Abstract
Evolutionary effects of fishing can have unwanted consequences diminishing a fishery's value and sustainability. Reserves, or no-take areas, have been proposed as a management tool for reducing fisheries-induced selection, but their effectiveness for migratory species has remained unexplored. Here we develop an eco-genetic model to predict the effects of marine reserves on fisheries-induced evolution under migration. To represent a stock that undergoes an annual migration between feeding and spawning grounds, we draw model parameters from Atlantic cod (Gadus morhua) in the northern part of its range. Our analysis leads to the following conclusions: (i) a reserve in a stock's feeding grounds, protecting immature and mature fish alike, reduces fisheries-induced evolution, even though protected and unprotected population components mix on the spawning grounds; (ii) in contrast, a reserve in a stock's spawning grounds, protecting only mature fish, has little mitigating effects on fisheries-induced evolution and can sometimes even exacerbate its magnitude; (iii) evolutionary changes that are already underway may be difficult to reverse with a reserve; (iv) directly after a reserve is created or enlarged, most reserve scenarios result in yield losses; and (v) timescale is very important: short-term yield losses immediately after a reserve's creation can give way to long-term gains.
Keywords: Atlantic cod, contemporary evolution, density-dependent growth, fisheries-induced adaptive change, marine protected area, marine reserve, migration, phenotypic plasticity
Introduction
Recent theoretical studies (e.g., Ernande et al. 2004; Thériault et al. 2008; Arlinghaus et al. 2009; Dunlop et al. 2009b; Enberg et al. 2009; Jørgensen et al. 2009) and empirical assessments (e.g., Ricker 1981; Grift et al. 2003; Olsen et al. 2004; Mollet et al. 2007) have provided compelling evidence that fishing can induce evolutionary changes in key life-history traits. For example, the most commonly observed fisheries-induced trend attributed to evolution is toward earlier ages and smaller sizes at maturation (see recent reviews by Dieckmann and Heino 2007; Jørgensen et al. 2007; Kuparinen and Merilä 2007; Hutchings and Fraser 2008; Dunlop et al. 2009a). If occurring, these evolutionary changes could cause reduced body sizes in the catch; diminish a stock's productivity, stability, and recovery potential; lead to economic loses; and take a long time to reverse (Kirkpatrick 1993; Heino 1998; Law 2000; Conover et al. 2009; Dunlop et al. 2009b; Enberg et al. 2009). Therefore, managers need viable options for mitigating the unwanted evolutionary consequences of fishing. Even though the evidence for fisheries-induced evolution has triggered some lively debate in the literature (Hilborn 2006; Conover and Munch 2007; Dieckmann and Heino 2007; Browman et al. 2008; Heino et al. 2008; Jørgensen et al. 2008b; Kuparinen and Merilä 2008; Swain et al. 2008), the precautionary approach to fisheries management requires that the potential consequences of evolution be carefully considered to ensure sustainable fisheries.
Marine reserves are seen as an important tool for bringing an ecosystem perspective to fisheries management, because they help preserve ecosystem structure and function, with positive effects (such as the prevention of overfishing) potentially also occurring outside the reserves (e.g., Costanza et al. 1998; Pauly et al. 2002; Lubchenco et al. 2003). Moreover, by protecting a certain segment of a population from harvest, marine reserves might also reduce, stop, or reverse the evolutionary consequences of fishing. This reasoning has led some to propose marine reserves as a potential tool for managing evolving fish stocks (Conover and Munch 2002; Law 2007). Marine reserves may be expected to reduce the overall selective pressures causing, for example, earlier maturation, because they could be expected to protect a proportion of the population's individuals with genotypes coding for delayed maturation (Trexler and Travis 2000). A study by Baskett et al. (2005) supports this hypothesis. Based on the analysis of a quantitative genetic model, Baskett et al. (2005) predict marine reserves to reduce fisheries-induced selection for smaller sizes at maturation, provided the reserves are large enough relative to the target species’ dispersal range. Similarly, a simple age-structured individual-based model by Miethe et al. (2009) also predicts the creation of reserves to reduce the evolution of smaller sizes at maturation. Marine reserves might furthermore offer additional evolutionary benefits, such as the protection of genetic diversity (Perez-Ruzafa et al. 2006).
In contrast to traditional management approaches (including size limits and effort limits), marine reserves for mobile or migratory species may not enhance fisheries or provide effective protection from the ecological consequences of overexploitation (Hannesson 1998; Hilborn et al. 2004; Kaiser 2005). As many commercially harvested species undergo seasonal migrations or are highly mobile, this possibility deserves careful consideration. Indeed, most documented cases of fisheries benefits derived from the implementation of a marine reserve are for coral-reef species, which have a more localized home range (Halpern and Warner 2002; Halpern 2003). However, even though reserves may be less effective for highly mobile species (Kramer and Chapman 1999; Botsford et al. 2001; Gerber et al. 2005), they may still offer much needed protection of life stages or locations that are particularly vulnerable to harvest (Gell and Roberts 2003; Roberts et al. 2005).
Migratory species give rise to additional complications when considering the effectiveness of reserves for reducing undesirable effects of fisheries-induced evolution. In particular, for the many commercially important fish stocks that undergo an annual migration between feeding grounds and spawning grounds (including many pelagic species such as tunas and clupeoids, and demersal species such as Atlantic cod and plaice), the selective pressures imposed by fishing can vary considerably depending on where fishing takes place. Fishing in the feeding grounds can be expected to cause evolution of earlier maturation, if both juveniles and adults are captured (Law and Grey 1989; Heino and Godø 2002; Heino et al. 2002b). In contrast, fishing in the spawning grounds favors individuals that delay maturation until they are larger and more fecund (Law and Grey 1989; Heino and Godø 2002). Because fishing of both juveniles and adults (e.g., above some minimum-size limit) could favor individuals that allocate energy away from growth and toward reproduction earlier in life, fishing in the feeding grounds may have undesirable consequences such as potentially altering biomass and yield (Law and Grey 1989). A marine reserve could therefore have very different effects depending on whether it is located in feeding or spawning grounds (Law 2007). In such cases, assessing the ideal placement and the expected effects of a marine reserve is not straightforward. Protection on the feeding grounds might dilute some of the benefits of implementing a marine reserve, because adults might fully mix in the spawning grounds. Conversely, protection on the spawning grounds might exacerbate evolution of earlier maturation caused by a feeding-ground fishery because individuals may gain higher fitness from maturing early to seek protection on the spawning grounds (Law 2007). So far, it is also unclear how soon after a reserve's establishment potentially mitigating evolutionary consequences might take effect, and how trade-offs between short-term and long-term reserve effects might complicate the evaluation of management strategies.
In this study, we present an eco-genetic model (Dunlop et al. 2009b; see also Dunlop et al. 2007; Thériault et al. 2008; Enberg et al. 2009; Okamoto et al. 2009; Wang and Höök 2009) to explore the effects of marine reserves on the evolutionary response to fishing in a migratory species. Our model is motivated by the life history of Atlantic cod (Gadus morhua). Many northern populations of Atlantic cod, most notably Northeast Arctic cod off northern Norway and Icelandic cod on the Icelandic Shelf, display a far-ranging annual migration between spawning and feeding grounds (Robichaud and Rose 2001, 2004; Godø 2003; Palsson and Thorsteinsson 2003). Northern populations of cod also share other life-history characteristics such as relatively slow growth to potentially large body size and relatively late maturation at large size. Moreover, cod is among the most valuable fishery targets in the North Atlantic, and there is evidence suggesting that significant fisheries-induced evolution has already occurred in many cod populations (Heino et al. 2002b; Barot et al. 2004; Olsen et al. 2004, 2005; Swain et al. 2007, 2008). Here we do not aim at precisely modeling any particular cod population, but instead develop and analyze a model representing the typical life history of cod in the northern parts of its range, as an example of a commercially exploited, long-lived, migratory fish.
The model developed here extends previous marine-reserve models (e.g., Guenette and Pitcher 1999; Baskett et al. 2005; Hart 2006; Miethe et al. 2009) by (i) considering the evolution of multiple life-history traits (for growth, maturation schedule, and reproductive investment), (ii) accounting for density dependence in growth and reproduction, and (iii) examining a migratory life history. The inclusion of density-dependent somatic growth is a particularly relevant extension, because it is known to play a critical role in determining the effectiveness of a reserve under conditions of crowding (Gårdmark et al. 2006).
Below, we first present an eco-genetic model for a migratory population harvested on spawning and feeding grounds. We then investigate scenarios in which a marine reserve is established either on the stock's spawning grounds or on its feeding grounds, by comparing life-history evolution, total yield, and fish size in the catch. Finally, we assess the sensitivity of our findings to assumptions about movement rates, presence or absence of natal homing or spawning migration, and displacement of fishing effort. Our results show that a reserve located on a stock's feeding grounds could mitigate fisheries-induced evolution, but that beneficial evolutionary effects on yield can only be expected long after the reserve's establishment.
Methods
We constructed an individual-based eco-genetic model (for an overview of eco-genetic modeling, see Dunlop et al. 2009b) to follow the evolution of four quantitative life-history traits: growth capacity, reproductive investment, and the intercept and slope of a linear probabilistic maturation reaction norm (PMRN; described in detail below). The core of the model conforms to an example analyzed in Dunlop et al. (2009b), except for the addition of a spatial dimension and annual migration. Events in our model occur in discrete annual time steps. In each time step, individuals can mature, grow, migrate, reproduce, and experience natural and fishing mortality, in this order (Fig. 1). For each individual, we follow its location (reserve or harvested area), length and age, and maturation status in time. We run the model for 2000 years prior to harvest, to ensure that population abundance and evolving traits have reached a stochastic equilibrium.
Figure 1.
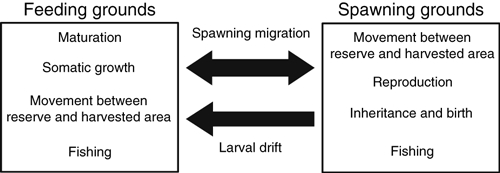
Schematic illustration of the eco-genetic model of Atlantic cod. Feeding grounds and spawning grounds are coupled through spawning migration and larval drift. Processes occurring in the two areas are indicated in the boxes.
We parameterize the model for Atlantic cod, Gadus morhua, in the northern part of its range (see Table 1 for parameter values and justifications) for three reasons: (i) Atlantic cod is one of the commercially most important fish species worldwide; (ii) several stocks of this species undergo substantial annual spawning migrations (Rose 1993; Jonsdottir et al. 1999; Comeau et al. 2002; Godø 2003); and (iii) several stocks have shown evidence of fisheries-induced evolution in maturation schedules and length-at-age (Heino et al. 2002b; Barot et al. 2004; Olsen et al. 2004, 2005; Swain et al. 2007, 2008). Parameter values were obtained from published data and were characteristic for stocks such as Icelandic cod, Northeast Arctic cod off Norway, and northern cod off the east coast of Canada (Table 1). No one stock allowed estimation of all parameter values and so we had to rely on multiple sources of data. Therefore, the model analyzed here is not appropriate for forecasting the effects of management decisions on one particular cod stock, but instead is meant to demonstrate expected trends and patterns for stocks and species with life histories similar to those investigated in this study.
Table 1.
Parameter values for the eco-genetic model of Atlantic cod
| Description | Symbol | Equations | Value | Source |
|---|---|---|---|---|
| Initial mean genetic PMRN intercept (cm) | 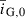 |
– | 93 (90.3) | 1 |
| Initial mean genetic PMRN slope (cm year−1) | 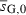 |
– | −0.052 (−0.052) | 1 |
| Initial mean genetic gonado-somatic index | 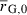 |
– | 0.12 (0.12) | 1 |
| Initial mean genetic growth capacity (cm) | 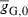 |
– | 12.8 (12.9) | 1 |
| Initial genetic coefficient of variation | CG,0 | – | 0.08 | 2 |
| Initial heritability | 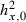 |
– | 0.2 | 2 |
| Default retention probability | q | 1a–d | 0.8 | 3 |
| PMRN width (cm) | w | 2c | 25.9 | 4 |
| Density-dependent growth constant (g−1) | b | 3a | 1.02 × 10−8 | 5 |
| Density-dependent growth exponent | c | 3a | 0.3 | 5 |
| Weight-specific oocyte density (g−1) | d | – | 4.4 × 103 | 6 |
| Conversion factor for gonado-somatic index | δ | 3c | 1.73 | 7 |
| Proportionality constant for weight (g cm−β) | α | 4a | 3.2 × 10−3 | 8 |
| Exponent of length–weight allometry | β | 4a | 3.24 | 8 |
| Density-independent stock-recruitment constant | k | 4b | 5.3 × 10−3 | 9 |
| Density-dependent stock-recruitment constant | j | 4b | 8.3 × 105 | 10 |
| Maximal growth increment (cm) | gmax | 5a | 80 | 11 |
| Background natural mortality probability | pB | – | 0.02 | 12 |
| Minimum-size limit on feeding grounds (cm) | lF | – | 60 | 13 |
Values in parentheses are mean prefishing equilibrium trait values, averaged over 30 independent model runs. PMRN, probabilistic maturation reaction norm.
Rationale and sources: (1) Set so that the prefishing equilibrium of evolving traits is reached within 2000 years and values are within empirical ranges for Atlantic cod reported for PMRNs (Heino et al. 2002b; Olsen et al. 2004), gonado-somatic indices (Lloret and Ratz 2000; McIntyre and Hutchings 2003; Rose and O'Driscoll 2002), and growth rates (ICES 2007; Marshall et al. 2004; Olsen et al. 2005). (2) Within the range reported by Houle (1992) and Mousseau and Roff (1987). (3) Model assumption. (4) Olsen et al. (2005). (5) Set so that the range of phenotypic growth rates predicted by the model is within the empirical range for Atlantic cod (ICES 2007; Marshall et al. 2004; Olsen et al. 2005). (6) Thorsen and Kjesbu (2001). (7) Lester et al. (2004). (8) From survey data for 1999–2007 collected by the Norwegian Institute of Marine Research (O.R. Kjesbu, pers. comm.). (9) Marshall et al. (2000). (10) Scaled from Marshall et al. (2000) so that population abundance at prefishing equilibrium is computationally manageable (ca. 20 000). (11) Set so that growth capacity at prefishing equilibrium produces phenotypic growth rates within the empirical range for Atlantic cod (ICES 2007; Marshall et al. 2004; Olsen et al. 2005). (12) Set so that the total natural mortality probability equals 0.18 (ICES 2007). (13) Model assumption as in Dunlop et al. (2009b).
Reserve design
All protected areas in the model are no-take reserves. At the time of reserve implementation, all individuals in the population are assumed to be randomly distributed in space. The reserve is then implemented by designating a proportion AL,R of the total area occupied by the population as no-take, where the location index L = F stands for a feeding-ground reserve and L = S for a spawning-ground reserve. For comparison, we also model populations with no separate feeding and spawning grounds, to test how this alters the effectiveness of a reserve.
We examined the effectiveness of each reserve location in two different reserve-establishment scenarios. In the first scenario, the reserve is established when fishing begins. This allows evaluation of the capacity of reserves to prevent fisheries-induced evolution from occurring in the first place. In the second scenario, fishing occurs for 50 years before the reserve is established. This allows examination of the propensity of reserves to slow, stop, or reverse fisheries-induced evolution once such evolution is already underway. For all scenarios, we investigated several different relative reserve sizes AL,R between 0 (no reserve) and 1 (entire area is protected).
Movement
All individuals have an annual probability of moving between the reserve and the harvested area. The conditional probability of movement is a function of the proportion AL,R of the total area in the reserve or the proportion AL,H = 1 − AL,R in the harvested area. The conditional movement probability also depends on the reserve's retention probability q, such that a proportion q of individuals remains within the reserve, whereas the remaining proportion 1 − q disperses globally, and therefore are equally likely to end up in the reserve R or in the harvested area H in strict proportion to their relative areas. Hence, the probabilities of remaining in an area and of moving, conditional upon the current location, are given by
| (1a) |
| (1b) |
| (1c) |
| (1d) |
where L = F refers to fish in the feeding grounds and L = S to fish in the spawning grounds. The amount of movement is likely to influence the efficacy of the reserve (Baskett et al. 2005) and we therefore vary q to test the influence of retention probability on model predictions.
Genetic structure
The genetic component of the model describes (i) the distribution of the evolving genetic traits in the initial population, (ii) inheritance of genetic traits from parents to offspring, and (iii) inter-individual environmental variation to determine the phenotypic expression of genetic traits. We use quantitative genetics to describe the changes in trait values (e.g., Falconer and Mackay 1996).
Following this framework, values for each of the four evolving traits (growth capacity, reproductive investment, and the intercept and slope of a linear PMRN) are assigned to the individuals in the initial population based on a normal distribution with a mean 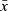 given by empirical data and a genetic standard deviation σG,x calculated from an assumed coefficient of genetic variation
given by empirical data and a genetic standard deviation σG,x calculated from an assumed coefficient of genetic variation 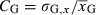 (Houle 1992), where xG indicates the value of the genetic trait in question (xG = iG for the PMRN intercept, xG = sG for the PMRN slope, xG = gG for growth capacity, and xG = rG for reproductive investment). Offspring inherit the genetic trait values of their parents from a normal distribution with a mean equal to the mid-parental value and a variance equal to half the genetic variance in the initial population (thus assuming a constant recombination–segregation–mutation kernel; see Roughgarden 1979; Dunlop et al. 2009b). All genetic traits evolve independently in this model, and we thus ignore any possible pleiotropy or genetic linkage between traits.
(Houle 1992), where xG indicates the value of the genetic trait in question (xG = iG for the PMRN intercept, xG = sG for the PMRN slope, xG = gG for growth capacity, and xG = rG for reproductive investment). Offspring inherit the genetic trait values of their parents from a normal distribution with a mean equal to the mid-parental value and a variance equal to half the genetic variance in the initial population (thus assuming a constant recombination–segregation–mutation kernel; see Roughgarden 1979; Dunlop et al. 2009b). All genetic traits evolve independently in this model, and we thus ignore any possible pleiotropy or genetic linkage between traits.
The phenotypic expression of any genetic trait xG occurs annually by drawing phenotypic trait values xP from a normal distribution with mean xG and inter-individual environmental variance 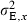 . The latter is parsimoniously held constant through time and is calculated as
. The latter is parsimoniously held constant through time and is calculated as 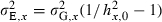 , where
, where 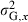 is the initial genetic variance of trait xG and
is the initial genetic variance of trait xG and 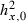 is the assumed heritability of xG in the initial population (Falconer and Mackay 1996). Therefore, each genetic trait value xG has a corresponding phenotypic trait value xP.
is the assumed heritability of xG in the initial population (Falconer and Mackay 1996). Therefore, each genetic trait value xG has a corresponding phenotypic trait value xP.
Maturation
We include phenotypic plasticity in the maturation process by modeling PMRNs (Heino et al. 2002a; Dieckmann and Heino 2007; Heino and Dieckmann 2008). Each individual is characterized by a PMRN that describes it genetic predisposition to mature as a function of its age and length. In our model, two traits describe the PMRN: its slope and its intercept. The slope is a measure of the type of growth-related phenotypic plasticity in maturation: a slope of zero describes a horizontal PMRN indicating that growth rates plastically influence maturation probability at age but not at length, whereas a slope approaching infinity describes a vertical PMRN indicating that growth rates plastically influence maturation probability at length but not at age. Together, the PMRN intercept and PMRN slope influence the lengths at which maturation is likely to occur for any particular age. Each year, the probability pm of an immature individual to mature is a function of its age a and length la,
| (2a) |
where 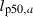 denotes the length at 50% maturation probability at age a (also known as the PMRN midpoint at age a) and is determined by an individual's phenotypic values for the PMRN intercept iP and slope sP,
denotes the length at 50% maturation probability at age a (also known as the PMRN midpoint at age a) and is determined by an individual's phenotypic values for the PMRN intercept iP and slope sP,
| (2b) |
The parameter that controls how the maturation probability pm at age a changes with the difference between the length la and 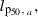
| (2c) |
is described by the PMRN width w, which measures the length difference at age a over which the maturation probability pm increases from pl to pu (Heino et al. 2002a). The two latter probabilities define the upper and lower bounds of what is called the maturation envelope (represented in our model by quartiles, pl = 25% and pu = 75%, so that z = w/ln 9 ≍ w/2.20). The PMRN width is assumed to be independent of age and constant in time. The latter assumption is underpinned by the prior investigation of models in which w was incorporated as an additional evolving trait, which showed that selective pressures on, and resultant evolutionary changes in, w were minimal.
Somatic growth
The somatic growth of individuals depends on multiple factors: (i) the individual's phenotypic growth capacity, i.e., the maximum possible growth in the absence of density dependence, but including inter-individual environmental variation; (ii) population biomass, because of density dependence in growth; (iii) inter-annual and inter-individual environmental variance in growth capacity; and, after maturation, on (iv) the individual's reproductive investment phenotype.
In our model, growth takes place in the feeding area and, for a given individual, therefore depends on the density of fish residing at the individual's location in the feeding area. This density naturally differs between the reserve and the harvested area, yielding an annual amount of energy available for growth measured by
| (3a) |
where b and c are constants, gP is the phenotypic growth capacity, BF,X and AF,X are the biomass in, and proportional area of, respectively, the feeding area in which the individual is located (X = R for the feeding-ground reserve or X = H for the feeding-ground harvested area).
Immature individuals invest all available energy into growth, growing from length la at age a to length la+1 at age a + 1 (Lester et al. 2004),
| (3b) |
with l0 = 0. Mature individuals, in contrast, partially utilize energy for reproduction that would have gone solely into the growth increment gd,X (Lester et al. 2004),
| (3c) |
where rP is the phenotypic reproductive investment, measured as the gonado-somatic index (GSI; the ratio of gonad mass to somatic mass), and δ is a conversion factor that accounts for the higher energy content of gonads relative to somatic tissue (Gunderson and Dygert 1988; Lester et al. 2004). If the rP of an individual in a given year would cause negative growth (la+1 < la), rP for that year is reduced such that la+1 equals la.
Reproduction
After the growing season, mature individuals migrate to the spawning grounds to reproduce. Following a common observation in many fish species (Kjesbu et al. 1998; Lloret and Ratz 2000; Oskarsson et al. 2002; Kennedy et al. 2007), gonad mass mG,a at age a, and therefore fecundity at that age, increase allometrically with body length, based on a proportionality constant α and an allometric exponent β,
| (4a) |
where rP is the individual's phenotypic reproductive investment, as measured by its GSI. The fecundity of each female is then equal to f = dmG,a, where d is the weight-specific oocyte density. The number Nr of recruits (i.e., of offspring surviving until the age of 1 year) produced by the population is determined by a Beverton–Holt stock-recruitment function (Hilborn and Walters 1992),
| (4b) |
where the total fecundity fT is obtained from summing fecundity over all mature females, k is the density-independent survival probability of offspring, and j is the total fecundity at which offspring survival is reduced to 50% because of density dependence.
Within a particular spawning area (reserve or harvested area in model designs with a spawning-ground reserve), males and females encounter and mate with each other at random, with the number of resultant offspring being proportional to each parent's gonad mass. We take this approach because individuals with large gonads are expected to possess larger numbers of gametes (eggs or sperm) and therefore will have a larger number of offspring. Also, a given female could mate with several males and a given male could mate with several females, in accordance with expectations for a batch-spawning species such as Atlantic cod (McEvoy and McEvoy 1992).
The probabilities of newly born offspring and first-time spawners to end up growing and feeding in the reserve or the harvested area equal the proportional areas, AF,R and AF,H, of those locations. This assumes that individuals choose their initial feeding and spawning site randomly.
Natal homing
Our default models assume feeding-site and spawning-site fidelity, but no natal homing. We also considered an alternative model with natal homing because (i) there is evidence that many marine species have spatially or genetically distinct local subpopulations (Hutchinson et al. 2001; Conover et al. 2006; Pampoulie et al. 2006), (ii) there is evidence for natal homing and spawning-site fidelity in cod and other species (Robichaud and Rose 2000; Thorrold et al. 2001; Hunter et al. 2003; Svedäng et al. 2007), and (iii) natal homing could be particularly important when designing or implementing spawning-ground reserves (Almany et al. 2007). Methodological details are provided in Appendix A.
Natural mortality
In addition to the offspring mortality described by the stock-recruitment relationship above, a classic growth-survival trade-off is assumed (Stearns 1992), causing a postrecruitment density-independent mortality probability of
| (5a) |
where gG is the genetic growth capacity and gmax is the annual length increment at which the survival probability drops to 0. The growth-survival trade-off assumes that individuals that have a high genetic propensity for growth, independent of the environment, have a higher mortality rate. We also impose a constant annual mortality probability pB on all individuals, so that the total natural mortality probability pT equals that used by ICES (2007) in their stock assessment of Atlantic cod, i.e., pB = 1 − (1 − pT)/(1 − pG). Mortality probabilities in the model are implemented by drawing a random number between 0 and 1; if that number is less than the mortality probability, the individual dies and is removed from the population.
Fishing mortality
Fishing occurs during the growing season on the feeding grounds and during the spawning season on the spawning grounds (e.g., Godø 2003). The fishery is regulated through an annually set total allowable catch BTAC,t, which is determined by the product of the harvest ratio γ and the total harvestable biomass, with the latter being defined as the total biomass of individuals in the population with lengths greater than the minimum-size limit lL of the fishery,
| (6a) |
where HF,t and HS,t are, respectively, the harvestable biomass in the feeding and spawning grounds. We employed a management regime that takes into account the potential displacement of effort by a marine reserve, implying that harvest probability for individuals outside a reserve become elevated in response to reserve establishment (e.g., Hilborn et al. 2006). As all mature individuals are considered to be fully recruited to fishing gear in many fisheries, in our model all mature fish on the spawning grounds are vulnerable to harvest and there is no minimum-size limit there (lS = 0). We also consider a fishery in which the displacement of effort does not occur and the total allowable catch therefore is given by the proportion of the harvestable biomass in the harvested area only (i.e., excluding the harvestable biomass in the reserve). To calculate biomass, the length of individuals is converted to weight as in eqn (4a), by raising length to the allometric exponent β and multiplying the result by the proportionality constant α.
The total allowable catch is then divided between catch in the spawning grounds (BS,t) and catch in the feeding grounds (BF,t). In each location, individuals in the harvested area that are larger than lL are randomly harvested until that area's allowable catch is reached. We analyzed several different ratios RF:(1 − RF) between feeding-ground catch and spawning-ground catch,
| (6b) |
where RF is the proportion of the total catch that is allocated to the feeding grounds. The cumulative yield or catch we report below is calculated as the total biomass of fish captured and killed in the fishery, measured over the 100 years during which fishing occurs, whereas the annual yield or catch is the biomass of fish captured and killed by the fishery in a given year.
Results
We start by establishing a baseline through investigating fisheries-induced evolution in the absence of a reserve. We then study the effects of reserves on evolutionary changes and on cumulative catches, before examining the effects of mobility and the annual spawning migration. Finally, we evaluate the expected impacts of reserves that are established only after a longer period of fishing.
Evolutionary responses to fishing in the absence of reserves
To determine the evolutionary effects of fishing in our model, we first explore outcomes without reserves. In the absence of reserves (Fig. 2, results shown along the vertical axes of each panel), extracting an increasing proportion of total catch in the feeding grounds relative to in the spawning grounds implies an increasing RF and causes the PMRN midpoint (Fig. 2A) and growth capacity (Fig. 2C) to decline and the GSI (Fig. 2E) to increase.
Figure 2.
Effects of a spawning-ground reserve (left) and feeding-ground reserve (right) on fisheries-induced evolution of maturation, growth, and reproductive investment. The feeding-ground ratio RF of catches describes the fraction of the total allowable catch that is permitted in the feeding grounds as opposed to in the spawning grounds. The thickness of lines and the size of symbols increase with RF between 0 (all fishing occurs in the spawning grounds) and 1 (all fishing occurs in the feeding grounds). Fishing occurred for 100 years with an annual harvest ratio of 0.5. The length at 50% maturation probability is the midpoint of the probabilistic maturation reaction norm (PMRN) for the mean age at maturation (8 years) in the initial population, lp50,8 = iG + sG 8 years, where iG is the genetic PMRN intercept and sG is the genetic PMRN slope. The genetic growth capacity gG describes the average juvenile growth increment in the absence of density dependence. The genetic gonado-somatic index rG is the average reproductive investment in the absence of allocation shortage. The horizontal dashed line indicates the value of the trait in the year before fishing is started when the population was at an evolutionary and ecological equilibrium. Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Relative to prefishing trait values (Fig. 2, dashed lines), reproductive investment always increases under fisheries-induced selection, but maturation probability and growth capacity may either increase or decrease, depending on where the larger part of catches are taken. If most of the catches are taken in the spawning grounds, no maturation evolution occurs relative to the prefishing equilibrium, but growth still evolves. Similarly, one could choose to split the catches in such a way that no growth evolution would occur.
Influence of reserves on fisheries-induced evolutionary changes
Next, we assess how evolutionary outcomes depend on reserve placement in feeding or spawning grounds. The creation of a spawning-ground reserve has no more than a small overall impact on the magnitude of evolution (Fig. 2A,C,E), whereas the protection of feeding grounds can exert a large influence on the magnitude of evolution (Fig. 2B,D,F). Not surprisingly, the influence of a spawning-ground or feeding-ground reserve is greatest when most fishing takes place in the spawning or feeding grounds, respectively. The influence of a reserve on maturation evolution is qualitatively different in feeding and spawning grounds: a reserve in the feeding grounds favors delayed maturation (Fig. 2C), whereas a reserve in the spawning grounds favors earlier maturation (Fig. 2A). Similar patterns apply to growth evolution (Fig. 2C,D), but not to reproductive investment, which declines with increasing areas of spawning-ground or feeding-ground reserves (Fig. 2E,F). For maturation and growth, the impact of creating a feeding-ground reserve is therefore the same as that of taking a larger proportion of catch in the spawning grounds, whereas the impact of creating a spawning-ground reserve is the same as that of taking a larger proportion of catch in the feeding grounds. In this sense, the spawning-ground reserve can be thought of as exacerbating evolution toward earlier maturation and slower growth caused by fishing in the feeding grounds.
We do not show results for the evolution of the PMRN slope because almost all of the evolutionary changes in the PMRNs are caused by evolution of the PMRN intercept: for example, fishing solely in the feeding grounds causes a large decrease in the PMRN intercept of 34%, but only a slight increase in the PMRN slope of 0.23%, with both changes expressed relative to the year before fishing (see also Dunlop et al. 2009b). Genetic variances were found to be little influenced by fishing and therefore, not surprisingly, by the creation of a reserve (results not shown). Variation of evolutionary outcomes among model runs was small (for example, in the year just prior to fishing the mean and standard deviation of the genetic PMRN intercept were 90.4 cm and 1.1 cm, respectively, amounting to a coefficient of sampling variation of no more than 1.2%).
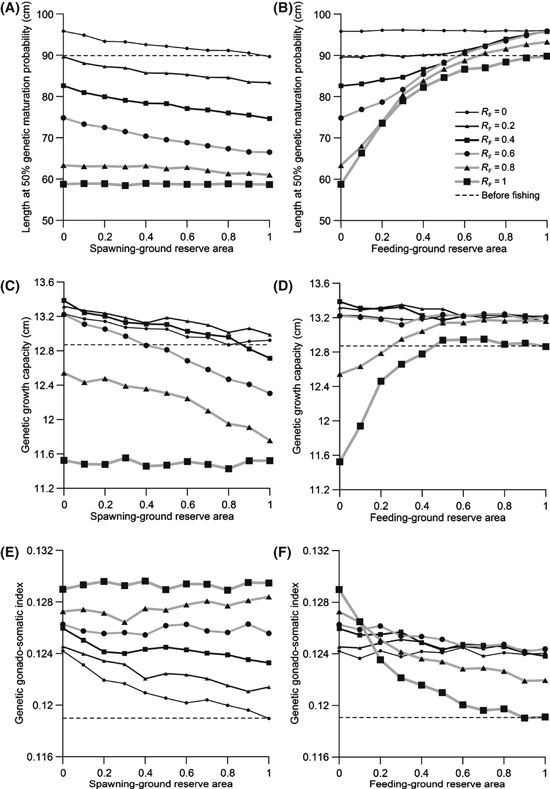
Influence of reserves on yields
To determine the effects of evolutionary changes and of reserves on cumulative catches, we investigate catches resulting under the different scenarios. Reserves alter the cumulative catch of the fishery (Fig. 3), as is apparent by comparing situations without a reserve (Fig. 3, results shown along the vertical axes) to those with a reserve (Fig. 3, results shown away from the vertical axes). In most cases, increasing the reserve size in one area (spawning grounds or feeding grounds) diminishes yield in that area (Fig. 3A,D), but improves yield in the other area (Fig. 3B,C); usually, however, the total yield decreases with reserve establishment, because the loss in one area is only imperfectly compensated by the gain in the other area. We find that the influence of a spawning-ground reserve on cumulative catches is close to linear (Fig. 3A,C), whereas the influence of a feeding-ground reserve becomes only apparent above a certain threshold (Fig. 3B,D); below this threshold, the reserve may slightly improve the total yield when all fishing occurs in the feeding grounds (RF = 1). Feeding-ground reserves often lead to a higher mean length of fish in the catch, whereas small spawning-ground reserves result in a lower mean length.
Figure 3.
Effects of a spawning-ground reserve (left) and feeding-ground reserve (right) on catch from the fishery. The feeding-ground ratio RF of catches describes the fraction of the total allowable catch that is permitted in the feeding grounds as opposed to in the spawning grounds. The thickness of lines and the size of symbols increase with RF between 0 (all fishing occurs in the spawning grounds) and 1 (all fishing occurs in the feeding grounds). Fishing occurred for 100 years with an annual harvest ratio of 0.5. Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Effects of mobility
To determine the influence on our results of the movement of fish among areas, we tested the sensitivity of our model results to the level of mobility, by changing the retention probability q: decreasing q results in an increase of movement between reserves and harvested areas. We find that greater individual movement lessens the effectiveness of a feeding-ground reserve in reducing fisheries-induced evolution (Fig. 4A–C). As there is little effect of a spawning-ground reserve on trait evolution, there also is little influence of mobility on the effectiveness of a spawning-ground reserve (Appendix A). Similar effects of movement were noted in populations with natal homing (Appendix A), indicating that natal homing had virtually no impact on the predictions of our model.
Figure 4.
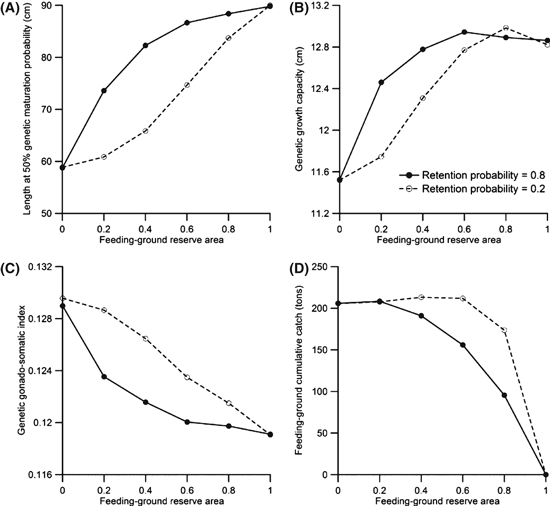
Effects of movement between the reserve and harvested area on the effectiveness of a feeding-ground reserve. The continuous line corresponds to the default retention probability of 0.8, whereas the dashed line refers to a retention probability of 0.2. All fishing occurs in the feeding grounds (RF = 1) for 100 years with an annual harvest ratio of 0.5. Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Effects of annual spawning migration
To quantify the effects of an annual migration between feeding grounds and spawning grounds, we compared results to a scenario in which the annual spawning migration was omitted (Appendix B). In the absence of a reserve, a nonmigratory population responds to fishing similarly to a migratory population harvested only on its feeding grounds, but the evolutionary response is less pronounced (Fig. B1). When a reserve is implemented, the evolutionary response of this population is almost indistinguishable from that of a migratory population with a feeding-ground reserve. On the other hand, the evolutionary response of a migratory population harvested on its spawning grounds differs starkly from that of a nonmigratory population, unless a large part of either population is protected by a reserve (Appendix B).
Effects of creating a reserve only after 50 years of fishing
In the investigations above, we implemented fishing and reserves simultaneously to explore the potential for reserves to reduce fisheries-induced selection. In a final step, we explore the potential for, and timescale of, fisheries-induced evolution to be reversed through reserve establishment. If 50 years of fishing pass by before a reserve is implemented, its effectiveness in slowing down evolution depends on harvest probability and reserve area (Fig. 5A,C,E). Populations that are fished more intensively show the largest reduction in the rate of evolution when a feeding-ground reserve is implemented (Fig. 5E), whereas implementing a small reserve for a lightly fished population has hardly any noticeable effect on the rate of evolution (Fig. 5A, thin line). The creation of a reserve always causes an initial reduction in annual yield, which may be followed by a short-term recovery in annual yield when the population approaches its new demographic equilibrium (Fig. 5B,D,F). On longer timescales, we see that fisheries-induced evolution continues despite a reserve, but also that the difference between the magnitude of evolution in a protected and a nonprotected population increases for a long period of time (Fig. 6A). More importantly, after decades to several hundred years, annual catches that can be extracted from a population protected by a reserve will be higher than if no reserve was created (Fig. 6B).
Figure 5.
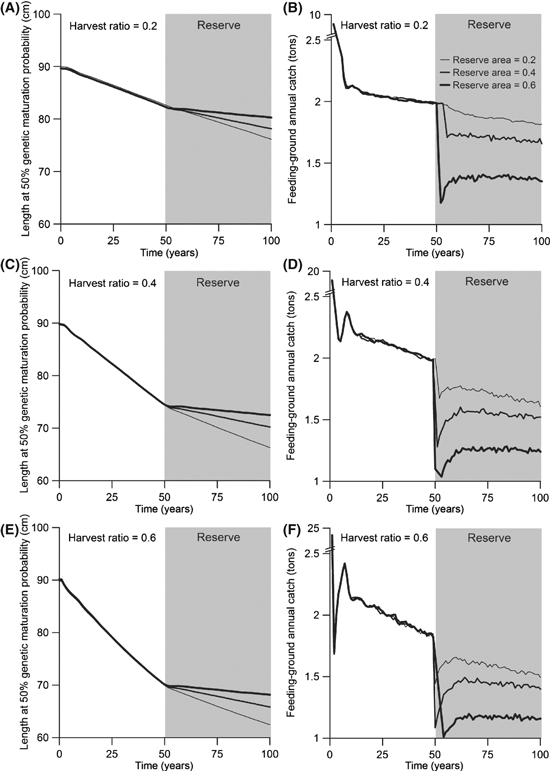
Effects of fishing for 50 years followed by the creation of a feeding-ground reserve. All fishing occurs in the feeding grounds (RF = 1). Results are shown for three different annual harvest ratios (0.2, 0.4, and 0.6) and reserve areas (0.2, 0.4, and 0.6). Fishing at these harvest ratios occurred before and after creation of the reserve. Reserve area increases with line thickness. Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Figure 6.
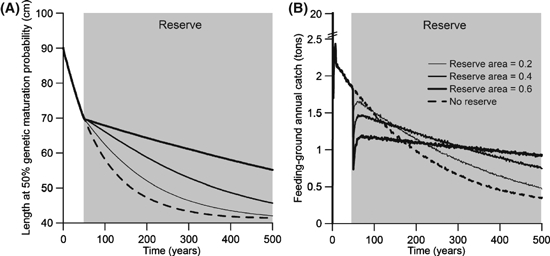
Effects of fishing for 50 years followed by the creation of a feeding-ground reserve. The annual harvest ratio was 0.6 in the stock's feeding grounds (RF = 1) and was applied before and after creation of the reserve. Results are shown for three different reserve areas (0.2, 0.4, and 0.6); reserve area increases with line thickness. The dashed lines describe a population that is not protected by a reserve. Values shown are means for 30 independent model runs. The legend in panel B applies to both panels.
Discussion
The central goal of this study was to evaluate the effectiveness of marine reserves in reducing the evolutionary effects of fishing in a species undergoing an annual spawning migration. The model presented here suggests that the selective pressures caused by fishing in a stock's feeding grounds are, for the most part, different than the selective pressures caused by fishing in the spawning grounds. This finding of differential selective pressures is in accordance with earlier studies relying on simpler models (Law and Grey 1989). We extend earlier analyses by considering the effects of reserve placement on fisheries-induced evolution in a migrating population and by incorporating density-dependent growth and the evolution of life-history traits beyond those affecting maturation. Some other novel features of our approach are discussed under the heading ‘Eco-genetic modeling’ below.
Effects of spatial stock structure
The reason for the selective pressures in our model to differ qualitatively between spawning grounds and feeding grounds is that when fishing occurs in the latter, both juveniles and adults are subject to being harvested above the minimum-size limit, so that evolution favors fish that mature earlier, have slower growth, and invest a higher proportion of energy in reproduction (Fig. 2, RF = 1). In contrast, when fishing occurs in the spawning grounds, only adults are harvested, so that individuals maturing later, when they are larger and more fecund, experience a higher reproductive success (Fig. 2A,B, RF = 0). Fast growth rates (Fig. 2C,D, RF = 0) and a higher investment in reproduction (Fig. 2E,F, RF = 0) are also favored by fishing in the spawning grounds.
It is interesting to note in this context that adding a conservative minimum-size limit to a spawning-ground fishery could favor early maturation (Jørgensen et al. 2009). In our model, we chose not to implement such a minimum-size limit on the spawning grounds, because mature size classes are often fully recruited to fisheries. Also, spawning-ground fisheries tend to be coastal, using traditional fishing methods (e.g., hand lines from smaller boats instead of trawling from open-ocean vessels) that are less selective for size; this is the case, for example, for the spawning-ground fishery for Northeast Arctic cod off Norway (Godø 2003).
Owing to the spatially distinct selective pressures, the success of marine reserves in reducing fisheries-induced evolutionary change is contingent upon the location of the reserve. The implementation of a marine reserve in the feeding grounds can have significant effects by protecting individuals before reproduction: the evolutionary response to fishing in the modeled life-history traits diminishes as the area of the reserve increases (Fig. 2B,D,F). However, the propensity of a marine reserve to reduce evolution is lessened when the reserve is located on the spawning grounds (Fig. 2A,C,E). As fishing in the feeding grounds causes the largest evolutionary change, a spawning-ground reserve can do little to curb these effects. Furthermore, by protecting spawning individuals that would otherwise be harvested, selection favoring delayed maturation and faster growth is lessened. In other words, we find that a spawning-ground reserve can aggravate the evolutionary response toward earlier maturation and slower growth that is induced by fishing in the feeding grounds (Fig. 2). Therefore, if the management goal is to reduce the magnitude of fisheries-induced evolution, the advisable location for a reserve is in a stock's feeding grounds.
Effects of reserve size
The size of a reserve that is most effective in reducing fisheries-induced evolution depends on the ratio between feeding-ground catch and spawning-ground catch, as well as on the mobility of individuals (Figs 2 and 4). When the total allowable catch in the feeding grounds is high, even a smaller reserve can offer benefits in terms of reducing the magnitude of evolutionary changes. In contrast, if fishing pressure in the spawning grounds is higher, only the very largest reserves are effective (Fig. 2; Appendix A) and there is so little fisheries-induced selection that it is perhaps not worthwhile to implement a reserve if its only goal is to prevent fisheries-induced evolution. We also see that as the mobility of individuals in the population is increased, the reserve needs to be increasingly larger to lessen evolutionary changes (Fig. 4); these results are related to arguments that reserves will be less effective, or need to be extremely large, in the case of mobile species (Hannesson 1998; Hilborn et al. 2004). Furthermore, when harvest pressure is low, the reserve needs to be slightly larger when there is an annual migration between spawning and feeding grounds; this is because of the gene flow that occurs among individuals while they reside on the spawning grounds (Appendix B). The results of our study underscore the idea that taking into account the selective pressures of fishing in different locations and the patterns of movement of species among those locations is crucial when assessing implementation options for marine reserves.
Effects of reserves on yield
Although our model suggests that a feeding-ground reserve can reduce the magnitude of fisheries-induced evolution, such a reserve has more complex effects on catch. The creation of a reserve almost always caused a reduction in cumulative catch (Figs 3–6). Yield increases were only noted for a few scenarios and tended to be small in magnitude. First, when a reserve was created and fishing started simultaneously, slight increases in cumulative catch (over 100 years) were observed when all fishing pressure was concentrated in the feeding grounds (Fig. 3); these increases were most obvious when movement rates between the reserve and the harvested areas were higher (Fig. 4). Second, creating a feeding-ground reserve enhanced catches in the spawning grounds, and creating a spawning-ground reserve could improve catches in the feeding grounds (Fig. 3). These effects are a consequence of changes that are in part demographic and in part evolutionary. Protecting fish in the feeding grounds can enable the rebuilding of size structure in the population, whereas protecting spawning individuals can enhance offspring production. Third, when a feeding-ground reserve was created after 50 years of fishing, there was always an initial reduction in yield (Fig. 5), but after some time, which in our example ranged from about 50 to several hundreds of years, yield could be enhanced relative to a population that was not protected (Fig. 6). The increases in catch that were observed in the three situations described above are probably not substantial enough to warrant creating a reserve solely based on the goal of enhancing yields.
Our results show that marine reserves can help to mitigate fisheries-induced evolution, but that this mostly implies reduced yield, especially in the short to medium term. Motivated by the discussion about fisheries benefits of marine reserves (Hannesson 1998; Hastings and Botsford 1999; Hilborn et al. 2004), one could ask whether the same benefits could have been achieved by simply reducing the harvest ratio, without implementing a reserve. Our results confirm that reducing harvest ratios can considerably lessen the magnitude of fisheries-induced evolution (as shown in Fig. 5, as well as in Appendices B and C; see also Law and Grey 1989; Heino 1998; Ernande et al. 2004; Dunlop et al. 2009b). As an option for future research, it will therefore be interesting to compare in detail the costs and benefits associated with the two alternative management strategies, of reducing harvest ratio and reducing harvest area, to establish whether, taking fisheries-induced evolution into account, reserves can offer a better benefit-to-cost ratio than traditional management strategies.
Other reserve benefits
There could be fisheries benefits to slowing down or reducing the magnitude of fisheries-induced evolution other than those accruing in the form of enhanced yields (e.g., Kirkpatrick 1993; Baskett et al. 2005). For example, fisheries-induced evolution can lead to reduced body sizes in the catch, a trend that can be alleviated through creating a feeding-ground reserve (Fig. 3). Also, there is some indication from our results that the creation of a reserve could improve yield stability: Fig. 5 shows that there is a steady reduction in yield in response to fishing, but that, after the strong initial decrease, the creation of a feeding-ground reserve can substantially slow the decline. Finally, evolution could have other effects, possibly altering species interactions, recovery potential, and migration patterns (Gårdmark et al. 2003; Jørgensen et al. 2007, 2008a; Thériault et al. 2008; Enberg et al. 2009). Protected areas could offer management options for mitigating such other effects, as our results show that feeding-ground reserves are capable of reducing the magnitude of evolutionary changes caused by fishing.
Effort displacement
The impact of effort re-allocation should be considered when designing a marine reserve (Hilborn et al. 2004). Our model can account for the often high harvest pressure that develops in areas outside the reserve, because the harvest ratio in our model is expressed as a proportion of the population's total harvestable biomass, which includes the biomass of individuals residing both inside and outside the reserve. Therefore, a build-up of biomass in the reserve while the harvest ratio is kept constant results in higher harvest probabilities per individual outside of the reserve.
We find that even with such a harvesting pattern reflecting effort displacement in the wake of a reserve's creation, feeding-ground reserves can reduce evolution and sometimes enhance yield. When creating a feeding-ground reserve, excluding effort displacement by setting the harvest ratio to be a proportion of the harvestable biomass in the harvested area only (thus not including the biomass inside the reserve), results in a slight reduction of fisheries-induced evolution, but only for low harvest ratios and for reserves of small to medium size (Appendix C). These results agree with findings by Baskett et al. (2005), who predicted that sufficiently large reserves may protect against strong fisheries-induced selection for earlier maturation irrespective of whether or not harvest rates outside the reserve were increased through effort displacement.
Eco-genetic modeling
The model used here for analyzing the evolutionary effects of marine reserves in migratory stocks builds upon previous eco-genetic models (Dunlop et al. 2007, 2009b). Our model permits the examination of multi-trait evolution and of density-dependent growth, features not included in previous marine-reserve models. We can also study evolutionary transients and assess their pace, something not possible with many other types of models, such as optimization models or adaptive dynamics models. Full integration of ecological and evolutionary timescales, as offered by eco-genetic modeling, is important in studies of marine reserves, as short-, medium-, and long-term consequences need to be properly evaluated and balanced. In our results, implementing a marine reserve always caused an initial reduction in yield, even though, as evolutionary effects emerge over time, the reserve could eventually enhance yield (Fig. 6). By examining the transients in Figs 5 and 6, we can discern three stages of this process. First is the immediate drop in yield that occurs with the displacement of effort. Second is the arched increase in yield that occurs approximately 55–70 years after reserve establishment, as biomass accumulates in the reserve and the stock's age and size structure build up. This second stage could be interpreted as an ecological response (Gaylord et al. 2005). Third is the long-term trend in yield that results from the evolutionary response. Without a simultaneous treatment of ecological and evolutionary timescales, these dynamics could not be discerned and examined.
Generalizations to other species
Our modeled population most closely resembles Atlantic cod stocks found in the northern part of the species range, including Icelandic cod, Northeast Arctic cod off Norway, or northern cod off the east coast of Canada. We focus on Atlantic cod because data are available to parameterize the model, the species is of considerable commercial and ecological importance, exploitation rates are often high, and many stocks of Atlantic cod undergo long spawning migrations resulting in the geographic separation of feeding and spawning grounds (Robichaud and Rose 2004). The parameter values we chose are validated in the sense that they result in emergent properties, including growth patterns and other life-history observables, that are very similar to those of northern populations of Atlantic cod (Table 1). In this manner, our study conforms to the pattern-oriented modeling approach described by Grimm and Railsback (2005).
Although we have not explored the effects of exploitation and marine reserves on species with other life histories, one simple generalization can be drawn. Our modeled cod population had a moderately high age at maturation of 8 years in the absence of fishing. Species or populations with shorter generation times – such as cod in the southern parts of its range and several key commercial targets such as herrings and flatfishes – will probably show faster evolutionary responses. As the evolutionary effects will then accrue more quickly, the benefits of implementing a reserve might also be observed on a shorter timescale. However, much more investigation is needed to determine the quantitative influence of life history on the combined effects of fisheries-induced evolution and marine-reserve implementation. We contend that the results reported here should foster the understanding that evolutionary impacts of marine reserves be assessed through the calibration of stock-specific models, before managers and stakeholders commit to costly implementation measures. For this, the framework laid out here can provide a template.
Model uncertainty
There is little empirical data with which to compare the predictions of our model. This is because the majority of previous studies have focused on the ecological effects of reserves, or examined timescales too short for evaluating evolutionary impacts. Some empirical evidence shows that increases in biomass and species diversity in marine reserves can be observed very quickly, with the potential for spillover to areas outside reserves, thereby suggesting that there could be significant demographic, nonevolutionary impacts (e.g., Roberts et al. 2001; Halpern and Warner 2002). However, evolutionary effects are slower and will take longer to observe, which obviously poses a challenge when trying to evaluate the efficacy of reserves in reducing the magnitude of fisheries-induced evolution. There is one study that does point to the possible genetic effects of marine reserves. Perez-Ruzafa et al. (2006) found higher intra-specific allelic diversity for sea bream inside two Mediterranean reserves than in neighboring nonprotected areas. At the time of sampling, the reserves were protected for 4 and 10 years. Although no data on life-history traits were reported, Perez-Ruzafa et al. (2006) suggest that the preservation of individuals with higher fecundity and faster growth reduced selective pressures induced by fishing, a mechanism that could have increased allelic diversity in the reserve.
While the numerical approach here limits our analysis to the parameter values used, in this study we tested the sensitivity of our predictions to several parameters, including retention probability, reserve area, harvest rate, time of reserve implementation, and the presence of natal homing. In another study (Dunlop et al. 2009b), the sensitivity of the base model was tested to changes in harvest rate, the minimum-size limit, the stock-recruitment relationship, density-dependent growth, genetic variation, and the growth-survival trade-off; that sensitivity analysis revealed that the speed of evolution depends on these functions, supporting their presence in the models, but the overall qualitative effects of exploitation remained the same: fishing caused most evolution in the PMRN toward earlier ages and smaller sizes at maturation. However, not all sensitivity analyses performed for the base model might be completely generalizable to this study because the base model did not include spatial structure.
The scarcity of empirical data on the potential long-term evolutionary effects of reserves underlines the vital role that carefully constructed and calibrated models ought to assume in addressing this question. We offer the analyses reported here as a step toward meeting this challenge. The various considerations above have hopefully made it clear that simple models featuring just a few variables and parameters are unlikely to do justice to the rich ecological settings that drive natural and anthropogenic evolutionary changes in nature. While we therefore believe that a model of the complexity studied here is indeed required for obtaining practically relevant results, this implies a trade-off with having to assess the adequacy of the adopted structural assumptions and parameter values. We therefore systematically explored the sensitivity of our model results to various assumptions and parameters, as summarized in Figs 2–6 and A1–C1.
Yet, there were several assumptions that, for the sake of brevity, we could not test here. For example, a simplifying assumption made in our model is that the four evolving traits are not subject to pleiotropy or constrained by linkage. This simplification was made because there is very little information available on wild stocks of Atlantic cod with which we could have parameterized such constraints or genetic covariances. Our model predicted that the PMRN midpoint (and specifically the PMRN intercept) underwent the largest evolutionary change among all four modeled life-history traits (see also Dunlop et al. 2009b), suggesting that the inclusion of genetic covariances may not have had a large effect on model predictions with regard to this central finding.
Other simplifying assumptions were implied by our modeling closed populations, excluding multi-species interactions, variable environmental conditions, or other evolving traits. One benefit of reserves is that they protect multiple species. Fisheries-induced evolution could alter species interactions (Gårdmark et al. 2003) and by only modeling a single species, we could be missing other possible reserve effects (Mangel and Levin 2005; Baskett et al. 2006, 2007a), especially when size- or location-specific predation affects the evolution of the traits explored here. Also, the spatial structure of our model was kept simple and could therefore not account for edge effects that develop when fishing is concentrated along reserve boundaries, or for localized fishing effort concentrating on previously untargeted areas, two spatial factors that can alter a reserve's effectiveness (Kaiser 2003; Roberts et al. 2005; Kellner et al. 2007). Finally, many other traits in addition to the traits we model here could evolve in response to fishing (Heino and Godø 2002; Walsh et al. 2006) and could be impacted differentially by the creation of a reserve. For example, population-level migration patterns or individual-level mobility may evolve in response to fishing (Jørgensen et al. 2008a; Thériault et al. 2008) or reserve implementation (Heino and Hanski 2001; Baskett et al. 2007b; Miethe et al. 2009), effects we have not modeled here.
Management implications
Several findings from this study have practical implications for fisheries management. First, reserves may reduce the evolutionary effects of fishing even in a migratory species. This is important because many commercially and ecologically important species migrate between feeding and spawning grounds. While it has been suggested that reserves would not be effective when individuals from reserves can spawn together with those from harvested areas, our results show that protection on the feeding grounds effectively reduces evolution. Second, feeding-ground reserves are capable of reducing fisheries-induced evolution, whereas spawning-ground reserves can exacerbate the evolutionary response toward earlier maturation. A clear management recommendation therefore is that if the goal is to reduce fisheries-induced maturation evolution, the reserve should not be placed in the stock's spawning grounds. Third, even when taking into account evolution caused by fishing, the implementation of reserves probably reduces yield over decadal timescales. It might have been thought that by mitigating yield-reducing evolutionary effects, implementing a reserve could improve yield, or at least keep it constant; our results show that this is mostly not the case, as such an effect only occurs in a narrow range of settings and only when a long-term perspective is taken. Fourth, evolutionary changes that are already well underway are difficult to reverse through implementing a reserve. Given that even stopping harvest altogether results only in a relatively slow recovery (Law and Grey 1989; Dunlop et al. 2009b; Enberg et al. 2009), a more effective management strategy is to prevent evolutionary changes from occurring in the first place, rather than trying to stop or reverse them once underway. Fifth, our results show that it is advisable to manage populations as a whole and account for potential stock structure, because fishing in one area may cause evolution that can drastically alter yield in another area.
How do the predictions of our model relate to current management practices of Atlantic cod and similar species? Protection of spawning aggregations of Atlantic cod has been proposed as an essential measure for ensuring the sustainability of exploited stocks (Vitale et al. 2008). Indeed, several closed areas currently implemented tend to focus protection on spawning grounds (Murawski et al. 2000; Hu and Wroblewski 2009). Although protection of spawning individuals may be important for demographic reasons, our results show that protecting individuals on feeding grounds is just as, if not more, important for safeguarding a stock against fisheries-induced evolution. This has implications for stocks such as Northeast Arctic cod, for which the introduction of industrial trawling has led to high rates of exploitation in the stock's feeding grounds (Law and Grey 1989; Heino et al. 2002b; Godø 2003). Our results suggest that protecting this stock's feeding grounds is highly advisable as a means of counteracting the fisheries-induced maturation evolution toward younger ages and smaller sizes.
As mentioned previously, marine reserves may have benefits that go beyond effects on single species. For example, reserves may provide protection of critical habitat that could sustain fish productivity. Our model, being a single-species model without habitat dynamics, obviously cannot account for these added reserve benefits. We therefore recommend that the approach to assessing the evolutionary impacts of fishing proposed here should be incorporated as one element of an ecosystem-based approach to fisheries management (Francis et al. 2007). Of the many model-based studies of marine reserves (for a review, see Gerber et al. 2003), only a few have considered evolution (e.g., Trexler and Travis 2000; Baskett et al. 2005; Miethe et al. 2009), so we really have only just begun to examine the full suite of potential benefits and consequences of mitigating fisheries-induced evolution through the creation of marine reserves.
Over mere decades, fishing can cause evolutionary changes in key life-history traits governing growth, maturation, and reproductive investment. Evolutionary changes induced by fishing can have far-reaching consequences, possibly altering yield, recovery potential, stock stability, profits from a fishery, species interactions, and migration patterns (Jørgensen et al. 2007). As these evolutionary effects may be slow or difficult to reverse (Conover et al. 2009; Dunlop et al. 2009b; Enberg et al. 2009; Stenseth and Dunlop 2009), the precautionary approach warrants that managers consider evolution when planning and implementing sustainable harvesting practices. In particular, the establishment of marine reserves may reduce the evolutionary effects of fishing, but appropriate reserve placement taking into account the spatial patterns of fisheries-induced selection pressures is crucial to their success.
Acknowledgments
Financial support for this project was provided to M.H. and E.D. by the Norwegian Research Council and the Bergen Research Foundation, to M.H. and U.D. by the European Research Network on Fisheries-induced Evolution (FinE), and to E.D., M.H., and U.D. by the European Research Training Network on Fisheries-induced Adaptive Changes in Exploited Stocks (FishACE). U.D. acknowledges additional support by the European Science Foundation, the Austrian Science Fund, and the Vienna Science and Technology Foundation. We thank members of the FishACE network and of the University of Bergen's EvoFish and Modeling groups for helpful discussion of this work.
Appendix A. Effect of natal homing on spawning-ground and feeding-ground reserves
In this appendix, we examine the influence of incorporating natal homing in our model. When natal homing is introduced, individuals in the population have a tendency to spawn in their area of birth. In other words, an individual born in a spawning-ground reserve will tend to return to that spawning-ground reserve for spawning. Individuals have only a ‘tendency’ to return, because there is movement between the harvested area and the reserve that introduces some variability in whether an individual actually returns to their area of birth (eqns 1a–d in the main text).
Results of this investigation show very little difference between situations with and without natal homing (there is little difference between the left and right columns in Fig. A1); this was true for both a spawning-ground reserve and for a feeding-ground reserve. Changing the retention probability q did influence predictions, but natal homing had little effect on those predictions. For a feeding-ground reserve, there was more evolution to smaller lengths at 50% genetic maturation probability (owing mainly to a decrease in the PMRN intercept), higher GSIs, and smaller genetic growth capacities (Fig. A1) when the retention rate was low (i.e., when there was more movement between the reserve and harvested area). For a spawning-ground reserve, the difference between results for the two retention probabilities was less than for a feeding-ground reserve. For a spawning-ground reserve, lower retention probabilities (and therefore more movement) led to evolution of larger length at 50% genetic maturation probability, higher genetic growth capacity, and higher genetic GSI (Fig. A1). Therefore, with the exception of the GSI, more movement coupled with a spawning-ground reserve had the opposite effect of more movement coupled with a feeding-ground reserve. This is perhaps not surprising given the different selective pressures acting when fishing occurs in the spawning grounds as opposed to in the feeding grounds (as discussed in more detail in the main text).
Figure A1.
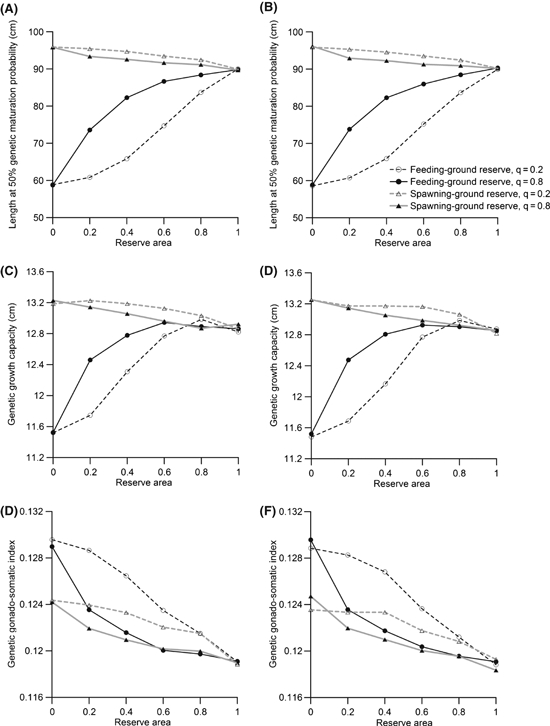
Influence of natal homing on the effectiveness of a reserve. Fishing occurs in the spawning grounds when the reserve is located in the spawning grounds, and fishing occurs in the feeding grounds when the reserve is located in the feeding grounds. Fishing occurs for 100 years with an annual harvest ratio of 0.5. Panels on the left (A, C, E) are for a population without natal homing (default) and panels on the right (B, D, F) are for a population in which there is a tendency for individuals to spawn in the area of their birth. The retention probability q was also varied (eqns 1a–d in the main text). Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Appendix B. Effect of a reserve on a population without annual spawning migration
In this appendix, we test the impact of a reserve on fisheries-induced evolution in a species that does not undergo an annual spawning migration. The harvestable biomass for this type of reserve is equal to the biomass of individuals above the minimum-size limit in the reserve and the harvested area. Everything else is equivalent to the model described in the main text.
Results of this investigation show that the difference between a population that annually migrates to spawning grounds and a population that does not migrate depends on the area of the reserve and on the annual harvest ratio (Fig. B1). For low annual harvest ratios and small to medium reserve areas, a reserve created for a nonmigrating population results in less evolution than a feeding-ground reserve created for a migrating population (Fig. B1). This is a likely result of the genetic mixing that occurs in the spawning grounds during reproduction when there is an annual spawning migration. An individual occupying the feeding-ground reserve could mate with an individual that occupies the feeding ground's harvested area, resulting in offspring trait values that will average between the two parental trait values.
Figure B1.
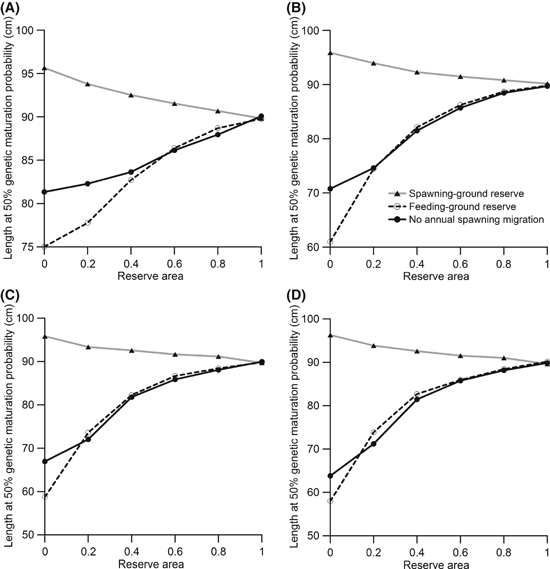
Influence of an annual spawning migration on the effectiveness of a reserve. Fishing occurs in the spawning grounds when the reserve is located in the spawning grounds, and fishing occurs in the feeding grounds when the reserve is located in the feeding grounds. Fishing occurs for 100 years with an annual harvest ratio of 0.2 (A), 0.4 (B), 0.5 (C), or 0.6 (D). Values shown are means for 30 independent model runs. The legend in panel B applies to all panels.
Generally, a feeding-ground reserve has an effect more similar to a reserve created for a nonmigrating population than to a spawning-ground reserve created for a migrating population (Fig. B1). The reason for the higher similarity is that harvest pressure on juveniles and adults causes selection for earlier maturation; this selection pressure can be reduced by protecting the juveniles and adults that reside in the reserve. The dissimilarity between situations with a spawning-ground reserve and with a nonmigrating population occurs because there is no targeted fishery of spawning individuals in the later case. A fishery of spawning individuals creates selection pressures mostly in the opposite direction than a fishery for juveniles and adults, and the subsequent protection of spawning individuals through the creation of a spawning-ground reserve has very different implications than protecting juveniles and adults above a minimum-size limit.
Appendix C. Effect of excluding effort displacement
In the model presented in the main text, harvestable biomass is determined as the biomass of all harvestable individuals in the reserve and the harvested area. This was to account for the effort displacement that can occur when a reserve is created. In this appendix, we test a scenario in which the harvestable biomass equals the harvestable biomass in the harvested area only, so that the former is unaffected by biomass in the reserve, and no effort displacement occurs.
We examine this scenario by considering fishing that occurs for 50 years prior to the creation of a feeding-ground reserve. Our results show that effort displacement generally causes little difference in the effect of a reserve on evolution (Fig. C1). The only difference occurs for low annual harvest ratios and small reserve areas (Fig. C1). In cases showing a difference, the reserve is less effective at curbing evolution when there is effort displacement (Fig. C1).
Figure C1.
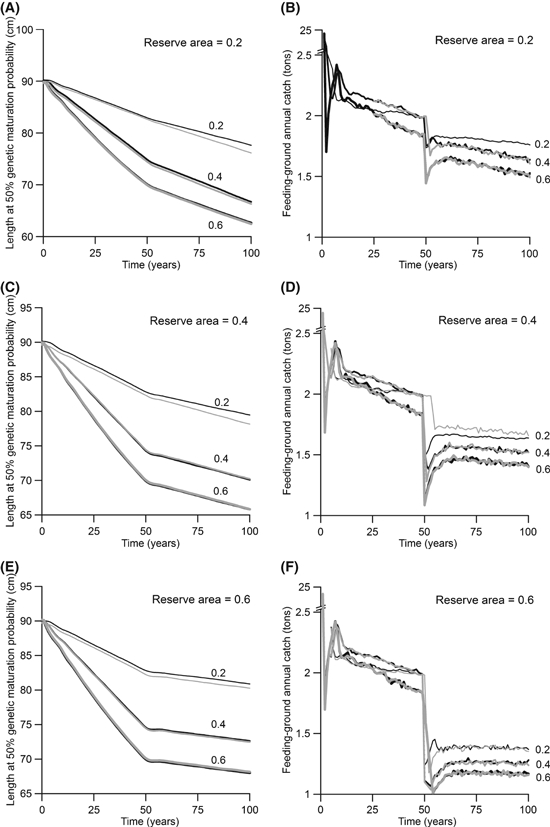
Effect of changing the measure of harvestable biomass. Gray lines describe settings with effort displacement, in which the harvestable biomass equaled the harvestable biomass in the reserve and the harvested area (default). Black lines describe settings without effort displacement, in which the harvestable biomass equaled the harvestable biomass in the harvested area alone. Line thickness increases with the annual harvest ratio (0.2, 0.4, and 0.6). Fishing occurs for 50 years followed by the creation of a feeding-ground reserve. Values shown are means for 30 independent model runs.
Literature cited
- Almany GR, Berumen ML, Thorrold SR, Planes S, Jones GP. Local replenishment of coral reef fish populations in a marine reserve. Science. 2007;316:742–744. doi: 10.1126/science.1140597. [DOI] [PubMed] [Google Scholar]
- Arlinghaus R, Matsumura S, Dieckmann U. Quantifying selection differentials caused by recreational fishing: development of modeling framework and application to reproductive investment in pike (Esox lucius. Evolutionary Applications. 2009;2:335–355. doi: 10.1111/j.1752-4571.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot S, Heino M, O'Brien L, Dieckmann U. Long-term trend in the maturation reaction norm of two cod stocks. Ecological Applications. 2004;14:1257–1271. [Google Scholar]
- Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecological Applications. 2005;15:882–901. [Google Scholar]
- Baskett ML, Yoklavich M, Love MS. Predation, competition, and the recovery of overexploited fish stocks in marine reserves. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:1214–1229. [Google Scholar]
- Baskett ML, Micheli F, Levin SA. Designing marine reserves for interacting species: insights from theory. Biological Conservation. 2007a;137:163–179. [Google Scholar]
- Baskett ML, Weitz JS, Levin SA. The evolution of dispersal in reserve networks. American Naturalist. 2007b;170:59–78. doi: 10.1086/518184. [DOI] [PubMed] [Google Scholar]
- Botsford LW, Hastings A, Gaines SD. Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecology Letters. 2001;4:144–150. [Google Scholar]
- Browman HI, Marshall CT, Law R. The role of fisheries-induced evolution. Science. 2008;320:47. doi: 10.1126/science.320.5872.47b. [DOI] [PubMed] [Google Scholar]
- Comeau LA, Campana SE, Chouinard GA. Timing of Atlantic cod (Gadus morhua L.) seasonal migrations in the southern Gulf of St Lawrence: interannual variability and proximate control. ICES Journal of Marine Science. 2002;59:333–351. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Conover DO, Munch SB. Faith, evolution, and the burden of proof. Fisheries. 2007;32:90–91. [Google Scholar]
- Conover DO, Clarke LM, Munch SB, Wagner GN. Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. Journal of Fish Biology. 2006;69:21–47. [Google Scholar]
- Conover DO, Munch SB, Arnott SA. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2009;276:2015–2020. doi: 10.1098/rspb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza R, Andrade F, Antunes P, Van Den Belt M, Boersma D, Boesch DF, Catarino F, et al. Principles for sustainable governance of the oceans. Science. 1998;281:198–199. doi: 10.1126/science.281.5374.198. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Dunlop ES, Shuter BJ, Dieckmann U. Demographic and evolutionary consequences of selective mortality: predictions from an eco-genetic model for smallmouth bass. Transactions of the American Fisheries Society. 2007;136:749–765. [Google Scholar]
- Dunlop ES, Enberg K, Jørgensen C, Heino M. Towards Darwinian fisheries management. Evolutionary Applications. 2009a;2:245–260. doi: 10.1111/j.1752-4571.2009.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop ES, Heino M, Dieckmann U. Eco-genetic modeling of contemporary life-history evolution. Ecological Applications. 2009b doi: 10.1890/08-1404.1. (in press) [DOI] [PubMed] [Google Scholar]
- Enberg K, Jørgensen C, Dunlop ES, Heino M, Dieckmann U. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evolutionary Applications. 2009;2:394–414. doi: 10.1111/j.1752-4571.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Essex, UK: Longman; 1996. [Google Scholar]
- Francis RC, Hixon MA, Clarke ME, Murawski SA, Ralston S. Fisheries management – ten commandments for ecosystem-based fisheries scientists. Fisheries. 2007;32:217–233. [Google Scholar]
- Gårdmark A, Dieckmann U, Lundberg P. Life-history evolution in harvested populations: the role of natural predation. Evolutionary Ecology Research. 2003;5:239–257. [Google Scholar]
- Gårdmark A, Jonzén N, Mangel M. Density-dependent body growth reduces the potential of marine reserves to enhance yields. Journal of Applied Ecology. 2006;43:61–69. [Google Scholar]
- Gaylord B, Gaines SD, Siegel DA, Carr MH. Marine reserves exploit population structure and life history in potentially improving fisheries yields. Ecological Applications. 2005;15:2180–2191. [Google Scholar]
- Gell FR, Roberts CM. Benefits beyond boundaries: the fishery effects of marine reserves. Trends in Ecology & Evolution. 2003;18:448–455. [Google Scholar]
- Gerber LR, Botsford LW, Hastings A, Possingham HP, Gaines SD, Palumbi SR, Andelman S. Population models for marine reserve design: a retrospective and prospective synthesis. Ecological Applications. 2003;13:S47–S64. [Google Scholar]
- Gerber LR, Heppell SS, Ballantyne F, Sala E. The role of dispersal and demography in determining the efficacy of marine reserves. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:863–871. [Google Scholar]
- Godø OR. Fluctuation in stock properties of north-east Arctic cod related to long-term environmental changes. Fish and Fisheries. 2003;4:121–137. [Google Scholar]
- Grift RE, Rijnsdorp AD, Barot S, Heino M, Dieckmann U. Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Marine Ecology Progress Series. 2003;257:247–257. [Google Scholar]
- Grimm V, Railsback SF. Individual-based Modeling and Ecology: Princeton Series in Theoretical and Computational Biology. Princeton, NJ, USA: Princeton University Press; 2005. [Google Scholar]
- Guenette S, Pitcher TJ. An age-structured model showing the benefits of marine reserves in controlling overexploitation. Fisheries Research. 1999;39:295–303. [Google Scholar]
- Gunderson DR, Dygert PH. Reproductive effort as a predictor of natural mortality rate. Journal du Conseil – Conseil International pour l'Exploration de la Mer. 1988;44:200–209. [Google Scholar]
- Halpern BS. The impact of marine reserves: do reserves work and does reserve size matter? Ecological Applications. 2003;13:S117–S137. [Google Scholar]
- Halpern BS, Warner RR. Marine reserves have rapid and lasting effects. Ecology Letters. 2002;5:361–366. [Google Scholar]
- Hannesson R. Marine reserves: what would they accomplish? Marine Resources Economics. 1998;13:159–170. [Google Scholar]
- Hart DR. When do marine reserves increase fishery yield? Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:1445–1449. [Google Scholar]
- Hastings A, Botsford L. Equivalence in yield from marine reserves and traditional fisheries management. Science. 1999;284:1537–1538. doi: 10.1126/science.284.5419.1537. [DOI] [PubMed] [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1971–1982. [Google Scholar]
- Heino M, Dieckmann U. Detecting fisheries-induced life-history evolution: an overview of the reaction norm approach. Bulletin of Marine Science. 2008;83:69–93. [Google Scholar]
- Heino M, Godø OR. Fisheries-induced selection pressures in the context of sustainable fisheries. Bulletin of Marine Science. 2002;70:639–656. [Google Scholar]
- Heino M, Hanski I. Evolution of migration rate in a spatially realistic metapopulation model. American Naturalist. 2001;157:495–511. doi: 10.1086/319927. [DOI] [PubMed] [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002a;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Reaction norm analysis of fisheries-induced adaptive change and the case of the Northeast Arctic codICES CM. 2002b;2002/Y:14. [Google Scholar]
- Heino M, Baulier L, Boukal DS, Dunlop ES, Eliassen S, Enberg K, Jørgensen C, et al. Evolution of growth in Gulf of St Lawrence cod? Proceedings of the Royal Society of London, Series B: Biological Sciences. 2008;275:1111–1112. doi: 10.1098/rspb.2007.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty. New York, NY, USA: Chapman & Hall; 1992. [Google Scholar]
- Hilborn R, Stokes K, Maguire JJ, Smith T, Botsford LW, Mangel M, Orensanz J, et al. When can marine reserves improve fisheries management? Ocean & Coastal Management. 2004;47:197–205. [Google Scholar]
- Hilborn R, Micheli F, De Leo GA. Integrating marine protected areas with catch regulation. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:642–649. [Google Scholar]
- Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LM, Wroblewski JS. Conserving a subpopulation of the northern Atlantic cod metapopulation with a marine protected area. Aquatic Conservation–Marine and Freshwater Ecosystems. 2009;19:178–193. [Google Scholar]
- Hunter E, Metcalfe JD, Reynolds JD. Migration route and spawning area fidelity by North Sea plaice. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2003;270:2097–2103. doi: 10.1098/rspb.2003.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson WF, Carvalho GR, Rogers SI. Marked genetic structuring in localised spawning populations of cod Gadus morhua in the North Sea and adjoining waters, as revealed by microsatellites. Marine Ecology Progress Series. 2001;223:251–260. [Google Scholar]
- ICES. 2007. Report of the Arctic Fisheries Working Group ICES CM 2007/ACFM:16.
- Jonsdottir ODB, Imsland AK, Danielsdottir AK, Thorsteinsson V, Naevdal G. Genetic differentiation among Atlantic cod in south and south-east Icelandic waters: synaptophysin (Syp I) and haemoglobin (HbI) variation. Journal of Fish Biology. 1999;54:1259–1274. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Dunlop ES, Opdal AF, Fiksen Ø. The evolution of spawning migrations: state dependence and fishing-induced changes. Ecology. 2008a;89:3436–3448. doi: 10.1890/07-1469.1. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. The role of fisheries-induced evolution – response. Science. 2008b;320:48–50. [Google Scholar]
- Jørgensen C, Ernande B, Fiksen Ø. Size-selective fishing gear and life history evolution in the Northeast Arctic cod. Evolutionary Applications. 2009;2:356–370. doi: 10.1111/j.1752-4571.2009.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MJ. Are closed areas the solution? Marine Conservation. 2003;5:11. [Google Scholar]
- Kaiser MJ. Are marine protected areas a red herring or fisheries panacea? Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:1194–1199. [Google Scholar]
- Kellner JB, Tetreault I, Gaines SD, Nisbet RM. Fishing the line near marine reserves in single and multispecies fisheries. Ecological Applications. 2007;17:1039–1054. doi: 10.1890/05-1845. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Witthames PR, Nash RDM. The concept of fecundity regulation in plaice (Pleuronectes platessa) tested on three Irish Sea spawning populations. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:587–601. [Google Scholar]
- Kirkpatrick M. The evolution of size and growth in harvested natural populations. In: Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Berlin, Germany: Springer-Verlag; 1993. pp. 145–154. [Google Scholar]
- Kjesbu OS, Witthames PR, Solemdal P, Walker MG. Temporal variations in the fecundity of Arcto-Norwegian cod (Gadus morhua) in response to natural changes in food and temperature. Journal of Sea Research. 1998;40:303–321. [Google Scholar]
- Kramer DL, Chapman MR. Implications of fish home range size and relocation for marine reserve function. Environmental Biology of Fishes. 1999;55:65–79. [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology & Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kuparinen A, Merilä J. The role of fisheries-induced evolution. Science. 2008;320:47–48. [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- Law R, Grey DR. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology. 1989;3:343–359. [Google Scholar]
- Lester NP, Shuter BJ, Abrams PA. Interpreting the von Bertalanffy model of somatic growth in fish: the cost of reproduction. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2004;271:1625–1631. doi: 10.1098/rspb.2004.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret J, Ratz HJ. Condition of cod (Gadus morhua) off Greenland during 1982–1998. Fisheries Research. 2000;48:79–86. [Google Scholar]
- Lubchenco J, Palumbi SR, Gaines SD, Andelman S. Plugging a hole in the ocean: the emerging science of marine reserves. Ecological Applications. 2003;13:S3–S7. [Google Scholar]
- Mangel M, Levin PS. Regime, phase and paradigm shifts: making community ecology the basic science for fisheries. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2005;360:95–105. doi: 10.1098/rstb.2004.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CT, Yaragina NA, Ådlandsvik B, Dolgov AV. Reconstructing the stock-recruit relationship for Northeast Arctic cod using a bioenergetic index of reproductive potential. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:2433–2442. [Google Scholar]
- Marshall CT, Needle CL, Yaragina NA, Ajiad AM, Gusev E. Deriving condition indices from standard fisheries databases and evaluating their sensitivity to variation in stored energy reserves. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:1900–1917. [Google Scholar]
- McEvoy LA, McEvoy J. Multiple spawning in several commercial fish species and its consequences for fisheries management, cultivation and experimentation. Journal of Fish Biology. 1992;41:125–136. [Google Scholar]
- McIntyre TM, Hutchings JA. Small-scale temporal and spatial variation in Atlantic cod (Gadus morhua) life history. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1111–1121. [Google Scholar]
- Miethe T, Pitchford J, Dytham C. An individual-based model for reviewing marine reserves in the light of fisheries-induced evolution in mobility and size at maturation. Journal of the Northwest Atlantic Fisheries Society. 2009;41:151–162. [Google Scholar]
- Mollet FM, Kraak SBM, Rijnsdorp AD. Fisheries-induced evolutionary changes in maturation reaction norms in North Sea sole Solea solea. Marine Ecology Progress Series. 2007;351:189–199. [Google Scholar]
- Mousseau TA, Roff DA. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–198. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Murawski SA, Brown R, Lai HL, Rago PJ, Hendrickson L. Large-scale closed areas as a fishery-management tool in temperate marine systems: the Georges Bank experience. Bulletin of Mathematical Biology. 2000;66:775–798. [Google Scholar]
- Okamoto K, Whitlock R, Magnan P, Dieckmann U. Mitigating fisheries-induced evolution in lacustrine brook charr (Salvelinus fontinalis) in southern Quebec, Canada. Evolutionary Applications. 2009;2:415–437. doi: 10.1111/j.1752-4571.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Lilly GR, Heino M, Morgan J, Brattey J, Dieckmann U. Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:811–823. [Google Scholar]
- Oskarsson GJ, Kjesbu OS, Slotte A. Predictions of realised fecundity and spawning time in Norwegian spring-spawning herring (Clupea harengus. Journal of Sea Research. 2002;48:59–79. [Google Scholar]
- Palsson OK, Thorsteinsson V. Migration patterns, ambient temperature, and growth of Icelandic cod (Gadus morhua): evidence from storage tag data. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1409–1423. [Google Scholar]
- Pampoulie C, Ruzzante DE, Chosson V, Jorundsdottir TD, Taylor L, Thorsteinsson V, Danielsdottir AK, et al. The genetic structure of Atlantic cod (Gadus morhua) around Iceland: insight from microsatellites, the Pan I locus, and tagging experiments. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2660–2674. [Google Scholar]
- Pauly D, Christensen V, Guenette S, Pitcher TJ, Sumaila UR, Walters CJ, Watson R, et al. Towards sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Perez-Ruzafa A, Gonzalez-Wanguemert M, Lenfant P, Marcos C, Garcia-Charton JA. Effects of fishing protection on the genetic structure of fish populations. Biological Conservation. 2006;129:244–255. [Google Scholar]
- Ricker WE. Changes in average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Roberts CM, Bohnsack JA, Gell F, Hawkins JP, Goodridge R. Effects of marine reserves on adjacent fisheries. Science. 2001;294:1920–1923. doi: 10.1126/science.294.5548.1920. [DOI] [PubMed] [Google Scholar]
- Roberts CM, Hawkins JP, Gell FR. The role of marine reserves in achieving sustainable fisheries. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2005;360:123–132. doi: 10.1098/rstb.2004.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud D, Rose GA. Multiyear homing of Atlantic cod. Canadian Journal of Fisheries and Aquatic Sciences. 2000;58:2325–2329. [Google Scholar]
- Robichaud D, Rose GA. Multiyear homing of Atlantic cod to a spawning ground. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:2325–2329. [Google Scholar]
- Robichaud D, Rose GA. Migratory behaviour and range in Atlantic cod: inference from a century of tagging. Fish and Fisheries. 2004;5:185–214. [Google Scholar]
- Rose GA. Cod spawning on a migration highway in the north-west Atlantic. Nature. 1993;366:458–461. [Google Scholar]
- Rose GA, O'Driscoll RL. Capelin are good for cod: can the northern stock rebuild without them? ICES Journal of Marine Science. 2002;59:1018–1026. [Google Scholar]
- Roughgarden J. Theory of Population Genetics and Evolutionary Ecology: An Introduction. New York, NY, USA: Macmillan Publishing Co., Inc; 1979. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- Stenseth NC, Dunlop ES. Unnatural selection. Nature. 2009;457:803–804. doi: 10.1038/457803a. [DOI] [PubMed] [Google Scholar]
- Svedäng H, Righton D, Jonsson P. Defining ‘natal homing’ in marine fish populations; need for inference in fishery science: reply to Bradbury & Laurel (2007) Marine Ecology Progress Series. 2007;349:309–310. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolution of growth in Gulf of St Lawrence cod: reply to Heino et al. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2008;275:1113–1115. doi: 10.1098/rspb.2007.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thériault V, Dunlop ES, Dieckmann U, Bernatchez L, Dodson JJ. The impact of fishing-induced mortality on the evolution of alternative life-history tactics in brook charr. Evolutionary Applications. 2008;1:409–423. doi: 10.1111/j.1752-4571.2008.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorrold SR, Latkoczy C, Swart PK, Jones CM. Natal homing in a marine fish metapopulation. Science. 2001;291:297–299. doi: 10.1126/science.291.5502.297. [DOI] [PubMed] [Google Scholar]
- Thorsen A, Kjesbu OS. A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. Journal of Sea Research. 2001;46:295–308. [Google Scholar]
- Trexler JC, Travis J. Can marine protected areas restore and conserve stock attributes of reef fishes? Bulletin of Marine Science. 2000;66:853–873. [Google Scholar]
- Vitale F, Borjesson P, Svedang H, Casini A. The spatial distribution of cod (Gadus morhua L.) spawning grounds in the Kattegat, eastern North Sea. Fisheries Research. 2008;90:36–44. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Wang H-Y, Höök TO. Eco-genetic model to explore fishing-induced ecological and evolutionary effects on growth and maturation schedules. Evolutionary Applications. 2009;2:438–455. doi: 10.1111/j.1752-4571.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


