Abstract
Brown trout (Salmo trutta) are extensively harvested and its habitat highly influenced by human encroachments. Using a 40-year time series of mark–recapture data we estimate vital rates for a piscivorous trout population. This population spawns upstream of a waterfall, which historically acted as a migration barrier for smaller trout. In 1966, the waterfall was dammed and a fish ladder constructed. All fish ascending the fish ladder were individually tagged and measured for a variety of traits. The fish ladder overall favoured access to upstream spawning areas for middle-sized trout, resulting in stabilizing selection acting on size at spawning. Over time, natural and fishing mortality have varied, with fishing mortality generally decreasing and natural mortality increasing. The average and, particularly, variance in size-at-first-spawning, and growth rates during the first years of lake residence have all decreased over the 1966–2003 period. These changes are all consistent with a shift from directional to stabilizing selection on age and size at spawning. Estimated rates of phenotypic change are relatively high, in particular for size at first spawning, adding further support for the growing notion that human interference may lead to rapid life-history trait evolution.
Keywords: contemporary evolution, evolutionary rates, human-induced evolution, life-history evolution, migration, Salmo trutta
Introduction
Many animal species perform extensive migrations between habitats that differ in quality, e.g. between habitats useful for rearing of young during summer and habitats useful for feeding and survival during winter (Dingle 1980; Stenseth and Lidicker 1994; Sutherland 1996; Clobert et al. 2001). Such migrations, whether long as in some birds and marine fishes and reptiles, or short as in some freshwater fishes and amphibians, are important life-history events having both large benefits and often also important costs. Benefits are usually related to the increased survival of progeny (in the rearing habitat) and growth and survival of adults (in the feeding habitat), whereas costs often are related to the migration itself (e.g. cost of movement, cost of being exposed to predators, etc.). These costs are often size and state dependent.
As migrations in salmonid fishes are often rather spectacular and associated with economic interest (Quinn 2005), the costs and benefits of such migrations have been extensively studied in these species (see Hendry et al. 2004a for a review). In brief, salmonid fish spawn and the young are reared in fresh water (usually rivers), after which the immature fish at some stage often migrate to a new habitat (the sea for the anadromous populations, a larger river or a lake for freshwater resident populations) for feeding (Elliott 1994; Quinn 2005). Strong natal homing opens the possibility for rapid local adaptation that may lead to strong population differentiation (Hendry et al. 2004b). In general, migration difficulty is a strong selective agent leading to large differences in body size between populations breeding in rivers of different size and steepness (Schaffer and Elson 1975; Fleming 1996; L’Abée-Lund et al. 2004). Swimming ability is directly related to size and is strongly influenced by morphology such as body depth (Videler and Wardle 1991; Videler 1993). In general, fish are larger-bodied in the larger and faster flowing rivers although several exceptions exist (see Quinn 2005).
Within rivers, there might be obstacles to migration that can function as phenotype-sorting mechanisms: for instance, some waterfalls may only allow the larger and stronger fish to ascend, effectively selecting against small size-at-maturity (selecting for swimming ability) (Brandt et al. 2005). In such rivers, we might expect population differentiation with larger and also more fast-growing fish above such a barrier and a more variable set of phenotypes below the barrier. Such consistent and strong selection favouring large body size should reduce levels of additive genetic variance and, thus, trait heritability over time. What will then happen if the selectivity of the barrier is changed following either natural or human disturbance? One possibility is that the consistent directional selection favouring large size is reduced. When selection for large size is reduced, we expect evolutionary changes in the population leading to an earlier age and smaller size at maturation.
One human activity that affects natural barriers is the construction of fish passage ladders – systems that are specifically built to help fish navigate impassable or difficult-to-pass stretches of a river. Herein, we utilize a unique long-term dataset collected following the construction of a dam and a fish ladder. In particular, we investigate the evolutionary effects of changing the size-dependent selection acting on a brown trout (Salmo trutta) population during their spawning migration.
Material and methods
The brown trout individuals spawning in the River Gulbrandsdalslågen, the Hunder trout, are famous for their large size. On average, they attain size at first spawning of 66–68 cm and weights of 3–5 kg, with the maximum size exceeding 17 kg (Aass et al. 1989). After spawning during autumn, the adult fish may stay in the river over the winter or migrate downstream to Lake Mjøsa where they typically remain for 2 years before making a new spawning attempt (Aass et al. 1989). The juveniles stay in the river for a period of 2–7 years, after which they migrate downstream, as smolt, during the spring into Lake Mjøsa at an average size of 25–27 cm (Aass et al. 1989). The fraction of individuals that do not migrate down stream as smolt (i.e. the residents) is not known, but it is very likely that some individuals, and in particular male individuals, develop into residents.
Lake Mjøsa (Fig. 1) is the largest lake in Norway, with a surface area of 365 km2, average depth of 153 m and maximum depth of 449 m. The lake contains 20 species of freshwater fish, the most common being vendace (Coregonus albula), whitefish (C. lavaretus), smelt (Osmerus eperlanus), roach (Rutilus rutilus), perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus) (Sandlund et al. 1985). During the 1950s and 1960s and culminating during the 1970s the lake was subject to strong cultural eutrophication caused by domestic sewage. Since that time, water treatment operations have returned the lake productivity to pre-1950 level (Fig. 2). The brown trout feed in the pelagic habitat and primarily on fish while in the lake, the most important prey species being, in that order, smelt, vendace and whitefish (Aass et al. 1989). The trout population is harvested both in the lake and during their breeding migration in the river. In the lake, trout are harvested using gill nets and through angling. The fishing effort has varied over time, with a decreased total effort in gillnet fisheries and an increasing angling effort over the last 15 years. Overall, the type of gear used has not changed much during this time period. In the river, trout have historically been captured by wood traps in the rapids, driftnets in the lower more slow-flowing parts, and by angling (Aass and Kraabøl 1999). The use of driftnets and traps was discontinued around the establishment of the dam, whereas angling may have increased somewhat. Angling is assumed to be less size-selective than at least the drift net fishery. Overall, we have no indication that the selectivity of the trout harvest has changed dramatically over the time period for which we have data, but this should be checked more carefully in the future.
Figure 1.
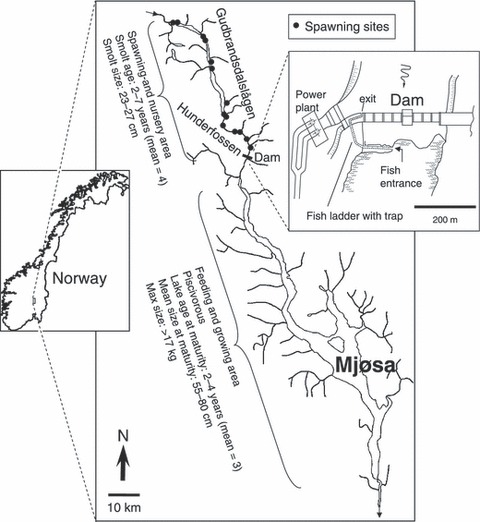
Location map of the study area, the river Gudbrandsdalslågen, indicating the location and dimensions of the dam and the fish ladder and also the location of the spawning grounds. Some key biological characteristics of the brown trout population are provided.
Figure 2.
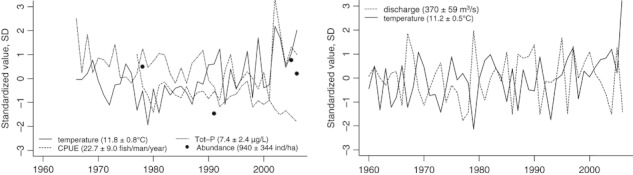
Environmental variables for the lake Mjøsa (left panel) and its main inlet river Gudbrandsdalslågen (right panel). Left: The water temperature in the 0–10 m depth layer in the lake Mjøsa has been measured at least seven times each year (March–November) over the 1972 to present period and is here presented as the mean. The plotted values resemble the June–October period arithmetic mean values from the same station at the deepest point in the lake that has been surveyed consistently over the period. The brown trout catch data constitute annual catches from a selected panel of local recreational fishermen. Under the assumption of similar behaviour of the trout over the period these catch data can be used as a proxy for the trout density in the lake. Finally, echo sounding surveys have been performed at four occasions over the study period. These data reveals that the prey fish density (mainly smelt and vendace) has varied a lot over the last three decades, but there is no overall trend. The river data, right panel, constitute mean mid day June-to-October water temperature and discharge (m3 s−1) values at the Hunderfossen dam. In order to enable presentation of the variables in the same figure, all values have been standardized so as to mean = 0 and standard deviation = 1 (estimates of means and standard deviations are given in parenthesis in figure legend).
The River Gulbrandsdalslågen drains a 17 000 km2 catchment area. The water discharge in the lower area is approximately 60–100 m3 s−1 during winter and 1000–2500 m3 s−1 during spring and summer floods (Arnekleiv et al. 2007). The Hunderfossen power station exploits a rapid with a total elevation of 46 m about 15 km upstream Lake Mjøsa (Fig. 1). Except for a fixed minimum flow, water is drained away from the first 4.4 km of the river downstream of the dam, thus reducing spawning opportunities severely (Aass et al. 1989) and also providing a poor incubation habitat with high risk of drought and/or freezing owing to the low water flow during winter. Before dam construction only the larger-sized brown trout were able to ascend the rapids and the main spawning activity were on the stretch of river downstream the dammed rapids (Aass et al. 1989; Aass and Kraabøl 1999). Following from this, the unique large individual size of the Hunder trout stock seem to be closely linked to the selective effect of the rapid. Indeed, the individual size of this brown trout population is significantly larger than any other of the more than 12 other piscivorous brown trout populations inhabiting Mjøsa (Skaala 1992).
The river was dammed in 1961, leading to fish having no access to upstream spawning areas in the period after dam construction and the completion of a fish ladder. The fish ladder was built and became functional in 1966 – this restored connectivity (Jensen and Aass 1995; Arnekleiv et al. 2007). Since 1966, all upstream migrating trout have been captured while navigating the fish ladder. Because large fish often are observed, in particular at low flow levels, to reside in the mouth area of the fish ladder and showing little interest in ascending the ladder, fish management personnel have long claimed that the ladder construction is disfavouring large individuals. A previous radio telemetry study demonstrated that the water flow was a critical variable for fish ladder ascent (Arnekleiv and Kraabøl 1996), but the individual size component remains an enigmatic issue.
All captured fish have been tagged each year since 1966 (193 individuals were captured and tagged in 1965), and these fish were later recaptured both in the river, in the lake during feeding, and/or on subsequent spawning migrations. These data were used to construct individual capture histories. Untagged fish that died during the capture or marking process (n = 232) are not included in the data set, whereas recaptured fish that died in the trap were right censored (registered as recaptures but excluded from later analyses, n = 93). Moreover, at capture a small number of scales were removed from each individual for analysis of individual growth histories. The data from the scale analyses were used to estimate each individual’s age- and length-at-first maturation as well as age- and length-at-each subsequent reproductive events (brown trout are iteroparous).
We here use the individual capture histories of 5346 mature wild brown trout spanning a time period of 40 years to estimate the survival and recapture probabilities during various time intervals, and to evaluate which factors best explain variation in these rates. We hypothesize that the changes in selection on size imposed by the new fish ladder (going from strong selection for large size to an assumed no selection; the assumption of no selection is also evaluated here) have resulted in evolutionary changes in the population structure and, specifically, that fish will be smaller and younger at their first breeding attempt than before the ladder was constructed.
Another human disturbance affecting this trout population is harvest. Harvest is often size selective (e.g. Carlson et al. 2007), which can drive evolutionary changes. Indeed, the evolutionary effects of harvesting have recently been acknowledged, though mostly with circumstantial genetic evidence, for various fish species (Haugen and Vøllestad 2001 (grayling); Olsen et al. 2004; Swain et al. 2007 (cod Gadus morhua); Edeline et al. 2007 (Northern pike Esox lucius); Quinn et al. 2007 [sockeye salmon Oncorhynchus nerka), Fukuwaka and Morita 2008 (chum salmon O. keta)]. Some of the highest rates of phenotypic change have been reported from harvested populations (Hendry and Kinnison 1999; Hendry et al. 2008). A change in fishing methods has been documented through the time period of study (Aass and Kraabøl 1999), which has the potential to alter the adaptive landscape. Consequently, we also ask to what extent size-at-first breeding and growth rates varied with time, and estimate rates of phenotypic change.
The data
This study is based on a capture–mark–recapture (CMR) data series that comprises 9095 brown trout individuals (of which 5346 were born in the wild) tagged at the Hunderfossen fish ladder (Fig. 1) between 1966 and 2003. All fish were tagged with individually numbered Carlin type tags (Laird and Stott 1978). The tagged fish were kept in tanks for recovery before being released back to the ladder, above the trap. During the tagging process the fish are weighed (g) and measured for total length (mm). At the same time, sex was determined and a small sample of scales (<10) was removed using forceps for age determination.
Every year since the dam was built a variable number of hatchery-reared smolt (fish ready for downstream migration) was stocked both above and below the dam. The numbers varied between 17 500 and 73 500 fish annually (Jensen and Aass 1995). The adipose fin of all hatchery-reared trout is clipped to aid separation from the wild fish. All hatchery-reared trout that were later caught in the ladder were tagged following the same procedure as for the wild fish. All hatchery fish are first generation progeny of wild-born parents and there has been no selection program in the hatchery (i.e. no intentional selection) and hence, we do not expect strong domestication effects. Nevertheless, to avoid potential artefacts due to domestication, we here use only data on wild trout in our analyses (a total of 5346 individuals). These fish have all been born in the wild, either from wild-born or F1-hatchery fish and have experienced the full set of natural selection processes throughout life.
The sampled scales were used for aging, back-calculation of growth and determination of spawning age and frequency. For such fast growing trout, analysis of scales provides information on the age-at-smolt transformation (transition between a riverine life feeding on benthos and a pelagic life feeding on fish) as well as age-at-spawning in much the same way as described in detail for Atlantic salmon (Salmo salar) (ICES 1984). During spawning the scale margins erodes, making it possible to identify years of spawning. The scales are also used to back-calculate length-at-age for each age using direct proportionality between fish growth and scale growth (Bagenal and Tesch 1978). As a large number of trout have been captured several times it was possible to use this information to increase the precision of the age determination and back-calculation methods (i.e. to use it as a learning tool) using scales sampled at different recapture events (i.e. at different ages). We estimated smolt age (age at downstream migration) and lake age (number of years in the lake), and test for differences in length-at-age using linear models.
Tagged trout are recaptured both in the trap and also by local fishermen both in the lake and in the river. Fish recaptured in the trap undergo the same sampling and release procedures as those that are tagged for the first time and are then released. All fish caught in the trap are carefully examined for tags and eventual indications of lost tags (i.e. scars). In total, 14 individuals have been identified to have evident tag loss. Hence, in accordance with other salmonid studies (e.g. Dietrich and Cunjak 2006) the retention rate is very high for these tags. Fish recaptured by anglers are usually killed; only recently have anglers started to release the caught fish.
Maturation and growth analyses
Based on the scale analysis, we used the information about age-at-first spawning and length-at-first spawning for estimating the probability of being a first-time spawner at a give age and length. The data available are somewhat unique as we have access to not only the individual growth history, but also to the individual spawning history. The data used in the model fitting was constructed such that for each individual immature ages coded with a ‘0’ response and the age at first spawning coded as ‘1’. Hence, this binominal response could be predicted from the simultaneous effects from age and back-calculated length at age, in addition to eventual external covariates and/or time. Furthermore, the models fit also included a random effect of individual so as to correct for using multiple data from the same individuals. The size-at-age maturation patterns were fit using mixed model generalized additive models, i.e. the GAMM procedure in R (mgcv-library, Wood and Augustin (2002), http://cran.r-project.org/). The reason for choosing the GAM approach was motivated by not forcing the reaction norm into any a priori response pattern. The core model fit was:
where s1 and s2 are smoothing effect functions of age and length, respectively. βt represents time-specific coefficients and b1 is the normally distributed random individual effect coefficient. The GAM estimates for this model were expressed as:
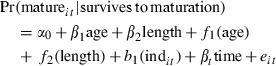 |
where f1 and f2 are linear-adjusted nonparametric estimates of the s1 and s2 effects.
This GAM approach enabled selection of the most parsimonious nonlinear model by finding the model explaining most variation in maturation probability that involved the lowest degrees of freedom of the smoothing functions. The model selection was performed using the generalized cross validation (GCV) criterion as proposed in Wahba (1990). In order to avoid complex response surfaces, we constrained each smoothing effect to a maximum of five degrees of freedom. The ‘estimated degrees of freedom’ (edf) that most optimally fit the data was selected from a cross validation routine implemented in the mgcv-package. The time effect was modelled as a decennium effect. The maturity model fitted in this study is somewhat related to the probabilistic maturity reaction norm concept introduced by Heino et al. (2002). However, in our approach we have not taken into account possible effects of size-biased mortality due to spawning. In order to do so we would need age-size data on immature individuals, data that are not available. Provided that the spawning-induced length-specific mortality pattern has not changed over the study period this should have little effect on the trends of the maturation pattern.
In order to analyse potential temporal trends in the growth pattern, we fit linear models to back-calculated length-at-lake-age data where the residual variance was corrected for temporal autocorrelation. The most optimal model (i.e. the most appropriate autocorrelation structure) was selected by using the corrected Akaike’s Information Criterion (AICc). When models have almost identical AICc-values (i.e. AIC-difference of <2) we perform model averaging so that all such models that have common parameters contribute to these parameter estimates according to their respective AIC weight (Burnham and Anderson 1998). The models were fit as a mixed effect linear model, using the lme-procedure in R version 2.5.1 (nlme library), allowing for correction for temporal autocorrelation as well as inclusion of random effects of individuals.
Survival, fishing mortality and fish ladder selectivity analyses
In order to assess estimates on size-specific natural and fishing mortality along with size-specific fish-ladder usage probability we performed capture–mark–recapture (CMR) analysis based on 5346 tagged wild fish covering the 1966–2003 period. The fish tagged comprised of 61% females and 39% males with an overall average size of 659 ± 115 (SD) mm, and lake-age distribution 5% (lake age = 1), 22% (2), 40% (3), 21% (4), 10% (5), and the remaining 2% for older age groups. Because the fish are encountered both alive (in the trap) and dead (caught by fishermen), we fit the combined mark–recapture model of Burnham (1993) as implemented in Program MARK (White and Burnham 1999) (see Fig. 3). This model structure is relevant since the exact time of death within a year is not always known. The model comprises four parameters that can be constrained depending on the hypothesis under consideration. The four parameters are survival (S), live encounter probability (p), dead encounter-and-reporting probability (r), and study-site fidelity (F). Because tagging and releasing only takes place in the river, we treat the lake and river as one study system (one stratum). Only five individuals were recaptured outside the lake and river proper over the 40 years since the tagging experiment was initiated, and so we considered the system as closed. Hence, F should be very close to 1. The capture occasions were defined as the peak period of brown trout trap captures (mid August, see histograms in lower part of Fig. 3 that display the pooled monthly frequencies of brown trout fish trap captures). Hence, meaning that 4–5 months of trap captures is collapsed into one capture occasion over which we assume no mortality (see Haugen et al. 2007; Juillard et al. 2001). The upper panel displays the fate diagram of brown trout tagged in either an even or odd year (c) at occasion k and the subsequent survival-, capture- and dispersal processes occurring over two capture occasions. Following the Burnham (1993) parameterization as implemented in program MARK (version 4.2, http://www.phidot.org/software/mark/index.html, White and Burnham 1999), Sk,c is the survival probability over the k to k + 1 period for individuals that have been tagged in a ‘c’ year (‘c’ is either ‘even year’ or ‘odd year’) and pk,c is the probability of being caught in the fish trap in the Hunderfossen fish ladder at occasion k. Because the fish trap catches all fish ascending the ladder, p can be interpreted as the probability of using the fish ladder. Fk,c is the probability, at a given capture occasion k, of remaining in the sampling area to the next capture occasion k + 1. The dead recovery encounter probability, rk,c, is the probability of being found dead and reported between occasion k and k + 1. Due to the biannual spawning cycle of brown trout from Hunderfossen, the interpretation of r is very different for years in which the individuals spawn and those in which they are not. In nonspawning years, r is a proxy for fishing mortality because dead encounters in the lake will almost exclusively relate to fisheries. However, r is just a proxy for fishing rate because the report rate is included in the parameter estimate. In spawning years, r constitutes the probability of an individual being found dead and reported between two capture occasions. In Fig. 3, encounter histories for the possible fates are provided in brackets to the right of each encounter trajectory; ‘0’ = not encountered, ‘1’ encountered, ‘−1’ encountered (see top-left for an example), but right censored (mostly due to death during stay in the fish trap). The encounter histories consist of two digits per year where the first digit keeps track with live encounters (i.e. recaptures in the fish ladder) and the second digit keeps track with dead encounters. Hence, the encounter histories comprise arrays of 0s and 1s at LD positions (see top left of Fig. 3 for a key) and these binominal histories form the basis for the multi-nominal parameter estimations under various model constraints. Owing to the biannual spawning life cycle of the trout under study (known based on preliminary analysis of the CMR data) all models have been fit with a tagging cohort structure that resembles fish tagged at even and odd years. As a consequence, time effects will not include just 37 survival intervals, but 74 intervals as even and odd year cohorts will have their own time estimates. In this study, we pay close attention to size-dependent survival and probability of using the fish ladder during the first spawning run.
Figure 3.
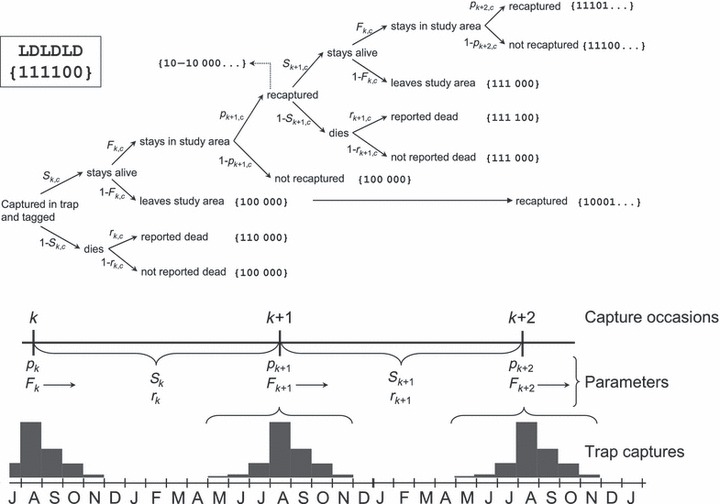
The definition of capture occasions (k), Burnham parameters and encounter histories. Lower panel: Capture probabilities (p) and site fidelities (F) were estimated for each capture occasion separated by an encounter period over which the survival probability (S) and dead recovery probability (r) could be estimated. The capture occasions were defined as the peak period of brown trout trap captures (mid August, see histograms). The upper panel displays the fate diagram and capture histories of brown trout tagged in either an even or odd year (c) at occasion k and the subsequent survival, capture and dispersal processes occurring over two capture occasions. For further details, see the main text.
We tested the overall fit of the model by using the bootstrap method implemented in MARK (300 bootstrap simulations), and evaluated the degree of over dispersion (ĉ =model χ2/model df). Following Lebreton et al. (1992), we adopted a model fitting strategy where capture probability was modelled under full temporal variation of survival and dead encounter probability (an estimate of fishing mortality), but keeping the site fidelity constant for lake periods and river periods. After finding the most supported capture probability model, we continued by fitting dead encounter probability models under the most supported capture probability model and with temporal variation in survival. Similarly, the most supported survival probability models were found under the most supported capture and dead encounter probability model structures, giving estimates of natural mortality. In the end, to test for model robustness, we once more fit capture probability models under the most supported survival and dispersal models (see e.g. Haugen et al. 2007). Because individual length effects on survival, fishing mortality and fish ladder use were of particular interest in this study, most models fit included length effects. In particular, we explored the support of stabilizing or disruptive effects (i.e. length2 coefficient≠0), and directional effects (length coefficient≠0) of size on the parameters S, r and p. We also explored if environmental variables modified these length effects by including length*environmental variable effects. All models were fit using the maximum likelihood procedure, as implemented in Program MARK version 4.2.
Estimating rates of phenotypic change
Rates of phenotypic change were estimated as both haldanes (h) and darwins (d) according to Hendry and Kinnison (1999):
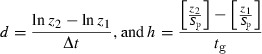 |
where z1 and z2 are mean trait values at time 1 and 2, Δt is the time period (in millions of years), Sp is the pooled standard deviation, tg is the number of generations. We assume a mean generation time of 6 years; mean smolt age of 4 years and mean lake age at first spawning equal to 3 years (Aass et al. 1989). Using linear regression it is possible to estimate the rates of phenotypic change. This was performed by using either lnzyear or z/(pooled SD) as y-values regressed on time (in millions of years) since first capture, and number of generations since first capture respectively. The pooled SD was calculated across years from individual ln-transformed data. The slopes of these two regressions produce darwin- and haldane estimates, respectively (Hendry and Kinnison 1999). Furthermore, this method provides confidence intervals directly, making it possible to test whether rate estimates differ significantly from zero (autocorrelation was corrected for as the autocorrelation structure was impemented in the lme-model, see description under growth modelling).
Results
Growth
The lme growth analyses revealed that there has been a significant linear decrease (t4188 = −11.03, P < 0.0001) in length of downstream migrating smolt over time (Supplementary Fig. S1), with smolt size decreasing by an average (±SE) of 0.52 ± 0.05 mm year−1. Also size-at-age during the first 3 years in the lake (lake age 1–3) has decreased significantly with time (annual decrease: −1.61 ± 0.15, −1.97 ± 0.20, −1.86 ± 0.27 mm; statistics: t5336,5168,4188 = −10.45, −9.74, 7.00, P < 0.0001, respectively) (Fig. 4). The autocorrelation-corrected estimated rates for change in size-at-age were high, consistent and significant (P < 0.0001 for all) for all lake-age classes (range across years: |darwins|: 2771–2946; |haldanes|: 0.115–0.132).
Figure 4.
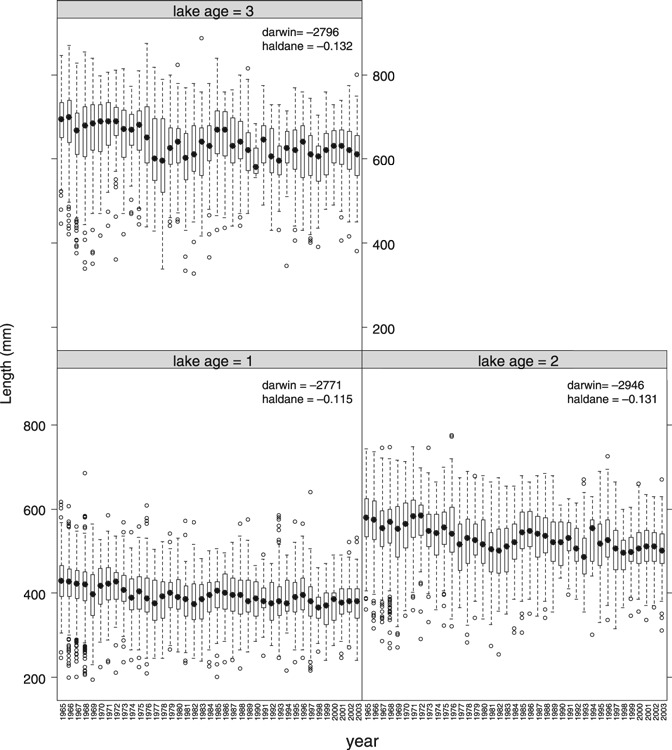
Box plots of length (mm) at lake-ages 1–3. The corresponding linear rates of phenotypic change (darwin and haldane) are given for each trait (all P < 0.0001). The boxes cover 50% of the observations, the whiskers 90% of the observations and the large black dot symbol mark the median.
Life-history transitions
The trout spawn for the first time after living in Lake Mjøsa in 1–7 years. Age at first maturation can be determined for all captured fish based on the analysis of the morphological scale structures, and their size at first maturation is then back-calculated from scale measurements. Mean size of spawning fish has varied over the time period (Fig. S2). The mean size at first spawning has decreased significantly for fish of different lake-age groups (Fig. 5, annual decrease in length = −0.73 ± 0.29, −1.44 ± 0.16, −1.59 ± 0.13, −1.78 ± 0.25 mm; statistics: t237,1239,2104,623 = −2.55, −9.17, −12.24, −7.29; for lake-age at maturity 1–4, respectively; P = 0.011 for lake-age = 1, P < 0.0001 for the remaining ages). The autocorrelation-corrected estimated rates of phenotypic change were high, consistent and significant (except for length at lake-age at maturity = 1, P = 0.13) among lake-age classes (|darwins|: 1667–2541; |haldanes|: 0.076–0.125). There was also a highly significant trend that the annual variability in length at spawning (estimated as the coefficient of variation and as standard deviation) has decreased with time (CVspawning length = −0.17 ± 0.04% per year, t36 = −3.71, P = 0.0007; stdspawning length = −1.16 ± 0.04 mm year−1, t36 = −5.38, P < 0.0001, Fig. 6).
Figure 5.
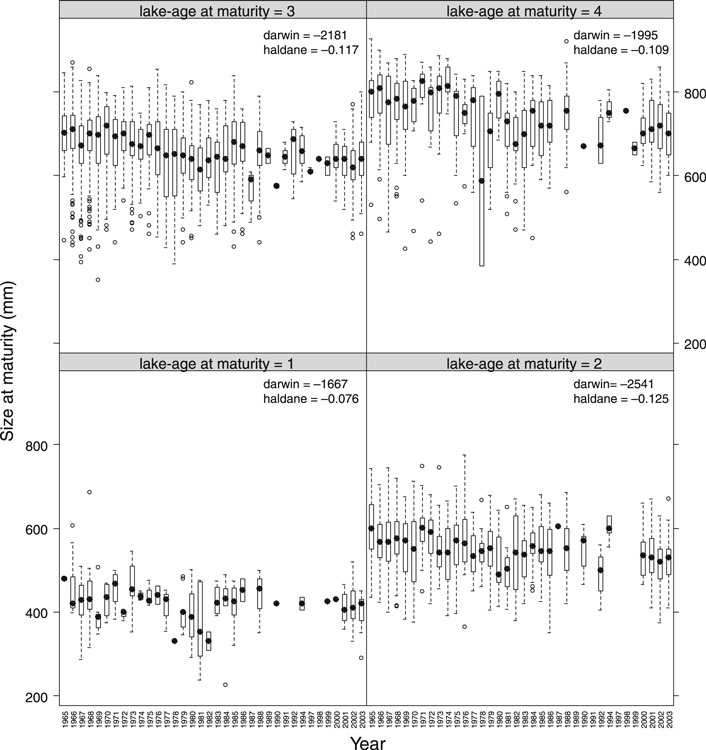
Box plots of length at maturation (mm) for trout maturing at various lake-ages (1–4). The corresponding linear rates of phenotypic change (darwin and haldane) are given for each trait (all P < 0.0001). The boxes cover 50% of the observations, the whiskers 90% of the observations and the large black dot symbol mark the median.
Figure 6.
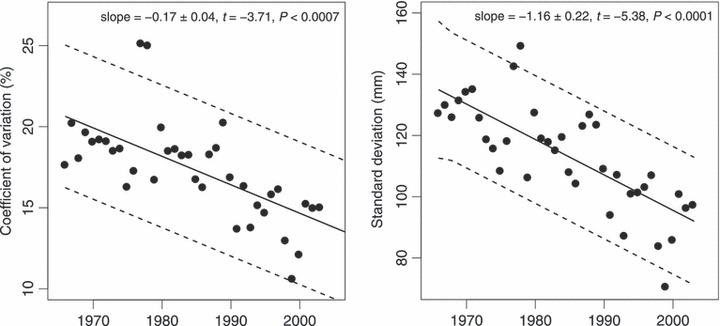
Temporal trend in the coefficient of variation (%) and standard deviation (mm) for mean annual length of mature fish caught in the Hunderfossen fish trap over the 1965–2003 period. The dotted lines correspond to autocorrelation-adjusted 95% confidence bounds, retrieved from the most AIC-supported autoregressive linear trend models (CVlength : PAR(1) = 0.032, stdlength : PAR(1)=0.054).
A GAMM analysis revealed that there has been a significant change in the length-at-spawning age pattern over the four decades covered by the analysis, where this reaction norm both has shifted down-left along the age- and size-at-maturation axes and the overall maturation space (distance between probability isoclines) has been dramatically reduced (Fig. 7). The cross validation procedure favoured simple linear effects of both age and length (and interaction effect) on the maturation probability (Table S1). Our analysis therefore implies that variation in length at maturation for a given age is expected to become smaller with time. The estimated 50% probability length at lake-age 3 changed from 621 mm in the 1960s to 572 mm, 543 mm and 520 mm over the following decades (Fig. 7, lower panel). This yielded haldane estimates ranging from −0.95 (1960s–1970s) to −0.21 (1980s–1990s) and corresponding darwin estimates between −11511 and −2885.
Figure 7.
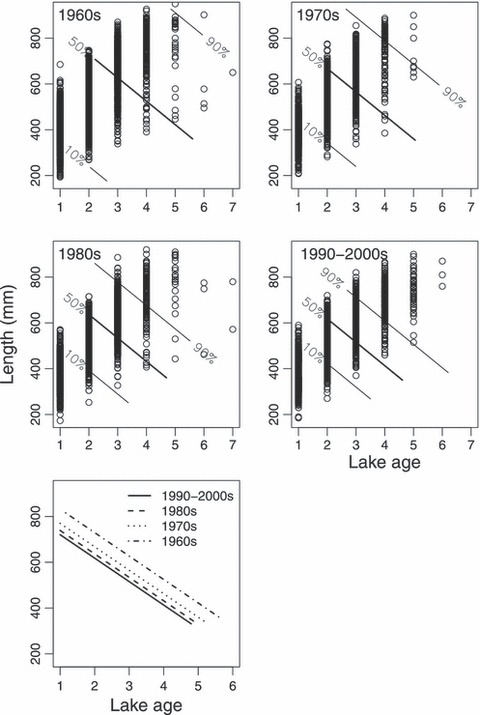
Decennium-wise maturation reaction norms. The probabilities are conditional on that the fish will survive to spawning. The lengths constitute back-calculated length (mm) at the end of winter for the respective ages (e.g. the length at end of winter prior to the year of first spawning). The model predictions presented are adjusted to the overall annual mean June–September 0–10 m water temperatures (11.8°C) in the lake. The model explains 59% of the deviance. The lower panel shows the 50% probability lines from all decades.
CMR-analysis
Model selection (Table S2) revealed that there is little support for between-year variation in fish ladder usage probability; whereas there is much support for time variation in both natural survival probability and fisheries catch probability (Table S1). There is much support for models including tagging-age effects over the two first years following tagging, and size effects were only evident over the first period following tagging. The Burnham model was found to adequately fit the data (bootstrapped P-value < 0.13; i.e. the observed model deviance for the model with full temporal variation corresponded to the 261st value of the 300 ranked simulated model deviances). Under the model constraints used, there was a slight over dispersion of ĉ = 1.93 (=1.54 using median ĉ approach). There was no evidence for a sex effect in any of the models.
The most supported Burnham model fit the CMR data is used for drawing further inferences (Fig. 8, corresponding to model 1 in Supplementary Table S2). This model revealed that the fish ladder favoured fish migration upstream at a medium size (60–70 cm, max probability = 67.9 cm, Fig. 8A), with both smaller and larger fish having a reduced probability of using the fish ladder. There was also a significant interaction effect of water discharge on fish size, indicating that the hydrological conditions in the fish ladder decides the ease with which variously sized fish can enter and migrate through the fish ladder. The mean June to October water temperature in the river had a highly significant positive effect on the probability of using the fish ladder (logit parameter coefficient ± SE: 0.35 ± 0.06). This model also estimated that postspawning natural survival increased with size, but decreased for sizes larger than 75 cm (Fig. 8B). Mature fish smaller than c. 60 cm seem to have a strongly reduced survival probability during the first winter and summer after spawning. The 0–10 m lake temperature had a positive additive effect on this postspawning survival rate, whereas the total phosphorus concentration had a negative additive effect on the same rate (Table S1). Further, during the first summer after spawning there was a weak tendency that larger fish were less exposed to being fished (Fig. 8C).
Figure 8.
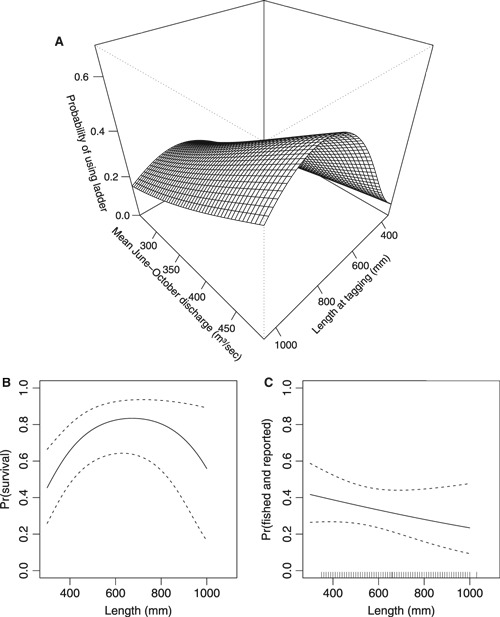
Estimated size-specific probability for (A) using the fish ladder (also includes the effect of water discharge and interaction effect of this variable with individual length), (B) surviving the first year in the lake following tagging, and (C) being caught in gillnets or by anglers in the lake. These predictions are based on the most supported Burnham model (model 1) in Supplementary Table S2. The inner tick marks in C indicate individual length observations (of which many are superimposed on each other). The A probabilities are adjusted for mean river water temperature of 11.2°C, and B probabilities are adjusted to a mean lake water temperature of 11.8°C and a mean total phosphorus level of 7.4 μg L−1.
The model for survival probability for brown trout more than 1 year after spawning (remember that most of the brown trout spawn every second year) suggests a decrease in lake survival probability with time (Fig. 9), though with a possible increased survival in the 2000s (low precision due to few observations). This was evident for both even- and odd-year spawners. Because the fishing rate probability (lake dead encounter and reporting rate, r), has decreased over the same period it is evident that natural survival must have decreased over the same period. The effect of variation in reporting rate is contained within our estimates of r.
Figure 9.
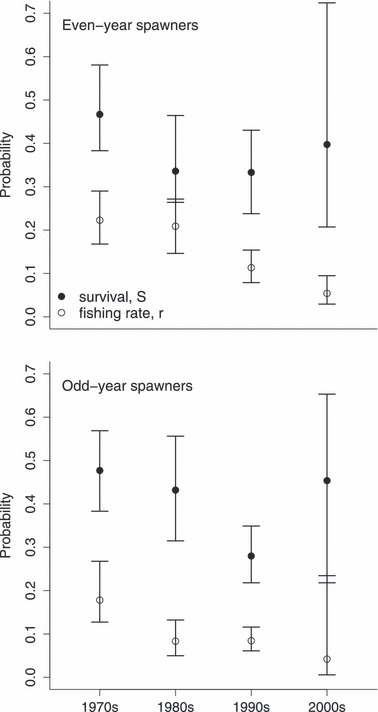
Annual survival and fishing rate as estimated for tagging ages >1 over four decenniums. The fishing rates represent dead encounter rates during nonspawning years. Even and odd year spawners have been given separate parameterization as they inhabit the lake (i.e. do not spawn) in different years. Note that the 2000 decennium estimates cover two survival periods only (data series used terminates in 2003). All estimates are adjusted to a mean lake water temperature of 11.8°C and a mean total phosphorus level of 7.4 μg L−1.
Discussion
We have documented a significant phenotypic decrease in size-at-smolt transformation (downstream migration), size-at-first spawning and a change in the maturation reaction norms in a brown trout population following a change in size-selectivity of an obstacle to migration. The fish ladder that was built to facilitate upstream migration of all mature fish clearly imposed a stabilizing selection regime on the fish, with both small and large mature fish finding it difficult to ascend the ladder. This clearly is a hydrological phenomenon, as the shape of the selection curve varied with overall water flow. Also, growth rate of the immature fish living in Lake Mjøsa decreased with time. The observed rates of phenotypic change are in the same order of magnitude as many such rates recently reported (Hendry and Kinnison 1999; Kinnison and Hendry 2001; Hendry et al. 2008). Both the mean haldane (0.115) and darwin (2413) estimates from this study were between the median and the 75% percentile as estimated for the supplementary tables given by Hendry et al. 2008 (medians – darwin: 1232, haldane: 0.0063). However, this study is, to our knowledge, the first contemporary study to document decreased trait variance. This decrease in trait variance is consistent with predictions following from a stabilizing selection regime, such as the one resulting from the way the Hunderfossen fish ladder operates. Other factors may also interact to produce trends such as the ones observed in this study, we will here argue for why we maintain that the observed phenotypic changes are due to the construction of the fish ladder and that they are mainly evolutionary.
It has long been discussed how fast animals can evolve in nature, the conventional wisdom being that significant evolutionary change will only rarely be observed at ecological time scales. A number of studies reporting rapid evolution in harvested fish populations have appeared during the last few decades (Haugen and Vøllestad 2001; Olsen et al. 2004; Conover et al. 2005; Walsh et al. 2006; Edeline et al. 2007; Quinn et al. 2007; Swain et al. 2007; see also Fukuwaka and Morita 2008 this volume) and have changed that perception: a consensus that evolution at times may be very rapid is now emerging implying that evolutionary changes may commonly be observed over short time periods (within few generations) (Carroll et al. 2007; Hendry et al. 2007). Trophy hunting in mammals has also been shown to induce rapid changes in sexually selected characters that are also selected by trophy hunters, and the associated genetic and population dynamic changes may be profound (Coltman et al. 2003; Hard et al. 2006; Loehr et al. 2007). Harvesting – an often strongly size-selective process (Carlson et al. 2007) – may evidently induce strong evolutionary responses on ecological time scales. Also changes in environmental conditions have been shown to induce evolutionary changes at very short times scales, exemplified with the changes in beak size of Darwin finches Geospiza sp. following events of rain or drought or human disturbance (Grant and Grant 2002; Hendry et al. 2006). Here we have shown evolution towards a smaller mature body size and a slower growth rate following a change in the size-selectivity of a partial barrier to migration in brown trout. The new partial barrier (the fish ladder) has acted as a phenotype sorting mechanism, and a new sorting function has induced clear changes in the population. This sorting has selected against both small and large size at migration, whereas before the fish ladder was in place there was a strong directional selection for being large. The changed fitness landscape has clearly led to a phenotypic movement of the population toward this new peak in the adaptive landscape.
The estimated rates of change in size at first maturation and in growth rate are rather high, and the haldane values estimated for maturation reaction norm mid points (|haldane|: 0.21–0.95) are amongst the highest ever reported and comparable to the high rates reported for Newfoundland Atlantic cod (Olsen et al. 2004). When adjusting for the time span over which the rates of phenotypic change were estimated the estimates are pretty much in line with what should be expected (Hendry et al. 2008). The estimated rates of phenotypic change (|haldane|: 0.076–0.125, |darwin|: 1667–2541) were observed over approximately 40 years (6–7 generations), and the maturation reaction norm midpoint rates covered periods down to 1.2 generations. The relatively rapid change may either be induced by strong selection or by phenotypic plasticity where the expressed phenotype is changed following a consistent environmental change. Potential environmental factors that might have induced plastic responses are temporal changes in growth opportunity due to changes in density of prey, density of competitors, or in temperature. The maturation reaction norm approach do take much of this into account (do also note that water temperature was adjusted for), but correction for all environmental variation is probably not possible. Selective forces inducing evolution may be consistent changes in survival probabilities, or selection against migrants of particular sizes.
There have been some changes to the lake and river environment throughout the study period. Among other factors, density of brown trout in Mjøsa has increased over the last 10–15 years [catch per unit of effort (CPUE) in the sport fishery used as a proxy for density; see Fig. 2]. In our analysis, we have used CPUE as a covariate in models for estimating survival rates. Neither of the models containing CPUE as a covariate received much statistical support, but in general all models containing CPUE as covariate indicated a negative effect (density dependence). Unfortunately, there is no good data on the availability of prey fish in the lake that could have been used as a covariate in the models. Data from hydro acoustic surveys show large variability among years, but no evident overall trend. Earlier data on vendace abundance (one important prey species) indicates 3–4 year cycles with alternating strong and weak year classes (Sandlund et al. 1991). The decreasing productivity trend found in Mjøsa (total phosphorus has decreased by 0.16 ± 0.02 μg L−1 year−1 (r = −0.82, P < 0.0001) over the 1966–2005 period, Fig. 2) could suggest that the production of important prey fish species also has decreased. However, the CMR-analysis indicated that the reduced productivity has had a positive effect on survival (Table S1). If the abundance of prey has been reduced by the reduced eutrophication this lowered density of prey might have been compensated by the increased visual range following from the decreased turbidity of the water. The average summer temperature in the lake has increased by 0.025 ± 0.007°C year−1 during the 1966–2003 period. Provided that there has been sufficient amount of prey during this period, the increase in temperature should result in an increased growth rate in the lake over this time period. Growth models predict maximum growth rates at temperatures around 12–14°C (Elliott et al. 1995), indicating an increased growth rate with time for trout in Mjøsa. Indeed, if there was a tight food limitation in Mjøsa one should have expected survival to decrease with temperature. However, the CMR analysis showed exactly the opposite to be the case (Table S1). Based on this evidence, we find it improbable that the observed phenotypic changes, and growth in particular, are due solely to plastic responses, and thus we suggest that the observed changes are mainly evolutionary in nature. Unfortunately, there are very few estimates of the heritability of growth for brown trout, and the one good estimate that is available (h2 = 0.28) is from aquaculture (reviewed by Carlson and Seamons 2008). There are, however, some estimates for salmonids from nature (mainly from Oncorhynchus spp.) indicting relatively high heritabilities (mean h2 = 0.46). If these estimates are somewhat close to the truth a rapid response to selection is to be expected. However, given that heritability estimates are extremely context dependent (Roff 1997), this ought to be investigated in detail in future studies.
There have been a number of changes in the selective environment of the brown trout population. The access to the spawning area is clearly size dependent, with the fish ladder selecting against both small and large fish. Our analysis of the CMR data clearly shows a stabilizing selection on length, with fish in the size interval 60–80 cm having the highest probability of using the ladder. Thus there will be selection against being too small (if small it pays to grow fast) and being very big (if already big it costs to grow much more). The reaction norm for age and size at maturity has changed in a manner consistent with this, as both the mean (same slope but difference intercept) and variance has decreased significantly with time (i.e. precision has increased). This trend should generate a reduced variance in length at maturity (or length at spawning) – this is indeed what we observe also when looking at the observed variation for length at spawning over time (Fig. 6). Kingsolver et al. (2001), when reviewing the strength of phenotypic selection in natural populations, noted that strong stabilizing selection was not very common. They also noted that large sample sizes were necessary to detect it. However, long-term mark–recapture data on pike (a top predator) do indeed show that natural selection favour the intermediate sized fish (Carlson et al. 2007), and also that imposed harvest selection on top of the natural selection leads to rapid shifts in growth rate in the population (Edeline et al. 2007). What seems important is that an induced (but not anticipated) strong stabilizing selection leads to significant reductions in phenotypic variation over relatively short time scales. This stresses the important of also quantifying the quadratic (non linear) selection differentials in studies of selection – and also for testing the effects of such selection in human altered systems.
A change in survival probability with time may influence age at maturity, and change in size-selective survival may be even more important (Stearns 1992; Roff 2002). We have in this study found that the total mortality rate was clearly size dependent with larger fish having higher survival probabilities, but fish larger than 80 cm experiencing higher mortalities. However, there was no indication that the shape of this selective landscape has changed with time (the survival versus size function has been stable over time). The impact of this selection may have increased over time, however, since overall natural mortality has increased with time. This increase in the natural mortality have, however, been counteracted by a reduced rate of fishing. Thus, in total, there is only a small change in the mortality rate or the overall selectivity due to mortality after spawning. The main change in the selective environment experienced by the trout spawning above the water fall is thus the introduction of a highly size-selective fish ladder leading to strong stabilizing selection for sizes around 60–80 cm. Based on these arguments, we conclude that the changes induced in the case with which differently sized fish may migrate upstream past a partial migration barrier have led to extensive changes in the demographic structure of the population and potentially in the population dynamics.
Conclusions
Our study documents how environmental changes (such as introduction of a fish ladder) may have evolutionary effects on vital life history traits such as age and size of maturation and growth rate. As such our study adds to the now growing literature documenting significant phenotypic and probably evolutionary changes caused by external environmental changes. Understanding such evolutionary impacts are important for managing our natural resources in a sustainable way.
Acknowledgments
The Directorate for nature management, Oppland and Hedmark County Environmental Administrations, University of Oslo, and the Norwegian Research Council have provided the funds needed for obtaining and analysing the data. Personnel at the Hunderfossen Hatchery helped with tagging and recapture of trout. Tore Qvenild and Ola Hegge have helped with data management, and Atle Rustadbakken and Espen Lund has curated the dataset and assisted with the analyses of the scale samples. Rustadbakken also helped with map construction together with Mette-Gun Nordheim. Jarl Eyvind Løvik, Finn Gregersen and Line Johanne Barkved have provided environmental data from Mjøsa and Gudbrandsdalslågen. Finally, thanks to Stephanie Carlson, Andrew Hendry and two anonymous referees for a number of good suggestions that significantly improved the quality of the paper.
Supporting Information
Literature cited
- Aass P, Kraabøl M. The exploitation of a migrating brown trout (Salmo trutta L.) population; change of fishing methods due to river regulation. Regulated Rivers: Research & Management. 1999;15:211–219. [Google Scholar]
- Aass P, Nielsen PS, Brabrand Å. Effects of river regulation on the structure of a fast-growing brown trout (Salmo trutta) population. Regulated Rivers: Research & Management. 1989;3:256–266. [Google Scholar]
- Arnekleiv JV, Kraabøl M. Migratory behaviour of adult fast-growing brown trout (Salmo trutta, L) in relation to water flow in a regulated Norwegian river. Regulated Rivers: Research & Management. 1996;12:39–49. [Google Scholar]
- Arnekleiv JV, Kraabøl M, Museth J. Efforts to aid downstream migrating brown trout (Salmo trutta L.) kelts and smolts passing a hydroelectric dam and a spillway. Hydrobiologia. 2007;582:5–15. doi: 10.1007/s10750-10006-10547-10758. [Google Scholar]
- Bagenal TB, Tesch FW. Age and growth. In: Bagenal T, editor. Methods for Assessment of Fish Production in Fresh Waters. Oxford: Blackwell Scientific Publications; 1978. pp. 101–136. International Biological Programme Handbook no 3. [Google Scholar]
- Brandt MM, Holloway JP, Myrick CA, Kondratieff MC. Effects of waterfall dimensions and light intensity on age-0 brook trout jumping performance. Transactions of the American Fisheries Society. 2005;134:496–502. doi: 10.1577/T03-175.1. [Google Scholar]
- Burnham KP. A theory for combined analysis of ring recovery and recapture data. In: Lebreton J-D, North PM, editors. Marked Individuals in the Study of Bird Populations. Basen: Birhäuser Verlag; 1993. pp. 199–213. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Inferences. New York: Springer-Verlag; 1998. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IA, Fletcher JM, James JB, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Clobert J, Danchin E, Dhondt AA, Nichols JD. Dispersal. Oxford: Oxford University Press; 2001. [Google Scholar]
- Coltman DW, O’Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. Undesirable evolutionary consequences of trophy hunting. Nature (London) 2003;426:655–658. doi: 10.1038/nature02177. [DOI] [PubMed] [Google Scholar]
- Conover DO, Arnott SA, Walsh MR, Munch SB. Darwinian fishery science: lessons from the Atlantic silverside (Menidia menidia. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:730–737. [Google Scholar]
- Dietrich JP, Cunjak RA. Evaluation of the impacts of Carlin tags, fin clips, and Panjet tattoos on juvenile Atlantic salmon. North American Journal of Fisheries Management. 2006;26:163–169. [Google Scholar]
- Dingle H. Ecology and evolution of migration. In: Gauthreaux SA, editor. Animal Migration, Orientation, and Navigation. New York: Academic Press; 1980. pp. 1–101. [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JM. Quantitative Ecology and the Brown Trout. Oxford Series in Ecology and Evolution. Oxford: Oxford University Press; 1994. [Google Scholar]
- Elliott JM, Hurley MA, Fryer RJ. A new, improved growth model for brown trout, Salmo trutta. Functional Ecology. 1995;9:290–298. [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Reviews in Fish Biology and Fisheries. 1996;6:379–416. [Google Scholar]
- Fukuwaka M, Morita K. Increase in maturation size after the closure of a high seas gillnet fishery on hatchery-reared chum salmon Oncorhynchus keta. Evolutionary Applications. 2008;1:376–387. doi: 10.1111/j.1752-4571.2008.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PB, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Hard JJ, Mills LS, Peek JM. Genetic implications of reduced survival of male red deer Cervus elaphus under harvest. Wildlife Biology. 2006;12:427–441. [Google Scholar]
- Haugen TO, Vøllestad LA. A century of life-history evolution in grayling. Genetica. 2001;112/113:475–491. [PubMed] [Google Scholar]
- Haugen TO, Winfield IJ, Vøllestad LA, Fletcher JM, James JB, Stenseth NC. Density dependence and density independence in the demography and dispersal of pike over four decades. Ecological Monographs. 2007;77:483–502. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Bohlin T, Jonsson B, Berg OK. To sea or not to sea? Anadromy versus non-anadromy in salmonids. In: Hendry AP, Stearns SC, editors. Evolution Illuminated. Salmon and Their Relatives. Oxford: Oxford University Press; 2004a. pp. 92–125. [Google Scholar]
- Hendry AP, Castric V, Kinnison MT, Quinn TP. The evolution of philopatry and dispersal: Homing versus straying in salmonids. In: Hendry AP, Stearns SC, editors. Evolution Illuminated. Salmon and Their Relatives. Oxford: Oxford University Press; 2004b. pp. 52–91. [Google Scholar]
- Hendry AP, Grant PB, Brant BR, Ford HA, Brewer MJ, Podos J. Possible human impacts of adaptive radiation: beak size bimodality in Darwin’s finches. Proceedings of the Royal Society of London, Series B. 2006;273:1887–1894. doi: 10.1098/rspb.2006.3534. doi: 10.1098/rspb.2006.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Nosil P, Reisenberg LH. The speed of ecological speciation. Functional Ecology. 2007;21:455–464. doi: 10.1111/j.1365-2435.2006.01240.x. doi: 10.1111/j.1365-2435.2007.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- ICES. Atlantic Salmon Scale Reading. Aberdeen: International Council for the Exploration of the Sea; 1984. [Google Scholar]
- Jensen AJ, Aass P. Migration of a fast-growing population of brown trout (Salmo trutta L.) through a fish ladder in relation to water flow and water temperature. Regulated Rivers: Research & Management. 1995;10:217–228. [Google Scholar]
- Julliard R, Stenseth NC, Gjøsaeter J, Lekve K, Fromentin JM, Danielssen DS. Natural mortality and fishing mortality in a coastal cod population: A release-recapture experiment. Ecological Applications. 2001;11(2):540–558. [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, et al. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hendry AP. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica. 2001;112–113:145–164. [PubMed] [Google Scholar]
- L’Abée-Lund JH, Vøllestad LA, Beldring S. Spatial and temporal variation in the grilse proportion of Atlantic salmon in Norwegian rivers. Transactions of the American Fisheries Society. 2004;113:743–761. [Google Scholar]
- Laird LM, Stott B. Marking and tagging. In: Bagenal T, editor. Methods for Assessment of Fish Production in Fresh Waters. Oxford: Blackwell Scientific Publications; 1978. pp. 84–100. International Biological Programme Handbook no. 3. [Google Scholar]
- Lebreton J-D, Burnham KP, Clobert J, Anderson DR. Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs. 1992;62:67–118. [Google Scholar]
- Loehr J, Carey J, Hoefs M, Suhonen J, Ylönen H. Horn growth rate and longevity: implications for natural and artificial selection in thinhorne sheep (Ovis dalli. Journal of Evolutionary Biology. 2007;20:818–828. doi: 10.1111/j.1420-9101.2006.01272.x. doi: 10.1111/j.1420-9101.2006.01272.x. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature (London) 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle, WA: University of Washington Press; 2005. [Google Scholar]
- Quinn TP, Hodgson S, Flynn L, Hilborn R, Rogers DE. Directional selection by fisheries and the timing of sockeye salmon (Oncorhynchus nerka) migrations. Ecological Applications. 2007;17:731–739. doi: 10.1890/06-0771. [DOI] [PubMed] [Google Scholar]
- Roff DA. Evolutionary Quantitative Genetics. New York: Chapman & Hall; 1997. [Google Scholar]
- Roff DA. Life History Evolution. Sunderland, MA: Sinauer Associates, Inc; 2002. [Google Scholar]
- Sandlund OT, Næsje TF, Klyve L, Lindem T. 1985;62:136–149. The vertical distribution of fish species in Lake Mjøsa, Norway, as shown by gill net catches and echo sounder. Report of the Institute of Freshwater Research Drottningholm. [Google Scholar]
- Sandlund OT, Jonsson B, Næsje TF, Aass P. Year-class fluctuations in vendace, Coregonus albula (Linnaeus): Who’s got the upper hand in intraspecific competition? Journal of Fish Biology. 1991;38:873–885. [Google Scholar]
- Schaffer WM, Elson PF. The adaptive significance of variations in life history among local populations of Atlantic salmon in North America. Ecology. 1975;56:577–590. [Google Scholar]
- Skaala O. Genetic population-structure of Norwegian brown trout. Journal of Fish Biology. 1992;41:631–646. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Stenseth NC, Lidicker WZ. Animal Dispersal. Small Mammals as a Model. London: Chapman & Hall; 1994. [Google Scholar]
- Sutherland WJ. From Individual Behaviour to Population Ecology: Oxford Series in Ecology and Evolution. Oxford: Oxford University Press; 1996. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited population. Proceedings of the Royal Society of London, Series B. 2007;274:1010–1022. doi: 10.1098/rspb.2006.0275. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videler JJ. Fish Swimming. London: Chapman & Hall; 1993. [Google Scholar]
- Videler JJ, Wardle CS. Fish swimming stride by stride: speed limits and endurance. Reviews in Fish Biology and Fisheries. 1991;1:23–40. [Google Scholar]
- Wahba G. Spline Models for Observational Data: CBMS-NSF Regional Conference Series in Applied Mathematics/Sponsored by Conference Board of the Mathematical Sciences; Supported by National Science Foundation, v. 59. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1990. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing; impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. doi: 10.1111/j.1461-1248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46:120–139. [Google Scholar]
- Wood SN, Augustin NH. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecological Modelling. 2002;157:157–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


