Abstract
Captive rearing often alters the phenotypes of organisms that are destined for release into the wild. Natural selection on these unnatural phenotypes could have important consequences for the utility of captive rearing as a restoration approach. We show that normal hatchery practices significantly advance the development of endangered Atlantic salmon (Salmo salar) fry by 30+ days. As a result, hatchery fry might be expected to face strong natural selection resulting from their developmental asynchrony. We investigated patterns of ontogenetic selection acting on hatchery produced salmon fry by experimentally manipulating fry development stage at stocking. Contrary to simple predictions, we found evidence for strong stabilizing selection on the ontogeny of unfed hatchery fry, with weaker evidence for positive directional selection on the ontogeny of fed fry. These selection patterns suggest a seasonally independent tradeoff between abiotic or biotic selection favoring advanced development and physiological selection linked to risk of starvation in unfed fry. We show, through a heuristic exercise, how such selection on ontogeny may exacerbate problems in restoration efforts by impairing fry productivity and reducing effective population sizes by 13–81%.
Keywords: Atlantic salmon, deleterious domestication, ontogeny, otoliths, phenology
Introduction
The coordination of events in a life cycle is theoretically shaped by both ontogenetic and phenological evolution. In other words, individuals that express the optimal developmental condition (i.e. ontogeny) at the optimal time (i.e. phenology) are expected to have higher lifetime fitness and to thus be favored by natural selection. Although, ontogeny and phenology are often considered jointly, they may often reflect distinct adaptations that interact to shape life-history synchrony. For example, when in an organism's development, and when seasonally, it undergoes a particular life cycle event, it may be affected by growth genes or circadian clock genes respectively. That said, time must to some degree constrain the apparent independence of these traits – a significant developmental delay may constrain whether, or how early, in a season some developmental processes might occur (Brannon 1987). Indeed, temporal linkages among life cycle events may mean that evolution of some ontogenetic and phenological traits are derived, in part, from constraints acting at other life stages (Stearns 1976; Moran 1994; Sinervo and Svensson 1998).
From these principles, one can hypothesize that natural or anthropogenic processes that disrupt the coordination of ontogeny or phenology may reduce the mean fitness of populations, cause population declines or drive further evolution (Bradshaw and Holzapfel 2001; Both et al. 2006). Here, we experimentally assess the strength and pattern of combined ontogenetic/phenological selection in the wild that results from anthropogenic alteration of ontogeny, and consider the potential significance of such selection for the recovery of endangered populations with partial captive propagation.
One common model of the evolution of life cycle synchrony is based on the premise that there is an optimal seasonal window within which individuals should undergo some key life cycle event. This is often referred to as the match–mismatch hypothesis; because individuals that fail to match some life cycle event to this window will suffer reduced performance and fitness (Cushing 1990; Frank and Leggett 1994). This model strongly emphasizes the importance of selection on seasonal timing (phenology), and does not clearly link to selection on ontogenetic variation. Indeed, tests of the match–mismatchhypothesis generally compare the performance of individuals that attain the same developmental state early or late in a season (Armstrong and Nislow 2006). By comparison, an orthogonal design would consider the relative performance of individuals of different developmental condition at the same seasonal time (i.e. controlling for phenology). Such a design would provide a test of ontogenetic selection. By analogy, to the match–mismatch hypothesis, we refer to this as the ‘ready-or-not hypothesis’ in recognition that an organism's developmental ‘readiness’ may often influence its performance and fitness under prevailing environmental conditions. In real life, these two hypotheses are likely more complementary than mutually exclusive. Nonetheless, we believe that match–mismatch and ready-or-not can lead to qualitatively different expectations for the selective consequences of processes that disrupt normal ontogeny.
Population ecologists are often concerned with particular ontogenetic transitions, sometimes referred to as critical periods (Werner and Gilliam 1984), which have the potential to impose especially high mortality on populations. In this sense, critical periods can be a key factor of cohort strength (Elliott 1989, 1990; Sirois and Dodson 2000; Nislow et al. 2004). In many organisms, the period of transition from parental resources (e.g. endosperm, yolk, provisioning) to independent feeding is thought to represent a critical period. Because of the potentially high mortality experienced in this critical period, it is no surprise that many species conservation programs either seek to: (i) greatly increase the total number of juveniles available to enter this period, or (ii) greatly improve juvenile survival through this period (Brown and Day 2002). In fishes, partial captive propagation, where some or all members of a population are bred in captivity and their offspring are released back into the wild (e.g. hatcheries), is often employed to increase numbers of individuals that enter and survive such critical periods or to avert such critical periods altogether.
Although captive propagation programs often seek to release juveniles into the wild at conducive times, captive rearing environments are often quite different from natural environments. Environmental differences may result in considerable disparities in ontogeny and phenology between captive reared individuals and their wild counterparts (Reisenbichler and Rubin 1999; Mackey et al. 2001). If we assume that natural populations are approximately ontogenetically and phenologically adapted, then we might presume that phenotypic asynchrony induced by artificial environments may increase mortality rates of captive individuals released into the wild. Likewise, anthropogenic asynchrony might alter which genotypes perform best in the wild, causing inadvertent selection with concomitant effects on genetic effective population sizes and adaptive diversity.
The objectives of the present study were threefold. First, we assess the degree to which captive propagation alters the ontogeny of endangered salmon fry and their phenology of exposure to stream environments in the wild. Second, we experimentally assess the strength and pattern of ontogenetic selection acting on captive bred salmon released into the wild during the critical period of transition from endogenous yolk to exogenous feeding (alevin to fry transition). In doing so, we directly assess the ready-or-not hypothesis and indirectly assess the match–mismatch hypothesis with respect to the fitness consequences of hatchery activities. Finally, we heuristically assess the potential for developmental asynchrony and natural selection on artificial phenotypes to confound conservation and restoration goals.
Methods
Study system
Atlantic salmon in Maine, USA, return to their natal rivers beginning in spring but the peak of spawning occurs during just a few weeks around late October. Successive spawning events of females usually occur over a few days (Fleming 1996) with effort distributed among several nest sites (redds). Hatching of eggs usually occurs in April, but the young remain in the gravel until the yolk is largely absorbed and emerge from the gravel in May or June of the year to establish feeding territories where they consume drifting stream invertebrates. The majority of emergence from a given redd occurs over a few days but emergence among redds may spread out over several weeks (Mackenzie and Moring 1988; Bardonnet et al. 1993). Although genetic variation and egg size can influence the ontogeny of the egg and larval development period (Beacham et al. 1985), by far the largest determining factors of juvenile development are adult spawning date (which is itself highly heritable –Quinn et al. 2000) and water temperature (Beacham and Murray 1990).
In 2000, the Gulf of Maine Distinct Population Segment (GOM DPS) of Atlantic salmon was listed under the U.S. Endangered Species Act (ESA) due to dangerously reduced spawning runs and low wild juvenile production. In 2009, a decision was made to list additional rivers in Maine under the GOM DPS. The new listing includes populations in Maine rivers from the Androscoggin to the Dennys. Seven of these populations (Machias, Narraguagus, Sheepscot, East Machias, Dennys, Pleasant and Penobscot) are part of a supplemental breeding program at the United States Fish and Wildlife Service's Craig Brook National Fish Hatchery (CBNFH) in East Orland, ME, where river-specific parents produce larvae (fry) that are stocked back into the rivers. A stated goal of this conservation program is to maximize retention of local genetic diversity. To maintain this genetic variability, conservation hatcheries generally strive to minimize variation in parental reproductive success in order to maximize effective population size (Ne) and resulting heterozygosity. Highly variable reproductive contributions among individuals reduce effective population size, resulting in a greater risk of loss of adaptive genetic variation (Ryman and Laikre 1991).
Hatchery rearing to the fry stage bypasses high egg and larval mortality that occurs in redds over winter, and stocking of fry seeks to increase the number that is available to enter the critical period of transition to exogenous feeding. Under standard CBNFH procedures, the fry stocked at any given location are usually derived from matings occurring on a single date and are thus of uniform developmental stage. Very limited efforts are made to synchronize fry ontogeny and seasonal phenology at stocking. Indeed, CBNFH obtains water for salmon rearing and egg incubation from Craig Pond and Alamoosook Lake. The lake sources result in incubation temperatures at CBNFH that are warmer than those encountered in local salmon streams. This is expected to result in hatchery-stocked fry that are advanced in ontological development relative to their wild counterparts (Fig. 1). Such advanced development may similarly lead to early stocking of fry to reduce the risk of fry starvation in captivity or the need for costly feeding. Although some fry may re-enter the gravel following stocking, most fry likely enter the free-flowing stream environment phenologically earlier than their wild counterparts that naturally emerge from the gravel.
Figure 1.
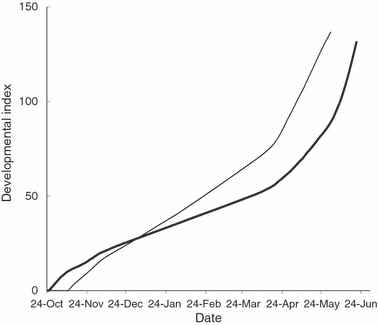
Developmental index of Atlantic salmon (Salmo salar) based on average daily water temperature at Craig Brook National Fish Hatchery 2003–2004 (light line) and at Shorey Brook (bold line), Narraguagus River for the same period.
Given this hatchery regiment, and the match–mismatch hypothesis, we might thus predict that early stocking of advanced fry would result in directional selection favoring the subset of the less developed fry. We predict this on the grounds that under very early stocking, fry that are less developed are expected to pass through critical developmental stages (e.g. initiation of exogenous feeding) at later times, closer to seasonal optima than their more advanced counterparts that might pass through such stages too early. This prediction somewhat assumes that optimal seasonal phenology is more closely approximated by that of wild fry than that of comparatively advanced hatchery fry, but it does not require that the phenology of wild fry be fully optimal itself. Under the ready-or-not hypothesis we might predict the exact opposite; at any given point in time, but particularly under the more stressful conditions of early stocking, natural selection may favor ontogenetically advanced fry that are larger, have superior swimming abilities, are less susceptible to predation, and dominate in competition for feeding territories. Manipulating hatchery rearing conditions allowed us to experimentally evaluate the relative importance of such phenological (match–mismatch) and ontogenetic (ready-or-not) selection under early stocking conditions.
Developmental disparity
To assess quantitatively the pattern and scope of ontogenetic and phenological asynchrony due to hatchery practices, we compared the developmental condition and stocking date of hatchery fry with the predicted developmental condition and emergence timing of fry spawned under normal seasonal chronology and thermal regimes in the wild. Most salmon hatchery programs quantify the developmental condition of fry with Developmental Index (DI) units (Kane 1988), a thermal sum notation in which a value of 100 approximately equates with the temperature units required for initiation of exogenous feeding. We used this convention in estimating fry development condition from hatchery and wild-thermal regimes. We used a date of 25 October for wild-salmon spawning, based on records of redd formation in the wild (E. Atkinson, unpublished data). We consider this a conservative spawn date for our purposes because it is at the beginning of redd building activities. 10 November was the average hatchery spawning date from 2003 to 2007. In fall 2003, both dates had water temperatures between 5 and 6°C. Water temperatures at CBNFH were recorded with a Common Sensing TBO-DL6F (Point Four Systems, Coquitlam, BC, Canada) and water temperatures at Shorey Brook (SB) were based on an in-stream YSI temperature probe (Yellow Springs, OH, USA).
Hatchery procedures and marking of otoliths
We obtained juvenile salmon for our experiments from adult salmon spawned in early- to mid-November of 2006 and 2007. Offspring were raised at CBNFH under typical hatchery practices, with the exception of our experimental manipulations. Eggs, larvae and fry from the same mixed batch of at least eight parental matings were split into 10 groups (SB 2006 only had eight groups). Each group was in turn raised under slightly varying temperature regimes to produce a broad ontogenetic distribution of fry of a similar genetic background. This scheme mimics the amount of family variation stocked at a given site, as well as the variation in developmental stages of hatchery fry that might be stocked out given the range of spawning dates, and thermal regimes present under current hatchery practices.
We distinguished thermally manipulated fry groups using artificial banding patterns on otoliths (Letcher and Terrick 1998). We created distinct banding patterns on otoliths by transferring fry in ambient hatchery temperature troughs/trays to troughs/trays with recirculating water that was either chilled or heated to a 4°C differential for a 24- or 48-h period. In addition, the extended exposure of fish to heated or chilled hatchery water for developmental manipulation also produced distinct banding. Before stocking, a sample of fry was collected for each selection trial to ensure that different banding schemes were distinguishable and to provide a library of reference marks for later comparison. Thermal marking does not influence subsequent fry mortality (Volk et al. 1990).
We thermally manipulated larvae/fry from both the Narraguagus and Penobscot populations. Narraguagus fry were from parents that were captured as parr and reared to maturity in captivity. Penobscot River fry were from a program that uses sea-run parents captured at a fishway. We followed routines consistent with normal hatchery practices for the two stocks. As a result, Penobscot River fry were fed for a short period in the hatchery and Narraguagus fry were not.
Fry stocking and recapture
Narraguagus fry were stocked into SB in 2 years (SB 2006 and SB 2007; Fig. 2). Shorey Brook was chosen because it was the site of an existing detailed mark–recapture study of juvenile salmon ecology. Penobscot River fry were stocked into three tributary sites in 2007: Alder Brook (AB 2007), Kingsbury Stream (KS 2007) and an unnamed tributary of Kingsbury Stream (KT 2007). We chose all study sites to avoid overlap with any natural salmon reproduction (based on redd survey records), and sites were inspected at stocking to verify absence of natural reproduction from the previous fall. No other salmon restoration stocking occurred within 1 km of these study sites.
Figure 2.
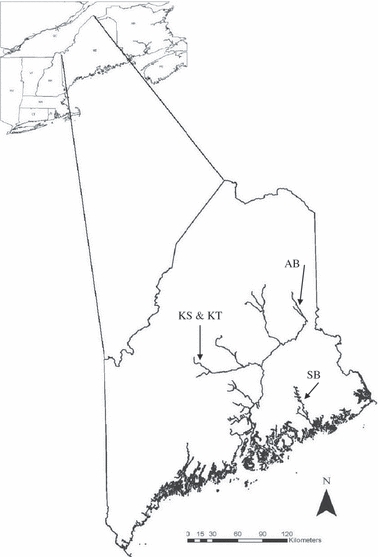
The State of Maine, USA, indicating the streams stocked with marked Atlantic salmon (Salmo salar) fry and later sampled for estimates of mortality.
Fry were stocked for the SB 2006 and SB 2007 trials on 5 May at a density of 50 per 100 m2. Fry were stocked for the AB 2007 trial on 15 May at a density of about 100 per 100 m2 and for the KS 2007 and KT 2007 trials on 17 May at a density of about 100 per 100 m2. Fry were scattered throughout the study sections at stocking to minimize microhabitat effects. Subsequently, fry were sampled approximately 6 weeks after stocking via intensive electrofishing (400–500 V unpulsed DC; Smith-Root Backpack electroshocker) in a haphazard pattern throughout each study site (Table 1). We used small-meshed dipnets to avoid size or age-biased captures. This method of capture is considered to be unbiased with respect to fry size or emergence time (Einum and Fleming 2000). Captured fry were euthanized in water with buffered MS-222 at concentrations of 1000 mg/L and were then transferred to 95% ETOH as a fixative.
Table 1.
Pearson's chi-squared tests of selection based on proportions of fish recaptured within each developmental group relative to proportions released at stocking. Proportions of fry of different DI within stocking groups were similar except for minor discrepancies due to mortality in the hatchery
| Stream | Groups | Total stocked | Days poststocking | Salmon recaptured | Otoliths assigned | Chi-squared P |
|---|---|---|---|---|---|---|
| SB 2006 | 8 | 4271 | 46 | 125 | 114 | <0.001 |
| SB 2007 | 10 | 3916 | 45 | 133 | 126 | <0.010 |
| KS 2007 | 10 | 23 671* | 45 | 115 | 101 | 0.430 |
| KT 2007 | 10 | 11 835* | 45 | 98 | 88 | 0.655 |
| AB 2007 | 10 | 16 341 | 45 | 131 | 82 | 0.117 |
KS and KT were roughly divided from original group of 35 506 2/3rd to KS and 1/3rd to KT.
Otolith processing
We removed both sagittal otoliths from each fish under a dissecting microscope with the aid of polarized light. Otoliths were cleaned and mounted with epoxy on a glass slide for polishing with lapping film. Photos were taken with a compound microscope at 250X magnification. These photos were used to assign individual fish to their original thermal manipulation group by comparing banding patterns with our library of known marks. We compared two independent readers’ interpretations of the otoliths. When these did not agree, the otoliths were resanded and reread. A small number of otoliths were removed from further analysis if readers could not reach agreement or if they were damaged by oversanding.
Detecting and quantifying selection
We did not employ a logistic regression approach to estimate selection coefficients (sensu Janzen and Stern 1998), because this would have required the assumption that any fry that were not recaptured had died. Our resampling was designed to be representative, but not exhaustive (i.e. relative survival versus absolute survival), and consistent with working with an endangered species. Rather, to evaluate overall statistical support for differential selection, the number of fish recaptured from each ontogenetic group was compared to the expected number of returns (based on the proportions released), using a Pearson chi-squared analysis. Deviations from a uniform probability of recapture were considered evidence of selection on DI. To quantify pattern or mode of selection, we used recapture rates (number recaptured/number released) to obtain values of relative fitness for each manipulation group. It is customary for relative fitness to be expressed as the fitness of a phenotype relative to the mean absolute fitness of the population (Lande and Arnold 1983). Hence, relative fitness was calculated by dividing each ontogenetic group's recapture rate by the weighted mean recapture rate for all groups combined (weighting by the number of fry initially released per group). Similarly, we standardized developmental indices to a mean of zero and a standard deviation of one, weighting by the number of fry in each ontogenetic group at release. We then estimated linear and quadratic coefficients of selection using linear regressions of relative fitness on standardized development indices and visualized the pattern of selection using the derived models (Lande and Arnold 1983; Stinchcombe et al. 2008).
Corrected Akaike's information criterion (AICc) was used to assess the best model of mode of selection without over-fitting the models. For this exercise we evaluated a pure linear model, a pure quadratic model (without linear term) and a linear-quadratic model (i.e. akin to a standard quadratic function used in estimating selection coefficients). We also quantified the opportunity for selection (Brodie et al. 1995). Meta-analysis reviews of selection in the wild often discern significant and nonsignificant estimates of selection based on P-values, hence in addition to AICc we also determined P-values for the linear and quadratic modes of selection. We used an alpha level of 0.10 for assessing statistical significance of selection coefficients. This alpha was decided upon a priori due to the conservative nature of our design (i.e. small number of ontogenetic groups available to fit the fitness functions: 8–10), and due to the common power limitations associated with detecting directional and quadratic selection in nature (Hoekstra et al. 2001). Other recent studies of selection have used a similar alpha; with recognition that P-values between 0.05 and 0.10 are considered somewhat less conclusive support for a hypothesis than P-values less than 0.05 (Head et al. 2007; D. Weese, S. Gordon, A. Hendry and M. Kinnison, unpublished manuscript).
Natural rearing versus hatchery rearing
We used a heuristic exercise to better understand the potential demographic and effective population size costs of developmental asynchrony produced by hatcheries. This exercise was conducted by comparing actual hatchery data on distributions of fry development at stocking to our empirical fitness functions for SB. Assuming that similar selection acts throughout the Narraguagus drainage, as supported by intradrainage patterns in other studies (Good et al. 2001) and river systems in Maine (M. M. Bailey and M. T. Kinnison unpublished data), we estimated the percent of population reduction due to mismatch of development at stocking. We did this for the five spawning years, 2003–2007, because we had temperature profiles, spawn date and stocking date data to estimate DI at stocking for all groups of fry stocked into the Narraguagus system in these years (similarly complete data were not available for the Penobscot River system). We subjected the DIs of each stocking group produced by the hatchery to a mean fitness function from the 2006 and 2007 SB trials (obtained by averaging the parameter estimates) to assess the relative performance of the different stocking groups under those selective conditions. We used the average fitness function for this heuristic exercise because the fitness functions in 2006 and 2007 were relatively similar in shape and because their peak performing DIs differed by only a small portion of the total DI stocking range (c. 4 DI vs nearly 40 DI). This mean function was somewhat wider than encountered in either 2006 or 2007, and is thus likely conservative with respect to the potential demographic costs of selection. After imposing selection on the Narraguagus fry, we estimated the expected variation among stocking groups in the mean number of offspring per parent, and used those variances in reproductive success to estimate the anticipated change in effective population size (Ne) relative to the theoretical optima of equal parental contributions across all stocking groups. We estimated Ne using the methods of Crow and Denniston (1988) simplified for diploid individuals and equal males and females (based on Wright's (1931) original concept).
where N is the number of individuals spawned and σ is the variation in fry contributions per parent following application of the fitness function. For our calculations of Ne, we used an average egg-to-female ratio from the Narraguagus River broodstock of 4865.5 eggs/female based on 221 individuals from 2002 to 2005 spawning from the CBNFH (N. F. Wilke and M. T. Kinnison, unpublished data).
Results
As expected, hatchery reared fry experienced substantially advanced development compared to wild fish. Based on recorded temperatures, hatchery reared fish spawned in CBNFH would reach 100 DI on 5 May and naturally reared fish spawned in SB would reach 100 DI on 7 June. Conversely, CBNFH fish would reach well over 135 DI by 7 June, whereas naturally reared fish in SB would reach only 67 DI by 5 May (Fig. 1). Phenologically, fry stocking was thus estimated to have occurred 43 days earlier than predicted natural fry emergence, resulting in a dramatically premature exposure of fry to the free-flowing stream environment. Ontogenetically, variation in spawning date and thermal regimes at the hatchery also resulted in stocking fry at a wide range of DI. For the 5 years of data available on the Narraguagus salmon, fry are estimated to have been stocked at anywhere from 87 to 122 DI.
All recaptured fry had thermal marks on their otoliths, and we were able to classify 86% of the fish to a distinct manipulation group (Table 1). Only SB trials in 2006 and 2007 showed highly biased recapture patterns suggestive of strong selection (2006: χ2 1.8 < 0.01; 2007: χ2 1.10 < 0.001). Nonetheless, linear and quadratic selection coefficients provided evidence of varying modes of selection. In general, linear terms were positive in nearly all trials, albeit only marginally significant for the trial in KS (P = 0.078; β = 0.037), indicating selective mortality against individuals of lower DI. Several trials provided significant evidence for negative quadratic terms (SB 2006, SB 2007 and KS 2007; P ≤ 0.10; Table 2; Fig. 3), suggesting stabilizing selection against developmental extremes at stocking. Shorey Brook models had greater quadratic selection terms than models for Penobscot River sites. Indeed, AICc indicated that pure quadratic selection was a better fit (ΔAICc < 4.0) than models of pure linear or linear plus quadratic selection in both SB trials. Models containing quadratic model were not identified as a better fit for any of the Penobscot River trials (ΔAICc < 4.0; Table 3).
Table 2.
Opportunity for selection (I), linear (β) and linear-quadratic (γ) selection coefficients for developmental index of Atlantic salmon (Salmo salar) fry at stocking in Maine
| Stream | I | β (SE) | P | r2 | γ (SE) | P | r2 |
|---|---|---|---|---|---|---|---|
| SB 2006 | 0.277 | −0.014 (0.068) | 0.850 | 0.006 | −0.091 (0.039) | 0.063 | 0.536 |
| SB 2007 | 0.199 | 0.011 (0.045) | 0.812 | 0.007 | −0.051 (0.022) | 0.056 | 0.432 |
| KS 2007 | 0.100 | 0.03 (0.018) | 0.078 | 0.336 | −0.012 (0.006) | 0.096 | 0.565 |
| KT 2007 | 0.086 | 0.031 (0.018) | 0.113 | 0.283 | 0.002 (0.008) | 0.727 | 0.297 |
| AB 2007 | 0.184 | 0.013 (0.029) | 0.663 | 0.025 | −0.010 (0.012) | 0.459 | 0.103 |
Figure 3.
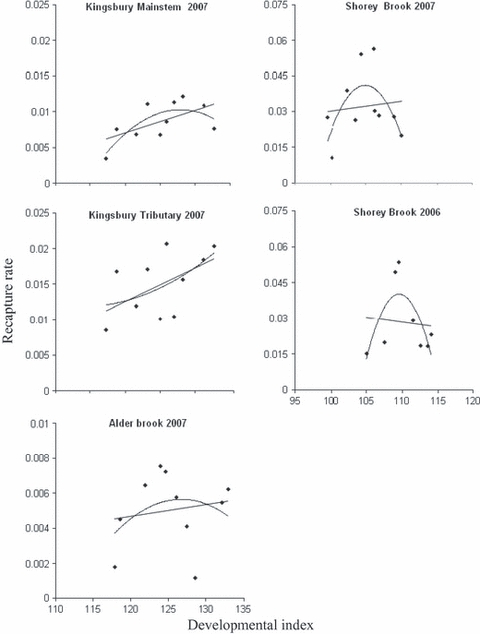
Relative recapture rates by developmental group, and estimated linear (β) and linear-quadratic (γ) selection functions for Atlantic salmon (Salmo salar) fry in Maine.
Table 3.
Models of mode of selection on Atlantic Salmon (Salmo salar) fry (ΔAICc < 4.0 used for model selection). Asterisk indicates model is significant at P < 0.01
| Stream | Linear | Quadratic | Linear-quadratic | Best model fit |
|---|---|---|---|---|
| SB 2006 | 23.318 | 19.241* | 26.562 | Quadratic |
| SB 2007 | 21.127 | 15.567* | 21.546 | Quadratic |
| KS 2007 | 10.206* | 11.298 | 11.977* | All similar |
| KT 2007 | 9.485 | 12.745 | 15.299 | Linear, quadratic |
| AB 2007 | 20.154 | 19.580 | 25.315 | Linear, quadratic |
Heuristically, we found that selection against the hatchery-produced phenotypes could theoretically result in substantial demographic and genetic costs (Fig. 4). Postselection, we estimate that stocked fry could suffer as much as a 24–81% reduction in overall survival (assuming hard selection). In addition, the nonrandom survival of offspring from various spawning groups increased variation in offspring production among adult salmon, and thus greatly reduced the estimated genetic effective population size contributing to the restoration effort (Table 3). Specifically, the average idealized Ne between 2003 and 2007 was 167.7 individuals (27.1 SD). After applying our selection function, average Ne dropped to 63.4 individuals (30.3 SD). This represents over a 2.5-fold reduction from the theoretical optimum (Table 4).
Figure 4.
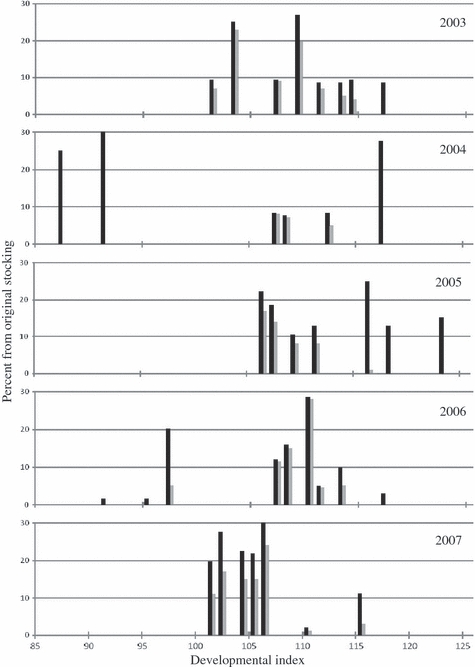
Percentage of Atlantic salmon fry (Salmo salar) stocked into the Narraguagus River (black bars) based on developmental index (2003–2007) and percentage of original numbers stocked (grey bars) remaining after applying an average selection function from 2006 to 2007.
Table 4.
Estimated effects of ontogenetic selection on fry abundance and effective population sizes for entire Narraguagus River population (2003–2007). These heuristic estimates assume a single pattern of hard selection throughout the Narraguagus system
| Estimated effective population size | |||||
|---|---|---|---|---|---|
| Year | Total no. fry stocked | Average DI | % Fry reduction | Before | After |
| 2003 | 350 000 | 108.4 | 24 | 144 | 61 |
| 2004 | 380 000 | 100.7 | 81 | 176 | 32 |
| 2005 | 485 000 | 111.6 | 41 | 192 | 62 |
| 2006 | 341 000 | 107.0 | 32 | 134 | 49 |
| 2007 | 484 000 | 105.0 | 13 | 192 | 113 |
Discussion
We found that captive propagation greatly advances the development of hatchery salmon fry relative to expectations for their wild counterparts. We also found evidence for selective mortality on the development stage of hatchery fry following release into the wild. However, contrary to simple predictions that selection would be strongly directional, due either to fry asynchrony with an optimal seasonal window (match–mismatch) or a general advantage for larger and more advanced fry (ready-or-not), we found stronger evidence for stabilizing selection (significant in two of five trials). That said, the general trend in the linear components of selection was positive in nearly all trials (statistically significant in one). We suggest that these findings for hatchery-stocked fish generally support a modified version of the ready-or-not hypothesis. In the remainder of this discussion, we consider the proximal basis for these patterns of selection, how they might compare with selection on wild fry, and the potential implications of such selection for salmon recovery in Maine.
We have demonstrated a wide disparity in the phenology of fry experiencing natural and hatchery rearing regimes (Fig. 1). Hatchery fry are stocked, and enter the stream environment, roughly a month earlier on average than is expected for their wild counterparts. Early May is typically a time of high and variable stream flows and variable temperatures, whereas June is dominated by more constant stream flow and temperature with an added factor of abundant food resources in the form of dramatic hatches of stream invertebrates. Also significant, we found that variation in hatchery and stocking practices can result in fry being stocked at anywhere from 87 to 122 DI. Although some of these fry may be able to seek shelter back in the gravel, abrupt exposure to stream environments would be expected to have very different consequences across such a large developmental range.
In both years of this study, fry released into SB faced strong stabilizing selective pressures over a relatively small window of development (Table 2). Although selective optima differed modestly between years (DI = 109 in 2006 and DI = 105 in 2007), observed patterns of selection were generally very similar. The slight difference in the optimum fry size between years may relate to higher than average flows during the spring of 2006. Larger fry may be better equipped to cope with such turbulent conditions. Flows in 2007 were more typical for both SB and the Penobscot. Regardless of these slight differences between years, our estimates of quadratic selection for SB fry fall close to the median of other estimates of quadratic selection values in the wild for a wide range of taxa (γ = −0.091 for 2006 = 40th percentile and γ = −0.051 for 2007 = 55th percentile; Kingsolver et al. 2001).
Penobscot fry do not appear to have encountered as strong of a stabilizing selection regime as SB fry. Rather, trends in the three Penobscot groups showed a greater linear component favoring larger fry. Shorey Brook (Narraguagus) and the Penobscot River are geographically disjunct and their fry originate from different parental sources; hence, divergent adaptations or parental rearing regimes may account for some of these differences in patterns of selection. However, we believe the most parsimonious explanation for different patterns of selection is simply that Penobscot fry were fed before stocking, whereas SB (Narraguagus) fry were not (consistent with usual hatchery practices). Affording developmentally advanced fry a chance to feed before stocking theoretically increases the probability of a successful transition from endogenous to exogenous resources (Nislow et al. 2004) and reduces the risk that advanced fry will starve when stocked into the wild. Under such conditions, larger and more advanced fry may fare better in predatory and competitive interactions than less advanced fry. Letcher and Terrick (2001) found that developmentally accelerated, but unfed, fry showed poor survival relative to fed fry or fry that were not fed but stocked at a lesser DI. Indeed, based on lab experiments they inferred that unfed fry were likely approaching starvation within a few days of stocking.
Another factor that might have influenced apparently divergent modes of selection was that Penobscot River fry were stocked at a nominally higher density (50 vs 100 per 100 m2 suitable habitat), which could theoretically place a greater premium on the competitive abilities of larger fry than in SB. However, we think this is less likely as an explanation given that our stocking densities are relatively low for this species, and probably overestimate the differences in functional densities actually experienced by fry. Nonetheless, future experiments can, and should, be designed to test these alternative hypotheses independent of river source and stocking site.
Although hatchery stocking of fry may be viewed as a manipulation of fry ontogeny and phenology in general, it may also be considered a model for ontogenetic selection on the stage at which fry undergo emergence from the gravel. To the degree that stocking mimics forced emergence into the stream environment, our results may reflect that there is moderate to strong selection for emerging at a relatively advanced stage, albeit this is at times balanced by a risk (e.g. starvation) for fry that delay emergence too late. Fry that developmentally delay emergence, or that are stocked unfed at a late stage, may have expended most of their yolk reserves, and may thus have difficulties learning to feed properly, hastening them to a point-of-no-return (Elliott 1989, 1990). Artificial redd experiments have been used to study selection on date (i.e. phenology) and size of fry under more natural conditions of emergence in the wild (Einum and Fleming 2000), but not selection on their ontogenetic stage at emergence.
Phenologically speaking, fry that emerge too early in the spring are often suggested to suffer severe costs (Elliott 1990; Kennedy et al. 2008). Stream-spawned fish that seasonally delay emergence from the gravel are protected from high flows (Erman and Ligon 1988) and predation (Brännäs 1995), and may enter the stream environment at a time closer to peak spring food abundance. Stream conditions postemergence can be a potent selective force on fry emergence timing (Einum and Fleming 2000). In our study system, stocked fry were developmentally advanced relative to their wild counterparts and normal seasonal phenology. However, we did not find support for the match–mismatch hypothesis prediction that selection would predominately favor stocked fry with lower DI, despite the fact that such fry should be in closer synchrony with normal seasonal phenology (and more similar to wild fry in this regard). Admittedly, this constitutes only an indirect test of the match–mismatch hypothesis, as we did not hold fry ontogeny constant while varying release timing. Nonetheless, a tendency for intermediate or advanced fry to be favored at any given point in time is more consistent with the ready-or-not perspective that developmental preparedness is particularly important to fry performance. This finding is consistent with the experimental findings of Einum and Fleming (2000) in which early emerging fry were favored over later emerging fry, albeit that study involved delaying emergence, rather than advancing it as hatcheries do in the present study.
This is not to say that there is not selection for an optimal seasonal window for wild or hatchery fry to enter the natural environment, indeed, other studies have found evidence to support such a window (Letcher and Terrick 2001; Jones et al. 2003; Kennedy et al. 2008). For that matter, it is quite possible that the very early phenology of stocked fry in this system places a premium on more advanced DI, and is in part responsible for the moderate to strong selection we observed. Overall, our findings merely imply that variation in fitness (i.e. selection) associated with ontogenetic preparedness (i.e. ready-or-not) at emergence/stocking (e.g. competitive ability, swimming ability etc) is likely greater than that associated with the precision of phenological synchrony (i.e. match–mismatch), particularly under the prevailingly advanced developmental and stocking conditions associated with hatchery rearing.
Implications for restoration
Within the last couple of decades, managers have seen an increase in evidence that captive propagation can change the genetic features of populations and inadvertently compromise their fitness and sustainability in the wild (reviewed in Reisenbichler and Rubin 1999; Araki et al. 2008). However, the specific selective mechanisms that underlie inadvertent domestication and fitness reductions are poorly understood (Araki et al. 2008). Most hypotheses have tended to focus on the role of inadvertent artificial selection occurring within the period of captive propagation (Reisenbichler and Rubin 1999; McLean et al. 2005; Araki et al. 2008). Very often, the prescription to minimize or counter these effects in most species management plans is to release individuals into the wild as early as possible to maximize exposure to natural patterns of selection (National Marine Fisheries Service and U.S. Fish and Wildlife Service 2005). Our findings highlight a different and potentially confounding source of potential inadvertent selection and fitness loss – natural selection on artificial phenotypes.
Captive rearing environments can significantly alter the phenotypes that are expressed by particular genotypes. Because these changes result from phenotypic plasticity, and are not necessarily heritable, they are usually not perceived as a long-term threat to the genetic health of populations. This perception might be shortsighted. Upon release, ‘artificial’ phenotypes induced by captive environments may face moderate to strong natural selection, as detected in this study. To the extent that artificial propagation disrupts normal phenotype/genotype relationships, exposure of these phenotypes to otherwise normal modes of natural selection could significantly and permanently alter underlying genetic distributions.
Given that (i) the relative timing and DI of fry stocking can be a largely arbitrary product of fry rearing, and (ii) that all of the offspring of particular males or females spawned in captivity usually experience the same date and DI at stocking; it seems very feasible that natural selection on artificial phenotypes may often favor or disfavor various genotypes in ways that bear little similarity to their performance under natural reproduction. Deleterious evolution similarly seems likely given that traits linked to ontogeny and phenology, such as adult spawning time, egg size, and emergence date are quite heritable (Quinn et al. 2000; Kinnison et al. 2001; Carlson and Seamons 2008). Moreover, in program's like Maine's, broodstock derives from stocked fry that are recollected from the wild, potentially compounding deleterious effects on adaptive genetic variation generation to generation. If hatchery operations consistently associate certain genotypes and artificial stocking phenotypes (e.g. early, middle or late spawners) then the natural selection, such as we observed, could ultimately favor the evolution of semi-domesticated genotypes adapted to a combination of early hatchery development and later wild rearing. Insidiously, such domestication may appear as an improvement in the performance of hatchery released fish over time, while masking fitness declines among the fully wild component of the population.
Importantly, this hypothetical outcome is relevant to more aspects of the phenotypes of salmon, and other species, than just phenology and ontogeny. Phenology and ontogeny are convenient traits for demonstrating dramatic phenotypic effects of captive propagation in salmon, but ample evidence shows that hatchery rearing influences phenotypic expression of numerous morphological (Fleming et al. 1994), behavioral (Fleming et al. 1996) and physiological (Fleming et al. 2002) characters in Atlantic salmon and other species (reviewed in Snyder et al. 1996). Further studies like the present one are needed to assess the scope for natural selection on these aspects of artificial phenotypes, and the associated severity of domestication.
Notably, even if the hatchery were largely random with respect to which genotypes experience a given DI in a given year, we have heuristically shown that natural selection on artificial phenotypes is still expected to increase variation in reproductive success and substantially reduce Ne relative to theoretical expectations. This is consistent with the theoretical effects of selection on Ne in general (Nunney and Elam 1994). This genetic cost would be in addition to a potentially significant demographic cost (e.g. 24–82%) of fry abundance. Admittedly, our heuristic demonstration of such effects is crude at best. We extrapolate selection at one site in two years to an entire drainage, extrapolate selective mortality beyond the bounds of our empirical data, and assume hard selection wherein frequency and density-dependent effects are not factored. However, these concerns can be addressed to a degree. We and others have found evidence to support that selection patterns on juvenile salmon can be relatively consistent within drainages in this region (Good et al. 2001; M. Bailey, unpublished data), somewhat justifying our extrapolation to the entire Narraguagus system. Likewise, hatchery experience suggests that unfed fry stocked at extremely low or high DI probably do not survive well in the wild. Although some of the selection that we quantified at particular sites may arise through competition of fry of different DI (i.e. soft selection; Wallace 1975), stocking densities were again notably modest in our selection trials. Moreover, current hatchery practices usually dictate that fry stocked at a given site are of uniform DI, which would largely negate local soft selection on DI and favor hard selection among stocking groups/sites. Regardless, our heuristic analyses should be considered as suggestive of potential demographic and genetic costs and not demonstrative.
How might the potential deleterious consequences of natural selection on artificial phenotypes be mitigated? One obvious measure in the present case would be to spawn adults, incubate eggs and larvae, and stock fry under a regime that more precisely mimics the natural ontogeny and phenology of various genotypes. This would likely take considerable knowledge of natural salmon systems, and a major revision of normal hatchery operations. Alternative rearing systems, such as spawning channels, stream-side incubator, or artificial redds might all aid such an objective. Alternatively, managers might partly mitigate the effects of natural selection on artificial phenotypes by substantially increasing phenotypic DI variation within families, while reducing mean DI variation among families. By spreading the phenotypes of all families out over a similar DI range, the variance in parental reproductive success due to selection on DI would be substantially reduced. Interestingly, such bet hedging may occur to some degree in nature due to the tendency for individuals to naturally spawn over a period of days to weeks (breeding ecology reviewed in Fleming and Reynolds 2004; Fleming 1996).
Acknowledgments
We thank the Craig Brook National Fish Hatchery for use of their facility to mark and raise our study fry. We thank many people from the University of Maine who participated in capturing fry and removing otoliths, especially Stephen Fernandes and Jennifer Bradbury. Funding was provided by a grant through NOAA's National Marine Fisheries Service-Northeast Fisheries Science Center and the Maine Department of Marine Resources Bureau of Sea-Run Fisheries and Habitat. This manuscript was greatly improved by comments from J. Dodson, J. Kocik, J. Trial and J. Zydlewski. This manuscript is contribution 3093 of the Maine Agricultural and Forest Experiment Station.
Literature cited
- Araki H, Berejikian BA, Ford MJ, Blouin MS. Fitness of hatchery-reared salmonids in the wild. Evolutionary Applications. 2008;1:342–355. doi: 10.1111/j.1752-4571.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JD, Nislow KH. Critical habitat during the transition from maternal provisioning in freshwater fish, with emphasis on Atlantic salmon (Salmo salar) and brown trout (Salmo trutta. Journal of Zoology. 2006;269:403–413. [Google Scholar]
- Bardonnet A, Gaudin P, Thorpe E. Diel rhythm of emergence and of first displacement downstream in trout (Salmo trutta), Atlantic salmon (S. salar) and grayling (Thymallus thymallus. Journal of Fish Biology. 1993;43:755–762. [Google Scholar]
- Beacham TD, Murray CB. Temperature, egg size, and development of embryos and alevins of five species of Pacific salmon: a comparative analysis. Transactions of the American Fisheries Society. 1990;119:927–945. [Google Scholar]
- Beacham TD, Withler FC, Morley RB. Effect of egg size on incubation time and alevin and fry size in chum salmon (Oncorhynchus keta) and coho salmon (Oncorhynchus kisutch. Canadian Journal of Zoology. 1985;63:847–850. [Google Scholar]
- Both C, Bouwhuis S, Lessells CM, Visser ME. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännäs E. First access to territorial space and exposure to strong predation pressure: a conflict in early emerging Atlantic salmon (Salmo salar L.) fry. Evolutionary Ecology. 1995;9:411–420. [Google Scholar]
- Brannon EL. Mechanisms stabilizing salmonid fry emergence timing. Canadian Special Publication of Fisheries and Aquatic Sciences. 1987;96:120–124. [Google Scholar]
- Brodie ED, Morre AJ, Janzen FJ. Visualizing and quantifying natural selection. TREE. 1995;10:313–318. doi: 10.1016/s0169-5347(00)89117-x. [DOI] [PubMed] [Google Scholar]
- Brown C, Day RL. The future of enhancements: lessons for hatchery practice from conservation biology. Fish and Fisheries. 2002;3:79–94. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptations to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF, Denniston C. Inbreeding and variance effective population numbers. Evolution. 1988;42:482–495. doi: 10.1111/j.1558-5646.1988.tb04154.x. [DOI] [PubMed] [Google Scholar]
- Cushing DH. Plankton production and year class strength in fish populations: an update of the match/mismatch hypothesis. Advances in Marine Biology. 1990;26:249–293. [Google Scholar]
- Einum S, Fleming IA. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar. Evolution. 2000;54:628–639. doi: 10.1111/j.0014-3820.2000.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Elliott JM. Mechanisms responsible for population regulation in young migratory trout, Salmo trutta I. The critical time for survival. Journal of Animal Ecology. 1989;58:987–1002. [Google Scholar]
- Elliott JM. Mechanisms responsible for population regulation in young migratory trout, Salmo trutta. II. Fish growth and size variation. Journal of Animal Ecology. 1990;59:171–185. [Google Scholar]
- Erman DC, Ligon FK. Effects of discharge fluctuation and the addition of fine sediment on stream fish and macroinvertebrates below a water-filtration facility. Environmental Management. 1988;12:85–97. [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Reviews in Fish Biology and Fisheries. 1996;6:379–416. [Google Scholar]
- Fleming IA, Reynolds JD. Salmonid breeding systems. In: Hendry AP, Stearns SC, editors. Evolution Illuminated: Salmon and Their Relatives. Oxford: Oxford University Press; 2004. pp. 264–294. [Google Scholar]
- Fleming IA, Jonsson B, Gross MR. Phenotypic divergence of sea-ranched, farmed and wild salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:2808–2824. [Google Scholar]
- Fleming IA, Jonsson B, Gross MR, Lamberg A. An experimental study of the reproductive behaviour and success of farmed and wild Atlantic salmon (Salmo salar. The Journal of Applied Ecology. 1996;33:893–905. [Google Scholar]
- Fleming IA, Augustsson T, Finstad B, Johnsson JI, Björnsson BT. Effects of domestication on growth physiology and endocrinology of Atlantic salmon (Salmo salar) Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:1323–1330. [Google Scholar]
- Frank KT, Leggett WC. Fisheries ecology in the context of ecological and evolutionary theory. Annual Review of Ecology and Systematics. 1994;25:401–422. [Google Scholar]
- Good SP, Dodson JJ, Meekan MG, Ryan DAJ. Annual variation in size-selective mortality of Atlantic salmon fry. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1187–1195. [Google Scholar]
- Head ML, Lindholm AK, Brooks R. Operational sex ratio and density do not affect directional selection on male sexual ornaments and behavior. Evolution. 2007;62:135–144. doi: 10.1111/j.1558-5646.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hoang A, Hill CE, Beerli P, et al. Strength and tempo of directional selection in the wild. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9157–9160. doi: 10.1073/pnas.161281098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen FJ, Stern HS. Logistic regression for empirical studies of multivariate selection. Evolution. 1998;52:1564–1571. doi: 10.1111/j.1558-5646.1998.tb02237.x. [DOI] [PubMed] [Google Scholar]
- Jones M, Laurila A, Peuhkuri N, Piironen J, Seppä T. Timing an ontogenetic niche shift: responses of emerging salmon alevins to chemical cues from predators and competitors. Oikos. 2003;102:155–163. [Google Scholar]
- Kane TR. Relationship of temperature and time of initial feeding of Atlantic salmon. The Progressive Fish-Culturist. 1988;50:93–97. [Google Scholar]
- Kennedy BP, Nislow KH, Folt CL. Habitat-mediated foraging limitations drive survival bottlenecks for juvenile salmon. Ecology. 2008;89:2529–2541. doi: 10.1890/06-1353.1. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, et al. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Unwin MJ, Hendry AP, Quinn TP. Migratory costs and the evolution of egg size and number in introduced and indigenous salmon populations. Evolution. 2001;55:1656–1667. doi: 10.1111/j.0014-3820.2001.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Letcher BH, Terrick TD. Thermal marking of Atlantic salmon otoliths. North American Journal of Fisheries Management. 1998;18:406–410. [Google Scholar]
- Letcher BH, Terrick TD. Effects of developmental stage at stocking on growth and survival of Atlantic salmon fry. Transactions of the American Fisheries Society. 2001;12:102–110. [Google Scholar]
- Mackenzie C, Moring JR. Estimating survival of Atlantic salmon during the intragravel period. North American Journal of Fisheries Management. 1988;8:45–49. [Google Scholar]
- Mackey G, McLean JE, Quinn TP. Comparisons of run timing, spatial distribution, and length of wild and newly established hatchery populations of steelhead in Forks Creek, Washington. North American Journal of Fisheries Management. 2001;21:717–724. [Google Scholar]
- McLean JE, Bentzen P, Quinn TP. Nonrandom, size- and timing-biased breeding in a hatchery population of steelhead trout. Conservation Biology. 2005;19:446–454. [Google Scholar]
- Moran NA. Adaptation and constraint in the complex life cycle of animals. Annual Review of Ecology and Systematics. 1994;25:573–600. [Google Scholar]
- National Marine Fisheries Service and U.S. Fish and Wildlife Service. Recovery Plan for the Gulf of Maine Distinct Population Segment of Atlantic Salmon (Salmo salar) Silver Spring, MD: National Marine Fisheries Service; 2005. [Google Scholar]
- Nislow KH, Einum S, Folt CL. Testing predictions of the critical period for survival concept using experiments with stocked Atlantic salmon. Journal of Fish Biology. 2004;65:188–200. [Google Scholar]
- Nunney L, Elam DR. Estimating the effective population size of conserved populations. Conservation Biology. 1994;8:175–184. [Google Scholar]
- Quinn TP, Unwin MJ, Kinnison MT. Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced Chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Reisenbichler RR, Rubin SP. Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES Journal of Marine Science. 1999;56:459–466. [Google Scholar]
- Ryman N, Laikre L. Effects of supportive breeding on the genetically effective population size. Conservation Biology. 1991;5:325–329. [Google Scholar]
- Sinervo B, Svensson E. Mechanistic and selective causes of life history trade-offs and plasticity. Oikos. 1998;83:432–442. [Google Scholar]
- Sirois P, Dodson JJ. Critical periods and growth-dependent survival of larvae of an estuarine fish, the rainbow smelt Osmerus mordax. Marine Ecology. Progress Series. 2000;203:233–245. [Google Scholar]
- Snyder NFR, Derrickson SR, Beissinger SR, Wiley JW, Smith TB, Toone WD, Miller B. Limitations of captive breeding in endangered species recovery. Conservation Biology. 1996;10:338–348. [Google Scholar]
- Stearns SC. Life-history tactics: a review of the ideas. The Quarterly Review of Biology. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution. 2008;62:2435–2440. doi: 10.1111/j.1558-5646.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- Volk EC, Schroder SL, Fresh KL. Inducement of unique otolith banding patterns as a practical means to mass mark juvenile Pacific salmon. American Fisheries Society Symposium. 1990;7:203–215. [Google Scholar]
- Wallace B. Hard and soft selection revisited. Evolution. 1975;29:465–473. doi: 10.1111/j.1558-5646.1975.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Werner EE, Gilliam JF. The ontogenic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics. 1984;15:393–426. [Google Scholar]
- Wright S. The statistical theory of evolution. In: Provine WB, editor. Evolution: Selected Papers. Chicago: University of Chicago Press; 1931. pp. 88–96. Reprinted In 1986. [Google Scholar]


