Abstract
Cardiac vagal neurons (CVNs) in the nucleus ambiguus (NA) are the major determinant of parasympathetic activity to the heart. Spontaneous GABAergic neurotransmission to CVNs is modulated by hypothalamic neuropeptide orexin-A in postnatal days 2-5 (P5) rats, however during early postnatal development orexin expression changes and the role of orexin-A in modulating CVN activity at other stages of development is unknown. In this study we compared changes in GABAergic inhibitory postsynaptic currents (IPSCs) in CVNs evoked by orexin-A in P5, P16-20 (P20), and P27-30 (P30) rats using an in-vitro brain stem slice preparation. Bath-applied orexin-A enhanced GABAergic IPSCs in all CVNs tested in P5 and P30 animals and in the majority of neurons tested in P20 pups. Focal application of orexin-A ejected from a pipette positioned within 30 μm of the patched CVN did not alter GABAergic signaling in P5 pups. In contrast, in both P20 and P30 rats focal application of orexin-A inhibited GABAergic IPSCs and this inhibition persisted in the presence of tetrodotoxin. These results indicate orexin-A facilitates GABAergic IPSCs likely by activating preceding GABAergic neurons that project to CVNs. Orexin-A also likely acts at GABAergic presynaptic terminals surrounding CVNs within the NA to inhibit GABA release. The latter mechanism is absent in P5 pups but occurs in P20 and P30 rats. In conclusion, this study elucidates an important maturation of the parasympathetic cardiac control system. Alterations in these developmental mechanisms may play a role in pathogenesis of disorders related to a specific stage of development maturation.
Keywords: orexin-A, cardiac vagal neurons, brainstem, development
Parasympathetic control of heart rate undergoes significant alterations after birth. Although parasympathetic innervation of the heart is functional in newborn rats and vagally-mediated cardio-inhibitory reflexes are present in postnatal days (P) 5/6, tonic cardiac vagal motor activity only reaches a mature level between the second and third weeks of postnatal age in rat pups (Adolph, 1967; Tucker and Johnson, 1984; Kasparov and Paton, 1997). Immature and/or altered autonomic functions have been implicated in some disorders prevalent at specific postnatal ages, such as sudden infant death syndrome (SIDS) (Leistner et al., 1980; Kluge et al., 1988; Schechtman et al., 1988; Southall et al., 1988; Hunt, 2006). It is of interest that SIDS incidence peaks during a narrow developmental time window, specifically between 2 and 4 month of infant age, which represents an unstable time for many physiologic systems (Willinger et al., 1991; Filiano and Kinney, 1994). However, little information is known regarding changes in synaptic function underlying the maturation of parasympathetic cardiac control.
Parasympathetic activity regulating heart rate and cardiac function primary originates from preganglionic cardiac vagal neurons (CVNs) within the nucleus ambiguus (NA) (Mendelowitz, 1999; Cheng and Powley, 2000; Wang et al., 2001a; Wang et al., 2001b). Focal electrical or chemical stimulation of the NA region elicits decreases in heart rate mediated by the activation of CVNs (Ciriello and Calaresu, 1982; Ciriello and de Oliveira, 2003). These neurons are intrinsically silent, and recent studies have identified major synaptic pathways including activation of GABAergic, glycinergic, and glutamatergic postsynaptic receptors in CVNs (Mendelowitz, 1998; Neff et al., 1998; Wang et al., 2001b).
The hypothalamic orexin-A and orexin-B neuropeptides, also called hypocretin-1 and hypocretin-2, play an important role in postnatal development influencing several aspects of brain maturation (Stoyanova et al., 2009). Orexins are expressed as early as embryonic days 18-20 in rats (de Lecea et al., 1998; Van Den Pol et al., 2001). Furthermore, postnatal expression of orexins undergoes significant changes. Orexin mRNA in hypothalamic tissue is detectable by the first day of birth, followed by an increase to the maximum at P20, and finally no significant differences exist between the adolescent and adult (Van Den Pol et al., 2001; Steininger et al., 2004). A transient increase of orexin expression is also observed around P20 in the rat brainstem (Volgin et al., 2002).
Compelling evidence links orexin-A to regulation of cardiovascular function (Ciriello and de Oliveira, 2003; Ciriello et al., 2003; Dergacheva et al., 2005; Dergacheva et al., 2011). Orexin fibers have been found in nuclei that are well known to be involved in cardiovascular regulation (Peyron et al., 1998; Ciriello and de Oliveira, 2003; Ciriello et al., 2003). Intracisternal or intrathecal administration of orexin-A as well as microinjection of orexin-A into the rostral ventral medulla and the nucleus tractus solitarius complex increases mean arterial pressure and heart rate in rats (Chen et al., 2000; Antunes et al., 2001; Smith et al., 2002). We have previously shown orexin-A strongly modulates the spontaneous GABAergic neurotransmission to CVNs and the GABAergic neurotransmission evoked by stimulation of the lateral paragigantocellular nucleus (LPGi) in P2-5 (P5) rats (Dergacheva et al., 2005; Dergacheva et al., 2011). In this study we examined changes in the maturation of central parasympathetic control, and more specifically, we compared changes in GABAergic neurotransmission to CVNs evoked by orexin-A in P5, P16-20 (P 20), and P27-30 (P30) rats.
Experimental Procedures
All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and any possible discomfort.
To identify CVNs in vitro a two-stage procedure was used. In an initial surgery, Sprague-Dawley rats (postnatal days 1–3; Hilltop, Scottdale, PA, USA) were anesthetized with hypothermia and received a right thoracotomy. The heart was exposed, and 0.05 ml of 1– 5% rhodamine (XRITC, Molecular Probes, Eugene, OR, USA) was injected into the pericardial sac to retrogradely label CVNs. On the day of experiment P5 and P20 animals were anesthetized with isoflurane and killed by rapid cervical dislocation. Using a dissection microscope the cerebellum was removed and the hindbrain was isolated. A single slice of the medulla (300 μm thickness) that included the NA was obtained and submerged in a recording chamber, which allowed perfusion (5–10 ml/min) of artificial cerebrospinal fluid (aCSF) at room temperature (25 °C) containing (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, and 5 HEPES equilibrated with 95% O2 and 5% CO2 (pH 7.4).
To obtain viable CVNs from juvenile (P30) animals we have adopted the methodology by Ye and colleagues (Ye et al., 2006). The key to this approach is using glycerol based aCSF (GaCSF), in which all NaCl is replaced with glycerol, an endogenously occurring three-carbon alcohol. GaCSF contained (in mM): 252 glycerol, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl, 2.4 CaCl2, 26 NaHCO3, and 11 glucose. GaCSF was perfused transcardially before sacrifice. A single slice of the medulla (300 μm thickness) that included the NA was obtained and transferred from the GaCSF to normal aCSF for at least one hour before experiments were conducted, and previous work has shown this approach preserves the electrophysiological and pharmacological properties in many types of neurons (Ye et al., 2006).
Individual CVNs in the NA were identified by the presence of the fluorescent tracer. These identified CVNs were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 MΩ) were filled with a solution consisting (in mM) of 150 KCl, 2 MgCl2, 2 EGTA, 10 HEPES, and 2 Mg-ATP, pH 7.4. With this pipette solution, the chloride current induced by activation of GABA receptors was recorded as an inward current. Voltage clamp whole-cell recordings were made at a holding potential of −80 mV with an Axopatch 200B and pClamp 8 software (Axon Instruments, Union City, CA, USA).
GABAergic inhibitory postsynaptic currents (IPSCs) were isolated by continuous focal application of strychnine (1 μM), d-2-amino-5-phosphonovalerate (50 μM), and 6-cyano-7-nitroquinoxaline-2,3-dione (50 μM) to block glycine, N-methyl-d-asparate, and non–N-methyl-d-asparate glutamatergic receptors, respectively. Drugs were focally released using a picrospritzer and pressure ejected from a patch pipette positioned within 30 μm of the patched CVN. The maximum range of drug application was determined previously to be 100–120 μm downstream from the drug pipette and was considerably less behind the drug pipette (Wang et al., 2002). Continual focal drug applications were performed using a pneumatic picopump pressure system (WPI, Sarasota, FL, USA). Other drugs used in this study included orexin-A (0.01, 0.1, and 1 μM), the orexin-1 receptor antagonist SB-334867 (10 μM), and tetrodotoxin (1 μM). After the end of each experiment the GABAergic IPSCs was abolished by application of gabazine (25 μM). Orexin-A and SB-334867 were purchased from Tocris Bioscience (Ellisville, MO, USA). All other drugs used in this study were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Analysis of action potential-dependent IPSCs and tetrodotoxin-insensitive miniature IPSCs (mIPSCs) was performed using MiniAnalysis (version 5.6.12; Synaptosoft, Decatur, GA, USA) with a threshold determined by the amplitude at which all synaptic events were blocked in the presence of gabazine (25 μM). Spontaneous GABAergic IPSC amplitude and frequency was analyzed from 200-second periods prior and during drug applications (orexin-A and/or SB-334867) and upon washout of orexin-A. Results are presented as mean ± SE. To test for differences in GABAergic IPSC amplitude and frequency between P5, P20, and P30 animals, the results were statistically compared using ANOVA and Kruskal-Wallis non-parametric test followed by Dunns post-test. To test for changes in IPSC amplitude and frequency evoked by orexin-A and/or SB-334867 as well as concentration-dependent effects of orexin-A the results were statistically compared using one-way repeated measures ANOVA and Dunnett's posttest. Amplitude and frequency distributions in single neurons were performed using the Kolmogorov-Smirnov test. Significant difference was set at p< 0.05. Only one experiment was performed in each preparation except experiments examining dose-dependent response of orexin-A when two doses of orexin-A were applied sequentially starting with the smallest concentration.
Results
There were no statistically significant differences (p>0.05) in either the amplitude or frequency of GABAergic IPSCs in CVNs between P5, P20, and P30 animals.
Effects of bath application of orexin-A on GABAergic IPSCs in CVNs
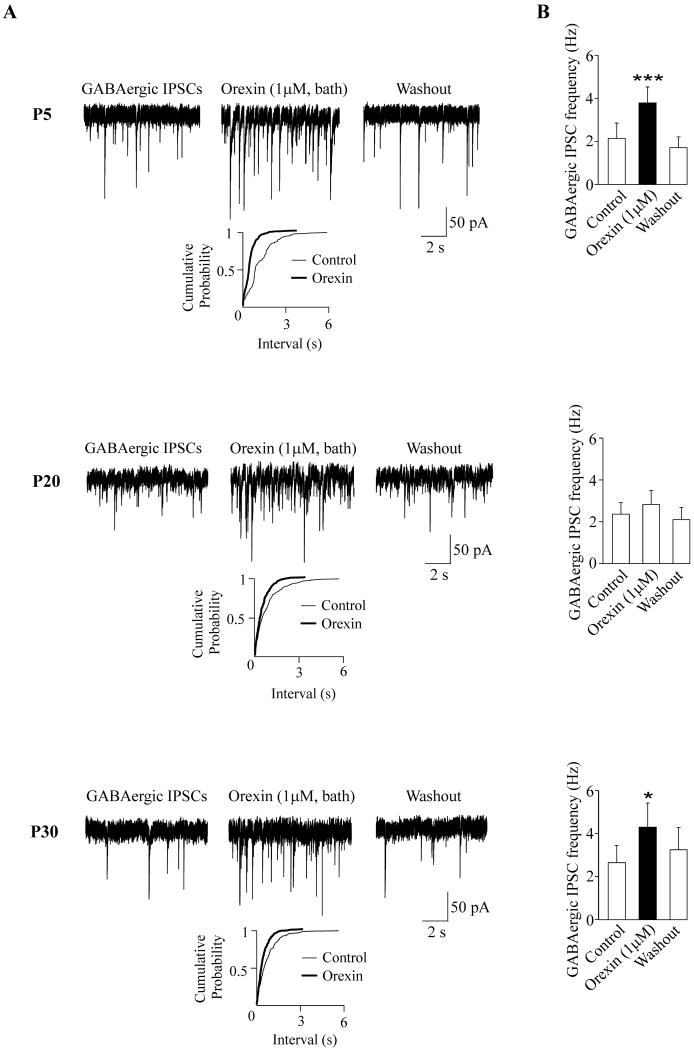
In agreement with previously published data (Dergacheva et al., 2005) bath application of orexin-A (1 μM) elicited a significant and reversible increase in the frequency of GABAergic IPSCs in CVNs in all neurons tested in P5 rats (from 2.1± 0.7 Hz to 3.8±0.7; p<0.001, n=8, see Fig. 1). In P20 pups bath-applied orexin-A (1 μM) evoked facilitation in the frequency of GABAergic IPSCs in the majority of CVNs (7 out of 10). The responses also included inhibition (n=2) of GABAergic IPSCs. In one CVN no significant alteration was observed in the presence of orexin-A (1 μM). Thus, upon statistic analysis of all 10 neurons combined orexin-A evoked no significant change in the frequency of GABAergic IPSCs in CVNs (2.4±0.6 Hz versus 2.8 ±0.7 Hz; p>0.05, see Fig. 1). The CVN responses to bath-applied orexin-A (1 μM) in P30 pups were similar to those observed in P5 pups. In P30 pups, bath application of orexin-A evoked a significant and reversible increase in the frequency of GABAergic IPSCs in all CVNs tested (from 2.7± 0.8 Hz to 4.3±1.1; p<0.05, n=8, see Fig. 1). Orexin-A (1 μM) had no effect on the amplitude of the GABAergic IPSCs in P5, P20, or P30 animals (see Table 1).
Figure 1.
Bath application of orexin-A elicited an alteration in the frequency of GABAergic IPSCs in CVNs at all ages (P5, P20 and P30) studied. Typical experiments along with cumulative fraction plots are shown in A whereas the summary data are illustrated in B. Orexin-A evoked a significant and reversible increase in the GABAergic IPSC frequency in all neurons tested in both P5 (n=8, top) and P30 (n=8, bottom) animals. However, at P20 the CVN responses were variable (middle). Although there was increase in GABAergic IPSC frequency in the majority of neurons tested (7 out of 10) following orexin-A application, but orexin-A evoked a decrease in 2 CVNs and no change in 1 CVN in P20 animals. Asterisks indicates statistically significant differences, * P < 0.05, and ***P < 0.001.
Table 1.
The mean values of the GABAergic IPSC amplitude (in pA) are shown before, during, and after orexin-A (1 μM) applications. Neither bath nor focal application of orexin-A (1 μM) significantly changed (P>0.05) the amplitude of IPSCs in P5, P20, or P30 animals.
| Experimental groups | Control | Orexin-A (1 μM) | Washout | |||
|---|---|---|---|---|---|---|
| Bath | Focal | Bath | Focal | Bath | Focal | |
| P5 | 51.2±5.6 | 54.2±7.4 | 64.1±10.4 | 53.2±6.6 | 51.4±3.8 | 53.5±6.7 |
| P20 | 50.4±5.2 | 56.5±5.4 | 47.8.±5.0 | 54.1±5.4 | 52.0±5.9 | 55.2±6.2 |
| P30 | 56.6±5.2 | 54.4±8.6 | 51.6±4.5 | 53.1±8.9 | 54.3±6.8 | 53.5±9.0 |
Effects of focal application of orexin-A on GABAergic IPSCs in CVNs
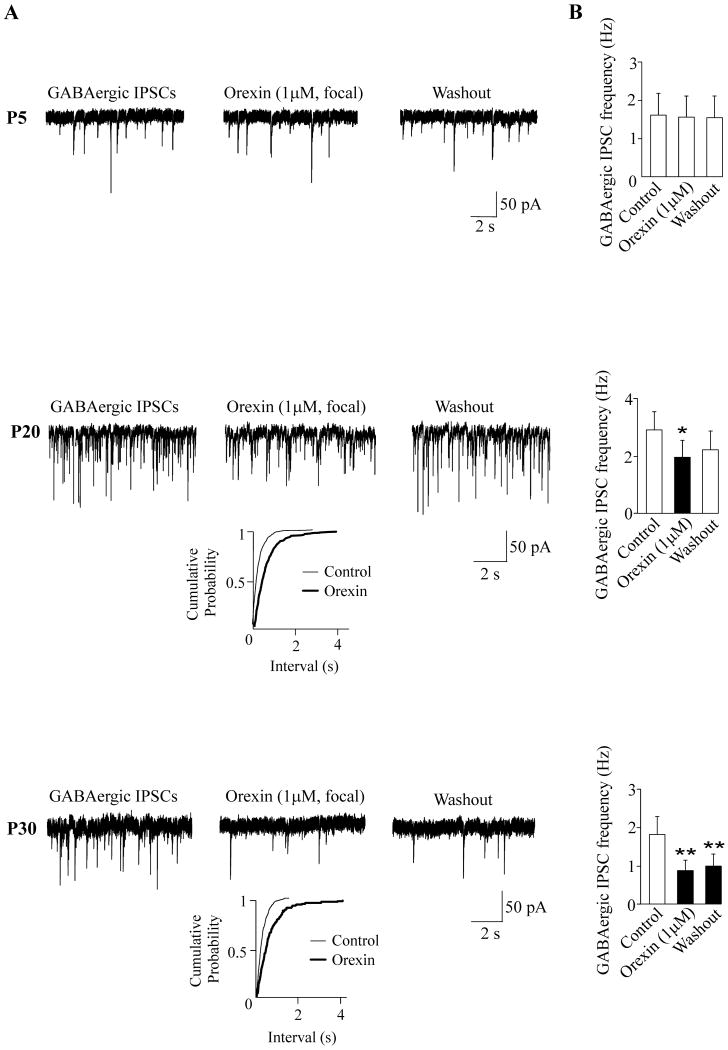
To test if orexin-A can affect GABAergic signaling in CVNs by activating sites located within the NA, focal application of orexin-A was performed in additional experiments. In contrast to the results upon bath application, focal application of orexin-A (1 μM) ejected from a pipette positioned within 30 μm of the patched CVN did not significantly alter the frequency of GABAergic IPSCs in CVNs in P5 animals (1.6±0.6 Hz versus 1.6 ±0.6 Hz; p>0.05, n=8, Fig. 2). However, in P20 pups focal application of orexin-A (1 μM) elicited a significant decrease in the frequency of GABAergic IPSCs in CVNs (from 2.9± 0.6 Hz to 2.0±0.6; p<0.05, n=8, see Fig. 2). In P30 pups focally-applied orexin-A (1 μM) elicited a significant inhibition in the frequency of GABAergic IPSCs (from 1.8±0.5 Hz to 0.8±0.3; p<0.01, n=8, Fig. 2). Focal application of orexin-A (1 μM) had no effect on the amplitude of the GABAergic IPSCs in CVNs in P5, P20, or P30 animals (see Table 1).
Figure 2.
In contrast to bath application, focally applied orexin-A did not alter the frequency of GABAergic GABAergic IPSCs in CVNs in P5 pups (n=8, top). However, focal application of orexin-A elicited a significant inhibition in the frequency of GABAergic GABAergic IPSCs in CVNs in both P20 (n=8, middle) and P30 (n=8, bottom) animals. Typical experiments along with cumulative fraction plots are shown in A whereas the summary data are illustrated in B. While the inhibition of GABAergic neurotransmission was reversible in the most of neurons in P20 pups (middle), the frequency of GABAergic IPSCs remained diminished during 10-min washout period in P30 animals (bottom). Asterisks indicates statistically significant differences, * P < 0.05, and **P < 0.01.
GABAergic mIPSCs in CVNs and orexin-A
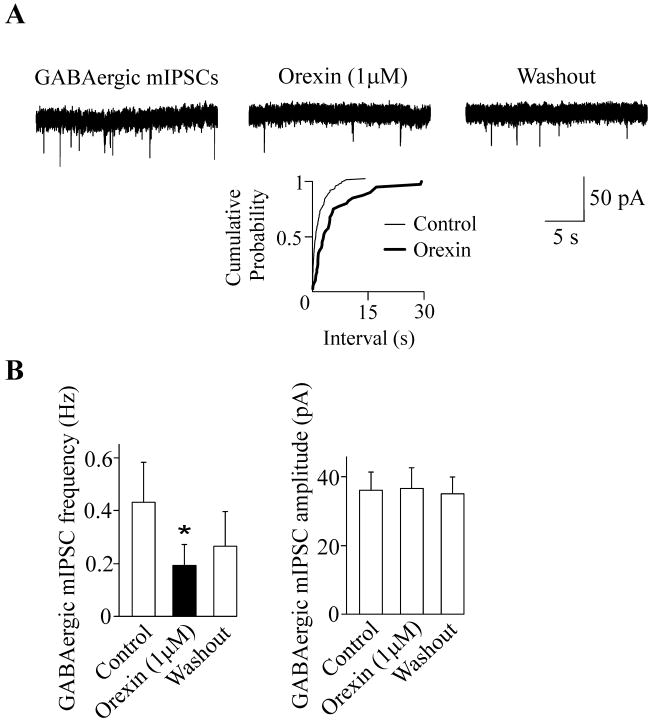
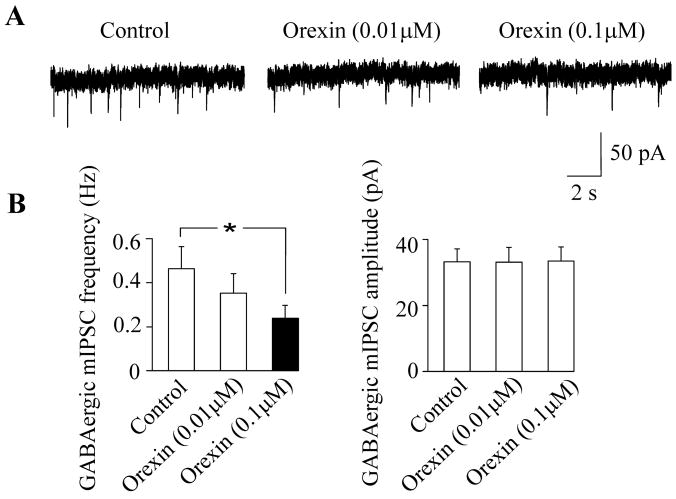
To further elucidate the cellular mechanisms of differential effects of bath and focal applications of orexin-A we examined the effect of orexin-A on mIPSCs in CVNs in the presence of tetrodotoxin, a voltage-gated sodium channel blocker. We have previously shown that orexin-A has no effect on GABAergic signaling in the presence of tetrodotoxin (1 μM) in P5 animals (Dergacheva et al., 2005). Since the effect of focal application of orexin-A on GABAergic IPSCs in CVNs was similar in P20 and P30 animals (Fig. 2) but more robust in P30 animals, we examined the effect of orexin-A on mIPSCs in CVNs exclusively in P30 animals. Unlike in P5 pups, in the presence of tetrodotoxin (1 μM) orexin-A (1 μM) elicited a decrease in the frequency of GABAergic mIPSCs in all CVNs tested in P30 pups (from 0.4±0.2 Hz to 0.2±0.08; p<0.05, n=8, Fig. 3). In additional sets of experiments we applied orexin-A at concentrations of 0.01 μM and 0.1 μM in the presence tetrodotoxin (1 μM) to examine the dose response relationship of orexin-A. At a concentration of 0.01 μM orexin-A had no significant effect of GABAergic mIPSCs in CVNs (see Fig. 4). Subsequent application of orexin-A on the same CVNs at a concentration of 0.1 μM elicited a significant inhibition in the frequency of GABAergic mIPSCs (from 0.5 ±0.1 Hz to 0.2±0.06; p<0.05, n=7, Fig. 4).
Figure 3.
In the presence of tetrodotoxin (1 μM), orexin-A (1 μM) elicited a significant decrease in the mIPSC frequency in P30 animals. However, the amplitude of the GABAergic mIPSCs was not altered by orexin-A application. A typical experiment along with cumulative fraction plot is shown in A whereas the summary data from 8 neurons are illustrated in B. Asterisk indicates a statistically significant difference, * P < 0.05.
Figure 4.
Bath-applied orexin-A altered the frequency of GABAergic mIPSCs in a dose-dependent manner. At a concentration of 0.01 μM orexin-A did not evoke a significant alteration in GABAergic mIPSC frequency. However, the frequency of mIPSCs was significantly decreased following orexin-A application at a concentration of 0.1 μM. The amplitude of GABAergic mIPSCs was not altered by orexin-A application at either concentration of 0.01 μM or 0.1 μM. Representative traces of GABAergic mIPSCs under control conditions and during orexin-A applications are shown in A whereas the summary data from 7 neurons are illustrated in B. Asterisk indicates a statistically significant difference, * P < 0.05.
GABAergic IPSCs in CVNs and orexin-1 receptor antagonist
To test if the effect of orexin-A was selectively mediated via orexin-1 receptors and to eliminate the possibility of non-orexin-1 receptor activation, SB-334867, an orexin-1 receptor antagonist, was used prior to and during orexin-A administration. In the absent of tetrodotoxin, bath application of SB-334867 at a concentration of 10 μM by itself did not evoke any significant change in either frequency or amplitude of GABAergic IPSCs in P30 pups (1.5±0.4 Hz versus 1.4 ±0.4 Hz and 50.5±4.7 pA versus 52.0±6.4 pA, respectively; p>0.05, n=8, Fig. 5). However, SB-334867 blocked the orexin-A-elicited alteration of GABAergic IPSCs since in the presence of SB-334867 (10 μM) orexin-A (1 μM) failed to significantly alter the frequency of GABAergic IPSCs in CVNs in P30 pups (1.5±0.4 Hz versus 1.3 ±0.5 Hz; p>0.05, n=8, Fig. 5).
Figure 5.
The orexin-1 receptor antagonist SB-334867 did not alter either frequency or amplitude of GABAergic IPSCs in CVNs. However, SB-334867 prevented the orexin-A-elicited alteration in the GABAergic IPSCs in CVNs as in the presence of SB-334867 orexin-A did not evoke any significant changes in the frequency of GABAergic IPSCs. A typical experiment is shown on the top (A) while the summary data from 8 neurons are demonstrated in the bottom (B).
Discussion
The results from this study indicate bath-applied orexin-A (1 μM) increased GABAergic IPSCs in all CVNs tested in P5 and P30 animals and in the majority of neurons tested in P20 animals. In contrast, focal application of orexin-A did not alter GABAergic IPSCs in P5 pups but decreased GABAergic IPSCs in both P20 and P30 rats. In addition, orexin-A evoked inhibition of GABAergic mIPSC frequency in P30 animals.
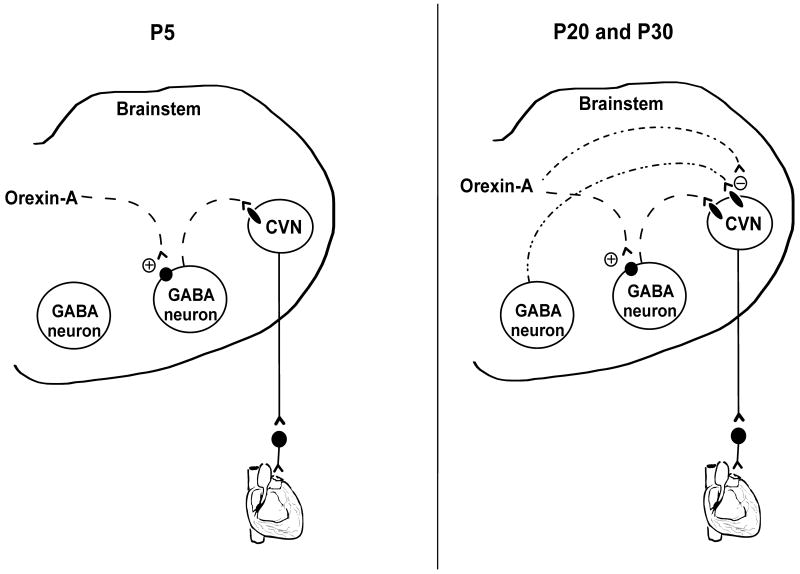
Although bath-applied orexin-A evokes varied responses in P20 pups, most of the responses (7 out of 10) include the facilitation of GABAergic IPSCs which are similar to those elicited in P5 and P30 animals. The transient variety of responses in P20 animals possibly reflects the developmental changes of parasympathetic cardiac control that occur in P20 animals. In other in-vitro studies, orexin has been shown to elicit heterogeneous responses to the same population of neurons including both increases and decreases in the GABAergic IPSC frequency (Van Den Pol et al., 1998; Burlet et al., 2002; Liu et al., 2002). We have previously shown the effect of bath-applied orexin-A on GABAergic IPSCs in P5 animals is prevented by tetrodotoxin (Dergacheva et al., 2005) indicating bath-applied orexin-A alters GABAergic IPSCs in CVNs in an action potential dependent manner and likely acts on preceding GABAergic neurons that project to CVNs (see Fig. 6, left). These preceding GABAergic neurons are likely located in the rostral ventral medulla, including the LPGi, as GABAergic neurotransmission to CVNs evoked by electrical stimulation of LPGi neurons is increased by orexin-A (Dergacheva et al., 2011).
Figure 6.
A proposed model for orexin-A modulation of GABAergic neurotransmission to CVNs in P5, P20 and P30 rats. Bath applied orexin-A facilitates GABAergic IPSCs in CVNs likely via activation of GABAergic neurons in the rostral ventral medulla that project to CVNs (left). This mechanism is also present in P20 and P30 animals (right). In addition, orexin-A inhibits GABAergic neurotransmission likely acting on presynaptic terminals surrounding CVNs in P20 and P30 rats (right), but not in P5 pups.
In contrast to bath application, focal application of orexin-A does not alter GABAergic IPSCs in P5 pups, supporting the hypothesis that orexin-A acts on neurons that located outside of the NA at this development age. However, in both P20 and P30 animals focal application of orexin-A inhibits GABAergic IPSCs. In addition, orexin-A dose-dependently inhibits GABAergic mIPSC frequency in the presence of tetrodotoxin in P30 animals. These data indicate that orexin-A, in addition to acting on GABAergic neurons that are outside of the NA, also may act at GABAergic presynaptic terminals surrounding CVNs within the NA to inhibit GABA release (Fig. 6, right). This latter mechanism is absent in P5 pups but occurs in P20 and P30 rats. Thus, the effects of orexin-A on GABAergic IPSCs in CVNs appear to be complex in older rats depending on which pathways and receptors are activated. It is possible orexin-A differentially acts on different populations of GABAergic neurons to evoke either inhibition or facilitation of GABAergic IPSCs in CVNs under different conditions and network responses (Fig. 6). The results from other studies suggest orexinergic neurons exert complex and opposing effects within the same site within the central nervous system, and the responses may depend on specific condition and/or physiological state. Indeed, the effect of orexin-A microinjected 1mm dorsal to the right lateral ventricle on food intake is both dose and time dependent (Rodgers et al., 2000). The results from in-vivo studies in adult rats indicate orexin-A can exert diverse physiological effects on brainstem parasympathetic cardiac activity. Microinjection of orexin-A into the rostral ventral medulla produces a tachycardia mediated in part by inhibition of parasympathetic activity to the heart (Ciriello et al., 2003) whereas microinjection of orexin-A into the NA elicits bradycardia mediated by excitation of parasympathetic activity to the heart (Ciriello and de Oliveira, 2003). The results from this study provide a possible explanation for these diverse in-vivo results. Bath-applied orexin-A facilitates GABAergic IPSCs in CVNs likely via activation of preceding GABAergic neurons that project to CVNs. Thus, tachycardia evoked by orexin-A microinjection into the rostral ventral medulla observed in vivo studies (Ciriello et al., 2003) is likely mediated by activation of GABAergic neurons in the rostral ventrolateral medulla that project to and inhibit CVNs in the NA. In accordance with this hypothesis, other in-vivo studies indicate that microinjection of orexin-A into the rostral ventral medulla causes an increase in both mean arterial pressure and heart rate (Machado et al., 2002), whereas no effect is observed in vagotomized rats (Young et al., 2005). In contrast, focal application of orexin-A can excite CVNs via disinhibition of GABAergic IPSCs in CVNs. This would be consistent with the bradycardia evoked upon microinjection of orexin-A into the NA (Ciriello and de Oliveira, 2003).
The results from this study indicate orexin-1 receptor antagonist SB-334867 by itself does not evoke any significant change in GABAergic neurotransmission to CVNs suggesting that there is no endogenous release of orexin-A within the slice preparation used in this study. This observation is consistent with the location of orexinergic neurons which have been exclusively found in the perifornical area and lateral hypothalamus but not in the medulla oblongata (de Lecea et al., 1998; Sakurai et al., 1998). However, SB-334867 blocked the orexin-A-elicited alteration of GABAergic IPSCs indicating the effect of orexin-A on GABAergic neurotransmission to CVNs is selectively mediated via orexin-1 receptors. Consistently, orexin-A-labeled axons and presumptive axonal terminals have been found in the rostral ventral medulla and within the ventral aspects of the NA where CVNs are located (Ciriello and de Oliveira, 2003; Ciriello et al., 2003).
Interestingly, the development of the orexinergic system occurs with a similar time course to the changes in orexinergic modulation of CVNs. Orexinergic levels are very low at parturition and begin to increase at P13 until P26 in rats, at which point no further changes occur from the adolescent to adult (Steininger et al., 2004). Tonic parasympathetic control of heart rate also reaches mature levels between the second and third weeks of postnatal age in rat pups with no additional changes from the adolescent to adult (Adolph, 1967; Tucker and Johnson, 1984; Kasparov and Paton, 1997). It is possible that the alteration in orexinergic modulation of CVN activity elucidated in this study play a role in the maturation of tonic cardiac vagal motor activity in rats. In contrast to P5 pups, orexin-A has an inhibitory effect on GABAergic IPSCs in P20 and P30 animals. This inhibition of GABAergic IPSCs would result in disinhibition of CVNs and activation of parasympathetic activity to the heart which may contribute to the maturation of tonic parasympathetic control of heart rate between the second and third weeks of postnatal age.
Conclusion
This study elucidates an important development differences in orexinergic modulation of CVN activity between P5 and older P20 and P30 rats.
Orexinergic modulation of cardiac vagal neuron activity changes during maturation
Orexin-A enhances GABAergic neurons that project to cardiac vagal neurons
In older animals orexin-A inhibits GABA release
Acknowledgments
This work was supported by NIH grants HL 59895, HL 49965, and HL 72006 to D.M.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CVNs
cardiac vagal neurons
- GaCSF
glycerol based artificial cerebrospinal fluid
- IPSCs
inhibitory postsynaptic currents
- LPGi
lateral paragigantocellular nucleus
- mIPSCs
miniature inhibitory postsynaptic currents
- NA
nucleus ambiguus
- SIDS
sudden infant death syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolph EF. Ranges of heart rates and their regulations at various ages (rat) Am J Physiol. 1967;212:595–602. doi: 10.1152/ajplegacy.1967.212.3.595. [DOI] [PubMed] [Google Scholar]
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 2000;424:588–606. [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Medullary origin of vagal preganglionic axons to the heart of the cat. J Auton Nerv Syst. 1982;5:9–22. doi: 10.1016/0165-1838(82)90086-8. [DOI] [PubMed] [Google Scholar]
- Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1611–1620. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Philbin K, Bateman R, Mendelowitz D. Hypocretin-1 (orexin A) prevents the effects of hypoxia/hypercapnia and enhances the GABAergic pathway from the lateral paragigantocellular nucleus to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011;175:18–23. doi: 10.1016/j.neuroscience.2010.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Hunt CE. Ontogeny of autonomic regulation in late preterm infants born at 34-37 weeks postmenstrual age. Semin Perinatol. 2006;30:73–76. doi: 10.1053/j.semperi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JF. Changes in baroreceptor vagal reflex performance in the developing rat. Pflugers Arch. 1997;434:438–444. doi: 10.1007/s004240050418. [DOI] [PubMed] [Google Scholar]
- Kluge KA, Harper RM, Schechtman VL, Wilson AJ, Hoffman HJ, Southall DP. Spectral analysis assessment of respiratory sinus arrhythmia in normal infants and infants who subsequently died of sudden infant death syndrome. Pediatr Res. 1988;24:677–682. doi: 10.1203/00006450-198812000-00005. [DOI] [PubMed] [Google Scholar]
- Leistner HL, Haddad GG, Epstein RA, Lai TL, Epstein MA, Mellins RB. Heart rate and heart rate variability during sleep in aborted sudden infant death syndrome. J Pediatr. 1980;97:51–55. doi: 10.1016/s0022-3476(80)80129-6. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Van Den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Nicotine excites cardiac vagal neurons via three sites of action. Clin Exp Pharmacol Physiol. 1998;25:453–456. doi: 10.1111/j.1440-1681.1998.tb02233.x. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Blundell JE. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96:71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11:413–424. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- Southall DP, Stevens V, Franks CI, Newcombe RG, Shinebourne EA, Wilson AJ. Sinus tachycardia in term infants preceding sudden infant death. Eur J Pediatr. 1988;147:74–78. doi: 10.1007/BF00442617. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Kilduff TS, Behan M, Benca RM, Landry CF. Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain. J Chem Neuroanat. 2004;27:165–181. doi: 10.1016/j.jchemneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Stoyanova II, Rutten WL, le Feber J. Orexin-A and orexin-B during the postnatal development of the rat brain. Cell Mol Neurobiol. 2009;30:81–89. doi: 10.1007/s10571-009-9433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DC, Johnson AK. Development of autonomic control of heart rate in genetically hypertensive and normotensive rats. Am J Physiol. 1984;246:R570–577. doi: 10.1152/ajpregu.1984.246.4.R570. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmenl expression and response to hypocretin (orexin) J Comp Neurol. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18(19):7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res. 2001a;889:78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Synaptic activation of hypoglossal respiratory motorneurons during inspiration in rats. Neurosci Lett. 2002;332:195–199. doi: 10.1016/s0304-3940(02)00957-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001b;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11:677–684. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]