Abstract
As policymakers and managers work to mitigate the effects of rapid anthropogenic environmental changes, they need to consider organisms’ responses. In light of recent evidence that evolution can be quite rapid, this now includes evolutionary responses. Evolutionary principles have a long history in conservation biology, and the necessary next step for the field is to consider ways in which conservation policy makers and managers can proactively manipulate evolutionary processes to achieve their goals. In this review, we aim to illustrate the potential conservation benefits of an increased understanding of evolutionary history and prescriptive manipulation of three basic evolutionary factors: selection, variation, and gene flow. For each, we review and propose ways that policy makers and managers can use evolutionary thinking to preserve threatened species, combat pest species, or reduce undesirable evolutionary changes. Such evolution-based management has potential to be a highly efficient and consistent way to create greater ecological resilience to widespread, rapid, and multifaceted environmental change.
Keywords: conservation biology, environmental management, evolution, gene flow, selection, variation
Introduction
Human activity has altered every ecosystem on the planet, and these impacts are likely to increase in the future (Vitousek et al. 1997). From the local to the global scale, human actions have influenced – and in many cases, disrupted – the structure and functioning of populations, communities, and ecosystems. In response, environmental scientists, decision makers, and managers have strived to mitigate these impacts and preserve biologic diversity and ecosystem function. The vast majority of these mitigations have focused on the numerical dynamics of populations (Mace and Purvis 2008). However, populations are rarely genetically uniform and thus have the potential for evolutionary change. In recent years, it has become increasingly clear that these evolutionary changes can occur rapidly (i.e. on ecological time scales), fast enough that management cannot safely ignore this possibility (Ashley et al. 2003; Stockwell et al. 2003). This means that considering evolutionary history, as well as current and future evolutionary processes, more generally will be important for achieving successful management outcomes.
Evolutionary principles have been central to the science of conservation biology since its inception (Hendry et al. 2010). In particular, genetic information has long been used to inform conservation priorities and strategies, such as through the delineation of ‘Evolutionarily significant units’ to protect genetically distinct populations (Crandall et al. 2000) and to monitor population genetic patterns that indicate population status (Schwartz et al. 2007). At the local scale, species recovery plans and reserve designs often explicitly seek to preserve genetic diversity within populations, in the hope that this will promote the population's ability to respond to future evolutionary pressures (Storfer 1996). These plans seek either to preserve past evolutionary conditions (e.g. preserving the genetic structure of meta-populations) or to ensure maximum evolutionary potential to meet future changing condition (Mace and Purvis 2008). Such plans are often locally focused, however, and can miss important factors at the global and (inter)national scale (Mace and Purvis 2008). Additionally, there have been increasing calls for directly incorporating contemporary selection and adaptation in conservation strategies (Kinnison et al. 2007; Mace and Purvis 2008). To date, most of the research has focused on the importance of incorporating evolutionary processes into predictions, for example, as to whether a population will persist or be especially vulnerable to an impending threat, and avoiding management actions that inadvertently select for undesirable traits (Kinnison and Hairston 2007; Mace and Purvis 2008). The necessary next step is to explore how managers and policy makers can proactively use evolutionary principles to achieve conservation goals (Kinnison et al. 2007; Mace and Purvis 2008; Schlaepfer et al. 2010). Incorporating evolutionary thinking into conservation decision making has the potential to both reduce the likelihood of ‘unpleasant surprises’ and offer novel avenues for management intervention.
In this synthesis, we seek to build upon this previous work by reviewing and where appropriate, proposing new ways in which evolutionary processes can be directly manipulated to meet management goals. These management actions span a wide range of spatial and political scales, from local actions that could be implemented by particular land managers (such as choosing plant seeds for restoration projects that are ‘pre-adapted’ to future climates) to policy decisions that involve cooperation between multiple national governments (such as the designation of no-take marine reserves in international waters to lessen harvest selection). These ideas are necessarily preliminary, and as such we hope our suggestions will inspire new research, both in tractable model systems and in adaptive management frameworks where appropriate, to test whether the benefits of direct management intervention in evolutionary processes will outweigh the risks inherent in such strategies.
Many conservation challenges stem from human-induced environmental changes that have placed many, perhaps most organisms, populations, and communities in rapidly changing, novel conditions. In the short term, some taxa are not responding well to these conditions and are declining, while others are doing so well that they become pests. The relative ability to respond well immediately to environmental change depends on the organisms’ traits (see Hendry et al. 2011). In addition, the long-term fate of these taxa likely depends on their evolutionary responses, which can be influenced by a range of interconnected processes (Fig. 1). Thus, policies and management strategies may benefit from understanding and manipulating these processes. We structure our consideration of evolution in management and policy making around four fundamental evolutionary principles. (i) Prior to the environmental change in question, populations have a phenotypic distribution and level of genetic variation in key traits that have been shaped by their past evolutionary history. These current phenotypes and their degree of plasticity will determine the population's initial response to the new environment. (ii) For populations in which the current phenotypic distribution is sufficiently different from the new optimum imposed by the environmental change, and plasticity is not great enough to overcome this difference, selection will favor those phenotypes that are closest to the new optimum. (iii) The adaptive potential of a population will be controlled by the level of genetic variation in relevant traits as well as the size and structure of the population. (iv) Finally, the level of gene flow to and from other populations, will determine whether favorable innovations will be able to spread across a landscape, and whether divergent selection pressures among populations will constrain the ability of any one population to adapt to its local conditions. Each of these steps may prove amenable to management intervention to promote or inhibit evolutionary change depending on the management goals (Table 1).
Figure 1.
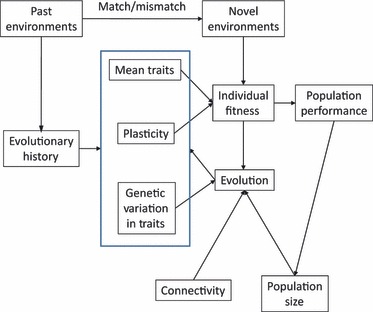
Past environments provide the evolutionary history that shapes traits, plasticity, and genetic variation. Traits and plasticity along with novel environments (that might match or mismatch past environments) influence individual fitness that governs population performance. Fitness and genetic variation along with population size and connectivity drive evolution that feeds back to determine future traits, plasticity, and genetic variation. These, in turn, loop back to influence future fitness and population performance.
Table 1.
Overview of how the basic principles of evolutionary history, genetic variation, selection, and population connectivity can be in environmental management. For each evolutionary principle, one or more management actions and practical examples are given. For selection and connectivity, actions and examples are divided into ones that reduce undesirable outcomes and ones that increase desirable outcomes, yet these are not mutually exclusive
| Evolutionary characteristic | Action | Example(s) |
|---|---|---|
| Evolutionary history | Population history can inform potential benefit from management strategies | Preserve populations from warm part of range under climate change (Sgró et al. 2011) |
| Genetic variation | Preserve (1) environmental gradients and (2) refuges to maintain functional variation | (1) Birds in the Andes and Africa (Thomassen et al. 2011) (2) Three frog species in South America (Bonin et al. 2007) |
| Selection | ||
| Reducing undesirable outcome | (1) Buffer or counter selection, (2) delay selection to after reproduction, (3) diversify selection | (1) Size limits or no-take zone in fisheries (Baskett et al. 2005), (2) Mosquito management (Koella et al. 2009), (3) pesticide cocktails (Georghiou and Wirth 1997) |
| Increasing desirable outcome | (1) Permit selection on some species at intermediate levels, (2) Reduce mortality from nonselective factor | (1) Native birds on Hawaii, rodent mortality and avian malaria resistance (Kilpatrick 2006) |
| Connectivity | ||
| Reducing undesirable outcome | Refuges with countered (e.g. neutralized) selection pressures. Avoid multiple sources of invasives | No-take refuges for fish populations (Baskett et al. 2005). Multiple introductions led to greater adaptability of invasive reed canary grass (Lavergne and Molofsky 2007) |
| Increasing desirable outcome | Adaptive introductions and corridors | Plant community restoration with seeds from populations adapted to invasives (Leger 2008) |
Evolutionary history
A population's first line of defense against extinction in the face of environmental change is having genotypes with traits that allow individuals to tolerate those changes. Because the current trait distribution of a population is often in large part a consequence of its past selective regime, understanding past selection may help predict which species or populations are likely to be especially vulnerable or resistant to particular environmental changes, and prioritize limited conservation resources accordingly. Additionally, managers may be able to use knowledge of a species’ evolutionary past to design interventions that will have the strongest (or weakest, depending on the goal) impact on the population, both numerically and evolutionarily.
History may be especially important for phenotypically plastic responses, in which an individual uses certain environmental cues to elicit a phenotypic change (in morphology, physiology, behavior, etc.). Adaptive plastic responses evolve when a cue is a reliable predictor of the future conditions, and the organism can respond by changing its phenotype in a way that increases fitness in the new conditions. Rapid environmental changes can alter the relationship between cue and future condition, such that the normal phenotypic response to certain cues is no longer adaptive, a phenomenon known as an ‘evolutionary trap’ (Schlaepfer et al. 2002). Knowledge of a species’ past evolutionary history, and how rapid environmental changes may alter the value of traits that evolved in past conditions, could provide a means of predicting the vulnerability of different species or populations to forecasted future conditions, allowing for preemptive action before drastic population declines are documented (see Schlaepfer et al. 2010; Sih et al. 2011). For instance, because most plant species have evolved in environments in which photoperiod was a strong predictor of future climatic conditions, many plant species use photoperiod changes to time phenological events like bud break or flowering. However, if the relationship between photoperiod and temperature is altered by climate change, relying on photoperiod may become maladaptive (Bradley et al. 1999). On the other hand, species that use temperature directly as their phenological cue may prove less vulnerable to rapid climate change. Policy makers tasked with prioritizing conservation resources may benefit from using this increased predictive power to identify and direct efforts toward particularly vulnerable taxa or situations.
More proactively, recognizing that the proximate cause of a species’ or population's decline may be attributed to a maladaptive behavioral response, rather than direct mortality or loss of resources because of the environmental change, is likely to offer alternative management options. For instance, recognizing that sea turtle hatchlings maladaptively move toward artificial light (which mimics moonlight reflected of the ocean water) allows for the management action of reducing artificial lights near nesting sites during nesting season (Schlaepfer et al. 2002). Introduced species often impose evolutionary traps on native species, leading to native predators that naively feed on toxic prey (Phillips and Shine 2006), native prey that fail to recognize introduced predators (Kiesecker and Blaustein 1997), or native herbivores that oviposit on toxic introduced plants (Chew 1980), for example. When a native animal population is supplemented through translocations or captive breeding programs, this offers an opportunity to mitigate an evolutionary trap, for instance by training the animals to avoid novel predators prior to releasing them into the wild (Griffin et al. 2000). Ultimately, long-term persistence will require populations to evolve appropriate behavioral responses, as has been seen for some native species affected by novel predators (Kiesecker and Blaustein 1997) or toxic prey (Phillips and Shine 2004). Management strategies can promote this evolution and encourage the spread of beneficial adaptations (see sections on Selection and Gene Flow below).
Managers of pest species can also benefit from using evolutionary history, but with the goal of intentionally mismatching evolved responses to cues and future conditions. To borrow an example from agricultural systems, crop rotation operates on this principle. For instance, the corn rootworm (Diabrotica virgifera), a major pest of maize, evolved under conditions in which host plants germinated close to their parents. By rotating maize with soybean (on which rootworms cannot feed) every year, farmers disrupted the cue used by adult females to predict the future environment of their offspring. Rootworms responded with two resistance strategies: some populations now oviposit at the base of soybean plants, which are now a good predictor of maize presence the following spring (Gray et al. 2009), while others wait two winters before hatching to avoid hatching during soybean cultivation (Levine et al. 1992). Thus, farmers may need to respond by introducing new sources of uncertainty and confusion.
Knowledge of evolutionary history can help managers understand and predict the response not only to current anthropogenic impacts, but also to future conditions. Evolutionary history can affect the vulnerability to future conditions at the individual, population, and species level. This has been seen most clearly in investigations into species vulnerabilities to future climate change. At an individual level, evidence suggests that organisms that evolved under variable climates (i.e. in temperate zones) tend to have much broader physiological tolerances for temperature than those that evolved in aseasonal zones (i.e. the tropics) (Tewksbury et al. 2008). At a population level, work with Drosophila species suggests that populations of tropical species tend to have very limited genetic variation for cold tolerance or desiccation resistance, while those from temperate or arid species are much more variable for these same traits (Hoffmann 2010). Finally, climate tolerances can show substantial levels of phylogenetic conservatism, in which closely related species tend to share similar levels of heat, cold, or desiccation tolerance. This implies that deep evolutionary history may create constraints on the evolutionary potential of extant species (Ackerly 2003; Kimura 2004; Donoghue 2008; Matzkin et al. 2009). Thus, tropical species, in addition to having narrow individual climate tolerances, may also be less able to evolve increased tolerances in the face of rapidly changing climates than related temperate species. Therefore, even though temperatures are expected to change more drastically in polar zones, the ecological impacts of climate change may be more severe in tropical zones because of the reduced ability of both individuals to acclimate and populations to adapt to new climates.
As species and populations will vary in their vulnerability to future conditions according to their evolutionary history, this raises the issue of whether resources should be directed toward protecting the most vulnerable populations (because they will be at the highest risk) or toward the less vulnerable ones (because this will have the best chance of success) (Game et al. 2008). While these decisions will be determined by the goal of a conservation strategy and the resources available, a case can be made that conservation strategies should capitalize on these differences in evolutionary history by prioritizing the preservation of populations that show some degree of adaptation or pre-adaptation to predicted future conditions. For instance, populations at the warm extreme of a species’ range may be pre-adapted to future climates (Sgró et al. 2011). Similarly, native populations that are currently coexisting with aggressive or predatory invaders, or emerging diseases may do so because they have particular trait distributions the confer resistance or tolerance to the new threat, due either to a past history with similar threats or rapid adaptive changes that have occurred since the introduction of the new species (Phillips and Shine 2004, 2006; Cox and Lima 2006; Leger 2008). These populations may harbor key genetic innovations that warrant protection from nonselective sources of mortality, such as urban development and agriculture.
Selection
Any human activity that affects the mortality or fecundity of a wild population may exert selection if individuals with different phenotypes differ in sensitivity to this activity. If the population harbors additive genetic variation in the selected traits, then the population can respond to this selection, causing the mean and variance of those traits to change across generations (see Marnocha et al. 2011 for an example with island lizards). From a management perspective, this can be desirable or not depending on the desirability of the new trait distribution. Selection can potentially lead to adaptation, and for many populations adaptation to altered environmental conditions created by human activity will be necessary if the population is to persist (Chevin et al. 2010). In these cases, management and policy should focus on ways to promote a population's ability to respond to this selection. In contrast, for pests, diseases, or invasive species, the management goal is often to induce extinction, at least locally. In these cases, we seek to reduce their level of adaptation. Additionally, in many instances the adaptive response of desirable species to human actions results in less desirable trait values (e.g. Baskett et al. 2005). In these cases, management and policy should strive to reduce this aspect of the adaptive response (Law 2000; Darimont et al. 2009).
For many species, persistence in the face of rapid environmental changes will require that the populations respond quickly to novel selection pressures. To adapt to a new selection pressure, populations must be exposed to the selection. While this may seem trivial, in fact many conservation management schemes are designed precisely to protect vulnerable populations from the forces that are driving down population size. Unfortunately, by protecting the population from these forces, we also eliminate the selection imposed by those forces and thus slow or eliminate the adaptation process. For example, many vulnerable species are threatened by invasive predators or competitors, and one strategy to protect them has been to establish populations free from the invaders [e.g. the takahe (Porphrio hochstetteri) in New Zealand (Grueber and Jamieson 2008)]. While sometimes a necessary last resort, caution should be taken with such strategies because they will offer no opportunity for native species to evolve the adaptations that will allow them to coexist with the invader. This is especially important for threats like invasive species, emerging infectious diseases, and climate change where there is little to no hope of eliminating the threat itself, and conservation depends on finding ways for species to persist in spite of the threat (Carroll 2011).
In the face of an environmental change that drives population declines, the persistence of the population will be determined by the relative rates of demographic decline and adaptation. For a given environmental threat (i.e. selection pressure), populations will persist only if adaptation occurs before the population declines to a size vulnerable to stochastic extinction (Bell and Gonzalez 2009). Therefore, persistence may be promoted both by speeding up the adaptive response (e.g. by maintaining or increasing genetic variation, see below) or by slowing the demographic decline. Thus, while it is important to avoid removing the selection pressure altogether (which would halt adaptation and could prevent long-term persistence), strategies that retain the direction of selection but lessen its magnitude may help tip the scales toward adaptation rather than extinction. Policies that expose vulnerable populations to predicted future threats (e.g. an encroaching invader or disease) in carefully controlled doses prior to their arrival could thus contribute to environmental management. This may not always be feasible, but Schlaepfer et al. (2005) suggest that managers can give adaptation a head start just before a novel threat establishes in a new population by inoculating naïve populations with individuals from more experienced populations.
Even when managers themselves are not able to address the dominant selection pressure (like climate change) directly, reducing other sources of mortality can allow populations to handle higher selective loads without declining to nonviable sizes. For instance, models suggest that controlling rodents on Hawai'i may facilitate the evolution of resistance to avian malaria in native birds (Kilpatrick 2006). Furthermore, multiple stressors may have negative synergistic effects on populations, so that mitigating one stress may additionally reduce the stress imposed by a different environmental change (Relyea and Mills 2001; Relyea 2009). This implies that policy or management action geared to reducing mortality sources that seem minor in the face of overwhelming impacts from forces like climate change may nevertheless play an important role in population persistence by creating the ‘breathing room’ needed to adapt to new circumstances.
For undesirable species, like agricultural pests or exotic invaders, management goals will often revolve around skewing the demography versus adaptation race toward extirpation. Imposing faster, nonselective demographic declines will give the population less time to adapt to the imposed selective pressure and could tip the scales toward extinction. Additionally, managers may also consider strategies that slow the adaptive response. This can be accomplished by reducing the strength of selection [e.g. by making the selective agent less discriminating among genotypes, (Baskett et al. 2005) or by interfering with the population's ability to respond to a given selection pressure].
Management strategies may also interfere with a population's ability to respond to selection, even if the strength of the selection is unchanged. One means to do this is to impose counter selection on a correlated trait. When traits are genetically correlated, selection acting on one trait will impose indirect selection on the other (Lande and Arnold 1983). When the direct selection on two correlated traits is opposing, this will slow or eliminate the population's ability to respond to either selection pressure. It is important to note, however, that this correlation must have an additive genetic basis; if, as with the brown rockfish behaviors studied by Lee and Bereijikian (2008), they are correlated only as plastic responses to similar conditions, selection will not act at the genetic level. Similarly, requiring a population to respond to multiple selection pressures simultaneously may slow the overall response, as adaptation will require not only a change in gene frequencies for the selected traits, but also the creation of genetic correlations between the traits. For instance, the use of multiple pesticides or antibiotics with different modes of action can slow the evolution of resistance (Georghiou and Wirth 1997). A similar strategy could be used to slow the development of resistance in invasive species to biocontrol agents; biological control may be self-defeating if the agent quickly selects for more resistant genotypes of the invader (Muller-Scharer et al. 2004; Stevens and Rizzo 2008). Simultaneous resistance to multiple biocontrol agents that attack the host in different ways is likely to be slower than if any one agent were released in isolation.
Another important way to manipulate the response to selection without affecting its overall strength is to adjust the timing of the selective pressure. Deleterious traits that are expressed after the bulk of reproduction occurs will have much less impact on fitness than those that are expressed before or during peak reproductive ages; this is considered a prime reason why chronic diseases tend to accumulate with age (Charlesworth 1994). Thus, any management change that pushes the selective event onto older individuals is likely to result in slower and smaller phenotypic changes in the affected population. For instance, mosquitoes will develop resistance to insecticides that act later in the life cycle much more slowly than those that act at the larval stage (Koella et al. 2009); As it takes some time for adult mosquitoes to develop a high enough malarial load to be effective vectors, later acting insecticides can provide equivalent disease control with less likelihood of resistance evolution in the insect (Koella et al. 2009).
The above discussion focused on changing the selective regime within a population. However, if there is gene flow, opposing selection pressures can be applied to different populations and still slow adaptation. We discuss this in more detail in the next two sections on Variation and Connectivity and gene flow.
Variation
In order for a population to adapt to a novel environment as discussed in the previous section, selection needs variation on which to operate. Specifically, a response to selection requires additive genetic variation in the key traits that govern fitness in the novel environment. Genetic variation in neutral markers, while useful for understanding demographic processes, may have little predictive power for adaptation, because neutral genetic diversity does not necessarily predict quantitative variation in key traits (Holderegger et al. 2006). Thus, conservation decisions that incorporate both neutral and functional genetic variation may make better predictions about the vulnerability of populations (Bonin et al. 2007).
Reductions in genetic variation can cause reductions in population fitness in the short term by combining deleterious recessive alleles or through the loss of diversity at self-compatibility loci (Edmands 2007). They can also have longer term consequences, if the loss of genetic variation through drift reduces the adaptability of the population to future environmental changes (Kinnison et al. 2007). Management plans need to address both timescales. Managers can increase, or at least conserve, genetic variation within populations by maintaining large population sizes and connectance among metapopulations. Conservation policies and management strategies use many methods to increase population sizes of threatened species, which results in both demographic and genetic benefits. Additionally, even with a constant number of individuals, more genetic variation can be maintained by increasing the effective population size (Traill et al. 2010), e.g. by increasing outbreeding, focusing on populations that span environmental gradients, or reducing reproductive skew. Captive breeding programs use this concept to maintain the most genetic diversity despite strict limitations on the number of individuals the facilities can house, through the careful manipulation of breeding (Fraser 2008). Note that managed breeding to maximize genetic variation may conflict with the organisms’ adaptive mate choice preferences. Thus, while managed breeding in domesticated populations might help to maintain genetic variation, it might simultaneously reduce mean fitness by allowing less preferred, less fit individuals to breed (Quader 2005). In wild populations, it is considerably more difficult to explicitly manage mating, so managers must strive to maintain large enough census populations to ensure adequate effective population sizes. While the relationship between census and effective population size varies according to a species’ life history, mating system, and history, a meta-analysis of estimates of minimum viable population sizes suggested that targets proposed by conservation agencies are usually far too small to maintain evolutionary potential (Traill et al. 2010).
One of the best ways to maintain genetic diversity and increase effective population size is gene flow, which we discuss in the next section. Protected area designs may also be optimized to preserve the most genetic variation. Populations that span environmental gradients are expected to harbor the most phenotypic and genetic variation, and thus, areas that span these gradients may warrant high conservation priority (Fig. 2, see Thomassen et al. 2011).
Figure 2.
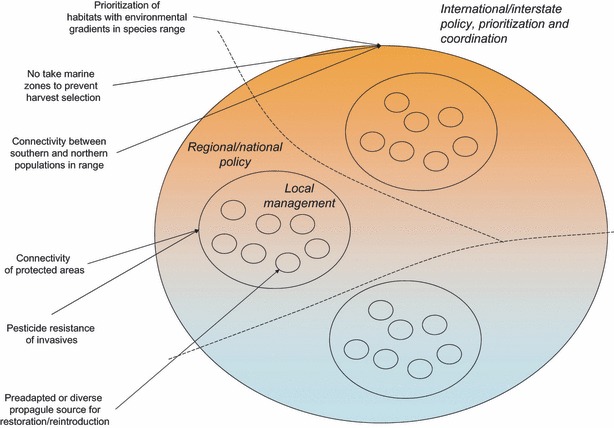
The link between the spatial scale of evolutionary environmental management actions and the level of decision making at which they initially should be considered is illustrated with examples from Table 1. An imagined species range is shown, consisting of three distinct regional populations and within these a set of local populations. The species range spans regional or national administrative boundaries (stippled lines), highlighting the need for international or interstate consideration of evolutionary management actions. The change in underlying color illustrates an environmental selection gradient, e.g. temperature.
Connectivity and gene flow
On a landscape scale, understanding the pattern of gene flow among populations can be vital to accurately predict the ability of both individual and meta-populations to persist. The importance of maintaining connections between individual populations has long been recognized for the viability of threatened species, both for ecological reasons (e.g. rescue effects) and for evolutionary reasons (Beier and Noss 1998; Garant et al. 2007). Well-connected populations are each likely to harbor more genetic variation within populations, although the populations will also be less genetically differentiated. Because of their greater variation, well-connected populations may have greater potential to adapt to novel conditions, especially to environmental changes that affect all of the sub-populations simultaneously (e.g. climate change). High gene flow between populations will also make it more likely that any new beneficial mutations that arise in one population will be able to spread. On the other hand, high gene flow may inhibit the process of local adaptation. Thus, gene flow may slow the evolutionary response of populations to local environmental changes if other sub-populations face divergent selection pressures (Storfer and Sih 1998; Lenormand 2002).
If some populations are better prepared genetically for future threats, then conservation decisions that promote the spread of these genetic innovations to other populations can promote the conservation of the species at large. For example, molecular genetic analysis revealed that the recovery of a lowland Hawai'ian honeycreeper occurred as a result of the evolution of resistance to avian malaria in small pockets and the subsequent spread of the resistant individuals (Foster et al. 2007). On the other hand, some managers will be able to use gene flow to slow down locally adaptive responses. For instance, refuges from pesticides can slow the development of resistance (Gassmann et al. 2009), while gene flow from fishing reserves can provide a steady influx of ‘large body’ alleles to counter the removal of those alleles through harvesting (Baskett et al. 2005).
Targeting land or water acquisitions and restoration projects to promote connectivity among protected areas, both for demographic and genetic reasons, has a long history in conservation thought and practice (Beier and Noss 1998; Chetkiewicz et al. 2006; Beier et al. 2008). For some pressing threats, however, certain connections may be more valuable than others (Fig. 2). Corridors connecting southern to northern (or low to high elevation) populations will likely play a more important role in facilitating migration of individuals and adapted genes in the face of warming climates than east–west corridors. Similarly, corridors connecting populations that have developed some resistance to invasive species or emerging diseases to naïve ones may prove beneficial as the invader or disease spreads (see Carroll 2011).
Corridors may not always be desirable, however. When natural movement is hampered by dispersal barriers or too slow to keep pace with environmental changes or when corridors would also allow the threat (e.g. disease) to spread, managed translocations provide another option for providing artificial gene flow, although this can be controversial. For instance, translocating individuals from distant populations runs the risk of introducing maladapted genes, which could potentially lower mean fitness in small populations (Edmands 2007). Fortunately, if the introduction represents a small percentage of the total population size, then presumably the desirable genes will spread quickly since they are favored by selection, while the maladapted genes of the introduced individuals will be eliminated because they are disfavored by selection. More research is needed to determine the optimum number of introduced individuals to ensure the rapid spread of target genes without unduly disrupting local adaptation in the rest of the genome.
Some conservation scientists have promoted the intentional transport of species beyond their current ranges, as they fear that the low dispersal abilities of many species and the numerous dispersal barriers in contemporary landscapes may prevent species from naturally tracking changing climate, which has inspired intense debate among scientists and policy makers (McLachlan et al. 2007). Translocations of individuals within the current range limits of species, to intentionally spread pre-adapted genotypes in the face of environmental change, may offer a compromise solution for some species (see Sgró et al. 2011). Forestry science has a long history of carefully determined seed transfer zones, based on the spatial structure of climatic adaptation of populations (Ying and Yanchuk 2006). Given the long lives of trees and rapid changes in future climate, there have recently been increasing calls for prospective seed zones, in which seeds from warmer provenances are intentionally used for reforestation projects to ensure that the resulting trees will grow optimally in future climates (Wang et al. 2010). A similar approach has been suggested for disease resistance in white pines, where white pine blister rust is devastating many stands, but natural resistance occurs in some populations. Introducing seeds from these resistant genotypes into artificial gaps in stands ahead of the disease front may jumpstart stand recovery once the disease hits (Schoettle and Sniezko 2007).
A similar opportunity to intentionally ‘pre-adapt’ populations exists in restoration and reintroduction efforts. Both restoration of plant communities and reintroductions of threatened and endangered species generally attempt to preserve or recreate past genetic conditions (Hedrick 1995; Hufford and Mazer 2003; McKay et al. 2005). However, in a rapidly changing world, the past genetic composition of a population may no longer be optimal. Rather than rely purely on local seed sources for restoration projects (a common strategy based on the assumption of local adaptation to current conditions), seeds may instead be chosen to reflect predicted future conditions as well (Rice and Emery 2003; Jones and Monaco 2009; Kramer and Havens 2009). This will frequently involve using seeds from populations adapted to warmer or drier conditions to create communities resilient to climate change (Rice and Emery 2003). Where invasive species or emerging diseases are expected to infest the restored community, including seeds from populations already coexisting with the invader or disease may provide resistance as well (Leger 2008). As in the case of genetic translocations, the best strategy will likely involve a mix of locally sourced seeds to provide genes locally adapted to constant aspects of the environment (like soil type) and seeds from populations pre-adapted to the predicted future environment. Including seeds from a wide array of genetically differentiated populations may provide additional evolutionary potential in the face of uncertain future changes. These genetically mixed restorations may require higher initial seeding rates and greater attention in the early stages to compensate for the selective load introduced by the process of sorting the available genetic variation to produce genotypes adapted to both local conditions and changing climate.
When supplementing populations with individuals derived from a captive bred population, managers have less opportunity to select pre-adapted genotypes based on geographic location. However, when possible, captive breeding programs designed to produce individuals for release into the wild may benefit from biasing breeding toward individuals with traits likely to be favored in future conditions. For species especially threatened by climate change, such a biased breeding program may be preferable even if it results in a reduction in neutral genetic diversity.
As landscapes continue to be fragmented by human development, maintaining gene flow between populations is often a primary goal of conservation strategies; however, there may be instances where managers will want to reduce rates of gene flow. For instance, gene flow among populations of invasive or pest species may lead to undesirable outcomes by increasing their ability to respond to selection. Reducing gene flow among populations of an invasive species can slow the rate of adaptation by lowering the additive genetic variation within populations. It can also prevent key evolutionary innovations that occur in one population from spreading to others. Reducing the flow of genes from the native range, by preventing multiple introductions, may be especially important. For instance, invasive populations of reed canary grass (Phalaris arundinacea) in North America are more genetically diverse than native populations because multiple introductions from different areas of Europe combined genes from differentiated regions of Europe. This increased genetic variation has led to greater fitness and phenotypic plasticity in the invasive populations (Lavergne and Molofsky 2007).
Evolutionary management and the scale of decision making
The diverse examples of evolutionary conservation actions given above illustrate that evolution needs to be considered not just in the later stages of the conservation effort, but when possible, also early on in the political and planning process (Fig. 2). This is especially true for actions that require coordination over large areas (e.g. across administrative boundaries), such as facilitating the movement of genes in response to climate change, designing networks of protected areas that capture adaptive genetic variation across a species range, or the preventing unwanted harvest induced selection by establishing marine no-take zones in international waters. Other actions such as the choice of a pre-adapted seed source for plant community restoration or a pest control strategy that prevents resistance evolution can be implemented locally with less need for large scale coordination. Thus, all players in the conservation field, from policy makers in national governments and strategists in international nongovernmental organizations down to land managers, tasked with managing particular parcels of land or water could benefit from incorporating evolutionary principles into their decision making process.
Conclusions
In the face of unprecedented rates of environmental alterations and species extinctions, conservation biologists, managers, practitioners, and policy makers cannot afford to ignore past, current, and future evolutionary processes. The evolutionary response of populations to human-induced environmental changes will be controlled by a few basic processes, namely the past evolutionary history of the population, the nature of selection imposed by the change, the level of genetic variation present in populations, and finally the connections between populations on a landscape. By understanding and targeting factors that affect these basic processes, conservation managers and policy makers should be able to improve the accuracy of their predictions, avoid unpleasant and unexpected outcomes, and even expand the available tool-kit for addressing pressing conservation dilemmas (Table 1). Active manipulations of natural processes always entail some risk of unanticipated consequences, and manipulation of evolutionary processes is no exception. However, it is now undeniable that human activity has already affected evolutionary processes in many species, and thus managers and policy makers will be ill equipped to respond to these effects without an explicit recognition of and potentially direct manipulation of the evolutionary processes in question. Species are almost never threatened by single forces acting in isolation. Thus, policies and management strategies must act to promote resilience and persistence in the face of multiple known and unknown threats. Evolutionary strategies may provide a powerful and efficient means to accomplish this end, by harnessing the power of the process that has ultimately generated the incredible diversity of life we are striving the conserve.
Acknowledgments
We thank Scott Carroll and the organizers and participants of the Applied Evolution Summit for the many thoughtful discussions behind these ideas. We also thank three anonymous reviewers for their many helpful suggestions. RAL was supported by NSF DEB grant # 0918450. PSJ acknowledges the Danish National Research Foundation for support to Center for Macroecology, Evolution and Climate. DJH acknowledges support from the US National Science Foundation and the UC Davis Center for Population Biology.
Literature cited
- Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences. 2003;164:S165–S184. [Google Scholar]
- Ashley MV, Willson MF, Pergams ORW, O'Dowd DJ, Gende SM, Brown JS. Evolutionarily enlightened management. Biological Conservation. 2003;111:115–123. [Google Scholar]
- Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecological Applications. 2005;15:882–901. [Google Scholar]
- Beier P, Noss RF. Do habitat corridors provide connectivity? Conservation Biology. 1998;12:1241–1252. [Google Scholar]
- Beier P, Majka DR, Spencer WD. Forks in the road: choices in procedures for designing wildland linkages. Conservation Biology. 2008;22:836–851. doi: 10.1111/j.1523-1739.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. Evolutionary rescue can prevent extinction following environmental change. Ecology Letters. 2009;12:942–948. doi: 10.1111/j.1461-0248.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- Bonin A, Nicole F, Pompanon F, Miaud C, Taberlet P. Population adaptive index: a new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Conservation Biology. 2007;21:697–708. doi: 10.1111/j.1523-1739.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- Bradley NL, Leopold AC, Ross J, Huffaker W. Phenological changes reflect climate change in Wisconsin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9701–9704. doi: 10.1073/pnas.96.17.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SP. Conciliation Biology: eco-evolutionary management of permanently invaded biotic systems. Evolutionary Applications. 2011;4:184–199. doi: 10.1111/j.1752-4571.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Evolution in Age-Structured Populations. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- Chetkiewicz CLB, Clair CCS, Boyce MS. Corridors for conservation: integrating pattern and process. Annual Review of Ecology Evolution and Systematics. 2006;37:317–342. [Google Scholar]
- Chevin LM, Lande R, Mace GM. Adaptation, plasticity, and extinction in changing environment: towards a predictive theory. PLoS Biology. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew FS. Foodplant preferences of Pieris caterpillars (Lepidoptera) Oecologia. 1980;46:347–353. doi: 10.1007/BF00346263. [DOI] [PubMed] [Google Scholar]
- Cox JG, Lima SL. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends in Ecology and Evolution. 2006;21:674–680. doi: 10.1016/j.tree.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Crandall KA, Bininda-Emonds ORP, Mace GM, Wayne RK. Considering evolutionary processes in conservation biology. Trends in Ecology & Evolution. 2000;15:290–295. doi: 10.1016/s0169-5347(00)01876-0. [DOI] [PubMed] [Google Scholar]
- Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace other agents of trait change in the wild. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:952–954. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ. A phylogenetic perspective on the distribution of plant diversity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands S. Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Molecular Ecology. 2007;16:463–475. doi: 10.1111/j.1365-294X.2006.03148.x. [DOI] [PubMed] [Google Scholar]
- Foster JT, Woodworth BL, Eggert LE, Hart PJ, Palmer D, Duffy DC, Fleischer RC. Genetic structure and evolved malaria resistance in Hawaiian honeycreepers. Molecular Ecology. 2007;16:4738–4746. doi: 10.1111/j.1365-294X.2007.03550.x. [DOI] [PubMed] [Google Scholar]
- Fraser DJ. How well can captive breeding programs conserve biodiversity? A review of salmonids. Evolutionary Applications. 2008;1:535–586. doi: 10.1111/j.1752-4571.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game ET, McDonald-Madden E, Puotinen ML, Possingham HP. Should we protect the strong or the weak? Risk, resilience, and the selection of marine protected areas. Conservation Biology. 2008;22:1619–1629. doi: 10.1111/j.1523-1739.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- Garant D, Forde SE, Hendry AP. The multifarious effects of dispersal and gene flow on contemporary adaptation. Functional Ecology. 2007;21:434–443. [Google Scholar]
- Gassmann AJ, Carriere Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annual Review of Entomology. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- Georghiou GP, Wirth MC. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae) Applied and Environmental Microbiology. 1997;63:1095–1101. doi: 10.1128/aem.63.3.1095-1101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO. Adaptation and invasiveness of Western corn rootworm: intensifying research on a worsening pest. Annual Review of Entomology. 2009;54:303–321. doi: 10.1146/annurev.ento.54.110807.090434. [DOI] [PubMed] [Google Scholar]
- Griffin AS, Blumstein DT, Evans C. Training captive-bred or translocated animals to avoid predators. Conservation Biology. 2000;14:1317–1326. [Google Scholar]
- Grueber CE, Jamieson IG. Quantifying and managing the loss of genetic variation in a free-ranging population of takahe through the use of pedigrees. Conservation Genetics. 2008;9:645–651. [Google Scholar]
- Hedrick PW. Gene flow and genetic restoration – the Florida panther as a case-study. Conservation Biology. 1995;9:996–1007. doi: 10.1046/j.1523-1739.1995.9050988.x-i1. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Lohmann LG, Conti E, Cracraft J, Crandall KA, Faith DP, Hauser C, et al. Evolutionary biology in biodiversity science, conservation, and policy: a call to action. Evolution. 2010;64:1517–1528. doi: 10.1111/j.1558-5646.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT, Heino M, Day T, Smith TB, Fitt G, Bergstrom CT, et al. Evolutionary principles and their practical application. Evolutionary Applications. 2011;4:159–183. doi: 10.1111/j.1752-4571.2010.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA. Physiological climatic limits in Drosophila: patterns and implications. Journal of Experimental Biology. 2010;213:870–880. doi: 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- Holderegger R, Kamm U, Gugerli F. Adaptive vs. neutral genetic diversity: implications for landscape genetics. Landscape Ecology. 2006;21:797–807. [Google Scholar]
- Hufford KM, Mazer SJ. Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology & Evolution. 2003;18:147–155. [Google Scholar]
- Jones TA, Monaco TA. A role for assisted evolution in designing native plant materials for domesticated landscapes. Frontiers in Ecology and the Environment. 2009;7:541–547. [Google Scholar]
- Kiesecker JM, Blaustein AR. Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs. Ecology. 1997;78:1752–1760. [Google Scholar]
- Kilpatrick AM. Facilitating the evolution of resistance to avian malaria in Hawaiian birds. Biological Conservation. 2006;128:475–485. [Google Scholar]
- Kimura MT. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia. 2004;140:442–449. doi: 10.1007/s00442-004-1605-4. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Kinnison MT, Hendry AP, Stockwell CA. Contemporary evolution meets conservation biology II: impediments to integration and application. Ecological Research. 2007;22:947–954. [Google Scholar]
- Koella JC, Lynch PA, Thomas MB, Read AF. Towards evolution-proof malaria control with insecticides. Evolutionary Applications. 2009;2:469–480. doi: 10.1111/j.1752-4571.2009.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AT, Havens K. Plant conservation genetics in a changing world. Trends in Plant Science. 2009;14:599–607. doi: 10.1016/j.tplants.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. Ices Journal of Marine Science. 2000;57:659–668. [Google Scholar]
- Lee JSF, Bereijikian BA. Stability of behavioral syndromes but plasticity in individual behavior: consequences for rockfish stock enhancement. Environmental Biology of Fishes. 2008;82:179–186. [Google Scholar]
- Leger EA. The adaptive value of remnant native plants in invaded communities: an example from the Great Basin. Ecological Applications. 2008;18:1226–1235. doi: 10.1890/07-1598.1. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends in Ecology and Evolution. 2002;17:183–189. [Google Scholar]
- Levine E, Oloumisadeghi H, Fisher JR. Discovery of multiyear diapause in Illionis and South Dakota norther corn rootworm (Coleoptera, Chrysomelidae) eggs and incidence of the prolonged diapause trait in Illinois. Journal of Economic Entomology. 1992;85:262–267. [Google Scholar]
- Mace GM, Purvis A. Evolutionary biology and practical conservation: bridging a widening gap. Molecular Ecology. 2008;17:9–19. doi: 10.1111/j.1365-294X.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- Marnocha E, Pollinger J, Smith TB. Human-induced morphological shifts in an island lizard. Evolutionary Applications. 2011;4:388–396. doi: 10.1111/j.1752-4571.2010.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin LM, Watts TD, Markow TA. Evolution of stress resistance in Drosophila: interspecific variation in tolerance to desiccation and starvation. Functional Ecology. 2009;23:521–527. [Google Scholar]
- McKay JK, Christian CE, Harrison S, Rice KJ. ‘How local is local?’– A review of practical and conceptual issues in the genetics of restoration. Restoration Ecology. 2005;13:432–440. [Google Scholar]
- McLachlan JS, Hellmann JJ, Schwartz MW. A frmework for debate of assisted migration in an era of climate change. Conservation Biology. 2007;21:297–302. doi: 10.1111/j.1523-1739.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- Muller-Scharer H, Schaffner U, Steinger T. Evolution in invasive plants: implications for biological control. Trends in Ecology & Evolution. 2004;19:417–422. doi: 10.1016/j.tree.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Shine R. Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17150–17155. doi: 10.1073/pnas.0406440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Shine R. An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proceedings. Biological sciences/The Royal Society. 2006;273:1545–1550. doi: 10.1098/rspb.2006.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader S. Mate choice and its implications for conservation and management. Current Science. 2005;89:1220–1229. [Google Scholar]
- Relyea RA. A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia. 2009;159:363–376. doi: 10.1007/s00442-008-1213-9. [DOI] [PubMed] [Google Scholar]
- Relyea RA, Mills N. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2491–2496. doi: 10.1073/pnas.031076198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KJ, Emery NC. Managing microevolution: restoration in the face of global change. Frontiers in Ecology and the Environment. 2003;1:469–478. [Google Scholar]
- Schlaepfer MA, Runge MC, Sherman PW. Ecological and evolutionary traps. Trends in Ecology & Evolution. 2002;17:474–480. [Google Scholar]
- Schlaepfer MA, Sherman PW, Blossey B, Runge MC. Introduced species as evolutionary traps. Ecology Letters. 2005;8:241–246. [Google Scholar]
- Schlaepfer MS, Sherman PW, Runge MC. Decision makng, environmental change, and population persistance. In: Westneat DF, Fox CW, editors. Evolutionary Behavioral Ecology. Oxford, UK: Oxford University Press; 2010. pp. 506–515. [Google Scholar]
- Schoettle AW, Sniezko RA. Proactive intervention to sustain high-elevation pine ecosystems threatened by white pine blister rust. Journal of Forest Research. 2007;12:327–336. [Google Scholar]
- Schwartz MK, Luikart G, Waples RS. Genetic monitoring as a promising tool for conservation and management. Trends in Ecology & Evolution. 2007;22:25–33. doi: 10.1016/j.tree.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Sgró CM, Lowe AJ, Hoffmann AA. Building evolutionary resilience for conserving biodiversity under climate change. Evolutionary Applications. 2011;4:326–337. doi: 10.1111/j.1752-4571.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Ferrari MCO, Harris DJ. Evolution and behavioural responses to human-induced rapid environmental change. Evolutionary Applications. 2011;4:367–387. doi: 10.1111/j.1752-4571.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Rizzo DM. Local adaptation to biocontrol agents: a multi-objective datadriven optimization model for the evolution of resistance. Ecological Complexity. 2008;5:252–259. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Storfer A. Quantitative genetics: a promising approach for the assessment of genetic variation in endangered species. Trends in Ecology & Evolution. 1996;11:343–348. doi: 10.1016/0169-5347(96)20051-5. [DOI] [PubMed] [Google Scholar]
- Storfer A, Sih A. Gene flow and ineffective antipredator behavior in a stream-breeding salamander. Evolution. 1998;52:558–565. doi: 10.1111/j.1558-5646.1998.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. Ecology – Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- Thomassen HA, Fuller T, Buermann W, Milá B, Kieswetter CM, Jarrín-V P, Cameron SE, et al. Mapping evolutionary process: a multi-taxa approach to conservation prioritization. Evolutionary Applications. 2011;4:397–413. doi: 10.1111/j.1752-4571.2010.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traill LW, Brook BW, Frankham RR, Bradshaw CJA. Pragmatic population viability targets in a rapidly changing world. Biological Conservation. 2010;143:28–34. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melilo JM. Human domination of Earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Wang TL, O'Neill GA, Aitken SN. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecological Applications. 2010;20:153–163. doi: 10.1890/08-2257.1. [DOI] [PubMed] [Google Scholar]
- Ying CC, Yanchuk AD. The development of British Columbia's tree seed transfer guidelines: purpose, concept, methodology, and implementation. Forest Ecology and Management. 2006;227:1–13. [Google Scholar]


