Abstract
Immune evasion is required for Mycobacterium tuberculosis to survive in the face of robust CD4+ T cell responses. We have shown previously that M. tuberculosis cell wall glycolipids, including mannose capped lipoarabinomannan (ManLAM), directly inhibit polyclonal murine CD4+ T cell activation by blocking ZAP-70 phosphorylation. We extended these studies to antigen-specific murine CD4+ T cells and primary human T cells and found that ManLAM inhibited them as well. Lck and LAT phosphorylation also were inhibited by ManLAM without affecting their localization to lipid rafts. Inhibition of proximal TCR signaling was temperature sensitive, suggesting that ManLAM insertion into T cell membranes was required. Thus, M. tuberculosis ManLAM inhibits antigen-specific CD4+ T cell activation by interfering with very early events in TCR signaling through ManLAM's insertion in T cell membranes.
Keywords: tuberculosis, immune evasion, T-cell, signal transduction, LAT, Lck, Zap70, lipid raft, glycolipid, ManLAM
1. Introduction
Mycobacterium tuberculosis infects and persists in a substantial portion of the world's population making it one of the world's most important pathogens (1). M. tuberculosis' ability to survive in the host despite eliciting strong innate and adaptive immune responses is dependent on mechanisms of immune evasion (2,3). These evasion mechanisms include resistance to macrophage killing, inhibition of phagosome maturation and indirectly suppressing CD4+ T cell recognition of M. tuberculosis infected cells by interfering with MHC-II antigen processing.
Recent reports have shown that M. tuberculosis also can directly inhibit T-cell function (4,5). We have demonstrated that glycolipids, specifically mannose-capped lipoarabinomannan (ManLAM) inhibit T-cell receptor signaling through suppression of ZAP-70 phosphorylation (6). These results are consistent with what has previously been reported (4,7) however the mechanism of inhibition is unknown. Although ManLAM binds host receptors including the mannose receptor, dendritic-cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN), and CD14, these receptors are not expressed on T cells (8). ManLAM can interact with host cells, including T cells, independent of receptor binding by directly inserting into cell membranes (9,10). Through their glycosylphosphatidylinositol (GPI)-anchor, M. tuberculosis glycolipids insert themselves within GPI rich domains of cellular membranes such as lipid rafts, rich in cholesterol and sphingolipids that act as a platform for cell signaling (11,12).
ManLAM insertion into GPI rich domains can modulate T cell and macrophage function (13). One study of LAM's effect on Th1 cytokine mRNA expression found LAM present in lipid rafts of Th1 cells resulting in increased activation of Lck and Cbp/PAG, a negative regulator of Lck (4). Others have shown that LAM insertion into lipid rafts contributes to blocking phagosome maturation in macrophages with a similar effect recently reported with lipophosphoglycan from Leishmania donovani (10,14).
In this study we extended our observation of direct inhibition of T cell activation by M. tuberculosis glycolipids in two directions. First we determined if ManLAM inhibition of murine primary CD4+ T cells could be extended to antigen-specific CD4+ T cell activation by antigen presenting cells and whether human CD4+ T cells were similarly inhibited. Second, we determined the mechanism of ManLAM-mediated inhibition of TCR signaling in terms of its effect on Lck and LAT phosphorylation and lipid raft integrity.
2. Materials and Methods
2.1 Mice
8–10-week-old female C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). DO11.10 TCR transgenic mice were that express TCRs specific for the OVA323–339 presented in the context of I-Ad (15). Mice were housed under specific-pathogen-free conditions. Studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
2.2 Cells and medium
Unless otherwise specified, all experiments were performed at 37°C in 5% CO2 atmosphere and serum-free HL-1 media (BioWhittaker, East Rutherford, NJ) supplemented with 1 μM 2-ME, 10 mM HEPES buffer, nonessential amino acids, 2 mM L-glutamine, 100 μg of streptomycin, and 100 U of penicillin (complete HL-1; BioWhittaker). Spleen cells from 8–10-week old wild-type C57Bl/6 mice, OVA-specific DO11.10 were isolated and red blood cells lysed in hypotonic lysis buffer (10 mM Tris-HCl and 0.83% ammonium chloride). Spleen cells were plated in 100 mm tissue culture plates and allowed to adhere for 1 h at 37°C. Untouched CD4+ T cells were purified from nonadherent spleen cells using CD4+ T cells negative isolation kits (Miltenyi Biotec, Germany) following manufacturer's instructions. Purity of CD4+ T cells was confirmed by flow cytometry and ranged between 88–95% (6). T-hybridoma cells, DB-1 and 1T1A, were generated as previously described (16) and maintained in DMEM (BioWhittaker, East Rutherford, NJ) supplemented as indicated for complete HL-1 with the addition of 10% heat-inactivated fetal bovine serum (Hyclone, Logan, Utah). Prior to use in a stimulatory assay T-hybridoma cells were washed and re-suspended in complete HL-1. Human T lymphoblasts were a gracious gift from Dr. Alan Levine and prepared as previously described (17,18). Briefly, PBMC were purified from the blood of healthy donors by Ficoll-Hypaque density separation (Sigma-Aldrich, St. Louis, MO). PBMC were stimulated with 0.5% PHA (Invitrogen Life Technologies, Carlsbad, CA) in the presence of 5 ng/ml IL-2 (R&D Systems, Minneapolis, MN) in RPMI 1640, 10% heat-inactivated FBS and 25 mM HEPES for 48 h. Cells were treated with 5 ng/ml IL-2 for 1 wk and rested for 24 h prior to use in T-cell activation assays.
2.3 Antibodies and reagents
The following mAbs and antibodies were purchased for murine CD4+ T-cell activation: hamster anti-mouse CD3 mAb (145-2C11), hamster anti-mouse CD28 mAb (L3T4) and mouse anti-hamster immunoglobulin G1 (IgG1) from BD Biosciences (San Jose, Ca). For human T cell activation, murine anti-human CD3 (OKT3) and anti-human CD28 were purchased from BD Bioscience and cross-linking sheep anti-mou)2 from Sigma-Aldrich. For immunoblotting rabbit mAbs or polyclonal antibodies against ZAP-70, phosphorylated ZAP-70 (Tyr319), Lck, phosphorylated Src-Tyr416 (recognizing phosphorylated Lck-Tyr394), phosphorylated Lck-Tyr505, LAT, phosphorylated LAT-Tyr171 and phosphorylated LAT-Tyr191 were purchased from Cell Signaling Technologies (Danvers, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used for detection. The pharmacological inhibitor 8-Bromoadenosine 3',5'-cyclic monophosphorothioate, Rpisomer was purchased from Sigma-Aldrich. Anti-ManLAM mAb producing hybridoma cell line CS35 and ManLAM from M. tuberculosis H37Rv were obtained through the Tuberculosis Vaccine Testing and Research Materials Contract (NIAID HHSN266200400091C) at Colorado State University (CSU).
2.4 ManLAM purification
ManLAM was also purified from M. tuberculosis H37Ra using CSU's method. M. tuberculosis cells were delipidated by organic extraction with 10:10:3 chloroform, methanol water (CHCl3:CH3OH:H2O). Delipidated cells were re-suspended in lysis buffer containing 8% TX-114 and lysed in a French press. Lysates were extracted with 8% TX-114 as described previously (19). The detergent phase was precipitated with cold ethanol overnight and digested with pronase. Remaining protein-free extract was separated with size exclusion columns connected in tandem (S-100, S-200, GE Healthcare) in an AKTA purifier system (Pharmacia Biotech, GE Healthcare). Fractions were analyzed in 15% Tris-glycine polyacrylamide gels and glycolipid bands revealed with acid-silver stain. Three pools of fractions containing 34-kDa (pool 1, ManLAM), 14-kDa (pool 2, LM) and 4-kDa (pool 3, PIM6) bands, respectively, were collected. Presence and purity of ManLAM in pool 1 were confirmed by Western blotting with mAb CS35. H37Ra ManLAM purified at CWRU had same bio-activity for TCR inhibition as H37Rv ManLAM obtained from CSU.
2.5 CD4+ T cell assays
For polyclonal activation resting murine CD4+ T cells or human lymphoblasts (1×106 cells/well) were activated in 96-well, flat-bottomed microtiter plates with 1 μg/ml of soluble CD28 mAb in wells coated with 1 μg/ml of CD3 mAb. Cells were stimulated in the presence or absence of ManLAM for 48 h. For antigen-specific activation assays, purified OVA-specific DO11.10 were treated with or without ManLAM and cultured in the presence of peptide or protein pulsed IFN-γ pretreated bone-marrow derived macrophages (BMM) for 24 h before collecting supernatants for IL-2 measurements as previously described. Supernatants were assayed for IL-2 production in Immulon 4HBX flat-bottomed microtiter plates (Thermo) coated with purified capture IL-2 mAb (1μg/ml) and detected with biotinylated IL-2 mAb (1 μg/ml), followed by alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch) and phosphatase substrate (Sigma-Aldrich). Plates were read with a Versa Max turntable microplate reader and data analyzed with Soft Max Pro LS analysis software.
2.6 Measurement of tyrosine phosphorylation
CD4+ T cells were rested overnight in complete DMEM supplemented with 1% FBS. Cells (3×106 cells/ml) were re-suspended in 1 ml of complete HL-1 medium in 1.5ml eppendorf tubes (LBS, Rochester, NY). Cells were incubated with ManLAM (10 μg/ml) for 1 h at 37°C before activation. Cells were washed (10 min at 4,000 rpm) and re-suspended in 100 μl of HL-1. The TCR complex was activated by the addition of a cross-linking mouse anti-hamster IgG1 (10 μg/ml) for 2 min before the addition of anti-CD3 (10 μg/ml) for 2 min. CD4+ T-cell activation was stopped by adding 100 μl of 2× Laemmli sample buffer and boiling samples for 10 min. Unstimulated cells, incubated with mouse anti-hamster IgG1 alone, served as control for non-specific TCR-CD3 activation.
2.7 Isolation of lipid rafts by sucrose gradient centrifugation
Lipid rafts were purified from T-hybridoma cells as previously described (20). T-hybridoma cells (~2.5×107) were suspended in 2 ml of complete HL-1 medium in a 15 ml conical tube. ManLAM (10 μg/ml) or medium alone was added and cells were incubated for 1 h at 37°C. Cells were washed (4,000 rpm for 10 min) and re-suspended in 100 μL of fresh HL-1. Cells were stimulated for 2 min with cross-linking hamster-anti-mouse IgG1 followed by 5 min with anti-CD3 as stated above and immediately placed on ice. Cells were spun down (4,000 rpm for 10 min) and supernatant re-suspended in 500 μL of TKM buffer (50 mM Tris-HCl, pH 7.4, 25 mM KCl, 5 mM MgCl2, and 1 mM EDTA) containing 1% Triton X-100 (Sigma-Aldrich) and a cocktail of protease inhibitors (Roche). Cell suspension was homogenized with a Dounce homogenizer (~25–30 strokes) and lysed on ice for 30 min. Post-nuclear supernatant was prepared by spinning lysate down (4,000 rpm for 10 min) twice. 500 μL of 80% wt/vol sucrose (Sigma Aldrich) in TKM buffer was added to lysate and transferred to SW41 tubes and overlaid with 6 ml of 38% wt/vol sucrose and topped with 3.5 ml of 5% sucrose in TKM buffer. Sucrose gradients were ultra-centrifuged at 39,000 rpm for 18 h in an SW41 rotor (Becton Dickinson, Franklin Lakes, NJ) and 1 ml fractions collected from the top of the gradient to the bottom. For Western Blot analysis, 20 μl of each fraction was mixed with an equal volume of 2× Laemmli buffer and boiled for 10 min.
2.8 Western Blotting
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel (Bio-Rad, Hercules, CA) under reducing conditions and electro-transferred to nitrocellulose membranes (Bio-Rad) in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. After transfer, membranes were incubated at room temperature for 1 h in SuperBlock (Thermo Scientific, Rockford, IL). Primary and secondary antibodies were diluted in 1% nonfat milk, 0.05% Tween-20 in PBS at the concentrations recommended by the manufacturer. For detection of ManLAM within lipid rafts, 1:2 dilution of anti-ManLAM hybridoma CS35 supernatant was incubated with nitrocellulose membranes at 4°C overnight. Following multiple washes with 0.05% Tween-20, membranes were incubated with conjugated secondary antibody for 1 h at room temperature. Detection of HRP-conjugated Abs was performed using West Pico Supersignal (Thermo Scientific). Chemiluminescence was detected with BioMax film (Kodak).
2.9 Statistical Analysis
Statistical analysis was performed by using a one-tailed student t test. A p value of < 0.05 was considered significant.
3. Results
3.1 ManLAM inhibits antigen-specific murine and polyclonal human T cell activation
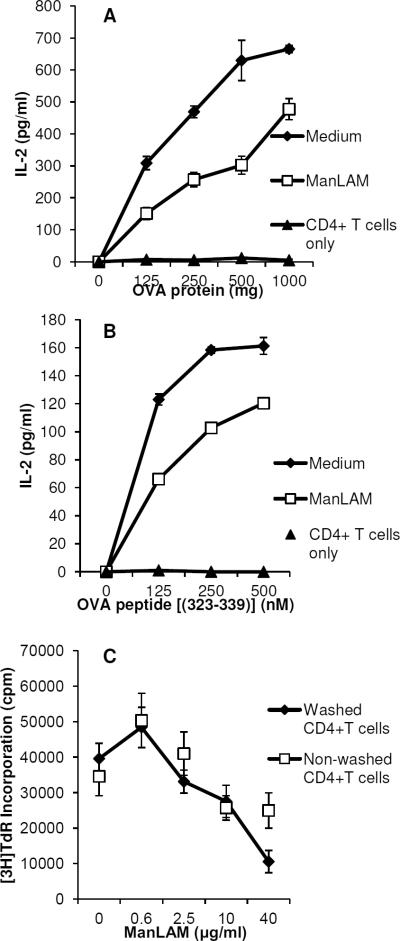
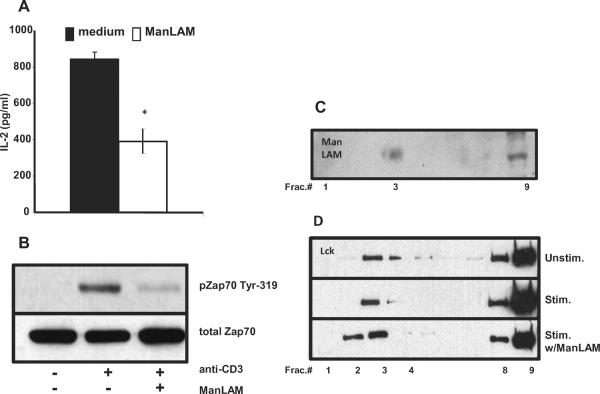
We previously demonstrated that M. tuberculosis glycolipids directly inhibit polyclonal activation of primary naïve and memory mouse CD4+ T cells in the absence of antigen presenting cells and that ManLAM as a major M. tuberculosis glycolipid was particularly potent (6). To determine if ManLAM inhibits antigen-specific activation, we performed experiments with DO11.10 CD4+ TCR transgenic T cells specific for OVA323–339 peptide in the context of I-Ad and IFN-γ activated BMM as APC. As shown in Fig. 1A, pretreatment of DO11.10 CD4+ T cells with 40 μg/ml ManLAM for 1 h before adding antigen pulsed APC resulted in decreased IL-2 production. To control for ManLAM carryover effects on APC, BMM were fixed with 1% paraformaldehyde before adding T cells and again inhibition of T cell activation was measured along a range of OVA323–339 peptide concentrations (Fig. 1B). Purity of CD4+ T cells for these and subsequent experiments in this manuscript was 88–95% by flow cytometry. Pretreatment of CD4+ T cells was sufficient to inhibit their subsequent activation by antigen, since extensive washing of DO11.10 cells after a 1 h incubation with a range of ManLAM concentrations did not affect ManLAM's ability to inhibit antigen-specific T cells activation (Fig. 1C).
Fig. 1. ManLAM inhibits antigen-specific CD4+ T cell activation.
A. Bone marrow-derived macrophages (1 × 105 cells/well) were activated with IFN-γ overnight and then incubated with ovalbumin for 4 h. Purified CD4+ T cells (5 × 104 cells/well) from DO11.10 TCR transgenic mice, pre-incubated with ManLAM (40 μg/ml) or medium alone for 1 h, were added to antigen-pulsed APC for 24 h. Purified CD4+ T cells (CD4+ T cells alone) were cultured with antigen to confirm functional depletion of APC from purified T cells. IL-2 production was measured by ELISA. B. IFN-γ treated macrophages were fixed with 1% paraformaldehyde and incubated with OVA323–339 peptide for 4 h. CD4+ T cells pre-incubated with or without ManLAM were added to peptide-pulsed fixed APC for 24 h, and supernatants harvested for IL-2 measurement. C. CD4+ T cells (2 × 106 cells) were incubated with ManLAM in increasing concentrations for 1 h, and divided into 2 groups (1×106 cells each). One was washed extensively and the other remained in ManLAM. 5 ×104 CD4+ T cells/well from each group were added to OVA323–339 peptide-pulsed, fixed APC for 72 h, and [3H]-thymidine incorporation measured. Data presented are the mean (+/−S.D.) for triplicate wells. Results are representative of at least three experiments.
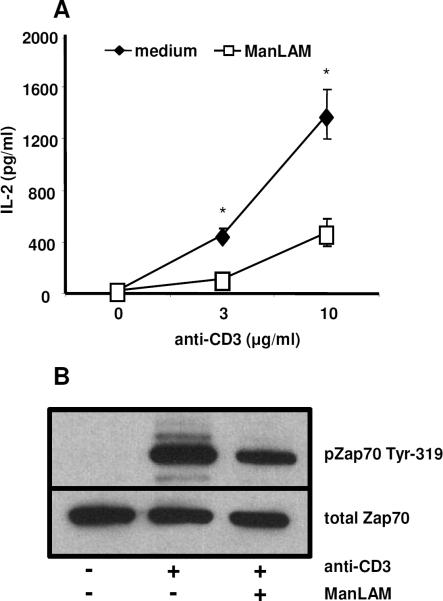
To extend these findings further, we determined whether human T cells were inhibited by ManLAM. Human T lymphoblasts, generated by activating PBMC with PHA and IL-2 for 7 d, were re-stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs in the presence or absence of 10 μg/ml of ManLAM. In the presence of ManLAM, IL-2 production by human lymphoblasts was inhibited by more than 50%, and this was associated with decreased ZAP-70 phosphorylation (Fig. 2). Overall, these results indicate that ManLAM not only inhibits the polyclonal activation of murine and human T cells but also directly interferes with antigen-specific activation of CD4+ T cells independent of its effects on APC.
Fig. 2. ManLAM inhibits the activation of human T cells.
A. In serum-free medium human T lymphoblasts (1×105 cells/well) were stimulated with increasing concentrations of plate-bound anti-CD3 (3–10 μg/ml) and soluble anti-CD28 (1 μg/ml) for 48 h. Cells were co-cultured in the presence or absence of ManLAM (10 μg/ml). Supernatants were harvested and IL-2 measured by ELISA. Data points and values are means +/−S.D. of triplicate wells, and representative of three experiments. *P < 0.01. B. 3×106 human T-lymphoblasts were incubated with 10 μg/ml of ManLAM or medium alone at 37°C for 1 h prior to activation. Cells were washed and incubated for 2 min with cross-linking sheep anti-)2 (10 μg/ml) followed by anti-human CD3 mAb (10 μg/ml) for 2 min. Western analysis was performed with antibodies to phosphorylated ZAP-70-Tyr319 (upper panel) and total ZAP-70 (lower panel). Results presented are representative of three independent experiments.
3.2 ManLAM inhibits phosphorylation of Lck Tyr-394 and LAT Tyr-191/-171 in addition to that of ZAP-70
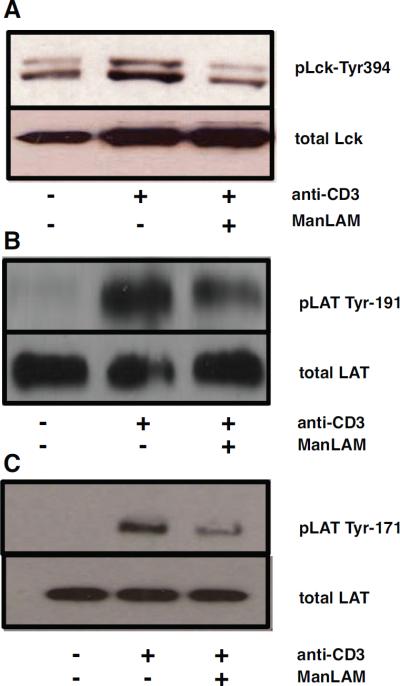
Our initial study demonstrated that ManLAM inhibits phosphorylation of ZAP-70, an early step in TCR signaling (6). To further characterize ManLAMs effect on TCR signaling, we analyzed signaling upstream and downstream of Zap-70. Upon binding of the TCR with peptide loaded MHC, Lck, a Src family kinase, is phosphorylated at Tyr-394 allowing the kinase domain to phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) found on the TCR complex. ZAP-70 binds to these motifs and is subsequently phosphorylated by Lck (21,22). As shown in Fig. 3A, phosphorylation of Lck at Tyr-394 was inhibited when murine CD4+ T cells were pretreated with ManLAM (10 μg/ml) before anti-CD3 activation. Phosphorylation of ITAMs was also inhibited (data not shown)
Fig. 3. ManLAM inhibits Lck and LAT phosphorylation.
CD4+ T cells (3×106) were pre-incubated with 10 μg/ml of ManLAM or medium alone for 1 h at 37°C. Cells were washed and cultured for 2 min with mouse anti-hamster IgG1 (10 μg/ml) followed by hamster anti-mouse CD3 mAb (10 μg/ml) for 2 min. Western analysis was performed with antibody to Lck-Tyr394 A., phosphorylated LAT-191 and LAT-171 B–C. Total Lck and LAT were detected to control for protein loading. Results presented are representative of at least three independent experiments.
Activated ZAP-70 phosphorylates adaptor molecule LAT at Tyr-171 and Tyr-191. Activated LAT binds several molecules, including PLC-γ1, Grb2 and SLP-76 that activate downstream secondary signaling pathways (23). ManLAM decreased phosphorylation of LAT at both Tyr-191 and Tyr-171 (Fig. 3B–C). These findings indicate that ManLAM blocks the entire proximal TCR signaling pathway.
3.3 ManLAM does not alter Lck Tyr-505 phosphorylation
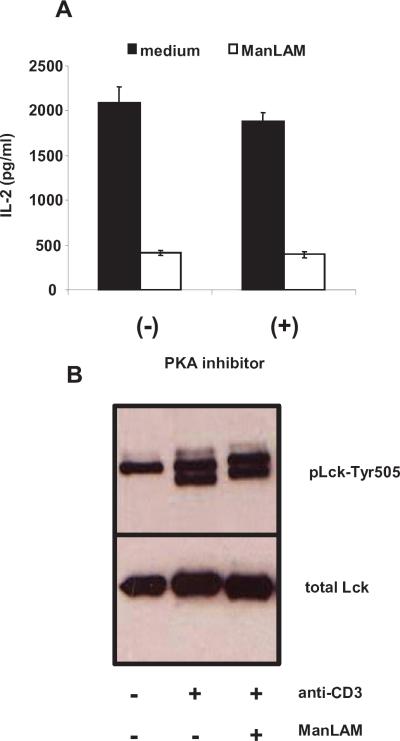
M. tuberculosis cell wall glycolipids can modulate Cbp/PAG, a transmembrane protein involved in regulating Lck (4). Cbp/PAG regulates membrane localization of Csk, a protein kinase that phosphorylates Lck at Tyr-505 inducing Lck's kinase domain into an inactive conformation. Csk binding to Cbp/PAG is regulated through phosphorylation of Cbp/PAG and involves cAMP/PKA (24–26). To determine if this pathway had a role, we utilized two approaches. First, we cultured T cells with ManLAM (10 μg/ml) in the presence or absence of PKA inhibitor, 8-bromoadenosine 3', 5'-cyclic monophosphorothioate, Rp-isomer (50 μM) for 48 h. PKA inhibitor did not reverse ManLAM's inhibition of IL-2 production (Fig. 4A). At higher concentrations (500 μM), there was a slight increase in IL-2 production due to non-specific activation effects. As a positive control for PKA inhibition, CD4+ T cells were stimulated in the presence prostaglandin E2, a known cAMP/PKA inhibitor, resulting in reversal (up to 50%) of 10−8 M prostaglandin E2-induced T cell inhibition (data not shown).
Fig. 4. ManLAM does not activate the cAMP/PKA pathway or inhibit Lck-Tyr505 phosphorylation.
A. In complete HL-1 medium, CD4+ T cells (1×105 cells/well) were activated with plate bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml) for 48 h in the presence of either 50 μM PKA inhibitor 8-bromoadenosine 3', 5'-cyclic monophosphorothioate, Rp-isomer or medium alone. Cells were co-cultured with ManLAM (10 μg/ml) or medium alone. Following incubation, supernatants were harvested for IL-2 measurement. Data points are expressed as means of triplicate samples +/−S.D. Results are representative of four independent experiments. B. CD4+ T cells (3×106 cells) were pre-incubated with ManLAM (10 μg/ml) or medium alone for 1 h at 37°C, and stimulated as in Fig. 3. Western analysis was performed with antibody to phosphorylated Lck-Tyr505 (upper panel) or total Lck (lower panel). Results are representative of at least three independent experiments.
Next we analyzed phosphorylation of Lck Tyr-505 in T cells stimulated with cross-linked anti-CD3 after pre-incubation with ManLAM. There was no difference in Tyr-505 phosphorylation between cells pre-treated with or without ManLAM. Thus, ManLAM does not appear to inhibit TCR signaling through the Cbp/PAG pathway.
3.4 ManLAM insertion into CD4+ T cell membranes is necessary for inhibition of proximal TCR signaling
Through their glycosylphosphatidylinositol (GPI)-anchor, ManLAM can insert into host cell membranes, including GPI-rich lipid rafts (4,9). Lipid rafts are organized microdomains of sphingolipids and cholesterol, and their integrity and reorganization is required for T cell activation (11,12). Inhibition of proximal TCR signaling by ManLAM required pre-incubation and inhibition of antigen-specific activation was not affected by washing ManLAM treated CD4+ T cells (Fig. 1C), suggesting that membrane insertion was required. ManLAM's insertion into host membranes is perturbed when cells are cooled to 4°C, due to loss of membrane fluidity (9). Murine CD4+ T cells were pretreated with ManLAM at either 4°C or 37°C. There was no decrease in ZAP-70 phosphorylation of CD4+ T cells pre-incubated with ManLAM at 4°C compared to cells pre-incubated at 37°C (Fig. 5).
Fig. 5. Inhibition of TCR signaling by ManLAM is temperature sensitive.
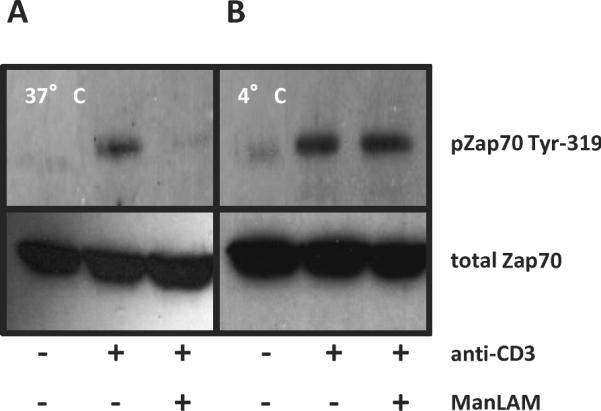
CD4+ T cells (3×106) were pre-incubated with 10 μg/ml of ManLAM for 1 h at either 37°C A. or 4°C B. and stimulated as in Fig. 3. Western analysis was performed for phosphorylated ZAP-70-Tyr319 (upper panels) or total ZAP-70 (lower panels). Results are representative of three independent experiments.
To determine the role of ManLAM insertion into lipid rafts, we used a MHC-II restricted T-hybridoma system due to the large numbers of cells required to purify lipid rafts. We first verified that ManLAM inhibited T hybridoma activation (Fig. 6A–B). To verify that ManLAM inserted into lipid rafts, rafts from T-hybridoma cells were isolated using sucrose gradient ultracentrifugation. Lysates from T-hybridoma cells treated with ManLAM (10 μg/ml) were ultra-centrifuged in a sucrose gradient and fractions (n=9, 1 ml) analyzed by Western blot. Lipid raft were in fractions 2 through 5, as determined by the presence of LAT and Lck. ManLAM was found in the lipid rafts of ManLAM treated T hybridoma cells, confirming previous studies (4,9–10) (Fig. 6C).
Fig. 6. ManLAM is found in T cell membranes and does not affect localization of Lck in lipid rafts of activated T cells.
A. T-hybridoma cells (1×105 cells/well) were stimulated with plate bound anti-CD3 (1 μg/ml) for 6 h in the presence or absence of ManLAM (10 μg/ml). Supernatants were for IL-2 measurement. Data points and values are means +/−S.D. of triplicate wells, and representative of three experiments. *P < 0.01. B. 3×106 T-hybridoma cells were incubated with 10 μg/ml of ManLAM or medium alone at 37°C for 1 h prior to activation. Cells were stimulated as in Fig 3. Western analysis was performed with antibody to phosphorylated ZAP-70-Tyr319 (upper panel) and total ZAP-70 (lower panel). C. T-hybridoma cells (2.5×107 cells) were incubated in the presence or absence of ManLAM (10 μg/ml) for 1 h at 37°C. Cells were subjected to discontinuous sucrose density gradient ultracentrifugation. 1 ml fractions (Fract.) were collected from the top to the bottom and ManLAM distribution within fractions analyzed by Western with anti- ManLAM mAb CS-35. Results are representative of two independent experiments. D. T-hybridoma cells (2.5×107 cells) were incubated in the presence or absence of ManLAM (10 μg/ml) for 1 h at 37°C. Cells were stimulated as in Fig. 3. Cells were lysed and subjected to discontinuous sucrose density gradient ultracentrifugation. 1 ml fractions were collected from top to the bottom from unstimulated (upper panel), stimulated (middle panel), and stimulated T cells in the presence of ManLAM (lower panel). Western analysis was performed for the distribution of Lck. Results are representative blots of four independent experiments.
3.5 ManLAM does not affect localization of LAT and Lck to lipid rafts in resting and activated CD4+ T cells
Modulating lipid raft integrity affects T cell activation (18,27). Next we determined if Lck and LAT levels in rafts were changed by ManLAM. In non-activated T cells, exposure to ManLAM did not affect Lck and LAT localization in raft and non-raft fractions (data not shown). For T cell activation, T-hybridoma cells were pre-incubated with ManLAM (10 μg/ml) for 1 h at 37°C, washed and stimulated for 5 min with cross-linked anti-CD3 mAb. Western blot analysis of sucrose gradient fractions of un-stimulated, stimulated and stimulated in the presence of ManLAM T cells showed no decrease or major alteration in the distribution of Lck (Fig. 6D) or LAT (data not shown) among the three experimental groups. In fact, rather than decreases, minimal but consistent increased LAT or Lck levels were observed in rafts of ManLAM treated cells. Thus, inhibition of TCR signaling by ManLAM was not due to major disruptions of Lck and LAT localization to lipid rafts.
4. Discussion
We previously reported that M. tuberculosis glycolipids, including ManLAM, directly inhibited polyclonal CD4+ T cell activation by blocking ZAP-70 phosphorylation. In this study, we focused on ManLAM, the major cell wall glycolipid of M. tuberculosis, and extended these observations to antigen-specific CD4+ T cell responses, and human T cells, while furthering the analysis of ManLAM's effect on proximal TCR signaling. Phosphorylation of signaling proteins were inhibited both upstream (Lck) and downstream (LAT) of ZAP-70, indicating that ManLAM inhibited TCR signaling at the site of triggering. ManLAM did not affect phosphorylation at Lck-Tyr505 and was independent of PKA indicating that negative regulator Csk was not involved. As reported by others, ManLAM inserted into T cell membranes including lipid rafts. In addition, temperature sensitivity and washing experiments suggest that membrane insertion by ManLAM is required for inhibition of proximal TCR signaling. ManLAM insertion did not affect the presence Lck and LAT in lipid rafts in T cells.
Others have postulated that ManLAM's insertion in membranes modulates host cell behavior (4,10). However, no study has definitively shown that membrane insertion itself is responsible for ManLAM's observed effects. Our results and others support two divergent hypotheses. The first is that insertion of ManLAM into T cell membranes is required and sufficient. In this model, membrane insertion activates an unknown signaling event(s) or inhibits the ability of membranes to reorganize them-selves upon stimulation. This hypothesis is supported by Shabaana et al. of increased kinase activity within lipid rafts of Th1 cells (4). However, we did not observe increased phosphorylation of Lck at either its positive or negative regulatory site. Additionally our data indicates that Csk, a negative regulator of Lck, is not activated by ManLAM. An alternative hypothesis is that membrane insertion is necessary but the effect is not dictated by lipid raft disruption. ManLAM insertion via its GPI motif acts as a tether allowing other components of ManLAM (such as its mannose cap) to bind or interfere with T cell surface molecule(s), possibly the TCR complex itself, and thus affect their function. This model has been proposed for ManLAM's ability to inhibit phagosome maturation after insertion into macrophage lipid rafts and is supported by our previous finding that PIM, while having the phosphoinositol backbone of ManLAM, is much less efficient on a per molecule basis in inhibiting T cell activation (6,10).
Lipid rafts are defined by their insolubility in detergent and their ability to float to low density in sucrose density gradient centrifugation (28,29). Upon TCR engagement, these rafts coalesce at the site of activation, bringing with them signaling molecules such as Lck and LAT required for transducing the TCR signal. This model has been criticized because of the method used to isolate lipid rafts (30,31). Sucrose density gradients give a static picture of rafts and only determine whether or not certain molecules are present. They do not address raft structure nor establish if proper microcluster formation is occurring properly at the T cell-APC synapse. Confocal microscopy and other more advanced imaging techniques have broadened our understanding of the dynamics and kinetics of T cell stimulation induced microcluster formation. Lillemeier et al. used a combination of photoactivated localization microscopy, fluorescence cross-correlation spectroscopy and transmission electron microscopy to show that TCR and LAT are found on separate protein islands that coalesce upon activation (32). Total internal reflection fluorescence microscopy has been used to detect TCR microcluster formations that include ZAP-70, Lck, and other signaling components that form within few seconds of TCR engagement (33,34). Further studies of ManLAMs activity within rafts may identify a T cell regulatory mechanism that ManLAM hijacks to benefit M. tuberculosis survival.
In addition to blocking microcluster formation, ManLAM could induce phosphatase activity. Numerous phosphatases negatively regulate T cell activation by dephosphorylating Lck, ZAP-70 and ERK (23,35–36). Studies of microbial inhibition of T cells have identified several instances where pathogens either through the induction of phosphatase activity or through their own phosphatases inhibit proximal TCR signaling (37–39). Knutson et al. has shown that LAM by increasing phosphatase activity suppressed MAPK signaling in macrophages (40). Phosphatase Src homology 2 containing tyrosine phosphatase (SHP-1) had a major role. SHP-1 regulates proximal TCR signaling and its activity itself is regulated at several points that ManLAM could take advantage of (23). ManLAM did not inhibit IL-2 production by anti-CD3/CD28 activated Jurkat T cells (data not shown) known to be missing inositol phosphatases SHIP-1 and PTEN (41). These phosphatases regulate phosphorylation downstream of proximal TCR signaling. PTEN is involved in regulation of phosphoinositide 3-kinase (PI3K) pathway, utilized by some co-stimulatory receptors.
Modulation of host immunity by glycolipids is uncommon except among intracellular pathogens such as Leishmania (lipophosphoglycan), Trypanosoma (glycoinositol phospholipids, GIPL) and mycobacteria. These pathogens block phagosome maturation, suppress cytokine production and modulate surface expression of MHC molecules (42–44). While these glycolipids have unique head groups, they share a glycosylphosphatidylinositol (GPI) anchor important for their effects on host immunity (45–47). Tachado et al. purified GPIs from Plasmodium, Trypanosoma and Leishmania and found that they induced protein kinase C activity (PKC) (48). T-cell studies by others have suggested that ManLAM can induce PKC signaling to modulate host immunity, however, in our earlier study PKC activation by phorbol myristate acetate (PMA) was not inhibited by ManLAM (6, 49). Besides ManLAM, the only other microbial glycolipid known to directly modulate T-cells is Trypanosoma GIPL. (50,51). Since microbial glycolipids have similar effects on other cell types, microbial lipophosphoglycan and glycolipids may have similar inhibitory effects on T cell activation as seen for ManLAM. To our knowledge we are the first to determine that microbial glycolipids such as ManLAM can directly inhibit proximal TCR signaling. These findings not only provide insight into how M. tuberculosis evades host immunity but also how TCR activation may be regulated by microbes.
Highlights
ManLAM directly inhibits both monoclonal and polyclonal T cell activation.
ManLAM inhibits entire proximal T-cell receptor signaling pathway.
Inhibition of proximal T-cell receptor signaling is temperature sensitive ManLAM inserts into lipid rafts of T-hybridoma cells.
Insertion of ManLAM does not affect Lck or LAT's presence in lipid raft.
Acknowledgements
This work was supported by National Institutes of Health Grants AI-27243 and HL-55967 (to W.H.B.), Contract No. HHSN266200700022C/NO1 AI-70022 for Tuberculosis Research Unit (TBRU) (to W.H.B.), NIH grants AI069085, AI034343 and AI035726 to C.V.H, American Lung Association grants RG48786N to R.E.R. We thank Qing Ling for her assistance in running and analyzing Western blots, Lopa Das and Jeffrey Meisch for their assistance in generating human T lymphoblasts, and Scott Reba and Scott Fulton for technical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- [2].Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Current Opin Immunol. 2003;15:450–455. doi: 10.1016/s0952-7915(03)00075-x. [DOI] [PubMed] [Google Scholar]
- [3].Hestvik AL, Hmama Z, Av-Gay Y. Mycobacterial manipulation of the host cells. FEMS Microbiol Rev. 2005;29:1041–1050. doi: 10.1016/j.femsre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [4].Shabaana AK, Kulangara K, Semac I, Parel Y, Ilangumaran S, Dharmalingam K, Chizzolini C, Hoessli DC. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signaling. Cell. Mol Life Sci. 2005;62:179–187. doi: 10.1007/s00018-004-4404-5. [DOI] [PubMed] [Google Scholar]
- [5].Wang X, Barnes PF, Dobos-Elder KM, Townsend JC, Chung Y, Shams H, Weis SE, Samten B. ESAT-6 inhibits production of IFN-γ by Mycobacterium tuberculosis-responsive human T cells. J Immunol. 2009;182:3668–3677. doi: 10.4049/jimmunol.0803579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mahon RN, Rojas RE, Fulton SA, Franko JL, Harding CV, Boom WH. Mycobacterium tuberculosis cell wall glycolipids directly inhibit CD4+ T cell activation by interfering with proximal T-cell receptor signaling. Infect Immun. 2009;10:4574–4583. doi: 10.1128/IAI.00222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moreno C, Mehlert A, Lamb J. The inhibitory effects of mycobacteria lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immmunol. 1988;74:206–210. [PMC free article] [PubMed] [Google Scholar]
- [8].Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- [9].Ilangumaran S, Arni S, Poincelet M, Theler JM, Brennan PJ, Nasirud D, Hoessli DC. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- [10].Welin A, Winberg ME, Abdalla H, Sarndahl E, Rasmusson B, Stendahl O, Lerm M. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882–2887. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alonso MA, Millan J. The role of lipid rafts in signaling and membrane trafficking in T lymphocytes. J Cell Sci. 2001;114:3957–3965. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- [12].Pizzo P, Viola A. Lymphocyte lipid rafts: structure and function. Curr Opin Immunol. 2003;15:255–260. doi: 10.1016/s0952-7915(03)00038-4. [DOI] [PubMed] [Google Scholar]
- [13].Mañes S, del Real G, Martinez-A C. Pathogens: Raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- [14].Winberg ME, Holm A, Särndahl E, Vinet AF, Descoteaux A, Magnusson K, Rasmusson B, Lerm M. Leishmania donovani lipophosphoglycan inhibits phagosomal maturation via action on membrane rafts. Microbes Infect. 2009;11:215–222. doi: 10.1016/j.micinf.2008.11.007. [DOI] [PubMed] [Google Scholar]
- [15].Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- [16].Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits γ-interferon-regulated HLA-DR and FcγR1 on human macrophages through Toll-like receptor 2. Infect. Immun. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Das L, Levine AD. TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J Immunol. 2008;180:1490–1498. doi: 10.4049/jimmunol.180.3.1490. [DOI] [PubMed] [Google Scholar]
- [18].Schade AE, Levine AD. Lipid raft heterogeneity in human peripheral blood T lymphoblasts: A mechanism for regulating the initiation of TCR signal transduction. J Immunol. 2002;168:2233–2239. doi: 10.4049/jimmunol.168.5.2233. [DOI] [PubMed] [Google Scholar]
- [19].Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- [20].Ilangumaran S, Arni S, van Echten-Deckert G, Borisch B, Hoessli DC. Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinase in T lymphocyte plasma membranes. Molec Biol Cell. 1999;10:891–905. doi: 10.1091/mbc.10.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Au-Yeung BB, Deindl S, Hsu L, Palacios EH, Levin SE, Kuriyan J, Weiss A. The structure, regulation, and function of ZAP-70. Immuno Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- [22].Iwashima M. Kinetic perspectives of T cell antigen receptor signaling: A two tier-model for T cell full activation. Immunol Rev. 2003;191:196–210. doi: 10.1034/j.1600-065x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- [23].Samelson LE. Signal transduction mediated by the T cell antigen receptor: The role of adaptor proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- [24].Mustelin T, Taskén K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;371:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, Skålhegg BS, Hansson V, Mustelin T, Taskén K. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Ex Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vang T, Abrahamsen H, Myklebust S, Horejsi V, Taskén K. Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase A inhibits T cell receptor signaling. J Biol Chem. 2003;278:497–507. doi: 10.1074/jbc.C300077200. [DOI] [PubMed] [Google Scholar]
- [27].Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- [28].Hoessli DC, Rungger-Brandle E. Isolation of plasma membrane domains from murine T lymphocytes. Proc Natl Acad Sci USA. 1983;80:439–433.1. doi: 10.1073/pnas.80.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- [30].Kenworthy AK. Have we become over reliant on lipid rafts: Talking point on the involvement of lipid rafts in T-cell activation. Euro Molec Biol Org. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shaw AE. Lipid rafts: now you see them, now you don't. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- [32].Lillemeier BF, Mörtelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–97. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Ex Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin M, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of ZAP-70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- [35].Saito T, Yamasaki S. Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol Rev. 2003;192:143–160. doi: 10.1034/j.1600-065x.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- [36].Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Molec Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- [37].Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- [38].Sloan DD, Han JY, Sandifer TK, Stewart M, Hinz AJ, Yoon M, Johnson DC, Spear PG, Jerome KR. Inhibition of TCR signaling by herpes simplex virus. J Immunol. 2006;176:1825–1833. doi: 10.4049/jimmunol.176.3.1825. [DOI] [PubMed] [Google Scholar]
- [39].Yao T, Mecsas J, Healy JI, Falkow S, Chien Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, YopH. J Ex Med. 1999;190:1343–1350. doi: 10.1084/jem.190.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knutson KL, Hamama Z, Herrera-Velit P, Rochford R, Reiner NE. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes: Role of the Src homology containing tyrosine phosphatase 1. J Biol Chem. 1998;273:645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- [41].Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signaling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- [42].Brodskyn C, Patricio J, Oliveira R, Lobo L, Arnholdt A, Mendonça-Previato L, Barral A, Barral-Netto M. Glycoinositolphospholipids from Trypanosoma cruzi interfere with macrophages and dendritic cell responses. Infect Immun. 2002;70:3736–2743. doi: 10.1128/IAI.70.7.3736-3743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Descoteaux A, Turco SJ, Sacks DL, Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991;146:2747–2753. [PubMed] [Google Scholar]
- [44].Desjardins M, Descoteaux A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J Ex Med. 1997;185:2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Almeida IC, Gazzinelli RT. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J Leuk Biol. 2001;70:467–477. [PubMed] [Google Scholar]
- [46].Arrighi RBG, Debierre-Grockiego F, Schwarz RT, Faye I. The immunogenic properties of protozoan glycosylphosphatidylinositiols in the mosquito Anopheles gambiae. Develop Comp Immunol. 2009;33:216–223. doi: 10.1016/j.dci.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [47].Chawla M, Vishwakarma RA. Alkylacyglycerolipid domain of GPI molecules of Leishmania is responsible for inhibition of PKC-mediated c-fos expression. J Lipid Res. 2003;44:594–600. doi: 10.1194/jlr.M200296-JLR200. [DOI] [PubMed] [Google Scholar]
- [48].Tachado SD, Gerold P, Schwarz R, Novakovic S, McConville M, Schofield L. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of inositolglycan and diacylglycerol moieties. Proc Natl Acad Sci USA. 1997;94:4022–4027. doi: 10.1073/pnas.94.8.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bernier R, Barbeau B, Olivier M, Tremblay MJ. Mycobacterium tuberculosis mannose-capped lipoarabinomannan can induce NF-κB-dependent activation of human immunodeficiency virus type 1 long terminal repeat in T cells. J. Gen. Virol. 1998;79:1353–1361. doi: 10.1099/0022-1317-79-6-1353. [DOI] [PubMed] [Google Scholar]
- [50].Bello M, Oliveira ASC, Mermelstein CS, Capella MAM, Viola JPB, Levraud J, Dosreis GA, Previato JO, Mendonça-Previato L. Costimulatory action of glycoinositolphospholipids from Trypanosoma cruzi: increased interleukin 2 secretion and induction of nuclear translocation of the nuclear factor of activated T cells. FASEB J. 1999;13:1627–1636. doi: 10.1096/fasebj.13.12.1627. [DOI] [PubMed] [Google Scholar]
- [51].Gomes NA, Previato JO, Zingales B, Mendonça-Previato L, DosReis GA. Down-regulation of T lymphocyte activation in vitro and in vivo induced by glycoinositolphospholipids from Trypanosoma cruzi: Assignments of the T-cell suppressive determinant to the ceramide domain. J Immunol. 1996;156:628–635. [PubMed] [Google Scholar]