Abstract
High-impact biological invasions often involve establishment and spread in disturbed, high-resource patches followed by establishment and spread in biotically or abiotically stressful areas. Evolutionary change may be required for the second phase of invasion (establishment and spread in stressful areas) to occur. When species have low genetic diversity and short selection history, within-generation phenotypic plasticity is often cited as the mechanism through which spread across multiple habitat types can occur. We show that trans-generational plasticity (TGP) can result in pre-adapted progeny that exhibit traits associated with increased fitness both in high-resource patches and in stressful conditions. In the invasive sedge, Cyperus esculentus, maternal plants growing in nutrient-poor patches can place disproportional number of propagules into nutrient-rich patches. Using the invasive annual grass, Aegilops triuncialis, we show that maternal response to soil conditions can confer greater stress tolerance in seedlings in the form of greater photosynthetic efficiency. We also show TGP for a phenological shift in a low resource environment that results in greater stress tolerance in progeny. These lines of evidence suggest that the maternal environment can have profound effects on offspring success and that TGP may play a significant role in some plant invasions.
Keywords: annual plants, competitive ability, environmental stress, inclusive fitness, maternal environmental effects, phenotypic plasticity, propagule dispersal, resource patch, seed size, spatial heterogeneity
Introduction
Post-invasion ecotypic variation in introduced environments (Rice and Mack 1991b; Sexton et al. 2002; Maron et al. 2004) can occur and may be the result of adaptation, genetic drift, within-generation plasticity, trans-generational plasticity (TGP; i.e. maternal environmental effects) or combinations of these evolutionary responses. In this study, we focus on the potential importance of TGP in facilitating plant invasions. Within-generation phenotypic plasticity (PP) is recognized as an important contributor to the establishment and spread of some invasive plant species (Baker and Stebbins 1965; Bradshaw 1965; Baker 1974; Novak et al. 1991; Rice and Mack 1991a; Williams and Black 1996; Sakai et al. 2001). The important role of plastic responses is not surprising given that most populations experience strong demographic contractions during the dispersal process. These demographic contractions are often, but not always, correlated with reductions in genetic diversity; post-invasion adaptive potential is predicted to be highly reduced as a direct consequence (Barrett and Kohn 1991; Ghalambor et al. 2007).
The period of population expansion during invasion is not a uniform process, but can be divided into two phases to make a distinction between two time periods differentially governed by demographic and evolutionary processes (Dietz and Edwards 2006). In the primary phase, spread of invasive species into high-resource environments is strongly influenced by the dynamics of dispersal, nutrient availability, disturbance and propagule pressure. In the secondary phase, further population expansion is constrained by biotic and abiotic factors and evolutionary changes might be necessary before further spread into stressful habitats is possible. In this study, we make a distinction between simple PP and TGP, and we show that TGP can be important in both phases of invasion by increasing offspring fitness in both high-resource and stressful environments.
While PP is a within-generation adjustment to current conditions, TGP is a mechanism by which parental responses may pre-condition offspring for the environment they are most likely to encounter (Mousseau and Fox 1998; Galloway 2005). TGP is a maternal environmental effect whereby plastic responses of individuals to environmental cues influence the phenotype and fitness of their progeny (Roach and Wulff 1987; Donohue and Schmitt 1998; Mousseau and Fox 1998; Agrawal et al. 1999). Thus, PP is the individual's response to variation in current environmental conditions, whereas TGP is a response to the maternal environment expressed in the progeny generation (Galloway 2005).
Phenotypic plasticity and TGP are two ways through which plants can achieve positive population growth in a landscape of heterogeneous resource patches. When encountering a new set of resource conditions (e.g. crossing a patch boundary), individuals may rely on PP for survival, but beyond the first generation, TGP will be of greater value because it is more efficient than PP. Both PP and TGP require sensing environmental cues and responding appropriately. However, in PP, the seedling performs this role, thus introducing a time delay in adaptive response (Weinig 2000). TGP allows maternal plants to provision 100% of offspring with the adaptive phenotypic state (Jablonka and Lamb 1989; but see Villa-Aiub et al. 2003), thus potentially eliminating the delay in adaptive response. In stressful environments, TGP can provision offspring adaptively so that the stress is only experienced by the maternal plant and is minimized for the progeny (Donohue and Schmitt 1998). In this study, we give an example of TGP for earlier flowering in Aegilops triuncialis; a phenological response that reduces drought stress in this annual grass invader.
Primary phase invasion will be facilitated by habitat disturbance that creates high-resource, low-competition patches (Dietz and Edwards 2006). One of the drivers of the primary phase of invasion into a high-resource patch is propagule fitness and dispersal. Both PP and TGP can increase plant fitness in these high-resource patches. Propagule size, nutrient content, dormancy and heteromorphism are all variables affected by the maternal plant that influence progeny growth and survival (Harper et al. 1970; Gutterman 2000). Clonal plants can preferentially place ramets and propagules in response to environmental signals correlated with high-resource patches (de Kroon and Hutchings 1995). A number of studies have shown that feedback from roots and rhizomes generate an integrated physiological response from maternal plants resulting in an adaptive phenotypic response (Alpert 1994; Gruntman and Novoplansky 2004). This plastic interaction between the maternal plant and the soil environment allows plants to modify internal resource allocation such that propagules can be optimally placed with respect to resource availability (Evans and Cain 1995; van Kleunen and Fischer 2001). In this way, the PP necessary for invading new habitats would be complemented by TGP to ensure reproductive output after the first generation. In this study, we present evidence for this type of dispersal TGP in the invasive sedge Cyperus esculentus.
Secondary phase invasion depends on dispersal across resource boundaries and into patches with much higher abiotic and biotic stress (Dietz and Edwards 2006). It is here that we suggest TGP can play an even more important role for invasive species. In this stage of invasion, evolutionary processes are important for population expansion in high stress areas. In contrast to several examples from animal species (Vellend et al. 2007), many invasive plant species exhibit low genetic diversity during initial colonization (Dlugosch and Parker 2008). In the face of this low genetic diversity, coupled with a short residence time during which selection can act (Antonovics 1976), TGP provides a mechanism by which maternal influences on progeny morphology or physiology can increase stress tolerance and increase fitness of progeny in stressful environments. A TGP response will result in more rapid population expansion in stressful habitats compared with a PP response because of the time delay in accruing fitness benefits of adaptive PP. Below, we show that TGP can confer greater fitness to progeny in the invasive species Aegilops triuncialis on stressful soils by (i) accelerating phenology such that seasonal resource stress is reduced in drier environments and (ii) down-regulating the photosynthetic rate which results in larger, more fecund plants.
The importance of maternal effects on the phenotypes of offspring has been documented in a number of previous studies (reviews in Mousseau and Fox 1998; Sultan et al. 2009), but the adaptive significance of TGP in plants has not been widely tested (Donohue and Schmitt 1998). We chose to utilize the term ‘transgenerational plasticity’ (Agrawal et al. 1999), rather than ‘maternal environmental effects’ (Roach and Wulff 1987) because (1) for the many invasive plant species that are entirely selfing or clonally reproducing, maternal and paternal environmental effects cannot be partitioned and (2) the particular parental environmental effects under study here persist long into the life-cycle of the next generation, which is usually not the case for maternal environmental effects (Roach and Wulff 1987). Parental environmental effects have traditionally been viewed as undesirable noise in the design and analysis of experiments, but they can have important implications for parental fitness. For example, adaptive TGP has been shown to occur in response to light availability (Galloway and Etterson 2007; Bell and Galloway 2008 and references therein) and drought stress (Sultan et al. 2009) in native and naturalized exotic species.
Demonstrating the adaptiveness of any trait is fraught with difficulty (Maynard Smith 1978) because very few traits are adaptive in all environmental conditions. Because we conducted TGP studies across environmental gradients, we are able to assess the potential for traits to influence fitness in multiple habitats (Donohue and Schmitt 1998) and to influence population expansion in primary and secondary phases of invasion. The sections that follow represent some of the best available data for adaptive TGP in propagule dispersal, phenology and photosynthetic efficiency and represent some of the best available data on adaptive TGP in invasive plant species. First we describe the experimental framework for each TGP outcome (dispersal, phenology and photosynthetic efficiency), followed by the methods for each study. We then present the results and discuss their implications for the ecology and evolution of some invasive species.
Differential propagule dispersal
We grew genetically identical individuals of Cyperus esculentus, a sedge that reproduces via belowground tubers, under several soil resource treatments to determine whether maternal plants preferentially place tubers into nutrient-rich patches via rhizomes. By eliminating variation among genotypes, we were able to test the response of the maternal plant to spatial resource variation. In sexually reproducing species, this design would be approximated by the use of full-sib families. Under uniform resource conditions, we predicted that the distribution of different measures of biomass should decrease linearly from the point of sowing away from the maternal plant as roots foraged only far enough to obtain needed resources. However, under heterogeneous resource conditions, we predicted that root density would be higher in areas with greater resource availability. If this form of dispersal TGP is adaptive, the maternal plant should place a greater proportion of propagules within higher resource patches.
Phenology
Summer drought stress is one of the most important constraints on plant survival and reproduction in Mediterranean systems (Aragon et al. 2008). In a mosaic of soil types and textures, the temporal availability of soil moisture is variable even if rainfall patterns are the same across the landscape. The ability to flower and set seed successfully under different soil drying rates is critical for annual plants in Mediterranean systems; and plants with accelerated phenologies are more likely to complete their reproductive cycle before soil water is depleted (Donohue 2003). Soil drying can significantly alter patterns of maternal allocation to seeds because plants dynamically assign resources to seeds over time (Aragon et al. 2008). Maternal plants abort ovules or reduce seed size as resources are depleted. Plasticity in flowering time can be adaptive if annual plants that sense a drier environment flower earlier, thus increasing the amount of time available to produce and fill seeds prior to death by drought (Volis et al. 2002a,b, 2004; Strauss et al. 2006). However, in general, and under less stressful conditions, plants that flower later have been found to produce larger seeds that, in turn, produce larger and more competitive progeny (Volis et al. 2002b, 2004). Plants that can respond dynamically to environmental cues are therefore more likely to reproduce successfully if they have the capacity to respond to resource variation by either producing small seeds earlier under maternal drought or fewer large seeds under less stressful maternal conditions.
We grew Aegilops triuncialis, an invasive annual grass, from serpentine and loam source populations in a common garden for two generations to assess PP and TGP effects related to performance in edaphically stressful conditions. This species is currently invading serpentine habitats (California Exotic Pest Plant Council 1999) that represent a mosaic of serpentine and loam soils. Serpentine soil dries more quickly than loam soil, and plants growing on serpentine are under greater water stress (Sambatti and Rice 2006). Positive population growth in the second phase of invasion, in stressful habitats, may be facilitated by a TGP response in flowering time that is more efficient than PP. We first tested whether earlier flowering is adaptive on serpentine in generation one and then looked for TGP response in generation two with earlier flowering in progeny from serpentine-grown maternal plants. If larger seeds are linked to delayed flowering time and reduced fitness on serpentine, we expected smaller seeds to be produced from progeny of maternal plants growing on serpentine soils.
Stress tolerance
Serpentine soils are characterized by very low levels of macronutrients, low Ca/Mg ratios and by high concentrations of metals. Overall, serpentine soils are an excellent example of a habitat type that should select for genotypes adapted to edaphic stress (i.e. ‘the stress tolerance syndrome’ of Chapin et al. 1993). As noted above, the secondary phase of a plant invasion may involve the development of tolerance to more stressful, invasion-resistant habitats (Dietz and Edwards 2006). A prediction of the stress tolerance syndrome is that photosynthesis will decrease as the photosynthate source:sink ratio increases (Chapin et al. 1989, 1993). Larger seeds typically produce seedlings with a high amount of photosynthetic leaf area relative to the mass of meristematic tissues. Larger seedling leaf size in progeny from mothers growing on serpentine soils should increase the source (leaf):sink (meristem) ratio and thus reduce photosynthetic rates. We used both common garden and reciprocal transplant approaches to test for TGP effects on photosynthesis and fitness in Ae. triuncialis genotypes exposed to stressful soil conditions.
Methods
Differential propagule dispersal
Cyperus esculentus L. (yellow nutsedge, Cyperaceae) is a widespread, annual, C4 species introduced to the United States from the Mediterranean and North Africa. Cyperus esculentus reproduces clonally from tubers produced at the terminal ends of spreading rhizomes that remain attached to the parent plant until senescence at the end of the growing season. Allocation to inflorescences is ≤2% of biomass (Williams 1982), and very little natural recruitment from seed is observed (Stoller and Sweet 1987). Genetic variation is very low with fewer than two genotypes on average per population (Ellstrand and Roose 1987; Horak et al. 1987). Consistent with species in the primary phase of invasion, C. esculentus is problematic in disturbed soils where it reproduces asexually in very large numbers (Tumbleson and Kommedahl 1961). It is highly competitive, particularly belowground (Li et al. 2001). The low population-level genetic variation and the ability for a few genotypes to dominate diverse habitats suggest that adaptive TGP is likely to be important to survival and establishment in this species, particularly in the primary phase of invasion.
We grew two pre-sprouted, C. esculentus tubers at one end of flat plastic trays (50 cm × 25 cm × 5 cm) in low-nutrient commercial top soil, 3 cm deep, in a glasshouse. We applied three treatments: no additional nutrients (no nutrients), ∼30 g of pelleted slow-release fertilizer (10–10–10 NPK) spread uniformly on the surface of the soil (uniform) and ∼5 g of pelleted fertilizer placed along the end of the tray opposite the tubers (patch). Treatments were replicated five times. Trays were watered as needed, but not saturated to avoid lateral movement of labile nutrients. After 8 weeks, we divided the trays lengthwise into three equal sections and collected shoot mass, root mass, tuber mass and tuber number from each section. To account for the effect of nutrients on plant size, we converted all individual measures to proportion of the total per tray and compared the transformed proportions (arcsine square root) using 2-way ANOVA comparing treatments within tray sections or 1-way ANOVA on data from the section furthest from point of sowing only.
Phenology
Aegilops triuncialis (barbed goatgrass, Poaceae) is a selfing, annual, allotetraploid, C3 grass with a native range throughout Europe, Asia and the Mediterranean Basin. In California, it is invasive and occurs on many soil types, but has highest population growth rates on well-drained, low fertility soils (K. J. Rice and J. M. McKay, unpublished data). Aegilops triuncialis has the unusual capacity to invade native plant communities on abiotically stressful serpentine soils (California Exotic Pest Plant Council 1999). Molecular analyses indicate an extreme post-introduction genetic bottleneck with only three multilocus genotypes currently identified in its California range (Meimberg et al. 2006; J. M. McKay and K. J. Rice, unpublished data). As a result of this bottleneck, adaptive TGP is likely to be of great importance for further range expansion in Ae. triuncialis.
Maternal generation
Seeds from nine serpentine and nine non-serpentine California populations were weighed and individually planted in containers (3.8 cm × 21 cm) into either serpentine or loam soil in Davis, CA, USA. Fifteen seeds per population were sown in each soil type. Plants were moved into the glasshouse at start of flowering. We reduced water application to serpentine pots to one-third of that supplied to loam pots at this time and we continued to water until flowering was completed.
Progeny generation
Thirty seeds per soil treatment from each population were individually weighed and planted in containers filled with loam soil. Plants were germinated in the glasshouse and then moved outdoors. Plants were moved into glasshouse at start of flowering.
Data collection
For each generation, we noted the date of flowering for each plant and at harvest we recorded the number of seeds produced and total seed mass (mg) and calculated average seed size produced (total seed mass/number of seeds). Seedling size was measured 4 weeks after sowing in generation two.
Data analysis
The variables total seed mass and number of seeds produced were significantly and highly correlated, and the experimental results for each of these variables were parallel. Therefore, we present only the analyses for total seed mass. Only plants that produced seeds are included in the analysis. Data were analysed using ANOVA in jmp 7.1 (SAS Institute, Inc., Cary, NC, USA). Dependent variables for first generation plants were flowering date, total seed mass and seed size. In addition to the effects of experimental treatments, we also included planted seed weight and plant genotype in the statistical models. Maternal effects are often caused by differences in seed weight; including seed mass as a covariate allowed us to identify effects beyond those related to variation in individual seed weight alone (Sambatti and Rice 2006). Each of the populations from which we collected seed belonged to one of three distinct genotypic groups as determined by microsatellite analysis (H. Meimberg, N. F. Milan, M. Karatassiou, E. K. Espeland, J. K. McKay, and K. J. Rice, unpublished data). Although all genotypes occur on all soil types, the three genotypes are not evenly distributed among source soil types; therefore, it was appropriate to include genotype group as an explanatory variable. In summary, the explanatory variables in the statistical model were block, genotype group, source soil, growing soil, the growing soil by genotype interaction and the growing soil by source soil interaction (Table 1A). Mass of the planted seed was used as a covariate. For the progeny generation, dependent variables were seedling size, date of flowering, total seed mass and seed size. Effects in the statistical model were block, genotype, source soil, maternal soil, the maternal soil by genotype interaction and the maternal soil by source soil interaction. Maternal family was used as the unit of replication.
Table 1.
Statistical tables for the Aegilops triuncialis phenology study, showing the effects of genotype and soil type on plant phenology and seed production P-values <0.05 are shown in bold. d.f. = degrees of freedom; SS = sums of squares
| (A) First generation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flowering date | Total seed mass | Seed size | ||||||||
| d.f. | SS | F-ratio | P-value | SS | F-ratio | P-value | SS | F-ratio | P-value | |
| Block | 1 | 43.6 | 0.60 | 0.441 | 161.3 | 2.02 | 0.156 | 5.6 | 0.35 | 0.553 |
| Genotype | 2 | 26698.1 | 182.28 | <0.0001 | 1880.6 | 11.76 | <0.0001 | 2040.6 | 64.49 | <0.0001 |
| Source soil | 1 | 366.6 | 5.01 | 0.026 | 235.7 | 2.95 | 0.087 | 113.2 | 7.14 | 0.008 |
| Growing soil | 1 | 18.1 | 0.25 | 0.620 | 727.5 | 9.10 | 0.003 | 13.0 | 0.82 | 0.366 |
| Growing soil × genotype | 2 | 449.6 | 3.07 | 0.048 | 143.6 | 0.90 | 0.408 | 46.0 | 1.45 | 0.235 |
| Growing soil × source soil | 1 | 172.5 | 2.36 | 0.126 | 148.1 | 1.85 | 0.175 | 1.6 | 0.10 | 0.749 |
| Planted seed weight | 1 | 7.3 | 0.10 | 0.752 | 260.8 | 3.26 | 0.072 | 56.7 | 3.46 | 0.063 |
| (B) Second generation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seedling size | Flowering date | Total seed mass | Seed size | ||||||||||
| d.f. | SS | F-ratio | P-value | SS | F-ratio | P-value | SS | F-ratio | P-value | SS | F-Ratio | P-value | |
| Genotype | 2 | 32.2 | 8.28 | 0.0003 | 3379.8 | 43.1 | <0.0001 | 5056.3 | 5.39 | 0.005 | 732.9 | 109.2 | 0.0001 |
| Source soil | 1 | 1.6 | 1.23 | 0.267 | 3.8 | 0.098 | 0.755 | 669.4 | 1.43 | 0.233 | 1.9 | 0.57 | 0.453 |
| Maternal soil | 1 | 0.03 | 0.018 | 0.893 | 1.0 | 0.098 | 0.873 | 1008.3 | 2.15 | 0.144 | 4.4 | 1.31 | 0.253 |
| Mat. soil × genotype | 2 | 0.07 | 0.025 | 0.976 | 23.1 | 0.295 | 0.744 | 482.8 | 0.52 | 0.598 | 5.5 | 0.83 | 0.439 |
| Mat. soil × source soil | 1 | 1.8 | 1.32 | 0.252 | 23.7 | 0.604 | 0.438 | 429.9 | 0.92 | 0.339 | 0.02 | 0.01 | 0.934 |
| Planted seed weight | 1 | 55.5 | 40.70 | <0.0001 | 603.2 | 15.38 | <0.0001 | 3395.3 | 8.52 | 0.038 | 11.6 | 3.4 | 0.065 |
(A) Serpentine and non-serpentine field-collected seed grown on loam and serpentine soil. (B) Seed from first generation grown only on loam soil.
Stress tolerance
Split family common garden design
Seeds were collected from 10 maternal families from each of four Ae. triuncialis populations located at sites of serpentine invasion in the Northern Coast range of California. The four populations were located at the U.C. McLaughlin Reserve (38º51′41″N; 122º24′28″W), Snell Valley Reserve (38º41′56″N; 122º24′24″W), Bear Valley Road (39º04′55″N; 122º24′38″W) and the U.C. Hopland Research and Extension Center (39º00′10″N; 122º06′03″W). A common garden with contrasting soil treatments was prepared at the Agronomy Farm of the University of California, Davis (38º32′23″N; 121º47′19″W). Planting holes were filled with serpentine soil (Henneke and Montara soil series from the McLaughlin site) or refilled with local soil (Reiff sandy loam). Ten maternal families from each site were split and planted in both serpentine and non-serpentine soil in a completely randomized design. Inflorescences were harvested as they matured. Inflorescences were air-dried (to maintain seed viability) for 6 months in the laboratory and then seeds were weighed.
Clone reciprocal transplant design
Seeds from 10 maternal families collected at the McLaughlin and Snell Valley serpentine sites were planted into peat-based soil and grown in a growth chamber with 15 h days at 23/13°C day/night. Plants were kept fertilized and well-watered. After 60 days of growth, tillers of an individual were divided to produce clones. Six randomly selected clones from both McLaughlin and Snell Valley were divided into separate tillers and grown under shorter days (12/12 h day/night) and cooler temperature (18/8°C day/night) to acclimate them to field conditions. After 20 days of growth, the clones were transplanted into both serpentine and loam soil sites at the McLaughlin and Snell Valley locations on 5 February 2003. Clones were planted only into the site of their origin (e.g. McLaughlin clones were planted only at the McLaughlin site). Growth and phenology of the reciprocal transplants were monitored throughout the spring. Inflorescences from these reciprocally transplanted clones were collected as they matured, dried at room temperature and seeds from each inflorescence were individually weighed.
Progeny generation common environment (growth chamber)
Seeds from both the split family common garden and the clone reciprocal transplant experiment were weighed and then planted into a growth chamber in a completely randomized design. To mimic the low-nutrient soils in the field, we used 1:1 sand:fritted clay containing only trace macronutrients and plants were only fertilized twice with 3 mL of a complete nutrient solution (Epstein and Bloom 2005). This resulted in adult plants that had similarly low mean leaf N concentration (0.81%) as field-collected plants (J. M. McKay and K. J. Rice, unpublished data). Light levels in the growth chamber were 370 μmol/m2/s PPFD, photoperiod was 12 h light/12 h dark and the temperature cycled 23/13°C day/night.
Photosynthetic capacity and plant fitness analyses
Photosynthetic rates of progeny were measured using a Licor 6400 with a narrow leaf chamber (LiCor Inc., Lincoln, NE, USA). A minimum of 20 repeated measures were averaged to obtain a single estimate of photosynthetic rate for each plant. Leaves were scanned and area determined using a series of macros for Photoshop (Adobe, Seattle, WA, USA) and Scion Image (Scion, Frederick, MD, USA). Aboveground plant material was dried at 60°C for 72 h and weighed. Total aboveground plant dry weight (family study) and total leaf area (clone study) were used as indices of fitness.
Photosynthesis, plant dry weight and total leaf area data were analysed in a hierarchical, mixed ANOVA model (SAS Institute, Inc.). The SAS MIXED procedure was not used because the PROC MIXED approach is inappropriate when the number of levels of the random factors in the model is relatively few (Littell et al. 2006). In the split family analysis, family was nested within population and maternal soil type was crossed with both population and family. Maternal family was considered a random factor while maternal soil type and population were treated as fixed factors. In the clone reciprocal transplant analysis, clone genotype was nested within population and maternal soil type was crossed with both clone genotype and population. Clone genotype was considered a random factor while maternal soil type and population were treated as fixed factors. In the clone analyses, photosynthetic rates were natural log transformed to reduce heterogeneity of variance. In both the split family and the clone analyses, individual planted seed weight was included as a covariate in the analysis. At Snell Valley all clones survived to reproduction while at McLaughlin only three of the original six clones produced seeds.
Results and discussion
Differential propagule dispersal in Cyperus esculentus
Clones growing in either uniform or no nutrient treatments showed no strong increase in the distribution of shoot, root, or tuber biomass, or the number of tubers in the section farthest from the maternal section (where plants were sown) (Fig. 1). The maternal section contained the majority of biomass and tubers and the third section the least. However, the patch treatment plants had lower proportions of reproductive biomass (tuber mass and tuber numbers) in the maternal section and significantly higher proportions in the patch section compared with the other treatments (treatment × section: tuber number, F4,36 = 6.248, P < 0.001; tuber mass, F4,36 = 2.8545, P < 0.05; Fig. 1). The treatment by section interaction was not significant for shoot (F4,36 = 1.217, P = 0.321) and root biomass (F4,36 = 1.406, P = 0.252), however, the ANOVA on root biomass showed the patch treatment root mass was significantly greater in the patch (F1,28 = 62.148, P < 0.001). In our patch treatment, more tubers and greater tuber biomass were produced in the enriched patch soil, compared with non-patch treatments, indicating that production of tuber-producing rhizomes were directed disproportionally from the maternal plant into the higher resource patch.
Figure 1.
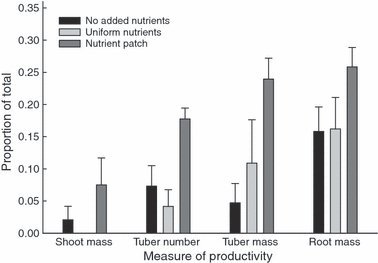
Response of Cyperus esculentus to slow-release fertilizer treatments of none, distributed uniformly, or distributed as a patch, and expressed as the mean proportion (±1 SE) of the total found in the section of the plot furthest from where tubers were sown. For the patch treatment, significantly more tubers (P < 0.01), greater tuber mass (P < 0.05) and root mass (P < 0.01) were located in the resource patch section of the plot (n = 5) compared with the other two treatments.
We interpret the directional placement of offspring as a maternally mediated response because root foraging and rhizome production are independent processes in C. esculentus. Roots forage for resources throughout the rhizosphere and proliferate in resource patches (Chapin 1980; Hodge 2004). However, rhizome production originates from the epicotyl of the maternal plant. The quantity of tubers produced by the maternal plant is dependent on soil nutrient availability (Barko and Smart 1979) and is an indirect, but integrated, response to root foraging. The spatial distribution of propagules from the maternal plant should follow a log-normal pattern (Cousens et al. 2008) unless there is a specific directional response to resource heterogeneity.
The placement of propagules in local resource patches is a potentially adaptive response by the maternal plant (sensuDonohue and Schmitt 1998) to biochemical cues from the roots. How the cues are processed and integrated into a plastic response is not well understood, but the response has been documented in other plant species (Hutchings and de Kroon 1994; Alpert and Simms 2002). In clonal plants, rhizomes also produce new ramets that are interconnected and provide information about the habitat to the maternal plant (de Kroon and Hutchings 1995) and effectively move the plant across the landscape.
In the early phases of invasion, most plants must rely on PP for establishment and survival. The response plasticity found in seedlings is often mediated by seed traits: seed size is correlated with higher relative growth rate, timing of germination, initial competitive ability and many other factors (Harper et al. 1970; Gutterman 1990). If resources in the invaded habitat are higher because of soil and habitat disturbance, those plants capable of producing propagules best able to take rapid advantage of the resource conditions are more likely to survive. The disproportional placement of tubers into nutrient-rich patches will lead predictably to more rapid population growth, size-mediated competitive ability and greater subsequent tuber production. Therefore, in the primary phase of invasion when resources are initially high, population growth may depend on TGP to increase resource acquisition within the patch. Those populations capable of facilitating the success of progeny by placing them in optimal locations will invariably dominate the patch over time.
Phenology in Aegilops triuncialis
Planted seed weight had direct fitness consequences in the progeny generation. The variation in seed mass among progeny was generated in large part by our experimental treatments in the maternal generation, thus demonstrating TGP effects on progeny fitness. In the progeny generation, planted seed weight was positively correlated with (1) seedling size (Table 1B; seedling size = 4.10 + 0.14 × planted seed size, R2 = 0.26), (2) flowering date (larger seeds resulted in plants that flowered later; Fig. 2A) and (3) total seed mass, which is highly correlated with seed number [P = 0.01; Table 1B; total seed mass (mg) = 78.11 + 0.86 × planted seed size, R2 = 0.04]. In the maternal generation, smaller seeds from serpentine sources (Fig. 2B) produced plants that flowered earlier (Fig. 2C) and made smaller seeds [serpentine source 10.3 ± 0.03 mg vs loam source 13.0 ± 0.04 mg (mean ± 1 SE)]. The number of seeds produced from each soil type was statistically equivalent (total seed mass was not predicted by source soil, P = 0.08). Thus, plants from each soil type produced equal numbers of progeny, but progeny from each soil type were preconditioned for adaptive phenology within the maternal soil environment: early flowering on serpentine soil and later flowering on loam soil.
Figure 2.
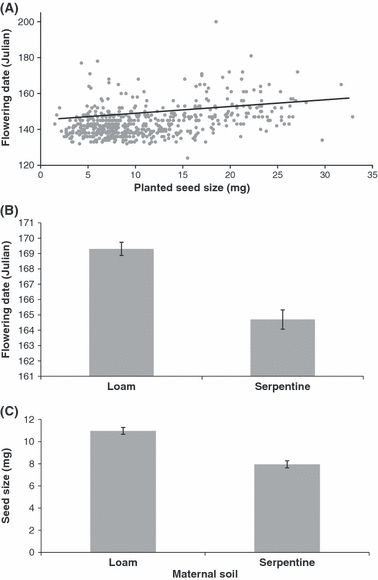
Trans-generational plasticity effects on Aegilops triuncialis phenology. (A) Effect of planted seed size of Ae. triuncialis on flowering date in generation two, flowering date = 137.99 + 0.55 × planted seed size, R2 = 0.16. (B) Effect of source soil type on flowering date in generation one (means significantly different, Tukey HSD, P < 0.5). (C) Effect of source soil type on seed size in generation one (mean value significantly different, Tukey HSD, P < 0.5). Bars indicate 1 SE.
Larger seeds have been shown to be adaptive in non-stressful environments (Volis et al. 2002b; Leger et al. 2009). In our study, large seeds produced plants with higher fitness under conditions of higher soil fertility and reduced moisture stress. The size of the seeds produced in the progeny generation was not strongly affected by planted seed weight (P = 0.065; Table 2B). This may have been because of the lack of heterogeneity in stress levels in the progeny generation: all progeny were grown on non-stressful soil. We might expect more variation in traits when the environment is stressful (Pigliucci et al. 1995; but see Funk 2008), and a lack of amplitude in responses to experimental treatments may have reduced our ability to detect treatment effects.
Table 2.
(A) Effects of maternal soil type, population source and family nested within population on Aegilops triuncialis photosynthetic rates and total aboveground plant dry weight. (B) Effects of maternal soil type, population source, and clone genotype nested with population Ae. triuncialis photosynthetic rates and total leaf area. P-values <0.05 are shown in bold. d.f. = degrees of freedom; SS = sums of squares
| (A) Split family ANOVA results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Photosynthetic rate (μmol CO2/m2/s) | Total aboveground dry weight (mg) | |||||||
| Source | d.f. | SS | F-ratio | P-value | d.f. | SS | F-ratio | P-value |
| Overall model | 32 | 322.8 | 3.81 | 0.004 (R2 = 0.89) | 52 | 0.4995 | 41.26 | <0.0001 (R2 = 0.83) |
| Maternal soil | 1 | 32.1 | 12.14 | 0.003 | 1 | 0.0031 | 14.51 | 0.0002 |
| Population source | 3 | 102.4 | 7.32 | 0.002 | 3 | 0.0120 | 14.78 | <0.0001 |
| Family within population | 24 | 141.3 | 2.22 | 0.056 | 44 | 0.0122 | 1.29 | 0.108 |
| Maternal soil × population | 3 | 11.99 | 1.51 | 0.252 | 3 | 0.0019 | 2.95 | 0.032 |
| Planted seed weight | 1 | 19.4 | 7.32 | 0.016 | 1 | 0.2874 | 1329.7 | <0.0001 |
| Error | 15 | 39.7 | 468 | 0.1012 | ||||
| (B) Reciprocal clone ANOVA results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Photosynthetic rate (μmol CO2/m2/s) | Total leaf area (cm2) | |||||||
| Source | d.f. | SS | F-ratio | P-value | d.f. | SS | F-ratio | P-value |
| Overall model | 18 | 1.547 | 4.53 | 0.015 (R2 = 0.69) | 18 | 1883.50 | 1 | 0.2840 (R2 = 0.24) |
| Maternal soil | 1 | 0.155 | 0.13 | 0.726 | 1 | 8.05 | 0.15 | 0.711 |
| Population source | 1 | 0.121 | 2.13 | 0.188 | 1 | 23.38 | 0.68 | 0.439 |
| Clone genotype | 7 | 0.388 | 0.52 | 0.796 | 7 | 247.94 | 0.64 | 0.714 |
| Maternal soil × population | 1 | 0.183 | 1.65 | 0.240 | 1 | 14.83 | 0.27 | 0.618 |
| Maternal soil × clone | 7 | 0.777 | 5.62 | 0.010 | 7 | 381.09 | 0.76 | 0.631 |
| Planted seed weight | 1 | 0.0005 | 0.002 | 0.960 | 1 | 413.80 | 5.80 | 0.039 |
| Error | 9 | 0.178 | 9 | 642.02 | ||||
Photosynthetic rate data were ln transformed before analysis.
Although progeny from small seeds produced fewer seeds (a potential reduction in fitness), we predicted that earlier flowering time would promote higher fitness in these plants under drought stress conditions. Flowering time can have both heritable and plastic components (Volis et al. 2002b), and we have shown that all the traits measured in this experiment have a genetic component (Table 1A,B). Genotype also had a significant interaction with growing soil for flowering date: one genotype flowered later on loam soils compared with serpentine (Julian date 177.2 ± 0.14 and 171.7 ± 0.10 respectively). Seeds collected from loam source populations produced offspring with larger seeds (i.e. seed source effect P < 0.01; Table 1A), but this effect of seed source neither carries over into the progeny (i.e. seed source effect P = 0.45) nor was there a maternal soil effect on progeny (maternal soil P = 0.25; Table 1B). The potential field maternal effect observed in the maternal generation could have been as a result of soil-correlated factors that are only present in situ. Alternatively, the degree to which the soil type of the original field-collected seeds expresses a maternal effect may be context-dependent (Miao et al. 1991; Van Zandt and Mopper 2004) in that detection of TGP may require progeny to be grown in both stressful and non-stressful environments. It is important to note that we cannot separate maternal and paternal TGP in this experiment: Ae. triuncialis is an almost entirely selfing species, thus maternal and paternal environments are likely the same. It has been noted previously that TGP in seed size can be affected by both maternal and paternal genomes (Etterson and Galloway 2002; Xiao et al. 2006).
Stress tolerance in Aegilops triuncialis
In the split family study, maternal soil environment had a significant effect on progeny photosynthetic rates (Table 2A). Progeny produced by maternal plants growing on serpentine soil exhibited significantly lower rates of photosynthesis than progeny produced by maternal plants growing on loam soils (Fig. 3A). This >30% average reduction in photosynthetic rate was consistent across all four populations tested. There was also a significant population effect that suggests genetic differentiation in photosynthetic rates among Ae. triuncialis populations. Analyses of aboveground dry biomass in the family study (Table 2B) indicated that progeny from maternal plants growing on serpentine soils exhibited greater plant size. Progeny from maternal plants that grew on serpentine were significantly larger than progeny from maternal plants that grew on loam soils (Fig. 3B). There were also significant population effects on plant size as well as a maternal soil by population interaction that suggested the TGP effects of maternal soil on progeny fitness varied among populations. The highly significant effect of seed size indicates a TGP effect of seed size as well as the effect of maternal soil on progeny fitness.
Figure 3.
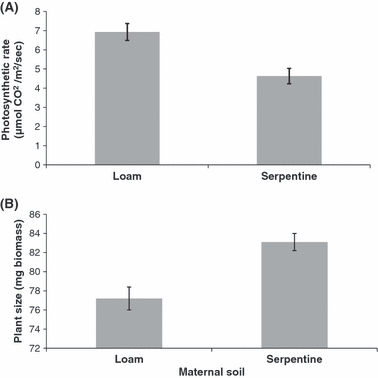
Effects of maternal soil environment on Aegilops triuncialis photosynthetic rates and plant size. (A) Plants grown in loam soil produced offspring with a higher photosynthetic rate (μmol CO2/m2/s) than the same genotypes grown in serpentine soil. (B) Progeny from plants grown on serpentine soil were larger than progeny from the same genotypes grown on loam soil. Bars indicate 1 SE.
In the clone reciprocal transplant study, the importance of TGP effects of maternal soil on progeny photosynthetic rates differed between the McLaughlin and Snell Valley field sites. At the McLaughlin site, the progeny from clones growing in serpentine sites exhibited significantly lower photosynthetic rates (3.50 ± 0.51 μmol CO2/m2/s; P < 0.05, a priori contrasts) than the progeny from maternal clones grown in non-serpentine sites (4.33 ± 0.52 μmol CO2/m2/s). In a similar comparison of progeny from clones growing at the Snell Valley site, there was no significant effect of maternal clone soil environment on progeny photosynthetic rates (P = 0.110, a priori contrasts). Given that the clones were unique to each study site, the differences between McLaughlin and Snell Valley in the effect of clone maternal soil environment may reflect genetic differences among populations, differences in maternal environments between sites, or both.
Both the split family and the clone reciprocal transplant studies demonstrate the importance of maternal soil environment on photosynthetic rates in progeny grown in a low-nutrient common garden. By using seed weight as a covariate, we are able to demonstrate persistent non-seed-size TGP effects on Ae. triuncialis photosynthesis. The reduced photosynthetic rates in progeny from maternal plants grown in serpentine soil is consistent with the concept that plants exposed to low resource environments may down-regulate physiological processes in response to stress. In the split family study, TGP effects on photosynthesis were adaptive; progeny from maternal plants grown on serpentine soil were larger at the end of the experiment despite exhibiting lower levels of photosynthesis. As is typical for many other annual species (Heywood 1986), plant size is highly correlated with seed output in this species (r = 0.93, P < 0.0001, N. Milan, unpublished data).
Summary
There is a pressing need for research to identify mechanisms leading to positive population growth of invasive species in both high-resource and stressful habitats (Dietz and Edwards 2006). We suggest that TGP provides a mechanism for increasing invasive plant fitness in both habitat types. In this study, we have shown that TGP can (1) place progeny in nutrient-rich patches, (2) change flowering phenology in an adaptive manner and (3) down-regulate photosynthesis under stressful conditions, resulting in the production of larger plants. We have indicated three distinct pathways through which TGP may increase reproductive rates when the parental generation is in a stressful environment. Increased reproduction is necessary for positive population growth and increasing population densities, two fundamental contributors to the spread of invasive species.
In harsh abiotic conditions, preferential placement of progeny into resource-rich patches may be extremely important for population persistence. Although plants are not able to sample the environment as animals do, the ability to place offspring preferentially in patches away from the maternal plant represents a form of directed movement across the landscape. This movement can increase fitness in the succeeding generation and reduce the risks associated with purely passive dispersal of propagules. For C. esculentus, directional growth of the rhizomes and placement of tubers into resource patches increased inclusive fitness because the maternal and tuber genotypes are identical. For invasive plants with clonal reproduction, this form of TGP will enhance the probability of establishment because of the increased probability of finding high-nutrient resource patches regardless of the nutrient conditions experienced by the maternal plant.
Variation in propagule size because of environmental influences is quite common (McGinley et al. 1987). The multigenerational effects of propagule size variation are rarely addressed in experiments with plants (but see Miao et al. 1991 and Case et al. 1996); however, multigenerational effects of egg size are commonly addressed in the entomological literature, particularly with Drosophila (e.g. Fox and Czesak 2000). Adaptive TGP relating to offspring sizes may therefore be a mechanism for increased invasiveness across taxa. For annual plants in a mosaic landscape of edaphic stresses, TGP relating to seed size may be a more efficient evolutionary trajectory than genetically fixed seed size allocation (McGinley et al. 1987). Larger seeds tend to have higher survivorship (Moles and Westoby 2004), and produce seedlings that are more competitive in some environments (Stratton 1989; Schmid and Dolt 1994; Turnbull et al. 2004). However, the advantage of large seed size can change with the environment (Bruun and ten Brink 2008). The fitness benefits of large seeds may be greater in competitive environments, while in edaphically stressful environments the rapid production of smaller seeds, before soil moisture becomes limiting, may confer higher fitness.
Smaller seeds produced by serpentine-grown Ae. triuncialis plants grew into seedlings with lower photosynthetic rates. The reduced photosynthetic rates in progeny of serpentine-grown plants are consistent with the concept that plants exposed to low resource environments may down-regulate metabolic processes in response to stress (Chapin et al. 1993). An important question in the study of TGP is whether these types of physiological changes induced in the progeny are adaptive. This appeared to be the case in the experiment reported here for Ae. triuncialis. In general, there is very little information on the effects of maternal environments on physiological traits (but see Sultan et al. 2009). Manipulation of maternal temperature regimes in the weedy grass Echinochloa crus-galli, resulted in contrasting metabolite composition in the seed of the next generation (Potvin and Charest 1991). Although progeny photosynthesis was not tested in that study, the authors proposed that differences in seed biochemical composition would be likely to affect a number of physiological processes in the progeny. Typically the expression of the ‘stress tolerance syndrome’ has been envisioned as a genetic or phenotypically plastic response (Chapin et al. 1993); however, our results suggest that TGP represents an additional pathway for the production of stress tolerant phenotypes within invasive plant species.
We are not suggesting that TGP is the sole mechanism acting in the primary and secondary phases of invasion into harsh habitats, as within generation plasticity and adaptation have also been shown to play important roles in many species. In fact, the relative importance of TGP in invasions and range expansion is unknown, mainly because of lack of experimental data. TGP in plants is well-documented (Roach and Wulff 1987; Shaw and Byers 1998), but the number of field studies on these effects is surprisingly few (Schmitt et al. 1992; Donohue and Schmitt 1998; Galloway 2005). In particular, a comprehensive demonstration of beneficial or adaptive TGP is difficult, as this requires examination of parental fitness in the parental environment as well as offspring fitness in the offspring environment (Donohue and Schmitt 1998; Wolf et al. 1998). If TGP is adaptive, this may either obviate the need for genetic adaptation, or allow the population to persist long enough for genetic adaptation to occur. Here we have shown detailed case studies of two invasive plants that are ‘pre-adapted’ to their new environment through beneficial TGP. Further studies are needed to determine if TGP is one of the general characteristics that distinguishes successful invaders from the very large number of species introduced.
As has long been argued for PP in invasive species (Baker 1965), we suggest that clonal or selfing invasive species with low genetic variation represent a set of conditions where TGP may be maintained initially (and perhaps indefinitely) over genetic differentiation. Initially, this is because several generations in a relatively constant environment are required for selection to effectively favour specific genotypes (Antonovics 1976; Lenormand 2002). In addition, the genetic variation necessary for response to selection is less likely to be available in clonal and selfing species where genetic variation is often very low, especially if the species is an invasive and has experienced a genetic bottleneck during introduction (Barrett and Kohn 1991). Therefore, for invasive species where the potential for recombination is low, population expansion across resource boundaries into more abiotically stressful habitats will be facilitated by TGP.
In species that are rapidly expanding their range, TGP provides an efficient way to pre-condition offspring for high reproductive rates in a spatially variable environment with predictable resource availability across generations. We have demonstrated that maternal plants can provision offspring adaptively in terms of spatial placement, phenology and photosynthetic efficiency. Thus, the appearance of adapted phenotypes in invasive species may arise from interactions between the genotype and the environment and will not always be dependent on recombination or the fixation of new beneficial mutations. For any species that has little genetic variation upon which selection can act, and little evolutionary history in a selective landscape, TGP may be especially important in early stages of invasion. In subsequent generations, this TGP that allowed the invasive to persist (and even have positive population growth rate) may be ‘replaced’ by genetic adaptation to the novel abiotic or biotic factors in the new range (Lee 2002).
Acknowledgments
The authors would like to thank Carol Lee, Kristina Schierenbeck and Robert Holt for organizing a symposium titled ‘Synthesis of the Ecology and Evolution of Invasive Species’ and Hsein Easlon, Angel Moseley and Neil Willits for help with data collection and analyses. This study benefited greatly from the keen editorial eyes of Carol Lee and two anonymous reviewers. The Aegilops triuncialis research was supported by USDA NRI grant #2005–35320–15314 to J.K. McKay and K.J. Rice.
Literature cited
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defenses in animals and plants. Nature. 1999;401:60–63. [Google Scholar]
- Alpert P. Effect of clonal integration on plant plasticity in Fragaria chiloensis. Plant Ecology. 1994;141:99–106. [Google Scholar]
- Alpert P, Simms EL. Relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evolutionary Ecology. 2002;16:285–297. [Google Scholar]
- Antonovics J. The nature of limits to natural selection. Annals of the Missouri Botanical Garden. 1976;63:224–247. [Google Scholar]
- Aragon CF, Escudero A, Valladares F. Stress-induced dynamic adjustments of reproduction differentially affect fitness components of a semi-arid plant. Journal of Ecology. 2008;96:222–229. [Google Scholar]
- Baker H. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The Genetics of Colonizing Species. New York: Academic Press; 1965. pp. 147–172. [Google Scholar]
- Baker HG. The evolution of weeds. Annual Review of Ecology and Systematics. 1974;5:1–14. [Google Scholar]
- Baker HG, Stebbins GL. The Genetics of Colonizing Species. New York: Academic Press; 1965. [Google Scholar]
- Barko JW, Smart RM. The nutritional ecology of Cyperus esculentus, an emergent aquatic plant. Aquatic Botany. 1979;6:13–28. [Google Scholar]
- Barrett SCH, Kohn JR. Genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Falk DA, Holsinger KE, editors. Genetics and Conservation of Rare Plants. New York: Oxford University Press; 1991. pp. 3–30. [Google Scholar]
- Bell DL, Galloway LF. Population differentiation for plasticity to light in an annual herb: adaptation and cost. American Journal of Botany. 2008;95:59–65. doi: 10.3732/ajb.95.1.59. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Bruun HH, Ten Brink DJ. Recruitment advantage of large seeds is greater in shaded habitats. Ecoscience. 2008;15:498–507. [Google Scholar]
- California Exotic Pest Plant Council. Exotic Pest Plants of Greatest Ecological Concern in California. San Juan Capistrano, CA: California Exotic Pest Plant Council; 1999. [Google Scholar]
- Case AL, Lacey EP, Hopkins RG. Parental effects in Plantago lanceolata L. 2. Manipulation of grandparental temperature and parental flowering time. Heredity. 1996;76:287–295. [Google Scholar]
- Chapin FS., III The mineral nutrition of wild plants. Annual Review of Ecology and Systematics. 1980;11:233–260. [Google Scholar]
- Chapin FS, III, Groves RH, Evans LT. Response of growth, photosynthesis, and phosphate absorption to phosphorus stress in wild, weedy, and cultivated Hordeum species. Oecologia. 1989;79:96–105. doi: 10.1007/BF00378245. [DOI] [PubMed] [Google Scholar]
- Chapin FS, III, Autumn K, Pugnaire F. Evolution of suites of traits in response to environmental stress. American Naturalist. 1993;142:S78–S92. [Google Scholar]
- Cousens R, Dytham C, Law R. Dispersal in Plants: A Population Perspective. New York: Oxford University Press; 2008. [Google Scholar]
- Dietz H, Edwards PJ. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology. 2006;87:1359–1367. doi: 10.1890/0012-9658(2006)87[1359:rtcpcd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Donohue K. Setting the stage: phenotypic plasticity as habitat selection. International Journal of Plant Science. 2003;164:S579–S592. [Google Scholar]
- Donohue K, Schmitt J. Maternal environmental effects: adaptive plasticity? In: Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. Oxford, UK: Oxford University Press; 1998. pp. 137–158. [Google Scholar]
- Ellstrand NC, Roose ML. Patterns of genotypic diversity in clonal plant species. American Journal of Botany. 1987;74:123–131. [Google Scholar]
- Epstein E, Bloom AJ. Mineral Nutrition of Plants: Principles and Perspectives. 2nd edn. Sunderland, MA, USA: Sinauer Associates; 2005. [Google Scholar]
- Etterson JR, Galloway LF. The influence of light on paternal plants in Campanula americana (Campanulaceae): pollen characteristics and offspring traits. American Journal of Botany. 2002;89:1899–1906. doi: 10.3732/ajb.89.12.1899. [DOI] [PubMed] [Google Scholar]
- Evans JP, Cain ML. A spatially explicit test of foraging behavior in a clonal plant. Ecology. 1995;76:1147–1155. [Google Scholar]
- Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- Funk JL. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology. 2008;96:1162–1173. [Google Scholar]
- Galloway LF. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytologist. 2005;166:93–100. doi: 10.1111/j.1469-8137.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- Ghalambor C, McKay JK, Carroll S, Reznick D. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gruntman M, Novoplansky A. Physiologically mediated self/non-self discrimination in roots. Proceedings of the National Academy of Sciences, United States of America. 2004;101:3863–3867. doi: 10.1073/pnas.0306604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman Y. Maternal effects on seeds during development. In: Fenner M, editor. Seeds: The Ecology of Regeneration in Plant Communities. Wallingford, UK: CABI Publishing; 2000. pp. 59–85. [Google Scholar]
- Harper JL, Lovell PH, Moore KG. The shapes and sizes of seeds. Annual Review of Ecology and Systematics. 1970;1:327–356. [Google Scholar]
- Heywood JS. The effect of plant size variation on genetic drift in populations of annuals. The American Naturalist. 1986;127:851–861. [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Horak MJ, Holt JS, Ellstrand NC. Genetic variation in yellow nutsedge (Cyperus esculentus. Weed Science. 1987;35:506–512. [Google Scholar]
- Hutchings MJ, De Kroon H. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research. 1994;25:1159–1238. [Google Scholar]
- Jablonka E, Lamb MJ. The inheritance of acquired epigenetic variations. Journal of Theoretical Biology. 1989;139:69–83. doi: 10.1016/s0022-5193(89)80058-x. [DOI] [PubMed] [Google Scholar]
- Van Kleunen M, Fischer M. Adpative evolution of plastic foraging responses in a clonal plant. Ecology. 2001;82:3309–3319. [Google Scholar]
- De Kroon H, Hutchings MJ. Morphological plasticity in clonal plants: the foraging concept revisited. Journal of Ecology. 1995;83:143–152. [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends in Ecology and Evolution. 2002;118:386–391. [Google Scholar]
- Leger EA, Espeland EK, Merrill KR, Meyer SE. Genetic variation and local adaptation at a cheatgrass (Bromus tectorum) invasion edge in western Nevada. Molecular Ecology. 2009;18:4366–4379. doi: 10.1111/j.1365-294X.2009.04357.x. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends in Ecology and Evolution. 2002;17:183–189. [Google Scholar]
- Li B, Shibuya T, Yogo Y, Hara T, Matsuo K. Effects of light quantity and quality on growth and reproduction of a clonal sedge, Cyperus esculentus. Plant Species Biology. 2001;16:69–81. [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for Mixed Models. 2nd edn. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Maron JL, Vila M, Bommarco R, Elmendorf S, Beardsley P. Rapid evolution of an invasive plant. Ecological Monographs. 2004;74:261–280. [Google Scholar]
- Maynard Smith J. Optimization theory in evolution. Annual Review of Ecology and Systematics. 1978;9:31–56. [Google Scholar]
- McGinley MA, Temme DH, Geber MA. Parental investment in offspring in variable environments – theoretical and empirical considerations. American Naturalist. 1987;130:370–398. [Google Scholar]
- Meimberg H, Hammond JI, Jorgensen C, Park TW, Gerlach JD, Rice KJ, McKay JK. Molecular evidence for an extreme genetic bottleneck during the introduction of barbed goatgrass, Aegilops triuncialis, to California. Biological Invasions. 2006;8:1355–1366. [Google Scholar]
- Miao SL, Bazzaz FA, Primack RB. Effects of maternal nutrient pulse on reproduction of two colonizing Plantago species. Ecology. 1991;72:586–596. [Google Scholar]
- Moles AT, Westoby M. What do seedlings die from and what are the implications for evolution of seed size? Oikos. 2004;106:193–199. [Google Scholar]
- Mousseau TA, Fox CW. Maternal Effects as Adaptations. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- Novak SJ, Mack RN, Soltis DE. Genetic variation in Bromus tectorum (Poaceae): population differentiation in its North American range. American Journal of Botany. 1991;78:1150–1161. [Google Scholar]
- Pigliucci M, Whitton J, Schlichting CD. Reaction norms of Arabidopsis.1. Plasticity of characters and correlations across water, nutrient and light gradients. Journal of Evolutionary Biology. 1995;8:421–438. [Google Scholar]
- Potvin C, Charest C. Maternal effects of temperature on metabolism in the C4 weed Echinochloa crus-galli. Ecology. 1991;72:1973–1979. [Google Scholar]
- Rice KJ, Mack RN. Ecological genetics of Bromus tectorum II. Intraspecific variation in phenotypic plasticity. Oecologia. 1991a;88:84–90. doi: 10.1007/BF00328407. [DOI] [PubMed] [Google Scholar]
- Rice KJ, Mack RN. Ecological genetics of Bromus tectorum: III. The demography of reciprocally sown populations. Oecologia. 1991b;88:91–101. doi: 10.1007/BF00328408. [DOI] [PubMed] [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- Sambatti JBM, Rice KJ. Local adaptation, patterns of selection, and gene flow in the Californian serpentine sunflower (Helianthus exilis. Evolution. 2006;60:696–710. [PubMed] [Google Scholar]
- Schmid B, Dolt C. Effects of maternal and paternal environment and genotype on offspring phenotype in Solidago altissima L. Evolution. 1994;48:1525–1549. doi: 10.1111/j.1558-5646.1994.tb02194.x. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Niles J, Wulff RD. Norms of reaction of seed traits to maternal environments in Plantago lanceolata. American Naturalist. 1992;139:451–466. [Google Scholar]
- Sexton JP, McKay JK, Sala A. Ecological genetics of the invasive shrub, Tamarix ramosissima, at latitudinal extremes. Ecological Applications. 2002;12:1652–1660. [Google Scholar]
- Shaw RG, Byers DL. Genetics of maternal and paternal effects. In: Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. New York: Oxford University Press; 1998. pp. 97–111. [Google Scholar]
- Stoller EW, Sweet RD. Biology and life cycle of purple and yellow nutsedge (Cyperus rotundus and C.esculentus. Weed Technology. 1987;1:66–73. [Google Scholar]
- Stratton DA. Competiton prolongs expression of maternal effects in seedlings of Erigeron annuus (Asteraceae) American Journal of Botany. 1989;76:1646–1653. [Google Scholar]
- Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecology Letters. 2006;9:354–371. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Barton K, Wilczek AM. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology. 2009;90:1831–1839. doi: 10.1890/08-1064.1. [DOI] [PubMed] [Google Scholar]
- Tumbleson ME, Kommedahl T. Reproductive potential of Cyperus esculentus by tubers. Weeds. 1961;9:646–653. [Google Scholar]
- Turnbull LA, Coomes D, Hector A, Rees M. Seed mass and the competition/colonization trade-off: competitive interactions and spatial patterns in a guild of annual plants. Journal of Ecology. 2004;92:97–109. [Google Scholar]
- Van Zandt PA, Mopper S. The effects of maternal salinity and seed environment on germination and growth in Iris hexagona. Evolutionary Ecology Research. 2004;6:813–832. [Google Scholar]
- Vellend M, Harmon LJ, Lockwood JL, Mayfield MM, Hughes AR, Wares JP, Sax DF. Effects of exotic species on evolutionary diversification. Trends in Ecology and Evolution. 2007;22:481–488. doi: 10.1016/j.tree.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Villa-Aiub MM, Martinez-Ghersa MA, Ghersa CM. Evolution of herbicide resistance in weeds: vertically transmitted fungal endophytes as genetic entities. Evolutionary Ecology. 2003;17:441–456. [Google Scholar]
- Volis S, Mendlinger S, Turuspekov Y, Esnazarov U. Phenotypic and allozyme variation in Mediterranean and desert populations of wild barley, Hordeum spontaneum Koch. Evolution. 2002a;56:1403–1415. doi: 10.1111/j.0014-3820.2002.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Volis S, Mendlinger S, Ward D. Differentiation along a gradient of environmental productivity and predictability in populations of Hordeum spontaneum Koch: multilevel selection analysis. Biological Journal of the Linnean Society. 2002b;75:313–318. [Google Scholar]
- Volis S, Verhoeven KJF, Mendlinger S, Ward D. Phenotypic selection and regulation of reproduction in different environments in wild barley. Journal of Evolutionary Biology. 2004;17:1121–1131. doi: 10.1111/j.1420-9101.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Weinig C. Plasticity vs. canalization: population difference in the timing of shade-avoidance responses. Evolution. 2000;54:441–451. doi: 10.1111/j.0014-3820.2000.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Williams RD. Growth and reproduction of Cyperus esculentus L. and Cyperus rotundus L. Weed Research. 1982;22:149–154. [Google Scholar]
- Williams DG, Black RA. Effects of nutrient amendment and environment on growth and gas exchange for introduced Pennisetum setaceum in Hawaii. Canadian Journal of Botany. 1996;74:268–275. [Google Scholar]
- Wolf JB, Brodie ED, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends in Ecology and Evolution. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
- Xiao WY, Brown RC, Lemmon BE, Harada JJ, Goldberg RB, Fischer RL. Regulation of seed size by hypomethylation of maternal and paternal genomes. Plant Physiology. 2006;142:1160–1168. doi: 10.1104/pp.106.088849. [DOI] [PMC free article] [PubMed] [Google Scholar]


