Abstract
Eco-genetic individual-based models involve tracking the ecological dynamics of simulated individual organisms that are in part characterized by heritable parameters. We developed an eco-genetic individual-based model to explore ecological and evolutionary interactions of fish growth and maturation schedules. Our model is flexible and allows for exploration of the effects of heritable growth rates (based on von Bertalanffy and biphasic growth patterns), heritable maturation schedules (based on maturation reaction norm concepts), or both on individual- and population-level traits. In baseline simulations with rather simple ecological trade-offs and over a relatively short time period (<200 simulation years), simulated male and female fish evolve differential genetic growth and maturation. Further, resulting patterns of genetically determined growth and maturation are influenced by mortality rate and density-dependent processes, and maturation and growth parameters interact to mediate the evolution of one another. Subsequent to baseline simulations, we conducted experimental simulations to mimic fisheries harvest with two size-limits (targeting large or small fish), an array of fishing mortality rates, and assuming a deterministic or stochastic environment. Our results suggest that fishing with either size-limit may induce considerable changes in life-history trait expression (maturation schedules and growth rates), recruitment, and population abundance and structure. However, targeting large fish would cause more adverse genetic effects and may lead to a population less resilient to environmental stochasticity.
Keywords: fishing induced evolution, maturation reaction norm, walleye, whitefish
Introduction
There is increasing evidence that evolution occurs at a time scale relevant to population dynamics and natural resource management (e.g., Hendry et al. 2000; Conover and Munch 2002). Further, ecological processes (e.g., intra-specific competition, interacting species dynamics) can influence genetic selection and phenotypic expression within a population. As individual phenotypic expression (e.g., growth, age or size at maturation) is a function of both individual genetics and environmental (both biotic and abiotic) interactions, it can be quite difficult to predict and discriminate between ecological versus genetic changes in life-history traits. Most life-history traits are plastic and spatio-temporal variation in these traits generally does not arise due to genetic selection alone.
Linkages between ecology and evolution are particularly relevant for fisheries management. Fish are generally selectively harvested based upon individual traits such as size, behavior, and location. Such selective harvesting can alter the genetic composition of the population and affect mean life-history traits, including growth rates and maturation schedules (Stokes et al. 1993; Heino 1998; Law 2000; Conover and Munch 2002; Heino and Godø 2002). In turn, as these traits have strong influence on a fish stock's harvest potential, effective management of fisheries for sustainable yields and genetic diversity requires consideration of both short- and long-term impacts (including both ecological and genetic effects) on fish growth and maturation schedules.
Previous research suggests that fishing may cause changes to a suite of fish population-level attributes (e.g., survival and recruitment) as well as various interrelated phenotypic expressions at the individual level (e.g., growth, and size and age at maturation; Stokes et al. 1993; Law 2000; Walsh et al. 2006). However, the mechanisms of these changes are often unclear. To illustrate these complex effects of fishing, consider that intensively harvested populations are often comprised of a high proportion of small and/or young fish (Berkeley et al. 2004), and that size-selective fishing harvest will lead to changes in mean size and age of both mature and immature fish by simply truncating the population. Moreover, fishing reduces population size, thereby relaxing intra-population competition of the harvested population. As a result, individuals may grow more rapidly and mature at younger ages or larger sizes (Trippel 1995). Simultaneously, selective harvest of larger, older individuals may over time select for genetically determined slower growth rates and maturation at younger ages and smaller sizes (Edeline et al. 2007; Jørgensen et al. 2007). Regardless of the mechanisms of action (genetic selection or ecological processes), changes in growth rates or maturation expression will undoubtedly feedback to affect each other, due to trade-offs between growth and reproduction (Hutchings 2005).
Clearly, fishing has a variety of effects on population- and individual-level expression, however, the relative influences of underlying mechanisms, e.g., ecological (plastic) versus evolutionary (genetic) pathways, are less clear. Many empirical studies support the notion that fishing may cause evolutionary changes in harvested populations (summarized in Law 2000; Jørgensen et al. 2007; Hard et al. 2008). Conover and Munch (2002) and Walsh et al. (2006) showed that experimentally harvesting large Atlantic silverside Menidia menidia in a laboratory setting could lead to genetic and phenotypic changes in several fitness-related traits (growth rates, egg size, feeding rates, etc.). However, these studies were criticized as overestimating the evolutionary effects of harvesting, as the authors applied unrealistically high harvest rates in these laboratory settings (Hilborn 2006, 2007; Brown et al. 2008). Using the parameters from Conover and Munch (2002) with more realistic fishing intensity, Brown et al. (2008) demonstrated that harvest-induced evolution suggested by Conover and Munch (2002) could occur in wild fish but at a much slower rate. In addition, fishing-induced evolutionary effects are probably impacted by simultaneous ecological effects induced by fishing and other environmental factors (Rijnsdorp 1993; Kuparinen and Merilä 2007), and may also be affected by seemingly stochastic environmental processes (Blanchard et al. 2005). Understanding the mechanisms through which fishing affects population dynamics in more complex, highly variable systems should aid in designing harvest practices with an eye toward long-term sustainability.
Quantitative population ecology models which take into account genetic inheritance represent a potentially useful tool to consider the evolutionary impacts of size-selective fisheries harvest (e.g., Martínez-Garmendia 1998; Jager 2001; Dunlop et al. 2007, 2009a). In particular, models which depict life-history traits as phenotypically plastic and density dependent and which represent the inherent trade-offs between current and future growth and reproduction (i.e., eco-genetic models, Dunlop et al. 2009a) should prove particularly insightful for evaluating the relative importance of genetic and ecological effects on fish life-history traits and stock productivity. In addition to constructing, parameterizing and applying such models for specific fish populations, we believe it is beneficial to evaluate the consequences of certain model approaches and assumptions on qualitative and quantitative model predictions.
Herein, we present an eco-genetic individual-based model to consider the evolution of genotypes describing growth and maturation schedules and consequences for fish population dynamics and individual phenotypic expression. While the model is not species specific, it is loosely based on two, similar-sized North American freshwater fish species, lake whitefish (Coregonus clupeaformis) and walleye (Sander vitreus). These two species can be classified as periodic (e.g., fish with long life span and high fecundity; Winemiller and Rose 1992) and display high across-system variation in growth and maturation schedules. We use our model to explore phenotypic expression and evolution. We consider consequences of growth and maturation schedule evolving separately versus concurrently and the effects of stochastic and density-dependent factors. Finally, we use the model to explore fishing-induced genetic and plastic effects on growth and maturation traits, recruitment, and harvest sustainability. We hypothesized that harvest via different size-limits and fishing intensities would induce differential plastic and genetic effects on growth and maturation traits and that environmental stochasticity might dilute both plastic and genetic effects of fishing. To test these hypotheses, we designed a series of simulations to evaluate evolving and observed life histories, recruitment traits and population abundance with respect to size-selective fishing mortality (i.e., targeting large or small fish and under different fishing mortality rates).
Model overview
Our model is multi-generational and tracks the simulated population at annual time steps. Although the model describes individual-level fish dynamics, the model actually tracks ‘super-individuals’ with each super-individual potentially representing a multitude of individual fish (e.g., Scheffer et al. 1995). This approach is useful as it does not necessitate the elimination of simulated fish whenever there is a mortality event (mortality simply causes a reduction in the number of individuals represented by a super-individual), thereby allowing for efficient simulation of large populations. In addition to the number of individual fish (NI) represented by a super-individual, other individual-level (i.e., I-state) variables include: sex (binary), age (years), maturation status (binary), total length (mm), somatic weight (g), gonadal weight (g) and heritable growth and maturation parameters. Each year individual fish grow, potentially mature, and experience some mortality. Between annual time steps (y and y + 1), mature individuals reproduce. The resulting new individuals (which are characterized by heritable growth and maturation parameters obtained from reproductively successful adults) enter the population as age 1 fish at the beginning of annual time step, y + 2. Our inheritance design for growth and maturation parameters is similar to previous applications (e.g., Strand et al. 2002) and based on Mendelian genetics. For each maturation and growth parameter, a new individual receives one random heritable value from both its mother and father. Parameter expression is based on co-dominance (i.e., each parameter is expressed as the mean of paired values). Our inheritance design facilitates modeling genetics without having to assume that traits are normally distributed. Also, while we did not define heritability of traits in the model, maturation and growth traits are subject to both genetic- and density-dependent effects, thereby heritability <1. Moreover, while some studies suggest that growth and maturation traits may co-evolve (e.g., Hard et al. 2008), empirical measures of genetic structure or correlation for these traits are lacking. Thus, we assumed no dependence among growth and maturation parameters or between parameters of each trait. Consequently, expression of evolving traits in our model might be more flexible than a model that imposed covariance among genetic parameters. We specified a maximum age of 50 years, but none of our results presented here depended on the maximum age.
Maturation
Individual fish can become mature at age 2 and older, and once an individual becomes mature, it will remain mature throughout its life. The maturation process is modeled via a deterministic maturation reaction norm (MRN) approach (i.e., a fish would mature if its length is above the threshold for maturation at a given age; Stearns and Koella 1986; Heino et al. 2002). This is different from previous eco-genetic models (Dunlop et al. 2009a) that involved modeling probabilistic maturation reaction norms (PMRNs; Dieckmann and Heino 2007), i.e., maturation is a probabilistic process based on a fish's length and age. Each individual is characterized by 18 pairs of heritable maturation values and means of each pair determine an individual's 18 maturation parameters (M1–M18; M1–M8 and M10–M17 relate to maturation of age 2–9 males and females, respectively; M9 and M18 relate to maturation of age ≥10 males and females, respectively). Each maturation parameter represents the minimum length an immature individual must reach to mature at a given age. For example, assume an immature 4-year-old female's 12th maturation parameter (M12) is 500 mm. If this individual is to mature at the end of the year, she must grow to at least 500 mm. While this discrete representation of MRNs requires tracking a multitude of individual maturation parameters [as opposed to a linear function, e.g., Dunlop et al. (2007), or some other parameter-sparse functions], it does not require us to presuppose the form of MRNs and allows each parameter to only directly influence maturation for a single age.
Growth
The growth model tracks two tissue types (somatic and gonadal) of equal energy density, i.e., growth of gonads occurs at a direct cost to growth of soma, and is a biphasic growth model (Quince et al. 2008a,b) building on the von Bertalanffy growth model. Spawning occurs at the end of each year, and consequently at the beginning of a year, individuals’ gonads weigh 0 g. Individual growth rates are dependent on population abundance, stochastic processes, and heritable growth parameters.
Each individual is characterized by 12 pairs of heritable growth values and means of each pair determine an individual's 12 growth parameters (X1–X12; six parameters relate to growth of males, X1–X6, and females, X7–X12, respectively). These growth parameters can take values between 0.1 and 1, and are initially drawn from a uniform distribution between 0 and 1. The lower bound, 0.1, is necessary to ensure positive growth (i.e., K > 0; see below). Growth parameters, in turn, determine an individual's initial somatic growth rate (K) and maximum length (Lmax). We assume a negative relationship between K and Lmax (e.g., Beauchamp et al. 2004) and these variables can take a defined range of values, i.e., for an individual male:
| (1) |
| (2) |
That is, increases in X1 and X2 (e.g., induced by selection) would correspond to a relatively large K and small Lmax for an individual male. We chose coefficients in eqns (1) and (2) such that K and Lmax would take values within the observed range for walleye and lake whitefish (e.g., Beauchamp et al. 2004).
During each annual time step, an individual's potential somatic and gonadal growth is a function of its current length and genetically determined growth parameters (K and Lmax). However, an individual's realized growth can be affected by stochastic and density-dependent processes which can cause realized growth to either exceed or fall below potential growth. Further, whereas mature or maturing individuals can allocate energy to both somatic and gonadal tissue, immature individuals can only allocate energy to somatic tissue. To facilitate these different growth possibilities, during each time step we calculate an individual's potential growth and then use this as a basis for calculating realized growth of both somatic and gonadal tissue.
We assume a base allometric relationship between fish length (L, mm) and somatic weight (WS, g):
| (3) |
Further, we assume the expected gonadal weight (WG, g) to be a proportion (P) of expected somatic weight:
| (4) |
with different parameter values for males and females. We select parameters for eqn (4) (Table 1) to allow for greater gonad weight for females as compared to males and increased gonadal investment with length, i.e., patterns observed for a plethora of fish species (e.g., walleye; Moles et al. 2008).
Table 1.
Parameters and coefficients used for baseline and initiation simulations
| Parameters or coefficients | Mean value (range) |
|---|---|
| MRNs (mm) | 400 (150–750) |
| K (year−1) | 0.3 (0.02–0.65)* |
| Lmax (mm) | 622 (400–800)* |
| a (g mm−b) | 0.00003 |
| b | 3.0 |
| c, d, and f (mm−1) for males | 0.03, 4.0, 0.008 |
| c, d, and f (mm−1) for females | 0.15, 20.0, 0.01 |
| Emax (g) | 0.035422 |
| G (g year−1.5) | 0.0005 |
| H (g−1.5) | 150 |
| α | 0.0008 |
| β | 5.5 × 10−12 |
| εR | 1.0 (0, 4.05)† |
| C (deterministic; g) | 2.0 × 1011 |
| C (stochastic; g) | 1.5 × 1011–2.5 × 1011 (5 × 1010, 3.5 × 1011) |
Maturation reaction norms (MRNs) comprise a set of 18 pairs of sex- and age-specific parameters. For simulations with inherited maturation, individual MRN values were randomly drawn from a normal distribution (mean = 400 mm and bounded within [150, 750]). Initial growth rate (K) and maximum attainable length (Lmax) are determined by a set of 12 pairs of sex-specific growth parameters (Xi). For simulations with inherited growth, Xi-values were randomly drawn from [0.1, 1].
Ranges of parameter values were calculated based on Xi = [0.1, 1].
Sampled from a right-skewed, log-normal distribution.
We assume that total (somatic and gonadal) annual potential growth by an individual is a function of individual length, and that maturation status dictates the proportion of growth allocated toward somatic and gonadal tissue. Each year, we calculate a mature individual's total growth potential (GPT, g) as the sum of potential somatic growth (GPS, g) and gonadal growth (GPG, g). A mature individual's length is expected to increase (ΔL) as a function of its length at the beginning of a year (Lt):
| (4) |
Thus, the potential somatic growth for a mature individual is,
| (5) |
and its potential gonadal growth is,
| (7) |
Total realized growth (GRT, g) is then calculated by multiplying an individual's total growth potential (GPT) by a term (ADJ) which encapsulates density dependent and stochastic processes:
| (8) |
where C is mean population carrying capacity (2 × 1011 g), εC is a uniform random number between 0.5 and 1.5, Wi and Ni are the somatic weight and number of individuals represented by super-individual i, respectively, and ψ is a fluctuating variable (a sine function varied from −5 × 1010 to +5 × 1010 g) representing inter-annual growth variation in 20-year cycles. With variation in ADJ, individual growth may be suppressed (ADJ < 1) or compensated (ADJ > 1). To bound potential growth rates, ADJ can take values between 0.25 and 2.5.
Total realized growth GRT is partitioned between somatic and gonadal growth. Allocation to somatic and gonadal growth is determined by (i) current length and weight of fish and (ii) total realized growth. If GRT is sufficient, growth of both soma and gonads occurs, the allometric relationship is maintained (eqn 3) and there is full allocation to gonadal growth (eqn 4). Otherwise, allocation to soma and/or gonads is reduced.
We compare the sum (WS+G, g) of an individual's expected somatic weight (WS, i.e., given its length at the beginning of the year, eqn 3) and gonadal weight (WG, g, i.e., given its length at the beginning of the year, eqn 4) versus its new total weight (WT, g), i.e., the sum of its weight at the beginning of the year and GRT. If growth is sufficient, i.e., WT ≥ WS+G, then an individual would grow in somatic weight [WS(t+1), g], gonadal weight before spawning [WG(′), g], and length [L(t+1)]:
| (9) |
| (10) |
| (11) |
If WT does not exceed WS+G, then somatic weight and gonadal weight must equal some fraction of WS and WG, respectively (see eqns 12–18). We assume that a fish would not reduce its length when receiving insufficient energy, i.e., if WT < WS+G, then
| (12) |
If energy intake is very low (WT ≤ WS − WG), no allocation to gonads takes place:
| (13) |
| (14) |
If energy intake is somewhat greater, i.e., if WS > WT > WS − WG, then gonads grow preferentially,
| (15) |
| (16) |
Finally, if energy intake is moderate, i.e., if WS+G > WT ≥ WS, then gonadal growth is prioritized followed by somatic growth:
| (17) |
| (18) |
Individuals which are immature at the beginning of a year can either remain immature or become mature during the year. Thus, we initially calculate growth of immature individuals identical to growth of mature individuals. If resulting Lt+1 exceeds the relevant MRN parameter, then the individual becomes mature [i.e., WS(t+1) and WG(′) are calculated as above]. Otherwise, the individual remains immature,
| (19) |
| (20) |
| (21) |
Mortality
Mortality is depicted as a random process and includes both size-independent, natural mortality and size-dependent, fishing mortality. Initially, to evaluate model behavior, we only include natural mortality and set Z (instantaneous total mortality) to 0.5 year−1 (i.e., a rate within the range observed for several fish species). Each year, the number of individuals represented by a super-individual is reduced by a random number drawn from a binomial distribution (probability = 1 − e−Z·1 year; number of trials = NI).
Reproduction
If the population contains at least one mature male and female fish, then reproduction occurs between annual time steps (y and y + 1), and the resulting new individuals enter the population as age 1 fish at the beginning of annual time step, y + 2. The number of new individuals (R) entering the population is a function of the population's viable egg production (SEP; i.e., the sum of all female super-individuals’ egg production; EPi) and follows a Ricker-type stock recruitment curve:
| (22) |
where εR is a log-normally distributed random variable with a mean of 1.0. We select Ricker parameters resulting in moderately over-compensatory recruitment across the population-level egg production observed in simulations (Table 1). Each year, R is divided amongst 1000 new super-individuals. If R ≤ 103, then each new super-individual represents one fish. Similarly, if R ≥ 109, then each new super-individual represents 106 fish. The parents for these new super-individuals are drawn from pools of mature female and male fish as a function of individuals’ egg and milt production. The probability that an individual mature female will be the mother for a given new super-individual is simply the proportion of the population's total egg production contributed by the individual (i.e., EPi/SEP), and the probability that an individual mature male will be the father for a given new super-individual is simply the proportion of the population's total milt production contributed by the individual.
A female super-individual's egg production (EPi) is calculated as a function of its WG(′),i, NIi, proportion of viable eggs (Vi), and mean egg weight (Ei; g),
| (23) |
As for several fish species (e.g., cod Gadus morhua; Marteinsdottir and Steinarsson 1998; black rockfish Sebastes melanops; Berkeley et al. 2004), we assume that Ei increases with female age (Ai),
| (24) |
that Ei reaches a maximum at Emax (Table 1) and that Vi increases with Ei (Vi = 1.0 when Ei = Emax),
| (25) |
A male super-individual's milt production (MPi) is calculated in a similar manner, but we assume that for males Vi = 1.0 (i.e., sperm viability is not a function of age), and thus,
| (26) |
New super-individuals enter the model as immature, either male or female (0.5 probability) age 1 fish each representing R/1000 individuals. Initial gonadal weight is by definition 0 g (i.e., immature fish), and initial length and somatic weight are determined based on an individual's inherited growth parameters.
Baseline simulations
To elucidate model behavior, we apply the model to simulate a series of scenarios. These baseline simulations can be grouped into four general categories: (i) maturation and growth parameters are fixed, (ii) only maturation parameters are heritable, (iii) only growth parameters are heritable, and (iv) both growth and maturation parameters are heritable. The initial population consists of 5000 super-individuals, with sex and age assigned randomly. The number of individuals initially represented by each super-individual is determined as a function of age and annual mortality rate. We specified distributions for heritable maturation and growth parameters to ensure that initial populations had suitable genetic variation. For simulations with heritable maturation parameters, initial parameter values for each super-individual are drawn from a normal distribution with a mean of 400 mm and a standard deviation of 300 mm (bounded by 150 and 750 mm). Similarly, for simulations with heritable growth parameters, initial parameter values for each super-individual are drawn from a uniform distribution with bounds of 0.1 and 1 (Table 1).
For baseline simulations with fixed maturation or growth parameters, we set M1–M18 to 400 mm and X1–X12 to 0.5, respectively. We also explore the effects of the magnitude of fixed maturation parameters on inheritance of growth, and vice versa, by running simulations with M1–M18 set at 300 or 500 mm and X1–X12 set at 0.3 or 0.7. Similarly, to explore the effects of mortality rate on inheritance patterns, we ran simulations with Z = 0.3, 0.5, or 0.7 year−1. We ran all simulations under three different scenarios: (i) to begin with, we ran all simulations assuming that recruitment is deterministic (εR = 1; recruitment is solely a function of spawner stock size) and carrying capacity is fixed (εC = 1 and ψ = 0); (ii) then, to explore the effects of stochasticity, we repeated simulations assuming that recruitment and carrying capacity vary stochastically from year to year (εR and εC vary stochastically and ψ fluctuates at 20-year cycles); and (iii) finally, to explore the effects of density-dependent individual growth on inheritance patterns we repeat all simulations with density-dependent growth effects removed from the model (i.e., ADJ = 1; a super-individual's growth is solely a function of its growth parameters). Each simulation tracks the population for 200 years, and we extract population-level information at 20-year intervals. For each scenario, we ran 10 replicate simulations.
Fishing simulations
We extended model analyses to consider the joint ecological and evolutionary effects of fishing. In so doing, we assumed that growth and recruitment are density dependent and that individuals’ growth and maturation parameters are simultaneously heritable.
Prior to simulating fishing-induced effects, we conducted deterministic initiation simulations to evaluate how heritable parameters would evolve in our simulated environment in the absence of fishing. Initial maturation and growth parameters for these simulations are presented in Table 1. The population initially consisted of 5000 super-individuals. Initiation simulations were run for 1000 years with 10 replicates, and the mean values of evolved growth and maturation parameters were extracted at 20-year intervals. We found that 1000 years was a sufficient number of time steps for the population to evolve fairly stable heritable growth and maturation. Final growth and maturation mean values from the 10 baseline simulation replicates were then used as initial mean trait values for fishing simulations.
In fishing simulations, total instantaneous annual mortality rate (Z) is the sum of natural (M) and fishing (F) mortality rates. We assumed a uniform M = 0.2 year−1 for all individuals to emphasize responses to variable F. Preliminary simulations suggested that comparisons of population responses between size-limits or across different levels of F were not sensitive to level of M or differential M with age (also see Dunlop et al. 2007).
We considered two types of size-selective mortality: (i) targeting ‘small’ (more precisely, intermediate size) fish, we allowed fish <250 mm or >650 mm to escape fully from harvest, fish between 250 and 400 mm to recruit fully to fishing gear, and fish between 400 and 650 mm to recruit partially (i.e., F decreased linearly with length within this size range) and (ii) targeting large fish, we let fish <250 mm escape harvest, fish between 250 and 400 mm partially recruit (i.e., F increased linearly with length within this size range), and fish ≥400 mm to recruit fully to fishing gear. Again, our preliminary analyses showed that simulation results were not sensitive to the exact shapes of size-dependent mortality functions (but were sensitive to the differences in length ranges harvested).
We first evaluated effects of fishing under deterministic conditions, applying two size-limits (targeting large and small fish, respectively), each for 200 years at F = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 year−1, with 10 replicates (with the same number of initial super-individuals). For all simulations, we kept track of population mean values of sex- and age-specific maturation parameters, sex-specific K and Lmax, female length-at-age and maturation schedules, recruitment, and population abundance at 20-year intervals. Finally, to consider the effects of a more variable environment, we repeated initiation (prefishing) and fishing simulations under stochastic carrying capacity and recruitment, and we compared population responses to fishing under deterministic versus stochastic conditions.
Results
Baseline simulations
Heritable maturation and growth parameters clearly influenced population abundance (Fig. 1) and expression of other population-level characteristics (e.g., recruitment and size-at-age; Figs S1 and S2). We found that expression of population-level characteristics depended upon assumptions regarding: (i) stochasticity and density-dependent growth (compare panels in Fig. 1) and (ii) whether maturation parameters, growth parameters, or both were heritable (compare line styles within each panel in Fig. 1). Obviously, with stochastic effects included in simulations, there was a high degree of variation in phenotypic expression, while under deterministic recruitment and fixed carrying capacity, phenotypic expression across simulation types was less variable. Further, under density-dependent growth, population abundance, recruitment, and size-at-age were all greater as compared to density-independent simulations. Over the course of 200-year baseline simulations with either no inheritance or with only heritable maturation parameters, trends in population abundance, recruitment, and size-at-age were not evident. However, when growth parameters were heritable (or when both growth and maturation parameters were heritable), simulated populations trended toward greater population abundance, recruitment, and size-at-age.
Figure 1.
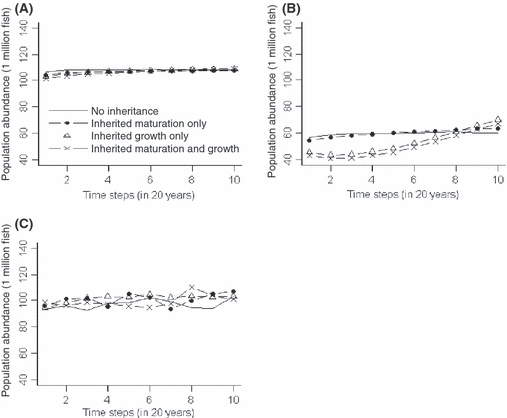
Population abundance in baseline simulations with (A) deterministic recruitment, fixed carrying capacity and density-dependent individual growth, (B) deterministic recruitment, fixed carrying capacity and density-independent individual growth, and (C) stochastic recruitment and carrying capacity, and density-dependent individual growth. For each plot, different line styles represent mean values of 10 simulations for each of the four designs: solid lines, no inheritance (i.e., fixed growth and maturation parameters); dotted-dashed lines, inherited maturation parameters; triangle-dashed lines, inherited growth parameters; cross-dashed lines, inherited growth and maturation parameters.
We also evaluated how stochastic and density-dependent mechanisms influenced the evolution of maturation and growth parameters. For brevity, herein we focus on evolution of maturation and growth parameters under deterministic, density-dependent growth and recruitment with a constant carry capacity (εR = 1, εC = 1, and ψ = 0 Fig. 1a). Evolution of maturation and growth parameters under stochasticity or density-independent growth was qualitatively similar. In the Supporting Information, we present some comparisons of maturation and growth parameter evolution under density-independent growth conditions (ADJ = 1; Figs S3 and S4).
In baseline simulations, selection on maturation parameters corresponding to younger ages favored lower threshold sizes for maturation (Fig. 2a). Such decreases in mean Mi-values were more dramatic for males than females. Further, decreases in mean Mi-values were most pronounced during initial simulation years, but continued to year 200. On the other hand, there was not significant selection on maturation parameters for older ages. As a mature super-individual remains mature until it is removed from the simulation, there is likely limited selection pressure exerted on maturation parameters corresponding to older ages.
Figure 2.
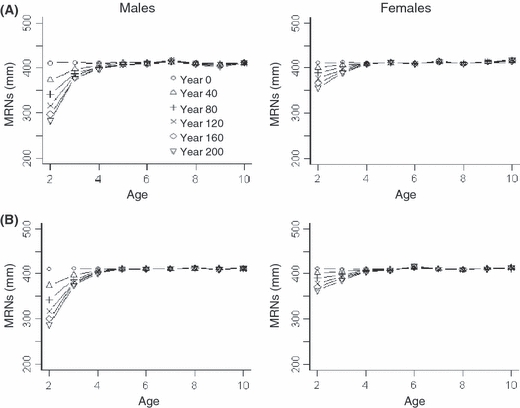
Evolution of maturation parameters for males (left) and females (right) in baseline simulations with (A) inherited maturation and fixed growth parameters (X1–X12 = 0.5), and (B) inherited maturation and growth parameters. Different symbols represent mean (from 10 separate simulations) maturation reaction norms (MRNs) at 40-year intervals.
Similar to selection on maturation parameters, selection on growth parameters (i) differed among males and females, (ii) led to larger changes in mean traits during initial simulation years, and (iii) continued to the end of simulations (Fig. 3A). In general, males evolved faster initial growth (increased mean Ki) with little change in their maximum attainable length (small increased mean Lmax,i), whereas females evolved in the opposite direction (decreased mean Ki; increased mean Lmax,i).
Figure 3.
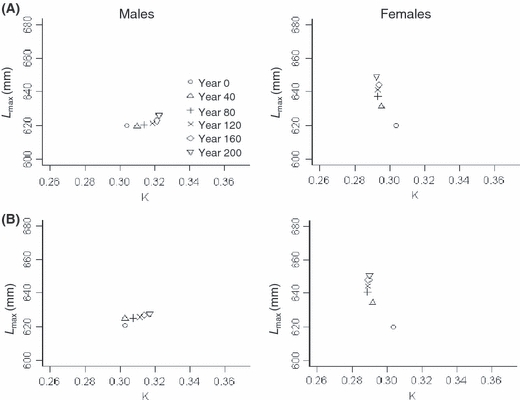
Evolution of growth parameters for males (left) and females (right) in baseline simulations with: (A) inherited growth and fixed maturation parameters (M1–M18 = 400 mm), and (B) inherited growth and maturation parameters. Different symbols represent mean (from 10 separate simulations) initial growth rate (K) and maximum attainable length (Lmax) at 40-year intervals.
Baseline simulations revealed a clear interaction between maturation and growth parameters. Comparisons of simulations with either heritable maturation or growth versus simultaneously heritable maturation and growth parameters, suggest that maturation and growth parameters evolved in a similar manner irrespective of whether the other type of genetic parameter was heritable (compare Figs 2A,B and 3A,B). However, evolution of growth parameters was influenced by maturation fixed at certain levels and vice versa (Fig. 4). When Xi was fixed at greater levels (i.e., increased initial growth rate, but decreased maximum attainable length), maturation thresholds at younger ages evolved to smaller length cut-offs. Similarly, when Mi was fixed at greater lengths, growth parameters evolved to maximize attainable length. This interaction between growth and maturation parameters was qualitatively similar across sexes to fixed maturation and growth parameters.
Figure 4.
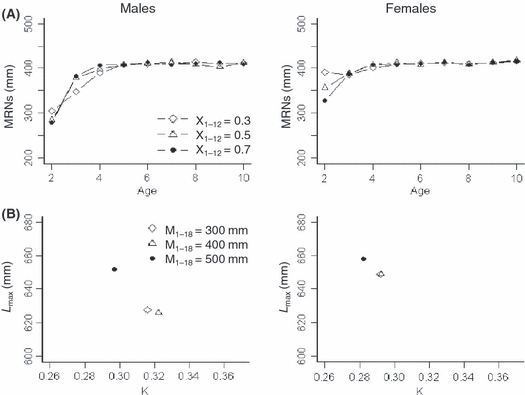
(A) Mean maturation reaction norms (MRNs) and (B) growth parameters [initial growth rate (K) and maximum attainable length (Lmax)] in year 200 for males (left) and females (right). Results are presented for baseline simulations with: (A) inherited maturation parameters and three levels of fixed growth parameters (X1–X12 = 0.3, 0.5, and 0.7, shown in different line styles; smaller Xi-values correspond to relatively small K and large Lmax) and (B) inherited growth parameters and three levels of fixed maturation parameters (M1–M18 = 300, 400, and 500 mm, shown in different symbols).
Finally, mortality rate also affected evolution of maturation and growth parameters (Fig. 5). Note that mortality rate acts on maturation and growth parameters both through selection by decreased survival and release of density-dependent controls. With Z > 0.3 year−1, Mi corresponding to age 2 males and females evolved to decrease greatly, while Mi corresponding to ages >2 did not trend strongly. Similarly, with Z > 0.5 year−1, both males and females evolved a greater maximum attainable length and a smaller K. It is noteworthy that growth and maturation parameters responded nonlinearly to different mortality rates (e.g., lower age 2 Mi at Z = 0.5 year−1 as compared to Z = 0.7 year−1).
Figure 5.
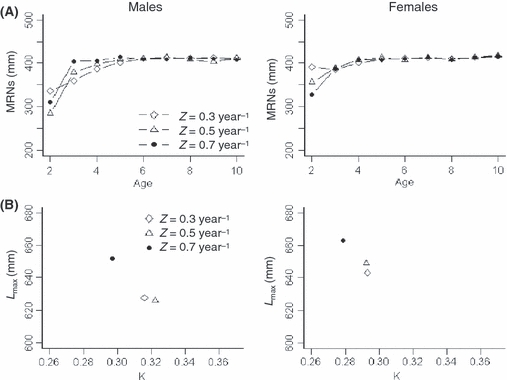
(A) Mean maturation reaction norms (MRNs) and (B) growth parameters [initial growth rate (K) and maximum attainable length (Lmax)] in year 200 for males (left) and females (right). Results are presented for baseline simulations with: (A) inherited maturation, fixed growth parameters (X1–X12 = 0.5), and three levels of mortality rates (Z = 0.3, 0.5, and 0.7 year−1, shown in different line styles) and (B) inherited growth parameters, fixed maturation parameters (M1–M18 = 400 mm), and three levels of mortality rates (Z, shown in different symbols).
Fishing simulations
Through initiation simulations (during which maturation and growth parameters evolved under the prefishing scenario for 1000 years), maturation parameters, K, and Lmax evolved differentially between sexes. Male maturation parameters generally decreased at younger ages, while female maturation parameters evolved less (Fig. S5). Further, by the end of initiation simulations, mean K decreased and Lmax increased more pronouncedly for females than males (Fig. S6). Resulting sex- and age-specific Mi (±200 mm) and Xi (±0.25) parameters were then used as starting conditions for fishing simulations.
Maturation parameters of both sexes evolved differentially in response to fishing. When targeting large fish with increasing F from 0 to 0.6 year−1, male maturation parameters differed little from the baseline (F = 0 year−1), while female maturation parameters at young ages decreased more considerably (Fig. 6). At high F (F = 1.0 year−1), evolving maturation parameters of both sexes varied among replicate simulations and fluctuated by age. This resulted in changes in mean maturation parameters at older ages (Fig. 6). Such patterns indicate a genetic drift due to small population size. When targeting small fish, maturation in males evolved little, but maturation thresholds in female increased at young ages (Fig. 6).
Figure 6.
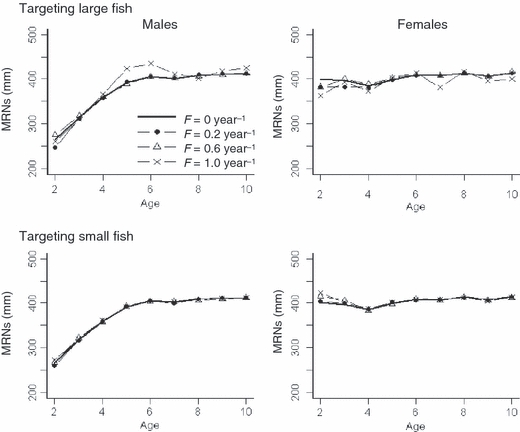
Final (after 200 years) mean evolving maturation reaction norms (MRNs) for males (left) and females (right) under deterministic conditions, with two size-limits (targeting large fish versus small fish) and various fishing mortality rates (to better discern changes in MRNs, we only show results at F = 0, 0.2, 0.6, and 1.0 year−1 in different line styles).
Growth traits responded to size-limits and fishing mortality rates pronouncedly. Increased fishing mortality rates under both size-limits led to decreases in K and corresponding increases in Lmax (Fig. 7). Compared to harvest of small fish, targeting on large fish led to lower K and higher Lmax-values at a given F. These individual-parameters responded dramatically at moderate to high F (e.g., F ≥ 0.6 year−1). Also notably, when targeting small fish, magnitudes of evolving K and Lmax for females did not follow fishing mortality rates; e.g., at highest fishing mortality (F = 1.0 year−1), K and Lmax did not evolve the most but achieved intermediate values.
Figure 7.
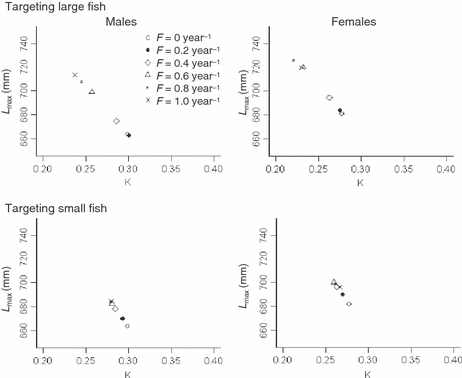
Final (after 200 years) mean initial growth rate (K) and maximum attainable length (Lmax) for males (left) and females (right) under deterministic conditions, with two size-limits (targeting large fish versus small fish) and various fishing mortality rates (F = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 year−1; shown in different symbols).
Different fishing mortality rates influenced recruitment levels and population abundance in a predictable manner (i.e., lower population abundance under greater fishing mortality; Fig. S7). Population characteristics and individual-level phenotypic traits responded more pronouncedly to size-selective fishing relative to evolving maturation and growth traits. For example, when targeting either large or small fish with increasing F, length-at-age, recruitment, and population abundance changed pronouncedly (Table 2). Furthermore, length-at-age patterns (based on female fish data) increased with fishing mortality rates under both size-limits, suggesting that fishing relaxed density-dependent growth constraints (Fig. 8). When targeting large fish, the population displayed greater length-at-age patterns, and age structure of the population appeared to be truncated at F ≥ 0.8 year−1. Similarly, maturation schedules (based on age-specific proportion of mature females) also varied based on size-limit and fishing mortality rate. While harvest under either size-limit induced shifts toward early maturation, this effect was more pronounced when targeting large fish (Fig. 8).
Table 2.
Mean and standard deviation (SD) of evolving maturation (Mi for age 2 males and females) and growth parameters (K and Lmax), female length-at-age (ages 2 and 7), and population-level recruitment and abundance under two size-selective fishing options (A: targeting large fish; B: targeting small fish)
| Deterministic | Stochastic | |||||||
|---|---|---|---|---|---|---|---|---|
| F = 0.2 | F = 0.6 | F = 0.2 | F = 0.6 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| (A) Targeting large fish | ||||||||
| Mi for age 2 (male; mm) | 247 | 3 | 276 | 7 | 251 | 7 | 281 | 7 |
| Mi for age 2 (female; mm) | 382 | 6 | 382 | 7 | 385 | 6 | 379 | 4 |
| K (male; year−1) | 0.30 | 0.003 | 0.26 | 0.003 | 0.30 | 0.003 | 0.26 | 0.003 |
| K (female; year−1) | 0.27 | 0.003 | 0.23 | 0.003 | 0.27 | 0.003 | 0.24 | 0.003 |
| Lmax (male; mm) | 663 | 3 | 699 | 2 | 665 | 2 | 698 | 3 |
| Lmax (female; mm) | 684 | 3 | 720 | 2 | 685 | 3 | 718 | 3 |
| Age 2 length (mm) | 260 | 2 | 340 | 3 | 257 | 19 | 340 | 12 |
| Age 7 length (mm) | 513 | 3 | 721 | 2 | 505 | 15 | 716 | 3 |
| Age 1 recruitment (1 million fish) | 53 | 0.03 | 23 | 0.29 | 53 | 5.64 | 17 | 3.08 |
| Population abundance (1 million fish) | 191 | 0.31 | 47 | 0.68 | 187 | 12.00 | 34 | 5.21 |
| (B) Targeting small fish | ||||||||
| Mi for age 2 (male; mm) | 260 | 5 | 267 | 3 | 264 | 4 | 267 | 5 |
| Mi for age 2 (female; mm) | 403 | 4 | 417 | 4 | 406 | 4 | 417 | 5 |
| K (male; year−1) | 0.29 | 0.002 | 0.28 | 0.002 | 0.29 | 0.003 | 0.28 | 0.002 |
| K (female; year−1) | 0.27 | 0.001 | 0.26 | 0.002 | 0.27 | 0.002 | 0.26 | 0.002 |
| Lmax (male; mm) | 670 | 2 | 683 | 1.28 | 672 | 2 | 683 | 2 |
| Lmax (female; mm) | 690 | 1 | 700 | 1.69 | 692 | 2 | 699 | 2 |
| Age 2 length (mm) | 253 | 3 | 276 | 2.3 | 247 | 14 | 295 | 26 |
| Age 7 length (mm) | 511 | 3 | 607 | 2.8 | 494 | 14 | 596 | 18 |
| Age 1 recruitment (1 million fish) | 51 | 0.16 | 53 | 0.05 | 55 | 13.00 | 50 | 9.64 |
| Population abundance (1 million fish) | 179 | 0.57 | 117 | 0.38 | 190 | 17.52 | 113 | 10.58 |
The mean and SD values were derived from population mean trait values by the end of ten 200-year simulations. For each fishing size-limit, we compared individual and population traits under deterministic versus stochastic conditions, and under relatively low versus high fishing mortality rates (F = 0.2 year−1 vs. F = 0.6 year−1).
Figure 8.
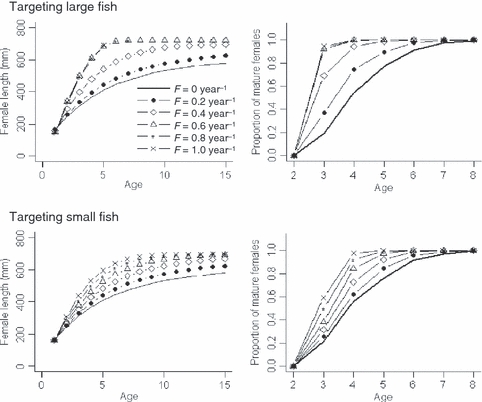
Final (after 200 years) mean female length-at-age (left) and maturation schedules (age-specific proportion of mature females; right) under deterministic conditions, with two size-limits (targeting large fish versus small fish) and various fishing mortality rates (F = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 year−1; shown in line styles).
In general, population characteristics and individual-level phenotypic traits responded similarly to fishing under deterministic and stochastic scenarios. While mean and standard deviation of recruitment and population abundance differed slightly among deterministic and stochastic fishing simulations, length-at-age generally was not sensitive to stochasticity (Table 2). Notably, both mean values and standard deviation of evolving traits did not differ among stochastic and deterministic simulations, thereby suggesting that similar patterns may evolve in both static and variable environments.
Discussion
Eco-genetic individual-based models are a potentially useful tool for considering how environmental and genetic processes may interact to shape phenotypic expression of populations (Dunlop et al. 2009a). Our simulation results demonstrate how a plethora of ecological factors may impact both plastic and genetic components of growth and maturation schedules. For instance, the magnitude of mortality rates and density-dependent processes may plastically affect growth and maturation and simultaneously feedback to exert selection pressure on these life-history traits. Further, we demonstrate how growth rates and maturation schedules interactively mediate each other's selection and plastic expression. Most past attempts to incorporate inheritance into fish IBMs have not simultaneously considered genetic and ecological processes (e.g., Martínez-Garmendia 1998; Jager 2001). Not accounting for ecological processes can compromise predictions on the direction and magnitude of genetic selection. For instance, Gårdmark et al. (2003) demonstrate that harvesting of large individuals will not necessarily favor earlier maturation if there is simultaneous natural predation directed toward small individuals.
Several recent studies have highlighted the potential for rapid evolutionary changes in genetically determined maturation schedules (e.g., Olsen et al. 2004, 2005) and growth rates (e.g., Conover and Munch 2002) of fish. However, relatively few studies have simultaneously focused on both heritable growth and maturation. This trend also holds for modeling approaches, which have primarily focused on either growth or maturation (e.g., Martínez-Garmendia 1998; Jager 2001; Dunlop et al. 2007); only recently, models that allow for evolution of multiple traits have started to appear; (Dunlop et al. 2009a,b; Enberg et al. 2009). Our simulations suggest that when size at maturation is fixed and growth rates are partially genetically determined, the maturation length threshold has potentially strong influence on the genetic component of growth. Similarly, when growth parameters are fixed and maturation parameters are heritable, fixed growth parameters exert selection (albeit relatively weak) on genetically determined maturation length thresholds. Simulations allowing for simultaneous selection on both growth and maturation parameters facilitate continuous co-selective feedback of these two life-history traits.
While several authors have developed species-specific eco-genetic models to evaluate selective pressures on fish populations (e.g., Dunlop et al. 2007; Okamoto et al. 2009), our model is not species specific. Ultimately, the development of species- and stock-specific eco-genetic models could be beneficial. However, we suggest that for many populations the ecological and genetic understanding necessary to develop a realistic stock-specific eco-genetic model is lacking. For instance, while maturation and growth rate may be partially genetically determined, the number and type of genes which exert control on these traits are less clear. Quantitative genetic approaches may facilitate analysis under such genetic uncertainties, however, such approaches require some simplifying assumptions. Herein, we use relatively simple and naïve genetic models (i.e., Mendelian inheritance, and no dependence among growth and maturation parameters) in part because the basis for assuming something more complex is lacking. Similarly, many of the trade-offs and potential costs that select for appropriate maturation and growth genotypes (e.g., energy exerted during spawning; relationship between egg viability and female age and size; behavioral hierarchy of spawning) are insufficiently understood to incorporate into a stock-specific model.
Some of our simulated sex-specific life histories were consistent with predictions based on life-history theory. For example, selection would favor females to delay maturation to maximize lifetime fecundity. Consistent with this prediction, in baseline simulations age 2–3 female MRNs evolved to decrease less than corresponding male reaction norms. Further, trajectories of evolving growth traits also varied, with females evolving a greater Lmax at the expense of lower K. This sexual differentiation arises due to two assumptions in our model: (i) females have larger gonads than males, and thus female gonadal development has a greater impact on somatic growth and future reproductive potential and (ii) while male sperm quality does not vary with size or age, female egg size and viability increase with female age. Through some preliminary model exploration, we applied a different set of coefficients to make a more severe trade-off between female reproductive age and egg quality. In so doing, we found that the trends for male and female evolving maturation and growth traits held, but with this more severe trade-off female maturation parameters corresponding to young ages evolved to increase from initial conditions.
It is noteworthy that when either growth or both growth and maturation were heritable, our simulated population expressed relatively high recruitment, population abundance, and growth patterns. On the other hand, when growth parameters were fixed, these population-level attributes were lower. It is manifest that important life-history traits are partially genetically determined and will feed back to influence population-level traits. We suggest that these results may stimulate further applications of eco-genetic models to enhance understanding of the relationships among inheritance of life-history traits and population dynamics.
Application of eco-genetic model to explore fishing-induced effects
Several studies have drawn attention to the notion that growth and maturation schedules are in part genetically determined, and that size-selective fisheries harvest can play a significant role in altering a fish population's growth and maturation genotypes (Stokes et al. 1993; Heino 1998; Law 2000; Conover and Munch 2002; Heino and Godø 2002). In turn, as these traits have strong influence on a fish stock's harvest potential, it is important to consider both short- and long-term impacts (including genetic effects) of size-selective harvest on fish growth and maturation schedules. By applying our eco-genetic model with various size-limits and fishing mortality rates, we demonstrated simultaneous fishing-induced evolutionary and ecological effects. We identified several aspects of fishing-induced evolution. For example, our model suggested that targeting large fish at intermediate to high fishing mortality could lead to small decreases in maturation parameters corresponding to young ages and considerable declines in genetically determined initial somatic growth rates; together these traits fostered a potential for early maturation and slow growth rate. In contrast, targeting smaller fish selected for slight increases or no changes in genetically determined maturation and less pronounced changes in genetically determined growth traits. The results of targeting large fish were consistent with previous studies that suggested declines in length-at-age and age at maturity of commercial fish stocks attributed to potential fishing-induced genetic selection (Olsen et al. 2004, 2005; Swain et al. 2007). While targeting on small fish is unusual, the results were intuitive; fish that grew fast might be able to escape fisheries, and a prolonged maturation schedule could help maximize somatic growth.
Second, our model suggested that fishing-induced genetic selection might be more pronounced for growth traits than MRNs (albeit some other models suggest an opposite pattern; Dunlop et al. 2009a; Enberg et al. 2009). This pattern was not due to unequal evolving potential between MRNs and growth traits, as we ensured that the initial population possessed sufficiently wide ranges of both maturation and growth parameters. While maturation and growth traits evolved independently, we observed that the magnitude of one trait might influence evolution of the other (based on baseline simulations), thereby evolution of these traits is likely not independent. In fact, genetic correlation between maturation and growth traits has been suggested (Dieckmann and Heino 2007; Hutchings and Fraser 2008). Along these lines, we observed that evolution of growth and maturation traits tended to compensate each other. Further, prediction of greater changes in evolving growth parameters than maturation parameters could be expected because fishing effects directly operated on length, not maturation schedules. Furthermore, Dunlop et al. (2005) showed that stability of MRNs had been maintained for two smallmouth bass Micropterus dolomieu populations (both were originally introduced from a single source) even though they experienced different mortality regimes for 100 years.
Third, as in the baseline simulations, under prefishing and fishing simulations the model predicted that female MRNs responded more pronouncedly to fishing, and K and Lmax achieved a lower and greater values, respectively, for females, as compared to males. Such differential genetic responses might arise from sexually differential reproductive trade-offs. Furthermore, as many fish display sexually dimorphic size patterns (females tend to be larger than males), fishing-induced effects likely will vary and result in differential responses between sexes.
In addition to fishing-induced evolution, our model demonstrated drastic plastic changes for mean growth rates, maturation schedules, and population dynamics. Fishing-induced plastic effects might occur by (1) affecting density-dependent growth and recruitment, (2) altering age and size structures, and (3) changing population maturation schedules via effects (1) and (2) (Nelson and Soulé 1987; Law 2000, 2007; Berkeley et al. 2004). It is notable that fishing-induced plastic effects on growth were in an opposing direction to evolutionary effects; i.e., while fishing-induced selection favored slow growth rates (lower K and higher Lmax), plastic effects increased growth rates. As the observed female length-at-age patterns increased with F, the plastic effects out-weighed evolutionary effects. On the other hand, both plastic effects on growth and decreased MRNs by fishing would promote early maturation schedules.
Our model predictions of fishing-induced evolutionary and plastic effects are comparable to recent modeling studies (Ernande et al. 2004; de Roos et al. 2006; Dunlop et al. 2007). Dunlop et al. (2007) constructed an eco-genetic model based on empirical data on smallmouth bass and explored fishing-induced plastic and evolutionary effects by simulating harvesting of individuals either (i) above a size-limit (18 cm) or (ii) age 0. While Dunlop et al.'s (2007) model predicted no effects on PMRNs when targeting age 0 fish (whereas Ernande et al. 2004 and this study showed detectable effects when targeting small fish), they found significant changes in PMRNs when targeting large fish and considerable plastic changes in growth and biomass under both size-limits, supporting our findings. Further, both our study and Dunlop et al.'s (2007) model showed that plasticity in growth rates might influence the result of fishing-induced evolution.
Our modeling results highlighted that environmental stochasticity could mediate individual and population responses to fishing-induced effects. Size-selective harvesting of large fish may result in populations with truncated age and size structures (Berkeley et al. 2004), and such populations tend to display high recruitment variability (Hsieh et al. 2006; Anderson et al. 2008). Further, in simulations targeting large fish at a relatively high F mean recruitment and population abundance were slightly lower under stochasticity. This suggests that environmental stochasticity could exacerbate reduction of a population (and its sustainability) under high harvest rates.
On the other hand, mean values of evolving traits and length-at-age as well as standard deviation of evolving traits were less sensitive to stochasticity. As stochasticity was modeled as random deviations from mean carrying capacity and per capita recruitment, it might have little effect on mean responses of evolving traits and length-at-age patterns. Dunlop et al. (2007) evaluated sensitivity of PMRNs to stochasticity and indicated consistent findings with ours.
In conclusion, eco-genetic models are a potentially useful tool for considering how environmental and genetic processes may interact to shape phenotypic expression of fish populations (Dunlop et al. 2009a). Our simulations demonstrate the potential influence of ecological factors (mortality rate, density-dependent growth) and the interactive effects of growth and maturation on the inheritance of these two life-history traits. Moreover, as suggested for many commercial and recreational fishes (Beard and Essington 2000; Law 2000; Kuparinen and Merilä 2007; Sharpe and Hendry 2009), our model predicts that intensively targeting large fish can affect strong plastic and evolutionary changes in growth and life-history traits. Furthermore, our model results imply that harvest-induced effects (both ecological and evolutionary) on life-history traits can then cascade to influence growth patterns and population dynamics in the direction of unsustainable fisheries.
Acknowledgments
Discussions with Jim Breck, Scott Peacor, and Carl Simon helped in the development of simulation models. This work was supported by the Great Lakes Fishery Trust, US Fish and Wildlife Service and NOAA's Great Lakes Environmental Research Laboratory (GLERL). This is NOAA-GLERL contribution 1518.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Age 1 recruitment in baseline simulations with (A) deterministic recruitment, fixed carrying capacity and density-dependent individual growth, (B) deterministic recruitment, fixed carrying capacity and density-independent individual growth, and (C) stochastic recruitment and carrying capacity, and density-dependent individual growth.
Figure S2. Mean total length of age 2 (left) and age 10 (right) female super individuals in baseline simulations with (A) deterministic recruitment, fixed carrying capacity and density-dependent individual growth, (B) deterministic recruitment, fixed carrying capacity and density-independent individual growth, and (C) stochastic recruitment and carrying capacity, and density-dependent individual growth.
Figure S3. Evolution of (A) maturation parameters for males (left) and females (right) in baseline simulations with inherited maturation and fixed growth parameters (X1−X12 = 0.5) and including density-independent growth. (B) Evolution of growth parameters with inherited growth and fixed maturation parameters (M1−M18 = 400 mm) including density-independent growth.
Figure S4. (A) Mean maturation reaction norms (MRNs) and (B) growth parameters [initial growth rate (K) and maximum attainable length (Lmax)] in year 200 for males (left) and females (right).
Figure S5. Mean evolving maturation reaction norms (MRNs) for (A) males and (B) females over a 1000-year initiation simulation under deterministic conditions.
Figure S6. Mean of ten, 1000-year initiation simulations of (A) evolving initial growth rate (K) and (B) maximum attainable length (Lmax) for males (left) and females (right) under deterministic conditions.
Figure S7. Mean of 10 replicates of 200-year simulations of age 1 recruitment (left) and population size (right) under deterministic conditions, with two size-limits (targeting large fish versus small fish) and various fishing mortality rates (F = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 year−1; shown in different line styles).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature cited
- Anderson CNK, Hsieh C, Sandin SA, Hewitt R, Hollowed A, Beddington JR, May RM, et al. Why fishing magnifies fluctuations in fish abundance. Nature. 2008;452:835–839. doi: 10.1038/nature06851. [DOI] [PubMed] [Google Scholar]
- Beard TD, Jr, Essington TE. Effects of angling and life history processes on bluegill size structure: insights from an individual-based model. Transactions of the American Fisheries Society. 2000;129:561–568. [Google Scholar]
- Beauchamp KC, Collins NC, Henderson BA. Covariation of growth and maturation of lake whitefish (Coregonus clupeaformis. Journal of Great Lakes Research. 2004;30:451–460. [Google Scholar]
- Berkeley SA, Hixon MA, Larson RJ, Love MS. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries. 2004;29:23–32. [Google Scholar]
- Blanchard JL, Dulvy NK, Jennings S, Ellis JR, Pinnegar JK, Tidd A, Kell LT. Do climate and fishing influence size-based indicators of Celtic Sea fish community structure? ICES Journal of Marine Science. 2005;62:405–411. [Google Scholar]
- Brown CJ, Hobday AJ, Ziegler PE, Welsford DC. Darwinian fisheries science needs to consider realistic fishing pressures over evolutionary time scales. Marine Ecology Progress Series. 2008;369:257–266. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Dunlop ES, Shuter BJ, Ridgway MS. Isolating the influence of growth rate on maturation patterns in the smallmouth bass (Micropterus dolomieu. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:844–853. [Google Scholar]
- Dunlop ES, Shuter BJ, Dieckmann U. Demographic and evolutionary consequences of selective mortality: predictions from an eco-genetic model for smallmouth bass. Transactions of the American Fisheries Society. 2007;136:749–765. [Google Scholar]
- Dunlop ES, Heino M, Dieckmann U. Eco-genetic modeling of contemporary life-history evolution. Ecological Applications. 2009a;???:????–????. doi: 10.1890/08-1404.1. [DOI] [PubMed] [Google Scholar]
- Dunlop ES, Baskett M, Heino M, Dieckmann U. Propensity of marine reserves to reduce the evolutionary effects of fishing in a migratory species. Evolutionary Applications. 2009b;2:371–393. doi: 10.1111/j.1752-4571.2009.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enberg K, Jørgensen C, Dunlop ES, Heino M, Dieckmann U. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evolutionary Applications. 2009;2:394–414. doi: 10.1111/j.1752-4571.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London, Series B (Biological Sciences) 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gårdmark A, Dieckmann U, Lundberg P. Life-history evolution in harvested populations: the role of natural predation. Evolutionary Ecology Research. 2003;5:239–257. [Google Scholar]
- Hard JJ, Gross MR, Heino M, Hilborn R, Kope RG, Law R, Reynolds JD. Evolutionary consequences of fishing and their implications for salmon. Evolutionary Applications. 2008;1:388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1971–1982. [Google Scholar]
- Heino M, Godø OR. Fisheries-induced selection pressures in the context of sustainable fisheries. Bulletin of Marine Science. 2002;70:639–656. [Google Scholar]
- Heino M, Dieckmann U, Godø O. Measuring probabilistic reaction norms for age and size at maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- Hilborn R. Faith, evolution, and the burden of proof – reply. Fisheries. 2007;32:91–92. [Google Scholar]
- Hsieh C, Reiss CR, Hunter JR, Beddington JR, May RM, Sugihara G. Fishing elevates variability in the abundance of exploited species. Nature. 2006;443:859–862. doi: 10.1038/nature05232. [DOI] [PubMed] [Google Scholar]
- Hutchings JA. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:824–832. [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Jager HI. Individual variation in life history characteristics can influence population extinction risk. Ecological Modeling. 2001;144:59–74. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology and Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- Marteinsdottir G, Steinarsson A. Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. Journal of Fish Biology. 1998;52:1241–1258. [Google Scholar]
- Martínez-Garmendia J. Simulation analysis of evolutionary response of fish populations to size-selective harvesting with the use of an individual-based model. Ecological Modeling. 1998;111:37–60. [Google Scholar]
- Moles MD, Johnston TA, Robinson BW, Leggett WC, Casselman JM. Is gonadal investment in walleye (Sander vitreus) dependent on body lipid reserves? A multipopulation comparative analysis. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:600–614. [Google Scholar]
- Nelson K, Soulé M. Genetical conservation of exploited fishes. In: Ryman N, Utter F, editors. Population Genetics and Fishery Management. Seattle: University of Washington; 1987. pp. 345–368. Washington Sea Grant Program. [Google Scholar]
- Okamoto K, Whitlock R, Magnan P, Dieckmann U. Mitigating fisheries-induced evolution in lacustrine brook charr (Salvelinus fontinalis) in southern Quebec, Canada. Evolutionary Applications. 2009;2:415–437. doi: 10.1111/j.1752-4571.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Lilly GR, Heino M, Morgan MJ, Brattey J, Dieckmann U. Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:811–823. [Google Scholar]
- Quince C, Abrams PA, Shuter BJ, Lester NP. Biphasic growth in fish I: theoretical foundations. Journal of Theoretical Biology. 2008a;254:197–206. doi: 10.1016/j.jtbi.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Quince C, Shuter BJ, Abrams PA, Lester NP. Biphasic growth in fish II: empirical assessment. Journal of Theoretical Biology. 2008b;254:207–214. doi: 10.1016/j.jtbi.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Rijnsdorp AD. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. [DOI] [PubMed] [Google Scholar]
- De Roos AM, Boukal DS, Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proceedings of the Royal Society of London, Series B (Biological Sciences) 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M, Baveco MJ, DeAngelis DL, Rose KA, Van Nes EH. Super-individuals a simple solution for modeling large population on an individual basis. Ecological Modeling. 1995;80:161–170. [Google Scholar]
- Sharpe D, Hendry A. Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evolutionary Applications. 2009;2:260–275. doi: 10.1111/j.1752-4571.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Stokes TK, McGlade J, Law R. Berlin: Springer-Verlag; 1993. The Exploitation of Evolving Resources. Lecture Notes in Biomathematics 99. [Google Scholar]
- Strand E, Huse G, Giske J. Artificial evolution of life history and behavior. American Naturalist. 2002;159:624–644. doi: 10.1086/339997. [DOI] [PubMed] [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society of London, Series B (Biological Sciences) 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippel EA. Age at maturity as a stress indicator in fisheries. BioScience. 1995;45:759–771. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Winemiller KO, Rose KA. Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:2196–2218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


