Abstract
Invasive species may establish in communities because they are better competitors than natives, but in order to remain community dominants, the competitive advantage of invasive species must be persistent. Native species that are not extirpated when highly invasive species are introduced are likely to compete with invaders. When population sizes and genetic diversity of native species are large enough, natives may be able to evolve traits that allow them to co-occur with invasive species. Native species may also evolve to become significant competitors with invasive species, and thus affect the fitness of invaders. Invasive species may respond in turn, creating either transient or continuing coevolution between competing species. In addition to demographic factors such as population size and growth rates, a number of factors including gene flow, genetic drift, the number of selection agents, encounter rates, and genetic diversity may affect the ability of native and invasive species to evolve competitive ability against one another. We discuss how these factors may differ between populations of native and invasive plants, and how this might affect their ability to respond to selection. Management actions that maintain genetic diversity in native species while reducing population sizes and genetic diversity in invasive species could promote the ability of natives to evolve improved competitive ability.
Keywords: coevolution, competition, contemporary evolution, invasive species, management, microevolution, restoration
The introduction of highly invasive plant species can represent a new and strong selective agent for native plants (Strauss et al. 2006; Carroll et al. 2007). The most invasive species, those that rapidly dominate and drastically change the abundance of native species in a community (Colautti and MacIsaac 2004), interact strongly with resident natives. For many native species, the outcome of these interactions is competitive exclusion, but some native species do persist and coexist with invaders (Gurevitch and Padilla 2004; Sax and Gaines 2008). Native species that remain in highly invaded areas, hereafter called ‘remnant natives,’ may persist for different reasons (Fig. 1A). First, they may be in the process of going locally extinct, just more slowly than species already displaced. Secondly, persistence of remnant natives may be promoted by ecological factors. For example, the inability of an invader to disperse to all available habitats, habitat heterogeneity, or effects of enemies/herbivores may promote coexistence between native species and highly invasive ones (Chesson and Warner 1981; Holt 1984; Huntly 1991; Chesson 2000; Amarasekare and Nisbet 2001; Hubbell 2006). Finally, the presence of remnant populations may be the result of a change in gene frequencies since the introduction of the invader (Fig. 1A). Remaining natives may be either better competitors or more tolerant of the impacts of invasive species than the population average at the time of the invasive species introduction, representing evolutionary change in remnant native populations in response to invasive species (e.g. Leger 2008). This type of evolutionary change within remnant populations is contingent upon native species having the demographic and genetic resources necessary to evolve in response to the exotic invader before going locally extinct (Strauss et al. 2006).
Figure 1.
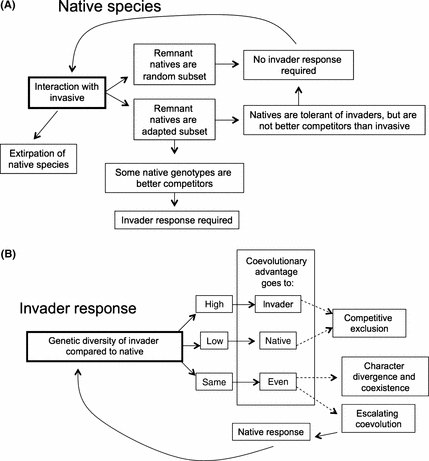
Interactions between native species and invaders may lead to coevolution, as either a transient or escalating dynamic. This depends on the response of the native species to the initial interaction (A), which determines if the invasive species is likely to evolve in response to the native (B). The outcome of coevolution between native and invasive species may rely in part on the relative diversity between interacting native and invasive populations for traits that affect fitness in invaded systems (B). Dotted lines indicate possible result of long-term consistency in coevolutionary advantage. This assumes that demographic parameters will support these interactions but it is, of course, quite possible that the native species may become locally extirpated if populations become too small.
Relatively small evolutionary changes in traits can be important for determining the distribution and abundance of species in natural communities (e.g. Johnson et al. 2009; Jones et al. 2009). Many examples of rapid evolutionary change have been observed in invasive species colonizing new habitats, and these evolutionary changes may be a key component of the invasion process (Hendry and Kinnison 1999; Reznick and Ghalambor 2001; Carroll et al. 2007; Whitney and Gabler 2008). There is also a growing body of evidence that native species can also evolve in response to invasive competitors (Lau 2006; Mealor and Hild 2006; Strauss et al. 2006; Leger 2008; Whitney and Gabler 2008). Here, we consider the possibility that strongly interacting native and invasive species can enter coevolutionary relationships, wherein a genetic change in one species is met with a reciprocal evolutionary change in the other (Thompson 1994; Fig. 1B). If we understand and anticipate the potential for coevolution to affect the relative abundance of co-occurring native and invasive species, deliberate management decisions may be able to tip the scales in favor of native species. We first discuss why coevolution between natives and invasive species might occur, and secondly discuss factors that may differentially affect the evolutionary capacity of native and exotic species, outlining experimental methods that should be used to identify the potential for coevolution to occur. Finally, we consider how management actions may favor one side or the other in continuing coevolution between native and invasive species.
Coevolution between competitors
Coevolutionary relationships between competitors are not as well-studied as coevolution among species of different trophic levels (such as plant/herbivore interactions or pathogen/host relationships), and most studies of coevolution between competitors have been conducted on organisms other than plants, such as birds (Diamond 1986; Grant 1986; Diamond et al. 1989; Grant and Grant 1989), fish (Schluter and McPhail 1992; Pritchard and Schluter 2001), and insects (Joshi and Thompson 1995, 1996, 1997). Plants have been found to be locally adapted at very small scales, including adaptations to their competitors (Turkington and Harper 1979; Aarssen and Turkington 1985; Reynolds et al. 1997), but the reciprocal genetic shifts between species that represent true coevolutionary process have not yet been demonstrated in plant communities. Continuing coevolution between competitors has historically been considered unlikely: character displacement was predicted to be the outcome of coevolution between competitors, with each species becoming the dominant competitor in a different niche (MacArthur and Levins 1964; Connell 1980). However, theoretical models have demonstrated that coevolution can result in either character displacement or character convergence (Aarssen 1983; Roughgarden 1983; Taper and Case 1992). Under some circumstances, such as limited resource availability, species are expected to evolve to use the same resource base, and species struggle to gain the upper hand in effectively competing for a limited, necessary resource (Roughgarden 1983; Taper and Case 1992; Futuyma 2005). This situation can lead to convergence, rather than divergence, of traits, which in turn can lead to continuing coevolution between competitors (i.e. ‘Red Queen dynamics’ as in Stenseth and Smith 1984; or ‘competition combining ability’ as in Aarssen 1983) while character displacement leads to coevolution only as a transient dynamic (Connell 1980; or ‘ecological combining ability’ as in Aarssen 1983). Whether coevolution is continuous or leads to character displacement may be an intrinsic aspect of resource availability, or, in the case of interacting native and exotic species, it may be an outcome of anthropogenic disturbance and management activities.
There are two main ways to determine if coevolution is occurring between species. The first method infers a history of coevolution from current character displacement by comparing traits in the species of interest when they do co-occur (sympatric populations) with areas where they do not co-occur (allopatric populations, e.g. Schluter and Grant 1982; Schluter and McPhail 1992). If species differ more from each other when they co-occur, but are more similar where they do not occur, this suggests that natural selection has favored reciprocal changes within interacting populations (e.g. Schluter and Grant 1982). However, coevolution is only one mechanism of many by which character displacement may occur (Thompson 2005a), and character displacement is only one possible outcome of coevolution (Roughgarden 1983; Taper and Case 1992). Additionally, this retrospective method may not be as useful for detecting coevolution between remnant native and invasive species as it is for species with a longer history of association for a few reasons. For one, coevolution between natives and recently introduced exotics might be in the intial stages. For another, imperfect knowledge about invasion history complicates efforts to compare invasions of known age. For these reasons, a prospective method might be more useful to examine the potential for coevolution between native and invasive species, wherein one gauges the level of heritable variation within each interacting species for traits that affect competitive ability. The stage is set for coevolution to occur when there are some native individuals that are particularly good competitors with invasive species, and when invasive individuals vary in their ability to compete with the best native competitors (Fig. 1B). Determining if this is the case involves quantifying the amount of heritable variation within each interacting species for traits that affect fitness under competition (e.g. Henter 1995; Henter and Via 1995) in field or greenhouse competition studies (Table 1A). We know of no such studies that examine these questions in interacting plant competitors, either native or invasive.
Table 1.
Examples of experimental questions and proposed methods that would address the potential for and contributing factors to coevolution between native and invasive plant species
| Experimental question | Method | Outcome |
|---|---|---|
| A. Do strongly interacting remnant native and invasive species have similar levels of heritable variation for competitive traits? | Competition studies that include family structure or parent/offspring regressions in experimental design | Predict if coevolution can occur; predict long-term competitive outcomes |
| B. Can targeted management actions decrease heritable variation in competitive traits in invasive, but not native, species? | Apply management treatments and measure changes in heritable variation pre- and post-treatment in native and exotic species | Identify management actions most likely to favor native species in coevolutionary interactions |
| C. Does isolating patches of invasive species increase or decrease ability of invasive species to evolve in response to remnant natives? | Observational studies of heritable variation in isolated versus connected patches of invaders; or, long-term manipulative experiments with different patch sizes | Determine if management should focus on interrupting connectivity between patches |
| D. Do restoration materials collected from wild populations have greater genetic variation for competitive traits than agriculturally produced seeds? | Compare levels of heritable variation for competitive ability between wild- and agriculturally produced seed sources | Determine which restoration material is more likely to be able to evolve in response to invasive species |
Why might coevolution occur between native and invasive plants?
Coevolutionary dynamics happen only when species co-occur and happen more quickly when each strongly affects the other's fitness (Thompson 1994). By definition, extremely invasive plants are widely distributed and locally abundant (Colautti and MacIsaac 2004), which ensures that any native species that remain in an invaded landscape have a high likelihood of direct interaction with the invasive. Additionally, in invaded communities, plant richness may be lower, and thus most interactions will be between the most common remnant native species and the invader, rather than with other native plant species, which may facilitate coevolution (Connell 1980).
When the criterion of co-occurrence is met, the next criterion is that interacting species affect each other's fitness. Interacting plants can affect each other by directly competing for space, light, water, nutrients, or mutualists such as pollinators. Negative indirect interactions are also possible through mechanisms such as altering soil microbial communities (Reinhart and Callaway 2006; Batten et al. 2008), pathogens or herbivores (DeWalt et al. 2004, Lau and Strauss 2005), fungal diversity (Hawkes et al. 2006), or soil chemistry (Batten et al. 2006). Many studies have shown that invasive plants can strongly affect the fitness of native plants (DiTomaso 2000; Mack et al. 2000; Mooney and Cleland 2001), and the idea that some native species or particular native genotypes can affect the fitness of invaders is the foundation of restoration activities in highly invaded systems (e.g. Seabloom et al. 2003; Corbin and D'Antonio 2004; Morghan and Rice 2005; Lulow 2006, 2008). Therefore, it is likely that there is opportunity for reciprocal fitness affects between interacting native and exotic species, though these effects may not be entirely symmetrical, due to differences in both the frequency of interactions and the strength of competitive ability between native and invasive species.
Capacity for evolution in native species and exotic species
Population size, growth rates, and genetic diversity
All the factors that increase the risk of extinction in small populations (genetic drift, Allee affects, and demographic stochasticity) also restrict the ability of remnant natives to adapt to invasive species, because there is an increased chance that small populations of natives will lose genetic diversity and go locally extinct prior to adaptation to the novel invader (Ellstrand and Elam 1993; Lande 1993; Groom 1998). However, in a coevolutionary relationship, genetic diversity may be most important as a relative phenomenon. Genetic diversity is typically linked to population size (Hedrick 2005), but these things may become decoupled in invasive species. For example, invasive species with a limited number of introductions may be genetically depauperate, despite having large population sizes (Dlugosch and Parker 2008; Ward et al. 2008). Even though remnant native species may have much smaller population sizes than the invader, they may retain more genetic diversity than exotics, simply because they have not gone through a recent introduction bottleneck, and they may have long-term seed banks that house significant genetic diversity (Table 2A; Nunney 2002; Waples 2006).
Table 2.
Population genetic traits and ecological processes important for coevolution, and factors that may differ between remnant native and invasive species. The ‘+’ and ‘−’ indicates whether a factor is likely to positively or negatively affect the ability of the species to evolve to a competitor
| Trait/process | Remnant native species | Invasive species |
|---|---|---|
| A. Genetic diversity | Long residence time, high diversity (+) Seed bank increases diversity (+) Small populations (−) | Bottleneck, low diversity (−) Multiple introductions (+) Large populations (+) |
| B. Growth rate | Slow or negative population growth (−) | Populations rapidly expanding (+) |
| C. Gene flow | Gene flow from other populations can increase genetic diversity (+) Gene flow could swamp adaptive traits (−) | Less chance of gene flow from native range (−) Little chance of gene flow swamping adaptation (+) |
| D. Co-occurrence rate | Frequent encounters with invaders (+) | Low encounter rates with any single native species (−) |
| E. Number of selective agents | Low initially (+) Greater over time if invasion modifies habitat (−) | High initially (−) Less over time if invasion modifies habitat (+) |
Because invasive species have very rapid growth rates, they may maintain higher diversity than one would expect based on their likely invasion histories alone (Dlugosch and Parker 2008) because a population with a rapid growth rate is likely to retain allelic diversity, even after experiencing a bottleneck (Nei et al. 1975). In contrast, population growth rates of remnant natives may at best increase slowly in the face of invasion, or they may hold steady or decline. A population with a low or declining growth rate is more susceptible to genetic drift (Hartl and Clark 2007). In addition to any differences in diversity caused by population sizes or the introduction history of the invader, differences in growth rates between native and invasive populations may lead to relatively lower genetic diversity in traits that affect fitness within remnant natives (Table 2B). Thus, while invasives may begin with low diversity, they can more easily increase and maintain that diversity while natives may start with high diversity yet easily lose it through genetic drift, or as a response to strong selection by the invader.
We expect that if genetic diversity is reasonably large, there will be heritable variation in competitive ability among individuals. Multiple studies in multiple plant species have shown genotype-specific competitive ability (Turkington and Harper 1979; Turkington and Mehroff 1990; Fridley et al. 2007; Crutsinger et al. 2008), thus variation for selection to act upon may be common. In some cases, resident natives may have higher genetic diversity than invaders, and in others, invaders may be more diverse than resident natives. The relative amounts of genetic diversity in these populations have implications for the long-term outcome of competition between native and exotic species, including whether one species is able to competitively exclude the other (Fig. 1B; Aarssen and Turkington 1985; Roscher et al. 2008). Studies comparing the phenotypic variability of co-occurring native and invasive plants often have a taxonomic element to them, with researchers comparing native and invasive congeneric pairs in order to control for phylogenetic relationships between species (e.g. Brock et al. 2005; Funk 2008). However, in order to predict whether remnant natives and invasive species are capable of reciprocal evolutionary change, it is necessary to compare the relative genetic diversity of co-occurring, strongly interacting native and invasive plant species (e.g. Nagel and Griffin 2001; Niinemets et al. 2003).
Gene flow and the coevolutionary processes
Gene flow may either speed up or inhibit coevolutionary dynamics, in a situation parallel to positive and negative contributions of gene flow to local adaptation (Holt and Gomulkiewicz 1997). Native and invasive plant species may experience very different amounts of gene flow (Table 2C). Because they are in their home environment, native species may be more likely to have the potential for gene flow from a diversity of outside populations. This may create novel genotypes upon which natural selection may act, however, gene flow from noninvaded areas may swamp specific adaptations occurring within invaded habitats (Bridle and Vines 2007). On the other hand, depending on invasion history, invasive species may lack gene flow from populations sufficiently divergent to introduce new genotypes, leaving mutation and recombination as the only sources of novel genotypes (e.g. Meimberg et al. 2005). This would slow the coevolutionary response time of the invader. However, if the invasion was initiated via multiple introductions, this process can bring together a large amount of genetic diversity, as previously isolated populations are combined in the invasive range (Ellstrand and Schierenbeck 2000; Sexton et al. 2002). Such diversity could produce a wide variety of novel genotypes, more than what one would expect from gene flow among populations of native species.
Co-occurrence rate
The likelihood of coevolution increases with increased interactions (Thompson 1994). The decrease in abundance of native species in invaded communities has the potential to make the interactions between remaining native species and invaders particularly focused. However, the rate at which native and exotic species encounter each other differs based on their relative abundance in the population (Table 2D), which affects the coevolutionary rate (Turkington and Harper 1979; Aarssen 1983; Vermeij 1994; Thompson 2005a). Native species are likely to have high encounter rates with invasive species, and in contrast, invasive species are likely to have more interactions with other conspecifics than with the remnant native species in a population. This is likely to increase the ability of natives to evolve competitive or tolerant traits in response to exotics, and to decrease the ability of the invader to respond in kind.
Number of new selection pressures
Native species have presumably evolved in a competitive environment with other residents of the plant community (Turkington and Harper 1979; Martin and Harding 1982; Aarssen and Turkington 1985; Evans et al. 1985), and have had the opportunity to adapt to the local abiotic conditions (Kawecki and Ebert 2004). Invasive species, on the other hand, are often introduced to a host of strong selective forces: new climate, soils, pathogens, herbivores, pollinators, and competitors. This array of new selective forces for introduced exotic species may in part create the biotic and abiotic resistance responsible for the lack of establishment of many introduced species (Levine et al. 2004). For native species, on the other hand, the most significant new selective force for native plants may be their novel, invasive competitor. Thus, the coevolutionary community context is different for invasives and natives. Differences in community context can speed or slow coevolutionary rates, depending on the population and species involved (Antonovics 1979; Thompson 2005b; Haloin and Strauss 2008). When natives are responding to a single new selective agent and exotics are responding to multiple, strong, new selective pressures, natives may have the upper hand in an escalating evolutionary trajectory (Connell 1980; Vermeij 1994). This scenario may change over time if the invader is capable of modifying habitat sufficiently, either through changes in the disturbance regime and/or shifts in ecosystem processes or with the addition of multiple new invaders, and what is initially ‘familiar’ to the native species might reverse and become more so to the invader (Table 2E).
Management actions
Both intentional and unintentional interactions between humans and plants have the potential to alter interactions between species. If management activities can influence the rate and direction of coevolution between native and invasive plants, we may have a powerful tool to increase the chances of native species persistence, and perhaps even decrease the dominance of invasive species, in highly invaded systems. Management actions can affect all of the factors (described above) that are likely to influence the outcome of coevolution between species. Specifically, we will discuss how human activities can change population sizes and genetic diversity, alter gene flow, and change selective regimes for both natives and exotic species. If we are cognizant of how our activities affect these factors, we may be able to intentionally favor native species over exotics by changing our management practices. We suggest that any management resulting in maintenance of heritable variation in fitness-related traits for native species in invaded systems, while decreasing such variation in invasive populations, is likely to shift the coevolutionary advantage in favor of native species. Focusing on the maintenance of adaptive genetic variation within natives in invaded communities could create more attainable management goals, and have the long-term effect of increasing the diversity and cover of native species in invaded communities.
Population sizes and genetic diversity
Many invasive species management practices such as herbicides, fire, grazing management, and biocontrol releases are intended to favor native species over exotic invaders. These actions are taken with the intention of replacing invasive species with native ones (Bakker and Wilson 2004), but this is not always successful. For example, it is quite common to reduce the population size of an invasive species, only to have it spread back into a treated area (Mack et al. 2000; Rinella et al. 2009). This instance is typically considered a management failure and a waste of resources. However, management practices also affect the genetic diversity of natives and exotics. From an evolutionary perspective, periodic reductions in the population sizes of invasives through targeted management may be a valuable contribution to the long-term diversity in an area. Specifically, causing population bottlenecks within invasive species, especially if the bottleneck is sustained for a period of time, and is in response to an unrelated selective agent such as herbicide, could greatly affect the ability of invasive species evolve to better compete with co-occurring native populations. Periodic disturbances that reduce invasive populations, but not native ones, may essentially set the coevolutionary clock for invasives back to zero, even if these disturbances only temporarily reduce invasive population sizes. On the other hand, periodic release of native species from competition with invasive and restoration with local genotypes could increase the growth rates and population sizes of native species, increasing their ability to maintain genetic variation within a population and maintain a viable population size by decreasing the loss of alleles to drift and the risk of extinction due to demographic stochasticity (Ellstrand and Elam 1993; Lande 1993). Management actions that favor natives over exotics could significantly affect the amount of diversity possessed by each species, and thus the outcome of coevolution (Fig. 1B, Table 1B). Even actions that do not completely exclude invaders from a site may be valuable for the long-term diversity of an invaded community.
Gene flow
Human activities affect gene flow within invasive species. First, continual re-introduction either from the native range or from other invasive populations can be a significant source of genetic diversity within invasive populations (Ellstrand and Schierenbeck 2000; Sexton et al. 2002). Keeping the genetic diversity of invasive populations low may limit their evolutionary potential. Conversely, a high rate of gene flow could swamp ongoing evolution within an invasive weed population, however we do not recommend this approach, because the risks of introducing genotypes that are even more invasive are too great. Thus, preventing multiple introductions should be a management goal, even for species that are currently widespread. Secondly, creating barriers between invasive populations, in the form of breaks between weed patches, could limit gene flow and keep effective population sizes smaller, increasing the likelihood of genetic drift and preventing the maintenance of incurred genetic diversity. Management resources are often not devoted to the largest invaded areas, because of the low chance of control. However, if remnant natives still co-occur in these areas, management activities that limit gene flow within relatively large stands of highly invasive species may be beneficial. Research should be undertaken to determine the evolutionary impact of creating multiple, smaller populations of invasive plants compared to large, uninterrupted stands of invasive plants (Table 1C).
Maintaining continuity between extant stands of natives will increase gene flow among populations and provide the opportunity for genetic diversity to be maintained in native species. Again, there is the possibility that too much gene flow between uninvaded populations and invaded populations of natives species could be harmful, although, we do not understand these dynamics well enough to suggest that management actively limit gene flow between remnant native populations. However, there is the possibility that gene flow caused by certain types of management activities could slow or reverse coevolutionary dynamics. Because of economic considerations, the agronomic approach has been the most effective at generating seed for restoration projects. This involves choosing one population, or in some cases, one genotype, and increasing it for use in a wide variety of environments. When native species are being actively restored in large areas, whole stands of natives may have extremely low genetic diversity (e.g. Poa secunda, Jones and Larson 2005), despite having large population sizes. This practice may put native species at a disadvantage in restored areas (Fig. 1B), and gene flow from restored populations into remnant populations may reduce the overall genetic diversity of native species, as well as swamp any ongoing coevolution within remnant populations. Because economic considerations are real, an alternative to the agronomic approach may be to collect restoration material from an invaded source population that is undergoing natural selection against the invasive of interest for agronomic seed increase (Leger 2008). This would be increase gene flow between invaded environments, but with a particular subset of genotypes that have proven to be effective at growing in invaded habitats.
Measuring success by measuring evolutionary change
Success in restoration or management is typically measured by empirical studies of plant abundance. If invasive species are mostly absent, and natives are mostly dominant, this is considered a success. However, a longer-term, evolutionary view of the interactions between natives and exotics could alter our measure of success. If a manager knows that native plant populations, while small, are increasing their competitive ability with invasive species, this is a considerably more hopeful situation than may appear when looking at abundance data alone. The effects of this greater competitive ability might take longer than a typical management cycle to manifest as a change in dominance on the ground, particularly in unproductive systems. However, in many highly invaded systems, we lack the resources to achieve eradication of invasive weeds, and focusing on long-term outcomes may be the most realistic scenario.
Conclusions
The long-term outcome of competitive interactions between invasive and remnant native plants probably depends on a number of population genetic and ecological factors, including genetic variation, population size and history, gene flow, encounter rates, and the number of additional selective agents acting on each species. It is possible for active management to affect all of these, and we suggest a set of experimental questions that directly assess the likelihood of and affects of management on coevolution between interacting species (Table 1). Adopting an evolutionary perspective while managing highly invaded systems may alter our invasive species control actions: it is possible that long-term community composition can be affected by relatively small management efforts. When eradication of invaders is not possible, we suggest considering an alternative goal: improving the competitive ability of native plants that persist in highly invaded systems.
Acknowledgments
The authors thank Nancy Emery, John Gaskin, Erin Goergen, and Andrew Lenssen for helpful comments on the manuscript.
Literature cited
- Aarssen LW. Ecological combining ability and competitive combining ability in plants – toward a general evolutionary-theory of coexistence in systems of competition. American Naturalist. 1983;122:707–731. [Google Scholar]
- Aarssen LW, Turkington R. Biotic specialization between neighboring genotypes in Lolium perenne and Trifolium repens from a permanent pasture. Journal of Ecology. 1985;73:605–614. [Google Scholar]
- Amarasekare P, Nisbet RM. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. American Naturalist. 2001;158:572–584. doi: 10.1086/323586. [DOI] [PubMed] [Google Scholar]
- Antonovics J. The nature of limits to natural selection. Annals of the Missouri Botanical Garden. 1979;63:224–247. [Google Scholar]
- Bakker JD, Wilson SD. Using ecological restoration to constrain biological invasion. Journal of Applied Ecology. 2004;41:1058–1064. [Google Scholar]
- Batten KM, Scow KM, Davies KF, Harrison SP. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biological Invasions. 2006;8:217–230. [Google Scholar]
- Batten KM, Scow KM, Espeland EK. Soil microbial community associated with an invasive grass differentially impacts native plant performance. Microbial Ecology. 2008;55:220–228. doi: 10.1007/s00248-007-9269-3. [DOI] [PubMed] [Google Scholar]
- Bridle JR, Vines TH. Limits to evolution at range margins: when and why does adaptation fail. Trends in Ecology & Evolution. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Brock MT, Weinig C, Galen C. A comparison of phenotypic plasticity in the native dandelion Taraxacum ceratophorum and its invasive congener T. officinale. New Phytologist. 2005;166:173–183. doi: 10.1111/j.1469-8137.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Chesson PL. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- Chesson PL, Warner RR. Environmental variability promotes coexistence in lottery competitive systems. American Naturalist. 1981;117:923–943. [Google Scholar]
- Colautti RI, MacIsaac HJ. A neutral terminology to define ‘invasive’ species. Diversity and Distributions. 2004;10:135–141. [Google Scholar]
- Connell JH. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos. 1980;35:131–138. [Google Scholar]
- Corbin JD, D'Antonio CM. Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology. 2004;85:1273–1283. [Google Scholar]
- Crutsinger GM, Souza L, Sanders NJ. Intraspecific diversity and dominant genotypes resist plant invasions. Ecology Letters. 2008;11:16–23. doi: 10.1111/j.1461-0248.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- DeWalt SJ, Denslow JS, Ickes K. Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology. 2004;85:471–483. [Google Scholar]
- Diamond J. Evolution of ecological segregation in the New Guinea montane avifauna. In: Diamond J, Case JJ, editors. Community Ecology. New York: Harper & Row; 1986. pp. 98–425. [Google Scholar]
- Diamond J, Pimm SL, Gilpin ME, Lecroy M. Rapid evolution of character displacement in myzomelid honeyeaters. American Naturalist. 1989;134:675–708. [Google Scholar]
- DiTomaso JM. Invasive weeds in rangelands: species, impacts, and management. Weed Science. 2000;48:255–265. [Google Scholar]
- Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Elam DR. Population genetic consequences of small population size: implications for plant conservation. Annual Review of Ecology and Systematics. 1993;24:217–242. [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DR, Hill J, Williams TA, Rhodes I. Effects of coexistence on the performance of white clover perennial ryegrass mixtures. Oecologia. 1985;66:536–539. doi: 10.1007/BF00379346. [DOI] [PubMed] [Google Scholar]
- Fridley JD, Grime JP, Bilton M. Genetic identity of interspecific neighbors mediates plant responses to competition and environmental variation in a species-rich grassland. Journal of Ecology. 2007;95:908–915. [Google Scholar]
- Funk JL. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology. 2008;96:1162–1173. [Google Scholar]
- Futuyma DJ. Evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Grant PR. Ecology and Evolution of Darwin's Finches. Princeton, NJ: Princeton University Press; 1986. [Google Scholar]
- Grant BR, Grant PR. Evolutionary Dynamics of a Natural Population: The Large Cactus Finch of the Galapagos. Chicago, IL: University of Chicago Press; 1989. [DOI] [PubMed] [Google Scholar]
- Groom MJ. Allee effects limit population viability of an annual plant. American Naturalist. 1998;151:487–496. doi: 10.1086/286135. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Padilla DK. Are invasive species a major cause of extinctions? Trends in Ecology & Evolution. 2004;19:470–474. doi: 10.1016/j.tree.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Haloin JR, Strauss SY. Interplay between ecological communities and evolution: review of feedbacks from microevolutionary to macroevolutionary scales. Annals of the New York Academy of Sciences. 2008;1133:87–125. doi: 10.1196/annals.1438.003. [DOI] [PubMed] [Google Scholar]
- Hartl DL, Clark AG. Principles of Population Genetics. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- Hawkes CV, Belnap J, D'Antonio C, Firestone MK. Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant and Soil. 2006;281:369–380. [Google Scholar]
- Hedrick PW. Genetics of Populations. Sudbury, MA: Jones and Bartlett Publishers; 2005. [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Henter HJ. The potential for coevolution in a host-parasitoid system. 2. Genetic-variation within a population of wasps in the ability to parasitize an aphid host. Evolution. 1995;49:439–445. doi: 10.1111/j.1558-5646.1995.tb02276.x. [DOI] [PubMed] [Google Scholar]
- Henter HJ, Via S. The potential for coevolution in a host-parasitoid system. 1. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution. 1995;49:427–438. doi: 10.1111/j.1558-5646.1995.tb02275.x. [DOI] [PubMed] [Google Scholar]
- Holt RD. Spatial heterogeneity, indirect interactions, and the coexistence of prey species. American Naturalist. 1984;124:377–406. doi: 10.1086/284280. [DOI] [PubMed] [Google Scholar]
- Holt RD, Gomulkiewicz R. How does immigration influence local adaptation? A reexamination of a familiar paradigm. American Naturalist. 1997;149:563–572. [Google Scholar]
- Hubbell SP. Neutral theory and the evolution of ecological equivalence. Ecology. 2006;87:1387–1398. doi: 10.1890/0012-9658(2006)87[1387:ntateo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Huntly N. Herbivores and the dynamics of communities and ecosystems. Annual Review of Ecology and Systematics. 1991;22:477–503. [Google Scholar]
- Johnson MTJ, Vellend M, Stinchcombe JR. Evolution in plant populations as a driver of ecological changes in arthropod communities. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1593–1605. doi: 10.1098/rstb.2008.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Larson SR. USDA Forest Service Proceedings RMRS-P-38. Fort Collins, CO: USDA Forest Service Rocky Mountain Station; 2005. Status and use of important native grasses adapted to sagebrush communities. [Google Scholar]
- Jones LE, Becks L, Ellner SP, Hairston NG, Yoshida T, Fussmann GF. Rapid contemporary evolution and clonal food web dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1579–1591. doi: 10.1098/rstb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Thompson JN. Alternative routes to the evolution of competitive ability in 2 competing species of Drosophila. Evolution. 1995;49:616–625. doi: 10.1111/j.1558-5646.1995.tb02298.x. [DOI] [PubMed] [Google Scholar]
- Joshi A, Thompson JN. Evolution of broad and specific competitive ability in novel versus familiar environments in Drosophila species. Evolution. 1996;50:188–194. doi: 10.1111/j.1558-5646.1996.tb04485.x. [DOI] [PubMed] [Google Scholar]
- Joshi A, Thompson JN. Adaptation and specialization in a two-resource environment in Drosophila species. Evolution. 1997;51:846–855. doi: 10.1111/j.1558-5646.1997.tb03666.x. [DOI] [PubMed] [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Lande R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. American Naturalist. 1993;142:911–927. doi: 10.1086/285580. [DOI] [PubMed] [Google Scholar]
- Lau JA, Strauss SY. Insect herbivores drive important indirect effects of exotic plants on native communities. Ecology. 2005;86:2990–2997. [Google Scholar]
- Lau JA. Evolutionary responses of native plants to novel community members. Evolution. 2006;60:56–63. [PubMed] [Google Scholar]
- Leger EA. The adaptive value of remnant native plants in invaded communities: an example from the Great Basin. Ecological Applications. 2008;18:1226–1235. doi: 10.1890/07-1598.1. [DOI] [PubMed] [Google Scholar]
- Levine JM, Adler PB, Yelenik SG. A meta-analysis of biotic resistance to exotic plant invasions. Ecology Letters. 2004;7:975–989. [Google Scholar]
- Lulow ME. Invasion by non-native annual grasses: the importance of species biomass, composition, and time among California native grasses of the Central Valley. Restoration Ecology. 2006;14:616–626. [Google Scholar]
- Lulow ME. Restoration of California native grasses and clovers: the roles of clipping, broadleaf herbicide, and native grass density. Restoration Ecology. 2008;16:584–593. [Google Scholar]
- MacArthur RH, Levins R. Competition, habitat selection, and character displacement in a patchy environment. Proceedings of the National Academy of Sciences of the United States of America. 1964;51:1207–1210. doi: 10.1073/pnas.51.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. [Google Scholar]
- Martin MM, Harding J. Estimates of fitness in Erodium populations with intraspecific and interspecific competition. Evolution. 1982;36:1290–1298. doi: 10.1111/j.1558-5646.1982.tb05498.x. [DOI] [PubMed] [Google Scholar]
- Mealor BA, Hild AL. Potential selection in native grass populations by exotic invasion. Molecular Ecology. 2006;15:2291–2300. doi: 10.1111/j.1365-294X.2006.02931.x. [DOI] [PubMed] [Google Scholar]
- Meimberg H, Hammond JI, Jorgensen CM, Park TW, Gerlach JD, Rice KJ, McKay JK. Molecular evidence for an extreme genetic bottleneck during introduction of an invading grass to California. Biological Invasions. 2005;8:1355–1366. [Google Scholar]
- Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morghan KJR, Rice KJ. Centaurea solstitialis invasion success is influenced by Nassella pulchra size. Restoration Ecology. 2005;13:524–528. [Google Scholar]
- Nagel JM, Griffin KL. Construction cost and invasive potential: comparing Lythrum salicaria (Lythraceae) with co-occurring native species along pond banks. American Journal of Botany. 2001;88:2252–2258. [PubMed] [Google Scholar]
- Nei M, Maruyama T, Chakraborty R. The bottleneck effect and genetic variability in populations. Evolution. 1975;29:1–10. doi: 10.1111/j.1558-5646.1975.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Valladares F, Ceulemans R. Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell and Environment. 2003;26:941–956. doi: 10.1046/j.1365-3040.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- Nunney L. The effective size of annual plant populations: the interaction of a seed bank with fluctuating population size in maintaining genetic variation. American Naturalist. 2002;160:195–204. doi: 10.1086/341017. [DOI] [PubMed] [Google Scholar]
- Pritchard JR, Schluter D. Declining interspecific competition during character displacement: summoning the ghost of competition past. Evolutionary Ecology Research. 2001;3:209–220. [Google Scholar]
- Reinhart KO, Callaway RM. Soil biota and invasive plants. New Phytologist. 2006;170:445–457. doi: 10.1111/j.1469-8137.2006.01715.x. [DOI] [PubMed] [Google Scholar]
- Reynolds HL, Hungate BA, Chapin FS, Dantonio CM. Soil heterogeneity and plant competition in an annual grassland. Ecology. 1997;78:2076–2090. [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. [PubMed] [Google Scholar]
- Rinella MJ, Maxwell BD, Fay PK, Weaver T, Sheley RL. Control effort exacerbates invasive-species problem. Ecological Applications. 2009;19:155–162. doi: 10.1890/07-1482.1. [DOI] [PubMed] [Google Scholar]
- Roscher C, Schumacher J, Weisser WW, Schulze ED. Genetic identity affects performance of species in grasslands of different plant diversity: an experiment with Lolium perenne cultivars. Annals of Botany. 2008;102:113–125. doi: 10.1093/aob/mcn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughgarden J. Competition and theory in community ecology. American Naturalist. 1983;122:583–601. [Google Scholar]
- Sax DF, Gaines SD. Species invasions and extinction: the future of native biodiversity on islands. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11490–11497. doi: 10.1073/pnas.0802290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Grant PR. The distribution of Geospiza difficilis in relation to Geospiza fuliginosa in the Galapagos islands – tests of three hypotheses. Evolution. 1982;36:1213–1226. doi: 10.1111/j.1558-5646.1982.tb05490.x. [DOI] [PubMed] [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. American Naturalist. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Seabloom EW, Harpole WS, Reichman OJ, Tilman D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13384–13389. doi: 10.1073/pnas.1835728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JP, McKay JK, Sala A. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecological Applications. 2002;12:1652–1660. [Google Scholar]
- Stenseth NC, Smith JM. Coevolution in ecosystems – red queen evolution or stasis. Evolution. 1984;38:870–880. doi: 10.1111/j.1558-5646.1984.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecology Letters. 2006;9:354–371. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Taper ML, Case TJ. Coevolution among competitors. Oxford Surveys in Evolutionary Biology. 1992;8:63–109. [Google Scholar]
- Thompson JN. The Coevolutionary Process, Chicago. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution, Chicago. Chicago, IL: University of Chicago Press; 2005a. [Google Scholar]
- Thompson JN. Coevolution: the geographic mosaic of coevolutionary arms races. Current Biology. 2005b;15:992–994. doi: 10.1016/j.cub.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Turkington R, Harper JL. Growth, distribution and neighbor relationships of Trifolium repens in a permanent pasture 4. Fine-scale biotic differentiation. Journal of Ecology. 1979;67:245–254. [Google Scholar]
- Turkington R, Mehroff LA. The role of competition in structuring plant communities. In: Grace JB, Tilman D, editors. Perspectives in Plant Competition. Caldwell, NJ: The Blackburn Press; 1990. pp. 308–340. [Google Scholar]
- Vermeij GJ. The evolutionary interaction among species – selection, escalation, and coevolution. Annual Review of Ecology and Systematics. 1994;25:219–236. [Google Scholar]
- Waples RS. Seed banks, salmon, and sleeping genes: effective population size in semelparous, age-structured species with fluctuating abundance. American Naturalist. 2006;167:118–135. doi: 10.1086/498584. [DOI] [PubMed] [Google Scholar]
- Ward SM, Gaskin JF, Wilson LM. Ecological genetics of plant invasion: what do we know? Invasive Plant Science and Management. 2008;1:98–109. [Google Scholar]
- Whitney KD, Gabler CA. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Diversity and Distributions. 2008;14:569–580. [Google Scholar]


