Abstract
The root systems of most agronomic crops are colonized by diverse assemblages of arbuscular mycorrhizal fungi (AMF), varying in the functional benefits (e.g. nutrient transfer, pathogen protection, water uptake) provided to hosts. Little is known about the evolutionary processes that shape the composition of these fungal assemblages, nor is it known whether more diverse assemblages are beneficial to crop productivity. In this review we aim to identify the evolutionary selection pressures that shape AMF diversity in agricultural systems and explore whether promotion of AMF diversity can convincingly be linked to increases in agricultural productivity and/or sustainability. We then ask whether farmers can (and should) actively modify evolutionary selection pressures to increase AMF functioning. We focus on three agriculturally imposed selection regimes: tillage, fertilization, and continuous monoculture. We find that the uniform nature of these practices strongly selects for dominance of few AMF species. These species exhibit predictable, generally non-beneficial traits, namely heavy investment in reproduction at the expense of nutrient scavenging and transfer processes that are beneficial for hosts. A number of focus-points are given based on empirical and theoretical evidence that could be utilized to slow down negative selection pressures on AMF functioning, therein increasing crop benefit.
Keywords: agriculture, cooperation, mutualism, punishment, selection pressures
Introduction
Interest in the functional role of biodiversity has burgeoned in recent years (Cardinale et al. 2007), including its potential to enhance the sustainability and output of agricultural systems (Tilman et al. 1996; Moonen and Bàrberi 2008). This interest stems from the often assumed, and sometimes demonstrated (Schlapfer and Schmid 1999), cause and effect relationship between particular components of biodiversity and long-term agricultural productivity. Biodiversity of organism groups such as decomposers, predators, pollinators, etc. are thought to increase the provision of specific agricultural services by providing an array of functionally complementary species. Such complementarity may raise agricultural productivity. The mechanisms are diverse, but in the broadest terms, biodiversity may act in an ‘insurance’ role, buffering systems against stresses or losses, while also increasing the multi-functionality of a system (Hector and Bagchi 2007), known as the ‘niche differentiation effect’ (Tilman 1999; Ptacnik et al. 2008; Marquard et al. 2009).
Many of the problems encountered in high-input global agriculture (e.g. nutrient runoff, pests, weeds and erosion) are among those that a diverse ecosystem is predicted to counteract. In particular, the functional role of soil microbial diversity in agroecosystems has received much attention to date. Higher nutrient use efficiency, increased soil aggregate stability and respiration, improved organic matter formation and increased water regulation, are among the soil-related processes that microbially-diverse systems are hypothesized to promote (Mäder et al. 2002; Brussaard et al. 2007).
Despite numerous ecologically-focused studies on the role of microbial diversity in agroecosytem function (Shennan 2008; Toljander et al. 2008), surprisingly little is known about: (i) the evolutionary selection pressures that promote or diminish microbial biodiversity in agricultural systems, (ii) whether promotion of microbial biodiversity can convincingly be linked to increases in agricultural productivity and/or sustainability (see e.g. Martini et al. 2004), and (iii) whether farmers can (and should) actively modify management practices to manipulate evolutionary selection pressures for increased service provisioning from soil microbes.
Here, we examine these questions by focusing on one critical group of soil microbes, abundant in both agricultural and natural ecosystems: arbuscular mycorrhizal fungi (AMF). In this 450-million-year-old symbiosis, the mycorrhizal fungal partner forms an obligate symbiosis with its host plant, exchanging nutrients from the soil for carbon from the host. This interaction is arguably the world's most abundant symbiosis, responsible for massive amounts of nutrient transfer globally (van der Heijden et al. 2008). In agricultural systems, arbuscular mycorrhizae form associations with almost all important crops (maize, wheat, soybean, but not: cabbage, mustard and beet), and are therefore an intricate component of the above- as well as the belowground ecosystem. Increasingly, the role of AMF in pathogen suppression (Lendzemo et al. 2005), pollination enhancement (Cahill et al. 2008), herbivore protection (Gange and West 1994; Bennett et al. 2009) and improved water relations are being recognized (Augé 2001; Wilson et al. 2009).
Diversity of AMF: How diverse is diverse?
The number of species currently recognized in the phylum of Glomeromycota (i.e. AMF, Schussler et al. 2001) is about 200 (Redecker et al. 2000). Recent evidence, however, suggests the number of species may actually be an order of magnitude higher (Vandenkoornhuyse et al. 2002). This number of species is still surprisingly low compared to other groups of fungi (e.g. Basidiomycota, Ascomycota; James et al. 2006). The relatively low number of species may reflect the fact that nearly all AMF species are compatible with almost all mycotrophic plant species (Smith and Read 1997), and thus the absence of speciation through host-specialization. However, a second explanation for low species number is a technical one: a bias towards easily culturable AMF species. Indeed, taxonomic delineation of AMF is a Herculean task. Current taxonomy is based on morphological characters of asexual resting spores. While this technique has revealed relatively low numbers of AMF species compared to other taxonomic groups, new molecular techniques applied at the community level are revealing unexpected high diversity of AMF in colonized roots (e.g. Vandenkoornhuyse et al. 2003; Santos-Gonzalez et al.2007; Öpik et al. 2009). This apparent discrepancy may be related to the long asexual evolution of AMF. A long asexual evolution can lead to substantial genetic diversity within single morphologically-recognized species (Croll et al. 2008; Rosendahl 2008; Rosendahl and Matzen 2008; Stockinger et al. 2009), potentially maintained by diversifying selection (i.e. selection for polymorphism at the population level in a heterogeneous environment in time and/or space; Corradi et al. 2009). Further, because AMF lack most forms of septation, multiple nuclei can be transmitted through sporulation with no distinction between soma and germ line (Jany and Pawlowska 2010). Therefore, there is variation within a single germline and life-history mutations that are transmitted to vegetative offspring are potentially evolutionarily important (Ehinger et al. 2009).
Increasingly, research is revealing more host specialization in AMF than had been previously recognized (Haas and Krikun 1985; Smith et al. 2000; van der Heijden and Scheublin 2007). This is indicated by the occurrence of non-random host-AMF assemblages (Vandenkoornhuyse et al. 2002; Johnson et al. 2004; Scheublin et al. 2004; Öpik et al. 2009) – we now know that AMF show significant preferences to different host plant species and vice versa (Sanders 2003). The trend for specificity even extends to different genotypes within a single AMF species (Croll et al. 2008). This means AMF genotypes differentially affect – as determined by plant biomass production and nutrient uptake in comparison to non-mycorrhizal controls – their host plants, in both positive (Munkvold et al. 2004; Koch et al. 2006) and negative ways (Koch et al. 2006). For instance, although plants may consistently experience increased phosphorus uptake in response to AMF, actual plant growth responses are not consistently positive (Sudova 2009). The interplay of biotic and abiotic conditions (e.g. host density, photosynthetic levels and available nutrients) will strongly determine the degree of benefits conferred to the host (Fitter 1991; Jones and Smith 2004).
From an applied point of view it is diversity of functional traits, more than species diversity (which may not coincide; Knapp et al. 2008; Prinzing et al. 2008), that will be of interest to agricultural management (Gamper et al. 2010). Do AMF differ functionally and how might this be important for crop plants? For example, differences in the average distance forged to nutrient sources by the fungal hyphae (Smith et al. 2000; Jansa et al. 2005), and species’ abilities to exploit nutrient patches (Cavagnaro et al. 2005) have been found, with species varying in their inorganic versus organic nitrogen uptake (Leigh et al. 2009). Other agriculturally important functions in which AMF species have been found to differ include the ability to stabilize soil aggregates (Wu et al. 2008), and their capacity to mediate water uptake (Marulanda et al. 2003).
It has been suggested that these types of functional traits, for instance the ability to protect host roots against pathogens (Fig. 1), are phylogenetically conserved (Maherali and Klironomos 2007; Powell et al. 2009). This increased protection against pathogen infection might explain why plants maintain interactions with particular AMF families, even when others may provide greater phosphorus (P) uptake benefits (Powell et al. 2009). Another class of functional traits, carbon allocation strategy, differs greatly between fungal species. Amount of carbon extracted from host (Pearson and Jakobsen 1993), the species’ sporulation investment (Oehl et al. 2004; Violi et al. 2007), and the allocation of host carbon to storage versus nutrient uptake (van Aarle and Olsson 2003), can influence the overall benefits received by the crop host over its lifetime.
Figure 1.
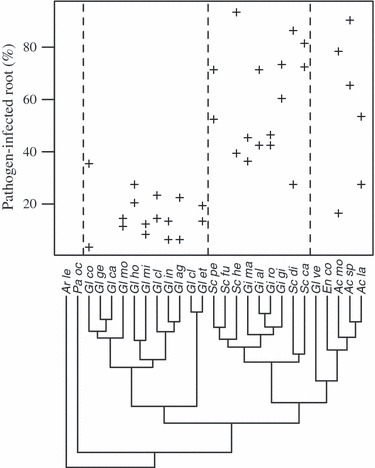
Example of a functional trait (pathogen protection) as it relates to AMF phylogeny. Percentage of roots colonized by soil pathogens, either fusarium oxysporum or pythium sp., (y-axis) is presented as a function of AMF species (x-axis) grown on Plantago lanceolata in a greenhouse experiment. Representatives of AMF families are divided with dashed lines (families represented by values: Gl = Glomeraceae; Sc, Gi = Gigasporaceae, Ac=Acaulosporaceae; AMF species where values are missing have not been tested). The trait of pathogen protection is relatively conserved within, but differs among families. Figure taken from Powell et al. (2009).
Complementarity of AMF functional groups
If AMF differ widely in their functional traits, then agricultural management may aim to utilize the selection pressures that best optimize AMF species mixtures. This would theoretically increase the multi-functionality of the system. It has been proposed that AMF species complement each other when simultaneously colonizing a root system by occupying different niches (Koide 2000; Maherali and Klironomos 2007). What do we know about complementarity and how does increasing AMF biodiversity affect the productivity of plant communities?
A widely cited diversity-productivity AMF experiment suggested that increasing the number of AMF species in simulated microcosms increased the biomass of the plant community tested (van der Heijden et al. 1998). This experiment involved increasing AMF richness from 1 up to 14 species and measuring AMF density, plant biomass and P uptake by a plant community consisting of 15 species. The authors proposed the biomass increase was the result of enhanced nutrient scavenging by the AMF community that, as a whole, exhibited functional complementarity in the utilization of a range of nutrient niches. Since then, multiple experiments have found increased P uptake and growth in plant species when they were simultaneously inoculated with mixtures of AMF species (Gustafson and Casper 2006; Jansa et al. 2008; Hoeksema et al. 2010), especially when the mixtures were composed of phylogenetically overdispersed AMF species (Koide 2000; Maherali and Klironomos 2007). A mechanistic explanation is provided in an experiment by Smith et al. (2000) in which one of two AMF species was grown on Medic (Medicago truncatula) in the presence of a heavy P isotope placed at a distance from the roots. Both AMF species increased P uptake, but one species increased the proportion of the heavy P isotope significantly more, indicating the AMF species differed strongly in spatial P-uptake patterns.
In theory, the complementarity phenomenon holds promise for agricultural soil management. Potentially, suites of functional groups may be co-inoculated to stimulate crop nutrient uptake. However, often particular combinations of AMF do no better (Farmer et al. 2007) or even worse (Jansa et al. 2008) than the best yielding AMF singly. For instance, in an experiment comparing seven inbred lines of maize grown with six AMF species, Mickelson and Kaeppler (2005) found that maize biomass was greatest when inoculated singly with one AMF species, and growth was actually depressed when maize was simultaneously inoculated with all six species.
On an evolutionary timescale, increasing the numbers of AMF strains can create a ‘tragedy of the commons’ (Hardin 1968). The tragedy is that less-mutualistic strains potentially share in the collective benefits (e.g. host assimilates), while paying fewer costs (e.g. energy expended to transfer nutrients; Kiers and Denison 2008). The costs for host plants of supporting symbionts can be high: the formation and maintenance of mycorrhizal structures can consume between 5% and 20% of the host's photosynthetically fixed carbon (Douds et al. 2000). Similarly, a number of energy-consuming steps are necessary to supply P to a plant host (e.g., P uptake by fungus, conversion to polyphosphate, transport and efflux to the plant; Ezawa et al. 2004; Viereck et al. 2004). As the cost of supplying these benefits to the crop host increases, cooperation becomes a less favourable strategy (Schwartz and Hoeksema 1998; Hoeksema and Schwartz 2003). When a host plant is colonized by multiple symbionts, theory predicts that ‘free-riding’ AMF strains can spread at the expense of more mutualistic strains (Denison et al. 2003), perhaps by ‘hiding’ among more beneficial strains (Bever et al. 2009). Indeed, in a microbial system (where cooperation was intra- not interspecific as in the mycorrhizal mutalism), relative fitness of a non-cooperative strategy was greater when non-cooperators were rare (see Ross-Gillespie et al. 2007), suggesting that hiding among mutualist strains may be a successful evolutionary strategy.
Costs of AMF ‘free-riders’ may be particularly acute in agricultural situations where increased nutrients can skew the benefit:cost ratio in favour of the less beneficial strains (Johnson 1993; Kiers et al. 2002; Egerton-Warburton et al. 2007; Johnson et al. 2008). In situations where nutrient availability is high, strategies to maximize nutrient scavenging become less crucial. Being colonized by a consortium of AMF strains may likewise become less crucial. In a recent experiment involving another type of rhizosphere mutualists (nitrogen (N)-fixing Rhizobium symbionts), it was found that in a high N environment, plants had the lowest fitness when inoculated with a mixture of strains than when inoculated with the worse single strain alone (Heath and Tiffin 2007). The authors suggested that when there is less to be gained from symbiosis, such as for plants under high nutrient conditions, it is more costly to harbour multiple strains than just a single one. This higher cost has been hypothesized to arise either directly (e.g. via the need to control resource allocation to multiple strains instead of one, as suggested by Heath and Tiffin 2007), or indirectly (e.g. via antagonism between the strains; Janouskova et al. 2009) potentially reducing their effectiveness over ecological and evolutionary time scales. More empirical data are needed to understand how interactions among symbionts are altered by resource availability.
In contrast, it could also be argued that competition among strains, at least in theory, has the potential to increase the evolutionary persistence of the mutualism (Ferriere et al. 2002) by imposing variation that reinforces host choice. Although partnering with a less-mutualistic strain is unlikely to directly benefit the host, colonization by several symbionts may allow the host to select the most beneficial strains among several competitors. If plant hosts have the ability to evaluate strains and preferentially supply more resources to more beneficial partners, as has been shown for some plants and their mutualists (Kiers et al. 2003; Kiers and van der Heijden 2006; Simms et al. 2006; Bever et al. 2009), then increasing the functional range of strains colonizing a host could be an advantage rather than a cost. In a theoretical study Ferriere et al. (2007) show that competition on one side of the symbiosis is actually critical for the evolutionary persistence of a (one-sided) obligate mutualism, and therefore plant benefit. Does this hold true for agricultural systems? In the next section, we discuss the unique selection pressures of agricultural systems and how these differences play a role in determining the functional benefits of AMF diversity.
Selection pressures in agricultural versus natural systems and their role in AMF diversity
Agricultural systems can be exposed to incredibly intense selection pressures over very short time scales. Microbial strategies or traits not typically found in natural habitats, can arise under intensive agricultural management. For example, large acreages of wheat, rye and barley likely facilitated the recombination of two distinct stem rust pathogens of wheat and rye, leading to the emergence of a new pest highly pathogenic to barley (Burdon and Thrall 2008). For AMF, sporulation strategy is an example of a trait strongly affected by agriculture; AMF isolated from intensively (no crop-rotation, high input) managed agricultural fields exhibit earlier onset of sporulation compared to the same species from extensively managed agricultural systems and grasslands (Oehl et al. 2003), suggesting strong selection for rapid reproduction. Similarly, AMF in cropping systems are required to endure long fallow periods in which hosts are absent, selecting for strong seasonality in the activity of AMF (Daniell et al. 2001; Hijri et al. 2006).
In understanding how agricultural selective pressures shape AMF communities, the concept of r-selection (Pianka 1970) as a framework for studying life-history evolution, particularly for microbial ecology (Fierer et al. 2007; Sykorova et al. 2007), can be useful. R-selection favours organisms adapted to environments requiring fast, copious reproduction and dispersal. Because intensive agricultural regimes can create unstable and unpredictable environmental conditions characterized by disruptive tillage regimes, high nutrient fluctuations, and large-scale removal of annual host plants, there is less advantage in adaptations that allow AMF to compete for limited resources. Instead, rapidly changing environments are likely to favour AMF employing r-strategies, such as the ability to quickly reproduce. This means less functional complementarity of AMF assemblages in agricultural systems than in natural, nutrient-limited ecosystems (Fig. 2). Therefore the positive ‘niche-partitioning effect’ of AMF (e.g. different AMF species occupying different and potentially complimentary nutrient niches in space and time; Reynolds et al. 2003), identified in numerous greenhouse experiments (Lekberg et al. 2007; Sikes et al. 2009) is not likely to be as prevalent under intensive field management dominated by r-selected microbial mutualists.
Figure 2.
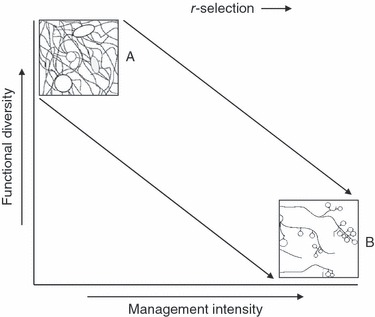
Conceptual relationship between agricultural management intensity and AMF functional diversity. Through strong r-selection imposed by intensive agricultural practices (e.g. high nutrient input, low crop diversity, and high tillage frequency) diverse AMF functional traits may be lost. As management intensity increases, AMF are predicted to shift from (A) functionally diverse communities with extensive hyphal mattes and large spores to (B) AMF communities with little investment in mycelium and fast production of numerous spores.
Agricultural management practices will differ in strength in which they select for or against multi-functionality. Below we ask, what are the characteristic selection pressures of agricultural systems that promote or diminish AMF biodiversity, and can these selection pressures be modified in ways that maximize the benefits of AMF to farming systems? Here we consider four regimes specific to agricultural management: (i) tillage, (ii) nutrient input, (iii) crop rotations, and (iv) crop diversity schemes.
Tillage
Tillage is arguably the most unique and strongest agricultural selection pressure for mycorrhizal symbionts. Although tillage practices can vary in intensity from ploughing (lower-intensity) to ripping (higher-intensity), most represent some form of intense disruption. In contrast, except for instances involving uprooted trees or movement of soil-burrowing animals, AMF are generally not exposed to soil disturbances of this scale. Their extensive hyphal matts, which can reach lengths up to tens of meters in one gram soil (Sanders et al. 1998), are the foundation for their nutrient transfer capability and are strongly correlated with, and responsible for, plant biomass production (Powell et al. 2009). Repeated destruction of this integral hyphal network in agricultural systems has the potential to radically alter the evolutionary trajectory of the organism.
It is well-established that tillage decreases mycorrhizal diversity at the family level (Jansa et al. 2002), and can lead to competitive dominance by only a few strains (e.g. Menendez et al. 2001). AMF species have been found to differ in their tolerance to hyphal disruption (de la Providencia et al. 2005, 2007) and ecological shifts in AMF community composition are often noted when high and low tillage regimes are compared (Boddington and Dodd 2000; Jansa et al. 2002, 2003; Castillo et al. 2006; Alguacil et al. 2008). Although AMF can use spores to infect new plants, there are species of AMF that can colonize through fragmented hyphal networks and infected rootpieces (Biermann and Linderman 1983); intense tillage regimes have been hypothesized to favour such species (Hamel 1996). AMF in the Gigasporaceae family (recently split into five distinct families; Oehl et al. 2008) do not utilize infected root pieces or hyphae fragments to colonize hosts, and a decrease of species in this family upon tillage is often reported (Daniell et al. 2001; Jansa et al. 2003; Castillo et al. 2006). When hyphal networks are disturbed, AMF in the Gigasporaceae repair broken linkages by reconnecting the broken ends (de la Providencia et al. 2007) suggesting the family is not completely maladapted to disturbance. Under natural conditions, reconnecting broken hyphal ends and their reliance on energy-rich spores may represent optimal proliferation strategies. Indeed, members of this family are often found in sand dunes (Cordoba et al. 2001; Kowalchuk et al. 2002), a habitat characterized by high natural disturbance. However, even higher levels of disturbances such as high tillage regimes and destructive harvesting, may decrease the success of these strategies for the Gigasporaceae. AMF in the Gigasporaceae have been shown to functionally complement other AMF families (e.g. Glomeraceae, Acaulosporaceae) in the P-nutrition of plants due to their higher soil-hyphal density (Maherali and Klironomos 2007), so their elimination from agricultural systems represents a potential loss of useful functional diversity for crop hosts.
In contrast, AMF in the Glomeraceae, a very cosmopolitan AMF family with species spreading across global agricultural systems (Rosendahl et al. 2009), are able to randomly connect hyphae in close proximity after disruption. This strategy is more energy intensive, and could represent a larger carbon cost for the host, but is arguably an optimal strategy for rapid recovery of the symbiont under large disturbance regimes.
Tillage will also modify the evolutionary trajectory of AMF by decreasing the spatial structure so that local fungal communities are no longer isolated (Rosendahl and Matzen 2008). In natural ecosystems, fungal dispersal may be limited to distances reached by hyphal extension. Spatial structure is likely to enhance the functional diversity of fungal communities by creating patches harbouring distinct populations thereby increasing total diversity of a given soil volume. Extreme spatial structuring is likewise predicted (Kiers and van der Heijden 2006) and shown (Bever et al. 2009) to be positive for the evolutionary persistence of mycorrhizal interactions as it increases the relatedness among strains within a host root, and among fungal conspecifics in nearby soil. Theoretically no-till systems would increase relatedness among strains by decreasing dispersal distances. This will increase the probability that benefits of cooperation will be shared with related kin (Hamilton 1964). This could promote the evolution of mutualistic interactions by isolating less mutualistic symbiont patches from more mutualistic ones (Wilson et al. 2003; Lion and van Baalen 2008), facilitating the ability of hosts to direct resources to patches of high-quality mutualists (Bever et al. 2009). However, benefits to cooperation may be offset by the increased competition generated between kin in patchy, unmixed populations (Griffin et al. 2004). In the case of mycorrhizal fungi, this means that competition for local resources among relatives could completely negate any potential kin-selection advantage (West et al. 2002).
Increasingly, research from natural systems is revealing high local diversity of AMF (e.g. within a single root; Vandenkoornhuyse et al. 2002) which testifies to relatively low spatial structure and weak genetic differentiation found among field populations (Croll et al. 2008). Studies are now needed to asses the relationship between genetic structuring of fungal communities and the effectiveness and functional diversity of these communities, as they relate to tillage regime. Studies should include an examination of different tillage types (e.g. mouldboard or chisel-disk plowing; Galvez et al. 2001), as these may have a different influence on the (intraspecific) diversity of AMF communities (Borstler et al. 2010). Although spatial structuring has been the subject of much theoretical work (West et al. 2001; Lion and van Baalen 2008), different tillage regimes can provide ideal empirical models for studying the consequences of how spatial structuring modifies competitive interactions and the evolution of functional diversity in microbial communities.
Nutrient input
Prevailing theory argues that positive species interactions are more likely to emerge and be maintained in poor-quality environments (Hochberg et al. 2000; Thrall et al. 2007). In rhizosphere mutualisms, this is because nutrient enrichment has the potential to ameliorate the nutrient limitations that make mutualists beneficial (Johnson 2010). Fertilization can make microbial partners costly, even parasitic (Hoeksema and Schwartz 2003; Ryan et al. 2005). Intensive agriculture is characterized by high N and P inputs. When exposed to high nutrients, host plants may severely decrease or cease resource allocation to their fungal partners, resulting in decreased AMF colonization (Mäder et al. 2000). As host plants reduce resource allocation to their roots, competition for limited carbon resources increases. This is predicted to shift the competitive balance among microbes, favouring more aggressive, antagonistic microbial genotypes in subsequent generations (Kiers et al. 2002; Thrall et al. 2007; Kiers and Denison 2008; Johnson 2010). Such competitive shifts have the potential to alter the evolution of AMF functional traits, for instance increased allocation to reproduction and/or storage structures and away from the hyphal networks (Johnson et al. 1997). One recent study found that AMF investment in storage vesicles increased four-fold in fertilized compared to control plots (Nijjer et al. 2010). These types of strategies will likely favour a subset of AMF that are highly competitive but less beneficial to the host crop.
Indeed, long-term studies monitoring the impact of fertilization on AMF communities have found significant shifts in species composition and negative impacts on mycorrhizal functioning (Thomson et al. 1992; Johnson 1993; Gryndler and Lipavsky 1995; Kahiluoto et al. 2009). Shifts towards reduced resource allocation to extraradical mycelium and arbuscules upon nutrient addition are common (Johnson et al. 2003), but experiments to test whether these are ecological (species replacement), evolutionary (individual genetic changes) or represent phenotypic plasticity of existing symbionts are scarce. In one study that excluded the plasticity effect, Johnson (1993) found roots inoculated with mycorrhizae from long-term N and P fertilized plots were dominated by vesicles (resource storage structures, suggested to be indicative of more parasitic strategy) rather than arbuscules (nutrient transfer structures). In another study, Johnson et al. (2010) found significant plant-AMF co-adaptation to local nutrient conditions, also showing a genetic rather then a phenotypic effect. Whether shifts stem from a replacement of AMF species or evolutionary changes within a species is not known, but studies are needed as this has great implications for the maintenance of AMF functioning.
Addition of N fertilizer can likewise alter functional diversity of AMF. High N-fertilization in P-rich soils was shown to decrease AMF community richness and diversity (Egerton-Warburton et al. 2007). Particular AMF taxa have been found to be more sensitive than others to heavy application of mineral N (Oehl et al. 2004; Toljander et al. 2008). In one long-term study, the addition of calcium nitrate was correlated with massive colonization of Glomus intraradices in maize roots (Toljander et al. 2008), a species previously reported as being ‘nitrophilic’ (Scheublin et al. 2004; Jumpponen et al. 2005). G. intraradices may be affiliated more generally with nutrient enrichment as the abundance of the species also strongly increased following 8 years of N and P additions, compared to non-fertilized controls (Johnson 1993). The dominance of particular species could indicate competitive exclusion of others, explaining negative correlations between, for example, soil mineral nitrogen and the number of AMF sequence groups detected (Santos et al. 2006). Whether AMF functioning was reduced as well, was not determined.
Understanding how high nutrient regimes affect selection for functional diversity in AMF communities is made more complicated because AMF harbour two levels of genetic diversity on which selection can act, among individuals and within individuals. Within individuals selection can occur because different nuclei are present in single AMF isolates (Hijri and Sanders 2005). Ehinger et al. (2009) recently studied genetically distinct G. intraradices individuals isolated from the same field. They found that the isolates exhibited different strategies when grown on different host plants and under various phosphorus levels. Most interestingly, they found that strains of the same origin developed a different (composite) genotype under different host or nutrient conditions. This means that abiotic factors, such as phosphate availability, can alter the genotype of an AMF isolate over multiple generations. Among the different nuclei present in a single isolate, some were found to proliferate under a given resource availability, while others disappeared. Similar dynamics have been found by Oliveira et al. (2010) upon cultivation of a Glomus geosporum isolate for one year in two soils with a different pH. They observed that the resulting lineages shared only one third of their genetic markers, and significantly differed in traits such as ability to increase phosphorus concentration in host plants, extraradical mycelium density and spore density when grown under the same conditions. Given that this fast genetic divergence coincides with fitness-related traits (such as spore density; Ehinger et al. 2009; Oliveira et al. 2010), studies are now needed to consider how uniform nutrient conditions will affect selection for greater or less mutualism and whether such conditions erode or increase genetic (and potentially functional) diversity both among and within AMF individuals.
If our goal is to maintain functional diversity of AMF communities to benefit crops (e.g. for other potential benefits such as water uptake or disease protection) despite high nutrient inputs, then research on the evolution of functional diversity of natural symbiont communities may unearth interesting approaches. Recent modelling work suggests that the evolutionary strategies used by plants to physically allocate resources to their (ecto-) mycorrhizal symbionts under different nutrient availabilities will influence the persistence of a functionally diverse symbiont community (Cowden and Peterson 2009). The authors suggest that the carbon allocation strategy of the host plant plays a critical role in maintaining mycorrhizal functional diversity. Of three evolutionary strategies investigated, a selective carbon allocation strategy, in which host plant directs carbon to root tips based on a cost:benefit analysis, was identified as the only strategy that maintained productive, multi-symbiont communities (Cowden and Peterson 2009). However, even when a selective carbon allocation strategy was simulated, high nutrient conditions tended to select against functionally diverse communities.
Whether host plants are physiologically able to selectively allocate carbon to their mycorrhizal mutualists is an important question (Kiers and van der Heijden 2006; Kiers and Denison 2008; Bever et al. 2009), and given that this ability is expected to decrease under high nutrient regimes (West et al. 2002), empirical data to test these theoretical predictions are needed. There is some evidence that legumes bred under high N-regimes may be less effective at controlling carbon distribution to their rhizobial symbionts (Kiers et al. 2007). Research also suggests that closely related citrus cultivars differ in their ability to control C-allocation to AMF, with those cultivars more dependent on AMF being less able to control costly colonization (Graham and Eissenstat 1994). Plant C-allocation traits may prove beneficial attributes to incorporate into breeding programs (Sawers et al. 2008) given that an effective carbon allocation strategy has been named as a key trait in selecting for a multifunctional mycorrhizal community (Cowden and Peterson 2009).
Cropping rotations: diversity in time
In contrast to the evolutionary forces that shape agricultural AMF communities, an agricultural crop in the field may escape evolutionary selection pressures. This is because a plant genotype can be re-planted year after year in an agricultural system, regardless of its actual fitness. Although some genotypes may be abandoned if agronomic performance is poor, a plant's individual fitness will have no effect on the genetic composition of subsequent crops. This can lead to strong asymmetry in the evolution of partners. Buckling and Rainey (2002) studied evolutionary asymmetry by measuring differences in infectivity and resistance of coevolved bacteria and their viral parasites, as compared to infectivity and resistance in their ancestral strains. In a series of infections and isolations, they found that when the latest (coevolved) viral parasites were allowed to infect the initial bacterial strains, the parasites were significantly more virulent.
This asymmetry may not be so different from plant-AMF interactions in agricultural systems. Continuous re-cropping of wheat, a crop not highly dependent on AMF for nutrient acquisition, is an interesting illustration. Although there are reports that wheat crops can benefit from mycorrhizal associations (Manske 1990; Tawaraya 2003), AMF colonization of wheat under field conditions has been documented to result in largely negative growth effects (Ryan and Graham 2002; Ryan et al. 2005). When wheat is re-cropped continuously, these negative AMF effects can begin to resemble parasitism, with increases in AMF colonization leading to decreases in soluble carbohydrates of wheat hosts (Ryan et al. 2005). Although these negative AMF effects could be the result of a straight density effect (e.g. negative effects of high fungal density), another theory is that the continuous cropping of a single host genotype will speed up the evolution of a mycorrhizal symbiont (Kiers et al. 2002). This could allow the symbiont to evolve measures to counter plant strategies to enforce cooperation, perhaps leading to a decrease in symbiont effectiveness over time (Kiers and Denison 2008). Alternatively, if hosts impose effective sanctions against less-mutualistic strains, despite being re-cropped year after year, then continuous monoculture would theoretically provide the strongest possible selection for mutualism.
The combination of continuous re-cropping of a single monoculture host combined with increased dispersal of AMF propagules from intensive tillage regimes has the potential to result in large population increases of a single, dominant AMF strain. Evolutionary research on the population dynamics of clonally reproducing organisms suggests that the larger the population size, the higher the rate of adaptation (De Visser and Rozen 2005; Handel and Rozen 2009). In wheat, to reduce parasitism and reduce mycorrhizal colonization, a non-mycorrhizal Brassica ‘break crop’ can be grown which results in superior growth of the subsequent wheat crop (Ryan and Angus 2003). Interestingly, Brassicas can sometimes be better ‘break crops’ than legumes – which are mycorrhizal (Kirkegaard et al. 2008) – and their ability to clean the ‘mycorrhizal commons’ (e.g. by reducing total populations of mycorrhizal fungi of which all are harmful) might be one explanation. Johnson et al. (1992) found consistent negative correlations between spore abundances of proliferating fungi and the performance of the crop on which they proliferated, providing correlative evidence that crop rotation has the potential to drive AMF communities to be less parasitic.
A parallel, but even more extreme example is the negative effects of Glomus macrocarpum on tobacco, Nicotiana tabacum. This mycorrhizal species can cause Tobacco stunt disease (Modjo and Hendrix 1986). However, by rotating tobacco with a fescue crop, higher tobacco yields were found, coinciding with ecological but not necessarily evolutionary changes in the species composition of the mycorrhizal communities (Hendrix et al. 1992). An et al. (1993) have shown that the proliferation of G. macrocarpum is crop specific, highlighting the importance of choosing an appropriate crop for rotation. Together these lines of evidence support a ‘negative feedback’ hypothesis in which AMF proliferation on a given host is negatively correlated with that host's benefit (Bever 2002b).
Crop rotation may act as a strong selective agent by preventing particular mycorrhizae from dominating the soil profile. However, is the opposite true? Does increasing frequency of rotation actually increase the functional diversity of the community? If so, is frequent rotation to the betterment of the crop host? Many studies have demonstrated the predominantly positive microbe-mediated fertility effects of rotation (e.g. Pypers et al. 2007), and even shown that crop-rotation is related to higher AMF diversity (Oehl et al. 2003; Hijri et al. 2006), and can lead AMF communities in agricultural systems to more closely resemble communities derived from natural sites (Verbruggen et al. 2010). However, it is difficult to demonstrate direct benefits of higher AMF diversity as related to rotation effects. Perhaps the most likely place to see an AMF evolutionary effect of crop rotation is when a long-standing perennial crop, such as a green fertilizer, is rotated into an annual cropping system. Legumes are strongly AMF dependent and the cropping of a perennial legume would eliminate asymmetrical evolution because the plant's fitness/growth of over subsequent growing years could have an effect on the genetic composition of the AMF community.
In a recent publication, Oehl et al. (2009) demonstrated that arable lands with an extensive crop rotation, including a perennial grass-clover mixture, were richer in AMF species than their continuous-monoculture counterparts, and even richer than natural grasslands. This demonstrates the potency of including a mycorrhizal green-manure in rotation as a means to increase AMF richness. What is striking is that many AMF species not detected in continuous monoculture fields, were detected in laboratory microcosms created from these monoculture field soils after 8 months of growth. Apparently, the growth of these slower sporulating genotypes had been strongly depressed, but they were able to increase in abundance again when associated with plants allowed to grow longer than the average crop season. These lines of evidence suggest that including perennial crops in rotation, apart from other benefits (e.g. Ernst and Siri-Prieto 2009), may be an effective strategy to counter genetic erosion and potentially clean the agricultural commons of dominant AMF genotypes.
Polycultures, diversity in space
One unique aspect of the AMF mutualism is that both host and symbiont are able to simultaneously interact with several partners; even a ‘single’ AMF may be connected to a multitude of host plants. This means that in natural communities, AMF will be exposed to selection pressures from several different plant species. Because AMF-plant species associations are non-random (Vandenkoornhuyse et al. 2002; Santos-Gonzalez et al. 2007), differential promotion of AMF strains by different plant species is likely to result in a correlation between the diversity of a plant community and the diversity of its AMF community. Indeed, correlations between plant and AMF diversity have been found (Landis et al. 2004), and lowest aboveground diversity in agricultural systems has been shown to correlate with lowest AMF soil diversity (Öpik et al. 2006), but tests are needed from more systems.
However, not all evidence supports the positive relationship between plant diversity and AMF diversity (e.g. Lovelock and Ewel 2005). For example, plants inoculated with soil from four sources: (i) bare fallow soil, (ii) soil from under non-mycorrhizal plants, (iii) soil from under a monoculture and (iv) soil from under a 12 plant species polyculture, differed strongly in the diversity of colonizing AMF species (Johnson et al. 2004). Surprisingly, bare fallow and non-mycorrhizal crops produced highest diversity followed by the polyculture soil. However, the lowest AMF diversity was derived from the monoculture. This suggests that conditions that strongly favour a particular AMF species or strain (e.g. abundance of a particular host) can lead to lower overall AMF diversity. This may be attributed to host-specific mycorrhizal growth rates (Bever 2002a), with host plants supporting the growth of a few select species.
Host identity likewise plays a clear role in structuring AMF communities. Recent work found unique AMF communities associated with a variety of single plant monocultures, but diversity effects were altered when heterospecific neighbor plants were included in the microcosoms (Hausmann and Hawkes 2009). In an agroforesty context, tree-based intercropping with soybean legumes showed higher AM fungal diversity, as expressed by the Shannon-Wiener indices, compared to typical forest plantation, suggesting that intercropping systems may enhance fungal richness (Chifflot et al. 2009). In an experiment of legume-intercropping, it was shown that AMF could mediate N-transfer to a non-legume (chicory), resulting in a gain between 15% and 77% of the receiver's shoot nitrogen balance (Martensson et al. 1998), and thus providing a clear benefit. This transfer, however, depended strongly on AMF isolate and plant variety combination.
The impracticality of managing polycultures from an agronomic perspective means it is unlikely that farmers will shift to growing diverse cropping systems simply to facilitate a ‘proposed’ increase in AMF functional diversity, not even if direct nutrient benefit are expected. This is especially true if the potential AMF-mediated crop gain from the intercropping is dependent on the presence of a functionally diverse AMF community. However, multiple cropping systems, such as grass-clover mixtures, are routinely used for green manure and live-stock feed. This polyculture combination is interesting to consider because legumes are more dependent on AMF for P-supply (but see Smith et al. 2009) than most other plants (Scheublin et al. 2007), whereas grasses have large, fine root systems reaching great depths (exceeding tilled zone; Canadell et al. 1996). Rooting depth may be an important factor in selecting for functionally diverse AMF communities, as the highest AMF diversity is typically below ploughing depth (Oehl et al. 2005). Therefore this particular polyculture combination may be one route toward selecting for a functionally diverse AMF community. Together with the fact that intercropping with legumes is found to increase stratification of nutrient uptake (Hauggaard-Nielsen and Jensen 2005), this could have the potential to benefit AMF-community at greater depth. In recent years, including less economically valuable crops in rotation to reduce pathogen pressure has gained popularity. Likewise, benefits of multi-cropping may prove to outweigh costs if the practice leads to selection for beneficial AMF traits.
Conclusion
The first two questions addressed in this review were: (i) What are the evolutionary selection pressures that promote or diminish microbial biodiversity in agricultural systems? And (ii) Can promotion of microbial biodiversity be convincingly linked to increases in agricultural productivity and/or sustainability? In regards to (i), studies on AMF have focused on documenting effects of various agricultural management schemes on AMF diversity. Although such studies are essential to understand the genetic hierarchy of AMF community response, the second question can only be answered through a shift of focus to ‘functional diversity’, not just ‘diversity’ of AMF. In theory, there are numerous AMF attributes (pathogen and herbivory protection, alleviation of water stress, tolerance to salinity, pH, toxins, etc.) with the potential to increase agricultural productivity and sustainability. However, we are seeing that current management practices are more likely to favour AMF with attributes less beneficial for crop hosts, such as fast, abundant sporulation and increased carbon acquisition from hosts. A less beneficial AMF community fails to provide optimal functioning (nutrient acquisition and otherwise) and so agricultural practices (e.g. higher fertilization) are required to maintain crop productivity, with the result that these practices continue to degrade the AMF community.
Calling for large-scale changes in management regimes is not practical, especially given that AMF functional attributes, at least at field scale, are still more theoretical than demonstrated. However, relatively small scale changes in agricultural practices may lead to a more functionally complex AMF community, potentially with the benefit of increasing productivity (Fig. 3).
Figure 3.
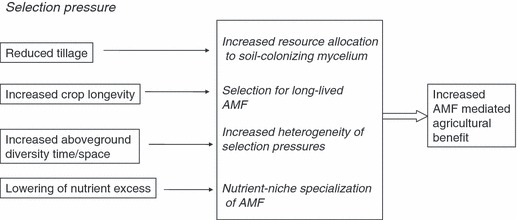
Schematic representation of selective environment imposed by ‘sustainable’ management practices (left) and their influence on the AMF community (middle). Implementation of more sustainable management regimes is predicted to increase the benefit of AMF to agricultural systems by facilitating a re-establishment of AMF with functionally diverse attributes.
The last question we aimed to answer with our review, is also the most urgent: what small-scale changes in management practices (e.g. particular crop rotations) will have large-scale benefits towards increasing the functioning of AMF communities?
One route towards gathering more concrete data on the selection pressures that modify functional traits in AMF is through the use of microcosms (e.g. Boddington and Dodd 2000) inoculated with AMF communities from agricultural fields. Multigenerational experiments in which treatments mimic agricultural selection pressures such as tillage, fertilizer regimes, crop rotations and crop polyculturing may begin to capture how agronomic-like manipulations modify functional traits of AMF communities over time. The small size of the experiments would allow the tracking of genetic diversity (ideally both among and within AMF individuals), as well as functional diversity. Benefits of specific functional attributes could be measured and followed over multiple generations. We could then begin to determine the specific AMF strategies favourable in an agricultural context and ask what selection pressures facilitated their spread in the AMF community. Beneficial strains surviving over several generations of strong agronomic selection pressures could be isolated, propagated and potentially introduced with their host crop into the field. Two major pitfalls in this approach are (i), problems of scaling up from microcosms dynamics to agronomic fields (Oehl et al. 2009), and (ii), the recently highlighted issues of introducing AMF inoculum strains into (albeit managed) ecosystems (Schwartz et al. 2006; Mummey et al. 2009).
From a purely evolutionary point of view, the incredibly high evolvability of AMF strains makes them an interesting model organism for investigations into rates of adaption. Ehinger et al. (2009) found that under laboratory conditions, AMF genetic composition could change within one propagation cycle upon nutrient or host selection pressures. Future research should focus on whether this change is random or directed. If random, local drift processes may occur but the population as a total may still harbour the same genetic information. If directed, however, this could mean rapid evolution of AMF strains with the likely loss (or gain) of valuable functions due to strong agronomic pressures. Ideally, molecular methods will be developed in the near future utilizing gene-expression as a way to approximate mutualistic benefit for specific functional traits (Gamper et al. 2010). These types of tools could prove to be useful for the future management of agroecosystems, ultimately allowing farmers to maximize mutualistic benefit of soil microbes.
Acknowledgments
We want to thank Ford Denison, Gerard Driessen and Matty Berg for critical readings of the manuscript. Suggestions and comments by Nancy C. Johnson and one anonymous reviewer are gratefully acknowledged. This research was supported by the Netherlands Organization for Scientific Research (EV by an ERGO grant 838.06.021 awarded to Marcel van der Heijden and ETK by an NWO ‘Veni’ grant).
Literature cited
- Van Aarle IM, Olsson PA. Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Applied and Environmental Microbiology. 2003;69:6762–6767. doi: 10.1128/AEM.69.11.6762-6767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alguacil MM, Lumini E, Roldan A, Salinas-Garcia JR, Bonfante P, Bianciotto V. The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops. Ecological Applications. 2008;18:527–536. doi: 10.1890/07-0521.1. [DOI] [PubMed] [Google Scholar]
- An ZQ, Guo BZ, Hendrix JW. Mycorrhizal pathogen of tobacco – cropping history and current crop effects on the mycorrhizal fungal community. Crop Protection. 1993;12:527–531. [Google Scholar]
- Augé RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Bennett AE, Bever JD, Bowers MD. Arbuscular mycorrhizal fungal species suppress inducible plant responses and alter defensive strategies following herbivory. Oecologia. 2009;160:771–779. doi: 10.1007/s00442-009-1338-5. [DOI] [PubMed] [Google Scholar]
- Bever JD. Host-specificity of AM fungal population growth rates can generate feedback on plant growth. Plant and Soil. 2002a;244:281–290. [Google Scholar]
- Bever JD. Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002b;269:2595–2601. doi: 10.1098/rspb.2002.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecology Letters. 2009;12:13–21. doi: 10.1111/j.1461-0248.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- Biermann B, Linderman RG. Use of Vesicular Arbuscular Mycorrhizal roots, intraradical vesicles and extraradical vesicles as inoculum. New Phytologist. 1983;95:97–105. [Google Scholar]
- Boddington CL, Dodd JC. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. II. Studies in experimental microcosms. Plant and Soil. 2000;218:145–157. [Google Scholar]
- Borstler B, Thiery O, Sykorova Z, Berner A, Redecker D. Diversity of mitochondrial large subunit rDNA haplotypes of Glomus intraradices in two agricultural field experiments and two semi-natural grasslands. Molecular Ecology. 2010;19:1497–1511. doi: 10.1111/j.1365-294X.2010.04590.x. [DOI] [PubMed] [Google Scholar]
- Brussaard L, De Ruiter PC, Brown GG. Soil biodiversity for agricultural sustainability. Agriculture, Ecosystems & Environment. 2007;121:233–244. [Google Scholar]
- Buckling A, Rainey PB. Antagonistic coevolution between a bacterium and a bacteriophage. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:931–936. doi: 10.1098/rspb.2001.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon JJ, Thrall PH. Pathogen evolution across the agro-ecological interface: implications for disease management. Evolutionary Applications. 2008;1:57–65. doi: 10.1111/j.1752-4571.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill JF, Elle E, Smith GR, Shore BH. Disruption of a belowground mutualism alters interactions between plants and their floral visitors. Ecology. 2008;89:1791–1801. doi: 10.1890/07-0719.1. [DOI] [PubMed] [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Cardinale BJ, Wrigh JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo CG, Rubio R, Rouanet JL, Borie F. Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biology and Fertility of Soils. 2006;43:83–92. [Google Scholar]
- Cavagnaro TR, Smith FA, Smith SE, Jakobsen I. Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell and Environment. 2005;28:642–650. [Google Scholar]
- Chifflot V, Rivest D, Olivier A, Cogliastro A, Khasa D. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems. Agriculture Ecosystems & Environment. 2009;131:32–39. [Google Scholar]
- Cordoba A, De Mendonça M, Stürmer S, Rygiewicz P. Diversity of arbuscular mycorrhizal fungi along a sand dune stabilization gradient: a case study at Praia da Joaquina, Ilha de Santa Catarina, South Brazil. Mycoscience. 2001;42:379–387. [Google Scholar]
- Corradi N, Ruffner B, Croll D, Colard A, Horak A, Sanders IR. High-Level Molecular Diversity of Copper-Zinc Superoxide Dismutase Genes among and within Species of Arbuscular Mycorrhizal Fungi. Applied and Environmental Microbiology. 2009;75:1970–1978. doi: 10.1128/AEM.01974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden CC, Peterson CJ. A multi-mutualist simulation: Applying biological market models to diverse mycorrhizal communities. Ecological Modelling. 2009;220:1522–1533. [Google Scholar]
- Croll D, Wille L, Gamper HA, Mathimaran N, Lammers PJ, Corradi N, Sanders IR. Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist. 2008;178:672–687. doi: 10.1111/j.1469-8137.2008.02381.x. [DOI] [PubMed] [Google Scholar]
- Daniell TJ, Husband R, Fitter AH, Young JPW. Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiology Ecology. 2001;36:203–209. doi: 10.1111/j.1574-6941.2001.tb00841.x. [DOI] [PubMed] [Google Scholar]
- De Visser J, Rozen DE. Limits to adaptation in asexual populations. Journal of Evolutionary Biology. 2005;18:779–788. doi: 10.1111/j.1420-9101.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Denison RF, Bledsoe C, Kahn M, O'Gara F, Simms EL, Thomashow LS. Cooperation in the rhizosphere and the “free rider” problem. Ecology. 2003;84:838–845. [Google Scholar]
- Douds DD, Pfeffer PE, Shachar-Hill Y. Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant and Soil. 2000;226:255–261. [Google Scholar]
- Egerton-Warburton LM, Johnson NC, Allen EB. Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecological Monographs. 2007;77:527–544. [Google Scholar]
- Ehinger M, Koch AM, Sanders IR. Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytologist. 2009;184:412–423. doi: 10.1111/j.1469-8137.2009.02983.x. [DOI] [PubMed] [Google Scholar]
- Ernst O, Siri-Prieto G. Impact of perennial pasture and tillage systems on carbon input and soil quality indicators. Soil & Tillage Research. 2009;105:260–268. [Google Scholar]
- Ezawa T, Cavagnaro TR, Smith SE, Smith FA, Ohtomo R. Rapid accumulation of polyphosphate in extraradical hyphae of an arbuscular mycorrhizal fungus as revealed by histochemistry and a polyphosphate kinase/luciferase system. New Phytologist. 2004;161:387–392. doi: 10.1046/j.1469-8137.2003.00966.x. [DOI] [PubMed] [Google Scholar]
- Farmer MJ, Li X, Feng G, Zhao B, Chatagnier O, Gianinazzi S, Gianinazzi-Pearson V, et al. Molecular monitoring of field-inoculated AMF to evaluate persistence in sweet potato crops in China. Applied Soil Ecology. 2007;35:599–609. [Google Scholar]
- Ferriere R, Bronstein JL, Rinaldi S, Law R, Gauduchon M. Cheating and the evolutionary stability of mutualisms. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:773–780. doi: 10.1098/rspb.2001.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriere R, Gauduchon M, Bronstein JL. Evolution and persistence of obligate mutualists and exploiters: competition for partners and evolutionary immunization. Ecology Letters. 2007;10:115–126. doi: 10.1111/j.1461-0248.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology. 2007;88:1354–1364. doi: 10.1890/05-1839. [DOI] [PubMed] [Google Scholar]
- Fitter AH. Costs and benefits of mycorrhizas – implications for functioning under natural conditions. Experientia. 1991;47:350–355. [Google Scholar]
- Galvez L, Douds DD, Drinkwater LE, Wagoner P. Effect of tillage and farming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize. Plant and Soil. 2001;228:299–308. [Google Scholar]
- Gamper HA, Van Der Heijden MGA, Kowalchuk GA. Molecular trait indicators: moving beyond phylogeny in arbuscular mycorrhizal ecology. New Phytologist. 2010;185:67–82. doi: 10.1111/j.1469-8137.2009.03058.x. [DOI] [PubMed] [Google Scholar]
- Gange AC, West HM. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago Lanceolata L. New Phytologist. 1994;128:79–87. doi: 10.1111/j.1469-8137.1994.tb03989.x. [DOI] [PubMed] [Google Scholar]
- Graham J, Eissenstat D. Host genotype and the formation and function of VA mycorrhizae. Plant and Soil. 1994;159:179–185. [Google Scholar]
- Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- Gryndler M, Lipavsky J. Effect of phosphate fertilization on the populations of arbuscular mycorrhizal fungi. Rostlinna Vyroba. 1995;41:533–540. [Google Scholar]
- Gustafson DJ, Casper BB. Differential host plant performance as a function of soil arbuscular mycorrhizal fungal communities: experimentally manipulating co-occurring Glomus species. Plant Ecology. 2006;183:257–263. [Google Scholar]
- Haas JH, Krikun J. Efficacy of endomycorrhizal-fungus isolates and inoculum quantities required for growth-response. New Phytologist. 1985;100:613–621. [Google Scholar]
- Hamel C. Prospects and problems pertaining to the management of arbuscular mycorrhizae in agriculture. Agriculture Ecosystems & Environment. 1996;60:197–210. [Google Scholar]
- Handel A, Rozen DE. The impact of population size on the evolution of asexual microbes on smooth versus rugged fitness landscapes. BMC Evolutionary Biology. 2009;9:10. doi: 10.1186/1471-2148-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin G. Tragedy of commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Hauggaard-Nielsen H, Jensen ES. Facilitative root interactions in intercrops. Plant and Soil. 2005;274:237–250. [Google Scholar]
- Hausmann NT, Hawkes CV. Plant neighborhood control of arbuscular mycorrhizal community composition. New Phytologist. 2009;183:1188–1200. doi: 10.1111/j.1469-8137.2009.02882.x. [DOI] [PubMed] [Google Scholar]
- Heath KD, Tiffin P. Context dependence in the coevolution of plant and rhizobial mutualists. Proceedings of the Royal Society B-Biological Sciences. 2007;274:1905–1912. doi: 10.1098/rspb.2007.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448:188–191. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden MGA, Scheublin TR. Functional traits in mycorrhizal ecology: their use for predicting the impact of arbuscular mycorrhizal fungal communities on plant growth and ecosystem functioning. New Phytologist. 2007;174:244–250. doi: 10.1111/j.1469-8137.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- Van Der Heijden MGA, Bardgett RD, Van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Hendrix JW, Jones KJ, Nesmith WC. Control of pathogenic mycorrhizal fungi in maintenance of soil productivity by crop-rotation. Journal of Production Agriculture. 1992;5:383–386. [Google Scholar]
- Hijri M, Sanders IR. Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature. 2005;433:160–163. doi: 10.1038/nature03069. [DOI] [PubMed] [Google Scholar]
- Hijri I, Sykorova Z, Oehl F, Ineichen K, Mäder P, Wiemken A, Redecker D. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Molecular Ecology. 2006;15:2277–2289. doi: 10.1111/j.1365-294X.2006.02921.x. [DOI] [PubMed] [Google Scholar]
- Hochberg ME, Gomulkiewicz R, Holt RD, Thompson JN. Weak sinks could cradle mutualistic symbioses – strong sources should harbour parasitic symbioses. Journal of Evolutionary biology. 2000;13:213–222. [Google Scholar]
- Hoeksema JD, Schwartz MW. Expanding comparative-advantage biological market models: contingency of mutualism on partners’ resource requirements and acquisition trade-offs. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:913–919. doi: 10.1098/rspb.2002.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters. 2010;13:394–407. doi: 10.1111/j.1461-0248.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- Janouskova M, Seddas P, Mrnka L, Van Tuinen D, Dvorackova A, Tollot M, Gianinazzi-Pearson V, et al. Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza. 2009;19:393–402. doi: 10.1007/s00572-009-0243-4. [DOI] [PubMed] [Google Scholar]
- Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossard E. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza. 2002;12:225–234. doi: 10.1007/s00572-002-0163-z. [DOI] [PubMed] [Google Scholar]
- Jansa J, Mozafar A, Kuhn G, Anken T, Ruh R, Sanders IR, Frossard E. Soil tillage affects the community structure of mycorrhizal fungi in maize roots. Ecological Applications. 2003;13:1164–1176. [Google Scholar]
- Jansa J, Mozafar A, Frossard E. Phosphorus acquisition strategies within arbuscular mycorrhizal fungal community of a single field site. Plant and Soil. 2005;276:163–176. [Google Scholar]
- Jansa J, Smith FA, Smith SE. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytologist. 2008;177:779–789. doi: 10.1111/j.1469-8137.2007.02294.x. [DOI] [PubMed] [Google Scholar]
- Jany JL, Pawlowska TE. Multinucleate spores contribute to evolutionary longevity of asexual Glomeromycota. American Naturalist. 2010;175:424–435. doi: 10.1086/650725. [DOI] [PubMed] [Google Scholar]
- Johnson NC. Can fertilization of soil select less mutualistic mycorrhizae? Ecological Applications. 1993;3:749–757. doi: 10.2307/1942106. [DOI] [PubMed] [Google Scholar]
- Johnson NC. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist. 2010;185:631–647. doi: 10.1111/j.1469-8137.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Copeland PJ, Crookston RK, Pfleger FL. Mycorrhizae-possible explanation for yield decline with continuous corn and soybean. Agronomy Journal. 1992;84:387–390. [Google Scholar]
- Johnson NC, Graham JH, Smith FA. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytologist. 1997;135:575–586. [Google Scholar]
- Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology. 2003;84:1895–1908. [Google Scholar]
- Johnson D, Vandenkoornhuyse PJ, Leake JR, Gilbert L, Booth RE, Grime JP, Young JPW, et al. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytologist. 2004;161:503–515. doi: 10.1046/j.1469-8137.2003.00938.x. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Rowland DL, Corkidi L, Allen EB. Plant winners and losers during grassland N-eutrophication differ in biomass allocation and mycorrhizas. Ecology. 2008;89:2868–2878. doi: 10.1890/07-1394.1. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proceedings of the National Academy of Sciences. 2010;107:2093–2098. doi: 10.1073/pnas.0906710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MD, Smith SE. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Canadian Journal of Botany-Revue Canadienne De Botanique. 2004;82:1089–1109. [Google Scholar]
- Jumpponen A, Trowbridge J, Mandyam K, Johnson L. Nitrogen enrichment causes minimal changes in arbuscular mycorrhizal colonization but shifts community composition-evidence from rDNA data. Biology and Fertility of Soils. 2005;41:217–224. [Google Scholar]
- Kahiluoto H, Ketoja E, Vestberg M. Contribution of arbuscular mycorrhiza to soil quality in contrasting cropping systems. Agriculture Ecosystems & Environment. 2009;134:36–45. [Google Scholar]
- Kiers ET, Denison RF. Sanctions, Cooperation, and the Stability of Plant-Rhizosphere Mutualisms. Annual Review of Ecology Evolution and Systematics. 2008;39:215–236. [Google Scholar]
- Kiers ET, Van Der Heijden MGA. Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology. 2006;87:1627–1636. doi: 10.1890/0012-9658(2006)87[1627:msitam]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kiers ET, West SA, Denison RF. Mediating mutualisms: farm management practices and evolutionary changes in symbiont co-operation. The Journal of Applied Ecology. 2002;39:745–754. [Google Scholar]
- Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- Kiers ET, Hutton MG, Denison RF. Human selection and the relaxation of legume defences against ineffective rhizobia. Proceedings of the Royal Society B. 2007;274:3119–3126. doi: 10.1098/rspb.2007.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard J, Christen O, Krupinsky J, Layzell D. Break crop benefits in temperate wheat production. Field Crops Research. 2008;107:185–195. [Google Scholar]
- Knapp S, Kuhn I, Schweiger O, Klotz S. Challenging urban species diversity: contrasting phylogenetic patterns across plant functional groups in Germany. Ecology Letters. 2008;11:1054–1064. doi: 10.1111/j.1461-0248.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Koch AM, Croll D, Sanders IR. Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecology Letters. 2006;9:103–110. doi: 10.1111/j.1461-0248.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- Koide RT. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytologist. 2000;147:233–235. [Google Scholar]
- Kowalchuk GA, De Souza FA, Van Veen JA. Community analysis of arbuscular mycorrhizal fungi associated with Ammophila arenaria in Dutch coastal sand dunes. Molecular Ecology. 2002;11:571–581. doi: 10.1046/j.0962-1083.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- Landis FC, Gargas A, Givnish TJ. Relationships among arbuscular mycorrhizal fungi, vascular plants and environmental conditions in oak savannas. New Phytologist. 2004;164:493–504. [Google Scholar]
- Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist. 2009;181:199–207. doi: 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- Lekberg Y, Koide RT, Rohr JR, Aldrich-Wolfe L, Morton JB. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. Journal of Ecology. 2007;95:95–105. [Google Scholar]
- Lendzemo VW, Kuyper TW, Kropff MJ, Van Ast A. Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crops Research. 2005;91:51–61. [Google Scholar]
- Lion S, Van Baalen M. Self-structuring in spatial evolutionary ecology. Ecology Letters. 2008;11:277–295. doi: 10.1111/j.1461-0248.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- Lovelock CE, Ewel JJ. Links between tree species, symbiotic fungal diversity and ecosystem functioning in simplified tropical ecosystems. New Phytologist. 2005;167:219–228. doi: 10.1111/j.1469-8137.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- Mäder P, Edenhofer S, Boller T, Wiemken A, Niggli U. Arbuscular mycorrhizae in a long-term field trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Biology and Fertility of Soils. 2000;31:150–156. [Google Scholar]
- Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U. Soil fertility and biodiversity in organic farming. Science. 2002;296:1694–1697. doi: 10.1126/science.1071148. [DOI] [PubMed] [Google Scholar]
- Maherali H, Klironomos JN. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science. 2007;316:1746–1748. doi: 10.1126/science.1143082. [DOI] [PubMed] [Google Scholar]
- Manske GGB. Genetical analysis of the efficiency of VA mycorrhiza with spring wheat. Agriculture, Ecosystems & Environment. 1990;29:273–280. [Google Scholar]
- Marquard E, Weigelt A, Temperton VM, Roscher C, Schumacher J, Buchmann N, Fischer M, et al. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology. 2009;90:3290–3302. doi: 10.1890/09-0069.1. [DOI] [PubMed] [Google Scholar]
- Martensson AM, Rydberg I, Vestberg M. Potential to improve transfer of N in intercropped systems by optimising host-endophyte combinations. Plant and Soil. 1998;205:57–66. [Google Scholar]
- Martini EA, Buyer JS, Bryant DC, Hartz TK, Denison RF. Yield increases during the organic transition: improving soil quality or increasing experience? Field Crops Research. 2004;86:255–266. [Google Scholar]
- Marulanda A, Azcon R, Ruiz-Lozano JM. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiologia Plantarum. 2003;119:526–533. [Google Scholar]
- Menendez AB, Scervino JM, Godeas AM. Arbuscular mycorrhizal populations associated with natural and cultivated vegetation on a site of Buenos Aires province, Argentina. Biology and Fertility of Soils. 2001;33:373–381. [Google Scholar]
- Mickelson SM, Kaeppler SM. Evaluation of six mycorrhizal isolates for their ability to promote growth of maize genotypes under phosphorus deficiency. Maydica. 2005;50:137–146. [Google Scholar]
- Modjo HS, Hendrix JW. The Mycorrhizal fungus Glomus macrocarpum as a cause of tobacoo stunt disease. Phytopathology. 1986;76:688–691. [Google Scholar]
- Moonen A-C, Bàrberi P. Functional biodiversity: an agroecosystem approach. Agriculture, Ecosystems & Environment. 2008;127:7–21. [Google Scholar]
- Mummey DL, Antunes PM, Rillig MC. Arbuscular mycorrhizal fungi pre-inoculant identity determines community composition in roots. Soil Biology & Biochemistry. 2009;41:1173–1179. [Google Scholar]
- Munkvold L, Kjoller R, Vestberg M, Rosendahl S, Jakobsen I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytologist. 2004;164:357–364. doi: 10.1111/j.1469-8137.2004.01169.x. [DOI] [PubMed] [Google Scholar]
- Nijjer S, Rogers WE, Siemann E. The impacts of fertilization on mycorrhizal production and investment in Western Gulf Coast Grasslands. American Midland Naturalist. 2010;163:124–133. [Google Scholar]
- Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Applied and Environmental Microbiology. 2003;69:2816–2824. doi: 10.1128/AEM.69.5.2816-2824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehl F, Sieverding E, Mäder P, Dubois D, Ineichen K, Boller T, Wiemken A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia. 2004;138:574–583. doi: 10.1007/s00442-003-1458-2. [DOI] [PubMed] [Google Scholar]
- Oehl F, Sieverding E, Ineichen K, Ris EA, Boller T, Wiemken A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytologist. 2005;165:273–283. doi: 10.1111/j.1469-8137.2004.01235.x. [DOI] [PubMed] [Google Scholar]
- Oehl F, De Souza FA, Sieverding E. Revision of Scutellospora and description of five new genera and three new families in the arbuscular mycorrhiza-forming Glomeromycetes. Mycotaxon. 2008;106:311–360. [Google Scholar]
- Oehl F, Sieverding E, Ineichen K, Mader P, Wiemken A, Boller T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agriculture Ecosystems & Environment. 2009;134:257–268. [Google Scholar]
- Oliveira RS, Boyer LR, Carvalho MF, Jeffries P, Vosatka M, Castro PML, Dodd JC. Genetic, phenotypic and functional variation within a Glomus geosporum isolate cultivated with or without the stress of a highly alkaline anthropogenic sediment. Applied Soil Ecology. 2010;45:39–48. [Google Scholar]
- Öpik M, Moora M, Liira J, Zobel M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. Journal of Ecology. 2006;94:778–790. [Google Scholar]
- Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytologist. 2009;184:424–437. doi: 10.1111/j.1469-8137.2009.02920.x. [DOI] [PubMed] [Google Scholar]
- Pearson JN, Jakobsen I. Symbiotic exchange of carbon and phosphorus between cucumber and 3 arbuscular mycorrhizal fungi. New Phytologist. 1993;124:481–488. [Google Scholar]
- Pianka ER. On r-selection and K-selection. American Naturalist. 1970;104:592–597. [Google Scholar]
- Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H. Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proceedings of the Royal Society B-Biological Sciences. 2009;276:4237–4245. doi: 10.1098/rspb.2009.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzing A, Reiffers R, Braakhekke WG, Hennekens SM, Tackenberg O, Ozinga WA, Schaminee JHJ, et al. Less lineages - more trait variation: phylogenetically clustered plant communities are functionally more diverse. Ecology Letters. 2008;11:809–819. doi: 10.1111/j.1461-0248.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- De La Providencia IE, De Souza FA, Fernandez F, Delmas NS, Declerck S. Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytologist. 2005;165:261–271. doi: 10.1111/j.1469-8137.2004.01236.x. [DOI] [PubMed] [Google Scholar]
- De La Providencia IE, Fernandez F, Declerck S. Hyphal healing mechanism in the arbuscular mycorrhizal fungi Scutellospora reticulata and Glomus clarum differs in response to severe physical stress. FEMS Microbiology Letters. 2007;268:120–125. doi: 10.1111/j.1574-6968.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- Ptacnik R, Solimini AG, Andersen T, Tamminen T, Brettum P, Lepisto L, Willen E, et al. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5134–5138. doi: 10.1073/pnas.0708328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pypers P, Huybrighs M, Diels J, Abaidoo R, Smolders E, Merckx R. Does the enhanced P acquisition by maize following legumes in a rotation result from improved soil P availability? Soil Biology and Biochemistry. 2007;39:2555–2566. [Google Scholar]
- Redecker D, Morton JB, Bruns TD. Molecular phylogeny of the arbuscular mycorrhizal fungi Glomus sinuosum and Sclerocystis coremioides. Mycologia. 2000;92:282–285. [Google Scholar]
- Reynolds HL, Packer A, Bever JD, Clay K. Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- Rosendahl S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytologist. 2008;178:253–266. doi: 10.1111/j.1469-8137.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- Rosendahl S, Matzen HB. Genetic structure of arbuscular mycorrhizal populations in fallow and cultivated soils. New Phytologist. 2008;179:1154–1161. doi: 10.1111/j.1469-8137.2008.02535.x. [DOI] [PubMed] [Google Scholar]
- Rosendahl S, McGee P, Morton JB. Lack of global population genetic differentiation in the arbuscular mycorrhizal fungus Glomus mosseae suggests a recent range expansion which may have coincided with the spread of agriculture. Molecular Ecology. 2009;18:4316–4329. doi: 10.1111/j.1365-294X.2009.04359.x. [DOI] [PubMed] [Google Scholar]
- Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency dependence and cooperation: theory and a test with bacteria. American Naturalist. 2007;170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- Ryan MH, Angus JF. Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant and Soil. 2003;250:225–239. [Google Scholar]
- Ryan MH, Graham JH. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant and Soil. 2002;244:263–271. [Google Scholar]
- Ryan MH, Van Herwaarden AF, Angus JF, Kirkegaard JA. Reduced growth of autumn-sown wheat in a low-P soil is associated with high colonisation by arbuscular mycorrhizal fungi. Plant and Soil. 2005;270:275–286. [Google Scholar]
- Sanders IR. Preference, specificity and cheating in the arbuscular mycorrhizal symbiosis. Trends in Plant Science. 2003;8:143–145. doi: 10.1016/S1360-1385(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Sanders IR, Streitwolf-Engel R, Van Der Heijden MGA, Boller T, Wiemken A. Increased allocation to external hyphae of arbuscular mycorrhizal fungi under CO2 enrichment. Oecologia. 1998;117:496–503. doi: 10.1007/s004420050685. [DOI] [PubMed] [Google Scholar]
- Santos JC, Finlay RD, Tehler A. Molecular analysis of arbuscular mycorrhizal fungi colonising a semi-natural grassland along a fertilisation gradient. New Phytologist. 2006;172:159–168. doi: 10.1111/j.1469-8137.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Santos-Gonzalez JC, Finlay RD, Tehler A. Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a seminatural grassland. Applied and Environmental Microbiology. 2007;73:5613–5623. doi: 10.1128/AEM.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJH, Gutjahr C, Paszkowski U. Cereal mycorrhiza: an ancient symbiosis in modern agriculture. Trends in Plant Science. 2008;13:93–97. doi: 10.1016/j.tplants.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Scheublin TR, Ridgway KP, Young JPW, Van Der Heijden MGA. Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Applied and Environmental Microbiology. 2004;70:6240–6246. doi: 10.1128/AEM.70.10.6240-6246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheublin TR, Van Logtestijn RSP, Van Der Heijden MGA. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. Journal of Ecology. 2007;95:631–638. [Google Scholar]
- Schlapfer F, Schmid B. Ecosystem effects of biodiversity: a classification of hypotheses and exploration of empirical results. Ecological Applications. 1999;9:893–912. [Google Scholar]
- Schussler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research. 2001;105:1413–1421. [Google Scholar]
- Schwartz MW, Hoeksema JD. Specialization and resource trade: Biological markets as a model of mutualisms. Ecology. 1998;79:1029–1038. [Google Scholar]
- Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecology Letters. 2006;9:501–515. doi: 10.1111/j.1461-0248.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- Shennan C. Biotic interactions, ecological knowledge and agriculture. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:717–739. doi: 10.1098/rstb.2007.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes BA, Cottenie C, Klironomos JN. Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. Journal of Ecology. 2009;97:1274–1280. [Google Scholar]
- Simms EL, Taylor DL, Povich J, Shefferson RP, Sachs JL, Urbina M, Tausczik Y. An empirical test of partner choice mechanisms in a wild legume-rhizobium interaction. Proceedings of the Royal Society B-Biological Sciences. 2006;273:77–81. doi: 10.1098/rspb.2005.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal Symbiosis. 2nd edn. London: Academic Press; 1997. [Google Scholar]
- Smith FA, Jakobsen I, Smith SE. Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytologist. 2000;147:357–366. [Google Scholar]
- Smith FA, Grace EJ, Smith SE. More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist. 2009;182:347–358. doi: 10.1111/j.1469-8137.2008.02753.x. [DOI] [PubMed] [Google Scholar]
- Stockinger H, Walker C, Schussler A. ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytologist. 2009;183:1176–1187. doi: 10.1111/j.1469-8137.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- Sudova R. Different growth response of five co-existing stoloniferous plant species to inoculation with native arbuscular mycorrhizal fungi. Plant Ecology. 2009;204:135–143. [Google Scholar]
- Sykorova Z, Ineichen K, Wiemken A, Redecker D. The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza. 2007;18:1–14. doi: 10.1007/s00572-007-0147-0. [DOI] [PubMed] [Google Scholar]
- Tawaraya K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Science and Plant Nutrition. 2003;49:655–668. [Google Scholar]
- Thomson BD, Robson AD, Abbott LK. The effect of long-term applications of phosphorus-fertilizer on populations of Vesicular-Arbuscular Mycorrhizal Fungi in pastures. Australian Journal of Agricultural Research. 1992;43:1131–1142. [Google Scholar]
- Thrall PH, Hochberg ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends in Ecology & Evolution. 2007;22:120–126. doi: 10.1016/j.tree.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Tilman D. The ecological consequences of changes in biodiversity: a search for general principles. Ecology. 1999;80:1455–1474. [Google Scholar]
- Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. [Google Scholar]
- Toljander JF, Santos-Gonzalez JC, Tehler A, Finlay RD. Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microbiology Ecology. 2008;65:323–338. doi: 10.1111/j.1574-6941.2008.00512.x. [DOI] [PubMed] [Google Scholar]
- Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JPW. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Molecular Ecology. 2002;11:1555–1564. doi: 10.1046/j.1365-294x.2002.01538.x. [DOI] [PubMed] [Google Scholar]
- Vandenkoornhuyse P, Ridgway KP, Watson IJ, Fitter AH, Young JPW. Co-existing grass species have distinctive arbuscular mycorrhizal communities. Molecular Ecology. 2003;12:3085–3095. doi: 10.1046/j.1365-294x.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- Verbruggen E, Röling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, Van Der Heijden MGA. Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytologist. 2010;186:968–979. doi: 10.1111/j.1469-8137.2010.03230.x. [DOI] [PubMed] [Google Scholar]
- Viereck N, Hansen PE, Jakobsen I. Phosphate pool dynamics in the arbuscular mycorrhizal fungus Glomus intraradices studied by in vivo P-31 NMR spectroscopy. New Phytologist. 2004;162:783–794. doi: 10.1111/j.1469-8137.2004.01048.x. [DOI] [PubMed] [Google Scholar]
- Violi HA, Treseder KK, Menge JA, Wright SF, Lovatt CJ. Density dependence and interspecific interactions between arbuscular mycorrhizal fungi mediated plant growth, glomalin production, and sporulation. Canadian Journal of Botany-Revue Canadienne De Botanique. 2007;85:63–75. [Google Scholar]
- West SA, Murray MG, Machado CA, Griffin AS, Herre EA. Testing Hamilton's rule with competition between relatives. Nature. 2001;409:510–513. doi: 10.1038/35054057. [DOI] [PubMed] [Google Scholar]
- West SA, Kiers ET, Simms EL, Denison RF. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:685–694. doi: 10.1098/rspb.2001.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WG, Morris WF, Bronstein JL. Coexistence of mutualists and exploiters on spatial landscapes. Ecological Monographs. 2003;73:397–413. [Google Scholar]
- Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecology Letters. 2009;12:452–461. doi: 10.1111/j.1461-0248.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Wu Q-S, Xia R-X, Zou Y-N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. European Journal of Soil Biology. 2008;44:122–128. [Google Scholar]


