Abstract
In Australia, monitoring Helicoverpa species for resistance to the Cry2Ab toxin in second generation Bacillus thuringiensis (Bt) cotton has precisely fulfilled its intended function: to warn of increases in resistance frequencies that may lead to field failures of the technology. Prior to the widespread adoption of two-gene Bt cotton, the frequency of Cry2Ab resistance alleles was at least 0.001 in H. armigera and H. punctigera. In the 5 years hence, there has been a significant and apparently exponential increase in the frequency of alleles conferring Cry2Ab resistance in field populations of H. punctigera. Herein we review the history of deploying and managing resistance to Bt cotton in Australia, outline the characteristics of the isolated resistance that likely impact on resistance evolution, and use a simple model to predict likely imminent resistance frequencies. We then discuss potential strategies to mitigate further increases in resistance frequencies, until the release of a third generation product. These include mandating larger structured refuges, applying insecticide to crops late in the season, and restricting the area of Bollgard II® cotton. The area planted to Bt-crops is anticipated to continue to rise worldwide; therefore the strategies being considered in Australia are likely to relate to other situations.
Keywords: agriculture, Bacillus thuringiensis, cotton, Helicoverpa species, pest management, resistance, transgenic
Introduction
An increasing problem in agriculture is the evolution of resistance by pests to the agents used to control them. The most significant and well documented case is the development of pest resistance to insecticides (McKenzie 1996). Various scientifically based strategies have been devised to retard the rate at which resistance might develop. The success of these strategies is usually monitored by determining changes in the susceptibility of field populations exposed to insecticides. This is normally achieved by measuring changes in LC50 or in survival of field insects when exposed to a ‘discriminating dose’ of toxin which indicates that genetic changes have occurred in the population (e.g., Forrester et al. 1993; Ali and Luttrell 2009). If the occurrence of resistance is increasing to a particular insecticide, then strategies can be adopted to try and curb further increases (Andow and Ives 2002). The ultimate aim is to prevent failures of the insecticide in the field. However the approach of measuring changes in LC50 is imprecise and the more sensitive technique of monitoring the actual frequencies of alleles that confer resistance is rarely used (Ali and Luttrell 2009). With this latter approach, increasing frequencies of resistance alleles can be used to trigger a more timely response to the impending threat of resistance.
Partly in response to increasing pest resistance to pesticides, insecticidal toxins have been engineered into plants. To date the most common approach to engineering crops for insect tolerance has been the addition of genes coding for insecticidal toxins from the soil bacterium Bacillus thuringiensis (Bt) (Romeis et al. 2006). The first generation of crops expressed a single Bt gene and was commercialized in 1996 in the USA and Australia and later in other countries (Krattiger 1997). Although there have been no measurable shifts in resistance frequencies in most targeted pest populations, there have been four recent claims of field-evolved resistance to proteins in the Cry1A class present in single toxin Bt crops (Busseola fusca, Cry1Ab maize, South Africa; Helicoverpa zea, Cry1Ac cotton, USA; Spodoptera frugiperda, Cry1F maize, Puerto Rico; H. armigera, Cry1Ac cotton, China; Tabashnik et al. 2009). In 2003, second generation Bt crops, containing two independently acting Bt genes, were introduced in the USA and Australia. There have been no published reports of resistance to the additional toxins employed in second generation crops but several studies have established base-line measures for future monitoring (Downes et al. 2010a).
Bt cotton has been widely adopted in Australia since 1996. The first generation product, INGARD® (called Bollgard® elsewhere and expressing Cry1Ac), was capped at 30% by area. This has risen to 85% of the cotton area since the addition of the second generation product, Bollgard II® (expressing Cry1Ac and Cry2Ab) and removal of cap in 2004/2005 (Fitt 2008). A significant benefit has been an 85% reduction in the amount of insecticide active ingredient applied to Bollgard II® (Fitt 2008). The reduction of insecticide usage has resulted in enhanced environmental outcomes and an improving perception of the cotton industry by the general public. The future value of this highly adopted technology is dependent on retaining the susceptibility of the target pest populations of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) to both of the Bt toxins.
The Resistance Management Plan (RMP) for Bt cotton in Australia was initially developed in 1996/1997 by the Monsanto Australia Limited Cotton Team in consultation with the Transgenic and Insecticide Management Strategy (TIMS) Committee of the industry body ‘Australian Cotton Growers Research Association’ (now ‘Cotton Australia’). A Bt Technical Panel of the TIMS Committee was formed in 1995 and since then versions of the RMP have been revised annually by that group. The RMP is based on the widely advocated ‘high dose plus refuge’ pre-emptive resistance management strategy (reviewed in Bates et al. 2005; see below for further details), though the ‘high dose’ component does not strictly hold (also see below). Bollgard II® is available in the full complement of varieties grown in Australia. A comprehensive program has monitored the susceptibility of field populations of the key targets, Helicoverpa armigera and H. punctigera, since 1994 (Downes et al. 2007).
A challenge for deploying the second generation Bt cotton in Australia has been the discovery of elevated frequencies of alleles that confer resistance to one of the toxins (Cry2Ab). Before the widespread adoption of two-gene Bt cotton, the frequency of Cry2Ab resistance alleles was at least 10−3 in H. armigera and H. punctigera (Mahon et al. 2007a; Downes et al. 2009). Of most concern is that in the past 5 years there has been a significant and apparently exponential increase in the frequency of alleles conferring Cry2Ab resistance in field populations of one of those species, H. punctigera, since the adoption of Bollgard II® (Downes et al. 2010a). Furthermore, the frequency of Cry2Ab resistance alleles in populations from cotton cropping areas is eightfold higher than that found in populations collected in the same year from non-cropping regions geographically isolated from cotton production (Downes et al. 2010a). These data suggest incipient evolution of resistance by H. punctigera to Cry2Ab (Downes et al. 2010a).
In response to these findings, the TIMS Bt Technical Panel Committee of Cotton Australia developed a document entitled ‘Contingency Plan for Mitigating Resistance to the Toxins Within Bollgard II® Cotton’. In this paper we outline strategies that may be deployed to delay further increases in Cry2Ab resistance in H. punctigera in Australia, to prevent increases in resistance in H. armigera, and to further build upon the Contingency Plan. Here we review the history of Bt cotton deployment in Australia, and the science behind the RMP for the single and dual gene transgenic Bt cotton technologies. We outline the characteristics of the isolated Cry2Ab resistance that are potentially important for resistance evolution, and use a simple model to predict likely trends in resistance frequencies. We then consider the role of future technologies that may become available in Australia before discussing possible mitigation strategies to contain increases in frequencies of resistance.
Our approach in this paper is defined according to scenarios of Cry2Ab resistance in H. armigera and H. punctigera. We have not found any shifts in the low (<10−3) frequencies of alleles conferring resistance to Cry1Ac in either Helicoverpa species (Mahon et al. 2007a; Downes et al. 2009) and so resistance to this toxin is not explicitly considered in the current Contingency Plan. However, it is envisaged that the document will be reviewed annually and adapted in light of new information on resistance frequencies and characteristics of resistance found in Helicoverpa species.
In 2008 there was more than 130 million hectares of Bt-crops planted for insect control in 25 countries throughout the world (James 2008). The use of transgenic insecticidal crops is predicted to continue to increase (James 2008), and thus adaptive resistance management will become increasingly important. While the finer detail of this article is specific to Australia the broader approach, including potential strategies for mitigating further increases in resistance frequencies, is likely to serve as a model that is relevant to other systems.
BT cotton in Australia
The prime target for Bt cotton in Australia is H. armigera because during the 1990s it became increasingly difficult to control with insecticide sprays (Fitt 2008). In contrast, the Australian endemic species H. punctigera, which is also a key pest, has not evolved resistance to insecticide sprays despite 40 years of pesticide exposure in cotton production (Forrester et al. 1993). This may reflect the different patterns of movement of the two species. In the cropping regions of South-Eastern Australia, H. armigera populations recruit adults in spring from overwintering pupae within the region (Fitt and Daly 1990). In contrast, H. punctigera populations typically peak in the spring, driven by large-scale migration into the region from inland Australia where they are not exposed to insecticides (Oertel et al. 1999). Any resistance that may accumulate in the small resident population of H. punctigera in the cropping regions that is exposed to insecticides is thought to be periodically diluted by susceptible individuals emigrating from these inland sources (Gregg et al. 1995).
The Cry1Ac toxin produced by INGARD® is very effective against moths of the genera Pectinophora and Heliothis (major pest targets in the New World) but is less potent to members of the genus Helicoverpa which are innately more tolerant of Cry toxins (Liao et al. 2002). In Australia, INGARD® varieties provided excellent protection against H. armigera and H. punctigera early in the season but once plants flowered, the promoter became progressively less effective (Olsen et al. 2005) and the reduced levels of toxin in the plant tissues allowed H. armigera and H. punctigera to survive. Clearly some toxin remained because the larvae grew more slowly on older INGARD® plants, but survival rates were comparable to those on non-Bt cotton (Fitt 2008). Season-long averages of pupae production, which indicate survival of larvae, under INGARD® were 55% of that under non-Bt cotton (Baker et al. 2008).
INGARD® varieties were grown for seven seasons in Australia and in the 2004/2005 season were removed from the market and replaced with Bollgard II® varieties. Bollgard II® cotton was produced by inserting a cry2Ab gene (together with an antibiotic marker gene) into the genomic DNA of INGARD® cotton (APVMA 2003). In Bollgard II®, the Cry2Ab toxin is expressed more uniformly throughout the season than Cry1Ac (Greenplate et al. 2003), while expression levels of Cry1Ac in Bollgard II® are similar to that in INGARD® (APVMA 2003). Bollgard II® provides almost season-long protection against H. armigera and H. punctigera, although based on knowledge of Cry1Ac expression in INGARD® it is presumed that in older plants only Cry2Ab provides effective control. However, Greenplate et al. (2003) showed that the combined actions of the toxins can be additive and therefore it is likely that on occasions Cry1Ac continues to contribute some level of mortality of larvae in the field late in the season, as suggested by the work of Mahon and Olsen (2009).
In any year around 15% of Bollgard II® crops may support >1 medium-large Helicoverpa larva/m during mid- to late-flowering (Whitburn and Downes 2009). For various reasons these larvae are not always treated or adequately controlled with insecticidal sprays. Some survive to pupation and emerge as healthy moths. Survival is not a consequence of a physiological resistance to Bt, as larvae collected (as eggs) from various crops are just as likely to carry Bt resistance genes as larvae collected as survivors on Bollgard II® plants (Whitburn and Downes 2009). Indeed, most larvae collected as survivors on Bollgard II® plants prove to be susceptible, and the few larvae that carry a Bt resistance gene are heterozygotes and should be killed by the plant because resistance is recessive (see below). Because survivors are physiologically susceptible to Bt toxins, we presume that larvae surviving on older plants are exposed to a non-lethal dose of toxin. This finding has relevance to resistance evolution because for a period in the season the high-dose target is compromised for both toxins (see below).
The resistance management plan for BT cotton in Australia
To slow the evolution of pest resistance, a pre-emptive RMP that proposed a ‘high-dose’ plus ‘refuge’ was adopted by the USA, India and Australia upon introducing insecticidal Bt transgenic crops (Tabashnik et al. 2009). When formulating this strategy, it was assumed that heterozygotes may be partially resistant to the toxin, and if so, would survive a low dose of the toxin but not a ‘high’ dose (Gould 1998). Ideally transgenic plants would produce toxin at concentrations that were several times greater than those which kill susceptible insects, to render heterozygotes functionally susceptible (Gould 1998). The survival rate of heterozygotes is important because they carry the vast majority of resistance alleles in the population and resistance evolves far more rapidly if they possess even a slight advantage over fully susceptible insects.
The role of the refuge is to produce large numbers of ideally homozygous susceptible insects that have not been exposed to Bt toxin. The large numbers of such insects from refuges presumably mate with the occasional homozygous resistant insects that survive in the transgenic Bt crop (Tabashnik et al. 2004). The offspring of such matings will be heterozygous and thus functionally susceptible to a high-dose of Bt toxin (Gould 1998).
When INGARD® was released in Australia, the conditions necessary for a ‘high-dose plus refuge’ RMP were not fully met because H. armigera and H. punctigera are innately moderately tolerant of Cry toxins, including Cry1Ac (Liao et al. 2002). A conservative RMP was introduced for INGARD® because it was envisaged that within a few years, an improved two-gene product would be available and the value of the combination would be diminished if resistance to Cry1Ac had already evolved.
The INGARD® RMP had several components based both on our understanding of the evolution of resistance and practical considerations:
A ‘cap’ was imposed that limited the area of INGARD® that could be grown on a farm and thus reduced the selection pressure placed on the technology (McKenzie 1996). The INGARD® area was 10% of the total cotton area in the year the technology was introduced (1996/1997) and gradually increased to a maximum of 30% of the total cotton area by 2000/2001.
A ‘planting window’ was imposed whereby sowing was effectively restricted to 42 days to limit the length of the season that Bt cotton could serve as a host for Helicoverpa spp. and thereby limit the number of generations of insects that were exposed to the toxin (McKenzie 1996).
Growers of INGARD® were required to plant a non-Bt refuge crop (Gould 1998; Tabashnik et al. 2008). Prior to deploying INGARD®, it was established that some crops produced more Helicoverpa moths per unit area of crop than others (Baker et al. 2008). The RMP defined the area of a refuge crop required for a given area of INGARD® depending on that crop's productivity for Helicoverpa moths. The reference crop for ‘productivity’ was cotton that was not sprayed for Helicoverpa (hereafter ‘unsprayed’), and modeling indicated that this refuge set at 10% of the area of the Bt crop should be adequate to retard the evolution of resistance. If the designated refuge was unsprayed pigeon pea, which produced the most moths, only 5% of the area of INGARD® was required (i.e., 100 ha of INGARD® required a 5 ha unsprayed pigeon pea refuge). If sprayed non-Bt cotton was chosen as the refuge, 50% of the INGARD® area was required. Other refuges were 15% of unsprayed sorghum and 20% of unsprayed corn that was planted in three sequential stages to extend the period over which they were attractive to moths such that it aligned with that of cotton. Resistance in H. punctigera was not considered to be a high risk; thus although corn and sorghum are rarely hosts for H. punctigera, they were permitted to be grown as a refuge (Baker et al. 2008).
After harvest it was mandatory to cultivate the soil where INGARD® had been grown to kill possibly resistant pupae undergoing winter diapause (Forrester et al. 1993).
Insecticidal products that contained Bt toxins could not be used in the sprayed non-Bt cotton refuge, to reduce exposure to Cry toxins (McKenzie 1996).
Volunteer Bt cotton plants were required to be removed because if they were present in a crop that is also a host for Helicoverpa, larvae could grow on that crop to a size that could tolerate Bt toxin and may then migrate to the volunteer Bt cotton plants. This situation could select for survival of heterozygous, Bt-resistant larvae. Volunteer host plants of non-Bt cotton in Bt cotton fields pose similar threats and were also required to be removed. Additionally, cotton is capable of outcrossing, and volunteer plants resulting from a cross between Bt and non-Bt varieties will result in plants heterozygous for the Bt gene, and thus express toxin at a reduced level which may favor heterozygote Bt-resistant insects.
Although not a formal component of the RMP, a complementary document advised that ‘for preventative resistance management of Heliothis (Helicoverpa) late-season larval populations must be controlled with an effective insecticide’ (e.g., Schulze and Tomkins 2002). The same guidelines advised that ‘INGARD® should not be utilized as a stand alone pest control measure’ and outlined principles of integrated pest management (IPM) including the selection of effective pesticides for Helicoverpa control that are the least disruptive to the beneficial insects and spiders present in the crop.
In theory, a two-gene transgenic crop is predicted to be significantly more robust than a single-gene transgenic crop in retarding the evolution of resistance because the rare insect that is resistant to one toxin will be killed by the second (Roush 1998; Zhao et al. 2005). Therefore, when Bollgard II® was introduced to Australia, INGARD® was removed from the market to reduce the threat of pest resistance developing to the Cry1Ac-toxin deployed in both of the Bt cotton technologies. However, the conservative RMP adopted for INGARD® was relaxed by the removal of the 30% cap, allowing for up to 95% of a grower's arable land to be planted to Bollgard II® if the smallest refuge option of 5% unsprayed pigeon pea was used. The RMP for Bollgard II® (Farrell 2008) retains all of the other elements of the INGARD® RMP but the area requirement for sprayed non-Bt cotton was increased to 100% of the Bollgard II® area to compensate for the potentially increased efficacy of new insecticide sprays. H. armigera and H. punctigera are also innately moderately tolerant of Cry2Ab (Liao et al. 2002), thus, as with INGARD®, the conditions necessary for a ‘high-dose plus refuge’ RMP are not fully met.
The effectiveness of the RMP for Bt cotton has been evaluated by a resistance monitoring program run by the Commonwealth Scientific and Industrial Research Organization (CSIRO) and funded by the Cotton Research and Development Corporation (Downes et al. 2007). As part of the stewardship of their technology, Monsanto also monitor for resistance. The screening method, known as F2 screens (Andow and Alstad 1998), generates isofemale lines that produce a proportion of individuals which are homozygous for haplotypes present in their field-derived parents and is thus capable of detecting both dominant and fully recessive forms of resistance. Once a resistant colony is established it becomes possible to employ it to assay the frequency of the resistance using an F1 screen which involves crossing a field insect (of unknown genotype) to a homozygous resistant insect and screening the F1 offspring for resistance (Gould et al. 1997; Liu et al. 2008; Yue et al. 2008). Both methods are currently used in Australia to estimate frequencies of Cry2Ab resistance alleles. The precise details of the methods used and results are detailed in Downes et al. (2007) and Mahon et al. (2007a, 2010). When INGARD® was grown, the focus for resistance testing was on H. armigera but in 2004/2005 H. punctigera was comprehensively incorporated into the program upon detecting an allele conferring resistance to Cry2Ab.
Characteristics that influence resistance evolution
To date, all unique Cry2Ab resistant isolates of H. armigera (15) and H. punctigera (6) that have been tested proved to be allelic (Mahon et al. 2008; Downes and Mahon, unpublished data); thus it is appropriate to treat the frequencies obtained from resistance testing as estimates of one common form of resistance in each species. Interestingly, colonies formed from the first isolated Cry2Ab resistant isofemale line of H. armigera (designated SP15) and H. punctigera (designated Hp4-13) display characteristics of resistance that are remarkably similar. Moreover, in both species, resistance results from an alteration to the target site for the toxin (a protein imbedded on the surface of midgut: Caccia et al. 2010).
Unless stated otherwise, the following information is relevant to both H. armigera and H. punctigera. Insects that are resistant to Cry2Ab are not cross resistant to Cry1Ac. At a concentration of 0.25 μg/cm2 all individuals in the colonies are susceptible to Cry1Ac, but not more so than susceptible insects (Mahon et al. 2007b; Downes et al. 2010b). Thus when Bollgard II® expresses Cry1Ac and Cry2Ab optimally, Cry2Ab-resistant insects will be controlled which will slow the rate of resistance evolution.
Resistance is due to a single gene, and is recessive across concentrations ranging from 0.01 to 1.0 μg/cm2 (Mahon et al. 2007b; Downes et al. 2010b). Thus all heterozygotes (RS) are functionally susceptible and only homozygous resistant insects can survive on Cry2Ab toxin. This result is of great significance, because even a small selective advantage to heterozygotes will greatly increase the rate of resistance evolution (McKenzie 1996). The degree of resistance is also important as resistant insects need to survive levels of toxin present in transgenic cotton if they are to pose a threat. Resistant colonies of both H. armigera and H. punctigera show no response to different doses of toxin and can tolerate doses of Cry2Ab toxin that approach 15 times the upper level in field-grown Bollgard II® in Australia (Downes et al. 2010b).
Fitness costs have been demonstrated in Lepidoptera resistant to Cry1Ac-expressing cotton (reviewed by Gould 1998; Gassmann et al. 2009). Such costs could retard the rate of evolution of resistance, and potentially prevent any increase in resistance frequency. It could be argued from first principles that any fitness costs associated with Cry2Ab resistance in Helicoverpa are likely to be recessive. However, it is unlikely that a dominant fitness cost of any importance could exist at frequencies much above the mutation rate (Hartl 1999) which is unlikely to be as high as the frequency of at least 0.001 found in both Helicoverpa species before widespread selection by Bollgard II®.
Investigations of individual components of the lifecycle for the H. armigera SP15 colony detected no evidence of dominant or recessive fitness costs. No differences in survival or growth rates of homozygous resistant, heterozygotes and homozygous susceptible genotypes were found when they were reared on non-Bt cotton (Mahon and Olsen 2009) or pigeon pea or at high or low temperatures or after enduring six months in diapause (Rodney Mahon, unpublished data). A search for the presence of fitness costs was also made using population cages where the resistance frequency was initially set at 0.5 and the populations were maintained for nine generations (>12 months) in the absence of selection (with Cry2Ab). If fitness costs were present (either dominant or recessive), the frequency of the resistance allele would decline over time. No such decline occurred (Mahon and Young 2010). Thus if any fitness costs are associated with SP15 resistance, they are unlikely to be of sufficient magnitude to retard the evolution of resistance.
In H. armigera and H. punctigera resistance to Cry2Ab was detected at a Bayesian frequency of 0.002 with a 95% credibility interval (CI) between 0.0006 and 0.005 and 0.0002 and 0.005, respectively, in F2 tests performed from 2002 to 2004 before the widespread adoption of Bollgard II® cotton expressing Cry2Ab (Mahon et al. 2007a; Downes et al. 2009). Across years that both F2 and F1 screen data are available, the mean difference in frequencies between the two types of screens is 4.5-fold and 4-fold for H. armigera and H. punctigera, respectively (Downes et al. 2010a; Mahon et al. 2010). If we assume this difference is constant, we can use this factor to extrapolate backwards in time to the periods when only F2 data are available to predict that before the widespread adoption of Bollgard II® the F1 screen frequency of Cry2Ab resistance genes in H. armigera and H. punctigera would have been around 0.01 and 0.008, respectively.
Previously we proposed that an agent other than Bt cotton may favor selection for alleles conferring resistance to Cry2Ab in Helicoverpa species (Mahon et al. 2007a). This notion is supported by the presence of Hp4-13-like resistance alleles in populations of H. punctigera from non-cropping areas of inland Australia (Downes et al. 2010a). However, to drive the increasing frequencies of Cry2Ab resistance alleles in H. punctigera, the hypothesized agent must have increased in efficacy within cropping regions at the time that Bollgard II® was introduced without doing so in the sampled non-cropping areas. This coincidence seems unlikely, though another selective agent may have raised the frequency of Cry2Ab resistance alleles to a relatively high level prior to Bollgard II® being introduced.
Furthermore, the resistance frequencies of populations from cropping areas are significantly greater than those of populations from non-cropping areas (Downes et al. 2010a). The current resistance frequencies in non-cropping populations (0.006, 95% CI 0.002 and 0.012) are perhaps similar to those predicted for cropping populations before opportunities for significant selection by Bollgard II® (0.008; see above). These sources of evidence strongly suggest that the Cry2Ab toxin expressed in Bollgard II® cotton is responsible for the increasing frequencies of resistance alleles reported in Downes et al. (2010a).
Modeling the development of resistance
The characteristics of the Cry2Ab resistance explored above have been incorporated as parameters in a simple simulation model. There are strengths and weaknesses in the setup of the model that require consideration before interpreting its predictions. It is important to note that the model is a guide and should not be used in a definitive sense.
The model incorporates our latest data on the frequencies of resistance alleles in both Helicoverpa species for Cry1Ac and Cry2Ab resistance. Such data indicate that alleles conferring resistance to Cry1Ac are extremely rare. The model assumes that when Cry1Ac levels are high (pre-flowering), no larvae survive on Bollgard II®, but a decline in Cry1Ac toxin after flowering allows susceptible Helicoverpa to survive. Specifically, this situation is represented in a simplified form such that Cry2Ab levels in the plant remain toxic throughout the season, whereas Cry1Ac is absent for the final generation. As mentioned above, the basis for this assumption depends on two sources of information. Firstly, the mean season-long pupae count under INGARD® was 55% of that under non-Bt cotton (Baker et al. 2008). Secondly, the cry1Ac gene and its promoter present in Bollgard II® have not changed from that in INGARD® ([APVMA] Australian Pesticides and Veterinary Medicines Authority 2003). It is also known that there are three to four generations of Helicoverpa on cotton per year and in contrast to Cry1Ac, the titer of Cry2Ab toxin remains relatively stable throughout the season (Greenplate et al. 2003).
The model accommodates the structured refuge by assuming that Bt cotton and non-Bt cotton are equally attractive thus we expect eggs from adults to be deposited on an area equivalent to the proportional size of the non-transgenic crop (10%). It ignores the impact of ‘non-structured’ refuge in the environment such as neighboring host crops or weed and remnant vegetation hosts. In some cotton-growing areas, this may underestimate the input of moths not exposed to toxin. However in other areas, during mid to late summer (the period when Cry1Ac production in INGARD® declined) cotton and the associated structured refuges may represent the bulk of healthy vegetation suitable to maintain Helicoverpa populations. Further, because Helicoverpa are highly mobile, if resistance evolves in one area, it is expected to rapidly diffuse through populations in all cropping areas, as occurred with H. armigera that were resistant to synthetic pyrethroids (Forrester et al. 1993). The remaining parameters in the model are; resistance to Cry2Ab is completely recessive, there are no fitness costs, resistance is due to a single gene, mating occurs at random throughout all populations and, population growth is not restricted by density dependent factors.
In Fig. 1, the model predicts the ‘durability’ of Cry2Ab toxin with different starting frequencies for the resistant allele. Durability is represented as generations until the frequency of the resistance allele (R) reaches 0.5 (50%). Given that there are approximately four generations a year, dividing the number of generations by four, estimates the number of cotton growing seasons that the model predicts the Helicoverpa species will remain largely susceptible to Cry2Ab toxin. The frequency of resistance found by the CSIRO monitoring program during 2008/2009 is highlighted in the figure separately for each species with a circle and the range of predicted generations until control failure drawn in a line above the predicted curve. The range was determined by adjusting the frequency of resistance applied in the model to the upper and lower CIs around the estimates of gene frequencies.
Figure 1.
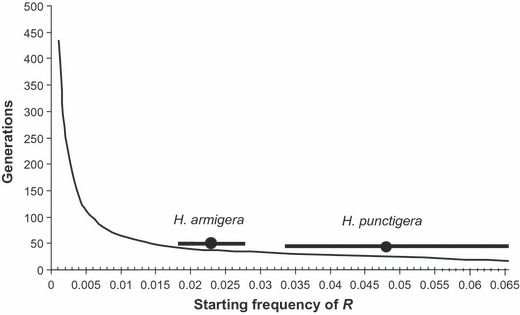
The output of a simple computer model depicting the number of generations of Helicoverpa species before field failures occur (R = 0.5), and indicating the current frequency of R for H. armigera and H. punctigera based on the 2008/09 CSIRO monitoring results using F1 screens (Downes et al. 2010a; Mahon et al. 2010). Bayesian frequencies are indicated separately for each species with a circle and the range of predicted generations until control failure, based on 95% credibility intervals around those frequencies, drawn in a line above the predicted curve.
The predicted longevity of the two-gene cotton is dependent on the frequency of resistance prior to the release of Bollgard II® and it would be greatly extended if, as it was reasonable to assume, the frequency of Cry2Ab resistance alleles was rare. Under the specific set of conditions described above, the model predicts resistance to evolve in 36 generations (around 9 years) with a range of 44–32 generations for H. armigera and 24 generations (around 6 years) with a range of 48–28 for H. punctigera. The model predicts a steady increase in resistant allele frequency similar to the gradual increase that is observed in H. punctigera (Downes et al. 2010a).
Future technologies for Australia
The toxins naturally produced by Bt, and particularly Cry toxins, have been the primary source of proteins used to produce insect-resistant crops. Other sources of toxins for insect-resistant crops are available from plants, other bacteria, insects and arachnids, but it is likely that there will be a considerable delay before they will be deployed (see review by Malone et al. 2008). There is also the potential to ‘silence’ vital genes of the insect using RNAi (Huvenne and Smagghe In Press). This technique involves inserting an artificial construct of DNA from an essential insect gene into the genome of the plant. The configuration of the construct ensures that a section of double stranded RNA is produced that is recognized by the plant as belonging to a virus and therefore sliced into short lengths. When ingested, those short lengths of RNA induce a response in the insect to deactivate its RNA for that gene and thereby ‘silence’ it such that the insect fails to thrive. This technique has considerable potential and has been shown to suppress the growth of H. armigera by preventing, or at least reducing, the ability of the larvae to breakdown gossypol, a secondary compound found in cotton (Mao et al. 2007).
Cry toxins other than Cry1Ac and Cry2Ab that have an impact on Lepidoptera are known, however the number that is sufficiently toxic to be useful is limited. In addition, insects resistant to one toxin may show cross resistance to closely related toxins, further limiting the number of useful toxins. For example, Cry2Ab resistant H. armigera are also resistant to Cry2Aa and Cry2Ae toxins (Mahon et al. 2007b; Caccia et al. 2010). Thus if resistance to Cry2Ab occurs in Australian populations, substituting existing transgenic cotton with others that produce another Cry toxin from the Cry2 class is unlikely to be useful.
A non-Cry Bt toxin that is effective against Lepidoptera, including Australian Helicoverpa (Llewellyn et al. 2007), is VIP (vegetative insecticidal protein). This protein does not form the crystals characteristic of Cry toxins. It is presently being developed by Syngenta Seeds, Inc. for cotton varieties in USA called VipCot™, which expresses Vip3A and Cry1Ab toxins. In January 2010 Syngenta Seeds, Inc. announced that it has licensed its transgenic cotton event, COT102, containing the novel Vip3A protein, to Dow AgroSciences, which plans to combine it with WideStrike® Insect Protection to develop a product expressing Cry1Ac, Cry1F and Vip3A (http://www.nk-us.com/).
In December 2009, another Syngenta licensee of the COT102 event, Monsanto Company, announced plans to stack COT102 with Genuity™ Bollgard® II to create a future insect control product, Bollgard III® (http://www.nk-us.com/). Monsanto expect to introduce Bollgard III® to the Australian market place in 2014/2015 (subject to regulatory approval). It is expected that a 2-year period will follow when both the new technology and Bollgard II® will be grown. Computer models predict that by the time Bollgard III® is released, unless further increases in frequencies of Cry2Ab resistance alleles are restricted, much of the longevity of a three-gene pyramid will be forfeited. After the discovery of the surprisingly elevated frequencies of resistance to Cry2Ab present in Helicoverpa species that threaten the future efficacy of Bollgard II® it is essential to determine the pre-existing resistance status of Helicoverpa species to Vip3A. A high priority should be placed on determining baseline levels of resistance to that toxin in order to set a responsible RMP prior to the release of Bollgard III® cotton. Any mitigation strategies employed before the release of this technology will be temporary – currently, deployment of Bollgard III® is the most effective available option for curbing further increases in Cry2Ab resistance alleles.
Possible mitigation strategies
In response to the current frequencies of Cry2Ab resistance alleles in both Helicoverpa species, and particularly the recent increases in frequencies of Cry2Ab resistance alleles in H. punctigera, the industry has adopted a number of measures to clarify aspects of the RMP. These include further defining existing conditions within the current RMP to ensure alignment with the original intentions of the document. For example, to better ensure that refuges are attractive to ovipositing moths during the period that Bollgard II® is attractive, the timing of planting of refuge crops has also been restricted to a shorter period. As part of the stewardship of their technology, Monsanto annually revise their auditing procedures to improve compliance with the current RMP.
Below, potential strategies for mitigating further increases in frequencies of Cry2Ab resistance alleles have been listed. We briefly mention for each proposed action the likely practical impact on growing Bollgard II® cotton; these comments apply to the majority of growing areas but may not be appropriate in all circumstances. It should be noted that because there is no evidence of fitness costs associated with Cry2Ab resistance in either Helicoverpa species, none of the proposed strategies are expected to result in a significant decline in frequency of individuals carrying resistance alleles. Rather, the objective is to contain any further increases in order to maximize the longevity of the third generation Bt cotton. However, a decline in Cry2Ab allele frequency in H. punctigera may occur through a fortuitous occurrence of a major immigration of susceptible moths from inland Australia. Long-term, pheromone trap records from the cropping region near Narrabri, N.S.W. show that the abundance of H. punctigera in the first generation (spring) is highly variable between years (Baker et al. 2010). This pattern suggests that recruitment of moths to cropping regions from inland areas is erratic. Since 1992, there have been 5 years in which large catches (overall means >10 male moths/trap/night) have been recorded at the Narrabri trapping grid for the spring generation (1992, 1993, 1995, 2000 and 2005).
Remove crops that are not hosts for all target pests as options for the structured refuge
Currently the RMP for irrigated Bollgard II® allows irrigated, unsprayed maize or sorghum in three sequential sowings (20% and 15% of the total crop, respectively) as a refuge. However, these crops are not hosts for H. punctigera (Zalucki et al. 1986). If such crops were removed as structured refuges, hence allowing only options that are suitable hosts for both Helicoverpa species, it is likely there would be a minor overall effect on resistance development since maize and sorghum are rarely chosen and together comprise <7% of the refuges grown in a season. Consequently, for the majority of growers, there is likely to be no practical changes involved with this mitigation strategy. In the following strategies we presume that from 2010/2011 maize and sorghum will no longer be refuge options. However, it is worth noting that irrespective of the structured refuge, in some regions there has been a significant increase in the acreage of maize and sorghum in recent years which likely contributed significantly to the unstructured refuge for H. armigera.
Structured refuges are not to be disturbed until the subsequent spring
Although the current RMP recommends this strategy, there is thought to be little, if any, compliance with the request. Consequently, potentially resistant pupae in soil below Bollgard II® and predominantly susceptible pupae below refuge crops are being killed through soil disturbance at the same rate. If pupae below refuges were allowed to remain undisturbed through winter and emerge after diapause then they would be more numerous relative to the numbers of potentially resistant pupae from Bollgard II® fields which are required to be disturbed as part of the RMP. Previous work indicates that overwinter survival of pupae is around 50% (Fitt and Daly 1990), whereas full cultivation of cotton fields can reduce survival to near zero. This change is likely to have a moderate impact on resistance through effectively enhancing the efficacy of refuges. A negative aspect of this practice is that it will result in an increased population of early season moths. With adequate planning, and the current abundance of fallow country (due to drought), this strategy would involve only a minor change of practice for growers.
Increase the area of structured refuges relative to the area of Bollgard II®
With this strategy the same refuge cropping options will be available, but a greater area of each would be grown. Obviously, the impact of this strategy on resistance development will be influenced by the magnitude of the increase in refuge size. As an example, if the proportional area of refuge was doubled from the current requirement, the impact on the development of Cry2Ab resistance in H. punctigera would shift from 24 generations down to 36 generations (based on the simple model described above). This option for growers would be costly through the need to dedicate more land and resources to refuge. In addition, at least initially, the availability of seed for refuge crops may be limiting.
Treat Bollgard II® with conventional insecticides
The application of an insecticide spray that targets Helicoverpa species on Bollgard II® should kill a high proportion of any resistant individuals and thus selectively reduce the frequency of this genotype in the population. If applied correctly it is believed that this approach could exert a moderate impact on the rate of evolution of resistance but the frequency and timing of applications would be critical. Based on the period of efficacy of Cry1Ac in INGARD®, the opportunity for selection of Cry2Ab resistant insects is likely to span a period of at least 6 weeks. Until resistance frequencies have increased to the point that field failures are imminent, sprays would need to be applied on fields that are not at threshold levels of larvae. Thus, any spray program must be largely prophylactic and applied over an extended period. There are obvious trade-offs with this approach, including the potential to cause resistance to conventional insecticides as well as the potential incompatibility of such an approach with IPM principles.
An attractant designed to lure foraging Helicoverpa moths is commercially available in Australia (Gregg et al. 2010). When used with insecticides, a single application will kill a high proportion (at least 50–80%) of predominantly female moths in the treated area for 4–6 days (Del Socorro et al. 2010; Magnet® label). Since these applications are applied to less than 2% of the crop and have a negligible impact on beneficial species, they could provide a way of killing moths from Bollgard II® without disrupting the beneficial fauna in the crop. This approach would also be substantially cheaper than broad scale applications of insecticide sprays. However, field studies have found that after an application of an attractant-insecticide mixture to a non-Bt cotton crop, egg densities on non-Bt cotton in areas several kilometers from the treated areas can be significantly reduced (P. Gregg and P. Grundy, unpublished data). Thus it is apparent that moths up to several kilometers away from the treated areas may ultimately be affected because of attraction to the treated area or via another mechanism. Irrespective of the reason, an application of the attractant-insecticide mixture on Bollgard II® will not only kill some of the moths emerging from Bollgard II® fields but also may kill a proportion of the (mostly) susceptible ‘refuge’ population. If so, the magnitude of the depletion of the refuge population relative to the Bollgard II® population, along with the absolute kill of moths on Bollgard II® and subsequent opportunities for selection of the larval stage, will be critical in determining the relative frequencies of resistant insects in the population. While substantial research has validated the efficacy of the noctuid moth ‘attracticide’ (reviewed in Gregg et al. 2010), further work is necessary to establish if it will assist in retarding the evolution of resistance and be assigned a role in resistance management.
Impose a 30% cap on the area of Bollgard II® cotton per farm
This strategy would likely have a high impact on resistance evolution through reducing the area of Bollgard II® and thus selection pressure. Even though control of Helicoverpa in the 70% of non-Bt cotton would require insecticide sprays, this would represent more than double the current area of non-Bt cotton relative to the area of Bollgard II® required as a structured refuge. However the practical implications of this measure are high. These include a presently unanticipated increase in the availability of seed of non-Bt cotton and insecticide; that land in sensitive areas set aside for Bollgard II® may not be suitable for growing non-Bt cotton (or other crops) requiring insecticide sprays; and lack of experience by newer growers in managing the agronomy and pest complex of non-Bt cotton.
Withdraw Bollgard II® cotton from the Australian market until Bollgard III is available commercially
This strategy would likely have a significant impact on resistance evolution because the major selection pressure would be removed. The practical implications, which are the same as those considered above for the imposition of a cap but to a greater degree, are significant.
Concluding comments
In Australia monitoring for resistance has provided an early warning of increases in frequencies that may lead to failures of the transgenic Bt technology. Until the release of a third generation Bt cotton, the main potential strategies to mitigate further increases in resistance frequencies include mandating larger structured refuges, applying insecticide to crops late in the season, and restricting the area of Bollgard II® cotton. The most immediate challenge for the Australian cotton industry is to identify which of these alternatives are feasible and appropriate in response to measured shifts in frequencies of Cry2Ab resistance.
The strategies proposed herein are considered primarily based on scientific merit. However, their implementation relies critically on practical and financial considerations. For example, imposing a 30% cap on the area of Bollgard II® cotton per farm ranks higher in terms of impact on resistance development than increasing the area of structured refuges relative to the area of Bollgard II®. However, although the former involves a considerable reversion of farming operations back towards managing non-Bt cotton, the latter may have a greater impact on the bottom line of operations through devotion of greater resources towards growing structured refuges.
While the strategies presently being considered in Australia are likely to be relevant to other situations, a necessary precursor is the capacity to score the frequency of recessive forms of resistance through genetic tests. Without that capacity, monitoring will rely on the use of phenotypic tests where a change in survival rates may be detected too late to attempt mitigation strategies (Andow and Ives 2002).
Acknowledgments
We thank the many industry stakeholders that provided comments on the TIMS document that formed the basis for this paper, especially the TIMS Bt Technical Panel members Andrew Parkes, Colin Tann, Lewis Wilson, Rick Roush, and Paul Grundy. CSIRO, Cotton Australia, and the Cotton Research and Development Corporation in Australia financed the work reviewed herein, and supported the authors to develop the ‘Contingency Plan for Mitigating Resistance to the Toxins Within Bollgard II® Cotton’ and write this article.
Literature cited
- Ali MI, Luttrell RG. Response estimates for assessing Heliothine susceptibility to Bt Toxins. Journal of Economic Entomology. 2009;102:1935–1947. doi: 10.1603/029.102.0526. [DOI] [PubMed] [Google Scholar]
- Andow DA, Alstad DN. F2 screen for rare resistance alleles. Journal of Economic Entomology. 1998;91:572–578. [Google Scholar]
- Andow DA, Ives AR. Monitoring and adaptive resistance management. Ecological Applications. 2002;12:1378–1390. [Google Scholar]
- [APVMA] Australian Pesticides and Veterinary Medicines Authority. Canberra, Australia: Australian Pesticides and Veterinary Medicines Authority; 2003. Evaluation of the New Active Bacillus thuringiensis var. kerstaki Delta-endotoxins as Produced by the Cry1Ac and Cry2Ab Genes and Their Controlling Sequences in the New Product Bollgard II Cotton Event 15985. [Google Scholar]
- Baker GH, Tann CR, Fitt GP. Production of Helicoverpa spp. (Lepidoptera, Noctuidae) from different refuge crops to accompany transgenic cotton plantings in eastern Australia. Australian Journal of Agricultural Research. 2008;59:723–732. [Google Scholar]
- Baker GH, Tann CR, Fitt GP. A tale of two trapping methods: Helicoverpa spp. (Lepidoptera, Noctuidae) in pheromone and light traps in Australian cotton production systems. Bulletin of Entomological Research. 2010 doi: 10.1017/S0007485310000106. [DOI] [PubMed] [Google Scholar]
- Bates SL, Zhao JZ, Roush RT, Shelton AM. Insect resistance management in GM crops: past, present and future. Nature Biotechnology. 2005;23:57–62. doi: 10.1038/nbt1056. [DOI] [PubMed] [Google Scholar]
- Caccia S, Hernández-Rodríguez C, Mahon R, Downes S, James W, Bautsoens N, Rie JV, et al. Target site alteration is responsible for field-derived resistance to Bacillus thuringiensis Cry2A insecticidal proteins in Helicoverpa spp. PLoS ONE. 2010;5:e9975. doi: 10.1371/journal.pone.0009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Socorro AP, Gregg PC, Hawes AJ. Development of a synthetic plant volatile based attracticide for female noctuid moths. III. Insecticides for adult Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Australian Journal of Entomology. 2010;49:31–39. [Google Scholar]
- Downes S, Mahon R, Olsen K. Monitoring and adaptive resistance management in Australia for Bt-cotton: current status and future challenges. Journal of Invertebrate Pathology. 2007;95:208–213. doi: 10.1016/j.jip.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Downes S, Parker T, Mahon R. Frequency of alleles conferring resistance to the Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in Australian populations of Helicoverpa punctigera (Lepidoptera: Noctuidae) from 2002 to 2006. Journal of Economic Entomology. 2009;102:733–742. doi: 10.1603/029.102.0234. [DOI] [PubMed] [Google Scholar]
- Downes S, Parker T, Mahon R. 2010a. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard ii cotton. PLoS ONE. In press.
- Downes S, Parker T, Mahon R. Characteristics of resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) isolated from a field population. Journal of Economic Entomology. 2010b doi: 10.1603/ec09289. In press. [DOI] [PubMed] [Google Scholar]
- Farrell T. Cotton Pest Management Guide 2008–2009. Orange, New South Wales: NSW Department of Primary Industries; 2008. [Google Scholar]
- Fitt GP. Have Bt crops led to changes in insecticide use patterns and impacted IPM? In: Romeis J, Shelton TM, Kennedy G, editors. Integration of insect-resistant GM crops within IPM programs. Berlin, Heidelberg, Germany: Springer-Verlag; 2008. pp. 303–328. Progress in Biological Control Series. [Google Scholar]
- Fitt GP, Daly J. Abundance of overwintering pupae and the spring generation of Helicoverpa spp. (Lepidoptera: Noctuidae) in northern New South Wales, Australia: consequences for pest management. Journal of Economic Entomology. 1990;83:1827–1836. [Google Scholar]
- Forrester NW, Cahill M, Bird LJ, Layland JK. Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bulletin of Entomological Research Supplement Series. 1993;Supplement No. 1:132. [Google Scholar]
- Gassmann AJ, Carriere Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annual Review of Entomology. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annual Review of Entomology. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- Gould F, Anderson A, Jones A, Sumerford D, Heckel DG, Lopez J, Micinski S, Leonard R, Laster M. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proceedings of the National Academy of Sciences USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenplate JT, Mullins JW, Penn SR, Dahm A, Reich BJ, Osborn JA, Rahn PR, et al. Partial characterization of cotton plants expressing two toxin proteins from Bacillus thuringiensis: relative toxin contribution, toxin interaction, and resistance management. Applied Entomology. 2003;127:340–347. [Google Scholar]
- Gregg PC, Fitt GP, Zalucki MP, Murray DAH. Insect migration in an arid continent: II. Helicoverpa spp. in eastern Australia. In: Drake VA, Gatehouse AG, editors. Insect migration: tracking resources through space and time. Cambridge/New York/Melbourne: Cambridge University Press; 1995. pp. 151–172. [Google Scholar]
- Gregg PC, Greive KA, Socorro APD, Hawes AJ. Research to realisation: The challenging path for novel pest management products in Australia. Australian Journal of Entomology. 2010;49:1–9. [Google Scholar]
- Hartl D. A Primer of Population Genetics. 3rd edn. Sunderland, MA: Sinauer Associates, Inc; 1999. [Google Scholar]
- Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. Journal of Insect Physiology. 2010;56:227–235. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- James C. Global status of commercialized biotech/GM crops: 2008. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications; 2008. ISAAA Briefs 39-2008. [Google Scholar]
- Krattiger AF. Insect Resistance in Crops: A Case Study of Bacillus thuringiensis (Bt) and Its Transfer to Developing Countries. Ithaca, NY: ISAAA; 1997. ISAAA Briefs No. 2. [Google Scholar]
- Liao CY, Heckel DG, Akhurst R. Toxicity of Bacillus thuringiensis insecticidal proteins for Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae), major pests of cotton. Journal of Invertebrate Pathology. 2002;80:55–63. doi: 10.1016/s0022-2011(02)00035-6. [DOI] [PubMed] [Google Scholar]
- Liu FY, Xu ZP, Chang JH, Chen J, Meng FX, Zhu YC, Shen JL. Resistance allele frequency to Bt cotton in field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) in China. Journal of Economic Entomology. 2008;101:933–943. doi: 10.1603/0022-0493(2008)101[933:raftbc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Mares CL, P FittG. Field performance and seasonal changes in the efficacy against Helicoverpa armigera; (Hubner) of transgenic cotton expressing the insecticidal protein vip3A. Agricultural and Forest Entomology. 2007;9:93–101. [Google Scholar]
- Mahon R, Olsen K. Limited survival of a Cry2Ab-resistant strain of Helicoverpa armigera (Lepidoptera: Noctuidae) on Bollgard II. Journal of Economic Entomology. 2009;102:708–716. doi: 10.1603/029.102.0232. [DOI] [PubMed] [Google Scholar]
- Mahon R, Young S. Selection experiments to assess fitness costs associated with Cry2Ab resistance in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Journal of Economic Entomology. 2010;103:835–842. doi: 10.1603/ec09330. [DOI] [PubMed] [Google Scholar]
- Mahon R, Olsen K, Downes S, Addison S. Frequency of alleles conferring resistance to the Bt toxins Cry1Ac and Cry2Ab in Australian populations of Helicoverpa armigera (Lepidoptera: Noctuidae) Journal of Economic Entomology. 2007a;100:1844–1853. doi: 10.1603/0022-0493(2007)100[1844:foacrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mahon R, Olsen K, Garsia K, Young S. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Journal of Economic Entomology. 2007b;100:894–902. doi: 10.1603/0022-0493(2007)100[894:rtbttc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mahon R, Olsen K, Downes S. Isolations of Cry2Ab resistance in Australian populations of Helicoverpa armigera (Lepidoptera: Noctuidae) are allelic. Journal of Economic Entomology. 2008;101:909–914. doi: 10.1603/0022-0493(2008)101[909:iocria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mahon R, Downes S, James W, Parker T. Why do F1 screens estimate higher frequencies of Cry2Ab resistance alleles in Helicoverpa armigera (Lepidoptera: Noctuidae) than do F2 screens? Journal of Economic Entomology. 2010;103:472–481. doi: 10.1603/ec09225. [DOI] [PubMed] [Google Scholar]
- Malone L, Gatehouse AMR, Barratt BIP. Beyond Bt: Alternative strategies for insect resistant genetically modified crops. In: Romeis J, Shelton AM, Kennedy G, editors. Integration of Insect Resistant Genetically Modified Crops within IPM Program. Netherlands: Springer; 2008. pp. 357–417. [Google Scholar]
- Mao YB, J Cai W, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nature Biotechnology. 2007;25:1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- McKenzie JA. Ecological and Evolutionary Aspects of Insecticide Resistance. Melbourne: Academic Press; 1996. [Google Scholar]
- Oertel A, Zalucki MP, Maelzer DA, Fitt GP, Sutherst R. Size of the first spring generation of Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) and winter rain in central Australia. Australian Journal of Entomology. 1999;38:99–103. [Google Scholar]
- Olsen KM, Daly JC, Holt HE, Finnegan EJ. Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae) Journal of Economic Entomology. 2005;98:1007–1017. doi: 10.1603/0022-0493-98.3.1007. [DOI] [PubMed] [Google Scholar]
- Romeis J, Meissle M, Bigler F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nature Biotechnology. 2006;24:63–71. doi: 10.1038/nbt1180. [DOI] [PubMed] [Google Scholar]
- Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1998;353:1777–1786. [Google Scholar]
- Schulze KJ, Tomkins AR. Cotton Pest Management Guide 2002–2003. New South Wales: Australian Cotton Cooperative Research Centre and NSW Agriculture Orange; 2002. [Google Scholar]
- Tabashnik BE, Gould F, Carriere Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. Journal of Evolutionary Biology. 2004;17:904–912. doi: 10.1111/j.1420-9101.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Gassmann AJ, Crowder DW, Carriere Y. Insect resistance to Bt crops: evidence versus theory. Nature Biotechnology. 2008;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Van Rensburg JBJ, Carriere Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. Journal of Economic Entomology. 2009;102:2011–2025. doi: 10.1603/029.102.0601. [DOI] [PubMed] [Google Scholar]
- Whitburn G, Downes S. Surviving Helicoverpa Larvae in Bollgard II: Survey Results. The Australian Cottongrower Magazine. 2009;30:12–16. [Google Scholar]
- Yue BS, Huang FN, Leonard BR, Moore S, Parker R, Andow DA, Cook D, Emfinger K, Lee DR. Verifying an F1 screen for identification and quantification of rare Bacillus thuringiensis resistance alleles in field populations of the sugarcane borer, Diatraea saccharalis. Entomologia Experimentalis Et Applicata. 2008;129:172–180. [Google Scholar]
- Zalucki MP, Daglish G, Firempong S, Twine P. The biology and ecology of Heliothis armigera (Hubner) and Heliothis punctigera Wallengren (Lepidoptera, Noctuidae) in Australia – what do we know? Australian Journal of Zoology. 1986;34:779–814. [Google Scholar]
- Zhao JZ, Cao J, Collins HL, Bates SL, Roush RT, Earle ED, Shelton AM. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]


