Abstract
Although contemporary trends indicative of evolutionary change have been detected in the life-history traits of exploited populations, it is not known to what extent fishing influences the evolution of alternative life-history tactics in migratory species such as salmonids. Here, we build a model to predict the evolution of anadromy and residency in an exploited population of brook charr, Salvelinus fontinalis. Our model allows for both phenotypic plasticity and genetic change in the age and size at migration by including migration reaction norms. Using this model, we predict that fishing of anadromous individuals over the course of 100 years causes evolution in the migration reaction norm, resulting in a decrease in average probabilities of migration with increasing harvest rate. Moreover, we show that differences in natural mortalities in freshwater greatly influence the magnitude and rate of evolutionary change. The fishing-induced changes in migration predicted by our model alter population abundances and reproductive output and should be accounted for in the sustainable management of salmonids.
Keywords: alternative tactics, eco-genetic model, evolution, fisheries-induced adaptive change, harvest, migration, reaction norm, recreational fishing
Introduction
Fishing is now acknowledged as a potential evolutionary force, described as a ‘massive uncontrolled experiment in evolutionary selection’ (Stokes and Law 2000). Whenever individuals with certain characteristics are more likely to survive harvest or to produce more viable offspring than others, fishing can induce evolutionary changes in life-history traits (Law and Grey 1989; Jørgensen 1990; Sutherland 1990; Law 1991, 2000; Smith 1994; Haugen and Vøllestad 2001; Conover and Munch 2002; Barot et al. 2004; Olsen et al. 2004; Reznick and Ghalambor 2005). That fishing can generate substantial selection differentials on phenotypic traits that are influenced by additive genetic variation is beyond doubt (Heino and Godø 2002; Swain et al. 2007). Yet, the rate of these changes and their consequences for stock viability, stability, yield, and recovery are less clear (Law 2000; Hutchings and Fraser 2008). Survivors of the harvesting process are likely to be genotypes with traits that confer relatively high fitness under fishing selection, but may be less than optimal with respect to natural selection (Conover 2000; Carlson et al. 2007). This may lead to slow recovery when fishing mortality is relaxed. Moreover, because cessation of fishing does not automatically produce equal selection pressures in the opposite direction, paying off this ‘Darwinian debt’ (Cookson 2004) may take a long time (Conover 2000; Law 2000).
Salmonids are well known for their diversity of life-history forms, with alternative mating tactics such as early maturing jacks in coho salmon, Oncorhynchus kisutch (Gross 1985), precocious parr in Atlantic Salmon, Salmo salar (Hutchings and Myers 1988), or various benthic and pelagic morphs in Artic charr, Salvelinus alpinus, and brook charr, Salvelinus fontinalis (Skúlason et al. 1996; Proulx and Magnan 2004). We follow Gross (1996) and Gross and Repka (1997, 1998) in using the term life-history tactics to refer to outcomes of life-history strategies, or decision rules, that determine how somatic and reproductive effort is allocated among alternative phenotypes. A common feature of many salmonid systems is the presence in sympatry of both anadromous (sea-run) and resident males and females, with resident fish completing their entire life cycle without migrating to sea (Jonsson and Jonsson 1993). Accumulating evidence suggests that these two forms may occur as alternative tactics within a single breeding population (Nordeng 1983; Morita et al. 2000; Olsson and Greenberg 2004; Thériault et al. 2007a). Individuals are understood to adopt a particular migration tactic by following a conditional life-history strategy involving energy thresholds, and various components of the energetic state of individuals (growth, lipid deposition, and metabolic rate) have been implicated in this process (Thorpe 1986; Bohlin et al. 1990; Hutchings and Myers 1994; Thorpe et al. 1998; Forseth et al. 1999; Morinville and Rasmussen 2003). Although influenced by environmental conditions (e.g. Olsson et al. 2006), the adoption of alternative life-history tactics in salmonids involves significant additive genetic variation, which has been demonstrated both in the laboratory (Silverstein and Hershberger 1992; Heath et al. 1994; Wild et al. 1994) and in the field (Garant et al. 2003; Thériault et al. 2007b). Moreover, whether an individual migrates or not will have critical consequences for its growth, survival, maturation, and reproduction. Survival is elevated in freshwater, but growth rates are reduced and resident individuals attain a smaller size at maturation (Gross 1987). As reproductive success is linked to body size in females (Fleming 1996; Morita and Takashima 1998; Thériault et al. 2007a), resident females experience decreased reproductive success relative to the bigger anadromous females. Reproductive success of males seems to be less affected by smaller size, as resident males employ alternative reproductive tactics, such as sneaking, to get access to mating opportunities (Hutchings and Myers 1988; Fleming 1996).
Owing to its size selectivity and temporally variable nature (Ricker 1995; Quinn et al. 2007), commercial fishing in salmonids has been shown to impact several life-history traits such as growth, age and size at maturation, and run timing. However, despite wide commercial and recreational interests in salmonids, evidence of evolutionary change caused by salmonid fisheries is still mostly circumstantial (Myers et al. 1986; Fukuwaka and Morita 2008, Hard et al. 2008). A fishery that targets only the migrant part of a population will inevitably be selective with respect to life-history tactics such as anadromy and residency. However, the consequences of such differential fishery-induced selection on the evolution of alternative life-history tactics have, to our knowledge, never been rigorously investigated.
Here, we used a recently developed modeling approach to predict the consequences of fishery-induced mortality on the evolution of anadromy and residency. The modeling approach (hereafter termed ‘eco-genetic’) incorporates both ecological and quantitative genetic processes, providing a mechanistically rich framework in which to predict the rate of evolutionary change on ecological timescales (Dunlop et al. 2007). In particular, our modeling approach enables distinguishing between plastic and evolutionary responses to fishing.
In the wild, salmonids show phenotypic plasticity in the age and size at migration. To account for such plasticity in the process of migration, we adopted a reaction norm approach. Reaction norms in the narrow sense describe how a single genotype is translated into different phenotypes depending on environmental conditions (Stearns 1992), while estimations of reaction norms in field studies must typically rely on the broader notion of population-level reaction norms (Sarkar and Fuller 2003; Hutchings 2004). Alternative tactics in salmonids have previously been described by reaction norms, based on the idea that the adoption of a particular tactic is governed by thresholds in growth rate (Myers and Hutchings 1986; Thorpe 1986; Bohlin et al. 1990; Hazel et al. 1990). Here, we extend this approach and consider the probability for the adoption of a particular migration tactic (anadromy or residency) as a function of size-at-age, where size-at-age has the helpful feature of integrating all environmental factors affecting growth. Such an approach has previously been used to model the evolution of maturation reaction norms (Ernande et al. 2004; Dunlop et al. 2007) and to tease apart phenotypically plastic responses from possible genetic changes in the age and size at maturation (Heino et al. 2002a; Grift et al. 2003; Barot et al. 2004, 2005; Olsen et al. 2004, 2005; Dunlop et al. 2005; Dieckmann and Heino 2007). Our study represents an extension of the maturation reaction norm approach so as to account for phenotypic plasticity in another fundamental life-history transition in the study of exploited populations.
We used data from a well-studied brook charr population in Québec, Canada, to parameterize our model. Recreational fishermen in the region are increasingly exploiting the sea-run components of this species as a result of the decline in Atlantic salmon stocks. Yet, anadromous populations of brook charr are not rigorously managed in many systems. Here, we examine the impact of various exploitation rates on the evolution of migration reaction norms, as well as on ecological and demographic characteristics of the population. We chose to model dynamics over a 100-year time horizon (approximately 30 generations) as this timeframe is commonly viewed as a manageable window from a conservation standpoint (Frankham et al. 2002).
The main purpose of our study was to address the following two questions: (i) Is fishing expected to induce evolutionary changes in the conditional migration strategy of salmonids? (ii) In a population with an evolving migration strategy, what are the effects of fishing on fecundity, abundance, and fishery yield? We also explored whether different freshwater mortality rates counteract or exacerbate the impact of fishing in saltwater on the evolution of anadromy and residency.
Methods
We constructed an individual-based eco-genetic model similar to that developed by Dunlop et al. (2007) to evaluate the effects of selective fishing mortality on the evolution of anadromy and residency within a conditional strategy framework. The model was built to reflect the life history of a sympatric population of brook charr in which anadromous and resident migration tactics coexist (Fig. 1) inhabiting a small tributary of the Ste-Marguerite River in Québec, Canada, named Morin Creek. The behavior and life history of brook charr in this system are well studied (Morinville and Rasmussen 2003, 2006, 2007; Thériault and Dodson 2003; Lenormand et al. 2004; Thériault et al. 2007a,b) and ample data from the years 1998–2004 were available to parameterize the model (Fig. 2, Table 1). The model follows evolution of the migration reaction norm, a quantitative trait that is passed on at the individual-level from parents to offspring. We assumed a closed population such that no new genetic variance was introduced by immigration. Model simulations were run for a total duration of 100 years in discrete, 1-year time steps and each simulation was repeated 30 times. As we consider the rate and amount of evolutionary change over the course of 100 years, our predictions represent transients and not evolutionary endpoints. Each year, individuals had the chance to experience the processes of migration to and from saltwater, growth, maturation, reproduction, and mortality (Fig. 1).
Figure 1.
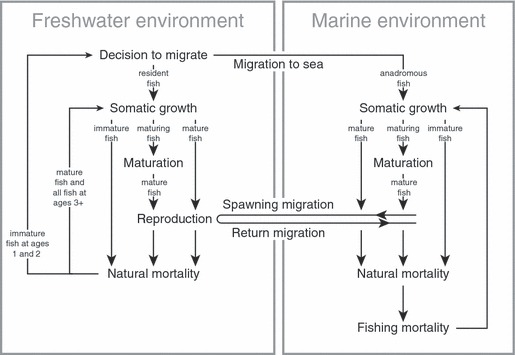
Schematic overview of the life cycle of brook charr, showing the sequence of events in the eco-genetic model.
Figure 2.
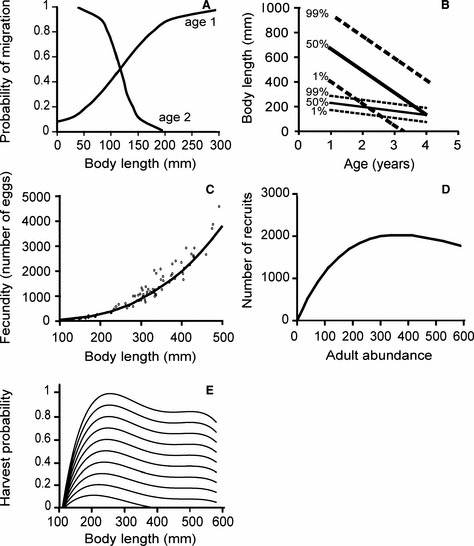
Empirically derived functions used in the model. (A) Probabilistic migration reaction norms estimated for Morin creek (data from Thériault and Dodson 2003). (B) Probabilistic maturation reaction norms estimated for anadromous individuals (thick lines) and resident individuals (thin lines), in each case showing the 50%, 1%, and 99% probability percentile curves. (C) Relationship between fecundity and body length for anadromous individuals (open circles) and resident individuals (filled circles) (data from Lenormand 2003). (D) Stock-recruitment relationship (based on Elliott 1993). (E) Selectivity curves for different maximal harvest probabilities increasing from 5% to 100% in increments of 10%.
Table 1.
Model parameters and their values.
| Symbol | Description | Equation | Source | Value |
|---|---|---|---|---|
| – | Initial mean body length (mm) | – | 1 | 82.98 |
| – | Initial standard deviation of body length (mm) | – | 1 | 13.68 |
| – | Mean emergence size (mm) | – | 1 | 31.71 |
| – | Standard deviation of emergence size (mm) | – | 1 | 5.52 |
| h2 | Initial heritability of evolving PMigRN traits | – | – | 0.5 |
| CV | Coefficient of variation for all evolving traits | – | – | 0.08 |
| c0 | Evolving PMigRN trait | 1 | 1 | −12.36 |
| c1 | Evolving PMigRN trait (mm−1) | 1 | 1 | 0.11 |
| c2 | Evolving PMigRN trait (year−1) | 1 | 1 | 9.69 |
| c3 | Evolving PMigRN trait (mm−1 year−1) | 1 | 1 | −0.08 |
| gs | Mean annual growth in saltwater (mm) | 2 | 2 | 95.53 |
| gf | Mean annual growth in freshwater (mm) | 2 | 1 | 35.35 |
| GSI | Mean gonado-somatic index | 2 | 2 | 0.147 |
| Sf | PMRN slope of resident morph (mm year−1) | 3 | 1 | −32.41 |
| if | PMRN intercept of resident morph (mm) | 3 | 1 | 259.72 |
| wf | PMRN width of resident morph (mm) | 3 | 1 | 114.53 |
| Ss | PMRN slope of anadromous morph (mm year−1) | 3 | 2 | −177.58 |
| is | PMRN intercept of anadromous morph (mm) | 3 | 2 | 843.38 |
| ws | PMRN width of anadromous morph (mm) | 3 | 2 | 532.9 |
| H1 | Constant in fecundity function (mm−1) | 4 | 2 | 0.04 |
| H2 | Constant in fecundity function | 4 | 2 | 2.86 |
| r | Constant in stock-recruitment function | 5 | 3 | 25.0 |
| b | Constant in stock-recruitment function | 5 | 3 | 0.0027 |
| mi,r | Immature natural mortality probability for resident morph under default conditions | – | 2 | 0.60 |
| mm,r | Mature natural mortality probability for resident morph under default conditions | – | 2 | 0.88 |
| mi,a1 | Immature natural mortality probability in 1st year for anadromous morph under default conditions | – | 2 | 0.80 |
| mi,a2 | Immature natural mortality probability in 2nd year for anadromous morph under default conditions | – | 2 | 0.60 |
| mm,a | Mature natural mortality probability for anadromous morph under default conditions | – | 2 | 0.55 |
| mi,r,poor | Immature natural mortality probability for resident morph under poor conditions | – | 2 | 0.80 |
| mm,r,poor | Mature natural mortality probability for resident morph under poor conditions | – | 2 | 0.95 |
| mi,r,good | Immature natural mortality probability for resident morph under good conditions | – | 2 | 0.20 |
| mm,r,good | Mature natural mortality probability for resident morph under good conditions | – | 2 | 0.70 |
PMRN, probabilistic maturation reaction norm; PMigRN, probabilistic migration reaction norm.
Data sources: (1) Morin Creek data from Thériault (2001) and Thériault and Dodson (2003), (2) Ste-Marguerite River data for anadromous fish and Morin Creek data for resident fish from Lenormand (2003), (3) based on Elliott (1993).
Migration
The migration reaction norm was represented by a logistic function, describing the probability P of migrating as a function of age a and body length l,
| 1 |
where 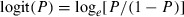 . This form was chosen because it allows the probability of migrating as a function of size to change its slope with age (Fig. 2A). Each individual was thus characterized by the four evolving parameters c0, c1, c2, and c3 describing its probabilistic migration reaction norm (PMigRN), which, together with its age and length, in turn determined its probability of migrating in a given year. An individual could migrate at either age 1 or 2 years only: if a fish did not migrate by age 2, we assumed that it would be a freshwater resident for all its life. This understanding is corroborated by field observations on this system (Thériault and Dodson 2003).
. This form was chosen because it allows the probability of migrating as a function of size to change its slope with age (Fig. 2A). Each individual was thus characterized by the four evolving parameters c0, c1, c2, and c3 describing its probabilistic migration reaction norm (PMigRN), which, together with its age and length, in turn determined its probability of migrating in a given year. An individual could migrate at either age 1 or 2 years only: if a fish did not migrate by age 2, we assumed that it would be a freshwater resident for all its life. This understanding is corroborated by field observations on this system (Thériault and Dodson 2003).
Somatic growth
Individuals grew according to the growth model introduced by Lester et al. (2004). Newborns in the model were given a random size at emergence, in accordance with the empirical mean and standard deviation estimated from back-calculations of the 1998-, 1999-, and 2000-year classes of fish captured in Morin creek (Thériault 2001; Thériault and Dodson 2003). Prior to maturation, individuals grew with an annual phenotypic growth increment ge determined by the environment in which they resided during that year (freshwater, e = f, or saltwater, e = s). The maximal growth increment is expressed in saltwater, whereas individuals living in freshwater grow slower due to the poorer growing environment they experience. The environment-specific growth rates gf and gs were empirically derived from immature individuals of the Ste-Marguerite River system and Morin Creek (Lenormand 2003; Thériault and Dodson 2003; Table 1).
Immediately following maturation, individuals devoted a proportion of energy to reproductive tissues, so that the body length la+1 at age a + 1 was given by
| 2 |
where GSI was the gonado-somatic index (gonadic mass divided by somatic mass) estimated for anadromous females. The gonado-somatic index was assumed to be similar and constant for all mature individuals in the population for simplicity. Growth rates were assumed to be density-independent, both in saltwater and in freshwater, to keep predictions simple. This simplifying assumption was further motivated by the following two reasons. First, in view of the high productivity of marine habitats, the small population sizes modeled here, and the importance of density-dependent predation mortality in saltwater, the density dependence of growth rates at sea must be expected to be weak. Second, results gathered from a creek adjacent and similar to Morin Creek failed to detect any density-dependence in freshwater growth for brook charr of age 0 and older (Centre Interuniversitaire de Recherche sur le Saumon Atlantique, CIRSA, unpublished data). When we tested the effects of relaxing a simplifying model assumption by adding density-dependent freshwater growth, there was little impact on the probability of migrating at age 1, but the probability of migrating at age 2 did evolve to be higher when the strength of density dependence was increased (Supplementary material, Section 3). We also evaluated the sensitivity of our results with regard to relaxing the simplifying assumption that growth rates were not evolving (Supplementary material, Section 2). We could thus confirm the robustness of our results under the incorporation of growth evolution.
Maturation
In any given year, an immature individual had a probability to mature during the upcoming year that was based on its environment (freshwater, e = f; or saltwater, e = s) and on its age a and body length l. These probabilities Pm were given by probabilistic maturation reaction norms (PMRNs, Fig. 2B, Heino et al. 2002a; Dieckmann and Heino 2007) with logistic length dependence, linear age dependence, and constant width,
| 3 |
where ie, se, and we were the environment-specific PMRN intercepts, slopes, and widths, respectively, and k = logit(99%) − logit(1%). To keep the model simple, and to focus on the evolution of migration, maturation tendency was not considered as an evolving trait in our model.
A logistic regression was applied to age-specific length distributions of immature and mature fish to provide an approximate estimation of the population’s PMRNs (Heino et al. 2002a; Dieckmann and Heino 2007). For anadromous fish, we used data gathered from the whole Ste-Marguerite River system (pooled from 1998 to 2001), whereas for resident fish, we used data gathered from Morin creek (pooled from 1998 to 2002). A linear regression of lengths at ages 2 and 3 (the two age classes for which sufficient data were available) at which the probability to mature in the next year was 50% was used to estimate the slopes and intercepts of linear PMRNs (Fig. 2B). We then used the 1% and 99% maturation probability percentiles of 3-year-old individuals to determine the PMRN widths. Our estimated PMRNs are only an approximation because, due to sampling constraints, we included fish that could have matured in a previous year in our mature length distribution. However, maturation occurs over such a narrow range in this population (the majority of individuals mature between ages 2 and 3), that the effect of including previously matured fish should be minimal relative to a population in which the range of maturation ages is larger. Owing to the uncertainty in our approximated PMRN, we performed sensitivity analyses and found that varying the PMRN slope and intercept had little impact on our model results (Supplementary material, Section 4).
Reproduction
There was no sex-structure in our model and reproduction occurred annually in freshwater between random pairs of mature individuals. The largest individual in the reproductive pair was chosen to be the mother, so as to account for frequently observed mating between anadromous females (bigger) and resident males (smaller) and the apparent absence of the reverse (big anadromous males are not expected to mate with small resident females, Thériault et al. 2007a). The number of eggs produced by a reproductive pair was estimated from the body length l of the mother according to an empirically derived relationship between fecundity and body length (Fig. 2C):
| 4 |
with allometric constants H1 and H2. The number of new individuals recruiting to the population at age 1 was determined from a Ricker stock-recruitment function (Fig. 2D):
| 5 |
where S is the number of adults, and r and b are constants. As the necessary data to derive such a stock-recruitment function specifically for our system were not available, we used constants estimated for brown trout (Elliott 1993), a species with life-history characteristics very similar to brook charr, and scaled them so as to yield realistic estimates of recruitment and spawner abundance in our system (Table 1).
Inheritance and expression
Genotype determination
Inheritance of the PMigRN was described by the infinitesimal model of quantitative genetics (Cavalli-Sforza and Feldman 1976). We assumed that phenotypic plasticity for migration was heritable by modeling genetically based reaction norms that were passed from parents to offspring (e.g. Brommer et al. 2005; Nussey et al. 2005; Dunlop et al. 2007). The four parameters c0, c1, c2, and c3 describing the PMigRN were thus considered as evolving traits. The genetic trait values of an offspring were drawn at random from normal distributions with mean values given by the mid-parental genetic trait values and variances that equaled half the corresponding genetic variances in the initial population. Modeling offspring variance in this way assumes equal variances of maternal and paternal traits and that the segregation and recombination of genes during reproduction introduce a constant amount of variation into the population (Roughgarden 1979).
Phenotype determination
The phenotypically expressed values of an individual’s four PMigRN traits were drawn randomly in each year from normal distributions with mean values given by the individual’s genetic trait values and variances that equaled the assumed environmental variances. The latter were calculated based on an assumed initial heritability h2 (see section on Initial population structure below) for the PMigRN traits and on an assumed initial genetic coefficient of variation. Based on the definition of heritability, h2 = VA/VP with VP = VA + VE (VA is the additive genetic variance; VP, total phenotypic variance; and VE, environmental variance), it is possible to calculate VE for each trait from the initial values of h2 and VA. While the environmental variances for each trait were kept constant in the model, the corresponding values of VA, and thus of h2, were free to evolve after the initial year (Supplementary material, Section 1).
Natural mortality
Default natural mortalities
Age-specific annual mortality probabilities were estimated from Morin Creek data for resident individuals, and from a larger mark-recapture experiment in the whole Ste-Marguerite River system for anadromous individuals (Lenormand 2003). Immature (mi) and mature (mm) mortality probabilities were applied annually to resident and anadromous individuals (Table 1). For anadromous fish, the mortality probability of an immature individual varied depending on whether it was the first or second year the individual spent in saltwater (mi,a1 and mi,a2, respectively, Table 1).
Alternative natural mortalities
In addition to the default values representing ‘normal’ freshwater mortality probabilities, we also simulated ‘poor’ freshwater survival conditions and ‘good’ freshwater survival conditions (Table 1), while keeping natural saltwater mortalities unchanged.
Fishing mortality
We applied fishing to anadromous individuals only, as they are the only targets of the recreational fishery. Length-dependent annual harvest probabilities for these fish were derived based on the observed sizes of fish caught and on data quantifying overall annual exploitation rates (Lenormand 2003; CIRSA, unpublished data, Fig. 2E). Medium-sized anadromous fish (with body lengths between 200 and 350 mm) are most likely to be caught, because they are abundant and, during the upstream migration of immature anadromous brook charr in early fall (Lenormand et al. 2004), concentrated in the river’s estuary, where their exploitation is little regulated. Smaller brook charr (with lengths between 110 and 200 mm) are not attractive to fishermen, whereas the bigger, mostly mature charr (with lengths larger than 350 mm) are under spatial and temporal regulations that prevent high fishing pressures on these larger fish. Size-selective fishing mortality was applied to individuals regardless of their maturation status. We varied the maximal harvest probabilities in the selectivity curves of anadromous fish between 0% and 100% in increments of 10% (Fig. 2E).
Initial population structure
Initial length distribution
The initial population in the model consisted of 5000 age-1 individuals with initial body lengths following a normal distribution with mean and standard deviation estimated for the 1998- to 2000-year classes of fish captured in Morin creek (Thériault and Dodson 2003; Table 1).
Initial migration reaction norms
The initial population-level PMigRN was estimated using data on size and age at migration from Morin Creek. Data on fish of ages 1 and 2 were analyzed for the years 1998–2000, as migration occurs almost exclusively at these two ages (Thériault and Dodson 2003). All individuals from the initial population were assigned genetic values for the four evolving traits c0, c1, c2, and c3 following normal distributions with mean values given by the trait values implied by the initial population-level PMigRN (Fig. 2A) and standard deviations given by the assumed initial genetic coefficient of variation.
Initial heritabilities
The initial heritability of each trait describing the PMigRN was assumed as 0.5. We do not know the actual value of heritability of plasticity for anadromy and residency in this system, but genetic variation and heritability have been demonstrated for plasticity in general (Scheiner 1993; Nussey et al. 2005) and have been assumed for migration in salmonids in particular (Hazel et al. 1990; Hutchings and Myers 1994; see also the review by Hutchings 2004). We chose the value 0.5 because the heritability of threshold traits that influence the adoption of alternative migration tactics varies between 0.52 and 0.56 for brook charr in this system (Thériault et al. 2007b), and between 0.12 and 0.98, with a mean of 0.53 for various other threshold traits (heritability of binomial threshold traits are reported on a ‘liability’ scale; Roff 1996). After initialization, heritabilities, genetic variances, and genetic covariances were free to evolve, and can thus be regarded as emerging properties of the model. Even though heritabilities directly scale the speed of evolution, so that we must expect slower or faster changes in reaction norms if we assume lower or higher heritabilities, the nature of predicted evolutionary changes remains unchanged as heritabilities are jointly increased or decreased (see, e.g., Dunlop et al. 2007).
Results
Impact of different fishing mortalities
Increasing the probability of harvest causes an evolutionary shift in the migration reaction norms for both age-1 and age-2 individuals: for an individual of the same size, the probability of migration is lowered as the maximal harvest probability increases (Fig. 3A,B). This translates into an overall probability of migration that is decreasing with increasing maximal harvest probability (Fig. 3C). The absolute number of fish that migrate decreases as the maximal harvest probability increases, and this trend is more pronounced for age-1 individuals than for age-2 individuals (Fig. 3D). Mean age at migration increases with maximal harvest probability (Fig. 3E), primarily reflecting the fact that the proportion of fish migrating at age 2 increases with harvest probability. Mean age at maturation did not change for residents, but decreased for anadromous individuals (Fig. 3F). Mean individual fecundity, highly dependent on size, decreased with the maximal harvest probability for anadromous fish, but showed no change for resident fish (Fig. 3G). Overall abundance of the population shows little change with increasing maximal harvest probability, because the number of fish in freshwater rose while the number in saltwater decreased to almost zero (Fig. 3H). The heritability of the migration reaction norm traits varied though time, but did not show a significant increase or decrease, either at low or at high maximal harvest probability (Fig. S1).
Figure 3.
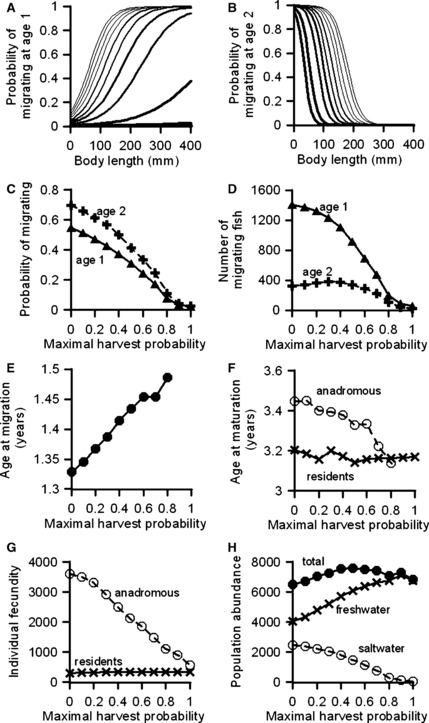
Model results after 100 years of fishing with different maximal harvest probabilities. Panels (A) and (B) show the resultant age-specific migration reaction norms (line thickness increases with increasing maximal harvest probability between 0% and 100% in increments of 10%). Panels (C–H) show how maximal harvest probabilities affect age-specific migration probabilities, age-specific numbers of migrating fish, ages at migration and maturation, individual fecundity, and population abundances. Results are averaged over 30 independent model runs.
Impact of different natural mortalities
Survival conditions in freshwater influenced the evolution of the migration reaction norm. After 100 years of fishing, low survival in freshwater associated with poor conditions leads to the evolution of a migration reaction norm that implies a higher probability of migrating for a given size than the reaction norm evolved under normal freshwater survival (Fig. 4A,B,C). In contrast, good survival conditions in freshwater leads to a lower probability of migrating for a given size than under normal conditions (Fig. 4A,B,C). Poor survival in freshwater thus offsets the evolutionary effect of fishing by increasing the probability of migrating (Fig. 4C) and the number of migrants (Fig. 4D), whereas good survival in freshwater had the opposite effect. The population’s abundance as a function of the maximal harvest probability is higher for poor than for normal freshwater survival conditions, whereas for good conditions, a nonmonotonic dependence on maximal harvest probability is found (Fig. 4E). The cumulative catch shows similar dome-shaped relationships for the three survival conditions in freshwater, but peaks at higher catches and higher maximal harvest probabilities as the survival conditions in freshwater are worsened (Fig. 4F).
Figure 4.
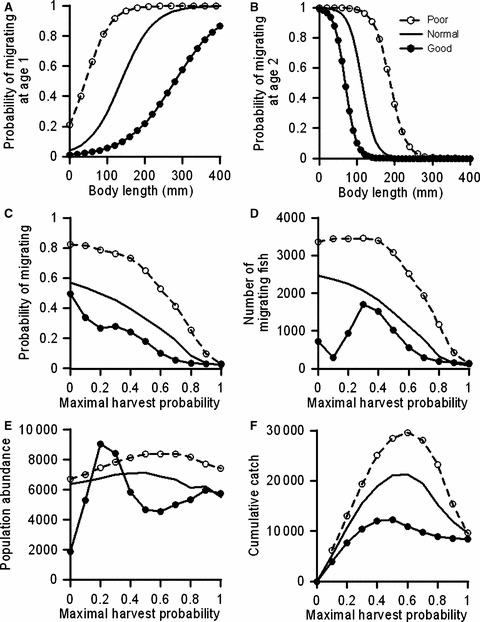
Model results after 100 years of fishing and different survival conditions in freshwater (poor, normal, or good). The maximum harvest probability was 0.5. Panels (A) and (B) show the resultant age-specific migration reaction norms. Panels (C–F) show how maximal harvest probabilities affect migration probabilities (for ages 1 and 2 combined), numbers of migrating fish (for ages 1 and 2 combined), total population abundances, and cumulative catches. Results are averaged over 30 independent model runs.
Discussion
Using an eco-genetic modeling approach, we explored the impact of recreational fishing on the evolution of anadromy and residency of a small population of brook charr. During a 100 years of fishing of anadromous individuals, we predicted evolution in this population’s migration reaction norm, with the average probability of migration decreasing with increasing harvest rate. These changes were accompanied by increases in the proportion of fish migrating at age 2, resulting in higher mean ages at migration. Our findings suggest that selective harvesting of anadromous fish results in a higher tendency for residency, through an increased fitness advantage of staying longer in freshwater and delaying migration. Shifts in the maturation reaction norms of several commercially important marine species, toward younger ages and smaller sizes at maturation, have been reported in the wake of heavy fishing pressures that selected against genotypes predisposing fish to mature later and larger (Atlantic cod, Gadus morhua, Heino et al. 2002b; Barot et al. 2004; Olsen et al. 2004, 2005; Baulier et al. 2006; plaice Pleuronectes platessa, Grift et al. 2003, 2007; American plaice, Hippoglossoides platessoides, Barot et al. 2005; sole, Solea solea, Mollet et al. 2006). Here, we have shown that evolutionary shifts in the reaction norm of another fundamental ontogenetic process – i.e., migration, an important life-history characteristic in salmonids – are also expected to result from elevated fishing mortality.
By changing the distribution of heritable traits, harvesting by humans can unintentionally select against the most desirable phenotypes (i.e. bigger individuals), which increase harvestable biomass (Law and Grey 1989; Coltman 2008; Hutchings and Fraser 2008). In a terrestrial context, trophy hunting of bighorn sheep over a period of 30 years has generated an undesired evolutionary response in weight and horn length, shown by the decrease in the mean breeding values of both of these traits (Coltman et al. 2003). Here, we have shown that the intensive harvesting of anadromous fish evolutionarily reduces the probability of migration. This ultimately leads to a reduced number of fish in saltwater, and thus to a situation in which less fish are available to the recreational fishery. We also found changes in the age at maturation for the anadromous part of the population. These changes, however, must not be interpreted as genetic changes, as the maturation tendency was not allowed to evolve in the model. Instead, these changes reflect the fact that at high harvest rates only the smallest and youngest anadromous fish are escaping harvest (as a result of the size-dependent harvest probabilities shown in Fig. 2E). The removal of large fish by the recreational fishery also causes a decline in the mean individual fecundity of anadromous fish at high harvest probabilities.
Genetic changes induced by fisheries, as any other selective force, are potentially reversible, given that sufficient heritable genetic variation remains and that adequate selection differentials in the opposite direction are generated once fishing is relaxed or stopped (Law 2000; Reznick and Ghalambor 2005). According to the body of work on commercially exploited marine species, however, it appears that such reversal is a difficult and slow process (Barot et al. 2004; Olsen et al. 2004; Swain et al. 2007; but see Fukuwaka and Morita 2008, who show an increase in the maturation threshold following cessation of fishing). Although we have not explored the extent of trait reversal following the cessation of fishing, our results suggest that heritable additive genetic variance in the migration reaction norm is preserved even at high harvest rates. The maintenance of genetic variation even under strong directional selection has been demonstrated for various traits (Houle 1992), including threshold traits (Roff 1994). In the latter case, variation remains ‘hidden’ by virtue of the threshold nature of the trait. This preservation of genetic variation may imply that evolutionary changes in threshold traits could be reversed after the return of favorable conditions. Lost alternative life-history tactics could be restored provided that the mechanisms for their phenotypic expression have not degenerated during a period of disuse (West-Eberhard 2003). Indeed, cases have been documented of nonanadromous salmonid fish stocks that maintained their capacity to migrate or that again gave rise to anadromous morphs after transplantation (Staurnes et al. 1992; Thrower et al. 2004).
Our study also aimed at assessing how variations in natural survival in freshwater could influence the evolution of migration. We found that shifts in migration reaction norms caused by selective harvesting were either impeded or exacerbated by low or high survival in freshwater, respectively. Fluctuating selection on body size has also been hypothesized as a factor favoring threshold variation, and thus ultimately maintaining alternative male life cycles, in Atlantic salmon (Aubin-Horth et al. 2005). Fluctuations in the direction of evolution have been demonstrated on the time scale of decades in Darwin finches, where selection on body size and beak shape has changed direction in time (Grant and Grant 2002). In the face of high temporal variability in ecological conditions, typical of northern temperate rivers inhabited by salmonids, predicting the rate and magnitude of fishing-induced life-history evolution thus becomes difficult over the long term.
This study deliberately focused on fishing-induced changes in the migration reaction norm of salmonids, as this phenomenon has received little, if any, attention in the published literature. To keep predictions simple, we did not allow for concomitant evolution of other life-history traits, such as growth capacity, the maturation reaction norm, or reproductive investment. While these other traits are also likely to evolve, most models published to date have focused, like our study here, on fishing-induced evolution in only one such character, such as maturation age or size, or the maturation reaction norm (Heino 1998; Ernande et al. 2004; Gårdmark and Dieckmann 2006; Dunlop et al. 2007). With this study being the first to address the fishing-induced evolution of a PMigRN, the inclusion of additional evolving traits would have unduly complicated the model, at least at this stage of investigation. We also confirmed that including growth capacity as an evolving trait did not significantly alter our predictions about migration evolution (Fig. S2). Future extensions of our model could explore the evolution of additional traits.
The effects of correlated responses to selection would also merit further investigation. Fishing-induced selection on one trait could generate responses in other genetically correlated traits: this could either diminish or amplify rates of evolutionary change, depending on the sign and magnitude of the correlation (Lynch 1999; Walsh et al. 2006; Dieckmann and Heino 2007; Hutchings and Fraser 2008). For example, smolting, maturation, and growth have all been shown to be genetically correlated, to varying degrees, in rainbow trout (Oncorhynchus mykiss,Thrower et al. 2004). Furthermore, the dynamic interactions of these traits with season-specific growth rates have been hypothesized to be a key factor maintaining genetic variation in smolting, despite complete selection against this phenotypic expression of migration (Thrower et al. 2004). Genes associated with smolting are thus conserved in the population, through selection for, or against, other genetically correlated traits. In brook charr, the probability of migrating at age 1 was found to be positively genetically correlated with body size at age 1 (Thériault et al. 2007b). One might expect that such a correlation would exacerbate the effect of fishing: selecting against anadromous individuals migrating at age 1 might indirectly select for smaller size at age 1, which in turn is associated with a higher probability of staying resident. However, a smaller size at age 1 could also translate into a smaller size at age 2, implying a higher probability of migrating. The effects of genetic correlations among traits related to the life-history tactics adopted in the face of fishing-induced selection remain to be explored.
Despite their important commercial and recreational implications, the effects of fishing on salmonids have rarely been demonstrated beyond the immediate consequences for abundance (see review by Hard et al. 2008). Genetic responses to commercial fisheries have been proposed for explaining a decrease in size of Pacific salmon (Ricker 1995), as well as changes in age and size at maturation in European grayling, Thymallus thymallus (Haugen and Vøllestad 2001), lake whitefish, Coregonus clupeaformis (Handford et al. 1977), and Atlantic salmon, Salmo salar (Bielak and Power 1986). However, these studies relied on indirect evidence as genetic changes were not demonstrated directly. By illustrating the impact of a sport fishery on the evolution of life-history tactics in salmonids, along with the associated ecological and demographic consequences, our present study contributes to the emerging view that evolution can be rapid enough to be an integral part of ecological interactions (Hendry and Kinnison 1999; Reznick and Ghalambor 2005; Jørgensen et al. 2007). The high harvest probabilities that caused the most significant demographic and evolutionary changes in this study are more common in commercial salmonid fisheries (such as in Atlantic salmon; Dempson et al. 2001) than in recreational fisheries (such as in the brook charr system studied here, where the mean harvest probability equaled 0.3; CIRSA, unpublished data). Recreational practices, however, still have the potential to contribute to the decline of fisheries (Cooke and Cowx 2006). We therefore recommend that managers and policy-makers increasingly complement traditional methods of fisheries management based on population dynamics alone with modeling approaches of the kind presented here.
Acknowledgments
We thank the CIRSA where data on the Ste-Marguerite River system were collected. The authors would also like to thank C. Jørgensen for helpful comments on earlier drafts of this manuscript. Funding of this project was provided to J.J.D. and L.B. by NSERC of Canada (Strategic Grant and Collaborative Special Projects), the Fondation de la Faune du Québec, the Government of Québec (FAPAQ), the Government of Canada (Economic development) and the financial partners of AquaSalmo R&D. This study is a contribution to the program of CIRSA and Québec-Océan. V.T. was financially supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds québécois de recherches sur la nature et les technologies (FQRNT). E.D. gratefully acknowledges financial support provided by the Research Council of Norway. U.D. acknowledges support by the European Community’s Sixth Framework Programme through the Marie Curie Research Training Network on Fisheries-induced Adaptive Changes in Exploited Stocks (FishACE) and through the Specific Targeted Research Project on Fisheries-induced Evolution (FinE).
Supporting Information
Literature cited
- Aubin-Horth N, Ryan DAJ, Good SP, Dodson JJ. Balancing selection on size: effects on the incidence of an alternative reproductive tactic. Evolutionary Ecology Research. 2005;7:1171–1182. [Google Scholar]
- Barot S, Heino M, O’Brien L, Dieckmann U. Long-term trend in the maturation reaction norm of two cod stocks. Ecological Applications. 2004;14:1257–1271. [Google Scholar]
- Barot S, Heino M, Morgan MJ, Dieckmann U. Maturation of Newfoundland American plaice (Hippoglossoides platessoides): long-term trends in maturation reaction norms despite low fishing mortality? ICES Journal of Marine Science. 2005;62:52–64. [Google Scholar]
- Baulier L, Heino M, Lilly GR, Dieckmann U. ICES CM. 2006. Body condition and evolution of maturation of Atlantic cod in Newfoundland; p. 19. 2006/H. [Google Scholar]
- Bielak AT, Power G. Changes in mean weight, sea-age composition, and catch-per-unit-effort of Atlantic salmon (Salmo salar) angled in the Godbout River, Quebec, 1859–1983. Canadian Journal of Fisheries and Aquatic Sciences. 1986;43:281–287. [Google Scholar]
- Bohlin T, Dellefors C, Faremo U. Large or small at maturity-theories on the choice of alternative male strategies in anadromous salmonids. Annales Zoologici Fennici. 1990;27:139–147. [Google Scholar]
- Brommer JE, Merilä J, Sheldon BC, Gustafsson L. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution. 2005;59:1362–1371. [PubMed] [Google Scholar]
- Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, Fletcher JM, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Evolution of continuous variation: direct approach through joint distribution of genotypes. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:1689–1692. doi: 10.1073/pnas.73.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman DW. Molecular ecological approaches to studying the evolutionary impact of selective harvesting in wildlife. Molecular Ecology. 2008;17:221–235. doi: 10.1111/j.1365-294X.2007.03414.x. [DOI] [PubMed] [Google Scholar]
- Coltman DW, O’Donoghue P, Jorgenson JT, et al. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. [DOI] [PubMed] [Google Scholar]
- Conover DO. Darwinian fishery science. Marine Ecology Progress Series. 2000;208:303–306. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Cowx IG. Contrasting recreational and commercial fishing: Searching for common issues to promote unified conservation of fisheries resources and aquatic environments. Biological Conservation. 2006;128:93–108. [Google Scholar]
- Cookson C. Over-harvesting leads to a Darwinian debt as only the smaller cod survive. The Financial Times 28 August 2004: 1. 2004. http://www.iiasa.ac.at/docs/HOTP/Sep04/article.pdf.
- Dempson JB, Schwarz CJ, Reddin DG, et al. Estimation of marine exploitation rates on Atlantic salmon (Salmo salar L.) stocks in Newfoundland, Canada. ICES Journal of Marine Science. 2001;58:331–341. [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Dunlop ES, Shuter BJ, Ridgway MS. Isolating the influence of growth rate on maturation patterns in the smallmouth bass (Micropterus dolomieu. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:844–853. [Google Scholar]
- Dunlop ES, Shuter BJ, Dieckmann U. The demographic and evolutionary consequences of selective mortality: predictions from an eco-genetic model of the smallmouth bass. Transactions of the American Fisheries Society. 2007;136:749–765. [Google Scholar]
- Elliott JM. A 25-year study of production of juvenile sea-trout, Salmo trutta, in an English Lake District stream. Canadian Special Publication of Fisheries and Aquatic Sciences. 1993;118:109–122. [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London Series B – Biological Sciences. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Reviews in Fish Biology and Fisheries. 1996;6:379–416. [Google Scholar]
- Forseth T, Nasje TF, Jonsson B, Harsaker K. Juvenile migration in brown trout: a consequence of energetic state. Journal of Animal Ecology. 1999;68:783–793. [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. New York: Cambridge University Press; 2002. [Google Scholar]
- Fukuwaka M, Morita K. Increase in maturation size after the closure of a high seas gillnet fishery on hatchery-reared chum salmon Oncorhynchus keta. Evolutionary Applications. 2008;1:376–387. doi: 10.1111/j.1752-4571.2008.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant D, Dodson JJ, Bernatchez L. Differential reproductive success and heritability of alternative reproductive tactics in wild Atlantic Salmon (Salmo salar L.) Evolution. 2003;57:1133–1141. doi: 10.1111/j.0014-3820.2003.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Gårdmark A, Dieckmann U. Disparate maturation adaptations to size-dependent mortality. Proceedings of the Royal Society London Series B – Biological Sicences. 2006;273:2185–2192. doi: 10.1098/rspb.2006.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-years study of Darwin’s finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Grift RE, Rijnsdorp AD, Barot S, Heino M, Dieckmann U. Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Marine Ecology Progress Series. 2003;257:247–257. [Google Scholar]
- Grift RE, Heino M, Rijnsdorp AD, Kraak SBM, Dieckmann U. Three-dimensional maturation reaction norms for North Sea plaice. Marine Ecology Progress Series. 2007;334:213–224. [Google Scholar]
- Gross MR. Disruptive selection for alternative life histories in salmon. Nature. 1985;313:47–48. [Google Scholar]
- Gross MR. Evolution of diadromy in fishes. American Fisheries Society Symposium. 1987;1:14–25. [Google Scholar]
- Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology & Evolution. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Gross MR, Repka J. Game theory and inheritance in the conditional strategy. In: Dugatkin LA, Reeve HK, editors. Game Theory and Animal Behavior. Oxford: Oxford University Press; 1997. pp. 168–187. [Google Scholar]
- Gross MR, Repka J. Stability with inheritance in the conditional strategy. Journal of Theoretical Biology. 1998;192:445–453. doi: 10.1006/jtbi.1998.0665. [DOI] [PubMed] [Google Scholar]
- Handford P, Bell G, Reimchen TE. A gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Hard JJ, Gross MR, Heino M, Hilborn R, Kope RG, Law R, Reynolds JD. Evolutionary consequences of fishing and their implications for salmon. Evolutionary Applications. 2008;1:388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T, Vøllestad LA. A century of life-history evolution in grayling. Genetica. 2001;112–113:475–491. [PubMed] [Google Scholar]
- Hazel W, Smock R, Johnsson MD. A polygenic model for the evolution and maintenance of conditional strategies. Proceedings of the Royal Society of London Series B – Biological Sciences. 1990;242:181–187. doi: 10.1098/rspb.1990.0122. [DOI] [PubMed] [Google Scholar]
- Heath DD, Devlin B, Heath JW, Iwana GK. Genetic, environmental and interaction effects on the incidence of jacking in Oncorhynchus tshawytacha (chinook salmon) Heredity. 1994;72:146–154. [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1971–1982. [Google Scholar]
- Heino M, Godø OR. Fisheries-induced selection pressures in the context of sustainable fisheries. Bulletin of Marine Science. 2002;70:639–656. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002a;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Reaction norm analysis of fisheries-induced adaptive change and the case of the Northeast Arctic cod. 2002b;2002/Y:14. ICES CM. [Google Scholar]
- Hendry AP, Kinnison MT. The pace of modern life: measuring the rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA. Norms of reaction and phenotypic plasticity in salmonids life histories. In: Hendry AP, Stearns SC, editors. Evolution Illuminated: Salmon and Their Relatives. New York: Oxford University Press; 2004. pp. 154–174. [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA. Mating success of alternative maturation phenotypes in male Atlantic salmon, Salmo salar. Oecologia. 1988;75:169–174. doi: 10.1007/BF00378593. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA. The evolution of alternative mating strategies in variable environments. Evolutionary Ecology. 1994;8:256–268. [Google Scholar]
- Jonsson B, Jonsson N. Partial migration: niche shift versus sexual maturation in fishes. Reviews in Fish Biology and Fisheries. 1993;3:348–365. [Google Scholar]
- Jørgensen T. Long-term changes in age at sexual maturity of Northeast Arctic cod (Gadus morhua L.) Journal du Conseil International pour l’Exploration de la Mer. 1990;46:235–248. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Law R. Fishing in evolutionary waters. New Scientist. 1991;1758:35–37. [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R, Grey RD. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology. 1989;3:343–359. [Google Scholar]
- Lenormand S. Québec, QC, Canada: Université Laval; 2003. Évolution de l’anadromie et stratégie de reproduction chez l’omble de fontaine (Salvelinus fontinalis. PhD thesis. [Google Scholar]
- Lenormand S, Dodson JJ, Ménard A. Seasonal and ontogenetic patterns in the migration of anadromous brook charr (Salvelinus fontinalis. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:54–67. [Google Scholar]
- Lester NP, Shuter BJ, Abrams PA. Interpreting the von Bertalanffy model of somatic growth in fishes: the cost of reproduction. Proceedings of the Royal Society of London Series B – Biological Sciences. 2004;271:1625–1631. doi: 10.1098/rspb.2004.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. Estimating genetic correlations in natural populations. Genetical Research. 1999;74:255–264. doi: 10.1017/s0016672399004243. [DOI] [PubMed] [Google Scholar]
- Mollet FM, Kraak SBM, Rijnsdorp AD. Fisheries-induced evolutionary changes in maturation reaction norms in North Sea sole (Solea solea. ICES CM. 2006;2006/H:14. [Google Scholar]
- Morinville GR, Rasmussen JB. Early juvenile bioenergetic differences between anadromous and resident brook trout (Salvelinus fontinalis. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:401–410. [Google Scholar]
- Morinville GR, Rasmussen JB. Does life-history variability in salmonids affect habitat use by juveniles? A comparison among streams open and closed to anadromy. Journal of Animal Ecology. 2006;75:693–704. doi: 10.1111/j.1365-2656.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- Morinville GR, Rasmussen JB. Distinguishing between juvenile anadromous and resident brook trout (Salvelinus fontinalis) using morphology. Environmental Biology of Fishes. 2007;81:171–184. [Google Scholar]
- Morita K, Takashima Y. Effect of female size on fecundity and egg size in white-spotted charr: comparison between sea-run and resident forms. Journal of Fish Biology. 1998;53:1140–1142. [Google Scholar]
- Morita K, Yamamoto S, Hoshino N. Extreme life history change of white-spotted charr (Salvelinus leucomaenis) after damming. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:1300–1306. [Google Scholar]
- Myers RA, Hutchings JA. Selection against parr maturation in Atlantic salmon. Aquaculture. 1986;53:313–320. [Google Scholar]
- Myers RA, Hutchings JA, Gibson RJ. Variation in male parr maturation within and among populations of Atlantic salmon, Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences. 1986;43:1242–1248. [Google Scholar]
- Nordeng H. Solution to the ‘Char Problem’ based on Artic char (Salvelinus alpinus) in Norway. Canadian Journal of Fisheries and Aquatic Sciences. 1983;40:1372–1387. [Google Scholar]
- Nussey DH, Postman E, Gienapp P, Visser ME. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–306. doi: 10.1126/science.1117004. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Heino M, Lilly GR, et al. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Lilly GR, Heino M, et al. Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:811–823. [Google Scholar]
- Olsson IC, Greenberg LA. Partial migration in a landlocked brown trout population. Journal of Fish Biology. 2004;65:106–121. [Google Scholar]
- Olsson IC, Greenberg LA, Bergman E, Wysujack K. Environmentally induced migration: the importance of food. Ecology Letters. 2006;9:645–651. doi: 10.1111/j.1461-0248.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Proulx R, Magnan P. Contribution of phenotypic plasticity and heredity to the trophic polymorphism of lacustrine brook charr (Salvelinus fontinalis M.) Evolutionary Ecology Research. 2004;6:503–522. [Google Scholar]
- Quinn TP, Hodgson S, Flynn L, Hilborn R, Rogers DE. Directional selection by fisheries and the timing of sockeye salmon (Oncorhynchus nerka) migrations. Ecological Applications. 2007;17:731–739. doi: 10.1890/06-0771. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata) Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:791–801. [Google Scholar]
- Ricker W. Trends in the average size of Pacific salmon in Canadian catches. In: Beamish RJ, editor. Climate Change and Northern Fish Population. Ottawa, Ontario: Canadian Special Publication of Fisheries and Aquatic Sciences; 1995. pp. 593–602. 121. [Google Scholar]
- Roff DA. Evolution of dimorphic traits: effect of directional selection on heritability. Heredity. 1994;72:36–41. [Google Scholar]
- Roff DA. The evolution of threshold traits in animals. Quarterly Review of Biology. 1996;71:3–35. [Google Scholar]
- Roughgarden J. Theory of Population Genetics and Evolutionary Ecology: An Introduction. New York: Macmillan Publishing Co. Inc; 1979. [Google Scholar]
- Sarkar S, Fuller T. Generalized reaction norms for ecological developmental biology. Evolution and Development. 2003;5:106–115. doi: 10.1046/j.1525-142x.2003.03016.x. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics. 1993;24:35–68. [Google Scholar]
- Silverstein JT, Hershberger WK. Precocious maturation in coho salmon (Oncorhynchus kisutch): estimation of heritability. Bulletin of the Aquaculture Association of Canada. 1992;92:34–36. [Google Scholar]
- Skúlason S, Snorrason SS, Noakes DLG, Ferguson MM. Genetic basis of life history variation among sympatric morphs of Artic char, Salvelinus alpinus. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:1807–1813. [Google Scholar]
- Smith PJ. Genetic Diversity of Marine Fisheries Resources: Possible Impacts of Fishing. Rome: FAO; 1994. FAO Fisheries Technical Paper No 344. [Google Scholar]
- Staurnes M, Lysfjord G, Berg OK. Parr-smolt transformation of a nonanadromous population of Atlantic salmon (Salmo salar) in Norway. Canadian Journal of Zoology. 1992;70:197–199. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- Stokes K, Law R. Fishing as an evolutionary force. Marine Ecology Progress Series. 2000;208:307–309. [Google Scholar]
- Sutherland WJ. Evolution and fisheries. Nature. 1990;344:814–815. [Google Scholar]
- Swain DP, Sinclair AF, Hanson MJ. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society Series B – Biological Sciences. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thériault V. Québec, QC, Canada: Université Laval; 2001. Anadromie et résidence chez l’omble de fontaine (Salvelinus fontinalis): présence d’une stratégie conditionnelle basée sur la croissance? Master of Science thesis. [Google Scholar]
- Thériault V, Dodson JJ. Body size and the adoption of a migratory tactic in brook charr. Journal of Fish Biology. 2003;63:1144–1159. [Google Scholar]
- Thériault V, Bernatchez L, Dodson JJ. Mating system and individual reproductive success of sympatric anadromous and resident brook charr, Salvelinus fontinalis, under natural conditions. Behavioral Ecology and Sociobiology. 2007a;62:51–65. [Google Scholar]
- Thériault V, Garant D, Bernatchez L, Dodson JJ. Heritability of life history tactics and genetic correlation with body size in a natural population of brook charr (Salvelinus fontinalis. Journal of Evolutionary Biology. 2007b;20:2266–2277. doi: 10.1111/j.1420-9101.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- Thorpe JE. Age at first maturity in Atlantic salmon, Salmo salar: freshwater period influences and conflicts with smolting. Canadian Special Publication of Fisheries and Aquatic Sciences. 1986;89:7–14. [Google Scholar]
- Thorpe JE, Mangel M, Metcalfe NB, Huntingford FA. Modeling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evolutionary Ecology. 1998;12:581–599. [Google Scholar]
- Thrower FP, Hard J, Joyce JE. Genetic architecture of growth and early life-history transitions in anadromous and derived freshwater populations of steelhead. Journal of Fish Biology. 2004;65:286–307. [Google Scholar]
- Walsh MR, Munch SB, Shiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Wild V, Simianer H, Gjoeen HM, Gjerde B. Genetic parameters and genotype × environment interaction for early sexual maturity in Atlantic salmon (Salmo salar. Aquaculture. 1994;128:51–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


