Abstract
Anthropogenic impacts increasingly drive ecological and evolutionary processes at many spatio-temporal scales, demanding greater capacity to predict and manage their consequences. This is particularly true for agro-ecosystems, which not only comprise a significant proportion of land use, but which also involve conflicting imperatives to expand or intensify production while simultaneously reducing environmental impacts. These imperatives reinforce the likelihood of further major changes in agriculture over the next 30–40 years. Key transformations include genetic technologies as well as changes in land use. The use of evolutionary principles is not new in agriculture (e.g. crop breeding, domestication of animals, management of selection for pest resistance), but given land-use trends and other transformative processes in production landscapes, ecological and evolutionary research in agro-ecosystems must consider such issues in a broader systems context. Here, we focus on biotic interactions involving pests and pathogens as exemplars of situations where integration of agronomic, ecological and evolutionary perspectives has practical value. Although their presence in agro-ecosystems may be new, many traits involved in these associations evolved in natural settings. We advocate the use of predictive frameworks based on evolutionary models as pre-emptive management tools and identify some specific research opportunities to facilitate this. We conclude with a brief discussion of multidisciplinary approaches in applied evolutionary problems.
Keywords: agro-ecosystem, biological control, environment, genetic modification, pathogens, pests, productivity, resistance, species interactions, weeds
Introduction
Anthropogenic impacts on all levels of biological organization in agricultural systems are occurring through fragmentation and simplification of natural ecosystems, global mixing of species and land-use change. Species interactions are also being altered through traditional breeding programmes and the introduction of novel genes into crops via genetic modification (GM) technologies. Increasingly, agriculture is also being impacted by climate variability and global pressures to increase food and bio-energy feedstock production and conserve biodiversity. Components of agricultural ecosystems will inevitably evolve in response to these trends, thus suggesting a central role for the application of evolutionary principles in dealing with the consequences of these changes (Thompson 2005). This includes, for example, the emergence of new pathogen and pest genotypes and the evolution of pesticide resistance (Fig. 1). Our track record of disrupting natural evolutionary processes in agro-ecosystems, and shifting them in new and often unpredictable directions emphasizes the importance of improving management of biotic interactions in production landscapes.
Figure 1.
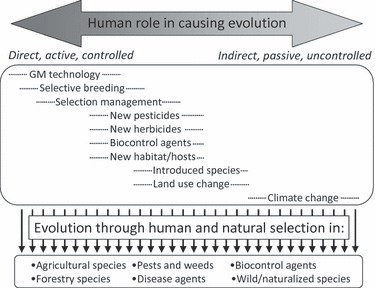
Overview of the role of humans in causing evolutionary change in agricultural systems. Effects range from actively driven genetic modifications and artificial selection to selection that arises as a by-product of anthropogenic activities. Intermediate to these situations are those exemplified by deliberate management of selection processes (e.g. the planting of susceptible crops to slow the evolution of resistance in pests). In many cases, selection is unintended but arises directly from the evolutionary opportunities that agricultural systems and changes therein offer for nonagricultural species. Evolution caused by unintended selection is often disadvantageous (e.g. pesticide resistance, introduced species adapting to local conditions), but it can also be relatively neutral (e.g. adaptations in wild species living in agricultural matrices, as long as they do not become weeds or pests) or advantageous (e.g. it is desirable that biocontrol agents adapt to local conditions).
The development of modern agriculture has resulted in simplified structures and species compositions, at least for above-ground biotic communities. However, agricultural production systems are rarely, if ever, isolated in landscapes. For example, cropping systems represent artificially selected plants interacting with a multitude of other organisms whose evolutionary history largely occurred in the context of natural systems. Thus, the processes that determine evolutionary trajectories in natural ecosystems also determine such trajectories in disease, weed and insect-pest populations in agro-ecosystems. It should be noted, however, that the relative importance of spatio-temporal patterning, population size, dispersal and isolation, reproductive system, life history and genetic variation is likely to differ in native and production landscapes.
We first briefly highlight some of the recent and likely future trends in agriculture as these will likely have significant consequences for ecological and evolutionary processes in agro-ecosystems. While our focus is primarily on biotic interactions such as invasive pests, pathogens and weeds in cropping situations, many of the issues we raise will have analogues in the animal industries and fisheries. We provide several compelling examples that highlight the value of an evolutionary perspective in agricultural research. Figure 1 outlines the range of direct and indirect effectors of evolutionary change in agro-ecosystems that we consider in this article.
Underlying economic and environmental drivers of change in agricultural production systems
Food security is a significant and growing global concern. Projections indicate that global food security will require producing more food in the next 50 years than has been produced in all of prior human history (World Bank 2008). Moreover, the demand for increasing yield will have to be met largely within existing agricultural land, because there is growing competition from other land-use options such as urbanization, conservation and, more recently, bio-energy feedstock production. At the same time, nutrient and energy resources are increasingly constrained, and water is limiting in many agricultural regions. Input costs are also increasing steadily, putting additional pressure on farmers to increase resource-use efficiencies, not only from an economic perspective, but also with regard to reducing environmental impacts (e.g. on water quality, on soil functional integrity, on fisheries affected by agricultural runoff, or more widely on native biodiversity). One consequence of these trends is the intensification of production, with the concomitant intensification of existing interactions between species and the introduction of new non-food crops and pests, and novel weed and disease control strategies. These trends all provide opportunities for novel ecological and evolutionary changes in agro-ecosystems.
Agriculture also faces several direct and indirect challenges in relation to climate change. Direct challenges include increases in variability and unpredictability that may alter how farmers manage their enterprises at a farm-scale, as well as where particular types of farming are possible (e.g. Sutherst et al. 2010). These changes will almost certainly have novel impacts for agricultural ecologies. Complex adaptive evolution among components of these ecosystems, including in agricultural-native interactions, is inevitable, with outcomes difficult to predict. Climate change will also challenge agriculture indirectly, because of mitigation actions and strategic responses required by this and other sectors of the economy. Such changes could affect, for example, access to public water resources, the use of nitrogenous fertilizers (the Haber process in fertilizer manufacture has a particularly heavy carbon footprint; Jenkinson 2001; Rafiqul et al. 2005; Snyder et al. 2009) and other non-renewable inputs, as well as requirements for carbon sequestration within production landscapes. Climate change will also lead to shifts in land use as future climates alter the spatial distribution of viable agricultural industries. All of these changes will affect the ecology of agro-ecosystems and thus potentially trigger evolutionary responses.
Notably, however, the greenhouse gas (GHG) issue is a ‘tragedy of the commons’ problem where an individual or country may obtain at least a short-term benefit from unsustainable activities (e.g. over-use of fertilizers) that may have a net negative effect on overall human welfare over time. Some kinds of land use may reduce future benefits to the communities responsible for managing that land, but future benefits are typically discounted relative to current ones. Conflict between short-term benefits to individual parties in agro-ecosystems and overarching longer-term benefits may retard effective implementation of climate adaptation or mitigation measures in agriculture.
There could also be conflict between the food security and environmental drivers affecting government and industry policies in agriculture. The latter include not only the GHG debate but also other broad issues such as land degradation, biodiversity conservation and the impacts of biological invasions. Where such conflicts arise, one might expect that food security and other direct human welfare and economic concerns may often prevail. Consequently, this article has a primary, but not exclusive, focus on the evolutionary problems arising from the widely agreed need for increased agricultural production yields with reduced environmental consequences, increased resource-use efficiencies, and the need to switch to renewable resources in an increasingly variable and unpredictable biophysical environment.
Future directions in agricultural practices
GM technology
Clearly, agriculture is in a state of transition. What will it look like in 30–40 years given the enormous need for increases in the quantity and types of food and bio-energy production? Given the constrained and in some cases declining resource base and the consequent need to significantly increase nutrient and water-use efficiencies, as well as the need to reduce input costs more generally, it seems likely that GM will become increasingly standard technology in crop improvement programmes, complementing ongoing efforts in conventional breeding. Notable here is the growing importance of such technologies to the developing world, where yield increases generally accruing through GM technology have generated massive uptake of the technology in the last decade (Carpenter 2010).
Regulatory and related costs associated with the introduction of new GM traits have been very substantial in the past, although the situation is now changing to some degree, at least outside of Europe. Some old patents on key technologies are coming to an end, which could also make GM technologies easier to implement. There remain ongoing issues with the deregulation arrangements to deal with GM volunteers or contamination in exported commodities (Stein and Rodriguez-Cerezo 2010). Moreover, at least for the next few years the regulatory costs of introducing GM traits into many minor crops are likely to remain prohibitively high. Nevertheless, the growing market for GM technologies, especially in the developing world, will continue to drive down the costs of development and implementation of such approaches. Concomitant rapid increases in our technical ability to insert or modify specific genes or gene pathways will further contribute to cost decreases.
The majority of GM traits commercialized thus far anywhere in the world have been input traits, conferring insect, disease and herbicide resistance to an increasingly wide variety of grain and horticultural crops. These traits have led to significant yield gains because they help to realize the yield potential of crop varieties. In many situations, GM traits have replaced inefficient management systems (e.g. Bt cotton in India and China) to enhance yield and reduce environmental impacts (Fitt 2008). Until now, the major direct increases in yield potential per se (e.g. semi-dwarf wheat and rice) and water-use efficiency (e.g. the Australian wheat variety ‘Drysdale’) have remained dependent on conventional plant breeding informed by agronomy and physiology. However, GM technologies can also be expected to make major contributions to future gains in yield, including in such traits as nitrogen-use and water-use efficiency.
There is also growing interest in a qualitatively different class of output traits. Rather than expanding development of current food, bio-energy feedstock and fibre products, some new technologies enhance in planta production of novel compounds that are of interest to the chemicals and materials manufacturing industries (Napier 2007). These ‘crop biofactories’ hold the prospect of significantly higher profit margins for growers and also offer potential GHG/environmental benefits in terms of substituting biomass for petrochemical feedstocks for manufacturing industries and bio-energy production. However, it is proving a challenge to integrate such crops into agricultural systems under increasing pressure to lift food commodity outputs (Tilman et al. 2009).
Associated with the increasing diversity of GM traits is an increasing diversity of underpinning transgenes. Researchers are moving rapidly to utilize genes from across biological kingdoms to introduce specific traits into crop plants. Thus, pest resistance in Bt cotton has been sourced from soil bacteria, genes necessary for Omega-3 oils have been isolated from marine algae, and genes allowing for the expression of unusual fatty acids in plants as renewable industrial feedstocks are being obtained from insects (Zhou et al. 2008; Napier and Graham 2010). Moreover, GM technology is rapidly moving into the realm of metabolic engineering where whole new transgenic pathways are being introduced. Thus, an ambitious project to convert rice from being a C3 to a C4 plant to increase photosynthetic efficiency has recently started (Mitchell and Sheehy 2006; Hibberd et al. 2008). Plans are also under development for a global consortium to ‘rework’ the biochemistry and architecture of wheat in an attempt to generate another quantum leap in yield (the International Wheat Genome Sequencing Consortium; http://www.wheatgenome.org/). Certainly, some of the proposed ideas will be extremely difficult to achieve with current technology (e.g. genetically engineering improved efficiency of rubisco in wheat), but rapid advances in de novo enzyme design provide tantalizing glimpses of a different future (Jiang et al. 2008).
Increasingly, the use of GM crops will require research agronomists, ecologists, farmers and policy makers alike to take more of a systems perspective which considers the broader evolutionary consequences of the traits in question. A precedent for such thinking has been the implementation of successful strategies aimed at controlling Bt resistance in cotton pests (e.g. the use of crop refuges) in response to the widespread uptake of Bt cottons (Fitt et al. 2009; Carriére et al. 2010; Downes et al. 2010). Likewise, there are already significant evolutionary consequences of the widespread deployment of herbicide-resistant crops (Zalucki and Lammers 2010). In particular, a strategic approach based on evolutionary concerns is being advocated in response to high use of specific herbicides that cause resistance evolution in weeds (Powles and Preston 2006; Powles 2008). Such a perspective will become even more important as the next generations of GM crops are introduced. Thus, if we design plants that contain genes allowing greater nutrient-use efficiency or which have different root architectures to improve the ability to capture scarce water, we need to understand how these will function in agricultural production contexts that potentially involve multiple management systems that each alter species competitive interactions. For example, there is potential for both positive and negative ecological and evolutionary feedbacks between novel crop types (e.g. varieties engineered to increase phosphorus uptake through the production of citric acid root exudates), herbivores and weeds, and soil microbial communities (Bais et al. 2006).
Agricultural intensification
The process of agricultural intensification is likely to accelerate given the need to better use existing land and other resources by optimizing inputs or shifting to renewable inputs and maximizing outputs. At the same time, it is likely that landholders will progressively adopt land management techniques that enhance carbon storage and long-term cycling through trees and other on-farm vegetation or through enhanced soil carbon sequestration. The pace at which land managers adopt such methods is at least partly dependent on economic concerns associated with the costs and benefits of adopting carbon trading schemes. With regard to soil carbon sequestration, it should be noted that it can be very difficult to change organic carbon reserves in soil. Recent analyses of hundreds of soils from around the world indicate constant ratios of organic carbon with other nutrients such as nitrogen, sulphur and organic phosphorus (Kirkby et al. 2010). This has clear practical implications for carbon sequestration schemes, as other nutrients would, of necessity, be tied up along with carbon in soil organic matter. The economic cost of these nutrients may be greater than the value received via carbon trading (M. Peoples, CSIRO Plant Industry, personal communication).
Land-use diversification
Overall, there is less biological complexity in agricultural than natural environments, and agricultural intensification may exacerbate this situation (e.g. Henle et al. 2007; Moller et al. 2008). However, agro-ecosystems are subject to many of the ecological and evolutionary processes that occur in natural plant and animal communities, albeit in response to more anthropogenic selection forces. There is likely to be, at least in some geographical regions or production systems, increasing shifts towards mixed farming systems to deal with greater climate variability. For example, in the grain cropping regions of southern Australia, there is a trend towards increased diversity in terms of the crop species deployed at regional and local, within-farm spatial scales (National Land & Water Resources Audit 2001). More broadly, it has been suggested that part of an integrated response to food security concerns should include deliberate enhancement of agro-ecosystem biodiversity at levels ranging from soil biota to crop varieties and cropping systems (Østergård et al. 2009). Such shifts are likely to introduce additional complexity into agro-ecological interactions that should be considered from an applied evolutionary perspective (including impacts on biodiversity and ecosystem function). Trade-offs between short-term costs of added infrastructure or potential increases in management complexity versus long-term impacts on productivity and profitability are likely to play a role in determining the extent to which such diversification in agricultural land use is adopted.
Predicting evolutionary outcomes in agro-ecosystems
One important, proactive way to address questions about evolutionary outcomes in agricultural environments is to assemble broad predictive frameworks. With such frameworks in place, we may be better able to anticipate and manage the consequences of our interventions in agro-ecosystems. Clearly, this is an ambitious goal because robust predictions depend on both underpinning theory and solid empirical information on a wide range of factors. However, agricultural systems have been under intense directional, human-driven selection, perhaps making the consequences of evolutionary selection pressures easier to predict than in natural communities. Moreover, agricultural ecosystems have been intensively studied for many decades, thus providing a unique opportunity to utilize evolutionary principles for our benefit. This is particularly the case, given recent advances in various genomic technologies and in population dynamical theory, particularly in spatial settings. There is a clear opportunity to apply such approaches to demographic and evolutionary problems in agro-ecosystems. For example, a number of recent studies have used spatially explicit models to investigate the population dynamics of pests, pathogens and biocontrol agents in agricultural settings (e.g. Thrall and Burdon 2004; Plantegenest et al. 2007; Bianchi et al. 2009; Saphoukhina et al. 2009; Vollhardt et al. 2010).
Sophisticated predictive frameworks are already used in several areas of agro-ecology. Noteworthy are predicted impacts of climate change on food production and future pest outbreaks (Baker et al. 2000; Garrett et al. 2006a; Sutherst et al. 2007), and the consequences of biofuel quotas on food prices (Geber et al. 2009) and global carbon emissions (Gibbs et al. 2008). Predictive frameworks based on evolutionary principles have been utilized less often than those based on ecological considerations but there are already some notable examples (see e.g. Onstad 2008 for an overview of various insect-related models). Many of these seek to address concerns regarding the potential evolution of resistance to various insecticides, herbicides and fungicides (e.g. Diggle et al. 2003; Mohammed-Awel et al. 2006; van den Bosch and Gilligan 2008). Moreover, model predictions can provide insights that aid in the development of appropriate agronomic management practices (e.g. rotational and mixture strategies). Often these models take account of the track record of resistance development, its genetic basis for the specific actives in question in a range of species, and the history of resistance development in the specific pests, weeds and diseases with respect to previously deployed counter-measures.
Perhaps the most sophisticated application of an evolutionary model thus far has been the highly successful industry-based management of resistance in heliothine pests to the first two generations of transgenic Bt cottons in the US and Australia (Carriére et al. 2010; Downes et al. 2010). These plans were based on detailed knowledge of pest population genetics and dynamics, the performance of the management strategies for pest resistances to various chemical insecticides and the genetics of the resistances to the Bt toxins emerging in other species. The latest versions of these plans involve the stacking of Bt toxin genes (analogous to the use of mixtures of chemical actives), provision of refuge crops for ongoing generation of susceptible subpopulations (particularly useful for recessively inherited resistance) and highly targeted use of supplementary chemical pesticide sprays (at times in crop growth when the Bt toxins are least efficacious). Many aspects of these approaches should be transferable to other cotton industries dealing with essentially the same pests. There are analogous, if not yet so thoroughly developed, plans for managing herbicide-resistant weeds (Powles and Preston 2006; Powles 2008). In these cases, the modelling again utilizes available data on the demography of the species in question and the genetics of the resistance traits (see Holst et al. 2007 for a review of modelling approaches to weed population dynamics).
We suggest that there are many more situations in agro-ecosystems where predictive modelling could be deployed (e.g. the impact of life history on the dynamics and impact of biological control agents; Thrall and Burdon 2004). While the necessary baseline data are currently missing for most of these situations, there are others where the requisite data are now within reach using current technology. Below we outline some cases where deployment of predictive evolutionary models could be particularly worthwhile and the underpinning data could be generated with targeted research activities. Two general situations considered are (i) evolutionary changes in the organisms involved in specific management interventions (e.g. in the pests or pathogens targeted by particular transgenes in GM crops); and (ii) broader effects in other components of the agricultural ecosystem, such as off-target effects of pesticides, herbicides and fungicides (e.g. the impact of agrochemical applications on host–parasite interactions in native communities; King et al. 2010).
Specific evolutionary responses
Insect pests and insecticide resistance
Along with pathogen responses to changes in the resistance of crop varieties (Burdon and Thrall 2008), the evolution of insect pest resistance to selection imposed by insecticides is one of the best known examples of an evolutionary response within agro-ecosystems to management interventions. Over 500 species of insects have developed resistance to one or more insecticides (Whalon et al. 2008). Most of the cases characterized to date involve specific biochemical changes to insect xenobiotic metabolizing systems or the molecular targets for insecticides (Li et al. 2007). There are now considerable data on the molecular and ecological genetics of target site and metabolic resistance mechanisms in many species, and it is becoming clear that the number of molecular genetic options available to evolve resistance to these chemistries is actually quite limited. Essentially, the same resistance mechanisms are now being found in a wide variety of species (Li et al. 2007; Russell et al. 2011). Thus, it should be possible to develop useful predictions about emergent and future resistance threats that can be broadly applied.
However some insect herbivores may also display other resistance mechanisms about which much less is known, and for which we are consequently much less able to predict future scenarios. There has been much speculation about the role of behavioural adaptation (Sparks et al. 1989), particularly in mosquitoes (Takken and Knols 1999) and one case of evolved behavioural resistance in a beetle in response to crop rotation has been well characterised in the Midwest of the USA (Spencer and Levine 2008). Thus in the western corn rootworm beetle (Diabrotica virgifera virgifera), behavioural resistance to maize/soybean rotations is because of an evolved change in host preference involving a loss of fidelity for maize in female beetles (Knolhoff et al. 2006; Gray et al. 2009). This behavioural change promotes better exploitation of crop rotations through increased ability to oviposit on soybean. Recent microarray analysis shows promise for identifying the genetic basis for this rotation resistance (Knolhoff et al. 2010). It is interesting to note that the northern rootworm D. barberi has also evolved resistance to maize rotations through physiological adaptations that change the dynamics of diapause rather than through behavioural adaptation per se. Other examples of pest adaptation to management include evolutionary shifts in a range of life history features. For example, Calcagno et al. (2010) explore evidence for genetically based changes in pupation behaviour of European corn borers (Ostrina spp.) in response to harvesting of maize.
Herbicide-resistant weeds
As with insecticide resistance above, there is now a large and rapidly growing body of data on the biochemical and population genetics of resistance to various major classes of herbicides in a range of weeds (Powles and Yu 2010). Thus, it should be possible to develop useful predictive models about future resistance threats. In this case, the issue is even more important because of the increasing range of transgenic herbicide resistance genes being deployed in major crops. An example is the widespread use of the GM Roundup Ready technology (e.g. in soybeans in the Americas), which has profoundly affected usage patterns of the broad-spectrum systemic herbicide glyphosate (Powles and Preston 2006; Powles 2008). Clearly, transgenic technology offers enormous prospects for improved weed control and the stacking of more resistances should offer increasing flexibility in the use of alternative herbicide options. However, responsible implementation of these new technologies should be based on robust predictive models of likely impacts on resistance evolution in the relevant weeds.
Biocontrol agents
Rapid micro-evolutionary changes have been observed in a number of plant–insect herbivore, pathogen–plant and arthropod predator–prey interactions (Carroll and Fox 2008). One might therefore expect that release of exotic biological control agents to control equally exotic pests would provide opportunities to observe and predict novel environmental drivers of rapid evolutionary change in agricultural and natural ecosystems (Roderick and Navajas 2003; Hufbauer and Roderick 2005). However, there is little evidence of rapid evolution in the interactions between biological control agents and the pest hosts they were introduced to manage (van Klinken and Edwards 2002; Roderick and Navajas 2003; Hufbauer and Roderick 2005). The only changes that have been observed are in genotype frequencies, as a result of the outcome of biotic interactions (Burdon et al. 1981) or some other biophysical drivers (Phillips et al. 2008). This lack of evidence is perhaps surprising given the more than 100-year time span over which such introductions have been made and that many introduced organisms have multiple generations a year.
Biocontrol species are introduced into novel environments because their coevolved hosts have become pests. Thus, significant evolutionary pressure is unlikely to be imposed upon the biocontrol agent and host interaction unless: (i) the introduced agents are poorly adapted to local environmental conditions or these lead to phenological asynchronies with their hosts (e.g. photoperiod, temperature; Woodburn 1996); (ii) the introduced organisms passed through a genetic bottleneck during the introductory process leading to reduced genetic variation associated with low fitness and poor performance, from which micro-evolution might generate some benefit; (iii) potentially suitable novel hosts are encountered in the new environment; or (iv) host abundance drops to very low levels as a result of biocontrol success, which in turn pressures biocontrol agents to expand their host range. Biocontrol practitioners avoid these circumstances by matching environments for populations selected for release (Zalucki and van Klinken 2006), minimizing risks of bottlenecks (Hopper et al. 1993) and selecting specialist agents by applying an internationally accepted and tested protocol for predicting and avoiding non-target impacts in the new range. All evidence regarding evolutionary change suggests that host range in specialized arthropods and pathogens (i.e. representative of those being sought for use in biological control) is very rigid and phylogenetically constrained (van Klinken and Edwards 2002). Agents used in early releases in the history of biological control were often not as specialized as those used in later releases, because early biocontrol practice was less precautionary. Earlier released agents may therefore be more likely to reveal micro-evolutionary changes after initial release. But the net result remains that biological control helps understand the limits for rapid evolution in key elements like host use ability when organisms have evolved high specialization. We can therefore predict that rapid evolution would most often be observed more explicitly in generalist species following changes in environmental selection pressures (Janz and Nylin 2008).
Host–pathogen interactions
Coevolution in host–pathogen relationships is the over-arching issue here. In many cereal grains, for example, there is a long history of pathogen control through the use of major gene resistance. During the mid-part of the 20th century the sequential release of individual wheat varieties each carrying a single novel rust resistance gene caused a process of ‘men-guided evolution in the rusts’ (Johnson 1961) as new virulent pathotypes of rusts emerged in response to novel selective forces. Similarly, the use of plant varietal mixtures for disease control has documented examples of reduced pathogen load and increased pathogen diversity associated with selection of pathogen strains with different fecundity rates on the individual host lines making up a mixture (Chin and Wolfe 1984). This includes interactions at the interface of agricultural and unmanaged ecosystems (Burdon and Thrall 2008), a pattern that is of central importance to understanding spatio-temporal patterns of disease incidence and severity (Hill 1998; Lockett et al. 2001). However, study of the genetic components of host–pathogen interactions has lagged far behind work documenting the demographic impacts of disease. Moreover, there has been remarkably little effort to directly investigate causal links between population genetic structure and disease dynamics, and even less work on factors influencing host–pathogen coevolution. The lack of empirical evidence is particularly surprising given the potential for such variation to affect the dynamics, prevalence and location of disease emergence (e.g. bacterial pathogens, Musser 1996; canine parvovirus, Parrish 1999). Increasingly, this lack of knowledge has led to calls for an integrated approach to disease management, incorporating both ecological and evolutionary processes (Ewald 1994; Schrag and Wiener 1995; Cupolillo et al. 1998; Stephens et al. 1998; Tibayrenc 1998; Real et al. 2005). The integration of traditional phenomenological approaches with studies that focus on the molecular genetics of host–pathogen interactions (Woolhouse et al. 2002) can address major knowledge gaps regarding disease evolution in agro-ecosystems.
More recently, the development of high-throughput sequencing technology has spawned a range of ‘ecogenomic’ technologies that allow for rapid assessment of the genetic composition of insect herbivore (Zheng and Dicke 2008) and pathogen communities (Garrett et al. 2006b). Such advances are particularly exciting given increasing evidence that plant defence responses may mediate the community dynamics of these antagonists (Stout et al. 2006). Specific disease-related questions that can now be addressed with these technologies include the following: (i) how agricultural management (e.g. crop spatial arrangement and extent and rotational sequences) influences host–pathogen population dynamics; (ii) how the relative proximity of wild host populations correlates with crop disease epidemiology and the rate at which new pathotypes appear (or where they first emerge); (iii) the extent to which the co-occurrence of crops and wild plants influences ecological and evolutionary processes in natural hosts (e.g. resistance variation, maintenance of sex); (iv) the influence of incursion of exotic weeds on the invasion and persistence of pathogens; (v) the role of spatial structure in determining host–pathogen dynamics in agro-ecosystems (can we detect the effects of variation in crop management and native community composition?); and (vi) the possibility of using mosaic landscape management approaches to control disease.
Transgenic disease resistance
One major new strategy that could be employed against crop diseases involves transgenic resistance. Several broad-spectrum antimicrobial and nematicidal genes have been identified now (e.g. Mi in tomato: Milligan et al. 1998; Rossi et al. 1998; Nombela et al. 2003) which could in theory be introduced in crops. However, there has been widespread scepticism regarding the long-term efficacy of such transgenes because targeted pathogens are expected to rapidly develop resistance. Given this potential outcome, there has been a general unwillingness to invest in transgenic strategies that may not remain commercial options long enough to recover the large upfront development and regulatory costs. However, several aspects of this situation are now changing and it may be timely to reconsider the viability of the approach. Relevant changes include the rapid increase in the number of candidate genes becoming available through various genome projects, ongoing improvements in the capacity of crop biotechnologists to insert multiple transgenes, and predicted decreases in the costs of the development and regulatory processes. As with the Bt cotton precedent, it seems timely to develop a broad predictive framework for managing pathogen responses in agro-ecosystems (Garrett et al. 2006a,b; Gilligan 2008), including those relating to transgenes and strategies for deployment in major crops.
Broader effects of interventions in agro-ecosystems
While some evolutionary responses to anthropogenic impacts will be confined to the components of the ecosystem directly impacted, others will involve a range of broader direct and indirect responses, requiring multifaceted, systems analysis.
Predicting future pests
There are now well over 20 insect species for which essentially whole genome sequences are available. Such sequence data can be used to build a profile of the metabolic scope for xenobiotic detoxification of a species. For example, sophisticated software is now available to predict the competence of an organism to carry out particular pathways in secondary metabolism on the basis of automated annotations of its genes. Similarly, it is quite straightforward to assess the levels of gene duplication and diversification it shows in key parts of these pathways. Several-fold differences in the contents of the main gene families involved in xenobiotic detoxification (glutathione-S-tranferases, cytochrome P450s and carboxylesterases) have been observed among the sequenced species and these differences correlate qualitatively with differences in both the degree of xenobiotic exposure in the natural habitats of these species and with species-specific sensitivities to chemical insecticides (Claudianos et al. 2006; Lee et al. 2010; Oakeshott et al. 2010). Advances in methodologies for genome sequencing and metabolomics and in the bioinformatics for developing profiles of metabolic potentials (see e.g. Dal'Molin et al. 2010; Young et al. 2010) suggest that strategic use of ‘-omics’ should add considerable explanatory power to investigations into host use ability and hence changes in pest potential in due course.
Evolutionary issues in emergent weeds
Evidence on the evolution of invasiveness in plants is still limited (Barrett et al. 2008; Prentis et al. 2008), but the consensus is that invasiveness in exotic species introduced outside their range can sometimes result from post-introduction evolutionary change (Schierenbeck and Ellstrand 2009). A range of studies have shown differences consistent with evolutionary change for exotic invasive plants over their congeneric counterparts in the native range that may provide them with an advantage in the introduced range (Ellstrand et al. 2010). These differences include broader eco-climatic ranges, higher physiological performance, altered size at flowering, high-performance parameters, greater phenotypic plasticity, changed reproductive/pollination and life history strategies and increases in polyploidy. Some of these changes may be consistent with escape from natural enemies (Keane and Crawley 2002; Bossdorf et al. 2005) or endophytes (Evans 2008) or other abiotic advantages encountered in the exotic range (Ellstrand et al. 2010). Such releases can also, at least in theory, be sufficient to generate selective pressure for evolutionary shifts in some of these parameters, such as competitive ability (Blossey and Nötzold 1995) or flowering size (Metcalf et al. 2009). Although empirical evidence remains relatively scarce, local adaptive evolution is also often invoked as an explanation of lag phases in plant invasions (Ellstrand and Schierenbeck 2000). One key issue that merits further investigation is to determine whether successful invasion is associated with pre-adaptation versus adaptive evolution. However, given that there remains the troubling question of what constitutes the ‘control’ home population in comparative ‘home and away’ studies (Bossdorf et al. 2005), it may be difficult to determine the extent to which adaptive evolution plays a role in ecological phenomena such as invasion lag phases.
There is strong evidence of intra- and inter-taxon hybridization following exotic plant introductions that has preceded invasion, suggesting genotype fitness advantages gained from introduction events (Prentis et al. 2008; Schierenbeck and Ellstrand 2009). Such mechanisms may also be driven by lower genetic variability or access to mates in the exotic range. There are also examples of evolution potentially leading to divergent life histories in crop-weed hybrids. Campbell et al. (2008) showed that artificially created crop-weed hybrids in Raphanus spp. exhibited rapid evolution of weedy traits from early generations of seemingly unfit hybrids. In Beta vulgaris, where cultivated beets also hybridize with wild relatives, there is evidence for variation in genetic structure and mating system that may contribute to the evolution of invasiveness in hybrids (Arnaud et al. 2010). Finally, many species are out-crossing where native but reproducing clonally or self-fertilizing where invasive (Barrett et al. 2008). Such alternate reproductive strategies are a form of pre-adaptation in the absence of mates or pollinators that also generates a fitness advantage in novel ecosystems where natural enemies are scarce. Overall, however, we still lack information on how frequently invasiveness results from hybridization relative to other changes. Weedy crop derivatives may provide useful models for investigating these issues, particularly given ecological similarities with their domesticated ancestors (Ellstrand et al. 2010).
Interestingly, plant breeders have, in many cases, increased crop yield potential by sacrificing individual-plant competitiveness (Denison et al. 2003), thus making crops potentially more susceptible to weed impacts. A recent paper by Weiner et al. (2010) suggests that there may be evolutionary potential to increase crop yields through the breeding of high density ‘cooperative’ varieties that can better suppress weeds. This is a good example of inadvertent evolutionary pleiotropism during a breeding programme, an issue we return to in relation to forest plantations below.
Linking soil biota to agro-ecological function
While soil community structure is likely also to have been substantially altered in managed systems, evidence for shifts in biological diversity and function are more equivocal (McCaig et al. 1999; Jesus et al. 2009; Wallis et al. 2010; A. Bissett, A. E. Richardson, G. Baker and P. H. Thrall, unpublished data). However, impacts of variation in microbial composition on ecosystem function are still poorly understood (Cohan 2006). Given that soil biota play fundamental roles in terrestrial ecosystem productivity and diversity (Reynolds et al. 2003; van der Heijden et al. 2008), understanding and assisting processes that maintain soil productivity are vital to our ability to increase agricultural production to meet the challenge of population growth.
New approaches that are fuelled by advances in molecular and analytical techniques offer the opportunity to develop a predictive framework for the ecological and evolutionary processes that drive soil communities at multiple spatio-temporal scales (van der Heijden et al. 2008). Development of predictive frameworks is critical to managing soil biology and its essential functions and services. Key research areas include the following: (i) causal links between soil biology and structure, physico-chemical factors and ecological processes (e.g. nutrient cycling, soil carbon sequestration) that contribute to plant community development and function; (ii) how soil communities respond to and impact on plant succession and weedy species; (iii) the role of plant–soil feedbacks in determining the evolutionary dynamics of soil mutualists and pathogens; and (iv) impacts of anthropogenic disturbance on soil diversity and function. Practical issues concerning soil health provide a clear example of where ecogenomic and metagenomic approaches can open opportunities to ask entirely new questions. For example, such approaches are now being specifically used to characterize and understand the phenomenon of disease suppression in cropping situations (e.g. Kyselková et al. 2009).
Off-target effects of pesticides and herbicides
One of the consequences of heavy use of pesticides, herbicides and fungicides in agricultural systems has been the recurrent inundation of soils with these chemistries, either deliberately to control soil pests or diseases, or incidentally, during the spraying of crops. In the case of the pesticides and fungicides, soil microbiota have adapted quickly to utilize this novel source of nutrients (carbon and often also phosphorus or sulphur), often reducing the efficacy of control of soil pests and pathogens (Mathiessen and Kirkegaard 2006; Russell et al. 2011). There are enough examples of this now described in the literature that it can be regarded as a predictable consequence of the application of pesticides, herbicides and fungicides to the soil, and it needs to be considered in their management.
Paradoxically, however, this example also represents a situation where it is possible to harness the consequences of an unplanned evolutionary response. Thus, one offset to the problem of enhanced biodegradation of soil-applied pesticides has been the discovery of many novel gene/enzyme systems responsible for degrading the various chemistries among the soil microflora. Some of these gene/enzyme systems have subsequently proven extremely useful in the development of transgenic herbicide-resistant crops (Bartsch and Tebbe 1989; Wehrmann et al. 1996; Castle et al. 2004). Others have been used in the development of bioremediation agents for the clean-up of contaminated environments (Sutherland et al. 2004; Scott et al. 2008; Russell et al. 2011).
Novel output traits in genetically modified crops
As discussed above, GM crops are perhaps the best example of a planned intervention where considerable thought, pre-emptive research and pre-emptive management systems have been applied to counter potential adverse evolutionary responses to GM deployment. To date, the traits in question have been input traits where the evolutionary issues directly relate to the insect pests or weeds which the trait is designed to control. However, future GM crops will involve new sets of traits, some of them (e.g. altered root architectures) affecting yields of existing products via fundamental alterations to plant physiologies and others (e.g. crop biofactories) producing fundamentally new products. The introduction of such traits may have broader but more subtle effects on the ecology of the production system. For example, plant root structure influences soil penetration and aeration, nutrient acquisition and root–rhizosphere interactions with mutualists and pathogens (Hodge et al. 2009). The capacity to model evolutionary responses in those systems within a predictive framework will be critically important.
Ecosystem engineering
An ability to predict changes to the ecological structure of agricultural ecosystems as they respond to changed climatic conditions will become increasingly important as the need to improve sustainability and change land uses intensifies (Garrett et al. 2006a; Schellhorn et al. 2008). We need to know how to build complex landscapes that can endure extremes and retain functional biodiversity while continuing to contribute to agricultural productivity (Lovell and Johnston 2008). A key issue is to understand what components are needed, not only to preserve evolutionary processes and biodiversity in changing agro-ecological landscapes, but to facilitate the development of community structure and function in revegetated sites. This is particularly critical given the large and growing investment in the revegetation and restoration of agricultural lands in many parts of the world (e.g. Ormerod 2003; Walker et al. 2004; Smith 2008). However, the success of these efforts is mixed, because design rules for re-establishing near-native ecosystems are essentially unknown. For example, there is some evidence that the use of beneficial soil microflora can significantly increase the success of revegetation efforts (Smith et al. 1998; Requena et al. 2001; Schwencke and Carú 2001; Thrall et al. 2005). At the same time, relatively little is known about the impacts of different agronomic management practices on the ecological and evolutionary dynamics of soil biota (Kiers et al. 2002), or how to maximize the ecosystem services they provide (e.g. maintenance of mutualisms, disease suppression, nutrient cycling).
Issues in forest ecosystems and plantations
Plantation forests are often grown adjacent to interfertile species and thus establish close ecological and genetic ties with neighbouring natural and managed ecosystems. In such cases, there is a risk that genotypes from breeding programmes or selected clones with deliberately or inadvertently selected traits will introgress into wild gene pools. This risk is greatest in species with high potential for hybridization, such as eucalypts, and has led to management strategies to minimize the genetic impacts of plantations on native forests in Australia (Barbour et al. 2005). Additional risks could arise if GM technology is used to introduce novel traits (e.g. insect resistance) into particular selections. This has prompted considerable societal regulation of research and commercial exploitation of GM trees (Brunner et al. 2007).
The widespread planting of forest trees outside of their natural environment is increasingly raising ecological and evolutionary questions. For example, until recent years exotic eucalypt plantations were virtually free of serious diseases and insect pests. However, increasing numbers of pests and pathogens are now emerging, some originating from Australia (Mendel et al. 2004) and others native to areas where eucalypts have been planted (Fig. 2), which have undergone sometimes surprising host jumps (Wingfield et al. 2008).
Figure 2.
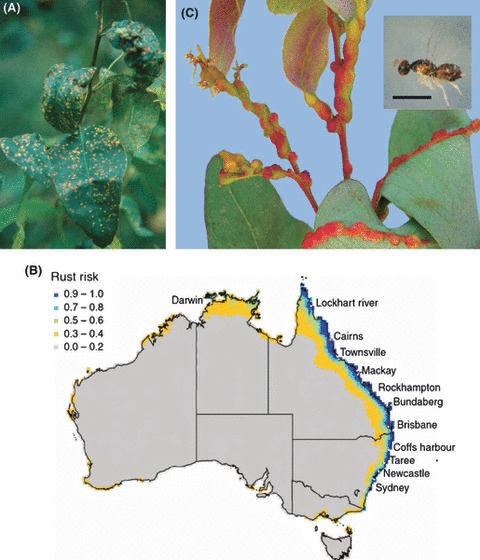
Eucalyptus is a major source of fibre and wood products. The majority of plantations are located outside of Australia and the number of native and exotic pests and pathogens is increasing. (A) Guava rust (Puccinia psidii) on Eucalyptus (photograph copyright CSIRO) is a serious disease threat to numerous native flora and eucalypt forests in Australia. The fungus is native to South America where it causes rust disease on a broad range of myrtaceous hosts and severe damage to introduced eucalypt plantations. It has spread to Florida and Hawaii and a member of the guava rust complex was detected on the Central Coast of NSW in April 2010 (Carnegie et al. 2010). (B) Bioclimatic predictions of guava rust disease regions in Australia span tropical, subtropical and some temperate plantation zones (Glen et al. 2007; map courtesy of T. H. Booth and T. Jovanovic, personal communication). Gall wasp (Leptocybe invasa) forms galls on leaf midribs, petioles and stems of new growth of several eucalypt species (Jacob and Kumar 2009). The wasp was first described after being detected in exotic eucalypt plantations in Israel in 2000 and has spread to most international plantation regions. Planting of eucalypts was temporarily suspended in Israel, and some of the most widely planted eucalypt clones in southern India are now unproductive. The emergence of gall wasp has highlighted the risks of reduced genetic diversity in clonal forestry operations. Bar = 1 mm. (Photo courtesy of John Jacob, Institute of Forest Genetics and Tree Breeding, Coimbatore, Tamil Nadu, India).
Climate change is also predicted to significantly alter patterns of disturbance from forest herbivores and pathogens. A striking illustration is the devastating expansion of mountain pine beetle into mature lodgepole pine forests over much of the interior of British Columbia (Logan and Bentz 1999). Mitigating future risks will require identification of focal species, regular abundance surveys and more accurate predictions of climate effects on the development and survival of herbivores and pathogens and concomitant physiological changes in tree defences (Ayres and Lombardero 2000; Garrett et al. 2006a).
In contrast to most crop plants or production animals, plantation trees are generally only a few generations removed from the wild state. There is thus potential for considerable contemporary, and quite possibly rapid, evolution for adaptation to domestication (Neale 2007). Aiding this evolution is the very low levels of linkage disequilibrium (LD) observed in most forest tree populations. Because of this, deliberate selection for traits of recognized advantage may not necessarily entrain large tracts of the genome in ‘selective sweeps’ which could disrupt other functional portions of the genome. Low LD also makes forest trees ideal organisms for association mapping. Association studies, employing high-throughput DNA sequencing and genotyping, combined with surveys of allelic and phenotypic variation in natural populations from different environments, can reveal powerful insights into adaptive processes in complex traits (Neale and Savolainen 2004; Savolainen et al. 2007).
Adoption of evolutionary principles in the management of agro-ecosystems
Regulatory mechanisms already exist in most countries for GM releases because of public concerns. For example, evolutionary aspects were explicitly considered with regard to Bt resistance in cotton pests. While not as advanced as Bt management, there has been some application of evolutionary principles to the management of glyphosate resistance in weeds (one factor which is both a long-term rationale and a short-term impediment of sorts is that glyphosate is far more cost-effective than any other chemical in many weed control scenarios). No doubt public awareness of the need to consider evolutionary issues will increase as GM technologies become more widespread in use (and in a broader range of crops).
Does this suggest that regulatory mechanisms based on evolutionary principles will emerge for the deployment of crop resistance to fungal pathogens, for example, with the goal of slowing rates of evolution of pathogen infectivity? Breeders now routinely seek to slow pathogen response times through the use of strategies that increase resistance durability. Results from spatially explicit models also indicate that it should be possible to design better strategies for the effective deployment of plant resistance genes (Saphoukhina et al. 2009). What about the deployment of biocontrol agents? A major impediment is that for many of the challenges facing agriculture, the benefits of a given solution (e.g. protection of native remnants on farms) are collective but the costs of implementation are borne by the individual. Thus, in general there is an economic barrier to acceptance and adoption of new practices (e.g. increasing resistance in insect pests is a cost borne by everyone, not just the farmer on whose property resistance first arose).
The likely extent to which ‘evolutionary’ shifts in management paradigms for agricultural landscapes will occur also depends partly on underlying tensions between economic (e.g. the need to maintain farm profitability) and ecological/evolutionary concerns and how these are articulated. For example, we still struggle with connecting biodiversity and ecosystem structure and function to ecosystem services in a way that is meaningful to farmers and other land managers. If we cannot establish meaningful connections to ecological processes associated with such obvious features of agro-ecological landscapes, then how likely is it that evolutionary concerns will get serious consideration with regard to the coordinated deployment of disease resistance genes, the evolution of weed life histories, or soil community structure and function? There are also conceptual, accounting and policy issues that continue to impede widespread adoption of market-based instruments for improving biodiversity and Natural Resource Management (NRM) outcomes in production landscapes. Similar complexities are likely to be a factor in developing management frameworks for agro-ecosystems that involve explicit consideration of many of the evolutionary issues we have outlined here.
General conclusions
Genetic modification of crops and introduced biocontrol agents are examples of planned interventions where considerable thought, pre-emptive research and pre-emptive management systems have been applied to counter potential evolutionary responses to their deployment. In large part, this level of rigour has been imposed by regulatory requirements, but is in contrast to the lack of focus applied to the evolutionary consequences of many other management tools utilized in agriculture. An obvious example is chemical pesticides where historically there was little regulatory focus on resistance risks. There is now evidence of increased rigour, but this is most often reactive rather than proactive. With a diminishing suite of target sites for future pesticides there is a real need to value and protect those available (Ishaaya et al. 2007). As GM crops become more complex, with multiple traits involved, and as multiple GM crops become established, the capacity to model evolutionary responses within a predictive framework will be critically important.
More generally, given the ever increasing potential for humans to impact the environment, and the complexities of land-use change and climate variability, it is likely that agro-ecological responses to management interventions will increase into the future, underscoring the importance of characterizing relevant biotic interactions in a community context (Thrall et al. 2007). It is therefore imperative that we do not waste the opportunity to learn from the natural experiments represented by changes in land use and agronomy, the introduction of new crops, biocontrol releases and pest control, large-scale revegetation, etc. This undertaking could involve, for example, the development of new monitoring and analytical approaches for such data, explicit design of initial changes on experimental farms, and conducting comparative analyses (Shea et al. 2000). An important element of this will be to include consideration of responses at spatial scales larger than individual paddocks and farms. Explicit recognition of the immense value of information from such initiatives could facilitate a quantum leap in our understanding of the ecological and evolutionary responses of agro-ecosystems.
Literature cited
- Arnaud JF, Fénart S, Cordellier M, Cuguen J. Populations of weedy crop-wild hybrid beets show contrasting variation in mating system and population genetic structure. Evolutionary Applications. 2010;3:305–318. doi: 10.1111/j.1752-4571.2010.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres MP, Lombardero MJ. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Science of the Total Environment. 2000;262:263–286. doi: 10.1016/s0048-9697(00)00528-3. [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco J. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Baker RHA, Sansford CE, Jarvis CH, Cannon RJC, MacLeod A, Walters KFA. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agriculture Ecosystems and Environment. 2000;82:57–71. [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE. Pollen dispersal from exotic eucalypt plantations. Conservation Genetics. 2005;6:253–257. [Google Scholar]
- Barrett SCH, Colautti RI, Eckert CG. Plant reproductive systems and evolution during biological invasion. Molecular Ecology. 2008;17:373–383. doi: 10.1111/j.1365-294X.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- Bartsch K, Tebbe CC. Initial steps in the degradation of phosphinothricin (glufosinate) by soil bacteria. Applied and Environmental Microbiology. 1989;55:711–716. doi: 10.1128/aem.55.3.711-716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi FJJA, Schellhorn NA, van der Werf W. Predicting the time to colonization of the parasitoid Diadegma semiclausum: the importance of the shape of spatial dispersal kernels for biological control. Biological Control. 2009;50:267–274. [Google Scholar]
- Blossey B, Nötzold R. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. Journal of Ecology. 1995;83:887–889. [Google Scholar]
- van den Bosch F, Gilligan CA. Models of fungicide resistance dynamics. Phytopathology. 2008;46:123–147. doi: 10.1146/annurev.phyto.011108.135838. [DOI] [PubMed] [Google Scholar]
- Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Li JY, DiFazio SP, Shevchenko O, Montgomery BE, Mohamed R, Wei H, et al. Genetic containment of forest plantations. Tree Genetics & Genomes. 2007;3:75–100. [Google Scholar]
- Burdon JJ, Thrall PH. Pathogen evolution across the agro-ecological interface: implications for management. Evolutionary Applications. 2008;1:57–65. doi: 10.1111/j.1752-4571.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon JJ, Groves RH, Cullen JM. The impact of biological control on the distribution and abundance of Chondrilla juncea in south-eastern Australia. Journal of Applied Ecology. 1981;18:957–966. [Google Scholar]
- Calcagno V, Bonhomme V, Thomas Y, Singer MC, Bourguet D. Divergence in behaviour between the European corn borer, Ostrinia scapulalis, and its sibling species Ostrinia nubilalis: adaptation to human harvesting? Proceedings of the Royal Society B. 2010;277:2703–2709. doi: 10.1098/rspb.2010.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LG, Snow AA, Sweeney PM, Ketner JM. Rapid evolution in crop-weed hybrids under artificial selection for divergent life histories. Evolutionary Applications. 2008;2:172–186. doi: 10.1111/j.1752-4571.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie A, Walker J, Lidbetter J, Tesoriero L, Horwood M, Glen M, Priest M. Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australasian Plant Pathology. 2010;39:463–466. [Google Scholar]
- Carpenter JE. Peer-reviewed surveys indicate positive impact of commercialised GM crops. Nature Biotechnology. 2010;28:319–321. doi: 10.1038/nbt0410-319. [DOI] [PubMed] [Google Scholar]
- Carriére Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evolutionary Applications. 2010;3:561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SP, Fox CW. Conservation Biology: Evolution in Action. New York: Oxford University Press; 2008. [Google Scholar]
- Castle LA, Siehl DL, Gorton R, Patten PA, Chen YH, Bertain S, Cho H-J, et al. Discovery and directed evolution of a glyphosate tolerance gene. Science. 2004;304:1151–1154. doi: 10.1126/science.1096770. [DOI] [PubMed] [Google Scholar]
- Chin KM, Wolfe MS. Selection on Erysiphe graminis in pure and mixed stands of barley. Plant Pathology. 1984;33:535–546. [Google Scholar]
- Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Feyereisen R, et al. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honey bee. Insect Molecular Biology. 2006;15:615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan FM. Towards a conceptual and operational union of bacterial systematics, ecology, and evolution. Philosophical Transactions of the Royal Society B. 2006;361:1985–1996. doi: 10.1098/rstb.2006.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupolillo E, Momen H, Grimaldi G., Jr Genetic diversity in natural populations of New World Leishmania. Memorias de Instituto Oswaldo Cruz. 1998;93:663–668. doi: 10.1590/s0074-02761998000500018. [DOI] [PubMed] [Google Scholar]
- Dal'Molin CGD, Quek LE, Palfreyman RW, Brumbley SM, Nielsen LK. A genome-scale reconstruction of the primary metabolic network in Arabidopsis. Plant Physiology. 2010;152:579–589. doi: 10.1104/pp.109.148817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison RF, Kiers ET, West SA. Darwinian agriculture: when can humans find solutions beyond the reach of natural selection? The Quarterly Review of Biology. 2003;78:145–168. doi: 10.1086/374951. [DOI] [PubMed] [Google Scholar]
- Diggle AD, Neve PB, Smith FP. Herbicides used in combination can reduce the probability of herbicide resistance in finite weed populations. Weed Research. 2003;43:371–382. [Google Scholar]
- Downes S, Mahon RJ, Rossiter L, Kauter G, Leven T, Fitt G, Baker G. Adaptive management of pest resistance by Helicoverpa species (Noctuidae) in Australia to the Cry2Ab Bt toxin in Bollgard II® cotton. Evolutionary Applications. 2010;3:574–584. doi: 10.1111/j.1752-4571.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Heredia SM, Leak-Garcia JA, Heraty JM, Burger JC, Yao L, Nohzadeh-Malakshah S, et al. Crops gone wild: evolution of weeds and invasives from domesticated ancestors. Evolutionary Applications. 2010;3:494–504. doi: 10.1111/j.1752-4571.2010.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC. The endophyte-enemy release hypothesis: implications for classical biological control and plant invasions. In: Julien MH, Sforza R, Bon MC, Evans HC, Hatcher PE, Hinz HL, Rector BG, editors. Proceedings of the XII International Symposium on Biological Control of Weeds. Wallingford, UK: CAB International; 2008. pp. 20–25. [Google Scholar]
- Ewald PW. Evolution of Infectious Diseases. Oxford: Oxford University Press; 1994. [Google Scholar]
- Fitt GP. Have Bt crops led to changes in insecticide use patterns and impacted IPM? In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of Insect-resistant GM Crops Within IPM Programs. Dordrecht: Springer; 2008. pp. 303–328. [Google Scholar]
- Fitt GP, Wilson L, Kelly D, Mensah R. Advances with integrated pest management as a component of sustainable agriculture: the case of the Australian cotton industry. In: Peshin R, Dhawan AK, editors. Integrated Pest Management: Dissemination and Impact. Dordrecht: Springer; 2009. pp. 507–524. [Google Scholar]
- Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE. Climate change effects on plant disease: genomes to ecosystems. Annual Review of Phytopathology. 2006a;44:489–509. doi: 10.1146/annurev.phyto.44.070505.143420. [DOI] [PubMed] [Google Scholar]
- Garrett KA, Hulbert SH, Leach JE, Travers SE. Ecological genomics and epidemiology. European Journal of Plant Pathology. 2006b;115:35–51. [Google Scholar]
- Geber N, van Eckert M, Breuer T. Biofuels and food prices: a review of recent and projected impacts. In: Krauter S, Baranowski F, Blandon NR, Kempf E, editors. RIO9 – World Climate & Energy Event 17–19 March 2009. Rio de Janeiro, Brazil: Rio Solar; 2009. pp. 139–146. [Google Scholar]
- Gibbs HK, Johnston M, Foley JA, Holloway T, Monfreda C, Ramankutty N, Zaks D. Carbon payback times for crop-based biofuel expansion in the tropics: the effects of changing yield and technology. Environmental Research Letters. 2008;3:34001. [Google Scholar]
- Gilligan CA. Sustainable agriculture and plant diseases: an epidemiological perspective. Philosophical Transactions of the Royal Society B. 2008;363:741–759. doi: 10.1098/rstb.2007.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen M, Alfenas AC, Zauza EAV, Wingfield MJ, Mohammed C. Puccinia psidii: a threat to the Australian environment and economy – a review. Australasian Plant Pathology. 2007;36:1–16. [Google Scholar]
- Gray ME, Sappington TW, Miller NJ, Moesner J, Bohn MO. Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annual Review of Entomology. 2009;54:303–321. doi: 10.1146/annurev.ento.54.110807.090434. [DOI] [PubMed] [Google Scholar]
- van der Heijden MGA, Bardgett RD, van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Henle K, Alard D, Clitherow J, Cobb P, Firbank L, Kull T, McCracken D, et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe – a review. Agriculture, Ecosystems & Environment. 2007;124:60–71. [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice – rationale and feasibility. Current Opinion in Plant Biology. 2008;11:228–231. doi: 10.1016/j.pbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hill AV. The immunogenetics of human infectious diseases. Annual Review of Immunology. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant and Soil. 2009;321:153–187. [Google Scholar]
- Holst N, Rasmussen IA, Bastiaans L. Field weed population dynamics: a review of model approaches and applications. Weed Research. 2007;47:1–14. [Google Scholar]
- Hopper KR, Roush RT, Powell W. Management of genetics of biological-control introductions. Annual Review of Entomology. 1993;38:27–51. [Google Scholar]
- Hufbauer RA, Roderick GK. Microevolution in biological control: mechanisms, patterns, and processes. Biological Control. 2005;35:227–239. [Google Scholar]
- Ishaaya I, Nauen R, Horowitz AR. Insecticides Design Using Advanced Technologies. New York: Springer; 2007. p. 314. [Google Scholar]
- Jacob JP, Kumar AR. Incidence of galls induced by Leptocybe invasa on seedlings of Eucalyptus camaldulensis and E. tereticornis from different seed sources in southern India. International Journal of Ecology and Environmental Sciences. 2009;35:187–198. [Google Scholar]
- Janz N, Nylin S. The oscillation hypothesis of host-plant range and speciation. In: Tilmon KJ, editor. Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects. Berkley, CA: University of California Press; 2008. pp. 203–215. [Google Scholar]
- Jenkinson DS. The impact of humans on the nitrogen cycle, with focus on temperate arable agriculture. Plant and Soil. 2001;228:3–15. [Google Scholar]
- Jesus EdaC, Marsh TL, Tiedje JM, de S Moreira FM. Changes in land use alter the structure of bacterial communities in Western Amazon soils. The ISME Journal. 2009;3:1004–1011. doi: 10.1038/ismej.2009.47. [DOI] [PubMed] [Google Scholar]
- Jiang L, Altoff EA, Clemente FR, Doyle L, Röthlisberger D, Zanghellini A, Gallaher JL, et al. De novo computational design of retro-aldol enzymes. Science. 2008;319:1387–1391. doi: 10.1126/science.1152692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. Man-guided evolution in plant rusts. Science. 1961;133:357–362. doi: 10.1126/science.133.3450.357. [DOI] [PubMed] [Google Scholar]
- Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. [Google Scholar]
- Kiers ET, West SA, Denison RF. Mediating mutualisms: farm management practices and evolutionary changes in symbiont co-operation. Journal of Applied Ecology. 2002;39:745–754. [Google Scholar]
- King KC, Mclaughlin JD, Boily M, Marcogliese DJ. Effects of agricultural landscape and pesticides on parasitism in native bullfrogs. Biological Conservation. 2010;143:302–310. [Google Scholar]
- Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G. Stable soil organic matter: a comparison of CNPS ratios in Australian and international soils. Geoderma. 2010 (in press) [Google Scholar]
- van Klinken RD, Edwards OR. Is host-specificity of weed biological control agents likely to evolve rapidly following establishment? Ecology Letters. 2002;5:590–596. [Google Scholar]
- Knolhoff LM, Onstad DW, Spencer JL, Levine E. Behavioral differences between rotation-resistant and wild-type Diabrotica virgifera virgifera (Coleoptera: Chrsomelidae) Environmental Entomology. 2006;35:1049–1057. [Google Scholar]
- Knolhoff LM, Walden KKO, Ratcliffe ST, Onstad DW, Robertson HM. Microarray analysis yields candidate markers for rotation resistance in the western corn rootworm beetle, Diabrotica virgifera virgifera. Evolutionary Applications. 2010;3:17–27. doi: 10.1111/j.1752-4571.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyselková M, Kopecký J, Frapolli M, Défago G, Ságová-Marečková M, Grundmann GL, Moënne-Loccoz Y. Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. The ISME Journal. 2009;3:1127–1138. doi: 10.1038/ismej.2009.61. [DOI] [PubMed] [Google Scholar]
- Lee SH, Min JS, Yoon KS, Strycharz JP, Johnson R, Mittapalli O, Margam VM, et al. Decreased detoxification genes and genome size makes the human body louse an efficient model to study insecticide resistance. Insect Molecular Biology. 2010;19:599–615. doi: 10.1111/j.1365-2583.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- Lockett SF, Robertson JR, Brettle RP, Yap PL, Middleton D, Brown AJ. Mismatched human leucocyte antigen alleles protect against heterosexual HIV transmission. Journal of Acquired Immune Deficiency Syndromes. 2001;27:277–280. doi: 10.1097/00126334-200107010-00010. [DOI] [PubMed] [Google Scholar]
- Logan JA, Bentz BJ. Model analysis of mountain pine beetle (Coleoptera : Scolytidae) seasonality. Environmental Entomology. 1999;28:924–934. [Google Scholar]
- Lovell ST, Johnston DM. Creating multifunctional landscapes: how can the field of ecology inform the design of the landscape? Frontiers in Ecology and the Environment. 2008;7:212–220. [Google Scholar]
- Mathiessen JN, Kirkegaard JA. Biofumigation and enhanced biodegradation: opportunity and challenge in soilborne pest and disease management. Critical Reviews in Plant Sciences. 2006;25:235–265. [Google Scholar]
- McCaig AE, Glover LA, Prosser JI. Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Applied and Environmental Microbiology. 1999;65:1721–1730. doi: 10.1128/aem.65.4.1721-1730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel Z, Protasov A, Fisher N, La Salle J. Taxonomy and biology of Leptocybe invasa gen. & sp n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Australian Journal of Entomology. 2004;43:101–113. [Google Scholar]
- Metcalf CJE, Rees M, Buckley YM, Sheppard AW. Seed predators and the evolutionarily stable flowering strategy in the invasive plant, Carduus nutans. Evolutionary Ecology. 2009;23:893–906. [Google Scholar]
- Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998;10:1307–1319. doi: 10.1105/tpc.10.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PL, Sheehy JE. Supercharging rice photosynthesis to increase yield. New Phytologist. 2006;171:688–693. doi: 10.1111/j.1469-8137.2006.01855.x. [DOI] [PubMed] [Google Scholar]
- Mohammed-Awel J, Kopecky K, Ringland J. A situation in which a local nontoxic refuge promotes pest resistance to toxic crops. Theoretical Population Biology. 2006;71:131–146. doi: 10.1016/j.tpb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Moller H, MacLeod CJ, Haggerty J, Rosin C, Blackwell G, Perley C, Meadows S, et al. Intensification of New Zealand agriculture: implications for biodiversity. New Zealand Journal of Agricultural Research. 2008;51:253–263. [Google Scholar]
- Musser JM. Molecular population genetic analysis of emerged bacterial pathogens: selected insights. Emerging Infectious Diseases. 1996;2:1–17. doi: 10.3201/eid0201.960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier JA. The production of unusual fatty acids in transgenic plants. Annual Review of Plant Biology. 2007;58:295–319. doi: 10.1146/annurev.arplant.58.032806.103811. [DOI] [PubMed] [Google Scholar]
- Napier JA, Graham IA. Tailoring plant lipid composition: designer oilseeds come of age. Current Opinion in Plant Biology. 2010;13:1–8. doi: 10.1016/j.pbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- National Land and Water Resources Audit. Land Use Change, Productivity and Diversification. Canberra: National Land and Water Resources Audit; 2001. [Google Scholar]
- Neale DB. Genomics to tree breeding and forest health. Current Opinion in Genetics & Development. 2007;17:539–544. doi: 10.1016/j.gde.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Neale DB, Savolainen O. Association genetics of complex traits in conifers. Trends in Plant Science. 2004;9:325–330. doi: 10.1016/j.tplants.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Nombela G, Williamson VM, Muñiz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Molecular Plant-Microbe Interactions. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- Oakeshott JG, Johnson RM, Berenbaum MR, Ranson H, Cristino AS, Claudianos C. Metabolic enzymes associated with xenobiotic and chemosensory responses in Nasonia vitripennis. Insect Molecular Biology. 2010;19:147–163. doi: 10.1111/j.1365-2583.2009.00961.x. [DOI] [PubMed] [Google Scholar]
- Onstad DW. Insect Resistance Management: Biology, Economics and Prediction. London: Elsevier; 2008. [Google Scholar]
- Ormerod SJ. Restoration in applied ecology: editor's introduction. Journal of Applied Ecology. 2003;40:44–50. [Google Scholar]
- Østergård H, Finckh MR, Fontaine L, Goldringer I, Hoad SP, Kristensen K, Lammerts van Bueren ET, et al. Time for a shift in crop production: embracing complexity through diversity at all levels. Journal of the Science of Food and Agriculture. 2009;89:1439–1445. [Google Scholar]
- Parrish CR. Host range relationships and the evolution of canine parvovirus. Veterinary Microbiology. 1999;69:29–40. doi: 10.1016/s0378-1135(99)00084-x. [DOI] [PubMed] [Google Scholar]
- Phillips CB, Baird DB, Iline II, McNeill MR, Proffitt JR, Goldson SL, Kean JM. East meets west: adaptive evolution of an insect introduced for biological control. Journal of Applied Ecology. 2008;45:948–956. [Google Scholar]
- Plantegenest M, Le May C, Fabre F. Landscape epidemiology of plant diseases. Journal of the Royal Society Interface. 2007;4:963–972. doi: 10.1098/rsif.2007.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles SB. Evolved glyphosate resistant weeds around the world. Pest Management Science. 2008;64:360–365. doi: 10.1002/ps.1525. [DOI] [PubMed] [Google Scholar]
- Powles SB, Preston C. Evolved glyphosate resistance in plants: biochemical and genetic basis of resistance. Weed Technology. 2006;20:282–289. [Google Scholar]
- Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Annual Review of Plant Biology. 2010;61:317–347. doi: 10.1146/annurev-arplant-042809-112119. [DOI] [PubMed] [Google Scholar]
- Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ. Adaptive evolution in invasive species. Trends in Plant Sciences. 2008;13:288–94. doi: 10.1016/j.tplants.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Rafiqul I, Weber C, Lehmann B, Voss A. Energy efficiency improvements in ammonia production – perspectives and uncertainties. Energy. 2005;30:2487–2504. [Google Scholar]
- Real LA, Henderson JC, Biek R, Snaman J, Jack TL, Childs JE, Waller L, et al. Unifying the spatial population dynamics and molecular evolution of epidemic rabies virus. Proceedings of the National Academy of Sciences of United States of America. 2005;102:12107–12111. doi: 10.1073/pnas.0500057102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena N, Perez-Solis E, Azcón-Aguilar C, Jeffries P, Bareal J-M. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Applied and Environmental Microbiology. 2001;67:495–498. doi: 10.1128/AEM.67.2.495-498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HL, Packer A, Bever JD, Clay K. Grassroots ecology: plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- Roderick GK, Navajas M. Genes in new environments: genetics and evolution in biological control. Nature Reviews Genetics. 2003;4:889–899. doi: 10.1038/nrg1201. [DOI] [PubMed] [Google Scholar]
- Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proceedings of the National Academy of Sciences of United States of America. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RJ, Scott C, Jackson CJ, Pandey R, Pandey G, Taylor MC, Coppin CW, et al. The evolution of new enzyme function: lessons from xenobiotic metabolising bacteria versus insecticide resistant insects. Evolutionary Applications. 2011;4:225–248. doi: 10.1111/j.1752-4571.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphoukhina N, Durel C-E, Le Cam B. Spatial deployment of gene-for-gene resistance governs evolution and spread of pathogen populations. Theoretical Ecology. 2009;2:229–238. [Google Scholar]
- Savolainen O, Pyhajarvi T, Knurr T. Gene flow and local adaptation in trees. Annual Review of Ecology Evolution and Systematics. 2007;38:595–619. [Google Scholar]
- Schellhorn NA, Pierce S, Bianchi FJJA, Williams D, Zalucki MP. Designing landscapes for multiple outcomes in broad-acre environments. Australian Journal of Experimental Agriculture. 2008;48:1549–1559. [Google Scholar]
- Schierenbeck KA, Ellstrand NC. Hybridization and the evolution of invasiveness in plants and other organisms. Biological Invasions. 2009;11:1093–1105. [Google Scholar]
- Schrag SJ, Wiener P. Emerging infectious disease – what are the relative roles of ecology and evolution? Trends in Ecology & Evolution. 1995;10:319–324. doi: 10.1016/s0169-5347(00)89118-1. [DOI] [PubMed] [Google Scholar]
- Schwencke J, Carú M. Advances in actinorhizal symbiosis: host plant-Frankia interactions, biology, and applications in arid land reclamation. A review. Arid Land Research and Management. 2001;15:285–327. [Google Scholar]
- Scott C, Pandey G, Hartley CJ, Jackson CJ, Cheesman MJ, Taylor MC, Pandey R, et al. The enzymatic basis for pesticide bioremediation. Indian Journal of Microbiology. 2008;48:65–79. doi: 10.1007/s12088-008-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea KS, Thrall PH, Burdon JJ. An integrated approach to management in epidemiology and pest control. Ecology Letters. 2000;3:150–158. [Google Scholar]
- Smith FP. Who's planting what, where and why – and who's paying?: an analysis of farmland revegetation in the central wheatbelt of Western Australia. Landscape and Urban Planning. 2008;86:66–78. [Google Scholar]
- Smith MR, Charvat I, Jacobson RL. Arbuscular mycorrhizae promote establishment of prairie species in a tallgrass prairie restoration. Canadian Journal of Botany. 1998;76:1947–1954. [Google Scholar]
- Snyder CS, Bruulsema TW, Jensen TL, Fixen PE. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agriculture, Ecosystems & Environment. 2009;133:247–266. [Google Scholar]
- Sparks TC, Lockwood JA, Byford RL, Graves JB, Leonard BR. The role of behavior in insecticide resistance. Pesticide Science. 1989;26:383–399. [Google Scholar]
- Spencer JL, Levine E. Resistance to crop rotation. In: Onstad DW, editor. Insect Resistance Management: Biology, Economics and Prediction. London: Elsevier; 2008. pp. 153–184. [Google Scholar]
- Stein AJ, Rodriguez-Cerezo E. International trade and the global pipeline of new GM crops. Nature Biotechnology. 2010;28:23–25. doi: 10.1038/nbt0110-23b. [DOI] [PubMed] [Google Scholar]
- Stephens DS, Moxon ER, Adams J, Altizer S, Antonovics J, Aral S, Berkelman J, et al. Emerging and reemerging infectious diseases: a multidisciplinary perspective. American Journal of Medical Science. 1998;315:64–75. doi: 10.1097/00000441-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Thaler JS, Thomma BPHJ. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annual Review of Entomology. 2006;51:663–689. doi: 10.1146/annurev.ento.51.110104.151117. [DOI] [PubMed] [Google Scholar]
- Sutherland TS, Horne I, Weir KM, Coppin CW, Williams MR, Selleck M, Russell RJ, et al. Enzymatic bioremediation: from enzyme discovery to applications. Clinical and Experimental Pharmacology and Physiology. 2004;31:817–821. doi: 10.1111/j.1440-1681.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- Sutherst RW, Baker RHA, Coakley SM, Harrington R, Kriticos DJ, Scherm H. Pests under global change: meeting your future landlords? In: Canadell JG, editor. Terrestrial Ecosystems in a Changing World. Berlin: Springer; 2007. pp. 211–225. [Google Scholar]
- Sutherst RW, Constable F, Finlay KJ, Harrington R, Luck J, Zalucki MP. Hoboken, NJ: Wiley; 2010. Adapting to Crop Pest and Pathogen Risks under a Changing Climate. Interdisciplinary Reviews, Climate Change. (in press) [Google Scholar]
- Takken W, Knols BGJ. Odor-mediated behaviour of afrotropical malaria mosquitoes. Annual Review of Entomology. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Thrall PH, Burdon JJ. Host-pathogen life-history interactions affect biological control success. Weed Technology. 2004;18:S1269–S1274. [Google Scholar]
- Thrall PH, Millsom DA, Jeavons AC, Waayers M, Harvey GR, Bagnall DJ, Brockwell J. Studies on land restoration: seed inoculation with effective root-nodule bacteria enhances the establishment, survival and growth of Acacia species. Journal of Applied Ecology. 2005;42:740–751. [Google Scholar]
- Thrall PH, Hochberg ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends in Ecology and Evolution. 2007;22:120–126. doi: 10.1016/j.tree.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. International Journal of Parasitology. 1998;28:85–104. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- Tilman D, Socolow R, Foley JA, Hill J, Larson E, Lynd L, Pacala S, et al. Beneficial biofuels – the food energy and environment trilemma. Science. 2009;325:270–271. doi: 10.1126/science.1177970. [DOI] [PubMed] [Google Scholar]
- Vollhardt IMG, Bianchi FJJA, Wäckers FL, Thies C, Tscharntke T. Spatial distribution of flower vs. honeydew resources in cereal fields may affect aphid parasitism. Biological Control. 2010;53:204–213. [Google Scholar]
- Walker KJ, Stevens PA, Stevens DP, Mountford JO, Manchester SJ, Pywell RF. The restoration and re-creation of species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biological Conservation. 2004;119:1–18. [Google Scholar]
- Wallis PD, Haynes RJ, Hunter CH, Morris CD. Effect of land use and management on soil bacterial diversity as measured by PCR-DGGE. Applied Soil Ecology. 2010;46:147–150. [Google Scholar]
- Wehrmann A, Van Vliet A, Opsomer C, Botterman J, Schulz A. The similarities of bar and pat gene products make them equally applicable for plant engineers. Nature Biotechnology. 1996;14:1274–1278. doi: 10.1038/nbt1096-1274. [DOI] [PubMed] [Google Scholar]
- Weiner J, Andersen SB, Wibke K-M, Griepentrog HW, Olsen JM. Evolutionary agroecology – the potential for cooperative, high density, weed-suppressing cereals. Evolutionary Applications. 2010;3:473–479. doi: 10.1111/j.1752-4571.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalon ME, Monte-Sanchez D, Hollingworth RM. Global Pesticide Resistance in Arthropods. London: CAB International; 2008. [Google Scholar]
- Wingfield MJ, Slippers B, Hurley BP, Coutinho TA, Wingfield BD, Roux J. Eucalypt pests and diseases: growing threats to plantation productivity. Southern Forests. 2008;70:139–144. [Google Scholar]
- Woodburn TL. Reduction of seed set in nodding thistle (Carduus nutans) by the seed-fly Urophora solstitialis, in Australia. In: McPheron BA, Steck GJ, editors. Fruit Fly Pests. A World Assessment of their Biology and Management. Florida: St. Lucie Press; 1996. pp. 165–169. [Google Scholar]
- Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nature Genetics. 2002;32:569–577. doi: 10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- World Bank. World Development Report 2008: Agriculture for Development. Herndon VA, USA: World Bank Publications; 2008. [Google Scholar]
- Young AL, Abaan HO, Zerbino D, Mullikin JC, Birrey E, Margulies EH. A new strategy for genome assembly using short sequence reads and reduced representation libraries. Genome Research. 2010;20:249–256. doi: 10.1101/gr.097956.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalucki MP, Lammers JH. Dispersal and egg shortfall in Monarch butterflies: what happens when the matrix is cleaned up? Ecological Entomology. 2010;35:84–91. [Google Scholar]
- Zalucki MP, van Klinken RD. Predicting population dynamics of weed biological control agents: science or gazing into crystal balls? Australian Journal of Entomology. 2006;45:331–344. [Google Scholar]
- Zheng S-J, Dicke M. Ecological genomics of plant-insect interactions: from gene to community. Plant Physiology. 2008;146:812–817. doi: 10.1104/pp.107.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XR, Horne I, Damcevski K, Haritos V, Green A, Singh S. Isolation and functional characterisation of two independently evolved fatty acid Delta 12 desaturase genes from insects. Insect Molecular Biology. 2008;17:667–676. doi: 10.1111/j.1365-2583.2008.00841.x. [DOI] [PubMed] [Google Scholar]


