Abstract
Prioritization of efforts to maintain biodiversity is an important component of conservation, but is more often applied to ecosystems or species than within species. We assessed distinctiveness among 27 populations of rainbow trout (Salmonidae: Oncorhynchus mykiss) from British Columbia, Canada, using microsatellite DNA variation (representing historical or contemporary demography) and morphology (representing adaptive variation). Standardized genetic scores, that is, the average deviation across individuals within populations from the overall genetic score generated by factorial correspondence analysis, ranged from 1.05 to 4.90 among populations. Similar standardized morphological scores, generated by principal components analysis, ranged from 1.19 to 5.35. There was little correlation between genetic and morphological distinctiveness across populations, although one population was genetically and morphologically the most distinctive. There was, however, a significant correlation (r = 0.26, P = 0.008) between microsatellite (FST) and morphological (PST) divergence. We combined measures of allelic richness, genetic variation within, and divergence among, populations and morphological variation to provide a conservation ranking of populations. Our approach can be combined with other measures of biodiversity value (habitat, rarity, human uses, threat status) to rationalize the prioritization of populations, especially for widespread species where geographic isolation across distinct environments promotes intraspecific variability.
Keywords: conservation prioritization, local adaptation, microsatellites, morphology, rainbow trout
Introduction
A challenging aspect of conservation biology is the prioritization of ecosystems, geographic areas, and individual species for actions to minimize biodiversity loss (Avise 2005; Brooks et al. 2006). Scientific investigations, and indeed those within the realm of evolutionary biology, are central to prioritization exercises (Meffe and Carroll 1994). Biological attributes such as the degree of endemism, per capita productivity, evolutionary history, rarity, and provision of ecosystem services are some of the criteria that can be used in isolation or in combination to assign conservation priority (see summary in Brooks et al. 2006). For example, Myers et al. (2000) identified 25 global ‘hotspots’ of endemism across several plant and animal groups that ranked highly in terms of the conservation of high numbers of species across relatively small areas.
There have been various attempts at developing criteria for identification and prioritization of intraspecific diversity for conservation. Prioritization of populations for conservation can be attempted by assembling information pertaining to: (i) the degree of threat to population persistence, and (ii) the biological consequences of loss of different populations (e.g., Given and Norton 1993; Allendorf et al. 1997). Threat status deals with issues related to the probability of extinction of specific populations and various criteria and guidelines are available for assessing status (e.g., Allendorf et al. 1997; International Union for the Conservation of Nature 2010). Similarly, the biological implications of the loss of populations can be aided by obtaining information about the ecological, genetic and evolutionary consequences of their loss (e.g., Allendorf et al. 1997; Crandall et al. 2000; Wood and Gross 2008). Initially, discussion on genetic and evolutionary legacy focused on how best to characterize and name such variation, that is, use of sub-specific or varietal designations, definition of ‘evolutionarily significant units’ (ESUs, Ryder 1986), ‘distinct population segments’ (Utter 1981), ‘designatable units’ (COSEWIC 2009), or other such descriptors. In many instances, single character types were proposed to define and prioritize intraspecific units for conservation whether they be genetic (Moritz 1994; Hedrick et al. 2001), morphological (e.g., Bush and Adams 2007; Seiler and Keeley 2009), or biogeographic (Myers et al. 2000) in nature. By contrast, it has long been recognized that concordance among a number of traits, especially among those that measure different aspects of organismal diversity, provide the strongest evidence of distinctiveness amongst a group of populations within taxa (e.g., Avise 1994; Crandall et al. 2000; Fraser and Bernatchez 2001) and this approach has also been extended across species to prioritize geographic areas for conservation (e.g., Moritz 2002). This is especially relevant for widespread species distributed across a variable landscape. In such instances, opportunities for physical isolation in distinct environments provide ideal conditions that promote intraspecific diversification. Without some method to capture such diversity in an efficient manner, however, it will be difficult to assign conservation priorities, and the typically scarce resources associated with such priorities, amongst a myriad of possibilities. Here, we describe a process to capture information on the ecological and evolutionary legacy relevant to conservation prioritization within species using a salmonid fish as a model system.
The rainbow trout, Oncorhynchus mykiss, is a salmonid fish (salmon, trout, char, whitefish, and grayling) native to the Pacific Basin, largely west of the continental divide in North America, including northern Mexico, and in the western Pacific in Kamchatka and south to the Amur River (Behnke 1992). A large portion of the species’ range occurs in British Columbia (BC), Canada, where it occurs in innumerable lakes and streams both as a freshwater-resident form (‘rainbow trout’) and as an anadromous (sea-run) form (‘steelhead trout’). The species is an extremely popular sportfish in BC and, indeed, worldwide, where it has been successfully introduced to all continents except Antarctica. Although globally a secure species (in most jurisdictions it is ranked as N5 and G5 –‘Secure’ by NatureServe 2009), in particular areas the species faces various threats from by-catch in salmon fisheries, habitat loss and degradation, and dams (e.g., Beacham et al. 1999; McKinney et al. 2001) and in the United States some ESUs of steelhead trout are listed as ‘Endangered’ or ‘Threatened’ under the Endangered Species Act (e.g., United States Department of Commerce 2006). As part of a program characterizing freshwater fish biodiversity in BC to effect conservation planning (e.g., Taylor et al. 1999; Parkinson et al. 2005) we have investigated the historical and contemporary factors that influence the extent and distribution of molecular and morphological variation in rainbow trout (e.g., Keeley et al. 2005; Tamkee et al. 2010). As a consequence of the biology of rainbow trout and their frequent interactions with human activities, there is a great need for characterizing biodiversity within the species and for developing prioritization methods useful in decision-making. In particular, in BC there have been several instances where specific populations of O. mykiss have been placed at potential risk owing to proposed resource developments and queries have been made as to the level of ‘uniqueness’ or ‘distinctiveness’ of these populations, yet no comparative analysis has been available to help objectively address such issues (E.B. Taylor, pers. observations).
In this study, we provide analyses that combine measures of genetic and morphological variation to be used to rank populations for conservation priority using data collected for O. mykiss populations in BC. We restrict the meaning of ‘conservation priority’ in the current context to mean generating priorities in terms of the genetic and evolutionary legacy of the species. We clearly recognize and support the importance and relevance of measures of threat status and ecological roles for defining priorities in certain contexts, but our analysis focuses on the situation where populations are all relatively pristine and/or where knowledge of ecological roles is either unknown or impractical to discern. We see our approach, therefore, as most applicable to situations where biodiversity managers may need to proactively evaluate populations in terms of which are most unusual or distinctive to establish reserves or assign them high conservation priority before they may be impacted. In addition, although quantitative methods for assessing distinctiveness for single data types have been developed (e.g., Crozier 1997; Petit et al. 1998; Bush and Adams 2007), and integrative approaches to setting conservation priorities have been proposed (e.g., Allendorf et al. 1997; Crandall et al. 2000), less attention has been paid to developing quantitative measures across data types. Given that molecular and morphological data are two of the easiest and most commonly collected kinds of data for many species, our approach should be applicable to a broad range of situations for other taxa.
Materials and methods
Collection of samples
Rainbow trout/steelhead were collected from 27 locations from throughout BC and for which both genetic and morphological data were available for individual fish (Fig. 1). The localities in this study ranged from multiple contiguous to noncontiguous habitats from the same watershed, to localities from different watersheds (Table 1) and totaled 1322 fish collected both from lakes and rivers. Samples were obtained from localities that contained only native and nonstocked rainbow trout (BC Ministry of Environment, stocking records unpublished data). A combination of angling, electroshocking, minnow trapping, and gill netting was used to collect fish as detailed in Keeley et al. (2005) and Tamkee et al. (2010). Most fish were freshwater-resident rainbow trout, but four steelhead trout populations were also sampled (Table 1). These samples represented replicate O. mykiss populations from six putative ecotype categories that were selected based on habitat characteristics (streams, lakes, sea-run/anadromous or freshwater resident) or the composition of fish species present (see definitions in Table S1).
Figure 1.
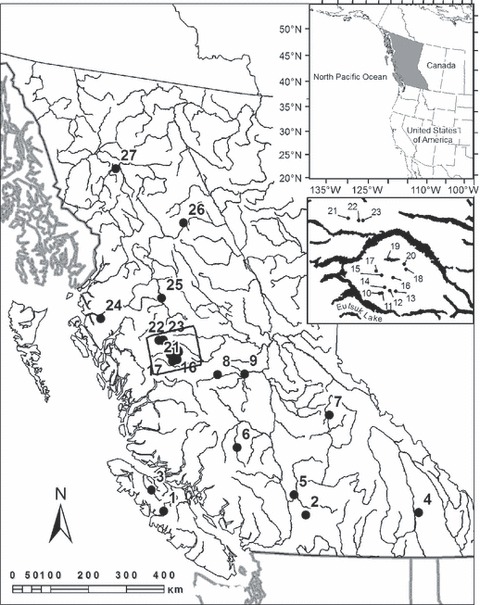
Map of collection localities for 27 populations of rainbow trout sampled from throughout British Columbia, Canada. Inset shows British Columbia (shaded area) in western North America. Names accompanying population number codes are given in Table 1.
Table 1.
Location of sampling localities and sample sizes used in genetic and morphological comparisons of rainbow trout (Oncorhynchus mykiss) populations. Also shown are allele richness, and expected heterozygosity for each locality. Population numbers correspond to localities in Fig. 1
| Waterbody* | Population number | Longitude (degree-decimal) | Latitude (degree-decimal) | Sample size morphology | Sample size genetics | Allelic richness† | Expected heterozygosity‡ |
|---|---|---|---|---|---|---|---|
| Gold River | 1 | 126°05.1′ | 49°50.2′ | 35 | 35 | 5.89 | 0.63 |
| Coldwater River | 2 | 120°50.6′ | 49°45.7′ | 35 | 35 | 4.80 | 0.55 |
| Nimpkish River | 3 | 126°35.1′ | 50°10.3′ | 35 | 35 | 5.17 | 0.62 |
| Fry Creek | 4 | 116°45.7′ | 50°00.6′ | 35 | 46 | 3.32 | 0.47 |
| Murray Creek | 5 | 121°22.2′ | 50°25.5′ | 35 | 38 | 2.69 | 0.39 |
| Fish Lake | 6 | 123°35.3′ | 51°25.4′ | 50 | 50 | 2.73 | 0.33 |
| Clearwater River | 7 | 120°10.2′ | 51°55.0′ | 50 | 54 | 4.30 | 0.49 |
| Kuyakuz Lake | 8 | 124°35.0′ | 53°05.7′ | 50 | 50 | 3.84 | 0.46 |
| Blackwater River | 9 | 123°30.9′ | 53°05.5′ | 50 | 50 | 5.20 | 0.59 |
| Blanchet Lake 3 | 10 | 126°25.3′ | 53°21.2′ | 50 | 50 | 2.38 | 0.21 |
| Blanchet Lake 2 | 11 | 126°23.1′ | 53°22.3′ | 50 | 50 | 2.50 | 0.35 |
| Blanchet Lake 1 | 12 | 126°18.0′ | 53°24.2′ | 50 | 50 | 2.67 | 0.36 |
| Tlutilias Lake | 13 | 126°14.4′ | 53°24.5′ | 50 | 50 | 2.35 | 0.31 |
| Grizzly Lake | 14 | 126°22.6′ | 53°24.5′ | 50 | 50 | 2.61 | 0.36 |
| Fenton Lake | 15 | 126°29.1′ | 53°30.0′ | 50 | 32 | 2.35 | 0.17 |
| Morgan Lake | 16 | 126°19.9′ | 53°30.0′ | 50 | 64 | 2.45 | 0.17 |
| Goodrich Lake | 17 | 126°31.8′ | 53°30.3′ | 50 | 32 | 2.06 | 0.19 |
| Theleteban Lake | 18 | 126°13.1′ | 53°35.3′ | 50 | 32 | 3.26 | 0.34 |
| Glatheli Lake | 19 | 126°20.1′ | 53°38.2′ | 50 | 160 | 3.24 | 0.36 |
| Ghitzeli Lake | 20 | 126°15.4′ | 53°38.0′ | 50 | 32 | 2.99 | 0.33 |
| Twinkle Lake | 21 | 127°01.1′ | 53°48.5′ | 50 | 95 | 3.12 | 0.37 |
| Skinny Lake | 22 | 126°53.6′ | 53°49.6′ | 50 | 50 | 3.18 | 0.43 |
| Horseshoe Lake | 23 | 126°50.4′ | 53°50.4′ | 50 | 32 | 3.64 | 0.46 |
| Khtada Lake | 24 | 129°25.5′ | 54°05.5′ | 50 | 35 | 2.04 | 0.20 |
| Canyon Creek | 25 | 126°45.6′ | 54°40.7′ | 35 | 32 | 2.16 | 0.22 |
| Moosevale Creek | 26 | 126°30.6′ | 56°35.8′ | 35 | 32 | 5.00 | 0.57 |
| Ealue Lake | 27 | 129°50.0′ | 57°45.3′ | 50 | 32 | 3.25 | 0.45 |
Geographic place names are from Canadian topographic maps of British Columbia. If no official place name was available from the map, we assigned an unofficial place name.
Mean across all 10 loci and for a minimum sample size of 32 individuals.
Mean across all 10 loci.
Microsatellite and morphological data collection
The genetic data used in our study consisted of allele frequency variation at 10 microsatellite DNA loci as described by Tamkee et al. (2010): Oneu14, Ssa197, Oneu8, Ssa85, Ssa456, Omy77, Ots3, Okia3, Ots100, and Ots103. Full details of DNA extraction and data collection, and basic population genetic analyses are described in Taylor et al. (2007) and Tamkee et al. (2010). Analyses presented in Tamkee et al. (2010) focused on individual population genetic and phylogeographic analyses. Our study used these data to provide a genetic distinctiveness score of each sample as described below. Raw allele frequency data are available at http://www.zoology.ubc.ca/~etaylor/nfrg/rbtr/evolapps/rbtrallfrevolapps.htm.
Similarly, morphological data were collected as described in Keeley et al. (2005). These morphological features represented a combination of external linear measurements, gill raker lengths and spacing, and internal organ masses (e.g., stomach, heart) related to swimming and feeding mechanics (Keeley et al. 2005; see also their Appendix 1). In addition, Keeley et al. (2007) demonstrated that differences in these morphological traits among these populations had a significant genetic component and these authors argued that such morphological distinction was, at least in part, a response to natural selection in contrasting environments.
Data analyses
Genetic data
We assessed conformance to Hardy-Weinberg and linkage equilibria using GENEPOP (version 4.0 updated from Raymond and Rousset 1995; Table S2). The microsatellite DNA and morphological analyses both were subject to summary ordination analyses. First, the microsatellite DNA allele frequency data were subject to factorial correspondence analysis (FCA), which is ideal for categorical allele frequency counts, using GENETIX 4.05.02 (Belkhir et al. 2004). Upon completion of the FCA, individual fish correspondence scores were used to calculate an overall mean, across all populations, across each of the three FCA axes (see below) using the general spreadsheet-based statistical software program PAST version 1.98 (Hammer et al. 2001). A genetic distinctiveness score (GDS) was calculated for each population by taking the average value of the absolute difference between each fish's score along a particular axis from the overall, across-population mean score, weighted by the percentage of variation accounted for by that axis, and summing these values across each of the three FCA axes. FCA values were standardized to normal Z-scores and a constant of 3 added to generate positive values and to facilitate comparison with morphological variation (see below). We also calculated Weir and Cockerham's (1984) pairwise θ as an estimate of FST (the proportion of the total microsatellite DNA allele frequency variation attributable to differences between populations) using FSTAT (Goudet 2001).
We also applied the approach of Petit et al. (1998) to assess the distinctiveness of rainbow trout based on microsatellite DNA variation in terms of variation within, and divergence between, populations simultaneously. The method of Petit et al. (1998) as implemented in the software CONTRIB (available at http://www.pierroton.inra.fr/genetics/labo/Software/) is a decompositional analysis that determines the contribution (C) of each population to the total genetic diversity (CT) in a sample of some n number of populations by comparing the total diversity including all populations to that after removing each population in turn. In addition, the individual population contributions to CT can be decomposed into components attributable to variation within a particular population (CS) and to its degree of divergence from all other populations (CD). Moreover, the method of Petit et al. (1998) can be applied to variation in allele richness (CTr = total allele richness, CSr = component of total allele richness attributable to within population allele richness and CDr = component of total allele richness attributable to among population variation) after adjusting for differences in sample size by rarefaction (El Mousadik and Petit 1996). The various values of C can be negative if a particular population's contribution to any measure of diversity or divergence is less than the overall average, or positive if its values are higher than the overall average (Petit et al. 1998).
Morphological data
As is common practice in studies of morphological shape variation, we used log10- transformed and size-adjusted data to minimize the effects of overall body size variation among samples on interpretations of body shape differences as described in Keeley et al. (2005). These data were then subject to a principal components analysis (PCA) on the inter-trait correlation matrix and an individual PCA score for each fish was calculated using PAST. A morphological distinctiveness score (MDS) was calculated for each population by taking the average value of the absolute difference between each fish's score along a particular axis from the overall, across-population mean score, weighted by the percentage of variation accounted for by that axis, and summing these values across the first three PCA axes. Results were summarized across the first three axes only as they contributed the most to the total morphological variance and analyzing up to five axes total did not affect the relative ranking of populations in morphological space (see Results). Principal Component scores were standardized to normal Z-scores and a constant of 3 added to generate positive values and to facilitate comparison with the FCA scores described earlier.
We calculated pairwise estimates of PST, or the proportion of the total morphological variance attributable to differences between populations, following Leinonen et al. (2006) and Phillimore et al. (2008) using multivariate analysis of variance (MANOVA) to estimate variance components in pairwise comparisons. The calculation of pairwise PST was based on the external morphological measurements only because internal organ sizes contributed relatively little to among population differences and because variation in these traits was not assessed for any genetic component (Keeley et al. 2005, 2007). Keeley et al. (2007) demonstrated some genetic basis to the morphological variation that we examined, but we have no empirical estimates of heritability (h2) for the populations or traits that we studied. Rainbow trout have, however, been subject to many quantitative genetic studies, typically for growth-related traits (e.g., Thorgaard et al. 2002), and Leary et al. (1985) estimated a mean (±SD) heritability of 0.66 (0.28) across eight meristic traits in a strain of rainbow trout. Consequently, we employed a h2 of 0.50 in our calculations of PST. Employing other values from did not affect our results involving PST (see Results). We tested the significance of an association between the pairwise PST (reflecting environmental and some adaptive divergence) and FST (reflecting neutral genetic divergence) using a Mantel test, with 5000 permutations of the matrices, using FSTAT.
Finally, we ranked each population in terms of its GDS, MDS, and the six C parameters. Most of the latter were strongly correlated with each other and with GDS (see Results). Consequently, we used only two measures of genetic variation, CDr and CT, which represented divergence in allelic richness among populations and the contribution of each population to total genetic diversity, respectively, because they were not correlated with each other, but were correlated with all other measures. We then calculated the mean rank across three measures of diversity (MDS, CDr, and CT) for each population.
Results
Microsatellite DNA variation
We collected microsatellite data for 27 populations (1322 individuals) for which we also had detailed morphological data. The number of alleles observed across all populations ranged from two (Ssa197) to 38 (Oki3a) with an average of 17.1 alleles per locus (Table S2). Observed heterozygosity averaged 0.42 across all loci and populations and ranged from 0.24 (Ots103) to 0.66 (Oki3a), respectively (Table S2).
Virtually all sample sites were in Hardy-Weinberg equilibrium with only 10 out of possible 270 (10 loci × 27 localities) tests showing statistically significant heterozygote deficits. These exceptions were found at several separate loci in 10 different populations (Table S2). Tests for linkage disequilibrium resulted in significant departures in four out of possible 1215 tests. Similarly, the significant departures were not concentrated on particular locus pairs or within specific populations.
The FCA summarized 41% of the total allele frequency variation across the first three axes and suggested the presence of three groups of populations: Khtada Lake and Murray Creek rainbow trout were distinct from each other and all other populations, populations from the upper Fraser River, and fish from a diversity of areas including the mid-Fraser and Thompson rivers, upper Columbia River, Skeena River, and Vancouver Island (Fig. 2A). When the standardized deviation of the average FCA score for each population from the overall average across all populations for the three axes was calculated it ranged from 1.05 (Blackwater River) to 4.90 (Murray Creek, Fig. 3A). Pairwise FST (θ) averaged 0.33 and ranged from 0.015 (between two steelhead populations – Gold River and Nimpkish River) to 0.79 (between Khtada Lake of the Skeena River system and Morgan Lake of the upper Fraser River system, Table 2).
Figure 2.
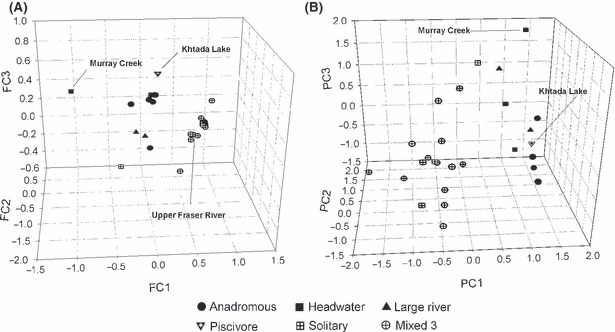
Mean population factorial correspondence (A) and principal component scores (B) for rainbow trout (Oncorhynchus mykiss) populations assayed at 10 microsatellite loci and 16 morphological and anatomical characters. Population ecotypes are defined in Table 3 and Table S1.
Figure 3.
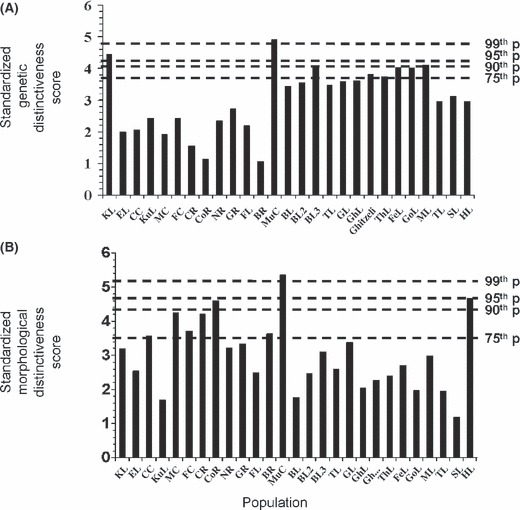
Relative values of standardized genetic distinctiveness score (GDS, A) and standardized morphological distinctiveness score (MDS, B) calculated for 27 populations of rainbow trout (Oncorhynchus mykiss). A constant of 3 was added to each score to make all values positive. Higher values indicate greater distinctiveness relative to the overall average score. Dashed horizontal lines represent indicated percentile values for GDS and MDS. Population codes are defined in Table 2.
Table 2.
Pairwise microsatellite-based FST (θ, upper diagonal) and morphological-based PST (lower diagonal) between 27 populations of rainbow trout (Oncorhynchus mykiss)
| KL | EL | CC | KuL | MC | FC | CR | CoR | NR | GR | FL | BR | MuC | BL | BL2 | BL3 | TL | GL | GlL | GhL | ThL | FeL | GoL | ML | TwL | SL | HL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KL | – | 0.58 | 0.74 | 0.54 | 0.52 | 0.51 | 0.48 | 0.47 | 0.41 | 0.40 | 0.65 | 0.42 | 0.57 | 0.61 | 0.60 | 0.73 | 0.67 | 0.61 | 0.61 | 0.69 | 0.68 | 0.77 | 0.78 | 0.79 | 0.63 | 0.62 | 0.63 |
| EL | 0.24 | – | 0.40 | 0.31 | 0.22 | 0.36 | 0.33 | 0.33 | 0.20 | 0.17 | 0.51 | 0.23 | 0.38 | 0.34 | 0.36 | 0.50 | 0.46 | 0.35 | 0.41 | 0.40 | 0.39 | 0.52 | 0.51 | 0.56 | 0.33 | 0.32 | 0.28 |
| CC | 0.28 | 0.42 | – | 0.48 | 0.37 | 0.49 | 0.49 | 0.47 | 0.42 | 0.37 | 0.63 | 0.42 | 0.57 | 0.53 | 0.53 | 0.69 | 0.62 | 0.53 | 0.47 | 0.54 | 0.50 | 0.59 | 0.63 | 0.64 | 0.44 | 0.42 | 0.43 |
| KuL | 0.28 | 0.37 | 0.22 | – | 0.38 | 0.37 | 0.31 | 0.30 | 0.27 | 0.27 | 0.54 | 0.22 | 0.43 | 0.22 | 0.22 | 0.40 | 0.31 | 0.21 | 0.36 | 0.34 | 0.31 | 0.38 | 0.39 | 0.44 | 0.33 | 0.27 | 0.27 |
| MC | 0.35 | 0.47 | 0.24 | 0.57 | – | 0.34 | 0.32 | 0.27 | 0.17 | 0.13 | 0.38 | 0.25 | 0.33 | 0.38 | 0.39 | 0.53 | 0.44 | 0.39 | 0.36 | 0.36 | 0.35 | 0.48 | 0.48 | 0.53 | 0.35 | 0.31 | 0.26 |
| FC | 0.22 | 0.31 | 0.19 | 0.41 | 0.16 | – | 0.16 | 0.19 | 0.23 | 0.22 | 0.41 | 0.15 | 0.36 | 0.43 | 0.44 | 0.53 | 0.49 | 0.43 | 0.48 | 0.45 | 0.44 | 0.54 | 0.54 | 0.59 | 0.42 | 0.40 | 0.39 |
| CR | 0.20 | 0.40 | 0.09 | 0.34 | 0.21 | 0.08 | – | 0.15 | 0.21 | 0.20 | 0.39 | 0.08 | 0.33 | 0.37 | 0.39 | 0.49 | 0.44 | 0.38 | 0.46 | 0.43 | 0.41 | 0.50 | 0.52 | 0.57 | 0.39 | 0.36 | 0.36 |
| CoR | 0.26 | 0.49 | 0.04 | 0.37 | 0.29 | 0.20 | 0.07 | – | 0.18 | 0.15 | 0.28 | 0.09 | 0.17 | 0.35 | 0.36 | 0.46 | 0.41 | 0.35 | 0.40 | 0.38 | 0.38 | 0.50 | 0.49 | 0.55 | 0.39 | 0.36 | 0.33 |
| NR | 0.31 | 0.49 | 0.21 | 0.51 | 0.30 | 0.31 | 0.24 | 0.25 | – | 0.14 | 0.36 | 0.12 | 0.21 | 0.30 | 0.31 | 0.44 | 0.36 | 0.30 | 0.36 | 0.35 | 0.35 | 0.47 | 0.46 | 0.52 | 0.36 | 0.32 | 0.29 |
| GR | 0.21 | 0.42 | 0.14 | 0.38 | 0.29 | 0.22 | 0.12 | 0.15 | 0.08 | – | 0.36 | 0.11 | 0.20 | 0.29 | 0.30 | 0.43 | 0.37 | 0.29 | 0.34 | 0.33 | 0.33 | 0.45 | 0.44 | 0.51 | 0.34 | 0.31 | 0.27 |
| FL | 0.35 | 0.49 | 0.27 | 0.11 | 0.61 | 0.47 | 0.39 | 0.37 | 0.54 | 0.43 | – | 0.31 | 0.36 | 0.56 | 0.56 | 0.68 | 0.59 | 0.57 | 0.50 | 0.56 | 0.55 | 0.66 | 0.66 | 0.69 | 0.52 | 0.51 | 0.49 |
| BR | 0.39 | 0.56 | 0.20 | 0.21 | 0.62 | 0.46 | 0.36 | 0.32 | 0.57 | 0.46 | 0.08 | – | 0.20 | 0.25 | 0.27 | 0.40 | 0.31 | 0.26 | 0.37 | 0.34 | 0.32 | 0.42 | 0.43 | 0.49 | 0.34 | 0.30 | 0.29 |
| MuC | 0.57 | 0.72 | 0.39 | 0.57 | 0.72 | 0.61 | 0.57 | 0.51 | 0.72 | 0.66 | 0.40 | 0.34 | – | 0.43 | 0.44 | 0.59 | 0.48 | 0.43 | 0.47 | 0.51 | 0.50 | 0.63 | 0.62 | 0.66 | 0.49 | 0.48 | 0.43 |
| BL | 0.27 | 0.14 | 0.40 | 0.32 | 0.53 | 0.42 | 0.44 | 0.50 | 0.46 | 0.40 | 0.45 | 0.55 | 0.71 | – | 0.03 | 0.35 | 0.16 | 0.01 | 0.24 | 0.26 | 0.22 | 0.34 | 0.33 | 0.37 | 0.33 | 0.30 | 0.30 |
| BL2 | 0.28 | 0.27 | 0.31 | 0.31 | 0.46 | 0.38 | 0.37 | 0.42 | 0.31 | 0.28 | 0.45 | 0.53 | 0.72 | 0.10 | – | 0.41 | 0.23 | 0.04 | 0.26 | 0.31 | 0.23 | 0.33 | 0.39 | 0.43 | 0.35 | 0.32 | 0.32 |
| BL3 | 0.41 | 0.27 | 0.54 | 0.61 | 0.56 | 0.49 | 0.56 | 0.65 | 0.55 | 0.54 | 0.68 | 0.74 | 0.83 | 0.22 | 0.25 | – | 0.47 | 0.34 | 0.44 | 0.42 | 0.46 | 0.63 | 0.55 | 0.58 | 0.43 | 0.44 | 0.46 |
| TL | 0.33 | 0.18 | 0.49 | 0.56 | 0.50 | 0.42 | 0.50 | 0.59 | 0.51 | 0.48 | 0.64 | 0.71 | 0.82 | 0.17 | 0.23 | 0.08 | – | 0.17 | 0.32 | 0.37 | 0.37 | 0.50 | 0.39 | 0.44 | 0.40 | 0.36 | 0.39 |
| GL | 0.40 | 0.25 | 0.54 | 0.58 | 0.62 | 0.53 | 0.58 | 0.66 | 0.60 | 0.57 | 0.66 | 0.74 | 0.84 | 0.12 | 0.20 | 0.07 | 0.09 | – | 0.23 | 0.27 | 0.22 | 0.33 | 0.36 | 0.31 | 0.32 | 0.29 | 0.30 |
| GlL | 0.40 | 0.35 | 0.40 | 0.52 | 0.43 | 0.42 | 0.46 | 0.51 | 0.38 | 0.39 | 0.61 | 0.66 | 0.78 | 0.25 | 0.15 | 0.23 | 0.19 | 0.22 | – | 0.08 | 0.07 | 0.22 | 0.18 | 0.18 | 0.23 | 0.20 | 0.20 |
| GhL | 0.40 | 0.34 | 0.36 | 0.47 | 0.46 | 0.42 | 0.45 | 0.50 | 0.41 | 0.40 | 0.57 | 0.63 | 0.76 | 0.22 | 0.12 | 0.26 | 0.24 | 0.25 | 0.08 | – | 0.05 | 0.30 | 0.16 | 0.16 | 0.18 | 0.16 | 0.15 |
| ThL | 0.53 | 0.49 | 0.45 | 0.60 | 0.51 | 0.53 | 0.57 | 0.59 | 0.53 | 0.54 | 0.67 | 0.71 | 0.80 | 0.42 | 0.33 | 0.40 | 0.41 | 0.43 | 0.24 | 0.14 | – | 0.15 | 0.19 | 0.19 | 0.18 | 0.14 | 0.15 |
| FeL | 0.30 | 0.23 | 0.31 | 0.25 | 0.44 | 0.39 | 0.38 | 0.39 | 0.33 | 0.30 | 0.37 | 0.45 | 0.61 | 0.07 | 0.05 | 0.23 | 0.21 | 0.16 | 0.15 | 0.10 | 0.25 | – | 0.35 | 0.35 | 0.31 | 0.27 | 0.31 |
| GoL | 0.26 | 0.27 | 0.28 | 0.19 | 0.47 | 0.38 | 0.35 | 0.36 | 0.31 | 0.26 | 0.31 | 0.39 | 0.60 | 0.11 | 0.07 | 0.33 | 0.30 | 0.27 | 0.23 | 0.19 | 0.38 | 0.04 | – | 0.03 | 0.28 | 0.25 | 0.30 |
| ML | 0.42 | 0.29 | 0.44 | 0.49 | 0.47 | 0.43 | 0.49 | 0.52 | 0.44 | 0.43 | 0.58 | 0.63 | 0.72 | 0.22 | 0.22 | 0.20 | 0.19 | 0.19 | 0.12 | 0.11 | 0.21 | 0.13 | 0.23 | – | 0.30 | 0.27 | 0.32 |
| TwL | 0.38 | 0.42 | 0.26 | 0.27 | 0.49 | 0.45 | 0.40 | 0.38 | 0.38 | 0.35 | 0.35 | 0.41 | 0.59 | 0.28 | 0.19 | 0.46 | 0.44 | 0.42 | 0.33 | 0.24 | 0.29 | 0.13 | 0.17 | 0.33 | – | 0.03 | 0.08 |
| SL | 0.35 | 0.33 | 0.31 | 0.46 | 0.35 | 0.34 | 0.36 | 0.42 | 0.32 | 0.32 | 0.56 | 0.60 | 0.73 | 0.25 | 0.15 | 0.24 | 0.21 | 0.27 | 0.09 | 0.05 | 0.15 | 0.16 | 0.23 | 0.16 | 0.25 | – | 0.06 |
| HL | 0.60 | 0.52 | 0.55 | 0.62 | 0.60 | 0.61 | 0.64 | 0.64 | 0.58 | 0.59 | 0.67 | 0.71 | 0.78 | 0.44 | 0.41 | 0.42 | 0.45 | 0.42 | 0.34 | 0.28 | 0.17 | 0.29 | 0.42 | 0.22 | 0.37 | 0.34 | – |
KL, Khtada Lake; EL, Ealue Lake; CC, Canyon Creek; KuL, Kuyakuz Lake; MC, Moosevale Creek; FC, Fry Creek; CR, Clearwater River; CoR, Coldwater River; NR, Nimpkish River; GR, Gold River; FL, Fish Lake; BR, Blackwater River; MuC, Murray Creek; BL, Blanchet Lake; BL2, Blanchet Lake 2; BL3, Blanchet Lake 3; TL, Tlutilias Lake; GL, Grizzly Lake; GhL, Glatheli Lake; ThL, Theleteban Lake; FeL, Fenton Lake; GoL, Goodrich Lake; ML, Morgan Lake; TwL, Twinkle Lake; SL, Skinny Lake; HL, Horseshoe Lake.
There was a wide range of contributions of various populations to the total microsatellite variation in allele frequency (Fig. 4A). For instance, Khtada Lake (no. 24, Fig. 4A) contributed the most to the total diversity (CT), and this was due to its high degree of divergence from other populations because in terms of variation within populations it was below average. By contrast, Gold and Nimpkish and rivers (nos. 1 and 3, Fig. 4A) contributed the next highest to CT, but this was a function of their high within-population diversities; they were below average in terms of their divergence from other populations. Some populations, such as Blanchet 2, Blanchet 1, and Grizzly lakes had negative contribution values because they were below the average in terms both of within population diversity and divergence from other populations (nos. 11, 12, and 14, Fig. 4A). Upon rarefaction to a minimum sample size of 50 alleles, allelic richness across loci varied from a low of 1.9 (Ssa197) to 8.6 (Oki3a). Populations had variable contributions to total allelic richness (CTr), although in general a smaller number of populations contributed to total allelic richness diversity (2) than to total genetic diversity (12, Fig. 4B). Typically, the Blackwater, Nimpkish, Gold rivers, Moosevale Creek, and Ealue Lake had the highest allelic richness within populations (usually greater than the mean across populations, black bars Fig. 4B). By contrast, most populations (with the exception of a few such as Khtada Lake and Canyon Creek) tend to show little divergence from each other in allelic richness resulting in overall low net values for allelic richness diversity (white bars, Fig. 4B). Finally, there were no occurrences of private alleles in the strict sense; that is, all alleles were found in at least two populations at a frequency of at least 1% (Table S3). One population, Fry Creek, did have three alleles at three different loci that were each present at a frequency of >0.25, but which were found at an average frequency of <0.02 across the other 26 populations (Table S3).
Figure 4.
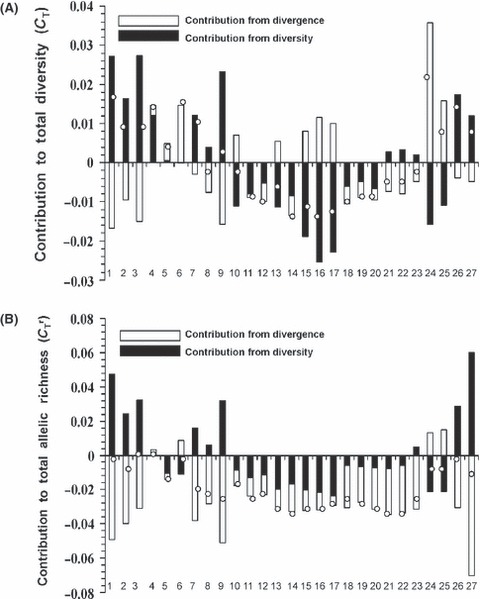
Contribution to (A) total microsatellite diversity (CT) and (B) total allelic richness (CTr) of each population of rainbow trout (Oncorhynchus mykiss), decomposed into a within population diversity (black bars) and among population divergence (white bars) component. The open circle indicates the total diversity/allelic richness of each population. The numbers 1–27 represent populations as ordered in Table 1.
Morphological variation
Sixty percent of the total morphological variation across the 27 populations was summarized across three principal components (Table S4). Contrasts included fish with large body parts and organs in general and those with high pyloric caecal mass and long gill rakers (PC1), large internal organs and those with deep bodies/caudal peduncles and long gill rakers (PC2), deep bodies, long gill rakers, large caecal and liver masses and fish with large eyes and mouths (PC3). To summarize, PC1 distinguished stream-dwelling fish and piscivorous fish as well as those from mixed 1 lake types from all other lake-dwelling ecotypes (Fig. 2B). Principal component 2 separated anadromous and headwater fish from all others, and PC3 separated all stream ecotypes from lake-dwelling fish (see also Keeley et al. 2005).
When the standardized deviation of the average PCA score for each population from the overall average across all populations for the three axes was calculated, it ranged from 1.19 (Skinny Lake) to 5.35 (Murray Creek, Fig. 3B). Calculation of PST indicated that there was considerable variability in the extent to which populations differed morphologically from one another; PST averaged 0.39 and ranged from a low of 0.038 (between Canyon Creek and Coldwater River) to a high of 0.84 (between Murray Creek and Grizzly Lake) with most pairwise comparisons between Murray Creek and all other populations being the highest, typically exceeding 0.70 (Table 2).
Comparisons between microsatellite and morphology
When the average genetic deviation score (GDS) and the average morphological deviation score (MDS) were compared among populations there was a slight, but insignificant, negative correlation between the two measures (Fig. 5, r = −0.23, P = 0.26). One population, however, was highly divergent both using microsatellites and morphology and when this population (Murray Creek) was removed, there was a moderate and significant negative correlation between the two measures of deviation (r = −0.44, P = 0.014); that is, greater morphological deviation from the ‘typical’ rainbow trout tended to be associated with lower microsatellite deviation (and vice versa). Furthermore, there were significant negative correlations between GDS and CT and CTr across populations (r = −0.56 and −0.51, both P < 0.005), but significant positive correlations between MDS and both CT and CTr (r = 0.48 and 0.58, P = 0.01 and 0.001, respectively, Table S5). Finally, a Mantel test comparison of the PST and FST matrices indicated that there was a broad tendency for high levels of pairwise morphological divergence to be associated with high values of pairwise microsatellite DNA divergence across a broad range of h2 values used in calculating PST (i.e., h2 = 0.25–0.75, Z = 45.6–76.5, r = 0.25–0.28, P = 0.01–0.008, e.g., Fig. 6).
Figure 5.
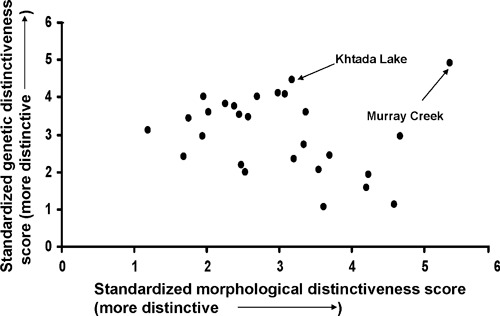
Biplot of genetic distinctiveness score (GDS) and morphological distinctiveness score (MDS) calculated for 27 populations of rainbow trout (Oncorhynchus mykiss).
Figure 6.
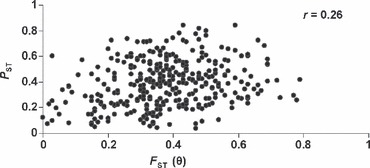
Bivariate plot of pairwise FST (θ) and PST calculated for 27 populations of rainbow trout (Oncorhynchus mykiss).
Populations were ranked based on their MDS, CT and CDr, and the average of these ranks calculated (Table 3). Canyon Creek, Fry Creek, Murray Creek, Moosevale Creek, Horseshoe Lake and Fish Lake, were the top five populations in terms of average ranks (two were tied), that is, top ranked populations were the most divergent morphologically, contributed the most to total genetic diversity and were the most divergent in terms of allelic richness.
Table 3.
Rankings of genetic distinctiveness score (GDS), divergence in allelic richness (CDr), total genetic diversity (CT), morphological distinctiveness score (MDS), mean rank of CDr, CT, and MDS, ecotype characterization, and fish community present in sympatry for 27 populations of rainbow trout (Oncorhynchus mykiss)
| Waterbody | GDS rank | CDr rank | CT rank | MDS rank | Mean rank | Ecotype | Fish species present |
|---|---|---|---|---|---|---|---|
| Khtada Lake | 2 | 22 | 1 | 12 | 11.7 | Piscivore | RB-KO-DV |
| Ealue Lake | 23 | 27 | 10 | 17 | 18.0 | Solitary | RB |
| Canyon Creek | 22 | 1 | 4 | 8 | 4.3 | Headwater | RB-BT-CT |
| Kuyakuz Lake | 19 | 19 | 14 | 26 | 19.6 | Mixed species–3 | RB-KO-LSU-LKC-MW-CA- NSC |
| Moosevale Creek | 24 | 20 | 4 | 4 | 9.3 | Anadromous | RB-CH |
| Fry Creek | 18 | 4 | 3 | 6 | 4.3 | Headwater | RB |
| Clearwater River | 25 | 23 | 8 | 5 | 12.0 | Large river | RB-CH |
| Coldwater River | 26 | 24 | 11 | 3 | 12.7 | Anadromous | RB-CH-CC |
| Nimpkish River | 20 | 21 | 6 | 11 | 12.7 | Anadromous | RB-CO-CH |
| Gold River | 17 | 25 | 7 | 10 | 14.0 | Anadromous | RB-CO-CH |
| Fish Lake | 21 | 3 | 2 | 18 | 7.7 | Solitary | RB |
| Blackwater River | 27 | 26 | 9 | 7 | 14.0 | Large river | RB-NSC-MW |
| Murray Creek | 1 | 5 | 12 | 1 | 6.0 | Headwater | RB |
| Blanchet Lake | 13 | 10 | 19 | 25 | 18.0 | Mixed species–3 | RB-LNC-LKC |
| Blanchet Lake 2 | 11 | 9 | 20 | 19 | 16.0 | Mixed species–3 | RB-LNC-LKC |
| Blanchet Lake 3 | 4 | 7 | 15 | 13 | 11.7 | Mixed species–3 | RB-LNC-LKC |
| Tlutilias Lake | 12 | 11 | 23 | 16 | 16.7 | Mixed species–3 | RB-LSC-LNC-LKC |
| Grizzly Lake | 10 | 14 | 26 | 9 | 16.3 | Solitary | RB |
| Glatheli Lake | 9 | 13 | 18 | 22 | 17.7 | Mixed species–3 | RB-MW-LNC-LSC-LKC |
| Ghitzeli Lake | 7 | 14 | 21 | 21 | 18.7 | Mixed species–3 | RB-KO-LNC-LSC-NSC-LKC |
| Theleteban Lake | 8 | 16 | 22 | 20 | 19.3 | Mixed species–3 | RB-KO-LNC-LSC-NSC |
| Fenton Lake | 5 | 12 | 24 | 15 | 17.0 | Mixed species–3 | RB-LKC-LNC |
| Goodrich Lake | 6 | 6 | 25 | 23 | 18.0 | Mixed species–3 | RB-LKC-LNC |
| Morgan Lake | 3 | 5 | 27 | 14 | 15.3 | Mixed species–3 | RB-LKC-LNC |
| Twinkle Lake | 16 | 17 | 17 | 24 | 19.3 | Mixed species–3 | RB-LSC-LNC-NSC-MW-BT- CC |
| Skinny Lake | 14 | 17 | 16 | 27 | 20.0 | Mixed species–3 | RB-KO-CA-LNC-LSC-MW- BB-NSC |
| Horseshoe Lake | 15 | 2 | 13 | 2 | 5.7 | Mixed species–3 | RB-MW-KO-LNC-LSC |
The top five populations in terms of mean ranking, and their characteristics, are indicated by boldface type. A higher mean rank (e.g., a rank of 1 versus a rank of 4) means that population was most divergent morphologically while displaying the greatest divergence from all others in allelic richness and contributing the most to total genetic diversity (a combination of diversity within populations and divergence from all other populations).
RB, rainbow trout (Oncorhynchus mykiss); KO, kokanee (O. nerka); LSC, largescale sucker (Catostomus macrocheilus); LNC, longnose sucker (C. catostomus); LKC, lake chub (Couesius plumbeus); RSC, redside shiner (Richardsonius balteatus); BT, bull trout (Salvelinus confluentus); DV, Dolly Varden (S. malma); CO, coho salmon (O. kisutch); CH, chinook salmon (O. tshawytscha); CT, cutthroat trout (O. clarkii); CA, prickly sculpin (Cottus asper); CC, unidentified sculpin species (Cottus spp.); MW, mountain whitefish (Prosopium williamsoni); BB, burbot (Lota lota); SU, unidentified sucker species (Catostomus spp.); NSC, northern pikeminnow (Ptychocheilus oregonensis); PL, Pacific lamprey (Entosphenus tridentatus).
Discussion
Measures of microsatellite and morphological variability
We examined presumptive neutral genetic variation using microsatellite DNA and presumptive adaptive variation in external morphology and internal anatomy in a geographically widespread species. Molecular variation in rainbow trout results from the impacts of: (i) isolation and postglacial dispersal from two main glacial refugia (a ‘coastal’ refuge and an ‘interior’ refuge), and (ii) contemporary restrictions in gene flow imposed, at least in part, by extrinsic landscape features such as distance, presence of waterfall migration barriers, isolated lake habitats, and underlying geomorphology (McCusker et al. 2000; Tamkee et al. 2010). Consequently, while not of obvious intrinsic value in-and-of-itself, neutral molecular variation represents a proxy measure of the history or ‘bioheritage’ of a taxon as well as reflecting aspects of its contemporary biology (e.g., demographic bottlenecks in population size) and future evolutionary potential – the three temporal scales of conservation (Bowen 1999).
By contrast, we have described patterns of morphological and anatomical variation among rainbow trout populations that appear, at least in part, to represent evolved differences of functional significance to persistence of specific populations in contrasting environments, that is, adaptive variation (Keeley et al. 2005, 2007). To a large degree, most of the variation occurred along an axis that differentiated stream-dwelling from lake-dwelling fish, and piscivorous populations from those with more generalist diets. These findings are consistent with the generally accepted importance of hydrodynamic and trophic environmental features in promoting adaptive variation in fishes (e.g., McGuigan et al. 2003; Kocher 2004; Langerhans 2008). Consequently, the morphological variation we have described might be important for population persistence in particular contemporary environments as well as providing a reservoir of evolutionary potential for adaptation to future changing environments. Langerhans (2009), for instance, demonstrated the importance of post-Pleistocene divergence in morphology to survival of Bahamas mosquitofish (Gambusia hubbsi) in environments that varied in predation pressure.
One challenge to using morphological variation in a diversity of taxa is in selecting which traits should be assayed. The value of using morphological variation in the present context lies in the evidence for its genetic basis and functional significance – the variation in the traits assayed reflect, in part, genetic variation that contributes to performance differences within specific environments which has promoted evolutionary divergence in these traits across populations (Keeley et al. 2005, 2007). More generally, when selecting morphological traits it will be critical to have a basic understanding of the genetics of variation and the possible role of plasticity in phenotypic expression (e.g., Pakkasmaa and Piironen 2000; Keeley et al. 2007). In addition, while some morphological traits may have relatively simple and common genetic architecture across populations (e.g., Colosimo et al. 2004), cases of multifarious morphological distinction that we have described will almost surely be considerably more complex in the number, identity, and action of genes that control such variation. Understanding the genetic architecture of such traits is challenging (Mackay 2003), but should not be necessary to detail as long as the basic requirement that aspects of phenotype are genetically controlled in a way that can respond to selection and lead to evolutionary change is met. Thus, it is also important to have some a priori basis for the functional significance of such variation in natural environments such that the traits examined reflect an assessment of the ecological exchangeability of populations (sensuCrandall et al. 2000). This will require background breeding studies, studies of phenotype-environment associations or experimental functional studies of the significance of morphological variation. Natural variation in traits as diverse as shell shape in mollusks (Conde-Padín et al. 2009) and pigmentation patterns in mice (Mullen and Hoekstra 2008) indicate that morphological variants across a diversity of taxa are amenable to experimental studies to assess their adaptive potential for use as a measure of ecological legacy as a component of conservation prioritization. Even in the absence of knowledge of the genetic control or architecture of specific traits, variation in morphology could still be used in conservation ranking schemes. For instance, extensive phenotypic plasticity may be important for population persistence in variable environments. In this case, phenotypic variants can signal important components of the habitat that are of high conservation value because they drive the expression of divergent phenotypes (e.g., Pakkasmaa and Piironen 2000; Holopainen et al. 2005).
The two measures of variation that we have examined in this study are also related biologically. For example, a strong degree of divergence at neutral microsatellite DNA loci between two adjacent populations implies either that there is restricted dispersal between these populations (and hence restricted potential gene flow) or that while interpopulation dispersal may be significant, these loci are linked to regions under divergent selection such that realized gene flow between localities is low. Under either scenario, the microsatellite divergence between localities suggests the potential (from isolation) or actual importance of natural selection promoting adaptive divergence between populations. The high PST values that we observed between many populations is consistent with environmental differences in their habitats (lakes vs streams, large rivers vs streams, etc.) and with the potential for the effects of genetic drift and divergent selection to promote morphological diversity in rainbow trout. Leinonen et al. (2006) came to a similar conclusion in a study of stream, lake, and anadromous populations of threespine stickleback (Gasterosteus aculeatus). We did, however, observe a positive correlation between FST and PST in our study and, overall, average FST and PST values were very similar (0.37 vs 0.39, respectively) which suggests a potential role of drift in driving some of the morphological diversity we have documented. Notwithstanding this general trend, there were clearly instances when comparing the same two populations showed that pairwise PST was either considerably less than FST (suggesting stabilizing selection) or considerably higher (suggesting divergent selection). For instance, several comparisons (e.g., Blanchet vs Skinny, Canyon Creek vs Clearwater River) had very high FST (0.34–0.49) in the face of very low PST (<0.1). By contrast, other comparisons (Skinny vs Horseshoe lakes; Nimpkish River vs Blackwater River) exhibited low FST (0.03–0.12), but relatively high PST (0.25 and 0.56, respectively). In summary, we suggest that our measures of microsatellite and morphological/anatomical variation within and between populations are of direct relevance to biodiversity conservation for the reasons discussed above. Our analysis of these data has attempted to consider both simultaneously in a population prioritization context. There are, however, other kinds of data that could be used in similar contexts. In fact, salmonid fishes in general show extensive variability in other aspects of phenotype, such as behaviour and life history, that may have more obvious adaptive significance than morphology (e.g., migratory behaviour, age and size at maturity –Taylor 1991; Garcia de Leaniz et al. 2007) and similar quantification and ranking procedures could be developed for these other traits in combination with assays of neutral variation.
Valuation of biodiversity
Avise (2005) listed three major contexts within which humanity rationalizes the conservation of biodiversity: aesthetic value, provision of ecosystem services, and ethics – a recognition of an intrinsic value to life. An additional consideration, in particular for species such as rainbow trout, is economic value – the recognition that biodiversity represents direct or indirect economic value to humans. Given these rationalizations for the value of biodiversity, jurisdictions responsible for the protection and or management of biodiversity are commonly faced with the difficult task of prioritizing effort, expenditures, regulatory initiatives, and opportunity costs towards various units of biodiversity whether they represent individuals, populations, or ecosystems (Brooks et al. 2006). One of the parameters that is considered in such prioritization exercises are the relative, actual, or perceived ‘values’ of the units being considered.
The valuation of biodiversity is a growing research area and has economic, cultural, and biological components which are not necessarily mutually exclusive. For instance, Rudd (2009) presented an analysis of nonmarket values for six species of vertebrates at risk in Canada that essentially ranked each of the species in terms of the amount of money that a sample (n = 2761) of people were willing to pay for conservation programs. By contrast, Redding and Mooers (2006) provided a ranking of 9546 species of birds based on threat status and a measure of the ‘genetic value’ of each taxon. The genetic value was based, essentially, on a measure of the evolutionary isolation of each species relative to all others and, hence, its biological value in terms of representing unique genetic variation. The former example is one in which the prioritization is based largely on the perceived societal values of the unit of biodiversity while the latter is an example of a more strictly biological/scientific prioritization (see also Meuser et al. 2009). Our analysis is more aligned with the latter approach, but is one that combines molecular and morphological aspects of diversity rather than relying on a single measure of distinctiveness (e.g., Bush and Adams 2007) which is akin to the idea of using character concordance to identify conservation units (e.g., Grady and Quattro 1999). Essentially, our analysis resulted in measures (MDS, GDS, CT, CDr,) of how divergent or ‘atypical’ each population was from the average rainbow trout both in terms of microsatellite and morphological characterization. In addition, for the genetic data we examined the relative levels of variation within each population (CS, CSr). Although we observed a positive correlation between FST and PST (see above) which are pairwise comparisons, we observed negative correlations between GDS, CTr, CT (and their components) and MDS. This implies that when evaluating population distinctiveness, use of single character types is problematic because distinctiveness in one character type is not necessarily accompanied by distinctiveness in another. We applied one solution to this possible outcome by taking the average rank of genetic diversity, allelic richness, and MDS to provide an overall rank of population distinctiveness. This procedure resulted in six of the 27 populations having the five highest average rankings with two of those populations tied with the highest average ranking.
In any system designed to rank populations based on biological attributes relevant to conservation ties will occur. One possible solution to this dilemma would be to weight the input variables differentially. Although this is easily achieved in a practical sense, the biological rationale for weighting morphological variation over neutral molecular variation (or vice versa), or within population variation over among population variation (or vice versa) is not obvious and a consensus would likely be difficult to reach (e.g., Petit et al. 1998). In the case of ties, it may be informative to include further ecological or habitat characteristics. For instance, the two creek populations (Canyon and Fry creeks) tied for the highest ranking are both classified ecologically as ‘headwater’. Given that these populations are similar in terms of their average morphological and genetic distinctiveness, one could further prioritize amongst them based on their ecology or fish communities. For instance, Canyon Creek fish are the only ones to co-exist with bull trout (Salvelinus confluentus) and coastal cutthroat trout (O. clarkii) whereas Fry Creek contains only rainbow trout, a similar ecological condition as Murray Creek (ranked 3rd). In this case, ranking Canyon Creek higher than Fry Creek could be justified because another headwater, rainbow trout-only population also occurs within the top five (Murray Creek). Allendorf et al. (1997) suggested that the ‘native assemblage’ of which a population is part of could be used to evaluate the ecological legacy of that population for conservation prioritization. Another possible ‘tie-breaker’ could be geographic representation. For instance, animal distributions can be mapped onto physical biogeoclimatic zones, ecozones, biogeographic zones, or in the case of aquatic organisms, major drainage systems (e.g., COSEWIC 2009). Ties in quantitative rankings could be broken by assigning the higher priority to that population which resides in a drainage system that is not yet represented.
Allendorf et al. (1997) used a points system in which populations were given either 1 or 0 ‘points’ for satisfying (or not) specific questions regarding their evolutionary and/or ecological character whereas our system provides a quantitative assessment of the degree to which populations differ from one another and are ranked thereafter. Perhaps a combination of such approaches would be fruitful. First, populations could be ranked based on quantitative measures of how much they differ from one another in molecular and morphological (or other quantifiable adaptive differences) traits. Second, any ties could be addressed by pairwise evaluation of populations of the same rank based on qualitative criteria such as community composition, habitat type, drainage basin occupancy, or ecological role or function. In this manner, a combination our system and others such as that of Allendorf et al. (1997) could objectively rank populations using a variety of criteria that are difficult to combine on the same quantitative scale. Another possible use of ranking systems is to utilize them in an iterative fashion. Initially, populations are quantitatively ranked based on molecular and morphological distinctiveness and selected for conservation priority based on these ranks subject to the limitation that there must be at least one population from each ecotype (six in the case of rainbow trout) or major drainage system (eight in the case of BC – see Taylor 2004). Then, the procedure is repeated to add a second population to each ecotype group or drainage system based on the quantitative rankings. Pressey and Nicholls (1989) proposed this kind of iterative process for selecting representative areas for conservation reserves based on different scoring criteria.
The scenarios discussed above are subject to the limitation that we sampled only 27 of what are likely 100s of populations of rainbow trout in BC. Consequently, the relative rankings of the populations that we have included in the current analysis could change with the addition of new populations, a limitation that is common to all prioritization schemes. Although the ranking of populations could easily be updated with new data, it would be preferable to sample as widely as possible such that all geographic areas, ecotypes, and putative genetic groups (perhaps inferred from geography) are represented in the initial study to minimize shifting ranking among populations (cf. Crandall et al. 2000).
In addition, these biological attributes can also be used in conjunction with additional criteria. For species-level prioritization, Avise (2005) suggested a multifaceted procedure where taxa are ranked by the sums of the weighted ranks of five criteria: rarity, distribution, ecological importance, ‘charisma’, and phylogeny. The criteria of rarity and distribution are also strongly tied to threat status of the unit of biodiversity being considered while the latter three are more related to inherent biological value. The use of the information that we have collected on each population is best viewed as a method of conservation priority in the absence of factors related to actual threat status, that is, they relate more closely to assessments of the genetic, evolutionary, and ecological legacy of the species (sensuAllendorf et al. 1997). For instance, our examples and protocol might be best applied in situations when conservation priority is a proactive exercise, that is, when attempting to set protective measures and rank populations that are healthy and exist under relatively pristine conditions (as most of ours do) or when evaluating the potential consequences of loss of such populations in response to proposed environmental changes. Further, a higher order analysis that factors in socio-economic real and opportunity costs to make proactive decisions on priorities for conservation planning, or reactively when challenges to specific populations arise, can augment prioritization schemes initiated with biological data (Avise 2005; Rudd 2009). For instance, Fish Lake (110 hectares) is located in the Chilcotin Region of southcentral BC and a gold-copper mine has been proposed for the area. There have been extensive environmental assessment and fish compensation studies related to this project (see http://www.ceaa.gc.ca/050/details-eng.cfm?cear_id=44811) which would, in the end, involve the loss of Fish Lake and its rainbow trout in their current state via the construction of a dam and conversion of Fish Lake into a tailings pond. Our analysis of Fish Lake compared to the other 26 populations in our study indicates that it ranked 5th overall with its distinctiveness driven largely by the measures of neutral genetic variation rather than by morphological distinctiveness (Table 3). The lake, however, supports a vigorous and popular recreational fishery for rainbow trout and the development has many other cultural, historical, and societal impacts all of which must be factored into a final decision on the whether or not the project proceeds and what kinds and levels of compensation are appropriate. Finally, when attempting to rank populations in terms of conservation and if those population are already compromised and/or susceptible to existing or future threats, then clearly other factors (e.g., rate of decline, population viability analyses, number and degree of threats) need to be considered in addition to measures of ecological and evolutionary legacy (cf. Allendorf et al. 1997).
Conclusions
Species with small geographic ranges pose particular problems in conservation owing to the risks to persistence inherent to such distributions. By contrast, species with large geographic ranges present challenges in terms of identifying populations or population assemblages at different risks of extirpation or of particular conservation value amongst potentially hundreds to thousands of individual populations depending on the taxon concerned (Hughes et al. 1997). Our analysis of genetic and morphological variability in a range of populations of rainbow trout has presented a general approach and specific protocol for assigning conservation value based on attributes that could easily be adapted to different taxa and characteristics and that could be combined with other, nonbiological attributes to help make conservation decisions. Finally, protocols and examples such as ours should be particularly valuable for considerations of intraspecific diversity in freshwater systems – one of the least explored aspects of conservation prioritization (Brooks et al. 2006).
Acknowledgments
We thank J. Scroggie, M. Phillpotts, S. Fancy, J. Hagen, A. Jenkins, Q. Tuckett, D. Piatt, M. Porter, J. Schultz, and W. McCleese for help in the lab or field. G. Reid, J. Leggett, B. Hooton, D. Atagi, D. Cadden, J. Hammond, D. McPhail, and D. Schluter provided helpful comments on our research. C. Primmer and three reviewers made especially thoughtful comments. We thank the staff of Tweedsmuir, Wells Gray, and Tatlatui Provincial Parks for assistance and permission to collect within park boundaries. Financial support was provided by grants from Forest Renewal British Columbia, by the Natural Sciences and Engineering Research Council of Canada, by The University of British Columbia, and by Idaho State University.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Ecotype categories and definingcriteria used in comparing rainbow trout (Oncorhynchusmykiss) genetic and morphological variation.
Table S2. Summary of allelic variation at 10microsatellite loci in rainbow trout (Oncorhynchusmykiss).
Table S3. The occurrence of rare alleles inpopulations of rainbow trout (Oncorhynchus mykiss).
Table S4. Coefficients from a principalcomponents analysis of morphological and internal anatomical traitsmeasured in rainbow trout (Oncorhynchus mykiss) from 27populations.
Table S5. Pairwise Spearman rank correlationsamong measures of genetic and morphological diversity in rainbowtrout (Oncorhynchus mykiss).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature cited
- Allendorf FW, Bayles D, Bottom DL, Currens KP, Frissell CA, Hankin D, Lichatowich JA, et al. Prioritizing Pacific salmon stocks for conservation. Conservation Biology. 1997;11:140–152. [Google Scholar]
- Avise JC. Molecular Markers, Natural History, and Evolution. 1st edn. Sunderland, MA: Sinauer Associates Ltd; 1994. [Google Scholar]
- Avise JC. Phylogenetic units and currencies above and below the species level. In: Purvis A, Gittleman JL, Brooks T, editors. Phylogeny and Conservation. Cambridge, UK: Cambridge University Press; 2005. pp. 76–101. [Google Scholar]
- Beacham TD, Pollard S, Le KD. Population structure and stock identification of steelhead in southern British Columbia, Washington, and the Columbia River based on microsatellite DNA variation. Transactions of the American Fisheries Society. 1999;128:1068–1084. [Google Scholar]
- Behnke RJ. Native Trout of Western North America. Bethesda, MD: American Fisheries Society; 1992. [Google Scholar]
- Belkhir K, Borsa P, Chikhi N, Raufaste N, Bonhomme F. 2004. GENETIX 4.05.2, logiciel sous Windows TM pour la genetique des populations. Laboratoire Genome, Populations, Interactions, CNRS UMR 5000, Universite de Montpellier II, Montpellier, France.
- Bowen BW. Preserving genes, species, or ecosystems? Healing the fractured foundations of conservation policy. Molecular Ecology. 1999;8:S5–S10. doi: 10.1046/j.1365-294x.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Brooks TM, Mittermeier RA, Da Fonesca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, et al. Global biodiversity conservation priorities. Science. 2006;213:58–61. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- Bush V, Adams CE. Using phenotypic variation to determine conservation value: application of a novel approach to Arctic charr. Ecology of Freshwater Fish. 2007;16:29–33. [Google Scholar]
- Colosimo PF, Peichel CL, Nereng K, Blackman BK, Shapiro MD, Schluter D, Kingsley DM. The genetic architecture of parallel armor plate reduction in threespine sticklebacks. Public Library of Science Biology. 2004;5:e109. doi: 10.1371/journal.pbio.0020109. doi: 10.1371/journal.pbio.0020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Padín P, Caballero A, Rolán-Alvarez E. Relative roles of genetic determination and plastic response during ontogeny for shell-shape traits subjected to diversifying selection. Evolution. 2009;63:1356–1363. doi: 10.1111/j.1558-5646.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- COSEWIC. 2009. Guidelines for recognizing designatable units below the species level. Committee on the Status of Endangered Wildlife in Canada, c/o Canadian Wildlife Service, Environment Canada, Ottawa, Ontario K1A 0H3.
- Crandall KA, Bininda-Emonds ORP, Mace GM, Wayne RK. Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution. 2000;15:290–295. doi: 10.1016/s0169-5347(00)01876-0. [DOI] [PubMed] [Google Scholar]
- Crozier RH. Preserving the information content of species: genetic diversity, phylogeny, and conservation worth. Annual Review of Ecology and Systematics. 1997;28:243–268. [Google Scholar]
- El Mousadik A, Petit RJ. High levels of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theoretical and Applied Genetics. 1996;92:832–839. doi: 10.1007/BF00221895. [DOI] [PubMed] [Google Scholar]
- Fraser DJ, Bernatchez L. Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Molecular Ecology. 2001;10:2741–2752. [PubMed] [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Given DR, Norton DA. A multivariate approach to assessing threat and for priority setting in threatened species conservation. Biological Conservation. 1993;64:57–66. [Google Scholar]
- Goudet J. FSTAT version 2.9.3.1 Updated from Goudet, J. 1995. Journal of Heredity. 2001;86:485–486. [Google Scholar]
- Grady JM, Quattro JM. Using character concordance to define taxonomic and conservation units. Conservation Biology. 1999;13:1004–1007. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 2001;4:9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- Hedrick PW, Parker KM, Lee RN. Using microsatellite and MHC variation to identify species, ESUs, and MUs in the endangered Sonoran topminnow. Molecular Ecology. 2001;10:1399–1412. doi: 10.1046/j.1365-294x.2001.01289.x. [DOI] [PubMed] [Google Scholar]
- Holopainen IJ, Aho J, Vornanen M, Huuskonen H. Phenotypic plasticity and predator effects on morphology and physiology of crucian carp in nature and in the laboratory. Journal of Fish Biology. 2005;50:781–798. [Google Scholar]
- Hughes JB, Dailey GC, Ehrlich PR. Population diversity: its extent and extinction. Science. 1997;278:689–691. doi: 10.1126/science.278.5338.689. [DOI] [PubMed] [Google Scholar]
- International Union for the Conservation of Nature. 2010. Guidelines for using the IUCN Red List of Threatened Species Categories and Criteria. Version 8.0. Available at http://www.iucn.org/knowledge/publications_doc/tools/
- Keeley ER, Parkinson EA, Taylor EB. Ecotypic differentiation of native rainbow trout (Oncorhynchus mykiss) populations from British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:1523–1539. [Google Scholar]
- Keeley ER, Parkinson EA, Taylor EB. The origins of ecotypic variation of rainbow trout: a test of environmental vs. genetically based differences in morphology. Journal of Evolutionary Biology. 2007;20:725–736. doi: 10.1111/j.1420-9101.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- Kocher T. Adaptive evolution and explosive speciation: the cichlid fish model. Nature Reviews Genetics. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- Langerhans BD. Predictability of phenotypic differentiation across flow regimes in fishes. Integrative and Comparative Biology. 2008;48:750–768. doi: 10.1093/icb/icn092. [DOI] [PubMed] [Google Scholar]
- Langerhans BD. Morphology, performance, fitness: functional insight into a post-Pleistocene radiation of mosquitofish. Biology Letters. 2009;5:488–491. doi: 10.1098/rsbl.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary RF, Allendrof FW, Knudsen KL. Inheritance of meristic variation and the evolution of developmental stability in rainbow trout. Evolution. 1985;39:308–314. doi: 10.1111/j.1558-5646.1985.tb05668.x. [DOI] [PubMed] [Google Scholar]
- Leinonen T, Cano JM, Mäkinen H, Meril J. Contrasting patterns of body shape and neutral genetic divergence in marine and lake populations of threespine sticklebacks. Journal of Evolutionary Biology. 2006;19:1803–1812. doi: 10.1111/j.1420-9101.2006.01182.x. [DOI] [PubMed] [Google Scholar]
- Mackay TF. The genetic architecture of quantitative traits. Annual Review of Genetics. 2003;35:303–339. doi: 10.1146/annurev.genet.35.102401.090633. [DOI] [PubMed] [Google Scholar]
- McCusker M, Parkinson E, Taylor EB. Phylogeography of rainbow trout (Oncorhynchus mykiss and its implications for taxonomy, biogeography, and conservation. Molecular Ecology. 2000;9:2089–2108. doi: 10.1046/j.1365-294x.2000.01121.x. [DOI] [PubMed] [Google Scholar]
- McGuigan K, Franklin CE, Moritz C, Blows MW. Adaptation of rainbow fish to lake and stream habitats. Evolution. 2003;57:104–118. doi: 10.1111/j.0014-3820.2003.tb00219.x. [DOI] [PubMed] [Google Scholar]
- McKinney T, Speas DW, Rogers RS, Persons WR. Rainbow trout in a regulated river below Glen Canyon Dam, Arizona, following increased minimum flows and reduced discharge variability. North American Journal of Fisheries Management. 2001;21:216–222. [Google Scholar]
- Meffe GK, Carroll CR. Principles of Conservation Biology. Sunderland, MA: Sinauer Associates, Inc; 1994. [Google Scholar]
- Meuser A, Harshaw HW, Mooers AØ. Public preference for endemism over other conservation-related species attributes. Conservation Biology. 2009;23:1041–1046. doi: 10.1111/j.1523-1739.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- Moritz C. Defining “Evolutionary Significant Units” for conservation. Trends in Ecology and Evolution. 1994;9:373–375. doi: 10.1016/0169-5347(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Moritz C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Systematic Biology. 2002;51:238–254. doi: 10.1080/10635150252899752. [DOI] [PubMed] [Google Scholar]
- Mullen LM, Hoekstra HE. Natural selection along an environmental gradient: a classic cline in mouse pigmentation. Evolution. 2008;62:1555–1570. doi: 10.1111/j.1558-5646.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- NatureServe Explorer. 2009. http://www.natureserve.org/explorer/
- Pakkasmaa S, Piironen J. Water velocity shapes juvenile salmonids. Evolutionary Ecology. 2000;14:721–730. [Google Scholar]
- Parkinson EA, Keeley E, Taylor EB, Pollard S, Tautz AF. 2005. Conservation units for steelhead base on phylogenetic and adaptive distinctions among populations. Fisheries Management Report 119. British Columbia Ministry of Water, Land and Air Protection. Biodiversity Branch, Victoria, BC.
- Petit RJ, El Mousadik A, Pons O. Identifying populations for conservation based on genetic markers. Conservation Biology. 1998;12:844–855. [Google Scholar]
- Phillimore AB, Owens IPF, Black RA, Chittock J, Burke T, Clegg SM. Complex patterns of genetic and phenotypic divergence in an island bird and the consequences for delimiting conservation units. Molecular Ecology. 2008;17:2839–2853. doi: 10.1111/j.1365-294X.2008.03794.x. [DOI] [PubMed] [Google Scholar]
- Pressey RL, Nicholls AO. Efficiency in conservation evaluation: scoring versus iterative approaches. Biological Conservation. 1989;50:199–218. [Google Scholar]
- Raymond M, Rousset F. GENEPOP Version 1.2: population genetics software for exact tests and ecumenism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Redding DW, Mooers AØ. Incorporating evolutionary measures into conservation prioritization. Conservation Biology. 2006;20:1670–1678. doi: 10.1111/j.1523-1739.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- Rudd MA. National values for regional aquatic species at risk in Canada. Endangered Species Research. 2009;6:239–249. [Google Scholar]
- Ryder OA. Species conservation and systematics: the dilemma of subspecies. Trends in Ecology and Evolution. 1986;1:9–10. [Google Scholar]
- Seiler MB, Keeley ER. Intraspecific taxonomy and ecology characterize morphological divergence among cutthroat trout (Oncorhynchus clarkii ssp. Richardson) populations. Biological Journal of the Linnean Society. 2009;96:266–281. [Google Scholar]
- Tamkee P, Parkinson EA, Taylor EB. The influence of Wisconsinan glaciation and contemporary stream hydrology on microsatellite DNA variation in rainbow trout (Oncorhynchus mykiss. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67 in press. [Google Scholar]
- Taylor EB. A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture. 1991;98:185–207. [Google Scholar]
- Taylor EB. An analysis of homogenization and differentiation in Canadian freshwater fish faunas with an emphasis on British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:68–79. [Google Scholar]
- Taylor EB, Pollard S, Louie D. Mitochondrial DNA variation in bull trout (Salvelinus confluentus) from northwestern North America: implications for zoogeography and conservation. Molecular Ecology. 1999;8:1155–1170. doi: 10.1046/j.1365-294x.1999.00674.x. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Tamkee P, Sterling G, Hughson W. Microsatellite DNA analysis of rainbow trout (Oncorhynchus mykiss) from western Alberta, Canada: native status and evolutionary distinctiveness of ‘‘Athabasca’’ rainbow trout. Conservation Genetics. 2007;8:1–15. [Google Scholar]
- Thorgaard GH, Bailey GS, Williams DS, Buhler DR, Kaattari SL, Ristow SS, Hansen JD, et al. Status and opportunities for genomics research with rainbow trout. Comparative Physiology and Biochemistry Part B Biochemistry and Molecular Biology. 2002;133:609–646. doi: 10.1016/s1096-4959(02)00167-7. [DOI] [PubMed] [Google Scholar]
- United States Department of Commerce. Endangered and threatened species: final listing determinations for 10 distinct population segments of west coast steelhead trout. US Department of Commerce, National Oceanic and Atmospheric Administration. Federal Register. 2006;71:834–862. Available at http://www.nmfs.noaa.gov/pr/pdfs/fr/fr71-834.pdf. [Google Scholar]
- Utter FM. Biological criteria for definition of species and distinct intraspecific populations of anadromous salmonids under the US Endangered Species Act of 1973. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1626–1635. [Google Scholar]
- Weir BS, Cockerham CS. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Wood CC, Gross MR. The elemental conservation unit: communicating risk without dictating targets for protection. Conservation Biology. 2008;22:36–47. doi: 10.1111/j.1523-1739.2007.00856.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


