Abstract
Industrial fishing has been identified as a cause for life history changes in many harvested stocks, mainly because of the intense fishing mortality and its size-selectivity. Because these changes are potentially evolutionary, we investigate evolutionarily stable life-histories and yield in an energy-allocation state-dependent model for Northeast Arctic cod Gadus morhua. We focus on the evolutionary effects of size-selective fishing because regulation of gear selectivity may be an efficient management tool. Trawling, which harvests fish above a certain size, leads to early maturation except when fishing is low and confined to mature fish. Gillnets, where small and large fish escape, lead to late maturation for low to moderate harvest rates, but when harvest rates increase maturation age suddenly drops. This is because bell-shaped selectivity has two size-refuges, for fish that are below and above the harvestable size-classes. Depending on the harvest rate it either pays to grow through the harvestable slot and mature above it, or mature small below it. Sustainable yield on the evolutionary time-scale is highest when fishing is done by trawling, but only for a small parameter region. Fishing with gillnets is better able to withstand life-history evolution, and maintains yield over a wider range of fishing intensities.
Keywords: energy allocation, evolutionary modeling, fisheries management, fishing-induced changes, life history evolution
Introduction
Exploitation of living resources can lead to evolutionary changes in harvested populations of plants (Law and Salick 2005) and mammals (Coltman et al. 2003), but because of the grand scale of commercial fishing most examples come from fish. The reviews by e.g. Jørgensen et al. (2007), Kuparinen and Merilä (2007), Fenberg and Roy (2008), Hutchings and Fraser (2008), and Sharpe and Hendry (2009) list phenotypic evidence of morphology and life history traits that changed over time in wild populations. Because these changes cannot be totally explained by environmental factors, part of the change is thought to represent contemporary evolution. The rates of change are furthermore rapid, and in general comparable to those observed in breeding programs (Reznick and Ghalambor 2001; Jørgensen et al. 2007). In a comparative study of anthropogenic causes for contemporary evolution, harvesting was found to result in quicker evolutionary change than other human influences (Darimont et al. 2009). Heritable changes in a number of behavioral and life history traits have also been observed in populations harvested experimentally (Conover and Munch 2002; Walsh et al. 2006; Biro and Post 2008). The problem of fishing-induced evolution requires attention because it might be widespread, as most commercially harvested fish stocks experience intense exploitation rates, with fishing mortality being up to four times higher than the natural mortality (Mertz and Myers 1998). In general, theoretical models predict less of an evolutionary response to harvesting if fishing mortality is lower (Law and Grey 1989; Ernande et al. 2004; Brown et al. 2008). This prediction is supported also by fisheries data, for example in Pink salmon where phenotypic change that could not be explained by environment was slower in regions with lower fishing mortality or less selective gear [Ricker 1981; see also the meta-analysis by Sharpe and Hendry (2009)].
Although there is general agreement that the world's fishing fleets are vastly oversized for a sustainable harvest practice, it has turned out difficult to down-regulate this overcapacity. The reason is a fundamental conflict between the short-term rewards that motivate individual players in the fishing industry versus the long-term goals of sustainability that would insure the viability of the very same industry. Any management solution has to acknowledge and address this trade-off (the following description of the state of fisheries management draws on Clark 2006). Catch quotas are often negotiated higher than advised because of lobbying by the fishing industry looking to capitalize quickly on investments in infrastructure and vessels, or small-scale fishermen eager to cover their expenses while competing with other fishermen for the same shared resource. A similar fate befalls effort control regulations, where technology creep leads to increasing ability to catch fish although the numbers of vessels or days at sea remain constant. The attempts by politicians and managers of more drastic methods, such as buyback programs to take vessels out of the fishery, have also failed. Fishermen are like other business owners, and either expect such buyback programs to take place and expand beforehand, or sell only the most inefficient boats so that there are only minor effects on overall fleet capacity. Individually owned quotas that give fishermen a long-term perspective have been proposed as a promising option to break the trade-off between short-term profit and long-term sustainability (see Costello et al. 2008). Under such regulations it becomes profitable for a fisherman to spare some fish since he has ownership of a fixed share of future harvest. Such individually transferable quotas (ITQs) avoid the tragedy of the commons but run into other problems because the public basically abandons ownership of a natural resource for free; at least the question of compensation remains largely unresolved. Given the immense difficulties in controlling effort and reducing harvest pressure, it seems that general advice of the type ‘reduce fishing mortality to one-quarter to lessen the evolutionary impact’ is unlikely to be effectively implemented in the near future. An alternative avenue may be to manage fishing gear and its size-selectivity (Law and Rowell 1993; Law 2000).
Most kinds of fishing gear do not catch all fish with equal probability but are selective for certain types of fish in one way or another. Often, this selectivity is based on body size. For example, small fish may slip through gillnets and large fish avoid getting caught, while fish with a girth close to the mesh size are most effectively harvested (Hamley 1975). For trawls, sorting grids and mesh size in the codend let small fish escape while larger fish are harvested. The pattern of size-selectivity may have large consequences for fishing-induced evolution, because the fish that survive and can pass on their genes to the next generation differ between gear types. For example, it has often been stated that if only fish above a certain size threshold are harvested, then it would become optimal to grow slower (Miller 1957) and mature earlier (Law and Grey 1989). These qualitative expectations have been confirmed by theoretical models (e.g. Favro et al. 1979; Law and Grey 1989; Ernande et al. 2004; Gårdmark and Dieckmann 2006) and found in harvesting experiments (Edley and Law 1988; Conover and Munch 2002). The most comprehensive experiment to date is on Atlantic silversides Menidia menidia (Conover and Munch 2002; Walsh et al. 2006). In that study, harvesting of the largest individuals, which is analogous to trawling, led to heritable changes towards smaller fish, slower growth, reduced fecundity, poorer viability of larvae, and diminished yield. Because of the potential for detrimental evolutionary effects caused by trawling-like size-selectivity, several authors have suggested that the bell-shaped selectivity curves of gillnets may be better from a sustainability perspective (Law and Rowell 1993; Law 2007). With a bell-shaped selectivity curve, fish that survive as they grow through the sizes vulnerable to the fishery may successfully reproduce, potentially repeatedly and at a large size. This may weaken selection toward early maturation, and thus lead to less of an evolutionary response compared to trawling (Law 2007).
Because there already is a tradition for mesh-size and gear-type regulation in fisheries management as well as routines for enforcement, it seems worthwhile considering gear regulation as a tool to manage evolutionary trait changes generated by fishing practices. This raises the question: what would a desirable harvesting regime look like from the perspective of an evolutionarily concerned fisheries manager? Ultimately, the goals of management are to be decided through a democratic and political process during which the views of the public, stakeholders, and interest groups are duly heard and considered (Jørgensen et al. 2007). From a biological perspective and for the sake of illustration, however, it can be worthwhile to focus on two relatively conservative aims initially. The first one is that the harvesting practice leads to little evolutionary change relative to the pre-harvesting situation (see also Hutchings, 2009). Currently, little is known about the potential consequences of fishing-induced evolution, but because life history traits are affected and these are central to population dynamics, many stock characteristics such as productivity, yield, and resilience might be altered. In general, the manager's tasks of predicting stock development and planning harvesting schemes would be easier if stock properties stayed as constant as possible. It might therefore be good for a manager to have a stock that undergoes little evolutionary change, so that sensitivity to environmental factors and responses to harvesting can be known or learnt from the stock's past behavior. Ecosystem relationships are also more likely to remain the same if the changes in stock characteristics are small. A second property that our evolutionary concerned manager might desire is that her choice of gear type is robust to excess harvesting, as the overall harvest rate has proven difficult to control as discussed above. By expecting that harvest rates might be higher than planned, she should choose a gear type that has minor consequences for the evolutionary outcome if harvest levels were to increase.
In this paper, we study the effects of gear type and its size-selectivity on expected evolutionary trait changes [see also the modeling study by Hutchings (2009)]. The life history model we use is designed for the Northeast Arctic stock of Atlantic cod Gadus morhua and is rich in ecological and physiological detail (Jørgensen and Fiksen 2006). Primarily we contrast different types of gillnets and trawls in search of evolutionarily desirable harvesting practices.
Model description
Life history model
The life history model we used finds the lifelong pattern of energy allocation between growth and reproduction that would maximize lifetime expected fecundity. The allocation decision depends on the individual's state, that is, its age (in months, thus also including seasonal patterns), body length (in cm), level of stored energy (percent of full energy stores), and the current state of the feeding environment that undergoes autocorrelated temporal variability (a graphical overview of the model is given in Fig. 1). As such, the model explicitly includes phenotypically plastic response to the internal state (age, body length, level of stored energy) and the external environment (food availability). The model optimizes the state-dependent energy allocation, with the result that age and size at maturation, growth rates, skipped spawning, and the level of energy stored are emergent properties arising from the optimal pattern of energy allocation. The description below is based on Jørgensen and Fiksen (2006) which is referred to for further details. The model has previously been used to investigate the potential for fishing-induced evolution to cause changes in life history traits and skipped spawning (Jørgensen et al. 2006) and in migration distance and large-scale geographical distribution (Jørgensen et al. 2008). Although selection from size-selective fishing gear has been quantified in earlier studies (e.g. Law and Rowell 1993), this is to our knowledge the first paper to systematically investigate the potential evolutionary consequences of size-selectivity harvesting on fish life histories.
Figure 1.
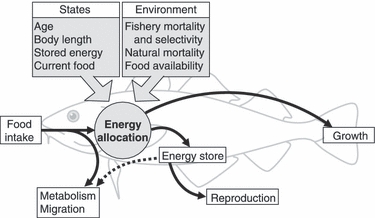
Graphical representation of the life-history energy-allocation model, parameterized for the Northeast Arctic cod (Gadus morhua). The central process is energy allocation toward growth or stores/reproduction. The energy allocation can take independent values depending on the individuals state (age, size, level of energy stored, and current food availability). The model is then solved with different forcing, here with focus on changing the size-selectivity and intensity of the fishing mortality. Black arrows denote energy flow. In periods when food intake is insufficient for metabolic demands, energy requirements can be met by stores (dotted line). The figure is modified from Jørgensen and Fiksen (2006).
Each month the individuals receive an amount of food that is stochastic and autocorrelated in time. Food intake scales allometrically with length L [cm] as L2.41 (based on Jobling 1988), and after energy to cover metabolic rate and basic activity has been spent, the remainder is available for allocation between somatic growth and storage as lipids and proteins for future reproduction.
The Northeast Arctic cod stock uses the Barents Sea as feeding area but spawns along the Norwegian coast, with the main spawning taking place in Lofoten after a migration of around 800 km. We assume it takes 5 months in total to migrate south to the spawning area, spawn (cod may produce up to 20 batches of eggs that each needs to mature; Kjesbu et al. 1996), and to migrate back north again. The energetic cost of migration is taken from the energy stores, and during spawning and migration cod only eat enough to cover their standard metabolism. The weight-specific energetic cost of migration decreases with fish size (see Ware 1978 and Alexander 2003 for general treatments of size-dependent swimming costs in fish). What is left of their energy stores after migration is used to produce eggs that are spawned. We model only females to avoid the problems of sexual selection and frequency-dependent competition among males.
Natural mortality M(L) is negatively size-dependent so that it is highest for small fish and then stabilizes at 0.25 year−1 for larger fish. Fishing takes place both at the spawning grounds (where there are only mature fish) and at the feeding grounds (where immature fish are all year round and mature fish the 7 months they are not migrating or spawning). Life history theory has shown that these two types of fishery act in opposite directions on age at maturation (Law and Grey 1989): while the feeder fishery favors early-maturing fish that manage to reproduce while still alive, a high mortality on the spawning grounds favor fish that are large when they risk that mortality to reproduce and therefore selects for late maturation. Studies have shown that maturation is much more sensitive to the mortality in the feeder fishery than the harvest rate at the spawning grounds (Law and Grey 1989; Jørgensen et al. 2006).
Evolutionary modeling approach
Optimal energy allocation patterns, i.e. energy allocation patterns that maximize individual fitness and thus result in optimal emergent life history strategies, were found using state-dependent dynamic programming (Houston and McNamara 1999; Clark and Mangel 2000). As fitness measure we used the expected lifetime reproductive success R0, i.e. the expected number of offspring produced in a lifetime. Theoretical studies have shown that evolution optimizes R0 as long as density dependence affecting the population acts only multiplicatively on the expected lifetime production of offspring (Mylius and Diekmann 1995), which is the case in our model (see section below on yield calculation). We therefore maximize reproductive value V, which at birth corresponds to R0 (Houston and McNamara 1999). For each age, reproductive value V has a future component that the individual can achieve if it survives, and this expected residual reproductive value depends on the new state the individual is in. Every year, the individual can also add to its reproductive value by reproducing. The algorithm finds the optimal allocation pattern by iterating backwards, starting at the maximum age of 25 years at which all individuals die and have no residual reproductive value. The model then compares the fitness consequences of all allocation values α between reproduction and growth and stores the allocation value that maximizes fitness. This is repeated for all lengths L, sizes of energy store E, and environmental food availability F, before the model moves one time-step backwards and repeats the process, assuming that energy allocation is optimal for the remainder of its life. The logic can be condensed to the dynamic programming equation, which can be written as:
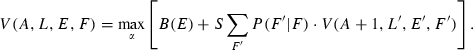 |
Here the left-hand side is the reproductive value for a given state-combination, and it is found by choosing the allocation α that maximizes fitness. Fitness is expressed within the square brackets, and it consists of (i) the current fecundity B which is a function of the energy available for reproduction, and (ii) the residual fitness if the individual survives (with probability S). The residual fitness depends on the new state at age A + 1 (1-month older) when the individual has grown to length L ′ and its energy store is E ′ (these state changes follow from α). The summation over F ′ finds the expected fitness over the possible levels of the feeding environment. Since F is autocorrelated in time, the conditional probability P(F ′|F) gives the probability of having food availability F ′ in the next month if the current level is F.
Optimization approaches rely on a static fitness measure, which is valid for populations experiencing simple density-dependent processes but means that frequency-dependent consequences on fitness cannot be included. Examples of such effects that need to be ignored are density dependence acting on growth, or mate competition based on relative size structure. The environmental influence is modeled as fluctuating and autocorrelated, but the environmental variance is constant over time.
The advantage of using state-dependent dynamic programming over other evolutionary modeling approaches, like optimality models (Stearns 1992) or selection gradient approaches (Abrams 2001) such as quantitative genetics (Lande 1976) or adaptive dynamics (Dieckmann and Law 1996), is that state dependence can be incorporated in great detail and that individual-level processes can be relatively complex. As such, the methodology needs to make fewer assumptions about how phenotypic plasticity should be constrained. The biological complexity of state-dependent strategies and phenotypic plasticity that dynamic programming models can deal with is generally out of reach for the other approaches. One limitation is that, although it is fully accounted for, the selection gradient cannot be computed explicitly.
When the optimal energy allocation pattern has been found for a given fishing regime, we simulate the population dynamics of this life history strategy using a state-structured population model in discrete time to record the emergent life history traits and long-term yield. The results we show are recorded during such forward simulations of optimal life history strategies.
The details of the model were published in Jørgensen and Fiksen (2006), and we refer the reader to that paper for further details on the physiological and ecological mechanisms included.
Genetic assumptions
The methodology we use finds phenotypes that optimize individual fitness given selection pressures generated by the ecological setting, which here originates from fishing and its size-selectivity but also from natural mortality, physiological constraints and environmental stochasticity. These optimal phenotypes are evolutionarily stable strategies. As such, they are evolutionary endpoints resulting from long-term evolution as dictated by selection pressures only. The underlying genetic assumptions of the model are thus according to the streetcar theory of evolution (Hammerstein 1996): there are no genetic constraints that delimit the long term evolutionary outcome. In other words, traits are heritable and genetic correlations do not prevent evolution to proceed (note that ecological and physiological constraints are specified in the model processes, though). The modeling approach does not rely on any particular value for heritability for the traits in question, as the heritability parameter would only scale the rate at which the optimal phenotype is approached but not change the long-term evolutionary endpoint itself. Heritability has been measured for many life history traits in fish and in cod (Gjedrem 1983; Carlson and Seamons 2008), suggesting the presence of genetic variability that is required for evolution to occur. Experiments that identify such a genetic basis for phenotypic variability are reviewed in Conover and Baumann (2009). It is worthwhile highlighting here that the model actually finds optimal state-dependent phenotypes and therefore incorporates explicitly both plasticity and evolution by considering the long-term evolution of plastic responses or multidimensional reaction norms.
Gear selectivity
In previous versions of this model, fishing mortality affected all individuals equally, regardless of their size or other individual states. The addition in this paper is that we introduce fishing selectivity curves U(L) that depend on an individual's length L and take values between 0 (the fish is unaffected by the fishery) and 1 (the fish is maximally selected by the fishery). The fishing mortality F(L) an individual of length L experiences is thus F(L) = U(L)·fmax, where fmax is the maximum annual harvest rate [year−1] when selectivity U(L) = 1. Total mortality is Z(L) = M(L) + F(L) and monthly survival probability P(L) is then P(L) = e–Z(L)/12. The results we present use different values of fmax for the feeder fishery and the spawner fishery, as indicated on the graphs. The focus is on drawing the management ‘map’ of how a choice of fisheries mortalities in the two fisheries might affect the evolutionary outcome in the longer term.
Because we use optimization, the methodology constrains us to use a fixed fishing intensity and size-selectivity while we find the optimal life history response to it. An inherent assumption is therefore that the fishing fleet continues to use the same gear while fish evolve life histories that allow them to escape the fishing mortality. This can result in a realized fishing mortality that is much lower than fmax as life histories evolve to sizes that are less vulnerable to the fishery. Alternative approaches could have been to determine a fixed total allowable catch or a harvest control rule with a given size-selectivity, and study the consequences of that management regime. In an optimization framework that would require an iterative procedure that first finds the optimal life history strategy, then simulates the catch resulting from this strategy, before modifying the fishing regime and repeating these steps in the next iteration, this until convergence. Such studies are better tackled through models where ecology and evolution take place on the same time-scale such as selection gradient approaches (Abrams 2001) or individual-based evolutionary models (Strand et al. 2002), although these models cannot include as much individual detail. We amend this by showing the fisheries yield that results from a given optimal life history, so that it becomes easier to interpret the evolutionary endpoint of a given harvest regime together with the long-term fisheries yield it would result in.
Size-selectivity curves for Atlantic cod have been determined for trawls and gillnets by Huse et al. (2000). Their findings agree with earlier studies where gillnets show bell-shaped selectivity curves as fish with a certain girth are captured with a higher probability than smaller fish that can slip through or larger fish that don't get far enough through the mesh to get stuck (reviewed by Hamley 1975). We modeled gillnet selectivity UG as a Gaussian function around a size of maximum selectivity Lmax:
Huse et al. (2000) found that the width parameter σ of the selectivity curves was 14% of the mean for the three mesh sizes they investigated. A mesh size regulation implemented in a real fishery would probably catch a wider size-range of fish than what was obtained in one scientific study (Huse et al. 2000), because of differences between boats, variable fishing practices, and variation in location and timing of fishing. We thus chose to double the width of the selectivity curve, such that σ = 0.28·Lmax. This makes the size-selectivity curves more similar to the empirical examples shown in Hilborn and Minte-Vera (2008). The conclusions and the qualitative results are the same if a narrower selectivity curve is used, but the quantitative predictions change somewhat. For cod, longlines (hook and bait) have a similar bell-shaped size-selectivity to gillnets (Huse et al. 2000).
In trawls, small fish can escape through the mesh in the codend or through specially designed sorting grids, whereas fish larger than a certain size are retained (Millar and Fryer 1999) resulting in sigmoid selectivity curves. To facilitate comparison, we modeled the trawl selectivity curve UT as the left half of the same Gaussian function as gillnets up to a size of Lmax, from which on selectivity was kept at 1:
We are aware of the tradition in fisheries science of using logistic functions for sigmoid selectivity curves. However for the sake of comparison, we preferred to keep the formulation of the selectivity curves as similar as possible for gillnet and trawl.
For both types of fishing gear, we show results for Lmax of 70, 90, and 110 cm; selectivity curves for gillnet and trawl with these parameters are shown in Fig. 2. The main aim of this study is to contrast the effects of gillnetting and trawling on fishing-induced life history evolution and its consequences for yield. To achieve this comparison, we applied the same fishing size-selectivity, either sigmoid or bell-shaped, at both the spawning and the feeding grounds. In contrast, the existing fishery for Northeast Arctic cod is dominated by trawling at the feeding grounds and longlines and gillnets at the spawning grounds. Therefore, we also ran the model with sigmoid selectivity, UT(L), at the feeding grounds and bell-shaped selectivity, UG(L), at the spawning grounds, both with Lmax = 90 cm, to see how a fishery with mixed gear types compares to fishing with only one gear type.
Figure 2.
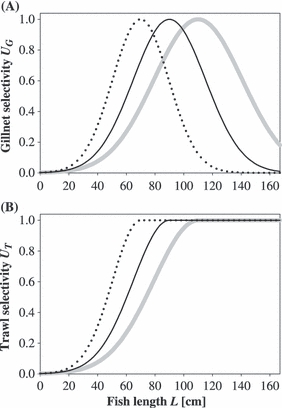
Size-selectivity curves used to impose selection on Northeast Arctic cod life histories. At maximum selectivity, fish of that size are harvested at the rate specified by the parameter fmax. (A) Bell-shaped size-selectivity curves for gillnets. The peaks of the Gaussian functions are at 70 cm (dotted line), 90 cm (thin black line), and 110 cm (thick grey line). In each case the standard deviation is 28% of the mean. (B) Sigmoid trawl selectivity was modeled based on the same probability distributions as for gillnets, but with maximum selectivity for all fish lengths larger than the peak.
Yield calculations
The population dynamics of individuals following the optimal life history strategies were simulated in a structured population model. Population size was regulated by a Beverton–Holt density-dependent function for recruit survival, which on its general form can be written as:
Here N2(t + 2) is the number of recruits introduced into the structured population model at age 2 at time t + 2, and B(t) is the population's total egg production at time t. The two parameters λ and γ determine the strength of density dependence, where λ is the recruit survival at low population densities and the ratio λ/γ is the asymptotic recruitment level. By choosing parameters so that λ = γ the asymptotic recruitment level becomes λ/γ = 1, implying that abundance N is scaled relative to this asymptotic level.
We used two versions of Beverton–Holt parameters in the population dynamics simulations, corresponding to different assumptions about how recruitment density dependence may change with population size. First, we assumed that the Beverton–Holt relationship remained constant whatever population size (λ = γ = 5.45 × 10−7; Fig. S1a). This would correspond to the situation where recruitment density dependence is regulated by purely external factors such as habitat availability, or by food (prey) abundance and predation that depend on static populations which do not respond to the size of our focal population (Walters and Korman 1999). In a second scenario, we assumed that the Beverton–Holt density dependent recruitment curve had constant curvature around the equilibrium population biomass. This would correspond to the assumption that density dependence is felt in the same way by individuals whatever the size of their population. This would occur if prey and predator populations were changing in size with our focal species, or if range contractions (or expansions) led to constant strength of predator-prey interactions as our focal population becomes smaller (or larger). This scenario was implemented using a calibrating procedure. First, the population dynamics was run with a constant recruitment 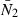 = 0.6. We then recorded the annual egg production in the population over time to estimate its mean
= 0.6. We then recorded the annual egg production in the population over time to estimate its mean 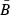 . The value of λ (=γ) that would let the Beverton–Holt curve go through the point (
. The value of λ (=γ) that would let the Beverton–Holt curve go through the point (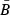 ,
,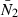 ) was then found by solving:
) was then found by solving:
which, when rearranged, gives
This value of λ was then used for the population dynamics simulations. Examples of this rescaling are shown in Fig. S1b.
Results
Our results depict the long-term effects of fishing-induced life history evolution on the stock as a function of fishing on all fish, i.e. immature and mature individuals (the feeder fishery), versus fishing only on mature fish (the spawner fishery). The results will be shown as 3D-surfaces representing age at maturation emerging from optimal energy allocation strategies as a function of maximum fishing mortality fmax in the feeder fishery (on the left-to-right axis) and in the spawner fishery (on the front-to-back axis). Age at maturation was chosen because this central life history trait is linked to population dynamics, stock productivity, and the stock's size-structure. It is also the trait for which most empirical evidence suggesting fishing-induced evolution has been analyzed and published (Jørgensen et al. 2007). We also show similar 3D-surfaces for yield. For a stock like the Northeast Arctic cod, where the mature component of the population is geographically separated for parts of the year and the two fisheries can be managed relatively independently, the surfaces represent a decision landscape for our evolutionarily concerned fisheries manager. In fish stocks where fishing intensity cannot be distributed between mature and immature individuals, the manager should look along the diagonal line where the fishing intensity is the same in the spawner and the feeder fishery.
We first illustrate the general effect of size-selectivity on evolution of maturation age (Fig. 3). If fishing is un-selective for size (fish of all sizes are harvested with equal probability), then the optimal age at maturation decreases strongly as mortality goes up in the feeder fishery, while it increases slightly as the mortality in the spawner fishery becomes more intense (Fig. 3A). This result agrees with the general prediction from life history theory (see also Law and Grey 1989; Ernande et al. 2004; and Jørgensen et al. 2006). This is in contrast to maturation evolution if the fishery is based purely on trawling, where almost all combinations of harvest rates lead to early maturation (Fig. 3B; sigmoid size-selectivity, Lmax = 70 cm). The only exception is when there is no or little fishing in the feeder fishery and just some fishing at the spawning grounds. If the fish were harvested by gillnets, yet another situation occurs (Fig. 3C; bell-shaped size-selectivity, Lmax = 90 cm). Now there are two plateaus for optimal age at maturation depending on the exploitation rate in the feeder fishery: when feeder fishery mortality is light to moderate then late maturation is optimal, whereas there is a sudden drop to early maturation if harvest rates become more intense at the feeding grounds. The spawner fishery has a weak effect of raising age at maturation.
Figure 3.
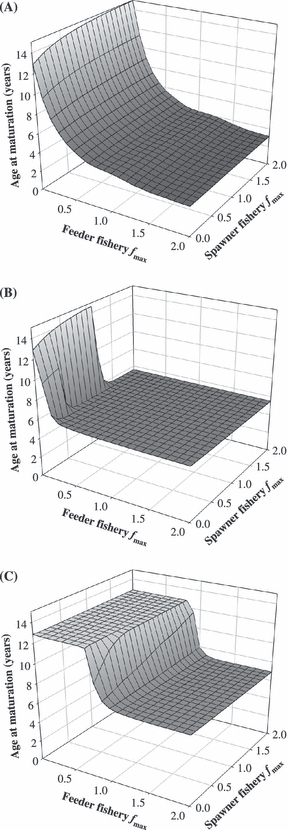
The effects of size-selective fishing gear on optimal age at maturation in the Northeast Arctic cod Gadus morhua. The left-to-right axis is the fishing mortality fmax [year−1] in the feeder fishery at the sizes where selectivity is 1, and the front-to-back axis is the fishing mortality fmax [year−1] at the spawning grounds. (A) Unselective fishing mortality; all sizes have the same probability of being caught (selectivity U is always 1). (B) Sigmoid trawl selectivity, where the size-selectivity of the fishery increases with the fish’ body size (here at maximum from Lmax = 70 cm onwards). (C) Bell-shaped size-selectivity is typical for gillnets (here with maximum selectivity at Lmax = 90 cm).
The evolutionary outcome for age at maturation in a gillnet fishery depends on its mesh size, which corresponds to the fish length for which selectivity is maximal, Lmax (Fig. 4A–C). Common for the different mesh sizes is that age at maturation is most sensitive to the fishing mortality in the feeder fishery. Below a threshold value for the intensity of the feeder fishery, the optimal life history matures late (at ∼12.8 years irrespective of mesh size). This threshold fishing mortality declines from ∼1.4 year−1 to ∼0.8 year−1 to ∼0.4 year−1 as the mesh size goes up from 70 to 90 to 110 cm, respectively. A stock adapted to a fishery with smaller mesh sizes can thus withstand higher fishing rates before selection leads to early maturation ages. On the other hand, if fishing were so intense that it exceeded this threshold and caused evolution toward earlier maturation, then a smaller mesh size would lead to a larger drop in maturation age. This is seen as the level of the rightmost plateau which increases with mesh size in the panels of Fig. 4A–C, where age at maturation is ∼5.7 years when Lmax is 70 cm (Fig. 4A), 7.0 years when Lmax is 90 cm (Fig. 4B), and 8.3 years when Lmax is 110 cm (Fig. 4C).
Figure 4.
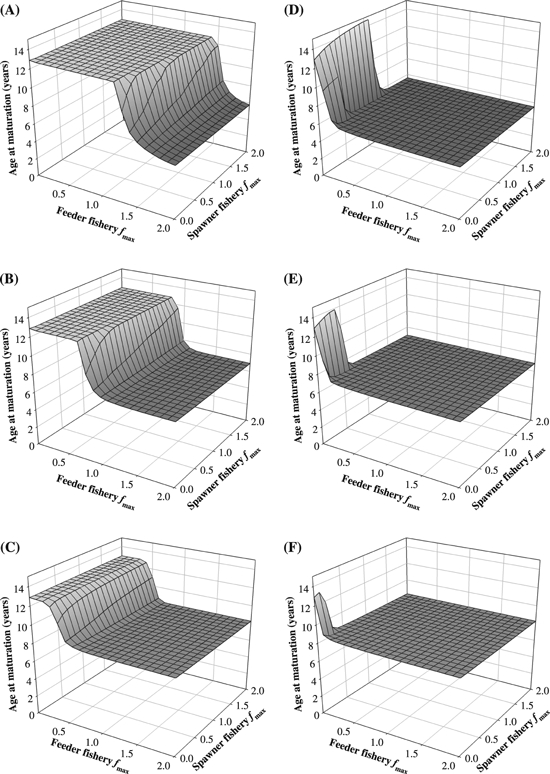
Optimal age at maturation in the Northeast Arctic cod, shown for increasing fishing mortality rates fmax [year−1] in the feeder and spawning fishery. (A–C) with bell-shaped size-selectivity curves as is typical for gillnets, and (D–F) with sigmoid size-selectivity curves for example with trawls. Maximum selectivity Lmax is at: (A,D) 70 cm; (B,E) 90 cm; and (C,F) 110 cm. See legend to Fig. 3 for further explanation of axes.
The outcome is different when fishing is conducted with a gear type that has a sigmoid size-selectivity, for example trawls (Fig. 4D–F). It is optimal for the cod to mature below the sizes at which vulnerability to harvesting is maximal, except when fishing is confined to the spawning grounds and is conducted at low intensities. The age and corresponding sizes are: 5.6 years and 55 cm when Lmax is 70 cm (Fig. 4D), 7.0 years and 72 cm when Lmax is 90 cm (Fig. 4E), and 8.3 years and 88 cm when Lmax is 110 cm (Fig. 4F).
The long-term equilibrium yield depends on the fisheries selectivity first because it determines which fish are caught, and second because the harvesting regime leads to life history evolution that changes size distributions and population dynamics and thereby which fish are available for the fishery. Whatever the scenario considered for Beverton–Holt density-dependent recruitment (Figs 5 and S2), maximum yield obtained with sigmoid size-selectivity is higher than with bell-shaped size-selectivity. However, high levels of yield for trawl selectivity are limited to a sharp peak around a small range of harvest rates in the spawner fishery only. Harvesting in the feeder fishery or at higher intensities in the spawner fishery leads to smaller yield than with gillnet selectivity.
Figure 5.
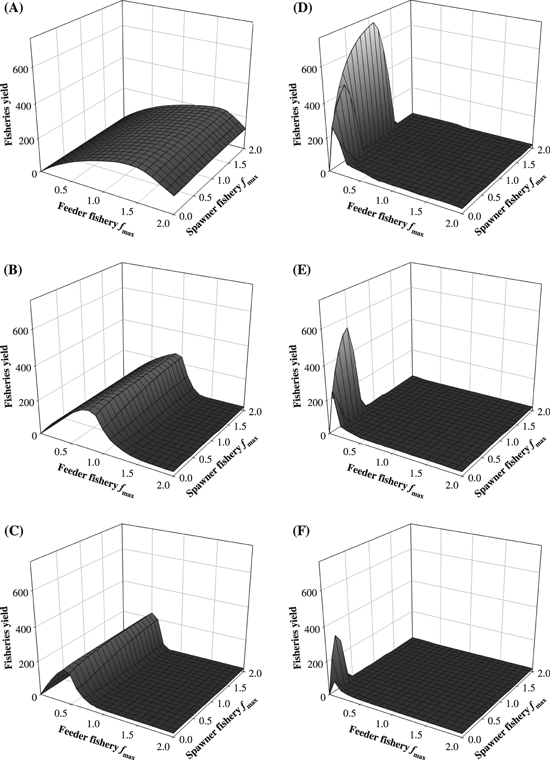
Long-term equilibrium yield, with the assumption that the Beverton–Holt total egg production-recruitment curve is rescaled so that the equilibrium population always produces the same number of recruits. The recruitment curve has thus increased in steepness as harvest becomes stronger, as in Fig. S1b). Bell-shaped size-selectivity: (A) Lmax = 70 cm; (B) Lmax = 90 cm; (C) Lmax = 110 cm. Sigmoid size-selectivity: (D) Lmax = 70 cm; (E) Lmax = 90 cm; (F) Lmax = 110 cm. Further legend is given in Fig. 3. Corresponding figures for yield but with the assumption of a constant Beverton-Holt recruitment curve are shown in Fig. S2.
In one of our scenarios for yield, we rescaled the Beverton–Holt recruitment curve for each optimal life history strategy so that the equilibrium population always produced the same number of recruits (see Fig. S1b in the online appendix). Under these conditions, the stock did not go extinct even at the highest exploitation rates (Fig. 5). In this case, yield when fishing is done by gillnets appears insensitive to the harvest rate in the spawner fishery and shows a dome-shaped relationship with harvest rate in the feeder fishery, peaking at fmax≍ 1.5 year−1 when Lmax is 70 cm, fmax≍ 0.8 year−1 90 cm, and fmax≍ 0.4 year−1 when Lmax is 110 cm.
When the Beverton–Holt stock recruitment curve was kept constant, harvest could bring about stock extinction (Fig. S2 in the online appendix). This happens with bell-shaped selectivity when the feeder fishery is intense (fmax > 1.0 year−1; Fig. S2a,b) and with sigmoid size-selectivity even when fishing is kept at low levels (fmax > 0.2 year−1 in the feeder fishery and fmax > 0.5 year−1 in the spawner fishery; Fig. S2d,e). When mesh size is large (Lmax = 110 cm; Fig. S2c,f), fishing does not lead to extinction as the stock is able to evolve life histories that mature and reproduce sufficiently at sizes smaller than those vulnerable to fishing, regardless of whether size-selectivity is bell-shaped or sigmoid. Otherwise, the surfaces that depict yield (Fig. S2) have the same qualitative characteristics as with a rescaled Beverton–Holt curve (Fig. 5). The main quantitative differences are that for bell-shaped size-selectivity, the maximum yield is obtained at lower fishing intensities, and for sigmoid size-selectivity, the peak of high levels of yield extends over a narrower range of harvest rates in the spawner fishery.
Finally, we ran the model with mixed gear types to better reflect how the fishery really operates nowadays. We used bell-shaped size-selectivity curves in Lofoten, as fishing for the spawning fish is done mostly with longlines and gillnets that both have similar size-selectivity (Huse et al. 2000). For the feeder fishery in the Barents Sea, mostly done by trawling, we used sigmoid size-selectivity. Both selectivity curves had a maximum selectivity size of Lmax = 90 cm. The resulting optimal age at maturation is similar to that when only sigmoid selectivity curves (trawling) were used in both fisheries, except for very low harvest rates in the feeder fishery (Fig. S3).
Discussion
In this paper, we used a life history optimization model for fish to investigate how the size-selectivity of fishing gear may affect fishing-induced evolution. We focused on two outcomes. First, we assessed the degree of expected life history evolution in a given harvest regime, quantified as the mean age at maturation emerging from the optimal state-dependent energy allocation pattern that would result from long-term evolution as dictated by selective pressures. Since we used state-dependent optimization, the energy allocation strategy that maximizes lifetime reproductive value corresponds to the evolutionarily stable strategies in the environment set by fishing, and the methodology includes phenotypic plasticity. Second, we quantified the effects of fishing-induced evolution on the long-term equilibrium yield. The harvesting strategy that maximizes long-term equilibrium yield after life-history evolution has been called the evolutionarily stable optimal harvesting strategy (ESOHS, Law and Grey 1989). Instead of focusing only on the single harvesting regime that optimizes yield, we show results for varying fishing mortalities in both the feeder and the spawner fishery to paint decision landscapes for how a harvesting strategy would affect long-term outcomes.
Maturation evolution under bell-shaped versus sigmoid size-selectivity
Our results show that whether fishing gear has bell-shaped or sigmoid size-selectivity has surprisingly strong effects on the qualitative outcomes both in terms of expected evolution of life history traits and in terms of the resulting fisheries yield. The ages and sizes at maturation that are optimal at intense fishing rates with gillnets are evolutionarily favored at even low harvest rates when trawling. It is also interesting how the gradual response in maturation age seen under random harvest disappears when fishing is size-selective, leading to a few life history outcomes that cover wide regions of parameter space and with sharp transitions between them. This can be explained by keeping in mind that fitness is defined as the expected lifetime reproductive success, which depends on the fecundity at age and the survival probability until that age.
With sigmoid size-selectivity when trawling, all fish above a certain size suffer reduced survival, which quickly erodes the advantages of maturing large with high fecundity. Even at low fishing intensities, fish that mature at sizes below the vulnerable ones have high survival until maturation, and will also survive better for the consecutive spawning seasons if they do not grow into the harvestable size classes. There is a size-refuge for small fish, which quickly becomes the best option even at low harvest intensities.
In the case of bell-shaped size-selectivity, such as with gillnets, the situation is more complex because there are size refugia for both small and large fish, while fish of intermediate size are harvested. Under intense fishing, few fish would survive as they grow through the harvestable size slot. Because the larger size-refuge is then practically unreachable due to the high fishing pressure, the optimal solution is to mature at sizes below those vulnerable, i.e. in the smaller size-refuge, as for trawling. If fishing is less intense, however, it can still pay to grow large and mature in the large size-refuge, although there is some probability of dying on the way. By maturing large, fecundity will increase but survival until maturation will be reduced. Depending on which effect is strongest, the expected lifetime reproductive success can be higher if maturation takes place at sizes either above or below the harvestable size slot. This explains the two plateaus for late and early maturation seen in optimal strategies with gillnet harvesting. Also, the transition between late and early maturation is so sudden in terms of increasing harvest rates because the optimal strategy is either to mature before or after the harvestable slot, but not in the middle of it. Hutchings (2009) suggests a new reference point, Fevol, which in his definition is the fishing rate at which early-maturing life history strategies have higher fitness than late-maturing strategies. An alternative interpretation of Fevol could be the fishing rate where the sharp transition between early- and late-maturing optimal life history strategies was observed in our results.
Ecological advantages of bell-shaped selectivity
Bell-shaped size-selectivity curves retain some of the older and larger fish in the population. The fraction retained and its size composition depends on harvest rate and the exact shape and width of the size-selectivity curve. There are, as Law (2007) pointed out, both good ecological and good evolutionary reasons to prefer bell-shaped over sigmoid size-selectivity curves (see also Berkeley et al. 2004b; Birkeland and Dayton 2005). As an example of a beneficial ecological effect, a diverse age-structure has been shown to lead to enhanced recruitment in Icelandic cod (Marteinsdottir and Thorarinsson 1998). In our model, the main ecological advantages of larger fish are that they have higher fecundity because of sheer size, and they also have more cost-effective migration to and from the spawning sites. We did not specifically implement parental effects, which have the potential to add further advantages to a diverse stock structure. One example of such an effect is the increased viability of larvae spawned by large or old mothers in rockfish (Berkeley et al. 2004a), although there is little evidence for maternal effects in cod (Busch et al. 2009). Similar correlations between parent size and offspring traits were found also in the artificial harvesting experiment reported in Walsh et al. (2006).
Another interesting feature of a broad age- or size-distribution in the population is that it buffers the population dynamical effects of environmental factors such as climate (Brander 2008). Ottersen et al. (2006) studied recruitment as a proxy for population dynamics of the Northeast Arctic cod, together with regional climate indices such as the North Atlantic Oscillation and sea temperature through the Kola transect in the Barents Sea. Their main finding was that the correlation between recruitment and climate grew stronger over time, in parallel with the truncation of the population's age- and size-structure. As the fish matured earlier, they became more tightly tied to climate. Along similar lines, Hsieh et al. (2008) reported that the geographical ranges of fished species fluctuated more with climate than unfished species, and they related this tightened coupling to the truncated population structure caused by fishing. For the Northeast Arctic cod, one possible mechanism can be the long spawning migration, which is relatively cheaper in energetic terms for larger fish. With a similar model as here but allowing migration distance to vary, Jørgensen et al. (2008) showed that because a population adapted to fishing will contain more early-maturing and smaller fish, the optimal migration distances are shorter, and they will spawn along a reduced geographical range compared to the pristine pre-fishing state. Because fish then would sample the environment over a narrower geographical range, one could expect that spawning areas that are particularly good in a given year may not be visited and that the population as a whole does not buffer climatic variation to the same degree as before. Any such effect where large or old individuals have beneficial consequences for population dynamics and recruitment would be preserved better with a bell-shaped size-selectivity curve whereby some large fish are retained, compared to sigmoid size-selectivity curves where also the big ones are fished out.
Fishing at the spawning grounds
The standard prediction from life history models without size-selective harvesting mortality is that mortality at the spawning grounds, which removes only mature fish, will increase age at maturation. This can be seen in the scenario with un-selective harvest (Fig. 3A) and has been shown also in other studies (Law and Grey 1989; Ernande et al. 2004; Gårdmark and Dieckmann 2006; Jørgensen et al. 2006). The underlying life history logic is that because fecundity increases with size, it becomes profitable to have large gonads when one accepts the extra mortality associated with spawning. Conversely, if mortality at the spawning grounds is lower than elsewhere, it would select for earlier maturation as spawning then offers a refuge from harvesting.
With size-selective harvesting, this result holds also under the bell-shaped size-selectivity typical for a gillnet fishery: although the effect is weaker, optimal maturation age goes up as the spawner fishery is increased (most easily seen in the sharp transition phase between the two plateaus in Fig. 4A–C). In contrast, under sigmoid size-selectivity, optimal age at maturation increases with increasing harvest mortality at the spawning grounds only up to a certain point, from which it suddenly drops to early maturation. The sigmoid size-selectivity creates a size-refuge for small fish also at the spawning grounds, and this refuge becomes more important as harvest rates go up. With little selection for early maturation in the feeder fishery and low harvest rates at the spawning grounds, the benefit of maturing large still outweighs early maturation (left corner of panels in Fig. 4D–F). However, increasing harvest rates in both the feeder and spawner fishery removes this size-advantage, consequently leading to early maturation below the harvestable size.
Effects of size-selectivity on evolutionarily stable yield
The model predicts that trawling can give the highest yield if it selects also small fish. High yield results from the same harvest rates that cause late maturation, but even a minor increase in harvest intensity causes early maturation to become optimal (Figs 4C–F and 5, Fig. S2). Thus, high evolutionarily stable yield is only achieved if three conditions are met: harvest rates have to be very low, mainly confined to the spawning grounds, and strictly controlled. Each of these three conditions are hard to meet with current fisheries management, meaning that the optimal solution is likely impossible in practice. If excess harvesting happens intermittently, it may be sufficient to cause maturation evolution and long-term loss of yield.
Gillnets produce more stable outcomes with respect to variation in fishing rates so that larger regions of parameter space give good yield (Fig. 5; see also Fig. S2). From a management perspective this means that a gillnet fishery is more robust to errors in the realized fishing mortality. Such errors can stem from uncertainty in stock assessments, politicized quota setting, or poor enforcement of management regulations. The stock's sensitivity to evolution may also vary with factors that were not included in the model. The major problem with a fishery based on gear types with bell-shaped size-selectivity is that if harvest rates become too high in the feeder fishery, then early maturation can become optimal, resulting also in a drop in the evolutionarily stable yield.
Comparing stock–recruitment curves
The two alternatives we investigated for the stock–recruitment relationship represent different scenarios for how the ecosystem will respond to changes in population abundance and demography. It is probably unlikely that the stock–recruitment curve stays exactly the same as the stock undergoes large changes (first scenario). It is probably also unlikely that the ecosystem fully compensates so that the curve becomes steeper as egg production diminishes, but with the same carrying capacity (second scenario). The ecosystem response will likely lie somewhere in between our two scenarios, so that they bracket the potential outcomes (although at least in theory, the stock–recruitment curve could also become less steep as adaptations take place). The major difference between the two scenarios is that a constant stock–recruitment relationship causes stock extinction at intense harvest levels.
Beyond stock extinction, the qualitative differences between the stock–recruitment mechanisms are minor and discrepancies are mostly quantitative. Studying yield consequences of fishing-induced changes is extremely difficult because it relies on critical assumptions about density dependence and how the rest of the ecosystem will react to harvest-induced changes in stock structure and life history traits (e.g. Gårdmark et al. 2003; Abrams and Matsuda 2005). For instance, we omitted other types of density dependence than the Beverton–Holt recruit survival, and many are known to act in the wild (e.g. on growth, Lorenzen and Enberg 2002; see also Enberg et al., 2009, and the role of size-dependent growth in Arlinghaus et al., 2009). The predictions we report for yield must therefore be interpreted with caution. Fully frequency-dependent models are better suited to include several sources of density dependence, and we look forward to studies that address effects on yield in further detail.
Robust management
If our evolutionarily concerned fisheries manager could manage gear size-selectivity, she would probably choose bell-shaped size-selectivity. One reason is that sigmoid size-selectivity easily leads to stock extinction or extremely low yield, depending on the stock–recruitment scenario, as soon as harvest rates increase to moderate levels. In contrast, population dynamics is much more robust under gillnet selectivity, which results in favorable outcomes over a larger area of parameter space.
However, when implementing a gillnet fishery, our manager is faced with a trade-off with respect to the choice of mesh size. For small mesh size, the population is evolutionarily relatively insensitive to harvest rates, and only with extreme fishing rates at the feeding grounds will it become optimal for cod to mature early. Yield is also high across many combinations of harvest rates on the feeding and spawning grounds. However, small mesh size also means that if harvest rates are so high as to lead to evolution, then the resulting life history strategy matures at a very early age. When mesh size is large, the late maturation plateau shrinks, evolution toward the early maturation plateau may take place at lower fishing intensities, but the early maturation plateau corresponds to a later maturation age. By choosing larger mesh sizes, the manager thus needs to exercise more caution in controlling the applied harvest rates, but she risks less of a reduction in maturation age if she fails. The optimal level of this trade-off depends on how important it is for management to prevent evolution and to what degree the fisheries management institution can influence quotas and control the fishing fleet.
Comparison with current fishing regime
Currently, Northeast Arctic cod is mostly fished by trawlers in the Barents Sea, represented by the feeder fishery in our model, with a harvest rate around 0.5 year−1. The potential to market fresh fish year round concentrates effort on the feeder fishery rather than the spawner fishery, where fish are only present for a few months. In contrast, the spawner fishery consists mostly of long-liners and gill-netters. Our model predicts that with these gear types, any harvest rate above 0.2 year−1 in the feeder fishery has the potential to cause early maturing fish, regardless of the mortality in the spawner fishery. Maturation ages have indeed declined in the Northeast Arctic cod (Jørgensen 1990), and the observed temporal trend can be related to changes in the probabilistic maturation reaction norm (Heino et al. 2002), which suggests that its cause is evolutionary. The observed changes in the stock are consistent with the predictions from our model, although we cannot assess evolutionary rates because optimization methods find evolutionary endpoints but do not answer whether these endpoints are attainable or at what rates they may be attained. On this issue, it is worth noting that the rate of change of maturation age in Northeast Arctic cod is comparable to observed rates in many other stocks thought to undergo fishing-induced evolution (quantified in the online appendix of Jørgensen et al. 2007). It would be interesting to look into what gear types have been used in these fisheries, how the use of gear and its selectivity have changed over time, what evolutionary outcomes one could expect, and finally compare those predicted outcomes to the observed life history changes for these stocks.
Acknowledgments
We thank participants of the EU-funded research training network FishACE for stimulating discussions, and two anonymous reviewers for comments. CJ thanks the Research Council of Norway for funding. BE acknowledges support by the European Marie Curie Research Training Network FishACE (Fisheries-induced Adaptive Changes in Exploited Stocks), funded through the European Community's Sixth Framework Programme (Contract MRTN-CT-2004-005578).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Rescaling of Beverton–Holt recruitment functions used in yield calculations.
Figure S2. Yield with constant recruitment function.
Figure S3. Age at maturation for optimal life histories with combination of trawls and gillnets.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature cited
- Abrams PA. Modelling the adaptive dynamics of traits involved in inter- and intraspecific interactions: an assessment of three methods. Ecology Letters. 2001;4:166–175. [Google Scholar]
- Abrams PA, Matsuda H. The effect of adaptive change in the prey on the dynamics of an exploited predator population. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:758–766. [Google Scholar]
- Alexander RM. Principles of Animal Locomotion. Princeton and Oxford: Princeton University Press; 2003. [Google Scholar]
- Arlinghaus R, Matsumura S, Dieckmann U. Quantifying selection pressures caused by recreational fishing. Evolutionary Applications. 2009;2:335–355. doi: 10.1111/j.1752-4571.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley SA, Chapman C, Sogard SM. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology. 2004a;85:1258–1264. [Google Scholar]
- Berkeley SA, Hixon MA, Larson RJ, Love MS. Fisheries Sustainability via protection of age structure and spatial distribution of fish populations. Fisheries. 2004b;29:23–32. [Google Scholar]
- Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends in Ecology & Evolution. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2919–2922. doi: 10.1073/pnas.0708159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander K. Tackling the old familiar problems of pollution, habitat alteration and overfishing will help with adapting to climate change. Marine Pollution Bulletin. 2008;56:1957–1958. doi: 10.1016/j.marpolbul.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Hobday AJ, Ziegler PE, Welsford DC. Darwinian fisheries science needs to consider realistic fishing pressures over evolutionary time scales. Marine Ecology Progress Series. 2008;369:257–266. [Google Scholar]
- Busch KET, Folkvord A, Otterå H, Hutchinson WF, Svåsand T. Effects of female spawning experience and larval size on feeding and growth of cod larvae Gadus morhua L. reared in mesocosms. Marine Biology Research. 2009;5:286–296. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CW. The Worldwide Crisis in Fisheries. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Clark CW, Mangel M. Dynamic State Variable Models in Ecology. New York: Oxford University Press; 2000. [Google Scholar]
- Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. [DOI] [PubMed] [Google Scholar]
- Conover DO, Baumann H. The role of experiments in understanding fishery-induced evolution. Evolutionary Applications. 2009;2:276–290. doi: 10.1111/j.1752-4571.2009.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Costello C, Gaines SD, Lynham J. Can catch shares prevent fisheries collapse? Science. 2008;321:1678–1681. doi: 10.1126/science.1159478. [DOI] [PubMed] [Google Scholar]
- Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace other agents of trait change in the wild. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:952–954. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann U, Law R. The dynamical theory of coevolution: a derivation from stochastic ecological processes. Journal of Mathematical Biology. 1996;34:579–612. doi: 10.1007/BF02409751. [DOI] [PubMed] [Google Scholar]
- Edley MT, Law R. Evolution of life histories and yields in experimental populations of Daphnia magna. Biological Journal of the Linnean Society. 1988;34:309–326. [Google Scholar]
- Enberg K, Jørgensen C, Dunlop E, Heino M, Dieckmann U. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evolutionary Applications. 2009;2:394–414. doi: 10.1111/j.1752-4571.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favro LD, Kuo PK, MacDonald JF. Population-genetic study of the effect of selective fishing on the growth rate of trout. Journal of the Fisheries Research Board of Canada. 1979;36:552–531. [Google Scholar]
- Fenberg PB, Roy K. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Molecular Ecology. 2008;17:209–220. doi: 10.1111/j.1365-294X.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- Gårdmark A, Dieckmann U. Disparate maturation adaptations to size-dependent mortality. Proceedings of the Royal Society B-Biological Sciences. 2006;273:2185–2192. doi: 10.1098/rspb.2006.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gårdmark A, Dieckmann U, Lundberg P. Life-history evolution in harvested populations: the role of natural predation. Evolutionary Ecology Research. 2003;5:239–257. [Google Scholar]
- Gjedrem T. Genetic variation in quantitative traits and selective breeding in fish and shellfish. Aquaculture. 1983;33:51–72. [Google Scholar]
- Hamley JM. Review of gillnet selectivity. Journal of the Fisheries Research Board of Canada. 1975;3:1943–1969. [Google Scholar]
- Hammerstein P. Darwinian adaptation, population genetics and the streetcar theory of evolution. Journal of Mathematical Biology. 1996;34:511–532. doi: 10.1007/BF02409748. [DOI] [PubMed] [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Reaction norm analysis of fisheries-induced adaptive change and the case of the Northeast Arctic cod. ICES CM. 2002;2002 Y:14. [Google Scholar]
- Hilborn R, Minte-Vera CV. Fisheries-induced changes in growth rates in marine fisheries: are they significant? Bulletin of Marine Science. 2008;83:95–105. [Google Scholar]
- Houston AI, McNamara JM. Models of Adaptive Behaviour: An Approach Based on State. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Huse I, Løkkeborg S, Soldal AV. Relative selectivity in trawl, longline and gillnet fisheries for cod and haddock. ICES Journal of Marine Science. 2000;57:1271–1282. [Google Scholar]
- Hsieh C-h, Reiss CS, Hewitt RP, Sugihara G. Spatial analysis shows that fishing enhances the climatic sensitivity of marine fishes. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:947–961. [Google Scholar]
- Hutchings JA. Avoidance of fisheries-induced evolution: management implications for catch selectivity and limit reference points. Evolutionary Applications. 2009;2:324–334. doi: 10.1111/j.1752-4571.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Jobling M. A review of the physiological and nutritional energetics of cod, Gadus morhua L, with particular reference to growth under farmed conditions. Aquaculture. 1988;70:1–19. [Google Scholar]
- Jørgensen T. Long-term changes in age at sexual maturity of Northeast Arctic cod (Gadus morhua L.) Journal Du Conseil. 1990;46:235–248. [Google Scholar]
- Jørgensen C, Fiksen Ø. State-dependent energy allocation in cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:186–199. [Google Scholar]
- Jørgensen C, Ernande B, Fiksen Ø, Dieckmann U. The logic of skipped spawning in fish. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:200–211. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing the world's evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Dunlop ES, Opdal AF, Fiksen Ø. The evolution of spawning migrations: the role of individual state, population structure, and fishing-induced changes. Ecology. 2008;89:3436–3448. doi: 10.1890/07-1469.1. [DOI] [PubMed] [Google Scholar]
- Kjesbu OS, Solemdal P, Bratland P, Fonn M. Variation in annual egg production in individual captive Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:610–620. [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology & Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lande R. Natural selection and random genetic drift in phenotypic evolution. Evolution. 1976;30:314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology-Progress Series. 2007;335:271–277. [Google Scholar]
- Law R, Grey DR. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology. 1989;3:343–359. [Google Scholar]
- Law R, Rowell CA. Cohort-structured populations, selection responses, and exploitation of the North Sea cod. In: Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Lecture Notes in Biomathematics. Berlin, Germany: Springer-Verlag; 1993. pp. 155–173. [Google Scholar]
- Law W, Salick J. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae) Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10218–10220. doi: 10.1073/pnas.0502931102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen K, Enberg K. Density-dependent growth as a key mechanism in the regulation of fish populations: evidence from among-population comparisons. Proceedings of the Royal Society B-Biological Sciences. 2002;269:49–54. doi: 10.1098/rspb.2001.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteinsdottir G, Thorarinsson K. Improving the stock-recruitment relationship in Icelandic cod (Gadus morhua) by including age diversity of spawners. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1372–1377. [Google Scholar]
- Mertz G, Myers RA. A simplified formulation for fish production. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:478–484. [Google Scholar]
- Millar RB, Fryer RJ. Estimating the size-selection curves of towed gears, traps, nets and hooks. Reviews in Fish Biology and Fisheries. 1999;9:89–116. [Google Scholar]
- Miller RB. Have the genetic patterns of fishing been altered by introductions or by selective fishing? Journal of the Fisheries Research Board of Canada. 1957;14:797–806. [Google Scholar]
- Mylius SD, Diekmann O. On evolutionarily stable life histories, optimization and the need to be specific about density dependence. Oikos. 1995;74:218–224. [Google Scholar]
- Ottersen G, Hjermann DØ, Stenseth NC. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fisheries Oceanography. 2006;15:230–243. [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112–113:183–198. [PubMed] [Google Scholar]
- Ricker WE. Changes in the average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Sharpe D, Hendry A. Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evolutionary Applications. 2009;2:260–275. doi: 10.1111/j.1752-4571.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Strand E, Huse G, Giske J. Artificial evolution of life history and behavior. American Naturalist. 2002;159:624–644. doi: 10.1086/339997. [DOI] [PubMed] [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Walters C, Korman J. Linking recruitment to trophic factors: revisiting the Beverton-Holt recruitment model from a life history and multispecies perspective. Reviews in Fish Biology and Fisheries. 1999;9:187–202. [Google Scholar]
- Ware DM. Bioenergetics of pelagic fish: theoretical change in swimming speed and ration with body size. Journal of the Fisheries Research Board of Canada. 1978;35:220–228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


