Abstract
The evolutionary processes that have enabled Bos taurus cattle to establish around the globe are at the core to the future success of livestock production. Our study focuses on the history of cattle domestication including the last 60 years of B. taurus breeding programmes in both favourable and unfavourable environments and its consequences on evolution and fitness of cattle. We discuss the emergence of ‘production diseases’ in temperate production systems and consider the evolutionary genetics of tropical adaptation in cattle and conclude that the Senepol, N'Dama, Adaptaur and Criollo breeds, among others with similar evolutionary trajectories, would possess genes capable of improving the productivity of cattle in challenging environments. Using our own experimental evidence from northern Australia, we review the evolution of the Adaptaur cattle breed which has become resistant to cattle tick. We emphasize that the knowledge of interactions between genotype, environment and management in the livestock systems will be required to generate genotypes for efficient livestock production that are both economically and environmentally sustainable. Livestock producers in the 21st century will have less reliance on infrastructure and veterinary products to alleviate environmental stress and more on the animal's ability to achieve fitness in a given production environment.
Keywords: adaptation, breeding, cattle, environment, genetic improvement, trait
Introduction
Whilst both natural evolutionary processes and modern genetic selection result in phenotypic changes, the mechanisms that determine the changes are quite different. Evolution is dynamic natural change in response to environmental pressure. Genetic selection is artificial directed change largely independent of environmental pressure. Within modern agriculture, genetic selection has been able to maximize directed change by minimizing environmental pressures. The environmental pressures are managed through intervention strategies such as providing high quality feeds, artificial housing or drugs to restrict disease. These intervention strategies are energy dense and enable agriculture to maximize outputs (productivism); however, they result in populations that are unable to withstand the pressure of more ‘natural’ environments. In some cases, the extreme selection for a single trait can result in physiological breakdown as resources are channelled towards particular production proteins. This study explores how evolutionary genetics might help inform modern cattle breeding programmes and shows some examples from tropically adapted cattle. We consider the importance of environmental stress for identifying robust genotypes in production systems that will increasingly need to focus on efficiency rather than total productivity.
Since the 1950s in the developed countries, the era of agricultural productivism emerged with a corresponding demise in the influence of natural selection in livestock production systems. Livestock productivism was characterized by a significant use of technology, both mechanization and biotechnology, to maximize productive output. This productivism was facilitated by relatively abundant cheap fossil fuels; environmental regulation removed environmental stressors to allow intensification, concentration and specialization of the systems (Ilbery and Bowler 1998). The genetic diversity of both Bos taurus and Bos indicus cattle was reduced with the concentration on breeds and sires within breeds that had the highest production potential. More recently, there has been a trend for a measured increase in productivity and growing pressure for cattle production systems to manage their total carbon budgets, which will require a reduction in their greenhouse gas emissions. The reduction in the use of fossil fuel derived energy and nutrients (direct: fuel and fertilizer; indirect: acaricide, anthelmintics and vaccine) will reduce the ability to manage directly environmental stress. A paradigm shift in priorities is therefore required – more diverse genotypes that are productive in a range of environments will need to be developed. The evolutionary path of cattle since domestication provides insight into the current fitness levels as well as a better understanding of the interactions between cattle genotypes, environments and management intervention strategies (hence forth called G × E × M interactions).
There has been a long history of domestication and implicit genetic selection; however, systematic breeding programmes have only been used over the last 60 years. These breeding programmes have enabled cattle to meet specific roles: predominantly supplying meat and milk for human consumption. There are hundreds of distinct cattle breeds around the world. However, a recent article published in Science (The Bovine HapMap Consortium 2009) looked at the process of cattle domestication. Their analyses (based on mutation rate, inbreeding rate, linkage disequilibrium levels, etc.) revealed that cattle have undergone a rapid recent decrease in effective population size from a very large ancestral population, possibly due to bottlenecks associated with domestication, selection and breed formation. This study also provided genetic relatedness amongst these breeds. Hence, any future proposal for evolutionary management of cattle domestication should consider this worldwide structure of the breeds to design conservation programmes and mating schemes. The reduction in the ancestral population diversity has important implications for breeding programmes in the 21st century.
The opportunity for modern cattle breeds to evolve in response to environmental stress is restricted as the diversity of the gene pool of cattle populations diminishes. However, there are examples of cattle breeds that have demonstrated rapid evolutionary change: in particular where B. taurus cattle have been reared in challenging tropical environments. The Adaptaur of northern Australia is a European B. taurus (interbred Hereford–Shorthorn) that has undergone a relatively rapid transition from extreme susceptibility to extreme resistance to cattle tick via a synergy between natural and artificial selection for tick resistance (Frisch 1981; Hetzel et al. 1990; Frisch et al. 2000). The evolution of the Adaptaur under tropical stress provides a model approach to cattle breeding, which, if applied within the broader livestock industries might determine more favourable genotypes especially in the advent of escalating environmental stress due to climate change.
This study explores the consequences of selection for production traits and demonstrates evolutionary genetics of taurine cattle in both temperate (favourable) and tropical (unfavourable) environments. As an example of evolutionary adaptation we focus on the Adaptaur from northern Australia; however, we also draw on examples from three other B. taurus breeds that all have evolutionary backgrounds in unfavourable environments: Senepol from the Caribbean, the Criollo breed group from Central and South America and the N'Dama from West Africa. We show how exposure to a specific environmental stress (cattle tick infestation in a tropical environment) demonstrates the potential for experimental evolution to generate new more robust genotypes that can perform in environmentally stressful environments. Genetic adaptation of cattle for a successful transition into the postproductivism era must incorporate alleles of economic importance from genotypes adapted to potentially more stressful environments. The principles of evolutionary genetics can be used to facilitate Darwinian fitness in future livestock breeding programmes to generate optimal genotypes for a low-cost and environmentally sustainable production system. In essence, there are complex interactions between genotype, environment and management (G × E × M) in the livestock systems and the implications relate to: (i) fitting the right genotypes to the right environments and production systems and (ii) determining the optimal genotype across a range of environments and production systems. We provide some perspectives on G × E × M and how this could contribute to evolution of future strains or breeds of cattle.
Historical movement of African and European Bos taurus
About 10 000 years bp (before present), Neolithic hunter-gathers began to domesticate simultaneously both B. taurus (Fertile Crescent and Saharan Africa) and B. indicus (Indian subcontinent) (Bradley et al. 1998; Bruford et al. 2003; Chen et al. 2010). The history of B. indicus in Asia (Harris 1992), and more recent introduction to Africa via the African east coast about 3500 years bp (Hanotte et al. 2002), is notable for the high levels of environmental stress associated with indicine evolution. The heterosis achieved from B. taurus × B. indicus crossbreeding is well documented (e.g. McDowell et al. 1996). However, some African taurine breeds, such as N'Dama, were able to survive in stressful tropical environments with little or no B. indicus hybridization1 (Frisch et al. 1997; Anderung et al. 2007).
In Europe, B. taurus accompanied Neolithic Man's conversion from nomadic hunter-gatherer to a sedentary agriculturalist and cattle evolved adaptations to temperate climates. Under various Celtic, Germanic and Nordic tribes, there was a general divergence of the larger continental breeds (e.g. Charolais, Simmental) from the medium-sized British breeds (e.g. Hereford, Red Poll, Murray Grey) and specialization into dairy (e.g. Holstein, Jersey), beef (e.g. Angus, Shorthorn) and dual purpose (e.g. Brown Swiss). In the developed regions of Europe, cattle increased their propensity to supply meat and milk through increased industrialization and dedicated breeding programmes.
European taurine cattle were also subjected to the stressors of tropical and semi-arid regions as these cattle were a significant component of the human colonization of Central and South America by the Spanish and Portuguese during the 16th century. Cattle from the colonization were collectively known as Creole or Criollo,2 and adapted to the environments that ranged from semi-arid to the wet tropics (Russell et al. 2000; Mariante et al. 2009). Also regarded as tropically adapted is the Senepol, a two-breed composite of N'Dama and Red Poll cattle, bred on the Caribbean island of St Croix in 1918 (Hammond et al. 1996). Moreover, northern Australia witnessed the formation of tropically adapted taurine composites. Herefords and Shorthorns, first imported into Australia from United Kingdom in 1826, along with the South African cattle breed, Africander imported from King Ranch, Texas, USA in 1953 formed part of a CSIRO research programme at the Belmont Research Station, Rockhampton in the 1950s (Kennedy and Turner 1959). Selection lines within a four-breed composite of Africander, Brahman, Hereford and Shorthorn, commercialized as Belmont Red, and a two-breed composite of Hereford and Shorthorn (HS line) were established. The HS line was partially commercialized as Adaptaur in 1985 (O'Neill et al. 1998).
Bos taurus breeding programmes in favourable environments – productivism and its consequences
Modern genetic selection programmes are capable of delivering cumulative gains of 1–2% per annum for the trait (or combination of traits) under selection (Simm et al. 2000). Quantitative genetic methods have been a crucial component of productivism: development of selection index principles in animal breeding programmes in early 1940s (Hazel and Lush 1943) followed by application of Best Linear Unbiased Prediction (BLUP) of animal's genetic merit from early 1970s: the animal model (Henderson 1975). These methods accurately quantify breeding potential (estimated breeding values or EBVs; expected progeny differences or EPDs) for economically important traits and are expressed in real units of measurement (e.g. kg for growth rate). To assess the relative superiority of a bull or a cow, the animal's EBV is compared with those reported by a breed average. With continuing advances in statistical and computing technology, quantitative geneticists successfully refined and modified BLUP methodology as new performance records (e.g. on daily growth) and associated new traits became available. Utilizing these sophisticated levels of technologies, livestock industries of the developed countries have achieved significant increases in the product quantity and quality within the optimal (i.e. stress-free) environments (Rauw et al. 1998; Bishop and Woolliams 2004; Maas et al. 2009). High performance under these stress-free environments does not, however, imply high performance in all environments. Whilst quantitative genetic techniques have successfully identified superior performing individuals and selectively bred from them, the selection process has been largely under increasingly optimal environments (i.e. stress-free). There is an emerging question of the costs of ‘stress-free selection’ as genotypes are exposed to more stressful environments. Whilst management intervention has increasingly reduced environmental stress the ability for an individual to respond has decreased as genetic selection has refined metabolic pathways towards product output at the expense of other traits such as disease resistance.
For the dairy industries the concern has been the emergence of ‘production diseases’ (e.g. lameness and metabolic and reproductive disorders), in parallel with increasing levels of milk production (Lucy 2001; Mulligan and Doherty 2008; Ward 2009). However, research and breeding programmes of dairy cattle have started to shift emphasis from production-only traits in the breeding goal to traits that are associated with robustness (e.g. resistance to clinical mastitis, lameness and milk fever) and sustainability (e.g. fertility, feed efficiency, conformation, optimal behaviour) (Distl 2000; Kadarmideen et al. 2000; Kadarmideen and Pryce 2001; Neuenschwander et al. 2005). Beef cattle breed societies have gone down a similar path to the dairy industries with the emphasis on performance recording production traits. In beef cattle breeding, growth, calving ease, reproduction and meat quality traits such as muscle and/or fat depth and tenderness are the main drivers of commercial operations. As with the dairy industries there has been an increase in persistent production diseases in the beef industries; namely respiratory diseases and metabolic disorders in the feedlot systems (e.g. Owens et al. 1998; Garcia et al. 2010). Continuing outbreaks of the ocular disease called infectious bovine keratoconjunctivitis (IBK) also generates significant economic and welfare issues for the cattle industries (Brown et al. 1998; Snowder et al. 2005). These problems have been addressed through research to develop animal management protocols, feed additives, pharmacological agents and vaccines to combat the diseases (Quigley et al. 1997; Galyean and Rivera 2003; McConnel et al. 2007; Carriquiry et al. 2009; Marcillac-Embertson et al. 2009) and demonstrates a classic ‘environment control’ strategy. However, genetic methods for the identification of host resistance/susceptibility for diseases such as IBK in the US Department of Agriculture reference cattle population (Casas and Stone 2006) and Australian Adaptaur cattle (Ali et al. 2010) are being explored.
The animal model can be used to evaluate genetic merit of animals for traits associated with the production diseases described above (Heringstad et al. 2000; Kadarmideen et al. 2000). It is not only possible to select animals for just one trait, but rather a combination of traits. Hence, animals’ EBVs for different traits (e.g. growth, reproductive success and some measures of adaptability like IBK resistance) are combined to form an aggregate or total breeding value (TBV). Therefore, a multi-trait breeding objective would appear as:
where 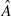 is the total breeding value, bi is an economic weight for the ith trait in breeding objective, EBVi is the EBV for the ith trait and n is the number of traits in the breeding goal. Breeders can modify their herd's genetics based on assigning different economic weights to different traits; for instance if adaptability or disease resistance is more important than other traits in tropical environments, then there needs to be higher weights for those traits in the breeding goal. Application of these novel methods has had major beneficial effects on the cost and quality of food, and on the efficiency, competitiveness and welfare status of the livestock industries concerned. The incorporation of resistance traits ensures that the TBV is also driven by evolutionary principles of adaptation.
is the total breeding value, bi is an economic weight for the ith trait in breeding objective, EBVi is the EBV for the ith trait and n is the number of traits in the breeding goal. Breeders can modify their herd's genetics based on assigning different economic weights to different traits; for instance if adaptability or disease resistance is more important than other traits in tropical environments, then there needs to be higher weights for those traits in the breeding goal. Application of these novel methods has had major beneficial effects on the cost and quality of food, and on the efficiency, competitiveness and welfare status of the livestock industries concerned. The incorporation of resistance traits ensures that the TBV is also driven by evolutionary principles of adaptation.
However, in any breeding programme the animal's physiological limits must be considered. In production systems of negligible environmental stress, the need for both energy and physiological mechanisms to alleviate stress are not required. In theory, the metabolic energy is therefore available for production traits but with continued selection for high production the dairy cow is unable to consume enough energy to meet this production potential and the cow's fertility (Butler and Smith 1989; Kadarmideen et al. 2003; Wathes et al. 2009) and health (Ingvartsen et al. 2003; Zuerner et al. 2007; Mulligan and Doherty 2008) suffer as a consequence. Moreover, a number of studies have shown a negative relationship between metabolic rate and resistance to stress (see Parsons 1990 and Hoffmann and Parsons 1991). Animals of low resistance to environmental stress have inherently higher rates of growth, appetite and fasting metabolism than animals relatively resistant to environmental stress (Frisch and Vercoe 1984). Thus, selection for production traits, disregarding physiological limits, fertility and resistance to environmental stress, would have serious negative consequences for future cattle herds as cows can only be maintained with high inputs that are unsustainable in the longer term.
The three main genetic processes involved in the evolution of animals during domestication are selection, genetic drift and inbreeding (Falconer and Mackay 1996), and inbreeding is posing additional problems for the intensive cattle industries. Inbreeding can lead to loss of fitness and even survival of a breed or species. For the dairy industry in the USA, Smith et al. (1998) found that the effects of inbreeding in the Holstein herd, years 1983–1993, were cumulative and larger on lifetime profit functions than on lactation traits. Techniques are now available to control inbreeding using conventional BLUP methods (Villanueva et al. 2006) or using molecular genetic markers in genetic improvement programmes (e.g. Li et al. 2008). Hence, any future breeding programmes must be designed to manage inbreeding depression (Kearney et al. 2004; Fernández et al. 2008).
In recent decades, cattle breeds of the developed countries have been subjected to breeding programmes that are not sustainable and inappropriate for determining genotypes that are productive in unfavourable environments. Stern and Orgogozo (2009) suggest that there is a need to identify and select specific mutations without negative consequences. We argue that for cattle of the 21st century it will be necessary to also explore the evolution of cattle in unfavourable environments, where there has been selection for alleles at many loci offering specific environmental adaptations. The next section provides some examples of breeding programmes that have identified desirable genetics contributing to adaptations of temperate cattle breeds to unfavourable tropical environments.
Bos taurus breeding programmes in unfavourable environments: tick resistance and tropical adaptation
Modern agricultural populations of plants and animals have shown rapid evolutionary adaptation to environmental stress in timeframes of decades (or less) (Thompson 1998; Bell and Collins 2008; Campbell et al. 2009). Environmental stress is defined as an environmental force, from either biotic or abiotic factors, that impairs an animal's regulatory mechanism to such an extent that fitness is compromised. Fitness, an animal's ability to grow and reproduce viable offspring will be adversely affected if the animal is unable to mount an appropriate response to the stressor via means of its physiology and/or behaviour. The degree of sensitivity to these stressors, or homeostasis, will be regarded as an aspect of fitness (Falconer and Mackay 1996). In a relatively short period of time, European B. taurus relocated from temperate to tropical regions and had to achieve higher fitness in the presence of tropical stressors. This higher fitness was achieved through the application of evolutionary principles within a genetic selection program.
The response to environmental stress involves a synergy between behaviour and physiological adaptation (Hart 1990; Hoffmann and Parsons 1991; Lomborg et al. 2008). Documenting the plethora of tropical environmental stressors and the corresponding responses of B. taurus to these stressors is beyond the scope of this study. Therefore, we present data from experiments that aimed to assess the evolutionary adaptation of B. taurus cattle to stress from infestations of the ectoparasite cattle tick Rhipicephalus (Boophilus) microplus. Cattle tick infestations have a substantial negative impact on host fitness (Frisch and Vercoe 1984; Lehmann 1993). By the end of last century, estimates of the yearly global costs of cattle tick and tick-borne diseases were between US$13.9 and US$18.7 billion (de Castro 1997). The global costs of ticks are set to escalate; increasing levels of acaricide resistance (Sangster 2001), limited efficacy of anti-tick vaccines and emergence of nonresponders to vaccination (Outteridge 1993; Sonenshine et al. 2006), the genetic antagonism on fitness traits from biased artificial selection for production traits (Frisch 1981; Kadarmideen et al. 2000) and the potential negative impact of climate change (White et al. 2003). Exploring the full repertoire of the animal's anti-tick mechanisms is warranted if genetic technologies that utilize naturally occurring adaptation to parasitic stress are going to contribute to sustainable livestock production.
European taurine cattle, such as the Criollo in the 1500s, were relocated to their respective tropical environments from temperate environments that were free of parasites endemic in the tropics. A similar case occurred in 1952 with the introduction of Herefords and Shorthorns (HS line) on the CSIRO Belmont Research Station, a property located 26 km NNW of Rockhampton, in north-eastern Australia. As a research station located in the tropics, acaricide was initially used routinely to treat tick infestations but from 1966 it was used only as an experimental treatment or to prevent mortalities from tick infestations (Frisch 1981). Thus on Belmont, there was a need to select European B. taurus cattle which had anti-parasitic mechanisms to enable them to survive and maintain fitness. Cattle, as with other herbivores, have evolved various anti-tick mechanisms: multiple immunological processors, hypersensitivity and exudate and grooming behaviour (Riek 1962; Roberts 1968a; Schleger et al. 1981; Brossard and Wikel 2004). Roberts (1968b) also showed that rejection of larvae on B. taurus occurred within 24 h of infestation and the effect was greater in animals resistant to tick. As pointed out by Roberts (1968b) the early mortality of larvae has implications for the restricted transmission of such tick-borne diseases as Babesia bigemina. A family within the HS line on Belmont did acquire extreme resistance to infestations of cattle tick (Frisch et al. 2000).
The origins of the Adaptaur began on Belmont in 1980 with the identification of a heifer (animal identity: 790546) of the 1979 calf crop of the HS line and after 1980 selection for both high growth tick resistance within the HS line focused on this family. The heifer acquired extreme resistance to field infestations of cattle tick soon after weaning (subsequent counts of zero or one regardless of tick counts of cohorts – see Fig. 1 for tick counts of cohorts). There was no evidence of hypersensitivity (J. E. Frisch and C. J. O'Neill, unpublished data) and a grooming response to remove larvae within 24 h of infestation (C. J. O'Neill and J. E. Frisch, unpublished data). Figure 1 presents a compilation of tick count data from various studies of the 1980s on adaptation, production or heterosis [see Frisch and Vercoe (1984), Frisch (1987), Frisch et al. (2000) for details of environment, breeding programmes experimental designs and various analyses of data]. These studies were conducted as a precursor to a major heterosis study conducted during the 1990s, involving breeds of European, Indian and African origins, to identify the optimal genotype for herd productivity in a stressful tropical environment (Frisch and O'Neill 1998a,b,c). Figure 1 illustrates that there is a strong tendency in low tick counts of progeny from sires of the 790546 family compared to progeny from sires of the remaining Adaptaur herd and progeny from the Brahman sires. The focus on the maternal lineage of 790546 not only assisted in rapid reduction in the phenotypic trend in tick count (Fig. 1), but also a corresponding linear (R2 = 0.93) genetic trend from 1983 to 1998 of a mean rate of 7 ticks/year of the Adaptaur herd (Frisch et al. 2000). A similar genetic trend was found by Henshall (2004).
Figure 1.
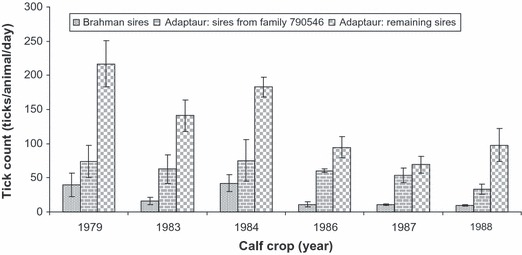
Arithmetic means (three highest counts for the calf crop) (±SEM) of field tick (Rhipicephalus microplus) infestations of heifer progeny of Brahman and Adaptaur sires for calf crops 1979, 1983–1984 and 1986–1988 on Belmont Research Station. Number of sires and progeny per group: Brahman, 24 sires with 159 progeny, Adaptaur maternal lineage 790546, five sires with 55 progeny, and remaining Adaptaur, 20 sires with 109 progeny.
Twenty-seven years after the identification of heifer 790546, in 2007 descendents from this heifer exhibited high tick resistance (Table 1) and with some straightbred animals (e.g. animal identities: 060017 and 060031) resembling the extreme phenotype of 790546 (Table 2). Tick count data from an experiment conducted on Belmont during November and December 2007, involving 2-year-old heifers are presented in Tables 1 and 2. This experiment was aimed at studying tick resistance of different breed types and observing host hypersensitivity to tick larvae. Animals were sourced for this experiment as follows: there were 15 Brahman heifers chosen at random from a calf crop of 140 heifers from Belmont Research Station and the Adaptaur and Adaptaur crossbreds were sourced locally from commercial Seedstock producers Vineree and Ben M'Cree. To restrict the immediate host grooming to remove tick larvae, we fitted a collar to the animal and taped a tube of larvae onto the collar. These cattle were artificially infested with 10 000 two-week-old tick larvae (R. microplus, Queensland Department of Primary Industries and Fisheries, Yeerongpilly, Australia). At 48-h postinfestation, all heifers were assessed for their degree of hypersensitivity. The Adaptaur straightbred has not lost its high resistance to cattle tick, even after 27 years (Table 1), and this high tick resistance appears to be conferred to other taurine breeds regardless of their genetic background for tick resistance (Table 2). The finding that some heifers with high counts at 6 months of age have progressively lower counts at 12 months of age and 24 months of age (Table 2) confirms that resistance is acquired. Although general hypersensitivity was not observed in this experiment, five of the most tick resistant B. taurus heifers had a small area (approx. 5 cm in diameter) of exudate ranging from minor to excessive at the site of larval release (Table 2), indicating a host immune response to the parasite. By day 15, there was no evidence of exudate. The relatively high weight gain on pasture of the Adaptaur straightbred and F1 compared to the Brahman (Table 1) would suggest that neither the imposition of 10 000 tick larvae nor the Adaptaur anti-tick mechanism was detrimental to their productivity. The results presented in Tables 1 and 2 suggest a relative advantage of Adaptaur and Adaptaur cross-over Brahmans in tick resistance at the same level of growth. However, if strong conclusions are to be made, a larger sample size in each of the breed classes would be required and a statistical analysis conducted.
Table 1.
Number of Bos indicus (straightbred Brahman) and Bos taurus (Adaptaur and F1 Adaptaur cross) 24-month-old heifers in each class of tick resistance from an artificial infestation of cattle tick (Rhipicephalus microplus) on Belmont Research Station November 2007*
| Tick resistance class (cumulative tick count)† | Number of heifers in tick class | ||
|---|---|---|---|
| Brahman | Adaptaur | Adaptaur F1 | |
| Very low (>150) | 0 | 0 | 0 |
| Low (81–150) | 0 | 0 | 0 |
| Average (31–80) | 3 | 1 | 0 |
| High (11–30) | 4 | 0 | 1 |
| Very high (1–10) | 8 | 3 | 2 |
| Extreme (0) | 0 | 4 | 3 |
| Daily weight gain (kg/day) during the tick infestation experiment | 1.03 ± 0.048 | 1.08 ± 0.066 | 1.13 ± 0.077 |
To confirm the viability of the tick larvae a straightbred Murray Grey heifer from Ben M'Cree of known high susceptibility (field counts >100 ticks/side at both 6 and 12 months of age at Ben M'Cree, personal communication P and L Quayle) of the same cohort was pastured with the above heifers at Belmont and produced a count of 233 mature ticks by day 21 of the artificial infestation.
Tick resistance class was based on number of maturing ticks from cumulative tick counts (/side) on days 19–21 from an artificial infestation of 10 000 larvae.
Table 2.
Bos taurus (Adaptaur straightbred and F1 Adaptaur cross) heifers with individual tick counts (Rhipicephalus microplus) at 6, 12 and 24 months of age and an assessment of the degree of exudate at the site of larval release from an artificial infestation of cattle tick at 24 months of age on Belmont Research Station, November 2007
| Tick count at age (months) | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Animal identity | Sire breed | Dam breed | 6* | 12* | 24† | Exudate‡ |
| F1 Adaptaur cross | 060001 | Simmental | Adaptaur | >100 | >100 | 24 | Nil |
| 060021 | Simmental | Adaptaur | >100 | 67 | 7 | Nil | |
| 060064 | Tuli | Adaptaur | >100 | 8 | 7 | Nil | |
| 060006 | Simmental | Adaptaur | 25 | 9 | 0 | Nil | |
| 060037 | Adaptaur | Murray Grey | 10 | 23 | 0 | Moderate | |
| 060003 | Senepol | Adaptaur | 0 | 0 | 0 | Excessive | |
| Adaptaur straightbred | 060044 | Adaptaur | Adaptaur | >100 | 17 | 2 | Nil |
| 060042 | Adaptaur | Adaptaur | >100 | 17 | 0 | Nil | |
| 060037 | Adaptaur | Adaptaur | 26 | 3 | 2 | Minor | |
| 060017 | Adaptaur | Adaptaur | 1 | 4 | 0 | Excessive | |
| 060031 | Adaptaur | Adaptaur | 1 | 2 | 0 | Excessive | |
Tick counts (/side) courtesy of P and L Quayle from field infestations at Ben M'Cree, Peachester, Queensland, Australia.
Cumulative tick counts (/side) on days 19–21 from the artificial infestation of 10 000 larvae.
Exudate assessed at the site of larval release 48-h postinfestation of the 10 000 larvae.
Evolutionary genetics of adaptation to environmental stressors in the tropics
Just as the African B. taurus evolved mechanisms for resistance to tropical stress so did the European B. taurus. There are a number of adaptation mechanisms that could explain the relatively high levels of resistance to tropical stressors for the Adaptaur, N'Dama, Senepol and Criollo breeds. The following scenarios highlight the complexity of disease resistance and pertain to evolutionary genetics: chance mutations, a synergy between natural selection and artificial selection and phenotypic plasticity. Moreover, adding to this complexity Colditz (2002) and Viney et al. (2005) contend that the host's optimum immune response to parasites and pathogens is one of immunosufficiency, because the former is a balance between energy loss due to pathogen infection and energy expenditure mounting an immune response that is conducive to fitness. Naessens (2006) has shown that it is the inappropriate response of the immune system that results in the mortality and morbidity of trypanosome-infected cattle. N'Dama not only possesses a genetic capacity to control parasitism (Claxton and Leperre 1991), but also to avoid anaemia and the severe pathology associated with trypanosome infection (Murray and Dexter 1988; Naessens 2006). A major gene for hair length ‘slick hair’ that contributes to improving the animal's thermoregulatory ability, has been described in both Senepol and Venezuelan Carora – a two-breed composite of Brown Swiss and local Criollo (Olson et al. 2003). It could be speculated that the Senepol also has the potential for high tick resistance given its N'Dama origin and slick coat (Claxton and Leperre 1991; Hammond et al. 1996). O'Kelly and Spiers (1983) using animals from the HS line, among other breeds, demonstrated that fewer ticks matured on clipped areas of coat compared to unclipped areas.
Relative to their temperate origins, the environmental assault on the HS line on Belmont was extreme, from solar radiation, heat stress, parasites and periods of poor nutrition, thus creating strong natural selection. A livestock population surviving with minimal intervention to remove environmental stress, such as not treating the Adaptaur with acaricide to remove parasites, means that the population was under natural selection pressure. Using both calf survival data and a multi-trait mixed model genetic analysis of growth rates from 1966 to 1981, Hetzel et al. (1990) concluded that the fitness of this population was increasing under natural selection. Moreover, with the artificial selection protocol in place, selecting sires for high growth rate in a stressful environment (Frisch 1981), was working in synergy with natural selection (Hetzel et al. 1990). Frisch (1981) also concluded that the increasing level of resistance to environmental stress was solely responsible for the improved growth rates for this population. Since 1983, the additional selection protocol of high tick resistance focusing on a single family with selection for high growth rate, confirmed the value of targeting components that positively impact on productivity in a stressful tropical environment. The 33% improvement in calving rates of Adaptaur cows from 1992 to 1996, compared to Adaptaurs before selection 1964–1972 (O'Neill and Frisch 1998), is further evidence that high selection intensity (high growth and high tick resistance) on fitness traits was successful for European B. taurus in a tropical environment. In a large Australian purebred-crossbreeding study from the Cooperative Research Centre for Cattle and Beef Quality, Newman et al. (2002) found that progeny from Belmont Red sires produced carcasses with the lowest sub-cutaneous fat and highest retail beef yield, desirable traits for human consumption. The above-mentioned research work with adapted genotypes has highlighted the importance of the synergy between natural and artificial selection for both adaptation and production traits.
The transition from unadapted to an adapted state for the N'Dama, Adaptaur, Senepol and Criollo could also involve elements of phenotypic plasticity, defined as the ability of a genotype to produce more than one phenotype in response to differing environments (Scheiner 1993; de Jong 2005; Fordyce 2006). Garland and Kelly (2006) point out that directional selection should support alleles that increase phenotypic plasticity in the direction of selection. When animal breeders are faced with multiple environments, models of phenotypic plasticity could contribute to the development of appropriate selection programmes for their production system (de Jong and Bijma 2002). It is conceivable that the Mexican Criollo from the Chihuahuan Desert3 possess alleles pertinent to plasticity for variations in water consumption, extreme fluctuations in ambient temperature, metabolism of a variety of herbaceous plants, and alternating between browsing and grazing. Existence of such alleles has implications for other semi-arid regions such as the livestock component in the management of dryland salinity of southern Australia as described by Masters et al. (2006). Given the extreme range of environments involved, plus the simultaneous natural and artificial selection pressure applied to achieve fitness, phenotypic plasticity could have contributed to the adaptation of the B. taurus to extreme and highly variable environmental parameters, and therefore future breeding programmes in variable challenging environments.
Extreme environmental conditions that result in high selection intensity on traits directly related to fitness have been shown to impact directly on the evolutionary process. Such high selection intensity tends to rapidly fix pleiotropic genes with predominantly positive effects (Lande 1982). Hoffmann and Parsons (1991) also point out that short periods (years, decades) of high selection intensity, rather than long periods (century or more) of low selection intensity, favour genes of major effect in the evolutionary process – hence the emergence of a major gene for ‘slick hair’ that contributes to heat resistance in the Senepol and Carora breeds (Olson et al. 2003). The identification and evidence of a putative major gene for tick resistance in the Adaptaur (Frisch 1994; Kerr et al. 1994) was not confirmed by Henshall (2004) with additional data and inclusion of polygenic effects in the statistical model. However, using complex segregation analysis, Kadarmideen et al. (2009) have shown evidence of major gene effects in this breed. The first large-scale gene expression profiling study (skin cDNA microarrays) was undertaken using resistant/susceptible Adaptaur cattle and identified several candidate mechanisms (e.g. antibody immune response day 1 of the infestation) that were involved in Adaptaur tick resistance (Wang et al. 2007). Nevertheless, because complex response mechanisms to multiple and variable stressors are involved, the contribution of quantitative genetics and molecular biology for the improvement of B. taurus to a stressful tropical and/or semi-arid environment remains to be fully assessed.
The completion of the bovine Genome Assembly (The Bovine Genome Sequencing and Analysis Consortium 2009) provides an opportunity to understand how cattle evolved and adapted to different geographical regions and production systems. The biological systems most affected by changes in the number and organization of genes in the cattle lineage include reproduction, immunity, lactation and digestion, all of which are important to our discussion on tropical adaptation. They concluded that these changes in the cattle lineage probably reflect metabolic, physiologic and immune adaptations due to microbial fermentation in the rumen, the environment and its influence on disease transmission, and the reproductive strategy of cattle. Indeed, understanding the taurine evolutionary trajectories also provides an insight into the predictability of cattle performance in cattle industries of the 21st century.
Genotype by environment by management interaction in cattle
Awareness of the taurine evolutionary trajectories in favourable environments will provide a guide to future breeding experiments. Breeding goals are enhanced by not only knowledge about the diseases associated with production and the stress they cause but also mechanisms of resistance to diseases. Global warming, with elevated ambient temperatures, altered rainfall patterns and changed distributions of parasites and pathogens (Sutherst 2001; Howden et al. 2008; Thornton et al. 2009), will also impact livestock evolutionary trajectories (Burdon and Thrall 2008) and provide new challenges for livestock producers.
In the previous sections, we reviewed and discussed the evolutionary history and genetics of B. taurus cattle and the selection pressures imposed by different environments and/or production systems using tick resistance as a case study. In a systems framework, it is the interactions between genotype, environment and management that contribute to evolution of different genotypes. Compared to the tropically adapted B. taurus, the evolutionary history of temperate (productivism) B. taurus possess inheritantly higher levels of production potential, the production achieved in the absence of all environmental stress, and attributed to traits such as higher metabolic rates and appetites of the temperate taurine breeds. Conversely, the evolution of B. taurus in environmentally stressful regions has ensured that those genotypes possess mechanisms of adaptation (e.g. resistance to cattle tick) unlike their temperate counterpart (see Hoffmann and Parsons 1991). Hence, the metabolic rates of breeds such as Charolais and Angus would be higher than the tropically adapted breeds N'Dama and Criollo. However, in terms of adaptation to environmental stress the reverse is true and the N'Dama and Criollo possess relatively higher levels of environmental adaptation than breeds synonymous with productivism. Historically in commercial livestock production systems environmental stress is alleviated with strategies such as parasite control and supplementary feeding. Figure 2 shows the G × E × M interactions in terms of relative production potential of temperate and tropical B. taurus (G) in the presence and absence of management (M) to negate the effect of increasing levels of environmental stress (E). The relative levels of production potential are shown on the vertical axis whilst the increasing levels of environmental stress are shown on the horizontal axis. Thus, as the level of environmental stress increases, the decline in production of the tropically adapted B. taurus is substantially less than the decline of the temperate B. taurus but if there is intervention to remove environmental stress then the response on production by the temperate taurine breeds would be greater than the response by the tropically adapted taurine breeds (Fig. 2). This implies that the costs associated with maintaining high productivity in temperate B. taurus might negate the profit obtained via high production. Hence, we emphasize that it is not high production, but rather ‘production efficiency’ that should be highest priority.
Figure 2.
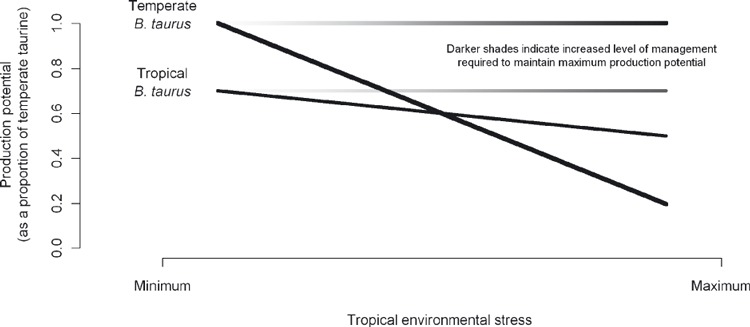
Diagrammatic representation of the G × E × M for Bos taurus adapted to a temperate environment (e.g. Angus) and B. taurus adapted to a tropical environment (e.g. N'Dama) showing the proportional decline from optimal production potential for growth when there is no intervention by management to alleviate increasing levels of tropical environmental stress. The two shaded horizontal lines indicate the level of management required to maintain production potential.
We have specifically avoided discussing G × E × M interactions that exist within breed between animals at the level of genes which respond to different environments and/or different management interventions. These are extensively discussed in Kadarmideen et al. (2006a,b) and are outside the scope of this study. In practice, the major hurdle to achieving economic sustainability will be the acknowledgement that no single genotype will outperform all other genotypes regardless of environmental conditions. Moreover, interactions between genotype and management (e.g. use of acaricide to control cattle tick) occur when performances of different genotypes are not equally affected by different management inputs. Let us consider the following examples. If N'Dama and Angus cattle are given the same dosage of acaricide in a region of tick infestation, Angus cattle responds better to treatment than N'Dama cattle (response as in growth and fertility). If, however, N'Dama and Angus cattle are not given any treatment of acaricide in the same region, then N'Dama cattle are likely to achieve higher production performance than Angus cattle (Fig. 2). When different genotypes perform differently in different management strategies, then there is G × M. Hence, a livestock system in essence is defined by the G × E × M interactions and understanding G × E × M and its impact on genetic, environmental and managerial strategies is crucial in future livestock systems.
Conclusion
Livestock production in the 21st century will be characterized by systems where both market (food and fibre output) and nonmarket (social, environmental and climate change) issues are given consideration. Thus, livestock producers will maintain their drive for maximization of output but with diminished reliance on industrialization and therapeutic agents to alleviate environmental stress and rely more on the genetic make-up of their animals to combat stress from heat, parasites, pathogens and poor nutrition. In other words, ‘production efficiency’ will become increasingly important. Various evolutionary trajectories of B. taurus since domestication suggest that the environmentally adapted breeds, along with B. indicus breeds, will play a pivotal role in the transition to sustainable production systems. The review of work on such breeds as the N'Dama, Senepol, Criollo and Adaptaur implies that both natural and artificial selection should be incorporated into selection decisions – assess potential breeding stock after a period of environmental stress. Once there is an understanding of the beef and dairy production diseases and the mechanisms of resistance to environmental stress then, as with the plant industries, molecular biology technology may be successfully applied to cattle. Corresponding breeding objectives for both production and adaptation traits could also be established via a combination of well-established quantitative genetic methodologies with emerging molecular biology technologies such as functional genomics.
We also conclude that the four adapted taurine breeds noted above, along with breeds that have undergone similar evolutionary trajectories, would possess candidate genes that could contribute to improving the productivity of cattle in challenging environments. However, the physiological antagonism between production potential and adaptation ensures no genotype achieves highest performance in all environments. The evolutionary history and knowledge of the genetic architecture will provide estimates of production potential (in a favourable environment) and level of resistance to environmental stress (in an unfavourable environment) and therefore estimates of how much production is realized when environmental stressors are controlled. Hence, the performance of a breed or combination of breeds in a given environment or degree of intervention by management to alleviate environmental stressors, are able to be predicted. Utilizing the full compliment of global cattle genetics and knowledge of G × E × M interactions will help ensure sustainable levels of beef and milk production in the 21st century.
Acknowledgments
This publication is from ‘G × E × M on Sustainable Grazing Management’ project led by Haja Kadarmideen and funded by CSIRO Sustainable Agricultural Flagship. Donation of the Adaptaur and Adaptaur cross heifers from Vineree and Ben M'Cree for the tick infestation study was much appreciated. Authors thank John Henshall and Dean Revell for their constructive comments on the earlier version of the manuscript.
Footnotes
African indicine and taurine crossbred cattle are generally referred to as Sanga cattle.
Creole or Criollo – Spanish words referring to descendents of Iberian livestock transported to Central and South America during colonization of these countries by the Spanish and Portuguese.
A semi-arid region of the USA–Mexico border with an annual rainfall of 235 mL.
Literature cited
- Ali AA, Kadarmideen HN, Thompson PC, O'Neill CJ. Genetic and phenotypic characteristics of infectious keratoconjunctivitis in tropical cattle. 2010. Proceedings of the 9th World Congress on Genetics Applied to Livestock Production PP4–156.
- Anderung C, Hellborg L, Seddon J, Hanotte O, Götherström A. Investigation of X- and Y-specific single nucleotide polymorphisms in taurine (Bos taurus) and indicine (Bos indicus) cattle. Animal Genetics. 2007;38:595–600. doi: 10.1111/j.1365-2052.2007.01663.x. [DOI] [PubMed] [Google Scholar]
- Bell G, Collins S. Adaptation, extinction and global change. Evolutionary Applications. 2008;1:3–16. doi: 10.1111/j.1752-4571.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SC, Woolliams JA. Genetic approaches and technologies for improving the sustainability of livestock production. Journal of the Science of Food and Agriculture. 2004;84:911–919. [Google Scholar]
- Bradley DG, Loftus RT, Cunningham P, MacHugh DE. Genetics and domestic cattle origins. Evolutionary Anthropology. 1998;6:79–86. [Google Scholar]
- Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129:S161–S176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- Brown MH, Brightman AH, Fenwick BW, Rider MA. Infectious bovine keratoconjunctivitis: a review. Journal Veterinary International Medicine. 1998;12:259–266. doi: 10.1111/j.1939-1676.1998.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Bruford MW, Bradley DG, Luikart G. DNA markers reveal the complexity of livestock domestication. Nature Reviews Genetics. 2003;4:900–910. doi: 10.1038/nrg1203. [DOI] [PubMed] [Google Scholar]
- Burdon JJ, Thrall PH. Pathogen evolution across the agro-ecological interface: implications for disease management. Evolutionary Applications. 2008;1:57–65. doi: 10.1111/j.1752-4571.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WR, Smith RD. Interrelationships between energy balance and postpartum reproductive function in dairy cattle. Journal of Dairy Science. 1989;72:767–783. doi: 10.3168/jds.S0022-0302(89)79169-4. [DOI] [PubMed] [Google Scholar]
- Campbell LG, Snow AA, Sweeney PM, Ketner JM. Rapid evolution in crop-weed hybrids under artificial selection for divergent life histories. Evolutionary Applications. 2009;2:172–186. doi: 10.1111/j.1752-4571.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriquiry M, Weber WJ, Dahlen CR, Lamb GC, Baumgard LH, Crooker BA. Production response of multiparous Holstein cows treated with bovine somatotropin and fed diets enriched with n-3 or n-6 fatty acids. Journal of Dairy Science. 2009;92:4852–4864. doi: 10.3168/jds.2008-1673. [DOI] [PubMed] [Google Scholar]
- Casas E, Stone RT. Putative quantitative trait loci associated with the probability of contracting infectious bovine keratoconjunctivitis. Journal of Animal Science. 2006;84:3180–3184. doi: 10.2527/jas.2006-200. [DOI] [PubMed] [Google Scholar]
- De Castro JJ. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Veterinary Parasitology. 1997;71:77–97. doi: 10.1016/s0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- Chen S, Lin B, Baig M, Mitra B, Lopes RJ, Santos AM, Magee DA, et al. Zebu cattle are an exclusive legacy of the South Asia Neolithic. Molecular Biology and Evolution. 2010;27:1–6. doi: 10.1093/molbev/msp213. [DOI] [PubMed] [Google Scholar]
- Claxton J, Leperre P. Parasite burdens and host susceptibility of zebu and N'Dama cattle in village herds in Gambia. Veterinary Parasitology. 1991;40:293–304. doi: 10.1016/0304-4017(91)90109-9. [DOI] [PubMed] [Google Scholar]
- Colditz IG. Effects of the immune system on metabolism: implications for production and disease resistance in livestock. Livestock Production Science. 2002;75:257–268. [Google Scholar]
- Distl O. Lameness. In: Axford RFE, Bishop SC, Nicholas FW, Owen JB, editors. Breeding for Disease Resistance in Farm Animals. Oxon: CABI Publishing; 2000. pp. 397–411. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4th edn. Essex: Pearson Education Limited; 1996. [Google Scholar]
- Fernández J, Toro MA, Caballero A. Management of subdivided populations in conservation programs: development of a novel dynamic system. Genetics. 2008;179:683–692. doi: 10.1534/genetics.107.083816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. The Journal of Experimental Biology. 2006;209:2377–2383. doi: 10.1242/jeb.02271. [DOI] [PubMed] [Google Scholar]
- Frisch JE. Changes occurring in cattle as a consequence of selection for growth rate in a stressful environment. Journal Agricultural Science (Cambridge) 1981;96:23–38. [Google Scholar]
- Frisch JE. Physiological reasons for heterosis in growth of Bos indicus × Bos taurus. Journal Agricultural Science (Cambridge) 1987;109:213–230. [Google Scholar]
- Frisch JE. Identification of a major gene for resistance to cattle ticks. Proceedings of the 5th World Congress on Genetics Applied to Livestock Production. 1994;20:293–295. [Google Scholar]
- Frisch JE, O'Neill CJ. Comparative evaluation of beef cattle breeds of African, European and Indian origins. 1. Liveweights and heterosis at birth, weaning and 18 months. Animal Science. 1998a;67:27–38. [Google Scholar]
- Frisch JE, O'Neill CJ. Comparative evaluation of beef cattle breeds of African, European and Indian origins. 2. Resistance to cattle ticks and gastrointestinal nematodes. Animal Science. 1998b;67:39–48. [Google Scholar]
- Frisch JE, O'Neill CJ. Preliminary estimates of comparative productivity of straightbred and crossbred cows of African, European and Indian origins. Proceedings of the 6th World Congress on Genetics Applied to Livestock Production. 1998c;25:231–234. [Google Scholar]
- Frisch JE, Vercoe JE. An analysis of growth of different cattle genotypes reared in different environments. Journal Agricultural Science (Cambridge) 1984;103:137–153. [Google Scholar]
- Frisch JE, Drinkwater R, Harrison B, Johnson S. Classification of the southern African sanga and East African shorthorned zebu. Animal Genetics. 1997;28:77–83. doi: 10.1111/j.1365-2052.1997.00088.x. [DOI] [PubMed] [Google Scholar]
- Frisch JE, O'Neill CJ, Kelly MJ. Using genetics to control cattle parasites – the Rockhampton experience. International Journal for Parasitology. 2000;30:253–264. doi: 10.1016/s0020-7519(00)00010-2. [DOI] [PubMed] [Google Scholar]
- Galyean ML, Rivera JD. Nutritionally related disorders affecting feedlot cattle. Canadian Journal of Animal Science. 2003;83:13–20. [Google Scholar]
- Garcia MD, Thallman RM, Wheeler TL, Shackelford SD, Casas E. Effect of bovine respiratory disease and overall pathogenic disease incidence on carcass traits. Journal of Animal Science. 2010;88:491–496. doi: 10.2527/jas.2009-1874. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA. Phenotypic plasticity and experimental evolution. The Journal of Experimental Biology. 2006;209:2344–2361. doi: 10.1242/jeb.02244. [DOI] [PubMed] [Google Scholar]
- Hammond AC, Olson TA, Chase CC, Jr, Bowers EJ, Randel RD, Murphy CN, Vogt DW, et al. Heat tolerance in two tropically adapted Bos taurus breeds, Senepol and Romosinuano, compared with Brahman, Angus, and Hereford cattle in Florida. Journal of Animal Science. 1996;74:295–303. doi: 10.2527/1996.742295x. [DOI] [PubMed] [Google Scholar]
- Hanotte O, Bradley DG, Ochieng JW, Verjee Y, Hill EW, Rege JEO. African pastoralism: genetic imprints of origins and migrations. Science. 2002;296:336–339. doi: 10.1126/science.1069878. [DOI] [PubMed] [Google Scholar]
- Harris M. The cultural ecology of India's sacred cattle. Current Anthropology. 1992;33:261–276. [Google Scholar]
- Hart BL. Behavioural adaptations to pathogens and parasites: five strategies. Neuroscience and Biobehavioral Reviews. 1990;14:273–294. doi: 10.1016/s0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- Hazel LN, Lush JL. The efficiency of three methods of selection. Journal of Heredity. 1943;33:393–399. [Google Scholar]
- Henderson CR. Best linear unbiased estimation and prediction under a selection model. Biometrics. 1975;31:423–427. [PubMed] [Google Scholar]
- Henshall JM. A genetic analysis of parasite resistance traits in a tropically adapted line of Bos taurus. Australian Journal of Agricultural Research. 2004;55:1109–1116. [Google Scholar]
- Heringstad B, Klemetsdal G, Ruane J. Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries. Livestock Production Science. 2000;64:95–106. [Google Scholar]
- Hetzel DJS, Quaas RL, Seifert GW, Bean KG, Aspden WJ, Mackinnon MJ. Evidence of natural selection in a herd of Hereford-Shorthorn cattle in the tropics. Proceedings of the Australian Association of Animal Breeding and Genetics. 1990;8:451–454. [Google Scholar]
- Hoffmann AA, Parsons PA. Evolutionary Genetics and Environmental Stress. Oxford: Oxford University Press; 1991. [Google Scholar]
- Howden SM, Crimp SJ, Stokes CJ. Climate change and Australian livestock systems: impacts, research and policy issues. Australian Journal of Experimental Agriculture. 2008;48:780–788. [Google Scholar]
- Ilbery B, Bowler I. From agricultural productivism to post-productivism. In: Ilbery B, editor. The Geography of Rural Change. Harlow: Addison Wesley Longman; 1998. pp. 57–84. [Google Scholar]
- Ingvartsen KL, Dewhurst RJ, Friggens NC. On the relationship between lactational performance and health: is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livestock Production Science. 2003;83:277–308. [Google Scholar]
- De Jong G. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytologist. 2005;166:101–118. doi: 10.1111/j.1469-8137.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- De Jong G, Bijma P. Selection and phenotypic plasticity in evolutionary biology and animal breeding. Livestock Production Science. 2002;78:195–214. [Google Scholar]
- Kadarmideen HN, Pryce JE. Genetic and economic relationships between somatic cell count and clinical mastitis and their use in selection for mastitis resistance in dairy cattle. Animal Science. 2001;73:19–28. [Google Scholar]
- Kadarmideen HN, Thompson R, Simm G. Linear and threshold model genetic parameters for disease, fertility and milk production in dairy cattle. Animal Science. 2000;71:411–419. [Google Scholar]
- Kadarmideen HN, Thompson R, Coffey MP, Kossaibati MA. Genetic parameters and evaluations from single- and multiple-trait analysis of dairy cow fertility and milk production. Livestock Production Science. 2003;81:183–195. [Google Scholar]
- Kadarmideen HN, Von Rohr P, Janss LLG. From genetical genomics to systems genetics: potential applications in quantitative genomics and animal breeding. Mammalian Genome. 2006a;17:548–564. doi: 10.1007/s00335-005-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadarmideen HN, Li Y, Janss LLG. Gene-environment interactions in complex diseases: genetic models and methods for qtl mapping. Genetical Research. 2006b;88:119–131. doi: 10.1017/S0016672306008391. [DOI] [PubMed] [Google Scholar]
- Kadarmideen HN, De Klerk B, Prayaga KC. Evidence of a major gene for tick- and worm resistance in tropical beef cattle via complex segregation analyses. 2009. Proceedings of the 60th European Association of Animal Production (EAAP) annual meeting, August 2009, Barcelona, Spain.
- Kearney JF, Wall E, Villanueva B, Coffey MP. Inbreeding trends and application of optimized selection in the UK Holstein population. Journal of Dairy Science. 2004;87:3503–3509. doi: 10.3168/jds.S0022-0302(04)73485-2. [DOI] [PubMed] [Google Scholar]
- Kennedy JF, Turner HG. A Project on Genetics of Adaptation in Cattle. Melbourne: CSIRO Division of Animal Health and Production; 1959. Division Report No. 8 (Series S.W.-3) [Google Scholar]
- Kerr RJ, Frisch JE, Kinghorn BP. Evidence for a major gene for tick resistance in cattle. Proceedings of the 5th World Congress on Genetics Applied to Livestock Production. 1994;20:265–268. [Google Scholar]
- Lande R. A quantitative genetic theory of life history evolution. Ecology. 1982;63:607–615. [Google Scholar]
- Lehmann T. Ectoparasites: direct impact on host fitness. Parasitology Today. 1993;9:8–13. doi: 10.1016/0169-4758(93)90153-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Kadarmideen HN, Dekkers JCM. Selection on multiple QTL with controlled gene diversity and inbreeding for long-term benefit. Journal of Animal Breeding Genetics. 2008;125:320–329. doi: 10.1111/j.1439-0388.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- Lomborg SR, Nielsen LR, Heegaard PMH, Jocobsen S. Acute phase proteins in cattle after exposure to complex stress. Veterinary Research Communication. 2008;32:575–582. doi: 10.1007/s11259-008-9057-7. [DOI] [PubMed] [Google Scholar]
- Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? Journal of Dairy Science. 2001;84:1277–1293. doi: 10.3168/jds.S0022-0302(01)70158-0. [DOI] [PubMed] [Google Scholar]
- Maas JA, Garnsworthy PC, Flint APF. Modelling responses to nutritional, endocrine and genetic strategies to increase fertility in the UK dairy herd. The Veterinary Journal. 2009;180:356–362. doi: 10.1016/j.tvjl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Marcillac-Embertson NM, Robinson PH, Fadel JG, Mitloehner FM. Effects of shade and sprinklers on performance, behaviour, physiology, and the environment of heifers. Journal of Dairy Science. 2009;92:506–517. doi: 10.3168/jds.2008-1012. [DOI] [PubMed] [Google Scholar]
- Mariante AS, Albuquerque MSM, Egito AA, McManus C, Lopes MA, Paiva SR. Present status of the conservation of livestock genetic resources in Brazil. Livestock Science. 2009;120:204–212. [Google Scholar]
- Masters D, Edwards N, Sillence M, Avery A, Revell D, Friend M, Sanford P, et al. The role of livestock in the management of dryland salinity. Australian Journal of Experimental Agriculture. 2006;46:733–741. [Google Scholar]
- McConnel CS, Shum L, House JK. Infectious bovine keratoconjunctivitis antimicrobial therapy. Australian Veterinary Journal. 2007;85:65–69. doi: 10.1111/j.1751-0813.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- McDowell RE, Wilk JC, Talbott CW. Economic viability of crosses of Bos taurus and Bos indicus for dairying in warm climates. Journal of Dairy Science. 1996;79:1292–1303. doi: 10.3168/jds.S0022-0302(96)76484-6. [DOI] [PubMed] [Google Scholar]
- Mulligan FJ, Doherty ML. Production diseases of the transition cow. The Veterinary Journal. 2008;176:3–9. doi: 10.1016/j.tvjl.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Murray M, Dexter TM. Anaemia in bovine African trypanosomiasis. Acta Tropica. 1988;45:389–432. [PubMed] [Google Scholar]
- Naessens J. Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? International Journal for Parasitology. 2006;36:521–528. doi: 10.1016/j.ijpara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Neuenschwander T, Kadarmideen HN, Wegmann S, De Haas Y. Genetics of parity-dependent production increase and its relationship with health, fertility, longevity, and conformation in Swiss Holsteins. Journal of Dairy Science. 2005;88:1540–1551. doi: 10.3168/jds.S0022-0302(05)72823-X. [DOI] [PubMed] [Google Scholar]
- Newman S, Reverter A, Johnson DJ. Purebred-crossbred performance and genetic evaluation of postweaning growth and carcass traits in Bos indicus × Bos taurus crosses in Australia. Journal of Animal Science. 2002;80:1801–1808. doi: 10.2527/2002.8071801x. [DOI] [PubMed] [Google Scholar]
- O'Kelly JC, Spiers WG. Observations on body temperature of the host and resistance to the tick Boophilus microplus (Acari:Ixodidae) Journal of Medical Entomology. 1983;20:498–505. doi: 10.1093/jmedent/20.5.498. [DOI] [PubMed] [Google Scholar]
- Olson TA, Lucena C, Chase CC, Jr, Hammond AC. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. Journal of Animal Science. 2003;81:80–90. doi: 10.2527/2003.81180x. [DOI] [PubMed] [Google Scholar]
- O'Neill CJ, Frisch JE. Consequences of selection in two Bos taurus breeds in the tropics. Proceedings of the 6th World Congress on Genetics Applied to Livestock Production. 1998;25:223–226. [Google Scholar]
- O'Neill CJ, Weldon GJ, Hill RA, Thomas JE. Adaptaur Association of Australia; a breed association based on the performance recording of Adaptaur cattle. Animal Production in Australia. 1998;22:229–232. [Google Scholar]
- Outteridge PM. High and low responsiveness to vaccines in farm animals. Immunology and Cell Biology. 1993;71:355–366. doi: 10.1038/icb.1993.42. [DOI] [PubMed] [Google Scholar]
- Owens FN, Secrist DS, Hill WJ, Gill DR. Acidosis in cattle: a review. Journal of Animal Science. 1998;76:275–286. doi: 10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- Parsons PA. The metabolic cost of multiple environmental stresses: implications for climate change and conservation. Trends in Ecology and Evolution. 1990;5:315–317. doi: 10.1016/0169-5347(90)90089-V. [DOI] [PubMed] [Google Scholar]
- Quigley JD, III, Drewry JJ, Murray LM, Ivey SJ. Body weight gain, feed efficiency, and fecal scores of dairy calves in response to galactosyl-lactose or antibiotics in milk replacers. Journal of Dairy Science. 1997;80:1751–1754. doi: 10.3168/jds.S0022-0302(97)76108-3. [DOI] [PubMed] [Google Scholar]
- Rauw WM, Kanis E, Noordhuizen-Stassen EN, Grommers FJ. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livestock Production Science. 1998;56:15–33. [Google Scholar]
- Riek RF. Studies on the reactions of animals to infestation with ticks VI. Resistance of cattle to infestation with tick Boophilus microplus (Canestrini) Australian Journal of Agricultural Research. 1962;13:532–549. [Google Scholar]
- Roberts JA. Acquisition by the host of resistance to the cattle tick, Boophilus microplus (Canestrini) The Journal of Parasitology. 1968a;54:657–662. [PubMed] [Google Scholar]
- Roberts JA. Resistance of cattle to the tick Boophilus microplus (Canestrini) II. Stages of the life cycle of the parasite against which resistance is manifest. The Journal of Parasitology. 1968b;54:667–673. [PubMed] [Google Scholar]
- Russell ND, Rios J, Erosa G, Remmenga MD, Hawkins DE. Genetic differentiation among geographically isolated populations of Criollo cattle and their divergence from other Bos taurus breeds. Journal of Animal Science. 2000;78:2314–2322. doi: 10.2527/2000.7892314x. [DOI] [PubMed] [Google Scholar]
- Sangster NC. Managing parasiticide resistance. Veterinary Parasitology. 2001;98:89–109. doi: 10.1016/s0304-4017(01)00425-3. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Reviews Ecological Systems. 1993;24:35–68. [Google Scholar]
- Schleger AV, Lincoln DT, Bourne AS. Arteriovenous anastomoses in the dermal vasculature of the skin of Bos taurus cattle, and their relationship with resistance to the tick, Boophilus microplus. Australian Journal of Biological Sciences. 1981;34:27–35. [PubMed] [Google Scholar]
- Simm G, Oldham JD, Coffey MP, Pryce JE, Kadarmideen HN. Future dairy cattle breeding strategies. Cattle Practice. 2000;8:391–400. [Google Scholar]
- Smith LA, Cassell BG, Pearson RE. The effects of inbreeding on the lifetime performance of dairy cattle. Journal of Animal Science. 1998;81:2729–2737. doi: 10.3168/jds.S0022-0302(98)75830-8. [DOI] [PubMed] [Google Scholar]
- Snowder GD, Van Vleck LD, Cundiff LV, Bennett GL. Genetic and environmental factors associated with incidence of infectious bovine keratoconjunctivitis in preweaned beef calves. Journal of Animal Science. 2005;83:507–518. doi: 10.2527/2005.833507x. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Kocan KM, De La Fuente J. Tick control: further thoughts on a research agenda. Trends in Parasitology. 2006;22:550–551. doi: 10.1016/j.pt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323:746–751. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherst RW. The vulnerability of animal and human health to parasites under global change. International Journal for Parasitology. 2001;31:933–948. doi: 10.1016/s0020-7519(01)00203-x. [DOI] [PubMed] [Google Scholar]
- The Bovine Genome Sequencing and Analysis Consortium. Elsik CG, Tellam RL, Worley KC. The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science. 2009;324:522–528. doi: 10.1126/science.1169588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Bovine HapMap Consortium. Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science. 2009;324:528–532. doi: 10.1126/science.1167936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JN. Rapid evolution as an ecological process. Trends in Ecology and Evolution. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- Thornton PK, Van De Steeg J, Notenbaert A, Herrero M. The impacts of climate change on livestock and livestock systems in developing countries: a review of what we know and what we need to know. Agricultural Systems. 2009;101:113–127. [Google Scholar]
- Villanueva B, Avenano S, Woolliams JA. Prediction of genetic gain from quadratic optimisation with constrained rates of inbreeding. Genetics Selection Evolution. 2006;38:127–146. doi: 10.1186/1297-9686-38-2-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney ME, Riley EM, Buchanan KL. Optimal immune responses: immuno-competence revisited. Trends in Ecology and Evolution. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wang YH, Reverter A, Kemp D, McWilliam SM, Ingham A, Davis CK, Moore RJ, et al. Gene expression profiling of Hereford Shorthorn cattle following challenge with Boophilus microplus tick larvae. Australian Journal of Experimental Agriculture. 2007;47:1397–1407. [Google Scholar]
- Ward WR. Why is lameness in dairy cows so intractable? The Veterinary Journal. 2009;180:139–140. doi: 10.1016/j.tvjl.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, Patton J, et al. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiological Genomics. 2009;39:1–13. doi: 10.1152/physiolgenomics.00064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N, Sutherst RW, Hall N, Whish-Wilson P. The vulnerability of the Australian Beef Industry to impacts of the cattle tick (Boophilus microplus) under climate change. Climate Change. 2003;61:157–190. [Google Scholar]
- Zuerner RL, Heidari M, Elliott MK, Alt DP, Neill JD. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Veterinary Microbiology. 2007;125:256–264. doi: 10.1016/j.vetmic.2007.06.001. [DOI] [PubMed] [Google Scholar]


