Abstract
Transgenic crops producing Bacillus thuringiensis (Bt) toxins are used worldwide to control major pests of corn and cotton. Development of strategies to delay the evolution of pest resistance to Bt crops requires an understanding of factors affecting responses to natural selection, which include variation in survival on Bt crops, heritability of resistance, and fitness advantages associated with resistance mutations. The two main strategies adopted for delaying resistance are the refuge and pyramid strategies. Both can reduce heritability of resistance, but pyramids can also delay resistance by reducing genetic variation for resistance. Seasonal declines in the concentration of Bt toxins in transgenic cultivars, however, can increase the heritability of resistance. The fitness advantages associated with resistance mutations can be reduced by agronomic practices, including increasing refuge size, manipulating refuges to increase fitness costs, and manipulating Bt cultivars to reduce fitness of resistant individuals. Manipulating costs and fitness of resistant individuals on transgenic insecticidal crops may be especially important for thwarting evolution of resistance in haplodiploid and parthenogenetic pests. Field-evolved resistance to Bt crops in only five pests during the last 14 years suggests that the refuge strategy has successfully delayed resistance, but the accumulation of resistant pests could accelerate.
Keywords: Bacillus thuringiensis, fitness cost, host-plant resistance, incomplete resistance, population dynamics, resistance management, transgenic crops
Introduction
Transgenic crops producing toxins from the soil bacterium Bacillus thuringiensis (Bt) are a major tool for controlling insect pests. Fourteen years after their commercialization, Bt corn and cotton are used in more than twenty developed and developing countries on six continents (James 2008). The predominant Bt toxins in Bt crops are crystalline (Cry) proteins that kill insects by binding to specific midgut membrane target sites and disrupting membrane integrity (Schnepf et al. 1998). Benefits of Bt crops vary temporally and spatially depending on pest abundance and crop traits, and sometimes include reduction in use of synthetic insecticides and increased yield (Cattaneo et al. 2006; Hunt et al. 2007; Buntin 2008; Showalter et al. 2009; Ma et al. 2009). Negative impacts on nontarget insects are generally lower for Bt crops than for broad-spectrum insecticides (Cattaneo et al. 2006; Romeis et al. 2006; Marvier et al. 2007; Wolfenbarger et al. 2008; Naranjo 2009). Yet, effects of Bt crops on population density of specific nontarget species can be negative, neutral, or positive (Bourguet et al. 2002; Dively 2005; Naranjo 2005; Sisterson et al. 2007a; Carrière et al. 2009a). Because insects are remarkably successful in adapting to toxins and other control tactics (Palumbi 2001; Onstad 2008), the evolution of resistance to Bt toxins by target pests threatens the continued efficacy of Bt crops (Tabashnik 1994; Gould 1998; Matten et al. 2008; Tabashnik and Carrière 2009).
Evolution of resistance to a Bt crop entails a genetically based decrease in susceptibility of a population to the Bt crop (National Research Council 1986; Tabashnik et al. 2009a). While most target pest populations remain susceptible to Bt crops, field-evolved resistance has been documented in some populations of five lepidopteran pests: cereal stem borer, Busseola fusca, in South Africa to Bt corn producing Cry1Ab (Kruger et al. 2009), fall armyworm, Spodoptera frugiperda, in Puerto Rico to Bt corn producing Cry1F (Matten et al. 2008), pink bollworm, Pectinophora gossypiella, in western India to Bt cotton producing Cry1Ac (Bagla 2010), cotton bollworm, Helicoverpa zea, in the southeastern United States to Bt cotton producing Cry1Ac and Cry2Ab (Luttrell et al. 2004; Tabashnik et al. 2008a, 2009a), and bollworm, Helicoverpa punctigera, in Australia to Bt cotton producing Cry1Ac and Cry2Ab (Downes et al. 2010). Field-evolved resistance was reported to be associated with increased field damage by B. fusca, S. frugiperda, P. gossypiella, and H. zea (Matten et al. 2008; Kruger et al. 2009; Tabashnik et al. 2008b, 2009a; Bagla 2010). The widespread use of Bt crops and evidence of evolution of resistance by pests provide an exceptional opportunity to test hypotheses about how responses to selection are mediated by various genetic and ecological factors.
Below we review the effects of Bt crops on pest metapopulation dynamics, the conditions required for evolution of resistance to Bt crops, and how various factors affect evolution of resistance to Bt crops. We conclude by considering the implications of what has been learned about the evolutionary ecology of insect resistance to Bt crops and future research directions for enhancing strategies to delay insect resistance.
Effects of Bt crops on pest metapopulation dynamics
The evolution of resistance to Bt crops unfolds in metapopulations. Pest populations typically increase in fields of non-Bt crops or patches of wild hosts, which are source habitats, while fields of Bt crops are population sinks where populations decline. Gene flow between source and sink habitats allows for repeated colonization of Bt fields and provides opportunities for local adaptation. However, under some conditions, gene flow into Bt fields from non-Bt host plants can delay evolution of resistance (Comins 1977; Georghiou and Taylor 1977). In particular, the refuge strategy adopted widely to delay resistance to Bt crops is based on the idea that susceptible insects produced on non-Bt host plants near Bt crops will mate with resistant pests surviving on Bt crops (Gould 1998; Matten et al. 2008; Tabashnik and Carrière 2009).
Source-sink theory predicts that widespread use of Bt crops could reduce regional pest populations and the source potential of refuges (Gould 1998; Peck et al. 1999; Caprio 2001; Carrière et al. 2003; Sisterson et al. 2005). Field data from long-term monitoring of insect pest populations support these predictions for Heliothis virescens (Micinski et al. 2008), Helicoverpa armigera (Wu et al. 2008), Helicoverpa zea (Adamczyk and Hubbard 2006; Micinski et al. 2008; Storer et al. 2008), Ostrinia nubilalis (Storer et al. 2008; Hutchison et al., unpublished data), and Pectinophora gossypiella (Carrière et al. 2003). Furthermore, the source potential of refuges increased as refuge width and the isolation between refuges and Bt crops increased (Caprio et al. 2004) or the regional abundance of Bt crops decreased (Carrière et al. 2004a). The source potential of refuges likely increased in these cases because fewer eggs were laid on Bt crops and thus population growth was larger in refuges.
Consistent with these empirical findings, results from metapopulation simulation models indicate that pest population dynamics and resistance evolution are affected by the distribution and abundance of Bt fields and refuges, as well as the management of refuges from year to year (Peck et al. 1999; Caprio 2001; Storer et al. 2003; Sisterson et al. 2004, 2005). Nevertheless, the regional spread of resistance is initiated by a local increase in resistance frequency where Bt fields are abundant compared to refuges (Peck et al. 1999; Storer et al. 2003; Sisterson et al. 2004, 2005). Thus, while a metapopulation approach yields important insights for understanding the evolution of resistance at a regional scale, a focus on local populations is useful to evaluate conditions influencing the evolution of resistance to Bt crops. Indeed, simple population genetic models considering, in effect, a single Bt field and refuge have been central for development of the refuge strategy and have successfully accounted for field changes in resistance frequencies in several pest species (Gould 1998; Carrière and Tabashnik 2001; Onstad and Guse 2008; Tabashnik et al. 2008a; Crowder and Carrière 2009).
Conditions for evolution of resistance to Bt crops
Three conditions are necessary and sufficient for local evolution of resistance to Bt crops by natural selection (Endler 1986): (i) variation among individuals in survival on Bt crops, (ii) inheritance of survival on Bt crops, and (iii) fitness differences consistently associated with the variation in survival on Bt crops. In what follows we summarize information about these factors and how they affect evolution of resistance to Bt crops.
Genetic variation in resistance
The first widely planted Bt crop cultivars were corn producing Bt toxin Cry1Ab and cotton producing Bt toxin Cry1Ac (Tabashnik et al. 2009a). Throughout the growing season, these cultivars caused virtually 100% larval mortality for highly susceptible pests such as Ostrinia nubilalis and Pectinophora gossypiella (Tabashnik et al. 2000; Archer et al. 2001). For such highly susceptible pests, the concentration of Bt toxin in Bt plants is much higher than the concentration needed to kill essentially all susceptible larvae (Fig. 1). In such cases, mutations that confer small decreases in susceptibility to Bt toxin provide little or no fitness advantage. Instead, under these conditions, selection for increased survival on Bt plants typically favors single mutations with effects large enough to boost survival on Bt plants (Fig. 1A). In contrast, for target pests that are less susceptible to Cry1Ab and Cry1Ac such as Helicoverpa armigera and Helicoverpa zea, mortality on Bt corn and cotton producing these toxins typically is lower than 95% and declines during the growing season (Storer et al. 2003; Showalter et al. 2009). Under these conditions, mutations conferring small or moderate decreases in susceptibility can boost survival on Bt crops, and thus polygenic resistance is more likely to evolve (Fig. 1B).
Figure 1.
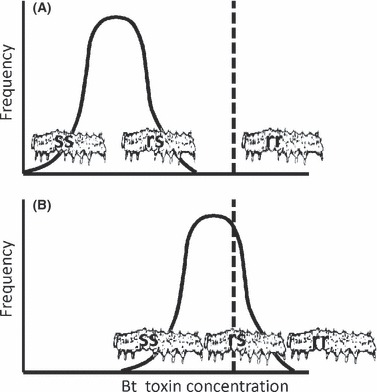
Distribution of insects from unexposed populations for which the given concentration of Bt toxin is the maximum tolerated. The dashed line shows the Bt toxin concentration produced by a Bt crop. The unexposed populations in (A) and (B) are composed almost entirely of susceptible (ss) and heterozygous (rs) individuals, while homozygous resistant (rr) individuals are extremely rare. (A) The insect population is highly susceptible. The toxin concentration in the Bt crop is higher than the concentration that kills virtually all larvae of the unexposed population. Mutations that cause major decreases in susceptibility are needed to boost survival on the Bt crop and thereby confer resistance. Survival is virtually 0% for both ss and rs, which yields recessive inheritance of resistance. (B) The unexposed population is inherently less susceptible and a substantial percentage can survive on the Bt crop. Mutations with small or moderate effects can boost survival on the Bt crop and thus confer resistance. Survival is greater for rs than ss, yielding non-recessive inheritance of resistance.
While the genetic basis of field-evolved resistance to Bt crops remains to be elucidated, genetic analysis of laboratory-selected resistance to Bt crops has usually identified single loci with major effects (Gahan et al. 2001; Morin et al. 2003; Yang et al. 2007; Pereira et al. 2008; Zhang et al. 2009). Based on these data and for simplicity, we will consider a theoretical framework in which resistance to each Bt toxin is controlled primarily by a single locus with two alleles, r for resistance and s for susceptibility.
Fitness costs occur when, in absence of Bt toxins, the fitness of individuals with r alleles is lower than the fitness of individuals without r alleles. Alleles conferring resistance to Bt often have negative pleiotropic effects that cause fitness costs, implying that r alleles will be maintained at a low frequency in populations not previously exposed to Bt crops (Gassmann et al. 2009). Fitness costs of resistance to Bt are usually recessive and involve a reduction in survival of about 25% in homozgyous resistant (rr) individuals compared to homozygous susceptible (ss) individuals (Gassmann et al. 2009). Using this information, we can apply mutation-selection equilibrium theory to predict the r allele frequency in populations not exposed to Bt toxins. For a mutation with recessive effects, the frequency of a r allele at equilibrium is q = (μ/S)0.5, where μ is the mutation rate from the s to r allele and S is the selection coefficient (Futuyma 1979). Assuming μ ranges from 10−6 to 10−4 (Drake et al. 1998; Russell 2000), the initial r allele frequency is expected to range from 0.002 to 0.02, which is similar to the observed range for several lepidopteran pests targeted by Bt crops (Table 1).
Table 1.
Estimated frequency of alleles conferring resistance to Bt crops in field populations of lepidopteran pests targeted by Bt corn or Bt cotton. Note that in most studies, frequency estimates were obtained after introduction of a Bt crop, which could have increased estimates of the resistance allele frequency. Insects were screened for resistance on Bt leaves or Bt crop plants unless noted otherwise
| Location | Crop | Toxin | 1st year* | Years sampled† | Frequency | Confidence limits‡ | Reference |
|---|---|---|---|---|---|---|---|
| Gelechiidae | |||||||
| Pectinophora gossypiella | |||||||
| AZ | Cotton | Cry1Ac | 1996 | 1997 | 0.16§ | 0.05–0.26 | Tabashnik et al. (2005a) |
| Cotton | Cry1Ac | 1996 | 1998 | 0.007§ | 0–0.02 | Tabashnik et al. (2005a) | |
| Cotton | Cry1Ac | 1996 | 2004 | 0.004§ | 0–0.01 | Tabashnik et al. (2005a) | |
| AZ, CA, TX | Cotton | Cry1Ac | 1996 | 2001–05 | 0¶ | 0–0.0003 | Tabashnik et al. (2006) |
| Noctuidae | |||||||
| Helicoverpa armigera | |||||||
| Australia | Cotton | Cry1Ac | 1996 | 2002–06 | 0** | 0–0.0009 | Mahon et al. (2007) |
| Australia | Cotton | Cry2Ab | 2004/05 | 2002–06 | 0.0033§ | 0.0017–0.0055 | Mahon et al. (2007) |
| China | Cotton | Cry1Ac | 1998 | 2006–07 | 0.094 | 0.044–0.145 | Liu et al. (2008) |
| China | Cotton | Cry1Ac | 1998 | 2003–05 | 0.0146 | 0.0084–0.0225 | Xu et al. (2009) |
| Heliothis virescens | |||||||
| LA, MS, NC, TX | Cotton | Cry1Ac | 1996 | 1993 | 0.0015†† | 0.0003–0.0041 | Gould et al. (1997) |
| LA, TX | Cotton | Cry1Ac | 1996 | 1996–2002 | 0¶ | 0–0.0021 | Gahan et al. (2007) |
| Sesamia nonagrioides | |||||||
| Spain | Corn | Cry1Ab | 1998 | 2004–05 | 0 | 0–0.0086 | Andreadis et al. (2007) |
| Greece | Corn | Cry1Ab | –‡‡ | 2004–05 | 0 | 0–0.0097 | Andreadis et al. (2007) |
| Pyralidae | |||||||
| Diatraea grandiosella | |||||||
| LA | Corn | Cry1Ab | 1996 | 2005 | 0 | 0–0.0035 | Huang et al. (2007a) |
| Diatraea saccharalis | |||||||
| LA | Corn | Cry1Ab | 1996 | 2004 | 0.0023 | 0.0003–0.0064 | Huang et al. (2007b) |
| LA | Corn | Cry1Ab | 1996 | 2005 | 0 | 0–0.0027 | Huang et al. (2008a) |
| TX | Corn | Cry1Ab | 1996 | 2007 | 0 | 0–0.0016 | Huang et al. (2009) |
| Ostrinia nubilalis | |||||||
| KS, TX | Corn | Cry1Ab | 1996 | 2000–01 | 0 | 0–0.0044 | Stodola et al. (2006) |
| France | Corn | Cry1Ab | –‡‡ | 1999–2000 | 0 | 0–0.00092 | Bourguet et al. (2003) |
| MN, SD, IA | Corn | Cry1Ab | 1996 | 1997–2000 | 0 | 0–0.00042 | Bourguet et al. (2003) |
| KS, TX | Corn | Cry1Ab | 1996 | 2000–01 | 0 | 0–0.0077 | Bourguet et al. (2003) |
First year Bt crop was grown commercially in the location monitored.
Year (s) insects were sampled to produce the frequency estimate.
The probability is 95% that the true mean is between the upper and lower limits.
Insects were screened on diet with high Bt toxin concentration and related work showed that insects surviving this concentration also survive on Bt cotton.
Insects were screened for cadherin mutations associated with survival on Bt cotton (P. gossypiella) or high resistance to Cry1Ac in diet (H. virescens).
Insects were tested on diet with Cry1Ac but not on Bt cotton.
Insects were not tested on Bt cotton but resistance to Cry1Ac in diet was very high.
Bt corn was not commercialized in region sampled.
Fitness costs are typically more prevalent and extensive in pests with high compared to low levels of Bt resistance (Gassmann et al. 2009). Also, in a selection experiment with H. armigera, a higher fitness cost was associated with increased levels of resistance within a single strain (Liang et al. 2008). In this experiment, resistance was quantified as a ‘resistance ratio’ calculated as the IC50 (concentration of Cry1Ac that inhibited larval growth by 50%) for the selected strain divided by the IC50 for an unselected strain. After 16, 34 and 87 generations of selection, the resistance ratios were 170, 210, and 2893, while costs affecting net replacement rate were respectively 29, 36, and 41%. The genetic basis of resistance also changed from primarily monogenic to polygenic during the course of selection. The spread of resistance alleles is expected to increase both the levels of resistance and fitness costs (Carrière et al. 1994; Carrière and Roff 1995). It is thus unclear whether the positive association between levels of Bt resistance and fitness costs, which was detected among pests and within the H. armigera strain, resulted from variation in resistance frequency or in costs associated with different resistance alleles. The latter factor, however, could have interesting implications for the evolution of resistance to Bt. As noted previously, for highly susceptible pests, r alleles with large effects are required to increase survival on Bt crops. Given that larger fitness costs could be associated with higher levels of resistance, larger fitness costs could be expected for r alleles with greater effects. In light of the mutation-selection balance that sets initial r allele frequency as described previously, r alleles with effects sufficiently large to confer survival on Bt crops could be rarer in highly susceptible pests than in less susceptible pests. Moreover, initial r allele frequency could be diminished by producing crops with higher toxin concentrations.
While the first Bt corn and cotton cultivars each produced one Bt toxin, some newer cultivars produce a ‘pyramid’ of two or more Bt toxins for the control of individual pest species (Tabashnik et al. 2009a). Furthermore, transgenic cultivars with two or more Bt toxins targeting individual pests are likely to become increasingly prevalent (Bravo and Soberón 2008; Matten et al. 2008). When resistance to each toxin is recessive and the genes conferring resistance to the various toxins segregate independently, the use of multiple toxins can significantly reduce the number of resistant phenotypes in a pest population. For example, with a r allele frequency of 0.003 at each locus conferring resistance to one toxin (i.e., the average upper limit of the r allele frequency in O. nubilalis, Table 1), the expected frequency of individuals with homozygous resistance at all relevant loci would be 0.0032, 0.0034, and 0.0036, for crops producing 1, 2, and 3 toxins, respectively. Thus, with 4 × 106 insects per Bt field (Sisterson et al. 2005), the expected number of individuals per field with homozygous resistance at the relevant loci would be 2360, 3.2 × 10−3, and 1.4 × 10−8, respectively. This suggests that genetic variation for resistance could be rare when two or more independently acting Bt toxins target the same pest and pest populations are small. In addition, fitness costs could be higher in pests that evolve resistance to crops producing many toxins, which could also contribute to the rarity of resistance alleles. The hypothesis of increased costs in insects resistant to crops with multiple toxins has not been evaluated, although it is supported by a study of the bacterium Pseudomonas aeruginosa where costs were greater in populations resistant to two antibiotics rather than one (Perron et al. 2007).
Although r allele frequency is low in populations of most pests (Table 1), genetic variation is often available to confer resistance to Bt crops. Some populations of five lepidopteran species have evolved resistance to a Bt crop in the field (see above). In addition, strains of four pest species, including three lepidopterans and one coleopteran (corn rootworm, Diabrotica virgifera virgifera), have been selected in the laboratory or greenhouse for higher survival on commercial Bt cultivars (Table 2). Also, the diamondback moth, Plutella xylostella, has been selected for resistance to a noncommercial cultivar of Bt broccoli that produces Cry1Ac and Cry1C (Zhao et al. 2005). In sum, documented cases of resistance to commercial and noncommercial cultivars of Bt crops selected in the field, laboratory, and greenhouse include at least nine species of insect pests, eight Bt toxins, and three Bt crops.
Table 2.
Survival on commercial Bt crops relative to survival on non-Bt crop counterparts for strains of four pest species selected in the laboratory or greenhouse for resistance to Bt toxins. Only studies reporting survival of lab- or greenhouse-selected strains from neonate to either pupa or adult are shown. Survival was measured in the greenhouse or using plants from the greenhouse unless noted otherwise. Diatraea saccharalis, Helicoverpa armigera, and Pectinophora gossypiella are lepidopterans; Diabrotica virgifera virgifera is a coleopteran
| Toxin(s), conditions | Insect species | Insect strain | Adjusted survival* | Reference |
|---|---|---|---|---|
| Corn | ||||
| Cry1Ab | D. saccharalis | B1F2 | 0.10 | Huang et al. (2007b) |
| Cry34Ab/Cry35Ab | D. v. virgifera | Rochelle-S | 0.18† | Lefko et al. (2008) |
| Cry34Ab/Cry35Ab | D. v. virgifera | York-S | 0.19† | Lefko et al. (2008) |
| Cry3Bb, field tested | D. v. virgifera | Constant-exposure | 0.44‡ | Meihls et al. (2008) |
| Cry3Bb, field tested | D. v. virgifera | Late-exposure | 0.14‡ | Meihls et al. (2008) |
| Cry3Bb, field tested | D. v. virgifera | Neonate-exposure | 0.015‡ | Meihls et al. (2008) |
| Cotton | ||||
| Cry1Ac | H. armigera | Cry1Ac-sel | 0.25 | Fan et al. (2000) |
| Cry1Ac, 5 to 6-leaf stage | H. armigera | ISOC4 | 0.37–0.39 | Bird and Akhurst (2004) |
| Cry1Ac, 15-leaf stage | H. armigera | ISOC4 | 0.91 | Bird and Akhurst (2005) |
| Cry1Ac + Cry2Ab, pre-square | H. armigera | SP15 | 0.029 | Mahon and Olsen (2009) |
| Cry1Ac + Cry2Ab, early square | H. armigera | SP15 | 0.044 | Mahon and Olsen (2009) |
| Cry1Ac + Cry2Ab, fruiting | H. armigera | SP15 | 0.52 | Mahon and Olsen (2009) |
| Cry1Ac + Cry2Ab, pre-square, field plants | H. armigera | SP15 | 0 | Mahon and Olsen (2009) |
| Cry1Ac + Cry2Ab, fruiting, field plants | H. armigera | SP15 | 0.17 | Mahon and Olsen (2009) |
| Cry1Ac | P. gossypiella | AZP-R | 0.46 | Liu et al. (2001) |
| Cry1Ac | P. gossypiella | AZP-R | 0.43 | Morin et al. (2003) |
| Cry1Ac | P. gossypiella | MOV97-R | 1.10 | Tabashnik et al. (2005b) |
| Cry1Ac | P. gossypiella | SAF97-R | 1.50 | Tabashnik et al. (2005b) |
| Cry1Ac | P. gossypiella | MOV97-H2, r1r3§ | 0.30 | Carrière et al. (2006) |
| Cry1Ac | P. gossypiella | MOV97-H2, r3r3§ | 0.38 | Carrière et al. (2006) |
| Cry1Ac | P. gossypiella | SAF97-H2, r1r2§ | 0.57 | Carrière et al. (2006) |
| Cry1Ac | P. gossypiella | SAF97-H2, r2r2§ | 0.20 | Carrière et al. (2006) |
Adjusted survival = survival of selected strain on Bt crop/survival of selected strain on non-Bt crop unless noted otherwise. Adjusted survival <1 indicates incomplete resistance (see text).
Survival of the selected Rochelle-S strain on Bt corn (0.137)/survival of the unselected parent strain (Rochelle-US) on non-Bt corn (0.762). Survival of the selected York-S strain on Bt corn (0.156)/survival of the unselected parent strain (York-US) on non-Bt corn (0.827). If survival on non-Bt corn was lower for the selected strain (data not reported) than for the unselected strain, these calculations would underestimate adjusted survival on Bt corn for the selected strains.
Adjusted survival of an unselected strain on Bt corn was 0.038. Survival was significantly higher than 0.038 in the constant-exposure and late-exposure strains, but not in the neonate-exposure strain.
Three cadherin alleles linked with resistance to Cry1Ac in P. gossypiella are denoted r1, r2, and r3. Adjusted survival is given for genotypes based on combinations of these alleles.
For some pests, however, it may sometimes prove difficult to select for levels of resistance high enough to confer survival on Bt crops. For example, many unsuccessfull attempts were made to select O. nubilalis for resistance to Cry1Ab corn in the laboratory, and only recent work suggests that this pest could achieve levels of resistance high enough for insects to survive to the adult stage on Bt corn (Pereira et al. 2008; Crespo et al. 2009). However, although survival of resistant larvae was high on Bt corn in these recent experiments, plants were dissected 15 days after infestation and survival to adulthood was not quantified. A lack of appropriate genetic variation or low frequency of recessive resistance alleles can constrain the evolution of resistance to insecticides (Peck et al. 1999; Brown and Hoffmann 2005; Labbé et al. 2007). There is a trend for the occurence of resistance alleles to be lower in the Pyrallidae than in Gelechiidae and Noctuidae, and so far no major alleles confering resistance to Cry1Ab have been found in O. nubilalis despite many screening attempts (Table 1). Thus, results from selection experiments and available data on frequency of resistance alleles indicate that the evolution of resistance to Cry1Ab corn could be constrained in O. nubilalis.
Control measures aimed at keeping pest populations low could be envisaged to manage the evolution of resistance (e.g., Carrière et al. 2001) in pests where the initial frequency of r alleles is low (e.g., O. nubilalis) and resistance is recessive. Interestingly, Bt crops can lower regional pest population density, as summarized above. Regional declines in pest population density are influenced by pest movement, reproductive rate in refuges, and abundance, distribution, and toxicity of Bt crops (Carrière et al. 2003; Sisterson et al. 2007b). Spatially explicit data on the abundance and distribution of Bt crops coupled with information on pest life-history and response to Bt crops could be useful to design measures to reduce pest population densities regionally (Carrière et al. 2004a; Marvier et al. 2008).
Heritability of resistance
An important factor underlying the refuge strategy is that the dominance of resistance is reduced by increasing the dose of Bt toxins. When the concentration of a Bt toxin is low, some heterozygous (rs) individuals typically survive exposure to a Bt toxin, but when the concentration is high, only rr can survive (Tabashnik et al. 2004; Crespo et al. 2009). Accordingly, Bt toxin genes inserted in transgenic plants were modified to produce high concentrations of Bt toxins, and genetically transformed plants with high levels of Bt toxins were selected to produce commercial transgenic cultivars (Showalter et al. 2009).
Even when r alleles are present in insect populations in Bt fields, movement of insects from refuges to Bt fields can significantly reduce the heritability of resistance. Resistance to crops that produce high concentrations of Bt toxins is recessive in highly susceptible pests, although this is not necessarily the case in pests less susceptible to Bt (Tabashnik et al. 2008a, 2009a). For refuges to be effective, the abundant susceptible insects produced on non-Bt host plants must mate with the rare resistant pests surviving on Bt crops. In such cases, when resistance is recessive, most hybrid offspring produced by resistant pests are killed by Bt crops. This reduces the heritability of resistance and delays the evolution of resistance (Gould 1998; Tabashnik and Carrière 2009). With time, however, movement of rr individuals from Bt fields to refuges can increase the frequency of r alleles in refuges (Sisterson et al. 2004), especially when fitness costs are absent (Carrière and Tabashnik 2001; Gould et al. 2006). Ultimately, some individuals with these r alleles will move back from refuges to Bt fields, which increases the heritability of resistance and accelerates the evolution of resistance (Comins 1977; Caprio and Tabashnik 1992; Sisterson et al. 2004).
The ‘pyramid’ strategy for delaying pest resistance is based on use of crops producing two or more distinct Bt toxins targeting individual pests. The pyramid strategy is expected to be most effective when resistance to each Bt toxin is recessive, fitness costs and refuges are present, and selection with any one of the Bt toxins does not cause cross-resistance to the others (Roush 1998; Zhao et al. 2005; Gould et al. 2006). Cross-resistance to Bt occurs when a genetically-based decrease in susceptibility to one toxin decreases susceptibility to other toxins (Tabashnik et al. 2009a,b). When resistance is recessive to both toxins in a pyramid, pests that bear two r alleles for survival to one toxin will nonetheless be killed unless they also bear two r alleles for survival to the second toxin. Thus when r alleles are rare, the only genotype with high survival on a crop that produces two or more Bt toxins is expected to be extremely rare. Accordingly, the refuge strategy is more effective for reducing the heritability of resistance when crops produce two or more Bt toxins than when crops produce a single Bt toxin (Gould 1998; Roush 1998).
Concentrations of Bt toxins in Bt corn and cotton typically decline as the growing season progresses, but seasonal changes in toxin concentration can vary among toxins and cultivars (Dutton et al. 2004; Nguyen and Ja 2009; Showalter et al. 2009). For example, in Bt cotton, Cry1Ac concentration usually decreases when plants start producing flowers and bolls (Showalter et al. 2009), while Cry2Ab concentration tends to spike in mid-season before declining (Adamczyk et al. 2001). Levels of a Bt vegetative insecticidal protein, Vip3A, are relatively stable throughout the season, although cotton plants producing Vip3A still lose some of their activity against H. armigera during mid-season (Llewellyn et al. 2007). The reasons for the seasonal reduction in Bt concentration remain unclear, but could be related to mRNA instability, declining promoter activity, reduced nitrogen metabolism, lower overall protein production, and toxin interactions (Showalter et al. 2009).
The seasonal decline in toxin concentrations may increase the dominance of resistance and accelerate the evolution of resistance, especially in pests less susceptible to Bt toxins. For example, resistance to Cry1Ac cotton was recessive in H. armigera fed Bt cotton in the 5–6 leaf stage (Bird and Akhurst 2004), but became partially dominant on cotton in the 15 leaf stage, which had concentrations of Cry1Ac 75% lower than in 4-week-old plants (Bird and Akhurst 2005). However, a seasonal decline in toxin concentrations does not always increase the dominance of resistance. When Diatraea saccharalis larvae fed on each of seven commercial Cry1Ab corn cultivars in 2005 and 2006 (Wu et al. 2007), average survival for each insect genotype was lower on vegetative corn than on older, reproductive corn (2005: 0.5% vs. 1.5% for ss, 1.4% vs. 3.4% for rs and 9.6% vs. 24.5% for rr; 2006: 0.0% vs. 2.7% for ss, 3.5% vs. 6.8% for rs, and 14.3% vs. 18.1% for rr). Nevertheless, in 2005, the average dominance of resistance (h = [survival of rs − survival of ss]/[survival of rr − survival of ss]) was slightly higher on vegetative corn (0.099) than on reproductive corn (0.083). In 2006, the average dominance of resistance was higher than in 2005, and it was slightly higher on reproductive (0.27) than on vegetative (0.24) corn. Thus, the higher survival on reproductive corn relative to vegetative corn, which presumably reflects lower Cry1Ab concentration in reproductive corn (Wu et al. 2007), did not produce consistent or large increases in the dominance of resistance.
Seasonal declines in Bt toxin concentrations could also reduce success of the pyramid strategy. Mahon and Olsen (2009) measured seasonal changes in survival of a H. armigera strain highly resistant to Cry2Ab on cotton producing Cry1Ac and Cry2Ab. Survival of rr individuals was respectively 0, 2.5, and 8.5% on field-grown cotton in the pre-square, early square and fruiting stages, while survival of ss was 0, 0, and 1.6%. Survival of rs on pre-square and fruiting cotton was respectively 0 and 1.7% and did not differ significantly from ss (survival of rs on early square cotton was not measured), showing that resistance remained recessive on the different cotton stages. Mahon and Olsen (2009) did not measure the change in concentrations of Cry1Ac and Cry2Ab in cotton plants but proposed that increased survival of the Cry2Ab-resistant insects was likely due to a decline in the concentration of Cry1Ac. As the oldest cotton was tested soon after fruiting, it is also possible that survival of rr individuals and the dominance of resistance could increase further on older cotton, or on cultivars where the concentration of the Bt toxins decline faster than in the experimental cultivar used. Accordingly, the seasonal decline in the concentration of one toxin in a pyramid (here Cry1Ac) could invalidate the fundamental assumption of the pyramid strategy (i.e., the killing of insects resistant to one toxin by another toxin), and thus accelerate evolution of resistance. It is noteworthy that H. zea, a pest in which seasonal changes in survival on Bt crops have been reported (Storer et al. 2003), has rapidly evolved resistance in the field to Cry1Ac and Cry2Ab produced by pyramided Bt cotton (Tabashnik et al. 2009a). There is a need to better evaluate and consider the consequences of seasonal declines in the concentrations of Bt toxins on the evolution of resistance to Bt crops (Brévault et al., unpublished data).
Selective advantage of resistance
Some field data suggest that refuges of non-Bt crops near Bt crops can delay the evolution of resistance (Tabashnik et al. 2008a, 2009a). More generally, in theory, the evolution of resistance can be delayed by any factors that reduce the selection coefficient between individuals with and without r alleles (Gould 1998; Carrière and Tabashnik 2001; Andow and Ives 2002; Crowder and Carrière 2009). Reducing the selection coefficient can be achieved through agronomic practices. For example, the selective advantage of resistance can be reduced by increasing refuge size, which increases the fitness of susceptible individuals relative to resistant individuals. Furthermore, the selective advantage of resistance can be reduced by implementing pest control practices such as pheromone mating disruption and elimination of crop residues containing insects in Bt fields, where resistant individuals are more abundant than susceptible individuals (Carrière et al. 2001; Andow and Ives 2002).
The selection coefficient between individuals with and without r alleles is also affected by environmental conditions and pest genetics, which interact to generate incomplete resistance and fitness costs. Incomplete resistance occurs when fitness of rr individuals is lower on Bt cultivars than on corresponding non-Bt cultivars (Carrière and Tabashnik 2001; Carrière et al. 2006; Crowder and Carrière 2009). Incomplete resistance reduces the selection coefficient favoring resistant individuals over susceptible individuals on Bt crops. On the other hand, fitness costs increase the selection coefficient favoring susceptible individuals in refuges. These factors influence the overall fitness of the r and s alleles across Bt fields and refuges (Carrière and Tabashnik 2001; Tabashnik et al. 2005a), which ultimately drives increases or decreases in the frequency of resistance (Fig. 2).
Figure 2.
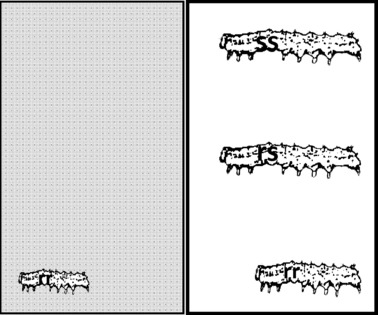
Fitness of resistance genotypes on a Bt crop (grey) and on a non-Bt host plant in a refuge (white). Fitness of each genotype in each habitat is proportional to the size of larvae in diagram. On the Bt crop, fitness of rr is 0.2 but rs and ss do not survive (resistance is recessive). In the refuge, fitness of ss and rs is 1 and fitness of rr is 0.8 (a recessive cost is present). On the Bt crop, the selection coefficient favoring rr over ss and rs is 0.2. In the refuge, the selection coefficient favoring ss and rs over rr is 0.2. Assuming that half of the larval population is exposed to each habitat, fitness of the r and s allele is equal and frequency of resistance is stable (see Tabashnik et al. 2005a, equation 6 for fitness calculation). However, if fitness of rr is 0.21 on the Bt crop, fitness of other genotypes is unchanged (thus smaller incomplete resistance), and half of the larval population is exposed to each habitat, fitness of r is higher than fitness of s and resistance frequency increases. On the other hand, if fitness of rr is 0.79 in the refuge, fitness of the other genotypes is unchanged (thus a higher cost), and half of the larval population is exposed to each habitat, fitness of r is smaller than fitness of s and resistance frequency declines. Thus, variation in costs and incomplete resistance has important effects on resistance evolution.
When r alleles are rare, which is typical before extensive exposure to Bt crops (Table 1), they are found primarily in rs individuals. Thus, in the initial stages of selection for resistance, nonrecessive costs expressed in rs individuals can delay the evolution of resistance more effectively than recessive costs that are only expressed in extremely rare rr individuals (Carrière and Tabashnik 2001). With nonrecessive costs, the fitness in refuges is higher for ss than rs individuals, which selects strongly against resistance (Carrière and Tabashnik 2001; Gould et al. 2006). Nonrecessive costs associated with Bt resistance were detected in 26% of fitness components assessed in 16 studies (Gassmann et al. 2009), suggesting that nonrecessive costs are more widespread than previously envisaged (Gould et al. 2006). Incomplete resistance, quantified as the fitness of rr on Bt crops divided by rr fitness on non-Bt crop counterparts, is common and averaged 0.63 (range 0.11–1.5) in seven pests (Crowder and Carrière 2009; see also Table 2).
Given the powerful effects of fitness costs and incomplete resistance on resistance evolution, manipulation of refuges and Bt cultivars could enhance resistance management (Gassmann et al. 2009; Crowder and Carrière 2009). Fitness costs are affected by environmental conditions, including natural enemies, intraspecific competition, and host plant (Gassmann et al. 2009). Thus, using refuges of host plants that magnify costs or make them less recessive, or treating refuges with natural enemies that have similar effects, could delay resistance (Gassmann et al. 2009).
Factors affecting incomplete resistance have not been critically evaluated, but a reasonable hypothesis is that individuals that survive on Bt crops are not completely impervious to high concentrations of Bt toxins. This hypothesis is supported by studies of H. armigera, where survival of resistant individuals on Bt cotton relative to non-Bt cotton was lower on young cotton plants with high concentrations of Cry1Ac than on older plants with lower concentrations of Cry1Ac (Table 2; Bird and Akhurst 2004, 2005). This suggests that the production of high concentrations of Bt toxins in Bt crops could not only contribute in increasing costs, but also could reduce fitness of resistant individuals on Bt crops relative to non-Bt crops. Cultivars with two or more toxins could also enhance this effect because insects resistant to one toxin could be killed by the other toxin (Crowder and Carrière 2009). This hypothesis is supported by a recent study of a H. armigera strain highly resistant to Cry2Ab (Table 2; Mahon and Olsen 2009). In this strain, survival of resistant individuals on Bt cotton producing Cry1Ac and Cry2Ab relative to non-Bt cotton was lower on younger than older cotton plants (Table 2), presumably because the concentration of Cry1Ac diminished in older plants and thus Cry1Ac did not kill insects resistant to Cry2Ab. Production of toxins bypassing known resistance mechanisms (Soberón et al. 2007) or inducing negative cross-resistance could also be envisaged to reduce fitness of resistant individuals on Bt crops (Crowder and Carrière 2009).
Manipulation of costs and incomplete resistance will be achieved more easily if the genetic basis of resistance is similar across populations of a target pest. For example, if multiple resistance genes or alleles with different pleiotropic effects occur in a pest metapopulation, different strategies for modifying costs might be needed to efficiently delay resistance in different regions. So far the molecular basis of resistance in the five pests that evolved resistance to Bt crops in the field is unknown. Of the species selected for higher survival on Bt crops in the laboratory (Table 2), the molecular basis of resistance is known in H. armigera and P. gossypiella. In both pests, resistance to Cry1Ac is linked with mutations in a gene encoding a cadherin protein that binds Cry1Ac (Morin et al. 2003; Yang et al. 2007). Three cadherin mutations were present in a H. armigera strain originating from the Hebei Province of China (Yang et al. 2007). A mutation in an aminopeptidase N gene also conferred high resistance to Cry1Ac in a H. armigera strain from the Henan Province of China (Zhang et al. 2009). In P. gossypiella, two mutant cadherin alleles denoted r1 and r3 occurred in western Arizona, whereas the mutant alleles r1 and r2 were found in eastern Arizona (Morin et al. 2003).
The effects of the cadherin and aminopeptidase N mutations on costs and incomplete resistance are unknown in H. armigera. However, these mutations could have different effects on costs and incomplete resistance, given that cadherin and aminopeptidase N proteins have different functions. In P. gossypiella, costs and incomplete resistance were largely similar in strains harboring different cadherin mutations or across genotypes when insects were reared on a single cotton cultivar (e.g., DP50 with or without Cry1Ac) (Carrière et al. 2005a, 2006). However, expression of costs and incomplete resistance sometimes changed markedly across different cultivars (Carrière et al. 2006). Because cadherin proteins may help to maintain integrity of the midgut membrane (Midboe et al. 2003; Carrière et al. 2009b), cadherin mutations may cause fitness costs by increasing permeability of the gut membrane to toxic phytochemicals (Carrière et al. 2004b). Each of the three cadherin mutations in P. gossypiella increased gut permeability to the cotton phytochemical gossypol, and costs were increased most by mutations inducing higher gut permeability (Williams 2009). Consistent expression of costs on particular cultivars, the pervasive presence of toxic phytochemicals in cotton, and the possibility that increased penetration of phytochemicals may magnify costs in individuals with cadherin mutations, indicate that refuges could be manipulated over a large scale by planting specific cultivars to enhance resistance management in P. gossypiella. More information on the consequences and distribution of molecular variation in resistance to Bt crops will be useful to improve our ability to manipulate costs and incomplete resistance.
So far the refuge strategy has been used to manage the evolution of resistance in diploid, sexually reproducing coleopterans and lepidopterans. However, recent developments in molecular biology and biotechnology suggest that future transgenic insecticidal crops could increasingly target haplodiploid and parthenogenic pests (Crowder and Carrière 2009). Simulation models have shown that as in diploid pests, refuges of nontransgenic host plants and recessive inheritance of resistance can delay resistance in haplodiploid and parthenogenetic pests. However, contrary to results with diploid pests, fitness costs and incomplete resistance are required to significantly delay the evolution of resistance in haplodiploid and parthenogenetic pests (Crowder and Carrière 2009).
In haplodiploid species, the evolution of resistance is often driven by selection for resistant males (Carrière 2003; Crowder et al. 2006; Crowder and Carrière 2009). The initial frequency of resistant haploid males is equal to the r allele frequency, while the frequency of diploid homozygous resistant females is lower (r2). The majority of r alleles in females of a haplodiploid species are present in rs individuals, although all r alleles in males are found in r individuals. Thus, recessive costs in females have a lower influence on resistance evolution than nonrecessive costs, while costs expressed in males are not affected by dominance and are always important (Crowder and Carrière 2009). Accordingly, decreasing the fitness of r males generally has the largest effect on delaying the evolution of resistance, unless costs in females are large and nonrecessive. Similarly, incomplete resistance that lowers the fitness of r males on transgenic crops delays resistance more than incomplete resistance affecting rr females. In parthenogenetic species, resistance can be delayed with refuges, fitness costs, and incomplete resistance, but incomplete resistance is more important than fitness costs (Crowder and Carrière 2009). Thus, in haplodiploid and parthenogenetic pests, while refuges cannot appreciably delay resistance by reducing heritability, they can delay resistance considerably when resistance is incomplete and fitness costs are present.
Conclusion
Since their commercial introduction 14 years ago, Bt crops have achieved great success. The deployment of Bt crops has decreased the regional density of several pests of corn and cotton. Contrary to expectations of widespread field control failures based on worst-case scenarios, field-evolved resistance has been documented only in some populations of five pest species. While empirical evidence from the field is still limited, patterns within and across species are generally consistent with predictions from evolutionary theory (Tabashnik et al. 2008a, 2009a). For example, in H. zea, resistance to Cry1Ac in Bt cotton evolved faster in areas with lower refuge abundance, both within and among states in the southeastern USA (Tabashnik et al. 2008a, 2009a). Furthermore, comparisons across pest species suggest that recessive inheritance of resistance and abundant refuges of non-Bt host plants are two key factors that delay the evolution of resistance (Tabashnik et al. 2008a, 2009a).
Refuges have been used widely for delaying the evolution of resistance, but compliance with the refuge strategy has varied in time and space. Compliance with refuge requirements was consistently high for Bt cotton in Arizona (Carrière et al. 2005b), but has declined for Bt corn in the USA (Jaffe 2009). In South Africa, the documented lack of compliance with the refuge strategy is suspected as a major factor contributing to rapid evolution of resistance to Bt corn in B. fusca (Kruger et al. 2009).
Even though few cases of resistance to Bt crops have been reported so far, a delay usually occurs between the introduction of a novel pesticide and the rise in the number of species that evolve resistance to the pesticide (Georghiou 1986). For example, for DDT used in cotton in the USA, the first case of resistance was documented more than 10 years after DDT was commercialized, followed by a rapid increase in the number of resistant species in the subsequent decade (Fig. 3). Bt cotton and DDT are similar in many ways with respect to resistance evolution (recessive inheritance of resistance, widespread adoption following commercialization, toxicity maintained for extended periods), but are different in other ways, including the much broader spectrum of insects killed by DDT and the lack of a refuge strategy for managing resistance to DDT. Nevertheless, the possibility remains that, as seen with DDT, the accumulation of pests resistant to Bt crops could accelerate. With increasing numbers of Bt cultivars commercialized, biotech companies are now in a better position to rapidly produce novel Bt cultivars that will delay the evolution of resistance, and biotech companies are already aggressively pursuing these technologies. Nevertheless, cautious resistance management approaches involving refuges continue to be important (Jaffe 2009; Tabashnik et al. 2009a).
Figure 3.
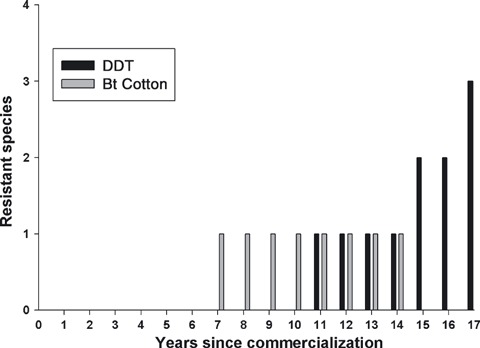
Cumulative number of species of cotton pests with documented field-evolved resistance to Bt cotton or DDT in years following their introduction in the US. Data on evolution of resistance to DDT are from APRD (2009). Some species categorized as evolving resistance to DDT between 1946 and 1963 in APRD (2009) were not included in the Figure (Lygus hesperus and Spodoptera exigua) because primary literature stated that populations tested for DDT resistance had not been collected in cotton.
We see several research directions that could provide a stronger scientific basis for managing resistance to Bt crops. Although theory, small-scale experiments, and retrospective analysis of resistance monitoring data suggest that the refuge strategy has been useful (Gould 1998; Tabashnik et al. 2008a, 2009a), a more detailed analysis of the spatial distribution of Bt crops and refuges in conjunction with patterns of field-evolved resistance could help to more rigorously test the effects of refuges. The demonstrated ability of Bt crops to cause regional pest suppression suggests that better understanding of source-sink dynamics could also be useful for enhancing resistance management strategies.
New cultivars of Bt corn have been released to control both coleopteran pests such as corn rootworms (Diabrotica spp.) and lepidopteran pests (Tabashnik et al. 2009a). While refuges of non-Bt corn used to delay resistance in lepidopteran pests were often planted as far as 1 km from Bt corn fields, refuges for the new cultivars effective against rootworms and lepidopteran pests are planted in or adjacent to Bt corn fields in regions where the rootworms are important pests (US EPA 2008). This might reduce isolation of refuges from Bt crop fields for the most dispersive lepidopteran pests, which could enhance regional declines in their populations (Carrière et al. 2003; Hutchison et al., unpublished data). Spatially-explicit studies linking the distribution of Bt corn fields and refuges to variation in population density of lepidopteran pests in corn-dominated agroecosystems could yield better understanding of the source-sink dynamics of Bt crops.
Whereas most research on fitness costs associated with resistance to Bt toxins has been done in the laboratory and greenhouse, field research is needed to enhance our ability to manipulate costs (Gassmann et al. 2009). Furthermore, RNA interference and other novel techniques will offer increasing flexibility to reduce pest fitness on transgenic insecticidal crops (Crowder and Carrière 2009). Research is also needed to better understand the physiological basis of incomplete resistance to design transgenic cultivars that will increasingly delay resistance. Innovative research and incorporation of knowledge gained from observed patterns of field-evolved resistance into future resistance management strategies will help maximize the benefits of current and future transgenic crops.
Acknowledgments
This work was supported by funding from USDA-NIFA grants 2007-35302-18225 and 2008-35302-0390. We thank Shannon Heuberger and Thierry Brévault for providing comments on this article.
Literature cited
- Adamczyk JJ, Hubbard D. Changes in populations of Heliothis virescens (F.) (Lepidoptera: Noctuidae) and Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in the Mississippi Delta from 1986 to 2005 as indicated by adult male pheromone traps. The Journal of Cotton Science. 2006;10:155–160. [Google Scholar]
- Adamczyk JJ, Jr, Adams LC, Hardee DD. Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. Journal of Economic Entomology. 2001;94:1589–1593. doi: 10.1603/0022-0493-94.6.1589. [DOI] [PubMed] [Google Scholar]
- Andow DA, Ives AR. Monitoring and adaptive resistance management. Ecological Applications. 2002;12:1378–1390. [Google Scholar]
- Andreadis SS, Álvarez-Alfageme F, Sánchez-Ramos L, Stodola TJ, Andow DA, Milonas PG, Savopooulos-Soultani M, et al. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in Greek and Spanish population of Sesamia nonagrioides (Lepidoptera: Noctuidae) Journal of Economic Entomology. 2007;100:195–201. doi: 10.1603/0022-0493(2007)100[195:fortbt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- APRD. 2009. Arthropod Pesticide Resistance Database. http://www.pesticideresistance.com/ (accessed April 2010)
- Archer TL, Schuler G, Patrick C, Cronjolm G, Bynum ED, Jr, Morrison WP. Whorl and stalk damage by European and Southwestern corn borers to four events of Bacillus thuringiensis transgenic maize. Crop Protection. 2001;19:181–190. [Google Scholar]
- Bagla P. Hardy cotton-munching pests are latest blow to GM crops. Science. 2010;327:1439. doi: 10.1126/science.327.5972.1439. [DOI] [PubMed] [Google Scholar]
- Bird LJ, Akhurst RJ. Relative fitness of Cry1A-resistant and -susceptible Helicoverpa armigera (Lepidoptera, Noctuidae) on conventional and transgenic cotton. Journal of Economic Entomology. 2004;97:1699–1709. doi: 10.1603/0022-0493-97.5.1699. [DOI] [PubMed] [Google Scholar]
- Bird LJ, Akhurst RJ. The fitness of Cry1A-resistant and -susceptible Helicoverpa armigera (Lepidoptera, Noctuidae) on transgenic cotton with reduced levels of Cry1Ac. Journal of Economic Entomology. 2005;59:1166–1168. doi: 10.1603/0022-0493-98.4.1311. [DOI] [PubMed] [Google Scholar]
- Bird LJ, Akhurst RJ. Variation in susceptibility of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) in Australia to two Bacillus thuringiensis toxins. Journal of Invertebrate Pathology. 2006;94:84–94. doi: 10.1016/j.jip.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Bourguet D, Chaufaux J, Micoud A, Delos M, Naibo B, Bombarde F, Marque G, et al. Ostrinia nubilalis parasitism and the field abundance of non-target insects in transgenic Bacillus thuringiensis corn (Zea mays) Environmental Biosafety Research. 2002;1:49–60. doi: 10.1051/ebr:2002005. [DOI] [PubMed] [Google Scholar]
- Bourguet D, Chauvaux J, Séguin M, Buisson C, Hinton JL, Stodola TJ, Porter P, et al. Frequency of alleles conferring resistance to Bt maize in French and US corn belt populations of the European corn borer, Ostrinia nubilalis. Theoretical and Applied Genetics. 2003;106:1225–1233. doi: 10.1007/s00122-002-1172-1. [DOI] [PubMed] [Google Scholar]
- Bravo A, Soberón M. How to cope with insect resistance to Bt toxins? Trends in Biotechnology. 2008;26:573–579. doi: 10.1016/j.tibtech.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Brown MW, Hoffmann AA. A reassessment of genetic limits to evolutionary change. Ecology. 2005;86:1371–1384. [Google Scholar]
- Buntin GD. Corn expressing Cry1Ab or Cry1F endotoxin for fall armyworm and corn earworm (Lepidoptera: Noctuidae) management in field corn for grain production. Florida Entomologist. 2008;91:523–530. [Google Scholar]
- Caprio MA. Source-sink dynamics between transgenic and nontransgenic habitats and their role in the evolution of resistance. Journal of Economic Entomology. 2001;94:698–705. doi: 10.1603/0022-0493-94.3.698. [DOI] [PubMed] [Google Scholar]
- Caprio MA, Tabashnik BE. Gene flow accelerates local adaptation among finite populations: simulating the evolution of insecticide resistance. Journal of Economic Entomology. 1992;85:611–620. [Google Scholar]
- Caprio MA, Faver MK, Hankins G. Evaluating the impacts of refuge width on source-sink dynamics between transgenic and non-transgenic cotton. Journal of Insect Science. 2004;4 doi: 10.1093/jis/4.1.3. 3available online: insectscience.org/4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y. Haplodiploidy, sex and the evolution of pesticide resistance. Journal of Economic Entomology. 2003;96:1626–1640. [PubMed] [Google Scholar]
- Carrière Y, Roff DA. Change in genetic architecture resulting from the evolution of insecticide resistance: a theoretical and empirical analysis. Heredity. 1995;75:618–629. [Google Scholar]
- Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. Proceedings of the Royal Society B. 2001;268:1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y, Deland J-P, Roff DA, Vincent C. Life history costs associated with the evolution of insecticide resistance. Proceedings of the Royal Society of London, B. 1994;58:35–45. [Google Scholar]
- Carrière Y, Ellers-Kirk C, Pederson B, Haller S, Antilla L. Predicting spring moth emergence in the pink bollworm: implications for managing resistance to transgenic cotton. Journal of Economic Entomology. 2001;94:1012–1021. doi: 10.1603/0022-0493-94.5.1012. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Sisterson MS, Antilla L, Whitlow M, Dennehy TJ, Tabashnik BE. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1519–1523. doi: 10.1073/pnas.0436708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y, Dutilleul P, Ellers-Kirk C, Pedersen B, Haller S, Antilla L, Dennehy TJ, et al. Sources, sinks, and the zone of influence of refuges for managing insect resistance to Bt crops. Ecological Applications. 2004a;14:1615–1623. [Google Scholar]
- Carrière Y, Ellers-Kirk C, Biggs R, Dennehy TJ, Tabashnik BE. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. Journal of Economic Entomology. 2004b;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Biggs R, Degain B, Holley D, Yafuso C, Evans P, et al. Effects of cotton cultivar on fitness costs associated with resistance of pink bollworm (Lepidoptera: Gelechiidae) to Bt cotton. Journal of Economic Entomology. 2005a;98:947–954. doi: 10.1603/0022-0493-98.3.947. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Kumar K, Heuberger S, Whitlow M, Antilla L, Dennehy TJ, et al. Long-term evaluation of compliance with refuge requirements for Bt cotton. Pest Management Science. 2005b;61:327–330. doi: 10.1002/ps.1039. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers Kirk C, Biggs RW, Nyboer ME, Unnithan GC, Dennehy TJ, Tabashnik BE. Cadherin-based resistance to Bt cotton in hybrid strains of pink bollworm: fitness costs and incomplete resistance. Journal of Economic Entomology. 2006;99:1925–1935. doi: 10.1603/0022-0493-99.6.1925. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Cattaneo MG, Yafuso CM, Antilla L, Huang C-Y, Rahman M, et al. Landscape effects of transgenic cotton on non-target ants and beetles. Basic and Applied Ecology. 2009a;10:597–606. [Google Scholar]
- Carrière Y, Showalter AM, Fabrick JA, Sollome J, Ellers-Kirk C, Tabashnik BE. Cadherin gene expression and effects of Bt resistance on sperm transfer in pink bollworm. Journal of Insect Physiology. 2009b;55:1058–1064. doi: 10.1016/j.jinsphys.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, Yafuso CM, Schmidt C, Huang C-Y, Rahman M, Olson C, Ellers-Kirk C, et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7571–7576. doi: 10.1073/pnas.0508312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comins HN. The development of insecticide resistance in the presence of migration. Journal of Theoretical Biology. 1977;64:177–197. doi: 10.1016/0022-5193(77)90119-9. [DOI] [PubMed] [Google Scholar]
- Crespo ALB, Spencer TA, Alves AP, Hellmich RL, Blankenship EE, Magalhães LC, Siegfried BD. On-plant survival and inheritance of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. Pest Management Science. 2009;65:1071–1081. doi: 10.1002/ps.1793. [DOI] [PubMed] [Google Scholar]
- Crowder DW, Carrière Y. Comparing the refuge strategy for managing the evolution of insect resistance under different reproductive strategies. Journal of Theoretical Biology. 2009;261:423–430. doi: 10.1016/j.jtbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Crowder DW, Carrière Y, Tabashnik BE, Ellsworth PC, Dennehy TJ. Modeling the evolution of resistance to pyriproxifen by the sweet-potato whitefly (Hemiptera: Aleyrodidae) Journal of Economic Entomology. 2006;99:1396–1406. doi: 10.1603/0022-0493-99.4.1396. [DOI] [PubMed] [Google Scholar]
- Dively GP. Impact of transgenic VIP3A × Cry1Ab Lepidopteran-resistant field corn on nontarget arthropods. Environmental Entomology. 2005;34:1267–1291. [Google Scholar]
- Downes S, Mahon RJ, Rossiter L, Kauter G, Leven T, Fitt G, Baker G. Adaptive management of pest resistance by Helicoverpa species (Noctuidae) in Australia to the Cry2Ab Bt toxin in Bollgard II cotton. Evolutionary Applications. 2010 doi: 10.1111/j.1752-4571.2010.00146.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutations. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A, D'alessandro M, Romeis J, Bigler F. Assessing expression of Bt-toxin (Cry1Ab) in transgenic maize under different environmental conditions. IOBC/WPRS Bulletin. 2004;27:49–55. [Google Scholar]
- Endler JA. Natural Selection in the Wild. Princeton: Princeton University Press; 1986. [Google Scholar]
- Fan X, Zhao J-Z, Fan Y, Shi X. Inhibition of transgenic Bt plants to the growth of cotton bollworm. Plant Protection. 2000;26:3–5. [Google Scholar]
- Futuyma DJ. Evolutionary Biology. Sunderland: Sinauer; 1979. [Google Scholar]
- Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- Gahan LJ, Gould F, López JD, Jr, Micinski S, Heckel DG. A polymerase chain reaction screen of field populations of Heliothis virescens for a retrotransposon insertion conferring resistance to Bacillus thuringiensis toxin. Journal of Economic Entomology. 2007;100:187–194. doi: 10.1603/0022-0493(2007)100[187:apcrso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gassmann AJ, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annual Review of Entomology. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- Georghiou GP, National Research Council . Pesticide Resistance: Strategies and Tactics for Management. Washington, DC: National Academies Press; 1986. The magnitude of the resistance problem; pp. 14–43. [Google Scholar]
- Georghiou GP, Taylor CE. Genetic and biological influences in the evolution of insecticide resistance. Journal of Economic Entomology. 1977;70:319–323. doi: 10.1093/jee/70.3.319. [DOI] [PubMed] [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annual Review of Entomology. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- Gould F, Anderson A, Jones A, Sumerford D, Heckel DG, Lopez J, Micinski S, et al. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F, Cohen MB, Bentur JS, Kennedy GG, Van Duyn J. Impact of small fitness costs on pest adaptation to crop varieties with multiple toxins: a heuristic model. Journal of Economic Entomology. 2006;99:2091–2099. doi: 10.1603/0022-0493-99.6.2091. [DOI] [PubMed] [Google Scholar]
- Huang F, Leonard BR, Cook DR, Lee DR, Andow DA, Baldwin JL, Tindall KV, et al. Frequency of alleles conferring resistance to Bacillus thuringiensis maize in Louisiana populations of southwestern corn borer (Lepidoptera: Crambidae) Entomologia Experimentalis et Applicata. 2007a;122:53–58. [Google Scholar]
- Huang F, Leonard BR, Andow D. Sugarcane borer (Lepidoptera: Crambidae) resistance to transgenic Bacillus thuringiensis maize. Journal of Economic Entomology. 2007b;109:164–171. doi: 10.1603/0022-0493(2007)100[164:sblcrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Huang F, Leonard BR, Moore S, Cook D, Baldwin J, Tindall KV, Lee DR. Allele frequency of resistance to Bacillus thuringiensis Cry1Ab corn in Louisiana populations of sugarcane borer (Lepidoptera: Crambidae) Journal of Economic Entomology. 2008a;101:492–498. doi: 10.1603/0022-0493(2008)101[492:afortb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Huang F, Leonard R, Moore S, Yue B, Parker R, Reagan T, Stout M, et al. Geographical susceptibility of Louisiana and Texas populations of the sugarcane borer, Diatracea saccharalis (F.) (Lepidoptera: Crambidae) to Bacillus thuringiensis Cry!Ab protein. Crop Protection. 2008b;27:799–806. [Google Scholar]
- Huang F, Parker R, Leonard R, Yong Y, Liu J. Frequency of resistance alleles to Bacillus thuringiensis-corn in Texas populations of sugarcane borer, Diatraea saccharalis (F.) (Lepidoptera: Crambidae) Crop Protection. 2009;28:174–180. [Google Scholar]
- Hunt TE, Bushman LL, Sloderbeck PE. Insecticide use in Bt and non-Bt field corn in the western corn belt: reported by crop consultants in a mail survey. American Entomologist. 2007;52:86–93. [Google Scholar]
- Jaffe G. 2009. Complacency on the farm. Center for Insect Science in the Public Interest. 19pp. http://cspinet.org/new/pdf/complacencyonthefarm.pdf. (Accessed April 2010)
- James C. Global Status of Commercialized Biotech/GM crops: 2008, ISAAA Brief No. 38. Ithaca, NY: International Service for the Acquisition of Agri-Biotech Applications (ISAAA); 2008. [Google Scholar]
- Kruger M, Van Rensburg JBJ, Van den Berg J. Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Protection. 2009;28:684–689. [Google Scholar]
- Labbé P, Berticat C, Berthomieu A, Unal S, Bernard C, Weill M, Lenormand T. Forty years of erratic insecticide resistance evolution in the mosquito Culex pipiens. PLoS Genetics. 2007;11:2190–2199. doi: 10.1371/journal.pgen.0030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefko SA, Nowatzki TM, Thompson SD, Binning RR, Pascual MA, Peters ML, Simbro EJ, et al. Characetrizing laboratory colonies of western corn rootworm (Coleoptera: Chrysomelidae) selected for survival on maize containing event DAS-59122-7. Journal of Applied Entomology. 2008;132:189–204. [Google Scholar]
- Liang G-M, Wu K-M, Yu H-K, Li K-K, Feng X, Guo Y-Y. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera:Noctuidae) along with its resistance evolution to Cry1Ac toxin. Journal of Invertebrate Pathology. 2008;97:142–149. doi: 10.1016/j.jip.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Liu Y-B, Tabashnik BE, Dennehy TJ, Patin AL, Sims MA, Meyer SK, Carrière Y. Effects of Bt cotton and Cry1Ac toxin on survival and development of pink bollworm (Lepidoptera: Gelechiidae) Journal of Economic Entomology. 2001;94:1237–1242. doi: 10.1603/0022-0493-94.5.1237. [DOI] [PubMed] [Google Scholar]
- Liu FX, Xu ZP, Chang JH, Meng J, Zhu FX, Shen YC, Shen JL. Resistance allele frequency to Bt cotton in field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) in China. Journal of Economic Entomology. 2008;101:933–943. doi: 10.1603/0022-0493(2008)101[933:raftbc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Mares CL, Fitt GP. Field performance and seasonal changes in the efficacy against Helicoverpa armigera (Hübner) of transgenic cotton expressing the insecticidal protein Vip3A. Agricultural and Forest Entomology. 2007;9:93–101. [Google Scholar]
- Luttrell RG, Ali I, Allen KC, Young SY, III, Szalanski A, Williams K, Lorenz G, et al. Resistance to Bt in Arkansas populations of cotton bollworm. In: Richter DA, editor. Memphis: National Cotton Council of America; 2004. pp. 1373–1383. Proceedings, 2004 Beltwide Cotton Conferences, 5-9 January 2004, pp. 5–9. [Google Scholar]
- Ma BL, Meloche F, Wei L. Agronomic assessment of Bt trait and seed or soil-applied insecticides on the control of corn rootworm and yield. Field Crop Research. 2009;111:189–196. [Google Scholar]
- Mahon RJ, Olsen KM. Limited survival of a Cry2Ab-resistant strain of Helicoverpa armigera (Lepidoptera: Noctuidae) on Bollgard II. Journal of Economic Entomology. 2009;102:708–716. doi: 10.1603/029.102.0232. [DOI] [PubMed] [Google Scholar]
- Mahon RJ, Olsen K, Downes SJ, Addison S. Frequency of alleles conferring resistance to the Bt toxins Cry1Ac and Cry2Ab in Australian populations of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Journal of Economic Entomology. 2007;100:1844–1853. doi: 10.1603/0022-0493(2007)100[1844:foacrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Marvier M, McCreedy C, Regetz J, Kareiva P. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science. 2007;316:1475–1477. doi: 10.1126/science.1139208. [DOI] [PubMed] [Google Scholar]
- Marvier M, Carrière Y, Ellstrand N, Gepts P, Kareiva P, Rosi-Marshall E, Tabashnik BE, et al. Harvesting data from genetically engineered crops. Science. 2008;320:452–453. doi: 10.1126/science.1154521. [DOI] [PubMed] [Google Scholar]
- Matten SR, Head GP, Quemada HD. How governmental regulation can help or hinder the integration of Bt crops into IPM programs. In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of Insect-resistant Genetically Modified Crops within IPM Programs. New York: Springer; 2008. pp. 27–39. [Google Scholar]
- Meihls LN, Higdon ML, Siegfried BD, Miller NJ, Sappington TW, Ellersiek MR, Spencer TA, et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19177–19182. doi: 10.1073/pnas.0805565105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micinski S, Blouin DC, Waltman WF, Cookson C. Abundance of Helicoverpa zea and Heliothis virescens in pheromone traps during the past twenty years in Northwestern Louisiana. Southwestern Entomologist. 2008;33:139–149. [Google Scholar]
- Midboe EG, Mehmet C, Bulla LA., Jr Expression of a midgut-specific cadherin BT-R1 during the development of Manduca sexta larva. Comparative Biochemistry and Physiology Part B. 2003;135:125–137. doi: 10.1016/s1096-4959(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, Higginson D, Holley D, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo SE. Long-term assessment of the effects of transgenic Bt cotton on the function of the natural enemy community. Environmental Entomology. 2005;34:1211–1223. [Google Scholar]
- Naranjo SE. Impacts of Bt crops on non-target invertebrates and insecticide use patterns. Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2009;011:1–11. [Google Scholar]
- National Research Council. Pesticide Resistance: Strategies and Tactics for Management. Washington, DC: National Academy Press; 1986. [Google Scholar]
- Nguyen HT, Ja J. Expression of Cry3Bb1 in transgenic corn MON88017. Journal of Agricultural and Food Chemistry. 2009;57:9990–9996. doi: 10.1021/jf901115m. [DOI] [PubMed] [Google Scholar]
- Onstad DW. Insect Resistance Management: Biology, Economics, and Prediction. San Diego: Elsevier; 2008. [Google Scholar]
- Onstad DW, Guse CA. Concepts and complexities of population genetics. In: Onstad DW, editor. Insect Resistance Management. Biology, Economics and Predictions. London: Academic Press; 2008. pp. 69–88. [Google Scholar]
- Palumbi SR. Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Peck S, Gould F, Ellner SP. Spread of resistance in spatially extended regions of transgenic cotton, implications for management of Heliothis virescens (Lepidoptera, Noctuidae) Journal of Economic Entomology. 1999;92:1–16. [Google Scholar]
- Pereira EJG, Storer NP, Siegfried BD. Inheritance of Cry1F resistance in laboratory-selected European corn borer and its survival on transgenic corn expressing the Cry1F toxin. Bulletin of Entomological Research. 2008;98:621–629. doi: 10.1017/S0007485308005920. [DOI] [PubMed] [Google Scholar]
- Perron GG, Gonzalez A, Buckling A. Source-sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness costs. Proceedings of the Royal Society B. 2007;274:2351–2356. doi: 10.1098/rspb.2007.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J, Meissle M, Bigler F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nature Biotechnology. 2006;24:63–71. doi: 10.1038/nbt1180. [DOI] [PubMed] [Google Scholar]
- Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philosophical Transactions of the Royal Society B: Biological Sciences. 1998;353:1777–1786. [Google Scholar]
- Russell PJ. Fundamentals of Genetics. 2nd edn. San Francisco: Benjamin-Cummings; 2000. [Google Scholar]
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Review. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Heuberger S, Tabashnik BE, Carrière Y. A primer for the use of insecticidal transgenic cotton in developing countries. Journal of Insect Science. 2009;9 doi: 10.1673/031.009.2201. 43, available online: insectscience.org/9.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisterson MS, Antilla L, Carrière Y, Ellers-Kirk C, Tabashnik BE. Effects of insect population size on evolution of resistance to transgenic crops. Journal of Economic Entomology. 2004;97:1413–1424. doi: 10.1093/jee/97.4.1413. [DOI] [PubMed] [Google Scholar]
- Sisterson MS, Carrière Y, Dennehy TJ, Tabashnik BE. Evolution of resistance to transgenic crops: interactions between insect movement and field distribution. Journal of Economic Entomology. 2005;98:1751–1762. doi: 10.1093/jee/98.6.1751. [DOI] [PubMed] [Google Scholar]
- Sisterson MS, Biggs RW, Manhardt NM, Carrière Y, Dennehy TJ, Tabashnik BE. Effects of transgenic Bt cotton on insecticide use and abundance of two generalist predators. Entomologia Experimentalis et Applicata. 2007a;124:305–311. [Google Scholar]
- Sisterson MS, Carrière Y, Dennehy TJ, Tabashnik BE. Non-target effects of transgenic insecticidal crops: implications of source-sink population dynamics. Environmental Entomology. 2007b;36:121–127. doi: 10.1603/0046-225x(2007)36[121:neotic]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Soberón M, Pardo-López L, López I, Gómez I, Tabashnik BE, Bravo A. Engineering modified Bt toxins to counter insect resistance. Science. 2007;318:1640–1642. doi: 10.1126/science.1146453. [DOI] [PubMed] [Google Scholar]
- Stodola TJ, Andow DA, Hyden AR, Hinton JL, Roark JJ, Buschman LL, Porter P, et al. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in southern United States corn belt population of European corn borer (Lepidoptera: Crambidae) Journal of Economic Entomology. 2006;99:502–507. doi: 10.1603/0022-0493-99.2.502. [DOI] [PubMed] [Google Scholar]
- Storer NP, Peck SL, Gould F, Van Duyn JW, Kennedy GG. Spatial processes in the evolution of resistance in Helicoverpa zea (Lepidoptera: Noctuidae) to Bt transgenic corn and cotton in a mixed agroecosystem: a biology-rich stochastic simulation model. Journal of Economic Entomology. 2003;96:156–172. doi: 10.1093/jee/96.1.156. [DOI] [PubMed] [Google Scholar]
- Storer NP, Dively GP, Herman RA. Landscape effects of insect-resistant genetically modified crops. In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of Insect-resistant Genetically Modified Crops within IPM programs, pp. New York: Springer; 2008. pp. 273–302. [Google Scholar]
- Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annual Review of Entomology. 1994;39:47–79. [Google Scholar]
- Tabashnik BE, Carrière Y. Insect resistance to genetically modified crops. In: Gatehouse A, Ferry N, editors. Environmental Impact of Genetically Modified and Novel Crops. Wallingford, UK: CABI; 2009. pp. 74–100. [Google Scholar]
- Tabashnik BE, Patin AL, Dennehy TJ, Liu YB, Carrière Y, Sims MA, Antilla L. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proceedings of the National Academy of Sciences of the United Sates of America. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik BE, Gould F, Carrière Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. Journal of Evolutionary Biology. 2004;17:904–912. doi: 10.1111/j.1420-9101.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Dennehy TJ, Carrière Y. Delayed resistance to trangenic cotton in pink bollworm. Proceedings of the National Academy of Sciences of the United States of America. 2005a;102:15389–15393. doi: 10.1073/pnas.0507857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik BE, Biggs RW, Higginson DM, Unnithan DC, Unithan GC, Ellers-Kirk C, Sisterson MS, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. Journal of Economic Entomology. 2005b;98:635–644. doi: 10.1603/0022-0493-98.3.635. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Fabrick JA, Henderson S, Biggs RW, Yafuso CM, Nyboer ME, Manhardt NM, et al. DNA screening reveals pink bollworm resistance to Bt cotton remains rare after a decade of exposure. Journal of Economic Entomology. 2006;99:1525–1530. doi: 10.1603/0022-0493-99.5.1525. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Insect resistance to Bt crops: evidence versus theory. Nature Biotechnology. 2008a;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Field-evolved resistance to Bt toxins. Nature Biotechnology. 2008b;26:1074–1976. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Van Rensburg JBJ, Carrière Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. Journal of Economic Entomology. 2009a;102:2011–2025. doi: 10.1603/029.102.0601. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Unnithan GC, Masson L, Crowder DW, Li X, Carrière Y. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106:11889–11894. doi: 10.1073/pnas.0901351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. 2008. Pesticide Fact Sheet. U.S. Environmental Protection Agency. http://www.epa.gov/oppbppd1/biopesticides/pips/smartstax-factsheet.pdf.
- Williams JL. University of Arizona; 2009. Fitness costs of resistance to Bacillus thuringiensis in the pink bollworm Pectinophora gossypiella. PhD Dissertation. [Google Scholar]
- Wolfenbarger LL, Naranjo S, Lundgren J, Bitzer R, Watrud L. Bt crop effects on functional guilds of non-target arthropods: a meta-analysis. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002118. Article Number e2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Huang F, Leonard BR, Moore SH. Evaluation of transgenic Bacillus thuringiensis corn hybrids against Cry1Ab-susceptible and –resistant sugarcane borer (Lepidoptera: Crambidae) Journal of Economic Entomology. 2007;100:1880–1886. doi: 10.1603/0022-0493(2007)100[1880:eotbtc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wu K-M, Lu Y-H, Feng H-Q, Jiang Y-Y, Zhao J-Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science. 2008;321:1676–1678. doi: 10.1126/science.1160550. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu F, Chen J, Huang F, Andow DA, Wang Y, Zhu YC, et al. Using an F2 screen to monitor frequency of resistance alleles to Bt cotton in field populations of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) Pest Management Science. 2009;65:391–397. doi: 10.1002/ps.1703. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen H, Wu Y, Yang Y, Wu S. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Applied Environmental Microbiology. 2007;73:6939–6944. doi: 10.1128/AEM.01703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cheng H, Gao Y, Wang G, Liang G, Wu K. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuring1ensis Cry1Ac toxin. Insect Biochemistry and Molecular Biology. 2009;39:421–429. doi: 10.1016/j.ibmb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Zhao JZ, Cao J, Collins HL, Bates SL, Roush RT, Earle ED, Shelton AM. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]


