Abstract
Context
Substantial uncertainty persists over the indications for radioactive iodine for thyroid cancer. Use of radioactive iodine over time and the correlates of its use remain unknown.
Objective
To determine practice patterns, the degree to which hospitals vary in their use of radioactive iodine, and factors that contribute to this variation
Design, Setting, Patients
We performed time trend analysis of radioactive iodine use in a cohort of 189,219 well-differentiated thyroid cancer patients treated at 981 hospitals associated with the National Cancer Database between 1990 and 2008. We used multilevel analysis to assess the correlates of patient and hospital characteristics on radioactive iodine use in the cohort treated from 2004–2008.
Main Outcome Measure
Use of radioactive iodine after total thyroidectomy
Results
Between 1990 and 2008, across all tumor sizes, there was a significant rise in the proportion of well-differentiated thyroid cancer patients receiving radioactive iodine (1373/3397, versus 11539/20620, P<0.001). Multivariable analysis of patients treated from 2004 to 2008 found that there was a statistical difference in radioactive iodine use between AJCC stage I and IV (odds ratios (OR) 0.34 (0.31–0.37) but not between stage II/III versus IV (OR 0.97 (0.88–1.07), 1.06 (0.95–1.17), respectively). In addition to patient and tumor characteristics, hospital volume was associated with radioactive iodine use. Wide variation in radioactive iodine use existed, and only 21.1% of this variation was accounted for by patient and tumor characteristics. Hospital type and case volume accounted for 17.1% of the variation. After adjusting for available patient, tumor, and hospital characteristics, much of the variance, 29.1%, was attributable to unexplained hospital characteristics.
Conclusions
Among patients treated for well-differentiated thyroid cancer at hospitals in the National Cancer Database, there was an increase in the proportion receiving radioactive iodine between 1990 and 2008; much of the variation in use was associated with hospital characteristics.
Over 40,000 Americans are diagnosed with thyroid cancer each year, and the overwhelming majority of cases are well-differentiated thyroid cancer. Standard treatment for well-differentiated thyroid cancer is thyroidectomy. To ensure full eradication of remnant thyroid tissue and to treat residual disease, in patients with visible inoperable iodine avid metastases, radioactive iodine is often administered after total thyroidectomy. Previous cohort studies have shown improved survival and reduced tumor recurrence when iodine-avid advanced stage well-differentiated thyroid cancer is treated with radioactive iodine.1–3 There is little controversy over the value of radioactive iodine for these patients. In contrast, for very low risk disease, in which the prognosis is typically excellent, treatment with radioactive iodine is of uncertain benefit.4–7
Indications for use of radioactive iodine following surgery for the majority of well-differentiated thyroid cancer are hotly debated.8–10 In the absence of randomized control trials evaluating the utility of radioactive iodine relative to disease severity, clinical guidelines have left radioactive iodine use to physician discretion in the majority of scenarios.11–16 Proponents argue that universal use of radioactive iodine increases the ease of following the tumor marker, thyroglobulin, and may destroy microscopic metastases. In contrast, opponents counter that the mortality secondary to thyroid cancer is sufficiently low negating the need for the unnecessary health risks17–29 and costs30 associated with universal radioactive iodine use.
The recent rise in the incidence of small, low risk thyroid cancers31,32 mandates an understanding of patterns of care in thyroid cancer. We hypothesized that there would be unwarranted variation in radioactive iodine use with factors other than disease severity predicting administration. In this study, we determined the recent change in practice patterns, examined the degree to which hospitals vary in their use of radioactive iodine, and assessed factors that contribute to this variation.
Methods
Data Source and Study Population
The National Cancer Database, a joint project of the American College of Surgeons Commission on Cancer and the American Cancer Society, is a nationwide, facility-based oncology data set that currently captures 70% of all newly diagnosed malignant cancers, including close to 85% of all thyroid cancers, in the United States.33 Once diagnosed and treated at a hospital with a Commission on Cancer-accredited cancer program, the remainder of the patient’s disease course and treatment are documented by the hospital registrar even when care is transferred to another facility.33 Data are coded and reported according to nationally established protocols coordinated under the auspices of the North American Association of Central Cancer Registries.34 No patient, physician, or hospital identifiers were examined in this study, and Institutional Review Board (IRB) exemption was granted for this study by the University of Michigan IRB.
Data from 314,039 patients diagnosed with primary thyroid cancers between January 1, 1990, and December 31, 2008 were queried from the National Cancer Database. To ensure a stable physician cohort over the time period reviewed in this study, only currently accredited Commission on Cancer programs that had reported cases in 14 of the 19 years to the National Cancer Database were included. The patients with tumor histologies of papillary, follicular, or Hurthle cell cancer types were retained for analysis. Finally, because total thyroidectomy is recommended before radioactive iodine treatment, only the patients who had undergone total thyroidectomy (n = 189,219) at the 981 Commission on Cancer-accredited programs were selected for analysis. Correlates of radioactive iodine use were evaluated in the 85,948 patients diagnosed between 2004 and 2008 in order to define the most contemporary practice patterns.
Measures
Patient age was stratified into three biologically-relevant groups: 44 and younger, 45–59, and 60 and older. Patient race/ethnicity was categorized by the National Cancer Database as non-Hispanic white, African American, and Hispanic, Asian/ Pacific Islanders, Native American. Due to smaller numbers, Hispanic, Asian/Pacific Islanders, and Native American were collapsed into Other. Race/ethnicity was included in the analysis because race/ethnicity has been shown to influence cancer treatment.35 With data drawn from the 2000 Census, we assigned 2008 100% poverty line, insurance type, percentage with college degree, and rural-urban continuum. We used the Charlson-Deyo Index to identify comorbid conditions within the cohort.36,37 Tumor size was categorized according to the definitions used by the American Joint Committee on Cancer (AJCC).38 Tumor histology was limited to International Classification of Diseases for Oncology (ICD-O) classification codes for papillary, follicular and Hurthle cell cancer types.39 Type of cancer program consisted of the following mutually exclusive categories: community hospitals, comprehensive community, teaching/research, and National Cancer Institute/National Comprehensive Cancer Network. Hospital volume was analyzed as a continuous and categorical variable. Case volume categories were created by computing a weighted average of the annual thyroid case volume at each reporting cancer program for the years 2004 to 2008 and dividing the distribution into equal-sized quintiles of hospitals: ≤ 6, 7–11, 12–19, 20–34, and ≥ 35 cases per year.
Statistical Analysis
We performed a time trend analysis of radioactive iodine use relative to tumor size between years 1990–2008. The Chi-square test was used to assess the statistical significance of temporal trends in radioactive iodine use.
Next, we selected data from the most recent five years in this cohort, 2004-2008, for univariate analysis and multivariable logistic regression. Univariate associations between radioactive iodine use and patient and tumor characteristics were evaluated with chi-square tests.
We used hierarchical generalized linear models40,41 to account for the clustering of thyroid cancer patients within hospitals while assessing the effect of comorbidity and sociodemographic (gender, age, race, poverty level, insurance, education, rural/urban continuum), tumor (histology, stage), and hospital (hospital type and case volume) characteristics. Specifically, we used a logit link to model the binary radioactive iodine use. Our model also included a random hospital-specific intercept to capture the heterogeneity across hospitals. Let Yij =1, if the jth patient seen at the ith hospital used radioactive iodine, and Yij = 0 otherwise. The probability of radioactive iodine use by the jth patient seen at the ith hospital can then be modeled as follows:
Level 1: between-patients (within hospitals):
Level 2: between-hospitals: µ0i = β00 + β0i + γ′-Zi
Combined model:
where β00 is the population-averaged log-odds of radioactive iodine use, β0i is the hospital-specific random effect, assumed to follow a normal distribution with mean zero and variance σ2hosp, Xij is the matrix of patient and tumor covariates, θ is the corresponding vector of fixed effects representing changes in the log-odds of radioactive iodine use corresponding to each unit change in the covariate values, Zi represents the vector of hospital-level covariates for the ith hospital, and γ is the corresponding vector of coefficients. Model estimates were obtained using likelihood based approach in SAS PROC GLIMMIX (SAS version 9.2; SAS Institute, Cary, NC). A hierarchical generalized linear model approach allows the estimation and partitioning of variance in radioactive iodine use between the patient and hospital levels. As a measure of the importance of the hospital effect on individual use of radioactive iodine, we estimated the percentage of the variance in radioactive iodine use attributable to hospital, using the intraclass correlation coefficient. The intraclass correlation coefficient was estimated based on the assumption of a threshold model that is appropriate for a binary outcome.40
Our initial null model contained only a hospital-specific random effect term. Next we fitted a series of adjusted models which, in addition to the hospital-specific random effect, included fixed patient characteristics (comorbidity, sociodemographic covariates), tumor characteristics and hospital covariates (each covariate group at a time). These models were used to calculate the percentage of total variance attributable to patient, tumor and hospital characteristics. The denominator for this calculation was the total variance, which included the variance attributable to random (unmeasured) hospital effects after adjustment for the corresponding fixed effect covariates in a given model, the variance attributable to the corresponding measured covariates (i.e. fixed effects), and the variance attributable to unmeasured patient or tumor characteristics plus error. In this way, the relative importance of each component could be examined. Finally, a fully adjusted model was fitted incorporating the available patient and hospital characteristics as fixed effects covariates in the model. The residual intraclass correlation coefficient was calculated based on the fully adjusted model and represents the percentage of variance attributable to hospital after adjustment for available patient and hospital characteristics. The denominator in the calculation of this percentage was composed of the variance attributable to unmeasured hospital effects, after adjustment for available patient and hospital variables, and the variance attributable to unmeasured patient or tumor characteristics plus error.
As another measure of hospital variation in use of radioactive iodine, hospital-specific radioactive iodine administration rates were calculated based on a hierarchical generalized linear model that was adjusted for patient and tumor characteristics. Hospital-specific rates were obtained using empirical Bayes predictions42 and then plotted by hospital rank, from lowest to highest according to the empirical Bayes predictions. This method shrinks the estimate of hospital-specific radioactive iodine administration rate towards the average rate, as a factor of the number of thyroid cancer patients treated at the hospital. Hospitals treating a large number of thyroid cancer patients will have less shrinkage whereas hospitals treating a small number of thyroid cancer patients will have more shrinkage towards the average rate.
All statistical analyses were performed using SAS software (SAS version 9.2; SAS Institute, Cary, NC). Two-sided tests were used, with p values <0.05 considered statistically significant.
Results
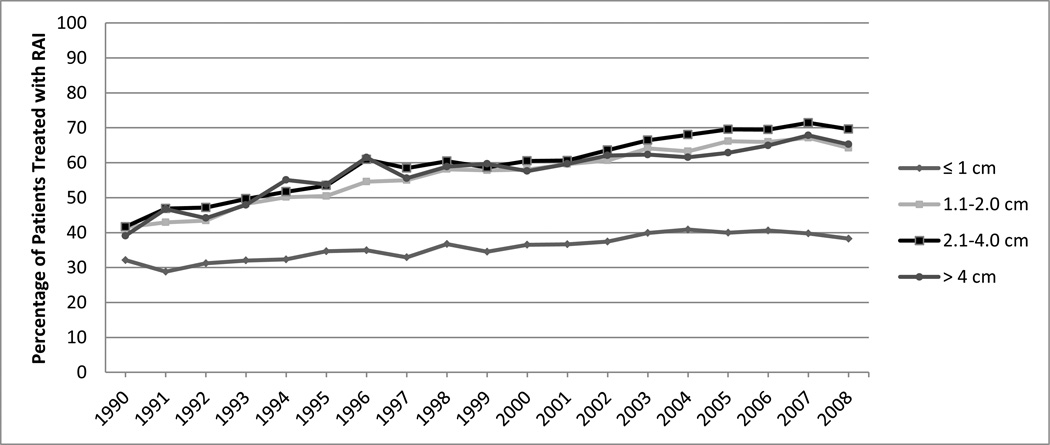
Between 1990 and 2008 there was a significant increase in the proportion of well-differentiated thyroid cancer patients receiving radioactive iodine as adjuvant therapy after total thyroidectomy (P<0.001). In 1990, 1,373/3,397 (40.4%) patients received radioactive iodine whereas in 2008 11,539/20,620 (56.0%) received radioactive iodine. For tumors 1.1 to 2 cm, 2.1 to 4 cm, and over 4 cm there was a 55–67% increase in the percentage of patients treated in 2008 compared to those treated in 1990. The proportion of tumors ≤ 1 cm treated with radioactive iodine was lower but has also climbed steadily over time (Figure 1).
Figure 1.
Proportion of patients treated with radioactive iodine (RAI) based on tumor size between 1990 and 2008.
Table 1 summarizes the study population and proportion receiving radioactive iodine as adjuvant therapy following total thyroidectomy in 2004–2008. In multivariable analyses, younger age and absence of comorbidity were associated with a small but significantly greater likelihood of receiving radioactive iodine after total thyroidectomy (odds ratio (OR) 2.15 (2.04–2.26), 1.19 (1.07–1.35), respectively). Female gender, African American race, and absence of private/government insurance was associated with significantly less likelihood of receiving radioactive iodine (OR 0.87 (0.84–0.91), 0.83 (0.77–0.89), and 0.84 (0.81–0.88), respectively). There was a statistical difference in radioactive iodine use between AJCC stage I and IV (OR 0.34 (0.31–0.37), but not between stage II and III versus stage IV (OR stage II, 0.97 (0.88–1.07), stage III, 1.06 (0.95–1.17)). When hospital case volume was analyzed as a categorical variable, there was an increased likelihood of radioactive iodine use as the volume category increased. There was a significant difference between low and low-medium versus high case volume (respectively OR 0.44 (0.33–0.58) and 0.62 (0.48–0.80)). The effect of continuous case volume was also statistically significant in both the unadjusted and adjusted models. The adjusted OR (95% CI) was 1.006 (1.003 – 1.008), p-value=0.0001 suggesting that with every one additional case a hospital treats, the odds of radioactive iodine use increases by 0.6% after adjusting for patient and tumor characteristics and hospital type.
Table 1.
Multivariate Analysis with Patient and Hospital Characteristics, 2004–2008
| Total No. (%) | No. treated with RAI (%) |
Unadjusted Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI) |
||
|---|---|---|---|---|---|
| Patient Characteristics | |||||
| Patient Gender | |||||
| Male | 19 754 (23.0) | 12 079 (61.2) | 1.00 | 1.00 | |
| Female | 66 194 (77.0) | 37 346 (56.4) | 0.82 (0.79–0.85) | 0.87 (0.84–0.91) | |
| Patient Age | |||||
| Age ≤44 years | 34 432 (40.1) | 21 090 (61.3) | 1.43 (1.38–1.48) | 2.15 (2.04–2.26) | |
| Age 45–59 years | 30 267 (35.2) | 17 159 (56.7) | 1.18 (1.14–1.22) | 1.19 (1.14–1.26) | |
| Age ≥ 60 years | 21 249 (24.7) | 11 176 (52.6) | 1.00 | 1.00 | |
| Charlson/Deyo Comorbidity Index Score | |||||
| 0 | 73 943 (86.0) | 42 942 (58.1) | 1.26 (1.15–1.39) | 1.19 (1.07–1.35) | |
| 1 | 10 303 (12.0) | 5 593 (54.3) | 1.08 (0.98–1.20) | 1.07 (0.95–1.21) | |
| ≥ 2 | 1 702 (2.0) | 890 (52.3) | 1.00 | 1.00 | |
| Patient Race/Ethnicity | |||||
| White | 67 528 (78.6) | 39 104 (57.9) | 1.00 | 1.00 | |
| African American | 5 539 (6.4) | 2 816 (50.8) | 0.75 (0.71–0.79) | 0.83 (0.77–0.89) | |
| Other | 12 881 (15.0) | 7 505 (58.3) | 1.02 (0.98–1.05) | 1.06 (1.00–1.12) | |
| Household Income | |||||
| Above 100% Poverty | 41 877 (52.4) | 24 011 (57.3) | 0.99 (0.96–1.02) | 1.04 (0.99–1.08) | |
| Below 100% Poverty | 38 054 (47.6) | 21 897 (57.5) | 1.00 | 1.00 | |
| Insurance | |||||
| Private/Gov | 64 070 (75.9) | 38 013 (59.3) | 1.00 | 1.00 | |
| Medicare/Medicaid/Uninsured | 20 359 (24.1) | 10 776 (52.9) | 0.77 (0.75–0.79) | 0.84 (0.81–0.88) | |
| Percentage with College Degree | |||||
| < 12% | 33 906 (42.4) | 19 335 (57.0) | 0.97 (0.94–1.00) | 1.01 (0.97–1.05) | |
| ≥ 12% | 46 021 (57.6) | 26 572 (57.7) | 1.00 | 1.00 | |
| Rural-Urban Continuum | |||||
| Metropolitan population | 67 852 (85.7) | 38 810 (57.2) | 0.94 (0.89–0.97) | 1.02 (0.96–1.08) | |
| Other | 11 364 (14.4) | 6 685 (58.8) | 1.00 | 1.00 | |
| Tumor Characteristics | |||||
| Tumor Histology | |||||
| Papillary | 78 651 (91.5) | 44 850 (57.0) | 1.00 | 1.00 | |
| Follicular | 4 893 (5.7) | 3 084 (63.0) | 1.29 (1.21–1.36) | 1.09 (1.01–1.18) | |
| Hurthle | 2 404 (2.8) | 1 491 (62.0) | 1.23 (1.13–1.34) | 1.03 (0.92–1.14) | |
| Tumor Size | |||||
| ≤ 1 cm | 29 941 (36.4) | 11 900 (36.4) | 0.36 (0.34–0.38) | - | |
| 1.1–2 cm | 24 771 (30.1) | 16 201 (65.4) | 1.04 (0.98–1.09) | - | |
| 2.1–4 cm | 20 024 (24.3) | 13 952 (69.7) | 1.26 (1.19–1.33) | - | |
| > 4 cm | 7 524 (9.1) | 4 862 (64.6) | 1.00 | - | |
| Lymph Node | |||||
| N0 | 66 137 (77.0) | 35 019 (53.0) | 1.00 | - | |
| N1 | 18 691 (21.7) | 13 570 (72.6) | 2.36 (2.72–2.44) | - | |
| Nx | 1 120 (1.3) | 836 (74.6) | 2.62 (2.29–3.00) | - | |
| Distant Metastases | |||||
| M0 | 85 388 (99.3) | 49 076 (57.5) | 1.00 | - | |
| M1 | 560 (0.7) | 349 (62.3) | 1.22 (1.03–1.45) | - | |
| AJCC TNM Stage | |||||
| I | 64 166 (75.6) | 34 539 (53.8) | 0.56 (0.52–0.59) | 0.34 (0.31–0.37) | |
| II | 9 018 (10.6) | 6 113 (67.8) | 1.01 (0.93–1.09) | 0.97 (0.88–1.07) | |
| III | 7 843 (9.2) | 5 407 (68.9) | 1.06 (0.98–1.15) | 1.06 (0.95–1.17) | |
| IV | 3 886 (4.6) | 2 630 (67.7) | 1.00 | 1.00 | |
| Hospital Characteristics | |||||
| Hospital type | |||||
| Community/Comprehensive Community | 48 532 (56.5) | 27 773 (57.2) | 0.93 (0.78–1.11) | 1.18 (0.97–1.44) | |
| Teaching-Research/NCI-NCCN | 37 416 (43.5) | 21 652 (57.9) | 1.00 | 1.00 | |
| Hospital case volume | |||||
| Low (<6 cases/year) | 2 415 (2.8) | 1 064 (44.1) | 0.49 (0.38–0.64) | 0.44 (0.33–0.58) | |
| Low-Med (7–11 cases/year) | 6 109 (7.1) | 3 098 (50.7) | 0.69 (0.54–0.86) | 0.62 (0.48–0.80) | |
| Medium (12–19 cases/year) | 10 261 (11.9) | 5 604 (54.7) | 0.87(0.69–1.09) | 0.82 (0.64–1.06) | |
| Med-High (20–34 cases/year) | 19 235 (22.4) | 11 440 (59.5) | 1.03 (0.82–1.29) | 0.99 (0.79–1.27) | |
| High (≥ 35 cases/year) | 47 928 (55.8) | 28 214 (58.9) | 1.00 | 1.00 | |
The subgroup analysis of patients treated between 2004 and 2008 demonstrates substantial variation in use of radioactive iodine. Patient characteristics explain 21.1% of this variance and measured hospital characteristics 17.1%. These partitioned variances were obtained from a series of adjusted models so the relative importance of each component could be examined. After controlling for gender, age, race, comorbidity, poverty level, insurance, education, degree of rural/urbanism, tumor histology, size, stage, and hospital type, case volume, the residual intraclass correlation coefficient was 29.1% indicating that substantial variation in radioactive iodine use still exists across hospitals.
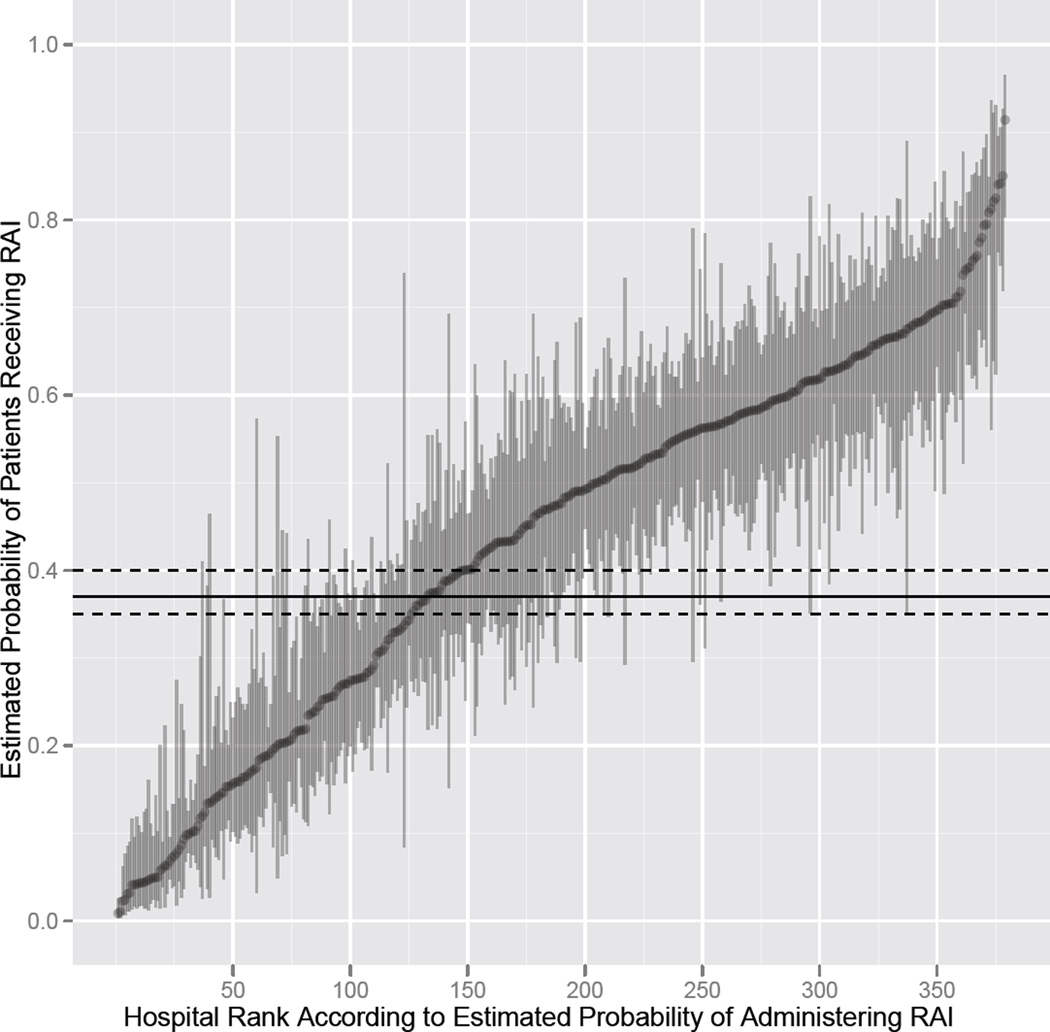
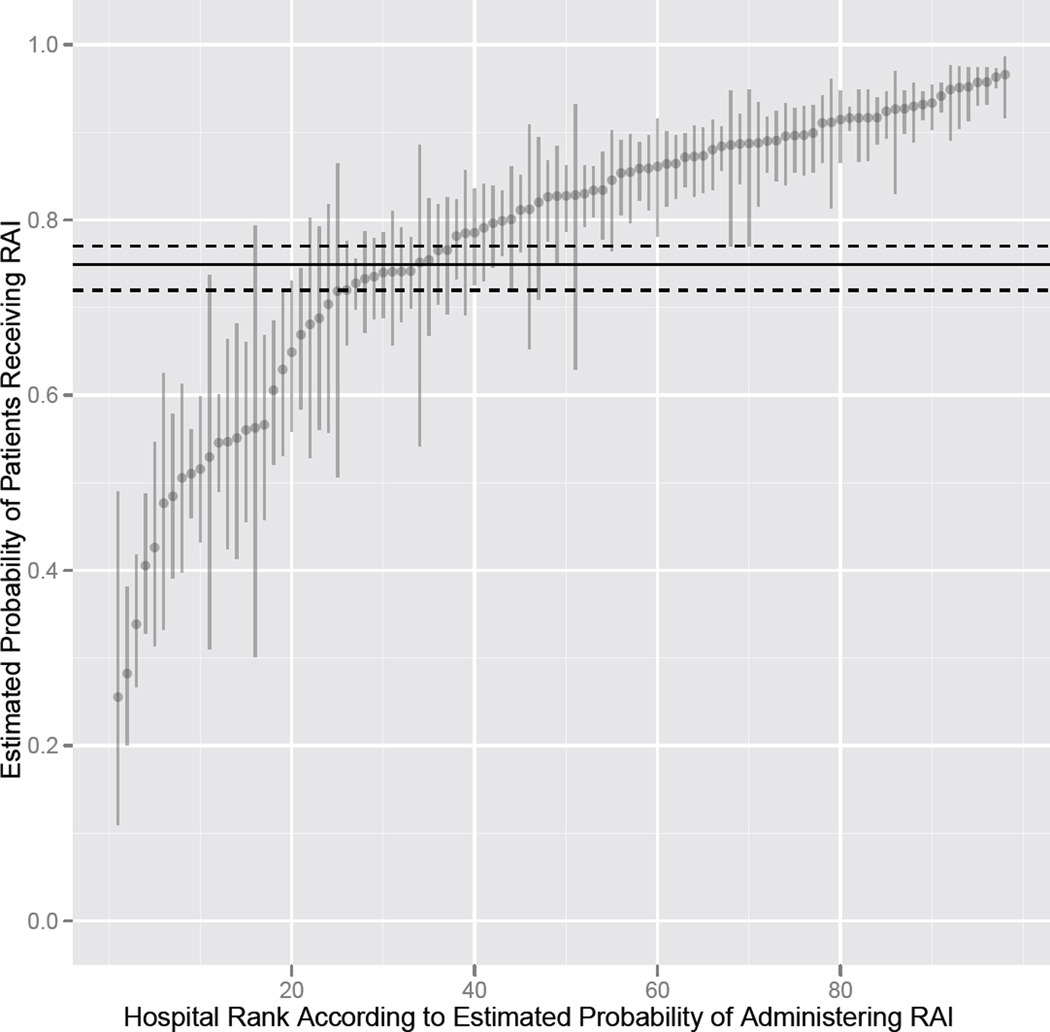
After selecting patients without comorbidity and with consistent sociodemographic variables (white race, income above 100% poverty, private insurance, areas where over 12% of the population have a college education, and metropolitan residence) evaluation of radioactive iodine use in both lower risk, young, female patients with tumor size ≤ 1 cm and stage I disease and in higher risk, older, male patients who have tumor size over 2 cm and have stage III or IV disease, showed wide hospital-level variation. For the lower risk profile, 246 (64.9%) of the 379 hospitals treating such patients had a radioactive iodine administration rate that was statistically significantly different from the average of 37.4%, with 79 (20.8%) of the 379 hospitals having a rate below the average rate, and 167 (44.1%) of the hospitals having a rate above the average (Figure 2). For the higher risk profile, 63 (64.3%) of the 98 hospitals treating such patients had a radioactive iodine administration rate that was statistically significantly different from the average rate of 74.9%, with 17 (17.4%) of the 98 hospitals having a rate below the average rate, and 46 (46.9%) of the hospitals having a rate above the average (Figure 3).
Figure 2.
When papillary thyroid cancer patients with characteristics associated with low risk of death were selected, there was tremendous variation in hospital-level radioactive iodine use. The horizontal line is the population average (37.4%) and the dashed lines represent the 95% confidence interval for the average. The vertical lines represent the 95% confidence intervals for the hospital-specific estimated probabilities of radioactive iodine use.
Figure 3.
When papillary thyroid cancer patients with characteristics associated with a higher risk of death were selected, variation in use of radioactive iodine still existed. The horizontal line is the population average (74.9%) and the dashed lines represent the 95% confidence interval for the average. The vertical lines represent the 95% confidence intervals for the hospital-specific estimated probabilities of radioactive iodine use.
Discussion
The results of this study provide insight into the use of radioactive iodine for management of well-differentiated thyroid cancer. Between 1990 and 2008, there was a rise in radioactive iodine use across all tumor sizes. In addition to tumor characteristics, other patient and hospital characteristics were also associated with radioactive iodine use. There was wide between-hospital variation in radioactive iodine use and much of the variance was attributable to unexplained hospital characteristics.
Previous studies have evaluated between-hospital variation in rates of surgical procedures43,44 and the role of discretionary decision making on treatment intensity.45,46 Germane to our study is a single-institution study that evaluated use of radioactive iodine over time and found a rise in use between 1940 and 199947 and a study with Surveillance, Epidemiology, and End Results data that found increased radioactive iodine use between 1973 and 2006.21 However, our study is novel as it investigates not only treatment trends but also correlates of radioactive iodine use and variation in use in a large and recently treated multicenter cohort of thyroid cancer patients.
The explanation for the rise in radioactive iodine use across all tumor sizes is not entirely clear, but it has been hypothesized that increased detection of low risk disease can lead to overestimation of treatment efficacy and a subsequent rise in use of therapy.48 We know from previous population studies that well-differentiated thyroid cancer is increasing at a faster rate than any other malignancy with a 2.4-fold rise in incidence over the past 30 years.31,32,49 The majority of the increase is due to detection of small, low risk tumors,31,50 and, in light of the 10–36% incidence of occult well-differentiated thyroid cancer in autopsy studies,51,52 over diagnosis of clinically irrelevant cancers may be occurring.48,53 Thus, there is potential for increased detection of low risk disease spurring a rise in thyroid cancer treatment intensity.
In addition to identifying trends in radioactive iodine use and correlates of use, this study also found large hospital-based variation with patient and tumor characteristics accounting for 21% of the variation and unknown hospital factors accounting for 29% of the variation. These findings suggest disease severity is not the sole determinant of radioactive iodine use.
Wide variation in radioactive iodine use was seen in both lower and higher risk patients. The low risk patient profile depicted in Figure 2 is a profile in which the use of radioactive iodine was left to physician discretion12–14,16 until the most recent clinical guidelines.11 In contrast, almost all clinical guidelines would strongly recommend radioactive iodine post thyroid surgery in the high risk patient profile depicted in Figure 3.11–16 The variation demonstrated in both low and high risk patients suggests clinical uncertainty.54,55 Some of this uncertainty may be explained by the lack of clinical trials evaluating the efficacy of radioactive iodine use for thyroid cancer and the conflicting single institution studies. Because of limited clinical evidence, clinical guidelines have left radioactive iodine use to physician discretion in many cases.11,12 A recent study has shown that when clinical guideline treatment recommendations are not supported by strong evidence there is less guideline-concordant care.56
Studies using a large database such as the National Cancer Database have inherent limitations. Specific to thyroid cancer, presence of extrathyroidal extension, post-operative serum thyroglobulin level, and tumor iodine-avidity are not recorded. In addition, treatment details such as dose of radioactive iodine and addition of prophylactic central lymph node dissection are not known. These missing details may be important as they can impact the indications for radioactive iodine57 and, in the case of radioactive iodine dosing, affect our assessment of intensity of care.
Even with the limitations inherent in a large database, the results of this study have implications for patients, physicians, and payers. Although appropriate therapy for select well-differentiated thyroid cancer, the benefit of radioactive iodine may not always exceed the risks. There is a clear role for adjuvant therapy with radioactive iodine in iodine-avid advanced stage well-differentiated thyroid cancer.1–3,58 however, there is unclear benefit to radioactive iodine use in low risk disease.4–6,59–64 as patients with low risk disease have an excellent prognosis regardless of intervention.5,65,66
In addition to clear cost saving benefits associated with not using radioactive iodine for low risk disease,30 limiting radioactive iodine use would decrease patients’ risks of side effects. Not only are there transient adverse effects on quality of life with the hypothyroidism typically required pre-radioactive iodine treatment,67 radioactive iodine has long term health risks. Recent studies have found increased risk for second primary malignancies after radioactive iodine treatment, even in the lowest risk patients,21 with the greatest risk for leukemia, which increases 2.5-fold.18,19,68,69 Radioactive iodine is also associated with additional adverse systemic effects, 26,17,27,70,29,16,71 and damage to local tissue, such as the salivary glands and nasolacrimal ducts.20,22,24,25 There are also potential public health risks if appropriate safety precautions are not taken at the time of radioactive iodine administration.72 In contrast to the potential for over treatment and greater harm than good when using radioactive iodine for low risk disease, the spectrum of radioactive iodine use in the high risk patient profile, suggest there may be under treatment of some high risk patients. This has potential implications for patient health, such as increased risk of disease recurrence and mortality.3,5
The fact that disease severity appears to have a small influence on radioactive iodine use after thyroid surgery is concerning. In the interest of curbing the rising health care costs and preventing both over- and under treatment of disease, indications for radioactive iodine should be clearly defined, and disease severity should become the primary driver of radioactive iodine use.
In summary, in the United States, the incidence of small, low risk thyroid cancers is growing at a faster rate than any other malignancy.49 Paradoxically, use of radioactive iodine is climbing in patients with all tumor sizes. The significant between-hospital variation in radioactive iodine use suggests clinical uncertainty over the role of radioactive iodine in thyroid cancer management. Of concern, for patients with thyroid cancer, the hospital where care is received has a substantial influence on treatment with radioactive iodine after total thyroidectomy, even after accounting for patient and tumor characteristics.
Acknowledgment
Dr. Haymart is funded by 1K07CA154595-01. Dr. Haymart had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Haymart, Stewart
Acquisition of data: Stewart, Birkmeyer
Analysis and interpretation of data: Haymart, Banerjee, Stewart, Koenig, Birkmeyer, Griggs Drafting of the manuscript: Haymart, Banerjee
Critical revision of the manuscript: Haymart, Banerjee, Stewart, Koenig, Birkmeyer, Griggs
Statistical analysis: Banerjee, Stewart
Administrative, technical, or material support: Haymart, Stewart
Supervision: Koenig, Birkmeyer, Griggs
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Varma VM, Beierwaltes WH, Nofal MM, Nishiyama RH, Copp JE. Treatment of thyroid cancer. Death rates after surgery and after surgery followed by sodium iodide I-131. JAMA. 1970 Nov 23;214(8):1437–1442. doi: 10.1001/jama.214.8.1437. [DOI] [PubMed] [Google Scholar]
- 2.Chow SM, Yau S, Kwan CK, Poon PC, Law SC. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer. 2006 Dec;13(4):1159–1172. doi: 10.1677/erc.1.01320. [DOI] [PubMed] [Google Scholar]
- 3.Jung TS, Kim TY, Kim KW, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J. 2007 Apr;54(2):265–274. doi: 10.1507/endocrj.k06-166. [DOI] [PubMed] [Google Scholar]
- 4.Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007 Jul 1;96(1):3–7. doi: 10.1002/jso.20656. [DOI] [PubMed] [Google Scholar]
- 5.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006 Dec;16(12):1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic's experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Masuoka H, Fukushima M, et al. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010 Jun;34(6):1285–1290. doi: 10.1007/s00268-009-0356-0. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferri EL. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 2009 Jun;23(7):579–588. [PubMed] [Google Scholar]
- 9.Jonklaas J, Cooper DS, Ain KB, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010 Dec;20(12):1423–1424. doi: 10.1089/thy.2010.0308. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID. Managing patients with a preoperative diagnosis of AJCC/UICC stage I (T1N0M0) papillary thyroid carcinoma: East versus West, whose policy is best? World J Surg. 2010 Jun;34(6):1291–1293. doi: 10.1007/s00268-010-0469-5. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009 Nov;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006 Feb;16(2):109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 13.Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2005 May;3(3):404–457. doi: 10.6004/jnccn.2005.0021. [DOI] [PubMed] [Google Scholar]
- 14.Sherman SI, Angelos P, Ball DW, Byrd D, Clark OH, Daniels GH, Dilawari RA, Ehya H, Farrar WB, Gagel RF, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey J, Olson JA, Ridge JA, Shah JP, Sisson JC, Tuttle RM, Urist MM. Thyroid Carcinoma Clinical Practice Guidelines in Oncology. Journal of National Comprehensive Cancer Network. 2007;5:568–621. doi: 10.6004/jnccn.2007.0052. [DOI] [PubMed] [Google Scholar]
- 15.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh Q-Y, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey JC, Olson JA, Parks L, Ridge JA, Shah JP, Sherman SI, Sturgeon C, Waguespack SG, Wang TN, Wirth L. Thyroid Carcinoma: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 16.Cobin RH, Gharib H, Bergman DA, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. American Association of Clinical Endocrinologists. American College of Endocrinology. Endocr Pract. 2001 May-Jun;7(3):202–220. [PubMed] [Google Scholar]
- 17.Raymond JP, Izembart M, Marliac V, et al. Temporary ovarian failure in thyroid cancer patients after thyroid remnant ablation with radioactive iodine. J Clin Endocrinol Metab. 1989 Jul;69(1):186–190. doi: 10.1210/jcem-69-1-186. [DOI] [PubMed] [Google Scholar]
- 18.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008 Feb;93(2):504–515. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 19.Sandeep TC, Strachan MW, Reynolds RM, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006 May;91(5):1819–1825. doi: 10.1210/jc.2005-2009. [DOI] [PubMed] [Google Scholar]
- 20.Solans R, Bosch JA, Galofre P, et al. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J Nucl Med. 2001 May;42(5):738–743. [PubMed] [Google Scholar]
- 21.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011 Mar 22; doi: 10.1002/cncr.26070. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malpani BL, Samuel AM, Ray S. Quantification of salivary gland function in thyroid cancer patients treated with radioiodine. Int J Radiat Oncol Biol Phys. 1996 Jun 1;35(3):535–540. doi: 10.1016/s0360-3016(96)80016-2. [DOI] [PubMed] [Google Scholar]
- 23.Sawka AM, Goldstein DP, Brierley JD, et al. The impact of thyroid cancer and postsurgical radioactive iodine treatment on the lives of thyroid cancer survivors: a qualitative study. PLoS One. 2009;4(1):e4191. doi: 10.1371/journal.pone.0004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JA, Morgenstern KE, Cahill KV, Foster JA, Jhiang SM, Kloos RT. Nasolacrimal obstruction secondary to I(131) therapy. Ophthal Plast Reconstr Surg. 2004 Mar;20(2):126–129. doi: 10.1097/01.iop.0000117340.41849.81. [DOI] [PubMed] [Google Scholar]
- 25.Morgenstern KE, Vadysirisack DD, Zhang Z, et al. Expression of sodium iodide symporter in the lacrimal drainage system: implication for the mechanism underlying nasolacrimal duct obstruction in I(131)-treated patients. Ophthal Plast Reconstr Surg. 2005 Sep;21(5):337–344. doi: 10.1097/01.iop.0000179369.75569.a8. [DOI] [PubMed] [Google Scholar]
- 26.Van Nostrand D, Neutze J, Atkins F. Side effects of "rational dose" iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. J Nucl Med. 1986 Oct;27(10):1519–1527. [PubMed] [Google Scholar]
- 27.Hyer S, Vini L, O'Connell M, Pratt B, Harmer C. Testicular dose and fertility in men following I(131) therapy for thyroid cancer. Clin Endocrinol (Oxf) 2002 Jun;56(6):755–758. doi: 10.1046/j.1365-2265.2002.t01-1-01545.x. [DOI] [PubMed] [Google Scholar]
- 28.Molinaro E, Leboeuf R, Shue B, et al. Mild decreases in white blood cell and platelet counts are present one year after radioactive iodine remnant ablation. Thyroid. 2009 Oct;19(10):1035–1041. doi: 10.1089/thy.2008.0430. [DOI] [PubMed] [Google Scholar]
- 29.Kloos RT. Protecting thyroid cancer patients from untoward effects of radioactive iodine treatment. Thyroid. 2009 Sep;19(9):925–928. doi: 10.1089/thy.2009.0236. [DOI] [PubMed] [Google Scholar]
- 30.Pace-Asciak PZ, Payne RJ, Eski SJ, Walfish P, Damani M, Freeman JL. Cost savings of patients with a MACIS score lower than 6 when radioactive iodine is not given. Arch Otolaryngol Head Neck Surg. 2007 Sep;133(9):870–873. doi: 10.1001/archotol.133.9.870. [DOI] [PubMed] [Google Scholar]
- 31.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006 May 10;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 32.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009 Aug 15;115(16):3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 33.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009 Jun 15;99(8):488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 34.Phillips JK, Stewart AK, et al., editors. Facility Oncology Data Standards. Chicago: Commission on Cancer; 2006. [Google Scholar]
- 35.Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: Confronting racial and ethnic disparities. Washington, D.C.: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 38.Greene FL, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 39.Percy CFA, et al., editors. ICD-O: International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 40.Snijders TBR. Multilevel Analysis. Thousands Oaks, Calif: SAGE Publications; 1999. [Google Scholar]
- 41.Subramanian SJK, Duncan C. Multilevel methods for public health research. New York: Oxford University Press; 2003. [Google Scholar]
- 42.Normand ST, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: Issues and applications. Journal of the American Statistical Association. 1997;92(439):803–814. [Google Scholar]
- 43.Birkmeyer JD, Sharp SM, Finlayson SR, Fisher ES, Wennberg JE. Variation profiles of common surgical procedures. Surgery. 1998 Nov;124(5):917–923. [PubMed] [Google Scholar]
- 44.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002 Apr 11;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 45.Griggs JJ, Sorbero ME, Ahrendt GM, et al. The pen and the scalpel: effect of diffusion of information on nonclinical variations in surgical treatment. Med Care. 2009 Jul;47(7):749–757. doi: 10.1097/MLR.0b013e31819748b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary decision making by primary care physicians and the cost of U.S. Health care. Health Aff (Millwood) 2008 May-Jun;27(3):813–823. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and longterm outcome in 2444 consecutively treated patients. World J Surg. 2002 Aug;26(8):879–885. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 48.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993 Apr 29;328(17):1237–1243. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- 49. [Accessed March 8, 2010];SEER Cancer Statistics Review. http://www.seer.cancer.gov/
- 50.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The Most Commonly Occurring Papillary Thyroid Cancer in the United States Is Now a Microcarcinoma in a Patient Older Than 45 Years. Thyroid. 2011 Mar;21(3):231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 51.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer. 1985 Aug 1;56(3):531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Tello FJ, Martinez-Cabruja R, Fernandez-Martin J, Lasso-Oria C, Ballestin-Carcavilla C. Occult carcinoma of the thyroid. A systematic autopsy study from Spain of two series performed with two different methods. Cancer. 1993 Jun 15;71(12):4022–4029. doi: 10.1002/1097-0142(19930615)71:12<4022::aid-cncr2820711236>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010 May 5;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 54.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–824. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 55.Wennberg JE. Understanding geographic variations in health care delivery. N Engl J Med. 1999 Jan 7;340(1):52–53. doi: 10.1056/NEJM199901073400111. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of Appropriate Surgical Care for Medicare Beneficiaries With Cancer. Arch Surg. 2011 Jun 20; doi: 10.1001/archsurg.2011.141. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009 Apr;94(4):1162–1167. doi: 10.1210/jc.2008-1931. [DOI] [PubMed] [Google Scholar]
- 58.Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004 Aug;89(8):3668–3676. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 59.Lin HW, Bhattacharyya N. Survival impact of treatment options for papillary microcarcinoma of the thyroid. Laryngoscope. 2009 Oct;119(10):1983–1987. doi: 10.1002/lary.20617. [DOI] [PubMed] [Google Scholar]
- 60.Brierley J, Tsang R, Panzarella T, Bana N. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin Endocrinol (Oxf) 2005 Oct;63(4):418–427. doi: 10.1111/j.1365-2265.2005.02358.x. [DOI] [PubMed] [Google Scholar]
- 61.Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006 Dec 15;94(8):692–700. doi: 10.1002/jso.20696. [DOI] [PubMed] [Google Scholar]
- 62.Roti E, degli Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008 Dec;159(6):659–673. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 63.Sisson JC. Applying the radioactive eraser: I–131 to ablate normal thyroid tissue in patients from whom thyroid cancer has been resected. J Nucl Med. 1983 Aug;24(8):743–745. [PubMed] [Google Scholar]
- 64.Hay ID, McDougall IR, Sisson JC. A Proposition for the Use of Radioiodine in WDTC Management. J Nucl Med. 2009 Jan 21; doi: 10.2967/jnumed.108.057141. [DOI] [PubMed] [Google Scholar]
- 65.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997 Feb 1;79(3):564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 66.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998 Dec 15;83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 67.Chow SM, Au KH, Choy TS, et al. Health-related quality-of-life study in patients with carcinoma of the thyroid after thyroxine withdrawal for whole body scanning. Laryngoscope. 2006 Nov;116(11):2060–2066. doi: 10.1097/01.mlg.0000240287.57704.01. [DOI] [PubMed] [Google Scholar]
- 68.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003 Nov 3;89(9):1638–1644. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009 May;19(5):451–457. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 70.Rosario PW, Borges MA, Purisch S. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. J Nucl Med. 2008 Nov;49(11):1776–1782. doi: 10.2967/jnumed.108.050591. [DOI] [PubMed] [Google Scholar]
- 71.Luster M, Clarke SE, Dietlein M, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008 Oct;35(10):1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 72.Sisson JC, Freitas J, McDougall IR, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine (1)(3)(1)i: practice recommendations of the american thyroid association. Thyroid. 2011 Apr;21(4):335–346. doi: 10.1089/thy.2010.0403. [DOI] [PubMed] [Google Scholar]