Abstract
Neotyphodium endophytic fungi, the asexual state of Epichloë species, protect cool-season grasses against stresses. The outcomes of Neotyphodium-grass symbioses are agronomically relevant as they may affect the productivity of pastures. It has been suggested that the mutualism is characteristic of agronomic grasses and that differential rates of gene flow between both partners’ populations are expected to disrupt the specificity of the association and, thus, the mutualism in wild grasses. We propose that compatibility is necessary but not sufficient to explain the outcomes of Neotyphodium-grass symbiosis, and advance a model that links genetic compatibility, mutualism effectiveness, and endophyte transmission efficiency. For endophytes that reproduce clonally and depend on allogamous hosts for reproduction and dissemination, we propose that this symbiosis works as an integrated entity where gene flow promotes its fitness and evolution. Compatibility between the host plant and the fungal endophyte would be high in genetically close parents; however, mutualism effectiveness and transmission efficiency would be low in fitness depressed host plants. Increasing the genetic distance of mating parents would increase mutualism effectiveness and transmission efficiency. This tendency would be broken when the genetic distance between parents is high (out-breeding depression). Our model allows for testable hypotheses that would contribute to understand the coevolutionary origin and future of the endophyte-grass mutualism.
Keywords: Epichloë, genetic specificity, mutualism effectiveness, Neotyphodium endophyte, species interactions, symbiosis, transmission efficiency
Introduction
Evolutionary perspectives have contributed to agriculture, for example, by providing elements to understand crop adaptation to low-input production systems and trade-offs between different plant traits (Sadras and Denison 2009). Evolutionary perspectives of relationships among organisms are critical to crop protection and, reciprocally, current theories of plant-microbe interactions are largely derived from agriculturally relevant symbioses including host-parasite or pathogen, legume-nitrogen fixing bacteria and plant-mycorrhizal fungi (Kiers et al. 2002; Thompson 2005; Thrall et al. 2007; Tikhonovich and Provorov 2009). Symbiotic interactions, antagonistic or mutualistic, may influence fitness and evolution of both plants and microbes (Douglas 1994; Thompson 2005). Antagonistic interactions between plants and predators or parasites are expected to dominate in productive environments, favoring mutualistic interactions with microbe protectors against enemies, whereas mutualism established with providers of essential elements are more likely in low-quality environments (Thrall et al. 2007). Conceptual frameworks explaining general patterns for the relative abundance of antagonistic/mutualistic interactions and protector/provider symbionts as driven by environmental quality and community complexity are essential in the context of increasing human interference in ecosystems (Saikkonen et al. 1998; Kiers et al. 2002; Thrall et al. 2007).
The hereditary symbiosis between cool-season grasses and Epichloë fungi (phylum Ascomycota, order Hypocreales, family Clavicipitaceae), commonly known as endophytes or Class I endophytes (Schardl et al. 2004; Rodriguez et al. 2009), is relatively common in wild and forage grasses and in many cereals and weeds (Saikkonen et al. 1998; Clay and Schardl 2002; Rudgers et al. 2009). Specifically, there is an increasing interest in the asexual states of Epichloë species, Neotyphodium fungi (Clay and Schardl 2002). In the last 25 years, endophyte-grass symbioses have been recognized as an important component in agro-ecosystems as they may affect host ability to invade new habitats, organic matter decomposition and energy flow through the food webs (Clay and Schardl 2002; Rudgers et al. 2009). However, the agricultural importance of endophytes became evident when their presence in forage grasses was associated with mammalian intoxication caused by endophyte-produced toxic alkaloids. Depending on the profile of loline, peramine, ergot and lolitrem alkaloids, host plants may acquire resistance to different groups of herbivores (Clay and Schardl 2002). As a result, Neotyphodium endophytes have been considered defensive mutualists of host grasses (i.e. protectors; Clay 1988; Selosse and Schardl 2007; Clay 2008).
Besides this anti-herbivore benefit, other effects of the endophyte-grass symbioses have been tested in a wide range of ecological conditions. Nonetheless, results from comparative studies of endophyte-infected versus noninfected plants have not been straightforward. The endophyte has been found to enhance plant tolerance to abiotic stresses such as drought, salinity, heavy metals and herbicides (Malinowski and Belesky 2000; Vila-Aiub et al. 2003). The infection may promote plant growth and enhance tolerance to nutrient and water shortage by enhancing nutrient uptake and increasing stomatal responsiveness to atmospheric demand (Malinowski and Belesky 2000). However, endophyte infection may reduce plant stress tolerance under extreme environmental conditions (Cheplick et al. 1989; Ahlholm et al. 2002). These negative effects have been usually explained in terms of fungus’ drain of plant resources, a cost that can outweigh the benefits that the fungus can provide to the host (Cheplick et al. 1989). Alternatively, in species that naturally occur in dry or semi-arid environments, the endophyte benefits were manifested under water restriction but not under well-watered conditions (Hesse et al. 2003). According with these results, it has been suggested that depending on the ecological context, there is a continuum of outcomes ranging from mutualistic to parasitic (Müller and Krauss 2005).
Recent studies have led to the generalization that the endophyte-grass symbiosis works as mutualism only in agronomic grasses (Faeth 2002; Faeth and Sullivan 2003; Saikkonen et al. 2004, 2006; Cheplick and Faeth 2009). In particular, Saikkonen et al. (2004, 2006) asserted that the protective mutualism in certain cool-season grasses has been gained through agricultural selection which favored genetic uniformity in populations of both host and endophyte. In fact, the endophyte-grass symbiosis has been mostly studied on agronomic grasses [(Schedonorus arundinaceus, formerly Festuca arundinacea (tall fescue) and Lolium perenne (perennial ryegrass)] that have had a long history of human selection under consistent selection pressures such as livestock grazing. Arguments against the mutualism in wild species rely on case studies with Festuca arizonica, a perennial bunchgrass native to North America for which it has been difficult to find positive effects of Neotyphodium spp. infection (Schulthess and Faeth 1998; Sullivan and Faeth 2004). It has been claimed that unlike agronomic grasses, wild species have not been subjected to breeding, are genetically more diverse and generally are grown in poorer environments (Saikkonen et al. 2006; Cheplick and Faeth 2009). Thus, the differential gene flow rate between both partners’ populations is seen as a disrupting force for the compatibility between the fungus and the host grass, which is a basic condition for the effectiveness and prevalence of mutualism (see Glossary; Saikkonen et al. 2004; Thompson 2005). Although these arguments are in accordance with the hypothesis that protectors are promoted in high-quality productive environments, they may contradict the idea that domestication and agricultural intensification may lead to loss of mutualistic interaction, or for mutualism to become parasitism (Kiers et al. 2002; Thrall et al. 2007).
In this article, we propose a more general framework to understand the underlying mechanisms that can explain the ubiquity and high endophyte infection level in grass populations in wild and agronomic contexts, even when there is not a clear mutualistic effect. We suggest that despite the selection pressure mediated by plant and animal husbandry in agronomic systems, endophyte-infected grasses maintain a large genetic variability and display gene flow among distant populations of the same species and populations of related species. In contrast to the model proposed by Saikkonen et al. (2004), our hypothesis is that genetic variability in host plant populations could promote stable, mutualistic interactions between plants and fungal endophytes whereby increased plant fitness indirectly benefits the endophyte. From an evolutionary perspective, we first explore how hybridization and gene flow may affect the persistence of endophyte-grass symbioses by stimulating host plant fitness and evolution. Emphasis is on life history traits involved in the determination of the compatibility and the intrinsic genetic variability of Neotyphodium endophyte and host grass populations. Second, we propose a conceptual framework that integrates fragmented knowledge and develops expectations for the relationship among endophyte-grass compatibility, mutualism effectiveness and efficiency of endophyte transmission (see Glossary) and the characteristic host plant genetic variability. Owing to the empirical limitations to explore all the possible combinations of these variables, we use a theoretical approach to develop testable predictions. Finally, we discuss the implication of these new perspectives and make suggestions for future research.
Evolutionary perspective for understanding the symbiosis between Neotyphodium endophytes and cool-season grasses
Life history traits of both partners and sources of genetic variability
Many mutualistic interactions are thought to derive from antagonistic relationships where coevolution has extended the ecological niche of both partners (Douglas 1994; Herre et al. 1999). Likely, symbionts’ life history traits in mutualistic interactions may include the complete loss of horizontal transmission and sexual reproduction, and the complete dependency of the host for multiplying and spreading (Herre et al. 1999; De Mazancourt et al. 2005). In this regard, different elements in the Epichloë/Neotyphodium complex can be identified as evidence of the evolutionary pathway from pathogenic toward mutualistic symbioses. Epichloë endophytes, the ancestors of Neotyphodium, can be transmitted both horizontally and vertically (Groppe et al. 2001; Rodriguez et al. 2009). But, remarkably, the pathogenic behavior is, during the sexual state, manifested as ‘choke disease’ in which the host's reproductive structures are replaced by the fungus's stroma (Groppe et al. 2001; Clay and Schardl 2002; Rodriguez et al. 2009). This pathogenic behavior, determines the ability of Epichloë endophyte for contagious spread. Alternatively, Neotyphodium fungi are only asexually and vertically transmitted from the host mother plant to offspring without disease symptoms (Fig. 1; Schardl et al. 2004; but see also Tadych et al. 2007). In spite of some exception (e.g. Neotyphodium lolii), Neotyphodium species are hybrids of Epichloë species and it has been suggested that the duplication of alkaloids’ gene copies, in addition to the complete loss of sexual reproduction and horizontal transmission, meant a crucial step toward the mutualism (Clay and Schardl 2002; Selosse and Schardl 2007). Therefore, since Neotyphodium endophytes are exclusively vertically transmitted and none of them are known to be transmitted by host pollen, endophytic fungi seem to be trapped within host plants, while host plants can live without the infection (Clay 1993, 2008; do Valle Ribeiro 1993; Ravel et al. 1997).
Figure 1.
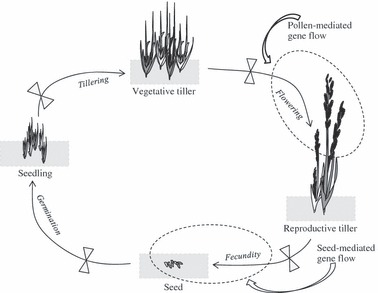
Annual life cycle in a grass population symbiotic with Neotyphodium endophyte with four life stages (Seed, Seedling, Vegetative and Reproductive tiller) and vital rates between two consecutive stages (Germination, Tillering, Flowering and Fecundity). Endophyte-infected and noninfected plants are represented in grey and white respectively. Control flow keys ( ) indicate points in the host life cycle in which population can be sieved by the biotic and abiotic environment selecting for genotypes of one of the partners or both, the host plant and the endophyte. In particular for the fungus, those transitions are known as partial transmission efficiencies of endophyte between two consecutive stages. Consequently, these transmissions control the proportion of endophyte-infected individuals in the populations. Indicated with dashed circles are those points in which gene flow can be mediated by pollen (only for host local population) and by seed (for both endophyte and host local population). The figure was built with information taken from different sources (e.g. Ravel et al. 1997; Clay and Schardl 2002; Schardl et al. 2004; Gundel et al. 2008; Rodriguez et al. 2009).
) indicate points in the host life cycle in which population can be sieved by the biotic and abiotic environment selecting for genotypes of one of the partners or both, the host plant and the endophyte. In particular for the fungus, those transitions are known as partial transmission efficiencies of endophyte between two consecutive stages. Consequently, these transmissions control the proportion of endophyte-infected individuals in the populations. Indicated with dashed circles are those points in which gene flow can be mediated by pollen (only for host local population) and by seed (for both endophyte and host local population). The figure was built with information taken from different sources (e.g. Ravel et al. 1997; Clay and Schardl 2002; Schardl et al. 2004; Gundel et al. 2008; Rodriguez et al. 2009).
Standing plant populations comprise a variable proportion of endophyte-infected and noninfected plants (Fig. 1; Clay and Schardl 2002; Gundel et al. 2009; Rudgers et al. 2009). Even though the transmission of endophyte from mother plants to offspring can be very efficient (Clay and Schardl 2002; Schardl et al. 2004), the prevalence of the symbiosis measured as the frequency of infected individuals in the population depends on the ability of the endophyte to be successfully transmitted during the whole life cycle in the host population (Ravel et al. 1997; Gundel et al. 2008). For example, the proportion of infected seeds can be different from the proportion of infected seedlings as a result of the differential survival rate of endophytes and seeds, and also, as a result of the relative fitness of endophyte-infected and noninfected seeds (Gundel et al. 2010). Since failures in the transmission of endophyte depend on the host stage, the species considered and the environmental condition (Afkhami and Rudgers 2008; Gundel et al. 2009), a similar analysis can be applied for each transition between successive host life stages (Fig. 1). Thus, considering that infected plants can produce a variable proportion of noninfected offspring, the local persistence and prevalence of the symbiosis requires certain levels of mutualism effectiveness and transmission efficiency above a given threshold (Clay 1993; Gundel et al. 2008; see also Saikkonen et al. 2002).
In most instances, one species of Neotyphodium is generally associated to one host grass species, indicating specificity of the association (Moon et al. 2000; Schardl et al. 2004; see exceptions in Iannone et al. 2009). As the fungus reproduces by growing hyphae into the host seeds, and seeds have a low primary dispersion rate in natural environments (Williams and Bartholomew 2005), genetic variability in endophyte populations is naturally low (Sullivan and Faeth 2004; van Zijll de Jong et al. 2008). Alternatively, the breeding system of the host grass species would promote high genetic diversity. Both seed- and pollen-mediated gene flow can contribute to genetic variability in host grass populations (Fig. 1). Festuca and Lolium species are among the most important hosts to endophytes, and despite of some exceptions (e.g. Lolium temulentum), most of them are self-incompatible and wind cross-pollinated (Busi et al. 2008; Yang et al. 2008). Hence, genetic variability at endophyte or cytoplasmatic level (seed-mediated gene flow) is lower than genetic variability at host or nuclear level (seed- and pollen-mediated gene flow). This pattern has been confirmed in studies of polymorphism among endophyte and host populations (Arroyo García et al. 2002; Brem and Leuchtmann 2003; Sullivan and Faeth 2004; Wäli et al. 2007) and using cytoplasmatic and nuclear DNA markers among populations (Balfourier et al. 1998, 2000).
A high level of specificity between the endophyte and grass species (i.e. the ability to establish the association) is expected from coevolutionary process of local populations (see Glossary; Kiers et al. 2002; Saikkonen et al. 2004; Thompson 2005; Easton 2007). The manipulation of partners’ genotype by means of hyphae inoculation or controlled crosses between host genotypes has been used to determine the level of compatibility (do Valle Ribeiro 1993; Christensen 1995; Chung et al. 1997; Brem and Leuchtmann 2003). Lack of compatibility would be manifested by cellular incompatibility reactions that can result in either the death of host tissue or the death of hyphae, as well as host stunted growth. In both cases, the fungus cannot persist within a host or the fungus finds problems to be transmitted (Koga et al. 1993; Christensen 1995; Wille et al. 1999). Genetic studies highlighted the existence of multiple determinants of specificity and compatibility between fungal endophytes and host plants (Chung et al. 1997; Schardl et al. 2004). However, the phenotypic and genotypic correspondence between the endophyte and the host grass populations (i.e. structured populations) is representative of specificity at the population level (Sullivan and Faeth 2004; Piano et al. 2005; van Zijll de Jong et al. 2008; Wäli et al. 2007).
A model of the Neotyphodium endophyte-grass symbiosis as integrated entity
Our model, presented in Fig. 2, integrates theoretical curves predicting prevalence of endophyte-grass symbiosis in relation to host plant genetic variability and the model by Ellstrand and Schierenbeck (2000) of plant fitness response to the genetic distance among parents (Box 1). Current models on the prevalence of hereditary symbionts, as fungal endophytes, stress the importance of the mutualism effectiveness and transmission efficiency (Clay 1993; Ravel et al. 1997; Saikkonen et al. 2002; Gundel et al. 2008). According to this view, we take into account the response of these two processes to the pollen-mediated genetic variability in the host population, considering the potential trade-off between the endophyte-grass compatibility at the population level and the fitness of host plants (Saikkonen et al. 2004). Even though a certain level of compatibility could be a requirement for the mutualism to work (Kiers et al. 2002; Reynolds et al. 2003; De Mazancourt et al. 2005), it does not imply that the mutualism is effective enough for the persistence of the symbiosis (Gundel et al. 2008).
Figure 2.
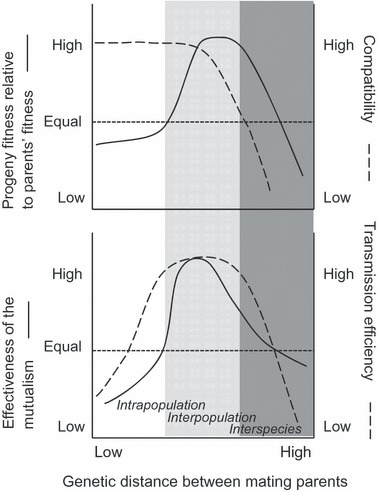
Predicted progeny fitness, compatibility, mutualism effectiveness and transmission efficiency as a function of genetic diversity in allogamous species. Upper panel: progeny fitness (continuous lines, left axis) and expected level of compatibility between Neotyphodium endophytes and host plants (dashed lines, right axis) in relation to genetic distance between mating parents. Lower panel: effectiveness of the mutualistic interaction between endophytes and host plants (continuous lines, left axis) and endophyte transmission efficiency (dashed lines, right axis) in relation to the genetic distance between mating parents. White, grey and dark-grey zones represent a progressive increment in the genetic distance between mating parents (x-axis) with the corresponding intra-population, inter-population and inter-species crossings.
Box 1. Hybridization as fitness stimulus for evolution.
Genetic variability is the raw material for populations to evolve and is associated to heterosis and hybrid vigor at the individual plant level (Ellstrand and Schierenbeck 2000; Radosevich et al. 2007). A theoretical model supported with experimental results predicts a nonlinear relationship between progeny fitness and the genetic distance between parents with a maximum at intermediate genetic distance (Fig. 2, upper panel, left y-axis, continuous line; Ellstrand and Schierenbeck 2000; Elam et al. 2007). Species have developed different strategies to deal with bottlenecks to genetic variability that could be related to life history traits and the demography at different spatial scales. The relationship observed in the first part of the model (Fig. 2, white section) that predicts a progressive increment of progeny fitness with the genetic distance of their parents can also be explained by the Allee Effect. The Allee effect is an increment in the individual plant fitness in response to the plant population density by enhancing the chances to get a suitable mating partner (Taylor and Hastings 2005). In the case of self-incompatible and wind pollinated species, this means an increment in the likelihood of successful pollination and, conversely, a reduction in fitness associated with small population patches, low plant population density or both (Elam et al. 2007). Plant populations usually face this kind of ecological scenarios after extreme events such as drought, flood, fire or herbicide treatment, as a consequence of habitat fragmentation or at the initial phases of an invasive process (Groppe et al. 2001; Armbruster and Reed 2005). The positive relationship between progeny fitness and genetic distance of parents continues growing as a result of the increment in heterosis and hybrid vigor (Fig. 2, grey section); however, this trend continues until a point in which progeny fitness begins to fall due to out-breeding depression (Fig. 2, dark-grey section; Ellstrand and Schierenbeck 2000).
Assuming self-incompatibility, wind pollination, and strong Allee effect (Box 1; Firestone and Jasieniuk 2007; Busi et al. 2008), intermediate genetic distance among mating parents would maximize progeny fitness of host plants relative to that of their parents (Fig. 2, solid line in upper panel). In contrast, the compatibility is expected to be maximized at lower genetic distance between parents (Fig. 2, dashed line in upper panel). After ovary fertilization, infected plants fertilized with genetically different pollen could increase host maternal fitness at the expense of compatibility between the host plant and the endophyte (Saikkonen et al. 2004). At this stage the mutualism could be destabilized because the same fungal genotype is transmitted into genetically distinct and variable seed progeny produced by out-crossing (Fig. 1). The probability of mismatching between fungal genotype and seed genotype will increase in proportion to the genetic distance between the mating parents. This theoretical curve of compatibility is supported by the emergent principles of the geographic mosaic of coevolution theory for microorganisms and hosts (Thompson 2005) as well as by the results obtained from hyphae cross inoculations and controlled plant crosses experiments (do Valle Ribeiro 1993; Christensen 1995; Chung et al. 1997; Brem and Leuchtmann 2003). At this point, however, it is surprising the lack of evidence supporting these mechanisms or processes at population level, considering that this conflict between both partners is physiologically interesting and evolutionarily relevant.
After simultaneous consideration of the host's genetic variability and compatibility of the association, the integrated model projects the performance of both partners now living in symbiosis (Fig. 2, lower panel). If the host plant incurs a material or energetic cost to maintain the endophyte under stressful conditions, we predict a reduction in the mutualism effectiveness derived from a reduction in the fitness of endophyte-infected plants at low level of genetic variability (i.e. inbreeding depression; Fig. 2, white zone). Inbreeding depressed plants have low performance in terms of biomass and seed production (Ellstrand and Schierenbeck 2000; Armbruster and Reed 2005; Elam et al. 2007). It is even possible that mutualism cannot be expressed in fitness-depressed host plants, notwithstanding the high genetic specificity (Fig. 2). At intermediate level of host genetic variability, we predict that endophyte-infected plants would have higher fitness than noninfected ones due to the mutualism (Fig. 2, grey zone). Thus, in this zone, plant fitness (regardless the infection status) and mutualism effectiveness both increase with genetic distance between parents. The vertical transmission efficiency would also be sensitive to the compatibility and host level of heterosis; however, it would be less sensitive (compared to relative fitness) to the compatibility (Fig. 2, dashed line in lower panel). But it is also possible to predict a reduction in the mutualism effectiveness with high transmission efficiency. This could be due to the increase in plant fitness associated with heterosis (i.e. hybrid vigor) and the relative decline in the importance of mutualism for both the host and the endophyte. However, although compatibility has started to fall, transmission efficiency remains high. In this specific zone, a vertically transmitted microorganism like the endophyte could behave as a free-rider, because the fitness of the host is highest in self-incompatible species (Denison et al. 2003). Finally, at high levels of host genetic variability, endophyte-infected plants would present equal or lower fitness than noninfected ones for two reasons: the disruption of compatibility/specificity and the overall low fitness of plants associated with out-breeding depression (Fig. 2, dark-grey zone). Out-breeding depressed plants and inter-specific hybrids have diminished biomass and seed production (Jauhar 1993; Ellstrand and Schierenbeck 2000). Essentially, the very low genetic specificity of inter-specific hybrids would prevent the expression of the endophyte positive effects on plant fitness.
Future challenges
Our theoretical model allows for testable hypotheses on the genetic bases of the endophyte-grass symbiosis at population level. As discussed above, the existence of structured populations or the genetic correspondence between both partners’ populations could be indicative of genetic specificity (Thompson 2005), but the impacts of gene flow on mutualism effectiveness, compatibility and endophyte transmission for the endophyte-grass symbiosis are largely unknown. To understand the genetic controls of the interaction between Neotyphodium endophytes and host grasses, we need to separate the effects of the genetic variability as a whole, from those specific genes controlling the compatibility. The positive relationship between genetic variability and host fitness can be accompanied by an enhanced probability of encountering incompatible genes between the host and the fungus. Following these directions, experiments could be designed to test the different sources of genetic variability on the symbiosis performance. The screening of populations varying in the frequency of endophyte infection could be used as a way to find variability in the time-span of local coevolution between the host plant and the fungal endophyte. Thus, we could manipulate the genetic background in host populations by performing reciprocal crosses to evaluate the mutualism effectiveness and the genetic specificity. The difference in the response of these two processes to genetic variability may be used to understand the existing discrepancies around the prevalence of this mutualism in nature.
The model considers the compatibility and the effectiveness of mutualism as processes that are not independent but can respond differently to the maintenance of sexual reproduction in the host plants. This is important in the light of the apparent lack of positive effects of endophytes in certain systems, focusing on factors that might reduce compatibility rather than mutualism effectiveness (Faeth and Sullivan 2003; Saikkonen et al. 2004; Cheplick and Faeth 2009). Genetic specificity is expected to arise as a basic condition for the functioning of the mutualistic interaction; but it cannot be interpreted as an evolved adaptation when studying mutualism effectiveness (Kiers et al. 2002; De Mazancourt et al. 2005). Previous studies have concluded that multiple genetic determinants are involved in the level of compatibility between the fungal endophyte and the host plant (Chung et al. 1997; Schardl et al. 2004). Nonetheless, data are needed to support that endophytes would be adapted to benefit from the common sources of genetic variability without potential for conflict in genetic specificity. Undoubtedly, the evolutionary stability and ubiquity of the endophyte-grass symbiosis should depend simultaneously on the capacity of endophytes to be effective, to tolerate rapid genetic changes in host plant populations, including the genetic changes in the same host plant after pollination, and to reproduce and spread efficiently through host seeds.
Although human interventions can aim at a specific target, unwanted secondary results are not rare, particularly when high-order interactions are neglected (Thrall et al. 2007; Radosevich et al. 2007). Vila-Aiub et al. (2003) proposed that vertically transmitted endophytes might help to rescue herbicide resistance genes in new environments through the enhancement of survival and fitness of selected plant phenotypes. The symbiosis would increase the initial frequency of resistant alleles and hence increasing the rate of host plant evolution toward herbicide resistance. Alternatively, this effect could be counterbalanced by reducing the herbicide selection pressure due to an enhanced survival in susceptible endophyte-infected plants. These two pathways are likely to work simultaneously depending on the ecological scenario. The increase in genetic variability may also be acquired if the impact of endophytes in reducing mortality under herbicide selection also affects gene flow with related species. However, this could confer higher herbicide resistance reducing genetic specificity. Thus, the host would survive but the mutualism would become extinct. It was found that annual ryegrass populations that evolved resistant to diclofop-methyl in Australia were endophyte free, but other resistant populations from Argentina had high rates of endophyte infection (Vila-Aiub et al. 2003). Annual ryegrass resistant populations from Italy were related to both, gene introgressions from related Festuca species and spontaneous mutations (Dinelli et al. 2004). Therefore, if resistance was acquired through gene flow from other species, our model would predict symbiosis extinction; this may explain why some resistant populations retain high endophyte infection levels (i.e. those in which resistance was caused by spontaneous mutation), while other loose it (i.e. those due to introgression).
In the context of climate global change, sustainable management of natural and agricultural systems requires knowledge of the adaptive mechanisms and evolutionary trajectories of plants and associated organisms (Thrall et al. 2007; Tikhonovich and Provorov 2009). As has been shown throughout the article, the interaction between leaf Neotyphodium fungal endophyte and cool-season grasses often challenges both the theoretical and the experimental evidence. The permanent and potential negative effects of gene flow on the compatibility were invoked to predict the unlikely development of Neotyphodium endophyte associations with annual plants (Saikkonen et al. 2004). However, Lolium multiflorum and Lolium rigidum are annual ryegrasses grasses with high levels of endophyte infection and genetic heterogeneity in diverse ecosystems worldwide (Balfourier et al. 1998, 2000; Moon et al. 2000; Vila-Aiub et al. 2003; Gundel et al. 2009). Thus, it is possible to expect lower levels of specificity for annual or allogamous than for perennial or autogamous host plant species. We are aware of the speculative character of our model; nonetheless it is proposed as a provisory stepping stone to inform urgently needed well-designed experiments to address the evolutionary and agronomic questions associated with this fascinating system.
Conclusions
The symbiosis between Neotyphodium endophyte fungi and cool-season grasses is a useful model system in ecology and evolution, and it is a challenge for the management of agricultural systems. Here, we present a general framework to interpret the underlying mechanisms and processes that regulate evolutionary stability of hereditary symbioses, taking into account that the interaction of endophytes has usually evolved in naturally allogamous host species for which hybridization is the rule. Based only on life history traits of both partners, our model applies equally to vertically transmitted endophytes housed in allogamous plant species, leaving without sense the controversy concerning the use of the term mutualism for wild or agronomic grasses. We incorporate a theoretical relationship between heterosis and plant fitness, and we offer perspectives for the mutualism effectiveness, the compatibility between both partners, and the efficiency of endophyte vertical transmission with respect to genetic distance among host parent plants. Theory and a few empirical studies support our predictions, but there are still more questions than answers. From this evolutionary perspective, the endophyte-grass symbiosis can be considered as an integrated entity able to face the environmental heterogeneity in space and time. Thus, major transitions in evolution might assure mutualism stability by diminishing the susceptibility to the gene flow challenges (Maynard Smith and Szathmáry 1995; Sadras and Denison 2009). Finally, we predict that natural selection and coevolution favor generalist rather than specialist endophytes. High plasticity to environmental and genetic changes in host populations together with the high adaptation to the apoplastic medium of the host plants (Easton 2007; Christensen et al. 2008), could be emergent properties of coevolution in the endophyte-grass symbioses.
Acknowledgments
We are especially grateful to M. A. Martínez-Ghersa for her fruitful discussion on early versions of the work. Three anonymous reviewers provided insightful comments that helped to improve the content and organization of the manuscript. This work was supported by the University of Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (FONCYT, PICT992) of Argentina.
Glossary
Endophyte transmission efficiency: the proportion of endophyte-infected individuals in the total progeny of an endophyte-infected plant (Gundel et al. 2008). Since endophytes are exclusively vertically transmitted and they can be lost in different stages of the host life cycle (Fig. 1), the transmission efficiency from plant to seeds is used as a summary measure of the efficiency during the whole cycle.
Compatibility: the result of different mechanisms of recognition between partners that allow hosts to select or reject between alternative symbionts (Douglas 1994; Christensen 1995). The specificity between the host plant and the fungal endophyte (i.e. the ability to form the association) depends on the level of compatibility however it does not necessarily imply mutualism strength or effectiveness (Kiers et al. 2002; Reynolds et al. 2003; De Mazancourt et al. 2005).
Mutualism effectiveness: the magnitude of the difference in fitness of endophyte-infected and noninfected plants measured in terms of biomass or seed production, also known as relative fitness (Clay 1993; Gundel et al. 2008). While genetic specificity and mutualism effectiveness are not completely independent, they could be not collinear (Kiers et al. 2002; Reynolds et al. 2003).
Progeny fitness relative to parents’ fitness: represents the fitness of the plants as determined by the level of heterosis resulting from the genetic distance between the mating parents (Ellstrand and Schierenbeck 2000; Elam et al. 2007; Radosevich et al. 2007).
Literature cited
- Afkhami ME, Rudgers JA. Symbiosis lost: imperfect vertical transmission of fungal endophytes in grasses. The American Naturalist. 2008;172:405–416. doi: 10.1086/589893. [DOI] [PubMed] [Google Scholar]
- Ahlholm JU, Helander M, Lehtimäki S, Wäli P, Saikkonen K. Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos. 2002;99:173–183. [Google Scholar]
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Arroyo García R, Martínez Zapater JM, García Criado B, Zabalgogeazcoa I. Genetic structure of natural populations of the grass endophyte Epichloë festucae in semiarid grasslands. Molecular Ecology. 2002;11:355–364. doi: 10.1046/j.0962-1083.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- Balfourier F, Charmet G, Ravel C. Genetic differentiation within and between natural populations of perennial and annual ryegrass (Lolium perenne and L. rigidum. Heredity. 1998;81:100–110. [Google Scholar]
- Balfourier F, Imbert C, Charmet G. Evidence for phylogeographic structure in Lolium species related to the spread of agriculture in Europe. A cpDNA study. Theoretical and Applied Genetics. 2000;101:131–138. [Google Scholar]
- Brem D, Leuchtmann A. Molecular evidence for host-adapted races of the fungal endophyte Epichloë bromicola after presumed host shifts. Evolution. 2003;57:37–51. doi: 10.1111/j.0014-3820.2003.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Busi R, Yu Q, Barrett-Lennard R, Powles S. Long distance pollen-mediated flow of herbicide resistance genes in Lolium rigidum. Theoretical and Applied Genetics. 2008;117:1281–1290. doi: 10.1007/s00122-008-0862-8. [DOI] [PubMed] [Google Scholar]
- Cheplick GP, Faeth S. Ecology and Evolution of the Grass-endophyte Symbiosis. New York, USA: Oxford University Press; 2009. [Google Scholar]
- Cheplick GP, Clay K, Marks S. Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytologist. 1989;111:89–97. [Google Scholar]
- Christensen MJ. Variation in the ability of Acremonium endophytes of Lolium perenne Festuca arundinacea and F. pratensis to form compatible associations in the three grasses. Mycological Research. 1995;99:466–470. [Google Scholar]
- Christensen MJ, Bennett RJ, Ansari HA, Koga H, Johnson RD, Bryan GT, Simpson WR, et al. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genetics and Biology. 2008;45:84–93. doi: 10.1016/j.fgb.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Chung K-R, Hollin W, Siegel MR, Schardl CL. Genetics of host specificity in Epichloë typhina. Phytopathology. 1997;87:599–605. doi: 10.1094/PHYTO.1997.87.6.599. [DOI] [PubMed] [Google Scholar]
- Clay K. Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology. 1988;69:10–16. [Google Scholar]
- Clay K. The ecology and evolution of grasses. Agriculture, Ecosystems and Environment. 1993;44:39–64. [Google Scholar]
- Clay K. Defensive mutualism and grass endophytes: still valid after all these years? In: White J, Torres M, editors. Defensive Mutualism in Microbial Symbiosis. Boca Raton, FL: Taylor & Francis Group Publishers; 2008. pp. 9–20. [Google Scholar]
- Clay K, Schardl C. Evolutionary origin and ecological consequences of endophyte symbiosis with grasses. The American Naturalist. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- De Mazancourt C, Loreau M, Dieckmann U. Understanding mutualism when there is adaptation to the partner. Journal of Ecology. 2005;93:305–314. [Google Scholar]
- Denison RF, Bledsoe C, Kahn M, O'Gara F, Simms EL, Thomashow LS. Cooperation in the rhizosphere and the “free rider” problem. Ecology. 2003;84:838–845. [Google Scholar]
- Dinelli G, Bonetti A, Marotti I, Minelli M, Catizone P. Characterization of Italian populations of Lolium spp. resistant and susceptible to Diclofop by inter simple sequence repeat. Weed Science. 2004;52:554–563. [Google Scholar]
- Douglas AE. Symbiotic Interactions. Oxford, UK: Oxford University Press; 1994. [Google Scholar]
- Easton HS. Grasses and Neotyphodium endophytes: co-adaptation and adaptive breeding. Euphytica. 2007;154:295–306. [Google Scholar]
- Elam DR, Ridley CE, Goodell K, Ellstrand NC. Population size and relatedness affect fitness of a self-incompatible invasive plant. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:549–552. doi: 10.1073/pnas.0607306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeth SH. Are endophytic fungi defensive plant mutualists? Oikos. 2002;98:25–36. [Google Scholar]
- Faeth SH, Sullivan TJ. Mutualistic asexual endophytes in a native grass, are usually parasitic. The American Naturalist. 2003;161:310–325. doi: 10.1086/345937. [DOI] [PubMed] [Google Scholar]
- Firestone JL, Jasieniuk M. 2007. Demographic and genetic Allee effects interact in depressing reproduction in a weedy grass (Lolium multiflorum). PS 30-124. The ESA/SER Joint Meeting, California, US.
- Groppe K, Steinger T, Schmid B, Baur B, Boller T. Effects of habitat fragmentation on choke disease (Epichloë bromicola) in the grass Bromus erectus. Journal of Ecology. 2001;89:247–255. [Google Scholar]
- Gundel PE, Batista WB, Texeira M, Martínez-Ghersa MA, Omacini M, Ghersa CM. Neotyphodium endophyte infection frequency in annual grass populations: relative importance of mutualism and transmission efficiency. Proceedings of the Royal Society of London B. 2008;275:897–905. doi: 10.1098/rspb.2007.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel PE, Garibaldi LA, Tognetti PM, Aragón R, Ghersa CM, Omacini M. Imperfect vertical transmission of the endophyte Neotyphodium in exotic grasses in grasslands of the Flooding Pampa. Microbial Ecology. 2009;57:740–748. doi: 10.1007/s00248-008-9447-y. [DOI] [PubMed] [Google Scholar]
- Gundel PE, Martínez-Ghersa MA, Batista WB, Ghersa CM. Dynamics of Neotyphodium endophyte infection in ageing seed pools: incidence of differential viability loss of endophyte, infected seed, and non-infected seed. Annals of Applied Biology. 2010;156:199–209. [Google Scholar]
- Herre EA, Knowlton N, Mueller UG, Rehner SA. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends in Ecology and Evolution. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. [DOI] [PubMed] [Google Scholar]
- Hesse U, Schoberlein W, Wittenmayer L, Forster K, Warnstorff K, Diepenbrock W, Merbach W. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Science. 2003;58:407–415. [Google Scholar]
- Iannone LJ, Cabral D, Schardl CL, Rossi MS. Phylogenetic divergence, morphological and physiological differences distinguish a new Neotyphodium endophyte species in the grass Bromus auleticus from South America. Mycologia. 2009;101:340–351. doi: 10.3852/08-156. [DOI] [PubMed] [Google Scholar]
- Jauhar J. Cytogenetics of the Festuca-Lolium Complex Relevance to Breeding. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Kiers ET, West SA, Denison RF. Mediating mutualisms: the influence of farm management practices on the evolutionary maintenance of symbiont cooperation. Journal of Applied Ecology. 2002;39:745–754. [Google Scholar]
- Koga H, Christensen MJ, Bennett RJ. Incompatibility of some grass/Acremonium endophyte associations. Mycological Research. 1993;97:1237–1244. [Google Scholar]
- Malinowski DP, Belesky DP. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science. 2000;40:923–940. [Google Scholar]
- Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford, UK: WH Freeman & Co. Ltd; 1995. [Google Scholar]
- Moon CD, Scott DB, Schardl CL, Christensen MJ. The evolutionary origins of Epichlöe endophytes from annual ryegrasses. Mycologia. 2000;92:1103–1118. [Google Scholar]
- Müller CB, Krauss J. Symbiosis between grasses and asexual fungal endophytes. Current Opinion in Plant Biology. 2005;8:450–456. doi: 10.1016/j.pbi.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Piano E, Bertoli FB, Romani M, Tava A, Riccioni L, Valvassori M, Carroni AM, et al. Specificity of host-endophyte association in tall fescue populations from Sardinia, Italy. Crop Science. 2005;45:1456–1463. [Google Scholar]
- Radosevich SR, Holt JS, Ghersa CM. Ecology of Weeds and Invasive Plants: Relationship to Agriculture and Natural Resource Management. 3rd edn. Hoboken, NJ, USA: John Wiley & Sons Inc; 2007. [Google Scholar]
- Ravel C, Michalakis Y, Charmet G. The effect of imperfect transmission on the frequency of mutualistic seed-borne endophytes in natural populations of grasses. Oikos. 1997;80:18–24. [Google Scholar]
- Reynolds HL, Packer A, Bever JD, Clay K. Grassroots ecology: plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytologist. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Afkhami ME, Rúa MA, Davitt AJ, Hammer S, Huguet VM. A fungus among us: broad patterns of endophyte distribution in the grasses. Ecology. 2009;90:1531–1539. doi: 10.1890/08-0116.1. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Denison RF. Do plant parts compete for resources? An evolutionary perspective. New Phytologist. 2009;183:565–574. doi: 10.1111/j.1469-8137.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ. Fungal endophytes: a continuum of interactions with host plants. Annual Review of Ecology and Systematics. 1998;29:319–343. [Google Scholar]
- Saikkonen K, Ion D, Gyllenberg M. The persistence of vertically transmitted fungi in grass metapopulations. Proceedings of the Royal Society of London B. 2002;269:1397–1403. doi: 10.1098/rspb.2002.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikkonen K, Wäli P, Helander M, Faeth SH. Evolution of endophyte-plant symbioses. Trends in Plant Science. 2004;9:275–280. doi: 10.1016/j.tplants.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Lehtonen A, Helander M, Koricheva J, Faeth SH. Model systems in ecology: dissecting the endophyte-grass literature. Trends in Plant Science. 2006;11:428–433. doi: 10.1016/j.tplants.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annual Review in Plant Biology. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- Schulthess FM, Faeth SH. Distribution, abundances and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica Vasey) Mycologia. 1998;90:569–578. [Google Scholar]
- Selosse M-A, Schardl CL. Fungal endophytes in grasses: hybrids rescued by vertical transmission? An evolutionary perspective. New Phytologist. 2007;173:452–458. doi: 10.1111/j.1469-8137.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- Sullivan TJ, Faeth SH. Gene flow in the endophyte Neotyphodium and implications for coevolution with Festuca arizonica. Molecular Ecology. 2004;13:649–656. doi: 10.1046/j.1365-294x.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- Tadych M, Bergen M, Dugan FM, White JF., Jr Evaluation of the potential role of water in spread of conidia of the Neotyphodium endophyte of Poa ampla. Mycological Research. 2007;111:466–472. doi: 10.1016/j.mycres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Hastings A. Allee effects in biological invasions. Ecology Letters. 2005;8:895–908. [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution. Chicago, IL: University of Chicago Press; 2005. [Google Scholar]
- Thrall PH, Hochberg ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends in Ecology and Evolution. 2007;22:120–126. doi: 10.1016/j.tree.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Tikhonovich IA, Provorov NA. From plant-microbe interactions to symbiogenetics: a universal paradigm for the interspecies genetic integration. Annals of Applied Biology. 2009;154:341–350. [Google Scholar]
- Do Valle Ribeiro MAM. Transmission and survival of Acremonium and the implications for grass breeding. Agriculture, Ecosystem and Environments. 1993;44:195–213. [Google Scholar]
- Vila-Aiub MM, Martínez-Ghersa MA, Ghersa CM. Evolution of herbicide resistance in weeds: vertically transmitted fungal endophytes as genetic entities. Evolutionary Ecology. 2003;17:441–456. [Google Scholar]
- Wäli PR, Ahlholm JU, Helander M, Saikkonen K. Occurrence and genetic structure of the systemic grass endophyte Epichloë festucae in fine fescue populations. Microbial Ecology. 2007;53:20–29. doi: 10.1007/s00248-006-9076-2. [DOI] [PubMed] [Google Scholar]
- Wille PA, Aeschbacher RA, Boller T. Distribution of fungal endophyte genotypes in doubly infected host grasses. Plant Journal. 1999;18:349–358. doi: 10.1046/j.1365-313x.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Williams R, Bartholomew P. Factors affecting Italian ryegrass (Lolium multiflorum L.) seed distribution. In: Murphy JJ, editor. Utilization of Grazed Grass in Temperate Animal Systems. Netherlands: Wageningen Academic Publishers; 2005. p. 248. [Google Scholar]
- Yang B, Thorogood D, Armstead I, Barth S. How far are we from unravelling self-incompatibility in grasses? New Phytologist. 2008;178:740–753. doi: 10.1111/j.1469-8137.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- Van Zijll de Jong E, Dobrowolski MP, Bannan NR, Stewart AV, Smith KF, Spangenberg GC, Forster JW. Global genetic diversity of the perennial ryegrass fungal endophyte Neotyphodium lolii. Crop Science. 2008;48:1487–1501. [Google Scholar]


