Abstract
Microsporidian parasites are being considered as alternatives to conventional insecticides for malaria control. They should reduce malaria transmission by shortening the lifespan of female mosquitoes and thus killing them before they transmit malaria. As the parasite replicates throughout the mosquito's life, it should have little detrimental effects on young mosquitoes, thus putting less selection pressure on the hosts to evolve resistance. Here, we examined these expectations for the microsporidian Vavraia culicis on Anopheles gambiae Giles sensu stricto mosquitoes under varying environmental conditions. Infection by the microsporidian delayed pupation by 10%, decreased fecundity by 23% and reduced adult lifespan by 27%, with higher infectious doses causing greater effects. The decrease of lifespan was mostly because of an increase of the mortality rate with age. Similarly, the parasite's effect on mosquito fecundity increased with the mosquitoes’ age. Neither infection nor food availability affected juvenile survival. Thus, as V. culicis reduced the longevity of A. gambiae (s.s.), yet affected mortality and fecundity of the young mosquitoes only slightly, the microsporidian is a promising alternative to insecticides for effective malaria control that will impose little evolutionary pressure for resistance.
Keywords: Anopheles gambiae, environmental variation, evolutionarily sustainable, host–parasite interactions, life-history traits, malaria control, microsporidia
Introduction
Malaria remains a major cause of human morbidity and mortality, with half of the world's population at risk of contracting malaria and an estimated 243 million cases leading to 863 000 deaths every year (WHO 2009). Currently, the best tools available and implemented by the World Health Organization and the Roll Back Malaria campaign against malaria are indoor residual spraying (IRS), insecticide-treated nets (ITNs) and artemisinin-combination therapy (ACT) (Enayati and Hemingway 2010). High insecticide coverage and efficient drug-management programs have led to significant decreases in malaria cases (Mabaso et al. 2004; Wakabi 2007; Ceesay et al. 2008, 2010). However, these improvements may not be sustainable if resistance of the malaria parasite to drugs and of the mosquito vector to insecticides continues to evolve. Indeed, the very effectiveness of the insecticides approved for IRS and ITNs makes such evolution likely. As they kill mosquitoes shortly after contact, they prevent not only transmission but also egg laying, thus exerting strong selection pressure for resistance (Hemingway and Ranson 2000; Nauen 2007; Ranson et al. 2009).
Evolutionarily sustainable alternatives to malaria control could be late life–acting biopesticides. By killing mosquitoes before the malaria parasite has completed its development and made the mosquito infectious, they block transmission; by letting the youngest mosquitoes survive, they enable them to lay eggs, thus decreasing selection pressure for resistance (Koella et al. 2009a,b; Read et al. 2009). Possibilities for such late life–acting insecticides include various biopesticides, such as entomopathogenic fungi (Thomas and Read 2007; Hancock et al. 2009) and microsporidia (Koella et al. 2009a). Their success will be determined by the balance between killing the mosquito early enough to block transmission, yet killing them and reducing their fecundity late enough to slow down evolution of resistance. As the parasites replicate throughout the period of infection and their density within the host increases with the host's age, their detrimental effects should also increase with age. Indeed, the mortality rate of mosquitoes infected with the fungus Beauveria bassiana is similar to that of uninfected individuals for the first several days after exposure and then increases substantially with time (Blanford et al. 2005). The age-specific effect of this parasite (or any other potentially late-acting biopesticide) on fecundity has, however, not been reported, despite the importance of fecundity for evolutionary success. Here, we estimate the age-specific effects on several life-history traits – including mortality rate and fecundity – in several environments for the microsporidian Vavraia culicis in the main vector of malaria in Sub-Saharan Africa, Anopheles gambiae Giles sensu stricto (s.s.).
Two of the environmental parameters that can influence parasite growth rates and, therefore, its harmful effects on the host are the parasite's infectious dose (Ebert et al. 2000; Timms et al. 2001; Brunner et al. 2005; de Roode et al. 2006) and the host's condition (which is affected by the quality of the host's environment, such as food availability, blood-feeding success or temperature). Hosts reared on high food levels store more resources and may, therefore, present a better environment for the parasite to proliferate than hosts reared in poor conditions (Bedhomme et al. 2004; Seppälä et al. 2008). Thus, well-fed hosts can have more parasites, which in turn can lead to more severe parasitic effects (Bittner et al. 2002). Alternatively, malnourished hosts may be weaker and therefore more susceptible to disease (Moret and Schmid-Hempel 2000; Krasnov et al. 2005; Mnyone et al. 2011), so that the lower the food availability, the higher the costs of parasitism (Jokela et al. 1999, 2005; Brown et al. 2000; Ferguson and Read 2002). In Aedes aegypti mosquitoes, for example, increasing infectious dose of a microsporidian and decreasing larval food availability lead to higher larval mortality and more parasitic spores (Agnew and Koella 1999). Thus, the epidemiology of a parasite and the expression of its virulence depend on a combination of the parasite's growth characteristics and the host's condition (e.g. Mitchell et al. 2005; de Roode et al. 2006, 2008; Vale and Little 2009).
Vavraia culicis parasitises several mosquito genera, for example Aedes, Culex and Anopheles and natural prevalence rates of infection range between 1% and 54% depending on the species and geographical location (Andreadis 2007). For example, prevalence of the microsporidian in A. gambiae mosquitoes was shown to be 6.6% in Senegal (Diarra and Toguebaye 1990). Vavraia culicis infects and can kill larvae. The surviving mosquitoes remain infected, and the parasite has various effects on the adult, including a shorter lifespan (Koella et al. 2009a) and reduced susceptibility to malaria (Bargielowski and Koella 2009). While the parasite has mainly been studied in the yellow-fever mosquito Ae. aegypti (for a summary see (Michalakis et al. 2008)), we consider its age-specific effects on A. gambiae (s.s.) with two experiments. In the first, we investigate how the parasite's infectious dose and its host's nutritional status affect parasite load and several of the host's life-history traits: pre-emergence survival, development time and adult lifespan. In the second, we restrict the design to estimate only the effect of one infectious dose and investigate how the host's nutritional status influences age-specific fecundity. Our results help to evaluate the potential of the microsporidian parasite V. culicis as a late life–acting candidate for malaria control under a range of environmental conditions.
Materials and methods
The mosquitoes came from a genetically diverse (based on SNP-analysis) (Mendes et al. 2008) colony established from A. gambiae (s.s.) caught in Yaoundé, Cameroon (G. Christophides, Imperial College, London, UK). Mosquitoes were reared under standard laboratory conditions (26 ± 1°C and 70 ± 5% relative humidity with 12 h light/dark cycles) at Silwood Park Campus (Imperial College, London, UK). The Vavraia culicis floridensis spores, which were originally provided by J.J. Becnel (USDA, Gainesville, USA) (Vàvra and Becnel 2007), were propagated in large groups of Ae. aegypti and A. gambiae (s.s.) mosquitoes at Silwood Park Campus. Although acknowledging the subspecies status of the Florida isolate (Vàvra and Becnel 2007), we continue to call the parasite species used in our experiments Vavraia culicis for consistency with earlier studies.
Experiment 1
Anopheles gambiae (s.s.) larvae were exposed to seven V. culicis doses: uninfected (= Control), 5000, 10 000, 20 000, 40 000, 80 000 and 160 000 spores per individual (dilution series from the same stock of V. culicis spores). For each dose, larvae were reared in two food environments in a fully balanced design. Larvae with high food obtained a standard amount of Tetramin fish food [Day 0 (hatching): 0.06 mg, Day 1: 0.12 mg, Day 2: 0.24 mg, Day 3: 0.36 mg, Day 4: 0.48 mg, Day 5 and any following day: 0.6 mg per individual], the low food contained half of this amount. Food levels were chosen on the basis of past experience in JCK's laboratory: The standard amount resulted in optimal larval development (approximately 8 days from larva to adult) and low levels of juvenile mortality, whereas half of this amount put larvae under increased nutritional and developmental stress, resulting in smaller and shorter-lived adults (unpublished data). Each treatment (dose by food) consisted of 48 larvae.
Larvae were reared individually in 2 mL de-ionised water in 12-well plates and fed every 24 h. They were infected when they were 2 days old. Larval mortality and age at pupation were recorded every 12 h. Pupae were transferred into individual emergence tubes (1.5 mL tubes filled with de-ionised water within 50 mL tubes). After adult mosquitoes had emerged, they were supplied daily with cotton soaked with 6% glucose solution. Age at adult death was recorded every 24 h. Dead adult mosquitoes were stored individually at −20°C. They were homogenised in 0.1 mL of de-ionised water, and V. culicis spores were counted under a phase-contrast microscope (400× magnification) with a haemacytometer.
Statistical analysis
Full models included V. culicis dose, larval food level and sex (except for the analysis of larval survival as larvae cannot be sexed), and all two-way interactions between them. In all analyses, dose was considered an ordinal response. The final models were selected by stepwise model simplification by comparing Akaike information criterion (AIC) values, and models with the smallest AIC values were selected as the minimum models (Crawley 2002). The significance level α was set to 0.05. Analyses were performed with JMP 8 (SAS Institute Inc 1989–2008).
The probability of emergence was analysed with logistic analysis. So that the analysis of age at pupation was not biased by the individuals that died before emergence, we analysed age at pupation with a survival analysis (proportional hazard), with the mosquitoes that died before pupation censored at their age at death. As all but about 1% of the mosquitoes pupated within a narrow range (6–9 days), we present the results as the proportion of mosquitoes that emerged before they were 8 days old. Adult lifespan was analysed with a survival analysis (proportional hazard). To explicitly evaluate age-specific mortality rates, we also analysed mortality rate as a function of age after emergence with a GLM with binomial distribution, corrected for overdispersion. The number of V. culicis spores was analysed with an ANOVA.
Experiment 2
In a separate experiment, we considered the age-specific effects of larval food and infection by the microsporidian on the mosquito's fecundity. As in the first experiment, we fed larvae with the 50% and 100% food regime. In contrast to aforementioned, we used only one infectious dose (an intermediate dose of 20 000 spores per larva). Larvae were reared and exposed to the parasite as described earlier. Adult females were held in cages according to treatment and age at pupation. Thirty males from the standard colony were introduced to the cage, and the mosquitoes were given 5 days to mate. The females were subsequently moved to individual tubes lined with wet filter paper and fed on the arms of JCK for 7 min. Three days later, the bottom of the tube was covered with water, enabling the mosquitoes to lay eggs. Five days after blood feeding, the mosquitoes were moved to a new tube, and the eggs in the previous tube were counted. We repeated this procedure four times, giving each mosquito the opportunity to lay eggs every 5 days for a total of four clutches. Throughout the experiment, the mosquitoes were given access to sugar (with a cotton ball soaked with a saturated sugar solution), except for the day before blood feeding.
Statistical analysis
We analysed the likelihood that mosquitoes laid eggs at any of the blood-feeding events with a logistic analysis including exposure to the parasite, larval food and their interaction. Using the mosquitoes that laid at least one egg after each blood feeding, we analysed the number of eggs laid at each clutch with a repeated analysis, including exposure to the parasite, larval food and their interaction as factors, and considering age (i.e. clutch) as an ordinal factor.
Results
Experiment 1
The analysis included 654 mosquitoes, with each dose by food treatment consisting of between 44 and 48 individuals. Forty-eight percent of the 470 emerging mosquitoes were female. Ninety-three percent of the 562 exposed mosquitoes harboured V. culicis spores at their death, irrespective of infectious dose (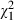 = 2.0, P = 0.153) and sex (
= 2.0, P = 0.153) and sex (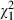 = 2.4, P = 0.118). Well-nourished individuals were less likely to be infected (90%) than poorly nourished individuals (97%;
= 2.4, P = 0.118). Well-nourished individuals were less likely to be infected (90%) than poorly nourished individuals (97%; 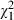 = 9.6, P = 0.002). When individuals were infected, their sex had the greatest effect on the log-transformed number of spores (F1, 435 = 11.2, P < 0.001), with females (450 000, SE 23 000) harbouring twice as many spores as males (230 000, SE 12 000). Neither food (F1, 435 = 1.2, P = 0.267) nor infectious dose (F1, 435 = 0.02, P = 0.774) affected the number of spores.
= 9.6, P = 0.002). When individuals were infected, their sex had the greatest effect on the log-transformed number of spores (F1, 435 = 11.2, P < 0.001), with females (450 000, SE 23 000) harbouring twice as many spores as males (230 000, SE 12 000). Neither food (F1, 435 = 1.2, P = 0.267) nor infectious dose (F1, 435 = 0.02, P = 0.774) affected the number of spores.
The probability of surviving up to emergence decreased from 85% of the high-food, uninfected individuals to 69% of the low-food individuals infected with the highest spore concentration (Fig. 1). However, although individuals exposed to the highest doses and reared at low food were least likely to survive, neither the difference between doses (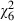 = 4.5, P = 0.611) nor the difference between food levels (
= 4.5, P = 0.611) nor the difference between food levels ( = 0.05, P = 0.830) was statistically significant.
= 0.05, P = 0.830) was statistically significant.
Figure 1.
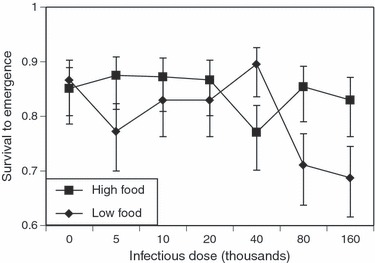
The effect of Vavraia culicis infectious dose and the mosquito's nutritional status on the survival of mosquitoes up to their emergence. Data points are presented as the proportion survival ± standard error of the proportion; diamonds represent mosquitoes reared on low food, squares mosquitoes reared on high food.
Female mosquitoes pupated later (38% at least 8 days after hatching) than males (23%; 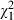 = 3.6, P = 0.058). Larvae reared with low food pupated later (38% at least 8 days after hatching) than those reared on high food (23%;
= 3.6, P = 0.058). Larvae reared with low food pupated later (38% at least 8 days after hatching) than those reared on high food (23%; 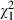 = 14.6, P < 0.001), and age at pupation increased with increasing V. culicis dose from 10% pupating at least 8 days after hatching when uninfected to 52% when infected with 160 000 microsporidian spores (
= 14.6, P < 0.001), and age at pupation increased with increasing V. culicis dose from 10% pupating at least 8 days after hatching when uninfected to 52% when infected with 160 000 microsporidian spores (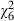 = 47.3, P < 0.001) The delay in pupation because of low food availability was more marked in uninfected and low-dose mosquitoes than in those infected with high spore doses (dose by food:
= 47.3, P < 0.001) The delay in pupation because of low food availability was more marked in uninfected and low-dose mosquitoes than in those infected with high spore doses (dose by food: 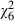 = 12.8, P = 0.046; Fig. 2).
= 12.8, P = 0.046; Fig. 2).
Figure 2.
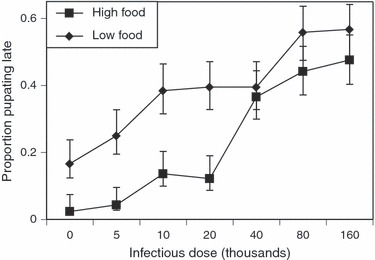
The effect of Vavraia culicis infectious dose and the mosquito's nutritional status on the proportion of Anopheles gambiae larvae pupating at least 8 days after hatching. Data points are presented as the mean ± standard error of the proportion; diamonds represent mosquitoes reared on low food, squares mosquitoes reared on high food.
On average, adult females lived 17.5 (± SE 0.3) days and males 15.4 (± SE 0.3) days (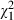 = 57.3, P < 0.001). Low-food mosquitoes lived 15.5 (± SE 0.3) days and high-food mosquitoes lived 16.2 (± SE 0.3) days (
= 57.3, P < 0.001). Low-food mosquitoes lived 15.5 (± SE 0.3) days and high-food mosquitoes lived 16.2 (± SE 0.3) days (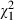 = 6.7, P = 0.010). Lifespan decreased with increasing dose from 19.1 (± SE 0.7) days in uninfected mosquitoes to 14.0 (± SE 0.5) days in adults infected with the highest spore concentration (
= 6.7, P = 0.010). Lifespan decreased with increasing dose from 19.1 (± SE 0.7) days in uninfected mosquitoes to 14.0 (± SE 0.5) days in adults infected with the highest spore concentration (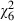 = 75.8, P < 0.001). The mortality rate of the youngest individuals was only slightly affected by the parasite, so that fewer than 20% of the mosquitoes died before they were 10 days old in any of the treatments (Fig. 3). Confirming the results of the survival analysis, the mortality rate increased with age (
= 75.8, P < 0.001). The mortality rate of the youngest individuals was only slightly affected by the parasite, so that fewer than 20% of the mosquitoes died before they were 10 days old in any of the treatments (Fig. 3). Confirming the results of the survival analysis, the mortality rate increased with age (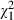 = 145.7, P < 0.001), was higher for males than for females (
= 145.7, P < 0.001), was higher for males than for females ( = 62.0, P < 0.001), was higher for low-food than for high-food mosquitoes (
= 62.0, P < 0.001), was higher for low-food than for high-food mosquitoes (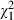 = 5.8, P = 0.017) and increased with dose (
= 5.8, P = 0.017) and increased with dose (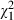 = 54.0, P < 0.001). Most importantly, the increase of mortality rate with age became more pronounced as the infectious dose increased (age by dose:
= 54.0, P < 0.001). Most importantly, the increase of mortality rate with age became more pronounced as the infectious dose increased (age by dose: 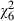 = 12.4, P = 0.054), confirming that the effect of infection was lower in young mosquitoes than in old ones.
= 12.4, P = 0.054), confirming that the effect of infection was lower in young mosquitoes than in old ones.
Figure 3.
Survival curves of adult (male and female) mosquitoes (age 0 is the day of emergence). Each curve represents an infectious dose. (A) Mosquitoes reared at high food (B) Mosquitoes reared at low food.
Experiment 2
Between 75% and 84% of the mosquitoes laid eggs after having been blood-fed. Neither exposure to the parasite ( = 4.6, P = 0.100) nor larval food level (
= 4.6, P = 0.100) nor larval food level ( = 0.3, P = 0.590) affected the likelihood that mosquitoes laid eggs at least once. When mosquitoes laid eggs, microsporidian infection decreased fecundity from 45 to 33 eggs (among mosquitoes component of repeated analysis: F1, 45 = 14.9, P < 0.001), and low larval food (50%) non-significantly decreased fecundity from 41 to 32 eggs (F1, 45 = 3.1, P = 0.087). The effect of food on fecundity was similar for the four ages at blood feeding (within mosquito component of repeated analysis: F3, 45 = 1.4, P = 0.250), but the effect of microsporidian infection increased with age from a difference of 16% in the first clutch to 45% in the fourth clutch (F3, 45 = 4.5, P = 0.008; Fig. 4).
= 0.3, P = 0.590) affected the likelihood that mosquitoes laid eggs at least once. When mosquitoes laid eggs, microsporidian infection decreased fecundity from 45 to 33 eggs (among mosquitoes component of repeated analysis: F1, 45 = 14.9, P < 0.001), and low larval food (50%) non-significantly decreased fecundity from 41 to 32 eggs (F1, 45 = 3.1, P = 0.087). The effect of food on fecundity was similar for the four ages at blood feeding (within mosquito component of repeated analysis: F3, 45 = 1.4, P = 0.250), but the effect of microsporidian infection increased with age from a difference of 16% in the first clutch to 45% in the fourth clutch (F3, 45 = 4.5, P = 0.008; Fig. 4).
Figure 4.
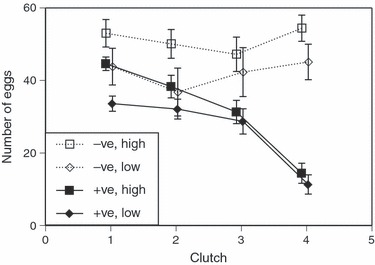
Effects of larval food and exposure to the microsporidian Vavraia culicis on the number of eggs laid at the first four clutches. Data points are presented as the mean ± standard error of the mean; diamonds represent mosquitoes reared at low food, squares represent mosquitoes reared at high food. Open symbols represent uninfected mosquitoes, solid symbols infected ones.
Discussion
In our experiments, the microsporidian Vavraia culicis had properties that make it a promising candidate for a late life–acting insecticide that could control malaria effectively. In particular, its detrimental effects were small in young mosquitoes and increased with the mosquitoes’ age, leading to a considerable reduction in adult lifespan. This is consistent with the continuous replication of the parasite within its host, leading to higher spore loads as mosquitoes become older.
Properties of the host–parasite relationship
As in other studies (Reynolds 1970; Agnew et al. 1999), V. culicis had little effect on the survival of mosquitoes up to their emergence under optimal host conditions. Although the increased mortality of the badly nourished individuals exposed to the highest infectious doses (Fig. 1) was not statistically significant, it corroborates experiments with Aedes aegypti, where lower food level and higher infectious doses led to lower levels of emergence (Bedhomme et al. 2004; Fellous and Koella 2009). The parasite probably has little effect on juveniles in laboratory conditions, where development is rapid even under low food conditions, because its spores do not proliferate enough during larval and pupal development (the spores of V. culicis usually mature 8–10 days after infection) to kill the mosquito before it emerges (Biron et al. 2005; Michalakis et al. 2008), unless larvae are weakened by stressful conditions and are exposed to a high concentration of infectious spores.
As generally observed (Bradshaw and Johnson 1995; Fellous and Koella 2010), the mosquitoes’ pupation was delayed by low food availability. Infection by V. culicis also delayed pupation. Microsporidia are intracellular, amitochondrial parasites that completely depend on their host's ATP (Weidner et al. 1999). They use their host's resources, so that, for example, V. culicis-infected Ae. aegypti larvae contain fewer lipids, sugars and glycogen than uninfected larvae (Rivero et al. 2007). Therefore, critical resources for rapid development, and thus, early pupation are lacking. The delay in pupation because of low food availability was especially marked in individuals infected with lower infectious doses, suggesting that the parasite utilises a larger proportion of the available resources of poorly nourished individuals than of well-nourished ones. That the parasite uses the mosquito's resources may also be responsible for the parasite's effect on fecundity. In the youngest mosquitoes, the parasite and low food availability had similar effects; a reduction of fecundity by about ten eggs. In the oldest mosquitoes tested, infection had a much greater effect on fecundity, whereas food had no impact. Again, as spore load increases (with age), the parasite may have used a greater proportion of the available resources, so that most of the observed effects are because of the parasite.
As in other mosquitoes (Bedhomme et al. 2004; Fellous and Koella 2009), V. culicis reduced the lifespan of adult A. gambiae (s.s.) mosquitoes by up to 5.1 days (27%), with shorter lifespans of individuals infected with higher V. culicis concentrations. The shorter life was almost completely because of increases in the mortality rates of the oldest mosquitoes; fewer than 20% of the mosquitoes died before they were 10 days old in any of the treatments. Mechanisms that could explain the shortened lifespan with increasing dose and decreasing host food are that microsporidia interact with other parasites present in the gut, which can lead to harmful effects of multiple infection (van Baalen and Sabelis 1995; Fellous and Koella 2009), or that they activate a costly immune system [the insect immune system produces cytotoxic substances, which can adversely affect the host; (Moret 2006) and references within]. Consistent with our findings on the effects of the other mosquito life-history parameters is the possibility that the parasite load in the oldest mosquitoes simply starves the mosquito so they die earlier than uninfected ones.
In the context of malaria control
Late life–acting agents for malaria control should have two properties (Koella et al. 2009b; Read et al. 2009). First, they should kill old mosquitoes to prevent the transmission of malaria. Vavraia culicis significantly reduced adult mosquito lifespan by up to 27%, with lower food availability and higher parasite concentrations causing higher adult mortality. As even in the most malarious areas, mosquitoes must bite several times before they are infected (Lyimo and Koella 1992), most mosquitoes become infected with Plasmodium parasites several days after emergence. It then takes 10–14 days for the mosquito to become infectious (Bradley et al. 1987; Killeen et al. 2000). Extrinsic mortality rates for the key vector species are very high – on average about 10% per day and ranging from 5% daily mortality in some areas to 20% in others (Costantini et al. 1996; Charlwood et al. 1997; Takken et al. 1998; Killeen et al. 2000; Okech et al. 2004; Midega et al. 2007). Therefore, most females die before becoming infectious, even in the absence of any public health measures. Decreasing longevity with the microsporidian thus has the potential to kill even more mosquitoes before they become infectious, thereby severely limiting the potential of transmission. [For a more detailed description of the effect of late-acting insecticides see (Koella et al. 2009b; Read et al. 2009).]. Second, late life–acting agents should have little detrimental effects on young mosquitoes, thus enabling them to lay eggs and minimising the pressure for the mosquitoes to evolve resistance against the microsporidian. This was the case in our experiments. Unlike the insecticides currently approved for ITN and IRS, V. culicis had little effect on juvenile survival, a much smaller effect on the fecundity of young adults than of old ones, and almost no effect on the mortality rate of up to 10-day-old mosquitoes.
Environmental conditions can change the pressure that the parasite exerts on its host. Poorly nourished larvae and those infected with higher parasite concentrations took longer to develop into adults, thus prolonging the mosquito's generation time, an important parameter of evolutionary fitness (Stearns 1992). Therefore, the highest spore concentration is not necessarily the best option for sustainable vector control, regardless of its impact on the mortality of adults. An essential part of the evaluation of a potential vector control agent should be the incorporation of ecological fluctuations probably experienced by mosquitoes in the field and their long-term impact on epidemiologically and evolutionarily important life-history parameters.
Finally, the impact of all potential control agents, including V. culicis, on the environment and nontarget organisms should be measured. While that is beyond the scope of this paper, we note that V. culicis infects not only the anopheline vectors of malaria but also several other genera of mosquitoes, e.g. Culex and Aedes (Weiser 1980). As the larvae of the other species live in different habitats, it should be possible to target the microsporidian to the larval sites containing anopheline mosquitoes. Even if the nontarget species are infected, the idea underlying the use of V. culicis is that evolutionary pressure is minimal, implying that its effect on population dynamics and densities should also be weak.
Acknowledgments
The authors thank Dr Maria-Gloria Basáñez and Dr Tom Little for comments on an earlier draft of this manuscript. This work was supported by a grant from the Natural Environment Research Council (grant number: NE/F011288/1).
Data archiving statement
Data for this study are available at Dryad: doi:10.5061/dryad.2s231.
Literature cited
- Agnew P, Koella JC. Life history interactions with environmental conditions in a host-parasite relationship and the parasite's mode of transmission. Evolutionary Ecology. 1999;13:67–89. [Google Scholar]
- Agnew P, Bedhomme S, Haussy C, Michalakis Y. Age and size at maturity of the mosquito Culex pipiens infected by the microsporidian parasite Vavraia culicis. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:947–952. [Google Scholar]
- Andreadis TG. Microsporidian parasites of mosquitoes. Journal of the American Mosquito Control Association. 2007;23:3–29. doi: 10.2987/8756-971X(2007)23[3:MPOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- van Baalen M, Sabelis MW. The dynamics of multiple infection and the evolution of virulence. American Naturalist. 1995;146:881–910. [Google Scholar]
- Bargielowski I, Koella JC. A possible mechanism for the suppression of Plasmodium berghei development in the mosquito Anopheles gambiae by the microsporidian Vavraia culicis. PLoS ONE. 2009;4:e4676. doi: 10.1371/journal.pone.0004676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Virulence reaction norms across a food gradient. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:739–744. doi: 10.1098/rspb.2003.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron DG, Agnew P, Marche L, Renault L, Sidobre C, Michalakis Y. Proteome of Aedes aegypti larvae in response to infection by the intracellular parasite Vavraia culicis. International Journal for Parasitology. 2005;35:1385–1397. doi: 10.1016/j.ijpara.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Bittner K, Rothhaupt K-O, Dieter E. Ecological interactions of the microparasite Caullerya mesnili and its host Daphnia galeata. Limnology and Oceanography. 2002;47:300–305. [Google Scholar]
- Blanford S, Chan BHK, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Newbold CI, Warrell DA. Malaria. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. Oxford Textbook of Medicine. Oxford: Oxford University Press; 1987. pp. 721–747. [Google Scholar]
- Bradshaw WE, Johnson K. Initiation of metamorphosis in the pitcher-plant mosquito: effects of larval growth history. Ecology. 1995;76:2055–2065. [Google Scholar]
- Brown MJF, Loosli R, Schmid-Hempel P. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos. 2000;91:421–427. [Google Scholar]
- Brunner JL, Richards K, Collins JP. Dose and host characteristics influence virulence of ranavirus infections. Oecologia. 2005;144:399–406. doi: 10.1007/s00442-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Ceesay SJ, Casals-Pascual C, Erskin J, Anya SE, O Duah N, Fulford AJC, Sesay SSS, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. The Lancet Infectious Diseases. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceesay SJ, Casals-Pascual C, Nwakanma DC, Walther M, Gomez-Escobar N, Fulford AJC, Takem EN, et al. Continued decline of malaria in The Gambia with implications for elimination. PLoS ONE. 2010;5:e12242. doi: 10.1371/journal.pone.0012242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JHET. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bulletin of Entomological Research. 1997;87:445–453. [Google Scholar]
- Costantini C, Li S-G, della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Medical and Veterinary Entomology. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Crawley M. Statistical Computing: An Introduction to Data Analysis Using S-Plus. Chichester: John Wiley and Sons; 2002. [Google Scholar]
- Diarra K, Toguebaye BS. Etude d'une infection microspoidienne naturelle chez Anopheles gambiae Giles (Diptera: Culicidae), moustique vecteur du paludisme au Senegal. Acta Protozoologica. 1990;29:163–168. [Google Scholar]
- Ebert D, Zschokke-Rohringer CD, Carius HJ. Dose effects and density-dependent regulation of two microparasites of Daphnia magna. Oecologia. 2000;122:200–209. doi: 10.1007/PL00008847. [DOI] [PubMed] [Google Scholar]
- Enayati A, Hemingway J. Malaria management: past, present, and future. Annual Review of Entomology. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- Fellous S, Koella JC. Infectious dose affects the outcome of the within-host competition between parasites. American Naturalist. 2009;173:E177–E184. doi: 10.1086/598490. [DOI] [PubMed] [Google Scholar]
- Fellous S, Koella JC. Cost of co-infection controlled by infectious dose combinations and food availability. Oecologia. 2010;162:935–940. doi: 10.1007/s00442-009-1535-2. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PA, Thomas MB, Godfray HCJ. An age-structured model to evaluate the potential of novel malaria-control interventions: a case study of fungal biopesticide sprays. Proceedings of the Royal Society B-Biological Sciences. 2009;276:71–80. doi: 10.1098/rspb.2008.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annual Review of Entomology. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively CM, Taskinen J, Peters AD. Effect of starvation on parasite-induced mortality in a freshwater snail (Potamopyrgus antipodarum. Oecologia. 1999;119:320–325. doi: 10.1007/s004420050792. [DOI] [PubMed] [Google Scholar]
- Jokela J, Taskinen J, Mutikainen P, Kopp K. Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. Oikos. 2005;108:156–164. [Google Scholar]
- Killeen GF, McKenzie FE, Fox BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. American Journal of Tropical Medicine and Hygiene. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella JC, Lorenz L, Bargielowski I. Microsporidians as evolution-proof agents of malaria control? Advances in Parasitology. 2009a;68:315–327. doi: 10.1016/S0065-308X(08)00612-X. [DOI] [PubMed] [Google Scholar]
- Koella JC, Lynch PA, Thomas MB, Read AF. Towards evolution-proof malaria control with insecticides. Evolutionary Applications. 2009b;2:469–480. doi: 10.1111/j.1752-4571.2009.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov BR, Khokhlova IS, Arakelyan MS, Degen AA. Is a starving host tastier? Reproduction in fleas parasitizing food-limited rodents. Functional Ecology. 2005;19:625–631. [Google Scholar]
- Lyimo EO, Koella JC. Relationship between body size of adult Anopheles gambiae s.l and infection with the malaria parasite Plasmodium falciparum. Parasitology. 1992;104:233–237. doi: 10.1017/s0031182000061667. [DOI] [PubMed] [Google Scholar]
- Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Tropical Medicine & International Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- Mendes AM, Schlegelmilch T, Cohuet A, Awono-Ambene P, De Iorio M, Fontenille D, Morlais I, et al. Conserved mosquito-parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathogens. 2008;4:e1000069. doi: 10.1371/journal.ppat.1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis Y, Bedhomme S, Biron DG, Rivero A, Sidobre C, Agnew P. Virulence and resistance in a mosquito-microsporidium interaction. Evolutionary Applications. 2008;1:49–56. doi: 10.1111/j.1752-4571.2007.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midega JT, Mbogo CM, Mwnambi H, Wilson MD, Ojwang G, Mwangangi JM, Nzovu JG, et al. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan coast by using mark-release-recapture methods. Journal of Medical Entomology. 2007;44:923–929. doi: 10.1603/0022-2585(2007)44[923:edasoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SE, Rogers ES, Little TJ, Read AF. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- Mnyone LL, Kirby MJ, Mpingwa MW, Lwetoijera DW, Knols BGJ, Takken W, Koenraadt CJM, et al. Infection of Anopheles gambiae mosquitoes with entomopathogenic fungi: effect of host age and blood-feeding status. Parasitology Research. 2011;108:317–322. doi: 10.1007/s00436-010-2064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1399–1405. doi: 10.1098/rspb.2006.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Management Science. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Knols BGJ, Kabiru EW, Killeen GF, Beier JC, Yan G, et al. Influence of indoor microclimate and diet on survival of Anopheles gambiae s.s. (Diptera: Culicidae) in village house conditions in western Kenya. International Journal of Tropical Insect Science. 2004;24:207–212. [Google Scholar]
- Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbe C, Yangalbe-Kalnone E, Sagnon N, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malaria Journal. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biology. 2009;7:e10000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DG. Laboratory studies of microsporidian Plistophora culicis (Weiser) infecting Culex pipiens-fatigans Wied. Bulletin of Entomological Research. 1970;60:339–349. doi: 10.1017/S0007485300040852. [DOI] [PubMed] [Google Scholar]
- Rivero A, Agnew P, Bedhomme S, Sidobre C, Michalakis Y. Resource depletion in Aedes aegypti mosquitoes infected by the microsporidian Vavraia culicis. Parasitology. 2007;134:1355–1362. doi: 10.1017/S0031182007002703. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Gold LR, Altizer S. Virulence determinants in a natural butterfly-parasite system. Parasitology. 2006;134:657–668. doi: 10.1017/S0031182006002009. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Pedersen AB, Hunter MD, Altizer S. Host plant species affects virulence in monarch butterfly parasites. Journal of Animal Ecology. 2008;77:120–126. doi: 10.1111/j.1365-2656.2007.01305.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. JMP 8. Cary, NC: SAS Institute Inc; 1989. –2008. [Google Scholar]
- Seppälä O, Liljeroos K, Karvonen A, Jokela J. Host condition as a constraint for parasite reproduction. Oikos. 2008;117:749–753. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- Takken W, Charlwood JD, Billingsley PF, Gort G. Dispersal and survival of Anopheles funestus and A. gambiae s.l. (Diptera: Culicidae) during the rainy season in southeast Tanzania. Bulletin of Entomological Research. 1998;88:561–566. [Google Scholar]
- Thomas MB, Read AF. Can fungal biopesticides control malaria? Nature Reviews Microbiology. 2007;5:377–383. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- Timms R, Colegrave N, Chan BHK, Read AF. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology. 2001;123:1–11. doi: 10.1017/s0031182001008083. [DOI] [PubMed] [Google Scholar]
- Vale PF, Little TJ. Measuring parasite fitness under genetic and thermal variation. Heredity. 2009;103:102–109. doi: 10.1038/hdy.2009.54. [DOI] [PubMed] [Google Scholar]
- Vàvra J, Becnel JJ. Vavraia culicis (Weiser, 1947) Weiser, 1977 revisited: cytological characterisation of a Vavraia culicis-like microsporidium isolated from mosquitoes in Florida and the establishment of Vavraia culicis floridensis subsp. n. Folia Parasitologica. 2007;54:259–271. [PubMed] [Google Scholar]
- Wakabi W. Africa counts greater successes against malaria. Lancet. 2007;370:1895–1896. doi: 10.1016/S0140-6736(07)61796-6. [DOI] [PubMed] [Google Scholar]
- Weidner E, Findley AM, Dolgikh V, Sokolova J. Microsporidian biochemistry and physiology. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. Washington, DC: American Society for Microbiology; 1999. pp. 172–195. [Google Scholar]
- Weiser J. Data Sheet on the Biological Control Agent Vavraia (Pleistophora) culicis (Weiser 1946) Geneva: World Health Organisation; 1980. pp. 1–5. [Google Scholar]
- WHO. 2009. WHO Malaria Report http://www.who.int/malaria/world_malaria_report_2009/en/index.html (accessed on 12 April 2010)


