Abstract
Ants are among the most damaging invasive species, and their success frequently arises from the widespread cooperation displayed by introduced populations, often across hundreds of kilometers. Previous studies of the invasive Argentine ant (Linepithema humile) have shown that introduced populations on different continents each contain a single, vast supercolony and, occasionally, smaller secondary colonies. Here, we perform inter-continental behavioral analyses among supercolonies in North America, Europe, Asia, Hawaii, New Zealand and Australia and show that these far-flung supercolonies also recognize and accept each other as if members of a single, globally distributed supercolony. Furthermore, populations also possess similar genetic and chemical profiles. However, these ants do show aggression toward ants from South Africa and the smaller secondary colonies that occur in Hawaii and California. Thus, the largest and most dominant introduced populations are likely descended from the same ancestral colony and, despite having been established more than 100 years ago, have diverged very little. This apparent evolutionary stasis is surprising because, in other species, some of the most rapid rates of evolutionary change have occurred in introduced populations. Given the spatial extent of the Argentine ant society we report here, there can be little doubt that this intercontinental supercolony represents the most populous known animal society.
Keywords: Cuticular hydrocarbon, invasive species, Linepithema humile, nestmate recognition, population genetics, supercolony
Introduction
Invasive species are one of the major threats to native communities and the scope and severity of biological invasions continues to grow with increasing global human commerce (Cox 1999; McNeely 2001). Invading populations often experience founder effects, genetic bottlenecks and novel selective pressures that can lead to evolutionary changes in important traits, such as tolerance, growth rate and behavior (Mooney and Cleland 2001; Sakai et al. 2001; Lee 2002). These forces can drive rapid evolution in the new range (Thompson 1998; Hendry and Kinnison 1999; Reznick and Ghalambor 2001) resulting in introduced populations that are markedly divergent from each other and from their ancestral source population. Invasive species with global distributions are interesting for studying evolution because the different introduced populations of global invaders can serve as natural replicates for studying the evolutionary responses to introduction.
The Argentine ant (Linepithema humile) is a particularly apt model system for evaluating the ecological and evolutionary consequences of a global invasion. The Argentine ant has been introduced from its native Argentina to six continents and many oceanic islands (Suarez et al. 2001; Wild 2004). The Argentine ant is highly invasive; it attains high population densities and is able to out-compete many native ant species (Holway et al. 2002). Introduced populations are remarkable because they are often comprised of a single, geographically vast ‘supercolony’ within which territorial behavior and intraspecific aggression are absent.
For many supercolonies around the world, colony boundaries have been determined at local or regional scales using bio-assays, with workers showing no aggression towards members of the same supercolony, but high aggression towards workers from different colonies (Tsutsui et al. 2000; Giraud et al. 2002; Wetterer and Wetterer 2006; Corin et al. 2007a,b; Bjorkman-Chiswell et al. 2008; Suhr et al. 2009; Sunamura et al. 2009). These studies have revealed that supercolonies can extend across large geographic distances. In New Zealand and California, for example, supercolonies span at least 700 and 900 km respectively (Tsutsui et al. 2000; Tsutsui and Case 2001; Corin et al. 2007a,b), and the main European supercolony extends for more than 4000 km, through Spain, Portugal, France and Italy (Giraud et al. 2002). On occasion, more than one supercolony can be found in parts of the introduced range. At least four smaller supercolonies occur in California (in addition to the main supercolony) (Tsutsui et al. 2003), and Europe is known to harbor one smaller supercolony, in Catalonia, Spain (Giraud et al. 2002).
Some evidence suggests that supercolonies often share a common origin. For example, mtDNA sequence data suggest that the supercolony in New Zealand originated from Australia (Corin et al. 2007b). Moreover, a study by Brandt et al., (2009) found that the four largest supercolonies, in California, Europe, Australia and Hawaii, are genetically and chemically similar to each other. In addition, a study by Sunamura et al. (2009) showed that workers from supercolonies in Japan, Europe and California are not aggressive towards each other, implying that workers of these colonies behave as if they belong to the same colony.
In this study, we conducted a global-scale analysis of the relationships among the world's largest supercolonies of Argentine ants. We performed inter-continental behavioral assays between nine previously described supercolonies in North America, Europe, Asia, Africa, Hawaii, New Zealand and Australia, to determine if these globally distributed introduced populations recognize each other as colony mates. We also performed genetic analyses of these populations using microsatellite loci to quantify their genetic relationships. Finally, we determined the chemical relationships among these supercolonies using gas chromatography and mass spectrometry (GC–MS) to analyze cuticular hydrocarbon profiles. Social insects use cuticular hydrocarbons to recognize ants from the same colony (Thomas et al. 1999; Wagner et al. 2000; Torres et al. 2007), and individuals within a colony often share a common hydrocarbon profile, or colony odor (Denis et al. 2006; Martin et al. 2008). This multidisciplinary approach allows us to describe the current patterns of behavior, genetics and chemical ecology, as well as make inferences about the historical processes that may have produced these patterns.
Methods
During 2008 and 2009, we collected L. humile from nesting sites belonging to the largest described supercolonies from North America, Asia, Europe Australia, New Zealand and Hawaii. In addition we collected from two smaller, secondary supercolonies from Hawaii and North America and from a supercolony in Africa (Table 1). Live ants were transported to the laboratory and maintained in quarantine at the University of California-Berkeley. The colonies were fed a diet of sugar water, chicken egg and protein solution (modified LB broth). Within a few days of the arrival, we performed standard behavioral assays to test for aggression between all of the supercolonies and a reference colony, the large supercolony that dominates California. We also conducted assays between many pairwise combinations of the supercolonies from the different continents. These assays were conducted within a few weeks of arrival.
Table 1.
Sampling locations
| Site | Latitude | Longitude |
|---|---|---|
| California, USA | ||
| Berkeley (US 1) | 37°52′22′′N | 122°15′52′′W |
| Lake Hodges, La Mesa (US 2) | 33°3′45′′N | 117°7′8′′W |
| Europe | ||
| Marseille (F) | 43°29′80′′N | 05°37′41′′W |
| Australia | ||
| Melbourne (AU) | 37°47′53′′N | 144°57′32′′W |
| New Zealand | ||
| Wellington (NZ) | 41°28′00′′N | 174°76′00′′W |
| Japan | ||
| Iwakuni City, Yamaguchi (J) | 34°06′15″N | 132°12′01″E |
| Hawaii, USA | ||
| Kipuka Nene (H 1) | 19°13′21′′N | 155°38′04′′W |
| KMC (H 2) | 19°26′01′′N | 155°16′25′′W |
| South Africa | ||
| Stellenbosch (SA) | 33°3′45′′N | 117°7′8′′W |
Behavioral assays
For each assay, we placed two arbitrarily chosen workers in a Petri dish (3.5 cm diameter) that was lined with Fluon (Northern Products Inc., Woonsocket, RI, USA). We observed the behavior of the ants for 3 min and scored the assay as aggressive if the marked worker showed one or more of the following behaviors; flaring of mandibles, recoil behavior, biting or grabbing. In addition to assays between each supercolony and the reference colony [the largest supercolony in California (US1)], we also conducted assays using the following pair combinations: US1–US2, F–US2, AU–US2, J–US2, F–J, AU–F, AU–J, H1–F, H2–F, H1–H2 (see Table 1 for abbreviations). It was not possible to test every pairwise comparison of supercolonies because the timing of collection abroad was not synchronous, and number of ants in some of the collections was too small to permit additional behavioral assays.
Cuticular hydrocarbon analysis
We extracted cuticular hydrocarbons from individual workers (12 per nest) from each nest by immersing each ant in 45 μL of hexane for 10 min. Each worker's profile was analyzed separately, using GC–MS. To detect quantitative differences in the hydrocarbon patterns of our samples, we injected 1 μL of each sample into a Agilent 7890 GC equipped with a 190915-433 capillary column (30 m × 250 mm × 0.25 μm; Agilent Technologies, Santa Clara, California). We used helium as carrier gas at 1 mL/min, the injector in splitless mode (1 min), and a temperature program of 2 min at 80°C, to 270°C at 20°C/min, then to 310°C at 3°C/min (Suarez et al. 2002). Injector temperature was set at 250°C. Electron impact mass spectra were obtained with an ionization voltage of 70 eV and a source temperature of 250°C. We integrated chromatograms in the program ACD SpecManager (version 10.0; Advanced Chemistry Development) and we calculated the relative proportions of each peak area to that of the total sample. We set up a method of selected ion monitoring, narrowing the mass range scanned to the three ions 99, 113 and 127. For each colony, we identified the cuticular compounds from a pool of 50 ants using full ion scans. Compounds were characterized using diagnostic ions and Kovats indices (Carlson et al. 1998) and were compared to published data to confirm assignment of the branching positions. We identified 73 peaks in all of the samples and for each peak calculated its peak area as a percentage of the total peak area.
We calculated pairwise Euclidian distances between individuals from the relative peak areas (z-transformed). From the resulting dissimilarity matrix, we visualized the chemical distances between individuals using two dimensional nonmetric multidimensional scaling (NMDS) in the program Genstat (version 11).
Genotyping
We genotyped 12 workers from each colony at 13 polymorphic microsatellite loci (GenBank accession numbers AF173164, AF093514, AF093515, AF093517, AF093520, AF093521, AF093522, AF093524, AF093525, AF093526, AF093527, AF093531 and AF093533; Krieger and Keller 1999; Suarez et al. 1999; Tsutsui et al. 2000). We estimated the inter-colony genetic distances according to Nei (1987) using the computer program GenAlEx (http://www.anu.edu.au/BoZo/GenAlEx/) and used the matrix of pairwise genetic distances as input for a two-dimensional MDS, as described above.
Supercolony distribution
We mapped the worldwide distribution of the supercolony based on data from this study as well as previously published aggression studies (Tsutsui et al. 2000; Giraud et al. 2002; Wetterer and Wetterer 2006; Corin et al. 2007a,b; Bjorkman-Chiswell et al. 2008; Suhr et al. 2009). Sampling locations were considered part of the transcontinental supercolony when cross tests between workers showed little or no aggression.
Results
We found no aggression between workers from the large Californian supercolony (US 1) and workers from the supercolonies in Japan (0 of 50 trials), Europe (0/50), New Zealand (0/50) and one of the two supercolonies in Hawaii (H 1) (0/20), and we saw little aggression between workers from the Californian supercolony and workers from the large Australian supercolony (4/50). Pairwise tests between these colonies also revealed no aggression (Japan versus Europe, 0/50; Japan versus Australia 0/50; Europe versus Hawaii 1, 0/20) or little aggression (Australia versus Europe, 5/50), indicating that workers from these widely distributed sites recognize each other as members of the same transcontinental supercolony. We did find high levels of aggression between workers of the Californian supercolony (US 1) and a smaller secondary supercolony in California (US 2) (42/50), a supercolony from South Africa (20/50), and one of the supercolonies in Hawaii (H 2) (31/40). We also found high levels of aggression between the smaller secondary supercolony in California (US 2) and the supercolonies from Europe (47/50), Japan (45/50) and Australia (38/45) and between one of the supercolony from Hawaii (H 2) and the other supercolony in Hawaii (H1) (15/20) and the supercolony from Europe (13/20). These data indicate that although many of these sites comprise a globally distributed, transcontinental supercolony, these ants are still capable of displaying aggression under the appropriate circumstances, and do not accept all conspecific ants as colony mates. Figure 1 depicts the global extent of the large supercolony and populations containing other, behaviorally distinct supercolonies.
Figure 1.

The global extent of the large supercolony (red, orange) and populations containing other, behaviorally distinct supercolonies (gray). Sites tested in this study and shown to belong to the same transcontinental supercolony are shown as red squares. Sites shown as orange circles also belong to this supercolony, based on data from this and other studies (Tsutsui et al. 2000; Giraud et al. 2002; Wetterer and Wetterer 2006; Corin et al. 2007a; Bjorkman-Chiswell et al. 2008; Suhr et al. 2009). Gray squares mark the locations that do not belong to this colony. Colonies in the native range, which typically show aggression at much smaller spatial scales, are shown as gray circles.
The behavioral responses concur with the chemical analyses. The chemical profiles of workers from the mutually amicable supercolonies in California (US 1), Australia, New Zealand, Japan, Europe and one of the supercolonies in Hawaii (H 1) were all very similar (Fig. 2A). However, the hydrocarbon profiles of these ants differ from the profiles of workers from South Africa, the smaller secondary supercolony in California (US 2) and the other supercolony in Hawaii (H 2), which also differ among each other (Fig. 2A). There was a significant relationship between the percentage of aggressive workers in the behavioral trials and the chemical distance between the hydrocarbon profiles of colony pairs (Fig. 3, F = 68.65, df = 13, P < 0.001).
Figure 2.
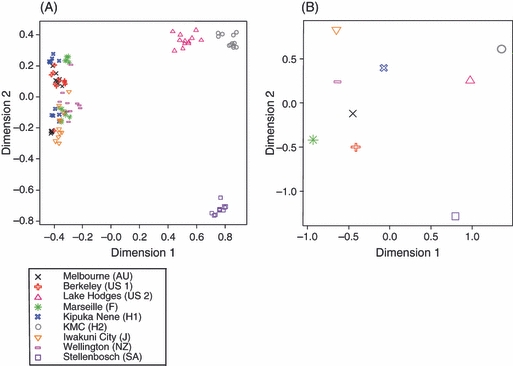
Multidimensional scaling visualization of the relationship between (A) all sampled Linepithema humile individuals based on differences in their cuticular hydrocarbon profiles (Euclidian distance), and (B) all sampled nests based on genetic distances (Nei). Distinct supercolonies are color coded; different symbols of the same color represent nesting sites within the same supercolony.
Figure 3.
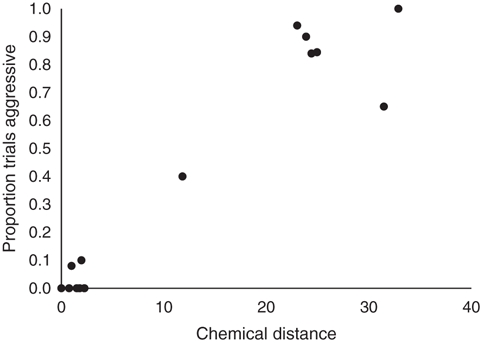
Relationship between the chemical distance between colonies and the proportion of trials that workers were aggressive (all P < 0.001).
The behavioral responses also match the results of the genetic analysis. The large supercolonies in California (US 1), Australia, New Zealand, Japan, Europe and one of the supercolonies in Hawaii (H 1) were genetically more similar to each other than they are to the supercolonies from South Africa, the smaller secondary supercolony in California (US 2) and the other supercolony in Hawaii (H 2) (Fig. 2B). There was a significant relationship between the percentage of aggressive workers in the behavioral trials and the genetic distance of colony pairs (Fig. 4, F = 35.28, df = 11.8, P < 0.001). Moreover, there was a strong relationship between the genetic similarity and the chemical similarity (Fig. 5, F = 41.72, df = 12.4, P < 0.001), suggesting that hydrocarbon profiles are genetically determined.
Figure 4.
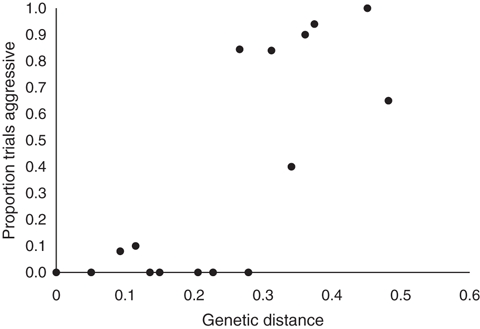
Relationship between the genetic distance (Nei) between colonies and the proportion of trials that workers were aggressive.
Figure 5.
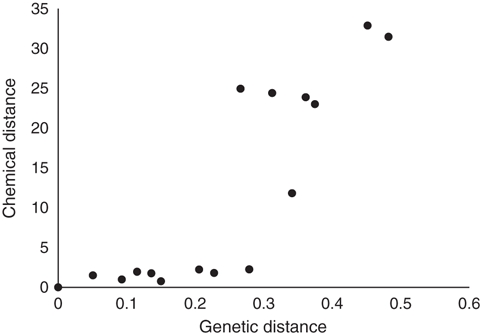
Relationship between the genetic (Nei) and chemical distances between colonies.
Discussion
Our data suggest that the largest Argentine ant supercolonies throughout the world behave as if they are members of a single, globally distributed supercolony. Included are the sole supercolony from both New Zealand and Australia, and the largest of the supercolonies that occur in Japan, Hawaii, California and Europe. Similar findings were reported recently by Sunamura et al. (2009), who showed that workers from the largest supercolony in Japan were not aggressive towards workers from the large supercolonies in Europe and California. Although the global distribution of this species appears to be dominated by the single major supercolony, we found high aggression between workers from this global supercolony and workers from a supercolony from South Africa, a smaller secondary supercolony from California and a smaller secondary supercolony from Hawaii, indicating that these colonies are likely of different origin. Our genetic and chemical data support the behavioral data: the supercolonies that are mutually tolerant are also genetically and chemically very similar, whereas those that are aggressive are genetically and chemically more different.
Our results show a striking similarity of Argentine ant populations at a global scale, despite the fact that many of these populations were established more than a century ago (Suarez et al. 2001). This is particularly surprising given that introduced populations often exhibit rapid evolutionary change over relatively short time scales (Huey et al. 2000; Koskinen et al. 2002; Dlugosch and Parker 2008; Edgell et al. 2009). For example, populations of Drosophila subobscura, introduced to North America evolved a cline in wing length in only 20 years (Huey et al. 2000). It has to be noted that, while we found no chemical and behavioral divergence, and only little genetical divergence there may have been divergence in other traits not measured in this study.
This slow pace of divergence among populations and the long-term persistence of behavioral, chemical and genetic homogeneity, remain unexplained. One potential explanation is that frequent exchange of individuals among distant populations has prevented the supercolonies from diverging and has constrained local adaptation. However, in this species, gene flow between populations can only occur if the introduced individuals are very similar to the established population, otherwise the introduced individuals would be rejected by the established population. While it is difficult to obtain a direct measure of the frequency with which introductions occur, multiple introductions into the same area appear to be common. For example, Corin et al. (2007a,b) collated data from interception records from New Zealand's border security and found that during a time period of 21 years, 37 interceptions of Argentine ants were made. Thus, frequent movement and introduction of Argentine ant propagules among distant parts of the global supercolony may promote homogeneity among these populations, thus facilitating the persistence of the observed behavioral patterns. From an applied perspective, the genetic similarity and apparently limited local adaptation of the Argentine ant may be useful when finding means to control or eradicate introduced populations. Genetically homogenous populations are less likely to evolve resistance to biocontrol agents (Van Driesche and Bellows 1996) and it may be possible to develop control methods that target these specific genotypes.
It is notable that the colonies that comprise the global supercolony are also each the largest supercolony recorded on the continent or island where it is found (Tsutsui et al. 2000; Giraud et al. 2002; Wetterer and Wetterer 2006; Corin et al. 2007a,b; Suhr et al. 2009; Sunamura et al. 2009). Why is this one globally dominant supercolony also locally dominant? One possible explanation is that this supercolony is a superior competitor, thus preventing the establishment of new populations and displacing previously established supercolonies. In accordance with this idea, Argentine ants from the large Californian supercolony are more likely to initiate and survive an attack than workers of the four secondary Californian supercolonies (Tsutsui et al. 2003). Tsutsui et al. (2003) suggested this polarity in aggressive behavior results from an asymmetry in genetic diversity; ants from colonies with low genetic diversity have fewer recognition cues and a more stringent recognition template making them more likely to initiate attacks than ants with a wide array of cues. Different introduced populations may thus have followed similar evolutionary trajectories, in which selection against genetically diverse colonies has led to the success of this one particular supercolony.
Alternatively, the expansive distribution of the global supercolony may be explained by invasion history. Under this scenario, the first colonists to become established in a new range are able to prevent the establishment of later-arriving propagules. Once this colony became established a new location, it may have prevented the establishment of other colonies, and served as a source of propagules that were dispersed and colonized new locations. This scenario fits with some aspects of the known introduction history of this species. Historical records suggest the earliest Argentine ant introduction occurred on Madeira Island in 1882 (Stoll 1898) and that the supercolony in Madeira gave rise to the main supercolony in continental Europe (Wetterer and Wetterer 2006). As we identified the main European supercolony as part of the transcontinental supercolony, it is feasible that many of supercolonies throughout the world descended from this apparent first introduction in Madeira, or from the same ancestral population. Interestingly, this would even include relatively recent colonizations, such as those in New Zealand and Japan, which were discovered in 1990 and 1993 respectively (Green 1990; Sugiyama 2000). However, it is also possible that the global supercolony was not one of the first introduced colonies per se, but rather that is was one of the first large supercolonies and that simply because of the extent of the colony this genotype was more likely to spread.
While the transcontinental supercolony we identified is already very extensive, it is likely we have only revealed the tip of the iceberg. There are at least 28 known separate introductions of the Argentine ants throughout the world (Suarez et al. 2001) and several other supercolonies not tested here probably also belong to this inter-continental supercolony. In addition, we expect some of the smaller supercolonies, which we did not identify as part of this transcontinental supercolony, to be related to each other.
It is well established that introduced populations of Argentine ants can achieve extraordinarily high population densities. For example, one study reported capturing over one million Argentine ant queens and 4.4 m3 of workers and brood from a 19-acre orchard in Louisiana (USA) (Horton 1918). Given the spatial extent of the Argentine ant society we report here, there can be little doubt that this intercontinental supercolony represents the most populous known animal society.
The origin and maintenance of cooperative social behavior on this scale remains a mystery. Closer examination of these populations will provide insights into the potential roles of common ancestry, convergent evolution, human-mediated gene flow, priority effects, and contingency in the evolutionary patterns of introduced species.
Acknowledgments
We would like to thank Eiriki Sunamura, Robert Peck, Andrew Suarez and Phil Lester for providing Argentine ants and Steve Franks for help in the field. This work was supported by the California Structural Pest Board (NDT), the United States Department of Agriculture (NDT) and the Australian Research Council (EvW and NDT).
Literature cited
- Bjorkman-Chiswell BT, Van Wilgenburg E, Thomas ML, Swearer SE, Elgar MA. Absence of aggression but not nestmate recognition in an Australian population of the Argentine ant Linepithema humile. Insectes Sociaux. 2008;55:207–212. [Google Scholar]
- Brandt M, Van Wilgenburg E, Tsutsui ND. Global-Scale analyses of chemical ecology and population genetics in the Argentine ant. Molecular Ecology. 2009;18:997–1005. doi: 10.1111/j.1365-294X.2008.04056.x. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Bernier UR, Sutton BD. Elution patterns from capillary GC for methyl-branched alkanes. Journal of Chemical Ecology. 1998;24:1845–1865. [Google Scholar]
- Corin SE, Abbott KL, Ritchie PA, Lester PJ. Large scale unicoloniality: the population and colony structure of the invasive Argentine ant (Linepithema humile) in New Zealand. Insectes Sociaux. 2007a;54:275–282. [Google Scholar]
- Corin SE, Lester PJ, Abbott KL, Ritchie PA. Inferring historical introduction pathways with mitochondrial DNA: the case of introduced Argentine ants (Linepithema humile) into New Zealand. Diversity and Distribution. 2007b;13:510–518. [Google Scholar]
- Cox GW. Alien species in North America and Hawaii: Impacts on natural ecosystems. Washington, DC: Island Press; 1999. [Google Scholar]
- Denis D, Blatrix R, Fresneau D. How an ant manages to display individual and colonial signals by using the same channel. Journal of Chemical Ecology. 2006;32:1647–1661. doi: 10.1007/s10886-006-9099-7. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecology Letters. 2008;11:701–709. doi: 10.1111/j.1461-0248.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Edgell TC, Lynch BR, Trussell GC, Palmer AR. Experimental evidence for the rapid evolution of behavioral canalization in natural populations. American Naturalist. 2009;174:434–440. doi: 10.1086/603639. [DOI] [PubMed] [Google Scholar]
- Giraud T, Pedersen JS, Keller L. Evolution of supercolonies: the Argentine ants of southern Europe. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6075–6079. doi: 10.1073/pnas.092694199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green OR. Entomologist sets new record at Mt. Smart, or Iridomyrmex humilis established in New Zealand. Weta. 1990;13:14–16. [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Holway DA, Lach L, Suarez AV, Ysutsui ND, Case TJ. The causes and consequences of ant invasions. Annual Review of Ecological Systems. 2002;33:181–233. [Google Scholar]
- Horton JR. 1918. The Argentine ant in relation to citrus groves: Bulletin/United States Department of Agriculture; no. 647, 74p.
- Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- Koskinen MT, Haugen TO, Primmer CR. Contemporary fisherian life-history evolution in small salmonid populations. Nature. 2002;419:826–830. doi: 10.1038/nature01029. [DOI] [PubMed] [Google Scholar]
- Krieger MJB, Keller L. Low polymorphism at 19 microsatellite loci in a French population of Argentine ants (Linepithema humile) Molecular Ecology. 1999;8:1078–1080. [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends in Ecology & Evolution. 2002;17:386–391. [Google Scholar]
- Martin SJ, Helantera H, Drijfhout FP. Colony-specific hydrocarbons identify nest mates in two species of Formica ant. Journal of Chemical Ecology. 2008;34:1072–1080. doi: 10.1007/s10886-008-9482-7. [DOI] [PubMed] [Google Scholar]
- McNeely JA. An introduction to human dimensions of invasive alien species. In: McNeely JA, editor. The Great Reshuffling. Human Dimensions of Invasive Alien Species. Gland, Switzerland and Cambridge, UK: IUCN; 2001. pp. 5–22. [Google Scholar]
- Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press; 1987. [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. [PubMed] [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- Stoll O. Zur Kenntniss der geographischen Verbreitung der Ameisen. Mitteilungen der Schweizerischen Entomologischen Gessellschaft. 1898;10:120–126. [Google Scholar]
- Suarez AV, Tsutsui NDT, Holway DA, Case TJ. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biological Invasions. 1999;1:43–53. [Google Scholar]
- Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez AV, Holway DA, Liang DS, Tsutsui ND, Case TJ. Spatiotemporal patterns of intraspecific aggression in the invasive Argentine ant. Animal Behaviour. 2002;64:697–708. [Google Scholar]
- Sugiyama T. Invasion of Argentine ant, Linepithema humile, into Hiroshima Prefecture, Japan. Japanese Journal of Applied Entomology and Zoology. 2000;44:127–129. [Google Scholar]
- Suhr EL, McKechnie SW, O'Dowd DJ. Genetic and behavioural evidence for a city-wide supercolony of the invasive Argentine ant Linepithema humile (Mayr) (Hymenoptera: Formicidae) in southeastern Australia. Australian Journal of Entomology. 2009;48:78–82. [Google Scholar]
- Sunamura E, Espadaler X, Sakamoto H, Suzuki S, Terayama M, Tatsuki S. Intercontinental union of Argentine ants: behavioral relationships among introduced populations in Europe, North America, and Asia. Insectes Sociaux. 2009;56:143–147. [Google Scholar]
- Thomas ML, Parry LJ, Allan RA, Elgar MA. Geographic affinity, cuticular hydrocarbons and colony recognition in the Australian meat ant Iridomyrmex purpureus. Naturwissenschaften. 1999;86:87–92. [Google Scholar]
- Thompson JN. Rapid evolution as an ecological process. Trends in Ecology & Evolution. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- Torres CW, Brandt M, Tsutsui ND. The role of cuticular hydrocarbons as chemical cues for nestmate recognition in the invasive Argentine ant (Linepithema humile. Insectes Sociaux. 2007;54:363–373. [Google Scholar]
- Tsutsui ND, Case TJ. Population genetics and colony structure of the Argentine ant (Linepithema humile) in its native and introduced ranges. Evolution. 2001;55:976–985. doi: 10.1554/0014-3820(2001)055[0976:pgacso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tsutsui ND, Suarez AV, Holway DA, Case TJ. Reduced genetic variation and the success of an invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui ND, Suarez AV, Grosberg RK. Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1078–1083. doi: 10.1073/pnas.0234412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driesche RG, Bellows TS., Jr . Biological Control. New York: Chapman and Hall; 1996. p. 539. [Google Scholar]
- Wagner D, Tissot M, Cuevas W, Gordon DM. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. Journal of Chemical Ecology. 2000;26:2245–2257. [Google Scholar]
- Wetterer JK, Wetterer AL. A disjunct argentine ant metacolony in Macaronesia and southwestern Europe. Biological Invasions. 2006;8:1123–1129. [Google Scholar]
- Wild AL. Taxonomy and distribution of the argentine ant (Linepithema humile) (Hymenoptera: Formicidae) Annals of the Entomological Society of America. 2004;97:1204–1215. [Google Scholar]


