salmon. Across a wide range of studies, the median heritability for life-history traits related to growth and development was 0.25 (Carlson and Seamons 2008). Although this is a modest value, it does provide ample opportunity for natural selection to operate. The other condition – a selective differential – is clearly met. Hydropower development has profoundly changed freshwater environmental conditions experienced by Snake River fall Chinook salmon, so ample reason exists to believe that selective pressures for age at smolting could have changed as well. Our analyses suggest that the possibility of rapid evolutionary change in juvenile migration tactic within the last 50–60 years. Credence is lent to this hypothesis by research that has concluded that evolutionary changes have been experienced by introduced Chinook salmon populations over a 90-year period in New Zealand. There, Unwin et al. (2000) documented a divergence in juvenile migratory timing associated with differences in water temperatures that had affected juvenile growth, an association similar to that observed in Snake River fall Chinook salmon. Further, warmer fall water temperatures have likely altered (delayed) the time of adult spawning (a trait that may respond rapidly to selection; Quinn et al. 2000) as a result of dam construction in Hells Canyon. The later spawning time may partially account for delayed emergence of juveniles in Snake River, thus decreasing growth opportunities. Finally, our overall conclusions are similar to those proffered by Quinn et al. (2001) who concluded that trait divergence in Chinook salmon initially resulted from plasticity, shortly after the fish were first introduced to New Zealand in the early 1900s, but that rapid evolutionary change occurred thereafter within, at most, 30 generations. On the other hand, just because selective pressures exist does not mean that evolutionary change will occur (Etterson and Shaw 2001; Merilä et al. 2001). Nonetheless, if the probability of survival in Lower Granite Reservoir has increased in recent years because of cool-water releases from an upstream storage reservoir (Connor et al. 2005) and if growth opportunities have increased in the reservoir (Connor et al. 2002), then the fitness advantages associated with the subyearling tactic would be expected to decrease. These changes to survival and growth, and their concomitant effects on fitness, might generate a selection response in the size threshold that would increase the probability that individuals would adopt the yearling tactic. If so, we would predict that selection would increase the smolt size threshold, leading to a reduction in the incidence of the subyearling tactic.
Thus, we can plausibly explain the recent shift in migration tactics of Snake River fall Chinook salmon by either phenotypic plasticity or evolution; quite likely, a combination of the two factors is responsible. We believe that the initial rapid change toward successful adult returns from smolts with a yearling tactic probably was primarily a plastic response to changed environmental conditions. Given the large shift in apparent selective pressures, some consideration of the implications of rapid evolution in this population is warranted.
Implications for recovery
It appears that the present river conditions favor fish with a yearling migration tactic, and possibly a component of the phenotypic change has resulted from evolution. This poses a number of interesting questions for applied evolutionary biology. Under the US ESA, Federal agencies must implement measures designed to recover a protected species to the point at which it no longer needs listing. If the species historically had juveniles that migrated to sea as subyearlings, but current conditions favor the yearling migration tactic, what part of the historical population should recovery efforts target? Should efforts focus on factors that will further enhance the yearling migration tactic, which seems to have an adaptive advantage under current (and substantially altered) conditions? Or should efforts focus on retaining the historical migration tactic of the population, even at (perhaps) a substantial demographic cost? Can a population be considered recovered if, in order to achieve productivity high enough to ensure sustainability, it is necessary to transform the key life history features of the population, to the extent that it loses some of the characteristics that historically made it distinctive? These questions raise normative issues and therefore have no simple scientific answers.
Additionally, if selection favoring a yearling migration tactic continues long enough to promote substantial evolution in the population, what would happen if the dams were ever removed? Two divergent possibilities might exist. If the population evolves toward slower growth rates that are incapable of producing a subyearling smolt, it might find itself in a desperate race to re-evolve historical life-history traits before going extinct (Waples et al. 2007). The population’s ability to repay this heavy Darwinian debt (Loder 2005) could depend critically on the amount of genetic diversity remaining for juvenile life-history traits. That is, if evolution drives the population to a point where it has strongly committed to the yearling migration tactic and has little flexibility to respond to rapid environmental changes, the short-term consequences of restoring the river to more ‘pristine’ conditions could be sobering. Alternatively, we might have only observed a change in allele frequencies. If the population has not lost substantial genetically based variation for this trait, then the frequencies might revert to those that occurred historically if dams were removed and migratory conditions also reverted back to historical conditions. Under this possibility, we might expect to see a fairly rapid shift back toward the subyearling migration tactic within a few generations. Although removal of dams is presently a contentious issue, in the long term they will fail and society will eventually have to confront the issue. Yet, even if the possibility that evolution has occurred, but is reversible, it might entail a large demographic cost in order to effect a rapid genetic change.
These issues are challenging scientifically and also illustrate the importance of integrating evolutionary considerations into conservation planning for species at risk. It has been generally recognized that such planning involves both technical and normative considerations (e.g. Vucetich et al. 2006; Waples et al. 2007). However, evolutionary thinking has been relatively neglected in such discussions. When thinking about conservation goals and the types of outcomes society would like to achieve, the above analyses indicate the importance of considering the possibility that evolutionary processes have changed historical populations to a new state, and reversion to former conditions may not occur easily.
Acknowledgments
We thank Nils Ryman, Steve Arnold and Jay Hesse who participated with this group of authors in a workgroup associated with the Evolutionary Changes and Salmon Symposium held in Seattle, WA, 7 December 2007, where we first began our discussions of evolutionary pressures on Snake River fall Chinook salmon. This paper would not have occurred without the collaboration there and follow-up discussions. We also thank many unknown people from the Washington Department of Fish and Wildlife for collecting adult fish, measuring them and taking scales; particularly John Sneva (for reading the scales) and Debbie Milks (for developing the database and providing us the demographic information on adult salmon). We thank field staff from the Nez Perce Tribe, USFWS and NOAA Fisheries Service for the PIT-tagging juvenile fish, and conducting many of the analyses on PIT-tagged juveniles subsequently detected at dams. Finally, the manuscript was considerably improved by addressing comments from three anonymous reviewers and the associate editor.
References
- Aubin-Horth N, Bourque J-F, Daigle G, Hedger R, Dodson JJ. Longitudinal gradients in threshold size for alternative male life history tactics in a population of Atlantic salmon (Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2067–2075. [Google Scholar]
- Beckman BR, Dickhoff WW. Plasticity of smolting in spring chinook salmon: relation to growth and insulin-like growth factor-I. Journal of Fish Biology. 1998;53:808–826. [Google Scholar]
- Bjornn TC. Report to Idaho Department of Fish and Game. 1960. The salmon and steelhead stocks of Idaho. Boise, Idaho. [Google Scholar]
- Brannon EL, Powell MS, Quinn TP, Talbot A. Population structure of Columbia River Basin chinook salmon and steelhead trout. Reviews in Fisheries Science. 2004;12:99–232. [Google Scholar]
- Bugert RM, Hopley CW, Busack CA, Mendel GW. Maintenance of stock integrity in Snake River fall Chinook salmon. In: Schramm HL Jr, Piper RG, editors. Uses and Effects of Cultured Fishes in Aquatic Ecosystems. Bethesda, MD: American Fisheries Society; 1995. pp. 267–276. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future in change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DW. Salmon and steelhead abundance in the Columbia River in the nineteenth century. Transactions of the American Fisheries Society. 1986;115:662–670. [Google Scholar]
- Clarke WC, Withler RE, Shelbourn JE. Genetic control of juvenile life history pattern in Chinook salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:2300–2306. [Google Scholar]
- Coltman DW. Molecular ecological approaches to studying the evolutionary impact of selective harvesting in wildlife. Molecular Ecology. 2008;17:221–235. doi: 10.1111/j.1365-294X.2007.03414.x. [DOI] [PubMed] [Google Scholar]
- Connor WP, Burge HL. Growth of wild subyearling fall chinook salmon in the Snake River. North American Journal of Fisheries Management. 2003;23:594–599. [Google Scholar]
- Connor WP, Bjornn TC, Burge HL, Marshall AR, Blankenship HL, Steinhorst RK, Tiffan KF. Early life history attributes and run composition of PIT-tagged wild subyearling Chinook salmon recaptured after migrating downstream past lower granite dam. Northwest Science. 2001;75:254–261. [Google Scholar]
- Connor WP, Burge HL, Waitt R, Bjornn TC. Juvenile life history of wild fall chinook salmon in the Snake and Clearwater rivers. North American Journal of Fisheries Management. 2002;22:703–712. [Google Scholar]
- Connor WP, Sneva JG, Tiffan KF, Steinhorst RK, Ross D. Two alternative juvenile life history types for fall Chinook salmon in the Snake River basin. Transactions of the American Fisheries Society. 2005;134:291–304. [Google Scholar]
- Cormack RM. Estimates of survival from the sightings of marked animals. Biometrika. 1964;51:429–438. [Google Scholar]
- Craig JA, Hacker RL. The history and development of the fisheries of the Columbia River. Bulletin of the Bureau of Fisheries. 1940;32:133–216. [Google Scholar]
- Diamond JM. Overview of recent extinctions. In: Western D, Pearl MC, editors. Conservation for the Twenty-first Century. New York: Oxford University Press; 1989. pp. 37–41. [Google Scholar]
- Dickhoff WW, Beckman BR, Larsen DA, Duan C, Moriyama S. The role of growth in endocrine regulation of salmon smoltification. Fish Physiology and Biochemistry. 1997;17:231–236. [Google Scholar]
- Ebel WJ, Koski CH. Physical and chemical limnology of Brownlee Reservoir, 1962–1964. Fishery Bulletin. 1968;67:295–335. [Google Scholar]
- Etterson JR, Shaw RG. Constraint to adaptive evolution in response to global warming. Science. 2001;294:151–154. doi: 10.1126/science.1063656. [DOI] [PubMed] [Google Scholar]
- Evermann BW. A preliminary report on salmon investigations in Idaho in 1894. Bulletin of the United States Fish Commission. 1896;15:253–284. [Google Scholar]
- Faulkner JR, Smith SG, Muir WD, Marsh DM, Williams JG. Report of Research by Fish Ecology Division. Portland, OR: Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration to Bonneville Power Administration; 2007. Survival estimates for passage of spring-migrating juvenile salmonids through Snake and Columbia River Dams and Reservoirs, 2006; p. 112. Project No. 1993-02900. Available online at: http://pisces.bpa.gov/release/documents/documentviewer.aspx?doc=P103914. [Google Scholar]
- Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Fulton LA. United States Fish and Wildlife Service Special Scientific Report-Fisheries No. 571. Washington DC, USA: United States Fish and Wildlife Service; 1968. Spawning areas and abundance of Chinook Salmon (Oncorhychus tshawytscha) in the Columbia River Basin – past and present; p. 26. [Google Scholar]
- Galbreath JL, Ridenour RL. Fecundity of Columbia River Chinook salmon. Research Briefs, Fish Commission of Oregon. 1964;10:16–27. [Google Scholar]
- Grant PR, Grant BR. Evolution of character displacement in Darwin’s finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology & Evolution. 1996;11:92–96. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Hazel WN, Smock R, Johnson MD. A polygenic model for the evolution and maintenance of conditional strategies. Proceedings of the Royal Society of London B. 1990;242:181–187. doi: 10.1098/rspb.1990.0122. [DOI] [PubMed] [Google Scholar]
- Healey MC. Life history of chinook salmon. In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. Vancouver, BC: University of British Columbia Press; 1991. pp. 311–393. [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA. The evolution of alternative mating strategies in variable environments. Evolutionary Ecology. 1994;8:256–268. [Google Scholar]
- Jolly GM. Explicit estimates from capture-recapture data with both death and immigration – stochastic model. Biometrika. 1965;52:225–247. [PubMed] [Google Scholar]
- Kareiva P, Marvier M, McClure M. Recovery and management options for spring/summer chinook salmon in the Columbia River Basin. Science. 2000;290:977–979. doi: 10.1126/science.290.5493.977. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hendry AP. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica 112. 2001;113:145–164. [PubMed] [Google Scholar]
- Krcma RF, Raleigh RF. Migration of juvenile salmon and trout into Brownlee Reservoir, 1962–65. Fishery Bulletin. 1970;68:203–217. [Google Scholar]
- Law W, Salick J. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae) Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10218–10220. doi: 10.1073/pnas.0502931102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson K, Lundberg P. The choice of reproductive tactics as mixed evolutionarily stable strategy: the case of male Atlantic salmon (Salmo salar L.) Report of the Institute for Freshwater Research. 1986:69–76. Vol. 63, Drottningholm. [Google Scholar]
- Loder N. Point of no return. Conservation in Practice. 2005;6:124–129. [Google Scholar]
- Lotka AJ. Principles of Mathematical Biology. New York: Dover; 1959. [Google Scholar]
- Mains EM, Smith JM. The distribution, size, time, and current preferences of seaward migrant chinook salmon in the Columbia and Snake Rivers. Fisheries research papers. Washington Department of Fisheries. 1964;2:5–43. [Google Scholar]
- Marine KR, Cech JJ., Jr Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook salmon. North American Journal of Fisheries Management. 2004;24:198–210. [Google Scholar]
- Merilä J, Sheldon BC, Kruuk LEB. Explaining stasis: microevolutionary studies in natural populations. Genetica. 2001;112-113:199–222. [PubMed] [Google Scholar]
- Mesa MG, Weiland LK, Wagner P. Effects of acute thermal stress on survival, predator avoidance, and physiology of juvenile fall Chinook salmon. Northwest Science. 2002;76:118–128. [Google Scholar]
- Miller RJ, Brannon EL. The origin and development of life history patterns in Pacific salmonids. In: Brannon EL, Salo EO, editors. Proceedings of the Salmon and Trout Migratory Behavioral Symposium. Seattle: University of Washington; 1982. pp. 296–309. [Google Scholar]
- Myers RA, Hutchings JA. Selection against parr maturation in Atlantic salmon. Aquaculture. 1986;53:313–320. [Google Scholar]
- Palumbi SR. Evolution – Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Parkhurst ZE. Survey of the Columbia River and Its Tributaries: Part 8. Area VII. Snake River, above Payette River to upper Salmon Falls. 1950. p. 19. U.S. Fish and Wildlife Service. Special Scientific Report-Fisheries, No. 57, Washington DC, USA. [Google Scholar]
- Prentice EF, Flagg TA, McCutcheon CS. Feasibility of using implantable passive integrated transponder (PIT) tags in salmonids. American Fisheries Society Symposium. 1990;7:317–322. [Google Scholar]
- Quinn TP, Unwin MJ, Kinnison MT. Evolution of temporal isolation in the wild: Genetic divergence in timing of migration and breeding by introduced chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Kinnison MT, Unwin MJ. Evolution of chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica. 2001;112:493–513. [PubMed] [Google Scholar]
- Raymond HL. Effects of dams and impoundments on migrations of juvenile chinook salmon and steelhead from the Snake River. 1966 to 1975. Transactions of the American Fisheries Society. 1979;108:505–529. [Google Scholar]
- Raymond HL. Effects of hydroelectric development and fisheries enhancement on spring and summer chinook salmon and steelhead in the Columbia River Basin. North American Journal of Fisheries Management. 1988;8:1–24. [Google Scholar]
- Seber GAF. A note on the multiple recapture census. Biometrika. 1965;52:249–259. [PubMed] [Google Scholar]
- Smith TB, Bernatchez L. Evolutionary change in human-altered environments. Molecular Ecology. 2008;17:1–8. doi: 10.1111/j.1365-294X.2007.03607.x. [DOI] [PubMed] [Google Scholar]
- Smith SG, Muir WD, Hockersmith EE, Zabel RW, Graves RJ, Ross CV, Connor WP, et al. Influence of river conditions on survival and travel time of Snake River subyearling fall Chinook salmon. North American Journal of Fisheries Management. 2003;23:939–961. [Google Scholar]
- Sterns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Taylor EB. Adaptive variation in rheotactic and agnostic behavior in newly emerged fry of chinook salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1988;45:237–243. [Google Scholar]
- Taylor EB. Phenotypic correlates of life-history variation in juvenile chinook salmon, Oncorhynchus tshawytscha. Journal of Animal Ecology. 1990;59:455–468. [Google Scholar]
- Thomkins JL, Hazel W. The status of the conditional evolutionarily stable strategy. Trends in Ecology & Evolution. 2007;22:522–528. doi: 10.1016/j.tree.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Thorpe JE. Age at first maturity in Atlantic salmon, Salmo salar, freshwater period influences and conflicts with smolting. In: Meerburg D, editor. Salmonid Age at Maturity. Ottawa: Canadian Special Publication of Fisheries and Aquatic Sciences 89, Canadian Department of Fisheries and Oceans; 1986. pp. 7–14. [Google Scholar]
- Thorpe JE, Mangel M, Metcalfe NB, Huntingford FA. Modelling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evolutionary Ecology. 1998;12:581–599. [Google Scholar]
- Unwin MJ, Quinn TP, Kinnison MT, Boustead NC. Divergence in juvenile growth and life history in two recently colonized and partially isolated chinook salmon populations. Journal of Fish Biology. 2000;57:943–960. [Google Scholar]
- Vigg S, Burley CC. Temperature-dependent maximum daily consumption of juvenile salmonids by northern squawfish (Ptychocheilus oregonensis) from the Columbia River. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:2491–2498. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Vucetich JA, Nelson MP, Phillips MK. The normative dimension and legal meaning of Endangered and Recovery in the US Endangered Species Act. Conservation Biology. 2006;20:1383–1390. doi: 10.1111/j.1523-1739.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- Waples RS. The effective size of fluctuating salmon populations. Genetics. 2002;161:783–791. doi: 10.1093/genetics/161.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples RS, Teel DJ, Myers KW, Marshall AR. Life-history divergence in Chinook salmon: historic contingencey and parallel evolution. Evolution. 2004;58:386–403. [PubMed] [Google Scholar]
- Waples RS, Zabel RS, Scheuerell MD, Sanderson BL. Evolutionary responses by native species to major anthropogenic changes to their ecosystems: Pacific salmon in the Columbia River hydropower system. Molecular Ecology. 2007;17:84–96. doi: 10.1111/j.1365-294x.2007.03510.x. [DOI] [PubMed] [Google Scholar]
- Williams JG, Smith SG, Zabel RW, Muir WD, Scheuerell MD, Sandford BP, Marsh DM, et al. Effects of the Federal Columbia River Power System on Salmon Populations. Seattle, NMFS-NWFSC-63: US Deparment of Commerce, NOAA Tech. Memo; 2005. p. 150. Available online at: http://www.nwfsc.noaa.gov/publications. [Google Scholar]
- World Commission on Dams (WCD) Dams and Development: a New Framework for Decision-Making. London: Earthscan Publications; 2000. [Google Scholar]
- Young FR, Robinson WL. 1974. Age, size, and sex of Columbia River chinook, 1960-69. Fish Commission of Oregon. Data Report Series, Report Number 4, Clackamas, OR.
- Zabel RW, Scheuerell MD, McClure MM, Williams JG. The interplay between climate variability and density dependence in the population viability of Chinook salmon. Conservation Biology. 2006;20:190–200. doi: 10.1111/j.1523-1739.2005.00300.x. [DOI] [PubMed] [Google Scholar]


