Abstract
We use a state dependent life history model to predict the life history strategies of female steelhead trout (Oncorhynchus mykiss) in altered environments. As a case study of a broadly applicable approach, we applied this model to the American and Mokelumne Rivers in central California, where steelhead are listed as threatened. Both rivers have been drastically altered, with highly regulated flows and translocations that may have diluted local adaptation. Nevertheless, evolutionary optimization models could successfully predict the life history displayed by fish on the American River (all anadromous, with young smolts) and on the Mokelumne River (a mix of anadromy and residency). The similar fitness of the two strategies for the Mokelumne suggested that a mixed strategy could be favored in a variable environment. We advance the management utility of this framework by explicitly modeling growth as a function of environmental conditions and using sensitivity analyses to predict likely evolutionary endpoints under changed environments. We conclude that the greatest management concern with respect to preserving anadromy is reduced survival of emigrating smolts, although large changes in freshwater survival or growth rates are potentially also important. We also demonstrate the importance of considering asymptotic size along with maximum growth rate.
Keywords: adaptation, anadromy, life history evolution, phenotypic plasticity, state-dependent model, steelhead, water management
Introduction
Due to phenotypic plasticity and contemporary evolutionary change (Kinnison and Hairston 2007), organisms can respond to changing environments in unexpected ways, and these unexpected responses present a great challenge to resource managers (Stockwell et al. 2003). Instances of rapid evolution are particularly well documented in salmonids (e.g. Hendry et al. 2000; Quinn et al. 2000, 2001), and actions meant to facilitate salmonid management have often yielded surprising results. For example, larger smolts are more likely to survive ocean entry (Ward et al. 1989). Therefore hatchery production meant to augment anadromous runs often focuses on producing rapidly growing fry that generate the largest smolts. However, hatcheries where fish grow very rapidly may disproportionately produce mature parr rather than anadromous fish (Schmidt and House 1979), as might have been predicted given more careful consideration of life history theory (Thorpe et al. 1998). As a result, the importance of evolutionary considerations in salmonid management is increasingly recognized (e.g. Williams et al. 2008).
Managers often face the challenge of environments already altered by previous actions and subjected to ongoing actions that may substantially change the selective regime. Such systems might have already experienced evolutionary change in response to the alteration in the environment, but may be far from evolutionary equilibrium due to lagged responses or ongoing environmental change. For example, steelhead/coastal rainbow trout (Oncorhynchus mykiss irideus) in the California Central Valley face a radically altered environment (McEwan 2001). Dams block access to historic spawning habitats, and highly regulated flows modify downstream habitats, changing water temperature and food availability and potentially impacting growth rates. In addition, compared to historic conditions on the American River, contemporary flows are less variable; with peak flows that are both lower overall and occur later in the year (Williams 2001). Variations in flow appear to have direct effects on food availability (Merz 2002) and growth in steelhead (Harvey et al. 2006), and have been directly linked to recruitment in brown trout (Lobón-Cerviá 2009). Variation in water releases can also affect water temperatures (U.S. Department of the Interior 2008), which can affect feeding activity (Merz and Vanicek 1996) and growth rates (Castleberry et al. 1991, 1993; Myrick and Cech 2000). Passage of anadromous fish to the ocean may be riskier now due to mortality associated with pumping in the Delta for water withdrawals (Baker and Morhardt 2001; Brandes and McLain 2001). Finally, due to repeated near extirpations, there have been extensive stocking efforts with multiple non-native genotypes (Williams 2006). Because steelhead are facultatively anadromous, and the anadromous fish may emigrate to the ocean at a wide range of ages, managers in these systems are particularly concerned with the potential impacts of management actions on life history variation. Although few baseline data are available, it is widely believed that life histories in Central Valley steelhead have already diverged substantially from their historic states and now include a greater proportion of fish with a resident life history (maturity in freshwater with no time spent in the ocean at any point) (Lindley et al. 2007; McClure et al. 2008). Given the potential for substantial ongoing change (e.g. VanRheenen et al. 2004), models that can predict evolutionary endpoints for different environments are of great utility.
Reservoirs behind dams on most rivers of the Central Valley in California provide limited cold water pools available for discharge to downstream rearing areas. Under current policy (U.S. Department of the Interior 2008) it is thought to be important to release some cold water for juvenile steelhead in the summer and early fall, whereas cool water in the late fall is important for adult Chinook salmon (Oncorhynchus tshawytscha) holding and spawning. There is, thus, a balancing act required with some incentive to minimize the amount of cold water released in summer and early fall so that more cool water is available for Chinook. Studies of geographic trends in residency versus anadromy have suggested that residency is more common when there are dependable flows and cool water in summer (Cramer and Beamesderfer 2006), suggesting that releasing too much cool water in summer and early fall may reduce the occurrence of anadromy in steelhead (U.S. Department of the Interior 2008).
Resolving such management issues requires a framework for predicting the evolutionary consequences of management actions. In this paper, we present a life history modeling framework that can predict evolutionary endpoints for steelhead life history in response to management actions that change stage-specific survival or growth rates. While the effects of some changes might seem obvious (e.g. increasing migration mortality should select against anadromy), the effects of changes in growth rate can be context-dependent and sometimes unexpected (Satterthwaite et al. 2009), with potentially complicated interactions between survival and growth rate.
Among the Pacific salmonids, O. mykiss is remarkable for intraspecific diversity in life history (Behnke 2002). Some individuals complete their entire life history in freshwater whereas others, sympatric at birth, spend variable amounts of time in freshwater, estuaries, and the ocean before returning to freshwater to reproduce. The expression of alternative life histories is the result of a complex interaction between genetic variation, including local adaptation, and environmental conditions. Atlantic salmon, Salmo salar, also exhibit a wide range of intraspecific life history variation and a relatively well developed conceptual and computational theory exists to describe this variation (see Mangel 1994; Thorpe et al. 1998; Mangel and Satterthwaite 2008). According to this life history theory, the developmental pathways (smolt transformation, maturation) followed by fish are determined by responses to growth conditions at particular times of year (called decision windows) and survival associated with the developmental pathway. The responses themselves are threshold traits and the thresholds are genetically determined (Piche et al. 2008). In this manner, there is a natural gene by environment interaction determining life history variation.
Although qualitatively general, the quantitative details of these predictions depend on fully parameterizing the model with site-specific growth, survival, and fecundity. This framework has been applied to Arctic charr Salvelinus alpinus (Rikardsen et al. 2004) and to steelhead in a small creek in coastal California (Satterthwaite et al. 2009) under relatively undisturbed, natural conditions. Modified rivers present a unique and challenging application for these models, especially given the probable multiple introductions of new genetic material (Williams 2006) and the short evolutionary histories of these populations under current environmental conditions. As such, this study provides important insights into the applicability of state-dependent evolutionary models to populations facing radically changed environments.
In this paper, we extend the life history modeling framework to steelhead in the California Central Valley, where steelhead are listed as threatened (Good et al. 2005). We advance the management utility of this modeling framework in two ways. First, we explicitly model growth as a function of environmental conditions. Second, as a rough assessment of the potential for human-induced evolutionary change, we present a comparison of the selective pressures and evolutionary endpoints expected in these highly modified systems with those in a more natural system that may a resemble potential source populations used in restocking efforts.
We address three questions about steelhead life histories and implications for management in two Central Valley rivers, the Lower American and Mokelumne (Fig. 1): (i) Are these populations currently displaying optimal life histories given the environment created by current water use patterns? (ii) Should we expect evolutionary changes in life history strategies, given current environmental conditions and water management policies on these rivers? (iii) What sort of evolutionary changes in life histories might we expect as environmental conditions are altered by human activities in the future?
Figure 1.
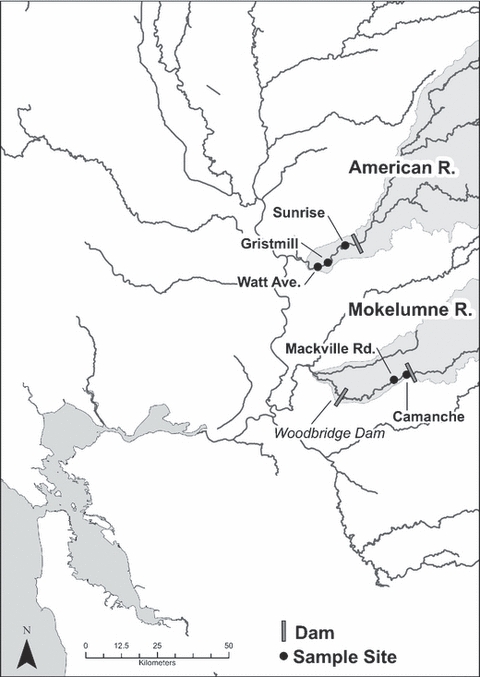
Map of California's Central Valley and delta, with our study sites on the Lower American and Mokelumne River marked.
Methods
Conceptual framework
Our models follow the state dependent life history model of female steelhead described in Satterthwaite et al. (2009) for coastal populations, except that we explicitly model fish growth as a function of environmental conditions as well as physiological state (parameters described in Table 1). Briefly, we model the expected lifetime fitness (lifetime egg production of a female fish) F as a function of state variables l– fork length (mm), g– sexual maturity indicator variable (1 = mature, 0 = immature), and e– smolting indicator variable (1 = smolt, 0 = parr) at all times t. We assume that smolting and maturing are mutually exclusive and that there are specific decision windows (Fig. 2) during which a fish may initiate sexual maturation or smolt transformation (Mangel 1994; Thorpe et al. 1998). Outside of these windows we assume life history trajectories are fixed; thus (as long as fish are not spawning or emigrating to the ocean at time t):
Table 1.
Definitions of all parameters and variables used in models (See Methods section for details)
| Symbol | Definition |
|---|---|
| t | Time (in days since January 1 of first year of fish's life) |
| (no symbol) | Julian day of emergence |
| ts | Julian day of resident spawning |
| te | Julian day of emigration |
| tw | Julian day of end of smolting window |
| tm | Julian day of end of maturity window |
| F | Expected lifetime egg output, given current state and time |
| l | Fork length (mm) |
| b | Fork length (mm) at the start of the decision window |
| g | Maturity switch: 1 = maturing, 0 = immature |
| e | Smolting switch, 1 = smolting, 0 = freshwater physiology |
| ϕ(l) | Length-specific egg production of resident female spawner |
| Φ | Expected lifetime egg production of an anadromous female |
| σ(l) | Size-specific marine survival from emigration to first spawning |
| l′ | Time and state dependent expected future size |
| l″ | Time, state, and recent growth dependent future size |
| s(t) | Freshwater survival from time t to time t + 1 |
| W(t) | Weight (g) at time t |
| T(t) | Temperature (°C) at time t |
| Ψ(T) | Effect of temperature on maximal consumption |
| c | Maximum weight of food (g) a 1 g fish can consume per day at its optimal feeding temperature |
| f | Relative energy density of food:fish tissue |
| a(t) | Foraging activity level of a fish on day t |
| κ(t) | Half-saturation constant of feeding – the activity level needed for a fish to reach half of its maximum daily consumption. Basically, a measure of the difficulty of acquiring food, or the inverse of food availability. |
| αe0.071T(t) | Catabolic energy costs (at rest) of 1 g of fish tissue, at ambient temperature T(t). α is essentially a measure of basal metabolic rate. |
Figure 2.
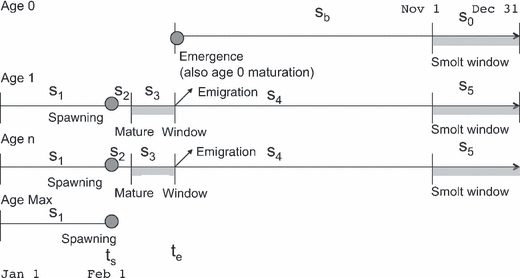
Model timeline. Some points do not have dates assigned, since their timing varies between rivers (see text in Methods).
| (1) |
where s(t) is freshwater survival from time t to time t + 1 and l′(l,g,e,t) describes the expected length at time t + 1, given expected growth from starting size l and physiological states g and e.
At the time of spawning ts, the fitness of sexually immature fish is updated as above (i.e. spawning time is no different from other times for immature fish), whereas sexually mature fish in the river receive an immediate fitness reward from egg production (ϕ(l)) (note that our analysis therefore directly applies to female fish only) along with their expected future fitness (thereby accounting for the possibility of iteroparity, which is common in O. mykiss):
| (2) |
At the time of emigration te, the fitness of nonsmolts is updated as in Equation 1, whereas smolts receive fitness based on their size-dependent probability of surviving emigration downstream and the ocean phase of their life history (σ(l)) along with the expected lifetime reproductive output (implicitly including the effects of iteroparity, based on rates of repeat spawning reported by Shapovalov and Taft 1954) Φ of a fish starting from its first return spawning trip. We assume that Φ is independent of l at the time of emigration, since there is little relationship between length at emigration and length at return (Sutherland 1973; Pearson 1993). Thus
| (3) |
During decision windows, we introduce an extra state variable b, the length of the fish at the beginning of the window. Together, b and l allow a calculation of growth rate during the decision window and thus an updated projection of future length l″(l,g,e,b,t) that accounts for recent growth conditions (see Satterthwaite et al. 2009 for details). At tw, the end of the smolting decision window, immature parr make a state-dependent selection of a life history pathway that maximizes their expected lifetime fitness:
| (4) |
At the end of the maturity decision window tm, a similar calculation is made for sexual maturity:
| (5) |
For each decision window, we can identify the combinations of l and b (i.e. size and recent growth rate) for which the optimal decision is to smolt, mature, or remain uncommitted, given the growth rates and survivals characteristic of each river. This allows the identification of threshold sizes, which can be compared against projections of expected sizes for fish growing under various conditions to predict expected age and size distributions of smolts and the balance between residency and anadromy on a population-wide scale. The threshold sizes and state-dependent decisions can also be compared against the range of sizes and growth rates seen in the field during the presumed decision windows to identify optimal distributions of life histories for a particular system. These decision rules can also predict the range of life histories associated with new sizes and growth rates expected under different management scenarios.
Study system
Our study sites on both rivers are below impassable dams, each with associated hatchery programs. The dams have blocked access to the majority of historic spawning areas, and the remainder has been radically altered in terms of substrate, scour, and floodplain area. The American River supports very rapid growth in juvenile steelhead, whereas growth on the Mokelumne River is more moderate (see Results sections for details, in particular Fig. 3). However, growth on both rivers is substantially faster than on the California coast (Hayes et al. 2008; Sogard et al. 2009).
Figure 3.
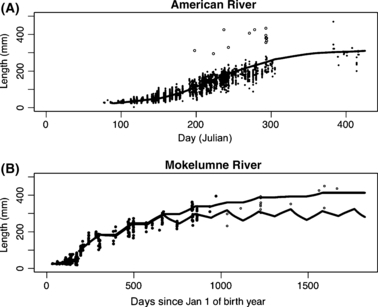
Growth trajectories of juvenile steelhead in the two watersheds, as a function of days since Jan 1 of birth year. See text in Methods section for data sources and explanation of the fitted lines. Note the different x-axis scales for each figure, and also note that the fitted trajectory is based on a model of changes in weight rather than changes in length, and thus apparent predictions of shrinkage in the Mokelumne are predictions of weight loss rather than actual shrinkage in length. For (B) The lower line represents the fit of the growth model to the data (allowing shrinkage), whereas the upper line shows the trajectory followed by a fish growing as allowed in our life history model (no shrinkage, note that this also results in a better fit to the sizes of the oldest fish). Solid circles are data points included in model fit, open circles are older fish that were not included when fitting the growth model.
We used a variety of methods to assess extant life histories on the two streams. During all sampling events in 2006–2008 (described in Appendix B), we examined fish visually for morphological features consistent with maturity and determined sex when possible. We determined the age distribution of emigrating smolts on the American River based on scales analyzed for 99 returning wild adults sampled during spawning at Nimbus Hatchery during the winters of 2001 through 2005. Scale samples were cleaned, dried, mounted between microscope slides, and viewed on a microfiche reader at 52× magnification. We determined age at ocean entry for each scale sample by counting the number of winter annuli formed on the scale up to the point of ocean entry. The drastic increase in scale circuli spacing that occurs as the smolt begins feeding in prey-rich ocean waters was used as the diagnostic for identifying the point of ocean entry. We only used data for which two independent scale readings were in agreement.
No data are available on absolute survival rates in either watershed, so we explore a wide range of survival values for both rivers. It is possible that emigration to the ocean from the Mokelumne rearing grounds entails greater mortality risks than emigration from the American, consistent with the apparent higher survival of Chinook smolts in the northern delta than in the central delta (Brandes and McLain 2001). This may be due to the lower water levels and pumping faced by Mokelumne fish on their route to the ocean, or increased predation risk associated with passage through the Woodbridge Dam and reservoir area downstream of our sample sites (Fig. 1), since dams and reservoirs are often associated with increased predation risk (Raymond 1979). Fish emigrating from the American River, in contrast, move within the relatively high flows of the Sacramento River and do not have to navigate through a dam prior to entry into the delta area.
Timing of decision windows
We assume that the smolting decision window lasts from the beginning of November until the end of December (Fig. 2), consistent with Satterthwaite et al. (2009). We assume that the maturity decision window spans the month before the major period of emigration, which begins in early March on the American River (Snider and Titus 2000) and mid-May on the Mokelumne (Merz and Saldate 2005). We place the maturity decision window further in advance of spawning (assumed to be February 1 for both rivers) than the smolting window is in advance of emigration because (especially for females) sexual maturation requires a more substantial physiological transformation than does smolting (Mangel 1994; Thorpe et al. 1998). We allow for a YOY maturity decision at the time of emergence, based on the date of emergence and initial growth rate. We assume that maturing slows growth in length by 18% (Satterthwaite et al. 2009) based on the mass of gonads in mature fish and length-weight allometries. We predict whether YOY mature or remain parr by first projecting the size expected from a given combination of emergence date and growth rate, and then comparing the fitness of mature versus immature fish of the expected size at the start of the YOY smolt decision window.
We assume that fish can commit to sexual maturity immediately after emergence, consistent with arguments by Mangel (1994) and Thorpe et al. (1998) that maturity is regulated by inhibition. We further assume that fish that initiate maturity as YOY can become competent spawners at the age 1 spawning event. We are unaware of documented cases of age 1 female O. mykiss spawning successfully, suggesting it may be physiologically impossible, but such cases have been documented in amago salmon O. masou ishikawai (Shimma and Kitamura 1987; Shimma et al. 1994) and a very small number (less than 0.1% of total hatchery stock) of sexually mature age 1 female steelhead have been observed in hatchery conditions (Schmidt and House 1979).
Model parameterization
We describe the details of model parameterization in Appendix A. Briefly, our state-dependent model requires the specification of growth and survival in two stages. As used in our model, freshwater survival s(t) refers to survival during the rearing period prior to downstream movement. Emigrant survival σ(l) includes survival during the downstream migration of smolts, the period of time spent in the ocean, and migration back to the spawning grounds. We model fecundity of spawning resident females ϕ(l) as an increasing function of size (Shapovalov and Taft 1954), and estimate the expected lifetime reproductive output Φ of a returning steelhead by applying ϕ(l) to the average size of at return in each stream, summing expected egg production over the first spawning and repeat spawnings discounted by expected kelt survival. We explore a range of plausible freshwater survival rates (Bley and Moring 1988), with freshwater survival either constant or size-dependent (Ward and Slaney 1993). We model emigrant survival as an increasing function of length at the time of migration (Shapovalov 1967; Bond et al. 2008), and use multiple rescalings of this function to explore different emigration survival scenarios.
Growth is an essential component of Equations 1–5 (captured in l′ and l″). We model fish growth using an energy-balance model conceptually similar to bioenergetic models previously applied to steelhead and rainbow trout (Rand et al. 1993; Railsback and Rose 1999) except that we explicitly tie consumption to activity levels and food availability (Mangel and Munch 2005). This approach was advocated to improve bioenergetic models by Andersen and Riis-Vestergaard (2004) and Bajer et al. (2004). We model the rate of change in weight (W) versus time in days (t) as
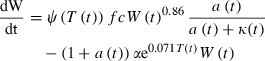 |
(6) |
We assume that growth reflects a balance between size- and temperature-dependent maximal consumption each day (Ψ(T(t))fcW(t)0.86) and catabolic costs (αe0.071T(t)W(t)) each day. The balance is also affected by how much effort fish expend on foraging is (a) compared to how difficult it is to acquire food (κ(t)), and we assume that fish optimize a given the other parameters. Our model predicts a food- and temperature-dependent asymptotic size as an emergent property, since metabolic needs increase faster than feeding ability as fish grow (catabolic costs scale with W1.0 while maximal consumption scales with W0.86).
Having parameterized Ψ, f, c and α, and assuming that a is chosen each day to maximize net energy gain, we fit this model to data collected in the field by inferring daily values of κ(t) that minimize the difference between observed and predicted growth given temperature T(t) and fish size W(t). We performed a least squares fit for a single growth trajectory passing through length data collected using various methods (described below) on the two rivers, assuming an emergence date of January 30 for the Mokelumne and April 1 for the American, based on the first appearance of small fish in our samples. Due to very early spawning by some fish, the earliest fry on the Mokelumne appear at the peak of spawning. We assume fish emerge at a length of 24 mm (Shapovalov and Taft 1954), and use an allometric equation fit to all of our length-weight data from each stream to convert between lengths and weights. At some sampling events only length data were collected, so we use length as our measure of size in the field even though our model predicts changes in weight. The collection of temperature and size data in the field is described in Appendix B.
Our growth model can predict weight loss, which is translated into a prediction of shrinkage in length if we assume a constant allometric relationship between length and weight. On the American River, our model never predicts shrinkage until fish have grown larger (and older) than any encountered in the field. On the Mokelumne, we do predict weight loss at times. In the growth projections used in our life history model, however, we do not allow shrinkage in length (i.e. we always force l′(l) ≥ l).
Baseline predictions and sensitivity analyses
We first predict optimal decision thresholds for fish given specified survival and growth rates. These thresholds may vary by river, due to different growth rates and timing of emigration. We then predict the observed distribution of life histories by using forward iteration (Mangel and Clark 1988; Clark and Mangel 2000) to determine the optimal life history pathways for fish of the sizes and recent growth rates observed empirically during the decision window time periods in each river. We compare these predictions to patterns currently displayed in each population.
As a sensitivity analysis, and to predict the effects of environmental change (also see Appendix C), we first repeat these analyses for all potential values of freshwater and emigrant survival rates as described earlier, while keeping the growth model constant. Second, we perform further simulations in which we allow growth rate to vary and determine optimal decision thresholds under these conditions, which might result from changes in temperature or food supply as a consequence of environmental change or new water management procedures. It is impossible to test all potential environmental perturbations. Thus, we present a few illustrative examples based on flow and temperature changes that are of potential interest to water managers in these systems. To demonstrate how our modeling approach can be used to address these management questions, we ask whether cooler water and increased food supply during the summer or fall (i.e. as a result of increased dam releases) are predicted to promote residency in scenarios where we now predict anadromy, and whether warmer water and reduced food supply are predicted to favor anadromy in scenarios where we now predict residency. To provide a powerful test, we chose a 3°C perturbation of temperature. This is a large change, but well within the range of water temperature perturbations predicted for managed versus unmanaged flows (Yates et al. 2008; e.g. their Fig. 6). We also examine the linked effects of increased temperature and reduced survival (due to direct effects of temperature and/or a temperature-predation risk link, e.g. McCullough 1999) or decreased temperature and increased survival.
Figure 6.
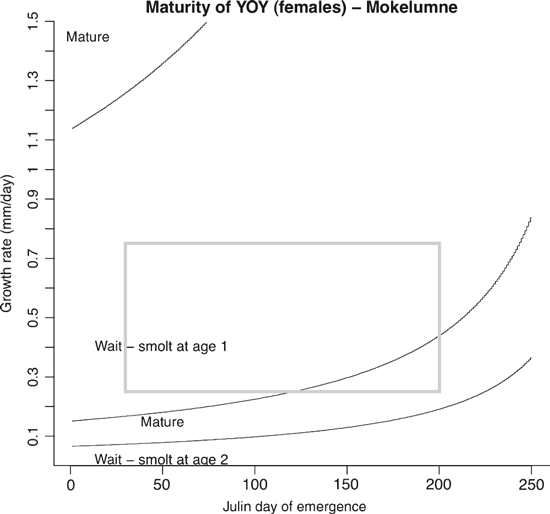
State-dependent decisions predicted in Mokelumne River fish (given low freshwater survival and high emigrant survival) for the YOY maturity decision window. The grey box indicates combinations of emergence time and initial growth rate observed in the field.
All models were coded in R (R Development Core Team 2007) using only the standard libraries and packages and are available from the lead author upon request.
Results
Inference of food availability
Our model predicts that to produce growth observed in the field (Fig. 3), the difficulty of acquiring food varies temporally in the two systems (Fig. 4) and in general is lower on the American River than on the Mokelumne. Food seems to be particularly easy to acquire in June-August on the American. For age 1+ fish on the Mokelumne, food appears more difficult to acquire in the summer and winter than spring and fall. However the low temporal resolution of data and pooling across years for the Mokelumne makes any conclusions about seasonal variation tentative. Furthermore the value of κ(t) associated with the emergence period on the Mokelumne is quite high, suggesting that most fish emerge later than the first emerging fish encountered in the dataset, since the average size of fish remains low due either to very slow growth or the continued emergence of small fish. This variability in emergence date does not impact later model predictions, since we explore a range of emergence dates in the YOY maturity decision and later decisions are determined only by size and recent growth, irrespective of emergence date.
Figure 4.
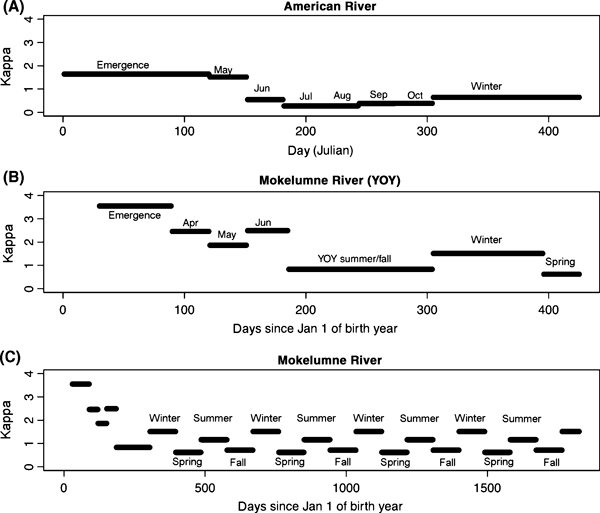
Growth model estimates of temporal variation of the difficulty of acquiring food (κ(t)) in the American (A) and Mokelumne (B-C, where B represents the first year of life and C represents multiple years) rivers. Higher values of κ(t) correspond to more difficult feeding.
Extant life histories
Our empirical observations indicate that the American River is dominated by anadromous fish smolting at young ages. In 3 years of sampling, we found only one fish identified as a mature male and none identified as mature females out of 629 fish total. Fish larger than 200 mm fork length were never encountered between February and June, suggesting all age 1 or older fish had left the system. From 99 scales examined from wild adult steelhead on the American River, it appears that 93 (94%) entered the ocean at age 1, five (5%) at age 2, and one (1%) at age 3.
In contrast, the Mokelumne contains a mix of resident and anadromous fish. Large fish were found year-round, and scale analysis (see Appendix B) revealed spawning checks (thereby verifying maturity) in 29 of 67 age 1 or older fish determined to be residents based on the absence of an ocean growth period in scale circuli. At the same time, the presence of anadromous steelhead on the Mokelumne is well documented (U.S. Department of the Interior 2008).
Life History Predictions
Due to high uncertainty in freshwater survival and recent trends in emigration survival, we are hesitant to identify any particular survival scenario as the baseline. However, to illustrate the application of our methods, we chose low freshwater survival (probably appropriate given the degraded nature of these rivers) and high emigrant survival (consistent with Shapovalov 1967, the geographically closest data source for the emigration survival of wild fish available) to present in full detail.
For the American River, we do not predict that female YOY initiate maturity for any of the combinations of emergence date and initial growth rate observed in the field, or for any plausible deviations outside the observed range. We predict that all YOY females are large enough by the end of the smolting decision window to initiate the smolt transformation and emigrate at age 1 (Fig. 5A). Although we predict no older fish would remain, any that do so are predicted to forego maturity (unless very large, Fig. 5B) and smolt at their next opportunity (Fig. 5C). This is consistent with what has been observed in the field, with the American River dominated by anadromous fish emigrating at age 1. The smolting threshold size predicted for these fish is larger (but more readily achieved in the field) than that predicted for fish on Scott Creek in coastal California (Satterthwaite et al. 2009).
Figure 5.
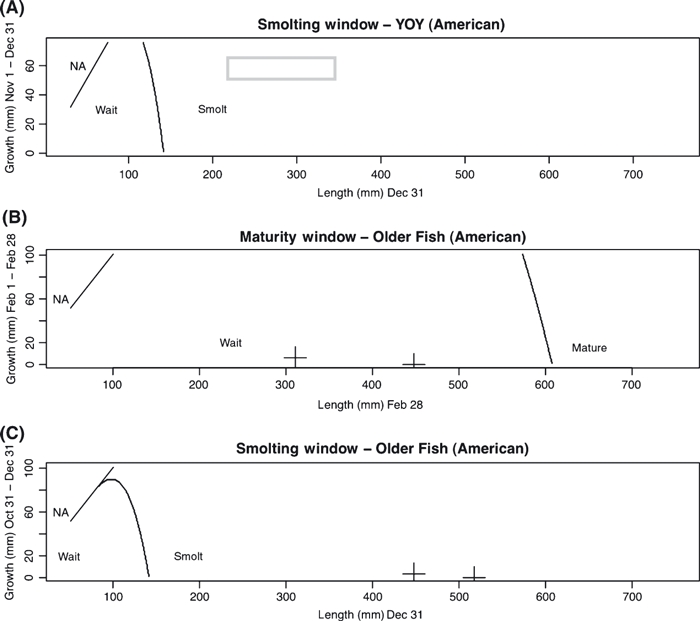
State-dependent decisions predicted in American River fish (given low freshwater survival and high emigrant survival) for the YOY smolting window (A), the age 1 maturity window (B), and the age 1 smolting window (C). For A, the grey box indicates combinations of size and recent growth rate observed in the field during the corresponding time periods, with size ranges determined from the length-frequency data from Fig. 3. For (B) and (C), observations in the field are not possible since we do not observe older fish, however the crosses indicate predicted sizes of parr at these times if growing according to our growth model. The region marked “NA” corresponds to impossible combinations of size and growth rate.
For the Mokelumne River, we predict that the slowest-growing and latest-emerging female YOY initiate maturity (Fig. 6), as should fish growing much faster and emerging earlier than any fish observed in the field. We predict that most if not all parr are large enough to initiate smolting at age 0 and emigrate at age 1 (Fig. 7A). Since the smallest fish in the field are close to our predicted size threshold for smolting, it is plausible that in some years (or with more extensive sampling to define the tails of size distributions) some fish would be too small to emigrate at age 1. Such fish that remain in the stream for another year and have not yet matured are predicted to forego their next chance at maturing (Fig. 7B) and then smolt (Fig. 7C). These predictions are fairly consistent with our empirical observations of a mix of resident and anadromous fish. However, if initiating maturity as a YOY and first spawning at age 1 is not possible, our baseline model would not predict any female residents.
Figure 7.
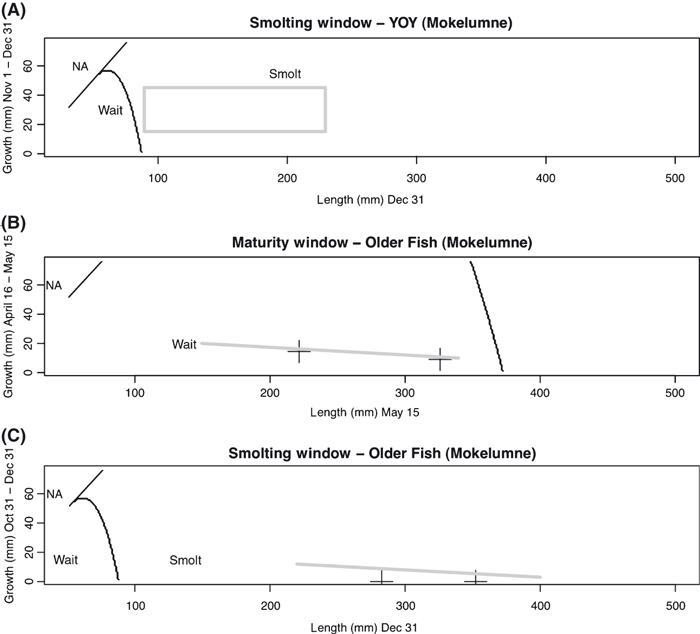
State-dependent decisions predicted in Mokelumne River fish (given low freshwater survival and high emigrant survival) for the YOY smolting window (A), the age 1 maturity window (B), and the age 1 smolting window (C). The grey box in (A) represents combinations of size and recent growth rate observed in the field during the corresponding time periods. For (B) and (C), crosses indicate the predicted size and growth rates of age 1 (left) and age 2 (right) fish during these time periods. The sloping lines cover the range of sizes observed in length-frequency data from the field for all older fish, with the growth rate associated with smallest and largest sizes inferred by the movement of the bounds of the length-frequency distribution. The region marked ‘NA’ corresponds to impossible combinations of size and growth rate.
To summarize, it appears that American River fish can readily reach a size associated with high probability of surviving emigration as age 1 smolts (Fig. 8A). Thus they forego maturing in freshwater at a young age and are not well served to wait and expose themselves to additional freshwater mortality risk by smolting at age 2 or older, or to wait and mature in freshwater at an older age. On the Mokelumne it appears that many fish can reach a size large enough to smolt at age 1, but the slower-growing fish are better served to mature as YOY and spawn at age 1 (Fig. 8B) rather than risk the extra freshwater mortality associated with waiting to smolt at age 2 (since much less time must elapse before the age 1 spawning opportunity compared to age 2 emigration). However, once the first spawning opportunity has passed and even slow growing fish are large enough to have a moderate chance of survival in the ocean, it takes too long and exposes fish to too much risk of freshwater mortality to grow to a large enough size to spawn with much success as a resident female at an even older age.
Figure 8.
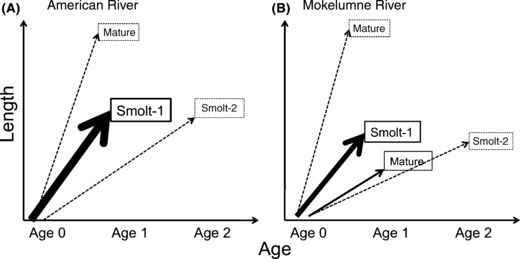
Predicted life histories as a function of size at age on the American (A) and Mokelumne (B) Rivers. Solid lines represent growth trajectories (with within year variability smoothed out) observed in the field, broken lines are outside the range of currently observed variability. The thicknesses of solid lines correspond to the proportion of fish following each trajectory. Smolt ages are at time of emigration.
The two rivers vary greatly in the relative fitness of optimal versus suboptimal strategies. We assessed this by comparing the expected lifetime fitness of fish on either an anadromous or resident track, with expected fitness calculated based on their size on the day of potential spawning at age 1. For fish on the American River, the fitness of anadromous fish is two to four times that of residents over the plausible range of fish sizes at that time (Fig. 9A). On the Mokelumne, small fish had higher fitness if on the resident pathway and large fish had higher fitness if on an anadromous pathway (Fig. 9B), consistent with earlier predictions. The fitnesses of the two strategies are very close over a wide range of sizes observed in the field, suggesting that a mixed strategy could more easily persist in the Mokelumne than in the American.
Figure 9.
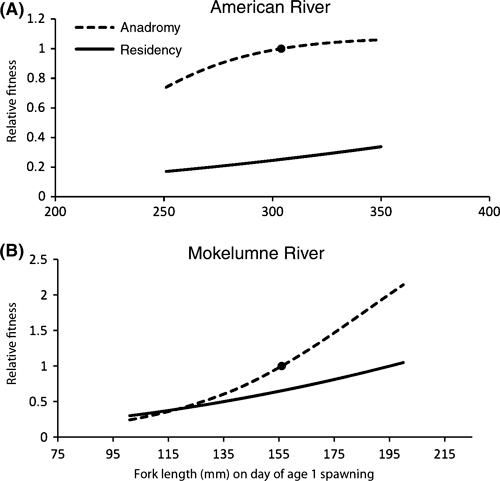
Relative expected lifetime fitness of fish committed to either a resident (solid line) or anadromous (dashed line) life history in the American (A) or Mokelumne (B) River, as a function of size at the time of potential age 1 spawning. Values are scaled so that the expected fitness of a fish growing according to the average trajectory of our growth model and following the optimal strategy for its size receives a relative fitness value of 1.0 (filled circles), and the x-axis scale corresponds to the range of sizes observed in the field at the time of spawning (Mokelumne) or projected from the last observed size range (American).
Sensitivity analyses
For the American River, we consistently predict that the vast majority of fish will smolt and emigrate at age 1 (as observed in the field) for almost all combinations of survival scenarios (Table 2). On the Mokelumne River, in contrast, our predictions are highly sensitive to freshwater survival, emigrant survival, and whether we assume it is physiologically possible for female fish to mature as YOY and spawn at age 1.
Table 2.
Life histories predicted for each river under baseline growth conditions for different survival scenarios, if female steelhead are physiologically capable of maturing as YOY and first spawning at age 1 (A) or if the first possible spawning comes at age 2 (B). When a mix of life histories is predicted, the most common phenotype is listed first. Asterisks denote the baseline scenario
| American River | Mokelumne River | |||||
|---|---|---|---|---|---|---|
| Emigrant/marine survival | ||||||
| Freshwater survival | Low | Medium | High* | Low | Medium | High* |
| (A) | ||||||
| Low* | Residents | Age 1 smolts | Age 1 smolts | Residents | Age 1 smolts and residents | Age 1 smolts and residents |
| Medium | Residents | Age 1 smolts | Age 1 smolts | Residents | Age 1 smolts, residents, and age 2 smolts | Age 1 smolts and age 2 smolts |
| High | Residents | Age 1 smolts | Age 1 smolts | Residents | Age 1 smolts, residents, and age 2 smolts | Age 1 smolts and age 2 smolts |
| Size-dependent | Residents | Residents | Residents | Residents | Residents and age 1 smolts | Age 1 smolts and residents |
| American River | Mokelumne River | |||||
|---|---|---|---|---|---|---|
| Emigrant/marine survival | ||||||
| Freshwater survival | Low | Medium | High* | Low | Medium | High* |
| (B) | ||||||
| Low* | Age 1 smolts | Age 1 smolts | Age 1 smolts | Age 1 smolts and residents | Age 1 smolts | Age 1 smolts |
| Medium | Age 1 smolts | Age 1 smolts | Age 1 smolts | Age 1 smolts and residents | Age 1 smolts and age 2 smolts | Age 1 smolts and age 2 smolts |
| High | Residents | Age 1 smolts | Age 1 smolts | Residents | Age 1 smolts and age 2 smolts | Age 1 smolts and age 2 smolts |
| Size-dependent | Age 1 smolts | Age 1 smolts | Age 1 smolts | Age 1 smolts | Age 1 smolts | Age 1 smolts |
The baseline scenario.
Sensitivity to emigrant survival
On both rivers, if emigrant survival is reduced to the low scenario, the model predicts that all fish mature as YOY (Table 2A), since there is a reduced reward associated with smolting even at large size. Under these conditions, maturing at a young age maximizes the number of potential lifetime spawning events. However, if it is physiologically impossible for females to first spawn at age 1, the predictions vary by watershed. In the American River, we predict that fish smolt and emigrate at age 1 unless freshwater survival is high and emigrant survival is low. At intermediate emigrant survival the size threshold for smolting is increased when freshwater survival is high and it is conceivable that a few slow-growing fish might not reach this size threshold as YOY. Any fish too small to smolt as YOY are predicted to mature rather than smolt as older fish. On the Mokelumne, we predict a mix of age 1 smolts and residents if freshwater survival is low to medium, all residents if freshwater survival is high, and all age 1 smolts if freshwater survival is size-dependent. In the case of size-dependent survival (14% annually for fish <150 mm, 75% annually for larger fish), we predict all smolts. This is because fish achieve 150 mm length shortly after the first spawning, thus there is little difference in cumulative mortality risk between waiting to spawn at age 1 and waiting to emigrate later that same year.
For intermediate emigration survival values, we predict an increase in the number of fish maturing (as YOY or older fish) relative to the high emigrant survival case, and thus we predict a mix of anadromous and resident fish with increasing representation of residents as survival in the ocean (and/or passage down the river to the ocean) declines. This prediction is consistent with the apparent high prevalence of residents on the Mokelumne, if passage from our study sites to the ocean has a greater risk of mortality compared to the American (Brandes and McLain 2001).
Sensitivity to freshwater survival
On the American River, freshwater survival has relatively little impact on predicted life histories. If YOY cannot mature and spawn at age 1, we always predict age 1 smolts unless emigrant survival is low and freshwater survival is high, in which case we predict all fish mature and become freshwater residents. If survival in freshwater is strongly size-dependent, we also predict maturity as YOY (if possible) for all values of emigrant survival, since fish are very likely to survive to repeat spawning in this scenario, and grow to large sizes where they are highly fecund.
The effects of freshwater survival on predicted life histories in the Mokelumne are quite complicated. Increased freshwater survival may favor increasing residency (e.g. the low emigrant survival where early maturity is not possible scenario in Table 2B), or it may favor smolting at older ages, potentially accompanied by reduced residency (e.g. the medium and high emigrant survival scenarios in Table 2A,B). In contrast with predictions for the American, high freshwater survival for large fish does not always favor early maturity.
This disparity between the effects of high freshwater survival in the American vs. the Mokelumne may reflect the asymptotic sizes achievable in each watershed, with these maximal sizes imposed by bioenergetic constraints. Fish on the American river are predicted to be able to grow to lengths of over 500 mm without going to sea, and to do so rapidly, whereas fish in the Mokelumne take several years to reach lengths over 300 mm and may have difficulty maintaining body weight through the fall at larger sizes (Fig. 3). Thus the potential reproductive output for a resident female is higher on the American than the Mokelumne, since it would be larger and thus produce more eggs.
Models with changing flow and temperature
To illustrate how our modeling framework can be used to predict the effects of changes in water management, we analyze perturbations that others have predicted to increase residency (U.S. Department of the Interior 2008) in selected scenarios where we currently predict all anadromy, and perturbations predicted to increase anadromy in scenarios where we currently predict all residency. We base this analysis on the suggestion that releasing too much cool water in summer and early fall may reduce the occurrence of anadromy, as discussed in the introduction. While we are constrained to evaluating only a small subset of potential environmental perturbations, we do so to provide specific examples of an approach with broad applicability.
For the American River, we might predict that cooler temperatures and increased food supply in the summer and fall would select for residency where we now see only anadromous fish. Since we already predict that food is easy to acquire in the summer on the American, we consider the effects of extending this easy food availability into the fall, reducing modeled temperatures by 3°C for October and November, along with extending the period of lowest κ(t) through the end of the year. For five out of six scenarios for which we predicted all anadromy under baseline conditions, we still predict all anadromy under altered growth conditions, although in one case we predict that the slowest growing parr might now wait and smolt at an older age (Table 3A). Only if freshwater survival is low and parr could mature as YOY do we predict a shift to the resident life history response to this changed environment, and we predict this shift to apply to only a small portion of the population.
Table 3.
Life histories predicted on the American River if the environment changes in ways that might be predicted to favor residency (relative to baseline conditions predicting pure anadromy). (A) Food is easier to acquire and water temperatures are cooler in the fall. (B) There is less mortality risk and the water is cooler in summer
| Cool, food-rich fall | Residents predicted? |
|---|---|
| (A) | |
| Low freshwater survival, medium emigrant survival | No |
| Medium freshwater survival, medium emigrant survival | No |
| High freshwater survival, medium emigrant survival | No |
| Low freshwater survival, high emigrant survival | Very few, only if fish can mature early |
| Medium freshwater survival, high emigrant survival | No |
| High freshwater survival, high emigrant survival | No (may get some age 2 smolts) |
| Cool, safer summer | Residents predicted? |
|---|---|
| (B) | |
| Low freshwater survival, medium emigrant survival | No |
| Medium freshwater survival, medium emigrant survival | No |
| High freshwater survival, medium emigrant survival | No |
| Low freshwater survival, high emigrant survival | No |
| Medium freshwater survival, high emigrant survival | No |
| High freshwater survival, high emigrant survival | No (may get some age 2 smolts) |
Alternatively, we might predict that cooler temperatures in the summer would reduce mortality due either to direct physiological effects and/or by decreasing predation risk. We simulated this scenario by reducing temperatures June 21–September 21 and increasing net survival over the summer by 30%. In this case, we never predicted freshwater maturity for any scenario examined (Table 3B), although if freshwater survival was already high some of the slowest growing parr might wait and smolt at older ages.
For the Mokelumne River, we ask whether making the fall harsher might lead to a prediction of anadromous fish for scenarios in which we predict only residents under the baseline growth conditions, since in our current model fall is a better time for growth than the summer. We first modeled a scenario in which the Mokelumne was 3° warmer in October and November and food availability in the fall was reduced to the average of summer and winter. In this situation we always predict that fish mature as YOY if physiologically possible, but if not we predicted at least some anadromy in three out of four scenarios examined (Table 4A). If instead of changing food availability we assumed that a warmer fall increased predation risk such that net survival through the fall was halved, we again predicted that all fish would mature as YOY if such early spawning is physiologically possible. If spawning at age 1 is not possible we predict at least partial anadromy in all scenarios examined, although only a few fish were predicted to be anadromous in one of the four cases (Table 4B). However, reducing survival for populations that have already been severely depleted is not a wise restoration strategy, and in all cases the predicted shift to anadromy only moderated an overall a decrease in fitness associated with the changed environment.
Table 4.
Life histories predicted on the Mokelumne River if the environment changes in ways that might be predicted to favor anadromy (relative to baseline conditions predicting pure residency). (A) Food is harder to acquire and water temperatures are warmer in the fall. (B) There is more mortality risk and the water is warmer in the fall
| Warm, food-poor fall | Smolts predicted? |
|---|---|
| (A) | |
| Low freshwater survival, low emigrant survival | Only if no option to mature early, then many smolts |
| Medium freshwater survival, low emigrant survival | Only if no option to mature early, then some smolts |
| High freshwater survival, low emigrant survival | No |
| Size-dependent freshwater survival, low emigrant survival | Only if no option to mature early, then many smolts |
| Warm, dangerous summer | Smolts predicted? |
|---|---|
| (B) | |
| Low freshwater survival, low emigrant survival | Only if no option to mature early |
| Medium freshwater survival, low emigrant survival | Only if no option to mature early |
| High freshwater survival, low emigrant survival | Only if no option to mature early, and then only for fastest growers |
| Size-dependent freshwater survival, low emigrant survival | Only if no option to mature early |
Discussion
Our modeling framework successfully predicts much of the observed variation in steelhead life history in these systems, and thus can make useful predictions of evolutionary endpoints when considering alternative management strategies. We conclude that the single most important factor in preserving the anadromous life history is survival during the period between emigration to the ocean and returning to spawn. While not unexpected, this result highlights the importance of removing or ameliorating impediments to passage up and down the rivers and through the Delta, and improving our understanding of environmental effects, including climate change, on ocean survival. Furthermore, our model provides additional and nonintuitive insights in suggesting that changes in freshwater growth rate will have more impact on life histories in the Mokelumne than on the American, and highlights the importance of considering both growth rate and asymptotic size limits in characterizing freshwater growth conditions. The extent to which changes in growth rate can favor mature female parr depends on their physiological capacity to spawn at age 1, a capacity that has not yet been adequately examined under natural or near-natural conditions. We also suggest that there may not be a strong conflict between steelhead and Chinook in terms of the optimal timing of cool water releases, but we caution that the analysis was applied only to the American and Mokelumne Rivers and only predicted the life history effects of alteration in growth rates, and not other direct or indirect effects of temperature on physiology and performance.
Using baseline parameter estimates, our model predicts currently displayed life histories on the American River with a high degree of accuracy. However, there appear to be a few older smolts on the American, which the model does not predict unless some parr grow more slowly than our observations imply. On the Mokelumne, our model successfully predicts a mix of resident and anadromous fish, although the number of residents observed in field sampling may be inconsistent with the model's prediction of a small proportion. However, our baseline emigration survival values may be too high if passage down and out of the Mokelumne River involves higher mortality risk compared to the American River; altering these rates leads to predictions of life histories consistent with what we observe on the Mokelumne. The very similar expected fitness for resident versus anadromous fish at smaller sizes (Fig. 9B) suggests there may be relatively little penalty for small or moderate sized fish selecting the resident strategy even if it is suboptimal. As shown by classic life history models (e.g. Cohen 1966; Slatkin 1974), a strategy with a slightly lower expected (arithmetic mean) fitness can be favored so long as it yields a corresponding reduction in the variance of fitness and a resultant increase in the geometric mean. If ocean survival is highly variable across years (and Ward et al. 1989 and Ward 2000 suggest that it is), we might therefore expect increased residency. We therefore suggest that considering variance as well as mean fitness will be important in predicting life histories in any system where the fitnesses of alternate strategies are similar.
The general match between model predictions and observed life histories is consistent with, but by no means unassailable evidence for, rapid evolution in these stocks. On the assumption that many of the O. mykiss in these rivers originated from transplants (Williams 2006), we might ask if they were pre-adapted to respond to their new environments in a way that produced optimal or near optimal behavior. We are not aware of any detailed studies of the Eel River putative source populations (Williams 2006) that would allow for developing a similar model to predict decision thresholds there. However, we can ask what life history decisions we would expect for fish with the sizes and growth rates realized in these Central Valley rivers if they behaved according to optimal decision rules for coastal California's Scott Creek (Satterthwaite et al. 2009). Under these conditions we predict a lower threshold size for smolting and predict YOY maturity for a very restricted range of emergence dates and growth rates that are slightly earlier and faster than those predicted to lead to maturity in the Mokelumne. Under Scott Creek decision rules, YOY on the American are all too big to be predicted to mature but are all are big enough to be predicted to smolt, meaning that our model predicts the observed life histories of American River fish if they were responding to optimal decision rules evolved in coastal California. For Mokelumne River fish following Scott Creek decision rules, we predict a small number of fish adopting the resident pathway, with most fish smolting and emigrating at either age 1 or 2. Thus, it appears there are more residents on the Mokelumne than we would predict for fish behaving according to rules evolved in a coastal stream. However, if the source populations came from far upstream where smolt migration entailed a higher mortality risk, we expect a higher tendency toward residency (Satterthwaite et al. 2009). Thus, it is unclear the extent to which the current life histories on these streams are best explained by a plastic response or a genetic change. Williams et al. (2008) noted a similar challenge in distinguishing plastic from genetic responses in explaining changes in Chinook life history.
We could produce more refined predictions if there were additional data on site-specific survival, temporal variability in freshwater and emigrant survival, and an explicit function to link changes in flow to changes in growth. Additional data on the frequency of resident and anadromous fish on the Mokelumne would help us to assess the skill of the model in predicting life histories on the Mokelumne. An even better test would be detailed data on the fates of individually marked fish that could be matched with their individual growth trajectories.
If apparent recent declines in marine survival (Ward 2000) represent an enduring trend in reduced smolt success, our models predict an eventual change in the distribution of life histories, with residency increasingly prevalent. Thus, water management decisions that make passage through the Bay-Delta more difficult may pose a threat to the conservation of the anadromous life history in Central Valley steelhead. In addition, changing ocean conditions may pose a threat to anadromy throughout the range of the species. This threat may only be realized over the long term: we predict that the evolutionary end point changes to a nonanadromous life history, but cannot predict how fast the populations would evolve toward this new endpoint. Data on the heritability of life histories (e.g. Carlson and Seamons 2008) in steelhead in combination with selection coefficients that could be estimated using our modeling approach could help us make this sort of prediction. However, in our framework it is decision thresholds rather than life histories per se we expect to be heritable, and estimating the heritability of decision thresholds of individual fish could be challenging.
Comparison with predictions of other models
We do not uniformly predict that the fastest growing parr will always mature, as a comparison of life history predictions between the American and Mokelumne Rivers for a given survival scenario reveals. Instead, increased growth rate may simply favor smolting as a large YOY with a high chance of surviving emigration as a large age 1 fish. Increased growth rate is only expected to select for residency if accompanied by a larger asymptotic size and poor emigrant survival of even large fish, or by very high survival in freshwater of older fish (c.f. Thorpe et al. 1998). Making particular times of year completely inhospitable will, of course, eliminate the resident life history, but in general we do not predict that a warm summer with low food availability will strongly favor anadromy over the baseline case in these rivers (c.f. Cramer and Beamesderfer 2006; U.S. Department of the Interior 2008), nor do we predict that a cool summer with high flow will strongly favor residency. In fact, while neither river has a particularly harsh summer, under current conditions we both predict and observe more resident fish in the Mokelumne, which appears to have a poorer growth conditions during summer relative to the American. Cramer and Beamesderfer (2006) propose that more hospitable summer and fall conditions may promote residency because anadromy is a response to poor conditions in the river, on the assumption that in a hospitable river resident spawners can achieve high fitness. By contrast, our model suggests a very large fecundity advantage due to the large size achieved by anadromous fish, an advantage that is exceedingly difficult to counter with good freshwater growth alone, especially since rapid freshwater growth also produces large smolts with increased emigrant survival. Instead, according to our model the costs of emigration (including both emigrant survival and cumulative mortality during the time spent growing to suitable smolt size) must be high to counter the fecundity advantage of large fish. However, we do not explicitly consider the effects of competition with adult residents on the growth of juvenile steelhead, which may affect age and size at smolting with negative impacts on net survival for anadromous fish (Cramer and Beamesderfer 2006).
Implications for understanding steelhead life history
Our understanding is that steelhead life history evolution is driven by an interacting network of growth rates, freshwater survival, and emigrant survival, along with limits on the asymptotic sizes achievable in freshwater. Thus, it is difficult and perhaps misleading to try to summarize the effects of any one of these variables in isolation on predicted changes in steelhead life history in response to management actions. It is also important to realize that smolting (or first maturing) at different ages leads to a substantial discontinuity in expected lifetime fitness. That is, there is a large difference between the expected lifetime fitness of a fish that emigrates at age 1 and a fish that emigrates at age 2. As a result, the change in fitness associated with a switch between the anadromous and resident life histories may be larger or smaller than that associated with a switching of smolt ages within the anadromous life history. It is, therefore, overly simplistic to make statements such as: the fastest growing parr are expected to mature, the next fastest growing to smolt as young fish, and the remainder to smolt as older fish. Sometimes the fastest growing fish are predicted to smolt immediately, slightly slower growing fish are predicted to mature as parr, and even slower growing fish are predicted to smolt at older ages (e.g. Fig. 8B), with the result that residency is associated with intermediate rather than fast or slow growth. Instead of a dichotomy between residency and anadromy, steelhead express a multitude of different, independent life histories, including sexual maturity as a resident at a variety of different ages or smolting at a variety of different ages. While some environmental conditions might be expected to favor the whole suite of resident strategies or the whole suite of anadromous strategies, in many other cases we should expect multiple switches between life histories of both types as we move along an environmental gradient. This is particularly likely to occur when resident and anadromous strategies lead to very similar expected fitness over a broad range of achievable sizes or even a size-dependent switch in the optimal strategy.
Acknowledgments
This material is based upon work supported by the CALFED Science Program under Grant SCI-05-140, Grantee Agreement U-05-SC-40. The work was completed with the support of the Center for Stock Assessment Research, a partnership between the Southwest Fisheries Science Center Santa Cruz Laboratory and UCSC. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the CALFED Science Program. We also thank W. Heady, J. Williams, M. Workman, and the East Bay Municipal Utility District for sharing unpublished data and ideas. We thank E. Mora for the map in Fig. 1. We thank L. Crozier and two anonymous reviewers for helpful comments.
Appendices
Appendix A – Modeling fecundity, survival, and growth
Fecundity
ϕ(l), the length-fecundity relationship for spawning resident females, comes from Shapovalov and Taft (1954, Figure 27)). Φ, the expected lifetime reproduction of a returning steelhead, comes from applying ϕ(l) to the average size of returning steelhead in each stream, with the average size of returning females on the American River (689 mm) calculated based on data from spawning fish collected for scale analysis as described in the main text and the average size of returning steelhead on the Mokelumne (575 mm) calculated from the average size of nonhalf-pounder females collected at the Mokelumne River Fish Hatchery (EBMUD unpublished data). We estimate fecundity for the first spawning and add the expected fecundity of repeat spawners, discounted by the proportion of repeat spawners and allowing for increased size of repeat spawners as reported on Waddell Creek by Shapovalov and Taft (1954), the closest stream for which we could find data on repeat spawning.
Survival
Data on freshwater survival are not available, and very difficult to obtain in large rivers where recapture rates are exceedingly low. We therefore repeat our analyses over a range of freshwater survivals spanning the upper and lower bounds reported for steelhead in the literature. We allow daily YOY survival to vary between values equivalent to 5–41% annual survival (Bley and Moring 1988), while allowing annual survival of larger (>150 mm FL) fish in freshwater to vary from the same lower bound up to 75% (c.f. Ward and Slaney 1993). We evaluated four scenarios for freshwater survival: low (5% annually), high (41% annually), medium (the geometric mean, 14% annually), and size-dependent (14% annually for fish <150 mm, 75% annually for larger fish).
We model length-dependent ocean survival of emigrating fish by
| (A1) |
with l measured in mm, based on a fit to Shapovalov (1967) and Bond et al. (2008) as described in Satterthwaite et al. (2009). As used in our model, σ(l) includes survival during the downstream migration of smolts, the period of time spent in the ocean, and migration back to the spawning grounds. We refer to this entire period as emigrant survival. Given evidence that marine survival has declined since 1990 at least for northern stocks (Ward 2000), we treat this as a high-end estimate of emigrant survival. We also analyze a low emigrant survival scenario using one-sixth of the values predicted by Equation A1, and a medium emigrant survival scenario using Equation A1 for small fish, but with the survival of the largest emigrants capped at 44%, matching the highest smolt to spawner survival reported by Ward et al. (1989).
Growth
We assume there is some maximum amount of energy a fish can potentially take in during a day (Ψ(T(t))fcW(t)0.86), which depends on its size and temperature. How close the fish comes to the maximal intake depends on how active it is (a) compared to how difficult it is to acquire food (κ(t)). The basal catabolic costs of the fish (αe0.071T(t)W(t)) also depend on its size and temperature. We assume that each day the fish maximizes its net rate of energy gain by optimizing a subject to the constraint that a is between 0 and 7, with increases in a increasing both consumption and total catabolic costs at different rates (Mangel and Munch 2005). Thus the term a(t)/(a(t)+κ(t)) is similar to the P in bioenergetic models (Rand et al. 1993; Railsback and Rose 1999), but a affects catabolic costs as well. Thus, the limit on consumption is set by the costs of acquiring food. These costs may include energy spent traveling and searching, swimming costs of maintaining station in flow (Fausch 1984), conflict with inter- and intraspecific competitors (Li and Brocksen 1977), or costs of vigilance associated with predation risk (Johnsson et al. 2004). Thus, κ(t) represents the combined effects of all of these factors that make acquiring food difficult. To a first approximation it might be viewed as the inverse of food availability, with the realization that food availability depends on more than the simple density of food items per se. The optimal value of a (given Equation 6 from the main text, and subject to the constraints mentioned above) is:
| (A2) |
The anabolic term contains terms that describe the relative energy density of food versus fish tissue (f, discounted for conversion efficiency), the daily maximum consumption (weight of food) of a 1 g fish under optimal temperature conditions (g), the allometric scaling of consumption with fish weight W(t)0.86, and a function (Ψ(T(t))) describing how maximum consumption scales with temperature (T). The basal catabolic term depends on a measure of weight-specific catabolic costs (α) and the effect of temperature (e0.071T(t), Brett and Groves 1979).
We estimate α (energy consumption per gram of fish, in grams of fish tissue equivalent, before incorporating temperature effects) as follows: Rand et al. (1993) report the oxygen consumption of a 1 g fish as 0.00264 g/day, applying a temperature correction very similar to ours (e0.06816T(t)). Assuming 13 560 J/g oxygen consumed (Elliott and Davison 1975) and 5900 J/g of fish tissue (Railsback and Rose 1999) yields α = (0.00264)(13560/5900) = 0.00607.
We model maximum possible consumption as a function of temperature Ψ(T(t)) (Thornton and Lessem 1978; as parameterized in Railsback and Rose 1999) and a weight- and temperature-specific maximum possible consumption ability for a 1 g fish c = 0.628 g (Rand et al. 1993), with maximal consumption by larger fish scaling with W0.86 (Moses et al. 2008), indicating that mass-specific maximum consumption decreases as fish grow larger whereas total consumption increases. This results in an asymptotic size limit above which fish must lose weight, with the asymptotic size dependent on temperature and food availability. To estimate growth potential, we scale consumption by the relative energy density of food versus fish tissue (discounted for waste and excretion) f. We calculate f in two steps as follows: Railsback and Rose (1999) report energy densities for trout prey in typical California streams as 2500 J/g and energy density of trout tissue of 5900 J/g, and we assume that 30% of energy intake is wasted in the sense of being unavailable for either growth or respiration (Brett and Groves 1979). Thus we estimate f as (2500/5900)(0.7) = 0.297.
Appendix B – Field methodology
On the American River, we assigned T(t) based on mean daily temperatures collected from a United States Geological Survey stream gage at Fair Oaks. Juvenile steelhead of natural origin were sampled on the American River by the California Department of Fish and Game (CDFG) during 2001–2004 (2003 excluded), along with less extensive sampling in 2006–2008. Sampling was primarily by 50-ft bag seine and secondarily by hook-and-line. Two sites of riffle-run habitats associated with gravel bars were sampled in each of three study reaches, from Paradise Beach (River Kilometer, RK 10) to lower Sunrise Bar (RK 31), thus including steelhead from the lower, middle, and upper production reaches downstream from Nimbus Dam. Sampling occurred on a bi-weekly or monthly basis from March through early November of each year, flow conditions allowing. Captured steelhead were anaesthetized with MS-222, measured for fork length (FL, nearest 1 mm) and wet weight (WW, nearest 0.1 g), checked for marks, tags, and ripe gonads, allowed to recover in a bucket of fresh river water, and then released back into their habitat unit of capture. Steelhead ≥65 mm FL were tagged with Passive Integrated Transponders (PIT tags) for mark–recapture assessment of individual growth rates; however, recapture rates were exceedingly low and we used only size-frequency data to infer growth for the model. Generally, very few steelhead of hatchery origin (distinguished with an adipose-fin clip) were encountered in these surveys, and these fish were excluded from analysis.
Scale analysis of a subset of fish suggested that fish older than age 1 were very rare in the American River. Thus, we assumed that only a single cohort is present at any one time, and our analysis excluded any fish more than 3 SD away from the mean size at its time of collection, assuming these outliers to be older fish.
For the period from emergence through October only the 2001–2004 data were used to fit growth trajectories due to the greater effort and temporal resolution of sampling during these years. Sampling in January and February occurred only in 2008. Thus we fit individual trajectories to the 2001, 2002, and 2004 data and estimated monthly variation in κ for each of those years separately. We then estimated the average monthly κ to yield an averaged trajectory from emergence through October, and estimated an additional κ term over the winter to yield a mean trajectory passing through the January and February size data.
For the Mokelumne River, temperature data came from a data logger operated by the East Bay Municipal Utilities District near Mackville Road. Sampling for fish on the Mokelumne consisted of quarterly hook and line sampling during 2006–2008 at two sites, one just upstream of Mackville Road and one at the day use area downstream of Camanche Dam. Captured fish were processed as described above. To supplement our data on YOY fish early in the year (through July), when they were generally too small to catch by hook and line sampling, we added data from electrofishing surveys carried out by the East Bay Municipal Utilities District between 2002–2004, assuming that all fish <90 mm FL caught during this time were YOY. Later in the year, there was not a clear distinction between the size distributions of fish of different age classes, and scale analysis revealed that multiple cohorts were present simultaneously. Thus, we estimated growth beyond July in the first year based on scale analysis from the hook and line sampling, where age was calculated in days as the sum of the Julian day of capture plus 365 times the age of the fish. Due to limited resources we aged only a subset of fish. We aged every recaptured fish, and aged supplemental fish selected via a haphazard approach that favored fish in size ranges where cohorts overlapped. This may have biased us toward larger YOY fish and smaller age 3 and 4 fish (which we therefore excluded from the model fitting algorithm), while likely inflating the variance of age 1 and 2 fish without obviously biasing the mean in either direction. Due to our small sample sizes on the Mokelumne, we combined data from all sample years into a single trajectory, with T(t) throughout the year calculated as the average for each date across the years 1997–2004 (data from later years was not available except from Camanche dam, where temperature data were less representative of the environment experienced by fish growing in our study site due to close proximity to the reservoir). We fit one trajectory from presumed emergence through July using the electrofishing data, estimating κ separately for each month. We fit a second trajectory (started from the mean size of fish at the start of the hook and line data) for older fish to avoid confounding our estimates of κ due to a change in mean sizes resulting from a change in sampling techniques. Due to the lower temporal resolution of the hook and line sampling, we estimated κ(t) for four seasons defined as winter: November–January, spring: February–April, summer: May–July, and fall: August–October as in Satterthwaite et al. (2009).
The values of κ(t) estimated for YOY during the emergence period (and months immediately thereafter, in the Mokelumne) are different from values predicted for the same seasons for older fish, based on the assumption that small, young fish feed on different food items than larger fish. In addition, fitting the value of κ(t) for the emergence period separately allows arbitrary specification of an emergence date without confounding values of κ(t). If we specify an earlier than appropriate emergence date, this will simply result in a higher than appropriate κ(t) in the period immediately following emergence.
Appendix C – Plasticity in response to temperature predicted by the growth model
Broadly defined, plasticity refers to a change in phenotype displayed by a constant genotype, given a change in the environment. When defined so broadly, plasticity may describe any number of biochemical, physiological, or behavioral responses, which need not be adaptive. Here we summarize how our growth model predicts plastic responses to changes in the environment at multiple levels. We illustrate predicted growth under an altered temperature regime for fish on the Mokelumne River. Because responses to temperature depend on food availability and fish size, we present responses under multiple levels of food supply and for both 100 mm (typical age 0) and 200 mm FL (typical age 1) fish. For illustrative purposes, we choose a 3°C warming or cooling, applied equally throughout the year. The actual effect of climate change is likely to be more variable seasonally (e.g. Meyer et al. 1996), but predicting water temperatures faces an added layer of difficulty because climate and dam releases interact to determine temperatures downstream. Thus rather than explore the full range of possible outcomes, we choose simple illustrative cases, demonstrating a method that is broadly applicable to different climate change scenarios.
An increase in temperature has two physiological effects. As temperature increases, the maximum amount of food a fish can eat increases but then decreases (Railsback and Rose 1999), with the strength of this response also dependent on the size of the fish (Fig. C1a,b). At the same time, increasing temperature always increases metabolic demands (Fig. C1c,d), and is size-dependent. These two changes are due to plasticity at the biochemical and physiological level. However we also predict an adaptive behavioral response as a result of these physiological changes. A fish behaving to maximize its net energy intake is predicted to alter its foraging activity level according to food supply, temperature, and its own size (Fig. C2). This results in growth rate peaking at a temperature lower than that which maximizes capacity to consume (Fig. C3, compare with Fig. C1a,b). The changes in daily growth rate can be projected over an entire lifetime of altered temperatures, as in Fig. C4. These changes in size at age might in some cases be predicted to lead to changes in life history (see Figs 5 and 6 in the main body of the manuscript), leading to a plastic change in life histories that should increase individual fitness, although the rules predicted to evolve under the old environment might no longer lead to optimal life history decisions in a changed environment. Thus over the long term we might expect a new evolutionary endpoint for decision rules (as inferred from re-running the models in the main text under the new environmental conditions), predicting a long term genetic change in genes controlling life history pathways.
Figure C1.
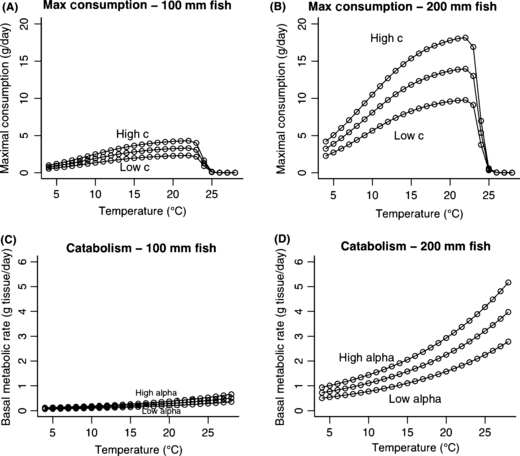
The plastic physiological responses (anabolism, panels A, B; catabolism, panels C, D) to temperature.
Figure C2.
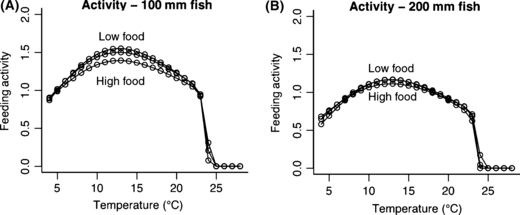
The adaptive behavioral response for 100 mm (A) and 200 mm (B) fish.
Figure C3.
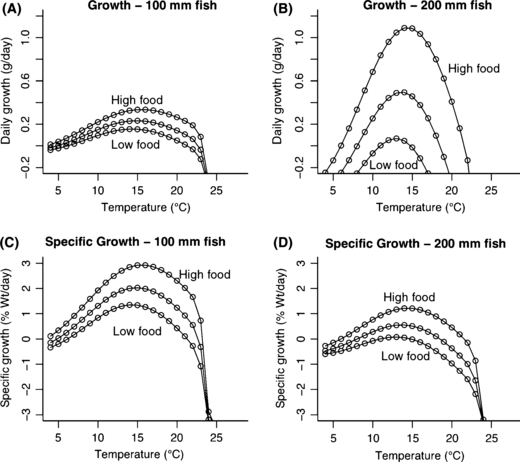
The adaptive emergent growth responses for 100 and 200 mm fish, using either specific growth rate (panels A, B) or daily growth rate (panels C, D).
Figure C4.
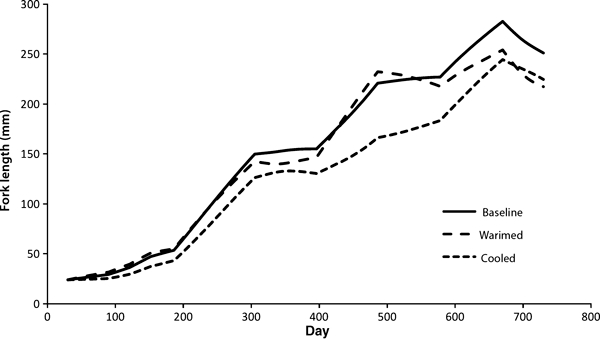
The expected growth under the current conditions for the Mokelumne River (solid line) and warmed (dashed line) or cooled (dotted line) by 3°C.
Literature cited
- Andersen NG, Riis-Vestergaard J. Alternative model structures for bioenergetics budgets of a cruising predatory gadoid: incorporating estimates of food conversion and costs of locomotion. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:2413–2424. [Google Scholar]
- Bajer PG, Whitledge GW, Hayward RS. Widespread consumption-dependent systematic error in fish bioenergetics models and its implications. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:2158–2167. [Google Scholar]
- Baker PF, Morhardt E. Survival of Chinook salmon smolts in the Sacramento-San Joaquin Delta and Pacific Ocean. In: Brown RL, editor. Fish Bulletin 179(2) Contributions to the Biology of Central Valley Salmonids. San Diego, CA: Scripps Institutions of Oceanography Library; 2001. pp. 163–182. Available at: http://repositories.cdlib.org/sio/lib/fb/179. [Google Scholar]
- Behnke RJ. Trout and Salmon of North America. New York: Free Press; 2002. [Google Scholar]
- Bley P, Moring JR. Freshwater and Ocean Survival of Atlantic Salmon and Steelhead: A Synopsis. Washington, DC: U.S. Department of the Interior; 1988. U.S. Fish and Wildlife Service, Biological Report 88(9) [Google Scholar]
- Bond MH, Hayes SA, Hanson CV, MacFarlane RB. Marine survival of steelhead (Oncorhynchus mykiss) enhanced by a seasonally closed estuary. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:2242–2252. [Google Scholar]
- Brandes PL, McLain JS. Juvenile Chinook salmon abundance, distribution, and survival in the Sacramento-San Joaquin estuary. In: Brown RL, editor. Fish Bulletin 179(2), Contributions to the Biology of Central Valley Salmonids. San Diego, CA: Scripps Institutions of Oceanography Library; 2001. pp. 39–138. Available at: http://repositories.cdlib.org/sio/lib/fb/179. [Google Scholar]
- Brett JR, Groves TDD. Physiological energetics. In: Hoar WS, Randall DJ, Brett JR, editors. Fish Physiology. VIII. New York: Academic Press; 1979. pp. 279–352. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleberry DT, Cech JJ, Jr, Saiki MK, Martin BA. Growth, Condition and Physiological Performance of Juvenile Salmonids From the American River, February Through June, 1991. Dixon, CA: U. S. Fish and Wildlife Service; 1991. [Google Scholar]
- Castleberry DT, Cech JJ, Jr, Saiki MK, Martin BA. Growth, Condition and Physiological Performance of Juvenile Salmonids From the American River, February Through July, 1992. Dixon, CA: U. S. Fish and Wildlife Service; 1993. [Google Scholar]
- Clark CW, Mangel M. Dynamic State Variable Models in Ecology. Methods and Applications. New York: Oxford University Press; 2000. [Google Scholar]
- Cohen D. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology. 1966;12:119–129. doi: 10.1016/0022-5193(66)90188-3. [DOI] [PubMed] [Google Scholar]
- Cramer SP, Beamesderfer RCP. Population Dynamics, Habitat Capacity, and a Life History Simulation Model for Steelhead in the Deschutes River, Oregon. Portland, OR: Prepared for Portland General Electric; 2006. [Google Scholar]
- Elliott JM, Davison W. Energy equivalents of oxygen consumption in animal energetics. Oecologia. 1975;19:195–201. doi: 10.1007/BF00345305. [DOI] [PubMed] [Google Scholar]
- Fausch KD. Profitable stream positions for salmonids: relating specific growth rate to net energy gain. Canadian Journal of Zoology. 1984;62:441–451. [Google Scholar]
- Good TP, Waples RS, Adams P, editors. Updated Status of Federally Listed ESUs of West Coast Salmon and Steelhead. U. S. Department of Commerce, NOAA Technical Memorandum NMFS-NWFSC-66; 2005. [Google Scholar]
- Harvey BC, Nakamoto RJ, White JL. Reduced streamflow lowers dry-season growth of rainbow trout in a small stream. Transactions of the American Fisheries Society. 2006;135:998–1005. [Google Scholar]
- Hayes SA, Bond MH, Hanson CV, Freund EV, Smith JJ, Anderson EC, Ammann AJ, et al. Steelhead growth in a small central California watershed: upstream and estuarine rearing patterns. Transactions of the American Fisheries Society. 2008;137:114–128. [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Johnsson JI, Rydeborg A, Sundstrom F. Predation risk and the territory value of cover: an experimental study. Behavioral Ecology and Sociobiology. 2004;56:388–392. [Google Scholar]
- Kinnison MT, Hairston NH. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Li HW, Brocksen RW. Approaches to the analysis of energetic costs of intraspecific competition for space by rainbow trout (Salmo gairdneri. Journal of Fish Biology. 1977;11:329–341. [Google Scholar]
- Lindley ST, Schick RS, Mora E, Adams PB, Anderson JJ, Greene S, Hanson C, et al. Framework for assessing viability of threatened and endangered Chinook salmon and steelhead in the Sacramento-San Joaquin basin. San Francisco Estuary and Watershed Science. 2007;5(1):1–26. Article 4. [Google Scholar]
- Lobón-Cerviá J. Why, when and how do fish populations decline, collapse and recover? The example of brown trout (Salmo trutta) in Rio Chaballos (northwestern Spain) Freshwater Biology. 2009;54:1149–1162. [Google Scholar]
- Mangel M. Climate change and salmonid life history variation. Deep Sea Research II. 1994;41:75–106. [Google Scholar]
- Mangel M, Clark CW. Dynamic Modeling in Behavioral Ecology. Princeton, NJ: Princeton University Press; 1988. [Google Scholar]
- Mangel M, Munch SB. A life-history perspective on short- and long-term consequences of compensatory growth. American Naturalist. 2005;166:E155–E176. doi: 10.1086/444439. [DOI] [PubMed] [Google Scholar]
- Mangel M, Satterthwaite WH. Combining proximate and ultimate approaches to understand life history variation in salmonids with application to fisheries, conservation, and aquaculture. Bulletin of Marine Science. 2008;83:107–130. [Google Scholar]
- McClure MM, Carlson SM, Beechie TJ, Pess GR, Jorgensen JC, Sogard SM, Sultan SE, et al. Evolutionary consequences of habitat loss for Pacific anadromous salmonids. Evolutionary Applications. 2008;1:300–318. doi: 10.1111/j.1752-4571.2008.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough DA. A Review and Synthesis of Effects of Alterations to the Water Temperature Regime on Freshwater Life Stages of Salmonids, With Special Reference to Chinook Salmon. Report to the U.S. Environmental Protection Agency, EPA 910-R-99-010; 1999. [Google Scholar]
- McEwan D. Central Valley steelhead. In: Brown RL, editor. Fish Bulletin 179(1), Contributions to the Biology of Central Valley Salmonids. San Diego, CA: Scripps Institutions of Oceanography Library; 2001. pp. 1–43. Available at: http://repositories.cdlib.org/sio/lib/fb/179. [Google Scholar]
- Merz JE. Seasonal feeding habits of steelhead trout in the lower Mokelumne River, California. California Fish and Game. 2002;88:95–111. [Google Scholar]
- Merz JE, Saldate MS. Lower Mokelumne River Fish Community Survey, 1 January 1997 Through 30 June 2004. 2005 Report to East Bay Municipal Utility District, 1 Winemaster Way Suite K2, Lodi, CA 95240. 22pp. [Google Scholar]
- Merz JE, Vanicek CD. Comparative feeding habits of juvenile Chinook salmon, steelhead, and Sacramento squawfish in the lower American River, California. California Fish and Game. 1996;82:149–159. [Google Scholar]
- Meyer GK, Orlob GT, Jokiel C. Effects of climate change on water quality in the Central Valley. In: Kaczmarek Z, editor. Water Resources Management in the Face of Climatic/Hydrologic Uncertainties. Dordrecht: Kluwer Academic Publishers; 1996. pp. 274–299. [Google Scholar]
- Moses ME, Hou C, Woodruff WH, West GB, Nekola JC, Zuo W, Brown JH. Revisiting a model of ontogenetic growth: estimating model parameters from theory and data. American Naturalist. 2008;171:632–645. doi: 10.1086/587073. [DOI] [PubMed] [Google Scholar]
- Myrick CA, Cech JJ. Temperature influences on California rainbow trout physiological performance. Fish Physiology and Biochemistry. 2000;22:245–254. [Google Scholar]
- Pearson GA. An Analysis of the Growth of Returning North Esk Atlantic Salmon (Salmo salar) Over the Period 1963-1993. Bangor: University of Wales; 1993. Master's Thesis. [Google Scholar]
- Piche J, Hutchings JA, Blanchard W. Genetic variation in threshold reaction norms for alternative reproductive tactics in male Atlantic salmon, Salmo salar. Proceedings of the Royal Society of London B. 2008;275:1571–1575. doi: 10.1098/rspb.2008.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TP, Unwin MJ, Kinnison MT. Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Kinnison MT, Unwin MJ. Evolution of chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica. 2001;112:493–513. [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Railsback SF, Rose KA. Bioenergetics modeling of stream trout growth: temperature and food consumption effects. Transactions of the American Fisheries Society. 1999;128:241–256. [Google Scholar]
- Rand PS, Stewart DJ, Seelbach PW, Jones ML, Wedge LR. Modeling steelhead population energetics in Lakes Michigan and Ontario. Transactions of the American Fisheries Society. 1993;122:977–1001. [Google Scholar]
- Raymond HL. Effects of dams and impoundments on migrations of juvenile Chinook salmon and steelhead from the Snake River, 1966 to 1975. Transactions of the American Fisheries Society. 1979;108:505–529. [Google Scholar]
- Rikardsen AH, Thorpe JE, Dempson JB. Modeling the life-history variation of Arctic charr. Ecology of Freshwater Fishes. 2004;13:305–311. [Google Scholar]
- Satterthwaite WH, Beakes MP, Collins E, Swank DR, Merz JE, Titus RG, Sogard SM, et al. Steelhead life history on California's central coast: insights from a state dependent model. Transactions of the American Fisheries Society. 2009;138:532–548. [Google Scholar]
- Schmidt SP, House EE. Precocious sexual development in hatchery-reared and laboratory-maintained male steelhead trout (Salmo gairdneri. Journal of the Fisheries Research Board of Canada. 1979;36:90–93. [Google Scholar]
- Shapovalov L. Biology and Management of Steelhead Trout in California. California: 1967. Dep. Fish Game Inland Fisheries Administrative Report No. 67-7. [Google Scholar]
- Shapovalov L, Taft A. The life histories of the steelhead rainbow trout (Salmo gairdneri) and silver salmon (Oncorhynchus kisutch) with special reference to Waddell Creek, California, and recommendations regarding their management. California Department of Fish and Game Fish Bulletin. 1954;98:1–373. [Google Scholar]
- Shimma H, Kitamura H. Maturation of female yearling amago salmon Oncorhynchus rhodurus and acquisition of fertilized eggs. Bulletin of National Research Institute of Aquaculture. 1987;12:93–96. [Google Scholar]
- Shimma H, Kagawa H, Hirose K. Effect of the fingerling size on the precocious maturation in amago salmon, Onchorhyncus [sic] masou ishikawai. Bulletin of National Research Institute of Aquaculture. 1994;23:55–63. [Google Scholar]
- Slatkin M. Hedging one's evolutionary bets. Nature. 1974;250:704–705. [Google Scholar]
- Snider B, Titus RG. Timing, Composition, and Abundance of Juvenile Anadromous Salmonid Emigration in the Sacramento River Near Knights Landing October1997-September 1998. 2000 California Department of Fish and Game Stream Evaluation Program Technical Report No. 00-5. [Google Scholar]
- Sogard SM, Williams TH, Fish H. Seasonal patterns of abundance, growth, and site fidelity of juvenile steelhead (Oncorhynchus mykiss) in a small coastal California stream. Transactions of the American Fisheries Society. 2009;138:549–563. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Sutherland DF. Distribution, seasonal abundance, and some biological features of steelhead trout, Salmo gairdneri, in the North Pacific Ocean. Fishery Bulletin. 1973;71:787–826. [Google Scholar]
- Thornton KW, Lessem AS. A temperature algorithm for modifying biological rates. Transactions of the American Fisheries Society. 1978;107:284–287. [Google Scholar]
- Thorpe JE, Mangel M, Metcalfe NB, Huntingford FA. Modeling the proximate basis of life history variation, with application to Atlantic salmon, Salmo salar L. Evolutionary Ecology. 1998;121:581–600. [Google Scholar]
- U. S. Department of the Interior. Central Valley Project an State Water Project Operations Criteria and Plan Biological Assessment. Mid-Pacific Region, Sacramento, CA: Bureau of Reclamation; 2008. [Google Scholar]
- VanRheenen NT, Wood AW, Palmer RN, Lettenmaier DP. Potential implications of PCM climate change scenarios for Sacramento-San Joaquin river basin hydrology and water resources. Climatic Change. 2004;62:257–281. [Google Scholar]
- Ward BR. Declivity in steelhead trout recruitment at the Keogh River over the past decade. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:298–306. [Google Scholar]
- Ward BR, Slaney PA. Egg-to-smolt survival and fry-to-smolt density dependence of Keogh River steelhead. In: Gibson RJ, Cutting RE, editors. Production of Juvenile Atlantic Salmon, Salmo salar, in natural waters. Ottawa: Canadian Special Publication of Fisheries and Aquatic Sciences 118; 1993. pp. 209–217. [Google Scholar]
- Ward BR, Slaney PA, Facchin AR, Land RW. Size-biased survival in steelhead trout (Oncorhynchus mykiss): back-calculated lengths from adults’ scales compared to migrating smolts at the Keogh River, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46:1853–1858. [Google Scholar]
- Williams JG. Chinook salmon in the lower American River, California's largest urban stream. In: Brown RL, editor. Fish Bulletin 179(2), Contributions to the Biology of Central Valley Salmonids. San Diego, CA: Scripps Institutions of Oceanography Library; 2001. pp. 1–38. Available at: http://repositories.cdlib.org/sio/lib/fb/179. [Google Scholar]
- Williams JG. Central Valley Salmon: a perspective on Chinook and steelhead in the Central Valley of California. San Francisco Estuary and Watershed Science. 2006;4(3):1–398. Article 2. [Google Scholar]
- Williams JG, Zabel RW, Waples RS, Hutchings JA, Connor WP. Potential for anthropogenic disturbances to influence evolutionary change in the life history of a threatened salmonid. Evolutionary Applications. 2008;1:271–285. doi: 10.1111/j.1752-4571.2008.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D, Galbraith H, Purkey D, Huber-Lee A, Sieber J, West J, Herrod-Julius S, et al. Climate warming, water storage, and Chinook salmon in California's Sacramento Valley. Climatic Change. 2008;91:335–350. [Google Scholar]


