Abstract
Evidence of fishery-induced evolution has been accumulating rapidly from various avenues of investigation. Here we review the knowledge gained from experimental approaches. The strength of experiments is in their ability to disentangle genetic from environmental differences. Common garden experiments have provided direct evidence of adaptive divergence in the wild and therefore the evolvability of various traits that influence production in numerous species. Most of these cases involve countergradient variation in physiological, life history, and behavioral traits. Selection experiments have provided examples of rapid life history evolution and, more importantly, that fishery-induced selection pressures cause simultaneous divergence of not one but a cluster of genetically and phenotypically correlated traits that include physiology, behavior, reproduction, and other life history characters. The drawbacks of experiments are uncertainties in the scale-up from small, simple environments to larger and more complex systems; the concern that taxons with short life cycles used for experimental research are atypical of those of harvested species; and the difficulty of adequately simulating selection due to fishing. Despite these limitations, experiments have contributed greatly to our understanding of fishery-induced evolution on both empirical and theoretical levels. Future advances will depend on integrating knowledge from experiments with those from modeling, field studies, and molecular genetic approaches.
Keywords: cogradient variation, common garden experiment, countergradient variation, local adaptation, natural selection, phenotypic plasticity, selection experiment
Introduction
Artificial selection is the essential tool for understanding the general evolvability of traits and the extent to which genetic correlations constrain evolution. (Fuller et al. 2005)
After more than a century of extensive exploitation the evidence is now overwhelming that various phenotypic traits have been altered substantially in many of the world's exploited fish stocks (Law 2000; Jørgensen et al. 2007). Some of these changes (e.g., decreasing size-at-age, earlier maturity) are consistent with predictions from evolutionary life history theory, but whether or not they are genetic remains uncertain for two principal reasons: (i) the traits in question are phenotypically plastic in response to the environment; and (ii) fishing causes a host of other confounding environmental changes including habitat alteration, the density of the targeted population, and the density of its forage, competitors, and predators. Shifts in climate over this time interval muddy the waters even further. Yet the question of fishery-induced evolution, if it exists, is exceedingly important because of its potential to decrease the yield and resilience of a population. This combination of uncertainty and consequence has sparked a lively and ongoing debate about whether fishery management needs to account for evolutionary consequences of fishing (Hilborn 2006; Conover and Munch 2007; Jørgensen et al. 2007; Browman et al. 2008; Hilborn and Minte-Vera 2008; Kuparinen and Merilä 2008).
An evolutionary response to the direct effects of fishing will occur if four general conditions are met. First, the trait under consideration must be phenotypically variable. Second, at least a portion of this phenotypic variation must have a genetic basis; i.e. it must be heritable. Third, fishing cannot be merely a thinning process, but must selectively remove the more susceptible genotypes as a function of their phenotypic expression. Note that these first three points are merely the basic conditions for Darwinian evolution except that the agent of selection is a human-induced source of selection imposed in the wild. Finally, the intensity of such fishery-induced selection must be sufficiently high so as to override natural selection operating at the same time (Carlson et al. 2007; Edeline et al. 2007). In addition, evolutionary responses may occur in response to the indirect effects of fishing on the environment such as habitat alteration or changes in the prey or predator community that may result from or influence density-dependent interactions (see Walsh and Reznick 2008). Such indirect effects may alter the selective landscape experienced by a harvested species.
The phenotypic trait most commonly targeted by harvest practices is body size. This poses a challenge to studies of fishery-induced evolution because size is not only an extremely plastic character, it is also the complex end product of numerous other physiological processes such as energy acquisition and allocation, digestion, conversion efficiency, metabolism, somatic tissue synthesis (growth), maturation, reproductive output, and behavioral correlates like activity and risk-taking. All these traits, including age and morphology, are not only phenotypically interrelated but likely also have genetic covariances. There are two consequences of this: (i) changes observed in one trait might solely be a by-product of changes induced in a correlated trait, and (ii) those changes might have occurred simply because of a plastic response to altered biotic and abiotic environmental conditions (e.g., temperature, food availability, competition, predator-prey overlap). Although life history theory provides a basis for predicting evolutionary change in harvested populations, interpreting phenotypic changes as an evolutionary response has been criticized by some as ‘adaptive story telling’ unless the genetic basis of these changes can be established (Kuparinen and Merilä 2008; but see Jørgensen et al. 2008 for a counterargument).
Four main approaches to disentangle the environmental and genetic components of observed phenotypic variations in harvested species have emerged over the recent past. Of these, indirect methods have so far received the most attention. They encompass (i) modeling approaches that try to mimic known ecological, physiological and/or genetic processes under imposed rates of fishing selectivity and environmental dynamics (e.g., de Roos et al. 2006;Savenkoff et al. 2007) and (ii) empirical analyses of long-term trends in exploited fish stocks, where statistical models attempt to control for environmental plasticity (Dieckmann and Heino 2007; Swain et al. 2007; Heino and Dieckmann 2008). Although the latter is necessarily restricted to relatively data rich situations, the majority of studies on fishery-induced evolution currently fall into this category. In contrast, direct methods exclude confounding environmental effects by either (iii) conducting experiments under controlled or manipulated environmental conditions (Silliman 1975; Conover and Munch 2002) or (iv) measuring genes that influence fitness directly at the molecular level, a line of attack that is just now emerging (Allendorf et al. 2008; Naish and Hard 2008). Each of these four approaches has strengths and weaknesses, and each has the potential to contribute uniquely to our understanding of fishery-induced evolution. None of them is self-sufficient. Further advancement will be achieved by fully exploiting the advantages of and combining the strengths across these methodologies.
The purpose of this paper is to review the findings that experimental approaches have so far contributed to our understanding of fishery-induced evolution, including the powers and limitations of this approach. In short, the greatest strengths of experiments are in standardizing environmental conditions so that genetic variation can be revealed and measuring the evolvability of and genetic correlations among traits (Fuller et al. 2005). We characterize what types of experimental designs can advance understanding and briefly review relevant examples from the literature. Finally, we make suggestions for future research and advocate for the integration of experimental and other approaches. We begin with a discussion of the three main types of knowledge gleaned from experiments to evaluate the potential for fishery-induced evolution.
Types of experiments and the evidence they provide
Selection experiments are irreplaceable tools for answering questions about adaptation and the genetic basis of adaptive trait clusters. (Fuller et al. 2005)
Common garden experiments: measuring extant natural genetic variation in adaptive traits
The first question that must be answered with respect to fishery-induced evolution is ‘what traits are capable of evolving?’ This question can be answered with a comparative approach that determines whether life history traits display adaptive genetic variation among extant stocks of a given species and, if so, which ones and in what manner? To be certain that any genetic variation measured is truly adaptive and not the result of stochastic processes such as drift, this approach works best when comparing variation across multiple locations spanning strong environmental gradients (Endler 1986). The process involves (i) asking to what extent phenotypic traits vary across an environmental gradient, (ii) determining whether trait plasticity or genetic differentiation is the source of this variability, and (iii) identifying the causal agents of selection. Simply put, only if we can demonstrate how and why traits have evolved in response to natural selection can we gain an understanding of how they might evolve in response to an agent of selection imposed by humans.
‘Common garden’ experiments play a crucial role in revealing adaptive genetic variation in the wild because they disentangle environmental from genetic influences across the gradient. In common garden experiments, offspring from different populations are reared under identical environmental conditions. Any among-population differences in phenotypes that persist under common garden conditions must be genetic and would thus prove that wild populations differ genetically. If such genetic variation is strongly correlated with environmental gradients, then it likely represents local adaptation, thus demonstrating that the traits in question are capable of evolving. Within the past decade, common garden approaches have been applied widely to many taxa (Conover et al. 2006). In fishes, such studies initially focused on isolated populations of freshwater species, while more recently they have also been expanded to many marine species. These studies have revealed that genetic adaptation to local environment conditions is common in fish populations and that the patterns of change are highly correlated with environmental gradients, e.g. latitude, temperature, seasonality, ice cover, migration costs, and predator abundance (Table 1).
Table 1.
Published common garden experiments on fish (teleosts, chondrichtyes) revealing countergradient adaptations in various traits along given environmental gradients
| Species | Common name | Trait(s) | Selection gradient | Source |
|---|---|---|---|---|
| Cynoscion nebulosus | Spotted weakfish | Larval growth rate | Temperature, season length | Smith et al. 2008 |
| Fundulus heteroclitus | Mummichog | Growth rate, embryo development | Seasonality | Schultz et al. 1996; DiMichele and Westerman 1997 |
| Gadus morhua | Cod | Growth rate, food conversion efficiency | Purchase and Brown 2001; Salvanes et al. 2004 | |
| Body shape | Temperature | Marcil et al. 2006 | ||
| Hippoglossus hippoglossus | Halibut | Growth rate, growth efficiency | Temperature | Jonassen et al. 2000 |
| Lepomis gibbosus | Pumpkinseed | Growth rate, cranial ossification | Competition, predation | Arendt and Wilson 1997, 1999, 2000 |
| Menidia menidia | Atlantic silverside | Metabolic rate, growth rate, swimming performance, foraging behavior Food consumption rate, growth efficiency, predator vulnerability | Season length | Arnott et al. 2006; Billerbeck et al. 2000, 2001; Chiba et al. 2007; Conover and Present 1990; Lankford et al. 2001; Munch and Conover 2003 |
| Menidia peninsulae | Tidewater silverside | Growth rate | Seasonality | Yamahira and Conover 2002 |
| Micropterus salmoides | Largemouth bass | Growth rate | Growing season? | Philipp and Whitt 1991 |
| Morone saxatilis | Striped bass | Growth rate | Seasonality | Brown et al. 1998; Conover et al. 1997; Secor et al. 2000 |
| Notropis atherinoides | Emerald shiner | Growth rate | Temperature | Pegg and Pierce 2001 |
| Oncorhynchus keta | Chum salmon | Body shape | Predation, food limitation | Tallman 1986; Tallman and Healey 1991 |
| Oncorhynchus nerka | Sockeye salmon | Breeding color | Sexual selection, carotenoid availability | Craig and Foote 2001; Craig et al. 2005 |
| Oncorhynchus tshawytscha | Chinook salmon | Ovarian mass | Migration cost | Kinnison et al. 2001 |
| Oryzias latipes | Japanese rice fish | Growth rate | Seasonality | Yamahira et al. 2007; Yamahira and Takeshi 2008 |
| Poecilia reticulata | Guppy | Sexual body coloration | Carotenoid availability | Grether et al. 2005 |
| Pomacentrus coelestis | Neon damselfish | Clutch size, egg size | Temperature? | Kokita 2003 |
| Salmo salar | Atlantic salmon | Growth rate, digestion rate | Light/ice cover | Nicieza et al. 1994a,b; Finstad and Forseth 2006 |
| Salmo trutta | Sea trout | Standard metabolic rate | River thermal regime | Alvarez et al. 2006 |
| Scophthalmus maximus | Turbot | Growth rate, growth efficiency | Temperature | Imsland et al. 2000, 2001 |
| Acipenser fulvescens | Lake sturgeon | Growth rate | Unknown | Power and McKinley 1997 |
Two common geographical patterns have emerged from these studies. The predominant pattern is countergradient variation (CnGV), which occurs when genetic variation in a phenotypically plastic trait is distributed such that it counteracts environmental influences on that trait, thereby making phenotypes appear to be similar when in fact their genotypes are not. Such genetic divergences, which have also been termed ‘genetic compensation’ (Grether 2005), can be revealed only by common garden experiments. CnGV has so far been detected in 21 fish species, including many from marine or estuarine environments that are extensively harvested (Table 1). It is common, too, in numerous other ectotherms including reptiles, amphibians, insects, and marine invertebrates (Conover et al. 2006). Most of the finfish examples involve temperate species in which growth rate has evolved to compensate for the reduction in temperature and length of the growing season that occurs at higher latitudes. Other traits that display CnGV are those mechanistically linked to growth rate such as metabolic rate, feeding rate, growth efficiency, foraging behavior, and body shape. The agent of selection that drives these differences, at least in some species, is size-selective first winter mortality that favors larger body sizes at higher latitudes (Munch et al. 2003; Hurst and Conover 1998; see review by Hurst 2007). Conversely, when genetic variation is distributed in nature such that it accentuates environmental influences on a plastic trait, the pattern is known as cogradient variation (CoGV; not shown in Table 1). CoGV has been documented in five fish species and primarily involves morphological characters (Day et al. 1994; Robinson and Wilson 1996; Billerbeck et al. 1997; Yamahira et al. 2006; Ghalambor et al. 2007), although at least one case of CoGV in growth rate has been documented (Arendt and Reznick 2005). For further details, an extensive review of the theory, prevalence, and evolutionary significance of CnGV and CoGV across all organisms, with implications for conservation of resource species, is provided by Conover et al. (2009a).
One of the most thoroughly studied cases of CnGV in growth is the Atlantic silverside, Menidia menidia. In this species, common garden experiments have demonstrated that the genetic capacity for growth increases greatly with latitude along the east coast of North America (Conover and Present 1990). Because this countergradient pattern almost exactly counteracts the threefold decrease in length of the growing season at higher latitudes, adult body size (at age one) is nearly the same at all latitudes. The principal agent of selection is size-selective winter mortality: i.e., there is strong directional selection that favors large body sizes in northern populations (Munch et al. 2003). Faster growth is positively correlated with a suite of covarying traits that together maximize energy acquisition including increased standard metabolism (Billerbeck et al. 2000; Arnott et al. 2006), food consumption and conversion efficiency (Present and Conover 1992), and foraging activity (Chiba et al. 2007). Also displaying a positive genetic correlation with growth rate is egg production rate (Conover 1992). However, there is a cost associated with higher rates of tissue synthesis. Fast growth is negatively correlated with swimming speed (Billerbeck et al. 2001; Munch and Conover 2004) and vulnerability to predation (Lankford et al. 2001), Hence, in the north where size selective winter mortality dominates, fast growth is favored despite the trade-offs with swimming performance and predation vulnerability. At southern latitudes, on the other hand, the time constraint on growing season length and the severity of winter is reduced, while predation intensity is increased. Under these conditions, genotypes that acquire energy and grow at lower rates and therefore have higher metabolic scope for swimming and evading predators have higher fitness (Arnott et al. 2006; Chiba et al. 2007).
How does knowledge of the prevalence of CnGV in growth rate and other physiological and behavioral traits help us understand fishery-induced evolution? The answer is three-fold. First, it proves that juvenile growth rate is not generally maximized by natural selection as was previously thought by early life history theorists (see review by Arendt 1997). Instead, growth is optimized by stabilizing selection and thereby is fine-tuned to the adaptive landscape in any given habitat. When an unfished stock is exploited, the imposed mortality on adults shifts the adaptive landscape, causing selection for a new phenotypic optimum, thereby disrupting the fine-tuning between growth rate and the natural environment. Second, it proves that despite the extreme plasticity of growth and metabolism in response to environmental factors such as temperature or food level in the wild, genetic variation remains a very important component of the growth rate expressed by individuals within a population and thereby the productivity among populations. Plasticity and genetic variation are not mutually exclusive and in fact may act antagonistically (as in CnGV) or synergistically (as in CoGV). Third, it demonstrates that size selective processes such as winter mortality are capable of driving growth rate evolution in the wild. These observations set the stage for the possibility of fishery-induced evolution.
Common garden experiments have several limitations. First, in order to confidently rule out confounding environmental effects, common garden experiments need to start with the earliest ontogenetic stage of a given species (usually fertilized eggs, preferably from parents that have been maintained in a common garden). This minimizes the possibility that irreversible effects of environment on phenotypes prior to the beginning of the experiment do not persist. While this may prove extremely difficult in species with high early life mortalities and special larval food requirements (e.g., many tropical reef fishes), it has also led to a bias in literature towards traits that are expressed relatively early during ontogeny (larval and juvenile stages) and thus require relatively short rearing protocols (weeks to months). For most fish species, attempts to investigate adult traits are not feasible because of very long rearing times (years). Relatively few common garden experiments have compared size and/or age at maturity among populations, even though this trait is strongly suspected to have evolved in many exploited fish stocks (Dieckmann and Heino 2007; Jørgensen et al. 2007; Heino and Dieckmann 2008). The argument for fisheries-induced evolution of age at maturity in cod, for example, would be greatly enhanced if there was a common garden study demonstrating a genetic basis for natural variation in this trait among populations. Experimental studies of age at maturity that do exist involve very short-lived species (swordtails: Kallman and Borkoski 1978; guppies: Reznick and Ghalambor 2005; platyfish: Sohn 1977). Second and most importantly, studies of the existing level of adaptive genetic variation among wild stocks are not informative about the rate at which such traits may have evolved or will evolve in the future. We know only that the divergence occurred sometime after at least partial separation from a common ancestor which in many cases may have been thousands of generations ago. To predict the potential for evolution in the future requires knowledge of the level of additive genetic variation currently existing within populations. An important exception involves recent introductions of species to novel environments (e.g., Haugen and Vøllestad 2000, 2001; Hendry et al. 2000) or as part of a planned field translocation as further discussed below. In these cases, rates of contemporary evolution are measurable. Finally, while common garden experiments on wild populations work well within the spatial domain, they are rarely feasible in the temporal domain. It is not possible to compare the genetic basis of extant trait variation in a given population today to what it was a century ago. However, common garden experiments at different points in time have been used to measure rates of evolution in field experiments that were planned in advance (see guppy studies described below).
Selection experiments
Fishes display an enormous diversity of life history patterns. We suspect most ecologists and evolutionists would agree that such divergent life histories likely evolved as a function of selection and adaptation operating throughout the long evolutionary history of fishes. There is valid scientific uncertainty, however, about the time frame required for such evolutionary divergences to transpire. Originally, the perception was that ecological dynamics operate on immediate to decadal time scales whereas evolutionary dynamics involve millennia, but there are now many examples of rapid evolution in nature occurring after only a few generations of selection (e.g., Hendry et al. 2000; Reznick and Ghalambor 2005). There is also uncertainty about the multivariate nature of selection. Not only must we contend with a tangled web of genetic and environmental covariance and interaction terms, but there is also a tangled web of positive and negative genetic covariances among traits that can constrain or accelerate rates of evolution. Selection experiments are the principal tool for measuring the rate of evolution of any given trait and, more importantly, the correlated evolution of trait clusters that are genetically linked to the target of selection (Fuller et al. 2005).
The main goals of a selection experiment are to (i) demonstrate that phenotypic selection on a given trait translates into genotypic selection, (ii) identify concomitant changes in correlated traits, and (iii) measure the rate of such evolutionary changes. The rate of evolutionary change is a product of the trait heritability (the extent to which phenotypes are determined by genes transmitted from parents) and the selection differential (the change in mean phenotype of parents caused by selection). The heritability is an intrinsic biological parameter while the selection differential varies with the intensity of selection which, in the case of fishing, is imposed by the harvest regime. Hence, many selection experiments purposely impose very severe selection differentials because this will require the fewest number of generations to provide an accurate measure of heritability. The rate of evolution imposed by some lesser selection differential is then easily calculated (e.g., Brown et al. 2008). Hence, the idea of fishery-induced selection experiments is not to directly mimic ‘real-world fisheries’ (at least initially) but to develop and refine theory from which testable predictions can be derived (see Benton et al. 2007).
Selection experiments on captive populations
There are two ways of carrying out selection experiments on captive populations (Fuller et al. 2005). The difference lies in the way the investigator intends to bring about phenotypic change in parental generations. In ‘artificial selection experiments,’ individual parents are directly chosen by the investigator for breeding and mated based on specific trait values. The offspring of such matings are reared and then sorted again by the investigator prior to the next breeding cycle. In such cases, selection is highly artificial so as to provide precise control over the parental phenotypes chosen for breeding. This approach is valuable primarily to test the heritability of a specific trait (e.g., coloration, number of gill rakers, etc.) and to measure correlated characters that are dragged along by selection on the targeted trait. In ‘natural selection experiments,’ on the other hand, genetically homogeneous populations are subdivided and assigned to two or more environmental treatments that differ only in the parameter of interest (e.g., temperature, density, predators, etc.). Selection is exerted by the differential effects of environmental parameters and the populations self reproduce. The investigator merely monitors the rate of divergence, if any, among populations over multiple generations. The advantage of this approach is that the responsible environmental agents are precisely known, yet the researcher does not directly control reproductive success or other components of selection except those imposed by the environment. Each treatment can be replicated with multiple populations so as to correct for other sources of variation that can cause divergence such as genetic drift. The drawback of this approach, as compared with artificial selection, is that it may prove difficult to distinguish between traits responding to selection and those that change indirectly because of genetic covariances (Fuller et al. 2005).
One of the first to conduct experimental simulations of fishery dynamics was Ralph Silliman. In a series of overlooked studies on captive guppy and tilapia populations, Silliman measured the ecological relationship between fishing mortality rate and yield, thereby providing an empirical basis for the basic stock production models that were emerging at that time (e.g., Silliman 1968, 1971, 1972). Then he turned his attention to evolutionary change. Silliman (1975) established two brood stocks of about 200 Tilapia mossambica derived from mixed source populations of unspecified origin. Once these captive populations were established, he subjected them to either a random or a large size-selective harvest scheme, removing 10–20% of the population every two months. The target of selection was body thickness, which was tightly correlated with length, and it was only large body thicknesses that were removed by fishing. After a period of only 3 years (roughly six generations), an evolutionary response was apparent. The selectively-fished population displayed diminished yield and male (but not female) fish grew slower and attained smaller body sizes on average than those from the control group (Fig. 1). Unfortunately, there are limitations in interpreting the outcome of Silliman's experiment. First, the mixed origin of the brood stocks may have introduced higher levels of genetic variation than would normally occur within a single population and this may have increased the likelihood of a rapid evolutionary response. Furthermore, because he did not interbreed the mixed origin brood stocks for several generations prior to the start of the exploitation trials, effects of genetic linkage disequilibrium may have influenced the results. Second, there was no replication of treatments (only one control and one harvested population) so it is not clear that the divergence was actually caused by fishing as opposed to genetic drift. Finally, the divergence occurred in only one gender, suggesting the possibility that sexual selection may have contributed to the outcome. Despite these issues, Silliman's work was ground-breaking in attempting to provide the experimental evidence of fishery-induced evolution. Sadly, his work has been virtually ignored (e.g., Silliman 1975 has been cited only nine times).
Figure 1.
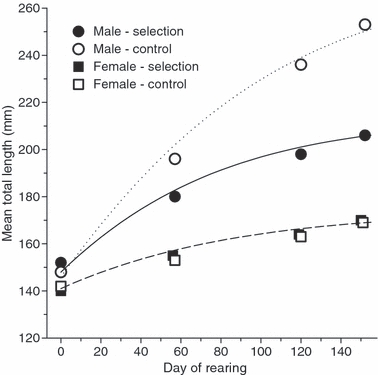
Outcome of a selection experiment on Tilapia mossambica (redrawn after Silliman 1975). A control population was harvested randomly while another was harvested selectively with respect to size (all individuals >25 mm body thickness) every 2 months over a period of 3 years. At the end, 46 size-matched fish each were reared for 150 days. Males from the selectively-fished population grew much slower than the control, while no such response was apparent in females.
Edley and Law (1988) used experimental captive populations of Daphnia magna (Crustacea, Branchiopoda) to track the evolutionary response to size-selective harvest. Their experiment involved replicated treatments of mixed clonal populations with culling based on the selective removal of either large or small body sizes. They observed rapid evolutionary responses to culling. Selective removal of large individuals resulted in lower population yields, decreased size and age at maturation, and lower individual growth rates. Selective removal of small individuals produced the opposite response. While this work has received a moderate amount of attention in the life history evolution literature (39 citations), it did not stimulate widespread concern about fishery-induced evolution probably because the taxon and its life history were viewed as being too far removed from that of harvested fishes.
Conover and Munch (2002) conducted the first fishery-induced selection experiment that involved a harvested marine fish, the Atlantic silverside. This experiment was motivated by the knowledge that this species displays CnGV in growth in the wild (Conover and Present 1990): i.e., growth rate is optimized at any given latitude by the tradeoff between the benefits of growing fast to ensure winter survival and the costs of rapid growth which entails diminished swimming speed and increased vulnerability to predation (as described above). A cluster of additional physiological and behavioral traits covary with growth rate in the wild (Table 1). The Conover and Munch (2002) harvest experiment was designed to measure the rate of evolution of these traits in response to severe size selection imposed by removing the largest 90% or the smallest 90% of the population each generation after the fish had grown to adult size. The results were striking. After only four generations the population yields and mean weights of fish diverged dramatically. Populations subjected to large size harvest quickly evolved lower growth rates, yields and mean fish weights while the small-size harvested populations did the reverse. Moreover, the same cluster of traits that vary with growth across latitudes in the wild (Table 1) also coevolved with growth rate in the fishing experiment (Walsh et al. 2006). Evolved differences in juvenile growth rate were positively correlated with changes in food consumption, growth efficiency, behavioral willingness to forage, fecundity, egg volume, larval size at hatch, larval viability, larval growth, and vertebral number (Walsh et al. 2006). Hence, the populations that evolved slower growth experienced correlated declines in a broad array of traits that collectively determine the per capita rates of energy flow and reproductive output, leading to an overall reduction in fitness. Because the experimental design included replicate populations for each fishing regime, as well as nonfished control populations, there is no question that size-selective harvest caused the genetic changes observed. The results of this experiment, combined with the knowledge of CnGV and its adaptive significance in the wild, provides irrefutable evidence that size selective mortality can cause evolutionary changes that influence the physiology, growth, behavior, and productivity of marine fish populations.
Once selected lines have been developed or identified, they provide the opportunity to test for the reversibility of fishery-induced evolution after fishing pressure is relaxed. If fishery managers are to be precautionary in their approach to conservation, the issue of reversibility is crucial because it determines the rate at which changes wrought by fishing selection might be undone by natural selection acting alone after fishing ceases. Many have speculated that the reversals will be very slow (Law and Grey 1989; Law 2000; de Roos et al. 2006; Dieckmann and Heino 2007; Swain et al. 2007) but, until recently, little hard data existed to address this question. Conover et al. (2009b) filled this empirical gap by extending the Menidia experiment for an additional five generations during which size selection at harvest was relaxed across all lines. They found that those populations evolving smaller size and lower productivity under fishing pressure displayed a very gradual but significant rebound in size after selective fishing ceased. This shows that harvested populations have an intrinsic capacity for reversal of the detrimental evolutionary effects of fishing but the rebound rate may be much slower (2.5 times longer in the Menidia experiment) than that caused initially by directional selection on fish size (Conover et al. 2009b).
Selection experiments in the field
Selection experiments can also be carried out in the field. Such experiments involve the introduction of a known composition of genotypes to a natural environment and tracking their relative fitness over time. This approach has the advantage of greater realism because environmental factors are not controlled by the investigator but has the disadvantage that agents of selection may be of multiple sources and therefore not always easy to identify. Such trials may involve tracing the fate of genotypes over a segment of the life history as they experience episodes of selection. Better still, self replenishing populations may be planted in contrasting environments or subjected to alternative forms of selection and then be allowed to diverge over multiple generations.
Biro and Post (2008) marked and released two genotypes of juvenile trout (Oncorhynchus mykiss) – slow growing/shy versus fast growing/bold – into replicate small, natural lakes, and four months later subjected them to intensive gillnet fishing that removed approximately 70% of the population. Independent of size, gillnets removed twice as many specimens of the fast/active genotype as compared with the slow/shy one. This experiment provided the first direct confirmation that fishing gear selectively alters the genotypic composition of a population. It also shows how behavioral differences associated with growth, rather than body size, may be the target of selection. However, because these populations were not self-reproducing the overall effect of such fishery-induced selection on life history evolution and stock productivity are unknown.
The pioneering experiments by David Reznick and colleagues on guppies (e.g., Endler 1980; Reznick and Bryga 1987; Reznick et al. 1990, 1996) were among the first to explicitly test predictions of life-history theory on multiple generations of natural populations. The authors took advantage of a stream system structured by waterfalls, in which isolated guppy (Poecilia reticulata) populations are adapted to high-predation (downstream) or low-predation (upstream) environments, depending on the abundance and species of co-occuring fish predators. After showing that guppies from high-predation environments consistently mature at smaller sizes and produce more and smaller offspring than their conspecifics in low predation environments, the authors experimentally transplanted guppies from high to low predation-environments (previously guppy free). Many generations later, common garden experiments on the transplanted versus founding populations demonstrated rapid evolution of life history traits in the directions predicted by theory, with significant 5–15% increases in male and female size-at-maturity after 4 and 7.5 years (7–12 generations), respectively. Offspring size, fecundity, and reproduction effort had significantly changed after 11 years (Reznick and Ghalambor 2005). While not specifically designed to measure fishery-induced evolution, these experiments are nonetheless highly relevant because they demonstrate the rapidity of life history evolution in a completely natural setting.
That the implications of these findings on guppy populations for fishery-induced evolution have been largely ignored by fishery scientists is puzzling. Reznick and Ghalambor (2005) argue that guppy populations are a realistic model for fisheries because, like harvested species, they have overlapping generations, the intensity of selection by predators is in the mid- to low range of that imposed by commercial fisheries, and the rate of phenotypic change in guppies is also comparable with that found in exploited fisheries. Moreover, the evolutionary response to predation in guppies involves changes in an array of behavioral and morphological traits similar to those in harvested species (Reznick et al. 2008) and also like those in the Menidia experiments cited above. Ignoring these lessons from guppies apparently reflects (i) a belief that natural predators can be agents of selection but not human predators, or (ii) a taxonomic bias, namely that guppies may evolve rapidly but not the taxons comprising commercially harvested species. Neither of these beliefs seem justifiable to us.
Recent insights from field and laboratory experiments on the Trinidadian killifish, Rivulus hartii, point out the importance of evolutionary responses to the indirect effects of predation-mediated mortality. Walsh and Reznick (2008) examined natural killifish populations adapted to high and low predation intensities. They observed that killifish from high predation localities displayed reduced size and age at maturity, increased reproductive investment and smaller hatchlings. These life history responses are similar to those of the guppies described above and might be attributable to the direct effect of predation. However when rearing second-generation-born killifish at two realistic food levels, the authors found significant interactions between (former) predator environment and food level for most life history traits (i.e., age and size at maturity, fecundity, egg size). These statistical interactions suggest that killifish evolution has not only been directly influenced by predation, but also indirectly by effects of elevated food availability in high predation environments.
Another experimental illustration of fishery-induced selection acting upon behavioral variation involves vulnerability to angling. Philipp et al. (2009) and Cooke et al. (2007) evaluated data from an experimental catch-and-release program involving largemouth bass (Micropterus salmoides) in a large reservoir in Illinois. Between 1977 and 1980, individually angled bass were creeled, tagged, and released. The lake was then drained and those fish angled and released four or more times in 1980 were stocked in ponds and allowed to breed to create a ‘high-vulnerability’ strain (HVF), while those never caught by angling were stocked and bred to produce a ‘low-vulnerability’ strain (LVF). These selected strains were maintained in experimental ponds and were further selected and bred for high-vulnerability or low vulnerability to fishing for three additional generations. The experiment enabled direct estimation of realized heritability for vulnerability, since both the selection differential and the response to selection could be quantified over multiple generations. The analysis of this experiment by Philipp et al. (2009) provided clear evidence that angling vulnerability is indeed a heritable trait (h = 0.15). Redpath et al. (2009) investigated growth and energy characteristics of the two strains, reporting that over a 6 month period LVF fish grew between 9–17% faster than HVF individuals. In addition, Cooke et al. (2007) found that the LVF had lower resting cardiac activities and lower metabolic requirements than the HVF, leading to an estimated 40% reduction in food requirements. Male LVF were also observed to invest less energy in parental care including decreased vigilance against predators. Hence, this selection experiment demonstrated not only that angling vulnerability has a genetic basis in largemouth bass but also that a suite of physiological and behavioral traits are genetically correlated with vulnerability. More importantly, some of these trait correlations were unexpected and would have been difficult to foresee without doing the experiment. For example, it is not obvious why angling vulnerability and parental care behavior should be genetically correlated but the fact that they are has important consequences. As Cooke et al. (2007) pointed out ‘if HVF are selectively harvested from a population, the remaining fish in that population may be less effective in providing parental care, potentially reducing reproductive output.’ They concluded that ‘strong angling pressure in many freshwater systems, and therefore the potential for this to occur in the wild, necessitate management approaches that recognize the potential evolutionary consequences of angling.’
Strengths and limitations of experimental approaches
An experimental, small-scale research program can easily be coupled with the development of theory and act as a stimulus to further research, thereby hastening both understanding of the issues and development of practical solutions. (Benton et al. 2007)
The principal strengths of experiments to understand fishery-induced evolution are well illustrated by the examples described above. First, by employing common garden techniques they excel at isolating the genetic component of phenotypic variation in nature thereby removing any doubt about the evolvability of any given trait(s). This is more than just a trivial exercise because without it, interpretation of field observations might be criticized as adaptive story telling (Kuparinen and Merilä 2008; but see Jørgensen et al. 2008). Moreover, rapid genetic changes may be hidden and remain undetectable because they are masked by simultaneous shifts in the environment, as occurs with CnGV. Second, selection experiments provide direct estimates of the rate of evolutionary change. Third, experimental approaches allow the effects of specific agents of selection to be isolated from other potential environmental factors. This is important because of the need to separate the direct evolutionary effects of fishing mortality from the indirect effects due to habitat alteration and other environmental variations such as climate change. Finally, experiments have shown repeatedly that it is not just single life history traits that evolve but a complex cluster of genetically and physiologically interconnected characters.
Of these contributions, the two most important in our view are the ones unanticipated by and unaccounted for in the current theory of fishery-induced evolution. The first is the propensity for CnGV in growth to evolve (Table 1), which may be of crucial significance in interpreting phenotypic responses due to fishing. Consider the possibility, for example, that fishing constitutes a form of countergradient selection. When a stock is harvested, the reduction in density creates an environment wherein higher food abundance would promote faster growth, at least according to density-dependent theory, but at the same time fishing mortality selects against fast growing individuals. These two conflicting influences may cancel each other out, leaving the appearance of little change in growth as a function fishing pressure, as observed in the meta-analysis by Hilborn and Minte-Vera (2008). Yet the constancy of growth in response to fishing potentially hides a considerable evolutionary change toward slower growing genotypes. While there are no certain examples of rapid evolution of cryptic CnGV in response to a temporally changing environmental in fishes, it has been documented over decadal time scales in a wild bird population (Merilä et al. 2001) and in wild Soay sheep (Wilson et al. 2007).
The second contribution is the multivariate nature of trait evolution as illustrated by the experiments on guppies, silversides, and largemouth bass. Genetic covariances among traits have the potential to accelerate or constrain rates of evolution depending on whether the correlations are positive and negative, respectively. If selective removal of fish from a population, for example, not only removes the fast growers but also those that have higher metabolism, growth efficiency, food consumption rates, reproductive output, stronger parental care ability, and more risky foraging behavior, the combined effect on evolutionary changes in productivity of the population will be much greater than if each trait were to vary in isolation of the others. This is an area of fishery-induced evolution theory that needs much further development.
Finally, an advantage of carefully designed experimental approaches is that they provide incontrovertible empirical results grounded in the biology of a particular species and constrained only by the environmental conditions in which they are conducted. Such empirical measurements, even if obtained within only a microcosm of nature, attract more interest than purely theoretical or numerical simulations that exist only in the abstract, or retrospective field studies that are open to multiple interpretations. We base this conclusion on the observation that early theoretical work on fishery-induced evolution (Law and Grey 1989; McAllister et al. 1992; Stokes et al. 1993) and case histories of phenotypic change in the field (e.g., Handford et al. 1977; Favro et al. 1979; Ricker 1981; Rijnsdorp 1993) were largely ignored for many years by mainstream fishery scientists. Until recently, textbooks on fisheries science and management did not discuss or reference the potential for fishery-induced evolution (see Hilborn and Walters 1992; King 1995; Wootton 1996). In contrast, the Conover and Munch (2002) experiment attracted a great deal of attention (cited 199 times so far) and controversy (e.g., Hilborn 2007; Brown et al. 2008; Hilborn and Minte-Vera 2008). Even though it provided little more than a proof of concept, the silverside empirical model succeeded in stimulating interest in fishery-induced evolution.
Experimental approaches also have major limitations with respect to understanding fishery-induced evolution. The first of these is the simplistic, self-contained environments in which laboratory experiments are typically carried out as compared with say the much more complex, open ocean environments where myriad environmental factors are changing simultaneously. Even the field experiments cited herein took place in relatively simple (i.e., none or only a few interacting fish species), small, enclosed ecosystems. A second problem is one of time scale and taxonomic bias. Because most investigators and funding agencies are not willing to wait decades for results to be obtained, only species with relatively short generation times are amenable for experimental analysis, and these frequently belong to families (e.g., guppies, silversides, tilapia) unrelated to those of the major harvested groups. Results from r-selected species with short life cycles may not translate directly to long-lived species that typically have K-selected or bet-hedging life history strategies.
Another problem for experiments is in adequately simulating the mortality imposed by fishing. The Conover and Munch (2002) experiment has been criticized, and rightly so, because it imposed knife-edged fishing mortality rates much higher than that occurring in most fisheries (e.g., Hilborn 2007; Brown et al. 2008; Hilborn and Minte-Vera 2008). Here again the logistical problem for experiments is one of time scale and also of statistical power. While it may be more realistic to impose moderate selection differentials in such experiments, the downside is that it would take much longer to obtain a statistically significant response. For this reason, it is a long standing practice in experimental research in general to include test levels of the treatment factor that exceed the highest and lowest found in nature. Fortunately, in the case of experiments on fishery-induced evolution, the problem of scaling back to realistic levels is not insurmountable because once an estimate of heritability has been obtained, it can be applied to any other level of fishing selectivity desired. For example, Brown et al. (2008) used the results of the Conover and Munch (2002) to demonstrate that had a more typical fishing mortality regime been applied in the silverside experiment, it would have taken about 30 generations to produce the same result. Few scientists or funding agencies would embark on or support financially an experiment lasting that long. However, Philipp et al. (2009) and Cooke et al. (2007) also found measurable genetic responses after only four generations of selection on vulnerability to angling so rapid evolutionary responses are not unique to the Conover and Munch (2002) experiment. Moreover, this angling experiment employed fishing gear like that used by the recreational fishers, so it closely mimicked selection due to fishing.
Summary and suggestions for future research
Experimental approaches have played a vital role in shaping our understanding of fishery-induced evolution. Their strengths lie in the ability to standardize confounding environmental factors so as to reveal genetic variation, test for the effect of specific agents of selection with replication, determine rates of evolution, measure the covariance among trait clusters, and study the evolution of complex characters like body size (Table 2). The weaknesses include the uncertainties associated with scaling up from simple environments and short-lived species to more complex systems and mimicking the actual selection differentials imposed by fishing. We are at the dawn of Darwinian fishery science, and so it is not surprising that the fishery-induced selection experiments conducted so far have been overly simplistic. The following are suggestions for future directions.
Table 2.
Summary of benefits and limitations of selection experiments to understand evolutionary responses in fish populations
| Benefits of selection experiments | Drawbacks of selection experiments |
|---|---|
| Standardize environmental variation Isolate agent of selection Measure rate of character evolution Control for genetic drift by replication of treated populations Monitor changes in variance Measure evolution of correlated characters Especially useful for complex characters like size Diverged lines become useful for additional tests of theory | Difficulty of maintenance and time required Taxonomic bias (short lived species required) – species not applicable Constant lab environments do not simulate variable conditions in the wild Field experiments involve simple or simplified environments Relatively small population sizes Difficulty of simulating fishing mortality |
There is a need for experimental investigations that establish the genetic basis of differences in age and size at maturity. Common garden experiments could be employed to examine variation among extant fish stocks in nature and selection experiments could be used to measure the rate of evolution and trait covariances under size-selective fishing. Such studies would validate that maturation reaction norms are capable of evolving and thus strengthen the argument that changes observed in field studies (Dieckmann and Heino 2007; Heino and Dieckmann 2008) truly represent evolution. There is also a need for a selection experiment analysis of the consequences of overlapping generations and density dependence on fishery-induced evolution of body size, growth rate, and yield. Because of the possibility that fishery-induced selection might act more strongly on behavior than body size (e.g., Cooke et al. 2007; Biro and Post 2008), experiments where selection is imposed on behaviors that influence vulnerability to fishing, such as rates of foraging, activity, and habitat selection, would also be valuable. We urge the pursuit of field experiments on closed freshwater populations, either by taking advantage of existing lakes where alternative forms of harvest regulation have been in place for many years, or by imposing experimental fishing regimes on a system of small natural lakes or ponds (e.g., Philipp et al. 2009).
We argue that the proper role of experiments is to demonstrate the capacity for evolution under various agents of selection and thereby contribute to improvements in theory, not to mimic any specific fishery or design a management plan. Two unique contributions to theory already provided directly from experimental approaches are the widespread occurrence of CnGV in growth (Table 1), and the realization that selection on body size influences not just growth rate or age at maturity but also a complex array of physiological, behavioral, and morphological traits. Theoretical models of fishery-induced evolution have not yet incorporated such biological complexity but the message from the experiments described herein is clear. Attempts to understand fishery-induced evolution by focusing on single traits such age at maturity or growth rate are biologically unrealistic.
To advance our knowledge of fishery-induced evolution, we urge the need for integrating knowledge across the four main research approaches outlined in the introduction to this paper. Rather than dismiss experiments because they don't mimic real fisheries (Hilborn 2007; Hilborn and Minte-Vera 2008), or discount probabilistic reaction norms because they do not completely eliminate confounding environmental factors (Marshall and McAdam 2007; Kuparinen and Merilä 2008), or fault analytical models or numerical simulations because they make untested assumptions and fail to incorporate biological realism, we need to build on the strengths of each of these approaches. Molecular genetics is the fourth approach which must now be brought more squarely into the picture. Genetic markers associated with phenotypic variation and thereby subject to selection can be monitored over time to detect fishery-induced evolution at the genomic level (Hendry et al. 2000; Allendorf et al. 2008). Artificially selected lines created through experimental fishing on captive populations can be instrumental in identifying candidate genes for analysis in the wild. For populations that are relatively small in size, quantitative genetic approaches can be applied in the wild by using neutral molecular markers to estimate relatedness via pedigree analysis coupled with phenotypic information obtained from captured individuals, thereby allowing the tracking of temporal changes in genetic composition of populations in terms of additive genetic (or breeding) values (e.g., see Coltman et al. 2003; DiBattista et al. 2009).
Despite their limitations, we submit that experimental analyses will continue to play a pivotal role in furthering our understanding of fishery-induced evolution. By exploiting the benefits of tractable species studied in common garden environments or in simple ecosystems in the wild, experimental analyses fill critical gaps in our knowledge that are unattainable by other methods. But it is when we combine this knowledge with data from other approaches, that we become the evolutionary detective, piecing together the evidence from various sources to comprehend the Darwinian dimensions of fishing.
Acknowledgments
We are grateful for many years of financial support from the US National Science Foundation (currently via grant OCE-0425830) and more recently from the Institute for Ocean Conservation Science at Stony Brook University. We thank the editor and two anonymous reviewers for suggestions that greatly improved this manuscript.
Literature cited
- Allendorf FW, England PR, Luikart G, Ritchie PA, Ryman N. Genetic effects of harvest on wild animal populations. Trends in Ecology & Evolution. 2008;23:327–337. doi: 10.1016/j.tree.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Cano JM, Nicieza AG. Microgeographic variation in metabolic rate and energy storage of brown trout: countergradient selection or thermal sensitivity? Evolutionary Ecology. 2006;20:345–363. [Google Scholar]
- Arendt JD. Adaptive intrinsic growth rates: an integration across taxa. The Quarterly Review of Biology. 1997;72:149. [Google Scholar]
- Arendt JD, Reznick DN. Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proceedings of the Royal Society B-Biological Sciences. 2005;272:333–337. doi: 10.1098/rspb.2004.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt JD, Wilson DS. Optimistic growth: competition and an ontogenetic niche-shift select for rapid growth in pumpkinseed sunfish (Lepomis gibbosus. Evolution. 1997;51:1946–1954. doi: 10.1111/j.1558-5646.1997.tb05116.x. [DOI] [PubMed] [Google Scholar]
- Arendt JD, Wilson DS. Countergradient selection for rapid growth in pumpkinseed sunfish: Disentangling ecological and evolutionary effects. Ecology. 1999;80:2793–2798. [Google Scholar]
- Arendt JD, Wilson DS. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:351–356. [Google Scholar]
- Arnott SA, Chiba S, Conover DO. Evolution of intrinsic growth rate: metabolic costs drive trade-offs between growth and swimming performance in Menidia menidia. Evolution. 2006;60:1269–1278. [PubMed] [Google Scholar]
- Benton TG, Solan M, Travis JMJ, Sait SM. Microcosm experiments can inform global ecological problems. Trends in Ecology and Evolution. 2007;22:516–521. doi: 10.1016/j.tree.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Billerbeck JM, Ortí G, Conover DO. Latitudinal variation in vertebrate number has a genetic basis in the Atlantic silverside, Menidia menidia. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1796–1801. [Google Scholar]
- Billerbeck JM, Schultz ET, Conover DO. Adaptive variation in energy acquisition and allocation among latitudinal populations of the Atlantic silverside. Oecologia. 2000;122:210–219. doi: 10.1007/PL00008848. [DOI] [PubMed] [Google Scholar]
- Billerbeck JM, Lankford TE, Conover DO. Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution. 2001;55:1863–1872. doi: 10.1111/j.0014-3820.2001.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proceedings of the National Academy of Sciences. 2008;105:2919–2922. doi: 10.1073/pnas.0708159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman HI, Law R, Marshall CT. The role of fisheries-induced evolution. Science. 2008;320:47. doi: 10.1126/science.320.5872.47b. [DOI] [PubMed] [Google Scholar]
- Brown JJ, Ehtisham A, Conover DO. Variation in larval growth rate among striped bass stocks from different latitudes. Transactions of the American Fisheries Society. 1998;127:598–610. [Google Scholar]
- Brown CJ, Hobday AJ, Ziegler PE, Welsford DC. Darwinian fisheries science needs to consider realistic fishing pressures over evolutionary time scales. Marine Ecology Progress Series. 2008;369:257–266. [Google Scholar]
- Carlson SM, Edeline E, Vollestad LA, Haugen TO, Winfield IJ, Fletcher JM, James JB, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Chiba S, Arnott SA, Conover DO. Coevolution of foraging behavior with intrinsic growth rate: risk-taking in naturally and artificially selected growth genotypes of Menidia menidia. Oecologia. 2007;154:237–246. doi: 10.1007/s00442-007-0825-9. [DOI] [PubMed] [Google Scholar]
- Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. [DOI] [PubMed] [Google Scholar]
- Conover DO. Seasonality and the scheduling of life history at different latitudes. Journal of Fish Biology. 1992;41:161–178. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Conover DO, Munch SB. Faith, evolution, and the burden of proof. Fisheries. 2007;32:90–91. [Google Scholar]
- Conover DO, Present TMC. Countergradient variation in growth-rate – compensation for length of the growing-season among Atlantic silversides from different latitudes. Oecologia. 1990;83:316–324. doi: 10.1007/BF00317554. [DOI] [PubMed] [Google Scholar]
- Conover DO, Brown JJ, Ehtisham A. Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:2401–2409. [Google Scholar]
- Conover DO, Clarke LM, Munch SB, Wagner GN. Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. Journal of Fish Biology. 2006;69:21–47. [Google Scholar]
- Conover DO, Duffy TA, Hice LA. The covariance between genetic and environmental influences across ecological gradients: reassessing the evolutionary significance of countergradient and cogradient variation. Annals of the New York Academy of Sciences. The Year in Evolutionary Biology 2009. 2009a doi: 10.1111/j.1749-6632.2009.04575.x. (in press) [DOI] [PubMed] [Google Scholar]
- Conover DO, Munch SB, Arnott SA. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proceedings of the Royal Society B: Biological Sciences. 2009b;276:215–220. doi: 10.1098/rspb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. Physiological and behavioral consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiological and Biochemical Zoology. 2007;80:480–490. doi: 10.1086/520618. [DOI] [PubMed] [Google Scholar]
- Craig JK, Foote CJ. Countergradient variation and secondary sexual color: phenotypic convergence promotes genetic divergence in carotenoid use between sympatric anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka. Evolution. 2001;55:380–391. doi: 10.1111/j.0014-3820.2001.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Craig JK, Foote CJ, Wood CC. Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biological Journal of the Linnean Society. 2005;84:287–305. [Google Scholar]
- Day T, Pritchard J, Schluter D. A comparison of two sticklebacks. Evolution. 1994;48:1723–1734. doi: 10.1111/j.1558-5646.1994.tb02208.x. [DOI] [PubMed] [Google Scholar]
- DiBattista JD, Feldheim KA, Garant D, Gruber SH, Hendry AP. Evolutionary potential of a large marine vertebrate: quantitative genetic parameters in a wild population. Evolution. 2009;63:1051–1067. doi: 10.1111/j.1558-5646.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- DiMichele L, Westerman ME. Geographic variation in development rate between populations of the teleost Fundulus heteroclitus. Marine Biology. 1997;128:1–7. [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, Ben James J, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edley MT, Law R. Evolution of life histories and yields in experimental populations of Daphnia magna. Biological Journal of the Linnean Society. 1988;34:309–326. [Google Scholar]
- Endler JA. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Endler JA. Natural Selection in the Wild. Princeton, NJ: Princeton University Press; 1986. p. 336. [Google Scholar]
- Favro LD, Kuo PK, MacDonald JF. Population-genetic study of the effects of selective fishing on the growth rate of trout. Journal of the Fisheries Research Board of Canada. 1979;36:552–561. [Google Scholar]
- Finstad AG, Forseth T. Adaptation to ice-cover conditions in Atlantic salmon, Salmo salar L. Evolutionary Ecology Research. 2006;8:1249–1262. [Google Scholar]
- Fuller RC, Baer CF, Travis J. How and when selection experiments might actually be useful. Integrative and Comparative Biology. 2005;45:391–404. doi: 10.1093/icb/45.3.391. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Grether GF. Environmental change, phenotypic plasticity, and genetic compensation. The American Naturalist. 2005;166:115–123. doi: 10.1086/432023. [DOI] [PubMed] [Google Scholar]
- Grether GF, Cummings ME, Hudon J. Countergradient variation in the sexual coloration of guppies (Poecilia reticulata): drosopterin synthesis balances carotenoid availability. Evolution. 2005;59:175–188. [PubMed] [Google Scholar]
- Handford P, Bell G, Reimchen T. A gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Haugen TO, Vøllestad LA. Population differences in early life history traits in grayling. Journal of Evolutionary Biology. 2000;13:897–905. [Google Scholar]
- Haugen TO, Vøllestad LA. A century of life-history evolution in grayling. Genetica. 2001;112:475–491. [PubMed] [Google Scholar]
- Heino M, Dieckmann U. Detecting fisheries-induced life-history evolution: an overview of the reaction-norm approach. Bulletin of Marine Science. 2008;83:69–93. [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- Hilborn R. Faith, evolution, and the burden of proof – the author responds. Fisheries. 2007;32:91–93. [Google Scholar]
- Hilborn R, Minte-Vera CV. Fisheries-induced changes in growth rates in marine fisheries: are they significant? Bulletin of Marine Science. 2008;83:95–105. [Google Scholar]
- Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment. New York: Chapman & Hall; 1992. [Google Scholar]
- Hurst TP. Causes and consequences of winter mortality in fishes. Journal of Fish Biology. 2007;71:315–345. [Google Scholar]
- Hurst TP, Conover DO. Winter mortality of young-of-the-year Hudson River Striped bass (Morone saxatilis): size-dependent patterns and effects on recruitment. Canadian Journal of Fisheries Aquatic Sciences. 1998;55:1122–1130. [Google Scholar]
- Imsland AK, Foss A, Naevdal G, Cross T, Bonga SW, Ham EV, Stefansson SO. Countergradient variation in growth and food conversion efficiency of juvenile turbot. Journal of Fish Biology. 2000;57:1213–1226. [Google Scholar]
- Imsland AK, Foss A, Stefansson SO. Variation in food intake, food conversion efficiency and growth of juvenile turbot from different geographic strains. Journal of Fish Biology. 2001;59:449–454. [Google Scholar]
- Jonassen TM, Imsland AK, Fitzgerald R, Bonga SW, Ham EV, Naevdal G, Stefansson MO, et al. Geographic variation in growth and food conversion efficiency of juvenile Atlantic halibut related to latitude. Journal of Fish Biology. 2000;56:279–294. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. The role of fisheries-induced evolution – Response. Science. 2008;320:48–49. [Google Scholar]
- Kallman KD, Borkoski V. A sex-linked gene controlling the onset of sexual maturity in female and male platyfish (Xiphopohorus maculatus), fecundity in females and adult size in males. Genetics. 1978;89:79–119. doi: 10.1093/genetics/89.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. Fisheries Biology: Assessment and Management. Oxford: Fishing News Booksa, Blackwell Science; 1995. p. 341. [Google Scholar]
- Kinnison MT, Unwin MJ, Hendry AP, Quinn TP. Migratory costs and the evolution of egg size and number in introduced and indigenous salmon populations. Evolution. 2001;55:1656–1667. doi: 10.1111/j.0014-3820.2001.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Kokita T. Potential latitudinal variation in egg size and number of a geographically widespread reef fish, revealed by common-environment experiments. Marine Biology. 2003;143:593–601. [Google Scholar]
- Kuparinen A, Merilä J. The role of fisheries-induced evolution. Science. 2008;320:47–48. [PubMed] [Google Scholar]
- Lankford TE, Billerbeck JM, Conover DO. Evolution of intrinsic growth and energy acquisition rates. II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution. 2001;55:1873–1881. doi: 10.1111/j.0014-3820.2001.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Law R, Grey DR. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology. 1989;3:343–359. [Google Scholar]
- Marcil J, Swain DP, Hutchings JA. Countergradient variation in body shape between two populations of Atlantic cod (Gadus morhua. Proceedings of the Royal Society B-Biological Sciences. 2006;273:217–223. doi: 10.1098/rspb.2005.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CT, McAdam BJ. Integrated perspectives on genetic and environmental effects on maturation can reduce potential for errors of inference. Marine Ecology Progress Series. 2007;335:301–310. [Google Scholar]
- McAllister MK, Peterman RM, Gillis DM. Statistical evaluation of a large-scale fishing experiment designed to test for a genetic effect of size-selective fishing on British Columbia Pink salmon (Oncorhynchus gorbuscha. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:1294–1304. [Google Scholar]
- Merilä J, Kruuk LEB, Sheldon BC. Cryptic evolution in a wild bird population. Nature. 2001;412:76–79. doi: 10.1038/35083580. [DOI] [PubMed] [Google Scholar]
- Munch SB, Conover DO. Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution. 2003;57:2119–2127. doi: 10.1111/j.0014-3820.2003.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Munch SB, Conover DO. Nonlinear growth cost in Menidia menidia: theory and empirical evidence. Evolution. 2004;58:661–664. [PubMed] [Google Scholar]
- Munch SB, Mangel M, Conover DO. Quantifying natural selection on body size from field data: winter mortality in Menidia menidia. Ecology. 2003;84:2168–2177. [Google Scholar]
- Naish KA, Hard JJ. Bridging the gap between the genotype and the phenotype: linking genetic variation, selection and adaptation in fishes. Fish and Fisheries. 2008;9:396–422. [Google Scholar]
- Nicieza AG, Reiriz L, Brana F. Variation in digestive performance between geographically disjunct populations of Atlantic salmon – countergradient in passage time and digestion rate. Oecologia. 1994a;99:243–251. doi: 10.1007/BF00627736. [DOI] [PubMed] [Google Scholar]
- Nicieza AG, Reyesgavilan FG, Brana F. Differentiation in juvenile growth and bimodality patterns between northern and southern populations of Atlantic salmon (Salmo Salar L) Canadian Journal of Zoology. 1994b;72:1603–1610. [Google Scholar]
- Pegg MA, Pierce CL. Growth rate responses of Missouri and Lower Yellowstone River fishes to a latitudinal gradient. Journal of Fish Biology. 2001;59:1529–1543. [Google Scholar]
- Philipp DP, Whitt GS. Survival and growth of Northern, Florida, and reciprocal F1-hybrid largemouth bass in Central Illinois. Transactions of the American Fisheries Society. 1991;120:58–64. [Google Scholar]
- Philipp DP, Cooke SJ, Claussen JE, Koppelman JB, Suski CD, Burkett DP. Selection for vulnerability to angling in Largemouth Bass. Transactions of the American Fisheries Society. 2009;138:189–199. [Google Scholar]
- Power M, McKinley RS. Latitudinal variation in lake sturgeon size as related to the thermal opportunity for growth. Transactions of the American Fisheries Society. 1997;126:549–558. [Google Scholar]
- Present TMC, Conover DO. Physiological basis of latitudinal growth differences in Menidia menidia: variation in consumption or efficiency? Functional Ecology. 1992;6:23–31. [Google Scholar]
- Purchase CF, Brown JA. Stock-specific changes in growth rates, food conversion efficiencies, and energy allocation in response to temperature change in juvenile Atlantic cod. Journal of Fish Biology. 2001;58:36–52. [Google Scholar]
- Redpath TD, Cooke SJ, Arlinghaus R, Wahl DH, Philipp DP. Life-history traits and energetic status in relation to vulnerability to angling in an experimentally-selected teleost fish. Evolutionary Applications. 2009;2:312–323. doi: 10.1111/j.1752-4571.2009.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Bryga H. Life history evolution in guppies (Poecilia reticulata): 1. Phenotypic and genetic changes in an introduction experiment. Evolution. 1987;41:1370–1385. doi: 10.1111/j.1558-5646.1987.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata) Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:791–801. [Google Scholar]
- Reznick DA, Bryga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. [Google Scholar]
- Reznick DN, Butler MJ, Rodd FH, Ross P. Life-history evolution in guppies (Poecilia reticulata) .6. Differential mortality as a mechanism for natural selection. Evolution. 1996;50:1651–1660. doi: 10.1111/j.1558-5646.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK, Crooks K. Experimental studies of evolution in guppies: a model for understanding the evolutionary consequences of predator removal in natural communities. Molecular Ecology. 2008;17:97–107. doi: 10.1111/j.1365-294X.2007.03474.x. [DOI] [PubMed] [Google Scholar]
- Ricker WE. Changes in the average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Rijnsdorp AD. Fisheries as a large-scale experiment on life-history evolution – disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Wilson DS. Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus. Evolutionary Ecology. 1996;10:631–652. [Google Scholar]
- De Roos AM, Boukal DS, Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvanes AGV, Skjaeraasen JE, Nilsen T. Sub-populations of coastal cod with different behaviour and life-history strategies. Marine Ecology Progress Series. 2004;267:241–251. [Google Scholar]
- Savenkoff C, Swain DP, Hanson JM, Castonguay M, Hammill MO, Bourdages H, Morissette L, et al. Effects of fishing and predation in a heavily exploited ecosystem: comparing periods before and after the collapse of groundfish in the southern Gulf of St. Lawrence (Canada) Ecological Modeling. 2007;204:115–128. [Google Scholar]
- Schultz ET, Reynolds KE, Conover DO. Countergradient variation in growth among newly hatched Fundulus heteroclitus: geographic differences revealed by common-environment experiments. Functional Ecology. 1996;10:366–374. [Google Scholar]
- Secor DH, Gunderson TE, Karlsson K. Effect of temperature and salinity on growth performance in anadromous (Chesapeake Bay) and nonanadromous (Santee-Cooper) strains of striped bass Morone saxatilis. Copeia. 2000;1:291–296. [Google Scholar]
- Silliman RP. Interaction of food level and exploitation in experimental fish populations. Fishery Bulletin. 1968;66:425–439. [Google Scholar]
- Silliman RP. Advantages and limitations of ‘simple’ fishery models in light of laboratory experiments. Journal of the Fisheries Research Board of Canada. 1971;28:1211–1214. [Google Scholar]
- Silliman RP. Effect of crowding on relation between exploitation and yield in Tilapia macrocephala. Fishery Bulletin. 1972;70:693–698. [Google Scholar]
- Silliman RP. Selective and unselective exploitation of experimental populations of Tilapia mossambica. Fishery Bulletin US. 1975;73:495–507. [Google Scholar]
- Smith NG, Jones CM, Van Montfrans J. Spatial and temporal variability of juvenile spotted seatrout Cynoscion nebulosus growth in Chesapeake Bay. Journal of Fish Biology. 2008;73:597–607. [Google Scholar]
- Sohn JJ. Socially induced inhibition of genetically determined maturation in the platyfish, Xiphophorus maculatus. Science. 1977;195:199–201. doi: 10.1126/science.831271. [DOI] [PubMed] [Google Scholar]
- Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society B-Biological Sciences. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman RF. Genetic differentiation among seasonally distinct spawning populations of Chum salmon, Oncorhynchus keta. Aquaculture. 1986;57:211–217. [Google Scholar]
- Tallman RF, Healey MC. Phenotypic differentiation in seasonal ecotypes of Chum salmon, Oncorhynchus keta. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:661–671. [Google Scholar]
- Walsh MR, Reznick DN. Interactions between the direct and indirect effects of predators determine life history evolution in a killifish. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:594–599. doi: 10.1073/pnas.0710051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Pemberton JM, Pilkington JG, Clutton-Brock TH, Coltman DW, Kruuk LEB. Quantitative genetics of growth and cryptic evolution of body size in an island population. Evolutionary Ecology. 2007;21:337–356. [Google Scholar]
- Wootton RJ. Ecology of teleost fishes. London, UK: Chapman and Hall; 1996. p. 404. [Google Scholar]
- Yamahira K, Conover DO. Intra- vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology. 2002;83:1252–1262. [Google Scholar]
- Yamahira K, Takeshi K. Variation in juvenile growth rates among and within latitudinal populations of the medaka. Population Ecology. 2008;50:3–8. [Google Scholar]
- Yamahira K, Lankford TE, Conover DO. Intra- and interspecific latitudinal variation in vertebral number of Menida spp. (Teleostei: Atherinopsidae) Copeia. 2006;3:431–436. [Google Scholar]
- Yamahira K, Kawajiri M, Takeshi K, Irie T. Inter- and intrapopulation variation in thermal reaction norms for growth rate: evolution of latitudinal compensation in ectotherms with a genetic constraint. Evolution. 2007;61:1577–1589. doi: 10.1111/j.1558-5646.2007.00130.x. [DOI] [PubMed] [Google Scholar]


