Abstract
Anthropogenic habitat alteration creates novel environments that can alter selection pressures. Construction of reservoirs worldwide has disturbed riverine ecosystems by altering biotic and abiotic environments of impounded streams. Changes to fish communities in impoundments are well documented, but effects of those changes on native species persisting in reservoirs, which are presumably subjected to novel selective pressures, are largely unexplored. I assessed body shape variation of a native stream fish in reservoir habitats and streams from seven reservoir basins in the Central Plains of the USA. Body shape significantly and consistently diverged in reservoirs compared with stream habitats within reservoir basins; individuals from reservoir populations were deeper-bodied and had smaller heads compared with stream populations. Individuals from reservoir habitats also exhibited lower overall shape variation compared with stream individuals. I assessed the contribution of genotypic divergence and predator-induced phenotypic plasticity on body shape variation by rearing offspring from a reservoir and a stream population with or without a piscivorous fish. Significant population-level differences in body shape persisted in offspring, and both populations demonstrated similar predator-induced phenotypic plasticity. My results suggest that, although components of body shape are plastic, anthropogenic habitat modification may drive trait divergence in native fish populations in reservoir-altered habitats.
Keywords: Cyprinella, fish body shape, geometric morphometrics, local adaptation, phenotypic plasticity, prairie streams, rapid evolution, reservoirs
Introduction
Species worldwide are subject to anthropogenic disturbances to ecosystems (Vitousek et al. 1997) and may consequently suffer extinction and contribute to the current unprecedented extinction rate (Pimm et al. 1995). The extent of environmental change and the subsequent responses of populations determine population viability in recently altered ecosystems. Stream impoundments are major contributors of habitat degradation and fragmentation in aquatic ecosystems (Baxter 1977; Dynesius and Nilsson 1994; Downing et al. 2006), threatening many imperiled freshwater organisms (Dudgeon et al. 2006). Generally, reservoirs have deleterious impacts on native biota, but for species that persist in these altered environments, they may serve as model systems to investigate population responses to rapid environmental disturbances because reservoirs are widespread, can be treated as replicated units, and potentially affect a wide range of taxa.
When streams are impounded, they rapidly change from relatively shallow flowing habitats to deep standing bodies of water, which most native stream fishes have likely not experienced during their evolutionary history (Baxter 1977). The presence and strength of novel biotic and abiotic selective pressures in reservoirs are evidenced by changes to historical structures of fish communities following impoundment: obligate stream fishes often suffer rapid extirpation or substantial declines in reservoirs of impounded streams (Taylor et al. 2001; Gido et al. 2009). Additionally, higher densities of native and non-native piscivorous fishes are facilitated in reservoirs by newly formed lentic habitats and stocking of game fish (Gido and Brown 1999; Taylor et al. 2001; Paller 2005). Although many native stream fishes cannot persist in these novel ecosystems (sensuHobbs et al. 2006), it is currently unclear how traits or evolutionary trajectories of resident populations may be impacted.
Intra- and interspecific phenotypic variation along natural environmental gradients of stream flow (Hubbs 1941; Walker 1997; Brinsmead and Fox 2002; Langerhans et al. 2003; McGuigan et al. 2003; Hendry et al. 2006; Langerhans 2008; Pavey et al. 2010; Tobler and Carson 2010) and predator regimes (Endler 1980; Reznick et al. 1997; Walker 1997; Langerhans et al. 2004; Hendry et al. 2006; Langerhans and Makowicz 2009; Pavey et al. 2010) can be used to generate a priori predictions of how fish morphologies may respond to reservoir habitats. Relationships between morphology and swimming performance likely constrain body shape variation along environmental gradients (Langerhans 2008; Tobler and Carson 2010). Specifically, selection on fishes in lotic habitats can result in fusiform body shapes that reduce drag and enable prolonged swimming, whereas increased body depth in lentic waters facilitates faster burst speeds and increased maneuverability (Gosline 1971; Alexander 1967, Langerhans 2008). However, these patterns are not ubiquitous as some fishes can display more streamlined body forms in lentic habitats compared with streams (e.g., Hendry et al. 2002; McGuigan et al. 2003). The presence of piscivorous fishes can also select for increased caudal depths of small-bodied fishes, presumably increasing predator escape through high burst-swimming speed (Domenici and Blake 1997; Langerhans et al. 2004; Hendry et al. 2006; Langerhans 2009). Therefore, both loss of flow and increased predator densities in reservoirs have the potential to drive predictable morphological trait divergence between ancestral stream populations and populations in these newly altered habitats.
While observational evidence suggests that variable predator and flow regimes can drive adaptive trait divergence in fishes, the relative contribution of genetic divergence and phenotypic plasticity to morphological variation of fishes in the field has largely been overlooked (Langerhans 2008). Indeed, environmentally contingent phenotypes (i.e., phenotypic plasticity) are widespread (West-Eberhard 1989; Schlichting and Pigliucci 1998), and fishes can exhibit predator-induced (Brönmark and Miner 1992; Chivers et al. 2008; Fine et al. 2011) and flow-induced (Pakkasmaa and Piironen 2001, Grünbaum et al. 2007; Keeley et al. 2007; Fischer-Rousseau et al. 2010) phenotypic plasticity. Given the plastic responses of some fishes to predators or variable flow regimes, phenotypic plasticity could be responsible for a portion of the morphological variation observed along these environmental gradients. Haas et al. (2010) demonstrated morphological divergence of a stream fish in reservoirs using field-collected specimens. However, it is currently unclear whether disparate morphologies are heritable, and how much body shape variation among populations could potentially be explained by phenotypic plasticity.
Here, I assessed whether newly formed lentic habitats drive morphological trait divergence of native stream fish populations and predicted that fish morphologies would demonstrate consistent divergence in replicated reservoir systems. I tested this prediction by quantifying body shape of a native small-bodied stream fish (Cyprinella lutrensis Baird and Girard) from field-collected individuals in streams near reservoirs and in reservoir habitats. Additionally, I assessed the relative contributions of genotypic variation and predator-induced phenotypic plasticity to morphological divergence in reservoirs by rearing laboratory-spawned offspring of a reservoir and stream population in a common garden experiment with and without predators present.
Materials and methods
Field collections
Cyprinella lutrensis, a small-bodied Cyprinid [<100 mm total length (TL)] native to and locally abundant in the Central Plains of the USA (Matthews 1987), were collected by seine in stream and reservoir habitats from seven reservoir basins in Oklahoma, USA (Table 1; Fig. 1). Specimens from five basins were collected between 1992 and 1999, and I obtained them from the Sam Noble Oklahoma Museum of Natural History in 2009 (Table 1). Specimens were fixed in 10% formalin in the field and transferred to 50% isopropyl alcohol for long-term preservation. I collected fish from the other two basins between 2007 and 2008 (Table 1) and preserved and stored them in 10% formalin before data acquisition (<2 weeks). Museum collections consisted of one reservoir population and one stream population either upstream or downstream of each impoundment, and recent collections included one reservoir population and several stream populations near each impoundment (Table 1; Fig. 1). I only used males in breeding condition (determined by the presence of tubercles on the forehead; Koehn 1965) for analyses to reduce potential body shape variation due to sexual dimorphism, or in females, gravidity.
Table 1.
Reservoir basin system (system ID in Fig. 1) and specific site (site ID in Fig. 1) data of Cyprinella lutrensis collected for geometric morphometric analysis to assess body shape divergence in reservoirs. Lot numbers of specimens obtained from the Sam Noble Oklahoma Museum of Natural History are indicated under Oklahoma Identification (OID)
| Basin system | Year impounded | Name of site | Year of collection | N | Latitude | Longitude | Distance (km) | OID |
|---|---|---|---|---|---|---|---|---|
| Canton (a) | 1986 | Canton Lake (1) | 1992 | 18 | 36.0813 | −98.6037 | 51521 | |
| Horse Creek (2) | 1993 | 13 | 35.6800 | −98.3810 | 66 | 67178 | ||
| Lake Arcadia (b) | 1948 | Lake Arcadia (3) | 1993 | 25 | 35.6102 | −97.4129 | 49306 | |
| Deep Fork River (4) | 1993 | 31 | 35.6720 | −97.1947 | 30 | 47771 | ||
| Grand Lake (c) | 1959 | Grand Lake (5) | 1994 | 15 | 36.6278 | −94.8642 | 48542 | |
| Neosho River (6) | 1993 | 9 | 36.8589 | −94.8757 | 26 | 49865 | ||
| Oogalah (d) | 1940 | Lake Oogalah (7) | 1993 | 14 | 36.6615 | −95.5989 | 48093 | |
| Verdigre River (8) | 1999 | 14 | 36.8401 | −95.5910 | 28 | 61628 | ||
| Fort Cobb (e) | 1963 | Fort Cobb (9) | 1992 | 11 | 35.2319 | −98.5179 | 53711 | |
| Cobb Creek (10) | 1998 | 10 | 35.2902 | −98.5942 | 8 | 63626 | ||
| Lake Texoma (f) | 1944 | Lake Texoma (11) | 2007–2008 | 39 | 33.8794 | −96.8021 | ||
| Caddo Creek (12) | 2008 | 9 | 34.2637 | −97.1643 | 80 | |||
| Walnut Bayou (13) | 2008 | 16 | 33.9166 | −97.2823 | 85 | |||
| Lake Thunderbird (g) | 1965 | Lake Thunderbird (14) | 2007–2008 | 68 | 35.2318 | −97.2133 | ||
| Bourbanais Creek (15) | 2008 | 19 | 35.1779 | −97.1421 | 10 | |||
| Clear Creek (16) | 2007 | 10 | 35.1788 | −97.2651 | 2 | |||
| Council Creek (17) | 2007 | 27 | 35.1569 | −97.0895 | 19 | |||
| Dave Blue Creek (18) | 2007–2008 | 29 | 35.1895 | −97.3470 | 4 | |||
| Elm Creek (19) | 2007 | 15 | 35.2908 | −97.3488 | 7 | |||
| Hog Creek (20) | 2007 | 18 | 35.3193 | −97.2496 | 2 | |||
| Pecan Creek (21) | 2007–2008 | 145 | 35.2031 | −97.1179 | 12 |
Figure 1.
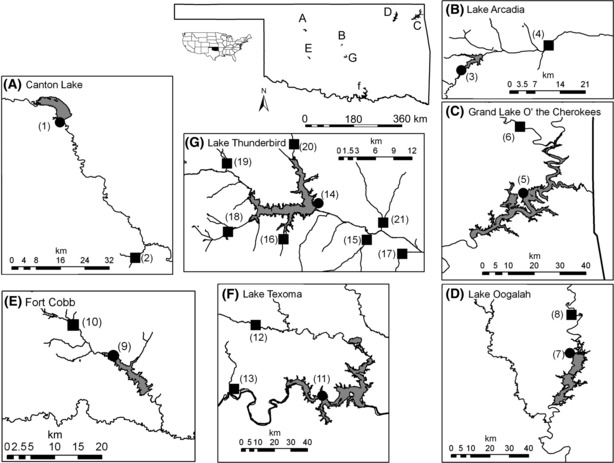
Reservoir basins and collection sites of Cyprinella lutrensis used for analyses. Reservoirs and sampling sites are coded as in Table 1. Reservoir habitats are denoted with filled circles and stream habitats as filled squares.
Morphological divergence and phenotypic plasticity
I assessed potential genotypic divergence and predator-induced phenotypic plasticity in morphology between reservoir and stream populations by spawning C. lutrensis adults from a reservoir and stream population in the laboratory and rearing their offspring in a split-cohort common garden experiment with or without a predator present. I collected adult C. lutrensis from a reservoir (Lake Thunderbird) and a stream population downstream of the reservoir (Pecan Creek; Table 1) in May 2009. I spawned individual breeding pairs from both populations (n = 4 pairs from stream population, n = 8 pairs from reservoir population) in 40-L aquaria (i.e., one male and one female from the same population per aquarium) in a greenhouse at the Aquatic Research Facility at the University of Oklahoma, starting July 1, 2009. One round gravel-filled plastic tray (140 diameter, 35 mm deep) in each aquarium served as spawning substrate. Every third day, I replaced trays and hatched eggs in separate aerated plastic trays. Hatched juveniles from the same cohort were then haphazardly split into two outdoor 380-L mesocosms (n = 24 mesocosms total). I allowed each parental pair to spawn until I consistently observed at least 20 juvenile C. lutrensis in each paired mesocosm.
After each parental pair was finished spawning (i.e., ≥20 offspring in each mesocosm pair), I randomly assigned predator and nonpredator treatments and introduced either a native piscivorous fish (Micropterus salmoides Lacepéde; Largemouth bass) or non-native nonpiscivorous fish (Cyprinus carpio Linnaeus; Common carp) to each mesocosm. Micropterus salmoides (hereafter termed predator) is native to this region and has likely shared an evolutionary history with C. lutrensis, whereas C. carpio (hereafter termed nonpredator) is an exotic. I included the nonpredator treatment in the paired mesocosms to control for the presence of a larger fish (i.e., the predator fish treatment) in the rearing environments. Parents did not successfully spawn simultaneously; therefore, although split cohorts received both predator and nonpredator treatments the same day, I sequentially added predator/nonpredator treatments through the summer. I placed hatched larval C. lutrensis in mesocosms between July 5 and August 26 and stocked the first treatments on July 21 and the last treatments on August 19. Predator treatment individuals were on average larger, mean (range) = 122 (90–180) mm TL, than nonpredator individuals, 92 (73–110) mm TL; therefore, I added more than one nonpredator to some mesocosms to approximate the length and biomass of the predator in the other paired mesocosm. The mean TL of predator and nonpredators in paired mesocosms did not differ significantly (paired t-test, n = 12, t = −0.238, P = 0.815). In addition, biomass estimated from published length–weight relationships of predator and nonpredator fish (Carlander 1969; Schneider et al. 2000) did not differ significantly between treatments (paired t-test, n = 12, t = −1.073, P = 0.304). I separated predator and nonpredator fish from juvenile C. lutrensis with a screen barrier (plastic window screen) held in place with silicone at ∼1/3 end of each mesocosm. Juvenile C. lutrensis were on average 13 days old (range = 1–22) before I stocked treatment fish, and juveniles were present with treatment fish on average 64 days (range = 45–77). I removed all juvenile C. lutrensis from mesocosms on October 3, 2009, euthanized, preserved, stored them in 10% formalin solution until data acquisition (<7 days), and only used individuals >10 mm standard length (SL) in analyses.
Geometric morphometric analysis
I quantified the body shape of C. lutrensis specimens using geometric morphometric analyses (Zelditch et al. 2004) with tps software (http://life.bio.sunysb.edu/morph/). I digitally photographed the left lateral side of each individual with a reference scale, randomized the order of digitized photographs (to reduce potential biases associated with the sequence specimens were photographed and subsequent landmarks placed on them) using tpsDig software (Rohlf 2004a), and set 10 homologous landmarks on each (Fig. 2). To account for bending of specimens, I unbent landmarks using the landmarks at the tip of the snout and middle of the eye and one temporary landmark set in the middle of the caudal peduncle (but removed in final analyses) using the ‘unbend specimens’ function in tpsUtil (Rohlf 2004b). I resized landmark coordinates using the reference scale and aligned landmark coordinates using least-squares superimposition to remove the effects of scale, translation, and rotation with the program tpsRelw (Rohlf 2004c). I calculated relative warps and uniform components (i.e., weight matrix; hereafter referred to as shape variables) and centroid size using tpsRelw. Because some shape variables often do not explain an appreciable amount of variation (Rohlf 1993), I retained only shape variables that explained ≥1% of the total variation in shape. Variation in shape was visualized using thin-plate spline transformation grids in tpsRegr (Rohlf 2004d).
Figure 2.
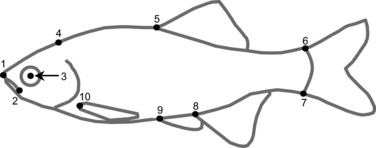
The 10 landmarks set on Cyprinella lutrensis photographs for geometric morphometric analyses: (1) tip of the snout, (2) corner of the mouth, (3) center of the eye, (4) back of the skull, (5) anterior insertion of the dorsal fin, (6) insertion of the last dorsal ray on the caudal fin, (7) insertion of the last ventral ray on the caudal fin, (8) anterior insertion of the anal fin, (9) anterior insertion of the pelvic fin, and (10) anterior insertion of the pectoral fin.
Data analysis
Field collections
I tested for differences in body shape between stream and reservoir habitats with multivariate analysis of covariance (MANCOVA). The MANCOVA model included 11 shape variables (explaining 96.9% of the variation in shape) as dependant variables, centroid size as a covariate (to test for effects of allometry), habitat type as a fixed factor (to test for effects of stream or reservoir habitats), basin as a fixed factor (to test for basin level effects), and population as a fixed factor nested within habitat by basin interaction (to test for unique population differentiation within habitat types). Heterogeneity of slopes was tested among basins and between habitat types by including centroid size in the respective interaction terms. F-values were approximated using Wilk's lambda and effect strengths by the use of partial eta squared (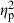 ). I calculated the relative variance as the partial variance for a given term divided by the maximum partial variance value in the model. All nonsignificant interaction terms were removed from the final model.
). I calculated the relative variance as the partial variance for a given term divided by the maximum partial variance value in the model. All nonsignificant interaction terms were removed from the final model.
Random nested terms are usually not applicable in a MANCOVA framework (i.e., matrix determinants can become negative, making the term untestable, and type I error rates may be inflated when nested terms in MANCOVA models are significant; see Rencher 2002, p. 162; and Langerhans and Makowicz 2009). Here, reservoir basin and population can be considered random factors (i.e., they are a sample of all reservoir basins and populations, and the nested population term was significant, see Results below); therefore, I also conducted a mixed-model ANCOVA to test the consistency and nature of morphological divergence in reservoir habitats. I first reduced the dimensionality of the 11 shape variables by calculating morphological divergence scores for each individual along the stream–reservoir gradient based on a divergence vector (referred to as habitat divergence vector hereafter) as defined by Langerhans (2009). This habitat divergence vector does not distort morphological space and summarizes the linear combination of shape variables that contribute to the greatest difference in body shape between reservoir and stream individuals (Langerhans 2009). To quantify this vector, I created a score for each specimen on the stream–reservoir shape axis by multiplying the eigenvector of the habitat term's sums of squares and cross products matrix from a preparatory MANCOVA (final model same as above) by the shape variables block to yield a column of habitat divergence vector scores for each individual. The resulting scores were used as the dependant variable in an ANCOVA with centroid size as a covariate, habitat as a fixed factor, basin as a random factor, and population as a random factor nested in the basin–habitat interaction. Heterogeneity of slopes was tested among basins and between habitat types. I also assessed individual landmark movement between habitat types by quantifying correlation coefficients between landmark positions and the habitat divergence vector scores of field-collected specimens.
Because reservoirs may have more homogenous biotic and abiotic conditions compared with natural stream systems, and thus have more consistent and similar selection pressures among reservoir populations, I also assessed shape diversity (i.e., total variation in shape space) of all specimens in the two habitat types. I first removed effects of size on the 11 shape variables using a preparatory MANCOVA with centroid size as a covariate and used the resulting residuals to quantify convex hull volumes of individuals from stream and reservoir habitats (Tobler and Carson 2010). The computational demand of quantifying convex hull volumes using all shape variables (n = 11) precluded their analysis; therefore, only residuals from the first six relative warps (explained 84% of the variation in shape) were analyzed. In addition, because I sampled more stream populations (n = 14) than reservoir (n = 7), there was potentially more shape variation present in stream individuals owing to more sampled genetic and thus phenotypic variation. Therefore, I randomly chose only one stream population from the Lake Thunderbird basin (Council Creek) and the Lake Texoma basin (Walnut Bayou) to include in the analysis. I randomly sampled (with replacement) 50 stream individuals and 50 reservoir individuals and quantified convex hull volumes of each sample over 10 000 iterations. I tested for differences in overall shape variation (i.e., convex hull volumes) between the two habitat types using independent samples t-test assuming unequal variances. Randomizations were conducted and convex hull volumes were calculated using the convhulln function in MATLAB v. 7.11.0.584 (The Mathworks Inc., Natick, MA, USA).
Morphologic divergence and phenotypic plasticity
I assessed genotypic differences in body shape between a reservoir and a stream population and tested for predator-induced phenotypic plasticity in reared offspring using MANCOVA. The MANCOVA model included 11 shape variables (explaining 97.5% of the variation in shape) as dependent variables, and covariates were log10 centroid size and the number of conspecifics (log10-transformed to approximate normality) to test for effects of density of fish in each mesocosm. Fixed factors were treatment (predator or nonpredator; to test for predator-induced phenotypic plasticity), population-of-origin (to test for genotypic differences between populations) and the interaction between population and treatment. Parents nested within population (to control for nonindependence of parents) served as a nested fixed factor.
Because parents were a random factor (i.e., the individuals spawned were a random sample of individuals in each population), I also conducted two ANCOVAs testing for effects of population and predator-induced phenotypic plasticity. Similar to above, I quantified two morphological divergence vectors, one for the population effect (population divergence vector) and one for the treatment effect (predator divergence vector). Each ANCOVA model used the divergent vectors as dependent variables with centroid size and density of conspecifics as covariates, population and treatment (i.e., predator, nonpredator) as fixed factors, and parents nested within population as a random factor. Population-of-origin could arguably be a random factor; however, in this instance, the two populations were chosen based on a priori knowledge of body shape differences between the populations (i.e., based on preliminary analysis, shape variation was greatest between these populations). Heterogeneity of slopes was tested between populations and treatment, and I removed nonsignificant interaction terms from each of the final models. I completed all analyses in SPSS v. 17.0 (SPSS Inc., Chicago, IL, USA) for Macintosh unless otherwise specified.
Results
Field collections
All terms had significant effects on body shape variation of field-collected C. lutrensis in the MANCOVA (Table 2). Centroid size had the strongest effect on shape (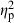 = 0.32), followed by basin (
= 0.32), followed by basin (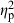 = 0.21), habitat (
= 0.21), habitat (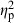 = 0.21), and population nested within the basin–habitat interaction (
= 0.21), and population nested within the basin–habitat interaction (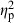 = 0.11; Table 2). The habitat–basin interaction was significant, but its effects were relatively weak in explaining shape variation (
= 0.11; Table 2). The habitat–basin interaction was significant, but its effects were relatively weak in explaining shape variation (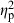 = 0.05).
= 0.05).
Table 2.
Results from body shape variation of individuals collected from stream and reservoir habitats using (a) MANCOVA model with shape variables (n = 11) as dependent variables and (b) ANCOVA model using reservoir and population as random factors and the habitat divergence vector as the dependant variable
| Significance | |||||
|---|---|---|---|---|---|
| Effect | Partial variance | Relative variance | F | df | P |
| (a) Body shape MANCOVA | |||||
| Centroid | 0.316 | 1.000 | 21.81 | 11, 519 | <0.001 |
| Basin | 0.214 | 0.677 | 13.03 | 66, 2782.55 | <0.001 |
| Habitat | 0.207 | 0.655 | 12.29 | 11, 519 | <0.001 |
| Population (Habitat × Basin) | 0.108 | 0.342 | 5.81 | 77, 3117.51 | <0.001 |
| Habitat × Basin | 0.049 | 0.155 | 2.42 | 66, 2782.55 | <0.001 |
| (b) Habitat vector ANCOVA | |||||
| Habitat | 0.372 | 1.000 | 8.23 | 1, 13.88 | 0.012 |
| Population (Habitat × Basin) | 0.215 | 0.578 | 11.13 | 13, 529 | <0.001 |
| Basin | 0.189 | 0.508 | 0.55 | 6, 14.16 | 0.763 |
| Centroid | 0.048 | 0.129 | 26.73 | 1, 529 | <0.001 |
The ANCOVA testing the habitat divergence vector demonstrated similar results (Table 2); however, habitat (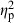 = 0.37) and population (
= 0.37) and population (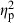 = 0.22) had the strongest effects followed by basin and centroid size. Conversely, the habitat–basin interaction was not significant. Body shape diverged consistently in reservoir habitats in the replicated reservoir basins; however, there was substantial variation in the replicated stream populations in one reservoir basin (Thunderbird) where several stream populations were collected (Fig. 3). Generally, C. lutrensis in reservoir habitats had shorter heads with deeper body depths compared with individuals from stream habitats (Fig. 4). Specifically, body shape divergence in reservoir habitats was attributed to posterior movement of the tip of the snout, dorsal movement of the corner of the mouth, posterior movement of the anal fin, ventral movement of the pelvic fin, and anterior movement of the pectoral fin (Table 3).
= 0.22) had the strongest effects followed by basin and centroid size. Conversely, the habitat–basin interaction was not significant. Body shape diverged consistently in reservoir habitats in the replicated reservoir basins; however, there was substantial variation in the replicated stream populations in one reservoir basin (Thunderbird) where several stream populations were collected (Fig. 3). Generally, C. lutrensis in reservoir habitats had shorter heads with deeper body depths compared with individuals from stream habitats (Fig. 4). Specifically, body shape divergence in reservoir habitats was attributed to posterior movement of the tip of the snout, dorsal movement of the corner of the mouth, posterior movement of the anal fin, ventral movement of the pelvic fin, and anterior movement of the pectoral fin (Table 3).
Figure 3.
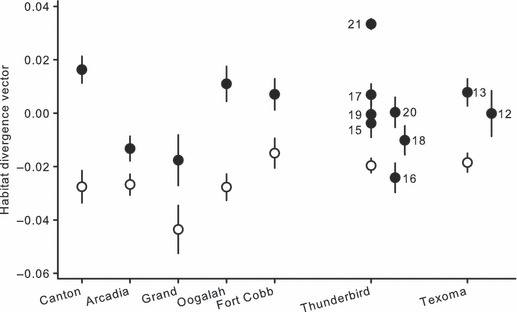
Mean ± SE morphological habitat divergence vector scores of stream populations (closed circles) and reservoir populations (open circles) from each reservoir basin. Stream populations from Lake Thunderbird and Lake Texoma are numbered according to Table 1.
Figure 4.
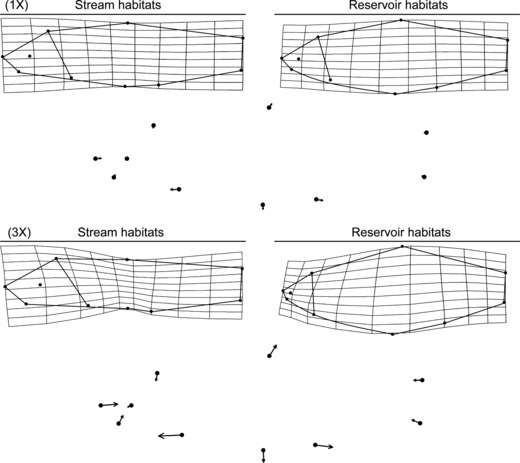
Morphological variation in Cyprinella lutrensis along the habitat divergence vector. Grids are thin-plate spline transformations from specimen means along the morphological divergence vector at the observed scale. Lines are drawn between landmarks to aid visualization. Landmark vectors below transformations reflect the direction and magnitude of each landmark movement between habitats. Vectors point in the direction landmarks moved from stream habitats to reservoir habitats. The top visualization is at the observed range and the bottom is magnified 3×.
Table 3.
Pearson's correlation coefficients between superimposed landmarks and morphological habitat divergence vector scores. Coefficients >0.40 are in bold, and the directionality of the landmark shifts is presented for stream habitats relative to reservoir habitats (i.e., movement of landmarks reflects the shifts from stream habitats to reservoir habitats)
| Landmark | Coefficient | Direction |
|---|---|---|
| X1 | +0.68 | Posterior |
| Y1 | +0.05 | – |
| X2 | +0.17 | – |
| Y2 | +0.52 | Dorsal |
| X3 | −0.27 | – |
| Y3 | −0.11 | – |
| X4 | −0.14 | – |
| Y4 | −0.35 | – |
| X5 | +0.29 | – |
| Y5 | +0.30 | – |
| X6 | −0.26 | – |
| Y6 | +0.03 | – |
| X7 | −0.29 | – |
| Y7 | +0.25 | – |
| X8 | +0.59 | Posterior |
| Y8 | −0.12 | – |
| X9 | −0.00 | – |
| Y9 | −0.41 | Ventral |
| X10 | −0.87 | Anterior |
| Y10 | −0.02 | – |
In tests for decreased shape diversity in reservoir habitats, on average stream fish demonstrated 43% greater variation in shape space compared with fish collected in reservoir habitats (t1,19998 = 87.7, P < 0.001; Fig. 5).
Figure 5.
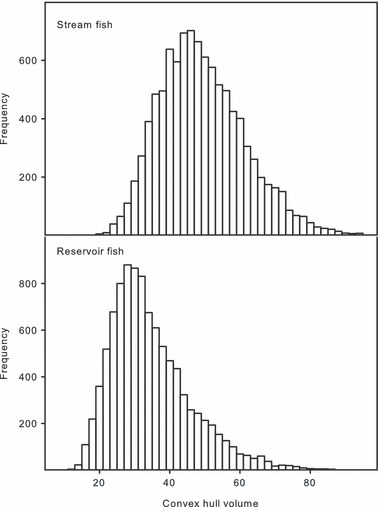
Frequency histograms of convex hull volumes calculated using the first six shape variables (i.e., relative warps) from 50 randomly sampled individuals over 10 000 iterations from stream habitats (top panel) and reservoir habitats (bottom panel).
Genetic divergence and phenotypic plasticity
Owing to low spawning success and high juvenile mortality, only four parental pairs from the reservoir population and eight parental pairs from the stream population were successfully spawned with offspring surviving in both predator and nonpredator treatments. Overall, 257 individuals were analyzed for shape variation and 10.7 individuals on average (range = 1–25) were analyzed from each mesocosm.
When testing for genotypic and predator-induced phenotypic plasticity effects on body shape of C. lutrensis offspring using MANCOVA, all terms had significant effects on body shape except for density of conspecifics in mesocosms. Centroid size (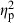 = 0.58) had the strongest effect, followed by treatment (
= 0.58) had the strongest effect, followed by treatment (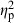 = 0.28, demonstrating predator-induced phenotypic plasticity), population-of-origin (
= 0.28, demonstrating predator-induced phenotypic plasticity), population-of-origin (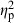 = 0.28, demonstrating population-level differences), and parents (
= 0.28, demonstrating population-level differences), and parents (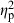 = 0.19), and the population–treatment interaction had the smallest significant effect (
= 0.19), and the population–treatment interaction had the smallest significant effect (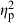 = 0.13; Table 4). The number of conspecifics in each mesocosm did not have a significant effect on shape variation.
= 0.13; Table 4). The number of conspecifics in each mesocosm did not have a significant effect on shape variation.
Table 4.
Results from the genotypic divergence and plasticity experiment. (a) MANCOVA model using shape variables (n = 11) as dependent variables, (b) ANCOVA model using parents as a random factor and the population divergence vector as the dependant variable, and (c) ANCOVA model using parents as a random factor and the predator divergence vector as the dependent variable
| Significance | |||||
|---|---|---|---|---|---|
| Effect | Partial variance | Relative variance | F | df | P |
| (a) Body shape MANCOVA | |||||
| Centroid | 0.583 | 1.000 | 29.33 | 11, 231 | <0.001 |
| Treatment | 0.284 | 0.487 | 8.34 | 11, 231 | <0.001 |
| Population | 0.279 | 0.479 | 8.14 | 11, 231 | <0.001 |
| Parents (population) | 0.186 | 0.319 | 5.016 | 110, 1741.9 | <0.001 |
| Population × Treatment | 0.084 | 0.144 | 1.92 | 11, 231 | 0.038 |
| Density | 0.061 | 0.105 | 1.37 | 11, 231 | 0.187 |
| (b) Population divergence ANCOVA | |||||
| Population | 0.366 | 1.000 | 5.88 | 1, 10.168 | 0.035 |
| Parents (population) | 0.195 | 0.533 | 5.87 | 10, 242 | <0.001 |
| Treatment | 0.057 | 0.156 | 14.71 | 1, 242 | <0.001 |
| Centroid | 0.016 | 0.044 | 3.91 | 1, 242 | 0.049 |
| Density | 0.002 | 0.005 | 0.37 | 1, 242 | 0.546 |
| (c) Plastic divergence ANCOVA | |||||
| Population | 0.435 | 1.000 | 7.85 | 1, 10.2 | 0.018 |
| Treatment | 0.187 | 0.430 | 55.47 | 1, 241 | <0.001 |
| Parents (population) | 0.144 | 0.331 | 4.07 | 10, 241 | <0.001 |
| Centroid | 0.085 | 0.195 | 22.39 | 1, 241 | <0.001 |
| Population × Treatment | 0.039 | 0.090 | 9.875 | 1, 241 | 0.002 |
| Density | 0.009 | 0.021 | 2.07 | 1, 241 | 0.152 |
The ANCOVAs testing effects on the population and predator divergence vectors offered similar results. Testing the population divergence vector revealed that population-of-origin had the strongest effect (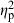 = 0.37) followed by parents (
= 0.37) followed by parents (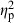 = 0.20). Treatment (
= 0.20). Treatment (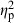 = 0.06) and centroid size (
= 0.06) and centroid size (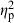 = 0.02) also had significant effects, but their contribution to shape variation was relatively small (Table 4). Similarly, population (
= 0.02) also had significant effects, but their contribution to shape variation was relatively small (Table 4). Similarly, population (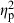 = 0.44), treatment (
= 0.44), treatment (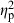 = 0.19), and parents (
= 0.19), and parents (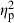 = 0.14) had significant effects on the predator divergence vector. Although there was a significant interaction between population and treatment, this effect was relatively weak (
= 0.14) had significant effects on the predator divergence vector. Although there was a significant interaction between population and treatment, this effect was relatively weak (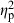 = 0.04), and populations generally responded to the presence of a predator in a similar manner (Fig. 6). Offspring from the stream population had larger caudal areas and smaller head regions compared with offspring from the reservoir population and resembled similar body shapes to adult male C. lutrensis collected from reservoir habitats (Fig. 7). Juvenile C. lutrensis reared with predators also had smaller heads and larger caudal areas compared with individuals reared with nonpredators (Fig. 7).
= 0.04), and populations generally responded to the presence of a predator in a similar manner (Fig. 6). Offspring from the stream population had larger caudal areas and smaller head regions compared with offspring from the reservoir population and resembled similar body shapes to adult male C. lutrensis collected from reservoir habitats (Fig. 7). Juvenile C. lutrensis reared with predators also had smaller heads and larger caudal areas compared with individuals reared with nonpredators (Fig. 7).
Figure 6.
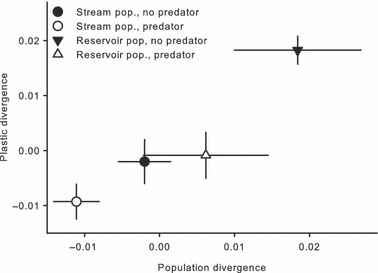
Mean ± SE population and predator divergence vector scores of mean offspring from each parent–treatment combination (i.e., each mesocosm) of offspring spawned from a reservoir (n = 4 parents) and stream population (n = 8 parents) and reared in predator and nonpredator treatments.
Figure 7.
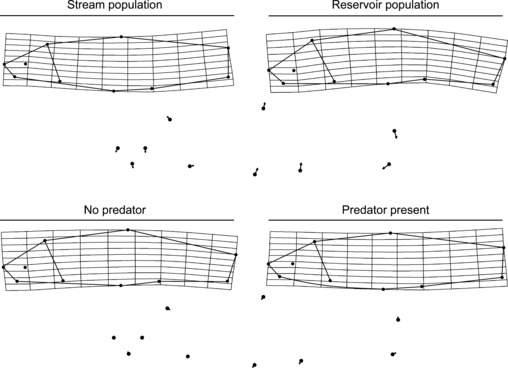
Morphological variation of Cyprinella lutrensis between stream population and reservoir population offspring and between offspring reared with and without a predator. Grids are thin-plate spline transformations from specimen means (observed range) along the population divergence vector (above) and the predator divergence vector (below). Lines are drawn between landmarks to aid visualization. Landmark vectors point in the direction landmarks move from the stream population to the reservoir population and from the nonpredator-reared offspring to the predator-reared offspring.
Discussion
My results suggest consistent morphological divergence of a native small-bodied fish in anthropogenically altered riverine systems. Experimental results from rearing offspring of a reservoir and stream population with and without predators verified that (i) shape variation between the two studied populations had a genetic basis and (ii) both populations exhibited similar predator-induced phenotypic plasticity in body shape.
Field collections
Consistent morphological divergence between stream and reservoir populations within reservoir basins suggests that habitat alteration by impoundments is driving predictable phenotypic variation in C. lutrensis. Body shape of C. lutrensis in reservoirs was less streamlined with deeper caudal areas and smaller heads. This morphological divergence was also qualitatively similar to morphological shifts found in reservoir-residing Cyprinella venusta (Haas et al. 2010), a small-bodied species ecologically similar to C. lutrensis. Such intraspecific trait divergence implies that different reservoirs create similar selective pressures on small-bodied fishes. In response, phenotypes are potentially adapting to maximize fitness in these habitats. It is unlikely that only one environmental factor is driving morphological divergence; a suite of novel selective pressures could potentially contribute to phenotypic differences between stream- and reservoir-resident populations.
Because conversion of riverine systems to reservoir habitats is associated with multiple biotic and abiotic environmental changes (e.g., turbidity, flow, temperature, biotic communities), it may be difficult to isolate one factor independently without experimental manipulation. However, phenotypic variation of C. lutrensis did match predicted morphologies thought to be adaptive in both low-flow conditions (Gosline 1971; Alexander 1967; Langerhans 2008) and habitats with high predator densities (Domenici and Blake 1997; Langerhans et al. 2004; Hendry et al. 2006; Langerhans et al. 2009). These two factors in concert could be driving observed morphological shifts of small-bodied fishes. The increased body depth and caudal area could increase predator escape performance (through increased burst speed; Langerhans 2008) and maneuverability for feeding on prey suspended in the water column (versus drifting prey in streams; Rincón et al. 2007) or through steady/unsteady swimming performance trade-offs (Langerhans 2008, 2009).
Assuming morphological divergence in reservoirs confers greater fitness to reservoir-resident individuals, divergent natural selection could lead to local adaptation in these habitats. Investigations of the morphologies of other fishes between lake–stream pairs suggest that local habitats can drive phenotypic variation in spite of close proximities of populations (Brinsmead and Fox 2002; Hendry et al. 2002; Berner et al. 2009; Haas et al. 2010). Currently, the extent of gene flow among stream and reservoir populations of C. lutrensis is unknown, but high migration rates among populations could limit the extent of local adaptation in reservoir habitats.
Reservoir basin explained a considerable amount of variation in shape in both the MANCOVA and ANCOVA models. Given the geographic distances among reservoir basins (Fig. 1), a significant basin effect would likely be expected assuming fish from different basins have unique evolutionary histories; however, the use of museum and more recently collected specimens likely confounded this result. Because museum specimens were in preservative for at least 10 years, significant preservation effects on body shape could have contributed to the basin effect. Indeed, both time and the type of long-term preservative solution (i.e., formalin or 50% isopropyl alcohol) can have significant effects on body shape of preserved C. lutrensis individuals (Appendix S1). Therefore, it was problematic to isolate basin effects versus preservation effects with this data set.
Physical barriers separating populations (Palkovacs et al. 2008), or geographic distances among populations (Moore et al. 2007; Berner et al. 2009; Langerhans et al. 2003), may influence morphological variation within reservoir basins. Although the low sample size precluded statistical analyses, the shape variation among the replicated stream populations within the Lake Thunderbird basin suggests there was a possible spatial component to morphological divergence. Indeed, two of the three stream populations that were morphologically most similar to reservoir individuals were collected from streams that flow directly into Lake Thunderbird (Fig. 1). Thus, the close spatial proximity of direct tributary populations to reservoirs could allow increased gene exchange with reservoir populations, or streams closer to the reservoir could have environmental conditions more similar to reservoirs (e.g., fish communities; Falke and Gido 2006).
Assessment of total shape variation between habitat types revealed lower morphological variation of C. lutrensis individuals in reservoirs compared with their stream counterparts. Unnatural reservoir habitats (both biotic and abiotic components) are likely more homogonous compared with natural stream systems (Wetzel 1990; Gido et al. 2009). Stream habitat heterogeneity could potentially create spatial and temporal variation in selection pressures or facilitate variable plastic morphological responses in stream fishes. Additionally, higher habitat complexity may also allow for increased individual specialization (Bolnick et al. 2003), increasing population-level morphological variation. While these explanations are speculative, further investigations will have to be implemented to derive the underlying mechanisms behind reduced morphological variation in reservoir habitats.
Genetic divergence and phenotypic plasticity
Results from rearing offspring from a reservoir and stream population with and without a predator present suggest that both genotypic variation and phenotypic plasticity contributed nearly equally to observed phenotypic differentiation between these two populations. Population-of-origin had strong effects on overall shape variation as well as the population and predator divergence vectors. Collectively, these results indicate that body shape variation among offspring was most strongly influenced by their population-of-origin, followed by predator-induced phenotypic plasticity. Although I was unable to assess heritability directly by comparing parental and offspring morphologies (parents were in very poor condition following spawning), or compare spawned offspring with field specimens (the size distributions of the two groups showed little overlap), results did support a heritable basis to body shape variation between the reservoir and stream populations.
When populations become isolated and divergent natural selection is strong, evolution of traits can occur over relatively short timescales (e.g., Reznick et al. 1997; Stockwell and Weeks 1999; Hendry et al. 2000). Because the reservoir and stream populations used here were separated by the physical stream impoundment, migration of individuals through the dam structure is improbable. Therefore, these two populations likely have had little or no gene flow since construction of the reservoir in 1965. Additionally, C. lutrensis can spawn during its first year of life (Marsh-Matthews et al. 2002), potentially allowing for over 80 generations since these two populations became isolated, far more than needed to observe evolution under experimental conditions (Reznick et al. 1997). This suggests that anthropogenic habitat alteration has potentially facilitated adaptive trait divergence. Nonetheless, the observed predator-induced phenotypic plasticity suggests that environmental contingent phenotypes could also contribute to observed phenotypic divergence in reservoirs.
When reared with predators, the offspring of both populations demonstrated similar predator-induced phenotypic plasticity (although significant, the population by treatment interaction explained comparatively little variation). However, based on the direction of the plastic shift in morphological space of both populations (Fig. 6), it is unlikely that the morphological divergence found in reservoirs was attributed to predator-induced phenotypic plasticity. Assuming reservoir phenotypes are adaptive and predator-induced plasticity was contributing to the observed phenotypic variation in reservoirs, the plastic shift in laboratory-reared individuals should have shifted in the direction of the reservoir population. However, when exposed to predators during development, offspring tended to resemble the stream population and not the reservoir population. Nonetheless, phenotypic plasticity along other environmental gradients observed between stream and reservoir habitats (e.g., flow regime) may contribute to observed phenotypic variation in the field.
The laboratory-reared offspring of the stream and reservoir populations exhibited disparate shape variation compared with their field-collected counterparts. However, this result needs to be interpreted with caution for several reasons. First, because C. lutrensis offspring were much smaller [mean SL (mm) = 22.3 ± 4.9 SD] than field-collected individuals [mean SL (mm) = 46.6 ± 6.6 SD], allometric shape variation may confound comparisons between such large size differences (Bookstein 1991; Zelditch et al. 2004). Indeed, centroid size had a significant effect on shape in all models tested, and therefore, direct comparison of field- and laboratory-reared phenotypes may be misleading. Second, sex and breeding condition of individuals could also confound comparisons between the two groups; shape analyses of field individuals were restricted to only males in breeding condition (i.e., individuals in breeding color with head tubercles), while laboratory-reared individuals were not sexed and none exhibited breeding colors or tubercles. Cyprinella lutrensis as small as 29 mm SL can reach sexual maturity (Marsh-Matthews et al. 2002); therefore, most laboratory-reared individuals were not of reproductive age. Whereas population-level differences were apparent in the laboratory-reared individuals, in light of these confounding effects, it is unclear whether the same shape differences observed in the field would be present in laboratory-reared individuals reared to a larger size.
While interpretations of morphological comparisons between field-collected and laboratory-reared individuals were likely confounded, it is also unclear whether the population-level morphological differences in the laboratory were driven by divergent selection in the reservoir or were merely a function of genetic differences due to geographic distance between populations. Moreover, the results of the plasticity experiment were limited by having only one reservoir replicate. Further experiments assessing population-level morphological divergence in other reservoir basins may elucidate the consistency of genetic divergence in replicated reservoir systems.
Conservation implications
The implications of rapid evolutionary change on conservation efforts have gained interest in recent years (Stockwell et al. 2003; Carroll et al. 2007). While reservoirs create novel environmental conditions, they are also relatively young on an evolutionary timescale. Yet evidence suggests that stream fishes that can persist in these habitats have undergone divergent evolution in <100 years (Haas et al. 2010; this paper). Assuming contemporary evolution of reservoir-resident fishes has adapted them to impounded habitats, these reservoir-adapted traits may not be adaptive in other environments. For example, reservoir-adapted phenotypes could have lower fitness in flowing water habitats compared with resident stream fishes. Therefore, reservoir-adapted individuals would potentially be poor candidates to re-colonize extirpated populations in streams that flow into a reservoir proper (i.e., direct tributaries of reservoirs). Matthews and Marsh-Matthews (2007) documented the near or complete extirpation of C. lutrensis from several direct tributaries of Lake Texoma, Oklahoma-Texas, USA, in spite of the fact C. lutrensis still inhabits the reservoir proper, whereas stream populations upstream of the reservoir remained intact. In addition, recent re-colonization of at least one direct tributary did not result in reestablishment of the species (Marsh-Matthews et al. 2011). Because the streams that historically harbored C. lutrensis flow directly into the reservoir, new colonists are likely to be derived from reservoir populations. Although other factors could have influenced the extirpation of C. lutrensis in these direct tributaries (e.g., habitat changes, increased predation pressure; Matthews and Marsh-Matthews 2007), reservoir-adapted individuals colonizing extirpated stream habitats are potentially ill-adapted to successfully reestablish viable populations. Moreover, introgression of reservoir-adapted genotypes into resident stream populations may also decrease the mean fitness of stream populations, increasing the likelihood of extirpations. However, experimental manipulation such as environmental transplanting or swimming performance estimates will be needed to assess whether reservoir individuals are ill-adapted to stream habitats.
Conclusions
This study documented consistent morphological divergence in body shape of a native stream fish in reservoirs of impounded riverine systems. A common garden experiment revealed that body shape differences between a reservoir and stream population had a genetic basis, and the rearing of offspring with and without predators induced phenotypic plasticity in body shape. However, based on the direction of the plastic shift in morphological space, increased predator densities in reservoirs are likely not driving the observed divergence (because of predator-induced phenotypic plasticity). Although this study provided evidence of genetic-based morphological divergence in reservoirs, assessment of several other lines of investigation are needed. First, migration levels among stream and reservoir populations will be needed to assess the extent to which gene flow may limit local adaptation to reservoir habitats. Second, although C. lutrensis demonstrated predator-induced plasticity, it may be fruitful to investigate the plastic responses to different flow regimes, the other obvious change to reservoir habitats. The relative contribution of plasticity versus genetic components in observed phenotypic variation will also elucidate the extent of local adaptation in these systems. Finally, relationships between body morphology and fitness in stream and reservoir habitats will need to be assessed to determine whether body shape influences fitness in various habitats.
Acknowledgments
M. Tobler, C. Tobler, E. Marsh-Matthews, W. Matthews, E. Remmel, R. Zamor, M. Dugas, and R. Cothran helped with specimen collection. The Sam Noble Oklahoma Museum of Natural History and E. Marsh-Matthews provided funding. A. Tarhule, C. Vaughn, G. Wellborn, E. Marsh-Matthews, W. Matthews, M. Tobler, A. Stiff, and OU ZEEB journal club made comments on the manuscript. M. Dugas and M. Tobler helped with data analyses and the preservation study. Three anonymous reviewers greatly improved the manuscript. This manuscript is in partial fulfillment of a Ph.D. degree from the Department of Zoology, University of Oklahoma. This project was approved by IACUC no. R08-004 from the University of Oklahoma.
Data archiving statement
Raw data for this study are available as Online Supporting Information on the Evolutionary Applications website.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Procrustes aligned landmark values for field-collected specimens.
Table S2. Procrustes aligned landmark values for lab reared specimens coded as parents, population of origin, and treatment.
Appendix S1. Preservation effects on body shape variation.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Literature cited
- Alexander RM. Functional Design in Fishes. London: Hutchinson; 1967. [Google Scholar]
- Baxter RM. Environmental effects of dams and impoundments. Annual Review of Ecology, Evolution and Systematics. 1977;8:255–283. [Google Scholar]
- Berner D, Grandchamp A, Hendry AP. Variable progress toward ecological speciation in parapatry: stickleback across eight lake-stream transitions. Evolution. 2009;7:1740–1753. doi: 10.1111/j.1558-5646.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. The ecology of individuals: incidence and implications of individual specialization. American Naturalist. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Brinsmead J, Fox MG. Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. Journal of Fish Biology. 2002;61:1619–1638. [Google Scholar]
- Brönmark C, Miner JG. Predator-induced phenotypic change in body morphology in Crucian carp. Science. 1992;258:1348–1350. doi: 10.1126/science.258.5086.1348. [DOI] [PubMed] [Google Scholar]
- Carlander KD. Handbook of Freshwater Fishery Biology. Vol. 1. Ames: The Iowa State University Press; 1969. [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Chivers DP, Zhao X, Brown GE, Marchant TA, Ferrari MCO. Predator-induced changes in morphology of a prey fish: the effects of food level and temporal frequency of predation risk. Evolutionary Ecology. 2008;22:561–574. [Google Scholar]
- Domenici P, Blake RW. Fish fast-start kinematics and performance. Journal of Experimental Biology. 1997;200:1165–1178. doi: 10.1242/jeb.200.8.1165. [DOI] [PubMed] [Google Scholar]
- Downing JA, Prairie YT, Cole JJ, Duarte CM, Tranvik LJ, Striegl RG, McDowell WH, et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnology and Oceanography. 2006;51:2388–2397. [Google Scholar]
- Dudgeon D, Arthington AH, Gessner MO, Kawabata Z, Knowler DJ, Lévêque C, Naiman RJ, et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological. Reviews. 2006;81:163–182. doi: 10.1017/S1464793105006950. [DOI] [PubMed] [Google Scholar]
- Dynesius M, Nilsson C. Fragmentation and flow regulation of river systems in the northern third of the world. Science. 1994;226:753–761. doi: 10.1126/science.266.5186.753. [DOI] [PubMed] [Google Scholar]
- Endler JA. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Falke JA, Gido KB. Effects of reservoir connectivity on stream fish assemblages in the Great Plains. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:480–493. [Google Scholar]
- Fine ML, Mayo MS, Newton SH, Sismour EN. Largemouth bass predators reduce growth, feeding, and movement in juvenile channel catfish. Ecology of Freshwater Fish. 2011;20:114–119. [Google Scholar]
- Fischer-Rousseau L, Chu KP, Cloutier R. Developmental plasticity in fish exposed to a water velocity gradient: a complex response. Journal of Experimental Zoology B Molecular and Developmental Evolution. 2010;314:67–85. doi: 10.1002/jez.b.21311. [DOI] [PubMed] [Google Scholar]
- Gido KB, Brown JH. Invasion of North American drainages by alien fish species. Freshwater Biology. 1999;42:387–399. [Google Scholar]
- Gido KB, Schaefer JF, Falke JA. Convergence of fish communities from the littoral zone of reservoirs. Freshwater Biology. 2009;54:1163–1177. [Google Scholar]
- Gosline WA. Functional Morphology and Classification of Teleostean Fishes. Honolulu: University of Hawaii Press; 1971. [Google Scholar]
- Grünbaum T, Cloutier R, Mabee PM, Le François NR. Early developmental plasticity and integrative responses in arctic charr (Salvelinus alpinus): effects of water velocity on body size and shape. Journal of Experimental Zoology B Molecular and Developmental Evolution. 2007;308:396–408. doi: 10.1002/jez.b.21163. [DOI] [PubMed] [Google Scholar]
- Haas TC, Blum MJ, Heins DC. Morphological responses of a stream fish to water impoundment. Biology Letters. 2010;6:803–806. doi: 10.1098/rsbl.2010.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Wenberg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Taylor EB, McPhail JD. Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the Misty system. Evolution. 2002;56:1199–1216. doi: 10.1111/j.0014-3820.2002.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kelly ML, Kinnison MT, Reznick DN. Parallel evolution of the sexes? Effects of predation and habitat features on the size and shape of wild guppies. Journal of Evolutionary Biology. 2006;19:741–754. doi: 10.1111/j.1420-9101.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, et al. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography. 2006;15:1–7. [Google Scholar]
- Hubbs CL. A Symposium on Hydrobiology. In: Needham JG, Sears PB, Leopold A, editors. Madison: Wisconsin Press; 1941. pp. 182–195. The relation of hydrological conditions to speciation in fishes. [Google Scholar]
- Keeley ER, Parkinson EA, Taylor EB. The origins of ecotypic variation of rainbow trout: a test of environmental vs. genetically based differences in morphology. Journal of Evolutionary Biology. 2007;20:725–736. doi: 10.1111/j.1420-9101.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- Koehn RK. Development and ecological significance of nuptial tubercles of the red shiner: Notropis lutrensis. Copeia. 1965;1965:462–467. [Google Scholar]
- Langerhans RB. Predictability of phenotypic differentiation across flow regimes in fishes. Integrative and Comparative Biology. 2008;48:750–768. doi: 10.1093/icb/icn092. [DOI] [PubMed] [Google Scholar]
- Langerhans RB. Trade-off between steady and unsteady swimming underlies predator driven divergence in Gambusia affinis. Journal of Evolutionary Biology. 2009;22:1057–1075. doi: 10.1111/j.1420-9101.2009.01716.x. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Makowicz AM. Shared and unique features of morphological differentiation between predator regimes in Gambusia caymanensis. Journal of Evolutionary Biology. 2009;22:2231–2242. doi: 10.1111/j.1420-9101.2009.01839.x. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Layman CA, Langerhans AK, DeWitt TJ. Habitat-associated morphological divergence in two Neotropical fish species. Biological Journal of the Linnaean Society. 2003;80:689–698. [Google Scholar]
- Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. Predator-driven phenotypic diversification in Gambusia affinis. Evolution. 2004;58:2305–2318. doi: 10.1111/j.0014-3820.2004.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. Predator-driven phenotypic diversification in Gambusia affinis. Evolution. 2009;58:2305–2318. doi: 10.1111/j.0014-3820.2004.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Marsh-Matthews E, Matthews WJ, Gido KB, Marsh RL. Reproduction by young-of-year red shiner (Cyprinella lutrensis) and its implications for invasion success. Southwestern Association of Naturalists. 2002;47:605–610. [Google Scholar]
- Marsh-Matthews E, Matthews WJ, Franssen NR. Can a highly invasive species re-invade its native community? The paradox of the red shiner. Biological Invasions. 2011 doi: 10.1007/s10530-011-9973-2. [Google Scholar]
- Matthews WJ. Geographic variation of Cyprinella lutrensis (Pisces: Cyprinidae) in the United States, with notes on Cyprinella lepida. Copeia. 1987;1987:616–637. [Google Scholar]
- Matthews WJ, Marsh-Matthews E. Extirpation of red shiner in direct tributaries of Lake Texoma (Oklahoma-Texas): a cautionary case history from a fragmented river-reservoir system. Transactions of the American Fisheries Society. 2007;136:1041–1062. [Google Scholar]
- McGuigan KA, Franklin CE, Moritz C, Blows MW. Adaptation of rainbow fish to lake and stream habitats. Evolution. 2003;57:104–118. doi: 10.1111/j.0014-3820.2003.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Moore JS, Gow JL, Taylor EB, Hendry AP. Quantifying the constraining influence of gene flow on adaptive divergence in the lake-stream threespine stickleback system. Evolution. 2007;61:2015–2026. doi: 10.1111/j.1558-5646.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- Pakkasmaa S, Piironen J. Water velocity shapes juvenile salmonids. Evolutionary Ecology. 2001;14:721–730. [Google Scholar]
- Palkovacs EP, Dion KB, Post DM, Caccone A. Independent evolutionary origins of landlocked alewife populations and rapid parallel evolution of phenotypic traits. Molecular Ecology. 2008;17:582–597. doi: 10.1111/j.1365-294X.2007.03593.x. [DOI] [PubMed] [Google Scholar]
- Paller MH. The influence of biomanipulation on fish community development in a southeastern United States cooling reservoir. Hydrobiologia. 2005;539:69–81. [Google Scholar]
- Pavey SA, Nielsen JL, Mackas RH, Hamon TR, Breden F. Contrasting ecology shapes juvenile lake-type and riverine Sockeye Salmon. Transactions of the American Fisheries Society. 2010;139:1584–1594. [Google Scholar]
- Pimm SL, Russel GJ, Gittleman JL, Brooks TM. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- Rencher AC. Methods of Multivariate Analysis. 2nd edn. New York: Wiley-Interscience; 2002. [Google Scholar]
- Reznick DN, Shaw FH, Rodd FH, Shaw RG. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticula. Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- Rincón PA, Bastir M, Grossman GD. Form and performance: body shape and prey-capture success in four drift-feeding minnows. Oecologia. 2007;152:345–355. doi: 10.1007/s00442-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Rohlf F. Relative warp analysis and an example of its application to mosquito wings. In: Marcus LF, Bello E, Garcia-Valdecasas A, et al., editors. Contributions to Morphometrics. Madrid, Spain: Musuo Nacionale de Ciencias Naturales; 1993. pp. 131–159. [Google Scholar]
- Rohlf F. Stony Brook: Department of Ecology and Evolution, State University of New York; 2004a. tpsDig. Version 2.1 [computer program] http://life.bio.sunysb.edu/morph/ (accessed on 15 August 2010) [Google Scholar]
- Rohlf F. Stony Brook: Department of Ecology and Evolution, State University of New York; 2004b. tpsUtil. Version 1.45 [computer program] http://life.bio.sunysb.edu/morph/ (accessed on 15 August 2010) [Google Scholar]
- Rohlf F. Stony Brook: Department of Ecology and Evolution, State University of New York; 2004c. tpsRelw. Version 1.46 [computer program] http://life.bio.sunysb.edu/morph/ (accessed on 15 August 2010) [Google Scholar]
- Rohlf F. Stony Brook: Department of Ecology and Evolution, State University of New York; 2004d. tpsRegr. Version 1.36 [computer program] http://life.bio.sunysb.edu/morph/ (accessed on 15 August 2010) [Google Scholar]
- Schlichting CD, Pigliucci M. Phenotypic Evolution: A Reaction Norm Perspective. Sunderland, Maryland: Sinauer Associates; 1998. [Google Scholar]
- Schneider JC, Laarman PW, Gowing H. Length-weight relationships. In: Schneider JC, editor. Manual of Fisheries Survey Methods II: With Periodic Updates. Fisheries Special Report 25, Ann Arbor: Michigan Department of Natural Resources; 2000. pp. 1–18. Chapter 17. [Google Scholar]
- Stockwell CA, Weeks SC. Translocations and rapid evolutionary responses in recently established populations of western mosquitofish (Gambusia affinis. Animal Conservation. 1999;2:103–110. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution. 2003;18:94–101. [Google Scholar]
- Taylor CA, Knouft JH, Hiland TM. Consequences of stream impoundment on fish communities in a small North American drainage. Regulated Rivers: Research and Management. 2001;17:687–698. [Google Scholar]
- Tobler M, Carson EW. Environmental variation, hybridization, and phenotypic diversification in Cuatro Ciénegas pupfishes. Journal of Evolutionary Biology. 2010;23:1475–1489. doi: 10.1111/j.1420-9101.2010.02014.x. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:492–499. [Google Scholar]
- Walker JA. Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biological Journal of the Linnean Society. 1997;61:3–50. [Google Scholar]
- West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics. 1989;20:249–278. [Google Scholar]
- Wetzel RG. Reservoir ecosystems: conclusions and speculations. In: Thornton KW, Kimmel BL, Payne FE, editors. Reservoir Limnology: Ecological Perspectives. New York: Wiley; 1990. pp. 227–238. [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets DH, Fink WL. Geometric Morphometrics for Biologists. San Diego, CA: Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


