Abstract
We examine the evolutionary history and speculate about the evolutionary future of three basic life history ecotypes that contribute to the biocomplexity of sockeye salmon (Oncorhynchus nerka). The ‘recurrent evolution’ (RE) hypothesis claims that the sea/river ecotype is ancestral, a ‘straying’ form with poorly differentiated (meta)population structure, and that highly structured populations of lake-type sockeye and kokanee have evolved repeatedly in parallel adaptive radiations between recurrent glaciations of the Pleistocene Epoch. Basic premises of this hypothesis are consistent with new, independent evidence from recent surveys of genetic variation in mitochondrial and microsatellite DNA: (1) sockeye salmon are most closely related to pink (O. gorbuscha) and chum (O. keta) salmon with sea-type life histories; (2) the sockeye life history ecotypes exist as polyphyletic lineages within large drainages and geographic regions; (3) the sea/river ecotype exhibits less genetic differentiation among populations than the lake or kokanee ecotypes both within and among drainages; and (4) genetic diversity is typically higher in the sea/river ecotype than in the lake and kokanee ecotypes. Anthropogenic modification of estuarine habitat and intensive coastal fisheries have likely reduced and fragmented historic metapopulations of the sea/river ecotype, particularly in southern areas. In contrast, the kokanee ecotype appears to be favoured by marine fisheries and predicted changes in climate.
Keywords: biocomplexity, conservation value, ecotype, kokanee, lake-type, niche model, recurrent evolution, river-type, sea-type
Biological complexity of populations is considered important for long-term sustainability of ecological goods and services. For example, the geographic and life history diversity of sockeye salmon (Oncorhynchus nerka, Walbaum, 1792) populations in Bristol Bay, Alaska have sustained a high aggregate productivity despite major changes in climatic conditions affecting the freshwater and marine environments during the last century (Hilborn et al. 2003). On the other hand, human activities increasingly threaten the persistence of such biocomplexity in the wild. Conservation decisions require scientific advice about adaptive diversity among populations – its scale and evolutionary origin, the likely evolutionary response to alternative management options, and the consequences of its loss (Wood and Gross 2008). In this paper, we examine the evolutionary origin of life history diversity in sockeye salmon, and speculate about its future in an environment that is being shaped dramatically by human society.
The species sockeye salmon comprises a multitude of reproductively isolated populations that can be grouped into three basic ecotypes based on differences in freshwater life history. The ‘lake ecotype’ is the typical anadromous form of sockeye salmon which spends about half its life in a nursery lake before migrating seaward (Burgner 1991). The ‘sea ecotype’ is also anadromous, but rears in fresh water for a shorter and more variable period (weeks or months) as it moves downstream to the estuary, typically inhabiting side channels and sloughs if these are available (Gilbert 1913). The term ‘river-type sockeye’ (Semko 1954) is sometimes used when closely spaced circuli (‘checks’) on scales indicate prolonged slow growth in fluvial or estuarine habitat. We consider the river-type form to be a special case of the sea-type life history because, by definition, neither sea-type nor river-type sockeye rear in lakes. For clarity, we will refer to them collectively as the ‘sea/river ecotype’. In contrast, the ‘kokanee ecotype’ is non-anadromous and found only in lakes (Nelson 1968).
These ecotypes exploit very different niches and exhibit corresponding adaptations. For example, common garden experiments have demonstrated that the sea/river ecotype can survive seawater at an earlier stage than the lake ecotype because of heritable differences in physiology and growth (Rice et al. 1994) and/or egg size which results in larger size and greater seawater adaptability at a given age (Wood 1995). Similar experiments with the lake and kokanee ecotypes have demonstrated heritable differences in the circannual cycle of seawater adaptability (Foote et al. 1992), gill raker morphology (Foote et al. 1999), size and age at maturity (Wood and Foote 1996), fecundity and egg size (Wood and Foote 1990, 1996), and carotenoid retention for spawning colour (Craig et al. 2005). The availability of suitable habitat varies greatly with latitude such that the sea/river ecotype is most common in glaciated rivers in northern and coastal areas of the species’ range (Wood et al. 1987; Halupka et al. 1993) whereas the kokanee ecotype is most common in southern and interior areas (Nelson 1968). The distribution of kokanee appears to be determined by lake productivity and the difficulty of anadromous migration, which in combination, likely determine fitness relative to the lake ecotype (Wood 1995). Under suitable conditions, kokanee occur sympatrically with lake-type sockeye as genetically distinct populations (Foote et al. 1989) that compete for food within the same rearing lake (Wood et al. 1999).
Wood (1995) proposed (but did not name) a ‘recurrent evolution’ (RE) hypothesis to explain the paradoxical pattern of allozyme variation in sockeye salmon. Briefly stated, sockeye salmon are presumed to have evolved as a cycle of alternating ecotypes driven by the 19 or 20 recurrent glaciations of the Pleistocene Epoch during which time each interglacial period lasted only 10 - 40 thousand years (Pielou 1991). Thus, present conditions are not typical of most of the period over which O. nerka evolved. Virtually all extant populations in Canada, southeast Alaska, and northern Washington State were established subsequent to the last glaciation which began 70–60 thousand years ago and reached its greatest extent 23–18 thousand years ago (Pielou 1991). Based on geological evidence and the geographical distribution of fish assemblages, McPhail and Lindsey (1970, 1986) concluded that Pacific salmon persisted during the last glaciation in isolated refuges in the Bering Sea region (Beringia) and south of the Cordilleran ice sheet in the Columbia River region (Cascadia). Patterns of allozyme variation in Canadian sockeye populations led Wood et al. (1994) to suggest that sockeye salmon also persisted in at least one other isolated refuge along the coast of British Columbia. This conclusion is consistent with the evidence of ‘deep structure’ in subsequent studies based on molecular markers, both in sockeye salmon (Beacham et al. 2005, 2006; Wood et al. unpubl. data, see Fig. 2), and O. kisutch (coho salmon, Smith et al. 2001), and with phylogeographic evidence in other taxa including plants (Lacourse et al. 2003) and terrestrial mammals (Byun et al. 1997).
Figure 2.
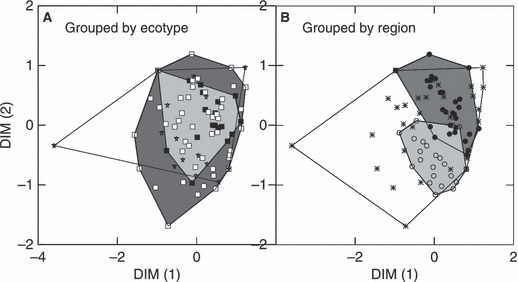
Multi-dimensional scaling plot of Cavalli-Sforza and Edwards (1967) chord distance between all populations (84) with a sample size of at least 20. A - the convex hulls group populations of the same ecotype (solid squares – sea/river ecotype, open squares – lake ecotype; stars – kokanee ecotype); B – the convex hulls group populations of the same region (open circles – southern, asterisks – coastal, solid circles – northern).
In the following paragraphs, we restate the RE hypothesis as six separate claims and review the arguments for each:
(1) The sea/river ecotype is an ancestral, ‘straying’ form of O. nerka which, like O. gorbuscha (pink salmon) and O. keta (chum salmon) typically exhibits a metapopulation1 structure. The genus Oncorhynchus evolved about 10 million years ago, likely from freshwater ancestors (McPhail 1997) although this point is open to debate (Waples et al. 2008), and speciation of O. nerka was probably complete by 7 million years ago (McKay et al. 1996; Fig. 1A). Phylogenetic studies of Pacific salmon (e.g., Stearley and Smith 1993; McKay et al. 1996; Domanico et al. 1997; Oakley and Phillips 1999) indicate that O. nerka is most closely related to O. gorbuscha and O. keta, both of which have exclusively sea-type life histories with minimal residence in fresh water after emergence. In contrast, the lake and kokanee ecotypes of O. nerka have adaptations for limnetic life (i.e., foraging on zooplankton) not seen elsewhere in the genus2 and their survival and abundance depend upon the productivity of a freshwater lake for rearing. Parsimony suggests that these limnetic adaptations evolved after O. nerka diverged from its common ancestor with O. gorbuscha and O. keta.
Figure 1.
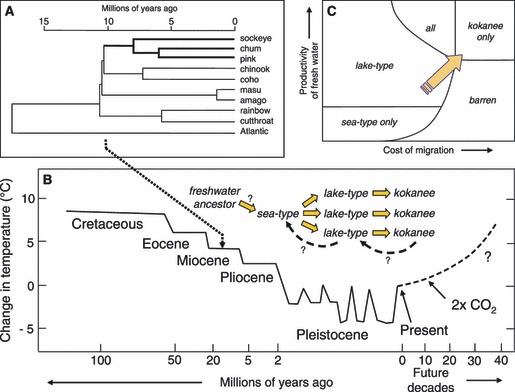
Evolution of sockeye salmon and the influence of climate change. A – Phylogeny of species in the genus Oncorhynchus and timescale for their evolution (after McKay et al. 1996). B – Trends in global temperature during the evolution of anadromous life histories in salmon and predictions for future decades (after Crowley and Kim 1995); C – Schematic niche model showing likely impact of global warming on the availability of habitat for sockeye ecotypes (modified from Wood 1995).
Tagging studies and genetic surveys of population structure (reviewed by Hendry et al. 2004) indicate that pink and chum salmon (with sea-type juvenile life histories) stray more than other Pacific salmon species (with freshwater-resident juvenile life histories). Surveys of genetic variation in allozymes (Beacham et al. 1985a, b; Kondzela et al. 1994; Phelps et al. 1994; Shaklee and Varnavskaya 1994; Wilmot et al. 1994) and mitochondrial DNA (Brykov et al. 1996; Sato 2004) all reveal less differentiation among spawning sites within countries or regions than is typical of other species in the genus.
(2) The sea/river ecotype is better adapted than the lake or kokanee ecotypes to persist in unproductive glaciated streams because it relies less on freshwater productivity for nutrition. Both sea-type and river-type sockeye populations currently exist where lake-type populations do not, typically in glaciated regions where lake habitat is absent or insufficiently productive (e.g., the Iskut and lower Stikine rivers, Wood et al. 1987; the East Alsek River, Geiger 2003). The sea/river ecotype is also relatively more abundant than the lake or kokanee ecotypes in many heavily glaciated drainages on the Yakutat coast of Alaska, and in northern British Columbia (Halupka et al. 1993; Wood 1995).
(3) The sea/river ecotype is better adapted than lake-type sockeye or kokanee ecotypes to colonize new freshwater habitat that becomes available as glaciers recede because of its proximity (greater persistence in glaciated habitat) and its greater tendency to stray from natal areas. Strong philopatry in lake-type sockeye appears to be a behavioral adaptation to find spawning sites that allow newly-emerged fry to reach the nursery lake despite their limited ability to overcome rapids; spawning sites are typically, but not always, situated upstream of the rearing lake (Wood 1995). This special requirement for precise homing to discrete areas of suitable spawning habitat promotes reproductive isolation and genetic differentiation of populations inhabiting even small lakes. For example, sockeye populations in six lakes in Washington State with surface areas of only 6–30 km2 were each judged sufficiently isolated and unique to be identified as distinct Evolutionarily Significant Units (Gustafson et al. 1997); similarly, sockeye populations in two small lakes (<10 km2) in British Columbia were each listed as endangered ‘wildlife species’ by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, Irvine et al. 2005). In contrast, sea/river-type sockeye are not constrained by the discontinuous nature of lake habitat. Wood (1995) speculated from allozyme survey data that sea/river-type sockeye stray more than lake-type sockeye, and thus, might more readily colonize newly accessible freshwater habitat. However, Pavey et al. (2007) document a counter-example in which a volcanic caldera lake was colonized recently by lake-type sockeye from an adjacent drainage rather than by sea-type sockeye from downstream within the same drainage.
(4) As limnetic habitat becomes accessible and sufficiently productive following glacial retreat, specialized, locally adapted populations of lake-type sockeye evolve in a parallel adaptive radiation from the proximate sea/river type ecotype metapopulation. To our knowledge, this specific claim has not yet been tested. However, genetic surveys have revealed extensive divergence of lake-type sockeye populations within drainages colonized since the last glaciation, a ‘mosaic’ pattern that is unique among Pacific salmon (Utter et al. 1984; Wood 1995; Winans et al. 1996; Nelson et al. 2003).
(5) Where the lake environment is sufficiently productive, or the seaward migration hazardous, the fitness of non-anadromous individuals can rival or exceed that of anadromous individuals, and consequently, populations of the kokanee ecotype evolve independently from proximate (often sympatric) lake-type sockeye populations. The kokanee ecotype is known to have arisen and persisted following unsuccessful introductions of the lake ecotype (Ricker 1940; Wood 1995; Quinn et al. 1998). In at least some lakes, conditions also meet the theoretical requirements for sympatric divergence of lake and kokanee ecotypes (Wood and Foote 1996). Existing genetic evidence for the independent evolution of kokanee from lake-type sockeye within separate drainages is generally compelling, although not unequivocal (Foote et al. 1989; Taylor et al. 1996).
Despite the remarkable extent of genetic and phenotypic divergence of lake-type and kokanee populations within recently colonized drainages, surprisingly little additional divergence is evident across larger geographic scales in allozymes (Guthrie et al. 1994; Varnavskaya et al. 1994; Winans et al. 1996) or mitochondrial DNA (Bickham et al. 1995; Wood et al. unpubl. data). Regional structuring is better revealed in studies of microsatellite DNA (because of greater allelic diversity), but as in other genetic markers, more variation is evident among lakes within drainages than among drainages (Beacham et al. 2006). This population structure is unusual. In chum salmon, as expected, genetic variation is greater among continents than among regions, and greater among regions within continents than among populations within regions (Sato 2004). Why then has there not been much greater divergence among sockeye salmon populations across the species’ range, given opportunities for continued evolution spanning recurrent glaciations?
(6) Many of the locally-adapted lake-type sockeye and kokanee populations are evolutionary dead ends because they are extirpated during the next glaciation which ‘resets’ the genetic structure of the species to that characterized by relatively undifferentiated metapopulations of sea/river-type sockeye. The lake ecotype is the most abundant and genetically diverse ecotype in the current interglacial period. Presumably it flourished during previous interglacial periods too, but most of the population structure and local adaptations associated with lakes in former interglacial periods would have been lost following resurgence of the ice sheets. The RE hypothesis claims that sea/river-type sockeye persisted through these glaciations, spawning in small streams in refuges at the margin of the Cordilleran ice sheet, just as they do today on the Yakutat coast of Alaska. We suggest that sea/river-type sockeye were more abundant then, freed from competition with lake-type sockeye and intense exploitation by humans, and that they existed in geographically and demographically large, relatively homogeneous metapopulations much like pink and chum salmon do today. Spatially extensive metapopulations of sea/river-type sockeye would have been less affected by random genetic drift or selection for adaptation at small spatial scales than small, isolated lake-type populations (Gustafson and Winans 1999). Persistence of such spatially extensive gene pools within glacial refuge areas could account for the relative homogeneity of allele frequencies over large distances following post-glacial dispersal. The ensuing interglacial period would afford new opportunities for the lake-type sockeye and kokanee ecotypes to re-evolve.
For the RE hypothesis to stand, we require corroborating evidence in support of three underlying assumptions: (1) sockeye salmon ecotypes are not monophyletic, but have evolved independently in different locations; (2) the sea/river ecotype strays more than the other ecotypes, resulting in a genetic metapopulation structure similar to that in pink and chum salmon; and (3) the sea/river ecotype is ancestral to the other ecotypes in drainages that were previously glaciated. We find that all three assumptions are generally consistent with new molecular genetic data.
Methods
Data Sources
We tested specific assumptions of the RE hypothesis by examining a wide variety of samples and independent genetic systems. We relied primarily on our mitochondrial DNA (mtDNA) survey data (Wood et al. unpubl. data summarized in Supplementary Table 1), but also considered evidence from other recent studies. We examined RFLP haplotype frequencies and genetic diversity indices for 123 spawning populations, including virtually all of the largest sockeye salmon populations in North America from the Yakutat coast and Alsek River south to the Columbia River. The survey includes three regions putatively colonized from different glacial refuges: ‘northern’ (from Yakutat to the Skeena River), ‘coastal’ (the mainland coast of British Columbia south of the Skeena River, including the lower Fraser River, Vancouver Island, and Haida Gwaii), and ‘southern’ (the upper Fraser River, the Columbia River and coast of Washington). All three ecotypes are represented by multiple (often sympatric or parapatric) populations in each region. Sample sizes are variable (range 3 to 140) but most populations (84) are represented by samples of at least 20 individuals. Samples of between 20 and 50 individuals are adequate to capture 95% or more of the existing mitochondrial DNA haplotypes for low and high levels of within-population diversity, respectively (Crandall and Templeton 1993).
Statistical Analyses
We partitioned variation in RFLP haplotype frequencies into 3-level hierarchies (‘among groups’, ‘among populations within group’, and ‘within populations’) based on alternative groupings by geography and/or ecotype (AMOVA, Excoffier et al. 1992; implemented in ARLEQUIN by Schneider et al. 2000). Fixation indices (FSC, FCT, and FST) based on the ratios of variance components were used to test the performance of each hierarchical structure against competing structures. Molecular differences among haplotypes were not considered in the AMOVA because most differences in haplotype frequency among populations have resulted from genetic drift rather than recent mutation (Wood et al. unpubl. data). To illustrate the grouping patterns, we used the multi-dimensional scaling (MDS) routine in SYSTAT (2004) based on pair-wise chord distances (Cavalli-Sforza and Edwards 1967) computed in PHYLIP (Felsenstein 2005). Chord distance is an appropriate measure of genetic distance when populations have diverged by genetic drift rather than mutation and population size has not remained constant (Felsenstein 2005). In the overall MDS plot where the number of replicate populations was large, we included only samples with at least 20 individuals to improve precision and clarity; for MDS plots within regions, all relevant samples were included regardless of sample size.
To compare genetic diversity among ecotypes, we examined two standardized indices of genetic diversity (Supplementary Table 1). The first, gene diversity, is not directly affected by sample size, and measures both the number of haplotypes and their relative frequencies. The second index is the number of haplotypes expected in a standard sample size of 10 individuals (determined by resampling); accordingly, in these comparisons, we included only samples with at least 10 individuals. We used 2-way Kruskal-Wallis tests (SYSTAT 2004) to test whether median values of gene diversity were higher in the sea/river-ecotype than in the other ecotypes combined, both coast-wide, and within each region.
Results
Parallel evolution of ecotypes
We found no evidence to suggest that ecotypes have evolved as monophyletic lineages (Fig. 2A). FCT fixation indices (the proportion of total variance explained by the grouping hypothesis) for groupings based only on ecotype were not statistically significant, regardless of whether populations were considered collectively coast-wide (P > 0.24), or separately within each region (from north to south, P > 0.12, P > 0.30, and P > 0.23). In contrast, the FCT index for grouping by region was highly significant (P < 0.001). We conclude that ecotype is not significant as a grouping variable independent of geography, and that regional differences must be taken into account to disentangle the recent effects of post-glacial dispersal and parallel evolution from deeper underlying structure arising from prolonged isolation in different refuges during the last glaciation. Even so, the regional differences associated with separate glacial refuges (Fig. 2B) are less than expected given the rate of recent divergence among populations within single drainages.
Separate analyses focusing on subsets of sympatric or parapatric populations confirm that, with few exceptions, ecotypes do not exist as monophyletic lineages at the scale of large drainages or regions. All FCT fixation indices were statistically significant (or nearly so, P < 0.06 for the northern region) when ecotypes were grouped within site, but never significant when sites were grouped within ecotype (P > 0.16 to P > 0.84) (Table 1). Overall FST values were as high or higher in all three regions when populations were grouped by site rather than ecotype.
Table 1.
Hierarchical FST analyses (AMOVA) of sympatric ecotypes by region.
| Number of | Variance component | Fixation indices (and probability of H0) | ||||||
|---|---|---|---|---|---|---|---|---|
| Hierarchical structure | Region* | units† | samples‡ | Within samples | Among samples within unit | Among units | Samples (Fst) | Units (Fct) |
| Ecotypes within site (site is unit) | Northern | 4 | 18 | 0.271 | 0.030 | 0.018 | 0.15 (<0.001) | 0.06 (<0.06) |
| Coastal | 4 | 12 | 0.177 | 0.083 | 0.133 | 0.55 (<0.001) | 0.34 (<0.01) | |
| Southern | 9 | 20 | 0.264 | 0.032 | 0.023 | 0.17 (<0.001) | 0.07 (0.02) | |
| Sites within ecotype (ecotype is unit) | Northern | 3 | 18 | 0.271 | 0.042 | 0.006 | 0.15 (<0.001) | 0.02 (0.16) |
| Coastal | 3 | 12 | 0.177 | 0.194 | −0.009 | 0.51 (<0.001) | −0.02 (0.63) | |
| Southern | 2 | 20 | 0.264 | 0.056 | −0.006 | 0.16 (<0.001) | −0.02 (0.84) | |
Bold font indicates that the fixation index is statistically > 0.
Regions putatively colonized from different glacial refuges (Wood 1995, Wood et al. unpublished data).
Units are defined as either sites or ecotypes (see column 1).
Samples refer to the individual populations in Supplementary Table 1.
Populations from the same or nearby sites typically clustered together regardless of ecotype (Fig. 3). The most parsimonious explanation for these results is that the same ecotypes have evolved independently on numerous occasions at the scale of individual rearing lakes, or within clusters of lakes in close proximity. Obvious exceptions include the parapatric sea/river-type population in Gingut Creek in the lower Nass River (Fig 3A), and the parapatric lake-type population in Pitt Lake in the lower Fraser River (Fig 3B), and the sympatric lake and kokanee ecotypes in Ozette Lake (Fig 3C).
Figure 3.
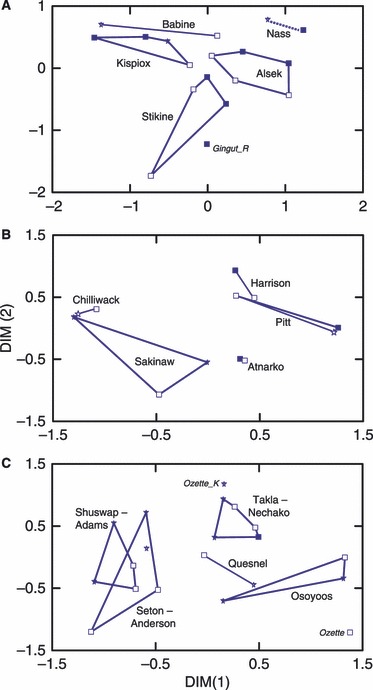
Multi-dimensional scaling plots of Cavalli-Sforza and Edwards (1967) chord distance between sympatric or parapatric populations shown separately by region. A – northern, B – coastal, and C – southern. Symbols denote ecotype (solid squares – sea/river ecotype, open squares – lake ecotype; stars – kokanee ecotype); convex hulls or lines group sympatric/parapatric populations; the dashed line indicates that one sea/river-type population (Gingut_R) did not fit within the cluster. Adjacent sites were grouped if they overlapped, as for the Stuart (Takla Lake) and Nechako rivers, Shuswap and Adams lakes, Anderson and Seton lakes, and upper and lower Alsek River sites.
Population differentiation within drainages and regions
Hierarchical analyses of geographic structuring within each ecotype based on all samples indicated that sea/river-type sockeye populations spawning in different tributaries within a drainage were genetically less differentiated than lake-type populations rearing in different lakes within a drainage, regardless of region (FSC values in Table 2). Significant variation could be attributed to additional differentiation across drainages in lake-type sockeye, but not in sea/river-type sockeye, except in the northern region (FCT values in Table 2); significant FCT values were higher in all regions in lake-type sockeye (range 0.08 to 0.13) than in sea/river-type sockeye (FCT = 0.05). Kokanee populations exhibited the highest differentiation among sites within drainages in both the southern (FSC = 0.22) and coastal (FSC = 0.55) regions, but no significant variation could be attributed to additional differentiation among drainages in either region (P > 0.06 for both FCT values). Too few kokanee samples were available from the northern region for a meaningful comparison.
Table 2.
Hierarchical FST analyses (AMOVA) by geographic region and ecotype.
| Number of | Variance component | Fixation indices (and probability of H0) | ||||||
|---|---|---|---|---|---|---|---|---|
| Geographic region* | Ecotype | drainages†ndrainage | sites‡npop | Within sites | Among sites (within drainage) | Among drainages | Sites (FSC) | Drainages (FCT) |
| Northern | Sea/river-type | 5 | 16 | 0.281 | 0.019 | 0.015 | 0.06 (<0.001) | 0.05 (0.04) |
| Lake-type | 5 | 23 | 0.233 | 0.070 | 0.046 | 0.23 (<0.001) | 0.13 (<0.01) | |
| Coastal | Sea/river-type | 4 | 7 | 0.247 | 0.105 | 0.054 | 0.30 (<0.001) | 0.13 (0.33) |
| Lake-type | 5 | 29 | 0.234 | 0.150 | 0.035 | 0.39 (<0.001) | 0.08 (0.03) | |
| Kokanee | 4 | 11 | 0.124 | 0.148 | 0.091 | 0.55 (<0.001) | 0.25 (0.06) | |
| Southern | Sea/river-type | 2 | 4 | 0.326 | −0.013 | 0.047 | −0.04 (0.96) | 0.13 (0.25) |
| Lake-type | 3 | 15 | 0.228 | 0.035 | 0.039 | 0.13 (<0.001) | 0.13 (<0.01) | |
| Kokanee | 3 | 13 | 0.263 | 0.074 | −0.008 | 0.22 (<0.001) | −0.02 (0.61) | |
Bold font indicates that the fixation index is statistically > 0.
Regions putatively colonized from different glacial refuges (Wood 1995, Wood et al. unpublished data).
Drainages are defined as in Table 1 except that the Fraser River drainage was divided between regions 1 (upper) and 2 (lower).
Samples refer to the individual populations in Supplementary Table 1.
We also tested whether the sea/river ecotype was consistently less differentiated than the lake ecotype in each major drainage for which separate analysis was possible (Alsek, Taku, Stikine, Skeena, Nass, lower Fraser, and Skagit rivers). To extend these comparisons, we included three additional coastal drainage areas comprising small proximate rivers (the central coast of British Columbia, the west coast of Vancouver Island, and the coast of Washington). Differentiation among lake-type sockeye populations within a drainage area was statistically significant in all rivers and drainage areas except coastal Washington; differentiation among sea/river-type sockeye populations was not statistically significant in 3 of the 5 northern rivers, or the Skagit River, or the Washington coast, but was significant in all three coastal drainage areas (Table 3). Moreover, in each possible pair-wise comparison, except in the lower Fraser river, the FST estimate of differentiation was less in sea/river-type sockeye than in lake-type sockeye.
Table 3.
Comparison of population differentiation (FST) in sea/river and lake ecotypes within large drainages or coastal drainage areas comprising proximate smaller rivers.
| Sea/river-type | Lake-type | ||||||
|---|---|---|---|---|---|---|---|
| Geographic region* | Drainage or coastal area | npop | FST | P | npop | FST | P |
| Northern | Alsek River | 2 | <0.01 | 0.42 | 3 | 0.78 | <0.05 |
| Taku River | 4 | 0.02 | 0.20 | 3 | 0.56 | <0.001 | |
| Stikine River | 3 | −0.02 | 0.74 | 3 | 0.46 | <0.001 | |
| Nass River | 3 | 0.09 | <0.01 | 4 | 0.23 | <0.001 | |
| Skeena River | 3 | 0.08 | <0.05 | 9 | 0.16 | <0.001 | |
| Coastal | BC Central Coast† | 3 | 0.11 | <0.01 | 9 | 0.27 | <0.001 |
| West Vancouver Island‡ | 2 | 0.24 | <0.001 | 5 | 0.57 | <0.001 | |
| Lower Fraser River | 2 | 0.60 | <0.001 | 5 | 0.40 | <0.001 | |
| Southern | Skagit River | 2 | −0.05 | 0.80 | 0 | – | – |
| Washington Coast§ | 3 | −0.04 | 0.95 | 3 | 0.08 | 0.17 | |
Bold font indicates that FST is statistically > 0.
Regions putatively colonized from different glacial refuges (Wood 1995, Wood et al. unpublished data).
Mainland coast from Kemano River south to Klinaklini River; population numbers 64 to 76 Supplementary Table 1.
West coast of Vancouver Island from Zeballos River south to Hobiton Lake; population numbers 45 to 51 Supplementary Table 1.
Puget Sound (including Skagit and Nooksack rivers) and Olympic Peninsula; population numbers 9 to 15 Supplementary Table 1.
Genetic diversity within ecotypes
Gene diversity distributions by ecotype overlapped broadly, but within each region, modal values were always highest in sea/river-type sockeye and lowest in kokanee (Fig. 4). Such a consistent result is statistically significant in itself (P = [1/3]3 < 0.04 given our a priori assumption). Pair-wise comparisons of median gene diversity in the sea/river ecotype and the other ecotypes combined were statistically significant overall (with regions combined, P < 0.01), but not in separate regional comparisons (P > 0.08).
Figure 4.
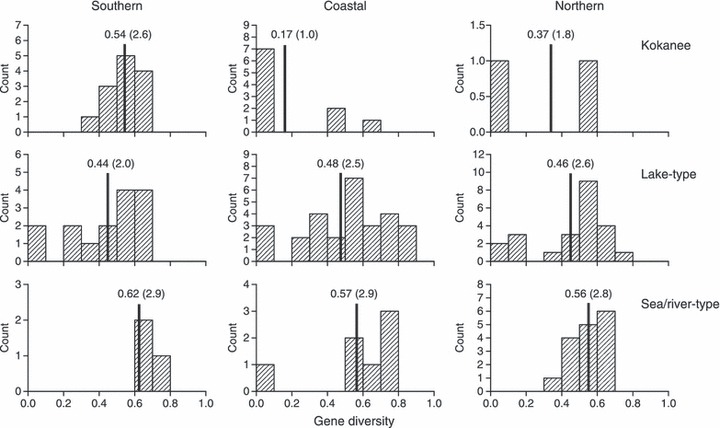
Comparison of gene diversity distributions for individual populations grouped by ecotype and geographic region. Vertical lines and numbers indicate median values; numbers in parentheses indicate the median number of haplotypes expected in standardized samples of 10 individuals.
Another measure of genetic diversity, the median number of haplotypes expected in standardized samples of 10 individuals ranged from 2.8 to 2.9 across regions in sea/river-type sockeye, 2.0 to 2.6 in lake-type sockeye, and 1.0 to 2.6 in kokanee. Again, the distributions overlapped broadly, but the median number of haplotypes was consistently highest in the sea/river-type populations, and lowest in kokanee in all but the southern region (Fig. 4).
Coast-wide, the percentage of populations fixed for a single haplotype was lowest in sea/river-type sockeye (1 of 27 populations or 3.7%), intermediate in lake-type sockeye (7 of 67 populations or 10%), and highest in kokanee (7 of 28 populations or 25%) (P < 0.05, Pearson’s χ2). No consistent pattern was evident within regions, likely because the number of populations sampled was insufficient to compare rare (fixation) events. The percentage of haplotypes that were unique (‘private’) to an ecotype was highest in the sea/river ecotype in both the northern (40%) and southern (50%) regions (despite the generally lower sampling rate for sea/river-type sockeye), but highest in the lake ecotype in the coastal region (47%), due primarily to a single large sample with atypically high diversity (Owikeno Lake) (Table 4).
Table 4.
Incidence of mitochondrial DNA haplotypes that are private to each ecotype by region.
| Number of | Number of haplotypes | ||||||
|---|---|---|---|---|---|---|---|
| Geographic region* | Ecotype | fish | sites† | Private | Not private | Total | Proportion Private |
| Northern | Sea/river-type | 372 | 16 | 4 | 6 | 10 | 0.40 |
| Lake-type | 525 | 23 | 0 | 6 | 6 | 0 | |
| Kokanee | 48 | 4 | 1 | 3 | 4 | 0.25 | |
| Coastal | Sea/river-type | 196 | 7 | 2 | 6 | 8 | 0.25 |
| Lake-type | 795 | 29 | 8 | 9 | 17 | 0.47 | |
| (excluding Owikeno) | (736) | (28) | (3) | (8) | (11) | (0.27) | |
| Kokanee | 538 | 11 | 2 | 4 | 6 | 0.33 | |
| Southern | sea/river-type | 67 | 3 | 3 | 3 | 6 | 0.50 |
| Lake-type | 342 | 15 | 2 | 4 | 6 | 0.33 | |
| Kokanee | 432 | 13 | 3 | 4 | 7 | 0.43 | |
Bold font indicates the highest values within each region.
Regions putatively colonized from different glacial refuges (Wood 1995, Wood et al. unpublished data).
Samples refer to the individual populations in Supplementary Table 1.
Discussion
Origin of ecotypes
The mtDNA survey data confirm that lake-type sockeye and kokanee ecotypes inhabiting the same nursery lake typically exist as reproductively isolated populations. We found significant differentiation between the sympatric ecotypes in 7 of 12 comparisons. Pair-wise genetic distances between sympatric lake-type sockeye and kokanee are typically less than among populations of the same ecotype in different lakes, consistent with parallel rather than monophyletic evolution of the kokanee ecotype. However, as in previous studies (Foote et al. 1989; Taylor et al. 1996; Winans et al. 1996), we cannot entirely rule out convergence through introgression of once-distinct lineages, and the spatial scale of parallel evolution seems to vary among cases. The ecotypes appear to have arisen separately within the same lake in at least 4 of the 12 comparisons, and within a cluster of proximate lakes within a major drainage in another 5 comparisons. The association between the proximate cluster of Seton and Anderson lakes with the more distant Shuswap and Adams lakes (Fig. 3C) is likely due to successful transplants of lake-type sockeye from Adams River to Seton Lake (Withler et al. 2000). An obvious exception was Ozette Lake, which is situated close to the southern limit of glaciation and likely represents a case of secondary invasion by anadromous sockeye salmon (Winans et al. 1996).
By definition, the sea/river ecotype is not sympatric with the lake or kokanee ecotypes so we have had to compare parapatric populations. In all 8 comparisons, the sea/river ecotype grouped closely with a parapatric lake or kokanee ecotype. The sea/river-type sockeye populations in both the Pitt and Nechako tributaries to the Fraser River clustered closely with a respective parapatric pair, consistent with parallel evolution at the tributary level. However, both populations were genetically distinct from other sea/river-type sockeye populations, which is inconsistent with our expectation of homogeneous metapopulation structure within the sea/river ecotype in the coastal and southern regions, respectively. Based on the pattern of shared haplotypes (Supplementary Table 1), we speculate that the small sea-type populations in the lower Fraser River (Widgeon Slough and Harrison Rapids) are fragmented remnants of a former metapopulation, but that the river-type population in the upper Fraser (Nechako River) is secondarily derived from the lake ecotype.
Post-glacial dispersal and metapopulation structure
Sea/river-type sockeye that are better able to persist in glaciated habitat and home less precisely than lake-type sockeye would be more likely to encounter newly accessible lake habitat, but lake-type sockeye would probably be better adapted for limnetic life in the new habitat. Thus, a trade-off exists between the potential number of colonists (encounter rate) and the fitness of colonists in new lake habitat (probability of successful reproduction after the encounter). This trade-off will depend on the relative proximity of the new habitat to potential colonizing sources of each ecotype. Lake-type sockeye are clearly able to extend their range into adjacent new habitat, as in Glacier Bay following the retreat of a glacier in recent times (Milner and Bailey 1989), and in the Aniakchak River following a volcanic eruption (Pavey et al. 2007). Similarly, lake-type sockeye might have colonized pro-glacial lakes at the southern margin of the ice sheets without benefit of the putative colonizing abilities of sea/river-type sockeye. Thus, sockeye ecotypes could have continued to evolve as separate lineages close to the limit of glaciation in the southern region, as indicated by the distinctiveness of the lake-type sockeye and kokanee ecotypes in Ozette Lake kokanee (Winans et al. 1996, corroborated in this study).
Gustafson and Winans (1999) propose that the sea/river ecotype in Washington State shares a common ancestry with northern populations of the sea/river ecotype. Allozyme variation indicates that sea/river-type populations in Washington are very distinct from all extant populations of the lake ecotype in the contiguous USA, yet similar to northern populations of the sea/river ecotype up to 2000 km distant. In general, more regional differentiation is evident among populations of the sea/river ecotype in both mtDNA and microsatellite DNA (Beacham et al. 2004, 2006) than in allozymes. However, our mtDNA data do corroborate the surprising results of Gustafson and Winans (1999). Sea/river-type sockeye in both the Skagit and Nooksack rivers are most similar to sea/river-type sockeye in the Upper Tatshenshini River within the Alsek River drainage, and sea/river-type sockeye in the Sauk River are most similar to sea/river-type sockeye in Zolzap Creek in the Nass drainage.
Extant populations of the sea/river ecotype exhibit sufficient genetic diversity to have founded populations of the other ecotypes by parallel evolution within regions. Gene diversity is typically as high or higher in sea/river-type sockeye than in lake-type sockeye based on allozymes (Wood 1995; Gustafson and Winans 1999), microsatellite DNA (Beacham et al. 2004, 2006) and mtDNA (this study). Thus, it is not possible to argue that the sea/river ecotype is generally derived from the lake ecotype, although this does seem likely in the Nechako River. Small population size is typically associated with reduced diversity, so how has diversity remained so high in the sea/river ecotype, which is widespread yet much less abundant than the lake ecotype? A plausible explanation is that the sea/river ecotype was once more abundant and less fragmented into remnant populations.
The evidence for greater gene flow among populations in the sea/river ecotype than in other ecotypes is most compelling in the northern region, especially in the Stikine and Taku rivers where the sea/river ecotype is still relatively abundant and suitable habitat is connected over large distances. Based on a survey of microsatellite DNA, Beacham et al. (2006) suggest that the “lack of significant differentiation among riverine populations is largely confined to comparisons between Taku River and Stikine River populations”. Beacham et al. (2004) report significant differentiation (P < 0.01) in microsatellite DNA within the Stikine, Nass, and Skeena rivers, but only marginally significant differentiation (P < 0.10) among sea/river-type populations within the Alsek and Taku drainages. Similarly, in mtDNA, we found significant differentiation among populations of the sea/river ecotype in the Nass, Skeena, and lower Fraser rivers, but not in the Alsek, Taku, Stikine, and Skagit rivers.
More relevant, however, is our finding that differentiation among populations within the same drainage is always less in the sea/river ecotype than in the lake ecotype, except in the lower Fraser River due to fixation of a single haplotype in the Widgeon Slough population. Like Beacham et al. (2004), we found statistically significant differentiation among drainages for sea/river-type sockeye populations in the northern region (FCT value in Table 2), but again, the level of differentiation is less in the sea/river ecotype than in the lake ecotype, and in all cases, less than attributed to differentiation among populations within drainages (FSC values in Table 2). Beacham et al. (2004, 2006) do not compare differentiation between ecotypes within drainages or regions.
Lower values of differentiation among populations within drainages in the sea/river ecotype might arise from greater proximity and greater continuity of spawning sites, often associated with the mainstem reaches of large rivers, rather than from differences in the distance that individuals stray from their natal sites. We did not explicitly compare isolation by distance as Gustafson and Winans (1999) do. Less genetic differentiation in neutral traits indicates a greater number of migrants among sites (i.e., greater historical or continuing gene flow denoted mNe where m is immigration rate and Ne is the genetically effective population size), but we cannot conclude that the rates of immigration or straying are typically higher in sea/river-type sockeye without taking historic population abundance into account. In most of our comparisons, current abundance is higher in the lake ecotype than in the sea/river ecotype, so the tendency for straying by individuals would be correspondingly higher in the sea/river ecotype - unless the abundance of the sea/river ecotype has declined substantially (as we argue below). Thus, the assumption that the sea/river ecotype has a greater tendency to stray remains difficult to test, but this uncertainty does not undermine the evidence for its greater metapopulation structure historically.
To have reset the regional pattern of genetic variation during successive glaciations, sea/river-type populations must have remained connected by substantial gene flow throughout previous interglacial periods. Extant populations of the sea/river ecotype have not met this requirement in south coastal BC and the US Pacific Northwest during the current interglacial period. These populations have likely suffered severe reductions in abundance over the last century because of human activities.
Vulnerability to human activities and climate change
Extensive loss of suitable river-rearing habitat can probably explain the small size of sea/river-type populations remaining in the southern extent of the range of sockeye salmon (Gustafson and Winans 1999). Populations of the sea/river ecotype still extant in coastal and southern regions are likely only fragments of much larger metapopulations that existed prior to European settlement. The maximum equilibrium abundance (carrying capacity) of a lake-type sockeye population is typically constrained by the surface area and productive capacity of the nursery lake (e.g., Koenings and Burkett 1987). Thus, carrying capacity could easily be greater for the sea/river ecotype inhabiting (once) large productive estuaries than for the lake ecotype inhabiting small lakes (Fig. 5A). However, intrinsic productivity is likely lower in the sea/river ecotype than in the lake ecotype because smaller smolts are more vulnerable to marine and estuarine predators.
Figure 5.
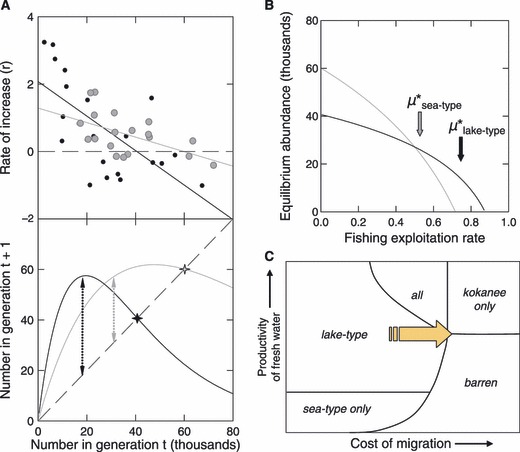
Comparison of productivity of the Tahltan lake-type (dots and darker lines) and mainstem Stikine sea/river-type (shaded circles and lighter lines) sockeye populations in the Stikine River. A – Ricker stock-recruitment curves (Ricker 1954) fitted to fisheries data showing intrinsic productivity (intercept in regression), maximum equilibrium abundance (crosses), and estimated maximum sustainable yield (vertical dotted arrows). The Ricker a parameter is 2.08 (se = 0.45) for the Tahltan (lake-type) population and 1.26 (se = 0.27) in the mainstem Stikine (sea/river-type) population. B – Equilibrium abundance as a function of sustained annual rate of fishing mortality for the Ricker curves fitted in Figure 5A; arrows indicate the exploitation rate (μ*) that would maximize sustainable yield for each ecotype. C – Schematic niche model showing the likely impact of intensive coastal fishing on the availability of habitat for sockeye ecotypes (modified from Wood 1995.)
Management of fisheries to achieve maximum sustainable yield (MSY) typically reduces the spawning abundance of a productive sockeye population to less than half the equilibrium level that would be attained if the population were not harvested. Unproductive populations suffer even greater reductions where they are captured in fisheries directed at more productive populations. For example, two lake-type populations (Cultus and Sakinaw) in south coastal British Columbia are now designated as endangered by COSEWIC, largely because of incidental fishing mortality (Irvine et al. 2005). In the Stikine River (northern British Columbia), an annual fishing mortality rate of 73% would provide maximum sustainable yield from the lake ecotype but would drive the sea/river ecotype to extinction (Fig. 5B). Because mainstem Stikine sea/river-type sockeye migrate upstream later than Tahltan lake-type sockeye, and can be identified during the fishing season, they have been fished selectively at an appropriately conservative harvest rate. However, this example illustrates how the sea/river ecotype could have been more abundant than the lake ecotype historically, but be reduced to low abundance (or extirpated) by fisheries that continued to target the most productive lake-type populations. Sea/river-type populations are so poorly documented that many may have disappeared already; those remaining in southern British Columbia and the US Pacific Northwest may be especially vulnerable to overexploitation.
Greenhouse gases are expected to elevate the global mean temperature by 3°C before 2100, and the North American mean temperature by 2 to 3°C before 2050 (IPCC 2007). Similar temperatures last occurred during the speciation of Oncorhynchus. Some climate modeling studies predict that beyond 2100 AD, global temperature could rise by 5 to 8°C, to levels experienced in the Eocene and Cretaceous (Crowley and Kim 1995). Warmer global temperatures will likely shift the distribution of salmon habitat northward geographically, increasing the productivity of most existing nursery lakes, but increasing the difficulty (fitness cost) of anadromous migration in southern areas as rivers warm and summer flows decrease. These changes would shift the relative fitness conferred by anadromous and nonanadromous life histories, and thus, would affect the future evolution of the life history ecotypes.
Future evolution and conservation value of ecotypes
To explore the implications of climate change and fisheries for the evolution of sockeye life history ecotypes, we modified a conceptual niche model suggested by Wood (1995) to include the sea/river ecotype. The predicted changes in climate will likely shift habitat (diagonally upwards in the niche diagram in Fig. 1C) such that the kokanee ecotype will gain habitat and the sea/river ecotype will lose habitat. Intensive coastal fisheries for sockeye salmon act to increase the cost of migration for both anadromous ecotypes (see Theriault et al., 2008), effectively shifting habitat towards kokanee (to the right in Fig. 5C). In both cases, we predict that the kokanee ecotype will prosper, but that anadromous sockeye, especially the sea/river ecotype, will be increasingly threatened.
Populations are real biological entities whereas sockeye life history ecotypes are abstract, polyphyletic classes. Even so, ecotype designations capture significant diversity in adaptive life history traits and should be considered when allocating conservation resources. Each ecotype has unique requirements for habitat and fisheries management and its relative abundance will vary with latitude, global temperature, and human impacts. We suggest that conservation priority over the next 100 years should be ranked highest for the lake ecotype, intermediate for the sea/river ecotype, and lowest for the kokanee ecotype. This ranking is based on the approach proposed by Wood and Gross (2008) which takes into account both biological risk (probability of loss) and four criteria for value (consequences of loss) including ecological specialization (non-exchangeability), value to ecosystems, evolutionary uniqueness (option value), and goods and services value to human society. The kokanee ecotype appears to be at least biological risk, has been transplanted with considerable success (Wood 1995), has relatively low evolutionary value in view of its multiple recent origins, is likely least valuable to ecosystems (no contribution of marine-derived nutrients), and to human society. The sea/river ecotype is likely at greatest biological risk, but it ranks intermediate on the other criteria. The lake ecotype likely ranks highest overall, in accordance with the history of designations under endangered species legislation in both Canada and the USA.
From a longer term perspective, we are uncertain whether extinction of local sea/river-type populations would necessarily compromise the persistence or evolution of sockeye salmon during future glaciations (if these occur despite global warming) more than would the extinction of lake-type populations. The sea/river and lake ecotypes are not monophyletic lineages, and consequently, the capacity to generate the phenotype of the ancestral sea/river ecotype might exist within some extant lake-type and kokanee populations. The sea/river ecotype in the Nechako River appears to have arisen in this way. However, with so little evidence of ecological or evolutionary exchangeability, we recommend a precautionary approach in conservation planning.
Acknowledgments
We thank P. Bentzen, M. Gross, A. Hendry, J. Reynolds, R. Waples, and an anonymous referee for their useful suggestions on earlier versions of the manuscript, and P. Etherton and K. Jensen for fisheries data from the Stikine River.
Footnotes
Metapopulation is used here to mean a group of partially isolated spawning demes which are not demographically or genetically isolated populations due to substantial migration across generations.
A possible exception is the pink salmon population in Lake Aleknagik, Alaska (Robins et al., 2005).
Supporting Information
Literature cited
- Beacham TD, Withler RE, Gould AP. Biochemical genetic stock identification of chum salmon (Oncorhynchus keta) in southern British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1985a;42:437–448. [Google Scholar]
- Beacham TD, Withler RE, Gould AP. Biochemical genetic stock identification of pink salmon (Oncorhynchus gorbuscha) in southern British Columbia and Puget Sound. Canadian Journal of Fisheries and Aquatic Sciences. 1985b;42:1474–1483. [Google Scholar]
- Beacham TD, McIntosh B, MacConnachie C. Population structure of lake-type and river-type sockeye salmon in transboundary rivers of northern British Columbia. Journal of Fish Biology. 2004;65:389–402. [Google Scholar]
- Beacham TD, McIntosh B, MacConnachie C. Population structure and stock identification of sockeye salmon (Oncorhynchus nerka) in coastal lakes in British Columbia, Canada. Canadian Journal of Zoology. 2005;83:834–844. [Google Scholar]
- Beacham TD, McIntosh B, MacConnachie C, Miller KM, Withler RE. Pacific rim population structure of sockeye salmon as determined from microsatellite analysis. Transactions of the American Fisheries Society. 2006;135:174–187. [Google Scholar]
- Bickham JW, Wood CC, Patton JC. Biogeographic implications of cytochrome b sequences and allozymes in sockeye. Journal of Heredity. 1995;86:140–144. doi: 10.1093/oxfordjournals.jhered.a111544. [DOI] [PubMed] [Google Scholar]
- Brykov A, Polyakova N, Skurikhina IA, Kuklevsky AD. Geographical and temporal mitochondrial DNA variability in populations of pink salmon. Journal of Fish Biology. 1996;48:899–909. [Google Scholar]
- Burgner RL. Life history of sockeye salmon (Oncorhynchus nerka. In: Groot C, Margolis L, editors. Pacific salmon life histories. Vancouver: University of British Columbia Press; 1991. pp. 1–117. [Google Scholar]
- Byun SA, Koop BF, Reimchen TE. North American black bear mtDNA phylogeography: Implications for morphology and the Haida Gwaii glacial refugium controversy. Evolution. 1997;51:1647–1653. doi: 10.1111/j.1558-5646.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Edwards WF. Phylogenetic analysis: models and estimation procedures. American Journal of Human Genetics. 1967;19:233–257. [PMC free article] [PubMed] [Google Scholar]
- Craig JK, Foote CJ, Wood CC. Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biological Journal of the Linnean Society. 2005;84:287–305. [Google Scholar]
- Crandall KA, Templeton AR. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics. 1993;134:959–969. doi: 10.1093/genetics/134.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Kim K. Comparison of longterm greenhouse projections with the geologic record. Geophysical Research Letters. 1995;22:933–936. [Google Scholar]
- Domanico MJ, Phillips RB, Oakley TH. Phylogenetic analysis of Pacific salmon (genus Oncorhynchus) using nuclear and mitochondrial DNA sequences. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1865–1872. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.6. Seattle: Department of Genome Sciences, University of Washington; 2005. Available from: http://evolution.genetics.washington.edu/phylip.html (accessed Nov. 2007) [Google Scholar]
- Foote CJ, Wood CC, Withler RE. Biochemical genetic comparison of sockeye salmon and kokanee, the anadromous and nonanadromous forms of Oncorhynchus nerka. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46:149–158. [Google Scholar]
- Foote CJ, Wood CC, Clarke WC, Blackburn J. Circannual cycle of seawater adaptability in Oncorhynchus nerka: genetic differences between sympatric sockeye salmon and kokanee. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:99–109. [Google Scholar]
- Foote CJ, Moore K, Stenberg K, Craig KJ, Wenburg JK, Wood CC. Genetic differentiation in gill raker number and length in sympatric anadromous and nonanadromous morphs of sockeye salmon, Oncorhynchus nerka. Environmental Biology of Fishes. 1999;54:263–274. [Google Scholar]
- Geiger HJ. Sockeye salmon stock status and escapement goals in Southeast Alaska. Regional Information Report Number 1J03-04: Juneau, Arkansas, USA: Alaska Department of Fish and Game; 2003. p. 132. and 10 co-authors. Available from http://www.cf.adfg.state.ak.us/region1/pdfs/salmon/bof/1j03-04.pdf. [Google Scholar]
- Gilbert CH. Age at maturity of the Pacific coast salmon of the genus Oncorhynchus. Report of the British Columbia Commissioner of Fisheries. 1913;1912:57–70. [Google Scholar]
- Gustafson RG, Winans GA. Distribution and population genetic structure of river- and sea-type sockeye salmon in western North America. Ecology of Freshwater Fish. 1999;8:181–193. [Google Scholar]
- Gustafson RG, Wainwright TC, Winans GA, Waknitz FW, Parker LT, Waples RS. Status review of sockeye salmon from Washington and Oregon. NOAA Technical Memorandum NMFS-NWFSC-33: Seattle, Washington, USA: U.S. Department of Commerce; 1997. p. 282. [Google Scholar]
- Guthrie CM, Helle JH, Aebersold P, Winans GA, Gharrett AJ. Preliminary report on the genetic diversity of sockeye salmon populations from southeast Alaska and northern British Columbia. Alaska Fisheries Science Center Processed Report 94-03, Seattle: U.S. National Marine Fisheries Service; 1994. [Google Scholar]
- Halupka KC, Troyer JK, Willson MF, Bryant MB, Everest FH. Identification of unique and sensitive sockeye salmon stocks of Southeast Alaska, Forestry Sciences Laboratory, Pacific Northwest Research Station. Portland, OR: U. S. Department of Agriculture; 1993. p. 235. [Google Scholar]
- Hendry AP, Castric V, Kinnison MT, Quinn TP. The evolution of philopatry and dispersal: homing versus straying in salmonids. In: Hendry AP, Stearns SC, editors. Evolution illuminated: salmon and their relatives. Oxford: Oxford University Press; 2004. pp. 53–91. [Google Scholar]
- Hilborn R, Quinn TP, Schindler DE, Rogers DE. Biocomplexity and fisheries sustainability. Proceedings of the National Academy of Sciences. 2003;100:6564–6568. doi: 10.1073/pnas.1037274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Climate change 2007: the physical science basis. Contributions of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007. Available from: http://195.70.10.65/ipccreports/ar4-wg1.htm (accessed November 2007) [Google Scholar]
- Irvine JR, Gross MR, Wood CC, Holtby LB, Schubert ND, Amiro PG. Canada’s Species At Risk Act: an opportunity to protect “endangered” salmon. Fisheries (Bethesda) 2005;30:11–19. [Google Scholar]
- Koenings JP, Burkett RD. Population characteristics of sockeye salmon (Oncorhynchus nerka) smolts relative to temperature regimes, euphotic volume, fry density, and forage base within Alaskan lakes. In: Smith HD, Margolis L, Wood CC, editors. Sockeye salmon (Oncorhynchus nerka) population biology and future management. Ottawa, Ontario, Canada: Canadian Special Publication of Fisheries and Aquatic Sciences 96; 1987. pp. 216–234. [Google Scholar]
- Kondzela CM, Guthrie CM, Hawkins SL, Russell CD, Helle JH, Gharrett AJ. Genetic relationships among chum salmon populations in southeast Alaska and northern British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl.1):50–64. [Google Scholar]
- Lacourse T, Mathewes RW, Fedje DW. Paleoecology of late-glacial terrestrial deposits with in situ conifers from the submerged continental shelf of western Canada. Quaternary Research. 2003;60:180–188. [Google Scholar]
- McKay SJ, Devlin RH, Smith MJ. Phylogeny of Pacific salmon and trout based on growth hormone type-2 and mitochondrial NADH dehydrogenase subunit 3 DNA sequences. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:1165–1176. [Google Scholar]
- McPhail JD. The origin and speciation of Oncorhynchus revisited. In: Stouder DJ, Bisson PA, Naiman RJ, editors. Pacific salmon and their ecosystems: status and future options. New York: Chapman and Hall; 1997. pp. 29–39. [Google Scholar]
- McPhail JD, Lindsey CC. Freshwater fishes of northwestern Canada and Alaska. Canada: Bulletin of the Fisheries Research Board; 1970. p. 381. 173. [Google Scholar]
- McPhail JD, Lindsey CC. Zoogeography of freshwater fishes of Cascadia (the Columbia system and rivers north to the Stikine) In: Hocutt CH, Wiley EO, editors. Zoogeography of North American freshwater fishes. New York: John Wiley & Sons; 1986. pp. 615–637. [Google Scholar]
- Milner AM, Bailey RG. Salmonid colonization of new streams in Glacier Bay National Park, Alaska. Aquaculture and Fisheries Management. 1989;20:179–192. [Google Scholar]
- Nelson JS. Distribution and nomenclature of North American kokanee, Oncorhynchus nerka. Journal of the Fisheries Research Board, Canada. 1968;25:409–414. [Google Scholar]
- Nelson RJ, Wood CC, Cooper G, Smith C, Koop B. Population structure of sockeye salmon of the central coast of British Columbia: implications for recovery planning. North American Journal of Fisheries Management. 2003;23:704–721. [Google Scholar]
- Oakley TH, Phillips RB. Phylogeny of salmonine fishes based on growth hormone introns: Atlantic (Salmo) and Pacific (Oncorhynchus) salmon are not sister taxa. Molecular Phylogenetics and Evolution. 1999;11:381–393. doi: 10.1006/mpev.1998.0599. [DOI] [PubMed] [Google Scholar]
- Pavey SA, Hamon TR, Nielsen JL. Revisiting evolutionary dead ends in sockeye salmon (Oncorhynchus nerka) life history. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:1199–1208. [Google Scholar]
- Phelps SR, LeClair LL, Young S, Blankenship HL. Genetic diversity patterns of chum salmon in the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl.1):65–83. [Google Scholar]
- Pielou EC. After the ice age: the return of life to glaciated North America. Chicago: University of Chicago Press; 1991. p. 366. [Google Scholar]
- Quinn TP, Graynoth E, Wood CC, Foote CJ. Genotypic and phenotypic divergence of sockeye salmon in New Zealand from their ancestral British Columbia populations. Transactions of the American Fisheries Society. 1998;127:517–534. [Google Scholar]
- Rice SD, Thomas RE, Moles A. Physiological and growth differences in three stocks of underyearling sockeye salmon (Oncorhynchus nerka) on early entry into seawater. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:974–980. [Google Scholar]
- Ricker WE. On the origin of kokanee, a fresh-water type of sockeye salmon. Transactions of the Royal Society of Canada. 1940;34:121–135. [Google Scholar]
- Ricker WE. Stock and recruitment. Journal of the Fisheries Research Board, Canada. 1954;11:559–623. [Google Scholar]
- Robins JB, Abrey CA, Quinn TP, Rogers DE. Lacustrine growth of juvenile pink salmon and a comparison with sympatric sockeye salmon. Journal of Fish Biology. 2005;66:1671–1680. [Google Scholar]
- Sato S. Genetic population structure of chum salmon in the Pacific Rim inferred from mitochondrial DNA sequence variation. Environmental Biology of Fishes. 2004;69:37–50. and 13 co-authors. [Google Scholar]
- Schneider S, Roessli D, Excoffier L. ARLEQUIN ver. 2.000: A software for population genetics data analysis. Switzerland: Genetics and Biometry Laboratory, University of Geneva; 2000. [Google Scholar]
- Semko RS. The stocks of West Kamchatka salmon and their commercial utilization. Izvestiia TINRO. 1954;41:3–109. Translated 1960, Fisheries Research Board of Canada Translation Series 288. [Google Scholar]
- Shaklee JB, Varnavskaya NV. Electrophoretic characterization of odd-year pink salmon (Oncorhynchus gorbuscha) populations from the Pacific coast of Russia, and comparison with selected North American populations. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl.1):158–171. [Google Scholar]
- Smith CT, Nelson RJ, Wood CC, Koop BF. Glacial biogeography of North American coho salmon (Oncorhynchus kisutch. Molecular Ecology. 2001;10:2775–2785. doi: 10.1046/j.1365-294x.2001.t01-1-01405.x. [DOI] [PubMed] [Google Scholar]
- Stearley RF, Smith GR. Phylogeny of the Pacific trouts and salmons (Oncorhynchus) and genera of the family Salmonidae. Transactions of the American Fisheries Society. 1993;122:1–33. [Google Scholar]
- SYSTAT. SYSTAT 11. Richmond, CA: SYSTAT Software, Inc; 2004. Available from: http://www.systat.com (accessed Nov. 2007) [Google Scholar]
- Taylor EB, Foote CJ, Wood CC. Molecular genetic evidence for parallel life-history evolution within a Pacific salmon (sockeye salmon and kokanee, Oncorhynchus nerka. Evolution. 1996;50:401–416. doi: 10.1111/j.1558-5646.1996.tb04502.x. [DOI] [PubMed] [Google Scholar]
- Theriault V, Dunlop ES, Dieckmann U, Bernatchez L, Dodson JJ. The impact of fishing-induced mortality on the evolution of alternative life-history tactics in brook charr. Evolutionary Applications. 2008;1:409–423. doi: 10.1111/j.1752-4571.2008.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter F, Aebersold P, Helle J, Winans G. Genetic characterization of populations in the southeastern range of sockeye salmon. In: Walton JM, Houston DB, editors. Proceedings of the Olympic Wild Fish Conference, 23-25 March 1983. WA: Port Angeles; 1984. pp. 17–32. [Google Scholar]
- Varnavskaya NV, Wood CC, Everett RJ. Genetic variation in sockeye salmon (Oncorhynchus nerka) populations of Asia and North America. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl. 1):132–146. [Google Scholar]
- Waples RS, Pess GR, Beechie T. Evolutionary history of Pacific salmon in dynamic environments. Evolutionary Applications. 2008;1:189–206. doi: 10.1111/j.1752-4571.2008.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot RL, Everett RJ, Spearman WJ, Baccus R, Varnavskaya NV, Putivkin SV. Genetic stock structure of Western Alaska chum salmon and a comparison with Russian Far East stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl.1):84–94. [Google Scholar]
- Winans GA, Aebersold PB, Waples RS. Allozyme variability in selected populations of Oncorhynchus nerka with special consideration of Redfish Lake, Idaho. Transactions of the American Fisheries Society. 1996;125:645–653. [Google Scholar]
- Withler RE, Le KD, Nelson RJ, Miller KM, Beacham TD. Intact genetic structure and high levels of genetic diversity in bottlenecked sockeye salmon (Oncorhynchus nerka) populations of the Fraser River, British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:1985–1998. [Google Scholar]
- Wood CC. Life history variation and population structure in sockeye salmon. American Fisheries Society Symposium. 1995;17:195–216. [Google Scholar]
- Wood CC, Foote CJ. Genetic differences in the early development and growth of sympatric sockeye salmon and kokanee (Oncorhynchus nerka), and their hybrids. Canadian Journal of Fisheries and Aquatic Sciences. 1990;47:2250–2260. [Google Scholar]
- Wood CC, Foote CJ. Evidence for sympatric genetic divergence of anadromous and non-anadromous morphs of sockeye salmon (Oncorhynchus nerka. Evolution. 1996;50:1265–1279. doi: 10.1111/j.1558-5646.1996.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Wood CC, Gross MR. Elemental conservation units: communicating extinction risk without dictating targets for protection. Conservation Biology. 2008;22:36–47. doi: 10.1111/j.1523-1739.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- Wood CC, Riddell BE, Rutherford DT. Alternative juvenile life histories of sockeye salmon (Oncorhynchus nerka) and their contribution to production in the Stikine River, northern British Columbia. In: Smith HD, Margolis L, Wood CC, editors. Sockeye salmon (Oncorhynchus nerka) population biology and future management. Ottawa, Ontario, Canada: Canadian Special Publication of Fisheries and Aquatic Sciences 96; 1987. pp. 12–24. [Google Scholar]
- Wood CC, Riddell BE, Rutherford DT, Withler RE. Biochemical genetic survey of sockeye salmon (Oncorhynchus nerka) in Canada. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl.1):114–131. [Google Scholar]
- Wood CC, Foote CJ, Rutherford DT. Ecological interactions between juveniles of reproductively isolated anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka) sharing the same nursery lake. Environmental Biology of Fishes. 1999;54:161–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


