Abstract
All plants, including crop species, harbor a community of fungal endophyte species, yet we know little about the biotic factors that are important in endophyte community assembly. We suggest that the most direct route to understanding the mechanisms underlying community assembly is through the study of functional trait variation in the host and its fungal consortium. We review studies on crop endophytes that investigate plant and fungal traits likely to be important in endophyte community processes. We focus on approaches that could speed detection of general trends in endophyte community assembly: (i) use of the ‘assembly rules’ concept to identify specific mechanisms that influence endophyte community dynamics, (ii) measurement of functional trait variation in plants and fungi to better understand endophyte community processes and plant–fungal interactions, and (iii) investigation of microbe–microbe interactions, and fungal traits that mediate them. This approach is well suited for research in agricultural systems, where pair-wise host–fungus interactions and mechanisms of fungal–fungal competition have frequently been described. Areas for consideration include the possibility that human manipulation of crop phenotype and deployment of fungal biocontrol species can significantly influence endophyte community assembly. Evaluation of endophyte assembly rules may help to fine-tune crop management strategies.
Keywords: assembly rules, community phylogenetics, functional traits, fungal endophytes, Fusarium, maize
Introduction
We now know that plants host a diversity of microbes. This is a paradigm shift away from conceptualizing plants as organisms beset by herbivores and pathogens – or engaged only in two-way mutualisms. We are now beginning to consider how the in planta microbial community figures into pair-wise models of host and pathogen. All wild and agricultural plant species surveyed to date harbor diverse communities of fungi, often at high density in tissue (e.g., Stone et al. 2000; Arnold 2007). Most members of these communities are endophytes, defined in this review as fungi documented to reside in living plant tissue that is apparently asymptomatic (Stone et al. 2000; Schulz and Boyle 2005). Designation as an endophyte therefore depends on an asymptomatic host–fungal interaction at the time of study. It does not, however, predict the endophyte's potential for shifts within the plant to biotrophic or necrotrophic pathogenesis, to mutualism or to commensalism. It follows that the influence of some endophyte species on host health is not static and can range from beneficial to detrimental. Some species provide benefits to the host including protection against pathogens and herbivores, while others impose costs, for example, by decreasing photosynthetic efficiency under drought conditions (Pinto et al. 2000; Clay and Schardl 2002; Arnold et al. 2003).
Although by definition an endophyte does not cause visible symptoms, its potential trophic and phenotypic range in the host plant is not stuck in neutral gear, but can change over both short and long time scales. Expression of symbiotic phenotypes may be plastic; for example, latent pathogens can reside in plant tissue as commensals until conditions are amenable to disease development (e.g., Dodd 1980). Alternatively, we could hypothesize that endophyte lineages may have evolved over time as commensals, for example, as a result of the loss of traits that confer pathogenicity. Endophytes may indirectly affect host health by altering the potential of other species in the community to act as pathogens or mutualists (e.g., Fravel et al. 2003). A plant individual can harbor anywhere from several to hundreds of fungal species, and with little known about the potential beneficial or detrimental effects of each species on the host, we can only speculate on the potential for single or synergistic interactions (Stone et al. 2000; Schulz and Boyle 2005; Arnold 2007).
One system in which host–endophyte relationships have been well studied is the interaction between fungal species in the family Clavicipitaceae and their grass hosts (for reviews see Clay and Schardl 2002; Belesky and Bacon 2009). Many species provide fitness benefits to the host, often increasing tolerance to environmental stressors, although the direction of this relationship can change with environmental conditions and plant–fungus genotype combinations (e.g., Meijer and Leuchtmann 2000; Clay and Schardl 2002). The primary mode of transmission for the Clavicipitaceous endophytes is vertical, in seed, and colonization is often extensive in host tissue. This interaction does not represent the majority of fungal endophyte–host plant interactions. Most plant species harbor a phylogenetically diverse assemblage of endophyte species, also often at high density in tissue, but are primarily transmitted horizontally, from plant to plant, rather than vertically (Schulz and Boyle 2005; Arnold 2007). For the purposes of our review, the focus is on nonmycorrhizal endophyte species outside of the Clavicipitaceace.
One area of endophyte biology that has advanced rapidly is description of endophyte communities associated with different host species (for wild host species see Carroll 1995; Stone et al. 2000; Wilberforce et al. 2003; Arnold 2007; for agricultural host species see Carter et al. 1999; Seghers et al. 2004; Manici and Caputo 2009; Saunders and Kohn 2009). That endophyte species diversity varies significantly among plant species, including crop species, is evident from the literature (Carter et al. 1999; Franke-Snyder et al. 2001; Arnold 2007; Hoffman and Arnold 2008). Our research focus has been on biotic factors influencing the assembly of endophyte communities in maize, where we have found that communities can also vary significantly among plant genotypes and phenotypes (Saunders and Kohn 2009). That communities differ predictably among plant species and genotypes suggests that plant traits can mediate endophyte community processes, but the specific mechanisms are poorly known.
We are intrigued by the experimentally accessible question of how host phenotype influences assembly of fungal endophyte communities. Plant traits such as defense compound production and tissue lignification can influence host colonization by known pathogens, and would therefore be expected to affect the species diversity of fungi within plants (VanEtten et al. 2001). Equally important to consider are the fungal traits that have putatively evolved in response to plant traits that discourage colonization. Interactions between microbial species are also expected to influence community processes. That microbial biocontrol agents can be deployed to control plant pathogens highlights the influence of one microbial species on the abundance of other species in the community. Effects of biocontrol agents on fungal community members other than the target pathogen are common (Brimner and Boland 2003), but the influence of biocontrol species on community processes is typically unknown.
Previous research in fungal community ecology and agricultural disease management has laid the foundation for asking exciting questions about the mechanisms that underlie fungal community dynamics, and the influence of the endophyte community on host health. In this review we examine fungal endophyte communities in agriculture with a focus on the following three areas of research that hold potential for describing general patterns in endophyte community ecology. (i) Application of the ‘assembly rules’ model to understand the dynamics of agricultural endophyte assemblages. This model provides a powerful framework with which to tease apart the specific mechanisms that contribute to community assembly. (ii) Use of plant and fungal functional trait variation to identify the phenotypes that significantly influence endophyte community processes. A given plant phenotype may effect the resident endophyte community, or may have a sphere of influence that extends to the endophyte communities of neighboring plants. The interplay between host and fungal phenotype is likely one of the most important factors in successful establishment for primary colonizing fungi. (iii) Identification of fungal traits important in mediating microbe–microbe interactions. Secondary colonizers will not only face challenges posed by the host, but also those presented by fellow members of the microbial community. Variation in fungal traits important in microbe–microbe interactions will influence endophyte community processes. We conclude by suggesting research questions that may help to describe mechanisms in endophyte community processes. Maize, an emerging model crop system for endophyte community research, will be a focal point for discussion.
Mechanisms that influence endophyte community dynamics: assembly rules, habitat filters and species interactions
Each individual plant harbors an endophyte community that is a subset of the species pool, the ambient fungal species aggregate in the environment outside of the plant. What determines which species co-occur within the community? Research indicates that communities can be the result of random assembly events, or the result of predictable, trait-based assembly processes, and the field is divided as to the importance of the two (Hubbell 2001; Fargione et al. 2003). For our purposes here, the ‘assembly rules’ concept in ecology offers a most useful perspective. Community ecology theory proposes that ‘filters’ mediate community assembly through a series of processes that result in the co-existence of particular species at a site (Diamond 1975; Weiher and Keddy 1999). These processes can be roughly divided into two categories: habitat filtering and species interactions. Specific environmental variables are expected to act as habitat filters by preventing establishment of species that lack the phenotype required to survive. If a species is able to tolerate the abiotic conditions in the environment, establishment may then depend on the outcome of interactions with other species in the community.
Typically, the distinction between habitat filters and species interactions is a useful way to discriminate between the influence of abiotic and biotic factors in community processes. For plant-associated fungi, habitat filters may also include biotic variables, because the environment being colonized is a living host. Therefore species are likely to be challenged by abiotic habitat filters and two distinct biotic factors: plant-imposed habitat filters and interactions with fellow microbial community members (Fig. 1). Potential abiotic habitat filters include water availability and UV exposure, while plant traits such as biochemical defenses and tissue lignification may act as plant-imposed filters. Interactions among microbial species may range from competitive to facilitative, with the outcome often influenced by the timing of colonization. Interestingly, once a fungal species is established in planta, it may prevent subsequent species from colonizing, acting in essence as a plant defense mechanism. These factors are likely to work on different scales, with abiotic habitat filters acting at the scale of the plant neighborhood and plant-imposed filters at the level of the individual plant and potentially at the level of the plant neighborhood. Fungal–fungal interactions are expected to occur between localized colonies within plant tissue. Abiotic filters, plant-imposed filters and fungal–fungal species interactions may or may not act in a nested fashion.
Figure 1.
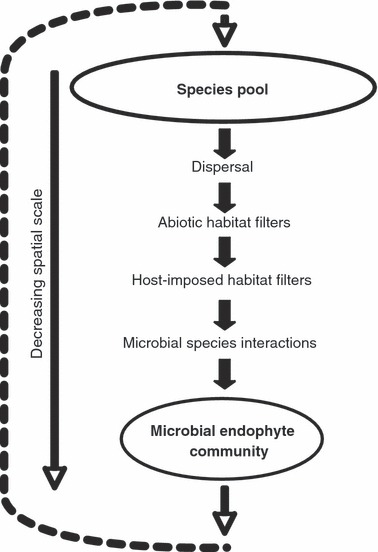
As species move from a regional species pool to become established as part of a community (the assemblage of fungi within an individual host), they must have the ability to disperse to the habitat and pass through environmental stressors, including abiotic habitat filters and plant-imposed habitat filters that prevent some species from colonizing. Once in the host environment, the species may experience competitive or facilitative interactions with other microbial species. The endophyte community assemblage may influence composition of the species pool; inoculum will reside on plant matter until it is dispersed by wind, water or animal to a new host (dotted line).
The environmental filtering hypothesis utilizes the ‘assembly rules’ concept to predict the formation of the community (Weiher and Keddy 1999). Under this hypothesis, species within communities are constrained by environmental factors; the overall species abundance within a community is driven to a trait average optimal for the specific environment. This hypothesis has found success in predicting community structure of plants, where functional traits such as specific leaf area, stem mass and height have proven informative (Shipley et al. 2006). Such an approach may also be useful in understanding endophyte community assembly, and would likely rely heavily on physiological traits. The choice of traits to be evaluated is important, and specific to the questions being asked. For example, Saunders and Kohn (2009) evaluated the role of host defense compounds in endophyte community assembly by comparing endophyte communities from host genotypes that either could or could not produce the compounds. Host genotypes that produce defense compounds had endophyte communities dominated by species with a higher level of tolerance to the toxins than did host genotypes that did not produce the compounds.
Community phylogenetics is an example of an ecological tool that could be widely useful in agricultural studies. Inference of community-wide phylogenies is a powerful approach that can be used to develop hypotheses about which fungal traits are most important in community assembly. It has long been noted that closely related species are ecologically similar, indicating that the traits important to success in a given environment are evolutionarily conserved (Darwin 1859). When functional traits are highly conserved, species are expected to compete when they co-occur (Cavender-Bares et al. 2009). Thus, community phylogenies can aid in the detection of community assembly processes. For example, if species within a community are phylogenetically clustered (more closely related than expected by chance), this may indicate that trait conservation has resulted in environmental filtering. For plant-associated fungi, phylogenetic clustering may be the result of similarities in responses to abiotic factors or similarities in host range. Conversely, over-dispersion of fungal species distributions (more distantly related than expected by chance) suggests competition between closely related species. Although relatedness of fungal pathogens is widely recognized in agricultural studies (e.g., Elmer et al. 2007; Ward et al. 2008), phylogenetic tools provide a statistical approach for hypothesis development. In agricultural systems, community phylogenetic data could be evaluated in concert with known mechanisms of fungal colonization to either reveal previously unrecognized fungal traits, or to assess the role of known fungal colonization mechanisms.
After phylogenetic analysis has revealed potential fungal traits of interest, experiments comparing fungal phenotypes will help to confirm the roles of various traits on community assembly. Such studies should expose traits important to endophyte success within a particular host species or genotype. In a previous review, Rodriguez et al. (2009) delimited four broad functional classes of endophytes based on life history characteristics such as host range, type of transmission (vertical or horizontal), and plant organs colonized. We suggest that investigation of community assembly rules will aid in describing additional functional classes. Such studies could inform hypotheses on life history strategies of endophyte species; for example, if the relative roles of abiotic stressors and microbial competition are of interest, the response of fungal species to microbial competition could be assessed along an environmental gradient. Experiments that isolate the influence of abiotic habitat filters, plant-imposed habitat filters and species interactions may reveal the relative importance of these factors in community assembly and in plant–fungal interactions.
Influence of plant-imposed habitat filters and the interplay between plant and fungal phenotype on endophyte community assembly
Which plant traits mediate endophyte community assembly? We know that variation in plant traits such as lignin quantity, cell wall thickness and production of defense compounds can influence the ability of fungal species to colonize and proliferate within plant tissue (Knogge 1996; VanEtten et al. 2001). Correspondingly, fungal species have evolved strategies to overcome colonization barriers, and species vary widely in both ability to colonize and the colonization strategies that are employed (Canhoto and Graca 1999; Osbourn 1999; Huckelhoven 2007; Kikot et al. 2009). For example, some plant pathogens are able to colonize by actively penetrating host tissue. Penetration is often mediated by the production of enzymes that degrade wax and cell walls, thus enabling penetration of host tissue (Kolattukudy 1985; Torto-Alalibo et al. 2009). Other species may colonize via open wounds or stomata and are able to successfully colonize without tissue penetration. Therefore, variation in plant traits such as leaf chemistry and susceptibility to herbivore damage are expected to influence the assemblage of fungal species that can successfully colonize the host.
How does the interplay between host and fungal traits influence fungal community dynamics? We know that host defense traits can select for colonization by a fungal species with an adaptive phenotype, but experiments have primarily been conducted on single host–fungal pairs, or on a single host and 2–3 fungal partners. A better understanding of how host trait variation influences fungal community assembly will be facilitated by studies that assess how plant functional trait variation affects both the species diversity and functional trait diversity of fungal communities. The ability of an individual plant to affect endophyte dynamics can also extend beyond its own resident endophyte community to affect community assembly of endophytes associated with neighboring plants. For example, antifungal compounds that are released into soil by one plant can influence the endophyte communities of surrounding plants, constituting a plant-imposed habitat filter that is mediated by neighbors of the host. In the next section we highlight several studies that either aim to identify plant traits that influence endophyte community dynamics, or hold promise for the screening and identification of important plant-imposed habitat filters.
Effects of plant traits on the resident endophyte community: plant defense compound production can impact assembly of maize endophyte communities
Endophyte communities from conspecific plant genotypes that differ in production of defense compounds can harbor predictably different endophyte communities, evidence that plant defense compounds can act as plant-imposed habitat filters (Bailey et al. 2005; Saunders and Kohn 2009). Secondary compounds produced by plants are incredibly diverse. Over 10 000 compounds have been described to date, and most that have been tested for biological activity have antifungal and/or antibacterial properties (Dixon 2001). Plant defense compounds are therefore likely to be one of the most common plant-imposed habitat filters encountered by plant-associated fungi. Predictably, fungal species commonly exhibit high tolerance to the toxins produced by their host species, evidence that defense compounds impose significant selective pressure on plant-associated fungi (Carter et al. 1999; Osbourn 1999; VanEtten et al. 2001; Bailey et al. 2005; Saunders and Kohn 2009). Detoxification of host defense compounds is one of the most common fungal tolerance mechanisms; in some host species, fungi that can detoxify can colonize and establish in greater abundance than those that cannot (Carter et al. 1999; VanEtten et al. 2001; Saunders and Kohn 2009).
Intraspecific variation in host defense compound production can significantly influence fungal endophyte community assembly in maize. Maize produces benzoxazinoids (BXs), leading to accumulation of a class of toxic byproducts, the benzoxazolinones, for example, 2-benzoxazolinone (BOA), in plant tissue and in soil (Krogh et al. 2006). BX byproducts have known toxicity against fungi, bacteria, insects and plants (Niemeyer and Perez 1995). Maize commonly harbors several species in the genus Fusarium that have relatively high levels of tolerance to BOA (Glenn et al. 2001; Saunders and Kohn 2008; Saunders and Kohn 2009). Many of these species, including Fusarium verticillioides (Sacc.) Nirenberg, F. subglutinans Wollenw. & Reinking and F. proliferatum (Matsush.) Nirenberg ex Gerlach & Nirenberg, present an economic and health risk; as endophytes in grain they produce mycotoxins (secondary compounds that have adverse effects on animals) that cause toxicosis in domesticated animals and may pose risks for cancers and other human health problems (Ueno et al. 1997; Marasas 2001). Concentration of BXs is a highly variable trait; maize has undergone selective breeding aimed at increasing BX concentrations to protect against damage from the European corn borer [Ostrinia nubilalis (Hübner)] and other herbivores (Barry and Darrah 1991).
Results from our field studies compared fungal endophyte communities from BX producing and nonproducing genotypes. While endophyte species diversity did not differ between the host genotypes, BX production provided a colonization advantage to Fusarium species (Fig. 2; Saunders and Kohn 2009) confirmed in second field study (M. Saunders, A.E. Glenn and L.M. Kohn, unpublished). Abundance of Fusarium species was up to 35 times higher in mature leaves of BX producers than of nonproducers. Production of BXs also influenced the distribution of BOA tolerance levels within endophyte communities. In mature leaves, plant genotypes that did not produce BXs had significantly more BOA sensitive isolates than the BX-producing genotypes, indicating that tolerance to BOA provided an ecological advantage not only to Fusarium species, but also to additional fungal endophyte species with high BOA tolerance. This plant-imposed habitat filter, in combination with the functional trait of BOA tolerance among some fungal species, was shown to shape the composition of the endophyte community. We suggest that breeding for elevated concentrations of BXs in maize may have unintentionally increased colonization by Fusarium species. Our results highlight endophyte functional traits and plant-imposed habitat filters as key factors in understanding fungal community assembly.
Figure 2.
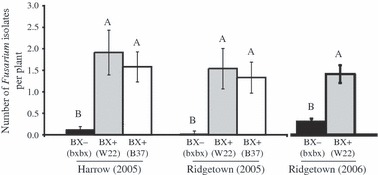
Mean number of Fusarium isolates per plant obtained from 9-week-old leaf tissue of maize genotypes that produce the benzoxazinoid defense compounds (BX+ genotypes = W22 and B37) and a genotype that does not produce BXs (BX− genotype = bxbx). In 2005, plants were grown in Harrow and Ridgetown, Ontario, Canada, and in 2006, plants were again grown in Ridgetown, Ontario. Tukey–Kramer HSD tests were conducted to compare mean number of isolates per plant obtained from bxbx (BX−), W22 (BX+), and B37 (BX+) genotypes. The same letter above two columns indicates no significant difference between means. Vertical bars, ±1 SE.
Does host variation in insect resistance influence composition of the fungal community?
Some of the same species of Fusarium that commonly reside in roots and shoots of maize as endophytes produce toxins that accumulate in the grain. Studies conducted over the past 10 years indicate that transgenic Bt maize accumulates significantly lower concentrations of Fusarium-produced mycotoxins in grain than do isogenic genotypes without the transgenes (Hammond et al. 2004). Bt maize hybrids contain the Cry1Ab protein originated from Bacillus thuringiensis Berliner. Presence of the protein protects plants from herbivore damage (Pilcher et al. 1997). Mycotoxin concentration is extremely variable among maize crops, and there are several known factors that contribute to the susceptibility of maize to infection and mycotoxin contamination. Insect damage in maize is positively correlated with infection by mycotoxin-producing Fusarium species, and mycotoxin contamination of grain. Thus, Bt maize suffers less insect damage ultimately resulting in lower levels of contamination with mycotoxins, such as the fumonisins, compared to isogenic maize hybrids (Munkvold and Desjardins 1997; Sobek and Munkvold 1999). The correlation is likely due to suppression of both herbivory and its collateral effects; the herbivores vector fungal spores, the inoculum, and they create an infection court in wounds resulting from herbivory. Deployment of Bt maize has led to a significant decrease in economic loss from mycotoxin contamination; in 2004 it was estimated that farmers in the US would recover $17 million dollars annually if Bt maize were deployed (Wu et al. 2004).
The impact of Bt maize on endophyte communities is unknown. We have included this example because wound-infecting endophyte species are common, and we hypothesize that plant variation in resistance to insect damage is likely to have a cascade of influence on the fungal community. We do not know which endophyte species, if any, occupy the niche space previous occupied by the wound-infecting Fusarium species, but it seems likely that a different suite of host-entry mechanisms is required for colonization in herbivore-resistant maize. The association between herbivore damage – or the lack of it – and penetration and subsequent colonization of endophyte species merits further investigation, especially where wound-infecting species are ‘keystone’ with respect to hindering or facilitating subsequent colonization by other species.
Host phylogeny may highlight plant functional traits important in endophyte community assembly
The evolutionary history of host plants should provide clues about plant traits regulating endophyte community processes. Use of community phylogenetic tools has proved useful for understanding dynamics of fungal pathogens. For example, Gilbert and Webb (2007) detected a phylogenetic signal in host range of foliar pathogens of tropical trees. The likelihood that a fungal species could infect two plant species decreased as phylogenetic distance between host species increased. In a parallel vein, ectomycorrhizal fungal communities from eight plant hosts were compared, and taxonomically close host species (e.g., congeneric pairs), had more similar mycorrhizal communities than did host species of different genera (Ishida et al. 2007). That closely related host species have similar relationships with their fungal inhabitants suggests that evolutionarily conserved traits act as plant-imposed habitat filters. Studies that explicitly test for a phylogenetic signal in the association of plants with their endophyte species can be used to develop hypotheses about which traits are important in mediating endophyte community assembly. For example, closely related hosts may share a chemical or physical defense trait that is not shared with a more distantly related plant species. Follow-up experiments that compare plant genotypes or species that vary in the traits of interest could then be conducted to determine if specific plant phenotypes are correlated with particular endophyte assemblages. This could be especially useful in explaining the endophyte community dynamics in closely related crop plants, where much is known about specific host phenotypes.
Beyond the resident endophyte community: influence of plant functional traits on endophyte assemblages within neighboring plants
Here, we present two conceptual models developed for pathogens and herbivores and consider their pertinence to fungal endophytes. The plant traits that act as habitat filters in pathogen colonization can have a spatial sphere of influence that extends beyond the individual plant to the neighbors of the focal host. Plants can experience ‘associational susceptibility’ or ‘associational resistance’ when presence of plant neighbors causes a change in the amount of herbivore- or pathogen-induced damage that is sustained (Power and Mitchell 2004; Burdon et al. 2006). An example of associational resistance in agriculture is the use of crop species mixtures to decrease pathogen transmission. Co-occurrence of such plants in a crop field can result in a spatial dilution of the unwanted pathogen. A mix of distantly related host species is usually deployed. For example, clover reduces infection of wheat by Septoria tritici Desm. by providing a barrier to water-dispersed propagules (Bannon and Cooke 1998). In contrast, some species mixtures result in associational susceptibility, such as when a ‘reservoir’ host accumulates a high density of infective propagules that are in turn transmitted to neighboring plants (Power and Mitchell 2004; Burdon et al. 2006).
Exudation of defense compounds from roots into soil is an example of a plant-imposed habitat filter that can have a spatial sphere of influence that extends to the endophyte communities of plant neighbors. Some root exudates can induce a defense response in neighboring plants, thereby providing a form of associational resistance. Root exudates can also cause associational susceptibility when the causative plant reduces the abundance of an organism that is a mutualist of its plant neighbors. Garlic mustard [Alliaria petiolata (M. Bieb.) Cavara & Grande], which is nonmycorrhizal, can dramatically reduce the activity of mycorrhizal fungi in soil by releasing fungitoxic compounds into soil, thereby suppressing the growth of surrounding tree species (Stinson et al. 2006; Wolfe et al. 2008). We tested the influence of BX producing maize plants on the endophyte communities of neighboring plants that cannot produce BXs (M. Saunders, A.E. Glenn and L.M. Kohn, unpublished). Maize genotypes that do not produce BXs were grown in a field with two BX-producing genotypes (triculture), as well as in monoculture. Communities in roots of BX nonproducing plants grown in monoculture had higher endophyte infection density and higher endophyte species diversity than those grown in triculture. Previous work has shown that BXs can be released into soil, where they persist (Krogh et al. 2006). Our results are consistent with what would be expected if BXs were present in soil, where they influenced the common microbial community. We did not measure BXs in the field and so can only conclude that the genetic composition and physiological activity of the host plants had a striking affect on endophyte species diversity.
Interactions between fungal species can modify endophyte assemblages
After a fungal species has become established within plant tissue it may pose an additional barrier to subsequent fungal colonizers. Therefore, for secondarily colonizing fungi, it is not only host plant traits that may preclude colonization, but also traits of the fungi that are already established in host tissue. Fungal species within plant tissue may either completely prevent colonization by additional fungi, or may interact with species once all are established in host tissue. It follows that the outcome of such species interactions will influence community assembly. Biocontrol research efforts have resulted in descriptions of several mechanisms of fungal competition (Baker and Cook 1974; Duffy and Raaijmakers 2003). We know that effects of fungal biocontrol species often extend beyond the target pathogen to nontarget fungal species (Brimner and Boland 2003). Trichoderma species are used to control soil-borne diseases such as Sclerotinia sclerotiorum (Lib.) de Bary and Fusarium oxysporum Schltdl., but can also penetrate the resting spores of the mycorrhizal species, Glomus intraradices Schenk and Smith (Sivan and Chet 1993; Inbar et al. 1996; Rousseau et al. 1996). The impact of biocontrol agents on nontarget fungal species is further evidence that microbe–microbe species interactions are likely to impact fungal community assembly, and that ‘keystone’ endophyte species can cause dramatic changes in fungal community structure as well as host health.
Fungal competition within host tissue can occur with direct or indirect contact. Within host tissue competition requires either direct contact, or residence within the same small area of substrate. For example, variation in fungal traits such as production of antifungal compounds and resource use are expected to modify competition within host tissue. Alternatively, species may engage in ‘long-distance’ competition by initiating the host defense response. Fungal–fungal interactions can also be facilitative, such as when one species mobilizes nutrients for another, or ameliorates a toxic environment (Lawrey 2000; Saunders and Kohn 2008). The following subsections describe several mechanisms of competition and facilitation. We draw attention to functional traits that have been documented to mediate the outcome of two- or three-way interactions and could therefore be interesting in a community interaction context. Research on the use of fungal species as biocontrol agents has provided a wealth of data on mechanisms of microbe–microbe competition, inviting further research aimed at impacts on endophyte community dynamics (e.g., Baker and Cook 1974; Mandeel and Baker 1991; Benitez et al. 2004; Minerdi et al. 2009).
Microbial competition can be mediated by production of antifungal compounds
The outcome of competitive interactions between fungal species is often attributed to the production of biologically active compounds produced by fungi. An in vitro study found that approximately 80% of fungal endophyte species produce secondary compounds with herbicidal, antibacterial or antifungal activity (Schulz et al. 2002). Overall, data from in vitro studies show that endophyte species can compete directly by producing antifungal compounds that diffuse in substrate, and prevent other species from encroaching. Some fungal species can detoxify the antifungal compounds produced by their microbial competitors, for example, Fusarium graminearum Schwabe can detoxify the toxic compound 6-pentyl-alpha-pyrone, produced by Trichoderma harzianum Rifai (Cooney et al. 2001). Ability to detoxify fungicides of fungal, bacterial, plant and synthetic origin varies widely among fungal species, and is likely to be important in community assembly. The response of fungi to plant and synthetic toxins is well documented and indicates three primary mechanisms of tolerance: detoxification, structural alteration of the target of the toxin, and activation of membrane transporters that exude toxins from the cell (VanEtten et al. 2001). It is likely that all three mechanisms are also employed in response to fungal-produced bioactive compounds. It is possible that the interplay between production of antifungal compounds by some fungal species and the ability to tolerate or resist them by others is a major driver of fungal–fungal competition. This is an experimentally tractable question that could be addressed by evaluating natural variation in antifungal compound production and resistance or by creating microcosm communities with characterized phenotypes.
Interestingly, some endophyte species produce volatile compounds with antifungal activity, resulting in direct competition at spatial scales expected to be larger than scales of toxicity from nonvolatiles within a host or substrate (Stinson et al. 2003; Steinebrunner et al. 2008). For example, the suite of low molecular mass, volatile, antifungal compounds produced by Muscodor albus Worapong, Strobel & W.M. Hess and related species influence microbial growth without direct contact between microbes (Stinson et al. 2003). Tolerance of fungal species to the volatiles is variable, and the influence of the compounds on whole fungal communities is unknown. To our knowledge, fungal mechanisms of defense against volatiles have not been studied in detail. As more cases of volatile defense compounds accrue, it appears that this mechanism of competition may be widespread (Stinson et al. 2003; Steinebrunner et al. 2008; Strobel et al. 2008; Minerdi et al. 2009). Identification of the mechanisms of volatile defense would be a first step in contrasting the importance of short- versus long-range fungal competition.
Competition for space or nutrients
Some fungal species may compete by simply occupying all of the tissue available at a particular site on the plant. Mandeel and Baker (1991) suggested that the root surface has a limited number of infection sites, and that protection of the root would increase with abundance of a fungal biocontrol species. There is some evidence that this can occur. For example a comparison between pathogenic and nonpathogenic strains of Fusarium oxysporum showed that as primary colonizers, root colonization was qualitatively the same among pathogens and nonpathogens with both extensively colonizing root tissue and decreasing or preventing colonization by additional fungi (Olivain and Alabouvette 1997, 1999). Resource competition is also expected to be prevalent among fungi, and can either occur when one species depletes available nutrients to the detriment of other species, or when one species blocks another species from access to nutrients. The later form of resource competition is inherently associated with other forms of competition. For example, nutrient availability of one species may be restricted by another species via production of antifungal compounds. This example illustrates the importance of colonization timing in community assembly, where priority effects are expected to play a significant role. A species also may demonstrate primary resource capture by growing on previously uncolonized substrate, or may obtain nutrients as a secondary colonizer, a strategy that likely involves interaction with a living tenant (Cooke and Rayner 1984; Boddy 2000). For the primary colonizing species, traits such as wide dispersal and rapid spore germination, mycelial growth and metabolism are important for success (Cooke and Rayner 1984). Competitive ability, such as that mediated by production of antifungal compounds, is crucial for survival of secondary colonizers, and is perhaps the most important type of interaction for many fungal species.
Competition via mycoparasitism
Fungal endophytes are subject to parasitism by other fungi, bacteria and viruses. Typical interactions characteristic of mycoparasitism are hyphal coiling, formation of a resistance sheath, and invasion into host hyphae. Ability to form these structures and penetrate fungal tissue can vary markedly within and among species. Several species in the fungal genus Trichoderma are exploited as biocontrol agents because they antagonize target pathogens via mycoparasitism, production of antifungal toxins and acquisition of space and nutrients (Benitez et al. 2004).
Relationships between endophytic fungi and ecto- and endosymbiotic bacteria can have broad impacts on the virulence, metabolism, and development of fungi. Of particular interest is the discovery of an avirulent Fusarium oxysporum strain that has biocontrol potential against plant disease caused by pathogenic strains of F. oxysporum. The ectosymbionts modulate the biology of the fungus, silencing its virulence and altering its developmental morphology (Minerdi et al. 2008). The association results in release of volatile organic compounds that may act as a long distance antagonism mechanism limiting the growth of pathogenic F. oxysporum strains (Minerdi et al. 2009). It is therefore possible that presence of the bacterium may alter the spatial scale at which F. oxysporum can influence endophyte community assembly. In contrast, the endofungal bacterium, Burkholderia rhizoxinica Partida-Martinez et al. confers phytopathogenicity to the fungal host (Rhizopus microsporus Tiegh.) by producing a phytotoxin (Partida-Martinez and Hertweck 2005; Partida-Martinez et al. 2007; Lackner et al. 2009). Thus, while the ectosymbiotic bacteria of F. oxysporum silence fungal virulence, the endosymbiotic Burkholderia confers the phytopathogenicity of R. microsporus, in both cases due to aspects of secondary metabolism. Similar interactions have been documented between fungi and endosymbiotic mycoviruses, which can render previously pathogenic fungal strains avirulent (Nuss 2005).
How do microbes that parasitize fungal endophytes influence endophyte community processes? Profound changes in fungal physiology mediated by parasitic microbes are likely to influence endophyte community processes, either by changing host suitability or by altering the ability of the parasitized fungus to directly compete with microbial community members. Some fungal species engage in active combat and mutual parasitism (Vajna 2003); such pairings could be used to investigate both the traits important in ability to parasitize and to resist invasion, and the influence of such traits on community assembly.
Fungal species indirectly influence endophyte community assembly by initiating the host defense response
A primary colonizing endophyte may also influence colonization of other microbes by inducing a ‘priming’ reaction in the plant (Trillas and Segarra 2009). Priming occurs when the plant immune system registers a microbial invader by detecting a well-conserved trait (e.g., chitin), which triggers induced resistance that is characterized by an increased capacity to activate a defense response to a later infection (Conrath et al. 2002). Fungal biocontrol species can instigate priming (Trillas and Segarra 2009). For example, Djonovic et al. (2007) found that a hydrophobin-like elicitor of the fungal endophyte Trichoderma virens (J.H. Mill., Giddens & A.A. Foster) Arx can induce systemic resistance in maize. We should anticipate that priming induced by an endophyte species used in biocontrol could have collateral effects on the endophyte community as well as on the target pathogen. A primary colonizer could gain a competitive advantage if it were able to first induce priming and then tolerate or avoid the plant resistance mechanisms that were induced. Many other studies have found that inoculation with a biocontrol endophyte protects against pathogen colonization in circumstances where endophyte and pathogen are very unlikely to interact directly, indicating that endophyte-stimulated induced resistance may be common (for review see Terry and Joyce 2004). Investigation of the interaction between priming, pathogen abundance and the microbial community may help to explain why the outcome of biocontrol treatments can be difficult to predict. It is possible that ability to colonize virgin host tissue and induce plant defenses is an adaptive colonization strategy. Is the identity of a primary colonizer simply a matter of chance or the result of adaptation to newly emerging tissue? Studies that investigate both the ability of different endophyte species to colonize and induce priming, and the downstream effects of endophyte-induced priming on the community would help to answer this question.
Facilitation between fungal endophyte species
Facilitation can occur between fungal species, although it is rarely documented (Lawrey 2000; Tiunov and Scheu 2005; Pan and May 2009). To evaluate if facilitation occurs between fungal endophyte species, we asked if detoxification of host defense compounds by one maize endophyte species could facilitate the growth of species less tolerant to the compounds. We found that under in vitro conditions, F. verticillioides could facilitate growth of less tolerant maize endophyte species (Saunders and Kohn 2008). F. verticillioides has a high level of tolerance to BOA, and converts it to a less toxic product. On synthetic medium, F. verticillioides was able to detoxify BOA and increase the growth rate of commonly co-occurring maize endophyte species. However, in field-grown maize F. verticillioides interacted competitively with community members by preventing species with lower BOA tolerance from colonizing root tissue (M. Saunders, A.E. Glenn, L.M. Kohn, unpublished manuscript). Interestingly, facilitation achieved via habitat amelioration has been documented for lichen-dwelling fungal species. The lichenicolous fungus Nectria parmeliae (Berk. & M.A. Curtis) D. Hawksw can only colonize the lichen Punctelia rudecta (Ach.) Krog after a lichenicolous Fusarium species has colonized and degraded the antifungal compounds produced by the lichen (Lawrey 2000).
Few studies have assessed the outcome of endophyte–endophyte species interactions across entire communities (Pan and May 2009). Evaluating endophyte communities associated with several genotypes of maize, Pan and May (2009) used null models to test for species that were found together more or less often than would be expected by chance, a hypothesized signal of facilitation and competition. Fungal communities in shoot tissue were assessed using both a culture-dependent and a culture-independent approach. Data from the culture-dependent approach suggest that interspecific facilitation is common, while data from the culture-independent approach, depending on the scale of analysis (individual leaf or individual plant), either did not detect significant pairwise interactions between species, or detected competition.
This highlights a unique challenge of working with microbial communities; natural communities can only be characterized by isolating microbes directly from tissue (culture dependent), or by isolating DNA from plant tissue and obtaining sequence data (culture independent). Both have the potential to bias the resulting community data: culture-based studies will likely yield those species that grow well on the same substrate, and molecular methods may exclude particular species due to PCR bias (Arnold et al. 2007; Pan and May 2009; Avis et al. 2010). A combination of culturing fungi directly from tissue and obtaining genomic DNA directly from plant tissue to use in next-generation sequencing will give the most comprehensive view of endophyte community dynamics by allowing for the saturation of species area curves while maintaining the option for manipulative experiments. Future studies that evaluate the net outcome of endophyte–endophyte species interactions across whole communities will help to determine the extent to which facilitation and competition influence community assembly.
Conclusions
Most plant species harbor a diverse assemblage of fungal endophytes, yet our knowledge of why particular species assemblages are found in specific host species is limited. It has been suggested that research in community ecology will be more likely to result in the development of general rules if quantification of functional trait variation is combined with data on variation in the abiotic environment and evolutionary history of the species of interest (McGill et al. 2006). Research documented here indicates that this approach will likely prove fruitful in understanding endophyte ecology. Assignment of fungal phenotype values assessed in vitro should be undertaken with care, however, because many environmental factors, such as temperature and light exposure can influence the phenotype being tested. For example, we found that starting population size, colony age, and nutrient availability influenced the growth rate of endophyte species grown in the presence of BOA, but none influenced the BOA tolerance threshold of isolates, suggesting that the binary trait of plus/minus growth was less plastic than growth rate.
Although somewhat beyond the scope of this review, it is notable that abiotic factors such as fertilizer application, temperature and seasonal moisture regimes can influence endophyte community assembly (Suryanarayanan et al. 2002; Seghers et al. 2004; Gonthier et al. 2006). The interaction between host genotype and environment can also significantly impact endophyte communities (Elamo et al. 1999; Pan et al. 2008; Pan and May 2009; Saunders and Kohn 2009). Variation in abiotic factors are likely an important component in any model of endophyte community assembly – and certainly a key set of variables to consider when assessing response to global change, predicting disease dynamics, or managing biocontrol efforts.
Much of the research presented here demonstrates that manipulation of host phenotype via crop breeding programs can have a huge impact on fungal endophyte colonization. Such influence can be desirable, as is seen in the reduction of mycotoxin contamination of Bt maize, or disadvantageous, as in the unanticipated increase in abundance of mycotoxin-producing Fusarium species that colonize BX-producing maize genotypes. In vitro studies indicate that microbe–microbe interactions can be mediated by the production of secondary compounds. Overall, production of toxins by plants and microbes has emerged as a significant player in these dynamics. Research on fungal communities associated with maize highlights a suite of factors that may impact community assembly, including production of defense compounds by the plant, herbivore resistance and presence of mycotoxin-producing endophytes in plant tissue. We suggest that answers to several questions will be particularly important in better understanding endophyte community dynamics:
Which host and fungal traits are important in endophyte community assembly? How does the distribution of host and fungal traits change with abiotic conditions, for example, across environmental gradients?
Which plant traits influence the available species pool of potential endophytes in a poly-species or poly-genotype plant neighborhood? What is the interaction between crop genetic diversity, endophyte colonization and pathogen abundance?
What is the influence of well-described mechanisms of fungal competition, such as mycoparasitism and fungicide production, on endophyte community assembly?
How can concurrent use of host and fungal phylogeny inform hypotheses about fungal community assembly rules?
To what extent and by what mechanisms do selective breeding and agronomic practices influence the assembly of endophyte communities? Can this information be used to optimize crop management and biocontrol strategies?
Community assembly dynamics will be key in the development of general models for endophyte biology in crop and wild plants. The field of microbial ecology has blossomed as molecular approaches such as next generation sequencing have facilitated more complete descriptions of endophyte communities. Use of such tools will be most fruitful when combined with trait-based studies that connect host and fungal phenotype. There is now a need to explore relationships between species diversity and functional traits, and to use phylogenetic tools to identify both the plant traits that act as plant-imposed filters and the fungal traits adaptive to such filters. We see this as the most immediate route to understanding host–fungal relationships and endophyte community ecology. We also predict that more studies will factor the endophyte community into investigations of plant disease epidemiology. Such an approach could improve biocontrol models toward the goals of predicting variation in treatment response and improving biocontrol practice.
Acknowledgments
M.S. would like to thank Profs. J.B. Anderson and P.M. Kotanen, as well as M. Andrew, S.B. Hill and A.A.M. MacDonald for engaging in valuable scientific discussion over the past several years. We also thank G.S. Gilbert, I.M. Parker, J. Cummings, C. Fresquez, S. Grove, S. Gulamhussein, J.L. Ohayon, D. Schweizer, J.A. Torres and an anonymous reviewer for helpful comments on drafts of this manuscript. Manuscript preparation was supported by a Discovery Grant (Natural Sciences and Engineering Research Council of Canada) to L.M.K., and a University of Toronto Graduate Fellowship (Department of Ecology and Evolutionary Biology) to M.S.
Literature cited
- Arnold AE. Understanding the diversity of foliar fungal endophytes: progress, challenges and frontiers. Fungal Biology Reviews. 2007;21:51–66. [Google Scholar]
- Arnold AE, Mejia LC, Kyllo LC, Rojas EI, Maynard Z, Robbins N, Herre EA. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AE, Henk DA, Eells RL, Lutzoni F, Vilgalys R. Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia. 2007;99:185–206. doi: 10.3852/mycologia.99.2.185. [DOI] [PubMed] [Google Scholar]
- Avis PG, Branco S, Tang Y, Mueller GM. Pooled samples bias fungal community descriptions. Molecular Ecology Resources. 2010;10:135–141. doi: 10.1111/j.1755-0998.2009.02743.x. [DOI] [PubMed] [Google Scholar]
- Bailey JK, Deckert R, Schweitzer JA, Rehill BJ, Lindroth RL, Gehring C, Whitham TG. Host plant genetics affect hidden ecological players: links among Populu, condensed tannins, and fungal endophyte infection. Canadian Journal of Botany. 2005;83:356–361. [Google Scholar]
- Baker KF, Cook RJ. Biological Control of Plant Pathogens. San Francisco: W.H. Freeman and Company; 1974. [Google Scholar]
- Bannon FJ, Cooke BM. Studies on dispersal of Septoria tritici pycnidiospores in wheat-clover intercrops. Plant Pathology. 1998;47:49–56. [Google Scholar]
- Barry D, Darrah LL. Effect of research on commercial hybrid maize resistance to European corn-borer (Lepioptera, Pyralidae) Journal of Economic Entomology. 1991;84:1053–1059. [Google Scholar]
- Belesky DP, Bacon CW. Tall fescue and associated mutualistic toxic fungal endophytes in agroecosystems. Toxin Reviews. 2009;28:102–117. [Google Scholar]
- Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. International Microbiology. 2004;7:249–260. [PubMed] [Google Scholar]
- Boddy L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiology Ecology. 2000;31:185–194. doi: 10.1111/j.1574-6941.2000.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Brimner TA, Boland GJ. A review of the non-target effects of fungi used to biologically control plant disease. Agriculture Ecosystems and the Environment. 2003;100:3–16. [Google Scholar]
- Burdon JJ, Thrall PH, Ericson L. The current and future dynamics of disease in plant communities. Annual Review of Phytopathology. 2006;44:19–39. doi: 10.1146/annurev.phyto.43.040204.140238. [DOI] [PubMed] [Google Scholar]
- Canhoto C, Graca MAS. Leaf barriers to fungal colonization and shredders (Tipula lateralis) consumption of decomposing Eucalyptus golubulus. Microbial Ecology. 1999;37:163–172. doi: 10.1007/s002489900140. [DOI] [PubMed] [Google Scholar]
- Carroll GC. Forest endophytes: pattern and process. Canadian Journal of Botany. 1995;73(S1):1316–1324. [Google Scholar]
- Carter JP, Spink J, Cannon PF, Daniels MJ, Osbourn AE. Isolation, characterization, and avenacin sensitivity of a diverse collection of cereal-root-colonizing fungi. Applied and Environmental Microbiology. 1999;65:3364–3372. doi: 10.1128/aem.65.8.3364-3372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecology Letters. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. American Naturalist. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B. Priming in plant-pathogen interactions. Trends in Plant Science. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Cooke RC, Rayner ADM. The Ecology of Saprotrophic Fungi. London: Longman; 1984. [Google Scholar]
- Cooney JM, Lauren DR, Di Menna ME. Impact of competitive fungi on trichothecene production by Fusarium graminearum. Journal of Agriculture and Food Chemistry. 2001;49:522–526. doi: 10.1021/jf0006372. [DOI] [PubMed] [Google Scholar]
- Darwin CR. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- Diamond JM. Assembly of species communities. In: Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. Cambridge: Harvard University Press; 1975. pp. 342–444. [Google Scholar]
- Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Djonovic S, Vargas WA, Kolomiets MV, Horndeski M, Wiest A, Kenerley CM. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiology. 2007;145:875–889. doi: 10.1104/pp.107.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J. The role of plant stresses in development of corn stalk rots. Plant Disease. 1980;64:533–537. [Google Scholar]
- Duffy B, Schouten A, Raaijmakers JM. Pathogen self-defense: mechanisms to counteract microbial antagonism. Annual Review of Phytopathology. 2003;41:501–538. doi: 10.1146/annurev.phyto.41.052002.095606. [DOI] [PubMed] [Google Scholar]
- Elamo P, Helander ML, Saloniemi I, Neuvonen S. Birch family and environmental conditions affect endophytic fungi in leaves. Oecologia. 1999;118:151–156. doi: 10.1007/s004420050713. [DOI] [PubMed] [Google Scholar]
- Elmer WH, Covert SF, O'Donnell K. Investigation of an outbreak of Fusarium foot and fruit rot of pumpkin within the United States. Plant Disease. 2007;91:1142–1146. doi: 10.1094/PDIS-91-9-1142. [DOI] [PubMed] [Google Scholar]
- Fargione J, Brown CS, Tillman D. Community assembly and invasion: an experimental test of neutral versus niche processes. Proceedings of the National Academy of Sciences USA. 2003;100:8916–8920. doi: 10.1073/pnas.1033107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke-Snyder M, Douds DD, Galvez L, Phillips JG, Wagoner P, Drinkwater L, Morton JB. Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in eastern Pennsylvania, USA. Applied Soil Ecology. 2001;16:35–48. [Google Scholar]
- Fravel D, Olivain C, Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytologist. 2003;157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- Gilbert GS, Webb CO. Phylogenetic signal in plant pathogen – host range. Proceedings of the National Academy of Sciences USA. 2007;104:4979–4983. doi: 10.1073/pnas.0607968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AE, Hinton DM, Yates IE, Bacon CW. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Applied and Environmental Microbiology. 2001;67:2973–2981. doi: 10.1128/AEM.67.7.2973-2981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonthier P, Gennaro M, Nicolotti G. Effects of water stress on the endophytic mycota of Quercus robur. Fungal Diversity. 2006;21:69–80. [Google Scholar]
- Hammond BG, Campbell KW, Pilcher CD, Dogooyer TA, Robinson AE, McMillen BL, Spangler SM, et al. Lower fumonisin mycotoxin levels in the grain of Bt corn grown in the United States in 2001–2002. Journal of Agricultural and Food Chemistry. 2004;52:1390–1397. doi: 10.1021/jf030441c. [DOI] [PubMed] [Google Scholar]
- Hoffman MT, Arnold AE. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycological Research. 2008;112:331–344. doi: 10.1016/j.mycres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. New Jersey: Princeton University Press; 2001. [Google Scholar]
- Huckelhoven R. Cell wall – associated mechanisms of disease resistance and susceptibility. Annual Review of Phytopathology. 2007;45:101–127. doi: 10.1146/annurev.phyto.45.062806.094325. [DOI] [PubMed] [Google Scholar]
- Inbar J, Menendez A, Chet I. Hyphal interactions between Trichoderma harzianum and Sclerotinia sclerotiorum and its role in biological control. Soil Biology and Biochemistry. 1996;28:757–763. [Google Scholar]
- Ishida TA, Nara K, Hogetsu T. Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests. New Phytologist. 2007;174:430–440. doi: 10.1111/j.1469-8137.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- Kikot GE, Hours RA, Alconada TM. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. Journal of Basic Microbiology. 2009;49:231–241. doi: 10.1002/jobm.200800231. [DOI] [PubMed] [Google Scholar]
- Knogge W. Fungal infection of plants. Plant Cell. 1996;8:1711–1722. doi: 10.1105/tpc.8.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE. Enzymatic penetration of the plant cuticle by fungal pathogens. Annual Review of Phytopathology. 1985;23:223–250. [Google Scholar]
- Krogh SS, Mensz SJM, Nielsen ST, Mortensen AG, Christophersen C, Fomsgaard IS. Fate of benzoxazinone allelochemicals in soil after incorporation of wheat and rye sprouts. Journal of Agriculture and Food Chemistry. 2006;54:1064–1074. doi: 10.1021/jf051147i. [DOI] [PubMed] [Google Scholar]
- Lackner G, Partida-Martinez LP, Hertweck C. Endofungal bacteria as producers of mycotoxins. Trends in Microbiology. 2009;17:570–576. doi: 10.1016/j.tim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lawrey JD. Chemical interactions between two lichen-degrading fungi. Journal of Chemical Ecology. 2000;26:1821–1831. [Google Scholar]
- Mandeel Q, Baker R. Mechanisms involved in biological control of Fusarium wilt of cucumber with strains of nonpathogenic Fusarium oxysporum. Phytopathology. 1991;81:462–469. [Google Scholar]
- Manici LM, Caputo F. Fungal community diversity and soil health in intensive potato cropping systems of the east Po valley, northern Italy. Annals of Applied Biology. 2009;155:245–258. [Google Scholar]
- Marasas WFO. Discovery and occurrence of the fumonisins: a historical perspective. Environmental Health Perspectives. 2001;109(Suppl. 2):239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Meijer G, Leuchtmann A. The effects of genetic and environmental factors on disease expression (stroma formation) and plant growth in Brachypodium sylvaticum infected by Epichloe sylvatica. Oikos. 2000;91:446–458. [Google Scholar]
- Minerdi D, Bossi S, Gullino ML, Garibaldi A. Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environmental Microbiology. 2009;11:844–854. doi: 10.1111/j.1462-2920.2008.01805.x. [DOI] [PubMed] [Google Scholar]
- Minerdi D, Moretti M, Gilardi G, Barberio C, Gullino ML, Garibaldi A. Bacterial ectosymbionts and virulence silencing in a Fusarium oxysporum strain. Environmental Microbiology. 2008;10:1725–1741. doi: 10.1111/j.1462-2920.2008.01594.x. [DOI] [PubMed] [Google Scholar]
- Munkvold GP, Desjardins AE. Fumonisins in maize, can we reduce their occurrence? Plant Disease. 1997;81:556–565. doi: 10.1094/PDIS.1997.81.6.556. [DOI] [PubMed] [Google Scholar]
- Niemeyer HM, Perez FJ. Potential of hydroxamic acids in the control of cereal pests, diseases, and weeds. In: Inderjit, Daksini KMM, Einhellig FA, editors. Allelopathy: Organisms, Processes and Applications. American Chemical Society, Washington DC, USA: ACS Symposium Series 582; 1995. pp. 260–270. [Google Scholar]
- Nuss DL. Hypovirulence: mycoviruses at the fungal-plant interface. Nature Reviews Microbiology. 2005;8:632–642. doi: 10.1038/nrmicro1206. [DOI] [PubMed] [Google Scholar]
- Olivain C, Alabouvette C. Colonization of tomato root by a non-pathogenic strain of Fusarium oxysporum. New Phytologist. 1997;137:481–494. doi: 10.1046/j.1469-8137.1997.00855.x. [DOI] [PubMed] [Google Scholar]
- Olivain C, Alabouvette C. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytologist. 1999;141:497–510. doi: 10.1046/j.1469-8137.1997.00855.x. [DOI] [PubMed] [Google Scholar]
- Osbourn AE. Antimicrobial phytoprotectants and fungal pathogens: a commentary. Fungal Genetics and Biology. 1999;26:163–168. doi: 10.1006/fgbi.1999.1133. [DOI] [PubMed] [Google Scholar]
- Pan JJ, May G. Fungal-fungal associations affect the assembly of endophyte communities in maize (Zea mays. Microbial Ecology. 2009;58:668–678. doi: 10.1007/s00248-009-9543-7. [DOI] [PubMed] [Google Scholar]
- Pan JJ, Baumgarten A, May G. Effects of host plant environment and Ustilago maydis infection on the fungal endophyte community of maize (Zea mays. New Phytologist. 2008;178:147–156. doi: 10.1111/j.1469-8137.2007.02350.x. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–888. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez LP, Groth I, Schmitt I, Richter W, Roth M, Hertweck C. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant pathogenic fungus Rhizopus microsporus. International Journal of Systematic and Evolutionary Microbiology. 2007;57:2583–2590. doi: 10.1099/ijs.0.64660-0. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Rice ME, Obrycki JJ, Lewis LC. Field and laboratory evaluations of transgenic Bacillus thuringiensis corn on secondary Lepodopteran pests (Lepidoptera: Noctuidae) Journal of Economic Entomology. 1997;90:669–678. [Google Scholar]
- Pinto LSRC, Azevedo JL, Pereira JO, Vieira MLC, Labate CA. Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytologist. 2000;147:609–615. doi: 10.1046/j.1469-8137.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- Power AG, Mitchell CE. Pathogen spillover in disease epidemics. American Naturalist. 2004;164:S79–S89. doi: 10.1086/424610. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytologist. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Rousseau A, Benhamou N, Chet I, Piche Y. Mycoparasitism of the extrametrical phase of Glomus intraradices by Trichoderma harzianum. Phytopathology. 1996;86:434–443. [Google Scholar]
- Saunders M, Kohn LM. Host-synthesized secondary compounds influence the in vitro interactions between fungal endophytes of maize. Applied and Environmental Microbiology. 2008;74:136–142. doi: 10.1128/AEM.01538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders M, Kohn LM. Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytologist. 2009;182:229–238. doi: 10.1111/j.1469-8137.2008.02746.x. [DOI] [PubMed] [Google Scholar]
- Schulz B, Boyle C. The endophytic continuum. Mycological Research. 2005;109:661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- Schulz B, Boyle C, Draeger S, Rommert A-K, Krohn K. Endophytic fungi: a source of biologically active secondary metabolites. Mycological Research. 2002;106:996–1004. [Google Scholar]
- Seghers D, Wittebolle L, Top EM, Verstraete W, Siciliano SD. Impact of agricultural practices on the Zea mays L. endophytic community. Applied and Environmental Microbiology. 2004;70:1475–1482. doi: 10.1128/AEM.70.3.1475-1482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley B, Vile D, Garnier E. From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science. 2006;314:812–814. doi: 10.1126/science.1131344. [DOI] [PubMed] [Google Scholar]
- Sivan A, Chet I. Integrated control of Fusarium crown and root rot of tomato with Trichoderma harzianum in combination with methyl bromide or soil solarization. Crop Protection. 1993;12:380–386. [Google Scholar]
- Sobek EA, Munkvold GP. European corn borer larvae as vectors of Fusarium moniliforme, causing kernel rot and symptomless infection of maize kernels. Journal of Economic Entomology. 1999;92:503–509. [Google Scholar]
- Steinebrunner F, Schiestl FP, Leuchtmann A. Ecological role of volatiles produced by Epichloe: differences in antifungal toxicity. FEMS Microbiology and Ecology. 2008;64:307–316. doi: 10.1111/j.1574-6941.2008.00452.x. [DOI] [PubMed] [Google Scholar]
- Stinson AM, Zidack NK, Strobel GA, Jacobsen BJ. Mycofumigation with Muscodor albus and Muscodor roseus for control of seedling diseases of sugar beet and Verticillium wilt of eggplant. Plant Disease. 2003;87:1349–1354. doi: 10.1094/PDIS.2003.87.11.1349. [DOI] [PubMed] [Google Scholar]
- Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, et al. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biology. 2006;4:727–731. doi: 10.1371/journal.pbio.0040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JK, Bacon CW, White JF. An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF, editors. Microbial Endophytes. New York: Marcel Dekker; 2000. pp. 3–30. [Google Scholar]
- Strobel GA, Spang S, Kluck K, Hess WM, Sears J, Livinghouse T. Synergism among volatile organic compounds resulting in increased antibiosis in Oidium sp. FEMS Microbiology Letters. 2008;283:140–145. doi: 10.1111/j.1574-6968.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan TS, Murali TS, Venkatesan G. Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Canadian Journal of Botany. 2002;80:818–826. [Google Scholar]
- Terry LA, Joyce DC. Elicitors of induced resistance in postharvest horticultural crops: a brief review. Postharvest Biology and Technology. 2004;32:1–13. [Google Scholar]
- Tiunov AV, Scheu S. Facilitative interactions rather than resource partitioning drive diversity-functioning relationships in laboratory fungal communities. Ecology Letters. 2005;8:618–625. [Google Scholar]
- Torto-Alalibo T, Meng SW, Dean RA. Infection strategies of filamentous microbes described with the gene ontology. Trends in Microbiology. 2009;17:320–327. doi: 10.1016/j.tim.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Trillas MI, Segarra G. Interactions between nonpathogenic fungi and plants. In: Van Loon LC, editor. Plant Innate Immunity. London: Academic Press Ltd., Elsevier Science Ltd; 2009. pp. 321–359. [Google Scholar]
- Ueno Y, Iijima K, Wang SD, Sugiura Y, Sekijima M, Tanaka T, Chen C, et al. Fumonisins as a possible contributory risk factor for primary liver cancer: a 3-year study of corn harvested in Haimen, China, by HPLC and ELISA. Food Chemistry and Toxicology. 1997;35:1143–1150. doi: 10.1016/s0278-6915(97)00113-0. [DOI] [PubMed] [Google Scholar]
- Vajna L. Hyperparasitic Stagonospora sp. on Botryosphaeria stevensii. Forest Pathology. 2003;33:375–382. [Google Scholar]
- VanEtten H, Temporini E, Wasmann C. Phytoalexin (and phytoanticipin) tolerance as a virulence trait: why is it not required by all pathogens? Physiological and Molecular Plant Pathology. 2001;59:83–93. [Google Scholar]
- Ward TJ, Clear RM, Rooney AP, O'Donnell K, Gaba D, Patrick S, Starkey DE, et al. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genetics and Biology. 2008;45:473–484. doi: 10.1016/j.fgb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Weiher E, Keddy PA. Ecological Assembly Rules: Perspectives, Advances, Retreats. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Wilberforce EM, Boddy L, Griffiths R, Griffith GW. Agricultural management affects communities of culturable root-endophytic fungi in temperate grasslands. Soil Biology and Biochemistry. 2003;35:1143–1154. [Google Scholar]
- Wolfe BE, Rodgers VL, Stinson KA, Pringle A. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. Journal of Ecology. 2008;96:777–783. [Google Scholar]
- Wu F, Miller JD, Casman EA. The economic impact of Bt corn resulting from mycotoxin reduction. Journal of Toxicology – Toxin Reviews. 2004;23:397–424. [Google Scholar]


